Summary
The extracellular matrix (ECM) provides essential cues to promote endothelial specification during tissue development in vivo; correspondingly, ECM is considered essential for endothelial differentiation outside of the body. However, systematic studies to assess the precise contribution of individual ECM proteins to endothelial differentiation have not been conducted. Further, the multi-component nature of differentiation protocols makes it challenging to study the underlying mechanisms by which the ECM contributes to cell fate. In this study, we determined that Laminin 411 alone increases endothelial differentiation of induced pluripotent stem cells over collagen I or Matrigel. The effect of ECM was shown to be independent of vascular endothelial growth factor (VEGF) binding capacity. We also show that ECM-guided endothelial differentiation is dependent on activation of focal adhesion kinase (FAK), integrin-linked kinase (ILK), Notch, and β-catenin pathways. Our results indicate that ECM contributes to endothelial differentiation through multiple avenues, which converge at the expression of active β-catenin.
Keywords: stem cells, endothelial cells, differentiation, cell signaling, signal transduction, extracellular matrix, laminin, vascular, focal adhesion, beta catenin
Graphical abstract
Highlights
-
•
Laminin 411 induces endothelial differentiation in human and mouse iPSCs
-
•
FAK, ILK, and Notch act downstream of laminin 411-induced endothelial differentiation
-
•
VEGF absorption by ECM alone does not induce endothelial differentiation
-
•
β-catenin signaling is necessary for ECM-induced endothelial differentiation
In this article, Ogle and colleagues demonstrate that the ECM protein laminin 411 induces a significant level of endothelial differentiation in both human and mouse iPSCs. Laminin 411-induced differentiation is dependent on focal adhesion kinase, integrin-linked kinase, Notch, and β-catenin, but not on differences in VEGF absorption into the ECM substrate.
Introduction
The extracellular matrix (ECM) plays a vital role in tissue development, providing structural support and cell adhesion domains, transducing mechanical cues, and sequestering soluble factors. Knockouts of many major ECM proteins are lethal in the embryonic or neonatal period, leading to disorganized and poorly functional tissues (Sugi and Markwald, 1996; Yang et al., 1999; Alpy et al., 2005; Majesky, 2018), which indicates an essential role for ECM in the organization, specification, and maturation of tissues in vivo. This, combined with changes in ECM composition with development (Brown, 2011; Jung et al., 2012; Rienks et al., 2014; Hortells et al., 2019), has led many groups to use decellularized tissue ECM to guide stem cell specification (Duan et al., 2011; Narayanan et al., 2014; Dzobo et al., 2015; Hoshiba et al., 2016). However, batch-to-batch variability is common with decellularized tissues, and they contain a large number of proteins, often in small quantities, which makes it difficult to obtain a thorough understanding of the role of ECM proteins in cell behavior. A number of studies have approached this problem from the opposing direction, utilizing individual ECM components, alone or in combination, to direct cell behavior (Martino et al., 2009; Jung et al., 2012, 2015, 2016). In this case, knowledge of the fundamental ECM components and relative abundance in native tissues informs the approach.
For endothelial cells, a special type of ECM sheet called the basement membrane supports cell health and function. Basement membrane is composed primarily of type IV collagen and laminins. Laminins are T-shaped heterotrimers composed of α, β, and γ chains, and are named based on the chains they contain. For example, laminin 521 is composed of the α5, β2, and γ1 chains. Laminin 111 is the most studied laminin due to its presence in the tumor-derived ECM Matrigel (Hansen et al., 2009; Hohenester, 2019). However, its expression in adult tissues is limited and it is not expressed in the vascular basement membrane (Wang et al., 2006b), which is instead composed primarily of laminins 411 and 511 (Thyboll et al., 2002). During development, laminin 411 is the most prevalent. Laminin α4-deficient mice typically survive until birth, although internal bleeding is present in all mice by the neonatal period and is associated with disorganized and discontinuous vascular basement membranes and reduced expression of type IV collagen (Col IV) and nidogen (Thyboll et al., 2002). The phenotype is likely non-embryonic lethal due to compensation from the laminin α5 chain, which is upregulated in the capillaries of laminin α4-deficient mice (Wang et al., 2006b). While laminin 411 is expressed in all vessels, beginning in development, laminin 511 begins to be expressed postnatally, predominantly in capillaries and venules (Hallmann et al., 2005; Wang et al., 2006b). Laminin α5-null mice die during embryogenesis, before it is expressed in blood vessels. However, cell-type-specific knockdowns have revealed essential roles in endothelial barrier properties and shear stress response (Di Russo et al., 2017; Song et al., 2017). These roles in mechanical transduction and barrier function as well as postnatal expression indicate a role for laminin 511 in the maturation of the endothelium.
That laminins 411 and 511 are essential for vessel formation and function has been established. Less well understood are the mechanisms by which laminins 411 and 511 promote endothelial specification and function. In vivo, endothelial cells are derived from mesoderm. A number of studies have shown that modulation of the Wnt β-catenin pathway produces an Flk-1+ mesodermal population, which mirrors the formation of mesoderm in vivo. Studies in vivo and in embryonic stem cells (ESCs) determined that Wnt and vascular endothelial growth factor(VEGF) were required for induction of an endothelial lineage from mesoderm (Carmeliet et al., 1996; Nostro et al., 2008). In human induced pluripotent stem cells (hiPSCs), activation of Wnt past the induction of mesoderm is sufficient to induce endothelial differentiation in approximately 20% of cells independent of exogenous VEGF (Lian et al., 2014; Bao et al., 2015). Like the majority of existing protocols for the differentiation of endothelial cells, this small-molecule protocol relies on an ECM substrate. In combination with the important role of ECM in development, this indicates that ECM may play a role during in vitro differentiation of endothelial cells as well. Ullah et al. demonstrated that decellularized kidney ECM supplemented with VEGF induced endothelial differentiation in the absence of other factors and that the differentiation was primarily due to the ECM's ability to sequester VEGF and other growth factors (Ullah et al., 2019). Hou et al. applied a different approach, using combinations of ECM proteins, including laminin 111, Matrigel, Col IV, gelatin, heparan sulfate, and fibronectin, to induce endothelial differentiation (Hou et al., 2016). However, a limitation of this study was that the majority of effective combinations included Matrigel, and examination of laminins was restricted to the non-vascular laminin 111. Ohta et al. did include laminins 411 and 511 in their study of matrices to promote endothelial differentiation and found that a fragment of laminin 411 increased the yield of CD31+ cells considerably (Ohta et al., 2016). However, the first several days of culture remained on Matrigel and, in addition, both a small-molecule Wnt activator and VEGF were added, meaning that, while they were able to achieve high purity, the complexity of the protocol made it difficult to untangle the effects of ECM from those of growth factors and changes induced by Wnt activation. Here we demonstrate the significant impact of the ECM protein laminin 411 alone on endothelial differentiation and identify key signaling pathways responsible for this effect.
Results
Laminin 411 promotes differentiation of human endothelial cells from hiPSCs
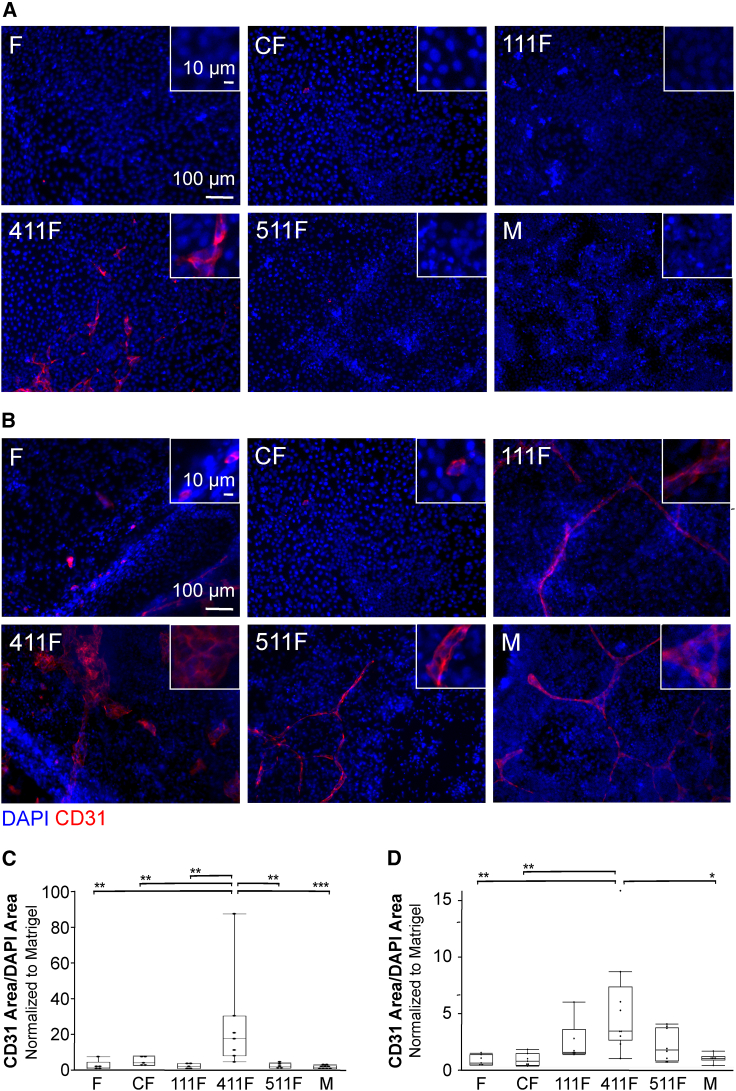
hiPSC specification to endothelial cells has been demonstrated using small-molecule agonists of Wnt signaling. Recently, more attention has been paid to the substrate on which endothelial differentiation can occur; specifically, Matrigel has been replaced with defined basement membrane proteins. Laminin 411 (LN411) was identified as a potent substrate to support endothelial differentiation when cells were switched to LN411-coated plates at day 3 of differentiation in concert with administration of the Wnt activator CHIR (Ohta et al., 2016). To determine whether basement membrane proteins could trigger endothelial specification without small-molecule Wnt agonists, hiPSCs were seeded directly onto plates coated with collagen I (Col I), laminin 111 (LN111), LN411, laminin 511 (LN511), or Matrigel. These proteins were selected because they are either present in the vascular basement membrane (LN411 and LN511) or commonly used substrates for endothelial differentiation (LN111) or appropriate for use as a negative control (Col I). Poor attachment of hiPSCs to Col I, LN411, and LN511 drove the addition of fibronectin (FN), which harbors the cell binding motif RGD, recognized by cell types, like iPSCs, that express integrin α4β1. An FN-negative control was therefore added. After 14 days of culture, cells were stained for CD31. A low but significant level of endothelial differentiation was detected on FN + LN411 (approximately 1.5%) (Figures 1A and 1C). No other condition showed any significant differentiation, indicating that LN411 promotes endothelial differentiation independent of small-molecule-driven Wnt activation.
Figure 1.
Evaluation of ECM coatings for endothelial differentiation of hiPSCs
(A) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation of hiPSCs on ECM-coated plates without the addition of small molecules to drive differentiation. F, fibronectin; C, collagen I; 111, laminin 111; 411, laminin 411; 511, laminin 511; M, Matrigel.
(B) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation of hiPSCs to endothelial cells using CHIR on ECM-coated plates.
(C) CD31 area normalized to DAPI area following differentiation of hiPSCs on ECM-coated plates in the absence of exogenous factors. Each condition was normalized to the Matrigel control. n ≥ 5 wells per condition, which includes at least three independent experimental replicates per condition. ∗∗p < 0.01; ∗∗∗p < 0.001.
(D) CD31 area normalized to DAPI area following differentiation of hiPSCs on ECM-coated plates with CHIR addition on days 0 and 1. Each condition was normalized to the Matrigel control. n ≥ 5 wells per condition, which includes at least three independent experimental replicates per condition. ∗p < 0.05; ∗∗p < 0.01.
In a subsequent experiment, the small-molecule Wnt activator CHIR was added on days 0 and 1 of differentiation to determine if the ECM and CHIR were working through the same or different mechanisms. Cells were fixed at day 14 and stained for the endothelial marker CD31. In the presence of CHIR, LN411 + FN showed significantly more differentiation than Matrigel, FN, or Col I + FN (approximately 17%) (Figures 1B and 1D). This result was confirmed by flow cytometry (Figure S4). This result indicates that without exogenous growth factors or matrix switching from Matrigel, LN411 has a positive effect on endothelial differentiation. The increased differentiation beyond that seen without CHIR and the difference in differentiation depending on matrix indicate that these effects are additive and can be combined to improve differentiation efficiency.
Laminin 411 promotes differentiation of endothelial cells from murine PSCs
Because the level of differentiation of hiPSCs was relatively low in the absence of CHIR, and all conditions required a possibly confounding FN base, we transitioned to murine iPSCs (miPSCs). miPSCs were selected owing to their ability to readily attach to the individual ECM components. While miPSCs are a useful model system in this context, it is important to note that mouse and human iPSCs do differ in gene expression (Schnerch et al., 2010; Paul, 2013). Notably, miPSCs express a higher level of integrin β1 than hiPSCs (Figure S3C) (Schnerch et al., 2010). This difference in adhesion protein expression is consistent with differences in matrix adhesion observed in culture, where miPSCs more readily adhere to a variety of substrates, including LN411 alone. The ability of miPSCs to adhere to LN411 without the addition of other ECM proteins to provide adhesion domains allows investigation of the role of LN411 without confounding factors. miPSCs were plated on tissue culture plates coated with ECM protein Col I, LN111, LN411, or LN511. After 14 days of culture in minimal medium, cells were stained for the endothelial marker CD31. Differentiation on LN111 or LN411 resulted in significantly more CD31+ cells (approximately 44%) than differentiation on Col I (Figure 2A) or Col IV (Figure S3A). This difference was not based on differing protein coating concentrations (Figure S1). The higher level of differentiation compared with hiPSCs could result from the absence of FN in the miPSC cultures. As no significant differentiation of hiPSCs is seen on FN, it may be providing signals that either inhibit endothelial differentiation or promote differentiation to another cell type. In either circumstance, the FN may have muted the signal for endothelial differentiation provided by LN411, resulting in less endothelial differentiation of the hiPSCs compared with miPSCs.
Figure 2.
Evaluation of ECM coatings for endothelial differentiation of miPSCs
(A) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation of miPSCs on ECM-coated plates. Graph shows CD31 area normalized to DAPI area following differentiation of miPSCs on ECM-coated plates. n ≥ 5 wells per condition, which includes at least three independent experimental replicates per condition. ∗p < 0.05; ∗∗∗p < 0.001. C, collagen I; 111, laminin 111; 411, laminin 411; 511, laminin 511.
(B) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation of miPSCs encapsulated in 3D PEG-ECM composites containing either Col I or LN411, and bright-field image showing tube formation seen in a PEG-LN411 composite gel. White arrowheads indicate the tube-like structures seen in the image. Graph shows CD31 area normalized to DAPI area following differentiation of miPSCs in PEG-ECM composites. n ≥ 3 gels per condition, which includes at least three independent experimental replicates per condition. ∗∗∗p < 0.001.
(C) Representative immunofluorescence images showing LDL (red) reuptake by cells differentiated on either Col I or LN411-coated plates. LDL area per DAPI area is shown for both conditions. No significant differences were observed. n ≥ 4 wells per condition, which includes at least three independent experimental replicates per condition.
(D) Representative bright-field images of a Matrigel tube formation assay performed with cells differentiated on Col I or LN411. Quantification of total tube length and number of branch points for both conditions are shown. No significant differences were observed. n ≥ 5 wells per condition, which includes at least three independent experimental replicates per condition.
To determine whether mPSC sourcing influenced endothelial differentiation, murine ESCs (mESCs) were plated on tissue culture plates coated with Col I or LN411 and cultured for 14 days in minimal medium, at which time they were stained for CD31. As in miPSCs, LN411 produced a significantly higher number of CD31+ cells. LN411-guided endothelial differentiation was conserved across mouse cell lines despite their different origins (iPSCs versus ESCs), indicating that LN411 could be a powerful tool for endothelial differentiation regardless of stem cell source (Figure S2).
A 3D culture environment enhances laminin 411-guided differentiation of miPSCs
During development, differentiation takes place in a dynamic, 3D environment. It has been previously reported that in most cases, 3D differentiation reflects that in 2D; however, 3D differentiation is magnified (Liu et al., n.d.; Martino et al., 2009; Riccio et al., 2010; Jung et al., 2016; Chandrasekaran et al., 2017; Fleischer et al., 2019). To determine how a 3D environment affects endothelial differentiation with ECM, miPSCs were encapsulated in polyethylene glycol (PEG)-ECM composite hydrogels containing Col I or LN411 and cultured in minimal medium for 14 days, at which time the gels were stained for the endothelial marker CD31. Mirroring what was seen in 2D, LN411 gels contained significantly more CD31+ cells than Col I-containing gels. Indeed, the effect of the ECM was magnified substantially in 3D, with up to 60% of cells becoming CD31+ in the PEG-LN411 composites (Figure 2B). In addition, tube-like structures were seen in some PEG-LN411 composites, indicating functionality of the endothelial cells differentiated in the composite gels.
Functional assessment of murine endothelial cells
The resulting murine endothelial cells were assessed for functional behavior: reuptake of low-density lipoprotein (LDL) and tube formation on Matrigel (Figures 2C and 2D). LDL reuptake was measured in the well directly following differentiation and without cell sorting. Despite the higher number of endothelial cells on LN411, LDL reuptake did not differ significantly between cells differentiated on Col I or LN411. However, LDL colocalization with CD31 was significantly higher in cells differentiated on LN411 (Figure S3B), indicating the reason for the lack of difference in LDL reuptake was contaminating cell types, which also take up LDL. Tube formation was performed with a total number of cells equal to the optimal cell density for the Matrigel tube formation assay multiplied by the fraction of cells differentiated to endothelial cells in that condition. No significant difference in tube length or number of branch points was observed between cells differentiated on Col I or LN411. Since numbers of endothelial cells were normalized between conditions, the absence of a significant difference between the conditions indicates that endothelial cells differentiated on either protein coating are equally functional.
VEGF stimulation enhances endothelial differentiation independent of matrix coating
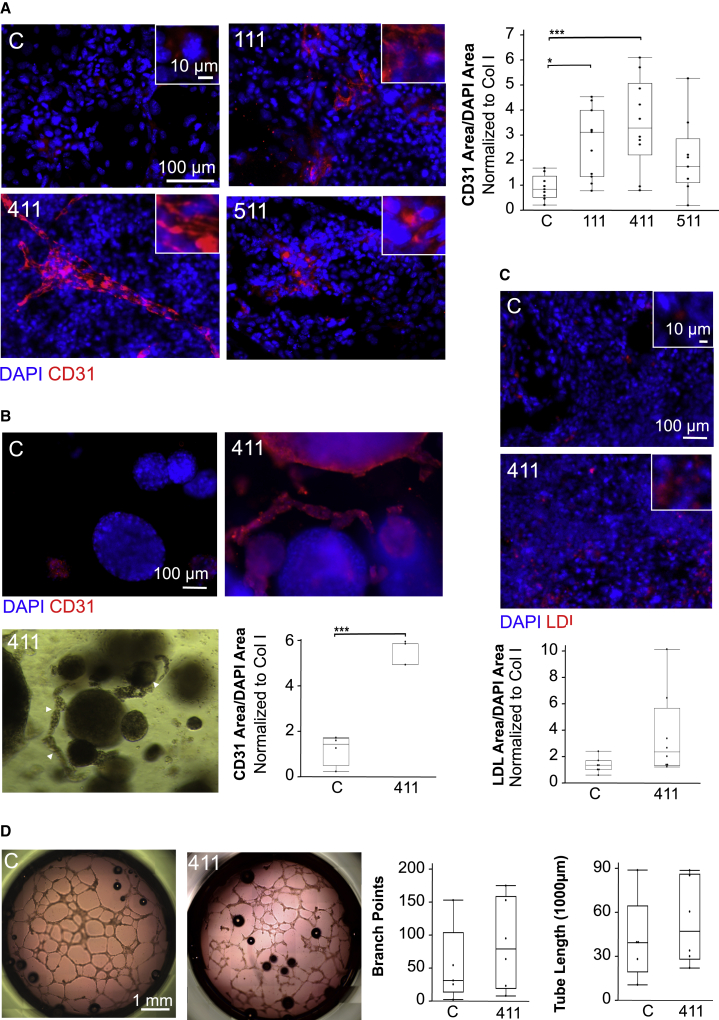
Vascular development has been shown to be dependent on VEGF, with VEGF-null or heterozygous mice showing severe defects in endothelial cell development and vascular morphogenesis (Shalaby et al., 1995; Ferrara et al., 1996). In vitro, VEGF is commonly utilized to promote endothelial differentiation (Yamashita et al., 2000; Nourse et al., 2010; Patsch et al., 2015; Ohta et al., 2016; Ge et al., 2018; Ullah et al., 2019). Because of this, it was important to understand how VEGF addition alters endothelial differentiation, as this would provide important insight into whether ECM-VEGF interactions were responsible for differences in ECM-guided endothelial differentiation. Tissue culture plates were coated with Col I and LN411. After the plates were washed with PBS, VEGF was incubated on the ECM-coated plates for 30 min before another wash with PBS. miPSCs were seeded onto the ECM-coated wells preincubated with VEGF and cultured for 14 days in minimal medium (without VEGF) before being stained for the endothelial marker CD31. VEGF incubation increased differentiation significantly, and to similar degrees, on both ECM proteins (Figures 3A and 3B).
Figure 3.
The effect of VEGF stimulation on ECM-guided endothelial differentiation
(A) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation on Col I or LN411 when either PBS (control) or VEGF was incubated with the ECM prior to cell seeding. C, collagen I; 411, laminin 411; VEGF, vascular endothelial growth factor.
(B) CD31 area per DAPI area following differentiation on Col I or LN411 with or without preincubation with VEGF. All conditions were normalized to the Col I control. n ≥ 5 wells per condition, which includes at least three independent experimental replicates per condition. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(C) Percentage VEGF absorbed compared with VEGF added when incubated with Col I or LN411, determined by ELISA. n = 4 for all conditions. No significant differences were observed.
Given the increase in differentiation seen in cell culture experiments with VEGF incubation, it was important to understand how efficiently VEGF binds to Col I and LN411. At the concentration used for the cell culture experiments, the VEGF in the supernatant was too dilute to be detected by ELISA, so a series of higher concentrations were incubated with Col I and LN411 for 30 min and the supernatants were reserved for ELISA. VEGF binding to Col I and LN411 was not significantly different at any of the concentrations tested (Figure 3C), which indicates that differential binding of VEGF found in serum or produced by the iPSCs to the ECM is likely not the cause of the observed difference in efficiency of endothelial differentiation between Col I and LN411.
Mechanism of ECM-guided endothelial differentiation
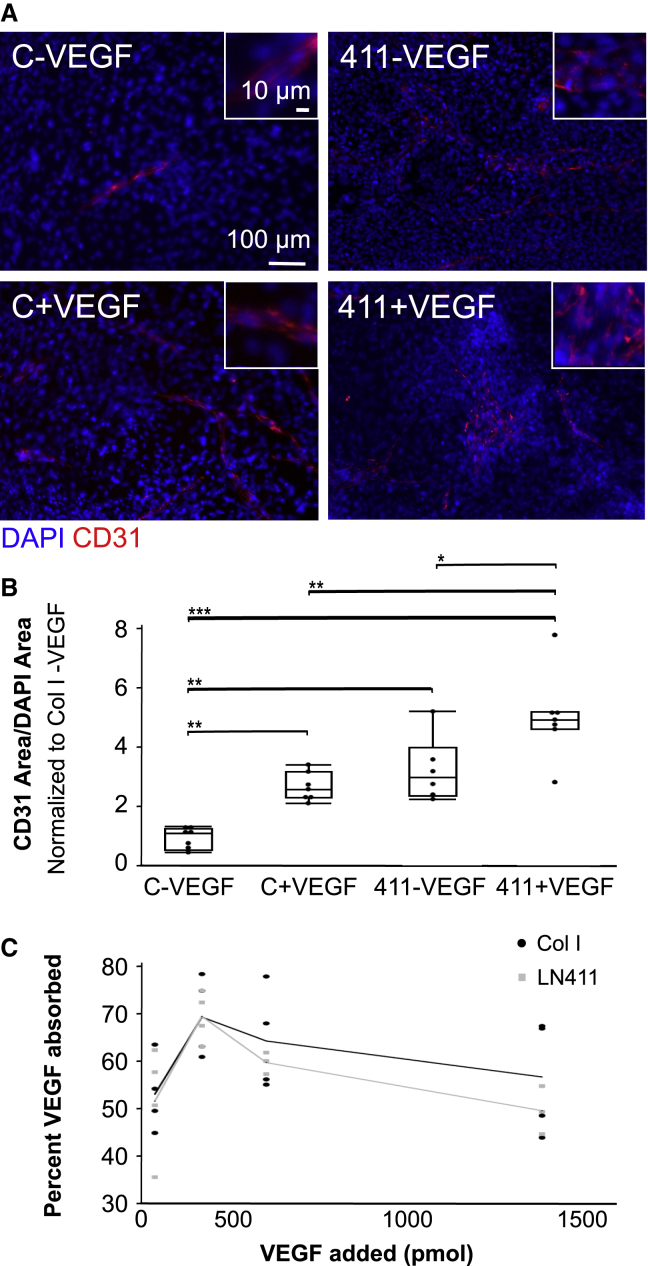
Cells interact with ECM through cell-surface receptor integrins, which cluster at focal adhesions. Integrin binding can activate a number of downstream pathways, beginning with activation of focal adhesion kinase (FAK) (Guan and Shalloway, 1992; Schaller et al., 1992; Zhao and Guan, 2011). This can lead to activation of another member of the focal adhesion complex, integrin-linked kinase (ILK). We hypothesized that LN411-induced endothelial differentiation is dependent on activation of integrins, and therefore FAK. ILK was considered as a possible component of the mechanism of differentiation, as links between ILK and β-catenin have been shown (de la Puente et al., 2015) and it is known that β-catenin activation promotes endothelial differentiation (Lian et al., 2014; Bao et al., 2015). To determine if FAK and ILK were involved in ECM-based endothelial differentiation, miPSCs were seeded onto Col I- or LN411-coated plates. On days 1–14, inhibitors of FAK and ILK were added to the cell culture medium. Analysis of CD31 staining revealed that addition of the inhibitors reduced differentiation on LN411 to the level seen on Col I without affecting cell viability (Figure S5C), which supported the idea that ECM engagement was activating integrin signaling through FAK and ILK (Figures 4A and 4B).
Figure 4.
Inhibition of focal adhesion kinase, integrin-linked kinase, Notch, and β-catenin eliminates the effect of ECM on endothelial differentiation
(A) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation on Col I or LN411 in the presence of DMSO or inhibitors of ILK (CPD22), FAK (PF573228), Notch (Fli-06), or β-catenin (IWP2). C, collagen I; 411, laminin 411.
(B) CD31 area per DAPI area following differentiation on Col I or LN411 in the presence of inhibitors. n ≥ 5 wells per condition, which includes at least three independent experimental replicates per condition. ∗∗∗p < 0.001. The large bracket indicates that all conditions within the bracket were significantly different from LN411 control and LN411 DMSO at p < 0.001.
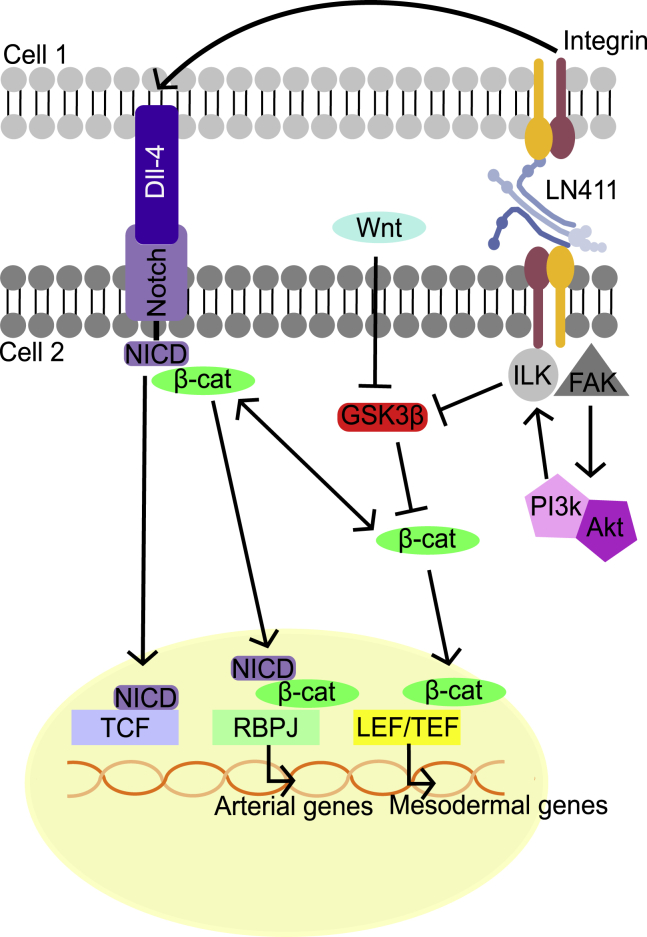
Laminins have previously been shown to increase expression of the Notch ligand Dll4 in endothelial cells (Estrach et al., 2011). Resulting cleavage of the Notch intracellular domain (NICD) is important in the context of endothelial differentiation because Notch can sequester active β-catenin at the membrane and because an NICD-β-catenin complex can form, which activates a set of genes associated with arterial specification (Hayward et al., 2005; Yamamizu et al., 2010; LaFoya et al., 2016; Park et al., 2018). Therefore, an inhibitor of Notch signaling was added on days 1–14 of differentiation to determine if Notch plays a role in LN411-guided endothelial differentiation. Similar to the inhibitors of ILK and FAK, the Notch inhibitor reduced endothelial differentiation on LN411 to the level seen in the Col I control without affecting cell viability (Figure S5C), indicating that Notch signaling does support endothelial differentiation on LN411 (Figures 4A and 4B).
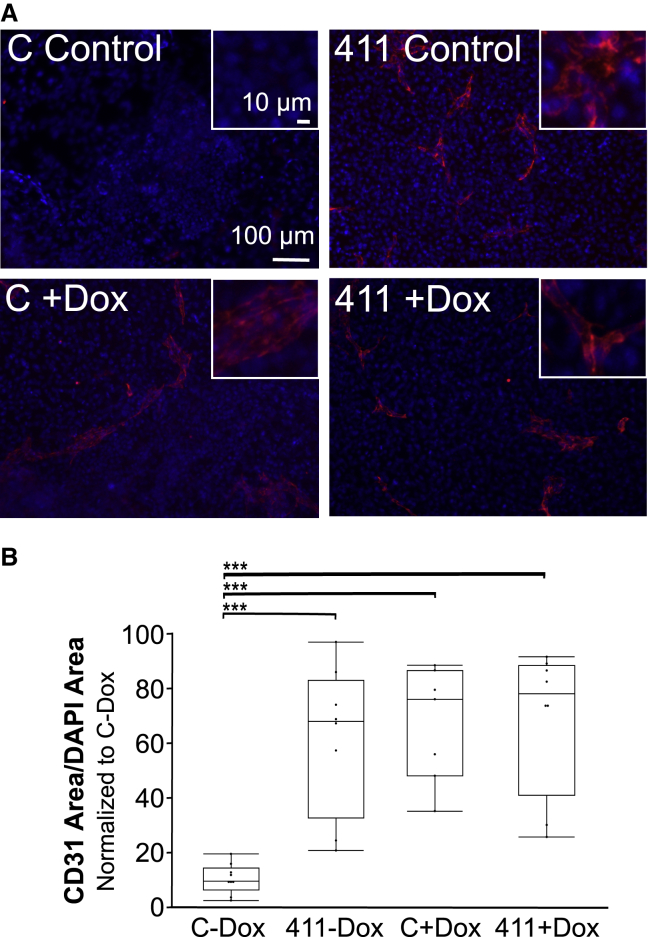
The role of β-catenin in endothelial differentiation has been well established (Lian et al., 2014; Bao et al., 2015), as have links to ILK and Notch signaling (Hayward et al., 2005; de la Puente et al., 2015; LaFoya et al., 2016). For this reason, an inhibitor of β-catenin was added on days 1–14 of differentiation to examine its effects on endothelial differentiation. Inhibitor of Wnt production-2 (IWP2) addition reduced the differentiation on LN411. Also, while the trend was not statistically significant, differentiation on either substrate in the presence of IWP2 was lower than that on Col I in the absence of IWP2 (Figures 4A and 4B). A low level of differentiation does occur in the Col I control and in the presence of the other three inhibitors; however, when IWP2 was present, no differentiation was found on either ECM, indicating that β-catenin signaling is required for endothelial differentiation regardless of ECM substrate used in this system. Addition of IWP2 did lower cell number at the end of the experiment (Figure S5C), likely because β-catenin signaling is important for many cell processes and indicating a need to confirm the involvement of β-catenin in the following targeted approach. When stabilized β-catenin (sβ-catenin) expression was induced with doxycycline in inducible stabilized β-catenin (isβ-catenin) mESCs, the difference in CD31 expression between ECM coatings was reduced, and the level of differentiation on Col I was no different from that observed on LN411 (Figures 5A and 5B). This outcome further supports the involvement of the β-catenin signaling pathway in the context of ECM-guided differentiation. This is also consistent with our findings in human iPSCs, where addition of the Wnt activator, CHIR, improved endothelial differentiation on ECM substrates.
Figure 5.
Effect of activation of doxycycline-inducible sβ-catenin on ECM-guided endothelial differentiation
(A) Representative immunofluorescence images showing CD31 staining (red) and DAPI (blue) following differentiation of isβ-catenin-inducible mESCs on collagen I or LN411 without and with doxycycline to induce isβ-catenin expression. C, collagen I; 411, laminin 411; Dox, doxycycline.
(B) CD31 area normalized to DAPI area following differentiation on Col I or LN411 with or without Dox to induce sβ-catenin expression. n ≥ 8 wells per condition, which includes at least three independent experimental replicates per condition. ∗∗∗p < 0.001.
Discussion
In this study, we evaluated ECM coatings for endothelial differentiation and found that vascular basement membrane-specific LN411 promotes increased endothelial differentiation over a Col I control in miPSCs. Similar results were seen using a 3D culture system, mESCs, and hiPSCs, indicating that LN411 promotes endothelial specification across species and cell lines. This builds upon prior studies that have used ECM as a substrate for small-molecule differentiation of endothelial cells (Lian et al., 2014; Bao et al., 2015; Ohta et al., 2016) and furthers our understanding of how ECM and cells interact to inform cell behavior.
ECM-dependent differentiation is potentially advantageous because insoluble ECM can be patterned though 3D printing to alter tissue specification in a location-dependent manner, which will be essential to the development of complex engineered tissues. For example, tissue vascularization would largely benefit from the ability to pattern endothelial differentiation with differentiation to another cell type (Du et al., 2014; Maiullari et al., 2018). In the case of cardiac tissue, this could be accomplished by patterning LN411 with a combinatorial ECM that was previously optimized for cardiomyocyte differentiation (Jung et al., 2015). Similarly, ECM-based differentiation could be extended to allow patterning of several cell types to re-create complex functional structures (Ker et al., 2011), like those found in the glomerulus of the kidney, the re-creation of which is a major challenge for tissue engineering due to the complexity of the structure and number of cell types (Takasato et al., 2014; Homan et al., 2016; Oxburgh et al., 2017; Hasegawa et al., 2019).
The Wnt-β-catenin pathway has been well studied in the context of endothelial differentiation (Wang et al., 2006a; Sumi et al., 2008; Woll et al., 2008; Patsch et al., 2015; Harding et al., 2017; Shao et al., 2019). In vitro, this insight has been used to modulate differentiation through the use of small-molecule Wnt activators (Lian et al., 2014; Bao et al., 2015). Because of the known link to endothelial differentiation, a Wnt activator, CHIR, was added to hiPSCs cultured on ECM at time points known to promote endothelial differentiation. This experiment revealed that Wnt activation improves ECM-guided endothelial differentiation in hiPSCs. In miPSCs, an inhibitor of β-catenin, which is downstream of Wnt, ablated all endothelial differentiation, indicating that it is a required factor. In addition, expression of stabilized β-catenin in mESCs ablated the ECM specificity of endothelial differentiation, bringing the level of differentiation on Col I to the level of LN411, supporting the involvement of β-catenin in ECM-guided endothelial specification. It is intriguing that, while in the mouse the activation of β-catenin did not further improve differentiation on LN411, the addition of CHIR, which will also lead to activation of β-catenin, did improve differentiation on LN411 + FN in human iPSCs. This difference could reflect (1) the differing systems used to activate β-catenin; (2) the fact that LN411 alone may allow for more complete activation than when combined with FN and, therefore, that β-catenin is already maximally activated by LN411 in mouse cells; (3) species-specific differences in non-ECM factors that contribute to differentiation; or (4) species-specific differences in the pathway linking LN411 engagement to β-catenin activation.
The primary way in which ECM and cells interact is through cell-surface integrins, which are clustered at focal adhesions. Activation of integrin β1 by binding to LN111 has been shown to induce endothelial differentiation in mESCs (Toya et al., 2015) and integrin β1 knockout studies in embryoid bodies have revealed disrupted vessel formation and increased apoptosis of endothelial progenitor cells (Malan et al., 2010). Integrin engagement stimulates FAK, which can phosphorylate many other components of the focal adhesion complex (Guan and Shalloway, 1992; Schaller et al., 1992; Zhao and Guan, 2011). FAK inhibition reduced endothelial differentiation on LN411 to the level seen on Col I, which indicates that ECM-integrin engagement leads to the variation in differentiation between the matrices. This is consistent with previous knockdown studies in mice, in which blood vessel development was disrupted (Toya et al., 2015). One of the focal adhesion components that FAK can activate is ILK, which phosphorylates GSK3β, thereby activating β-catenin (de la Puente et al., 2015). Inhibition of ILK during ECM-guided endothelial differentiation also returned differentiation to baseline levels. This pathway, ECM → integrin → FAK → ILK → β-catenin → endothelial differentiation, represents one avenue for ECM to induce endothelial differentiation (Figure 6).
Figure 6.
Proposed mechanism for ECM-guided endothelial differentiation
A schematic showing the proposed mechanism of endothelial differentiation on laminin 411 (LN411). Signaling through focal adhesion kinase (FAK), integrin-linked kinase (ILK), and Notch is linked to active β-catenin and its activation of transcription factors, which lead to the expression of endothelial genes.
A second avenue for promotion of endothelial differentiation involves both cell-ECM and cell-cell interactions. LN-integrin interactions have been shown to upregulate the Notch ligand Dll4 in endothelial cells (Estrach et al., 2011). Cell-cell interactions then lead to cleavage of the NICD. Active β-catenin can be sequestered at the membrane by bound NICD (Hayward et al., 2005; LaFoya et al., 2016), and this can form a complex that translocates to the nucleus and activates a set of genes important for arterial specification (Yamamizu et al., 2010; Park et al., 2018). Since inhibition of Notch signaling also reduces LN411-guided endothelial differentiation to baseline, this pathway, ECM → integrin → Dll4 → Notch → NICD-β-catenin → upregulation of arterial genes, represents a second avenue for ECM to induce endothelial differentiation (Figure 6).
In this study, it was determined that ECM-guided endothelial differentiation was not a function of differential binding of VEGF to particular ECM proteins, as both Col I and LN411 bound the same percentage of incubated VEGF at low concentration, and ECM-VEGF incubation increased differentiation similarly on both substrates. However, VEGF signaling may still contribute to the difference in endothelial differentiation observed between ECM coatings. For example, this study did not account for possible differences in VEGF expression induced by ECM. In addition, VEGF has been shown to interact with both Notch (Lawson et al., 2002; Liu et al., 2003; Williams et al., 2006; Funahashi et al., 2011; Li et al., 2017) and Wnt (Easwaran et al., 2003; Lian et al., 2014; Wu et al., 2015; Zhang et al., 2016) signaling. Further investigation of the cross talk between these pathways is necessary for a full understanding of the link between ECM engagement and expression of endothelial genes. Taken together, these pathways represent multiple possible avenues of endothelial differentiation converging on β-catenin, which all appear to play a role in ECM-guided differentiation, and whose components may interact with one another in complex ways to regulate endothelial gene expression.
Of the ECM proteins used in this study, two, LN411 and LN511, are human recombinant proteins. The others are isolated from human (FN), mouse (LN111), or bovine (Col I) tissues. While use of human recombinant proteins is ideal from an immunogenicity and purity perspective, there are associated challenges that must be considered in this context. ECM proteins undergo extensive posttranslational modifications, including glycosylation, phosphorylation, acetylation, and cross-linking, to create their complex 3D structures (Chang et al., 2016). While recombinant protein technology has moved closer to production of proteins with posttranslational modifications, they still remain a major challenge (Yang et al., 2018; Walimbe and Panitch, 2020). Especially given the relative dearth of research on LN411 and LN511, it must be considered that the recombinant forms of these proteins may not be as active as the native states, or that typically inactive sites may be accessible due to altered final protein structures. Further understanding of the posttranslational modifications of laminin subtypes will be essential to future studies.
In conclusion, this study directly evaluated ECM coatings for endothelial differentiation and found that LN411 alone induces endothelial differentiation. The differentiation uses human recombinant LN411 and defined medium formulations, making it a xeno-free protocol for differentiation of hiPSCs. The advantages of insoluble ECM as a signaling molecule for differentiation allow for potential applications in patterning of tissue types for tissue engineering and microphysiological systems, and a better understanding of this signaling molecule could lead to improved differentiation of endothelial cells for a variety of applications.
Limitations of the study
Further investigation into the mechanism of ECM-guided endothelial differentiation and the role of ECM-ECM interactions will be required to fully understand how ECM and cells interact to lead to this change in cell behavior. hiPSC experiments required FN to support cell adhesion, which could potentially confound the results, as the effects of ECM on cell behavior are not always additive. For example, ECM proteins can interact with one another to form new binding motifs or hide binding motifs. For this reason, an opportunity to advance the field lies in the investigation of the effects of combinations of ECM proteins on endothelial specification. In addition, while inhibition of key pathways provides strong evidence for the involvement of ILK, FAK, Notch, and β-catenin in this differentiation, more comprehensive pathway analysis is needed in the future and may lead to discovery of additional key factors in the differentiation. This may include PCR or western blot studies to further confirm the proposed pathways as well as analysis of the required timing of these signals. Finally, as sex differences in endothelial differentiation have been reported (Randolph et al., 2019), it will be important to determine how this differentiation is affected by the sex of the iPSCs.
Experimental procedures
Experimental model and subject details
hiPSCs were maintained with mTESR1 (STEMCELL) on Matrigel-coated plates at 37°C. hiPSCs were passaged every 3–5 days using ReLeSR (STEMCELL). hiPSCs were used between passages 40 and 70. hiPSCs were derived from male adult cardiac fibroblasts.
miPSCs (Van Laake et al., 2010) were maintained in maintenance medium (Glasgow modified essential medium [GMEM] + 10% fetal bovine serum [FBS], 1% non-essential amino acids [NEAA], 1% β-mercaptoethanol, 1% L-glutamine, 0.02 μg/mL leukemia inhibitory factor [LIF]). Cells were fed on day 2 of culture and passaged at day 3 to maintain optimal cell density for pluripotency. At passage, differentiated colonies were removed and cells were lifted from the plate with 0.05% trypsin/EDTA. Cells were then seeded at 3,645 cells/cm2 onto gelatinized tissue culture plates and kept at 37°C. miPSCs were derived from an 8-week-old male mouse.
ES-D3 mESCs were maintained identical to miPSCs with the addition of 10 μg/mL of BMP-4 to maintenance medium. mESCs were derived from blastocysts of a 129S2/SvPas mouse. Sex was not reported.
The isβ-catenin mouse ESC line was generated by electroporating ZX1 mouse ESCs (Iacovino et al., 2011) with p2Lox-sβ-catenin (Lindsley et al., 2006) and selecting under G418. The isβ-catenin inducible mESCs were maintained in maintenance medium (DMEM knockout + 15% FBS, 1% Glutamax, 1% Pen/Strep, 1% NEAA, 0.7% β-mercaptoethanol, 0.02 μg/mL LIF) with irradiated mouse embryonic fibroblasts (MEFs). MEFs were removed prior to seeding on ECM.
Method details
ECM coating
Col I coatings were prepared in 0.1% acetic acid at a concentration of 35.5 μg/mL. FN was diluted to 75 μg/mL in deionized (DI) water. Laminins 111, 411, and 511 were diluted in DPBS with calcium and magnesium to concentrations of 15.8, 0.8, and 0.8 μg/mL. All solutions were kept on ice during preparation. A solution volume of 200 μL was added to each well of a 96-well plate, which was then incubated at 4°C for 24 h. The wells were then washed twice with PBS and cells seeded immediately to prevent drying of the coatings. ECM coating was confirmed via immunostaining. Primary antibodies were mouse anti-FN, Millipore Sigma, Cat. No. 32160702; rabbit anti-laminin β1, abcam, Cat. No. 109293; and rabbit anti-Col I, abcam, Cat. No. 138492. Secondary antibodies were goat anti-rabbit Alexa Fluor 647, Invitrogen, Cat. No. A21244, and goat anti-mouse Alexa Fluor 647, Invitrogen, Cat. No. A32728 (Figure S6).
Author contributions
Conceptualization, B.M.O. and M.L.H.; methodology, M.L.H.; investigation, M.L.H., S.G., N.S, and M.I. writing – original draft, M.L.H.; writing – review & editing, B.M.O., M.L.H., S.G., M.K., and N.S.; visualization, M.L.H.; supervision, B.M.O.; funding acquisition, B.M.O.
Declaration of interests
The authors declare no competing interests
Acknowledgments
We would like to thank the Srivastava lab, University of California, San Francisco, for providing the miPSCs used in this study. We would also like to thank the Zhang Lab at the University of Alabama at Birmingham for providing the cell line hciPSC-MHC-CCND2 (human cardiac fibroblast-derived induced pluripotent stem cells expressing cyclin D2 under the myosin heavy chain promoter) used for all hiPSC experiments. This research was funded by grants from National Institutes of Health R01 HL137204 to B.M.O.; National Institutes of Health R01 AR075413 to M.K.; National Institutes of Health Stem Cell Biology T32 GM113846-09 to M.L.H. Dinnaken Fellowship Funding by PACCS Division, Department of Medicine, University of Minnesota to M.L.H. and National Science Foundation GRFP to S.G.
Published: February 3, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.01.005.
Supplemental information
References
- Alpy F., Jivkov I., Sorokin L., Klein A., Arnold C., Huss Y., Kedinger M., Simon-Assmann P., Lefebvre O. Generation of a conditionally null allele of the laminin α1 gene. Genesis. 2005;43:59–70. doi: 10.1002/gene.20154. [DOI] [PubMed] [Google Scholar]
- Bao X., Lian X., Dunn K.K., Shi M., Han T., Qian T., Bhute V.J., Canfield S.G., Palecek S.P. Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res. 2015;15:122–129. doi: 10.1016/j.scr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N.H. Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol. 2011;3:a005082. doi: 10.1101/cshperspect.a005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C., et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran A., Avci H.X., Ochalek A., Rösingh L.N., Molnár K., László L., Bellák T., Téglási A., Pesti K., Mike A., et al. Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2017;25:139–151. doi: 10.1016/j.scr.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Chang C.W., Dalgliesh A.J., López J.E., Griffiths L.G. Cardiac extracellular matrix proteomics: challenges, techniques, and clinical implications. Proteomics - Clin. Appl. 2016;10:39–50. doi: 10.1002/prca.201500030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Puente P., Weisberg E., Muz B., Nonami A., Luderer M., Stone R.M., Melo J.V., Griffin J.D., Azab A.K. Identification of ILK as a novel therapeutic target for acute and chronic myeloid leukemia. Leuk. Res. 2015;39:1299–1308. doi: 10.1016/j.leukres.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo J., Luik A.L., Yousif L., Budny S., Oberleithner H., Hofschröer V., Klingauf J., van Bavel E., Bakker E.N., Hellstrand P., et al. Endothelial basement membrane laminin 511 is essential for shear stress response. EMBO J. 2017;36:183–201. doi: 10.15252/embj.201694756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C., Narayanan K., Leong M.F., Wan A.C.A. Induced pluripotent stem cell-derived hepatocytes and endothelial cells in multi-component hydrogel fibers for liver tissue engineering. Biomaterials. 2014;35:6006–6014. doi: 10.1016/j.biomaterials.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Duan Y., Liu Z., O'Neill J., Wan L.Q., Freytes D.O., Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J. Cardiovasc. Transl. Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzobo K., Vogelsang M., Parker M.I. Wnt/β-Catenin and MEK-ERK signaling are required for fibroblast-derived extracellular matrix-mediated endoderm differentiation of embryonic stem cells. Stem Cell Rev. Rep. 2015;11:761–773. doi: 10.1007/s12015-015-9598-4. [DOI] [PubMed] [Google Scholar]
- Easwaran V., Lee S.H., Inge L., Guo L., Goldbeck C., Garrett E., Wiesmann M., Garcia P.D., Fuller J.H., Chan V., et al. Β-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 2003;63:3145–3153. [PubMed] [Google Scholar]
- Estrach S., Cailleteau L., Franco C.A., Gerhardt H., Stefani C., Lemichez E., Gagnoux-Palacios L., Meneguzzi G., Mettouchi A. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ. Res. 2011;109:172–182. doi: 10.1161/CIRCRESAHA.111.240622. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'shea K.S., Powell-Braxton L., Hillan K.J., Moore M.W., et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Jahnke H.G., Fritsche E., Girard M., Robitzki A.A. Comprehensive human stem cell differentiation in a 2D and 3D mode to cardiomyocytes for long-term cultivation and multiparametric monitoring on a multimodal microelectrode array setup. Biosens. Bioelectron. 2019;126:624–631. doi: 10.1016/j.bios.2018.10.061. [DOI] [PubMed] [Google Scholar]
- Funahashi Y., Shawber C.J., Sharma A., Kanamaru E., Choi Y.K., Kitajewski J. Notch modulates VEGF action in endothelial cells by inducing Matrix Metalloprotease activity. Vasc. Cell. 2011;3:2. doi: 10.1186/2045-824X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Zhang H., Hou J., Wan L., Cheng W., Wang X., Dong D., Chen C., Xia J., Guo J., et al. VEGF secreted by Mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2018;17:1667–1675. doi: 10.3892/mmr.2017.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J.L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Hallmann R., Horn N., Selg M., Wendler O., Pausch F., Sorokin L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. http://physrev.physiology.org/content/85/3/979.long [DOI] [PubMed] [Google Scholar]
- Hansen K.C., Kiemele L., Maller O., O'Brien J., Shankar A., Fornetti J., Schedin P. An in-solution ultrasonication-assisted digestion method for improved extracellular matrix proteome coverage. Mol. Cell Proteomics. 2009;8:1648–1657. doi: 10.1074/mcp.M900039-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A., Cortez-Toledo E., Magner N.L., Beegle J.R., Coleal-Bergum D.P., Hao D., Wang A., Nolta J.A., Zhou P. Highly efficient differentiation of endothelial cells from pluripotent stem cells requires the MAPK and the PI3K pathways. Stem Cells. 2017;35:909–919. doi: 10.1002/stem.2577. [DOI] [PubMed] [Google Scholar]
- Hasegawa S., Tanaka T., Nangaku M. Recent advances in renal regeneration [version 1; referees: 2 approved] F1000Res. 2019;8 doi: 10.12688/f1000research.17127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P., Brennan K., Sanders P., Balayo T., DasGupta R., Perrimon N., Martinez Arias A. Notch modulates Wnt signalling by associating with Armadillo/β-catenin and regulating its transcriptional activity. Development. 2005;132:1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E. Structural biology of laminins. Essays Biochem. 2019;63:285–295. doi: 10.1042/EBC20180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan K.A., Kolesky D.B., Skylar-Scott M.A., Herrmann J., Obuobi H., Moisan A., Lewis J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016;6:34845. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortells L., Johansen A.K.Z., Yutzey K.E. Cardiac fibroblasts and the extracellular matrix in regenerative and nonregenerative hearts. J. Cardiovasc. Dev. Dis. 2019;6:29. doi: 10.3390/jcdd6030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiba T., et al. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cell Int. 2016;2016:6397820. doi: 10.1155/2016/6397820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Coller J., Natu V., Hastie T.J., Huang N.F. Combinatorial extracellular matrix microenvironments promote survival and phenotype of human induced pluripotent stem cell-derived endothelial cells in hypoxia. Acta Biomater. 2016;44:188–199. doi: 10.1016/j.actbio.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovino M., Bosnakovski D., Fey H., Rux D., Bajwa G., Mahen E., Mitanoska A., Xu Z., Kyba M. Inducible cassette exchange: a rapid and efficient system enabling conditional gene expression in embryonic stem and primary cells. Stem Cells (Dayton, Ohio) 2011;29:1580. doi: 10.1002/stem.715. pmc/articles/PMC3622722/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.P., Squirrell J.M., Lyons G.E., Eliceiri K.W., Ogle B.M. Imaging cardiac extracellular matrices: a blueprint for regeneration. Trends Biotechnol. 2012;30:233–240. doi: 10.1016/j.tibtech.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.P., Hu D., Domian I.J., Ogle B.M. An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices. Sci. Rep. 2015;5:18705. doi: 10.1038/srep18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.P., Bache-Wiig M.K., Provenzano P.P., Ogle B.M. Heterogeneous differentiation of human mesenchymal stem cells in 3D extracellular matrix composites. BioResearch Open Access. 2016;5:37–48. doi: 10.1089/biores.2015.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ker E.D.F., Chu B., Phillippi J.A., Gharaibeh B., Huard J., Weiss L.E., Campbell P.G. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials. 2011;32:3413–3422. doi: 10.1016/j.biomaterials.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFoya B., Munroe J.A., Mia M.M., Detweiler M.A., Crow J.J., Wood T., Roth S., Sharma B., Albig A.R. Notch: a multi-functional integrating system of microenvironmental signals. Dev. Biol. 2016;418:227–241. doi: 10.1016/j.ydbio.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N.D., Vogel A.M., Weinstein B.M. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell. 2002;3:127–136. doi: 10.1016/S1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Li G.J., Yang Y., Yang G.K., Wan J., Cui D.L., Ma Z.H., Du L.J., Zhang G.M. Slit2 suppresses endothelial cell proliferation and migration by inhibiting the VEGF-Notch signaling pathway. Mol. Med. Rep. 2017;15:1981–1988. doi: 10.3892/mmr.2017.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X., Bao X., Al-Ahmad A., Liu J., Wu Y., Dong W., Dunn K.K., Shusta E.V., Palecek S.P. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley R.C., Gill J.G., Kyba M., Murphy T.L., Murphy K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/DEV.02551. [DOI] [PubMed] [Google Scholar]
- Liu Z.-J., Shirakawa T., Li Y., Soma A., Oka M., Dotto G.P., Fairman R.M., Velazquez O.C., Herlyn M. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell Biol. 2003;23:14–25. doi: 10.1128/mcb.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- n.d. Liu, W. et al. (n.d.) Comparison of osteogenic cell differentiation within 2D and 3D culture systems. Available at: www.3dbiotek.com (Accessed: 1 November 2020).
- Maiullari F., Costantini M., Milan M., Pace V., Chirivì M., Maiullari S., Rainer A., Baci D., Marei H.E., Seliktar D., et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018;8:13532. doi: 10.1038/s41598-018-31848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M.W. Vascular development. Arterioscler. Thromb. Vasc. Biol. 2018;38:e17–e24. doi: 10.1161/ATVBAHA.118.310223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan D., Wenzel D., Schmidt A., Geisen C., Raible A., Bölck B., Fleischmann B.K., Bloch W. Endothelial β1 integrins regulate sprouting and network formation during vascular development. Development. 2010;137:993–1002. doi: 10.1242/DEV.045377. [DOI] [PubMed] [Google Scholar]
- Martino M.M., Mochizuki M., Rothenfluh D.A., Rempel S.A., Hubbell J.A., Barker T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K., Lim V.Y., Shen J., Tan Z.W., Rajendran D., Luo S.C., Gao S., Wan A.C., Ying J.Y. Extracellular matrix-mediated differentiation of human embryonic stem cells: differentiation to insulin-secreting beta cells. Tissue Eng. Part A. 2014;20:424–433. doi: 10.1089/ten.TEA.2013.0257. [DOI] [PubMed] [Google Scholar]
- Nostro M.C., Cheng X., Keller G.M., Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourse M.B., Halpin D.E., Scatena M., Mortisen D.J., Tulloch N.L., Hauch K.D., Torok-Storb B., Ratner B.D., Pabon L., Murry C.E. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler. Thromb. Vasc. Biol. 2010;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta R., Niwa A., Taniguchi Y., Suzuki N.M., Toga J., Yagi E., Saiki N., Nishinaka-Arai Y., Okada C., Watanabe A., et al. Laminin-guided highly efficient endothelial commitment from human pluripotent stem cells. Sci. Rep. 2016;6:35680. doi: 10.1038/srep35680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxburgh L., Carroll T.J., Cleaver O., Gossett D.R., Hoshizaki D.K., Hubbell J.A., Humphreys B.D., Jain S., Jensen J., Kaplan D.L., et al. (Re) Building a kidney. J. Am. Soc. Nephrol. 2017;28:1370–1378. doi: 10.1681/ASN.2016101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.K., Lee T.W., Do E.K., Moon H.J., Kim J.H. Role of Notch1 in the arterial specification and angiogenic potential of mouse embryonic stem cell-derived endothelial cells. Stem Cell Res. Ther. 2018;9:197. doi: 10.1186/s13287-018-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch C., Challet-Meylan L., Thoma E.C., Urich E., Heckel T., O'Sullivan J.F., Grainger S.J., Kapp F.G., Sun L., Christensen K., et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C. Stem cell research using mouse models. Mater. Methods. 2013;3 doi: 10.13070/mm.en.3.184. [DOI] [Google Scholar]
- Randolph L.N., Bao X., Oddo M., Lian X.L. Sex-dependent VEGF expression underlies variations in human pluripotent stem cell to endothelial progenitor differentiation. Sci. Rep. 2019;9:16696. doi: 10.1038/s41598-019-53054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio M., Resca E., Maraldi T., Pisciotta A., Ferrari A., Bruzzesi G., De Pol A. Human dental pulp stem cells produce mineralized matrix in 2D and 3D cultures. Eur. J. Histochem. 2010;54:205–213. doi: 10.4081/ejh.2010.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienks M., Papageorgiou A.P., Frangogiannis N.G., Heymans S. Myocardial extracellular matrix: an ever-changing and diverse entity. Circ. Res. 2014;114:872–888. doi: 10.1161/CIRCRESAHA.114.302533. [DOI] [PubMed] [Google Scholar]
- Schaller M.D., Borgman C.A., Cobb B.S., Vines R.R., Reynolds A.B., Parsons J.T. pp125(FAK), a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc. Natl. Acad. Sci. U S A. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnerch A., Cerdan C., Bhatia M. Distinguishing between mouse and human pluripotent stem cell regulation: the best Laid Plans of mice and men. Stem Cells. 2010;28:419–430. doi: 10.1002/stem.298. [DOI] [PubMed] [Google Scholar]
- Shalaby F., Rossant J., Yamaguchi T.P., Gertsenstein M., Wu X.F., Breitman M.L., Schuh A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shao Y., Chen J., Freeman W., Dong L.J., Zhang Z.H., Xu M., Qiu F., Du Y., Liu J., Li X.R., Ma J.X. Canonical Wnt signaling promotes neovascularization through determination of endothelial progenitor cell fate via metabolic profile regulation. Stem Cells. 2019;37:1331–1343. doi: 10.1002/stem.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhang X., Buscher K., Wang Y., Wang H., Di Russo J., Li L., Lütke-Enking S., Zarbock A., Stadtmann A., et al. Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell Rep. 2017;18:1256–1269. doi: 10.1016/j.celrep.2016.12.092. [DOI] [PubMed] [Google Scholar]
- Sugi Y., Markwald R.R. Formation and early morphogenesis of endocardial endothelial precursor cells and the role of endoderm. Dev. Biol. 1996;175:66–83. doi: 10.1006/dbio.1996.0096. [DOI] [PubMed] [Google Scholar]
- Sumi T., Tsuneyoshi N., Nakatsuji N., Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance canonical Wnt/β-catenin, activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Takasato M., Maier B., Little M.H. Pediatric Nephrology. NIH Public Access; 2014. Recreating kidney progenitors from pluripotent cells; pp. 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyboll J., Kortesmaa J., Cao R., Soininen R., Wang L., Iivanainen A., Sorokin L., Risling M., Cao Y., Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol. Cell. Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya S.P., Wary K.K., Mittal M., Li F., Toth P.T., Park C., Rehman J., Malik A.B. Integrin α6β1 expressed in ESCs instructs the differentiation to endothelial cells. Stem Cells (Dayton, Ohio) 2015;33:1719–1729. doi: 10.1002/stem.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I., Abu-Dawud R., Busch J.F., Rabien A., Erguen B., Fischer I., Reinke P., Kurtz A. VEGF – supplemented extracellular matrix is sufficient to induce endothelial differentiation of human iPSC. Biomaterials. 2019;216:119283. doi: 10.1016/j.biomaterials.2019.119283. [DOI] [PubMed] [Google Scholar]
- Van Laake L.W., Qian L., Cheng P., Huang Y., Hsiao E.C., Conklin B.R., Srivastava D. Reporter-based isolation of induced pluripotent stem cell-and embryonic stem cell-derived cardiac progenitors reveals limited gene expression variance. Circ. Res. 2010;107:340–347. doi: 10.1161/CIRCRESAHA.109.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walimbe T., Panitch A. Proteoglycans in biomedicine: resurgence of an underexploited class of ECM molecules. Front. Pharmacol. 2020;10:1661. doi: 10.3389/fphar.2019.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Charles P.C., Wu Y., Ren R., Pi X., Moser M., Barshishat-Kupper M., Rubin J.S., Perou C., Bautch V., Patterson C. Gene expression profile signatures indicate a role for Wnt signaling in endothelial commitment from embryonic stem cells. Circ. Res. 2006;98:1331–1339. doi: 10.1161/01.RES.0000220650.26555.1d. [DOI] [PubMed] [Google Scholar]
- Wang J., Hoshijima M., Lam J., Zhou Z., Jokiel A., Dalton N.D., Hultenby K., Ruiz-Lozano P., Ross J., Tryggvason K., Chien K.R. Cardiomyopathy associated with microcirculation dysfunction in laminin α4 chain-deficient mice. J. Biol. Chem. 2006;281:213–220. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- Williams C.K., Li J.L., Murga M., Harris A.L., Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll P.S., Morris J.K., Painschab M.S., Marcus R.K., Kohn A.D., Biechele T.L., Moon R.T., Kaufman D.S. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 2008;111:122–131. doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen J., Chen C., Wang W., Wen L., Gao K., Chen X., Xiong S., Zhao H., Li S. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci. Rep. 2015;5:16151. doi: 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizu K., Matsunaga T., Uosaki H., Fukushima H., Katayama S., Hiraoka-Kanie M., Mitani K., Yamashita J.K. Convergence of Notch and β-catenin signaling induces arterial fate in vascular progenitors. J. Cell Biol. 2010;189:325. doi: 10.1083/JCB.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Itoh H., Hirashima M., Ogawa M., Nishikawa S., Yurugi T., Naito M., Nakao K., Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yang J.T., Bader B.L., Kreidberg J.A., Ullman-Culleré M., Trevithick J.E., Hynes R.O. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev. Biol. 1999;215:264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- Yang A., Cho K., Park H.S. Chemical biology approaches for studying posttranslational modifications. RNA Biol. 2018;15:427–440. doi: 10.1080/15476286.2017.1360468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Nör F., Oh M., Cucco C., Shi S., Nör J.E. Wnt/β-Catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells. 2016;34:1576–1587. doi: 10.1002/stem.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Guan J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011;63:610–615. doi: 10.1016/j.addr.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.