Graphical abstract
Abbreviations: IBD, Inflammatory bowel disease; EVs, Extracellular vesicles; MVs, Membrane vesicles; IECs, Intestinal epithelial cells; GI, Gastrointestinal; UC, Ulcerative colitis; CD, Crohn’s disease; SCFA, Short-chain fatty acid; FMT, Fecal microbiota transplantation; DSS, Dextran sulfate sodium; MSC, Marrow-derived mesenchymal stromal cells; NEAT1, Nuclear paraspeckle assembly transcript 1; Treg, Regulatory T cell; TNBS, 2,4,6-trinitrobenzenesulfonic acid; E-cadherin, Epithelial cadherin. EMT, Epithelial-mesenchymal transition; BMSC, bone marrow mesenchymal stem cell; TJs, tight junctions; ANXA1, Annexin A1; TLR, Toll-like receptor; E. coli, Escherichia coli; OMVs, Outer-membrane vesicles; CMVs, Cytoplasmic membrane vesicles; LPS, lipopolysaccharide; ECN, E. coli strain Nissle 1917; CRC, Colorectal cancer; PSMA7, proteasome subunit alpha type 7; hucMSC, Human umbilical cord MSC
Keywords: Inflammatory bowel disease, Extracellular vesicles, Microbiota, Intestinal barrier, Therapy
Highlights
-
•
EVs derived from different sources play modulatory functions in the intestine, especially interaction associated with microbiota.
-
•
An EV-mediated interaction system was established to describe the possible mechanism of IBD pathogenesis and its cure.
-
•
EVs-based treatments show great potential of clinical applications in IBD diagnosis and therapy.
Abstract
Background
The intestinal tract is a complicated ecosystem with dynamic homeostasis via interaction of intestine and microbiota. Inflammatory bowel disease (IBD) is chronic intestinal inflammation involving dysbiosis of intestinal microenvironment. Extracellular vesicles (EVs), as vital characteristics of cell–cell and cell-organism communication, contribute to homeostasis in intestine. Recently, EVs showed excellent potential for clinical applications in disease diagnoses and therapies.
Aim of Review
Our current review discusses the modulatory functions of EVs derived from different sources in intestine, especially their effects and applications in IBD clinical therapy. EV-mediated interaction systems between host intestine and microbiota were established to describe possible mechanisms of IBD pathogenesis and its cure.
Key Scientific Concepts of Review
EVs are excellent vehicles for delivering molecules containing genetic information to recipient cells. Multiple pieces of evidence have illustrated that EVs participate the interaction between host and microbiota in intestinal microenvironment. In inflammatory intestine with dysbiosis of microbiota, EVs as regulators target promoting immune response and microbial reconstruction. EVs-based immunotherapy could be a promising therapeutic approach for the treatment of IBD in the near future.
Introduction
The gut hosts a complex microbiota that is currently considered to be a new organ that is strongly associated with host health and multiple diseases [1], [2]. The microbiota in the intestine is initially comprised of microbes from the mother and then shaped by feeding and other environmental contacts [3]. Previous studies have shown that the contacts between the host and gut microbiota principally rely on metabolites [4], [5], nucleic acids[6] and proteins [7], [8].
Extracellular vesicles (EVs) and membrane vesicles (MVs) derived from host cells and microbiota, respectively, also participate in interactions in the intestinal microenvironment [9], [10]. Accumulated evidence has proven that vesicles, as important means of cell–cell and cell-organism communication, are essential for intestinal homeostasis [11], [12].
At present, inflammatory bowel disease (IBD) had affected more than 3.5 million people, with an increasing incidence worldwide [13]. The etiology and pathogenesis of IBD were very complex and remain unclear. In addition to genetic susceptibility and environmental pollution [14], [15], several studies reported that dysbiosis of the gut microbiota of IBD patients with abnormal metabolism of intestinal epithelial cells (IECs) is associated with a changing abundance of cargo in EVs and MVs [16]. Moreover, EVs are involved in the formation of IBD and they have roles in the regulation of the intestinal mucosa and epithelial barrier function [17] and the export of cellular metabolites [10], as well as being important regulators of immune cell recruitment [18].
Here, we will review the possible role of EVs in the interaction between the gastrointestinal (GI) tract and gut microbiota in IBD patients, especially the mechanism of action and the effects of extracellular vesicles and bacterial membrane vesicles on IBD pathogenesis. In addition, we summarized the potential utility of EVs as diagnostic markers and as a novel therapeutic approach, as well as the application of modified EVs in IBD patients.
An overview of IBD and gut microbiome
IBD is a chronic inflammatory condition of the GI tract with two main subtypes, ulcerative colitis (UC) and Crohn’s disease (CD), which are characterized by debilitating and chronic relapsing and remitting inflammation in the colon and GI tract, respectively. IBD patients have unique but inordinate microbial signatures [19], which commonly show reduced bacterial diversity [20] and a decreased abundance of Firmicutes [21], [22], [23], [24]. Meanwhile, a significantly increased abundance of Bacteroidetes and Proteobacteria, mainly Escherichia coli [25], has been reported [22], [23], as well as a decrease in obligate anaerobic producers, including Faecalibacterium prausnitzii and Roseburia intestinalis, which are specifically associated with short-chain fatty acid (SCFA) [26] production, in CD [27]. Decreased levels of SCFAs can inhibit histone deacetylase activity and modulate gene expression, cell proliferation and even the immune response [28]. In particular, reduced butyrate can aggravate colitis by increasing Treg cell production and decreasing the antibacterial activity of macrophages in GI [29].
In addition, some previous studies reported that the fungal microbiota was skewed [30] in IBD with the expansion of bacteriophages [31]. In pediatric IBD patients, the diversity of fungi also decreased, while Pacific Candida taxa were found to be increased in abundance [32]. The same results were also found in mature patients with an increased abundance of Basidiomycota/Ascomycota and decreased levels of Saccharomyces cerevisiae [30]. Another study indicated that the intestinal microenvironments of Crohn’s disease-specific bacteria might favor the proliferation of fungi at the expense of bacteria. For bacteriophages, some previous reports indicated that bacteriophage levels were significantly different in IBD patients when compared to controls [33], [34], such as Caudovirales [35], which was associated with a reduction in bacterial diversity by increasing the abundance of bacteriophage-exacerbated colitis via Toll-like receptor (TLR) 9 and IFN-g [31].
Under stimulation from dysbiosis and a disordered GI environment, the immune system and mucosal barrier integrity are impaired, leading to chronic inflammation and aberrant immune responses [36]. For instance, increasing numbers of pathogenic bacteria affect the permeability of the intestine and they are involved in the regulation of inflammatory gene expression [20], [37], resulting in intestinal inflammation[38]. Accordingly, many therapies targeting microbiota have been developed to restore the health of a diseased microbiome through diet [39], [40], prebiotics [41], antibiotics [42] and fecal microbiota transplantation (FMT) [28], [43]. However, on account of the complexity of IBD, more detail should be elucidated in many more clinical experiments.
Origin, compositions and general functions of EVs
EVs were initially described nearly 35 years ago [44] and they play a crucial role in cell-to-cell communication. EVs are classified based on their cellular origin and/or biological function or biogenesis. According to their biogenesis, EVs are divided into three main classes: exosomes, microvesicles and apoptotic bodies, with characteristic markers respectively (Table 1) [45]. Exosomes are ~40 to ~200 nm (or ~30 to ~150 nm), single-membrane, endosome-derived sEVs secreted by most cells. Briefly, exosomes are a homogeneous population of vesicles formed with the intraluminal budding of the multivesicular body membrane [45]. MVs are ~200 to ~1000 nm EVs formed by budding of the cell membrane, and apoptotic bodies (500–2000 nm) are products of apoptotic cell disassembly [46], [47]. Both microvesicles and exosomes are excellent vehicles for delivering molecules containing genetic information to recipient cells. Thus, in the following discussion, we mainly concentrate on the role of microvesicles and exosomes in IBD.
Table 1.
Three major types of EVs.
| Vesicle | Size(nm) | EV class | Origin | Protein markers |
|---|---|---|---|---|
| Exosomes | 40–200 (30–150) | Small EVa | Endosomes | CD63 (CD81 CD9) |
| Microvesicles | 200–1000 | Large EVb | Plasma membrane | Annexin A1 |
| Apoptotic bodies | 500–2000 | Large EV | Plasma membrane, endoplasmic reticulum | Annexin V |
Small EVs are <200 nm in diameter.
Large EVs are >200 nm in diameter.
There is a characteristic subset of cell-derived components packaged in EVs, including proteins, nucleic acids, lipids, and glycoconjugates [48]. As one of the important components of EV components, proteins are abundant in variety and complex in structure and can be mainly divided into two types: structural proteins (such as Alix, TSG101, CD9, CD63, and Hsp90) and specific proteins (such as MCH-Ⅰ, MCH-Ⅱ, CD80, CD86, FasL, and TGF-β) [49]. In addition, nucleotides, approximately 200 bp in size, in EVs are also protagonists for EV functions, such as genomic DNA, mitochondrial DNA and RNA. These extracellular nucleic acids can be transferred to other tissues and cells by EVs. The number of species of RNA in EVs are abundant, mainly including mRNAs and small regulatory RNAs [50], among which small noncoding RNAs are particularly enriched in exosomes [51], [52]. Compared to RNA, EVs containing DNAs are more controversial. Accumulating evidence has indicated that EVs contain various DNAs, including single- and double-stranded DNA, genomic DNA and mitochondrial DNA, and even reverse-transcribed complementary DNAs [53], [54], [55], [56], which may participate in the regulation of inflammation and are regarded as biomarkers of cancer, viral infection, and chemotherapeutic resistance [9].
However, the hypothesis that exosomes contain DNA was negated in a recent report [57]. In this research, investigators directly captured EVs by immunoaffinity to precisely characterize their constituents by virtue of high-resolution density gradient fractionation and found that small EVs are not vehicles of active DNA release. Under this circumstance, further exosomal DNA research needs more in-depth and comprehensive research with a high purity separation protocol.
In GI, EVs are mainly secreted by immune cells and IECs [58], [59]. Abundant research has convincingly shown that EVs are important contributors to the communication between the microbiota [60], IECs [58], endothelial cells [61], and immune cells [59]. EVs are the main participators in vascular and epithelial barrier function in inflamed intestines and wound healing, as well as being important regulators of immune cell recruitment. Thus, in the following sections, we will discuss in detail the role of EVs in intestinal homeostasis, intestinal barrier integrity and immune cell function in the IBD-GI tract.
Extracellular vesicles from host cells in IBD-GI
Extracellular vesicles in GI contain different molecular particles according to their parental cells, which determine the function of exosomes. Exosomes from immune cells contribute to evasion of the immune system, and IEC-derived exosomes have been proven to be important regulators of IEC-induced immune tolerance (Fig. 1). These functions are critical in exosome-mediated immune responses, intestinal barrier modulation and gut microbiota shaping in IBD.
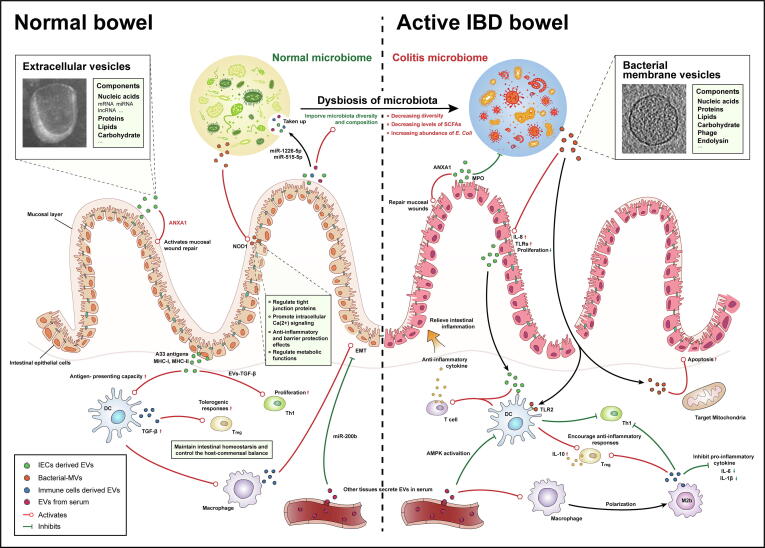
Fig. 1.
The EVs-mediated interaction in intestinal microenvironment. In intestinal microenvironment, EVs from host and commensal contribute to intestinal homeostasis maintaining, maturation of immune cells and regulation of metabolic functions. Within inflammatory bowel disease, the balance of host-commensal is destroyed, accompany by dysbiosis of microbiota, dysfunctional immune response and intestinal barrier. Intestinal EVs directly or indirectly interact with immune cells, intestinal epithelial cells and gut microbiota, participating regulation of anti-inflammation response, restoring of mucosal barrier integrity and reconstitution of microbiota composition.
Extracellular vesicles-mediated immune responses
Extracellular vesicles are involved in immunomodulation via the functional transfer of nucleotides, proteins and other cargos between immune cells. In the active IBD intestinal microenvironment, increased recruitment of innate immune cells such as macrophages, monocytes, dendritic cells (DCs), neutrophils and T-cells, takes place. Under these circumstances, EVs are tightly related to macrophages in IBD (Table 2). For example, dextran sulfate sodium (DSS)-induced colitis mice treated with exosomes from human bone marrow-derived mesenchymal stromal cells (MSC-Exos) act mainly on colonic macrophages [62], which polarize M2b macrophages without favoring intestinal fibrosis [63]. In addition, nuclear paraspeckle assembly transcript 1 (NEAT1) was also proven to be involved in the inflammatory response by polarization of macrophages [64]. Polarized M2b macrophage secretes exosomes to increase number of regulatory Treg cells and inhibit pro-inflammatory cytokine, resulting in attenuation of colitis [65] (Fig. 1). Exosomes from DSS-induced colitis mouse serum increase the phosphorylation of p38 and ERK and the production of tumor necrosis factor by macrophages [66]. Epithelial cells exposed to adherent-invasive Escherichia coli and extracellular vesicles from IBD patients’ intestinal lumen induce increasing proinflammatory responses and the migration of a significantly greater number of macrophages [67], [68]. In contrast, exosomes from visceral adipose tissue predispose the intestine to inflammation by promoting M1 macrophage polarization [69]. These studies support the hypothesis that exosomes are involved in the activation of macrophages. Furthermore, EVs have been found to have immunosuppressive effects, such as suppressing the activation of DCs to induce immune tolerance and participating in regulatory T cell (Treg) activation [18]. Activated Treg also secretes exosomes containing miR-195-3p, targeting pro-apoptotic Caspase 12, to alleviate IBD in mice [70]. IEC-derived exosomes are crucial regulators among sections, mediating the modulation of resident immune cells. In IBD development, IECs secrete epithelial cell adhesion molecule-dependent EVs with increased levels of TGF-β1 in an ERK-dependent manner to maintain the intestinal tract immune balance and decrease IBD severity [11]. In addition, IEC-derived exosomes are capable of binding to HLA-DR4 molecules and inducing productive T-cell activation by preferentially interacting with DCs [71]. These EVs also contain abundant immunomodulatory molecules, such as higher levels of major histocompatibility complex class I and class II molecules, in IBD [58], [72]. DCs are the only APCs capable of activating naive T cells, which contribute significantly to IBD pathogenesis [16]. Exosomes derived from DCs treated with IL-10 upregulate the levels of Tregs in the colonic lamina propria and relieve 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colites [73]. These immunomodulatory molecules are also essential in initiating adaptive immunity, including consolidating B-cell maturation [74].
Table 2.
A summary of EVs and their components in studies of IBD.
| EVs component | Source and type of EVs | Target | Disease model | Effects and mechanisms | References |
|---|---|---|---|---|---|
| Immune response modulation | |||||
| – | BMSC-EVs | Macrophage | DSS-induced colitis | Promote M2-like macrophage polarization, regulate the JAK1/STAT1/STAT6 signaling pathway | [62] |
| – | BMSC-Exos | Macrophage | DSS-induced colitis | Exosomal-metallothionein-2 suppress NF-κB activation | [63], [136] |
| NEAT1 | Blood serum-Exos | Macrophage | DSS-induced colitis | NEAT1 inhibition promote macrophage polarization | [64] |
| – | M2b macrophage- Exos | Tregs | DSS-induced colitis | Suppress Inflammatory cytokine, regulate CCL1/CCR8 axis | [65] |
| – | Colitis blood serum-Exos | Macrophage | – | Upregulated phosphorylation of both p38 and ERK | [66] |
| – | IBD blood serum-Exos | Colonic epithelial cells | – | Increase translation of IL-8 protein in recipient cells, induced migration of macrophages | [67] |
| miR-155 | Visceral adipose tissue-Exos | Macrophage | HFD/DSS-induced colitis | Promote M1 differentiation via transferring miR-155 | [69] |
| miR-195a-3p | Treg-Exos | Caspase 12 | DSS-induced colitis | Promote colonic epithelial cells proliferation and inhibited cell apoptosis | [70] |
| TGF-β1 | IECs-EVs | Tregs | DSS-induced colitis | EpCAM-dependent IECs-EVs with increased levels of TGF-β1 alleviate IBD | [11] |
| – | IECs-EVs | DCs | – | Induce productive T-cell activation by interacting with DCs | [71] |
| IL-10 | DCs-Exos | Tregs | TNBS-induced colitis | Increased levels of anti-Inflammatory cytokine down-regulates mRNA expression of pro-Inflammatory cytokines | [73] |
| miR-155 | DCs-Exos | – | LPS-induced IBD | miR-155 enhances inflammatory gene expression | [76] |
| miR-146a | DCs-Exos | – | LPS-induced IBD | miR-146a reduces inflammatory gene expression | [76] |
| Intestinal barrier modulation | |||||
| miR-146b | – | NF-κB pathway | DSS-induced colitis | miR-146b-mediated inhibition of the ubiquitination of TRAF proteins upstream of NF-κB. | [81] |
| miR-200b | BMSC-Exos | EMT | TNBS-induced colitis | Suppress the development of EMT by targeting ZEB1 and ZEB2. | [82], [83] |
| miR-223 | HMCs-Exos | CLDN8 | TNBS-induced colitis | miR-223 interacts with the IL23 pathway by inhitbiting CLDN8 | [85], [86] |
| ANXA1 | IECs-EVs | Epithelial FPRs | DSS-induced colitis | Target epithelial FPRs (FPR1 and FPR2/ALX) to promote wound repair | [91] |
| Gut microbial structure shaping | |||||
| – | TLR 2-deficient mouse serum EVs | TLR2/6 in probiotics | – | Inhibit the activity of Toll-like receptor (TLR)2/6 in probiotics leading microbial dysbiosis | [92] |
| miR-515-5p /miR-1226-5p | Fecal EVs | Bacterial gene transcripts | – | Enter F. nucleatum and E. coli to regulate bacterial gene transcripts and affect bacterial growth | [6] |
IBD: Inflammatory bowel disease; EVs: Extracellular vesicles; Exos: Exosomes; BMSC: Bone marrow mesenchymal stem cell; DSS: Dextran sulfate sodium; HFD: High fat diet; DCs: Dendritic cells; TNBS: 2,4,6-trinitrobenzenesulfonic acid; EMT: Epithelial-mesenchymal transition; HMCs: Human mast cells; CLDN8: Claudin 8; ANXA1: Annexin A1; FPRs: Formyl peptide receptors; TLR: Toll-like receptor.
Exosomal miRNAs are also important regulators of intestinal immune responses, modulating immune cell function and thereby coordinating the immune system in IBD (Table 2) [75]. For instance, exosomal miR155 and miR146a enter recipient DCs, mediating the repression of target genes [76]. In addition, T cells are activated by miR-1246 by activating proinflammatory nuclear factor in active IBD [77], [78]. The NF-kB signaling pathway is an important factor in the immune response activated in UC by targeting exosomal miRNAs, which promote the transcription of various proinflammatory cytokine genes, such as miR-16, an adenosine A2a receptor inhibitor mediating NF-kB signaling pathway activation [79].
In summary, the functional molecules in exosomes that express anti- and proinflammatory factors mediate immune responses in IBD pathogenesis (Fig. 1).
Extracellular vesicles-mediated intestinal barrier modulation
Intestinal barrier dysfunction is another pivotal factor in IBD pathogenesis. The mechanisms of gut barrier maintenance are disrupted in the inflammatory intestine, including downregulating epithelial cadherin (E-cadherin), reduced the thickness of the mucus layer, and dysfunction of goblet cells and Paneth cells [80]. IECs, a basic component of the intestinal barrier, are mainly affected by nucleotides in EVs from immune cells, the gut microenvironment and the ingesta. DC exosomes activate the NF-κB signaling pathway to improve intestinal barrier function in DSS-induced colitis via exosomal miR-146b [81]. lncRNA NEAT1 modulates the intestinal epithelial barrier to suppress the inflammatory response in IBD, including downregulating TNF-α and increasing the expression of CD206, IL-10, and arginase-1 [64]. In addition, epithelial-mesenchymal transition (EMT) occurs in the process of inflammation and cancer metastasis, while miR-200b plays an antifibrotic role in the inhibition of EMT by targeting Zeb1 and Zeb2 [82]. Jia and his group used bone marrow mesenchymal stem cell (BMSC)-derived microvesicles overexpressing miR-200b to prevent colon fibrosis induced by TNBS in colitis models [83]. On the contrary, another study showed a stimulating effect of exosomal miR-200b on colorectal cancer cell-derived xenografts to effective tumor growth, which indicated different roles of miRNA in the pathogenesis of IBD and colon cancer [84]. Exosomal miR-223 is a unique miRNA in IBD pathogenesis that breaches the integrity of intestinal tight junctions (TJs) by interacting with the IL-23 pathway by targeting Claudin-8 to promote IBD progression [85], [86]. miR-21, a characteristic marker in colonic cancer, is also regarded as one of the most dysregulated miRNAs with highly enriched expression in exosomes derived from various intestinal cells in IBD pathogenesis [87]. Several studies have proven that the expression of miR-21 is significantly increased and this has important implications in different phases of IBD pathogenesis [18], [87]. Deletion of miR-21 resulted in the exacerbation of both the TNBS-induced and T-cell transfer models of colitis [88], whereas decreased levels of miR-21 in T cells obtained from colonic mucosa were reported during the recovery stage of UC [87]. These evidences proved that partial same pathogenesis was found in the process of IBD and gastrointestinal cancers, according with that the occurrence of IBD increases the risk of gastrointestinal tumorigenesis. Hence, some markers of colorectal cancer, such as the levels of exosomal miR-21, should also be considered in IBD pathogenesis.
Proteins packaged in exosomes have also been demonstrated to mediate modulation of intestinal barrier function. Cellular prion protein, released from platelet-derived exosomes, maintains the function of the TJ barrier and protects lateral junctional complexes in IBD [89]. In addition, exosomal glucose-regulated protein 78 participates in cell–cell communication between colon cancer cells [90]. IECs secrete EVs containing Annexin A1 (ANXA1), a protein activating wound repair circuits, which was found to be significantly increased in sera from active IBD patients, accelerating the recovery of intestinal barrier function [91].
The gut microenvironment and ingesta are also important factors in intestinal barrier modulation, and we will discuss them in a following section. In summary, exosomal nucleotides and proteins from host cells show crucial functions in maintaining intestinal barrier function and activating intestinal wound repair circuits in IBD pathogenesis (Fig. 1, Table 2).
Extracellular vesicles-mediated gut microbial structure shaping
The importance of gut microbiota in the process of intestinal inflammation is undisputed. There are abundant conjectures about crosstalk between host and intestinal microbes. However, to date, a completely clear interaction network has not been described in IBD-GI. Several previous studies have proven that EVs from host cells participate in this network and play an important role in microbial reconstruction and dysbiosis (Fig. 1, Table 2). The activity of TLR 2/6 in probiotics is significantly reduced by EVs from TLR 2-deficient mouse sera, such as Bifidobacterium and Lactobacillus, leading to microbial dysbiosis [92]. Heat shock protein 70 (HSP70), a common component of exosomes, was able to stimulate proinflammatory responses by utilizing gram-positive (TLR2) and gram-negative bacteria (TLR4) receptors in a CD14-dependent fashion [93]. In addition, exosomal nucleic acids have also been proven to participate in microbial structure shaping. Liu investigated fecal miRNAs between humans and mice and found that several miRNAs are enriched in fecal samples as well as in intestinal extracellular vesicles. These miRNAs, such as miR-515-5p and miR-1226-5p, can enter Fusobacterium nucleatum and Escherichia coli (E. coli) to regulate bacterial gene transcripts and affect bacterial growth [6]. Moreover, fecal miRNA from WT mouse transplantation reconstitutes the colonic microbes and ameliorates colitis in IEC-miRNA-deficient mice [6].
Compared to extracellular vesicle-mediated immune responses and intestinal barrier modulation, studies about exosomal functions in the gut microbiome are scarce. Nevertheless, exosome-mediated gut microbial reconstruction is a potential strategy for manipulating the microbiome, which may become an important application in IBD and other intestinal disease therapies.
Bacterial membrane vesicles in IBD-GI
In addition to EVs from host cells, numerous MVs from gram-negative and gram-positive bacteria, fungi, and protozoa play a significant role in the GI. In general, MVs are often referred to as outer-membrane vesicles (OMVs) because they were first found to originate from controlled blebbing of the outer membrane of gram-negative bacteria [94]. However, a recent review indicated that MVs can be categorized by their different parent cells, structure and composition, including OMVs, outer-inner membrane vesicles from gram-negative bacteria and cytoplasmic membrane vesicles (CMVs) from gram-positive bacteria (Table 3) [10]. Regrettably, the vast majority of previous studies focused on OMV-related host pathology and immune tolerance.
Table 3.
Three major types of bacterial MVs.
| Vesicle | Origin | Cargo |
|---|---|---|
| OMV | Outer- membrane blebbing | Outer-membrane proteins, plasmids |
| OIMV | Explosive cell lysis and cell budding | Outer-membrane proteins, cytoplasmic (or inner) membrane proteins, RNA, DNA, plasmids |
| CMV | ‘Bubbling cell death’ and bacterial autolysins | Cytoplasmic (or inner) membrane proteins, RNA, DNA, plasmids |
OMV: outer-membrane vesicle; OIMV: outer-inner membrane vesicle; CMV: cytoplasmic membrane vesicles.
Types and general functions of MVs
Different MVs have different structures and compositions depending on the diverse routes of the formation of MVs. OMVs are bilayered lipid membrane nanostructures 20–250 nm in diameter with an outer leaflet of lipopolysaccharide (LPS) and an inner leaflet of phospholipids, and they are purposefully secreted by gram-negative bacteria [94], [95]. Their cargo can include biological components found within the parent bacterium, including LPS, periplasmic and membrane-bound proteins, DNA, RNA, enzymes, toxins and peptidoglycan [96]. Interactions between OMVs and epithelial cells can modulate the intestinal tract barrier [97], [98] and cellular mechanisms that control proliferation [99] and apoptosis [100], [101], and ultimately immune responses.
However, MVs were also observed in gram-positive bacteria without outer membranes and were thus named CMVs [10]. CMVs contain membrane and cytoplasmic components as well, also including DNA, RNA, enzymes, toxins and peptidoglycan, but no LPS.
Bacterial-MVs regulation of IBD-GI metabolism and immunity
Alterations in the intestinal microbiota have been involved in the pathogenesis and development of many diseases, as well as in IBD [102]. Homeostasis between the host and microbial communities in the intestine is implicated in the maturation and functions of IECs and various immune cells. Recent studies reported that an abundance of LPS-positive bacterial EVs was observed in patients with intestinal barrier dysfunction [103]. Consequently, bacterial EVs are also important regulators in IBD pathogenesis to modulate immunomodulation and the corresponding signaling pathways [98], [104] (Fig. 1). E. coli is mainly a pathogenic bacterium that has been observed to be significantly enriched in IBD-GI. OMVs from E. coli stimulate transcriptional upregulation of TLRs in the IEC lines HT29-19A and Caco-2, leading to a mild proinflammatory response [105] and reducing the proliferation of HT-29 cells [106]. In addition, enterohemorrhagic E. coli O157-derived OMVs stimulate TLR5 and the TLR4/MD-2 complex signaling pathway to increase the production of IL-8 in IECs [107] while targeting mitochondria to induce endothelial and epithelial apoptosis [108]. On the other hand, probiotic E. coli strain Nissle 1917 (ECN) secretes OMVs that contribute to intestinal homeostasis by activating NOD1 signaling pathways and regulating the expression of TJ proteins to enhance barrier function in IECs [109], [110]. Moreover, ECN-derived OMVs were proven to mediate anti-inflammatory and barrier protection effects and to relieve UC in experimental colitis [111].
Probiotics are crucial components in the intestine that demonstrate significantly decreased abundance in IBD pathogenesis. In the case of Bacteroides fragilis, OMVs from B. fragilis can interact with DCs via TLR2 to induce the differentiation of IL‐10‐producing regulatory T cells and suppress TNBS-induced colitis [112]. OMVs also interact within DCs to induce IL‐10 expression by regulatory T cells via the IBD‐associated gene ATG16L1 and could relieve intestinal inflammation in a colitis model [12]. Note, some regulations of bacterial-MVs are found only in healthy individuals rather than IBD, such as Bacteroides thetaiotaomicron [113]. The bacterium Akkermansia muciniphila is a vital strain in the healthy human intestinal tract that has a crucial role in the regulation of gut barrier homeostasis and metabolic functions [114]. OMVs from A. muciniphila are involved in gut permeability via regulation of intestinal TJs to improve metabolic functions [97]. Stentz reported that Bacteroides thetaiotaomicron-derived mammalian InsP6 phosphatase was packaged inside OMVs, defending gastrointestinal proteases and interacting with intestinal epithelial cells to promote intracellular Ca2+ signaling [115].
Moreover, enteric pathogenic bacteria take advantage of OMVs to achieve bacterial invasion. For instance, OMVs from Campylobacter jejuni, a major cause of bacterial food-borne gastroenteritis, increase levels of bacterial adhesion and invasion via cleavage of IEC E-cadherin and occluding [116]. Vibrio cholerae, a major virulence factor of the diarrheal disease cholera, secretes OMVs with cholera toxin. These OMVs are internalized via the cholera toxin receptor on IECs [117] and activate the MAPK and NF-κB signaling pathways in a NOD1-dependent manner, resulting in increased expression of proinflammatory cytokines. In addition, IECs interacting with OMVs activate DCs to promote T cell polarization toward an inflammatory Th2/Th17 response [118].
Accumulating evidence has demonstrated the importance of bacterial MVs in intestinal homeostasis and IBD pathogenesis (Fig. 1). However, the vast majority of studies have concentrated on the function of MVs from gram-negative rather than gram-positive bacteria. In reality, the abundance of obligate anaerobic Clostridia is significantly decreased in both CD and UC (introduced in chapter 2). Thus, the association between probiotic such as Clostridia-secreting CMVs and intestinal diseases needs more attention from researchers to demonstrate the mechanisms of probiotics and to develop new therapies related to CMVs and OMVs for IBD pathogenesis.
The interacted network of intestinal microenvironments
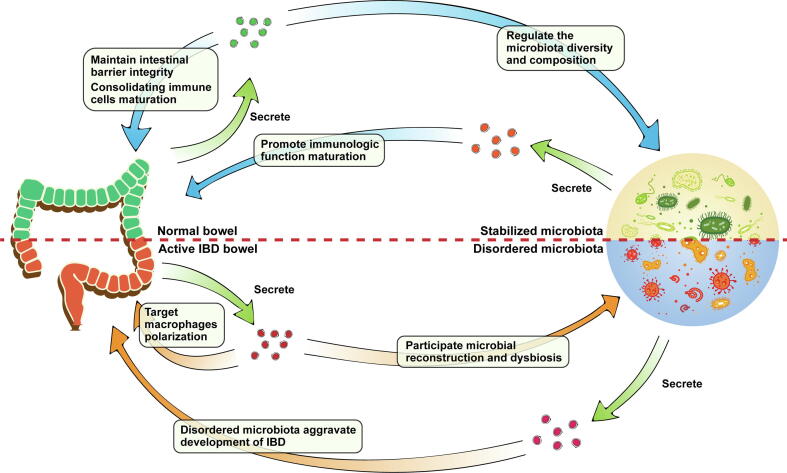
The interacted between host and gut commensal had been demonstrated in abundant previous stuides. However, the roles of EVs-based interacted network in inflammatory intestine is not clear. We summarized a compendious interacted network to show the importance of EVs in intestinal microenvironments base on existing work and above description (Fig. 2). In normal bowel, EVs contribute maturation of immune cells and maintaining intestinal barrier integrity. Meanwhile, EVs regulate the microbiota diversity and composition to promote intestinal homeostasis indirectly. In this microenvironment, microbiota with stabilized community structure secrete beneficial MVs to promote immunologic function perfection. When the intestines are under inflammatory conditions induced by stress from intestinal microenvironments, cyclic EVs are transported to intestine targeting macrophages polarization and associated with microbial reconstruction. These EVs contain various biomarkers of IBD and promote expressing anti- or pro-inflammatory factors mediate immune responses. On the contrary, MVs from microbiota with dysbiosis aggravate dysfunctions of intestinal mucosal barrier and levels of inflammation.
Fig. 2.
A compendious host-EVs-microbiota loop in normal and inflammatory intestine. In normal bowel, EVs-based interaction between host and microbiota to maintain intestinal homeostasis. Under inflammatory conditions, intestinal EVs target macrophages to promote anti- or pro-inflammatory factors expression mediating immune responses. MVs from disordered microbiota aggravate development of IBD.
In summary, EVs-based interacted loop within intestine contributes homeostasis of normal intestine, whereas show double roles in IBD. However, the EVs-based interacted network in intestinal microenvironment, an extremely enormous ecosystem, is difficultly described clearly without abundant in-depth work. Consequently, more systematic work associated with functional EVs in intestinal microenvironment need to be reported to demonstrate the irreplaceable roles of host-EVs-microbiota loop.
The clinical potential of EVs and MVs in IBD
EVs play a basic role in cell–cell and cell-organism communication and have shown strong potential in clinical diagnosis and therapies. Therefore, a large number of studies have explored their therapeutic value with high expectations from academia and industries.
EVs as biomarkers in IBD clinical diagnosis
The advantages of EV-dependent diagnosis have been demonstrated in numerous studies. Compared to traditional histopathology diagnosis, regarding EVs as biomarkers in various diseases, they have higher targeting, provide more abundant biological information and have a reduced invasiveness of collection, which is more acceptable to patients, especially in cancer and inflammation diagnoses [119]. In human colorectal cancer (CRC), the proteomics and miRNA transcriptomics of serum have shown that multiple miRNAs and proteins are significantly altered in patients with gastrointestinal cancer [120], [121], [122]. Exosomal miR-19a with a significantly increased expression level was identified as a prognostic biomarker for the recurrence of CRC [121]. Likewise, SPARC and LRG1, extracellular matrix-related proteins, were found to promote metastasis in right-sided colon cancer serum [120]. The risk of intestinal cancer is markedly increased in IBD patients, as indicated in a number of studies [123], [124], [125]. Currently, many biomarkers are extensively used in the clinic, such as serum C-reactive protein levels, the expression of the NOD2 gene, and inflammatory cytokines, while none can be used separately for an accurate diagnosis of IBD due to nonspecificity [126]. Therefore, it is essential to establish an EV-dependent diagnostic system with high accuracy and convenience from the IBD patients’ serum, luminal contents or feces. Calcium-dependent phospholipid-binding protein ANXA1 is an important proresolving mediator associated with epithelial wound repair via Rac2-dependent NADPH oxidase-1 and it is an inhibitor of inflammation [127], [128], [129]. Subsequently, ANXA1 was observed packaged in EVs derived from IECs and had elevated levels in sera from active IBD patients [91]. ANXA1 mimetic peptide encapsulated within targeted polymeric nanoparticles accelerated murine colonic wound healing and the recovery from experimental colitis [91]. Accordingly, increasing levels of ANXA1 in IEC-derived EVs may become a specific diagnostic approach for IBD clinical diagnosis. In addition, Zheng demonstrated that exosomes isolated from saliva contained proteasome subunit alpha type 7 (PSMA7), showed significant differences between IBD patients and healthy controls [130]. Compared to existing diagnostic methods, sampling saliva PSMA7 is more convenient and painless, avoiding the pain of colonoscopy in clinical diagnosis. However, the expression of PSMA7 was also found to be overexpressed in colorectal cancer [131], which demonstrated that higher purity is essential for a comprehensive analysis of EVs as potential biomarkers in IBD. Moreover, EVs containing nucleotides also have the potential to be biomarkers of IBD, such as exosomal miR214 and NEAT1 lncRNA, which are both found at high levels in active IBD [64], [132].
With the development of methods for EV purification and omics analysis, a large number of exosomal biomarkers for IBD have been reported, including proteins and nucleotides [133]. Regrettably, there is no purification technology that is convenient, efficient, steady, and has a high rate of recovery and operability for EVs targeting clinical samples, which seriously restricts the development of clinical applications. On the other hand, there is still a lack of research on screening different cell- or tissue-specific EV markers, and the sorting mechanism of EVs in cells is not clear. Consequently, multiple problems of EVs associated with separation, preservation and application need further study.
The therapeutic effect of exogenous EVs on IBD
Recently, accumulated evidence about therapeutic methods associated with EVs from different cells and tissues has adequately demonstrated the unique advantage of EVs in the treatment of multiple diseases. In colorectal cancer therapy, tumor derived EVs were eliminated to inhibited tumor metastasis and immune escape in a large number of studies [134]. In inflammatory diseases therapy, EV-related treatment methods can be mainly divided into two categories, regarding EVs from stem cells, immune cells, ingesta and microenvironment as a new remedy, or considered nanocarriers to accomplish the purpose of targeted therapy.
Stem cells derived EVs
EVs from stem cells contribute to the self-renewal, immunomodulation, expansion and damage repair abilities of stem cells [135]. Compared to stem cell therapy, the application of SC-EVs avoids the potential risk of stem cell transplantation and is more convenient for transport and storage. Currently, MSCs-EVs have been proven in a number of experimental and clinical studies as potentially new remedies for the therapy of cancer and autoimmune and inflammatory diseases [136], [137], [138], [139], including IBD. Several previous studies demonstrated that MSC therapy of colitis was mainly dependent on inhibition of colon macrophages, downregulation of proinflammatory cytokine levels and the suppression of NF-κB signaling pathways in BMSC-EV treatment [62], [140], which was especially amplified after inflammatory exposure [141]. Similar therapeutic effects were also observed for Adipose-, Olfactory ecto- and human umbilical cord MSC (hucMSC)-derived EVs injection of DSS-induced colitis [142], [143], [144]. In addition, Wu and colleagues showed that hucMSC-exosomes modulate inflammatory levels in DSS-induced colitis by regulating the ubiquitin modification level (Fig. 3) [145], [146]. Moreover, MSCs-EVs are also excellent nanocarriers to transport small molecule drugs, which is elaborated on in the following section.
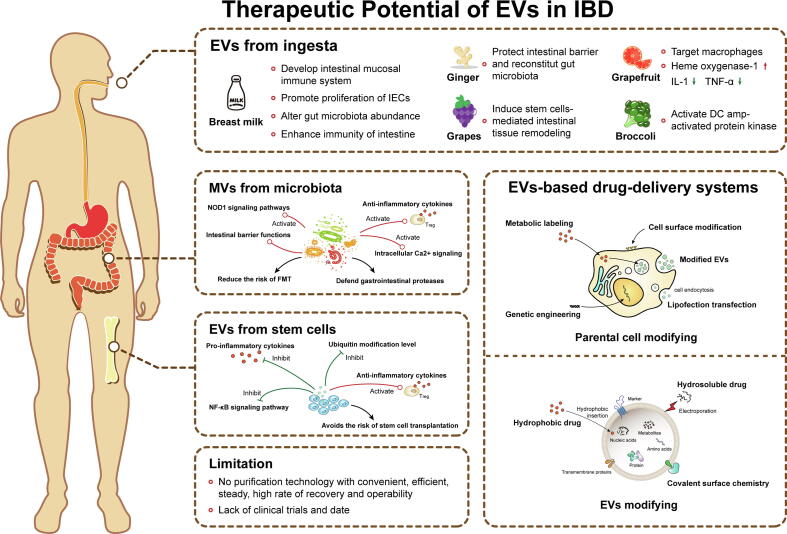
Fig. 3.
The therapeutic potential of EVs in IBD. Both of modified and unmodified EVs have showed great clinical application potential in IBD therapy. Unmodified EVs are mainly secreted from stem cells, microbiota and daily ingested food. These EVs can be taken up by intestinal cells and bacteria to relieve IBD via anti-inflammation response regulation and balance of intestinal homeostasis. The technology of EVs modification can be divided in two mainly types, parental cell modifying and direct EVs modifying. Both of two modified methods can obtain EVs containing small molecule drug and functional nucleic acid. The EVs-based therapy can reduce the risk of existing clinical therapy for IBD, but a large number of experimental and clinical studies need to be done before these therapies could be widely used in clinical treatment.
Consequently, therapy associated with MSCs-EVs is part and parcel of SCT, which represents a new, cell-free version of SCT, and has been investigated in various related diseases. Nevertheless, as a novel remedy for inflammatory diseases, a large number of experimental and clinical studies need to be done before MSC-EVs could be widely used in clinical therapy.
EVs from ingesta
In addition to EVs from host cells and the microenvironment, daily intake of EVs, such as from plants and milk, is also one of the primary sources of EVs, which are generally considered safe [18], [147]. These ingesta-derived EVs possess anti-inflammatory potential and are more acceptable to the public owing to the popularity of dietetic therapy.
Breast milk is a sole nutritional supply to newborn infants and plays a crucial role in the development of the infant intestinal mucosal immune system and microenvironment [148]. Breast milk is also known to be a rich source of EVs, with early milk containing more EVs than mature milk [149], and these EVs participate in the maturation of the infant’s immune system [150]. In Gl, breast milk-derived EVs and their components (miRNAs) survive digestion in vitro and are taken up by intestinal cells [151], [152], [153]; numerous studies have also indicated that EVs, regardless of the origin (human or different animals’ breast milk), promote the proliferation of intestinal epithelial cells, alter gut microbiota and enhance immunity of the intestine [154], [155], [156], [157], [158]. Hence, fresh milk may have the potential to prevent IBD [159] (Fig. 3).
Daily ingested food, such as fruits and vegetables, and derived exosome-like nanoparticles, also interact with the intestinal mucosa by osmosis (Fig. 3). Wang and colleagues demonstrated that exosome-like vesicles released from grapefruit were selectively taken up by intestinal macrophages and modulated increasing levels of heme oxygenase-1 and inhibition of IL-1 and TNF-α [160]. Consequently, they further developed an EV-based oral delivery system to realize targeted drug delivery to macrophages [160].
Moreover, grape and broccoli secrete exosome-like nanoparticles to relieve DSS-induced colitis by inducing stem cell-mediated intestinal tissue remodeling and activating DC amp-activated protein kinase, respectively [161], [162]. Ginger, the rhizome of Zingiber officinale, is one of the most widely used natural products and has been used as a spice and medicine for the treatment of some digestive tract problems [163]. Ginger-derived exosome-like nanoparticles were proven to be taken up by IECs and macrophages, contributing to protection of the intestinal barrier and reconstitution of the gut microbiota [163], [164].
In summary, food-derived EVs are closely related to homeostasis of the intestine and microbiota (Fig. 3). Studies associated with these vesicles have achieved encouraging outcomes in experimental animal models and have been accepted by the public. However, no clinical trials have been published to date.
EVs as nanocarriers
With the development of nanotechnology, nanobased drug-delivery systems have attained considerable importance. Multitudinous nanobased drug formulations have been applied in experimental and clinical therapy to improve therapeutic efficacy. For instance, macrophage-targeted siRNA and 5-aminosalysilic acid are packaged in nanoparticles and delivered to inflamed areas [165], [166]. However, the nanoparticles could not cross the border of the cytomembrane to realize efficient utilization of the drugs, as well as suffering from a deficiency of precise targeting.
Compared to other nanocarriers, EVs are highly regarded as vectors in the clinic, especially in cancer therapy, due to their nanoscale size, ideal native structure and characteristics [167], [168], [169]. The methods of modified EVs manufacture divided in two mainly types, parental cell modifying and direct EVs modifying (Fig. 3). In IBD, various effective proteins, RNA and small molecule drugs can be directly or indirectly packaged in transformed EVs from IECs, human embryonic kidney cells and immune cells, and then taken up by the intestine [64], [76]. MSCs-EVs are also excellent carriers. miRNA-200b and miRNA-146a were packaged in MSC-EVs via recombinant lentivirus construction and showed attenuation of experimental colitis [83], [170]. In addition, bone marrow-derived DCs were transfected with miRNA-155 and miRNA-146a mimics to secrete miRNA-overexpressing EVs, which were subsequently taken up by recipient DCs to regulate inflammation in LPS-injected mice [76]. Antibiotics are frequently used in inflammatory diseases to protect against bacterial infection. Researchers loaded antibiotics in exosomes from IECs to resist intracellular infections of methicillin-resistant Staphylococcus aureus, which is also an excellent application in IBD therapy [171].
The application of EVs as nanocarriers in IBD is not intensive and comprehensive. Some data have reported that the delivery ability of EV carriers can be increased via modification of their surface. For instance, exosomes fused with liposomes possess less immunogenicity and have superior colloidal stability [168]. In summary, as competitive nanocarriers for drug delivery, modified EVs have shown numerous possibilities in novel IBD therapy. However, it is not sufficient to accumulate evidence about experimental studies without clinical trials (Fig. 3). Ultimately, the wide application of EV-based drug delivery systems in clinical treatment depends on standardized production of either derived exosomes or modified exosomes as drug delivery systems.
MVs from microbiota
FMT is a popular novel therapy that uses intestinal flora reconstruction to repair disruption of the normal microbial communities, but it has a higher risk for IBD patients [172]. In contrast, transplantation of microbiota-derived MVs shows gentler growth and lower risk. The importance of bacterial MVs in intestinal homeostasis and IBD pathogenesis was minutely described in the previous section (Fig. 3). In addition, GI parasites, hookworms in particular, and their derived EVs, interact with host cells and bacteria. Hookworms have been used in clinical trials to treat IBD and celiac disease. Trichinella spiralis EVs was proved significantly ameliorating colitis via targeting inhiation of macrophage polarization [173], [174]. Eichenberger and colleagues indicated that helminth EVs show great potential applications in the development of remedies for dysregulated immune system-induced chronic noninfectious disease treatment [175]. Evidence regarding EVs from microbiota as a remedial approach for IBD is comparatively scarce. More mechanisms of interaction associated with EV-based communication between the host and intestinal microenvironment need to be identified and applied in clinical therapy.
Future directions
Obviously, the highly potent modulation and therapeutic effects of EVs within the IBD intestinal microenvironment are clearly evident and well documented. Notwithstanding, there is still massive work to be done in future studies. EVs have been regarded as ideal vectors for drugs transfer and delivery, and has more prospects in the applications of EVs. Accordingly, it is necessary to formulate standardized production, as MISEV 2018 [176], with regard to EVs modification, clinical preparation method, administration route and dosage, among other challenges. In addition, more details associated with mechanisms of EVs within the IBD need to be provided, so as to propose the possible pathogenesis related to EVs. As for the research of clinical application, future studies should try to combine with the clinical trials on the use of EVs-based diagnostic and therapeutic tool in IBD. Moreover, we believe that it is a prospective direction to modulate microbial structure or intestinal homeostasis targeting EVs-based interactive system in future studies.
Conclusion
In the current review, we mainly discussed the roles of EVs from different origins in the pathogenesis and progression of IBD, as well as their potential in clinical diagnosis and therapy. The development and study of EVs will build new understandings of cell–cell and cell-organism interactive communication and help develop novel therapeutic methods integrating safe, efficient and accurate targeting for the treatment and management of various conditions.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Qichen Shen: Conceptualization, Writing - original draft, Visualization. Zhuizui Huang: Data curation, Writing - review & editing. Jiachen Yao: Visualization. Yuanxiang Jin: Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21777146).
Biographies

Qichen Shen is a doctoral degree research scholar at College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310032, Zhejiang Province, China. He already published lots of scientific research articles associated with intestinal microbiota on the international recognized peer-review journal. For the last two years, He was doing researches on the interaction within intestinal microenvironment in inflammatory bowel disease under Professor. E-mail: shenqc1028@163.com

Zhuizui Huang is a master degree research scholar at College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310032, Zhejiang Province, China. She has published several scientific research articles related to the gut microbiota. Recently, under the guidance of the professor, she has been engaged in the study of the environmental pollution-mediated interaction between host and gut microbiota. E-mail: 654753668@qq.com

Jiachen Yao completed her bachelor degree from Faculty of Technology, University of Turku, Turku, 20014, Finland, and has gained admission of Master’s Programme in Health Informatics, Karolinska Institutet, Stockholm, Sweden. She has abundant experience on academic article writing. She is currently focusing on metabolomics and transcriptomic analysis. E-mail: jiayao@utu.fi

Yuanxiang Jin is professor of College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310032, Zhejiang Province, China. He has more than 126 research and review articles in the global recognized peer-review journal with high impact factor (h-Index=38). He is currently focusing research on environmental pollution-mediated interaction between host and gut microbiota in intestinal microenvironment. He has reviewed lots of articles with most cited in the recognized international scientific journals. E-mail: jinyx@zjut.edu.cn
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Findley K., Williams D.R., Grice E.A., Bonham V.L. Health disparities and the microbiome. Trends Microbiol. 2016;24:847–850. doi: 10.1016/j.tim.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mändar R.M.M. Transmission of mother’s microflora to the newborn at birth. Biol Neonate. 1996:30–35. doi: 10.1159/000244275. [DOI] [PubMed] [Google Scholar]
- 4.Cani P.D. Gut microbiota: Changes in gut microbes and host metabolism: squaring the circle? Nat Rev Gastroenterol Hepatol. 2016;13:563–564. doi: 10.1038/nrgastro.2016.135. [DOI] [PubMed] [Google Scholar]
- 5.Turroni S., Brigidi P., Cavalli A., Candela M. Microbiota-host transgenomic metabolism, bioactive molecules from the inside. J Med Chem. 2018;61:47–61. doi: 10.1021/acs.jmedchem.7b00244. [DOI] [PubMed] [Google Scholar]
- 6.Liu S., da Cunha A.P., Rezende R.M., Cialic R., Wei Z., Bry L., et al. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe. 2016;19:32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sberro H., Fremin B.J., Zlitni S., Edfors F., Greenfield N., Snyder M.P., et al. Large-Scale analyses of human microbiomes reveal thousands of small. Novel Genes. Cell. 2019;178:1245–1259. doi: 10.1016/j.cell.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Fernandez I.A., Qi Y., Jasper H. Loss of a proteostatic checkpoint in intestinal stem cells contributes to age-related epithelial dysfunction. Nature Commun. 2019;10:1050. doi: 10.1038/s41467-019-08982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 10.Toyofuku M., Nomura N., Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13–24. doi: 10.1038/s41579-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 11.Jiang L., Shen Y., Guo D., Yang D., Liu J., Fei X., et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. 2016;7:13045. doi: 10.1038/ncomms13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu H.T., Khosravi A., Kusumawardhani I.P., Kwon A.H.K., Vasconcelos A.C., Cunha L.D. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 14.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin C., Yuan X., Wang C., Fu Z., Jin Y. Maternal exposure to imazalil disrupts intestinal barrier and bile acids enterohepatic circulation tightly related IL-22 expression in F0, F1 and F2 generations of mice. J Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123668. [DOI] [PubMed] [Google Scholar]
- 16.Chang X., Wang S.-L., Zhao S.-B., Shi Y.-H., Pan P., Gu L., et al. Extracellular vesicles with possible roles in gut intestinal tract homeostasis and IBD. Mediators Inflamm. 2020;2020:1–14. doi: 10.1155/2020/1945832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bui T.M., Mascarenhas L.A., Sumagin R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers. 2018;6 doi: 10.1080/21688370.2018.1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocansey D.K.W., Zhang L., Wang Y., Yan Y., Qian H., Zhang X., et al. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc. 2020;95:1287–1307. doi: 10.1111/brv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huttenhower C., Kostic A.D., Xavier R.J. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014;40:843–854. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzosa E.A., Sirota-Madi A., Avila-Pacheco J., Fornelos N., Haiser H.J., Reinker S., et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shim J.O. Gut microbiota in inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:17–21. doi: 10.5223/pghn.2013.16.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker Alan W, Sanderson Jeremy D, Churcher Carol, Parkes Gareth C, Hudspith Barry N, Rayment Neil, et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non- inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knights D., Silverberg M.S., Weersma R.K., Gevers D., Dijkstra G., Huang H.L., et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014:107. doi: 10.1186/s13073-014-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V., et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Bio. 2012:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gevers D., Kugathasan S., Denson L.A., Vazquez-Baeza Y., Van Treuren W., Ren B., et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane E.R., Zisman T.L., Suskind D.L. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63–73. doi: 10.2147/JIR.S116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokol H., Leducq V., Aschard H., Pham H.P., Jegou S., Landman C., et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gogokhia L., Buhrke K., Bell R., Hoffman B., Brown D.G., Hanke-Gogokhia C., et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25(285–99) doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chehoud C., Albenberg L.G., Judge C., Hoffmann C., Grunberg S., Bittinger K., et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1948–1956. doi: 10.1097/MIB.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman J.M., Handley S.A., Baldridge M.T., Droit L., Liu C.Y., Keller B.C., et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepage P., Colombet J., Marteau P., Sime-Ngando T., Dore J., Leclerc M. Dysbiosis in inflammatory bowel disease: a role for bacteriophages? Gut. 2008;57:424–425. doi: 10.1136/gut.2007.134668. [DOI] [PubMed] [Google Scholar]
- 35.Wagner J., Maksimovic J., Farries G., Sim W.H., Bishop R.F., Cameron D.J., et al. Bacteriophages in gut samples from pediatric Crohn's disease patients: metagenomic analysis using 454 pyrosequencing. Inflamm Bowel Dis. 2013;19:1598–1608. doi: 10.1097/MIB.0b013e318292477c. [DOI] [PubMed] [Google Scholar]
- 36.Schirmer M., Smeekens S.P., Vlamakis H., Jaeger M., Oosting M., Franzosa E.A., et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(1125–36) doi: 10.1016/j.cell.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schirmer M., Franzosa E.A., Lloyd-Price J., McIver L.J., Schwager R., Poon T.W., et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol. 2018;3:337–346. doi: 10.1038/s41564-017-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed I., Roy B.C., Khan S.A., Septer S., Umar S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms. 2016;4:20. doi: 10.3390/microorganisms4020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis J.D., Chen E.Z., Baldassano R.N., Otley A.R., Griffiths A.M., Lee D., et al. Inflammation, antibiotics, and diet as environmental stressors of the gut microbiome in pediatric crohn's disease. Cell Host Microbe. 2015;18:489–500. doi: 10.1016/j.chom.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng S.C., Ananthakrishnan A.N. New approaches along the IBD course: diet, tight control and stem cells. Nat Rev Gastroenterol Hepatol. 2018;16:82–84. doi: 10.1038/s41575-018-0088-4. [DOI] [PubMed] [Google Scholar]
- 41.Mack D.R. Probiotics in inflammatory bowel diseases and associated conditions. Nutrients. 2011;3:245–264. doi: 10.3390/nu3020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perencevich Molly, Burakoff R. Use of antibiotics in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:651–664. doi: 10.1097/01.MIB.0000225330.38119.c7. [DOI] [PubMed] [Google Scholar]
- 43.Paramsothy S., Paramsothy R., Rubin D.T., Kamm M.A., Kaakoush N.O., Mitchell H.M., et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11:1180–1199. doi: 10.1093/ecco-jcc/jjx063. [DOI] [PubMed] [Google Scholar]
- 44.Pan BT, RM J. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983;33:967–78. [DOI] [PubMed]
- 45.Ela S., Mager I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 46.Gurunathan S., Kang M.H., Jeyaraj M., Qasim M., Kim J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 49.He C., Zheng S., Luo Y., Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–255. doi: 10.7150/thno.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y., El Andaloussi S., Wood M.J. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 51.Wei Z., Batagov A.O., Schinelli S., Wang J., Wang Y., El Fatimy R., et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 2017;8:1145. doi: 10.1038/s41467-017-01196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shurtleff M.J., Yao J., Qin Y., Nottingham R.M., Temoche-Diaz M.M., Schekman R., et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A. 2017;114:E8987–E8995. doi: 10.1073/pnas.1712108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balaj L., Lessard R., Dai L., Cho Y.J., Pomeroy S.L., Breakefield X.O., et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kahlert C., Melo S.A., Protopopov A., Tang J., Seth S., Koch M., et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sansone P., Savini C., Kurelac I., Chang Q., Amato L.B., Strillacci A., et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114:E9066–E9075. doi: 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., et al. Reassessment of exosome composition. Cell. 2019;177(428–45) doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Niel G., Raposo G., Candalh C., Boussac M., Hershberg R., Cerf-Bensussan N., et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterol. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 59.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Nedawi K., Mian M.F., Hossain N., Karimi K., Mao Y.K., Forsythe P., et al. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J. 2015;29:684–695. doi: 10.1096/fj.14-259721. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto S., Niida S., Azuma E., Yanagibashi T., Muramatsu M., Huang T.T., et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci Rep. 2015;5:8505. doi: 10.1038/srep08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao L., Xu H., Wang G., Liu M., Tian D., Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol. 2019;72:264–274. doi: 10.1016/j.intimp.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Liu H., Liang Z., Wang F., Zhou C., Zheng X., Hu T., et al. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu R., Tang A., Wang X., Chen X., Zhao L., Xiao Z., et al. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int J Mol Med. 2018;42:2903–2913. doi: 10.3892/ijmm.2018.3829. [DOI] [PubMed] [Google Scholar]
- 65.Yang R., Liao Y., Wang L., He P., Hu Y., Yuan D., et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi: 10.3389/fimmu.2019.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong W.Y., Lee M.M., Chan B.D., Kam R.K., Zhang G., Lu A.P., et al. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 2016;16:1131–1145. doi: 10.1002/pmic.201500174. [DOI] [PubMed] [Google Scholar]
- 67.Mitsuhashi S., Feldbrugge L., Csizmadia E., Mitsuhashi M., Robson S.C., Moss A.C. Luminal extracellular vesicles (EVs) in inflammatory bowel disease (IBD) exhibit proinflammatory effects on epithelial cells and macrophages. Inflamm Bowel Dis. 2016;22:1587–1595. doi: 10.1097/MIB.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carriere J., Bretin A., Darfeuille-Michaud A., Barnich N., Nguyen H.T. Exosomes released from cells infected with crohn's disease-associated adherent-invasive Escherichia coli activate host innate immune responses and enhance bacterial intracellular replication. Inflamm Bowel Dis. 2016;22:516–528. doi: 10.1097/MIB.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 69.Wei M., Gao X., Liu L., Li Z., Wan Z., Dong Y., et al. Visceral adipose tissue derived exosomes exacerbate colitis severity via pro-inflammatory MiRNAs in high fat diet fed mice. ACS Nano. 2020;14:5099–5110. doi: 10.1021/acsnano.0c01860. [DOI] [PubMed] [Google Scholar]
- 70.Liao F., Lu X., Dong W. Exosomes derived from T regulatory cells relieve inflammatory bowel disease by transferring miR-195a-3p. IUBMB Life. 2020 doi: 10.1002/iub.2385. [DOI] [PubMed] [Google Scholar]
- 71.Mallegol J., Van Niel G., Lebreton C., Lepelletier Y., Candalh C., Dugave C., et al. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterol. 2007;132:1866–1876. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 72.Mallegol J., van Niel G., Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol Dis. 2005;35:11–16. doi: 10.1016/j.bcmd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Yang X., Meng S., Jiang H., Chen T., Wu W. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol. 2010;45:1168–1177. doi: 10.3109/00365521.2010.490596. [DOI] [PubMed] [Google Scholar]
- 74.Merkenschlager J., Eksmond U., Danelli L., Attig J., Young G.R., Nowosad C., et al. MHC class II cell-autonomously regulates self-renewal and differentiation of normal and malignant B cells. Blood. 2019;133:1108–1118. doi: 10.1182/blood-2018-11-885467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sameena Wani I.K.M.L., Pothoulakis Charalabos. Role and mechanisms of exosomal miRNAs in IBD pathophysiology. Am J Physiol Gastrointest Liver Physiol. 2020;319:G646–G654. doi: 10.1152/ajpgi.00295.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexander M., Hu R., Runtsch M.C., Kagele D.A., Mosbruger T.L., Tolmachova T., et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cooks T., Pateras I.S., Jenkins L.M., Patel K.M., Robles A.I., Morris J., et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krissansen G.W., Yang Y., McQueen F.M., Leung E., Peek D., Chan Y.C., et al. Overexpression of miR-595 and miR-1246 in the sera of patients with active forms of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:520–530. doi: 10.1097/MIB.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 79.Tian T., Zhou Y., Feng X., Ye S., Wang H., Wu W., et al. MicroRNA-16 is putatively involved in the NF-kappaB pathway regulation in ulcerative colitis through adenosine A2a receptor (A2aAR) mRNA targeting. Sci Rep. 2016;6:30824. doi: 10.1038/srep30824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramos G.P., Papadakis K.A. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nata T., Fujiya M., Ueno N., Moriichi K., Konishi H., Tanabe H., et al. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-kappaB and improving epithelial barrier function. J Gene Med. 2013;15:249–260. doi: 10.1002/jgm.2717. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y., Xiao Y., Ge W., Zhou K., Wen J., Yan W., et al. miR-200b inhibits TGF-beta1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J., Zhou C.Z., Zhu R., Fan H., Liu X.X., Duan X.Y., et al. miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. J Gastroenterol Hepatol. 2017;32:1966–1974. doi: 10.1111/jgh.13797. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z., Xing T., Chen Y., Xiao J. Exosome-mediated miR-200b promotes colorectal cancer proliferation upon TGF-β1 exposure. Biomed Pharmacother. 2018;106:1135–1143. doi: 10.1016/j.biopha.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 85.Wang H., Chao K., Ng S.C., Bai A.H., Yu Q., Yu J., et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016;17:58. doi: 10.1186/s13059-016-0901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M., Zhao J., Cao M., Liu R., Chen G., Li S., et al. Mast cells-derived MiR-223 destroys intestinal barrier function by inhibition of CLDN8 expression in intestinal epithelial cells. Biol Res. 2020;53:12. doi: 10.1186/s40659-020-00279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ando Y., Mazzurana L., Forkel M., Okazaki K., Aoi M., Schmidt P.T., et al. Downregulation of MicroRNA-21 in colonic CD3+ T cells in UC remission. Inflamm Bowel Dis. 2016;22:2788–2793. doi: 10.1097/MIB.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 88.Wu F., Dong F., Arendovich N., Zhang J., Huang Y., Kwon J.H. Divergent influence of microRNA-21 deletion on murine colitis phenotypes. Inflamm Bowel Dis. 2014;20:1972–1985. doi: 10.1097/MIB.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 89.Petit C.S., Barreau F., Besnier L., Gandille P., Riveau B., Chateau D., et al. Requirement of cellular prion protein for intestinal barrier function and mislocalization in patients with inflammatory bowel disease. Gastroenterol. 2012;143(122–32) doi: 10.1053/j.gastro.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 90.Li Z., Zhuang M., Zhang L., Zheng X., Yang P., Li Z. Acetylation modification regulates GRP78 secretion in colon cancer cells. Sci Rep. 2016;6:30406. doi: 10.1038/srep30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leoni G., Neumann P.A., Kamaly N., Quiros M., Nishio H., Jones H.R., et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125:1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Bergenhenegouwen J., Kraneveld A.D., Rutten L., Kettelarij N., Garssen J., Vos A.P. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0089121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asea A., Rehli M., Kabingu E., Boch J.A., Bare O., Auron P.E., et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 94.Kulp A., Kuehn M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaparakis-Liaskos M., Ferrero R.L. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 97.Chelakkot C., Choi Y., Kim D.K., Park H.T., Ghim J., Kwon Y., et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50 doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao X.J., Li T., Wei B., Yan Z.X., Hu N., Huang Y.J., et al. Bacterial outer membrane vesicles from dextran sulfate sodium-induced colitis differentially regulate intestinal UDP-glucuronosyltransferase 1A1 partially through toll-like receptor 4/mitogen-activated protein kinase/phosphatidylinositol 3-kinase pathway. Drug Metab Dispos. 2018;46:292–302. doi: 10.1124/dmd.117.079046. [DOI] [PubMed] [Google Scholar]
- 99.Li Z.T., Zhang R.L., Bi X.G., Xu L., Fan M., Xie D., et al. Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microb Pathog. 2015;81:46–52. doi: 10.1016/j.micpath.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 100.Kunsmann L., Ruter C., Bauwens A., Greune L., Gluder M., Kemper B., et al. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci Rep. 2015;5:13252. doi: 10.1038/srep13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mondal A., Tapader R., Chatterjee N.S., Ghosh A., Sinha R., Koley H., et al. Cytotoxic and inflammatory responses induced by outer membrane vesicle-associated biologically active proteases from vibrio cholerae. Infect Immun. 2016;84:1478–1490. doi: 10.1128/IAI.01365-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu T.C., Stappenbeck T.S. Genetics and pathogenesis of inflammatory bowel disease. Annu Rev Pathol. 2016;11:127–148. doi: 10.1146/annurev-pathol-012615-044152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tulkens J., Vergauwen G., Van Deun J., Geeurickx E., Dhondt B., Lippens L., et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69:191–193. doi: 10.1136/gutjnl-2018-317726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alpdundar Bulut E., Bayyurt Kocabas B., Yazar V., Aykut G., Guler U., Salih B., et al. Human gut commensal membrane vesicles modulate inflammation by generating M2-like macrophages and myeloid-derived suppressor cells. J Immunol. 2020;205:2707–2718. doi: 10.4049/jimmunol.2000731. [DOI] [PubMed] [Google Scholar]
- 105.Patten D.A., Hussein E., Davies S.P., Humphreys P.N., Collett A. Commensal-derived OMVs elicit a mild proinflammatory response in intestinal epithelial cells. Microbiology (Reading) 2017;163:702–711. doi: 10.1099/mic.0.000468. [DOI] [PubMed] [Google Scholar]
- 106.Canas M.A., Gimenez R., Fabrega M.J., Toloza L., Baldoma L., Badia J. Outer membrane vesicles from the probiotic escherichia coli nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bielaszewska M., Marejkova M., Bauwens A., Kunsmann-Prokscha L., Mellmann A., Karch H. Enterohemorrhagic Escherichia coli O157 outer membrane vesicles induce interleukin 8 production in human intestinal epithelial cells by signaling via Toll-like receptors TLR4 and TLR5 and activation of the nuclear factor NF-kappaB. Int J Med Microbiol. 2018;308:882–889. doi: 10.1016/j.ijmm.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Bielaszewska M., Ruter C., Kunsmann L., Greune L., Bauwens A., Zhang W., et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alvarez C.S., Badia J., Bosch M., Gimenez R., Baldoma L. Outer membrane vesicles and soluble factors released by probiotic escherichia coli nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front Microbiol. 2016;7:1981. doi: 10.3389/fmicb.2016.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canas M.A., Fabrega M.J., Gimenez R., Badia J., Baldoma L. Outer membrane vesicles from probiotic and commensal escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Front Microbiol. 2018;9:498. doi: 10.3389/fmicb.2018.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fabrega M.J., Rodriguez-Nogales A., Garrido-Mesa J., Algieri F., Badia J., Gimenez R., et al. Intestinal anti-inflammatory effects of outer membrane vesicles from escherichia coli nissle 1917 in DSS-experimental colitis in mice. Front Microbiol. 2017;8:1274. doi: 10.3389/fmicb.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shen Y., Giardino Torchia M.L., Lawson G.W., Karp C.L., Ashwell J.D., Mazmanian S.K. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Durant L., Stentz R., Noble A., Brooks J., Gicheva N., Reddi D., et al. Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease. Microbiome. 2020;8:88. doi: 10.1186/s40168-020-00868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Collado M.C., Derrien M., Isolauri E., de Vos W.M., Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stentz R., Osborne S., Horn N., Li A.W., Hautefort I., Bongaerts R., et al. A bacterial homolog of a eukaryotic inositol phosphate signaling enzyme mediates cross-kingdom dialog in the mammalian gut. Cell Rep. 2014;6:646–656. doi: 10.1016/j.celrep.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Elmi A., Nasher F., Jagatia H., Gundogdu O., Bajaj-Elliott M., Wren B., et al. Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell E-cadherin and occludin. Cell Microbiol. 2016;18:561–572. doi: 10.1111/cmi.12534. [DOI] [PubMed] [Google Scholar]
- 117.Chatterjee D., Chaudhuri K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011;585:1357–1362. doi: 10.1016/j.febslet.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 118.Chatterjee D., Chaudhuri K. Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. J Biol Chem. 2013;288:4299–4309. doi: 10.1074/jbc.M112.408302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Console L., Scalise M., Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–171. doi: 10.1016/j.cca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 120.Zhong M.E., Chen Y., Xiao Y., Xu L., Zhang G., Lu J., et al. Serum extracellular vesicles contain SPARC and LRG1 as biomarkers of colon cancer and differ by tumour primary location. EBioMedicine. 2019;50:211–223. doi: 10.1016/j.ebiom.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsumura T., Sugimachi K., Iinuma H., Takahashi Y., Kurashige J., Sawada G., et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113:275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nedaeinia R., Manian M., Jazayeri M.H., Ranjbar M., Salehi R., Sharifi M., et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24:48–56. doi: 10.1038/cgt.2016.77. [DOI] [PubMed] [Google Scholar]
- 123.Bojesen R.D., Riis L.B., Hogdall E., Nielsen O.H., Jess T. Inflammatory bowel disease and small bowel cancer risk, clinical characteristics, and histopathology: a population-based study. Clin Gastroenterol Hepatol. 2017;15(1900–7) doi: 10.1016/j.cgh.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 124.Yoon Suk Jung M.H., Kim Won Ho, Park Sohee, Cheon Jae Hee. Cancer risk in the E arly S tages of Inflammatory Bowel Disease in Korean patients A Nationwide Population based Study. J Crohns Colitis. 2017;11:954–962. doi: 10.1093/ecco-jcc/jjx040. [DOI] [PubMed] [Google Scholar]
- 125.Kim B.J., Yang S.K., Kim J.S., Jeen Y.T., Choi H., Han D.S., et al. Trends of ulcerative colitis-associated colorectal cancer in Korea: a KASID study. J Gastroenterol Hepatol. 2009;24:667–671. doi: 10.1111/j.1440-1746.2008.05730.x. [DOI] [PubMed] [Google Scholar]
- 126.Mohsen Norouzinia V.C., Alizadeh Amir Houshang Mohammad, Zali Mohammad Reza. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench. 2017;10:155–167. [PMC free article] [PubMed] [Google Scholar]
- 127.Martin G.R., Perretti M., Flower R.J., Wallace J.L. Annexin-1 modulates repair of gastric mucosal injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G764–G769. doi: 10.1152/ajpgi.00531.2007. [DOI] [PubMed] [Google Scholar]
- 128.D’Acquisto M.P.a.F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 129.Leoni G., Alam A., Neumann P.A., Lambeth J.D., Cheng G., McCoy J., et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zheng X., Chen F., Zhang Q., Liu Y., You P., Sun S., et al. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell. 2017;8:686–695. doi: 10.1007/s13238-017-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang L., Tang Z., Zhang H., Kou W., Lu Z., Li X., et al. PSMA7 directly interacts with NOD1 and regulates its function. Cell Physiol Biochem. 2013;31:952–959. doi: 10.1159/000350113. [DOI] [PubMed] [Google Scholar]
- 132.Polytarchou C., Hommes D.W., Palumbo T., Hatziapostolou M., Koutsioumpa M., Koukos G., et al. MicroRNA214 is associated with progression of ulcerative colitis, and inhibition reduces development of colitis and colitis-associated cancer in mice. Gastroenterol. 2015;149(981–92) doi: 10.1053/j.gastro.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang X., Deeke S.A., Ning Z., Starr A.E., Butcher J., Li J., et al. Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nature Commun. 2018;9 doi: 10.1038/s41467-018-05357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lafitte M., Lecointre C., Roche S. Roles of exosomes in metastatic colorectal cancer. Am J Physiol Cell Physiol. 2019;317:C869–C880. doi: 10.1152/ajpcell.00218.2019. [DOI] [PubMed] [Google Scholar]
- 135.Li Y., Altemus J., Lightner A.L. Mesenchymal stem cells and acellular products attenuate murine induced colitis. Stem Cell Res Ther. 2020;11:515. doi: 10.1186/s13287-020-02025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nargesi A.A., Lerman L.O., Eirin A. Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther. 2017;17:29–42. doi: 10.2174/1566523217666170412110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tang X.D., Shi L., Monsel A., Li X.Y., Zhu H.L., Zhu Y.G., et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by ang-1 mRNA. Stem Cells. 2017;35:1849–1859. doi: 10.1002/stem.2619. [DOI] [PubMed] [Google Scholar]
- 138.Stone M.L., Zhao Y., Robert Smith J., Weiss M.L., Kron I.L., Laubach V.E., et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18:212. doi: 10.1186/s12931-017-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harrell C.R., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8:1605. doi: 10.3390/cells8121605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang J., Liu X.X., Fan H., Tang Q., Shou Z.X., Zuo D.M., et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Harting M.T., Srivastava A.K., Zhaorigetu S., Bair H., Prabhakara K.S., Toledano Furman N.E., et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36:79–90. doi: 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 142.Mao F., Wu Y., Tang X., Kang J., Zhang B., Yan Y., et al. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017;2017:5356760. doi: 10.1155/2017/5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heidari N., Abbasi-Kenarsari H., Namaki S., Baghaei K., Zali M.R., Ghaffari Khaligh S., et al. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021 doi: 10.1002/jcp.30275. [DOI] [PubMed] [Google Scholar]
- 144.Tian J., Zhu Q., Zhang Y., Bian Q., Hong Y., Shen Z., et al. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and treg cell responses. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.598322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yunbing Wu WQ, Xinwei Xu, Jingjing Kang, Jingyan Wang, Yingying Wen, Xudong Tang, Yongmin Yan, Hui Qian, Xu Zhang, Wenrong Xu, Fei Mao. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res 2018;10:2026–36. [PMC free article] [PubMed]
- 146.Wang G., Yuan J., Cai X., Xu Z., Wang J., Ocansey D.K.W., et al. HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin Transl Med. 2020;10 doi: 10.1002/ctm2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Di Gioia S., Hossain M.N., Conese M. Biological properties and therapeutic effects of plant-derived nanovesicles. Open Med (Wars) 2020;15:1096–1122. doi: 10.1515/med-2020-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Le Doare K., Holder B., Bassett A., Pannaraj P.S. Mother's milk: a purposeful contribution to the development of the infant microbiota and immunity. Front Immunol. 2018;9:361. doi: 10.3389/fimmu.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Torregrosa Paredes P., Gutzeit C., Johansson S., Admyre C., Stenius F., Alm J., et al. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014;69:463–471. doi: 10.1111/all.12357. [DOI] [PubMed] [Google Scholar]
- 150.Admyre C., Johansson S.M., Qazi K.R., Filen J.J., Lahesmaa R., Norman M., et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 151.Liao Y., Du X., Li J., Lonnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201700082. [DOI] [PubMed] [Google Scholar]
- 152.Kahn S., Liao Y., Du X., Xu W., Li J., Lonnerdal B. Exosomal MicroRNAs in milk from mothers delivering preterm infants survive in vitro digestion and are taken up by human intestinal cells. Mol Nutr Food Res. 2018;62 doi: 10.1002/mnfr.201701050. [DOI] [PubMed] [Google Scholar]
- 153.Shandilya S., Rani P., Onteru S.K., Singh D. Small interfering RNA in milk exosomes is resistant to digestion and crosses the intestinal barrier in vitro. J Agric Food Chem. 2017;65:9506–9513. doi: 10.1021/acs.jafc.7b03123. [DOI] [PubMed] [Google Scholar]
- 154.Chen T., Xie M.Y., Sun J.J., Ye R.S., Cheng X., Sun R.P., et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep. 2016;6:33862. doi: 10.1038/srep33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hock A., Miyake H., Li B., Lee C., Ermini L., Koike Y., et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. 2017;52:755–759. doi: 10.1016/j.jpedsurg.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 156.Gao H.N., Guo H.Y., Zhang H., Xie X.L., Wen P.C., Ren F.Z. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J Dairy Sci. 2019;102:985–996. doi: 10.3168/jds.2018-14946. [DOI] [PubMed] [Google Scholar]
- 157.Martin C., Patel M., Williams S., Arora H., Brawner K., Sims B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018;24:278–284. doi: 10.1177/1753425918785715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tong L., Hao H., Zhang X., Zhang Z., Lv Y., Zhang L., et al. Oral administration of bovine milk-derived extracellular vesicles alters the gut microbiota and enhances intestinal immunity in mice. Mol Nutr Food Res. 2020;64 doi: 10.1002/mnfr.201901251. [DOI] [PubMed] [Google Scholar]
- 159.Reif S., Elbaum-Shiff Y., Koroukhov N., Shilo I., Musseri M., Golan-Gerstl R. Cow and human milk-derived exosomes ameliorate colitis in DSS murine model. Nutrients. 2020;12:2589. doi: 10.3390/nu12092589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang B., Zhuang X., Deng Z.B., Jiang H., Mu J., Wang Q., et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ju S., Mu J., Dokland T., Zhuang X., Wang Q., Jiang H., et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z., et al. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Teng Y., Ren Y., Sayed M., Hu X., Lei C., Kumar A., et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(637–52) doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiao B., Laroui H., Ayyadurai S., Viennois E., Charania M.A., Zhang Y., et al. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-alpha RNA interference for IBD therapy. Biomaterials. 2013;34:7471–7482. doi: 10.1016/j.biomaterials.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Davoudi Z., Peroutka-Bigus N., Bellaire B., Wannemuehler M., Barrett T.A., Narasimhan B., et al. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. J Biomed Mater Res A. 2018;106:876–886. doi: 10.1002/jbm.a.36305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hood J.L., Wickline S.A. A systematic approach to exosome-based translational nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:458–467. doi: 10.1002/wnan.1174. [DOI] [PubMed] [Google Scholar]
- 168.Bunggulawa E.J., Wang W., Yin T., Wang N., Durkan C., Wang Y., et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16:81. doi: 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Luan X., Sansanaphongpricha K., Myers I., Chen H., Yuan H., Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wu H., Fan H., Shou Z., Xu M., Chen Q., Ai C., et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204–212. doi: 10.1016/j.intimp.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 171.Yang X., Shi G., Guo J., Wang C., He Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int J Nanomedicine. 2018;13:8095–8104. doi: 10.2147/IJN.S179380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dailey F.E., Turse E.P., Daglilar E., Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. 2019;49:29–33. doi: 10.1016/j.coph.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 173.Yang Y., Liu L., Liu X., Zhang Y., Shi H., Jia W., et al. Extracellular vesicles derived from trichinella spiralis muscle larvae ameliorate TNBS-induced colitis in mice. Front Immunol. 2020;11:1174. doi: 10.3389/fimmu.2020.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gao X., Yang Y., Liu X., Wang Y., Yang Y., Boireau P., et al. Extracellular vesicles derived from Trichinella spiralis prevent colitis by inhibiting M1 macrophage polarization. Acta Trop. 2021;213 doi: 10.1016/j.actatropica.2020.105761. [DOI] [PubMed] [Google Scholar]
- 175.Eichenberger RM, Ryan S, Jones L, Buitrago G, Polster R, Montes de Oca M, et al. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front Immunol 2018;9:850. [DOI] [PMC free article] [PubMed]
- 176.Thery C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]