Graphical abstract
Abbreviations: BC, breast cancer; GBD, global burden of diseases; CFR, case-fatality rates; CFP, case-fatality-percent; SDI, sociodemographic index; YLDs, years lived with disability; YLLs, years of life lost; DALYs, disability adjusted life years; DR, death rates; IR, incidence rates; ASIR, age-standardized incidence rates; ASDR, age-standardized death rates; APC, age-period-cohort
Keywords: Breast cancer, Incidence, Case fatality, World regions, Age-period-cohort
Highlights
-
•
Breast cancer (BC) is the most widely studied disease due to its higher prevalence, heterogeneity and mortality.
-
•
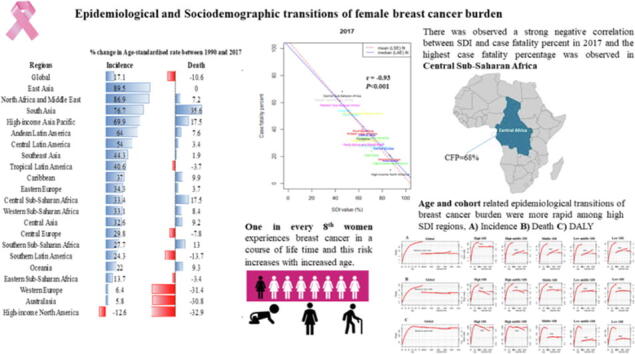
Age-standardised rate of BC incidence was high in high-income North America, Western Europe and Australia in 2017. Whereas it was significantly increased by 89.5% between 1990 and 2017 in East Asia.
-
•
There was a strong negative correlation between SDI and case fatality percent (r2017= −0.93) in 2017 around the globe.
-
•
Substantially high case-fatality-percent (CFP) was observed in six-world regions (CFP>40%), and the highest was in Central Sub-Saharan Africa (68%).
-
•
Overall, the risk of case-fatality rate tended to decrease most noticeably in high middle SDI countries, and the reduction of the risk of case-fatality rate in the recent cohort was the lowest in the low SDI countries.
Abstract
Introduction
Breast cancer (BC) is the most widely studied disease due to its higher prevalence, heterogeneity and mortality.
Objectives
This study aimed to compare female BC trends among 21 world regions and globally over 28 year of data and to assess the association between sociodemographic transitions and female BC risks.
Methods
We used Global burden of disease study data and measure the female BC burden according to 21 world regions and sociodemographic indices (SDI). Age-period-cohort (APC) analysis was used to estimate time and cohort trend of BC in different SDI regions.
Results
By world regions, age-standardised rate of female BC incidence were high in high-income-North America (ASR, 92.9; (95 %UI, 89.2, 96.6)), Western Europe (84.7; (73.4, 97.2)) and Australia (86; (81.7, 90.2)) in 2017. Whereas this rate was significantly increased by 89.5% between 1990 and 2017 in East Asia. We observed negative association between SDI and death, and DALYs in 25th and below percentiles of death and DALYs for the worldwide regions. Further, there was observed a strong negative correlation between SDI and case fatality percent (r2017 = −0.93; r1990 = −0.92) in both 2017 and 1990 worldwide and highest case fatality percentage was observed in Central Sub-Saharan Africa. Overall, the risk of case-fatality rate tends to decrease most noticeably in high middle SDI countries, and the reduction of the risk of case-fatality rate in the recent cohort was the lowest in the low SDI countries.
Conclusions
Remarkable variations exist among various regions in BC burden. There is a need to reduce the health burden from BC in less developed and under developing countries, because under-developed countries are facing higher degree of health-related burden. Public health managers should execute more classified and cost-effective screening and treatment interferences to lessen the deaths caused by BC, predominantly among middle and low SDI countries having inadequate healthcare supplies.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer among women and linked with considerable years of life lost (14.9 million DALYs), leading to increased cancer-related morbidity and mortality worldwide [1], [2]. The burden of the BC is still increasing in form of incidence and mortality both in developing and developed countries characterizing it as one of the most severe burdensome cancer worldwide [3], [4]. This increasing trend may be an outcome of the obesity epidemic together with dwindling parity [5]. BC incidence rate is higher in high-income countries as compared to the middle- and low-income ones. Recent literature has shown that awareness campaigns about BC associated risk factors and accessibility to advanced medical treatment have led to significantly decreased BC incidence and mortality rates among high income countries. But still BC incidence is greater in high SDI countries due to greater population size and lifestyle. However, not only the high-income countries are facing BC incidence burden, but it is equally devastating to middle- and low-income countries. Moreover, changes in reproduction pattern, improper awareness along with delayed disease diagnosis and treatment may contributes to low incidence among middle- and low-income countries [6], [2].
Regardless of advances in medical science, BC is still diagnosed in the advanced stages in the countries with inadequate resources as early detection, diagnosis, and proper treatment cannot be proficiently endorsed. Overall, the 5-year survival rate in BC has been improved in high-income countries (79%) as compared to middle- and low-income ones (57%) possibly due to logistical and economical constraints [7], [8]. Clinicians suggested that overall BC survival can be improved by focusing these four major domains including cancer recurrence and development of new cancers, late and long–term disease effects, coordination of care and modifiable health performances [9]. Moreover, molecular profiling, improved drugs and personalized medicine is another emerging area to cure the disease with better survival outcomes [10], [11].
Studying regional variations related to spatial–temporal trends in BC (incidence, mortality and DALYs) can be helpful in identifying the background factors contributing to these variations and can provide indication based and regional intervention of public health policy. In this regard, the age period cohort (APC) model is a common sociological, demographic, and epidemiological model used to assess the age, period, and cohort effects independently on disease incidence and mortality rate. The three different time related variations on disease incidence and mortality rate are distinguished in the APC model. The application of APC methods may facilitate the assessment of descriptive data, which can be confounded by age, calendar-period, and/or birth-cohort effects. Age effects reflect age-associated genetic events and/or carcinogenic exposures. Period effects may be observed due to changes in screening practices, diagnostic techniques, or classification definitions. Cohort effects are “cross-sectional” since they span or cut across all age groups and birth-cohorts within a given study period. Birth-cohort effects are longitudinal because they suggest the net impact of risk factors on the incidence and death rates within one generation or birth cohort [12], [13], [14]. Contrasting the rates by regions and age-related studies across birth and period cohorts may support in separating functioning of aforementioned factors and expose fluctuations in risk patterns that leads toward future etiological findings.
Various national and regional reports on BC incidence and mortality have been prepared, but the findings of these multifactorial studies display an inconclusive picture [1], [3], [7], [15]. To the best of our knowledge no research has been done in context, to compare the age period cohort trends among SDI regions and has address effects of socioeconomic development on BC variations during the present study period. Our study not only extend the current literature on application of APC model on differently regional population data but also provide the current statistics of BC in world regions and globally.
Materials and methods
Data source
The Global Burden of Diseases (GBD) is the most complete systematic epidemiological study in the world to date. It provides a thorough assessment of 359 injuries and diseases, 84 risk factors and 282 reported reasons of death among hundred and ninety-five countries, twenty-one regions, and seven super-regions. The GBD presents the 2017 estimates and updates for 1990–2016 data via innovative estimation methodologies and supplementary sources mentioned in previous studies [16], [17], [18], [19]. Information on BC has collected from the Global Health Data Exchange (GHDx) online data collection tool (http://ghdx.healthdata.org/gbd-results-tool). All the details about data sources were defined in “Availability of data and materials” statement at end of the article. Data on BC were collected from 1990 to 2017 on the annual incidence, death, disability adjusted life years (DALYs), and age standardized rate (ASR, per 100 k). The sociodemographic index (SDI) was built to categorize countries into five quintiles (high SDI, high middle SDI, middle SDI, low middle SDI, low SDI) based on national per capita income, average years of education among persons above 15 years of age and total fertility rate. This index ranges from 0 to 1 i.e., least to most developed ones. Further, SDI classification, distribution and list of countries has added as supplemental file.
According to GBD study, BC incidence estimates based on individual cancer registries or integrated cancer registry databases. Four independent DisMod-MR2.1 inputs were applied to the proportion of data obtained from the systematic literature review [20]. DisMod-MR2.1 based on Bayesian meta-regression tool, as the principal approach for estimation on prevalence and incidence of 354 injuries and diseases in 195 countries and territories. BC mortality data from vital registration systems and mortality projections were used as input data into CODEm (Death Ensemble Model) [21]. The CODEm estimates mortality based on available data and covariates, including education, SD1, smoking, lagging distribution income, and alcohol consumption. Using the CodCorrect procedure, the single cause estimates have been modified to fit all-cause mortality calculated separately [22], [23]. Years lived with disability (YLDs) were determined by multiplying the prevalence of each sequela by its weight of disability and adding the clinical morbidity associated with a BC diagnosis. Years of life lost (YLLs) caused by BC have been estimated using global standard life expectancy and number of deaths by age [22]. DALYs for BC have been computed as the sum of YLDs and YLLs. Specific calculation methods are mentioned in the series of articles published by GBD [18], [24], [25], [26]. As, current study was carried out using Global Burden of Disease Study (GBD) 2017 database and sources are mentioned in “Availability of data and materials” statement. The GBD study’s protocol has been approved by the research ethics board at the University of Washington (UW). The GBD studies must be conducted in full compliance with UW policies and procedures, as well as applicable federal, state, and local laws. Therefore, all ethical standards are justified by properly citing the respective sources (http://ghdx.healthdata.org/gbd-results-tool). Consequently, ethical approval is not required for this study.
Statistical analysis
Geographical wise assessment of BC variations was examined using map construction, stratified by time and age group. We calculated percent change in age-standardised and crude rate of BC incidence, mortality and DALYs between 1990 and 2017 and presented the 2017 estimates with 95% uncertainty interval (UI) for 21 world regions separately. The case-fatality percent (CFP) was calculated by dividing the age-standardised death rate (ASDR) by the age-standardised incidence rate (ASIR) and multiplying by 100 [27]. Correlation between SDI and CFP was tested using spearman correlation coefficient (r). The limited cubic splines with three knots centiles were used to flexibly model the relationship of incidence, mortality, and DALY with SDI values. Further quantile regression was applied to determine whether the influence of SDI on BC burden varied in their conditional distributions across quantiles or not [28], [29]. BC data of all regions from 1990 to 2017 was considered for quantile regression analysis.
In the framework of the age-period-cohort model, the relationship between multiple aspects of BC like incidence, death, case fatality and DALYs and each of the three primary sources for spatial and temporal variability: age, period (year), and cohort (year of birth) were analyzed for each SDI region as well as globally. In the age-period-cohort analysis, age-specific rates of BC from 20 to 80 years with successive 5-year age interval, and calendar time including consecutive period from 1990 to 2017, and successive cohort (period-age) from 1910 to 1997 were considered for statistical analysis. We performed the APC analysis using R package (Epi, version 2.44) developed by Carstensen et al. 2021 [30]. Rate ratio was estimated using maximum likelihood (ML) of APC-model Poisson with log (Y) based on natural-spline function. Median date of birth and median date of diagnosis among cases was chosen for reference cohort and period respectively. The goodness of fit was evaluated using the deviance table of estimated models.
Results
Geographical wise distribution of BC stratified by age group and time
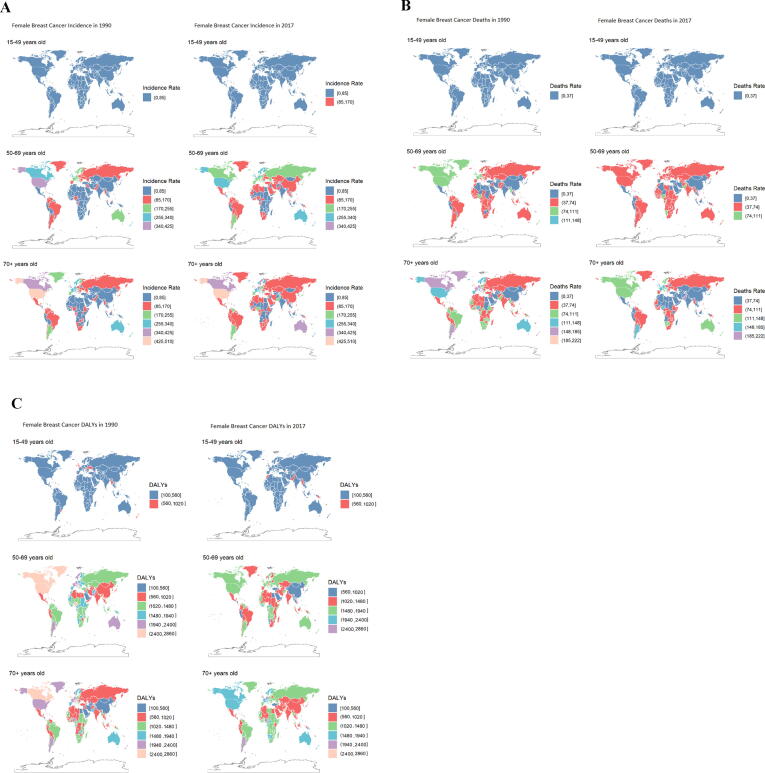
Age-specific geographical distribution of BC incidence, death and DALYs between 1990 and 2017 were depicted in Fig. 1. Globally, the incidence rate was increased in all regions between 1990 and 2017 among female for the age group of 50–69 years old and above. Particularly, these rates were higher in high SDIs with the largest increases in High-income North America and Australasia (Fig. 1A). Besides, death and DALYs rates showed a relatively consistent trend. From 1990 to 2017, both rates declined among female aged 50- to 69-year-old and above in high-income North America, Australasia, high-income Canada, North America and South America or Argentina but remained among the highest in the world (Fig. 1 B & C).
Fig. 1.
Female breast cancer incidence, death and DALYs (rate per 100 k) in 1990 and 2017 stratified by age group, 15–49years, 50–69years, and 70+years (A) Incidence in 1990 and 2017, (B) death in 1990 and 2017 (C) DALYs in 1990 and 2017.
Change in age-standardized and crude estimates of BC at global and regional level
Globally, BC among women accounted for 1,937,574 (95% UI: 1868019–2000363) cases in 2017, with an age standardised rate (per 100,000) of 45.9 (95% UI: 44.2–47.4), which increased significantly by 17.1% (95% UI: 6.2–25.5) between 1990 and 2017. It was also reported as the cause of death among 600,728 (95% UI: 578725–629932) female in 2017 with an age-standardised death rate (per 100,000) of 14.1 (95% UI: 13.6–14.8), which decreased by 10.6% (-20.8− -1.4) between 1990 and 2017. Furthermore, BC accounted for 17,423,143 (16617465–18378225) DALYs in 2017, with an age-standardised rate (per 100,000) of 414.7 (95% UI: 395.5–437.6), which was identified as a decrease of 9.3% (-21.9–2.3) globally between 1990 and 2017. Whereas the death and DALYs for crude rate was significantly increased by 22.5% (8.3–35.2) and 18.9% (2.3–34.3) between 1990 and 2017 (Table 1). Out of 21 world regions, only high-income North America had decreased age-standardised incidence rate between 1990 and 2017, and highest age-standardised incidence change between 1990 and 2017 was observed in East Asia. While, deaths and DALYs were significantly reduced in Australasia, Central Europe, High-income North America, Southern Latin America, and Western Europe between 1990 and 2017. Moreover, the incidence, death and DALYs change in crude rate was raised between 1990 and 2017 in all regions except for High-income North America and Western Europe where death and DALYs were declined in 2017 than 1990 (Table 1).
Table 1.
Incident cases, Deaths, and DALYs for female breast cancer in 2017 and percentage change of age standardized rates and crude rates by location.
| 2017 Counts | Age-standardised rate (per 100 000 population), 2017 | % change in Age-standardised rate between 1990 and 2017 | Crude rate (per 100 000 population), 2017 | % change in crude rate between 1990 and 2017 | |
|---|---|---|---|---|---|
| Regions | Incidence (95% UI) | ||||
| Global | 1,937,574 (1868019, 2000363) | 45.9(44.2,47.4) | 17.1(6.2,25.5) | 50.9(49.1,52.6) | 56.6(41.7,68) |
| East Asia | 380,032 (319781, 410357) | 35.9(30.2,38.8) | 89.5(30.6,125.9) | 52.2(43.9,56.4) | 223.3(124.6,286.5) |
| North Africa and Middle East | 90,417 (84474, 99766) | 36.9(34.5,41.5) | 86.9(28.7,152.7) | 31.3(29.3,34.6) | 159(77.1,247.8) |
| South Asia | 207,969 (179497, 246149) | 27.6(23.8,32.6) | 76.7(22.1,138.4) | 23.8(20.6,28.2) | 141.1(65.4,228) |
| High-income Asia Pacific | 91,510 (85434, 97448) | 52.4(49,55.8) | 69.9(54,86.4) | 96.3(89.9,102.6) | 149(125.4,173.5) |
| Andean Latin America | 8216 (7172, 9507) | 28.5(24.9,33) | 64(28,109.3) | 26.8(23.4,31) | 142.5(88.3,210.3) |
| Central Latin America | 51,179 (48491, 53899) | 39.5(37.4,41.6) | 54(42.6,65.9) | 39.3(37.2,41.4) | 149.6(130.8,168.8) |
| Southeast Asia | 124,436 (114126, 134729) | 35.6(32.7,38.5) | 44.3(8.2,86.4) | 37.6(34.5,40.8) | 115.1(59,181.7) |
| Tropical Latin America | 53,787 (51962, 55667) | 41.6(40.2,43.1) | 40.6(32.7,49.2) | 48.1(46.5,49.8) | 128.7(115.5,142.8) |
| Caribbean | 14,041 (12497, 15796) | 52.6(46.7,59.3) | 37(11,65.5) | 59.9(53.3,67.4) | 97.2(59.8,138.1) |
| Eastern Europe | 96,422 (92737, 100065) | 53.2(51.1,55.4) | 34.3(23.2,46.9) | 85.6(82.3,88.8) | 65.1(52.1,79.3) |
| Central Sub-Saharan Africa | 8001(6084, 10539) | 24.4(19.3,31.4) | 33.4(−29.8,131.8) | 13.1(9.9,17.2) | 38(−32.7,158.7) |
| Western Sub-Saharan Africa | 45,786 (34747, 60607) | 39.4(30.2,51.7) | 33.1(−27.6,150.7) | 20.7(15.7,27.4) | 38.6(−26,164.1) |
| Central Asia | 16,504 (15399, 17691) | 35.9(33.6,38.4) | 32.6(20,46.5) | 35.9(33.5,38.5) | 66.5(50.1,84.4) |
| Central Europe | 59,660 (56933, 62745) | 59.5(56.8,62.6) | 29.8(20.5,40.5) | 101.3(96.7,106.5) | 76.8(64,91.1) |
| Southern Sub-Saharan Africa | 10,320 (9350, 11228) | 30.3(27.4,32.7) | 27.7(4.2,51) | 26(23.6,28.3) | 68.9(39.8,100.2) |
| Southern Latin America | 22,499 (20093, 25353) | 52.8(47.1,59.6) | 24.3(6.6,45.9) | 67(59.8,75.5) | 56.5(34.5,83.2) |
| Oceania | 1704 (1265, 2367) | 40.7(31.9,53.9) | 22(−30,95.4) | 27.9(20.7,38.7) | 42.2(−25.6,150.5) |
| Eastern Sub-Saharan Africa | 24,819 (21561, 28701) | 24.1(21.1,27.9) | 13.7(−27.8,66.6) | 12.5(10.9,14.5) | 20.9(−24.1,81.2) |
| Western Europe | 338,131 (321227, 355134) | 86(81.7,90.2) | 6.4(−0.8,13.6) | 153.4(145.7,161.1) | 31(22.3,40) |
| Australasia | 18,659 (16261, 21292) | 84.7(73.4,97.2) | 5.8(−11.6,25.5) | 129.6(113,147.9) | 36.8(15.3,61.2) |
| High-income North America | 273,480 (263517, 284405) | 92.9(89.2,96.6) | −12.6(−17.4, −7.6) | 149.2(143.8,155.2) | 9.1(3.5,15.4) |
| Death (95% UI) | |||||
| Global | 600,728 (578725, 629932) | 14.1(13.6,14.8) | −10.6(−20.8, −1.4) | 15.8(15.2,16.6) | 22.5(8.3,35.2) |
| South Asia | 106,914 (91392, 129439) | 15(12.8,18.1) | 35.6(−8.4,85.7) | 12.3(10.5,14.8) | 89(29,162.2) |
| Central Sub-Saharan Africa | 4968 (3861, 6447) | 16.5(13.4,20.7) | 17.5(−35.2,93.6) | 8.1(6.3,10.5) | 20.4(−38.8,118.6) |
| High-income Asia Pacific | 18,426 (17651, 19249) | 9(8.6,9.4) | 17.5(10.6,24.8) | 19.4(18.6,20.3) | 98.3(87.1,110.3) |
| Southern Sub-Saharan Africa | 5357 (4832, 5767) | 16.5(14.9,17.8) | 13(−10.9,36) | 13.5(12.2,14.5) | 52.4(22.3,81.2) |
| Caribbean | 5065 (4442, 5769) | 18.8(16.4,21.5) | 9.9(−15.8,36.7) | 21.6(18.9,24.6) | 63.8(25.6,103.7) |
| Oceania | 836 (644, 1140) | 23.2(18.9,29.5) | 9.3(−33.6,64.9) | 13.7(10.5,18.7) | 25.8(−31.3,114.2) |
| Central Asia | 6095 (5733, 6471) | 13.9(13,14.7) | 9.2(−0.2,18.7) | 13.3(12.5,14.1) | 32(20.6,43.8) |
| Western Sub-Saharan Africa | 25,063 (19362, 32643) | 23.9(18.6,30.6) | 8.4(−39.6,97.3) | 11.3(8.7,14.7) | 7.9(−40.8,100.4) |
| Andean Latin America | 3212 (2832, 3687) | 11.3(10,13) | 7.6(−15.5,36.1) | 10.5(9.2,12) | 66(30,110.8) |
| North Africa and Middle East | 27,315 (25489, 30742) | 11.8(11,13.5) | 7.2(−27.1,47.4) | 9.5(8.8,10.6) | 49(0.3,103.8) |
| Eastern Europe | 32,216 (31323, 33174) | 16(15.6,16.5) | 3.7(−1.9,9.7) | 28.6(27.8,29.4) | 32.2(25.2,39.4) |
| Central Latin America | 15,548 (14815, 16320) | 12.2(11.6,12.8) | 3.4(−3.2,10.3) | 11.9(11.4,12.5) | 75.6(64.4,87.6) |
| Southeast Asia | 50,199 (46473, 54402) | 14.9(13.8,16.2) | 1.9(−23.3,31.4) | 15.2(14.1,16.5) | 57.1(17.2,105.1) |
| East Asia | 90,649 (76202, 97221) | 8.6(7.2,9.2) | 0(−32.1,18.2) | 12.5(10.5,13.4) | 81(23.6,114) |
| Eastern Sub-Saharan Africa | 14,460 (12611, 16779) | 15.7(13.7,18) | −3.4(−38,39.3) | 7.3(6.4,8.5) | 0(−37,49.2) |
| Tropical Latin America | 18,886 (18410, 19367) | 14.7(14.3,15) | −3.7(−7.9,0.8) | 16.9(16.5,17.3) | 66.5(59.1,74.4) |
| Central Europe | 20,243 (19436, 21083) | 17.5(16.8,18.3) | −7.8(−13.8, −1.8) | 34.4(33,35.8) | 38.7(29.6,47.6) |
| Southern Latin America | 8948 (8035, 10059) | 19.6(17.6,22.1) | −13.7(−24.9, −0.5) | 26.6(23.9,29.9) | 15(0.4,32.6) |
| Australasia | 4094 (3629, 4576) | 16.7(14.8,18.8) | −30.8(−40, −20.5) | 28.4(25.2,31.8) | −4.4(−17.1,9.5) |
| Western Europe | 87,430 (83303, 91614) | 18.1(17.2,18.9) | −31.4(−35.3, −27.3) | 39.7(37.8,41.6) | −6.8(−12.2, −1.2) |
| High-income North America | 54,802 (53111, 56588) | 17(16.4,17.6) | −32.9(−35.8, −29.8) | 29.9(29,30.9) | −12.6(−16.3, −8.8) |
| DALYs (95% UI) | |||||
| Global | 17,423,143 (16617465, 18378225) | 414.7(395.5,437.6) | −9.3(−21.9,2.3) | 457.8(436.6,482.9) | 18.9(2.3,34.3) |
| South Asia | 3,432,683 (2941453, 4164191) | 445.4(381.3,540.1) | 35.4(−7.7,88.8) | 393.6(337.3,477.5) | 79.3(21.4,151.8) |
| Central Sub-Saharan Africa | 165,332 (123344, 218998) | 475.7(365.6,620.6) | 16.3(−41.9,113.1) | 269.8(201.3,357.4) | 19.1(−43.6,129.6) |
| High-income Asia Pacific | 470,843 (441005, 501092) | 288.9(270.9,306.5) | 11.5(0.8,22.1) | 495.5(464.1,527.4) | 53(38.2,68.2) |
| Caribbean | 143,012 (122890, 166457) | 541.8(465.6,632.4) | 10.3(−18.8,43) | 609.9(524,709.8) | 55(13.7,100.8) |
| Western Sub-Saharan Africa | 841,439 (643411, 1109259) | 694.2(534.4,906.1) | 9.4(−40.3,103.1) | 380.1(290.7,501.1) | 14.4(−38.2,116.5) |
| Oceania | 30,752 (22374, 43891) | 692.5(530.1,947.9) | 7.3(−41.4,83.8) | 503.4(366.3,718.5) | 25.5(−36.9,132.2) |
| North Africa and Middle East | 958,621 (889519, 1059683) | 379.1(353.1,420.3) | 7.2(−28.2,45.8) | 332(308.1,367) | 48.9(−1,102.8) |
| Southern Sub-Saharan Africa | 157,518 (144145, 170965) | 452.7(413.1,489.9) | 5.3(−12.6,24.2) | 397.1(363.4,431) | 38.9(15.1,64.8) |
| Andean Latin America | 94,683 (82634,109651) | 327.7(286.1,380.2) | 4.1(−19.4,34.2) | 308.3(269.1,357.1) | 50.8(16.5,94) |
| Central Latin America | 470,478 (445493, 496143) | 359.1(340.2,378.5) | 3.7(−4,11.7) | 361.3(342.1,381) | 63.7(51.4,76.5) |
| Central Asia | 196,394 (183615, 210934) | 421.1(394,450.8) | 2.5(−7,12.7) | 427.6(399.8,459.2) | 30.5(18.3,44) |
| Southeast Asia | 1,684,759 (1553411,1823089) | 474.9(438.1,513.4) | 0.2(−25.9,31.7) | 509.7(470,551.6) | 47.9(8.6,97) |
| Tropical Latin America | 557,150 (539687, 574935) | 429.7(416.2,443.4) | −2.1(−7.4,3.5) | 498.7(483.1,514.6) | 54.9(46.4,63.8) |
| Eastern Europe | 866,215 (834927, 899837) | 481(462.5,500.3) | −2.5(−9.3,4.6) | 768.9(741.2,798.8) | 19.5(11.7,27.6) |
| East Asia | 2,744,523 (2347940, 2966189) | 257.5(219.3,278.5) | −5.5(−33.4,12.9) | 377.1(322.6,407.5) | 61(14.5,92.6) |
| Eastern Sub-Saharan Africa | 505,189 (439276, 586266) | 465.2(405.7,540.2) | −5.8(−40.7,41.3) | 255.1(221.8,296) | −0.9(−38.3,51.8) |
| Central Europe | 481,691 (459305, 506173) | 482.8(459.5,508.8) | −14(−20.3, −7) | 818(780,859.5) | 16.6(8.1,25.7) |
| Southern Latin America | 217566.5(193204, 246424) | 521(462,591.2) | −16.3(−28, −2.5) | 647.6(575.1,733.5) | 3.7(−10.6,20.7) |
| Australasia | 103,825 (90975, 117310) | 484.2(422.4,548.1) | −31.5(−42.4, −20) | 721.2(632,814.9) | −11.7(−25.4,3) |
| Western Europe | 1,909,719 (1788436, 2025117) | 498.5(466.8,530.4) | −33.3(−39.3, −27.3) | 866.2(811.2,918.5) | −17.7(−24.9, −10.5) |
| High-income North America | 1,390,749 (1316999, 1473569) | 492.1(465,521.6) | −34.4(−40.2, −28.2) | 759(718.7,804.2) | −17.4(−24.5, −9.5) |
UR, Uncertainty interval; values are ordered by most to least age-standardised percentage change (excluding global) for each breast cancer indicator (incidence, death and DALYs) separately.
Influence of sociodemographic transitions on BC incidence, mortality, case-fatality and DALYs
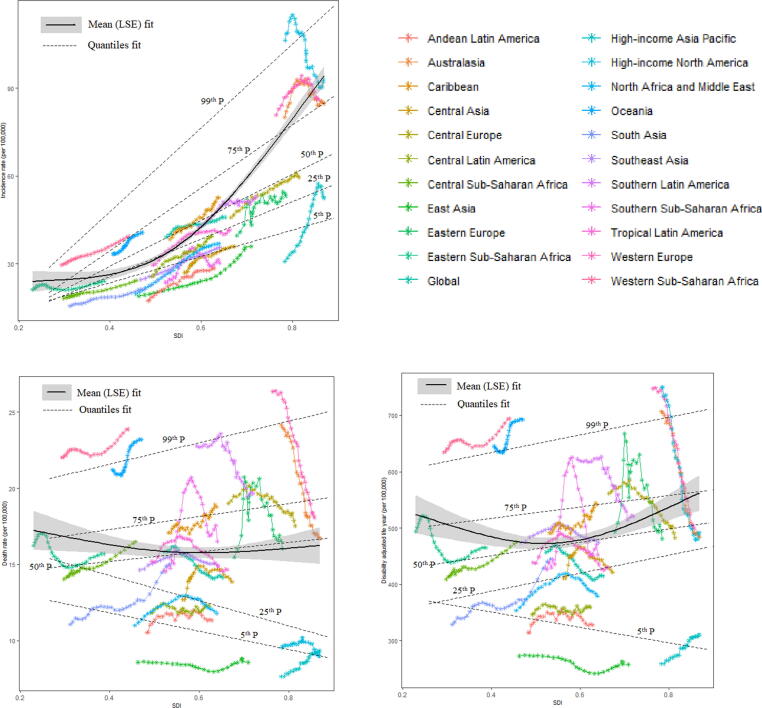
When age standardized incidence rate (ASIR), age standardized death rate (ASDR), and DALYs in each world region from 1990 to 2017 were plotted against an index of that region’s sociodemographic status in the same year, different patterns of epidemiological variations were observed in Fig. 2. In addition to High-income North America, Western Europe and Australia, the incidence of BC in different years increase with the increase of SDI, but the mortality and DALYs rates showed significant fluctuations. A finite cubic spline with three nodes was used to flexibly simulate the relationship between age-standardized rates (ASR) with SDI. The estimated relationship between SDI and ASR of BC incidence, mortality, and DALYs, shown as the black line in Fig. 2, was a gradual increase for ASIR as SDI increases, with more rapid growth at the highest levels of SDI. The reverse trend in ASDR and DALYs was found, decreasing first and then increasing, but this trend was not obvious. We observed negative association between SDI and death in 25th and below percentiles of death rate of countries. While for countries with DALYs in 25th and above percentiles have increase in DALYs with the increase of SDI but for countries with DALYs in 5th percentile, DALYs has decrease with increase of SDI. The change in BC parameter due to SDI in specific percentiles has depicted in Table 2. The incidence, death and DALY had increase by 88.46 (95 %CI: 77.62, 93.75), 3.5 (0.6, 4.63) and 135.75 (95 %CI: 116.62, 194.99) respectively due to unit increase in SDI on 50th percentile of parameters.
Fig. 2.
Relationship between the age-standardized rates (ASRs, per 100 k) of female breast cancer and SDI over time (1990–2017) by globally and by 21 GBD regions. Each colored line represents a time trend of the relationship for the specified world regions. Each point represents a specific year for that region. The black line with 95% confidence band represents the average expected relationship between SDI and ASRs for breast cancer based on values from all countries from 1990 to 2017. The dashed lines represents the relationship between SDI and ASRs on conditional distributions across quantiles of breast cancer; SDI, social-demographic index; SDI ranges from 0 (less developed) to 1 (most developed).
Table 2.
Quantile regression estimates predicting change in breast cancer due to SDI on conditional distributions across quantiles for the worldwide regions.
| Case fatality rate | Incidence rate | Death rate | DALY | ||
|---|---|---|---|---|---|
| Percentiles | Coefficient (95 %CI) | Coefficient (95 %CI) | Coefficient (95 %CI) | Coefficient (95 %CI) | |
| 5th percentile | Intercept | 1* (0.95,1.04) | −4.79 (−10.84,1.92) | 13.39* (9.27,14.31) | 382.26* (278.14,418.47) |
| SDI | −1.13* (−1.21, −1.04) | 50.63* (37.48,64.3) | −6.81* (−8.33, −1.78) | −156.14* (−217.76, −111.82) | |
| 25th percentile | Intercept | 1.02* (1,1.04) | −10.25* (−17.87, −5.09) | 17.42* (16.68,17.84) | 297.32* (251.93,443.87) |
| SDI | −1.06 (−1.1, −1.02) | 72.08* (61.3,87.38) | −9.34* (−9.97, −8.17) | 183.31* (−109.94,261.9) | |
| 50th percentile | Intercept | 1.01* (1,1.02) | −11.98* (−15.77, −6.27) | 13.71* (13.16,14.72) | 394.9* (362.68,410.74) |
| SDI | −0.95* (−0.97, −0.93) | 88.46* (77.62,93.75) | 3.5* (0.6,4.63) | 135.75* (116.62,194.99) | |
| 75th percentile | Intercept | 1.02* (1,1.06) | −17.82* (−20.23, −13.72) | 15.82* (14.98,23.19) | 486.27* (438.65,666.34) |
| SDI | −0.91* (−0.97, −0.87) | 121.1* (112.69,125.36) | 4.86 (−5.57,6.15) | 116.25* (−12.36,167.92) | |
| 99th percentile | Intercept | 0.97* (0.95,1.51) | −16.148 (−16.3, −15.07) | 20.06* (20.02,21.41) | 609.2* (606.7,627.68) |
| SDI | −0.64* (−1.49, −0.59) | 161.26* (157.29,162.02) | 7.97* (5.72,8.33) | 179.55* (154.7,185.17) |
DALY, disability adjusted life years; CI, confidence interval; SDI, sociodemographic index; *significant at 1% level of significance.
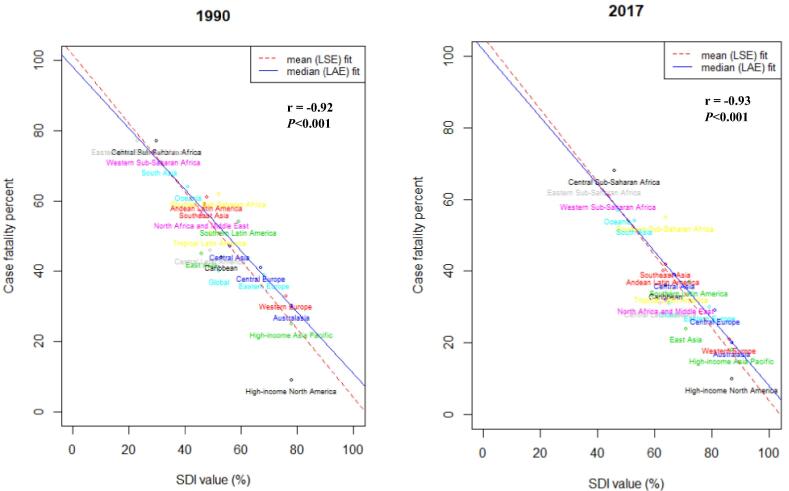
Additionally, there was observed a strong negative correlation between SDI and case fatality percent (r2017 = −0.93, P < 0.001; r1990 = −0.92, P < 0.001) in both 2017 and 1990 around the globe and the highest case fatality percentage was observed in Central Sub-Saharan Africa (Fig. 3).
Fig. 3.
Time specific correlation between socio-demographic index (SDI) and female breast cancer case-fatality percent (CFP) in 1990 and 2017 worldwide. Case-fatality percent was calculated by dividing age-standardised death rate by age-standardised incidence rate and multiplied by 100, r represent the correlation coefficient between SDI and CFP among world regions, LSE, Least Square Error fit; LAE, Least Absolute Error fit; SDI(%) ranges from 0 (less developed) to 100 (most developed).
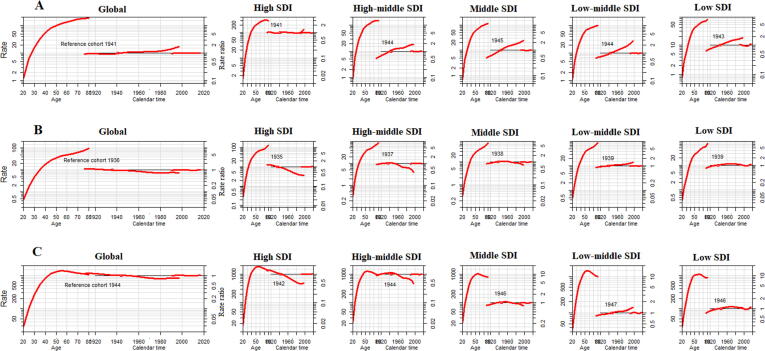
Age-period-cohort effects on BC incidence, death, DALYs and case-fatality
We estimated the age period cohort trends in BC for each SDI region and globally, which captures age, period and cohort effects. Each of these effects, holding all other variables constant at their sample mean values, as shown in Fig. 4. For each SDI region and the global age-related impacts, it can be easily seen that BC burden ((A) incidence, (B) death and (C) DALYs) almost in all regions continue to grow with age, and the risk of BC burden tend to increase most remarkably in low SDI regions. The analysis exposed increase incidence risk trend with increase birth cohorts from the median date of birth among cases, in each SDI regions. The risk value for death and DALYs was decreasing in high-SDI to middle-SDI regions, and it was increasing in low-middle to low-SDI regions from the reference cohort (median date of birth among cases) to onward (Fig. 4).
Fig. 4.
Age period cohort related female breast cancer trends in (A) incidence rate (B) death rate and (C) DALYs from 1990 to 2017 with ages 20 to 80. Rate ratio was estimated using ML of APC-model Poisson with log(Y) based on natural-spline function, for each SDI region separately. DALYs, disability adjusted life years; ML, maximum likelihood; APC, age-period-cohort; Reference cohort for age-effects was chosen as the median date of birth among cases; Median date of diagnosis among cases was selected as reference period.
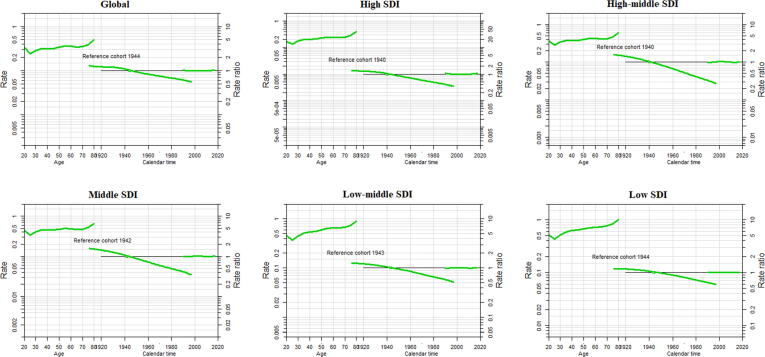
We also conducted APC analysis on case-fatality rates (CFR) in different SDI regions. As shown in Fig. 5, overall, risk of case-fatality rate tends to decrease most noticeably in high middle SDI countries, and the reduction of the risk of case-fatality rate in the recent cohort was the lowest in the low SDI countries. The effect separation was more obvious between different SDI regions on age greater than 50 years and for cohort 1960 to forward. The period effect remained consistent during the entire duration in all SDI regions and globally (Fig. 5).
Fig. 5.
Age period cohort trends in case-fatality rate of female breast cancer from 1990 to 2017 with ages 20 to 80. Rate ratio was estimated using ML of APC-model Poisson with log(Y) based on natural-spline function, for each SDI region separately. ML, maximum likelihood; APC, age-period-cohort; Reference cohort for age-effects was chosen as the median date of birth among cases; Median date of diagnosis among cases was selected as reference period.
Discussion
GBD-based studies reveal the most up-to-date patterns and trends of the incidence, mortality, DALYs and related socioeconomic risk factors associated with the onset and development of BC worldwide. Breast carcinogenesis involves a complex interplay of various genetic, environmental, socioeconomic and lifestyle risk factors. Since 1990, ssocioeconomic development linked disparities in the global BC incidence have continued to decrease. However, as per SDI, highly developed regions showed greater BC incidence burden by 2017. In the recent years, consistently the reverse trends in mortality rates among high and low SDI countries showed that the mortality intensity indicators become negative amongst women aged 15–49 and 50–69 years [31]. These circumstances are possibly due to shift in the burden of BC mortality from high-income to middle- and low-income countries. In the current study we have examined the age period and cohort trends in multiple aspects of BC including incidence, death, case fatality and DALYs among females and assess the association of these aspects with sociodemographic transition across 21 world regions based on GBD data from 1990 to 2017.
We have observed an increased incidence of BC with age standardised rate (per 100 k) of 45.9 in 2017 all over the world. Whereas a decrease is observed in the age standardised death rate (per 100 k) of BC cases between 1990 and 2017. Moreover, we have found that the death and DALYs for crude rate was significantly increased by 22.5% and 18.9% respectively between 1990 and 2017. Out of twenty-one regions, only high-income North America had reduced age-standardised incidence rate (per 100 k), while deaths and DALYs were significantly decreased in Australasia, Central Europe, High-income North America, Southern Latin America, and Western Europe in the said time duration.
Besides, an increase in the incidence, death and DALYs was observed among crude rate between 1990 and 2017 in all twenty-one regions except for High-income North America and Western Europe where a declining trend was found in death and DALYs in 2017 than 1990. Global cancer burden 2017 attributed the variations in BC incident rates to population growth and aging process [32]. Various other studies have also supported the findings that being a complex chronic systemic disease, BC likely to have significant association with age, as disease incidence increases with increased age [33], [34], [35], [36], [37]. Decreased deaths and DALYs among developed countries were attributed to the adoption of widespread mammographic screening of the disease [15], [33]. When we stratified the geographical distribution of BC by time and age group it was found that it is more prevalent among females above the age of 50 years all around the world being hormonally sensitive cancer [38]. Additionally, disease was more common in high SDI countries specifically in North America and Australasia with declining death and DALYs trends possibly due to advancement in BC treatment methodologies.
Universally, with higher than 2 million new BC diagnoses every year, attempts to improve early detection and treatment resonate deeply with cases, clinicians and surgeons alike. Though the five year survival rate of female BC cases has increased, notably in high SDI regions, over the last 3 decades, the disease burden of BC among women still remained elevated in most of the regions [3]. This is mainly attributed to the constantly increased BC incidence among women. In the current study, we have found that ASIR of BC among women continued to increase in most of the high SDI countries along with Asian and African regions with previously lesser BC rates [39]. This increasing ASIR trends are alarming and thought to signify the increased prevalence of known BC related risk factors like adopting western lifestyle, diet changes, reduced physical activity and various reproductive patterns. Another possible reason behind this increasing trend might be the availability of routinely and extensively performed mammography, awareness campaigns and access to health care systems. Mammographic screening unavoidably necessitates increased BC incidence due to early detection of BC among women that would otherwise have been diagnosed in the later stages of life or remain unidentified throughout the life [40]. One of the studies has shown that BC diagnosis at early stages has been doubled in the age group of 50–69 years due to the introduction of mammographic screening during 2003–2014 [41]. We have also found that ASIRs decline or remain stable in some of the countries and this declining trend was also obvious for ASDR and DALYs. These declining trends might be attributed to the lesser use of postmenopausal hormonal treatment after the availability of Women’s Health Initiative Trial as this hormonal usage is linked with elevated BC risk [42].
Additionally, this continuous decline or stabilization has been characterized to advancement in mammographic screening, BC diagnosis at earlier stages and better treatment facilities in terms of chemotherapeutics, radiation therapy and targeted approaches [6]. Moreover, we have observed that case fatality burden keeps on declining from 1990 to 2017 in most of the high and high middle SDI regions like North America, Asia pacific, Austrasia and Western Europe illustrating the fact that high income regions have better treatment facilities. Whereas Central Sub-Saharan Africa, Eastern Sub-Saharan Africa, Western Sub-Saharan Africa, Oceania, Southern Sub-Saharan Africa and South Asia have high case fatality rate being less developed regions with limited disease diagnosis and treatment resources.
APC analysis recognizes patterns of cancer incidence/mortality rates from population-based count and data. Group of people born in the same generation is called as age cohort. An aging effect is described as a shift in variable values that happens among all the cohorts separately of time period, as each cohort grows older. A cohort effect is an alteration which exemplifies different populations born at a specific point in time, but it is independent of aging procedure. Whereas a period effect is a change which occurs at a specific time, influencing equally cohorts and all age groups. Results for each SDI region and the global impact of age showed that BC burden (incidence, mortality and DALYs) increases with age in all regions and this trend was more pronounced in the high SDI regions. The period trend keeps on fluctuating in all populations, with higher instabilities in low SDI areas, but the overall results are not much variable. Else, inconsistent trend among the incidence, death and DALYs rates of BC was displayed by the cohort effects. Globally, declining death trends of BC were depicted by cohort. The effect in high SDI quantile was similar to the global trend, but the downward trend was more obvious, followed by high-middle and middle SDI regions, which showed a downward risk trend as a whole, whereas in low-middle and low SDI regions, overall, this risk for BC increased slightly. All these findings again highlight the potential development of breast diagnosis, prognosis and treatment and suggesting that better diagnostic tools along with easy access of treatment modalities all over the world can help in decreasing the global BC burden [6], [43], [44].
To enhance our findings, we have conducted APC analysis on case-fatality rates (CFR) separately for different SDI areas. Consistently, the age, period, and cohort effects of CFR were greater in low SDI as compared to high SDI, but the effect separation was found to be more evident amongst different SDI regions with age greater than 50 years and for cohort 1960 to onward. Furthermore, the overall case fatality risk trend was escalating with increased age and risk value was obviously greater in low SDI countries with reverse findings in high SDI regions. Our findings have shown that the overall cohort pattern gradually decreases from early to late cohort. Whereas the period effect continued to be consistent globally during the entire duration.
We have suggested that country specific information on cancer burden is required for the policy makers to evaluate the influence of cancer registries, control programs, standard of national progress, limited access to health care systems and provide a better plan to combat with the disease. Bearing in mind the fact that prevailing data in many countries is missing or of low quality, and most of the data was based on individual hospital registries and records as they lack national level registries, results from the GBD study can be used by stakeholders to explore the disease pattern of different countries in their corresponding locations.
GBD studies comes up with comprehensive quality global disease burden estimates yet they have some limitations depending upon the data collection procedures, treatment methodologies and individual biasness. Differences or ambiguities in data collection procedures its coding, handling and quality of the sources from where data has been collected (hospital records / national registries) remains unavoidable in any analysis pattern. In addition, variations in the incidence and mortality rates may partially represents the detection biasness linked with the screening and diagnostic protocol’s modifications rather than factual changes in age specific rates. Besides this another inevitable limitation is the unavailability of proper data from many of the undeveloped or under developing countries causing misleading findings, although GBD keeps on filling the gaps in the data throughout the year to provide accurate disease patterns.
Conclusion
BC remains a major health issue among women around the globe and responsible for highest number of cancer-related morbidity and mortality. Globally, BC burden continues to increase from 1990 to 2017 and linked with increasing age as well, whereas the age standardized death rates and DALYs rates has been declining. In the recent years, disease burden has been reduced in most of the high SDI regions, but incremental disease burden has been observed in middle and low SDI regions and there may be a continual growth in the coming years as, BC screening is becoming popular in under developing regions. Hence, preventive measure along with detrimental steps against concerned BC risk factors should be taken to reduce the disease burden specifically among lower SDI countries. There is a need to reduce the health burden from BC in less developed and under developing countries, because under-developed countries are facing higher degree of health-related burden. Public health managers should execute more classified and cost-effective screening and treatment interferences to lessen the premature deaths caused by BC, predominantly among middle and low SDI countries having inadequate healthcare supplies.
Ethics approval
Not applicable.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2018YFC1315302, 2017YFC 1200502); the National Natural Science Foundation of China (Grant No. 81773552).
Compliance for ethical requirement
Ethical requirement is not required as the data has been collected from online source.
CRediT authorship contribution statement
Sumaira Mubarik: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing - original draft, Writing - review & editing. Yong Yu: Formal analysis, Investigation, Software, Visualization. Fang Wang: Investigation, Writing - review & editing. Saima Shakil Malik: Visualization, Writing - original draft, Writing - review & editing. Xiaoxue Liu: Validation, Visualization, Data curation. Muhammad Fawad: Data curation, Methodology Visualization. Fang Shi: Investigation, Resources, Validation. Chuanhua Yu: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This project was supported by the National Key R&D Program of China and the National Natural Science Foundation of China, we would like to thank the funding foundations for their support.
Availability of data and materials
The dataset analyzed during the current study are available in the Institute for Health Metrics and Evaluation (IHME): http://ghdx.healthdata.org/gbd-results-tool.
Biographies

Ms. Sumaira Mubarik is a final year PhD researcher at Wuhan University, China. She has published more than 40 research papers in well-reputed peer-reviewed international journals, including, The Lancet, The Lancet Public Health, Journal of the American College of Cardiology, International journal of epidemiology, Frontiers in Medicine, Scientific Reports, Cancer Communications, Clinical Epidemiology etc. She’s also collaborator in Global burden of disease study projects, University of Washgonton USA.

Prof. Yong Yu is a postgraduate supervisor in the school of public health at Hubei university of Medicine. He graduated from Wuhan University of technology in June 2007. His research interests include environmental epidemiology and disease burden research, software development, data mining and processing, proficient in PHP, R, Python, C#, VB, and published more than 30 papers in well-reputed journals.

Dr. Fang Wang has received her PhD degree in Statistics from Wuhan University, Wuhan, China in the year 2021. Currently, she is working as a researcher at Xuzhou Medical University, China. She has published more than 30 research papers in well-reputed journals, including International journal of epidemiology, Journal of the American College of Cardiology, The Lancet, Cancer Communications, and Scientific Reports etc.

Ms. Xiaoxue Liu is final year PhD Scholar at Wuhan University, Wuhan, China. She has many important contributions towards cancer science and epidemiology published by various well-known publishers.

Dr. Saima Shakil Malik is a biologist and a postdoctoral researcher at University of Alabama at Birmingham. She received her PhD degree in Cancer genetics from Fatimah Jinnah Women University, Pakistan, in 2020. She has developed research in cancer genetics, epidemiology, pathology, DNA repair mechanisms and post translational modifications. She is an editor of a book, author of several book chapters and various scientific publications.

Dr. Muhammad Fawad has received his PhD degree in Mathematical Statistics from Central China Normal University, Wuhan, China in 2019. Currently, he is working as a Postdoctoral researcher at Zhengzhou Key Laboratory of Big Data Analysis and Application, Henan Academy of Big Data, and School of Mathematics and Statistics, Zhengzhou University, Zhengzhou, China. His research interests include Applied Probability, Robust Estimation Methods, Mathematical Statistics and developed various research articles within these research areas.

Ms. Fang Shi is a final year PhD Scholar at Wuhan University, Wuhan, China. She has published more than 10 research papers in well-reputed journals, including, Preventive Veterinary Medicine, Cancer Communications, International journal of epidemiology, Frontiers in Medicine, and Cancer Management and Research etc.

Prof. Dr. Chuanhua Yu is currently working at Department of Epidemiology and Biostatistics, School of Health Sciences, Wuhan University, Wuhan, China. He completed his PhD in Biostatistics at The Fourth Military Medical University, Xi'an, China. His main research interests include Method and application for Global Burden of Disease and Statistical methods in decision making for medical research. He has developed various research projects, have many national and international grants, and produced several postgraduate and PhD dissertations. He has published dozens of research articles in peer-reviewed international journals and authored or co-authored various books.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.07.012.
Contributor Information
Sumaira Mubarik, Email: sumaira@whu.edu.cn.
Chuanhua Yu, Email: yuchua@whu.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R., Allen C., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 2017; 390(10100): 1151–1210. [DOI] [PMC free article] [PubMed]
- 3.Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mubarik S., Hu Y., Yu C. A multi-country comparison of stochastic models of breast cancer mortality with P-splines smoothing approach. BMC Med Res Method. 2020;20(1):1–16. doi: 10.1186/s12874-020-01187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer J Clinicians 2019; 69(1): 7–34. [DOI] [PubMed]
- 6.Youlden D.R., Cramb S.M., Dunn N.A.M., Muller J.M., Pyke C.M., Baade P.D. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237–248. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Ginsburg O., Bray F., Coleman M.P., Vanderpuye V., Eniu A., Kotha S.R., et al. The global burden of women’s cancers: a grand challenge in global health. The Lancet. 2017;389(10071):847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira R.A., Biller G., Uemura G., Ruiz C.A., Curado M.P. Breast cancer screening in developing countries. Clinics. 2017;72(4):244–253. doi: 10.6061/clinics/2017(04)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfano C.M., Leach C.R., Smith T.G., Miller K.D., Alcaraz K.I., Cannady R.S., et al. Equitably improving outcomes for cancer survivors and supporting caregivers: a blueprint for care delivery, research, education, and policy. CA Cancer J Clin. 2019;69(1):35–49. doi: 10.3322/caac.21548. [DOI] [PubMed] [Google Scholar]
- 10.Malik SS, Akhtar N, Fatima I, Akram Z, Masood N. Molecular profiling of breast cancer in clinical trials: A perspective. 'Essentials of Cancer Genomic, Computational Approaches and Precision Medicine. Springer; 2020. p. 313–32.
- 11.Malik S.S., Masood N., Sherrard A., Bishop P.N. Elsevier; 2019. Small non-coding RNAs as a tool for personalized therapy in familial cancers. AGO-Driven Non-Coding RNAs; pp. 179–208. [Google Scholar]
- 12.Anderson W.F., Chen B.E., Brinton L.A., Devesa S.S. Qualitative age interactions (or effect modification) suggest different cancer pathways for early-onset and late-onset breast cancers. Cancer Causes Control. 2007;18(10):1187–1198. doi: 10.1007/s10552-007-9057-x. [DOI] [PubMed] [Google Scholar]
- 13.Clayton D., Schifflers E. Models for temporal variation in cancer rates. I: age–period and age–cohort models. Stat Med. 1987;6(4):449–467. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y., Land K.C. CRC Press; 2013. Age-period-cohort analysis: New models, methods, and empirical applications. [Google Scholar]
- 15.Mubarik S., Wang F., Malik S.S., Shi F., Wang Y., Nawsherwan, et al. A hierarchical age–period–cohort analysis of breast cancer mortality and disability adjusted life years (1990–2015) attributable to modified risk factors among Chinese women. Int J Environ Res Public Health. 2020;17(4):1367. doi: 10.3390/ijerph17041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyu H.H., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gakidou E., Afshin A., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vos T., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foreman K.J., Lozano R., Lopez A.D., Murray C.J. Modeling causes of death: an integrated approach using CODEm. Population Health Metrics. 2012;10(1):1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H., Abajobir A.A., Abate K.H., Abbafati C., Abbas K.M., Abd-Allah F., et al. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1084–1150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou L., Deng Y., Li N.a., Zheng Y.i., Tian T., Zhai Z., et al. Global, regional, and national burden of hodgkin lymphoma from 1990 to 2017: estimates from the 2017 Global Burden of Disease Study. J Hematol Oncol. 2019;12(1) doi: 10.1186/s13045-019-0799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray C.J.L., Acharya A.K. Understanding DALYs. J Health Econ. 1997;16(6):703–730. doi: 10.1016/s0167-6296(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 25.Murray C.J. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429. [PMC free article] [PubMed] [Google Scholar]
- 26.Salomon J.A., Haagsma J.A., Davis A., de Noordhout C.M., Polinder S., Havelaar A.H., et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Global Health. 2015;3(11):e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 27.Heer E., Harper A., Escandor N., Sung H., McCormack V., Fidler-Benaoudia M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Global Health. 2020;8(8):e1027–e1037. doi: 10.1016/S2214-109X(20)30215-1. [DOI] [PubMed] [Google Scholar]
- 28.Jung Y., Lee Y., MacEachern S.N. Efficient quantile regression for heteroscedastic models. J Stat Comput Simul. 2015;85(13):2548–2568. [Google Scholar]
- 29.Hu K, Lou L, Tian W, Pan T, Ye J, Zhang S. The outcome of breast cancer is associated with national human development index and health system attainment. PloS one 2016; 11(7). [DOI] [PMC free article] [PubMed]
- 30.Carstensen B, Plummer M, Laara E, Hills M, Carstensen MB. Package ‘Epi’. 2021.
- 31.Hu K., Ding P., Wu Y., Tian W., Pan T., Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ open. 2019;9(10):e028461. doi: 10.1136/bmjopen-2018-028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N.a., Deng Y., Zhou L., Tian T., Yang S.i., Wu Y., et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1) doi: 10.1186/s13045-019-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arshi A., Sharifi F.S., Khorramian Ghahfarokhi M., Faghih Z., Doosti A., Ostovari S., et al. Expression analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in breast cancer tissues from young women and women over 45 years of age. Mol Therapy-Nucleic Acids. 2018;12:751–757. doi: 10.1016/j.omtn.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malik S.S., Zia A., Rashid S., Mubarik S., Masood N., Hussain M., et al. XPC as breast cancer susceptibility gene: evidence from genetic profiling, statistical inferences and protein structural analysis. Breast Cancer. 2020;27(6):1168–1176. doi: 10.1007/s12282-020-01121-z. [DOI] [PubMed] [Google Scholar]
- 35.Ma X., Liu C., Xu X., Liu L., Gao C., Zhuang J., et al. Biomarker expression analysis in different age groups revealed age was a risk factor for breast cancer. J Cell Physiol. 2020;235(5):4268–4278. doi: 10.1002/jcp.29304. [DOI] [PubMed] [Google Scholar]
- 36.Mubarik S., Wang F., Fawad M., Wang Y., Ahmad I., Yu C. trends and projections in Breast cancer Mortality among four Asian countries (1990–2017): Evidence from five Stochastic Mortality Models. Sci Rep. 2020;10(1):1–12. doi: 10.1038/s41598-020-62393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mubarik S., Malik S.S., Wang Z., Li C., Fawad M., Yu C. Recent insights into breast cancer incidence trends among four Asian countries using age-period-cohort model. Cancer Manage Res. 2019;11:8145. doi: 10.2147/CMAR.S208323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z., Xu L.u., Shi W., Zeng F., Zhuo R., Hao X., et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990–2017. Breast Cancer Res Treat. 2020;180(2):481–490. doi: 10.1007/s10549-020-05561-1. [DOI] [PubMed] [Google Scholar]
- 39.DeSantis C.E., Bray F., Ferlay J., Lortet-Tieulent J., Anderson B.O., Jemal A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1495–1506. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 40.Kearney A.J. Viewpoint: It is time to reconsider policy for population-based mammography screening. J Public Health Policy. 2015;36(3):259–269. doi: 10.1057/jphp.2015.19. [DOI] [PubMed] [Google Scholar]
- 41.Katalinic A., Eisemann N., Kraywinkel K., Noftz M.R., Hübner J. Breast cancer incidence and mortality before and after implementation of the German mammography screening program. Int J Cancer. 2020;147(3):709–718. doi: 10.1002/ijc.32767. [DOI] [PubMed] [Google Scholar]
- 42.Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 43.Malik S.S., Mubarik S., Aftab A., Khan R., Masood N., Asif M., et al. Correlation of MSH2 exonic deletions and protein downregulation with breast cancer biomarkers and outcome in Pakistani women/patients. Environ Sci Pollut Res. 2021;28(3):3066–3077. doi: 10.1007/s11356-020-10717-z. [DOI] [PubMed] [Google Scholar]
- 44.Mubarik S., Liu X., Malik S.S., Wang L., Yu Y., Yu C. Evaluation of lifestyle risk factor differences in global patterns of breast cancer mortality and DALYs during 1990–2017 using hierarchical age-period-cohort analysis. Environ Sci Pollut Res. 2021:1–13. doi: 10.1007/s11356-021-14165-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during the current study are available in the Institute for Health Metrics and Evaluation (IHME): http://ghdx.healthdata.org/gbd-results-tool.