Abstract
Background and Aims
We compared lung function parameters in nonalcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated fatty liver disease (MAFLD), and examined the association between lung function parameters and fibrosis severity in MAFLD.
Methods
In this cross-sectional study, we randomly recruited 2,543 middle-aged individuals from 25 communities across four cities in China during 2016 and 2020. All participants received a health check-up including measurement of anthropometric parameters, biochemical variables, liver ultrasonography, and spirometry. The severity of liver disease was assessed by the fibrosis (FIB)-4 score.
Results
The prevalence of MAFLD was 20.4% (n=519) and that of NAFLD was 18.4% (n=469). After adjusting for age, sex, adiposity measures, smoking status, and significant alcohol intake, subjects with MAFLD had a significantly lower predicted forced vital capacity (FVC, 88.27±17.60% vs. 90.82±16.85%, p<0.05) and lower 1 s forced expiratory volume (FEV1, 79.89±17.34 vs. 83.02±16.66%, p<0.05) than those with NAFLD. MAFLD with an increased FIB-4 score was significantly associated with decreased lung function. For each 1-point increase in FIB-4, FVC was diminished by 0.507 (95% CI: −0.840, −0.173, p=0.003), and FEV1 was diminished by 0.439 (95% CI: −0.739, −0.140, p=0.004). The results remained unchanged when the statistical analyses was performed separately for men and women.
Conclusions
MAFLD was significantly associated with a greater impairment of lung function parameters than NAFLD.
Keywords: MAFLD, NAFLD, Lung function, Liver fibrosis score
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease, affecting ∼30% of adults worldwide.1 Convincing evidence indicates that NAFLD is a “multisystem” disease that affects multiple extrahepatic organ systems, including the respiratory system, cardiovascular system, endocrine system, and others.2–6 Jung et al.7 first reported that NAFLD was associated with decreased lung function. Although decreased lung function is associated with older age, obesity, smoking, and air pollution,8 recent observational studies have reported an association between NAFLD and lung function.7,9–17 Lonardo et al.18 also recently proposed that it would be the time to “cross the diaphragm between NAFLD and chronic obstructive pulmonary disease (COPD)”. A meta-analysis of six observational studies including 133,707 participants of predominantly Asian ethnicity reported a significant association between NAFLD and impaired lung function.19 Moreover, it has been reported that individuals with NAFLD had significant reductions in both FVC and FEV120,21 that worsened with the severity of NAFLD, especially with higher fibrosis stage.9,16,22 A Korean cohort study also showed that the risk for incident NAFLD increased with decreasing quartiles of both FEV1 (%) and FVC (%), regardless of smoking history, over a mean follow-up of ∼6 years.15
The current definition of NAFLD requires the exclusion of significant alcohol consumption and other secondary causes of chronic liver disease. More recently, an international panel of experts has proposed to change the terminology from NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD), and also proposed a new set of diagnostic criteria to better define this common liver disease.23–25 Therefore, MAFLD has been proposed as a more suitable definition to describe the fatty liver disease related to underlying metabolic dysfunction,26–28 and a more accurate definition for identifying those patients, who are at increased risk of developing extrahepatic complications, such as cardiovascular disease and chronic kidney disease.29,30
However, to the best of our knowledge, whether the renaming of NAFLD to MAFLD better identifies patients who are also at increased risk of impaired lung function is currently unknown. Therefore, the primary aim of this large cross-sectional study was to compare lung function parameters in NAFLD and MAFLD populations, and to examine the associations between lung function parameters and fibrosis severity in MAFLD.
Methods
Study design and data collection
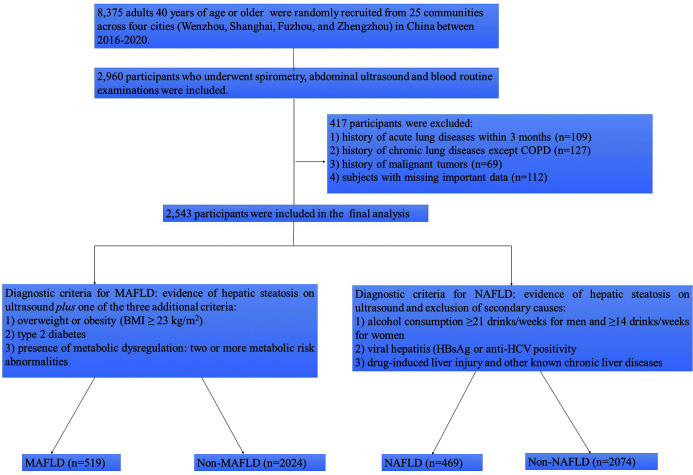
Between 2016 and 2020, we ran a national noncommunicable and chronic-disease management program that we developed to identify risk factors and pathogenesis of COPD based on clinical bioinformatics technology and epidemiology.31 The program randomly recruited a total of 8,375 individuals 40 years of age or older from 25 communities across four cities (Wenzhou, Shanghai, Fuzhou, and Zhengzhou) in Central and Southeast China. In the program, there were 2,960 individuals, who had undergone both spirometry and liver ultrasound examinations. As shown in Figure 1, we excluded those (1) who had acute or chronic lung diseases, except COPD, (2) with lung cancers or other extrapulmonary malignancies, and (3) who were missing important clinical and laboratory data from the study. The remaining 2,543 adults were included in the final analysis. The study was approved by the Institutional Review Board at each hospital involved in the study (no. 2016134). All participants signed an informed consent to participate in the study.
Fig. 1. Flowchart of subject recruitment.
At each visit, standardized self-administered questionnaires were administered to collect detailed information on demographic characteristics, alcohol consumption, smoking habits, physical activity, medical history, and any clinical symptoms associated with lung diseases. Serum lipids, liver enzymes, glucose, and other biochemical blood measurements were determined in all participants after an overnight fast by standard laboratory procedures. Body mass index (BMI, kg/m2) was calculated. Waist circumference was measured while standing, with the measurement taken horizontal to the floor at the midpoint between the lowest rib and the iliac crest. Hypertension was defined as a systolic blood pressure (SBP) ≥130 mmHg and diastolic blood pressure (DBP) ≥85 mmHg and/or use of antihypertensive drugs. Type 2 ddiabetes mellitus (T2DM) was defined by a fasting glucose ≥7.0 mmol/L (≥126 mg/dL) or glycated hemoglobin (HbA1c ≥6.5% or ≥48 mmol/mol) and/or use of any glucose-lowering agents.
Criteria for diagnosing NAFLD and MAFLD
Experienced radiologists performed liver ultrasonography in all participants. The radiologists were blinded to the clinical and laboratory details of participants and captured liver images in a standard manner. Diagnosis of hepatic steatosis was mainly based on the increased echogenicity of the liver relative to the echogenicity of the renal cortex or spleen parenchyma.32 The diagnosis of NAFLD was based on the evidence of hepatic steatosis and exclusion of significant alcohol consumption defined as ≥21 drinks/week for men and 14 drinks/week for women and other competing causes for hepatic steatosis, as shown in Figure 1.33 The diagnosis of MAFLD was based on the evidence of hepatic steatosis on ultrasonography and presence of at least one of the following three metabolic risk factors: (1) overweight or obesity (i.e., a BMI ≥23 kg/m2 for Asian people; (2) established T2DM according to the diagnostic criteria described above; or (3) metabolic dysregulation. Metabolic. Dysregulation included at least two of the following seven metabolic risk abnormalities: (1) a waist circumference ≥90 cm for men, and ≥80 cm for women; (2) blood pressure ≥130/85 mmHg or drug treatment; (3) triglycerides ≥1.70 mmol/L (≥150 mg/dL) or drug treatment; (4) high-density lipoprotein (HDL) cholesterol <1.0 mmol/L for men and <1.3 mmol/L for women; (5) prediabetes status defined as a fasting glucose between 5.6 and 6.9 mmol/L, or HbA1c between 5.7 and 6.4%; (6) a homeostasis model assessment-estimated insulin resistance (HOMA-IR) score ≥2.5; and (7) a plasma C-reactive protein >2 mg/L.24
Lean participants with NAFLD who did not have T2DM or metabolic dysregulation (as above) were defined as having nonmetabolic dysfunction-associated (non-MD)-NAFLD.30 The fibrosis 4 (FIB-4) score = age × AST (IU/L) / [platelet count (×109/L) × ALT (IU/L)0.5] was used to noninvasively assess the presence of advanced liver fibrosis.34,35
Spirometry
Trained medical personnel who were blinded to the clinical and laboratory details of participants, performed the spirometry following American Thoracic Society criteria.36 Forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1) were recorded and the predicted FEV1 and FVC were calculated as previously described.37
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD); categorical variables were expressed as numbers or percentages (%). Differences of continuous variables were compared by one-way analysis of variance and differences of categorical variables were compared by chi-squared tests. Separate analyses were performed by sex. Multivariable linear regression analysis was performed to examine the independence of associations between MAFLD and lung function tests after adjusting for age, sex, adiposity measures, smoking status, presence of significant alcohol intake (≥21 drinks/week for men and 14 drinks/week for women), and other potential confounding variables. The results were reported as beta coefficients and 95% confidence intervals (CIs). All statistical tests were two-sided and a p-values <0.05 (two-tailed) was considered to be statistically significant. SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
Results
Participant characteristics
In total, 2,543 middle-aged Chinese adults were included and their clinical and biochemical characteristics and lung function parameters are shown in Table 1. There were 519 individuals with MAFLD (20.4%) and 469 (18.4%) with NAFLD. Compared with the non-MAFLD group, those with MAFLD were younger and had higher levels of serum liver enzymes, white blood cell (WBC) counts, and total hemoglobin as well as a significant impairment of lung function, mainly FVC, predicted FVC (%), and predicted-FEV1 (%). Individuals with MAFLD also had a greater prevalence of metabolic syndrome, T2DM, dyslipidemia, and hypertension. The two groups of individuals did not significantly differ by sex, smoking status, presence of prior COPD, and FEV1/FVC ratio. Individuals with NAFLD also had a lower FVC, predicted FVC (%), predicted-FEV1 (%) and a greater prevalence of metabolic syndrome, T2DM, and dyslipidemia compared with the non-MAFLD group. Differences of FEV1, and FEV1/FVC in the two groups were not significant.
Table 1. Clinical and biochemical characteristics and lung function in participants with and without MAFLD and NAFLD.
| Characteristic | Non-MAFLD | MAFLD | p* | Non-NAFLD | NAFLD | p** |
|---|---|---|---|---|---|---|
| 2,024 (79.6) | 519 (20.4) | 2,074 (81.6) | 469 (18.4) | |||
| Age, years | 65.87±7.68 | 64.89±8.00 | 0.038 | 65.97±7.73 | 64.39±7.72 | 0.006 |
| Male sex | 1,245 (61.5) | 299 (57.6) | 0.107 | 1,275 (61.5) | 269 (57.4) | 0.099 |
| Ever-smoker | 943 (46.6) | 231 (44.6) | 0.220 | 958 (46.8) | 205 (43.8) | 0.322 |
| Alcohol use | 309 (15.3) | 136 (26.2) | <0.001 | 334 (16.1) | 111 (23.6) | <0.001 |
| BMI, kg/m2 | 23.59±2.63 | 27.81±2.60 | <0.001 | 23.63±2.75 | 27.66±2.63 | <0.001 |
| Waist circumference, cm | 84.16±8.70 | 94.2±9.98 | <0.001 | 84.63±8.75 | 93.30±11.36 | <0.001 |
| Systolic blood pressure, mmHg | 136.84±20.24 | 141.01±18.05 | <0.001 | 137.27±20.31 | 139.52±17.62 | 0.015 |
| Diastolic blood pressure, mmHg | 80.77±11.86 | 83.06±11.15 | <0.001 | 80.91±11.89 | 82.69±10.95 | 0.013 |
| Lung function tests | ||||||
| FVC, L | 2.80±0.88 | 2.60±0.82 | <0.001 | 2.78±0.88 | 2.69±0.80 | 0.038 |
| FVC, % predicted value | 95.18±19.43 | 88.19±17.66 | <0.001 | 94.36±19.70 | 91.07±16.97 | 0.001 |
| FEV1, L | 2.24±0.66 | 2.07±0.67 | <0.001 | 2.21±0.67 | 2.16±0.64 | 0.172 |
| FEV1, % predicted value | 86.53±18.29 | 79.95±17.87 | <0.001 | 85.57±18.73 | 83.28±16.80 | 0.015 |
| FEV1 /FVC | 81.03±8.43 | 80.65±11.03 | 0.225 | 80.74±9.08 | 80.23±8.78 | 0.417 |
| Comorbidities | ||||||
| COPD | 225 (11.1) | 68 (13.1) | 0.218 | 231 (11.3) | 58 (12.4) | 0.470 |
| Type 2 diabetes | 169 (8.6) | 132 (26.3) | <0.001 | 196 (9.5) | 126 (26.9) | <0.001 |
| Metabolic syndrome | 204 (10.1) | 206 (39.7) | <0.001 | 237 (11.4) | 173 (36.9) | <0.001 |
| Hypertension | 680 (33.6) | 199 (38.6) | 0.044 | 712 (34.3) | 167 (35.6) | 0.599 |
| Dyslipidemia | 641 (31.7) | 250 (48.2) | <0.001 | 665 (32.1) | 226 (48.2) | <0.001 |
| Laboratory tests | ||||||
| WBC count, 109/L | 6.05±1.71 | 6.52±1.66 | <0.001 | 6.04±1.71 | 6.44±1.62 | <0.001 |
| Hemoglobin, g/L | 141.99±14.31 | 144.08±14.70 | 0.003 | 141.24±14.31 | 144.05±14.63 | 0.002 |
| Platelet count, 109/L | 220.06±56.78 | 220.39±55.96 | 0.901 | 218.70±55.43 | 224.78±56.74 | 0.262 |
| FPG, mmol/L | 5.73±1.55 | 6.53±2.00 | <0.001 | 5.70±1.38 | 6.56±2.14 | <0.001 |
| ALT, U/L | 20.29±10.80 | 32.43±15.32 | <0.001 | 19.86±10.25 | 34.55±24.28 | <0.001 |
| AST, U/L | 24.61±12.02 | 26.78±15.66 | 0.001 | 24.58±12.09 | 26.69±15.14 | <0.001 |
| TC, mmol/L | 4.96±1.31 | 4.79±1.39 | 0.013 | 4.95±1.31 | 4.81±1.39 | 0.066 |
| TG, mmol/L | 1.66±1.18 | 2.22±1.50 | <0.001 | 1.67±1.17 | 2.26±1.57 | <0.001 |
| LDL, mmol/L | 3.10±0.95 | 2.91±0.94 | <0.001 | 3.08±0.95 | 2.93±0.95 | 0.002 |
| HDL, mmol/L | 1.38±0.39 | 1.19±0.30 | <0.001 | 1.38±0.39 | 1.22±0.34 | <0.001 |
*p for non-MAFLD vs. MAFLD; **p for non-NAFLD vs. NAFLD. Data are n (%) or mean±standard deviation, as indicated. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second of exhalation; FVC, forced vital capacity; FPG, fasting plasma glucose; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; WBC, white blood cells.
Lung function in MAFLD and NAFLD non-MD-NAFLD participants
Table 2 shows the comparisons of lung function tests between participants with NAFLD and different groups with MAFLD. Differences in lung function tests were adjusted for age, sex, adiposity, smoking status and, significant alcohol intake (by analysis of covariance). Compared with the NAFLD or non-MD-NAFLD groups, individuals with MAFLD had the lowest FVC, predicted FVC (%), FEV1, and predicted-FEV1 (%) values. In addition, those with MAFLD and coexistent T2DM had lower predicted FVC (%) and predicted FVC (%) than MAFLD patients without T2DM,. Similarly, lung function tests of patients with MAFLD were worse even after stratification by obesity status.
Table 2. Comparison of age, sex, BMI, smoking status, and lung function tests between subjects with NAFLD and different groups of subjects with MAFLD.
| Variable | Age, years | BMI, kg/m2, mean±SD | Men, n (%) | Current smoker, n (%) | FVC, L, mean±SD | FVC, % of predicted, mean±SD | FEV1, L, mean±SD | FEV1, % of predicted, mean±SD | FEV1/FVC, %, mean±SD |
|---|---|---|---|---|---|---|---|---|---|
| MAFLD (n=519) | 64.89±8.00 | 27.81±2.59 | 292 (58.1) | 127 (24.5) | 2.61±0.67 | 88.27±17.60* | 2.08±0.57* | 79.89±17.34* | 80.04±10.23 |
| NAFLD (n=469) | 64.34±7.73# | 27.66±2.67 | 269 (57.4) | 116 (24.8) | 2.67±0.75# | 90.82±16.85 | 2.16±0.53# | 83.02±16.66 | 81.08±8.32 |
| Non-MD-NAFLD (n=177) | 62.75±9.06& | 27.37±2.92 | 98 (55.3) | 42 (23.9) | 2.86±0.73& | 94.24±16.68& | 2.24±0.65 | 84.70±19.58& | 80.35±10.60 |
| P for trend$ | 0.006 | 0.150 | 0.097 | 0.592 | <0.001 | <0.001 | 0.010 | 0.006 | 0.008 |
| MAFLD without T2DM (n=379) | 64.41±6.53 | 28.04±2.59 | 211 (55.7) | 89 (23.5) | 2.63±0.74 | 89.69±15.64 | 2.09±0.59 | 81.40±17.24 | 80.00±10.53 |
| MAFLD with T2DM (n=140) | 66.16±6.21 | 27.19±2.49 | 88 (65.9) | 35 (25.2) | 2.49±0.67 | 85.16±16.45 | 2.01±0.51 | 77.04±19.12 | 81.20±11.45 |
| P | 0.027 | 0.001 | 0.161 | 0.528 | 0.0.004 | 0.009 | 0.190 | 0.008 | 0.251 |
| MAFLD with BMI <23 (n=55) | 63.49±9.05 | 23.48±1.31 | 19 (34.5) | 13 (24.1) | 2.33±0.67 | 79.08±18.01 | 1.81±0.56 | 75.45±19.21 | 78.67±12.23 |
| MAFLD with BMI ≥23 (n=464) | 65.06±7.86 | 28.32±2.20 | 280 (60.3) | 107 (23.1) | 2.65±0.76 | 89.00±17.26 | 2.10±0.23 | 80.28±17.12 | 80.42±10.34 |
| P | 0.169 | <0.001 | <0.001 | 0.571 | 0.019 | 0.008 | 0.009 | 0.030 | 0.437 |
Differences in lung function tests (FVC, FEV1 and FEV1/FVC ratio) were adjusted for age, sex, adiposity (BMI and waist circumference), smoking status and alcohol intake by analysis of covariance. *p<0.05 for MAFLD vs. NAFLD; #p<0.05 for NAFLD vs. Non-MD-NAFLD; &p<0.05 for MAFLD vs. Non-MD-NAFLD; $p<0.05 for comparisons among the NAFLD, MAFLD and Non-MD-NAFLD groups. BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume measured in the first second of exhalation; T2DM, Type 2 diabetes; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; non-MD-NAFLD, nonmetabolic dysfunction-NAFLD.
Relation between lung function tests and fibrosis severity
Table 3 shows the lung function tests stratified by increasing quartiles of FIB-4 score in participants with MAFLD or NAFLD. The mean values of most lung function tests progressively decreased across FIB-4 quartiles, in both NAFLD and in MAFLD.
Table 3. Lung function tests stratified by increasing FIB-4 quartiles in patients with MAFLD or NAFLD.
| MAFLD (n=519) | FIB-4 quartiles |
||||
|---|---|---|---|---|---|
| Quartile 1 (0–0.38, n=130) | Quartile 2 (0.38–0.49, n=129) | Quartile 3 (0.49–0.67, n=131) | Quartile 4 (0.67–3.91, n=129) | p | |
| Lung function tests | |||||
| FVC, L | 2.92±0.81 | 2.72±0.82 | 2.49±0.78 | 2.26±0.72 | <0.001 |
| FVC, % of predicted value | 90.60±17.03 | 89.24±16.64 | 87.72±17.41 | 84.80±16.21 | 0.056 |
| FEV1, L | 2.29±0.69 | 2.19±0.68 | 2.02±0.69 | 1.77±0.59 | <0.001 |
| FEV1, % of predicted value | 86.12±12.16 | 81.14±16.43 | 80.18±16.63 | 76.72±19.82 | <0.001 |
| FEV1/FVC, % | 81.10±7.41 | 80.94±10.24 | 79.46±8.16 | 78.89±12.99 | 0.227 |
| NAFLD (n=469) | Quartile 1 (0–0.36, n=115) | Quartile 2 (0.36–0.48, n=114) | Quartile 3 (0.48–0.64, n=115) | Quartile 4 (0.64–3.16, n=115) | p |
|---|---|---|---|---|---|
| Lung function tests | |||||
| FVC, L | 2.92±0.82 | 2.78±0.83 | 2.61±0.82 | 2.36±0.69 | <0.001 |
| FVC, % of predicted value | 93.43±17.48 | 91.43±15.56 | 89.06±17.67 | 89.37±16.93 | 0.152 |
| FEV1, L | 2.36±0.64 | 2.29±0.65 | 2.11±0.64 | 1.91±0.56 | <0.001 |
| FVC, % of predicted value | 85.54±16.55 | 84.09±15.56 | 82.06±17.79 | 80.97±17.17 | 0.155 |
| FEV1/FVC, % | 81.30±9.42 | 81.20±9.45 | 80.95±12.46 | 79.86±10.62 | 0.548 |
*One-way analysis of variance. FIB-4, fibrosis 4 score; FVC, forced vital capacity; FEV1, forced expiratory volume measured in the first second of exhalation; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic dysfunction-associated fatty liver disease; non-MD-NAFLD, nonmetabolic dysfunction-NAFLD.
Table 4 shows the unadjusted and adjusted changes in lung function tests for a 1-point increase in FIB-4 score in patients with MAFLD. Univariable linear regression (unadjusted model 1) found that the severity of liver fibrosis (the FIB4 score) was inversely related to FVC, FEV1, and predicted FVC (%). For each one-point increase in the FIB-4 score, FVC (%) decreased by 6.441 (95% CI: −12.032, −0.851, p=0.024), FVC decreased by 0.748 (95% CI: −1.002, −0.495, p<0.001), and FEV1 decreased by 0.587 (95% CI: −0.796, −0.378, p<0.001). As shown in Table 4, the results remained unchanged in multivariable regression after adjusting for sex, age, prior COPD, smoking status, and significant alcohol intake (adjusted model 2), as well as in regression models additionally adjusting for adiposity, blood pressure, WBC count, total hemoglobin, serum transaminases, fasting glucose, and the plasma lipid profile (adjusted models 3). For instance, in adjusted model 2, we found that each one-point increase in the FIB-4 score was associated with a significant reduction in both FVC (−0.507 95% CI: −0.840, −0.173, p=0.003) and FEV1 (−0.439 95% CI: −0.739, −0.140, P=0.004).
Table 4. Changes in lung function tests for 1-point increase in FIB-4 score in participants with MAFLD (n=519).
| Unadjusted model 1 | p | Adjusted model 2 | p | Adjusted model 3 | p | |
|---|---|---|---|---|---|---|
| FVC, L | −0.748 (−1.002, −0.495) | <0.001 | −0.420 (−0.753, −0.088) | 0.014 | −0.507 (−0.840, −0.173) | 0.003 |
| FVC, % of predicted value | −6.441 (−12.032, −0.851) | 0.024 | −1.816 (−7.564, −3.932) | 0.535 | −8.824 (−19.554, −1.906) | 0.107 |
| FEV1, L | −0.587 (−0.796, −0.378) | <0.001 | −0.245 (−0.380, −0.042) | 0.015 | −0.439 (−0.739, −0.140) | 0.004 |
| FEV1, % of predicted value | −4.039 (−9.724, 1.646) | 0.163 | −1.342 (−7.083, 4.435) | 0.652 | −6.053 (−16.621, 4.515) | 0.260 |
| FEV1 /FVC, % | 0.996 (−2.524, 4.516) | 0.579 | 1.563 (−1.949, 5.074) | 0.382 | 1.299 (−7.671, −5.073) | 0.688 |
Data are beta coefficients (95% CI). Model 1: univariable linear regression analysis (unadjusted model); Model 2: adjusted for age ≥65 years, sex, prior COPD, smoking status and significant alcohol intake; Model 3: adjusted for the same covariates of model 2 plus adiposity measures (BMI and waist circumference), blood pressure, WBC count, hemoglobin, serum transaminases, fasting glucose, and plasma lipid profile.
Lung function tests in MAFLD and NAFLD non-MD-NAFLD participants stratified by sex
The aforementioned results remained essentially unchanged even when we performed separate statistical analyses by sex. As shown in Supplementary Tables 1–4, we found that the trends in impairment of lung function were comparable in men and women with MAFLD, but that the impairment in lung function appeared to be greater in men than in women, after adjusting for age ≥65 years, pre-existing COPD, smoking status, significant alcohol intake, adiposity measures, and other potential confounding factors (Table S4, adjusted model 3).
Discussion
The main findings of this large cross-sectional study were that both MAFLD and NAFLD were significantly associated with decreased lung function tests (i.e., FVC and FEV1) in middle-aged Chinese adults after adjustment for multiple potential confounding factors. The observed decreases in lung function were significantly greater in participants with MAFLD than in those with NAFLD. Furthermore, the reductions in FEV1 and FVC in those with MAFLD increased progressively with the severity of liver fibrosis, as noninvasively assessed by the FIB-4 score.
To the best of our knowledge, this is the first observational study that aimed to compare lung function parameters in NAFLD and MAFLD populations and to examine the association between lung function parameters and MAFLD fibrosis severity as assessed noninvasively by FIB-4 score. As summarized in Supplementary Table 5, previous observational studies reported that NAFLD was associated with impaired lung function tests,9,12,19–21,38 and that the association worsened as the histological severity of NAFLD progressed to a higher fibrosis stage.16 Moreover, some studies have shown a significant association between NAFLD and the presence of COPD or chronic restrictive pulmonary diseases.12,15,17 Although most of the aforementioned studies were cross-sectional and NAFLD was diagnosed by ultrasonography, they support the existence of an association between NAFLD and COPD.
The precise pathogenic mechanisms underpinning the association between MAFLD and reduced lung function tests remain poorly understood. The lungs and the liver are both highly vascular organs with dual blood supplies, and both are involved in antigen processing and are master regulators of energy homeostasis.18 It might thus be hypothesized that MAFLD and its associated metabolic disorders, principally obesity and T2DM, may promote systemic chronic inflammation, increased oxidative stress, increased insulin resistance, and lipotoxicity,10,39,40 which may induce lung function impairment in the long-term, possibly by activation of bronchial inflammation, lung fibrosis, and hypotrophy of airway respiratory muscles. As low-grade chronic inflammation is associated with both impaired lung function and NAFLD,41,42 some studies have shown that increased plasma C-reactive protein concentrations are associated with impaired lung function.43–45 Impaired lung function may subsequently promote increased insulin resistance, oxidative stress, and low-grade chronic inflammation, all of which may contribute to NAFLD progression.46,47 Nevertheless, future prospective cohort studies are required to better elucidate the direction of the relationship between impaired lung function and fatty liver disease.
Recently, an international panel of experts proposed a change in name and definition of NAFLD to MAFLD.48 However, the criteria for diagnosing MAFLD and NAFLD are distinct, and to the best of our knowledge, there is currently no published research related to MAFLD and lung function. Thus, given the emerging worldwide epidemic of this metabolic liver disease, it is important to ascertain if the newly proposed definition of MAFLD is more able to identify subjects with impaired lung function compared to the old NAFLD definition. In our study, we made some key observations. Firstly, both MAFLD and NAFLD definitions were associated with significant reductions in lung function parameters in Chinese people, but the reductions was significantly greater in patients with MAFLD than in those with NAFLD. Secondly, patients with MAFLD and T2DM or overweight/obesity had greater decreases in lung function than their counterparts without T2DM or overweight/obesity. Thirdly, patients with MAFLD and increased FIB-4 scores had worse lung function, even after adjusting for sex, age, smoking status, significant alcohol intake, BMI, pre-existing T2DM, and other potential confounding factors. Fourthly, patients with non-MD-NAFLD had the best lung function parameters compared with the MAFLD and NAFLD groups. As patients with MAFLD have various metabolic abnormalities, especially overweight/obesity and T2DM, that may adversely affect lung function, it is clinically important to manage and control the underlying metabolic disorders related to MAFLD.
Our study has some important limitations that should be mentioned. Firstly, we used liver ultrasonography and FIB-4 scores for noninvasive diagnosing and staging of NAFLD or MAFLD. Secondly, the participants recruited from community hospitals were mostly middle-aged and elderly, which may have contributed to worse lung function compared with the normal population. Thirdly, the cross-sectional design of the study did not allow any temporal and causal inferences of the observed significant associations between decreased lung function volumes and MAFLD or MAFLD-related liver fibrosis. Finally, the findings should be confirmed in different ethnic groups.
In conclusion, the results of our study show that both MAFLD and NAFLD were associated with significant reductions of FEV1 and FVC in a large hospital-based cohort of middle-aged Chinese adults. The reductions in lung volumes were significantly greater in participants with MAFLD than in those with NAFLD. Reduced lung function parameters were also associated with higher FIB-4 scores in MAFLD. We suggest that future prospective and mechanistic studies are needed to decipher the existing but complex links between MAFLD and impaired lung function.
Supporting information
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- BUN
blood urea nitrogen
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- eGFR
estimated glomerular filtration rate
- FEV1
forced expiratory volume measured in the first second of exhalation
- FIB-4
fibrosis 4
- FVC
forced vital capacity
- FPG
fasting plasma glucose
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment of insulin resistance
- LDL
low-density lipoprotein
- MAFLD
metabolic associated fatty liver disease
- NAFLD
nonalcoholic fatty liver disease
- NFS
NAFLD fibrosis score
- non-MD-NAFLD
nonmetabolic dysfunction-associated NAFLD
- SBP
systolic blood pressure
- SD
standard derivation
- T2DM
type 2 diabetes mellitus
- TC
total cholesterol
- TG
triglyceride
Data sharing statement
No additional data are available.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6(7):578–588. doi: 10.1016/S2468-1253(21)00020-0. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Schattenberg JM, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. 2020:gutjnl-2020-323082. doi: 10.1136/gutjnl-2020-323082. [DOI] [PubMed] [Google Scholar]
- 5.Ballestri S, Mantovani A, Nascimbeni F, Lugari S, Lonardo A. Extra-hepatic manifestations and complications of nonalcoholic fatty liver disease. Future Med Chem. 2019;11(16):2171–2192. doi: 10.4155/fmc-2019-0003. [DOI] [PubMed] [Google Scholar]
- 6.Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 7.Jung DH, Shim JY, Lee HR, Moon BS, Park BJ, Lee YJ. Relationship between non-alcoholic fatty liver disease and pulmonary function. Intern Med J. 2012;42(5):541–546. doi: 10.1111/j.1445-5994.2011.02644.x. [DOI] [PubMed] [Google Scholar]
- 8.Talaminos Barroso A, Márquez Martín E, Roa Romero LM, Ortega Ruiz F. Factors Affecting Lung Function: A Review of the Literature. Arch Bronconeumol (Engl Ed) 2018;54(6):327–332. doi: 10.1016/j.arbres.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 9.Peng TC, Kao TW, Wu LW, Chen YJ, Chang YW, Wang CC, et al. Association Between Pulmonary Function and Nonalcoholic Fatty Liver Disease in the NHANES III Study. Medicine (Baltimore) 2015;94(21):e907. doi: 10.1097/MD.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viglino D, Jullian-Desayes I, Minoves M, Aron-Wisnewsky J, Leroy V, Zarski JP, et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur Respir J. 2017;49(6):1601923. doi: 10.1183/13993003.01923-2016. [DOI] [PubMed] [Google Scholar]
- 11.Qin L, Zhang W, Yang Z, Niu Y, Li X, Lu S, et al. Impaired lung function is associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in middle-aged and elderly Chinese. BMC Endocr Disord. 2017;17(1):18. doi: 10.1186/s12902-017-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwak MS, Kim E, Jang EJ, Lee CH. The association of non-alcoholic fatty liver disease with lung function: A survey design analysis using propensity score. Respirology. 2018;23(1):82–88. doi: 10.1111/resp.13127. [DOI] [PubMed] [Google Scholar]
- 13.Moon SW, Kim SY, Jung JY, Kang YA, Park MS, Kim YS, et al. Relationship between obstructive lung disease and non-alcoholic fatty liver disease in the Korean population: Korea National Health and Nutrition Examination Survey, 2007-2010. Int J Chron Obstruct Pulmon Dis. 2018;13:2603–2611. doi: 10.2147/COPD.S166902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CH, Choi SH, Chung GE, Park B, Kwak MS. Nonalcoholic fatty liver disease is associated with decreased lung function. Liver Int. 2018;38(11):2091–2100. doi: 10.1111/liv.13860. [DOI] [PubMed] [Google Scholar]
- 15.Song JU, Jang Y, Lim SY, Ryu S, Song WJ, Byrne CD, et al. Decreased lung function is associated with risk of developing non-alcoholic fatty liver disease: A longitudinal cohort study. PLoS One. 2019;14(1):e0208736. doi: 10.1371/journal.pone.0208736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HW, Lee DH, Lee JK, Lee S, Koo BK, Joo SK, et al. Pulmonary function is associated with fibrosis severity in patients with biopsy-proven nonalcoholic fatty liver disease. Liver Int. 2020;40(12):3008–3017. doi: 10.1111/liv.14626. [DOI] [PubMed] [Google Scholar]
- 17.El Amrousy D, El Ashry H, Maher S, Ganna S, Hasan S. Pulmonary function test abnormalities in children and adolescents with non-alcoholic fatty liver disease. Eur J Pediatr. 2021;180(6):1693–1699. doi: 10.1007/s00431-021-03941-3. [DOI] [PubMed] [Google Scholar]
- 18.Lonardo A, Nascimbeni F, Ponz de Leon M. Nonalcoholic fatty liver disease and COPD: is it time to cross the diaphragm? Eur Respir J. 2017;49(6):1700546. doi: 10.1183/13993003.00546-2017. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Lonardo A, Vinco G, Zoppini G, Lippi G, Bonora E, et al. Association between non-alcoholic fatty liver disease and decreased lung function in adults: A systematic review and meta-analysis. Diabetes Metab. 2019;45(6):536–544. doi: 10.1016/j.diabet.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Qin L, Zhang W, Yang Z, Niu Y, Li X, Lu S, et al. Impaired lung function is associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in middle-aged and elderly Chinese. BMC Endocr Disord. 2017;17(1):18. doi: 10.1186/s12902-017-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song JU, Jang Y, Lim SY, Ryu S, Song WJ, Byrne CD, et al. Decreased lung function is associated with risk of developing non-alcoholic fatty liver disease: A longitudinal cohort study. PLoS One. 2019;14(1):e0208736. doi: 10.1371/journal.pone.0208736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CH, Choi SH, Chung GE, Park B, Kwak MS. Nonalcoholic fatty liver disease is associated with decreased lung function. Liver Int. 2018;38(11):2091–2100. doi: 10.1111/liv.13860. [DOI] [PubMed] [Google Scholar]
- 23.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 25.Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, et al. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J (Engl) 2020;133(19):2271–2273. doi: 10.1097/CM9.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng KI, Sun DQ, Jin Y, Zhu PW, Zheng MH. Clinical utility of the MAFLD definition. J Hepatol. 2021;74(4):989–991. doi: 10.1016/j.jhep.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40(6):1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 28.Lonardo A. Renaming NAFLD to MAFLD: Could the LDE System Assist in This Transition? J Clin Med. 2021;10(3):492. doi: 10.3390/jcm10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng KI, Eslam M, George J, Zheng MH. When a new definition overhauls perceptions of MAFLD related cirrhosis care. Hepatobiliary Surg Nutr. 2020;9(6):801–804. doi: 10.21037/hbsn-20-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, et al. MAFLD and risk of CKD. Metabolism. 2021;115:154433. doi: 10.1016/j.metabol.2020.154433. [DOI] [PubMed] [Google Scholar]
- 31.Zheng H, Hu Y, Dong L, Shu Q, Zhu M, Li Y, et al. Predictive diagnosis of chronic obstructive pulmonary disease using serum metabolic biomarkers and least-squares support vector machine. J Clin Lab Anal. 2021;35:e23641. doi: 10.1002/jcla.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathiesen UL, Franzén LE, Aselius H, Resjö M, Jacobsson L, Foberg U, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis. 2002;34(7):516–522. doi: 10.1016/s1590-8658(02)80111-6. [DOI] [PubMed] [Google Scholar]
- 33.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107(6):811–826. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 34.Itakura J, Kurosaki M, Setoyama H, Simakami T, Oza N, Korenaga M, et al. Applicability of APRI and FIB-4 as a transition indicator of liver fibrosis in patients with chronic viral hepatitis. J Gastroenterol. 2021;56(5):470–478. doi: 10.1007/s00535-021-01782-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhou YJ, Zheng KI, Targher G, Byrne CD, Zheng MH. Non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis. Lancet Gastroenterol Hepatol. 2021;6(1):9–10. doi: 10.1016/S2468-1253(20)30308-3. [DOI] [PubMed] [Google Scholar]
- 36.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 37.Vukoja M, Bokan A, Vujasinovic G, Kopitovic I. The Differences in Spirometry Predictive Equations in Classifying Presence and Degree of Lung Function Impairment: Which Suit Fits the Best? Lung. 2018;196(1):87–92. doi: 10.1007/s00408-017-0065-7. [DOI] [PubMed] [Google Scholar]
- 38.Peng TC, Kao TW, Wu LW, Chen YJ, Chang YW, Wang CC, et al. Association Between Pulmonary Function and Nonalcoholic Fatty Liver Disease in the NHANES III Study. Medicine (Baltimore) 2015;94(21):e907. doi: 10.1097/MD.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao H, Dennery PA, Yao H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;314(4):L544–L554. doi: 10.1152/ajplung.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan SMH, Selemidis S, Bozinovski S, Vlahos R. Pathobiological mechanisms underlying metabolic syndrome (MetS) in chronic obstructive pulmonary disease (COPD): clinical significance and therapeutic strategies. Pharmacol Ther. 2019;198:160–188. doi: 10.1016/j.pharmthera.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136(2):304–311.e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2008;31(4):741–746. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalhan R, Tran BT, Colangelo LA, Rosenberg SR, Liu K, Thyagarajan B, et al. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5(7):e11431. doi: 10.1371/journal.pone.0011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasmussen F, Mikkelsen D, Hancox RJ, Lambrechtsen J, Nybo M, Hansen HS, et al. High-sensitive C-reactive protein is associated with reduced lung function in young adults. Eur Respir J. 2009;33(2):382–388. doi: 10.1183/09031936.00040708. [DOI] [PubMed] [Google Scholar]
- 45.Baines KJ, Backer V, Gibson PG, Powel H, Porsbjerg CM. Impaired lung function is associated with systemic inflammation and macrophage activation. Eur Respir J. 2015;45(2):557–559. doi: 10.1183/09031936.00187514. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Ballantyne CM. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res. 2020;126(11):1549–1564. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardet JC, Ash S, Kusa T, Camargo CA, Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48(2):403–410. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 2020;17(7):387–388. doi: 10.1038/s41575-020-0316-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.