Abstract
Cognitive impairment is a prevailing event in hypertensive patients and in frail older adults. Endothelial dysfunction has been shown to underlie both hypertension and cognitive dysfunction. Our hypothesis is that L-Arginine, which is known to ameliorate endothelial dysfunction, could counteract cognitive impairment in a high-risk population of hypertensive frail older adults. We designed a clinical trial to verify the effects of 4-weeks oral supplementation of L-Arginine on global cognitive function of hypertensive frail older patients. The study was successfully completed by 35 frail hypertensive elderly patients assigned to L-Arginine and 37 assigned to placebo. At follow-up, we found a significant difference in the Montreal Cognitive Assessment (MoCA) test score between the L-Arginine treated group and placebo (p: 0.0178). Moreover, we demonstrated that L-Arginine significantly attenuates Angiotensin II-induced mitochondrial oxidative stress in human endothelial cells. In conclusion, our findings indicate for the first time that oral L-Arginine supplementation significantly improves cognitive impairment in frail hypertensive older adults.
Clinical Trial Registration
www.ClinicalTrials.gov, identifier: NCT04962841.
Keywords: cardiac rehabilitation, endothelial (dys)function, L-Arg, L-Arginine, frail adults, frailty, cognitive impairment
Background
Hypertension is linked to endothelial dysfunction contributing to atherosclerosis, inflammation, and oxidative stress in the arterial wall (1–8). Physical frailty (hereafter defined as frailty) is a biological syndrome of decreased physiological reserves with increased susceptibility to stressors; stressors are classified as acute or chronic diseases that lead frail patients to mortality, hospitalization, disability, functional and cognitive impairment (9–15). In this scenario, hypertension is considered a common stressor; additionally, frail older adults display endothelial dysfunction as a consequence of the aging process; therefore, frailty and hypertension synergistically increase the risk of adverse events (16–18).
Cognitive impairment is often observed in hypertensive patients as well as in frail older adults (19–21). Hence, tackling cognitive impairment is crucial in order to delay and/or prevent adverse events, complications, and death. We and others have demonstrated that endothelial dysfunction is present in patients with hypertension and cognitive impairment (6, 22–25).
L-Arginine is an amino acid involved in a number of biological processes and is a substrate of two enzymes: nitric oxide (NO) synthase (NOS) and arginase (NOA) (26–28). L-Arginine is fundamental for NO production by endothelial cells, regulating vascular tone and cardiovascular homeostasis (28–32). We hypothesized that L-Arginine could counteract cognitive impairment in a high-risk population such as frail older adults with hypertension. To test this hypothesis, we designed a study to investigate the effects of 4-weeks supplementation of L-Arginine on global cognitive function in hypertensive frail older adults.
Methods
We designed a placebo-controlled clinical trial to study hypertensive frail older patients presenting from March 2021 to October 2021 at ASL (local health unit of the Italian Ministry of Health) Avellino, Italy. All of them met the following inclusion criteria: a previous diagnosis of primary hypertension (with no clinical or laboratory evidence of secondary causes); age >65 years; a frailty status; Montreal Cognitive Assessment (MoCA) test score <26.
Exclusion Criteria were: age <65 years; presence of neurodegenerative diseases; absence of frailty status; absence of hypertension; left ventricular ejection fraction <25%, with previous myocardial infarction or previous coronary revascularization.
Patients were randomly assigned to the L-Arginine (Bioarginina®, 1.66 g, twice a day) or placebo (n = 37) parallel groups and followed-up for 4-weeks. The dose of L-Arginine was based on previously published clinical trials (33, 34).
Assessment of Cognitive Function
Global cognitive function was assessed using the MoCA test, with scores ranging from 0 to 30 (lower scores indicate cognitive impairment), as we previously described (35); this cognitive test covers the main cognitive areas: immediate and delayed memory (free and cued recall), language, visuospatial and visuoperceptual capacities, motor planning, executive function, attention, and cognitive judgment (36–38).
Frailty Evaluation
A physical frailty assessment was performed following the Fried Criteria (11) as we previously described (35); a diagnosis of frailty status was made with at least three points out of the following five:
- Weight loss (unintentional loss of ≥4.5 kg in the past year).
- Weakness (handgrip strength).
- Exhaustion (poor endurance and energy, self-reported).
- Slowness (walking speed under the lowest quintile).
- Low physical activity level (lowest quintile of kilocalories of physical activity during the past week).
Cell Culture and Mitochondrial Reactive Oxygen Species (ROS) Detection
Human umbilical vein endothelial cells (HUVECs) were cultured in EGM-2 medium (Lonza, CC4147) and incubated at 37°C and 5% CO2 (39). Experiments on HUVECs were performed at passages 3–7. After reaching a 60–70% confluency, HUVECs were plated on glass bottom culture dishes and treated with Angiotensin II (Ang II) (Merck, 05-23-0101, 1 μM) and Ang II with L-Arginine (Fisher BioReagents, BP372-100, 500 μM) in EGM-2 medium for 24 h. ROS generation was quantified using MitoSOX™ Red (Molecular Probes Inc, M36008), incubating cells for 10 min at 37°C and 5% CO2, as we previously described (6, 40).
Study Approval
Informed consent was obtained by all patients before testing, and the experimental protocol was approved by the Ethical Committee of Campania Nord. The trial was registered in clinicaltrials.gov (NCT04962841).
Statistical Analysis
Data are presented as group mean ± SD or SE or numbers and percentages. Based on our preliminary findings, we calculated the minimum number of patients required for the study to reject the null hypothesis 95% of the time using G*POWER. A multivariable linear regression analysis, while adjusting for likely confounders, was used to investigate the association between L-Arginine treatment and MoCA score. Statistical significance was determined by a p value <0.05. All calculations were performed using SPSS 26 (IBM, Armonk, NY).
Results
Baseline Characteristics of Our Study Population
Seventy two frail hypertensive elderly patients, randomly assigned to the L-Arginine (n = 35) or placebo (n = 37) parallel groups, successfully completed the study (Figure 1). Their anthropometric features are described in Table 1. There were no significant differences in the mean age, BMI, and sex distribution between the two groups (Table 1). The use of diuretics, angiotensin-converting enzyme inhibitors, beta-blockers, and calcium-channel blockers was similar between the two groups (Table 1).
Figure 1.
Flow chart of the study.
Table 1.
Baseline clinical characteristics of our population.
| Parameter | L-Arginine | Placebo | |
|---|---|---|---|
| N | 35 | 37 | |
| Age (years) | 78.0 ± 6.6 | 77.0 ± 4.9 | |
| Female sex, n (%) | 21 (60) | 23 (62.1) | |
| BMI (kg/m2) | 29.1 ± 3.4 | 29.3 ± 3.6 | |
| SBP (mmHg) | 132.8 ± 10.9 | 129.2 ± 11.8 | |
| DBP (mmHg) | 82.0 ± 9.4 | 79.2 ± 6.3 | |
| Heart rate (bpm) | 73.7 ± 9.1 | 73.9 ± 8.9 | |
| Global cognitive evaluation | |||
| MoCA | 18.8 ± 3.8 | 18.97 ± 4.0 | |
| Anti-hypertensive treatments | |||
| β-blockers, n (%) | 20 (57.0) | 22 (60.0) | |
| ACE inhibitors, n (%) | 27 (77.0) | 29 (78.0) | |
| Angiotensin receptor blockers, n (%) | 8 (23.0) | 9 (24.0) | |
| Calcium channel blockers, n (%) | 22 (63.0) | 25 (66.0) | |
| Diuretics, n (%) | 11 (32.0) | 12 (33.0) | |
| Laboratory analyses | |||
| Plasma glucose (mg/dl) | 157.1 ± 52.4 | 154.6 ± 55.3 | |
| Creatinine (mg/dl) | 1.0 ± 0.2 | 1.0 ± 0.1 | |
| Total Cholesterol (mg/dl) | 142.0 ± 14.7 | 141.8 ± 15.0 | |
| LDL Cholesterol (mg/dl) | 93.8 ± 8.4 | 93.4 ± 8.6 | |
| HDL Cholesterol (mg/dl) | 42.5 ± 5.6 | 42.2 ± 5.8 | |
| Triglycerides (mg/dl) | 112.3 ± 6.0 | 112.7 ± 5.6 | |
| Comorbidities | |||
| Dyslipidemia (%) | 25 (72.0) | 27 (73.0) | |
| Diabetes (%) | 19 (54.0) | 19 (51.0) | |
| COPD (%) | 15 (43.0) | 17 (46.0) | |
| CKD (%) | 16 (46.0) | 16 (43.0) | |
| Previous Stroke (%) | 5 (15.0) | 6 (16.0) | |
| Anemia (%) | 8 (23.0) | 9 (24.0) | |
| AFib (%) | 11 (32.0) | 12 (33.0) | |
| Fried criteria | |||
| Slowness (%) | 27 (77.0) | 29 (78.0) | |
| Weakness (%) | 28 (80.0) | 28 (76.0) | |
| Low Physical Activity (%) | 24 (69.0) | 22 (60.0) | |
| Exhaustion (%) | 10 (29.0) | 13 (35.0) | |
| Weight Loss (%) | 16 (46.0) | 19 (51.0) |
Data are means ± SD or percentages. AFib, Atrial Fibrillation; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MoCA, Montreal Cognitive Assessment; SBP, systolic blood pressure.
MoCA Score at Follow-Up
At follow-up, we found a significant difference in the MoCA score between the L-Arginine treated group and placebo (Figure 2). In order to better identify explanatory variables that were associated with MoCA in our trial, we measured the association between L-Arginine treatment and MoCA in a multivariable linear regression model where MoCA was the dependent variable (Table 2). The association between L-Arginine treatment and MoCA score in frail hypertensive patients remained statistically significant after multivariable adjustment for age, BMI, blood glucose, serum creatinine, and blood pressure.
Figure 2.
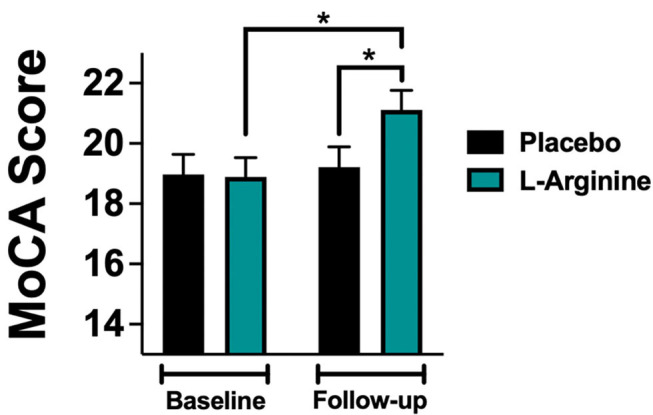
MoCA (Montreal Cognitive Assessment) score evaluated at baseline and at follow-up in the placebo and in the L-Arginine arms. Data are means ± SE; *: p < 0.05.
Table 2.
Multivariable linear regression analysis using the MoCA Score at follow-up as dependent variable.
| B | Standard error | Beta | t | p | 95.0% Confidence interval | ||
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Age | −0.150 | 0.069 | −0.309 | −2.175 | 0.039 | −0.291 | −0.008 |
| BMI | −0.199 | 0.078 | −0.180 | −2.562 | 0.016 | −0.359 | −0.040 |
| Blood Glucose | −0.020 | 0.012 | −0.237 | −1.765 | 0.082 | −0.043 | −0.003 |
| Serum Creatinine | 0.267 | 1.236 | 0.016 | 0.216 | 0.831 | −2.270 | 2.803 |
| SBP | −0.081 | 0.043 | −0.235 | −1.879 | 0.071 | −0.170 | 0.007 |
| DBP | 0.087 | 0.055 | 0.233 | 1.591 | 0.123 | −0.025 | 0.200 |
| L-Arginine | −2.569 | 0.837 | −0.319 | −3.077 | 0.003 | −4.237 | −0.900 |
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
L-Arginine Attenuates Endothelial Oxidative Stress
To provide insights into the physiological mechanisms of protection elicited by L-Arginine, we assessed its effect on human endothelial cells in vitro. For this purpose, we used HUVECs, incubated for 24 hours with Ang II (to simulate the hypertensive state) with or without addition of L-Arginine, measuring mitochondrial ROS generation via MitoSOX. We found that the co-treatment of HUVECs with Ang II and L-Arginine significantly attenuated mitochondrial ROS production compared to Ang II alone (Figure 3).
Figure 3.
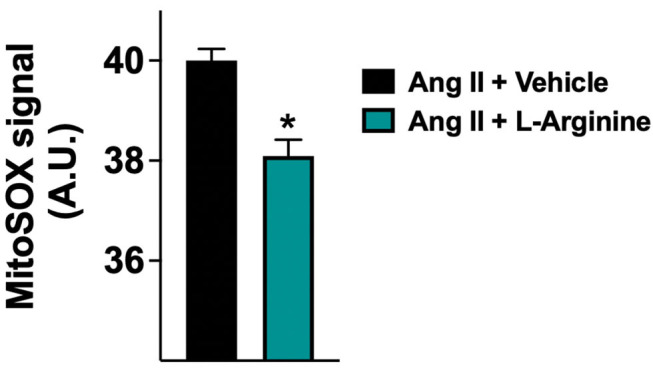
Mitochondrial production of reactive oxygen species (ROS) evaluated in human endothelial cells treated with Angiotensin II (Ang II) (1 μM) + vehicle or Ang II + L-Arginine (500 μM) for 24 hours; A.U., arbitrary units. Data are means ± SE; *: p < 0.01.
Discussion
The management of frailty in older adults is very debated (41–46). Comorbidities such as hypertension are known to play a key role in increasing the risk of mortality, hospitalization, disability and cognitive impairment. Indeed, hypertension determines alterations of endothelium causing inflammation, atherosclerosis and oxidative stress (47–51). L-Arginine supplementation in elders can equilibrate the L-Arginine/asymmetric dimethylarginine ratio, recovering the production of NO; in fact, the increased L-Arginine availability, resulting from supplementation, competes with asymmetric dimethylarginine in binding NOS (28, 52–54). Furthermore, L-Arginine has anti-inflammatory properties (55–58). In this context, we posited that L-Arginine treatment could improve cognitive impairment in frail older adults for its beneficial action on endothelial dysfunction. We tested this hypothesis in a clinical trial. Our results indicate that adding L-Arginine to standard therapy significantly improves the MoCA Score, indipendently of likely confounders including age, BMI, blood glucose, serum creatinine, and blood pressure.
Global cognitive function was assessed with the MoCA test, which was preferred to the Mini-Mental State Examination (MMSE), because the latter has been shown to be conditioned by demographic variables including age and years of education (59–61). Moreover, the MoCA test is generally considered the best test to detect mild cognitive impairment (62–65).
To mechanistically confirm our results, we examined in vitro the effects of L-Arginine on mitochondrial function in human endothelial cells, showing that L-Arginine significantly attenuates the generation of mitochondrial ROS induced by Ang II. Intriguingly, this finding is consistent with recent observations linking frailty and cognitive decline to mitochondrial dysfunction (66–68), which is also a well-recognized determinant of hypertension and vascular aging (69–72).
Several limitations of our study warrant consideration: first, the small sample size; second, we do not know the exact duration of the hypertensive disease; third, the evaluation of the effects of nutraceutical treatment would have benefited from a longer follow-up.
In conclusion, to the best of our knowledge, our study is the first to demonstrate that oral L-Arginine supplementation significantly improves cognitive impairment in hypertensive older adults. Further studies are warranted to verify whether these results can be extended to other populations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical approval was obtained from the Ethics Committee of Campania Nord. The patients/participants or their legal representatives provided a written informed consent to participate in this study.
Author Contributions
PM and GS designed the study, drafted the manuscript, approved its final version, and made the decision to submit and publish the manuscript. FV, UK, VT, and SJ revised the manuscript's intellectual content and approved the final version. AL, AP, and SF acquired the data, revised the manuscript's intellectual content, and approved the final version. PM is the guarantor of this work and, as such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved this submission.
Funding
The Santulli's Laboratory is supported in part by the National Institutes of Health (NIH): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259 and R01-DK033823), National Heart, Lung, and Blood Institute (NHLBI: R01-HL146691 and T32-HL144456), National Institute on Aging (NIA: R56-AG066431) to GS, by the Irma T. Hirschl and Monique Weill-Caulier Trusts (to GS), and by the Diabetes Action Research and Education Foundation (to GS). SJ and FV hold postdoctoral fellowships from the American Heart Association (AHA-22POST915561 and AHA-21POST836407, respectively). Both placebo and L-Arginine (Bioarginina®) were kindly provided by Farmaceutici Damor S.p.A., Naples, Italy, which had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, et al. AKT participates in endothelial dysfunction in hypertension. Circulation. (2004) 109:2587–93. 10.1161/01.CIR.0000129768.35536.FA [DOI] [PubMed] [Google Scholar]
- 2.Santisteban MM, Ahn SJ, Lane D, Faraco G, Garcia-Bonilla L, Racchumi G, et al. Endothelium-macrophage crosstalk mediates blood-brain barrier dysfunction in hypertension. Hypertension. (2020) 76:795–807. 10.1161/HYPERTENSIONAHA.120.15581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Montgolfier O, Pincon A, Pouliot P, Gillis MA, Bishop J, Sled JG, et al. High systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension. (2019) 73:217–28. 10.1161/HYPERTENSIONAHA.118.12048 [DOI] [PubMed] [Google Scholar]
- 4.Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. (2010) 55:1210–6. 10.1161/HYPERTENSIONAHA.109.143123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno PR, Purushothaman KR, Fuster V, O'Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. (2002) 105:2504–11. 10.1161/01.CIR.0000017265.52501.37 [DOI] [PubMed] [Google Scholar]
- 6.Mone P, Gambardella J, Pansini A, de Donato A, Martinelli G, Boccalone E, et al. Cognitive impairment in frail hypertensive elderly patients: role of hyperglycemia. Cells. (2021) 10:2115 10.3390/cells10082115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santulli G. Tirzepatide versus semaglutide once weekly in type 2 diabetes. N Engl J Med. (2022) 386:e17. 10.1056/NEJMc2114590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. (2019) 124:1045–60. 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381:752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho YY, Matteini AM, Beamer B, Fried L, Xue QL, Arking DE, et al. Exploring biologically relevant pathways in frailty. J Gerontol A Biol Sci Med Sci. (2011) 66:975–9. 10.1093/gerona/glr061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–156. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB, et al. Brief clinical instrument to classify frailty in elderly people. Lancet. (1999) 353:205–6. 10.1016/S0140-6736(98)04402-X [DOI] [PubMed] [Google Scholar]
- 13.Mone P, Pansini A. Gait speed test and cognitive decline in frail women with acute myocardial infarction. Am J Med Sci. (2020) 360:484–8. 10.1016/j.amjms.2020.03.021 [DOI] [PubMed] [Google Scholar]
- 14.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. (2010) 58:248–55. 10.1111/j.1532-5415.2009.02671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. (1995) 332:556–61. 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conroy SP, Westendorp RGJ, Witham MD. Hypertension treatment for older people-navigating between Scylla and Charybdis. Age Ageing. (2018) 47:505–8. 10.1093/ageing/afy053 [DOI] [PubMed] [Google Scholar]
- 17.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. (2016) 15:934–43. 10.1016/S1474-4422(16)30029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bromfield SG, Ngameni CA, Colantonio LD, Bowling CB, Shimbo D, Reynolds K, et al. Blood pressure, antihypertensive polypharmacy, frailty, and risk for serious fall injuries among older treated adults with hypertension. Hypertension. (2017) 70:259–66. 10.1161/HYPERTENSIONAHA.116.09390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of Hypertension on cognitive function: a scientific statement from the american heart association. Hypertension. (2016) 68:e67–94. 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panza F, Seripa D, Solfrizzi V, Tortelli R, Greco A, Pilotto A, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. (2015) 47:793–813. 10.3233/JAD-150358 [DOI] [PubMed] [Google Scholar]
- 21.Solfrizzi V, Scafato E, Lozupone M, Seripa D, Schilardi A, Custodero C, et al. Italian Longitudinal Study on Aging Working G: Biopsychosocial frailty and the risk of incident dementia: The Italian longitudinal study on aging. Alzheimers Dement. (2019) 15:1019–28. 10.1016/j.jalz.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 22.Rizzoni D, Rizzoni M, Nardin M, Chiarini G, Agabiti-Rosei C, Aggiusti C, et al. Vascular aging and disease of the small vessels. High Blood Press Cardiovasc Prev. (2019) 26:183–9. 10.1007/s40292-019-00320-w [DOI] [PubMed] [Google Scholar]
- 23.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. (2021) 17:639–54. 10.1038/s41581-021-00430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uiterwijk R, Huijts M, Staals J, Rouhl RP, De Leeuw PW, Kroon AA, et al. Endothelial activation is associated with cognitive performance in patients with hypertension. Am J Hypertens. (2016) 29:464–9. 10.1093/ajh/hpv122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikbov B, Soler MJ, Pesic V, Capasso G, Unwin R, Endres M, et al. Albuminuria as a risk factor for mild cognitive impairment and dementia-what is the evidence? Nephrol Dial Transplant. (2021) 37:ii55–62. 10.1093/ndt/gfab261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab. (2012) 303:E1177–1189. 10.1152/ajpendo.00284.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agapie T, Suseno S, Woodward JJ, Stoll S, Britt RD, Marletta MA, et al. formation by a catalytically self-sufficient bacterial nitric oxide synthase from Sorangium cellulosum. Proc Natl Acad Sci USA. (2009) 106:16221–6. 10.1073/pnas.0908443106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and Endothelial Function. Biomedicines. (2020) 8:277 10.3390/biomedicines8080277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Physiological cyclic stretch directs L-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. FASEB J. (2000) 14:1775–83. 10.1096/fj.99-0960com [DOI] [PubMed] [Google Scholar]
- 30.Morris CR, Kuypers FA, Kato GJ, Lavrisha L, Larkin S, Singer T, et al. Hemolysis-associated pulmonary hypertension in thalassemia. Ann N Y Acad Sci. (2005) 1054:481–5. 10.1196/annals.1345.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adebayo A, Varzideh F, Wilson S, Gambardella J, Eacobacci M, Jankauskas SS, et al. l-Arginine and COVID-19: An Update. Nutrients. (2021) 13:3951 10.3390/nu13113951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mone P, Izzo R, Marazzi G, Manzi MV, Gallo P, Campolongo G, et al. L-Arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J Pharmacol Exp Ther. (2022). 10.1124/jpet.122.001149. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorentino G, Coppola A, Izzo R, Annunziata A, Bernardo M, Lombardi A, et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine. (2021) 40:101125. 10.1016/j.eclinm.2021.101125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gambardella J, Fiordelisi A, Spigno L, Boldrini L, Lungonelli G, Di Vaia E, et al. Effects of chronic supplementation of L-Arginine on physical fitness in water polo players. Oxid Med Cell Longev. (2021) 2021:6684568. 10.1155/2021/6684568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mone P, Pansini A, Frullone S, de Donato A, Buonincontri V, De Blasiis P, et al. Physical decline and cognitive impairment in frail hypertensive elders during COVID-19. Eur J Intern Med. (2022). 10.1016/j.ejim.2022.03012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Yu KH, Lee BC, Kim BC, Kang Y. Validity of the Montreal Cognitive Assessment (MoCA) index scores: a comparison with the cognitive domain scores of the Seoul Neuropsychological Screening Battery (SNSB). Dement Neurocogn Disord. (2021) 20:28–37. 10.12779/dnd.2021.20.3.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holm H, Bachus E, Jujic A, Nilsson ED, Wadstrom B, Molvin J, et al. Cognitive test results are associated with mortality and rehospitalization in heart failure: Swedish prospective cohort study. ESC Heart Fail. (2020) 7:2948–55. 10.1002/ehf2.12909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mone P, Gambardella J, Lombardi A, Pansini A, De Gennaro S, Leo AL, et al. Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc Diabetol. (2022) 21:10. 10.1186/s12933-021-01442-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matarese A, Gambardella J, Sardu C, Santulli G. miR-98 Regulates TMPRSS2 Expression in Human Endothelial Cells: Key Implications for COVID-19. Biomedicines. (2020) 8:462 10.3390/biomedicines8110462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA. (2015) 112:11389–94. 10.1073/pnas.1513047112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura Y, Omura T, Toyoshima K, Araki A. Nutrition management in older adults with diabetes: a review on the importance of shifting prevention strategies from metabolic syndrome to frailty. Nutrients. (2020) 12:3367. 10.3390/nu12113367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. (2021) 25:824–53. 10.1007/s12603-021-1665-8 [DOI] [PubMed] [Google Scholar]
- 43.Gabbard J, Pajewski NM, Callahan KE, Dharod A, Foley KL, Ferris K, et al. Effectiveness of a nurse-led multidisciplinary intervention vs usual care on advance care planning for vulnerable older adults in an accountable care organization: a randomized clinical trial. JAMA Intern Med. (2021) 181:361–9. 10.1001/jamainternmed.2020.5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Neill DE, Forman DE. Cardiovascular care of older adults. BMJ. (2021) 374:n1593. 10.1136/bmj.n1593 [DOI] [PubMed] [Google Scholar]
- 45.Jyvakorpi SK, Ramel A, Strandberg TE, Piotrowicz K, Blaszczyk-Bebenek E, Urtamo A, et al. The sarcopenia and physical frailty in older people: multi-component treatment strategies (SPRINTT) project: description and feasibility of a nutrition intervention in community-dwelling older Europeans. Eur Geriatr Med. (2021) 12:303–12. 10.1007/s41999-020-00438-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivasi G, Tortu V, D'Andria MF, Turrin G, Ceolin L, Rafanelli M, et al. Hypertension management in frail older adults: a gap in evidence. J Hypertens. (2021) 39:400–7. 10.1097/HJH.0000000000002685 [DOI] [PubMed] [Google Scholar]
- 47.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. (2006) 71:247–58. 10.1016/j.cardiores.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 48.Xu S, Ilyas I, Little PJ Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. (2021) 73:924–67. 10.1124/pharmrev.120.000096 [DOI] [PubMed] [Google Scholar]
- 49.D'Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. (2018) 28:711–32. 10.1089/ars.2017.7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Almeida A, de Almeida Rezende MS, Dantas SH, de Lima Silva S, de Oliveira J, de Lourdes Assuncao Araujo de., Azevedo F, et al. Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid Med Cell Longev. (2020) 2020:1954398. 10.1155/2020/1954398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santulli G, Cipolletta E, Sorriento D, Del Giudice C, Anastasio A, Monaco S, et al. CaMK4 Gene Deletion Induces Hypertension. J Am Heart Assoc. (2012) 1:e001081. 10.1161/JAHA.112.001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Notsu Y, Nabika T, Bokura H, Suyama Y, Kobayashi S, Yamaguchi S, et al. Evaluation of asymmetric dimethylarginine and homocysteine in microangiopathy-related cerebral damage. Am J Hypertens. (2009) 22:257–62. 10.1038/ajh.2008.346 [DOI] [PubMed] [Google Scholar]
- 53.Pizzarelli F, Maas R, Dattolo P, Tripepi G, Michelassi S, D'Arrigo G, et al. Asymmetric dimethylarginine predicts survival in the elderly. Age (Dordr). (2013) 35:2465–75. 10.1007/s11357-013-9523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sibal L, Agarwal SC, Home PD, Boger RH. The Role of Asymmetric Dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. (2010) 6:82–90. 10.2174/157340310791162659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. (1993) 329:2002–12. 10.1056/NEJM199312303292706 [DOI] [PubMed] [Google Scholar]
- 56.Hnia K, Gayraud J, Hugon G, Ramonatxo M, De La Porte S, Matecki S, et al. L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am J Pathol. (2008) 172:1509–19. 10.2353/ajpath.2008.071009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, et al. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism. (2009) 58:1270–6. 10.1016/j.metabol.2009.03.029 [DOI] [PubMed] [Google Scholar]
- 58.Fritz JH. Arginine cools the inflamed gut. Infect Immun. (2013) 81:3500–2. 10.1128/IAI.00789-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carpinelli Mazzi M, Iavarone A, Russo G, Musella C, Milan G, D'Anna F, et al. Mini-mental state examination: new normative values on subjects in Southern Italy. Aging Clin Exp Res. (2020) 32:699–702. 10.1007/s40520-019-01250-2 [DOI] [PubMed] [Google Scholar]
- 60.Limongi F, Noale M, Bianchetti A, Ferrara N, Padovani A, Scarpini E, et al. The instruments used by the Italian centres for cognitive disorders and dementia to diagnose mild cognitive impairment (MCI). Aging Clin Exp Res. (2019) 31:101–7. 10.1007/s40520-018-1032-8 [DOI] [PubMed] [Google Scholar]
- 61.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 62.Kang JM, Cho YS, Park S, Lee BH, Sohn BK, Choi CH, et al. Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. (2018) 18:261. 10.1186/s12877-018-0951-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Junior AL, Costa MLG, Ximenes RCC, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's Disease (AD) in the elderly? Int Psychogeriatr. (2019) 31:491–504. 10.1017/S1041610218001370 [DOI] [PubMed] [Google Scholar]
- 64.O'Driscoll C, Shaikh M. Cross-Cultural Applicability of the Montreal Cognitive Assessment (MoCA): A Systematic Review. J Alzheimers Dis. (2017) 58:789–801. 10.3233/JAD-161042 [DOI] [PubMed] [Google Scholar]
- 65.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 66.Allali G, Montembeault M, Brambati SM, Bherer L, Blumen HM, Launay CP, et al. Brain Structure Covariance Associated With Gait Control in Aging. J Gerontol A Biol Sci Med Sci. (2019) 74:705–13. 10.1093/gerona/gly123 [DOI] [PubMed] [Google Scholar]
- 67.Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. (2021) 590:122–8. 10.1038/s41586-020-03160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El Assar M, Angulo J, Rodriguez-Manas L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic Biol Med. (2020) 149:72–7. 10.1016/j.freeradbiomed.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 69.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. (2018) 123:849–67. 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varzideh F, Jankauskas SS, Kansakar U, Mone P, Gambardella J, Santulli G. Sortilin drives hypertension by modulating sphingolipid/ceramide homeostasis and by triggering oxidative stress. J Clin Invest. (2022) 132:e156624. 10.1172/JCI156624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rao KNS, Shen X, Pardue S, Krzywanski DM. Nicotinamide nucleotide transhydrogenase (NNT) regulates mitochondrial ROS and endothelial dysfunction in response to angiotensin II. Redox Biol. (2020) 36:101650. 10.1016/j.redox.2020.101650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robert P, Nguyen PMC, Richard A, Grenier C, Chevrollier A, Munier M, et al. Protective role of the mitochondrial fusion protein OPA1 in hypertension. FASEB J. (2021) 35:e21678. 10.1096/fj.202000238RRR [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.