Graphical abstract

Keywords: Organophosphorus pesticides, Enzyme inhibition, Au nanomaterials, Rapid detection
Highlights
-
•
The review systematically and completely collated the enzyme inhibition method based on Au nanomaterials for organophosphorus pesticide detection method in the last 20 years.
-
•
The significance of the optical properties of Au nanomaterials is outlined with different shapes, sizes, and surface modifiers in enzyme inhibition methods.
-
•
The principles, classification and application of enzyme inhibition methods based on Au nanomaterials are comprehensively summarized from a new perspective in agricultural and environmental samples, including colorimetric method, fluorometric method, electrochemical biosensor method.
-
•
Unlike traditional enzyme inhibition method, the merits of enzyme inhibition method based on Au nanomaterials were elaborated in this review.
-
•
Combined with the research progress of enzyme inhibition method, this review predicts the future research direction of enzyme inhibition method, providing a theoretical reference for researchers.
Abstract
Background
Organophosphorus pesticides (OPs), as insecticides or acaricides, are widely used in agricultural products to ensure agricultural production. However, widespread use of OPs leads to environmental contamination and significant negative consequences on biodiversity, food security, and water resources. Therefore, developing a sensitive and rapid method to determine OPs residues in different matrices is necessary. Originally, the enzyme inhibition methods are often used as preliminary screens of OPs in crops. Many studies on the characteristic of Au nanomaterials have constantly been emerging in the past decade. Combined with anisotropic Au nanomaterials, enzyme inhibition methods have the advantages of high sensitivity, durability, and high stability.
Aim of Review
This review aims to summarize the principles and strategies of gold (Au) nanomaterials in enzyme inhibition methods, including colorimetric (dispersion, particle size of Au nanomaterials) and fluorometric (fluorescence energy transfer, internal filtration effect) detection, and electrochemical sensing system (shape of Au nanomaterials, Au nanomaterials combined with other nanomaterials). The application of enzyme inhibition in agricultural products and research progress was also outlined. Next, this review illustrates the advantages of Au nanomaterial-based enzyme inhibition methods compared with conventional enzyme inhibition methods. The detection limits and linear range of colorimetric and fluorometric detection and electrochemical biosensors have also been provided. At last, key perspectives, trends, gaps, and future research directions are proposed.
Key Scientific Concepts of Review
Herein, we introduced the technology of enzyme inhibition method based on Au nanomaterials for onsite and infield rapid detection of organophosphorus pesticide.
Introduction
Organophosphorus pesticides (OPs) are widely used in agricultural production; however, they may pose health hazards [1]. Many countries have banned the use of methamidophos, parathion, methyl parathion, monocrotophos, and other highly toxic OPs [2] to protect vulnerable population groups from the negative impacts of chemicals. Some OPs are poorly soluble and tend to get adsorbed onto soil particles and enter water bodies [3]. OPs inhibit acetylcholinesterase irreversibly by preventing the breakdown of acetylcholine during nerve impulse transmission in the central nervous system of mammals and insects. Mammals and insects in a continuous state of neuronal excitation produce a range of toxic symptoms, including decreased heart rate, pinpoint eye pupils, and seizures. Respiratory failure (RF) is the major cause of the high morbidity and mortality from OPs poisoning [4]. In this regard, the development of rapid, simple, and reliable screening methods for detecting OPs is warranted for food and agricultural products and human health [5], [6].
Methods for detecting OPs, include gas chromatography (GC) [7], high-performance liquid chromatography (HPLC) [8], mass spectrometry (MS) [9], gas chromatography-mass spectrometry (GC–MS) [10], and liquid chromatography-mass spectrometry (LC-MS) [11]. These methods are sensitive to trace levels; however, they are time-consuming, tedious, laborious, require expensive instruments, and restricted experimental conditions. Therefore, rapid detection of OPs has become a topic of particular research interest; and the number of annual publications containing the term ‘rapid detection of OPs has increased five times over the past 20 years (Fig. 1).
Fig. 1.
The progress of rapid detection of OPs (Data from the Web of Science with keywords of ‘rapid detection” and “organophosphorus pesticides”).
So far, the main techniques used for rapid detection of OPs include immunoassay, high-performance thin-layer chromatography (HPTLC), and enzyme inhibition assay. HPTLC is a simple and inexpensive technique that requires only a small amount of stationary phase, mobile phase, and chromogenic reagents to detect OPs rapidly [12]. HPTLC could qualitatively analyze and quantitatively detect OPs by visulatization and UV techque or fluorometric techque, respectively [13]. Although HPTLC can achieve rapid quantitative detection, it lacks the specificity to identify a class of OPs compared with enzyme inhibition and immunoassays. Immunosorbent assays mainly rely on specific recognition of antigen and antibody to detect the target analyte. Although immunoassays are sensitive and specific, the method requires the preparation of antibodies with high affinity. In contrast, enzyme inhibition methods do not involve a complex preparation process and can directly use natural enzymes for detecting OPs [14].
Enzyme inhibition-based colorimetric and fluorometric methods indirectly detect OP residues through colorimetric or fluorometric signals. Notably, some chemical reagents are still used for enzyme inhibition methods, leading to low sensitivity and false-positive results [15]. Noble metal nanomaterials, such as Au nanoparticles (NPs), display different colors in aggregation and dispersion states, are used in combination with enzyme inhibition. Products of acetylcholine can induce AuNPs from aggregation to dispersion to produce color changes, thus overcoming the disadvantages of low sensitivity and false positives to some extent. Similarly, biosensor methods are based on enzyme inhibition and metal NPs. The transducer is a key element in a biosensor, generating either optical or electrical signals proportional to the number of analytes–bioreceptor interactions [16], [17]. Biosensors have the merits of high sensitivity, short response time, simple-to-operate, and good selectivity.
Herein, we focused on enzyme inhibition methods, including colorimetric, fluorometric, and biosensor detection based on Au nanomaterials for rapidly detecting OPs over the past decades. Details on the classification, principles, and other properties of these enzyme inhibition methods based on Au nanomaterials have been addressed, as shown in Fig. 2. We comprehensively reviewed the development of enzyme inhibition methods based on Au (Fig. 3) and the classification of enzyme inhibition methods (Fig. 4).
Fig. 2.
Schematic illustration of enzyme inhibition methods is highlighted in this review.
Fig. 3.
A brief timeline of enzyme inhibition methods for rapid detection of OPs [18], [19], [20], [21], [22], [23].
Fig. 4.
Methods for rapid detection of OPs.
The principles of enzyme inhibition methods based on Au nanomaterials
Enzyme inhibition methods involve quantifying the degree to which AChE reduces the generation of thiocholine to detect the OPs residual levels. These methods are typically colorimetric, fluorometric, or biosensing (Fig. 4). George Ellman first proposed colorimetric detection for measuring AChE activity in biological tissues in 1960. Acetylthiocholine was hydrolyzed and generate thiocholine, which then reacts with dithiodinitrobenzoic acid ions. The intensity of the yellow color measures the rate of enzyme activity. OPs can reduce the production of thiocholine, thus yielding a light yellow color for a qualitative analysis [19]. Other natural enzymes used for enzyme inhibition assays, include butyrylcholinesterase, carboxylesterase, alkaline phosphatase (ALP), and tyrosinase. OPs can inhibit these enzymes, and residues can be detected by measuring the capacity of the enzyme to hydrolyze. Some synthetic enzymes have also been proposed for inhibition assays [14].
Metal NPs are materials with at least one dimension in the three-dimensional space in the range of 1–100 nm [24]. Metal nanomaterials have unique electrical, optical, and thermal properties and a high specific surface area used to immobilize various biomolecules to enhance the signal of bioenzyme sensors [25], [26]. AuNPs were the first metallic nanomaterials used in analytical assays as they are relatively simple to prepare, stable, and biocompatible [27], [28]. Au provides a large surface area for contact with target reactants, which improves the reaction efficiency. It can also react with some compounds or moieties [29], [30].
Au nanomaterials have good optical properties, red in the well-dispersed state, and turn blue after aggregation. This property can be directly applied in the enzyme inhibition method based on colorimetric detection of OPs. Further, the surface of Au nanomaterials is easily modified, and the sensitivity of its colorimetric detection method can be improved using chemical functional groups. The colorimetric detection method was based on the principle of local surface plasmon resonance of Au nanomaterials. According to the aspect ratios of Au nanomaterials, the colorimetric analysis can be used to measure the color difference. The Au nanomaterials have good fluorometric properties and can be quenched using quenched fluorescent substrates with wide absorption peaks to achieve the purpose of detecting Ops.
Au nanomaterials have a large specific surface area in the biosensing system, which provides more sites for the enzyme in the surface attachment. Au nanomaterials have an excellent electrical conductivity that can improve the sensitivity of the detection.
Classification and application of enzyme inhibition methods based on Au nanomaterials
Colorimetric detection
Dispersion of Au nanomaterials
Generally, AuNPs are red solutions in the dispersed state and can be aggregated to a blue solution under the induction of thiocholine. Sun and colleagues established a label-free colorimetric method in which AuNPs modified with lipoic acid was induced by thiocholine and changed from red to blue when aggregated [31]. The limit of detection (LOD) was 4.52 × 104 pmol/L for paraoxon. The method was applied to quantify the residues in apple juice with a LOD as low as 1 pmol/L. Similarly, Li and co-workers used citrate-modified AuNPs as probes to detect methamidophos; AuNPs' color changed from red to purple or gray [32]. Under optimal conditions, AuNPs' maximum absorbance at 522 nm exhibited linearity over the range of 0.02–1.42 µg/L with a LOD of 1.40 ng/mL. Although citrate is the most popular reducing agent for synthesizing Au nanomaterials, it is susceptible to environmentally induced aggregation; in turn, it is not considered a good choice [33]. In this context, Bala et al. used a colorimetric method to detect ethyl parathion using cysteine-modified AuNPs, where AChE hydrolyzes acetylthiocholine to thiocholine, leading to the aggregation of AuNPs and changing the solution from red to blue (Fig. 5a). The method is highly sensitive, with an observable LOD of 0.081 µg/L. The LOD using cit-AuNPs is 3 orders of magnitude lower than the LOD using cys-AuNPs [34].
Fig. 5.
(a) OPs detection using enzyme inhibition and Au nanoparticles. (b) Highly sensitive colorimetric detection of OPs using copper (Cu)-catalyzed click chemistry. Adapted with permission from Ref. [39]. (c) AuNP dissolution-based colorimetric method for highly sensitive detection of OPs. Adapted with permission from Ref. [41]. (d) Thiol-inhibited iodine (I2) etching of Au nanorods (AuNRs) to detect OPs. Adapted with permission from Ref. [43].
The reaction of either cysteine or citrate-modified AuNPs with thiocholine leads to the aggregation of AuNPs. Hydrolysis of acetylthiocholine by AChE generates thiocholine-containing SH groups. Due to electrostatic interaction and strong Au-S interaction, AuNPs can be aggregated and eventually change color [35], [36], [37].
The methods mentioned above rely on the aggregation of AuNPs to detect OPs, and a high concentration of pesticides was generally required to induce particle aggregation and generate a signal [38]. To overcome this challenge, Fu and colleagues introduced click chemistry into the reaction to achieve intermediate colorimetric signal amplification between the AChE-acetamido thiocholine system and the probe [39]. The team used copper functionalized with azide terminal alkyne groups to establish a rapid colorimetric method to determine OPs. In the presence of the reducing agent, sodium ascorbate, the acetic acid generated by the AChE-acetamido thiocholine system excites copper oxide NPs to release the reducing agent required as a catalyst for the cycloaddition between the azide and terminal alkyne groups on the AuNP surface. Copper-mediated signal amplification ensures that even a small change in copper ions would greatly affect the efficiency of the reaction and resultant color (Fig. 5b).
The particle size of Au
Colorimetric detection of OPs was based on the dispersion state of Au nanomaterials, which is determined by the reaction of enzymatic products with Au nanomaterials. However, particle aggregation is not highly specific and is affected by salt concentration. Most importantly, the suspensions of these aggregates were unstable in solution and their color changes over time [40], precluding an accurate detection of the target. To reduce the effects of salt concentration and aggregate instability on Au, Wu and co-workers developed a simple and sensitive colorimetric method using Au3+ and cetyl trimethyl ammonium bromide solutions to dissolve AuNPs [41]. In the absence of OPs, thiocholine reduced Au3+ and protected the Au3+-cetyl trimethyl ammonium bromide complex from solubilizing AuNPs. However, in the presence of OPs, AChE cannot produce thiocholine or produce only small quantities, yielding residual Au3+-solubilized AuNPs instead and changing the color of the solution from red to light pink to transparent. Under optimal conditions, the minimum colorimetric concentration observed was 0.7 µg/L, substantially improving detection sensitivity (Fig. 5c).
Further, Wu and colleagues adapted a semi-quantitative colorimetric method based on Au nanorods' localized surface plasmon excitonic resonance. In the presence of AChE, cetyl trimethyl ammonium bromide can form complexes with Au3+ to adjust the aspect ratio of the nanorods, resulting in a color change from brown to green, gray, purple, cyan, blue, red, and transparent. The method has a LOD of 1.2 µg/L for parathion [42]. To improve the sensitivity of the colorimetric detection, Qing et al. used the same method to change the aspect ratio of Au nanorods through inhibition of iodine etching on the nanorods by the sulfhydryl group on thiocholine and tested the method with triazophos [43]. Under optimum conditions, the linear range was between 0 and 117 nmol/L (R2 = 0.9908) with a LOD of 4.69 nmol/L for triazophos (Fig. 5d).
Other methods
Liao and co-workers developed a method of enzyme inhibition based on AuNPs for detecting OPs [44]. Thiocholine reduces AuCl4− in solution to generate AuNPs, which are large enough to break the orientation and arrangement of the molecules in liquid crystal, thus enhancing the optical signal. The sensitivity of the method was 0.3 nmol/L. The catalytic product of ALP in a silver (Ag) solution can reduce Ag to Ag monomers attached to the surface of AuNPs, detected by colorimetric or spectrophotometric methods [45]. ALP, one of the substrate enzymes of the enzyme inhibition method, catalyzes the generation of pamiphenol (p-AP) from the substrate, which reduces Ag (I) ions to metallic Ag on the surface of Au forming Au@Ag nanoparticles. As the Ag shell thickens, the color of the colloidal solution changes from red to yellow to gray. Under optimum conditions, the absorbance of Au@Ag nanoparticles at 370 nm was linearly correlated with the concentration of methamidophos in the range of 0.05–500 µg/L with a LOD of 0.025 µg/L.
Fluorometric detection
Fluorometric detection is more sensitive than colorimetric one, has a lower detection limit, is less affected by the matrix, and the fluorescent material is stable over time [14]. Many substances (fluorescent substrates, organic fluorescent dyes, quantum dots, carbon dots, and metallic nanomaterials) are used as probes with low background noise, high sensitivity, and selectivity [46]. For example, as a common organic fluorescent dye, rhodamine B has been widely used for the fluorescence detection of OPs, owing to its good water solubility, light stability, and strong fluorescence properties [47]. In addition, indophenol acetate has no fluorescence effect; however, its hydrolyzed product has a fluorescence effect for the indirect detection of organophosphorus pesticides. Fluorescence emitted by fluorescent substances can be quenched by Au nanoparticles, thus enabling fluorometric detection. The following is a classification of fluorometric detection from the perspective of the fluorescence quenching mechanism.
Fluorescence resonance energy transfer (FRET)
A fluorescence resonance transfer system typically includes a fluorescent probe and a nanoquencher [48]. FRET induces fluorescence quenching when the quencher absorption band overlaps with the emission band of the fluorescent probe. AuNPs have large extinction coefficients and wide absorption bands that overlap with the light emitted from fluorescent probes, making them ideal nanoquenchers for FRET applications [49].
Rhodamine B is easily adsorbed to the surface of NPs through electrostatic interaction. In this context, Liu and colleagues used rhodamine B-modified Au to detect OPs by colorimetric and fluorescent double-reading methods [50]. The solution changed from red to purple under the action of thiocholine because thiocholine was strongly adsorbed onto AuNP surfaces than rhodamine B. Electrostatic interaction between thiocholine and AuNPs caused the aggregation of AuNPs, thereby changing the color of the solution. The lowest detectable diazinon, malathion, and phosphate concentrations were 0.1, 0.3, and 1 µg/L, respectively. Further, Wu and co-workers used carbon quantum dots as they have the advantages of low toxicity, easy synthesis, and do not require near-infrared light [51]. Butyrylcholinesterase catalyzes acetylcholine's hydrolysis to choline, which causes the aggregation of AuNPs and the recovery of the fluorescence of carbon quantum dots. The method could detect paraoxon at a concentration less than 0.05 µg/L.
Lanthanide-doped up-conversion nanoparticles (UCNPs) can emit intense visible light under near-infrared excitation (typically 980 nm) [52]. Unlike conventional fluorescent probes, they have unique optical and chemical properties, strong tissue penetration, high resistance to photobleaching, and low cytotoxicity [53]. The first application of UCNPs to enzyme inhibition-based fluorescence analysis with AuNPs and FRET used the UCNPs as donors and AuNPs as acceptors [54]. OPs inhibit the production of thiocholine, leading to a fluorescence burst of AuNPs on UCNPs by electrostatic adsorption. In the absence of OPs, the electrostatic gravitational force between thiocholine and AuNPs disintegrates the AuNP/UCNP assemblies and disperses AuNPs. The detection limit of this method for parathion was 0.67 ng/L (Fig. 6a).
Fig. 6.
(a) Fluorescence resonance energy transfer between AuNPs and fluorescent substances. (b) Mechanism of action of coumarin 1 and AuNPs using fluorescent probes in enzyme inhibition. (c) Schematic illustration of rapid analysis of OPs using enzyme inhibition and IFE of AuNPs on the fluorescence of cadmium telluride (CdTe) quantum dots or graphitized carbon nitride (g-C3N4). (d) Schematic illustration of rapid analysis of OPs using enzyme inhibition and IFE of AuNCs on the fluorescence of manganese dioxide (MnO2). Adapted with permission from Ref. [60]. (e) AChE mutants on the surface of yeast cells to detect paraoxon. Aggregation of AuNCs quenched the fluorescence. Adapted with permission from Ref. [63].
Kamelipour and colleagues used organophosphorus hydrolase (OPH) to design a fluorometric method, whereby AuNPs were covalently bound to organophosphorus hydrolase, and coumarin 1was added as a competitive inhibitor [55]. AuNPs quench the fluorescence of coumarin 1 in the presence of FRET. In the presence of paraoxon, coumarin 1 dissociates from the complex, leading to enhanced fluorescence. The linear range of the methods ranged from 50 to 1050 nmol/L (R2 = 0.9908), and the LOD was 5 × 10−11 mol/L (as shown in Fig. 6b).
Inner filter effect (IFE)
A fluorescence probe based on primary IFE is an effective strategy in fluorescence spectroscopy [56], [57]. Unlike FRET, IFE does not require intermolecular interactions, thereby enhancing fluorometric detection sensitivity [58]. Au has a high extinction coefficient in the ultraviolet–visible region and a broad absorption band in visible light. The optical properties of AuNPs render them effective absorbers in IFE systems [59].
On this occasion, Xie et al. used graphitized carbon nitride as a fluorescent probe for IFE and combined it with Au to achieve a dual-signal detection for OPs [60]. In the AChE-acetylthiocholine system without pesticides, the generated thiocholine caused aggregation of AuNPs to change the color from red to blue and restore the fluorescence of graphitic carbon nitride. The presence of OPs prevents Au aggregation and produces a fluorescence burst. The detection limit of this method was 6.9 × 10−12 M for chlorpyrifos. Further, Guo and colleagues developed a rapid detection method for methamidophos using the IFE of Au on cadmium telluride quantum dots, with a detection limit of 2 µg/kg [61] (Fig. 6c).
Additionally, Cai et al. immobilized Au nanoclusters (NCs) on the surface of silicon dioxide particles by electrostatic adsorption, and the aggregated AuNCs emit strong fluorescence. Since the excitation spectra of AuNCs overlap with the absorption peaks of manganese dioxide nanosheets, the team added manganese dioxide to the nanocomposite to achieve a fluorescence burst based on the IFE [62]. In the presence of ALP, the decomposition product of sodium 1-ascorbyl-2-phosphate, ascorbic acid, can decompose manganese dioxide and recover the fluorescence. OPs inhibit the activity of ALP, and the method had a LOD of 0.09 µg/L (Fig. 6d).
Other methods
The previous methods modulate fluorescent probes or analytical strategies to improve the sensitivity of OPs detection. In this context, Liang and Han mutated AChE in yeast cells and combined the AChE mutant with protein-directed negative fluorescent AuNCs for fluorescence detection of parathion. The LOD of the method was 3.33 × 10−14 mol/L, which is much lower than other methods [63] (Fig. 6e). Further, Sharma and colleagues used bimetallic nanoclusters of Au and Ag capped with bovine serum albumin to bind copper and cause a fluorescence burst for detecting ethyl parathion [64]. In the AChE-acetylthiocholine reaction system, choline generated from the reaction between AChE and acetylcholine competes with copper, leading to strong fluorescence. Sensitive detection of ethyl parathion using BSA@AuAgNCs was achieved with a LOD value up to 2.40 pmol/L.
As shown in Table 1, this review summarizes the application of the colorimetric and fluorescence detection of OPs using enzyme inhibition methods based on Au nanomaterials.
Table 1.
The colorimetric and fluorescence detection of OPs using enzyme inhibition methods based on Au nanomaterials.
| Methods | Classification | Nanomaterial | Enzyme | Pesticide | Matrix | LOD | Reference |
|---|---|---|---|---|---|---|---|
| Colorimetric detection | Dispersion of Au | AuNPs/LA | AChE | Paraoxon | Apple juice | 4.52 × 104 pmol/L | [31] |
| AuNPs/LA | AChE | Methamidophos | Chinese cabbage | 1.40 ng/mL | [32] | ||
| AuNPs/Cys | AChE | Ethyl parathion | Spiked water | 0.081 μg/L | [34] | ||
| AuNPs/1,4-dimethyl-1H-1,2,3-triazole | AChE | Paraoxon | Apple juice | 10−6–10−4 g/L | [39] | ||
| The particle size of Au | AuNPs | AChE | Parathion | Apple washing solution; tap water; seawater | 0.7 μg/L | [41] | |
| AuNRs | AChE | Parathion | Cabbage washing solution; seawater | 1.2 μg/L | [42] | ||
| Others | AuNPs | AChE | OPs | – | 0.3 nmol/L | [44] | |
| AuNPs@Ag | ALP | Methamidophos | Spiked water | 0.025 μg/L | [45] | ||
| Fluorometric detection | FRET | AuNPs/ Rhodamine B | AChE | Diazinon, malathion phosphate | Tomato; apple; lake water | 0.1 μg/L 0.3 μg/L 1 μg/L |
[47] |
| AuNPs/ CQDs | BChE | Paraoxon | River water | 0.05 μg/L | [51] | ||
| AuNPs/UCNPs | AChE | Parathion | Capsicum; cucumber | 0.67 ng/L | [54] | ||
| Coumarin 1@AuNPs | OPH; EC3.1.8.1 | Paraoxon | – | 0.5 nmol/L | [65] | ||
| IFE | g-C3N4/AuNPs | AChE | Chlorpyrifos | Juice | 6.9 nmol/L | [60] | |
| CdTe /AuNPs | AChE | Methamidophos | Chinese cabbage | 2 μg/kg | [61] | ||
| MnO2-AuNCs-SiO2 | ALP | OPs | Baby cabbage | 0.09 μg/L | [62] | ||
| Others | AuNCs | AChE | Paraoxon | Cucumber juice; tap water; seawater; sewage | 3.33 × 10−2 nmol/L | [63] | |
| BSA@AuAgNCs | AChE | Ethyl parathion | Orange juice; tap water; soil water; apple juice |
2.40 pmol/L | [64] |
* LOD: Limit of detection, LA: lipoic acid, Cys: cysteine, CdTe: cadmium telluride, g-C3N4: graphitized carbon nitride, UCNPs: up-conversion nanoparticles, OPH: organophosphorus hydrolase, ALP: alkaline phosphatase, BSA: bull serum albumin, CQDs: carbon quantum dots.
Electrochemical biosensors
Electrochemical biosensors convert the signal generated by the chemical reaction into a measurable electrical signal to achieve specificity, reliability, and repeatability [6], [66]. Nanomaterials exhibit excellent electron transfer capability, which enhances the electrocatalytic ability of the sensor and accelerates its operating rate [24]. AuNPs can be synthesized as a stable nanomaterial-based sensor and have good quantum size effect, catalytic properties, and biocompatibility [67].
Au nanomaterials
Enzyme biosensors generally immobilize enzymes directly onto the electrode surface. In this context, Albareda-Sirvent and colleagues immobilized AChE directly onto the surface of a copper wire linked carbon paste electrode to detect parathion with a LOD (0.1 nmol/L) [68]. This detector suffers from weak enzyme immobilization and rapid enzyme inactivation. To resolve these defects, Grace et al. electrochemically deposited AuNPs, which in turn can covalently immobilize AChE on a screen-printed carbon electrode to detect OPs with a LOD of 0.6 µg/L [69].
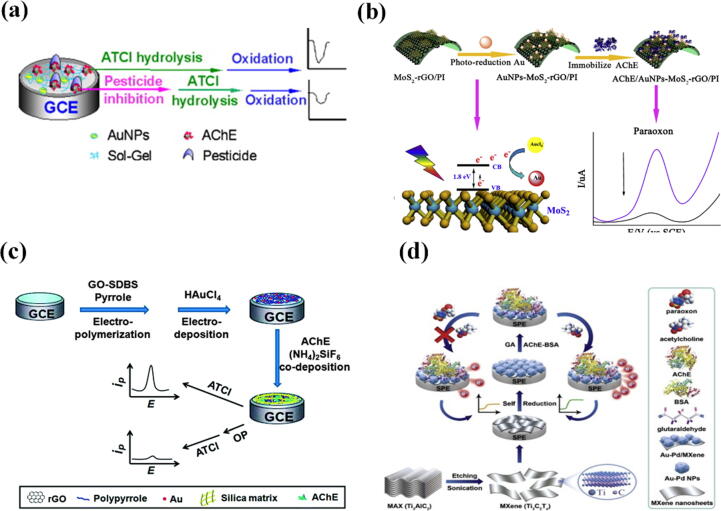
Apart from electrostatically driven adsorption onto the electrode surface, Au can facilitate the transfer of electrons. On this occasion, Du et al. used multi-walled carbon nanotube-coated Au in the sensor, and the composite hydrophilicity could facilitate AChE binding. The electrical conductivity of AuNPs improved the sensitivity of detection, with a LOD of 0.6 ng/mL for malathion [70]. The team developed another biosensor based on AchE that was assembled with AuNPs using a sol–gel-derived silicate network. AuNPs improved the electrochemical response by covalently immobilizing AChE at a lower potential, enabling sensitive detection of OPs. The large number of hydrogen bonds formed between the matrix material and AChE ensures stable bioactivity. The matrix material provides a biocompatible microenvironment and prevents leakage of AChE from the interface [71] (Fig. 7a).
Fig. 7.
(a) Schematic illustration of signaling strategy for OPs using AChE, AuNPs, and a sol–gel-derived silicate network (SiSG). Adapted with permission from Ref [70]. (b) Schematic illustration of signaling strategy for OPs using a flexible film containing AChE, AuNPs, molybdenum disulfide (MoS2), and reduced graphene oxide/polyimide (rGO/PI). Adapted with permission from Ref [74]. (c) Schematic illustration of signaling strategy for OPs using a composite of Au, sulfonated reduced graphene oxide (rGO), and pyrrole. Adapted with permission from Ref [76]. (d) Schematic illustration of signaling strategy for OPs using MXene nanosheets with Au and palladium (Pd) NPs. Adapted with permission from Ref [80].
*Au combined with organic or inorganic materials
Au in enzyme biosensors can be combined with nanomaterials to increase the rate of electron transfer. In this way, Wei and Wang developed an AChE biosensor using a honeycomb hierarchical ionic liquid-AuNP-porous carbon composite with a boron-doped diamond electrode as an immobilization matrix to enhance biosensor performance synergistically and improve the adsorption of AChE [72]. Lettuce leaves samples were analyzed using this biosensor, and the LOD was 0.661 pg/mL. Combining AuNPs with other nanomaterials can also harness other Au properties. For instance, Zhao et al. constructed a platform for AChE by gradually depositing electrochemically reduced graphene oxide, AuNPs, cyclodextrin, and Prussian blue chitosan on a glassy carbon electrode. The graphene oxide and AuNPs enhanced the electrochemical oxidation of thiocholine by synergistically promoting electron transfer between Prussian blue and glassy carbon electrodes [73]. AuNPs enhanced the electrical conductivity of graphene and prevented its agglomeration. The biosensor achieves a LOD of 4.41 pg/mL for malathion.
Moreover, Du et al. deposited AuNPs on a multi-walled carbon nanotube membrane by a multi-electrical stepwise technique using a glassy carbon electrode and methyl parathion-degrading enzyme immobilized on the modified electrode covalently linked to cadmium telluride quantum dots [74]. AuNPs and multi-walled carbon nanotubes significantly increased the surface area. They exhibited a synergistic effect on the enzyme, and the quantum dots were used as carriers for the enzyme with a LOD of 1.0 ng/mL. Methyl parathion-degrading enzyme can hydrolyze pesticides containing a P-S bond. It has a high selectivity for methyl parathion; other pesticides or oxygenated inorganic ions do not interfere with the detection. Additionally, Jia et al. synthesized a flexible membrane biosensor for AChE using an AuNP-molybdenum disulfide-reduced graphene oxide/polyimide electrode [75]. AuNPs were uniformly dispersed on the electrode by strong binding of Au-sulfur bonds. Unlike other groups, the team reduced AuNPs directly on the monolayer membrane under illumination, thus overcoming the tendency of AuNPs to peel off when coated on the electrode surface (Fig. 7b). The LOD of the sensor was 0.0014 µg/mL for paraoxon.
Au can bind to various materials to enhance enzyme binding and increase the binding area. In this way, Dhull et al. deposited a paste of AuNPs and multi-walled carbon nanotubes on an Au electrode. AChE was immobilized on carboxylated single-walled carbon nanotubes, and a Nafion layer was applied to prevent enzyme leaching on the electrode [76]. Moreover, Yang and colleagues co-deposited sulfonated reduced graphene oxide and pyrrole bound to prevent aggregation [77]. AuNPs were then electrodeposited onto the surface, and the nanocomposite provided a large conductivity platform for AChE and exhibiting a strong affinity for thiocholine (Fig. 7c). Under optimal conditions, the inhibition rate was proportional to the concentration of paraoxon in the range of 1.0 nmol/L − 5 µmol/L, with a low limit of detection of 0.5 nmol/L. Further, Chauhan et al. developed AuNPs and calcium carbonate composites to modify Au electrodes on which AChE was immobilized. Citric acid around the AuNPs conferred a negative charge that enhanced the loading of AChE [78].
*Au combined with other metal NPs
Bimetallic NPs are also used in enzyme biosensors. For instance, Upadhyay and colleagues electrodeposited Au-platinum bimetallic NPs onto 3-aminopropyltrioxysilane-modified glassy carbon electrodes; AChE/choline oxidase was immobilized on the modified electrode by cross-linking with glutaraldehyde [79]. The synergism of the NPs exhibited excellent electrocatalytic activity. Similarly, Chen and co-workers used Au and AgNPs to prepare a composite sensor with a LOD of 9.1 pmol/L for methyl parathion [80]. Further, Zhao and colleagues prepared an AChE biosensor platform by combining ultrathin MXene nanosheets with Au-palladium bimetallic NPs and achieved a LOD of 1.75 ng/L for parathion [81] (Fig. 7d).
*Au combined with metal–organic framework (MOFs) derivatives
Au nanomaterials can also be designed with metal–organic framework (MOFs) derivatives to bind other nanomaterials and enhance performance. In this context, Song et al. prepared highly conductive MOFs and deposited them onto glassy carbon electrodes as an AChE-based biosensor with a large surface area, enhanced AChE biocompatibility, and a LOD of 1.34 × 10−13 mol/L for methamidophos [82].
Au combined with other materials
Complex-shaped Au nanomaterials
AuNPs are widely used in electrochemical sensors; however, their fixed specific surface area limits their performance to some extent. Researchers have used other morphologies in enzyme sensors to increase the surface area and electrocatalytic activity to overcome this challenge. On this occasion, Sun and co-workers prepared a highly sensitive AChE biosensor by combining hollow Au nanospheres with chitosan-modified electrodes by electrostatic adsorption and assembling L-cysteine on the nanospheres [83] (Fig. 8a). The nanospheres enable a larger current response and higher selectivity, and their high specific surface area and good biocompatibility facilitate the immobilization of cholinesterase on the electrode surface. The LOD of the sensor was 0.06 µg/L for chlorpyrifos.
Fig. 8.
(a) Transmission electron microscopy of hollow Au nanospheres. Adapted with permission from Ref. [82]. (b) Transmission electron microscopy of coral-like Au nanostructures. Adapted with permission from Ref. [89].
Au nanorods have excellent optical properties [84], [85], [86]. In this way, Cui and colleagues developed an electrochemical acetylcholine esterase biosensor using the high scattering cross-section of Au nanorods and their capacity to form core–shell nanostructures with mesoporous silica [87]. The LOD for dichlorvos and dithiophos were 1.2 and 0.36 µg/L, respectively. Lang and co-workers synthesized an AChE/Au and Ag nanorods/glassy electrode with a LOD of 4.3 nmol/L for paraoxon [89].
More enzyme-binding sites are desirable structural characteristics in Au nanomaterials. For instance, Ju and colleagues synthesized nanocomposites of coral-like Au nanostructures on reduced graphene oxide and immobilized AChE on a glassy carbon electrode [88] (Fig. 8b). The structure enhanced electron transfer efficiency by increasing the AChE attachment point with excellent electrical conductivity. The inhibition of the enzyme by triazophos can be determined, with a LOD of 0.35 μg/L.
The examples above summarize the role and significance of Au nanomaterials in enzyme biosensors. Table 2 summarizes data from the literature on electrochemical enzyme biosensors that use Au to detect Ops.
Table 2.
Detection of OPs using electrochemical biosensors of enzyme inhibition based on Au nanomaterials.
| Materials | Electrode material/immobilization matrix | Pesticides | Matrix | LOD | Linearity | Reference |
|---|---|---|---|---|---|---|
| AuNPs | AChE-Au-MWNTs/GC | Paraoxon | – | 0.1 nmol/L | 0.1–7 nmol/L | [94] |
| AChE-Au/SPCE | Methyl parathion | – | 0.6 μg/L | 0.2–1 μg/L | [90] | |
| Au/VNSWCNTs/AuNPs/AChE | Malathion; Methyl parathion; Chlorpyrifos |
Cabbages | 1.96 × 10−6 μg/L; 3.04 × 10−6 μg/L; 2.06 × 10−6 μg/L |
1.00 × 10−5–1.00 μg/L; 1.00 × 10−5–1.00 μg/L; 1.00 × 10−5–1.00 μg/L | [91] | |
| AChE–Au-SiSG/GCE | Monocrotophos; Methyl parathion |
– | – | – | [71] | |
| Au combined with organic or inorganic materials | AChE-MWCNTs-Au-CHIT/GCE | Malathion | Garlic | 0.6 ng/mL | 1.0–1000 ng/mL; 2–15 μg/mL | [70] |
| AChE-[BSmim]-HSO4 -AuNPs-Porous carbon/BDD | Dichlorvos | Lettuce leaves | 0.661 pg/mL | 4.5 × 10−13–4.5 × 10 −9 mol/L | [72] | |
| CS/AChE/PB-CS/ERGO-AuNPs-β-CD/GCE | Malathion; Carbaryl |
Vegetables | 4.14 pg/mL 1.15 pg/mL | 7.98–2.00 × 103 pg/mL 4.3–1.00 × 103 pg/mL |
[73] | |
| AChE/SPE/AuNPs/MoS 2 | Paraoxon | Apple; pakchoi | 0.013 μg/L | 1.0–1000 μg/L | [95] | |
| MPDE-CdTe/Cys/AuNPs/ MWCNTs/GCE | Methyl parathion | Garlic | 1.0 ng/mL | 5.0 ng/mL–200 ng/mL; 200 ng/mL–1000 ng/mL | [74] | |
| AChE-AuNPs-MoS2-rGO/PI | Paraoxon | Vegetables | 0.0014 μg/mL | 0.005–0.150 μg/mL | [75] | |
| Nafion/AChE-cSWCNT/MWCNT/Au | OPs | – | 0.01 µmol/L | 0.1–130 µmol/L | [76] | |
| Au-PPy-rGO/AChE/(NH4)2SiF6 | Paraoxon-ethyl | Water | 0.5 nmol/L | 1.0 nM–5 μmol/L | [77] | |
| AChE-AuNPs-CaCO3/Au | Malathion; Chlorpyrifos |
River water | 0.1 nmol/L | 0.1–100 nmol/L; 0.1–70 nmol/L |
[78] | |
| AChE-Au-PPy/GCE | Methyl parathion | – | 0.005–0.12 μg /mL 0.5–4.5 μg/mL |
2 ng/mL | [92] | |
| Au combined with metal NPs | AChE/ChOx/Au-PtNPs/3-APTES/GC | Paraoxon ethyl | – | 150 nmol/L | 150–200 nmol/L | [79] |
| GCE/RGO-PDA-AuNPs-AgNPs-AChE-CS | Methyl parathion | River water | 9.1 pmol/L | 0.076–3040 nmol/L | [80] | |
| MXene/Au-Pd | Paraoxon | Pear, Cucumber | 1.75 ng/L | 0.1–1000 μg/L | [81] | |
| AChE/Au-Pd/IL-GR-CHI/GCE | Phorate | Apple juice | 2.5 × 10−16 mol/L | 5.0 × 10−16–2.5 × 10−13 mol/L; 4.9 × 10−13–9.5 × 10−6 mol/L |
[93] | |
| Au combined with MOFs |
AChE-Chit/MXene/AuNPs/ MnO2/Mn3O4/GCE | Methamidophos | Fruit | 1.34 × 10−13 mol/L | 10−12–10−6 mol/L | [82] |
| AChE/AuNCs/GO-CS/SPCE | Chlorpyrifos | – | 3 ng/L | 0.01 μg/L–500 μg/L | [96] | |
| AChE/AuDMBG/RGO/GCE | Triazophos | – | 0.35 μg/L | 0.50–210 μg/L | [89] | |
| AChE/Lcys/HGNs/Chits/GCE | Chlorpyrifos | Cabbage | 0.06 μg/L | 0.1–150 μg/L | [83] | |
| AChE-AuNRs@MS@TiO2-CS | Dichlorvos; Fenthion |
vegetables | 1.2 μg/L; 0.36 μg/L |
0.018 μmol/L–13.6 μmol/L | [87] | |
| AChE/Au@AgNRs/GCE | Paraoxon | River water | 0.7 nmol/L | 5 nmol/L–1 μmol/L | [88] |
*LOD: Limit of detection, SPCE: screen printed carbon working electrode, VNSWCNTs: vertical nitrogen-doped single-walled carbon nanotubes, SiSG: sol–gel-derived silicate network, CHIT/CS: chitosan, [BSmim]-HSO4: honeycomb-like hierarchically ion liquids, BDD: boron-doped diamond, ERGO: electrochemical reduced graphene oxide, β-CD: β-cyclodextrin, MPDE: methyl parathion degrading enzyme, PI: polyimide flexible film, PPy: polypyrrole, ChOx: choline oxidase, 3-APTES: 3-aminopropyltriethoxy silane, PDA: polydopamine, MXene: multi-dimensional nanocomposites, DMBG: dimethylbiguanide, Lcys: L-cysteine, HGNs: hollow gold nanospheres, MS: mesoporous SiO2
Conclusions and perspectives
This review summarized methods for rapidly detecting OPs by introducing enzyme inhibition methods based on Au nanomaterials, including colorimetric, fluorometric, and biosensor detection. Colorimetric and fluorometric detections are completely new classifications from a mechanistic point of view. By screening Au nanomaterials of different shapes, sizes and functionalized modifications on the surface of Au nanomaterials, researchers have further improved the sensitivity and accuracy of detection and reduced the generation of false positives. Electrochemical biosensors are classified in terms of material applications. Researchers combined Au with other metallic nanomaterials to improve detection sensitivity, such as inorganic and organic materials, to obtain better conductive efficiency and catalytic and optical properties. However, natural enzymes still have many problems, such as poor tolerance and stability. Commercially available enzyme inhibition methods based on Au nanomaterials can simultaneously semi-quantitatively detect OPs and carbamate pesticides. However, they cannot achieve quantitative detection of pesticides, and enzyme inhibition methods based on Au nanomaterials require sophisticated pretreatment. The forthcoming enzyme inhibition may be improved as follows. On the one hand, biological and genetic engineering technology can be used to modify the natural enzymes to obtain well-tolerated enzyme sources or use artificial mimic enzymes to avoid false positives in the testing process. On the other hand, existing pre-treatment methods could be modified and/or optimized to recognize the detection of OPs based on enzyme inhibition using Au nanomaterials for pre-treatment methods.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Rongqi Zhai: Investigation, Writing – original draft. Ge Chen: Investigation, Writing – original draft. Guangyang Liu: Investigation, Visualization. Xiaodong Huang: Investigation, Visualization. XiaoMin Xu: Visualization, Supervision. Lingyun Li: Visualization, Supervision. Yanguo Zhang: Visualization, Supervision. Jing Wang: Validation, Writing – review & editing. Maojun Jin: Validation, Writing – review & editing. Donghui Xu: Validation, Writing – review & editing. A.M. Abd El-Aty: Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the Agricultural Science and Technology Innovation Program of CAAS (CAAS-ZDRW202011), Central Public-interest Scientific Institution Basal Research Fund, Chinese Academy of Agricultural Sciences (IVF-BRF2021020), and Supported by the China Agriculture Research System of MOF and MARA (CARS-23-E03), the National Key Research Development Program of China (2020YFD1000300).
Biographies

Rongqi Zhai, is a M.S. candidate at Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS). His research focused on quality safety of agricultural product.

Ge Chen received her M.S. degree major in food science in 2017, and received her Doctor degree major in Quality of Agro-products and Food Safety from institutue of quality standard & testing technology for agro-products, Chinese Academy of Agriculutural Sciences in 2020. She is an assistant researcher of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS). Her research focused on quality safety of agricultural product.

Guangyang Liu received his Doctor degree major in Chemical Engineering and Technology from School of Chemistry and Chemical Engineering, Harbin Institute of Technology in 2016. He is an assistant researcher of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS). His research focused on agricultural nanotechnology R&D and applications.

Xiaodong Huang is an associate professor of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS). His current research is mainly focused on pesticides residue and environmental analysis. He majored in Pesticide Science and holds Ph.D. degree from College of Science, China Agricultural University (CAU).

Xiaomin Xu received her Doctor degree from School of Chemistry and Chemical Engineering, Hunan University in 2014. She is an assistant researcher of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS). Her research focused on quality safety of agricultural product.

Lingyun LI received her M.S. degree from State Key Laboratory of Elemento-organic Chemistry, Nankai University in 2009. She is an assistant researcher of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS).

Yanguo Zhang is an associate researcher of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS). His current research is mainly focused on pesticide residue detection. He received his M.S. degree from the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences (IVF CAAS).

Jing Wang received B.S. degree from Heilongjiang University in 1985. she was working in the Northeast Agriculture University between 1985 and 1996.After obtained her PhD, she was appointed to be an associate professor, professor of the Northeast Agriculture University and of Harbin Institute of Technology from 1996 to 2005. She is currently a professor and director of residues research department of the Institute of Quality Standards & Testing Technology for Agro-products, CAAS. She has engaged in studies of food safety and testing technology/screening novel products with bioactivities.

Maojun Jin received his BSc degree in plant protection in 2004, and received his Doctor degree majored in pesticide science in Zhejiang University in 2009. He is currently the associate professor of Institute of Quality Standards & Testing Technology for Agro-Products, Chinese Academy of Agricultural Sciences. He mainly focuses on the development of immunoassay for the trace detection of pollutants in food. Until now, he published more than 40 papers, 20 of which were cited by SCI.

Donghui Xu received his M.S. degree from School of Horticulture, Northeast Agricultural University in 2004 and received his Doctor degree major in Molecular breeding from the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences in 2007. He is researcher of the Institute of Vegetables and flowers, Chinese Academy of Agricultural Sciences. His research focused on quality safety of agricultural product.

A. M. Abd El-Aty is a Professor of Pharmacology, Cairo University, Egypt and currently (from Nov 2019 to date) appointed as a high-level foreign talent Professor, State Key Laboratory of Biobased Material and Green Papermaking, College of Food Science and Engineering, Qilu University of Technology, Shandong Academy of Science, Jinan and a Foreign Professor at Pharmacology Department, Faculty of Medicine, Ataturk University, Erzurum, Turkey (from Jan 2018 – to date). From 2013 till Jan 2018 he was a Brain Pool fellow in Chonnam National University, Gwangju and a Foreign Professor in Konkuk University, Seoul, Republic of Korea. His era of interest is “Food Science and Technology”; in particular, xenobiotic analysis using various extractions as well as analytical methods. He published > 335 articles in prestigious journals, with current h-index= 33. At the Editorial level, he is the EIC of Lipids in Health and Disease, was the Managing Editor for Journal of Advanced Research for the last 10 years and currently one the journal Associate Editor; Academic Editor – PLOS ONE; Associate Editor – Frontiers in Nutrition – Food Chemistry Section; Advisory board member of Biomedical Chromatography and Separation Science Plus. He is also a member of 2017 – 2021 Expert Roster of the Joint (FAO/WHO) Expert Committee on Food Additives.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Ge Chen, Email: chenge@caas.cn.
Donghui Xu, Email: xudonghui@caas.cn.
A.M. Abd El-Aty, Email: abdelaty44@hotmail.com.
References
- 1.Kumar S., Kaushik G., Villarreal-Chiu J.F. Scenario of organophosphate pollution and toxicity in India: a review. Environ Sci Pollut Res Int. 2016;23:9480–9491. doi: 10.1007/s11356-016-6294-0. http://doi:10.1007/s11356-016-6294-0 [DOI] [PubMed] [Google Scholar]
- 2.Wee S.Y., Omar T.F.T., Aris A.Z., Lee Y. Surface water organophosphorus pesticides concentration and distribution in the Langat River, Selangor, Malaysia. Exposure Health. 2016;8:497–511. http://doi:10.1007/s12403-016-0214-x [Google Scholar]
- 3.Pundir C.S., Chauhan N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: a review. Anal Biochem. 2012;429:19–31. doi: 10.1016/j.ab.2012.06.025. http://doi:10.1016/j.ab.2012.06.025 [DOI] [PubMed] [Google Scholar]
- 4.Amir A., Raza A., Qureshi T., Mahesar G.B., Jafferi S., Haleem F., et al. Organophosphate poisoning: demographics severity scores and outcomes from national poisoning control Centre, Karachi. Cureus. 2020;12 doi: 10.7759/cureus.8371. http://doi:10.7759/cureus.8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y., Fang J., Wang B., Zhang F., Shao G., Liu Y. A signal-on detection of organophosphorus pesticides by fluorescent probe based on aggregation-induced emission. Sensors Actuators B: Chem. 2019;292:156–163. http://doi:10.1016/j.snb.2019.04.123 [Google Scholar]
- 6.Sidhu G.K., Singh S., Kumar V., Dhanjal D.S., Datta S., Singh J. Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit Rev Environ Sci Technol. 2019;49:1135–1187. http://doi:10.1080/10643389.2019.1565554 [Google Scholar]
- 7.Van D., Zoonen P.V. Trace analysis of pesticides by gas chromatography. J Chromatogr A. 1999;843:301. doi: 10.1016/s0021-9673(99)00511-7. [DOI] [PubMed] [Google Scholar]
- 8.Mitobe H., Ibaraki T., Tanabe A., Kawata K., Yasuhara A. High performance liquid chromatographic determination of pesticides in soluble phase and suspended phase in river water. Toxicol Environ Chem. 2001;81:97–110. http://doi:10.1080/02772240109359023 [Google Scholar]
- 9.Hernandez F., Sancho J.V., Pozo O., Lara A., Pitarch E. Rapid direct determination of pesticides and metabolites in environmental water samples at sub-microg/l level by on-line solid-phase extraction-liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2001;939:1–11. doi: 10.1016/s0021-9673(01)01334-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Duan H.L., Fan L., Lin Y.M., Zhang Z.Q. Magnetic tetraethylenepentamine modified multi-walled carbon nanotubes as matrix clean-up materials for organophosphorus pesticide residue analysis in cucumber. Food Control. 2021;124 [Google Scholar]
- 11.Acosta-Dacal A., Rial-Berriel C., Díaz-Díaz R., Suárez M., Luzardo O.P. Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci Total Environ. 2020;753 doi: 10.1016/j.scitotenv.2020.142015. [DOI] [PubMed] [Google Scholar]
- 12.Pawar U.D., Pawar C.D., Kulkarni U.K., Pardeshi R.K. Development method of high-performance thin-layer chromatographic detection of synthetic organophosphate insecticide profenofos in visceral samples. JPC – J Planar Chromatogr – Modern TLC. 2020;33:203–206. http://doi:10.1007/s00764-020-00015-2 [Google Scholar]
- 13.Sanganalmath P.U., Nagaraju P.M., Sreeramulu K. Determination of quinalphos in human whole blood samples by high-performance thin-layer chromatography for forensic applications. J Chromatogr A. 2019;1594:181–189. doi: 10.1016/j.chroma.2019.02.003. http://doi:10.1016/j.chroma.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 14.Cao J., Wang M., Yu H.e., She Y., Cao Z., Ye J., et al. An overview on the mechanisms and applications of enzyme inhibition-based methods for determination of organophosphate and carbamate pesticides. J Agric Food Chem. 2020;68(28):7298–7315. doi: 10.1021/acs.jafc.0c01962. [DOI] [PubMed] [Google Scholar]
- 15.Rhee I.K., van Rijn R.M., Verpoorte R. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem Anal. 2003;14:127–131. doi: 10.1002/pca.675. http://doi:10.1002/pca.675 [DOI] [PubMed] [Google Scholar]
- 16.Windmiller J.R., Wang J. Wearable electrochemical sensors and biosensors: a review. Electroanalysis. 2013;25:29–46. http://doi:10.1002/elan.201200349 [Google Scholar]
- 17.Sharma H., Mutharasan R. Review of biosensors for foodborne pathogens and toxins. Sens Actuators B: Chem. 2013;183:535–549. http://doi:10.1016/j.snb.2013.03.137 [Google Scholar]
- 18.Turkevich J., Stevenson P.C., Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11 [Google Scholar]
- 19.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 20.Guilbault G.G., Kramer D.N. Fluorimetric system employing immobilized cholinesterase for assaying anticholinesterase compounds. Anal Chem. 1965;37:1675–1680. doi: 10.1021/ac60232a011. [DOI] [PubMed] [Google Scholar]
- 21.Bernabei M., Cremisini C., Mascini M., Palleschi G. Determination of organophosphorus and carbamic pesticides with a choline and acetylcholine electrochemical biosensor. Anal Lett. 1991;24(8):1317–1331. [Google Scholar]
- 22.Willner I, Xiao Y, Pavlov V. Inhibition of the acetycholine esterase-stimulated growth of Au nanoparticles: Nanotechnology-based sensing of nerve gases. [DOI] [PubMed]
- 23.Dan D., Chen S., Jie C., Zhang A. Immobilization of acetylcholinesterase on gold nanoparticles embedded in sol-gel film for amperometric detection of organophosphorous insecticide. Biosens Bioelectron. 2008;23:130–134. doi: 10.1016/j.bios.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Dang X., Hu H., Wang S., Hu S. Nanomaterials-based electrochemical sensors for nitric oxide. Microchim Acta. 2014;182:455–467. http://doi:10.1007/s00604-014-1325-3 [Google Scholar]
- 25.Prashant K., Jain X., Huang I., et al. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008 doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 26.Katz E., Willner I. Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew Chem Int Ed Engl. 2004;43(45):6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 27.Li N., Zhao P., Astruc D. Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angew Chem Int Ed Engl. 2014;53:1756–1789. doi: 10.1002/anie.201300441. http://doi:10.1002/anie.201300441 [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Wei Q., Wu C., Hu Z., Ji J., Wang P. The Escherichia coli O157:H7 DNA detection on a gold nanoparticle-enhanced piezoelectric biosensor. Science Bulletin. 2008;53:1175–1184. http://doi:10.1007/s11434-007-0529-x [Google Scholar]
- 29.Pingarrón J.M., Yáñez-Sedeño P., González-Cortés A. Gold nanoparticle-based electrochemical biosensors. Electrochim Acta. 2008;53:5848–5866. http://doi:10.1016/j.electacta.2008.03.005 [Google Scholar]
- 30.Zhou F., Yuan L., Wang H., Li D., Chen H. Gold nanoparticle layer: a promising platform for ultra-sensitive cancer detection. Langmuir. 2011;27:2155–2158. doi: 10.1021/la1049937. http://doi:10.1021/la1049937 [DOI] [PubMed] [Google Scholar]
- 31.Sun J., Guo L., Bao Y., Xie J. A simple, label-free AuNPs-based colorimetric ultrasensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosens Bioelectron. 2011;28:152–157. doi: 10.1016/j.bios.2011.07.012. http://doi:10.1016/j.bios.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 32.Li H., Guo J., Ping H., Liu L., Zhang M., Guan F., et al. Visual detection of organophosphorus pesticides represented by mathamidophos using Au nanoparticles as colorimetric probe. Talanta. 2011;87:93–99. doi: 10.1016/j.talanta.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 33.Giljohann D.A., Seferos D.S., Daniel W.L., Massich M.D., Patel P.C., Mirkin C.A. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49:3280–3294. doi: 10.1002/anie.200904359. http://doi:10.1002/anie.200904359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bala R., Sharma R.K., Wangoo N. Highly sensitive colorimetric detection of ethyl parathion using gold nanoprobes. Sensors Actuators B: Chem. 2015;210:425–430. http://doi:10.1016/j.snb.2014.12.123 [Google Scholar]
- 35.Aryal S., B K.C.R., Dharmaraj N., Bhattarai N., Kim C.H., Kim H.Y. Spectroscopic identification of S-Au interaction in cysteine capped gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63(1):160–163. doi: 10.1016/j.saa.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 36.Mocanu A., Cernica I., Tomoaia G., Bobos L.-D., Horovitz O., Tomoaia-Cotisel M. Self-assembly characteristics of gold nanoparticles in the presence of cysteine. Colloids Surf Physicochem Eng Aspects. 2009;338:93–101. http://doi:10.1016/j.colsurfa.2008.12.041 [Google Scholar]
- 37.Lee S.B., Martin C.R. pH-switchable, ion-permselective gold nanotubule membrane based on chemisorbed cysteine. Anal Chem. 2001;73(4):768–775. doi: 10.1021/ac0008901. [DOI] [PubMed] [Google Scholar]
- 38.Pavlov V., Xiao Y., Willner I. Inhibition of the acetycholine esterase-stimulated growth of Au nanoparticles: nanotechnology-based sensing of nerve gases. Nano Lett. 2005;5:649–653. doi: 10.1021/nl050054c. [DOI] [PubMed] [Google Scholar]
- 39.Fu G., Chen W., Yue X., Jiang X. Highly sensitive colorimetric detection of organophosphate pesticides using copper catalyzed click chemistry. Talanta. 2013;103:110–115. doi: 10.1016/j.talanta.2012.10.016. http://doi:10.1016/j.talanta.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 40.Guo L., Xu Y., Ferhan A.R., Chen G., Kim D.H. Oriented gold nanoparticle aggregation for colorimetric sensors with surprisingly high analytical figures of merit. J Am Chem Soc. 2013;135:12338–12345. doi: 10.1021/ja405371g. http://doi:10.1021/ja405371g [DOI] [PubMed] [Google Scholar]
- 41.Wu S., Li D., Wang J., Zhao Y., Dong S., Wang X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sensors Actuators B: Chem. 2017;238:427–433. http://doi:10.1016/j.snb.2016.07.067 [Google Scholar]
- 42.Wu S., Li D., Gao Z., Wang J. Controlled etching of gold nanorods by the Au(III)-CTAB complex, and its application to semi-quantitative visual determination of organophosphorus pesticides. Microchim Acta. 2017;184:4383–4391. http://doi:10.1007/s00604-017-2468-9 [Google Scholar]
- 43.Qing Zhihe, Li Yacheng, Li Younan, Luo Guoyan, Hu Jinlei, Zou Zhen, et al. Thiol-suppressed I2-etching of AuNRs: acetylcholinesterase-mediated colorimetric detection of organophosphorus pesticides. Mikrochim Acta. 2020;187(9) doi: 10.1007/s00604-020-04486-2. [DOI] [PubMed] [Google Scholar]
- 44.Liao Shuzhen, Qiao Yanan, Han Wenting, Xie Zhaoxia, Wu Zhaoyang, Shen Guoli, et al. Acetylcholinesterase liquid crystal biosensor based on modulated growth of gold nanoparticles for amplified detection of acetylcholine and inhibitor. Anal Chem. 2012;84(1):45–49. doi: 10.1021/ac202895j. [DOI] [PubMed] [Google Scholar]
- 45.Lv B., Wei M., Liu Y., Liu X., Wei W., Liu S. Ultrasensitive photometric and visual determination of organophosphorus pesticides based on the inhibition of enzyme-triggered formation of core-shell gold-silver nanoparticles. Microchim Acta. 2016;183:2941–2948. http://doi:10.1007/s00604-016-1939-8 [Google Scholar]
- 46.Rhee In Kyung, Appels Natalie, Luijendijk Teus, Irth Hubertus, Verpoorte Robert. Determining acetylcholinesterase inhibitory activity in plant extracts using a fluorimetric flow assay. Phytochem Anal. 2003;14(3):145–149. doi: 10.1002/pca.695. [DOI] [PubMed] [Google Scholar]
- 47.Luo Q.-J., Li Y.-X., Zhang M.-Q., Qiu P., Deng Y.-H. A highly sensitive, dual-signal assay based on rhodamine B covered silver nanoparticles for carbamate pesticides. Chin Chem Lett. 2017;28:345–349. http://doi:10.1016/j.cclet.2016.10.024 [Google Scholar]
- 48.Sapsford Kim E., Berti Lorenzo, Medintz Igor L. Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations. Angew Chem Int Ed Engl. 2006;45(28):4562–4589. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]
- 49.Moores A., Goettmann F.d.r. The plasmon band in noble metal nanoparticles: an introduction to theory and applications. New J Chem. 2006;30 http://doi:10.1039/b604038c [Google Scholar]
- 50.Liu D., Chen W., Wei J., Li X., Wang Z., Jiang X. A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal Chem. 2012;84:4185–4191. doi: 10.1021/ac300545p. http://doi:10.1021/ac300545p [DOI] [PubMed] [Google Scholar]
- 51.Wu X., Song Y., Yan X., Zhu C., Ma Y., Du D., et al. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens Bioelectron. 2017;94:292–297. doi: 10.1016/j.bios.2017.03.010. http://doi:10.1016/j.bios.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 52.Auzel F. Upconversion and anti-Stokes processes with f and d ions in solids. Cheminform. 2004;35:139. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 53.Liu C., Wang Z., Jia H., Li Z. Efficient fluorescence resonance energy transfer between upconversion nanophosphors and graphene oxide: a highly sensitive biosensing platform. Chem Commun (Camb) 2011;47:4661–4663. doi: 10.1039/c1cc10597c. http://doi:10.1039/c1cc10597c [DOI] [PubMed] [Google Scholar]
- 54.Long Q., Li H., Zhang Y., Yao S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens Bioelectron. 2015;68:168–174. doi: 10.1016/j.bios.2014.12.046. http://doi:10.1016/j.bios.2014.12.046 [DOI] [PubMed] [Google Scholar]
- 55.Kamelipour Nahid, Mohsenifar Afshin, Tabatabaei Meisam, Rahmani-Cherati Tavoos, Khoshnevisan Kamyar, Allameh Abdolamir, et al. Fluorometric determination of paraoxon in human serum using a gold nanoparticle-immobilized organophosphorus hydrolase and coumarin 1 as a competitive inhibitor. Microchim Acta. 2014;181(1-2):239–248. [Google Scholar]
- 56.Zhai Y., Jin L., Wang P., Dong S. Dual-functional Au-Fe3O4 dumbbell nanoparticles for sensitive and selective turn-on fluorescent detection of cyanide based on the inner filter effect. Chem Commun (Camb) 2011;47:8268–8270. doi: 10.1039/c1cc13149d. http://doi:10.1039/c1cc13149d [DOI] [PubMed] [Google Scholar]
- 57.Yan X., Li H., Li Y., Su X. Visual and fluorescent detection of acetamiprid based on the inner filter effect of gold nanoparticles on ratiometric fluorescence quantum dots. Anal Chim Acta. 2014;852:189–195. doi: 10.1016/j.aca.2014.09.008. http://doi:10.1016/j.aca.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 58.Zheng Min, Xie Zhigang, Qu Dan, Li Di, Du Peng, Jing Xiabin, et al. On-off-on fluorescent carbon dot nanosensor for recognition of chromium(VI) and ascorbic acid based on the inner filter effect. ACS Appl Mater Interfaces. 2013;5(24):13242–13247. doi: 10.1021/am4042355. [DOI] [PubMed] [Google Scholar]
- 59.Shang Li, Dong Shaojun. Design of fluorescent assays for cyanide and hydrogen peroxide based on the inner filter effect of metal nanoparticles. Anal Chem. 2009;81(4):1465–1470. doi: 10.1021/ac802281x. [DOI] [PubMed] [Google Scholar]
- 60.Xie H., Bei F., Hou J., Ai S. A highly sensitive dual-signaling assay via inner filter effect between g-C3N4 and gold nanoparticles for organophosphorus pesticides. Sensors Actuators B: Chem. 2018;255:2232–2239. http://doi:10.1016/j.snb.2017.09.024 [Google Scholar]
- 61.Guo Jiajia, Li Hongkun, Xue Meng, Zhang Minwei, Cao Xianyi, Luo Yeli, et al. Highly sensitive detection of organophosphorus pesticides represented by methamidophos via inner filter effect of au nanoparticles on the fluorescence of CdTe quantum dots. Food Anal Meth. 2014;7(6):1247–1255. [Google Scholar]
- 62.Cai Yue, Qiu Ziyin, Lin Xubin, Zeng Wei, Cao Yiran, Liu Weipeng, et al. Self-assembled nanomaterials based on aggregation-induced emission of AuNCs: fluorescence and colorimetric dual-mode biosensing of organophosphorus pesticides. Sensors Actuators B: Chem. 2020;321:128481. doi: 10.1016/j.snb.2020.128481. [DOI] [Google Scholar]
- 63.Liang B., Han L. Displaying of acetylcholinesterase mutants on surface of yeast for ultra-trace fluorescence detection of organophosphate pesticides with gold nanoclusters. Biosens Bioelectron. 2020;148 doi: 10.1016/j.bios.2019.111825. http://doi:10.1016/j.bios.2019.111825 [DOI] [PubMed] [Google Scholar]
- 64.Sharma D., Wangoo N., Sharma R.K. Sensing platform for pico-molar level detection of ethyl parathion using Au-Ag nanoclusters based enzymatic strategy. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121267. http://doi:10.1016/j.talanta.2020.121267 [DOI] [PubMed] [Google Scholar]
- 65.Karami R., Mohsenifar A., Mesbah Namini S.M., Kamelipour N., Rahmani-Cherati T., Roodbar Shojaei T., et al. A novel nanobiosensor for the detection of paraoxon using chitosan-embedded organophosphorus hydrolase immobilized on Au nanoparticles. Prep Biochem Biotechnol. 2016;46:559–566. doi: 10.1080/10826068.2015.1084930. http://doi:10.1080/10826068.2015.1084930 [DOI] [PubMed] [Google Scholar]
- 66.Pundir C.S., Malik Ashish, Preety Bio-sensing of organophosphorus pesticides: a review. Biosens Bioelectron. 2019;140:111348. doi: 10.1016/j.bios.2019.111348. [DOI] [PubMed] [Google Scholar]
- 67.Daniel M.C., Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004 doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 68.Albareda-Sirvent Miquel, Merkoçi Arben, Alegret Salvador. Pesticide determination in tap water and juice samples using disposable amperometric biosensors made using thick-film technology. Anal Chim Acta. 2001;442(1):35–44. [Google Scholar]
- 69.Grace Rini, Sundar Keerthana, Mohan N., Selvakumar D., Kumar N.S. Fabrication of an enzymatic biosensor based on gold nanoparticles modified electrochemical transducer for the detection of organophosphorus compounds. Nano Hybrids Compos. 2017;12:67–73. [Google Scholar]
- 70.Du D., Wang M., Cai J., Qin Y., Zhang A. One-step synthesis of multiwalled carbon nanotubes-gold nanocomposites for fabricating amperometric acetylcholinesterase biosensor. Sensors Actuators B: Chem. 2010;143:524–529. http://doi:10.1016/j.snb.2009.09.051 [Google Scholar]
- 71.Du D., Chen S., Cai J., Zhang A. Electrochemical pesticide sensitivity test using acetylcholinesterase biosensor based on colloidal gold nanoparticle modified sol-gel interface. Talanta. 2008;74:766–772. doi: 10.1016/j.talanta.2007.07.014. http://doi:10.1016/j.talanta.2007.07.014 [DOI] [PubMed] [Google Scholar]
- 72.Wei M., Wang J. A novel acetylcholinesterase biosensor based on ionic liquids-AuNPs-porous carbon composite matrix for detection of organophosphate pesticides. Sensors Actuators B: Chem. 2015;211:290–296. http://doi:10.1016/j.snb.2015.01.112 [Google Scholar]
- 73.Zhao H., Ji X., Wang B., Wang N., Li X., Ni R., et al. An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-Au nanoparticles-beta-cyclodextrin/Prussian blue-chitosan nanocomposites for organophosphorus pesticides detection. Biosens Bioelectron. 2015;65:23–30. doi: 10.1016/j.bios.2014.10.007. http://doi:10.1016/j.bios.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 74.Du D., Chen W., Zhang W., Liu D., Li H., Lin Y. Covalent coupling of organophosphorus hydrolase loaded quantum dots to carbon nanotube/Au nanocomposite for enhanced detection of methyl parathion. Biosens Bioelectron. 2010;25:1370–1375. doi: 10.1016/j.bios.2009.10.032. http://doi:10.1016/j.bios.2009.10.032 [DOI] [PubMed] [Google Scholar]
- 75.Jia L., Zhou Y., Wu K., Feng Q., Wang C., He P. Acetylcholinesterase modified AuNPs-MoS2-rGO/PI flexible film biosensor: towards efficient fabrication and application in paraoxon detection. Bioelectrochemistry. 2020;131 doi: 10.1016/j.bioelechem.2019.107392. http://doi:10.1016/j.bioelechem.2019.107392 [DOI] [PubMed] [Google Scholar]
- 76.Dhull V. A Nafion/AChE-cSWCNT/MWCNT/Au-based amperometric biosensor for the determination of organophosphorous compounds. Environ Technol. 2020;41:566–576. doi: 10.1080/09593330.2018.1505964. http://doi:10.1080/09593330.2018.1505964 [DOI] [PubMed] [Google Scholar]
- 77.Yang Y., Asiri A.M., Du D., Lin Y. Acetylcholinesterase biosensor based on a gold nanoparticle-polypyrrole-reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst. 2014;139:3055–3060. doi: 10.1039/c4an00068d. http://doi:10.1039/c4an00068d [DOI] [PubMed] [Google Scholar]
- 78.Chauhan N., Narang J., Pundir C.S. Immobilization of rat brain acetylcholinesterase on porous gold-nanoparticle–CaCO3 hybrid material modified Au electrode for detection of organophosphorous insecticides. Int J Biol Macromol. 2011;49:923–929. doi: 10.1016/j.ijbiomac.2011.08.006. http://doi:10.1016/j.ijbiomac.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 79.Upadhyay S., Rao G.R., Sharma M.K., Bhattacharya B.K., Rao V.K., Vijayaraghavan R. Immobilization of acetylcholineesterase-choline oxidase on a gold-platinum bimetallic nanoparticles modified glassy carbon electrode for the sensitive detection of organophosphate pesticides, carbamates and nerve agents. Biosens Bioelectron. 2009;25:832–838. doi: 10.1016/j.bios.2009.08.036. http://doi:10.1016/j.bios.2009.08.036 [DOI] [PubMed] [Google Scholar]
- 80.Chen X., Wang J., Liu Z., Li Y., Huang J., Tao C.-A. One pot fabrication of graphene-bimetallic nanoparticles-based acetylcholinesterase electrochemical biosensor with ultralow detection limit toward methyl parathion. Mater Res Express. 2019;6 http://doi:10.1088/2053-1591/ab3e84 [Google Scholar]
- 81.Zhao Fengnian, Yao Yao, Jiang Chengmei, Shao Yuzhou, Barceló Damià, Ying Yibin, et al. Self-reduction bimetallic nanoparticles on ultrathin MXene nanosheets as functional platform for pesticide sensing. J Hazard Mater. 2020;384:121358. doi: 10.1016/j.jhazmat.2019.121358. [DOI] [PubMed] [Google Scholar]
- 82.Song Dandan, Jiang Xinyu, Li Yanshan, Lu Xiong, Luan Sunrui, Wang Yuanzhe, et al. Metal-organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection. J Hazard Mater. 2019;373:367–376. doi: 10.1016/j.jhazmat.2019.03.083. [DOI] [PubMed] [Google Scholar]
- 83.Sun X., Zhai C., Wang X. A novel and highly sensitive acetyl-cholinesterase biosensor modified with hollow gold nanospheres. Bioprocess Biosyst Eng. 2013;36:273–283. doi: 10.1007/s00449-012-0782-5. http://doi:10.1007/s00449-012-0782-5 [DOI] [PubMed] [Google Scholar]
- 84.Chang Y.T., Liao P.Y., Sheu H.S., Tseng Y.J., Cheng F.Y., Yeh C.S. Near-infrared light-responsive intracellular drug and siRNA release using au nanoensembles with oligonucleotide-capped silica shell. Adv Mater. 2012;24:3309–3314. doi: 10.1002/adma.201200785. http://doi:10.1002/adma.201200785 [DOI] [PubMed] [Google Scholar]
- 85.Choi W.I., Sahu A., Kim Y.H., Tae G. Photothermal cancer therapy and imaging based on gold nanorods. Ann Biomed Eng. 2012;40:534–546. doi: 10.1007/s10439-011-0388-0. http://doi:10.1007/s10439-011-0388-0 [DOI] [PubMed] [Google Scholar]
- 86.Wang H., Huff T.B., Zweifel D.A., He W., Low P.S., Wei A., et al. In vitro and in vivo two-photon luminescence imaging of single gold nanorods. Proc Natl Acad Sci. 2005 doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui H.F., Zhang T.T., Lv Q.Y., Song X., Zhai X.J., Wang G.G. An acetylcholinesterase biosensor based on doping Au nanorod@SiO2 nanoparticles into TiO2-chitosan hydrogel for detection of organophosphate pesticides. Biosens Bioelectron. 2019;141 doi: 10.1016/j.bios.2019.111452. http://doi:10.1016/j.bios.2019.111452 [DOI] [PubMed] [Google Scholar]
- 88.Lang Q., Han L., Hou C., Wang F., Liu A. A sensitive acetylcholinesterase biosensor based on gold nanorods modified electrode for detection of organophosphate pesticide. Talanta. 2016;156–157:34–41. doi: 10.1016/j.talanta.2016.05.002. http://doi:10.1016/j.talanta.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 89.Ju Ke-Jian, Feng Jin-Xia, Feng Jiu-Ju, Zhang Qian-Li, Xu Tian-Qi, Wei Jie, et al. Biosensor for pesticide triazophos based on its inhibition of acetylcholinesterase and using a glassy carbon electrode modified with coral-like gold nanostructures supported on reduced graphene oxide. Microchim Acta. 2015;182(15-16):2427–2434. [Google Scholar]
- 90.Grace R., Sundar K., Mohan N., Selvakumar D., Kumar N.S. Fabrication of an enzymatic biosensor based on gold nanoparticles modified electrochemical transducer for the detection of organophosphorus compounds. Nano Hybrids Compos. 2016;12:67–73. http://doi:10.4028/www.scientific.net/NHC.12.67 [Google Scholar]
- 91.Xu M., Jiang S., Jiang B., Zheng J. Organophosphorus pesticides detection using acetylcholinesterase biosensor based on gold nanoparticles constructed by electroless plating on vertical nitrogen-doped single-walled carbon nanotubes. Int J Environ Anal Chem. 2019;99:913–927. http://doi:10.1080/03067319.2019.1616714 [Google Scholar]
- 92.Gong J., Wang L., Zhang L. Electrochemical biosensing of methyl parathion pesticide based on acetylcholinesterase immobilized onto Au-polypyrrole interlaced network-like nanocomposite. Biosens Bioelectron. 2009;24:2285–2288. doi: 10.1016/j.bios.2008.11.012. http://doi:10.1016/j.bios.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 93.Zhan H., Li J., Liu Z., Zheng Y., Jing Y. A highly sensitive electrochemical OP biosensor based on electrodeposition of Au–Pd bimetallic nanoparticles onto a functionalized graphene modified glassy carbon electrode. Anal Methods. 2015;7:3903–3911. http://doi:10.1039/c5ay00702j [Google Scholar]
- 94.Jha N., Ramaprabhu S. Development of Au nanoparticles dispersed carbon nanotube-based biosensor for the detection of paraoxon. Nanoscale. 2010;2:806–810. doi: 10.1039/b9nr00336c. http://doi:10.1039/b9nr00336c [DOI] [PubMed] [Google Scholar]
- 95.Zhao F., Yao Y., Li X., Lan L., Jiang C., Ping J. Metallic transition metal dichalcogenide nanosheets as an effective and biocompatible transducer for electrochemical detection of pesticide. Anal Chem. 2018;90:11658–11664. doi: 10.1021/acs.analchem.8b03250. http://doi:10.1021/acs.analchem.8b03250 [DOI] [PubMed] [Google Scholar]
- 96.Yao Y., Wang G., Chu G., An X., Guo Y., Sun X. The development of a novel biosensor based on gold nanocages/graphene oxide–chitosan modified acetylcholinesterase for organophosphorus pesticide detection. New J Chem. 2019;43:13816–13826. http://doi:10.1039/c9nj02556a [Google Scholar]