Abstract
Small proteins specifically refer to proteins consisting of less than 100 amino acids translated from small open reading frames (sORFs), which were usually missed in previous genome annotation. The significance of small proteins has been revealed in current years, along with the discovery of their diverse functions. However, systematic annotation of small proteins is still insufficient. SmProt was specially developed to provide valuable information on small proteins for scientific community. Here we present the update of SmProt, which emphasizes reliability of translated sORFs, genetic variants in translated sORFs, disease-specific sORF translation events or sequences, and remarkably increased data volume. More components such as non-ATG translation initiation, function, and new sources are also included. SmProt incorporated 638,958 unique small proteins curated from 3,165,229 primary records, which were computationally predicted from 419 ribosome profiling (Ribo-seq) datasets or collected from literature and other sources from 370 cell lines or tissues in 8 species (Homo sapiens, Mus musculus, Rattus norvegicus, Drosophila melanogaster, Danio rerio, Saccharomyces cerevisiae, Caenorhabditis elegans, and Escherichia coli). In addition, small protein families identified from human microbiomes were also collected. All datasets in SmProt are free to access, and available for browse, search, and bulk downloads at http://bigdata.ibp.ac.cn/SmProt/.
Keywords: Ribosome profiling, Small open reading frame, Upstream open reading frame, Variants, Disease
Introduction
Genome annotation is fundamental to life science. In recent years, it has been found that small open reading frames (sORFs) widely exist in genomes of many organisms including humans [1] and human microbiomes [2], and some are able to be translated into small proteins [3], [4], [5]. Small proteins are proteins with less than 100 amino acids, which may be derived from untranslated regions (UTRs) of mRNAs [6] or non-coding RNAs (ncRNAs) [7], [8] including primary microRNAs (pri-miRNAs) [9], [10], long ncRNAs (lncRNAs) [11], and circular RNAs (circRNAs) [12]. Small proteins were usually missed in previous coding sequence annotation, while their significance has been revealed in current years for diverse functions [13], such as embryonic development [14], [15], cell apoptosis [16], muscle contraction [17], and antimicrobial activity [18]. Some small proteins play roles in multiple diseases [19], [20] including tumors [9], [11], [12]. Despite the abundance of sORFs in genome, the number of well-studied small proteins is very limited. Annotation of numerous small proteins will contribute to studies on various physiological and pathological processes.
Identification of small proteins at proteomic level is challenging. Mass spectrometry (MS) can provide direct evidence of small proteins, but it relies much on the coverage of existing libraries, which mainly focus on large proteins rather than small proteins. Protease cleavage sites are lacking in small proteins due to the limited length. Besides, small proteins are usually of low abundance, and tend to be filtered out during enrichment process [21]. Ribosome profiling (also named as ribosomal footprinting or Ribo-seq) provides a more sensitive way for global detection of translation events based on the deep sequencing of ribosome-protected mRNA fragments (RPFs) [22], [23], which allows for identifying the location of translated ORFs and translation initiation sites (TISs), the distribution of ribosomes on mRNA, and the speed of translating ribosomes [24]. Reference libraries for MS can also be constructed with Ribo-seq data. The regular Ribo-seq (rRibo-seq) utilizes cycloheximide (CHX) [25], a drug bound at the ribosome E-site [26], as a translation elongation inhibitor to freeze the translating ribosomes. Translation is principally regulated at the initiation stage. Translation initiation sequencing (TI-seq) is a variation of rRibo-seq technique that uses different translation inhibitors, usually lactomidomycin (LTM) [25] or harringtonine (HARR) [27], which can induce ribosome stasis at TISs. TI-seq enables the global mapping of TISs, and is more accurate in prediction of non-ATG start codons. Many sORFs are proved to use non-classical ATG start codons [28], which is also an important mechanism for generating protein isoforms [29], [30]. rRibo-seq data usually show clear triplet periodicity [26]. Different computational analysis strategies [31], [32], [33], [34], [35], [36], [37], [38] have been developed to identify translated sequences using Ribo-seq data.
Emerging evidence shows that many upstream ORFs (uORFs) act in cis to regulate the translation of downstream ORFs by leaky scanning [39], reinitiation [40], and ribosome stalling [41]. Recently, variants creating new upstream start codons or disrupting stop sites of existing uORFs (uORF-perturbing) are found to be under strong negative selection [42]. uORF-perturbing variants have been demonstrated as an under-recognized functional class that contribute to human disease.
Given the great importance of small proteins, in-depth investigations of small proteins across various species are in need. SmProt is dedicated to integrating knowledge of small proteins translated from various sources, especially for those from UTRs and ncRNAs. The annotation information and functional sections in the current release are much richer than those in the initial release [43], and the data volume and reliability are also greatly improved.
Data collection and processing
Data sources
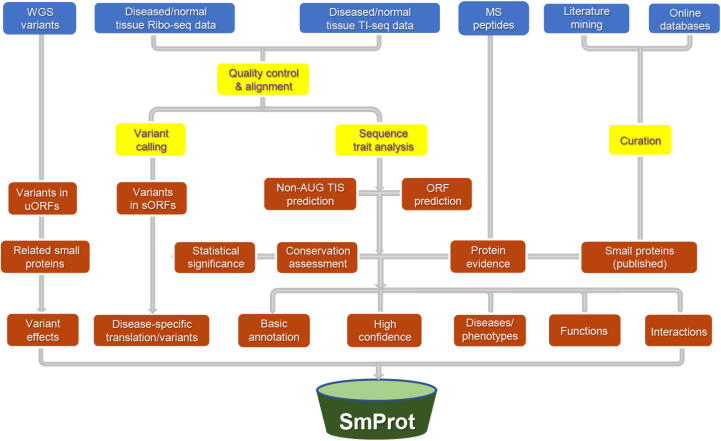
rRibo-seq and TI-seq datasets derived from diverse tissues/cell lines were collected from GEO [44] and European Nucleotide Archive (ENA) [45] databases. The latest reference genomes and gene annotations were download from Ensembl [46], GENCODE [47], and NCBI-Genome database. Whole-genome sequencing (WGS) variants were collected from various websites. The construction pipeline of SmProt is summarized in Figure 1.
Figure 1.
Construction of SmProt. Items in blue background represent data sources. Items in yellow background represent management processes. Items in red background represent results. WGS, whole genome sequencing; MS, mass spectrometry; TIS, translation initiation site; ORF, open reading frame; sORF, small ORF; uORF, upstream ORF.
Ribo-seq data processing
The fastq files of 547 Ribo-seq datasets were downloaded from GEO and ENA databases. Each dataset was checked manually to confirm the sequencing adapters. The adapters were removed using cutadapt 1.18 [48] and only reads with 25−35 bp in length were retained. Then the sequences were mapped to the latest genome using STAR 2.5.2a [49] using EndToEnd mode with allowance of up to 2 mismatches.
Ribo-seq quality and P-site offsets were assessed by Ribo-TISH [34] quality module. For TI-seq data, more attention was put on TIS quality (-t). Manual checks were then carried out to verify offset values and eliminate datasets without obvious triplet periodicity. After the quality control, 419 Ribo-seq datasets (Table S1) were retained.
Translated ORFs were predicted by Ribo-TISH predict module. Biological and technical duplication data under the same treatment in one dataset were merged. Minimum amino acid length of candidate ORFs was set to 5. Considering both ATG and near-cognate start codons (with one base different from ATG), rRibo-seq datasets using only CHX without matched TI-seq data were analyzed twice. One is prediction of ORFs with canonical ATG start codon, the other is prediction of ORFs with near-cognate start codons (--alt). Preferring data evidence instead of prior assumption in our database, only the best frame test results from multiple candidate start codons in the same ORF were reported (--framebest). For datasets containing TI-seq data, alternative start codons were included (--alt), and different parameters were set for LTM-based TI-seq and HARR-based TI-seq (--harr).
sORFs with less than 100 amino acids were filtered from the prediction results above. Furthermore, we removed some prediction results that may be supported by RPFs from other classic proteins with more than 100 amino acids. These include ORFs marked as known (i.e., TIS annotated in another transcript), CDSFrameOverlap (i.e., ORF overlapping with annotated CDS in another transcript in the same reading frame), and Truncated (i.e., ORF as a part of annotated CDS in the same transcript) without translation initiation evidence (i.e., no significant results identified from paired TI-seq datasets).
In-frame reads of sORFs were counted and normalized by library sequencing depth (in-frame total reads count) and sORF length, a similar method with reads per kilobase per million mapped reads (RPKM) in RNA-seq but using ribosome profiling data that represents the translation levels.
Finally, 3,060,793 records (i.e., unmerged primary results from all datasets and tissues) were retained. Results with the identical genome loci in one species were merged as the same small protein, generating 577,206 unique IDs, while information derived from multiple datasets were retained, a similar integration method as for piRBase [50].
Variants from ribosome profiling data
We performed germline variants detection on 96 human ribosome profiling datasets, referring to the workflow for processing RNA data for germline short variant discovery with GATK v4.1.8 [51], [52], [53], [54]. Duplicate reads were identified using MarkDuplicates tool after alignment, then reads with unidentified nucleotide (N) in Cigar were split using SplitNCigarReads tool. Base quality score recalibration was carried out based on true sites in training sets using BaseRecalibrator tool and applied using ApplyBQSR tool. Variants were called individually in each sample using the HaplotypeCaller tool. Variants with QualByDepth (QD) < 2 were removed using VariantFiltration tool. Germline single nucleotide variants (SNVs) were linked to small proteins in SmProt according to genomic positions.
Variants from WGS data
Variants from 1KGP3 [55], GAsP [56], TOPMed [57], gnomAD3 [42], [58], and NyuWa [59] were collected. VCF files were lifted over from old genome version to GRCh38 using LiftoverVcf tool of GATK with allowance to recover swapped reference and alternative alleles.
Variants in 5′ UTRs were evaluated for their effects on uORFs using VEP [60] with plugin UTRannotator [42], [61], and classified by their functional consequences. Translation evidence of uORFs was based on small proteins recorded in SmProt.
Disease-specific small proteins
Small proteins identified only from diseased cell lines/tissues but not from corresponding normal cell lines/tissues were predicted as disease-specific translation events. If there were matched data of normal and diseased groups in the same dataset, small proteins derived uniquely from diseased group were screened as disease-specific ones. If there was no matched control group in the same dataset, the same type of healthy tissue/cell line in other datasets were used as control. If there was no matched same tissue/cell line, all data from diverse normal tissues/cell lines were merged for comparisons (Table S2), and small proteins identified only from the diseased cell lines/tissues were predicted as tissue-specific. Disease-specific or tissue-specific translation events require Ribo P value in disease groups lower than 0.01 while similar proteins with different TISs at the same loci in control group not detected (Ribo P value higher than 0.05).
SNVs in diseased cell lines/tissues derived from ribosome profiling data and located within the genomic region of small proteins were regarded as diseased variant sets. SNVs detected only in diseased variant sets but not in normal sets were predicted as disease-specific SNVs. SNVs in corresponding normal cell lines/tissues (Table S2) derived from ribosome profiling data were combined with all variants derived from multiple WGS projects, as control variant sets for comparison.
Function domain prediction
Besides function of small proteins collected from literature mining, we used InterProScan [62] to predict function domain of small proteins, which focuses on combination of protein family membership and the functional domains/sites, and has been extensively used by genome sequencing projects and the UniProt Knowledgebase [63]. Default thresholds and additional parameters -goterms -pa were adopted for gene oncology and pathway annotations.
PhyloCSF calculation
Pre-calculated BigWig data of PhyloCSF [64] scores at each base across the whole genome were downloaded from Broad Institute (https://data.broadinstitute.org/compbio1/PhyloCSFtracks/), and the score for genomic region of each small protein was extracted with our script using pyBigWig (https://github.com/deeptools/pyBigWig).
Database implementation
Database website was organized with HTML (https://html.spec.whatwg.org/), JavaScript (https://www.javascript.com/), PHP (https://www.php.net/), and MYSQL (https://www.mysql.com/). UCSC Genome Browser (http://genome.ucsc.edu/) was used to visualize the small proteins and variants. NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used for sequence similarity searches.
Database content and usage
Overview
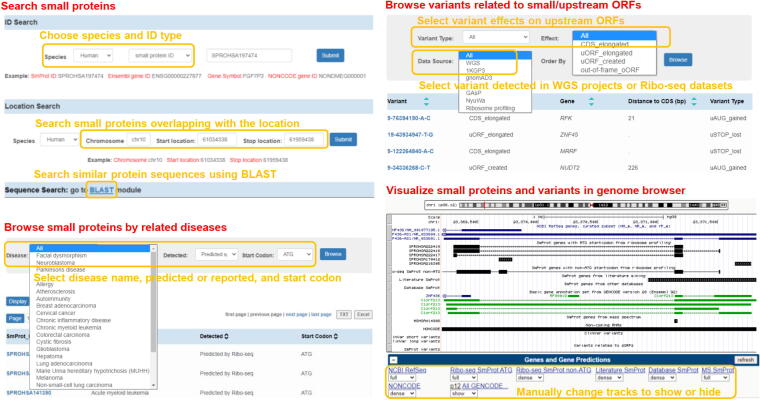
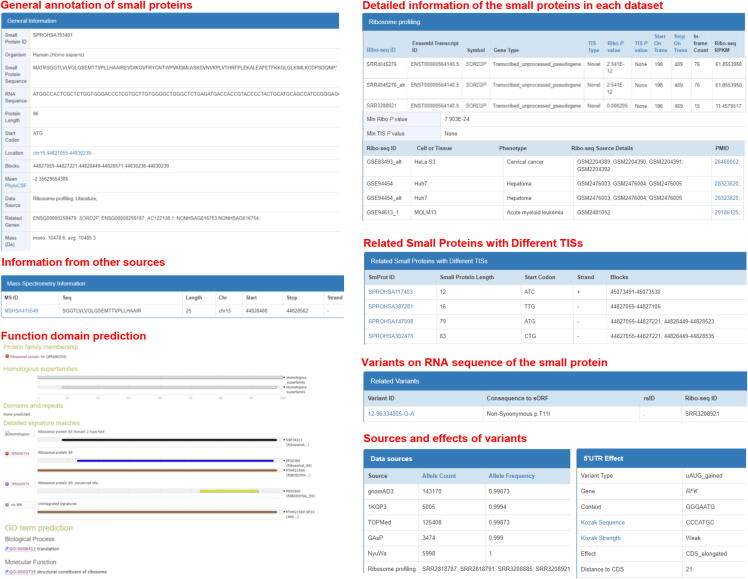
SmProt was constructed by pipeline described in Figure 1. Multiple ways were provided to search, browse, visualize, and study small proteins (Figure 2). Small proteins were found mainly from rRibo-seq and TI-seq data. All information for small proteins from different data sources and datasets were integrated. General information for small proteins was provided such as sequence, mass, location, blocks, tissue or cell line, predicted functions, conservation, and multiple IDs including small protein ID, Ensembl ID, and NONCODE [65] ID. Translation level (in frame counts and Ribo RPKM) of small proteins identified from each dataset and record was provided. Details for their related variants and diseases were also provided (Figure 3). SmProt now has 638,958 unique small proteins and 3,165,229 small protein records in total (Table 1; Table S3).
Figure 2.
Usage of SmProt. SmProt provides multiple ways to search, browse, and visualize small proteins, as well as related diseases and variants.
Figure 3.
Contents of SmProt. Detailed information for small proteins are provided, including general annotation, information from ribosome profiling data, literature, other databases, MS, function domain prediction, related diseases, and related variants from WGS projects, as well as corresponding effects.
Table 1.
Statistics of unique small proteins in SmProt
| Source | Start codon | Human | Mouse | Fruit fly | Rat | C. elegans | Yeast | E. coli | Zebrafish | All species examined |
|---|---|---|---|---|---|---|---|---|---|---|
| Ribo-seq | ATG | 70,931 | 48,909 | 5269 | 3560 | 4334 | 4535 | 1881 | 1924 | 141,343 |
| Near-cognate codons | 229,653 | 133,037 | 29,679 | 9910 | 9894 | 12,339 | 10,004 | 1347 | 435,863 | |
| Literature | ATG and near-cognate codons | 38,157 | 8875 | 22,228 | 163 | 4 | 355 | 296 | 3612 | 73,690 |
| Databases | ATG and near-cognate codons | 786 | 797 | 100 | 271 | 120 | 336 | 955 | 64 | 3429 |
| MS | ATG and near-cognate codons | 768 | 51 | 66 | 38 | 0 | 3 | 0 | 1 | 927 |
| All IDs examined | ATG and near-cognate codons | 327,995 | 189,433 | 56,574 | 13,829 | 14,255 | 17,312 | 12,881 | 6679 | 638,958 |
Note: Small protein families from human microbiomes are not included. Near-cognate codons refer to non-ATG start codons that differ from the canonical ATG start codon by a single base but are able to initiate translation, such as TTG, GTG, CTG, AAG, AGG, ACG, ATA, ATT, and ATC. ID refers to a unique entry with identical genomic loci in one species. Ribo-seq, ribosome profiling; MS, mass spectrometry.
Reliability of small proteins
SmProt emphasizes reliability of small proteins, which is ensured mainly by the significance of 3-nt periodicity in RPF P-site profile.
Firstly, we constructed new pipeline based on independently published toolkit Ribo-TISH [34], which allows for accurate detection of ORFs and TISs using rRibo-seq and TI-seq. Ribo-TISH uses rank sum test to detect 3-nt periodicity, and uses negative binomial test to detect TISs, which outperforms other established methods in prediction accuracy.
Secondly, in addition to the quality control based on Ribo-TISH quality module, manual checks were also carried out to ensure clear triplet periodicity and unambiguous offset of Ribo-seq data, which further eliminates noises.
Thirdly, we provided several evaluations as supporting evidence. These include 1) P values of small proteins called from multiple ribosome profiling datasets, which indicate the confidence in different samples and conditions; 2) PhyloCSF conservation of genomic regions, which reflects coding potential; and 3) peptide evidence derived from mass spectrum data. All these lines of evidences are exhibited in the small protein page. Moreover, a set with evidence of both translation events and protein fragments is provided on download page.
In addition, information of small protein derived from multiple sources is also integrated in small protein information page.
Variants related to small proteins
In total, 25,475 variants located on translated sORFs were provided, which are on display in the related small protein page. Given that uORF-perturbing variants are likely to impact translation of downstream proteins [42], variants from multiple WGS projects and ribosome profiling data were evaluated for their effects on uORFs. These include creating a new start codon ATG, removing an existing start codon ATG, creating a new stop codon within an existing uORF, removing the stop codon of an existing uORF, and creating a frameshift mutation in an existing uORF, which can be found at variants page.
Disease-specific small proteins
Disease-specific small proteins are potential candidates of molecular markers or targets for diagnosis and treatment. Disease-specific translation events as well as disease-specific SNVs of small proteins in 16 types of diseases were identified (see “Data collection and processing” section) (Table S4). Besides, small proteins that have been verified experimentally in certain diseases were also documented through literature mining.
Human microbiome small proteins
Over 4000 conserved small protein families identified from human microbiomes were collected [2]. A new section HumanMicroBio was created to integrate and display selected information of these small protein families.
Other sources
We use a set of keywords (File S1) to search articles about small proteins in PubMed database. High-confidence small proteins in CCDS [66] and Swiss-Prot [67] were also integrated. Literature mining is processed in stages, and the newly-published data from other sources will be released continuously after completion of manual review and curation.
Function domain prediction
For successfully predicted functions of small proteins derived from ribosome profiling and literature mining, SmProt provides graph for visualization and prediction details including Gene Oncology (GO) and pathway annotations. Users can choose predicted functions on Browse page to filter the results with function domain prediction.
Inner BLAST
The abundant small proteins across multiple species allow for sequence similarity searches at both nucleotide and protein levels. Users can search for sequences of interests using BLASTp and BLASTx (NCBI BLAST 2.2.24 release) online.
Visualization using UCSC Genome Browser
SmProt incorporates UCSC Genome Browser [68] to visualize all the information including genomic loci of small proteins, as well as variants from ribosome profiling data and multiple WGS projects related to small proteins, MS data, and gene annotations. The latest genome versions including hg38, mm10, rn6, dm6, ce11, sacCer3, and danRer11 were provided.
Comparison with other databases
SmProt currently includes 419 Ribo-seq datasets derived from 116 cell lines/tissues, compared to 60 datasets derived from 37 cell lines/tissues in the initial version. The number of small protein records identified from ribosome profiling in the current release is 60 times that of the initial release (3 million vs. 0.05 million). The current release of SmProt combined a large amount of duplicate records in the initial release [43], and Ribo-seq analysis pipeline was optimized to ensure the reliability of our results. Variants in translated sORFs identified from Ribo-seq data as well as uORF-perturbing variants identified from WGS projects were provided. Disease-specific small proteins may provide new perspectives for clinical studies.
Currently, there are a few databases for small proteins such as ARA-PEPs [69], PsORF [70], and sORFs.org [71]. ARA-PEPs and PsORF only harbor small proteins in plants. sORFs.org developed simple inner TIS-calling algorithm not based on triplet periodicity, which should be the most important feature of Ribo-seq. SmProt emphasizes high confidence using our Ribo-TISH pipeline that is more accurate than previous methods. In total, 419 Ribo-seq datasets have been analyzed in SmProt, while there were only 78 Ribo-seq datasets in sORFs.org. Moreover, SmProt pays special attention to function, variants, and related diseases of small proteins. Furthermore, WGS data resources are also integrated in SmProt, which are not covered in other databases.
Other proteomic databases such as UniProt, neXtProt [72], and OpenProt [73] are not specifically designed for small proteins. neXtProt only harbors proteins of humans while SmProt harbors small proteins of 8 species. Simialr to SmProt, OpenProt also used ribosome profiling and mass spectrum to predict proteins including some small proteins longer than 30 amino acids. Nonetheless, SmProt has analyzed many more ribosome profiling datasets (419), which are about 5 times that in OpenProt (87), and provides information for small proteins longer than 5 amino acids.
Conclusion
In brief, SmProt integrates small proteins from large amount of ribosome profiling data, and provides more abundant details. We strongly believe that SmProt will provide valuable and accurate information on small proteins for scientific community. Moreover, SmProt provides a new resource for users interested in functional and mechanistic studies, and a reference for construction of MS libraries of small proteins.
Data availability
SmProt is publicly available at http://bigdata.ibp.ac.cn/SmProt/.
Competing interests
The authors have declared no competing interests.
Handled by Zhang Zhang
2021 The Authors. Published by Elsevier B.V. and Science Press on behalf of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nd-nd/4.0/).
Orcid
0000-0001-5256-6696 (Yanyan Li)
0000-0001-7409-3092 (Honghong Zhou)
0000-0002-0633-2984 (Xiaomin Chen)
0000-0003-4936-8407 (Yu Zheng)
0000-0001-6790-5259 (Quan Kang)
0000-0003-0082-0730 (Di Hao)
0000-0002-3601-0150 (Lili Zhang)
0000-0003-2967-7704 (Tingrui Song)
0000-0001-9944-0345 (Huaxia Luo)
0000-0003-1384-4176 (Yajing Hao)
0000-0001-6049-8347 (Runsheng Chen)
0000-0001-9303-1639 (Peng Zhang)
0000-0002-7294-0865 (Shunmin He)
CRediT authorship contribution statement
Yanyan Li: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Software, Visualization. Honghong Zhou: Investigation, Data curation, Funding acquisition. Xiaomin Chen: Investigation, Data curation. Yu Zheng: Data curation, Software, Visualization. Quan Kang: Software, Visualization. Di Hao: Data curation, Software. Lili Zhang: Visualization. Tingrui Song: Visualization. Huaxia Luo: Writing – review & editing. Yajing Hao: Writing – review & editing. Runsheng Chen: Resources, Supervision, Funding acquisition. Peng Zhang: Conceptualization, Methodology, Investigation, Software, Writing – review & editing, Visualization, Project administration, Funding acquisition. Shunmin He: Conceptualization, Methodology, Resources, Investigation, Writing – review & editing, Supervision, Funding acquisition.
Acknowledgments
This work was supported by the National Key R&D Program of China (Grant No. 2016YFC0901702); National Natural Science Foundation of China (Grant Nos. 81902519, 91940306, 31871294, 31701117, and 31970647); the National Key R&D Program of China (Grant Nos. 2017YFC0907503, 2016YFC0901002, and 2018YFA0106901); the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB38040300); the 13th Five-year Informatization Plan of Chinese Academy of Sciences (Grant No. XXH13505-05); Special Investigation on Science and Technology Basic Resources, Ministry of Science and Technology, China (Grant No. 2019FY100102); and the National Genomics Data Center, China. We thank Center for Big Data Research in Health (http://bigdata.ibp.ac.cn/), Institute of Biophysics, Chinese Academy of Sciences, for supporting data analysis and computing resource. We thank Prof. Yiwen Chen from The University of Texas MD Anderson Cancer Center, USA for thoughtful discussions and valuable comments on the database.
Handled by Zhang Zhang
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gpb.2021.09.002.
Contributor Information
Runsheng Chen, Email: crs@ibp.ac.cn.
Peng Zhang, Email: zhangp@ibp.ac.cn.
Shunmin He, Email: heshunmin@ibp.ac.cn.
Supplementary material
The following are the Supplementary data to this article:
Keywords used for mining small proteins from literature.
References
- 1.Basrai M.A., Hieter P., Boeke J.D. Small open reading frames: beautiful needles in the haystack. Genome Res. 1997;7:768–771. doi: 10.1101/gr.7.8.768. [DOI] [PubMed] [Google Scholar]
- 2.Sberro H, Fremin BJ, Zlitni S, Edfors F, Greenfield N, Snyder MP, et al. Large-scale analyses of human microbiomes reveal thousands of small, novel genes. Cell 2019;178:1245–59.e14.. [DOI] [PMC free article] [PubMed]
- 3.Bazzini A.A., Johnstone T.G., Christiano R., Mackowiak S.D., Obermayer B., Fleming E.S., et al. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014;33:981–993. doi: 10.1002/embj.201488411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith J.E., Alvarez-Dominguez J.R., Kline N., Huynh N.J., Geisler S., Hu W., et al. Translation of small open reading frames within unannotated RNA transcripts in Saccharomyces cerevisiae. Cell Rep. 2014;7:1858–1866. doi: 10.1016/j.celrep.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Heesch S, Witte F, Schneider-Lunitz V, Schulz JF, Adami E, Faber AB, et al. The translational landscape of the human heart. Cell 2019;178:242−60.e29. [DOI] [PubMed]
- 6.Calvo S.E., Pagliarini D.J., Mootha V.K. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci U S A. 2009;106:7507–7512. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu S., Wang J., He Y., Meng N., Yan G.R. Peptides/proteins encoded by non-coding RNA: a novel resource bank for drug targets and biomarkers. Front Pharmacol. 2018;9:1295. doi: 10.3389/fphar.2018.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L.J., Leng R.X., Fan Y.G., Pan H.F., Ye D.Q. Translation of noncoding RNAs: focus on lncRNAs, pri-miRNAs, and circRNAs. Exp Cell Res. 2017;361:1–8. doi: 10.1016/j.yexcr.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Fang J., Morsalin S., Rao V.N., Reddy E.S.P. Decoding of non-coding DNA and non-coding RNA: pri-micro RNA-encoded novel peptides regulate migration of cancer cells. J Pharm Sci Pharmacol. 2017;3:23–27. [Google Scholar]
- 10.Razooky B.S., Obermayer B., O'May J.B., Tarakhovsky A. Viral infection identifies micropeptides differentially regulated in smORF-containing lncRNAs. Genes (Basel) 2017;8:206. doi: 10.3390/genes8080206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang JZ, Chen M, Chen D, Gao XC, Zhu S, Huang H, et al. A peptide encoded by a putative lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell 2017;68:171−84.e6. [DOI] [PubMed]
- 12.Zhang M., Zhao K., Xu X., Yang Y., Yan S., Wei P., et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9:4475. doi: 10.1038/s41467-018-06862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couso J.P., Patraquim P. Classification and function of small open reading frames. Nat Rev Mol Cell Biol. 2017;18:575–589. doi: 10.1038/nrm.2017.58. [DOI] [PubMed] [Google Scholar]
- 14.Freyer L., Hsu C.W., Nowotschin S., Pauli A., Ishida J., Kuba K., et al. Loss of Apela peptide in mice causes low penetrance embryonic lethality and defects in early mesodermal derivatives. Cell Rep. 2017;20:2116–2130. doi: 10.1016/j.celrep.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galindo M.I., Pueyo J.I., Fouix S., Bishop S.A., Couso J.P. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo B., Zhai D., Cabezas E., Welsh K., Nouraini S., Satterthwait A.C., et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 17.Anderson D.M., Anderson K.M., Chang C.L., Makarewich C.A., Nelson B.R., McAnally J.R., et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knappe D., Goldbach T., Hatfield M.P.D., Palermo N.Y., Weinert S., Strater N., et al. Proline-rich antimicrobial peptides optimized for binding to Escherichia coli chaperone DnaK. Protein Pept Lett. 2016;23:1061–1071. doi: 10.2174/0929866523666160719124712. [DOI] [PubMed] [Google Scholar]
- 19.Wen Y., Liu Y., Xu Y., Zhao Y., Hua R., Wang K., et al. Loss-of-function mutations of an inhibitory upstream ORF in the human hairless transcript cause Marie Unna hereditary hypotrichosis. Nat Genet. 2009;41:228–233. doi: 10.1038/ng.276. [DOI] [PubMed] [Google Scholar]
- 20.Cheng W., Wang S., Mestre A.A., Fu C., Makarem A., Xian F., et al. C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2alpha phosphorylation. Nat Commun. 2018;9:51. doi: 10.1038/s41467-017-02495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu P.Y., Benfey P.N. Small but mighty: functional peptides encoded by small ORFs in plants. Proteomics. 2018;18 doi: 10.1002/pmic.201700038. [DOI] [PubMed] [Google Scholar]
- 22.Ingolia N.T., Ghaemmaghami S., Newman J.R.S., Weissman J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingolia N.T., Lareau L.F., Weissman J.S. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. doi: 10.1016/j.cell.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss R.B., Atkins J.F. Translation goes global. Science. 2011;334:1509–1510. doi: 10.1126/science.1216974. [DOI] [PubMed] [Google Scholar]
- 25.Schneider-Poetsch T., Ju J., Eyler D.E., Dang Y., Bhat S., Merrick W.C., et al. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–217. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calviello L., Ohler U. Beyond read-counts: Ribo-seq data analysis to understand the functions of the transcriptome. Trends Genet. 2017;33:728–744. doi: 10.1016/j.tig.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Ingolia N.T., Brar G.A., Rouskin S., McGeachy A.M., Weissman J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc. 2012;7:1534–1550. doi: 10.1038/nprot.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S., Liu B., Lee S., Huang S.-X., Shen B., Qian S.-B. Global mapping of translation initiation sites in mammalian cells at single-nucleotide resolution. Proc Natl Acad Sci U S A. 2012;109:E2424–E2432. doi: 10.1073/pnas.1207846109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochetov A.V., Sarai A., Rogozin I.B., Shumny V.K., Kolchanov N.A. The role of alternative translation start sites in the generation of human protein diversity. Mol Genet Genomics. 2005;273:491–496. doi: 10.1007/s00438-005-1152-7. [DOI] [PubMed] [Google Scholar]
- 30.Oyama M., Kozuka-Hata H., Suzuki Y., Semba K., Yamamoto T., Sugano S. Diversity of translation start sites may define increased complexity of the human short ORFeome. Mol Cell Proteomics. 2007;6:1000–1006. doi: 10.1074/mcp.M600297-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Calviello L., Mukherjee N., Wyler E., Zauber H., Hirsekorn A., Selbach M., et al. Detecting actively translated open reading frames in ribosome profiling data. Nat Methods. 2016;13:165–170. doi: 10.1038/nmeth.3688. [DOI] [PubMed] [Google Scholar]
- 32.Fields A.P., Rodriguez E.H., Jovanovic M., Stern-Ginossar N., Haas B.J., Mertins P., et al. A regression-based analysis of ribosome-profiling data reveals a conserved complexity to mammalian translation. Mol Cell. 2015;60:816–827. doi: 10.1016/j.molcel.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Z, Song R, Regev A, Struhl K. Many lncRNAs, 5′ UTRs, and pseudogenes are translated and some are likely to express functional proteins. eLife 2015;4:e08890. [DOI] [PMC free article] [PubMed]
- 34.Zhang P., He D., Xu Y., Hou J., Pan B.F., Wang Y., et al. Genome-wide identification and differential analysis of translational initiation. Nat Commun. 2017;8:1749. doi: 10.1038/s41467-017-01981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malone B., Atanassov I., Aeschimann F., Li X., Grosshans H., Dieterich C. Bayesian prediction of RNA translation from ribosome profiling. Nucleic Acids Res. 2017;45:2960–2972. doi: 10.1093/nar/gkw1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raj A, Wang SH, Shim H, Harpak A, Li YI, Engelmann B, et al. Thousands of novel translated open reading frames in humans inferred by ribosome footprint profiling. eLife 2016;5:e13328. [DOI] [PMC free article] [PubMed]
- 37.Chun S.Y., Rodriguez C.M., Todd P.K., Mills R.E. SPECtre: a spectral coherence–based classifier of actively translated transcripts from ribosome profiling sequence data. BMC Bioinformatics. 2016;17:482. doi: 10.1186/s12859-016-1355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crappe J., Ndah E., Koch A., Steyaert S., Gawron D., De Keulenaer S., et al. PROTEOFORMER: deep proteome coverage through ribosome profiling and MS integration. Nucleic Acids Res. 2015;43 doi: 10.1093/nar/gku1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X.Q., Rothnagel J.A. 5'-Untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation. Nucleic Acids Res. 2004;32:1382–1391. doi: 10.1093/nar/gkh305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunišová S., Valášek L.S. Fail-safe mechanism of GCN4 translational control–uORF2 promotes reinitiation by analogous mechanism to uORF1 and thus secures its key role in GCN4 expression. Nucleic Acids Res. 2014;42:5880–5893. doi: 10.1093/nar/gku204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishimura R., Nagy G., Dotu I., Zhou H., Yang X.L., Schimmel P., et al. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science. 2014;345:455–459. doi: 10.1126/science.1249749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whiffin N., Karczewski K.J., Zhang X., Chothani S., Smith M.J., Evans D.G., et al. Characterising the loss-of-function impact of 5' untranslated region variants in 15,708 individuals. Nat Commun. 2020;11:2523. doi: 10.1038/s41467-019-10717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao Y., Zhang L., Niu Y., Cai T., Luo J., He S., et al. SmProt: a database of small proteins encoded by annotated coding and non-coding RNA loci. Brief Bioinform. 2018;19:636–643. doi: 10.1093/bib/bbx005. [DOI] [PubMed] [Google Scholar]
- 44.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvester N., Alako B., Amid C., Cerdeno-Tarraga A., Clarke L., Cleland I., et al. The European Nucleotide Archive in 2017. Nucleic Acids Res. 2018;46:D36–D40. doi: 10.1093/nar/gkx1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zerbino D.R., Achuthan P., Akanni W., Amode M.R., Barrell D., Bhai J., et al. Ensembl 2018. Nucleic Acids Res. 2018;46:D754–D761. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frankish A., Diekhans M., Ferreira A.M., Johnson R., Jungreis I., Loveland J., et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. [Google Scholar]
- 49.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Zhang P., Lu Y., Li Y., Zheng Y., Kan Y., et al. piRBase: a comprehensive database of piRNA sequences. Nucleic Acids Res. 2019;47:D175–D180. doi: 10.1093/nar/gky1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poplin R, Ruano-Rubio V, DePristo MA, Fennell TJ, Carneiro MO, Van der Auwera GA, et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 2018:201178.
- 52.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A., et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11.10.1−33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DePristo M.A., Banks E., Poplin R., Garimella K.V., Maguire J.R., Hartl C., et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sudmant P.H., Rausch T., Gardner E.J., Handsaker R.E., Abyzov A., Huddleston J., et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.GenomeAsia100K Consortium The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature. 2019;576:106–111. doi: 10.1038/s41586-019-1793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taliun D., Harris D.N., Kessler M.D., Carlson J., Szpiech Z.A., Torres R., et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature. 2021;590:290–299. doi: 10.1038/s41586-021-03205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P., Luo H., Li Y., Wang Y., Wang J., Zheng Y., et al. NyuWa Genome resource: a deep whole-genome sequencing-based variation profile and reference panel for the Chinese population. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.110017. [DOI] [PubMed] [Google Scholar]
- 60.McLaren W., Gil L., Hunt S.E., Riat H.S., Ritchie G.R.S., Thormann A., et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X., Wakeling M., Ware J., Whiffin N. Annotating high-impact 5'untranslated region variants with the UTRannotator. Bioinformatics. 2021;37:1171–1173. doi: 10.1093/bioinformatics/btaa783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones P., Binns D., Chang H.Y., Fraser M., Li W., McAnulla C., et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.UniProt Consortium UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin M.F., Jungreis I., Kellis M. PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics. 2011;27:i275–i282. doi: 10.1093/bioinformatics/btr209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He S., Liu C., Skogerb ø G, Zhao H, Wang J, Liu T, et al. NONCODE v2.0: decoding the non-coding. Nucleic Acids Res. 2008;36:D170–D172. doi: 10.1093/nar/gkm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pujar S., O'Leary N.A., Farrell C.M., Loveland J.E., Mudge J.M., Wallin C., et al. Consensus coding sequence (CCDS) database: a standardized set of human and mouse protein-coding regions supported by expert curation. Nucleic Acids Res. 2018;46:D221–D228. doi: 10.1093/nar/gkx1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haeussler M., Zweig A.S., Tyner C., Speir M.L., Rosenbloom K.R., Raney B.J., et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019;47:D853–D858. doi: 10.1093/nar/gky1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hazarika R.R., De Coninck B., Yamamoto L.R., Martin L.R., Cammue B.P.A., van Noort V. ARA-PEPs: a repository of putative sORF-encoded peptides in Arabidopsis thaliana. BMC Bioinformatics. 2017;18:37. doi: 10.1186/s12859-016-1458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y., Li D., Fan W., Zheng X., Zhou Y., Ye H., et al. PsORF: a database of small ORFs in plants. Plant Biotechnol J. 2020;18:2158–2160. doi: 10.1111/pbi.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olexiouk V., Van Criekinge W., Menschaert G. An update on sORFs.org: a repository of small ORFs identified by ribosome profiling. Nucleic Acids Res. 2018;46:D497–D502. doi: 10.1093/nar/gkx1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaudet P., Michel P.A., Zahn-Zabal M., Britan A., Cusin I., Domagalski M., et al. The neXtProt knowledgebase on human proteins: 2017 update. Nucleic Acids Res. 2017;45:D177–D182. doi: 10.1093/nar/gkw1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brunet M.A., Brunelle M., Lucier J.F., Delcourt V., Levesque M., Grenier F., et al. OpenProt: a more comprehensive guide to explore eukaryotic coding potential and proteomes. Nucleic Acids Res. 2019;47:D403–D410. doi: 10.1093/nar/gky936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Keywords used for mining small proteins from literature.
Data Availability Statement
SmProt is publicly available at http://bigdata.ibp.ac.cn/SmProt/.
Competing interests
The authors have declared no competing interests.
Handled by Zhang Zhang
2021 The Authors. Published by Elsevier B.V. and Science Press on behalf of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nd-nd/4.0/).