Abstract
Nonalcoholic fatty liver disease (NAFLD) is a multisystemic clinical condition that presents with a wide spectrum of extrahepatic manifestations, such as obesity, type 2 diabetes mellitus, metabolic syndrome, cardiovascular diseases, chronic kidney disease, extrahepatic malignancies, cognitive disorders, and polycystic ovarian syndrome. Among NAFLD patients, the most common mortality etiology is cardiovascular disorders, followed by extrahepatic malignancies, diabetes mellitus, and liver-related complications. Furthermore, the severity of extrahepatic diseases is parallel to the severity of NAFLD. In clinical practice, awareness of the associations of concomitant diseases is of major importance for initiating prompt and timely screening and multidisciplinary management of the disease spectrum. In 2020, a consensus from 22 countries redefined the disease as metabolic (dysfunction)-associated fatty liver disease (MAFLD), which resulted in the redefinition of the corresponding population. Although the patients diagnosed with MAFLD and NAFLD mostly overlap, the MAFLD and NAFLD populations are not identical. In this review, we compared the associations of key extrahepatic diseases between NAFLD and MAFLD.
Keywords: Metabolic diseases; Fatty liver; Liver fibrosis; Diabetes mellitus, type 2; Cardiometabolic risk factors
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become the leading cause of chronic liver disease worldwide, with an estimated global prevalence of 25%, placing a significant burden on the healthcare system.1 NAFLD is defined as the presence of hepatic steatosis, detected either by imaging or histology when secondary causes for hepatic fat accumulation are excluded.2 Given the close association of NAFLD with its causative drivers, such as obesity, metabolic syndrome (MS), and type 2 diabetes mellitus (T2DM), the increasing trend of these metabolic diseases is also expected to cause an increasing tendency in NAFLD incidence.3,4 As such, an international panel of experts from 22 countries proposed a change in terminology and definition that more accurately reflects the pathogenesis of the disease.5,6 The suggested terminology of metabolic (dysfunction)-associated fatty liver disease (MAFLD) is defined as hepatic steatosis entity in addition to the presence of overweight or obesity, diabetes mellitus, or evidence of metabolic dysfunction.6 The semantic modification of “NAFLD” as “MAFLD” highlights the role of metabolic factors in the disease etiology, which would hopefully facilitate understanding of the disease and patient-physician communication.7
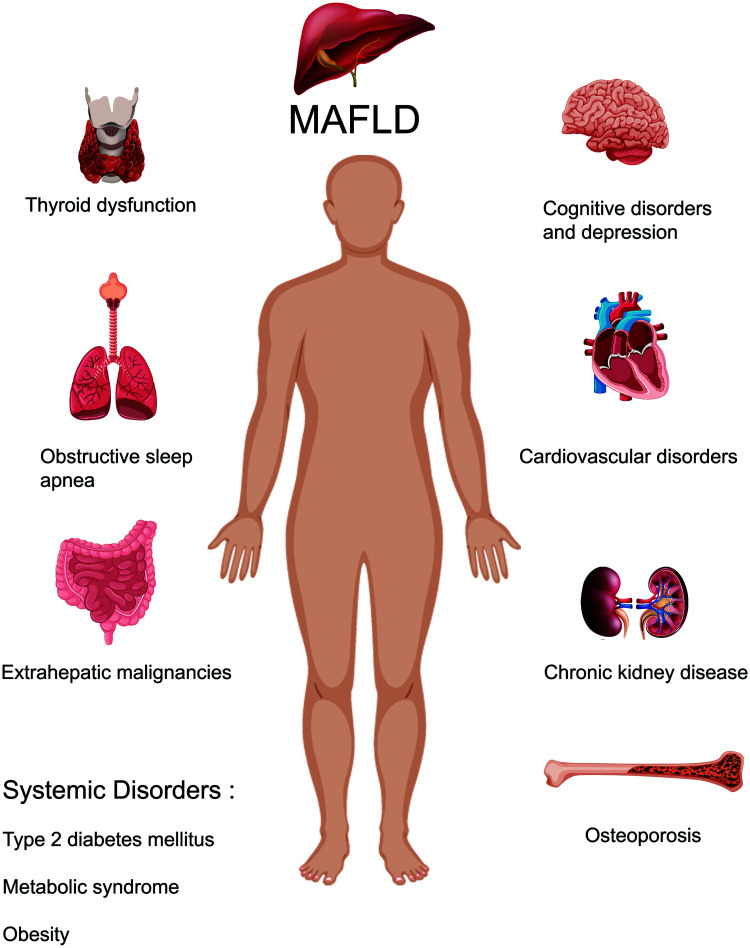
NAFLD patients are at higher risk of not only liver-related complications but also cardiovascular and all-cause mortality. Indeed, cardiovascular disorders are the leading cause of mortality in patients with NAFLD, followed by extrahepatic malignancies and liver-related complications, indicating the multisystemic involvement in the disease.8 In addition to cardiovascular disorders, NAFLD is also associated with other extrahepatic diseases, such as MS, obesity, T2DM, chronic kidney disease (CKD), polycystic ovarian syndrome (PCOS), obstructive sleep apnea (OSA), extrahepatic malignancies, osteoporosis, and cognitive disorders (Fig. 1).9–11 As such, the possible extrahepatic involvements of NAFLD need to be screened and treated.12
Fig. 1. MAFLD and its association with extrahepatic diseases.
MAFLD, Metabolic (dysfunction)-associated fatty liver disease.
However, despite increasing evidence on the strong relationship between extrahepatic diseases and NAFLD, studies on the effect of the redefinition of the patient population as “MAFLD” on the clinical reflection of extrahepatic diseases in this population are still lacking. This review will focus on the association of NAFLD with various extrahepatic diseases and discuss the influence of the semantic change to “MAFLD” in reanalyzing extrahepatic diseases in this newly defined group of patients.
Impact of redefinition on selected patients in studies
As is known, the redefinition of “NAFLD” as “MAFLD” is more than a single-letter change.7 Recently, this change was validated in the Third National Health and Nutrition Examination Surveys (NHANES-III 1988–1994) database, which defined hepatic steatosis according to ultrasonographic examination. It was concluded that MAFLD was able to identify patients with a high risk of disease progression more practically and accurately compared to NAFLD. This statement was concluded following a comparison of non-invasive scores including the fibrosis-4 index (FIB-4), NAFLD fibrosis score (NFS), and BARD score.13 Accordingly, in that study, MAFLD patients were older (48.79±15.06 vs. 46.81±15.77, p<0.001), mostly male (1959 [50.42%] vs. 2014 [46.33%], <0.001), higher body mass index (BMI) level (31.14±6.05 vs. 29.49±6.69, p<0.001) higher proportions of T2DM (1171 [30.14%] vs. 1092 [25.12%], p<0.001) and hypertension (1405 [36.16%] vs. 1343 [30.89%], p<0.001) compared to NAFLD patients. Moreover, MAFLD patients showed higher insulin resistance, serum lipid, and transaminases.13 On the other hand, in a smaller population who underwent vibration controlled transient elastography (VCTE) examinations from 2017–2018, advanced fibrosis showed similar proportions in the MAFLD and NAFLD populations (7.5% vs. 7.4%). Moreover, the two definitions showed a high rate of agreement, with a Kappa coefficient of 0.92.14 The NHANES-III database from 1988–1994, which diagnosed hepatic steatosis with ultrasonography, showed that MAFLD and NAFLD overlapped mostly, with an agreement coefficient of 0.76.15 These data suggest that the two definitions are able to define mostly similar but not identical populations.14,15 When the NHANES-III 1988–1994 database was analyzed with 2015 mortality data, it was revealed that both MAFLD and NAFLD showed similar rates of overall, cardiovascular- and neoplasm-related mortality, when age and sex were adjusted (hazard ratio [HR]: 1.27 (confidence interval [CI]: 1.16, 1.41) vs. 1.05 (0.87, 1.28), 1.17 (0.96, 1.42) vs. 1.07 (0.89, 1.30), and 1.16 (0.94, 1.42) vs. 1.01 (0.82, 1.25), respectively). On the other hand, after adjustment according to age, sex and ethnicity, T2DM-related mortality was markedly elevated in MAFLD patients compared to NAFLD patients (4.57 (2.63, 7.97) vs. 2.54 (1.49, 4.34)).15
Similar analyses were conducted in population-based cohorts. Yamamura et al.16 enrolled a total of 765 Japanese patients with fatty liver defined by ultrasonography. In their cohort, MAFLD patients were mostly male (308 (50.6%) vs. 138 (33.8%), p<0.001), had higher BMI (median [minimum-maximum]: 25.0 [23.2–26.9] vs. 24.0 [21.7–26.1], p<0.001) and higher rates of hypertension and dyslipidemia (252 (41.4%) vs. 160 (29.6%), p<0.001 and 289 (47.5%) vs. 210 (38.8%), p=0.003, respectively). On the other hand, the prevalence of T2DM did not significantly differ between MAFLD and NAFLD (89 (14.6%) vs. 59 (10.9%), p=0.060). Similar to the analysis with the data from the NHANES-III database,13 MAFLD patients had increased parameters indicating increased risk for fibrosis. In addition, those patients showed a more severe metabolic profile, with high serum creatinine, uric acid, alanine transaminase, aspartate transaminase, and gamma-glutamyl transferase levels.16
Wong et al.17 evaluated 1,016 Chinese patients with intrahepatic triglyceride accumulation >5% defined by magnetic resonance imaging proton density fat fraction (MRI-PDFF). In total, 25.9% and 25.7% of the patients were classified as MAFLD and NAFLD cases, respectively. Of the 277 subjects with evidence of MRI-PDFF-based hepatic steatosis, 247 (89.2%) showed overlaps with both MAFLD and NAFLD. Meanwhile, 14 patients (5.1%) were not diagnosed with MAFLD, despite having evidence of hepatic steatosis on MRI-PDFF. Follow up of patients without fatty liver indicated that the incidence of MAFLD is 25% lower than that of NAFLD in this population. The authors attributed this lower incidence to the lower metabolic burden of these patients. Following the study by Wong et al.,17 Angelico et al.18 evaluated an Italian cohort of 795 NAFLD patients diagnosed by ultrasonography. In total, 96.5% of the patients had both MAFLD and NAFLD; however, 3.5% of the patients remained with the NAFLD diagnosis and not MAFLD. They also observed higher non-invasive scores in patients with MAFLD than in those with NAFLD.
Genetic aspects of NAFLD and MAFLD
NAFLD progression is associated with at least five different genes, namely patatin-like phospholipase domain-containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), glucokinase regulator, membrane-bound O-acyltransferase domain containing 7, and 17β-hydroxysteroid dehydrogenase type 13.19,20 Meanwhile, the understanding of MAFLD seems to be challenging, owing to the limited number of genetic studies in patients with MAFLD. Further, the authors if the related papers have tended to define MAFLD according to the diagnostic criteria for NAFLD. Lee et al.21 compared the association of genetic alleles such as NCAN, glucokinase regulator, LYPLAL1, PNPLA3, PPP1R3B, FDFT1, COL13A1, EFCAB4B, PZP, and TM6SF2 between MAFLD patients and patients without hepatic steatosis in their Chinese cohort. They found that the PNPLA3 homozygous GG allele, higher BMI, and hypertriglyceridemia were independent predictors of MAFLD. However, they had recruited their population following the criteria for NAFLD. Similarly, Gu et al.22 showed that variations in fat mass and obesity-associated genes are associated with a higher risk of MAFLD in their Chinese cohort. However, they also defined MAFLD according to the NAFLD criteria.
Obesity, diabetes mellitus, and metabolic syndrome
The modification of “NAFLD” to “MAFLD” emphasized the role of metabolic dysfunction and highlighted the underlying metabolic drivers for the development of the disease.23 Obesity is a well-known risk factor of NAFLD.24,25 Indeed, in the most recent decade, the prevalence of NAFLD showed a similar increasing trend, parallel to the increasing prevalence of obesity.26,27 More than 90% of morbidly obese patients who undergo bariatric surgery have NAFLD.28,29 On the other hand, lean patients with evidence of hepatic steatosis were also to no less a degree. In a recent meta-analysis by Young et al.,30 “lean NAFLD” was prevalent in 11% and 25% of the general and NAFLD populations, respectively. Lean NAFLD patients presented with a more abnormal metabolic profile than healthy individuals and showed a higher prevalence of hypertension, insulin resistance, dyslipidemia, and metabolic syndrome and higher levels of inflammatory parameters.30,31 On the other hand, obese NAFLD patients showed higher levels of blood pressure, homeostatic model assessment for insulin resistance, hemoglobin A1C, alanine transaminase, and serum creatinine and albumin.31 Lean NAFLD patients also have higher levels of hemoglobin, hematocrit, and ferritin than overweight NAFLD patients.32,33,34 However, these patients also have a more favorable metabolic and histologic presentation than obese NAFLD patients.31
Notably, there are recommended ethnicity-specific BMI cutoffs to define “lean NAFLD”. The BMI cutoffs for defining lean are <25 kg/m2 for Caucasians and <23 kg/m2 for Asians.32 Therefore, the ethnicity of the study population should be identified when defining lean NAFLD. In general, lean NAFLD is prevalent in 5–45% of Asians and in 5–20% of Europeans.33 However, applying the proposed cutoff for Asians would lower the lean NAFLD prevalence. Therefore, accurate definition of obesity is crucial for an accurate MAFLD diagnosis. In this regard, the recent MAFLD guidelines recommend a BMI cutoff of 23 kg/m2 for Asians.35 Weight reduction and dietary interventions are considered the cornerstone of NAFLD treatment, both in obese and lean patients.36,37 The beneficial effects of lifestyle modifications targeting weight reduction and exercise have been consistently shown to be beneficial in the resolution of hepatic steatosis in both lean Asian and Caucasian NAFLD populations.38,39,40
The new definition as “MAFLD” emphasized the importance of metabolic dysfunction criteria or T2DM crucial for the diagnosis of the disease. In this concept, there are also patients who are classified with NAFLD but not MAFLD according to the new terminology. In the analysis performed by Angelico et al.,18 3.5% of the 795 NAFLD patients did not fulfill the MAFLD criteria due to not being overweight or obese and not having diabetes or metabolic dysfunction criteria. Wong et al.17 also reported that the lower prevalence of MAFLD than NAFLD among lean patients is due to the lack of other metabolic dysfunction criteria among lean patients. In line with these data, it appears that the new terminology would result in a non-classified group, that is, “NAFLD but not MAFLD”. However, further studies investigating the characteristics of newly defined lean MAFLD populations are needed.
T2DM accelerates disease progression, as evidenced by the higher rates of advanced fibrosis and adverse outcomes in diabetes patients with NAFLD.36 Moreover, the coexistence of NAFLD and T2DM is associated not only with increased liver-related mortality but also with cardiovascular and all-cause mortalities. Notably, microvesicular diabetic complications, such as retinopathy, nephropathy, and polyneuropathy, more frequently occur in diabetes patients with coexisting NAFLD, independent of confounding factors.37,41,42 Therefore, a first-line stratification algorithm with non-invasive diagnostic scores was recently recommended for T2DM patients with NAFLD.43 The FIB-4 can be easily calculated in ambulant clinical settings, and identifying patients with an FIB-4 >1.3 might be useful in the timely recognition of severe disease.44
Insulin resistance also plays a major role in disease development.45 Traditionally, insulin resistance is the first hit of the “two-hit hypothesis”, followed by oxidative stress, lipid peroxidation, and mitochondrial dysfunction as the second hit.46 Although there are multiple pathways in disease development, insulin resistance constitutes a major pathway in NAFLD. Patients with insulin resistance have more severe liver histology. Further, insulin resistance is an independent risk factor for advanced fibrosis.47,48
The new definition of MAFLD has led to an increase in its prevalence in T2DM patients.13 However, there are scarce data supporting the possibility that there is no significant difference in T2DM prevalence between NAFLD and MAFLD.16 Further studies are needed to highlight the possible impact of this terminology change. Recent MAFLD guidelines recommend that T2DM populations be screened for MAFLD.35 There also seems to be a bidirectional relationship between metabolic syndrome and NAFLD,49 as NAFLD itself is associated with MS and each of its components, namely abdominal obesity, hyperglycemia, hypertension, and dyslipidemia.50 In the meta-analysis performed by Ballesteri et al.,51 NAFLD was associated with incident MS in the 5-year follow-up. Similarly, another study found that patients with metabolic components were at higher risk of developing incident NAFLD.52 Lin et al.13 compared MAFLD with traditional NAFLD and found higher proportions of metabolic comorbidities in patients with MAFLD, indicating the impact of positive diagnostic criteria.
Cardiovascular diseases (CVDs)
Increasing evidence supports that CVD is a matter of debate in NAFLD. Patients with NAFLD have been reported to have a higher risk of CVD-related death than of liver-related death.53,54 A meta-analysis by Targher et al.55 involving 34,000 individuals followed-up for 6.9 years found an increased risk of fatal and non-fatal cardiovascular events (odds ratio [OR:] 1.64, 95% CI: 1.26–2.13). When the analysis was adjusted for the conventional cardiovascular risk factors, severe NAFLD increased the risk of a cardiovascular event (OR: 2.58, 95% CI: 1.78–3.75). Moreover, in the meta-analysis by Oni et al.,56 NAFLD was significantly associated with increased carotid artery intimal-medial thickness, impaired flow-mediated vasodilation, increased arterial stiffness, and coronary artery calcification, which are the main indicators of subclinical atherosclerosis. This association was found to be independent of traditional risk factors and metabolic components.
The risk of cardiovascular events and mortality pertained to NAFLD patients who were on the waiting list for liver transplantation and in the post-transplantation period.57,58 Even in primary care settings, there is an independent association between myocardial infarction and NAFLD.59 Advanced fibrosis is the most important prognostic factor in NAFLD. Therefore, initiating and planning therapy is of paramount importance.54,60 Among biopsy-proven NAFLD patients with incident cardiovascular events, advanced fibrosis on liver histology is an independent predictor of cardiovascular events.61 In a recent nationwide study from Korea, the multivariable-adjusted HRs for developing a cardiovascular event among patients with NAFLD and MAFLD were 1.09 (95% CI: 1.03–1.15) and 1.43 (95% CI: 1.41–1.45), respectively. This finding highlights that the redefinition of the disease may identify more patients with a high risk of developing cardiovascular events.62 Guerreiro et al.63 investigated 109 patients with hepatic steatosis, and 90% and 64% fulfilled the MAFLD criteria and NAFLD criteria, respectively. CVD occurred in 20% and 13%, respectively (p=0.137). These rates could be due to the inclusion of metabolic dysfunction criteria, which are associated with increased CVD rates, in the definition.64
NAFLD has also been reported to be associated with cardiac arrhythmias, including atrial fibrillation (AF), prolongation of the corrected QT interval (QTc), some cardiac conduction defects, cardiac autonomic dysfunction, and sudden cardiac death.65,66 NAFLD doubles the risk of AF (OR: 2.07; 95% CI: 1.38–3.10) independent of the traditional risk factors for AF.66 This relationship was confirmed in another meta-analysis involving a larger population of more than 600,000 individuals.67 Increasing evidence suggests an association between prolongation of QT interval (QTc) and ventricular arrhythmias in patients with MAFLD.66,67 The increased risk of QTc interval prolongation is in line with increased severity of ultrasonographically-defined NAFLD. After adjusting for factors associated with QTc prolongation, all types of NAFLD were associated with an increased risk of QTc prolongation in women, with ORs (95% CIs) of 1.11 (1.01–1.21) for mild, 1.61 (1.36–1.9) for moderate, and 1.31 (1.16–2.24) for severe. The corresponding ORs (95% CI) for men were 1.11 (1.01–1.21), 1.39 (1.22–1.59), and 1.87 (1.16–2.24), respectively. This significant association remained in the subgroup analysis of the diabetes and non-diabetes populations.68 In another retrospective analysis, the rates of nonsustained ventricular tachycardia and >30 premature ventricular complexes per hour were significantly higher in T2DM patients with an ultrasonographically-proven NAFLD diagnosis compared to non-NAFLD patients with diabetes. In the adjusted analysis for age, sex, BMI, smoking, hypertension, ischemic heart disease, valvular heart disease, chronic kidney disease, chronic obstructive pulmonary disease, serum gamma-glutamyl transferase levels, medication use, and left ventricular ejection fraction, NAFLD patients had a 3.5-fold higher risk of ventricular arrhythmias compared to non-NAFLD patients.69
NAFLD also appears to be associated with further structural cardiac pathologies. NAFLD was found to be significantly associated with a nearly 3-fold higher risk (OR: 2.70, 95% CI: 1.23–7.38) of aortic valve sclerosis and mitral annulus calcification, which are predictors of adverse cardiac outcomes.70 Moreover, a case-control study found that both hepatic steatosis and fibrosis were are associated with subclinical myocardial dysfunction on fluorodeoxyglucose-positron emission tomography.71 NAFLD severity assessed by the FIB-4 score is also independently associated with left ventricular diastolic dysfunction, larger atrial volume, and higher all-cause mortality in patients with known heart failure.72 This positive association between NAFLD severity and myocardial abnormalities was also confirmed in smaller studies of biopsy-proven NAFLD.73,74
The relation between increased cardiovascular burden and NAFLD is multifactorial. Abnormal blood glucose levels triggered by insulin resistance are a common pathophysiological condition. Another shared mechanism is endothelial dysfunction that is significantly associated with atherosclerosis. NAFLD patients have a decreased balance in procoagulant metabolism, which also plays a significant role in the development of cardiovascular events.75 Ectopic fat accumulation enhances the development of atherosclerosis. Moreover, it leads to the secretion of proinflammatory adipokines that results in atrial and ventricular fibrosis.76 Collectively, these induce systolic and diastolic dysfunction or cardiac arrhythmias.77 Meanwhile, although they are significantly associated with NAFLD and NAFLD severity, genetic variants of PNPLA3 and TM6SF2 were shown to be cardioprotective. This is probably due to the strong association of those variants with lower levels of triglycerides and low density lipoproteins.78
Collectively, these data support the significantly higher risk of cardiovascular-related morbidity and mortality in the NAFLD population, indicating the importance of first-line screening for cardiovascular disorders independent of traditional cardiovascular risk factors in these patients. MAFLD patients also have a higher risk of developing cardiovascular events, and this issue has gained more importance.
CKD
There is growing evidence supporting a close relationship between NAFLD and CKD. This association could be related to a high prevalence of both diseases or an independent occurrence.79 Nevertheless, NAFLD and CKD share common risk factors, including abdominal obesity, insulin resistance, atherogenic dyslipidemia, and hypertension.80 Moreover, recent studies have consistently demonstrated that NAFLD is independently associated with a higher prevalence of CKD.81–83 However, a cross-sectional study by Akahane et al.84 showed that the link between NAFLD and CKD was mediated by shared risk factors rather than an independent association. Similarly, the association of NAFLD with CKD appears to arise from increased adipose tissue-related inflammation. Therefore, a CKD-focused screening of NAFLD patients with obesity, hypertension, and hyperuricemia is recommended.85
T2DM is an important driving factor for both NAFLD and CKD. However, the prevalence of CKD was higher in NAFLD patients, regardless of diabetes status, than in patients without NAFLD (50% vs. 5–30%).86–89 Moreover, patients with severe liver histology were more likely to develop incident CKD.90 In contrast, hepatic steatosis has no adverse impact on renal function.91 In the meta-analysis by Musso et al.,92 advanced fibrosis and inflammation were associated with more severe kidney dysfunction than hepatic steatosis itself. They concluded that the more severe the liver histology, the more severe the CKD. Even early kidney dysfunction was proposed to be associated with more severe liver histology in biopsy-proven NAFLD patients, highlighting the importance of early screening for the timely management of kidney disease.93,94 MAFLD patients have been found to have a higher burden of CKD. Data from NHANES-III 1988–1994 revealed that CKD is more prevalent in MAFLD patients than in NAFLD patients. This suggested that MAFLD could identify more patients with a higher risk of CKD in addition to other comorbidities.95
Extrahepatic malignancies
Extrahepatic malignancies are one of the leading causes of mortality in NAFLD.8,9 Thus, hepatocellular cancer (HCC) surveillance is recommended in MAFLD patients with cirrhosis.35 The possible mechanism of carcinogenesis in NAFLD is derived from proinflammatory and procarcinogenic aspects of insulin resistance through activation of the insulin growth factor-1 axis. These induce antiapoptotic effects and adipose tissue dysfunction that increase inflammation and tumor proliferation. Gut dysbiosis was also found to be a possible contributing factor in promotion of tumorigenesis.96 A meta-analysis by Musso et al.97 found that extrahepatic malignancies were the most common cause for mortality, accounting for 28% of all deaths, followed by ischemic heart disease (25%) and liver-related diseases (13%). In a large Korean cohort, the prevalence rate of cancer was higher in the NAFLD group than in the non-NAFLD group (782.9 vs. 592.8 per 100,000 person-years; HR: 1.32; 95% CI: 1.17–1.49; p<0.001). Accordingly, the most common cancer type was HCC in the general population, colorectal carcinoma in males, and breast cancer in females.98
Allen et al.99 recently investigated the incidence of cancer in a large community cohort within a median follow-up of 8 years and found that NAFLD patients had a 2-fold higher risk of developing cancer. The most common cancer type was HCC, followed by uterine, gastric, pancreatic, and colonic cancers. Moreover, they showed that NAFLD was more strongly associated with cancer development than obesity. In the absence of NAFLD, obesity was less strongly associated with cancer development, which indicates the possible role of NAFLD in promoting cancer development in obese patients. Unlike in obese women, NAFLD was associated with breast cancer, independent of traditional risk factors, in non-obese women.100
In a recent meta-analysis of studies published between 1996 and January 2020, NAFLD was significantly associated with gastrointestinal cancers (colorectal cancer and colorectal adenoma), cholangiocarcinomas, and other cancers (including breast, gastric, pancreatic, prostate, and esophageal cancers), indicating that NAFLD is a potential influencing factor in extrahepatic diseases.101 A systemic review and meta-analysis suggested that NAFLD increased the risk of cholangiocarcinoma, and the increased risk was more pronounced for intrahepatic cholangiocarcinoma than for extrahepatic cholangiocarcinoma.102 A meta-analysis also found that NAFLD is significantly associated with extrahepatic cholangiocarcinoma but not with intrahepatic cholangiocarcinoma after excluding confounding factors.103 Another meta-analysis found that NAFLD increased the risk of colorectal adenoma and carcinoma.104,105 Among patients with NAFLD, the overall risk was higher for right colon tumors than for left colon tumors.106 Fukugana et al.107 recently demonstrated that MAFLD more accurately identifies colorectal adenoma than does NAFLD. Specifically, non-obese MAFLD is an independent factor for the presence of colorectal adenomas. As such, the authors recommended colonoscopy screening in patients with MAFLD.
Depression and cognitive disorders
Depression and cognitive impairment have been recently shown to be associated with NAFLD;108 however, this relationship remains unclear. Tomeno et al.109 detected major depressive disorder in 12% of their patients with biopsy-proven NAFLD. Moreover, liver histology was more severe in patients with depression than in patients without depression, in line with the findings by Youssef et al.110 In contrast, Lee et al.111 did not identify this association. In a large population-based study, hepatitis C infection was the chronic liver disease significantly associated with depression.
In line with this, there have been attempts to compare total brain volume, gray and white matter volumes, and lateral ventricle volume between patients with and without NAFLD. The results showed a greater risk for cognitive disorders in patients with NAFLD.112 Further studies found that MAFLD patients were at higher risk for early or subtle cognitive dysfunction than healthy individuals, but this relationship was not shown to be significantly correlated with the presence of metabolic syndrome. Cognition is mainly regulated by visuospatial and executive function domains associated with the prefrontal cortex.113 Weinstein et al.114 suggested that patients at higher risk for advanced fibrosis but not MAFLD were also at higher risk for cognitive impairment development. The association between Alzheimer’s disease and NAFLD remains to be clarified. However, increasing evidence from various animal studies suggested a possible significant association,115,116 although further investigations are still needed. The underlying mechanism between these associations was suggested to be dysfunction in lipid metabolism and insulin resistance, which have been previously proposed to trigger the development of Alzheimer’s disease.108 However, to our best knowledge, no study has compared between the impact of MAFLD and NAFLD on cognitive disorders.
PCOS
NAFLD and PCOS share several common comorbidities, such as obesity, insulin resistance, diabetes mellitus, hypertension, and metabolic syndrome.11,117 Accordingly, more than half of patients with PCOS also have NAFLD.118 A recent large population-based study found that PCOS patients were more likely to be diagnosed with NAFLD (OR: 4.3) even after adjusting for confounding factors. Thus, the risk of developing NAFLD has also been reported to increase independent of metabolic factors.119 Hyperandrogenism is another factor associated with an increased risk of MAFLD.120 In a study by Jones et al.,121 patients with hyperandrogenism showed significantly higher hepatic fat content in magnetic resonance spectroscopy examinations than did patients without hyperandrogenism and controls, even after adjusting for BMI.
Additionally, the severity of PCOS, in addition to the disease itself, appeared to be a significant factor influencing the prevalence of NAFLD. In a group of biopsy-proven NAFLD patients, women with PCOS had more severe liver histology and higher prevalence of advanced fibrosis than women without PCOS. They also had more advanced disease at a younger age.122 A FibroScan study in PCOS patients with and without NAFLD revealed that liver stiffness, as an indicator of liver fibrosis, was significantly higher among PCOS patients with NAFLD than in those without NAFLD.123 Despite the lack of exclusive studies on the MAFLD population, the common metabolic comorbidities indicate that the prevalence of PCOS may be higher in the MAFLD population.
Osteoporosis and sarcopenia
NAFLD has been shown to be associated with non-obesity-related diseases, such as osteoporosis and sarcopenia.124,125 In Chinese cohorts, bone mass density was negatively correlated with the presence of NAFLD in both sexes.126,127 Sarcopenia is also associated with severe NAFLD histology.128 As such, an increase in body muscle mass results in the resolution of NAFLD.129 The underlying pathological mechanisms seem to be associated with vitamin D deficiency, altered growth hormone/insulin-like growth factor 1 axis, and chronic inflammation.130
Hypothyroidism
Even if there is not enough evidence on cost-effectiveness of screening hypothyroid patients in terms of NAFLD, both subclinical and overt hypothyroidism are known to be associated with increased NAFLD prevalence.131,132 The relationship between hypothyroidism is both indirect and direct. Indirectly, due to association of hypothyroidism with increased visceral obesity, impaired lipid metabolism and induction of metabolic syndrome. However, increased levels of thyroid-stimulating hormone also directly affect hepatocytes and contribute to development of NAFLD.133 In biopsy-proven NAFLD cohorts, hypothyroidism was significantly more prevalent.134,135 In a population-based study conducted with USA NHANES data from 2007 to 2012, low-normal thyroid function and subclinical hypothyroidism increased the advanced fibrosis risk 1.9-fold and 2.1-fold, respectively.136
OSA
OSA plays a significant role in the development and progression of NAFLD.137 The parameters determining OSA severity, such as the apnea-hypopnea index, oxyhemoglobin desaturation index, and nocturnal oxyhemoglobin saturation, are associated with hepatic steatosis.138,139 In addition to the high prevalence of NAFLD in OSA, OSA severity was found to be an independent predictor of the presence and severity of liver fibrosis.140
The relationship between OSA and NAFLD remains controversial, owing to the confounding effects of obesity. However, the combination of early time sleepiness and OSA remains to have a significant impact in the development of NAFLD even after adjusting for visceral fat area.141 Chronic intermittent hypoxia has been suggested to play a significant role in the development of liver inflammation in OSA patients.142 Accordingly, an effective continuous positive airway pressure was proposed to be associated with improvement and even complete reversion of hepatic steatosis.143
Psoriasis
NAFLD and psoriasis co-exist frequently, with approximately half of psoriasis patients also having NAFLD.144,145 In a meta-analysis by Candia et al.,146 patients with psoriasis had a 2-fold higher risk of developing NAFLD (OR: 2.15, 95% CI: 1.57–2.94). Further, the severity of psoriasis influenced the prevalence of NAFLD. The underlying mechanism explaining the association between NAFLD and psoriasis is not fully understood; however, increased expression of pro-inflammatory cytokines, such as interleukin-6, interleukin-17 and tumor necrosis factor-alpha, is known to influence the development of insulin resistance and, finally, NAFLD.147
Both psoriasis and NAFLD are associated with MS, insulin resistance, and an increased cardiovascular risk.148 Psoriasis patients with NAFLD have a significantly higher 10-year risk of cardiovascular events (OR: 6.0, 95% CI: 3.3–11.1).149 Despite the lack of studies in biopsy-proven NAFLD patients, data suggest an increased risk of advanced fibrosis in NAFLD patients with psoriasis.150,151 Accumulating evidence also suggests that patients with psoriasis have a higher risk of hepatic complications. Therefore, regular follow-up should be considered, especially for psoriasis patients undergoing treatment.147
Conclusion
MAFLD must be evaluated as a multisystemic disease affecting many extrahepatic organs. The disease burden extends beyond liver-related complications, underlining the importance of multidisciplinary screening and disease management. Routine screening for MAFLD is recommended in patients with obesity/overweight, T2DM, or MS. Moreover, patients with MAFLD should also be examined for CVD and cardiovascular risk. Further, treatment of dyslipidemia, T2DM, and hypertension is recommended to decrease the risk of cardiovascular and kidney diseases. Importantly, the high rate of co-existing CVD, CKD, OSA, hypothyroidism, osteoporosis, and PCOS indicates that MAFLD patients should be evaluated for these extrahepatic diseases.
Abbreviations
- AF
atrial fibrillation
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- FIB-4
fibrosis-4 index
- HCC
hepatocellular cancer
- HR
hazard ratio
- MAFLD
metabolic (dysfunction)-associated fatty liver disease
- MRI-PDFF
magnetic resonance imaging proton density fat fraction
- MS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- NFS
NAFLD Fibrosis Score
- NHANES
third National Health and Nutrition Examination Surveys
- OR
odds ratio
- OSA
obstructive sleep apnea
- PCOS
polycystic ovarian syndrome
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- QTc
corrected QT interval
- T2DM
type 2 diabetes mellitus
- TM6SF2
transmembrane 6 superfamily member 2
- VCTE
vibration controlled transient elastography
References
- 1.Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 4.Kaya E, Yilmaz Y. Non-alcoholic Fatty Liver Disease: A Global Public Health Issue. In: Faintuch J, Faintuch S, editors. Obesity and Diabetes. Cham: Springer; 2020. pp. 321–333. [Google Scholar]
- 5.Eslam M, Sanyal AJ, George J, International Consensus Panel MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol. 2021;15(4):345–352. doi: 10.1080/17474124.2021.1860019. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154170. doi: 10.1016/j.metabol.2020.154170. [DOI] [PubMed] [Google Scholar]
- 9.Liu SS, Ma XF, Zhao J, Du SX, Zhang J, Dong MZ, et al. Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids Health Dis. 2020;19(1):118. doi: 10.1186/s12944-020-01288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li AA, Ahmed A, Kim D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver. 2020;14(2):168–178. doi: 10.5009/gnl19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colognesi M, Gabbia D, De Martin S. Depression and Cognitive Impairment-Extrahepatic Manifestations of NAFLD and NASH. Biomedicines. 2020;8(7):229. doi: 10.3390/biomedicines8070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godinez-Leiva E, Bril F. Nonalcoholic Fatty Liver Disease (NAFLD) for Primary Care Providers: Beyond the Liver. Curr Hypertens Rev. 2020 doi: 10.2174/1573402116999201209203534. [DOI] [PubMed] [Google Scholar]
- 13.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 14.Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021;41(6):1290–1293. doi: 10.1111/liv.14828. [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: Which Has Closer Association With All-Cause and Cause-Specific Mortality?-Results From NHANES III. Front Med (Lausanne) 2021;8:693507. doi: 10.3389/fmed.2021.693507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40(12):3018–3030. doi: 10.1111/liv.14675. [DOI] [PubMed] [Google Scholar]
- 17.Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, et al. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin Gastroenterol Hepatol. 2021;19(10):2161–2171.e5. doi: 10.1016/j.cgh.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 18.Angelico F, Pastori D, Del Ben M. Impact of the New Metabolic-Associated Fatty Liver Disease (MAFLD) on NAFLD Patients Classification in Italy. Clin Gastroenterol Hepatol. 2021:S1542-3565(21)00149-X. doi: 10.1016/j.cgh.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med. 2018;378(12):1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68(2):268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Lee GH, Phyo WW, Loo WM, Kwok R, Ahmed T, et al. Validation of genetic variants associated with metabolic dysfunction-associated fatty liver disease in an ethnic Chinese population. World J Hepatol. 2020;12(12):1228–1238. doi: 10.4254/wjh.v12.i12.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu Z, Bi Y, Yuan F, Wang R, Li D, Wang J, et al. FTO Polymorphisms are Associated with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Susceptibility in the Older Chinese Han Population. Clin Interv Aging. 2020;15:1333–1341. doi: 10.2147/CIA.S254740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demirtas CO, Yilmaz Y. Metabolic-associated Fatty Liver Disease: Time to integrate ground-breaking new terminology to our clinical practice? Hepatology Forum. 2020;3(1):79–81. doi: 10.14744/hf.2020.2020.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016;17(6):510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz Y, Younossi ZM. Obesity-associated nonalcoholic fatty liver disease. Clin Liver Dis. 2014;18(1):19–31. doi: 10.1016/j.cld.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9•1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki A, Nitta H, Otsuka K, Umemura A, Baba S, Obuchi T, et al. Bariatric surgery and non-alcoholic Fatty liver disease: current and potential future treatments. Front Endocrinol (Lausanne) 2014;5:164. doi: 10.3389/fendo.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11(1):137–141. doi: 10.1016/j.soard.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Young S, Tariq R, Provenza J, Satapathy SK, Faisal K, Choudhry A, et al. Prevalence and Profile of Nonalcoholic Fatty Liver Disease in Lean Adults: Systematic Review and Meta-Analysis. Hepatol Commun. 2020;4(7):953–972. doi: 10.1002/hep4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R, Rastogi A, Sharma MK, Bhatia V, Gard H, Bihari C, et al. Clinicopathological characteristics and metabolic profiles of non-alcoholic fatty liver disease in Indian patients with normal body mass index: do they differ from obese or overweight non-alcoholic fatty liver disease? Indian J Endocrinol Metab. 2013;17(4):665–671. doi: 10.4103/2230-8210.113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Obesity: preventing and managing the global epidemic. Report on a WHO consultation on obesity, Geneva, 3-5 June, 1997. WHO/NUT/NCD/98.1. Technical Report Series Number 894. Geneva, Switzerland: World Health Organization; 2000. [PubMed]
- 33.Eslam M, Chen F, George J. NAFLD in Lean Asians. Clin Liver Dis (Hoboken) 2021;16(6):240–243. doi: 10.1002/cld.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akyuz U, Yesil A, Yilmaz Y. Characterization of lean patients with nonalcoholic fatty liver disease: potential role of high hemoglobin levels. Scand J Gastroenterol. 2015;50(3):341–346. doi: 10.3109/00365521.2014.983160. [DOI] [PubMed] [Google Scholar]
- 35.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14(6):889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 36.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372–382. doi: 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 38.Bernhardt P, Kratzer W, Schmidberger J, Graeter T, Cruener B, EMIL Study Group Laboratory parameters in lean NAFLD: comparison of subjects with lean NAFLD with obese subjects without hepatic steatosis. BMC Res Notes. 2018;11:101. doi: 10.1186/s13104-018-3212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamurcu Varol P, Kaya E, Alphan E, Yilmaz Y. Role of intensive dietary and lifestyle interventions in the treatment of lean nonalcoholic fatty liver disease patients. Eur J Gastroenterol Hepatol. 2020;32(10):1352–1357. doi: 10.1097/MEG.0000000000001656. [DOI] [PubMed] [Google Scholar]
- 40.Brunner KT, Henneberg CJ, Wilechansky RM, Long MT. Nonalcoholic fatty liver disease and obesity treatment. Curr Obes Rep. 2019;8:220–228. doi: 10.1007/s13679-019-00345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14:99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 43.Younossi ZM, Corey KE, Alkhouri N, Noureddin M, Jacobson I, Lam B, et al. Clinical assessment for high-risk patients with non-alcoholic fatty liver disease in primary care and diabetology practices. Aliment Pharmacol Ther. 2020;52(3):513–526. doi: 10.1111/apt.15830. [DOI] [PubMed] [Google Scholar]
- 44.Yilmaz Y, Kaya E, Eren F. Letter: the use of Fibrosis-4 score in primary care and diabetology practices-Occam’s razor applied to advanced fibrosis screening. Aliment Pharmacol Ther. 2020;52(11-12):1759–1760. doi: 10.1111/apt.16034. [DOI] [PubMed] [Google Scholar]
- 45.Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40(5):1367–1393. doi: 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- 46.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 47.Demirtas CO, Sapmaz A, Gurel BA, Kizmaz H, Ulu T, Gorcin Karaketir S, et al. A disquiet find: The clinical and histological characteristics of patients with biopsy-proven non-alcoholic fatty liver disease in the absence of insulin resistance. Hepatology Forum. 2020;1(3):101–108. doi: 10.14744/hf.2020.2020.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujii H, Imajo K, Yoneda M, Nakahara T, Hyogo H, Takahashi H, et al. Japan Study Group of Nonalcoholic Fatty Liver Disease. HOMA-IR: An independent predictor of advanced liver fibrosis in nondiabetic non-alcoholicfatty liver disease. J Gastroenterol Hepatol. 2019;34(8):1390–1395. doi: 10.1111/jgh.14595. [DOI] [PubMed] [Google Scholar]
- 49.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 50.Kim D, Touros A, Kim WR. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin Liver Dis. 2018;22(1):133–140. doi: 10.1016/j.cld.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 52.Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390–397. doi: 10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor fordisease-specific mortality in NAFLD after up to 33 years of follow-up.Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 54.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fattyliver disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258–267. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 57.VanWagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 58.Piazza NA, Singal AK. Frequency of cardiovascular events and effect on survival in liver transplant recipients for cirrhosis due to alcoholic or nonalcoholic steatohepatitis. Exp Clin Transplant. 2016;14:79–85. doi: 10.6002/ect.2015.0089. [DOI] [PubMed] [Google Scholar]
- 59.Sinn DH, Kang D, Chang Y, Ryu S, Cho SJ, Paik SW, et al. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: A cohort study. J Gastroenterol Hepatol. 2020;35:833–839. doi: 10.1111/jgh.14856. [DOI] [PubMed] [Google Scholar]
- 60.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51(7):728–736. doi: 10.1111/apt.15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19(10):2138–2147.E10. doi: 10.1016/j.cgh.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Guerreiro GTS, Longo L, Fonseca MA, de Souza VEG, Álvares-da-Silva MR. Does the risk of cardiovascular events differ between biopsy-proven NAFLD and MAFLD? Hepatol Int. 2021;15(2):380–391. doi: 10.1007/s12072-021-10157-y. [DOI] [PubMed] [Google Scholar]
- 64.Dongiovanni P, Paolini E, Corsini A, Sirtori CR, Ruscica M. Nonalcoholic fatty liver disease or metabolic dysfunction-associated fatty liver disease diagnoses and cardiovascular diseases: From epidemiology to drug approaches. Eur J Clin Invest. 2021;51(7):e13519. doi: 10.1111/eci.13519. [DOI] [PubMed] [Google Scholar]
- 65.Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2018;15:425–439. doi: 10.1038/s41575-018-0010-0. [DOI] [PubMed] [Google Scholar]
- 66.Mantovani A, Dauriz M, Sandri D, Bonapace S, Zoppini G, Tilg H, et al. Association between non-alcoholic fatty liver disease and risk of atrial fibrillation in adult individuals: an updated meta-analysis. Liver Int. 2019;39:758–769. doi: 10.1111/liv.14044. [DOI] [PubMed] [Google Scholar]
- 67.Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. 2020;40:1594–1600. doi: 10.1111/liv.14461. [DOI] [PubMed] [Google Scholar]
- 68.Hung CS, Tseng PH, Tu CH, Chen CC, Liao WC, Lee YC, et al. Nonalcoholic fatty liver disease is associated with qt prolongation in the general population. J Am Heart Assoc. 2015;4:e001820. doi: 10.1161/JAHA.115.001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantovani A, Rigamonti A, Bonapace S, Bolzan B, Pernigo M, Morani G, et al. Nonalcoholic fatty liver disease is associated with ventricular arrhythmias in patients with type 2 diabetes referred for clinically indicated 24-hour holter monitoring. Diabetes Care. 2016;39:1416–1423. doi: 10.2337/dc16-0091. [DOI] [PubMed] [Google Scholar]
- 70.Mantovani A, Pernigo M, Bergamini C, Bonapace S, Lipari P, Valbusa F, et al. Heart valve calcification in patients with type 2 diabetes and nonalcoholic fatty liver disease. Metabolism. 2015;64(8):879–887. doi: 10.1016/j.metabol.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Lee YH, Kim KJ, Yoo ME, Kim G, Yoon HJ, Jo K, et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J Hepatol. 2018;68(4):764–772. doi: 10.1016/j.jhep.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 72.Sato Y, Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, et al. Liver stiffness assessed by Fibrosis-4 index predicts mortality in patients with heart failure. Open Heart. 2017;4(1):e000598. doi: 10.1136/openhrt-2017-000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petta S, Argano C, Colomba D, Camma C, Di Marco V, Cabibi D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62:928–933. doi: 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 74.Sunbul M, Kivrak T, Durmus E, Akin H, Aydin Y, Ergelen R, et al. Nonalcoholic steatohepatitis score is an independent predictor of right ventricular dysfunction in patients with nonalcoholic fatty liver disease. Cardiovasc Ther. 2015;33:294–299. doi: 10.1111/1755-5922.12145. [DOI] [PubMed] [Google Scholar]
- 75.Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110(7):921–937. doi: 10.1007/s00392-020-01709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lechner K, McKenzie AL, Kränkel N, Von Schacky C, Worm N, Nixdorff U, et al. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab Syndr Relat Disord. 2020;18(4):176–185. doi: 10.1089/met.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruzdeva O, Uchasova E, Dyleva Y, Borodkina D, Akbasheva O, Antonova L, et al. Adipocytes Directly Affect Coronary Artery Disease Pathogenesis via Induction of Adipokine and Cytokine Imbalances. Front Immunol. 2019;10:2163. doi: 10.3389/fimmu.2019.02163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat Genet. 2017;49(12):1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72:785–801. doi: 10.1016/j.jhep.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13(5):297–310. doi: 10.1038/nrneph.2017.16. [DOI] [PubMed] [Google Scholar]
- 81.Jang HR, Kang D, Sinn DH, Gu S, Cho SJ, Lee JE, et al. Nonalcoholic fatty liver disease accelerates kidney function decline in patients with chronic kidney disease: a cohort study. Sci Rep. 2018;8(1):4718. doi: 10.1038/s41598-018-23014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park H, Dawwas GK, Liu X, Nguyen MH. Nonalcoholic fatty liver disease increases risk of incident advanced chronic kidney disease: A propensity-matched cohort study. J Intern Med. 2019;286:711–722. doi: 10.1111/joim.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaps L, Labenz C, Galle PR, Weinmann-Menke J, Kostev K, Schattenberg JM. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United European Gastroenterol J. 2020;8(8):942–948. doi: 10.1177/2050640620944098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Akahane T, Akahane M, Namisaki T, Kaji K, Moriya K, Kawaratani H, et al. Association between Non-Alcoholic Fatty Liver Disease and Chronic Kidney Disease: A Cross-Sectional Study. J Clin Med. 2020;9(6):1635. doi: 10.3390/jcm9061635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Targher G, Bertolini L, Rodella S, Zoppini G, Lippi G, Day C, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444–450. doi: 10.1007/s00125-007-0897-4. [DOI] [PubMed] [Google Scholar]
- 87.Targher G, Bertolini L, Chonchol M, Rodella S, Zoppini G, Lippi G, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and retinopathy in type 1 diabetic patients. Diabetologia. 2010;53(7):1341–1348. doi: 10.1007/s00125-010-1720-1. [DOI] [PubMed] [Google Scholar]
- 88.Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol. 2010;5(12):2166–2171. doi: 10.2215/CJN.05050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2018;79:64–76. doi: 10.1016/j.metabol.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 90.Hsieh MH, Wu KT, Chen YY, Yang JF, Lin WY, Chang NC, et al. Higher NAFLD fibrosis score is associated with impaired eGFR. J Formos Med Assoc 2020;119(1 Pt. 3):496–503. doi: 10.1016/j.jfma.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Choudhary NS, Saraf N, Kumar N, Rai R, Saigal S, Gautam D, et al. Nonalcoholic fatty liver is not associated with incident chronic kidney disease: a large histology-based comparison with healthy individuals. Eur J Gastroenterol Hepatol. 2016;28(4):441–443. doi: 10.1097/MEG.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 92.Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med. 2014;11(7):e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun DQ, Ye FZ, Kani HT, Yang JR, Zheng KI, Zhang HY, et al. Higher liver stiffness scores are associated with early kidney dysfunction in patients with histologically proven non-cirrhotic NAFLD. Diabetes Metab. 2020;46(4):288–295. doi: 10.1016/j.diabet.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism. 2010;59(9):1327–1330. doi: 10.1016/j.metabol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 95.Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi: 10.1016/j.metabol.2020.154433. [DOI] [PubMed] [Google Scholar]
- 96.Sanna C, Rosso C, Marietti M, Bugianesi E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int J Mol Sci. 2016;17(5):717. doi: 10.3390/ijms17050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 98.Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2018;68(1):140–146. doi: 10.1016/j.jhep.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 99.Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J Hepatol. 2019;71:1229–1236. doi: 10.1016/j.jhep.2019.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwak MS, Yim JY, Yi A, Chung GE, Yang JI, Kim D, et al. Nonalcoholic fatty liver disease is associated with breast cancer in nonobese women. Dig Liver Dis. 2019;51(7):1030–1035. doi: 10.1016/j.dld.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 101.Liu SS, Ma XF, Zhao J, Du SX, Zhang J, Dong MZ, et al. Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids Health Dis. 2020;19(1):118. doi: 10.1186/s12944-020-01288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wongjarupong N, Assavapongpaiboon B, Susantitaphong P, Cheungpasitporn W, Treeprasertsuk S, Rerknimitr R, et al. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17:149. doi: 10.1186/s12876-017-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corrao S, Natoli G, Argano C. Nonalcoholic fatty liver disease is associated with intrahepatic cholangiocarcinoma and not with extrahepatic form: definitive evidence from meta-analysis and trial sequential analysis. Eur J Gastroenterol Hepatol. 2021;33(1):62–68. doi: 10.1097/MEG.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 104.Mantovani A, Dauriz M, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Association between nonalcoholic fatty liver disease and colorectal tumours in asymptomatic adults undergoing screening colonoscopy: a systematic review and meta-analysis. Metab Clin Exp. 2018;87:1–12. doi: 10.1016/j.metabol.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Jin C, Dongxue B, Shufei Z, Yang ZX, Tian GY, Luo Y, et al. The association between nonalcoholic fatty liver disease and risk of colorectal adenoma and cancer incident and recurrence: a meta-analysis of observational studies. Expert Rev Gastroenterol Hepatol. 2019;13:385–395. doi: 10.1080/17474124.2019.1580143. [DOI] [PubMed] [Google Scholar]
- 106.Lin X, You F, Liu H, Fang Y, Jin S, Wang Q. Site-specific risk of colorectal neoplasms in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One. 2021;16(1):e0245921. doi: 10.1371/journal.pone.0245921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fukunaga S, Nakano D, Kawaguchi T, Eslam M, Ouchi A, Nagata T, et al. Non-Obese MAFLD Is Associated with Colorectal Adenoma in Health Check Examinees: A Multicenter Retrospective Study. Int J Mol Sci. 2021;22(11):5462. doi: 10.3390/ijms22115462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colognesi M, Gabbia D, De Martin S. Depression and Cognitive Impairment-Extrahepatic Manifestations of NAFLD and NASH. Biomedicines. 2020;8(7):229. doi: 10.3390/biomedicines8070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomeno W, Kawashima K, Yoneda M, Saito S, Ogawa Y, Honda Y, et al. Non-alcoholic fatty liver disease comorbid with major depressive disorder: The pathological features and poor therapeutic efficacy. J Gastroenterol Hepatol. 2015;30(6):1009–1014. doi: 10.1111/jgh.12897. [DOI] [PubMed] [Google Scholar]
- 110.Youssef NA, Abdelmalek MF, Binks M, Guy CD, Omenetti A, Smith AD, et al. Associations of depression, anxiety and antidepressants with histological severity of nonalcoholic fatty liver disease. Liver Int. 2013;33(7):1062–1070. doi: 10.1111/liv.12165. [DOI] [PubMed] [Google Scholar]
- 111.Lee K, Otgonsuren M, Younoszai Z, Mir HM, Younossi ZM. Association of Chronic Liver Disease with Depression: A Population-Based Study. Psychosomatics. 2013;54:52–59. doi: 10.1016/j.psym.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 112.Filipović B, Marković O, Đurić V, Filipović B. Cognitive Changes and Brain Volume Reduction in Patients with Nonalcoholic Fatty Liver Disease. Can. J Gastroenterol Hepatol. 2018;2018:9638797. doi: 10.1155/2018/9638797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Celikbilek A, Celikbilek M, Bozkurt G. Cognitive assessment of patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2018;30(8):944–950. doi: 10.1097/MEG.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 114.Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int. 2019;39:1713–1721. doi: 10.1111/liv.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim DG, Krenz A, Toussaint LE, Maurer KJ, Robinson SA, Yan A, et al. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation. 2016;13:1. doi: 10.1186/s12974-015-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pinçon A, De Montgolfier O, Akkoyunlu N, Daneault C, Pouliot P, Villeneuve L, et al. Non-Alcoholic Fatty Liver Disease, and the Underlying Altered Fatty Acid Metabolism, Reveals Brain Hypoperfusion and Contributes to the Cognitive Decline in APP/PS1 Mice. Metabolites. 2019;9:104. doi: 10.3390/metabo9050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol. 2018;132(2):321–336. doi: 10.1097/AOG.0000000000002698. [DOI] [PubMed] [Google Scholar]
- 118.Kelley CE, Brown AJ, Diehl AM, Setji TL. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J Gastroenterol. 2014;20:14172–14184. doi: 10.3748/wjg.v20.i39.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asfari MM, Sarmini MT, Baidoun F, Al-Khadra Y, Ezzaizi Y, Dasarathy S, et al. Association of non-alcoholic fatty liver disease and polycystic ovarian syndrome. BMJ Open Gastroenterol. 2020;7(1):e000352. doi: 10.1136/bmjgast-2019-000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu J, Yao XY, Shi RX, Liu SF, Wang XY. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reprod Health. 2018;15(1):77. doi: 10.1186/s12978-018-0519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97(10):3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 122.Sarkar M, Terrault N, Chan W, Cedars MI, Huddleston HG, Duwaerts CC, et al. Polycystic ovary syndrome (PCOS) is associated with NASH severity and advanced fibrosis. Liver Int. 2020;40(2):355–359. doi: 10.1111/liv.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shengir M, Krishnamurthy S, Ghali P, Deschenes M, Wong P, Chen T, et al. Prevalence and predictors of nonalcoholic fatty liver disease in South Asian women with polycystic ovary syndrome. World J Gastroenterol. 2020;26(44):7046–7060. doi: 10.3748/wjg.v26.i44.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Poggiogalle E, Donini LM, Lenzi A, Chiesa C, Pacifico L. Nonalcoholic fatty liver disease connections with fat-free tissues: A focus on bone and skeletal muscle. World J Gastroenterol. 2017;23(10):1747–1757. doi: 10.3748/wjg.v23.i10.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yilmaz Y. Non-alcoholic fatty liver disease and osteoporosis—clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36(4):345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 126.Xia MF, Lin HD, Yan HM, Bian H, Chang XX, Zhang LS, et al. The association of liver fat content and serum alanine aminotransferase with bone mineral density in middle-aged and elderly Chinese men and postmenopausal women. J Transl Med. 2016;14:11. doi: 10.1186/s12967-016-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cui R, Sheng H, Rui XF, Cheng XY, Sheng CJ, Wang JY, et al. Low bone mineral density in chinese adults with nonalcoholic Fatty liver disease. Int J Endocrinol. 2013;2013:396545. doi: 10.1155/2013/396545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 129.Kim G, Lee SE, Lee YB, Jun JE, Ahn J, Bae JC, et al. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A 7-year longitudinal study. Hepatology. 2018;68:1755–1768. doi: 10.1002/hep.30049. [DOI] [PubMed] [Google Scholar]
- 130.Kumar R, Priyadarshi RN, Anand U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J Clin Transl Hepatol. 2020;8(1):76–86. doi: 10.14218/JCTH.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung GE, Kim D, Kim W, Yim JY, Park MJ, Kim YJ, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150–156. doi: 10.1016/j.jhep.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 132.Chung GE, Kim D, Kwak MS, Yim JY, Ahmed A, Kim JS. Longitudinal change in thyroid-stimulating hormone and risk of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;19:848–849.e1. doi: 10.1016/j.cgh.2020.02.039. [DOI] [PubMed] [Google Scholar]
- 133.Lonardo A, Ballestri S, Mantovani A, Nascimbeni F, Lugari S, Targher G. Pathogenesis of hypothyroidism-induced NAFLD: Evidence for a distinct disease entity? Dig Liver Dis. 2019;51(4):462–470. doi: 10.1016/j.dld.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 134.Pagadala MR, Zein CO, Dasarathy S, Yerian LM, Lopez R, McCullough AJ. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:528–534. doi: 10.1007/s10620-011-2006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim D, Kim W, Joo SK, Bae JM, Kim JH, Ahmed A. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123–131. doi: 10.1016/j.cgh.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 136.Kim D, Yoo ER, Li AA, Fernandes CT, Tighe SP, Cholankeril G, et al. Low-normal thyroid function is associated with advanced fibrosis among adults in the United States. Clin Gastroenterol Hepatol. 2019;17:2379–2381. doi: 10.1016/j.cgh.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mesarwi OA, Loomba R, Malhotra A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am J Respir Crit Care Med. 2019;199(7):830–841. doi: 10.1164/rccm.201806-1109TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trzepizur W, Boursier J, Mansour Y, Le Vaillant M, Chollet S, Pigeanne T, et al. Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group. Association between severity of obstructive sleep apnea and blood markers of liver injury. Clin Gastroenterol Hepatol. 2016;14:1657–1661. doi: 10.1016/j.cgh.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 139.Cakmak E, Duksal F, Altinkaya E, Acibucu F, Dogan OT, Yonem O, et al. Association Between the Severity of Nocturnal Hypoxia in Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Damage. Hepat Mon. 2015;15(11):e32655. doi: 10.5812/hepatmon.32655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krolow GK, Garcia E, Schoor F, Araujo FBS, Coral GP. Obstructive sleep apnea and severity of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2021;33(8):1104–1109. doi: 10.1097/MEG.0000000000001920. [DOI] [PubMed] [Google Scholar]
- 141.Yu JH, Ahn JH, Yoo HJ, Seo JA, Kim SG, Choi KM, et al. Obstructive sleep apnea with excessive daytime sleepiness is associated with non-alcoholic fatty liver disease regardless of visceral fat. Korean J Intern Med. 2015;30:846–855. doi: 10.3904/kjim.2015.30.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paschetta E, Belci P, Alisi A, Liccardo D, Cutrera R, Musso G, et al. OSAS-related inflammatory mechanisms of liver injury in nonalcoholic fatty liver disease. Mediators Inflamm. 2015;2015:815721. doi: 10.1155/2015/815721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Buttacavoli M, Gruttad’Auria CI, Olivo M, Virdone R, Castrogiovanni A, Mazzuca E, et al. Liver steatosis and fibrosis in OSA patients after long-term CPAP treatment: A preliminary ultrasound study. Ultrasound Med Biol. 2016;42:104–109. doi: 10.1016/j.ultrasmedbio.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 144.Miele L, Vallone S, Cefalo C, La Torre G, Di Stasi C, Vecchio FM, et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. 2009;51:778–786. doi: 10.1016/j.jhep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 145.Roberts KK, Cochet AE, Lamb PB, Brown PJ, Battafarano DF, Brunt EM, et al. The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology clinic. Aliment Pharmacol Ther. 2015;41:293–300. doi: 10.1111/apt.13042. [DOI] [PubMed] [Google Scholar]
- 146.Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656–662. doi: 10.1111/jdv.12847. [DOI] [PubMed] [Google Scholar]
- 147.Mantovani A, Gisondi P, Lonardo A, Targher G. Relationship between non-alcoholic fatty liver disease and psoriasis: a novel hepato-dermal axis? Int J Mol Sci. 2016;17:217. doi: 10.3390/ijms17020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE. Nonalcoholic fatty liver disease: Evolving paradigms. World J Gastroenterol. 2017;23:6571–6592. doi: 10.3748/wjg.v23.i36.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Romero-Pérez D, Belinchón-Romero I, Bellot P, Francés R, Marco F, Ramos-Rincón JM. Nonalcoholic fatty liver disease puts patients with psoriasis at greater cardiovascular risk. Australas J Dermatol. 2019;60(4):e304–e310. doi: 10.1111/ajd.13098. [DOI] [PubMed] [Google Scholar]
- 150.Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol Venereol. 2015;30:282–287. doi: 10.1111/jdv.13456. [DOI] [PubMed] [Google Scholar]
- 151.van der Voort EA, Koehler EM, Nijsten T, Stricker BH, Hofman A, Janssen HL, et al. Increased Prevalence of Advanced Liver Fibrosis in Patients with Psoriasis: A Cross-sectional Analysis from the Rotterdam Study. Acta Derm Venereol. 2016;96(2):213–217. doi: 10.2340/00015555-2161. [DOI] [PubMed] [Google Scholar]