Abstract
Background
The blood neutrophil-to-lymphocyte ratio (NLR) has recently emerged as a powerful predictor of adverse outcomes in some cardiovascular and lung diseases. Pulmonary arterial hypertension (PAH) is a lethal vasculopathy associated with increased inflammation. Although PAH exhibits a higher prevalence among women, men have a poorer prognosis. We investigated the NLR as an independent predictor of transplant-free survival in PAH.
Methods
We performed a retrospective analysis of 78 PAH patients from the Quebec PAHBiobank (71% female). We used univariate and multivariate (adjusted for age, sex, renal function, and disease severity) Cox regression analyses to assess the relationship between the NLR and transplant-free survival, in the whole sample, and according to sex. The NLR was categorized as high (≥ 4.8) or low (< 4.8) using receiver operating characteristic analysis. Unadjusted Kaplan-Meier analysis estimated survival per NLR category.
Results
The NLR was higher in patients who died, compared to that in patients who had transplant-free survival (P < 0.05). The NLR was an independent predictor of event-free survival in PAH (unadjusted hazard ratio: 1.11, 95% confidence interval: 1.04-1.18, which remained significant after adjustment for covariates). The high-NLR group had lower 1-, 3-, and 5-year survival compared to those with a low NLR (P < 0.001). The NLR remains a predictor of survival in women.
Conclusions
The NLR is an independent predictor of transplant-free survival in PAH. We report a potential sexual dimorphism in the ability of the NLR to predict mortality in PAH, emphasizing the importance of considering sex-related differences in the development of biomarkers in PAH.
Résumé
Contexte
Le rapport neutrophiles/lymphocytes (RNL) s’est récemment imposé comme un puissant facteur prédictif de l’issue défavorable de certaines maladies cardiovasculaires et pulmonaires. L’hypertension artérielle pulmonaire (HTAP) est une vasculopathie mortelle associée à une inflammation accrue. Bien que sa prévalence soit plus élevée chez les femmes, son pronostic est plus défavorable chez les hommes. Nous avons étudié le RNL en tant que prédicteur indépendant de la survie sans greffe chez des sujets atteints d’HTAP.
Méthodologie
Nous avons effectué une analyse rétrospective du matériel sur l’HTAP de la Biobanque du Québec se rapportant à 78 patients (71 % de femmes). Des analyses de régression de Cox univariées et multivariées (ajustées en fonction de l’âge, du sexe, de la fonction rénale et de la gravité de la maladie) nous ont permis d’évaluer la relation entre le RNL et la survie sans greffe dans l’ensemble de l’échantillon et selon le sexe. Le RNL a été classé comme élevé (≥ 4,8) ou faible (< 4,8) au terme d’une analyse des caractéristiques de fonctionnement du récepteur. Une analyse non ajustée effectuée selon la méthode de Kaplan-Meier a servi à estimer la survie par catégorie de RNL.
Résultats
Le RNL était plus élevé chez les patients décédés que chez les patients ayant survécu sans greffe (p < 0,05). Le RNL était un prédicteur indépendant de la survie sans événement chez les patients atteints d’HTAP (rapport des risques instantanés non ajusté : 1,11, intervalle de confiance à 95 % : 1,04-1,18, demeuré significatif après ajustement en fonction des covariables). La survie à 1, 3 et 5 ans a été inférieure au sein du groupe présentant un RNL élevé comparativement au groupe présentant un RNL faible (p < 0,001). Le RNL demeure un prédicteur de la survie chez les femmes.
Conclusions
Le RNL est un facteur prédictif indépendant de la survie sans greffe chez les sujets atteints d’HTAP. Selon nos observations, un dimorphisme sexuel potentiel le caractérise en tant que prédicteur de la mortalité chez les sujets atteints d’HTAP. Il importe donc de tenir compte des différences liées au sexe dans le développement des biomarqueurs de l’HTAP.
Pulmonary arterial hypertension (PAH) is a lethal vasculopathy hemodynamically characterized by increased mean pulmonary arterial pressure (> 20 mm Hg) and pulmonary vascular resistance (> 3 Wood units).1 Increased pulmonary vascular resistance in PAH reflects obliterative remodeling, vasoconstriction, and increased stiffness of the distal pulmonary arteries. PAH is further classified as idiopathic (IPAH), hereditary, and PAH associated with various conditions, including connective tissue diseases (CTDs). The 7-year survival rates of IPAH and CTD-PAH approximate 56% and 35%, respectively.2 High mortality rates in PAH are a consequence of late diagnosis, the lack of biomarkers for early disease detection, and the absence of a curative treatment.3,4
Systemic inflammation and increased circulatory levels of inflammatory markers are common to virtually all PAH patients.5 Interesting to note is that the neutrophil-to-lymphocyte ratio (NLR) is a simple, minimally invasive, and readily accessible measure of inflammation that tends to emerge as a powerful predictor of survival and poor outcome in coronary artery disease and heart failure, but more studies are needed to confirm its utility in a clinical setting.6 In PAH, the NLR has been found to correlate with the functional status,7, 8, 9 but conflicting results suggest that the NLR predicts event-free survival in PAH patients.7,9
PAH is a disease with a high prevalence in women, with a female-to-male ratio in incidence of 4:1.10 Although the incidence of PAH is higher in women, the prognosis is worse for men. In the Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management (REVEAL) registry, the estimated 5-year survival rate from diagnosis was ∼52% for men, compared to 62% for women.11 Surprisingly, this aptly named “sex paradox,” with women more likely to have PAH, but males having higher mortality, remains unexplained and is rarely considered in studies investigating biomarkers as predictors of PAH mortality. Interesting to note is that in other fields such as oncology, the NLR was shown to predict survival in only female patients.12
Our exploratory work aimed to assess the role of the NLR as an independent predictor of transplant-free survival in PAH and to determine a threshold that can adequately identify a subgroup of patients at higher risk of adverse events. We also aimed to evaluate how sex modulates the association between the NLR and transplant-free survival.
Methods
The Quebec PAH Biobank cohort includes PAH patients from the Quebec Heart and Lung Institute who underwent right heart catheterization (RHC) for suspected pulmonary hypertension or follow-up. PAH was diagnosed according to the current guidelines.1 Blood samples were drawn during the RHC procedure after written informed consent was obtained, and approval was received from the Laval University and l’Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) Biosafety and Ethics Committees (CER#20735). Blood samples included a whole blood count and differential blood count (absolute count for neutrophils, lymphocytes), as well as biochemistry analysis (levels of urea, creatinine, and N-terminal of the prohormone brain natriuretic peptide [NT-proBNP]). The NLR was calculated using the absolute neutrophil and lymphocyte counts, whereas the estimated glomerular filtration rate (eGFR) was determined using the modification of diet in renal disease equation (ml/min per 1.73 m2).13 Demographics, medical history, pulmonary function tests, and markers of disease severity (functional class, exercise capacity, pulmonary hemodynamics) were collected contemporarily, whereas incidences of morbidity and mortality events were collected prospectively.
Statistical analysis
All statistical analyses were conducted using the R statistical package (http://www.rproject.org, version 4.0.5), with P < 0.05 considered statistically significant. Continuous variables were reported as means ± standard deviations (SDs), and categorical variables were reported as frequencies (percentages). Between-group comparisons (men vs women and high vs low NLR) were made using either the unpaired t test (parametric), the Mann-Whitney U test (nonparametric), or the χ2 test, as applicable.
Univariate Cox regression models were used to identify factors associated with transplant-free survival, using the “survival” and “survminer” R packages. The date of initiation of RHC was the starting point to determine follow-up duration. The cut-off date was May 20, 2021. Patients lost to follow-up were censored as of the date of the last medical visit. Covariates of clinical significance and with P values < 0.05 in the univariate models and n > 70 observations were included in the multivariate Cox regression model. Collinearity was assessed using a correlation matrix (Pearson or Spearman, according to the distribution). Due to the limited number of events, we generated 6 regression models considering clinical variables (age, sex, and eGFR) and one variable reflecting disease severity (NT-proBNP, mixed venous oxygen saturation, right atrial pressure, cardiac index, world health organization [WHO] functional class, and PAH type, respectively, in models 1, 2, 3, 4, 5, and 6). The Kaplan-Meier method was also used to estimate the transplantation-free survival for patients with high (≥ 4.8) or low (< 4.8) NLR, defined as the value with the highest sum of sensitivity and specificity to predict outcomes following receiver operating characteristic (ROC) curve analysis (Wilson/Brown method to a 95% confidence interval). Survival distributions were compared using the log-rank (Mantel-Cox) test and the Gehan-Breslow-Wicoxon test.
Results
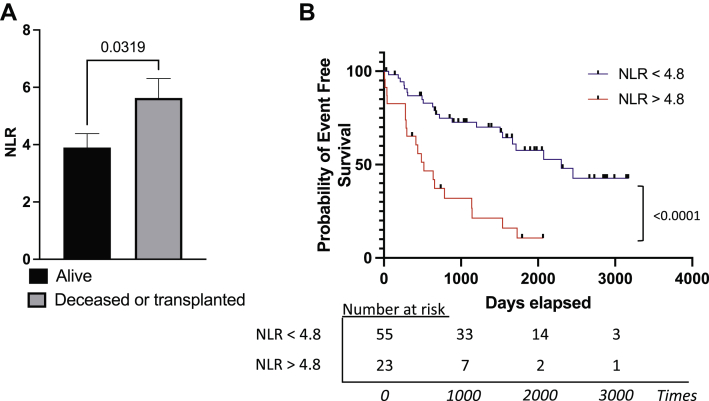
The characteristics of the study population are presented in Table 1. Most patients were WHO functional class III IPAH. During a median follow-up period of 1154 days (range: 7-3170 days), 41 PAH patients died (n = 39) or were referred for transplantation (n = 2). The NLR was significantly higher in patients who died or were referred for transplantation (P = 0.0319; Fig. 1A). The ROC curve analysis suggested 4.8 as the most discriminative value to predict poor outcomes (area under the curve: 0.64; P = 0.03; Supplemental Fig. S1). Patients in the high-NLR group were older, and had increased disease severity and decreased lung function compared to those in the low-NLR group (Table 1). The estimated 1-, 3-, and 5-year transplant-free survival rates were 65.2%, 21.3.4%, and 10.6%, respectively, in the high-NLR group, compared with 86.8%, 72.7% and 57.6% in the low-NLR group, respectively (Fig. 1B). This association was confirmed using univariate Cox regression models, which showed that the NLR was associated with an increased risk of death/transplant referral (hazard ratio [HR]: 1.11; 95% confidence interval [CI]: 1.04-1.18; Table 2). In multivariate analyses, the NLR remained independently associated with an increased risk of mortality/transplantation after adjusting for age, sex, eGFR, and disease severity, as defined by the NT-proBNP level (model 1; HR: 1.12; 95% CI: 1.04-1.21), SvO2 level (model 2; HR: 1.17; 95% CI: 1.08-1.27), right atrial pressure (model 3; HR: 1.13; 95% CI: 1.04-1.23), cardiac index (model 4; HR: 1.15; 95% CI: 1.06-1.24), and WHO functional class (model 5; HR: 1.12; 95% CI: 1.04-1.21; Table 3).
Table 1.
Baseline characteristic of the Quebec Pulmonary Arterial Hypertension (PAH) Biobank cohort
| Variable | PAH (n = 78) | Low NLR < 4.8 (n = 55) | High NLR > 4.8 (n = 23) |
|---|---|---|---|
| Age, y (no. missing) | 66 ± 17 (0) | 65 ± 17 (0) | 70 ± 12 (0)∗ |
| BMI, kg/m2 (no. missing) | 28± 7 (0) | 27 ± 8 (0) | 28 ± 6 (0) |
| Male/female | 26 (33.3)/52.0 (66.7) | 18 (32.7)/37 (67.3) | 8 (34.8)/15 (65.2) |
| Race | |||
| Caucasian | 77.0 (98.7) | 54 (98.2) | 23 (100) |
| African | 1.0 (1.3) | 1 (1.8) | 0 (0) |
| PAH type | |||
| IPAH | 50.0 (64.1) | 39 (70.9) | 11 (47.8) |
| APAH | 25.0 (32.0) | 15 (27.3) | 10 (43.6) |
| SSc-PAH | |||
| Heritable PAH | 2.0 (2.6) | 1 (1.8) | 1 (4.3) |
| Other | 1.0 (1.3) | 0 (0) | 1 (4.3) |
| Treatment | |||
| ERA | 15 (19.2) | 12 (21.8) | 3 (13.0) |
| PDE5 inhibitor | 25 (32.0) | 16 (29.1) | 9 (39.1) |
| Prostacyclin | 4 (5.1) | 2 (3.6) | 2 (8.6) |
| Calcium blocker | 24 (30.8) | 18 (32.7) | 6 (26.1) |
| Non-treated | 29 (37.2) | 21 (38.2) | 8 (34.8) |
| WHO functional class | |||
| I | 0 (0) | 0 (0) | 0 (0) |
| II | 16.0 (20.5) | 12 (21.9) | 4 (17.4) |
| III | 49.0 (62.8) | 35 (63.6) | 14 (60.9) |
| IV | 11.0 (14.1) | 6 (10.9) | 5 (21.7) |
| II or III | 1.0 (1.3) | 1 (1.8) | 0 (0) |
| Unknown | 1.0 (1.3) | 1 (1.8) | 0 (0) |
| 6MWD, m (no. missing) | 323 ± 171(13) | 341 ± 169 (6) | 272 ± 170 (7)∗ |
| Hemodynamic parameters (no. missing) | |||
| Mean PAP, mm Hg | 47 ± 14 (1) | 48.± 15 (1) | 44 ± 14 (0) |
| RAP, mm Hg | 7.0 ± 7.0 (1) | 7.0 ± 6.0 (1) | 10.5 ± 6.2 (0)∗ |
| CI, L/min per m2 | 2.4 ± 1.1 (1) | 2.4 ± 1.0 (1) | 2.1 ± 1.2 (0)∗ |
| PVRI, dynes/s/cm5 per m2 | 1271 ± 846 (2) | 1248 ± 866 (1) | 1412 ± 984 (0) |
| PCWP, mm Hg | 9.0 ± 5.0 (2) | 9.0 ± 4.8 (1) | 8.0 ± 7.0 (0) |
| SVO2, % | 65 ± 14 (1) | 66 ± 10 (2) | 56 ± 13 (1)† |
| TAPSE, mm | 18.0 ± 14 (57) | 20.0 ± 10.0 (44) | 14.0 ± 8.2 (13)∗ |
| Blood count, % (no. missing) | |||
| WBC, × 109 cells/L | 8.4 ± 3.0 (1) | 7.9 ± 3.1 (0) | 9.3 ± 3.5 (1)‡ |
| ALC, × 109 cells/L | 1.5 ± 1.1 (1) | 1.8 ± 0.8 (0) | 0.9 ± 0.5 (1)† |
| ANC, × 109 cells/L | 5.8 ± 2.6 (1) | 5.1 ± 2.1 (1) | 7.5 ± 3.6 (1)† |
| NLR | 3.3 ± 2.5 (1) | 3.0 ± 1.2 (0) | 8.1 ± 5.2 (1)† |
| Biochemistry | |||
| NT-ProBNP, pg/ml (no. missing) | 1207 ± 2666 (0) | 725 ± 2078 (1) | 2602 ± 5840 (0)‡ |
| eGFR, mL/min per 1.73m2 (no. missing) | 68 ± 32 (0) | 72 ± 33 (0) | 61 ± 28 (0)∗ |
Values are n (%), or median ± interquartile range, unless otherwise specified. P values determined by unpaired t test, Mann Whitney, or χ2 test.
ALC, absolute lymphocytes count; ANC, absolute neutrophil count; APAH, associated PAH; BMI, body mass index; CI, cardiac index; eGFR, estimated glomerular filtration rate; ERA, endothelin receptor antagonist; IPAH, idiopathic PAH; NLR, neutrophil-to-lymphocyte ratio; NT-pro-BNP, N-terminal pro–brain natriuretic peptide; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PDE5, phosphodiesterase 5; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SSc-PAH, scleroderma-associated PAH; SVO2, central mixed venous oxygen saturation; TAPSE, tricuspid annular plane systolic excursion; WBC, white blood cell count; WHO, World Health Organization. 6MWD, 6-minute walk distance.
P < 0.05.
P < 0.0001.
P < 0.01.
Figure 1.
The neutrophil-to-lymphocyte (NLR) ratio predicts events-free survival among patients with pulmonary arterial hypertension. (A) The NLR is significantly greater in deceased or transplanted patients (n = 41), compared with that in survivors (n = 37); ∗P < 0.05 (unpaired t test). (B) Kaplan-Meier curves depicting transplant-free survival in PAH patients with a low NLR (< 4.8) vs a high NLR (≥ 4.8; P < 0.001 by log-rank [Mantel-Cox] test and Gehan-Breslow-Wilcoxon test).
Table 2.
Cox regression models
| Variable | HR | 95% CI | P | Sample size |
|---|---|---|---|---|
| Age | 1.05 | 1.02–1.09 | < 0.001 | 78 |
| Sex (male) | 2.61 | 1.41–4.83 | 0.002 | 78 |
| BMI | 1.01 | 0.96–1.06 | 0.683 | 78 |
| PAH type | 1.20 | 0.89–1.64 | 0.235 | 78 |
| WHO functional class | 2.79 | 1.45–5.39 | 0.002 | 77 |
| 6MWD, m | 0.99 | 0.99–1.00 | 0.003 | 65 |
| Hemodynamic parameters | ||||
| Mean PAP, mm Hg | 1.00 | 0.98–1.03 | 0.770 | 77 |
| RAP, mm Hg | 1.08 | 1.01–1.15 | 0.025 | 77 |
| CI, L/min per m2 | 0.57 | 0.37–0.87 | 0.010 | 77 |
| PVRI, dynes/s/cm5 per m2∗ | 1.01 | 0.99–1.01 | 0.058 | 76 |
| PCWP, mm Hg | 1.02 | 0.92 – 1.14 | 0.674 | 77 |
| SvO2, % | 0.92 | 0.88–0.96 | < 0.001 | 75 |
| Blood counts | ||||
| WBC (× 109 cells/L) | 1.17 | 1.01–1.35 | 0.032 | 77 |
| ANC (× 109 cells/L) | 1.22 | 1.06–1.41 | 0.006 | 77 |
| ALC (× 109 cells/L) | 0.51 | 0.29–0.88 | 0.016 | 77 |
| NLR | 1.11 | 1.04–1.18 | 0.002 | 77 |
| Biochemistry | ||||
| eGFR, mL/min per 1.73 m2) | 0.98 | 0.96–0.99 | 0.001 | 78 |
| NT-proBNP (pg/ml)† | 1.01 | 1.00–1.01 | 0.009 | 77 |
ALC, absolute lymphocytes count; ANC, absolute neutrophil count; BMI, body mass index; CI, cardiac index; eGFR, estimated glomerular filtration rate; HR, hazard ratio; NLR, neutrophil-lymphocytes ratio; NT-pro-BNP, N-terminal pro–brain natriuretic peptide; PAP, pulmonary artery pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PVRI, pulmonary vascular resistance index; RAP, right atrial pressure; SvO2, central mixed venous oxygen saturation; WBC, white blood cell count; WHO, World Health Organization; 6MWD, 6-minute walk distance.
For each 10 units of PVRI.
For 100 units of NT-proBNP.
Table 3.
Multivariate Cox regression models
| Adjusted NLR∗ | HR | 95% CI | P | Sample size |
|---|---|---|---|---|
| Model 1 (NT-proBNP) | 1.12 | 1.04–1.21 | 0.002 | 76 |
| Model 2 (SvO2) | 1.17 | 1.08–1.27 | < 0.001 | 74 |
| Model 3 (RAP) | 1.13 | 1.04–1.23 | 0.003 | 76 |
| Model 4 (Cardiac Index) | 1.15 | 1.06–1.24 | < 0.001 | 76 |
| Model 5 (WHO functional class) | 1.12 | 1.04–1.21 | 0.003 | 76 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; NT-pro-BNP, N-terminal pro–brain natriuretic peptide; RAP, right atrial pressure; SvO2, central mixed venous oxygen saturation; WHO, World Health Organization.
All models were adjusted for age, sex, and estimated glomerular filtration rate. Additional variables for which the models were adjusted are indicated in parentheses.
We investigated whether the PAH subtype affects the NLR. We observed that NLR is significantly higher in scleroderma-associated PAH patients, compared with IPAH patients (P = 0.0135; Supplemental Fig. S2A), and can be used to successfully discriminate between these 2 PAH subtypes (area under the curve = 0.679; P = 0.0142; Supplemental Fig. S2B). Both IPAH and CTD-PAH patients with NLR > 4.8 exhibit lower survival, compared with patients with NLR < 4.8 (Supplemental Fig. S2C and D). Surprising to note is that PAH subtype was not associated with transplant-free survival (univariate analysis HR 1.22; 95% CI: 0.89-1.67; P = 0.206; Table 1), and NLR remains an independent predictor of survival when corrected for PAH type (model 6; Supplemental Table S1).
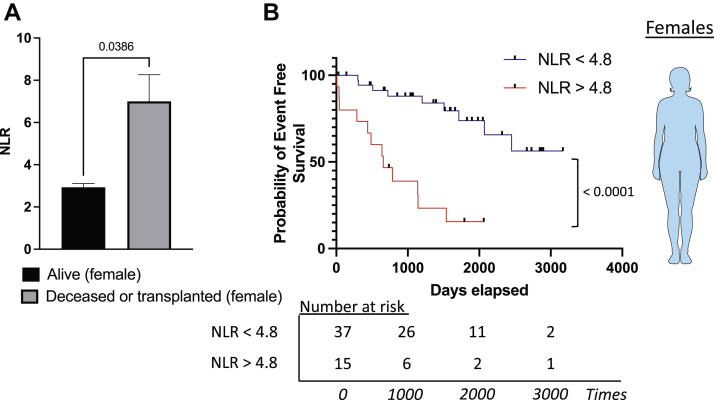
Lastly, we explored whether a sexual dimorphism was observed in PAH. Men and women had similar disease severity and NLR (Supplemental Table S2). An intriguing point is that the NLR was significantly higher in female PAH patients who died (7.0 ± 5.6. vs 2.9 ± 0.9; P = 0.0386) but not in male patients (4.2 ± 1.3 vs 4.3 ± 1.9; P = 0.8425; Fig. 2A; Supplemental Fig. S3A). Consistently, the NLR predicted transplantation-free survival in women only, with an estimated 1-, 3-, and 5-year transplant-free survival rate of 73.3%, 38.9%, and 15.5%, respectively, compared with 97.2%, 88.0%, and 73.9% in the high- and low-NLR groups, (Fig. 2B). We observed no evidence of an association between the NLR and survival in men (Supplemental Fig. S3B)
Figure 2.
Sexual dimorphism and the ability of the neutrophil-to-lymphocyte (NLR) ratio to predict mortality in patients with pulmonary arterial hypertension. (A) The NLR in blood of deceased or transplanted patients (n = 20), compared with that in alive female patients with pulmonary arterial hypertension (n = 32). (B) Survival curve in female patients with low NLR (< 4.8) and high NLR (≥ 4.8). Comparison between survival curve, log-rank (Mantel-Cox) test, and Gehan-Breslow-Wilcoxon test. ∗∗∗∗P < 0.0001.
Discussion
In this retrospective analysis of the Quebec PAH-biobank cohort, we observed that the NLR was a successful and independent predictor of transplant-free survival in PAH patients. We also identified an NLR threshold (≥ 4.8 vs < 4.8) that categorizes a subgroup of PAH patients with higher disease severity and higher risk of death/transplant. The association between the NLR and transplant-free survival in PAH appears to be greater for women, compared to men, thereby suggesting a potential sexual dimorphism in the ability of the NLR to predict transplant-free survival in PAH. However, the strength of this finding may be mitigated by the small number of men available for the analysis. Furthermore, we also report that an increased NLR can discriminate between patients with scleroderma-associated PAH vs IPAH.
The NLR has been shown to predict mortality in various populations.14, 15, 16, 17 In retrospective studies involving patients with various malignancies (breast, colorectal, esophageal, liver, melanoma, ovarian, pancreatic, prostate), and with lung diseases (eg, chronic obstructive pulmonary disease), the NLR successfully predicted survival.15,16,18,19 Furthermore, population-based studies demonstrated that an increased NLR was an independent predictor of heart faillure and cardiovascular mortality.14,17 Interesting to note is that the NLR was an independent predictor of right-ventricular dysfunction in patients with inferior ST-segment elevation myocardial infarction.20 We observed that PAH patients with a high NLR had a lower tricuspid annular plane systolic excursion (Table 1). These findings suggest that a high NLR could reflect the propensity for right-ventricular dysfunction, which is of particular relevance in PAH, in which right-ventricular failure remains the main cause of death. However, such hypotheses remain speculative, and further studies looking at the relationship between NLR and right-ventricular function are needed.
Our findings supporting the role of the NLR as a predictor of event-free survival in PAH are in accordance with those of previous work.7,9 In an exploratory cohort study of 77 PAH patients, Harbaum et al. also concluded that an increased NLR was an independent predictor of death or transplantation.9 However, these results contrast with those of other studies that failed to identify NLR as an independent predictor of death in PAH patients, despite its ability to adequately discriminate PAH patients from healthy controls (NLR = 2.44 in PAH patients vs 1.55 in controls).7,8 Nonetheless, the average NLR value observed in their cohorts was substantially lower than the one reported in our study (2.44 vs 4.90).7,8 Normal NLR values in a non-geriatric and otherwise healthy adult population range between 0.78 and 3.53.21 Thus, an NLR above the upper limit of the normal range (> 3.5) most likely reflects an abnormal state of increased systemic inflammation and physiological stress. Furthermore, age, sex, and race have also been known to influence the NLR.18,22 Genetic ancestry could thus explain these differences, as studies reporting a higher NLR (> 3.5) included mostly Caucasians from Canada and Germany, whereas those with lower NLR values originated from Turkey.7, 8, 9 Thus, discrepancies in the reported NLR values for PAH patients may stem from underlying patient characteristics.
Although NLR is moderately increased in transplanted or deceased patient groups (Fig. 1A; Supplemental Fig. S1), patients with a high NLR (NLR < 4.8) exhibit a significantly lower survival rate on a median follow-up period of 1154 days. The NLR threshold identified in our study (4.8) is strikingly similar to the NLR threshold previously reported by Harbaum et al. (> 4.14), using a similar approach based on an ROC curve analysis.9 However, Harbaum et al. reported a lower level of circulating neutrophils and lymphocytes in their PAH sample (absolute neutrophil count: 5.46 × 109/L, vs 6.12 × 109/L in our study; absolute lymphocyte count: 1.55 × 109/L, vs 1.6 × 109/L in our study). This discrepancy could be explained by technical factors related to the timing of the collection of blood samples. In the Harbaum et al. study, they collected their samples at least 1 week before RHC, whereas in our study, samples were collected during RHC. Invasive procedures are well known to induce stress and modify the level of circulating white blood cells, particularly neutrophils (also called neutrophil demargination).23,24 Despite these differences in levels of circulating neutrophiles and lymphocytes, the reported NLRs in our study were similar (average NLR value = 4.9 in both PAH cohorts), suggesting that it is a reproducible measurement regardless of timing and sample-collection methods.
A well described sex paradox occurs in PAH—the prevalence of the disease is substantially higher in women, compared with that in men, but men have a worse prognosis.10 Corroborating this observation, women seem to be more resistant to right-ventricular dysfunction and failure.25 According to the literature, male sex is associated with a significantly greater risk of mortality/transplant referral (HR: 2.6). We observed that deceased female PAH patients had a higher NLR, compared with that of female PAH survivors. However, despite a similar baseline NLR and disease severity, male survivor vs deceased PAH patients exhibit no NLR difference. Furthermore, men with an NLR < 4.8 exhibit a low survival rate, compared with that of women (1- and 5-year transplant-free survival rates of 72.2% and 29.6%, respectively, in men, compared with 94.7% and 89.1% in women). This finding suggests that underlying sex-related differences may affect the ability of the NLR to predict event-free survival in PAH patients. This observation is in line with previous observations made in cancer patients.12 In a cohort study of 1222 patients with head and neck squamous cell carcinoma, no significant differences were seen in baseline NLR between male and female patients. After patients were categorized according to NLR quartiles, the NLR successfully predicted mortality, with patients in the upper quartile having lower survival. However, when the sample was further categorized according to sex, the NLR predicted survival in only female patients.
Jung and colleagues showed that NLR is associated with connective tissue diseases.26 They observed an increased NLR in patients with scleroderma, compared with that in control patients, and reported that patients with scleroderma and interstitial lung disease had a higher NLR than those without interstitial lung disease. As expected, we observed that NLR is increased in patients with an immunologic etiology for PAH, such as CTD (scleroderma), compared with that in IPAH patients. However, in our cohort, PAH type is not associated with survival, and the ability of the NLR to predict survival is independent of PAH type. This observation might be a consequence of the low survival rate observed in our cohort. For example, we observed a 5-year survival rate of 43% in our cohort, compared to 61% in the REVEAL cohort.27 This high mortality rate might be explained by the higher age of the patients in our cohort (62.7 years, vs 51.7 years in the REVEAL cohort). Indeed, in the REVEAL cohort, the 5-year survival decreased as PAH patients aged (70.7%, 52.4%, 42.8%, respectively, in patients aged 35-54, 55-64, and > 64 years). Furthermore, patients enrolled in our cohort exhibited relatively severe disease, with 77% of the patients being in WHO functional class III-IV (vs 53% of patients in WHO class III-IV in the REVEAL cohort).
Although inflammation is a well established determinant of PAH development and progression, no inflammatory-based biomarker is available for the clinical management of PAH. Use of the NLR would allow integration of inflammation into PAH risk stratification. Biomarkers used in the clinic for risk stratification and outcome prediction include clinical signs of right heart failure, 6-minute walk test distance, cardiopulmonary exercise testing, imaging (echocardiography, cardiac magnetic resonance), hemodynamics (right heart catheterization), and NT-proBNP levels, which are not readily available in nonspecialized centres.28 The NLR has the advantages of being easily available from complete blood count as well as cost-efficient. Finally, the NLR might represent the first inflammatory-based biomarker for prediction of clinical outcome in PAH.
Limitations
Limitations of our study include the limited sample size, especially with the number of male PAH patients. This low number may have impaired our ability to detect statistically significant associations between the NLR and transplant-free survival in this group. The Quebec PAH Biobank cohort comprises 98.7% Caucasians. This limitation precludes any extrapolation of our findings to other ethnicities (eg, Hispanic). Due to the modest sample size, we did not investigate the NLR level in “heritable” and “other” PAH subtypes. We did not validate our observations in an independent validation cohort.
Conclusions
We demonstrated that the NLR is an independent predictor of event-free survival in PAH, supporting the role of the NLR as a reliable, minimally invasive, and easily accessible biomarker in PAH. Furthermore, we report a sexual dimorphism in the ability of NLR to predict mortality, further underlining the importance of integrating sex as a variable in studies evaluating the development of novel biomarkers in PAH.
Acknowledgements
The authors greatly appreciate the intellectual input from members of the Quebec Heart and Lung Institute Pulmonary Hypertension Research Group.
Funding Sources
F.P. receives grants from the Quebec Heart and Stroke Foundation and the Quebec Respiratory Health Research Network.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Blood samples were drawn during the right heart catheterization procedure after written informed consent was obtained and approval was received from the Laval University and l’Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) Biosafety and Ethics Committees (CER#20735).
See page 362 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.11.010.
Supplementary Material
References
- 1.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.01913-2018. 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benza R.L., Miller D.P., Barst R.J., et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.Thenappan T., Ormiston M.L., Ryan J.J., Archer S.L. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirani N., Brunner N.W., Kapasi A., et al. Canadian Cardiovascular Society/Canadian Thoracic Society position statement on pulmonary hypertension. Can J Cardiol. 2020;36:977–992. doi: 10.1016/j.cjca.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovitch M., Guignabert C., Humbert M., Nicolls M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angkananard T., Anothaisintawee T., McEvoy M., Attia J., Thakkinstian A. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. 2018;2018 doi: 10.1155/2018/2703518. 2703518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Özpelit E., Akdeniz B., Özpelit M.E., et al. Prognostic value of neutrophil-to-lymphocyte ratio in pulmonary arterial hypertension. J Int Med Res. 2015;43:661–671. doi: 10.1177/0300060515589394. [DOI] [PubMed] [Google Scholar]
- 8.Yıldız A., Kaya H., Ertaş F., et al. Association between neutrophil to lymphocyte ratio and pulmonary arterial hypertension. Turk Kardiyol Dern Ars. 2013;41:604–609. doi: 10.5543/tkda.2013.93385. [DOI] [PubMed] [Google Scholar]
- 9.Harbaum L., Baaske K.M., Simon M., et al. Exploratory analysis of the neutrophil to lymphocyte ratio in patients with pulmonary arterial hypertension. BMC Pulm Med. 2017;17:72. doi: 10.1186/s12890-017-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mair K.M., Johansen A.K., Wright A.F., Wallace E., MacLean M.R. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol. 2014;171:567–579. doi: 10.1111/bph.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro S., Traiger G.L., Turner M., et al. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141:363–373. doi: 10.1378/chest.10-3114. [DOI] [PubMed] [Google Scholar]
- 12.Lin C.Y., Kwon H., Rangel Rivera G.O., et al. Sex differences in using systemic inflammatory markers to prognosticate patients with head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2018;27:1176–1185. doi: 10.1158/1055-9965.EPI-18-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zabell J.R., Larson G., Koffel J., et al. Use of the modification of diet in renal disease equation for estimating glomerular filtration rate in the urologic literature. J Endourol. 2016;30:930–933. doi: 10.1089/end.2016.0143. [DOI] [PubMed] [Google Scholar]
- 14.Fest J., Ruiter T.R., Groot Koerkamp B., et al. The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: The Rotterdam Study. Eur J Epidemiol. 2019;34:463–470. doi: 10.1007/s10654-018-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Li J., Wang X. The neutrophil-lymphocyte ratio is associated with postoperative mortality of cardiac surgery. J Thorac Dis. 2021;13:67–75. doi: 10.21037/jtd-20-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paliogiannis P., Fois A.G., Sotgia S., et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. 2018;27 doi: 10.1183/16000617.0113-2017. 170113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S., Eliot M., Koestler D.C., Wu W.C., Kelsey K.T. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson Heart Study and modification by the Duffy antigen variant. JAMA Cardiol. 2018;3:455–462. doi: 10.1001/jamacardio.2018.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard R., Kanetsky P.A., Egan K.M. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9:19673. doi: 10.1038/s41598-019-56218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan T.P., Arekapudi A., Metha J., Prasad A., Venkatraghavan L. Neutrophil-lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: a systematic review. ANZ J Surg. 2015;85:414–419. doi: 10.1111/ans.13036. [DOI] [PubMed] [Google Scholar]
- 20.Yaylak B., Ede H., Baysal E., Altıntas B., et al. Neutrophil/lymphocyte ratio is associated with right ventricular dysfunction in patients with acute inferior ST-segment elevation myocardial infarction. Cardiol J. 2016;23:100–106. doi: 10.5603/CJ.a2015.0061. [DOI] [PubMed] [Google Scholar]
- 21.Forget P., Khalifa C., Defour J.P., et al. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10:12. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azab B., Camacho-Rivera M., Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabuchi Y., Shinka S., Ishida H. The effects of anesthesia and surgery on count and function of neutrophils. J Anesth. 1989;3:123–131. doi: 10.1007/s0054090030123. [DOI] [PubMed] [Google Scholar]
- 24.Romeo C., Cruccetti A., Turiaco A., et al. Monocyte and neutrophil activity after minor surgical stress. J Pediatr Surg. 2002;37:741–744. doi: 10.1053/jpsu.2002.32268. [DOI] [PubMed] [Google Scholar]
- 25.Keen J., Prisco S.Z., Prins K.W. Sex differences in right ventricular dysfunction: insights from the bench to bedside. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.623129. 623129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung J.-H., Lee Y.-M., Lee E.-G., Yoo W.-H., Lee W.-S. Neutrophil-to-lymphocyte ratio in diagnosis of systemic sclerosis for prediction of interstitial lung disease. J Respir Dis. 2017;24:138–142. [Google Scholar]
- 27.Farber H.W., Miller D.P., Poms A.D., et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 28.Galiè N., Channick R.N., Frantz R.P. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.01889-2018. 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.