Abstract
Background
Trials have addressed the combined use of direct oral anticoagulants (DOACs) and antiplatelets in atrial fibrillation (AF) patients undergoing percutaneous coronary intervention (PCI). These trials may have changed prescribing patterns.
Methods
This administrative audit of Albertans with AF undergoing PCI described antithrombotic therapy before vs after publication of the PIONEER AF-PCI (An Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention) trial results.
Results
Cohorts were similar before (n = 597) and after (n = 708) trial publication: median age 72 years; 23% female; 63% with acute coronary syndrome; and 22% with bleeding history. Anticoagulant use increased by 7.0% (P = 0.01) after; with DOAC use increasing by 24.9% and warfarin use decreasing by 17.5% (P < 0.0001). DOAC use was associated with being in the “after” cohort (odds ratio 5.42, 95% confidence interval 3.75-7.82, P < 0.0001).
Conclusions
Significantly more patients were prescribed anticoagulation therapy after the publication of the results of the PIONEER AF-PCI trial than before, and the choice of agent favoured DOAC over warfarin. Almost half of patients were not on anticoagulants, a situation that requires further investigation, to ensure that AF patients are being optimally managed post-PCI.
Résumé
Contexte
Des essais se sont penchés sur l’utilisation combinée d’anticoagulants oraux directs (AOD) et d’antiplaquettaires chez les patients atteints de fibrillation auriculaire (FA) qui subissent une intervention coronarienne percutanée (ICP). Il est possible que ces essais aient donné lieu à des modifications des habitudes de prescription.
Méthodologie
Cet audit interne portant sur des Albertains atteints de FA subissant une ICP fournit une description des traitements antithrombotiques prescrits avant et après la publication des résultats de l’essai PIONEER AF-PCI (AnOpen-label, Randomized, Controlled, Multicenter Study Exploring TwoTreatment Strategies ofRivaroxaban and a Dose-Adjusted Oral Vitamin K AntagonistTreatment Strategyin Patients WithAtrialFibrillation Who UndergoPercutaneousCoronaryIntervention).
Résultats
Les caractéristiques des cohortes étudiées avant (n = 597) et après (n = 708) la publication étaient semblables : âge médian, 72 ans; 23 % des femmes; 63 % ayant des antécédents de syndrome coronarien aigu; 22 % ayant des antécédents de saignements. Après la publication, on a observé une augmentation de 7,0 % (p = 0,01) de l’utilisation d’anticoagulants, dont une augmentation de 24,9 % de l’utilisation d’AOD et une diminution de 17,5 % de l’utilisation de warfarine (p < 0,0001). Une corrélation a été établie entre le recours aux AOD et l’appartenance à la cohorte prise en charge « après » la publication des résultats de l’essai (rapport de cotes : 5,42; intervalle de confiance à 95 % : 3,75 – 7,82; p < 0,0001).
Conclusions
Après la publication des résultats de l’essai PIONEER AF-PCI, les prescriptions d’anticoagulants ont sensiblement augmenté, et ce, en faveur des AOD plutôt que de la warfarine. Près de la moitié des patients ne prenaient pas d’anticoagulants, une situation qui nécessite un examen plus approfondi afin de s’assurer que les patients atteints de FA sont pris en charge de manière optimale après une ICP.
Management of patients with both atrial fibrillation (AF) and coronary artery disease requiring percutaneous coronary intervention (PCI) requires clinicians to find a delicate balance. To prevent stroke and systemic embolism, AF guidelines recommend direct oral anticoagulants (DOACs) over vitamin K antagonists (warfarin).1 In those who have undergone PCI with stenting, dual antiplatelet therapy (DAPT) is recommended to prevent major cardiac events.2 Combining anticoagulants and antiplatelets increases bleeding risk. For this reason, randomized controlled trials have been conducted to establish regimens that minimize bleeding.
The Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) trial, published in November 2016, the first of these trials,3 showed decreased bleeding with rivaroxaban- vs warfarin-based regimens. Similar results were found with other DOACs.4, 5, 6 We sought to describe changes in the use of DOACs, warfarin, and P2Y12 inhibitors in the province of Alberta, Canada, following the publication of the PIONEER AF-PCI trial results.
Methods
This retrospective study used administrative data from Alberta, Canada, with abstraction and linkage completed by Strategy for Patient Oriented Research (SPOR). Patients were identified by their Alberta personal health number (PHN) and PCI date. A combination of International Classification of Diseases, 10th edition (ICD-10) and Canadian Classification of Health Intervention (CCI) codes was used to identify relevant conditions, events, and procedures.
Albertans undergoing angiography were identified through the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) database,7,8 which contains procedure data, clinical variables, and comorbidities. Data enhancement and verification are performed yearly through linkage with other databases. The National Ambulatory Care Reporting System (NACRS, which captures data from ambulatory and emergency visits) and the Discharge Abstract Database (DAD, which captures all hospitalizations) were used to collect diagnosis and procedure data pre- and post-PCI. Prescription fills were identified through the Pharmaceutical Information Network (PIN), which records drug dispensations (date, dose, drug name and drug identification number, and quantity dispensed) for prescriptions from 98% of outpatient pharmacies in the province. DOACs included apixaban, rivaroxaban, and dabigatran; P2Y12 inhibitors included clopidogrel and ticagrelor. Acetylsalicylic acid obtained as an over-the-counter product is not captured, so it was not included.
Alberta residents 18 years of age or older with documented nonvalvular AF who underwent PCI with stenting between January 1, 2015, and December 31, 2018 were included. Patients were excluded if they had valvular AF (defined by the 2020 Canadian Cardiovascular Society atrial fibrillation guidelines1), coronary artery bypass graft surgery or death during index admission, or any non-AF indication for anticoagulation.
The primary outcome was a comparison of the use of DOACs, warfarin, and P2Y12 inhibitors for patients whose index PCI was performed before vs after the PIONEER AF-PCI trial, with the 2 groups separated by the online publication date of November 14, 2016. The antithrombotic regimen was anticoagulants and P2Y12 inhibitors filled within 30 days after PCI. For those with no anticoagulant prescription filled within this time frame, fill records up to 120 days prior to PCI were examined for anticoagulants that may have been continued with an existing supply. To ensure that patients had an adequate supply to continue after PCI, the time between the last medication fill and PCI could not exceed 1.2 times the day supply dispensed, corresponding to a medication possession ratio (MPR) of 80%.9 P2Y12 inhibitors were recorded based only on fills within 30 days of PCI, to ensure that P2Y12 inhibitors discontinued peri-procedure were not included erroneously. Additionally, annual breakdowns spanning the study timeline for use vs non-use of anticoagulant, and DOAC or warfarin, were performed.
Patient characteristics were reported as median (interquartile range) or percentage. Comparisons of the before and after cohorts were performed using analysis of variance for continuous variables, and χ2 or Fisher’s exact test for categorical variables. Multivariable logistic regression was conducted to determine the association between PIONEER AF-PCI trial publication and the use of DOACs vs warfarin. Other covariates included patient demographics (age, sex, weight) and comorbidities (congestive heart failure, previous myocardial infarction [MI], dyslipidemia, hypertension, diabetes, stroke/transient ischemic attack [TIA], and bleeding history). Annual breakdown of anticoagulant use was evaluated using the Cochran-Armitage trend test. P-values < 0.05 were considered significant. All analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC). Ethics approval was granted by the University of Alberta Health Research Ethics Board, study ID Pro00095669.
Results
In our population of 1305 patients, 597 were in the “before” cohort, and 708 were in the “after” cohort (Table 1). The median age was 72 years; approximately 23% were female patients, and 63% had acute coronary syndrome. Approximately 49% in each group had undergone prior revascularization, and 22% had prior bleeding history. More patients in the before cohort had previous MI. CHADS2 (Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack [doubled]) and HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly [> 65 Years], Drugs/Alcohol Concomitantly) scores were similar between groups. The before and after cohorts were similar with respect to clinical factors that would affect anticoagulant use and choice of anticoagulant.
Table 1.
Baseline characteristics of “before” and “after” cohorts
| Variable | Before n = 597 |
After n = 708 |
P |
|---|---|---|---|
| Age, y | 72 (64, 79) | 72 (64, 79) | 0.33 |
| Sex (% female) | 129 (21.6) | 170 (24.0) | 0.3 |
| Patient weight, kg | 86 (75, 100) | 86 (74, 100) | 0.95 |
| Indication for cardiac catheterization | 0.9 | ||
| Acute coronary syndrome | 370 (63.0) | 442 (63.1) | |
| Other∗ | 217 (36.3) | 259 (36.6) | |
| Bare-metal stent | 88 (14.7) | 19 (2.7) | < 0.0001 |
| Drug-eluting stent | 514 (86.1) | 691 (97.6) | < 0.0001 |
| Current smoker | 87 (14.6) | 100 (14.1) | 0.5 |
| Dyslipidemia | 348 (73.3) | 379 (68.4) | 0.11 |
| Hypertension | 511 (85.6) | 604 (85.3) | 0.88 |
| Diabetes | 235 (39.4) | 278 (39.3) | 0.97 |
| Congestive heart failure | 199 (33.3) | 228 (32.2) | 0.66 |
| Previous myocardial infarction | 394 (66.0) | 415 (58.6) | 0.01 |
| Previous revascularization | 295 (49.4) | 351 (49.6) | 0.9 |
| Stroke/TIA | 91 (15.2) | 122 (17.2) | 0.33 |
| Renal impairment† | 81 (13.6) | 83 (11.7) | 0.32 |
| Bleeding history‡ | 130 (21.8) | 156 (22.0) | 0.91 |
| CHADS2 score | 0.14 | ||
| 0 | 54 (9.0) | 70 (9.9) | |
| 1 | 168 (28.1) | 170 (24.0) | |
| 2 | 190 (31.8) | 248 (35.0) | |
| 3 | 124 (20.8) | 147 (20.8) | |
| 4 | 43 (7.2) | 48 (6.8) | |
| 5 | 11 (1.8) | 23 (3.2) | |
| 6 | 7 (1.2) | 2 (0.3) | |
| Mean (SD) | 2.0 (1.2) | 2.0 (1.2) | |
| Median (IQR) | 2 (1, 3) | 2 (1, 3) | |
| HAS-BLED | 0.71 | ||
| 0 | 36 (6.0) | 47 (6.6) | |
| 1 | 131 (21.9) | 134 (18.9) | |
| 2 | 267 (44.7) | 330 (46.6) | |
| 3 | 123 (20.6) | 147 (20.8) | |
| 4 | 37 (6.2) | 43 (6.1) | |
| 5 | 3 (0.5) | 7 (1.0) | |
| Mean (SD) | 2.0 (1.0) | 2.0 (1.0) | |
| Median (IQR) | 2 (1, 3) | 2.0 (1,3) |
Values are n (%), or median (interquartile range [IQR]), unless otherwise indicated. Boldface indicates significance.
CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly; SD, standard deviation; TIA, transient ischemic attack.
Other: stable angina, atypical pain, congenital heart disease, congestive heart failure, non-ischemic cardiomyopathy, pulmonary hypertension, serious arrhythmia, valvular heart disease, and unknown.
Renal impairment defined as serum creatinine level > 200 mmol/L, renal transplant, dialysis, or any recorded diagnosis of renal dysfunction or chronic kidney disease.
Combination of ISTH (International Society on Thrombosis and Hemostasis) major and clinically relevant non-major bleeding, modified to include any transfusion as # units transfused is not reliably recorded in the databases available.
After the PIONEER AF-PCI trial, overall use of anticoagulants increased, regardless of agent (49.1% vs 56.1%, P = 0.01; Table 2). In addition, a significant change in the choice of anticoagulant occurred, with the prevalence of DOACs increasing (21.3% vs 46.2%, P < 0.0001), and use of warfarin decreasing (27.8% vs 10.3%, P < 0.0001). In particular, the use of both rivaroxaban and apixaban approximately doubled (11.9% to 30.2%, P < 0.0001 and 5.53% to 11.7%, P < 0.0001, respectively). Clopidogrel was the most commonly used P2Y12 inhibitor both before and after (66.5% and 61.6%) the trial publication. For those patients not receiving an anticoagulant, 88.5% were on a P2Y12 inhibitor.
Table 2.
Anticoagulant use before vs after publication of the PIONEER AF-PCI trial results
| Anticoagulant | Before n = 597 | After n = 708 | Total n = 1305 | P |
|---|---|---|---|---|
| No anticoagulant∗ | 304 (50.9) | 311 (43.9) | 615 (47.1) | 0.01 |
| Warfarin | 166 (27.8) | 73 (10.3) | 239 (18.3) | < 0.0001 |
| DOAC | 127 (21.3) | 324 (46.2) | 451 (34.6) | < 0.0001 |
| Rivaroxaban | 71 (11.9) | 214 (30.2) | 288 (22.1) | < 0.0001 |
| Apixaban | 33 (5.53) | 83 (11.7) | 116 (8.9) | < 0.0001 |
| Dabigatran | 23 (3.85) | 27 (3.81) | 50 (3.8) | 0.97 |
DOAC, direct oral anticoagulant; PIONEER AF-PCI, An Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention.
38 patients in the before group and 33 in the after group had records in the Pharmaceutical Information Network but did not have either anticoagulant or P2Y12 inhibitor fills recorded.
In multivariable regression, PCI after the PIONEER AF-PCI trial was significantly associated with DOAC use over warfarin use (odds ratio 5.42, 95% confidence interval 3.75-7.82, P < 0.0001). Increasing patient weight was also associated with DOAC use, and previous MI was associated with warfarin use (Table 3).
Table 3.
Logistic regression of patients receiving anticoagulants, with odds of direct oral anticoagulant use vs warfarin use
| Odds ratio (95% CI) | P | |
|---|---|---|
| Sex (female vs male) | 1.384 (0.86, 2.23) | 0.1801 |
| Increased patient age (per 1 y) | 1.007 (0.99, 1.03) | 0.5187 |
| PIONEER AF-PCI (after vs before) | 5.415 (3.75, 7.82) | < 0.0001 |
| Patient weight (per 1-kg increase) | 1.017 (1.01, 1.03) | 0.0018 |
| Previous MI | 0.643 (0.43, 0.95) | 0.0278 |
Additional factors tested that were not significant included the following: congestive heart failure, dyslipidemia, hypertension, diabetes, stroke/transient ischemic attack, CHADS2 score, and bleeding history.
CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); CI, confidence interval; MI, myocardial infarction; PIONEER AF-PCI, An Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Patients With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention.
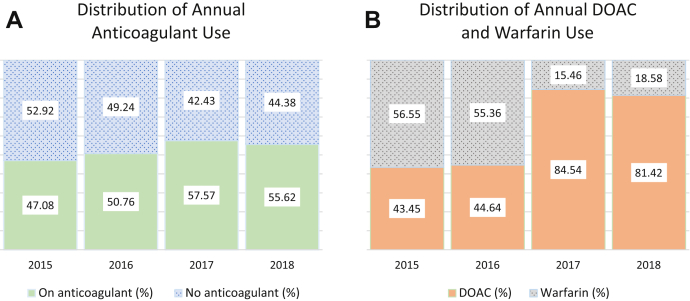
Significant differences were seen from year to year in the proportion of patients on anticoagulants—47.08% in 2015; 50.76% in 2016; 57.57% in 2017; 55.62% in 2018; P = 0.01 (Fig. 1A). A similar but more dramatic trend occurred in the preference for DOAC vs warfarin use among those who were on anticoagulants (Fig. 1B), with DOAC percentages increasing from 43.45% in 2015, to 44.64% in 2016, to 84.54% in 2017, to 81.42% in 2018 (P < 0.001).
Figure 1.
(A) Yearly comparison of the proportion of patients on an anticoagulant or no anticoagulant (n = 1305). Anticoagulant includes both warfarin and direct oral anticoagulant (DOAC). (B) Yearly comparison of the proportion of patients who were anticoagulated who were on warfarin or DOAC (n = 690). Significant differences were seen in each (P = 0.01 and < 0.001, respectively, using Cochrane-Armitage trend test).
Discussion
Our study found that the rate of DOAC use increased, and that of warfarin use decreased following the publication of the PIONEER AF-PCI trial results. Our findings are consistent with those of a contemporary Canadian single-centre cohort study that showed significantly increased DOAC use (9% to 63%) and decreased warfarin use (25% to 12%), in relation to the PIONEER AF-PCI trial. In their post–PIONEER AF-PCI cohort (2017), 25% were not on anticoagulation therapy,10 compared to 44% in our study. In their further 2019 cohort, 89% of the population was on an anticoagulant, still with a strong preference for a DOAC (84%) over warfarin (5%). Had we extended our time frame further, how the trend in our province compared to what they found would have been interesting to see.
Interesting to note is the increase in the rate of DOAC vs warfarin use, as well as the fact that the increase in anticoagulant use was larger between 2016 and 2017 than between other consecutive years. This difference aligns with the publication of the PIONEER AF-PCI study.
Although overall anticoagulant use increased, a significant proportion in both cohorts (51% before; 44% after) did not have prescriptions for anticoagulants. This lack of anticoagulant use in a population that otherwise should be eligible for it raises questions about the rationale for deviation from guideline-recommended therapy.1 The gap seen in our more specialized population is consistent with that reported in large observational studies of AF without PCI. Introduction of DOACs improved but did not eliminate this underuse.11,12
Limitations of this study include the lack of reliable data on acetylsalicylic acid use, given its availability without prescription, and the inability to determine the rationale behind prescribing choices. We collected data based on prescription fills, and thus we were unable to capture true medication adherence information. We also were unable to capture medications provided as samples, which may have been important, especially in the before cohort, as DOAC coverage through insurance was more limited at that time. Additionally, factors other than the publication of the PIONEER AF-PCI trial results may have contributed to the increased frequency of DOAC use seen with time, given the publication of updated Canadian Cardiovascular Society AF guidelines in 2016, which supported DOAC use over warfarin use for patients with AF and coronary artery disease,13 as well as concentrated efforts by industry to encourage DOAC use.
Conclusion
This study demonstrates rapid uptake of new scientific evidence into cardiology practice, as exemplified by the increased frequency of use of anticoagulation therapy and a shift in preference for DOAC use following the publication of the PIONEER AF-PCI trial results. However, there remains a large proportion of nonvalvular AF patients who are still not receiving anticoagulants, a situation that presents an opportunity for quality-improvement initiatives to optimize antithrombotic therapy in these individuals.
Acknowledgements
The authors acknowledge the contributions of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) in providing data for initial population selection.
Funding Sources
The authors have no funding sources to declare.
Disclosures
T.J.B. has received unrestricted research grants from Pfizer, not related to this research. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Ethics approval was granted by the University of Alberta Health Research Ethics Board, Study ID Pro00095669.
See page 382 for disclosure information.
References
- 1.Andrade J.G., Verma A., Mitchell L.B., et al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2018;34:1371–1392. doi: 10.1016/j.cjca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Mehta S.R., Bainey K.R., Cantor W.J., et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi: 10.1016/j.cjca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Gibson C.M., Mehran R., Bode C., et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 4.Vranckx P., Valgimigli M., Eckardt L., et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 5.Cannon C.P., Bhatt D.L., Oldgren J., et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 6.Lopes R.D., Heizer G., Aronson R., et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 7.Ghali W.A., Knudtson M.L. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. Can J Cardiol. 2000;16:1225–1230. [PubMed] [Google Scholar]
- 8.Norris C.M., Ghali W.A., Knudtson M.L., Naylor C.D., Saunders L.D. Dealing with missing data in observational health care outcome analyses. J Clin Epidemiol. 2000;53:377–383. doi: 10.1016/s0895-4356(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 9.Andrade S.E., Kahler K.H., Frech F., Chan K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu M.C., Boivin-Proulx L.A., Matteau A., et al. Evolution of antithrombotic management of atrial fibrillation after percutaneous coronary intervention over 10 years and guidelines uptake. CJC Open. 2021;3:1025–1032. doi: 10.1016/j.cjco.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubitz S.A., Khurshid S., Weng L.-C., et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J. 2018;200:24–31. doi: 10.1016/j.ahj.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzec L.N., Wang J., Shah N.D., et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. doi: 10.1016/j.jacc.2017.03.540. [DOI] [PubMed] [Google Scholar]
- 13.Macle L., Cairns J., Leblanc K., et al. 2016 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol. 2016;32:1170–1185. doi: 10.1016/j.cjca.2016.07.591. [DOI] [PubMed] [Google Scholar]