Summary
The horizontal basal cells (HBCs) of olfactory epithelium (OE) serve as reservoirs for stem cells during OE regeneration, through proliferation and differentiation, which is important in recovery of olfactory function. However, the molecular mechanism of regulation of HBC proliferation and differentiation after injury remains unclear. Here, we found that yes-associated protein (YAP) was upregulated and activated in HBCs after OE injury. Deletion of YAP in HBCs led to impairment in OE regeneration and functional recovery of olfaction after injury. Mechanically, YAP was activated by S1P/S1PR2 signaling, thereby promoting the proliferation of HBCs and OE regeneration after injury. Finally, activation of YAP signaling enhanced the proliferation of HBCs and improved functional recovery of olfaction after OE injury or in Alzheimer's disease model mice. Taken together, these results reveal an S1P/S1PR2/YAP pathway in OE regeneration in response to injury, providing a promising therapeutic strategy for OE injury.
Keywords: yes-associated protein, olfactory epithelium, horizontal basal cells, regeneration
Graphical abstract
Highlights
-
•
YAP is upregulated and activated in HBCs after OE injury
-
•
YAP deletion impairs OE regeneration and recovery of olfaction after injury
-
•
S1P/S1PR2/YAP signaling promotes OE regeneration
-
•
YAP activation promotes OE regeneration after acute injury or in AD model mice
In this article, Huang and colleagues reveal that YAP is upregulated and activated in horizontal basal cells (HBCs) after olfactory epithelium (OE) injury, YAP deletion in HBCs impairs OE regeneration after injury, and S1P/S1PR2/YAP signaling promotes OE regeneration after acute injury or in Alzheimer’s disease model mice, providing a promising therapeutic strategy for OE injury by selectively enhancing the S1P/S1PR2/YAP signaling pathway.
Introduction
The adult mammalian olfactory epithelium (OE) has long been known for its unique characteristic of actively generating neurons throughout life from basal stem cells (Choi and Goldstein, 2018; Schwob et al., 2017). Olfactory sensory neurons (OSNs) in the nasal cavity are highly vulnerable to the insults of external environments such as inspired chemicals, toxins, and viruses including SARS-Cov-2 (Zhang et al., 2021). Basal stem cells in the basal layer of OE, including horizontal basal cells (HBCs) and globose basal cells (GBCs), possess robust regenerative ability to replenish injury-induced loss of OSNs. HBCs are mitotically quiescent under physiological conditions but activated after severe OE injury (Iwai et al., 2008; Leung et al., 2007; Schwob et al., 1995), whereas GBCs are mitotically active and responsible for OE regeneration under most conditions (Huard and Schwob, 1995). Under OE injury, such as exposure to methyl bromide or ablation with methimazole, HBCs act as stem cells and can be activated to facilitate differentiation, proliferation, and migration of OE cells during regeneration (Herrick et al., 2017; Joiner et al., 2015; Schnittke et al., 2015). HBCs are marked by the expression of p63, keratin5/14 (K5/14), and intercellular adhesion molecule 1 (Carter et al., 2004; Fletcher et al., 2011; Holbrook et al., 1995), and in vitro experiments have shown that they have multipotent potential to generate diverse cell types of OE (Carter et al., 2004). Recent studies have further demonstrated that engrafted HBCs produce all OE cell types, confirming HBC’s multipotency (Peterson et al., 2019). However, the regenerative potential of OE is diminished during aging, leading to anosmia in some diseases, such as Alzheimer’s disease (AD) (Attems et al., 2015; Doty, 2018). Although recent studies based on genetic strategies have shown that transcription factors such as p63 (Fletcher et al., 2011; Schnittke et al., 2015), Wnt signaling (Chen et al., 2014; Fletcher et al., 2017), Notch signaling (Herrick et al., 2017), and inflammation signaling (Chen et al., 2017, 2019) contribute to OE regeneration after injury, the mechanism underlying the proliferation and differentiation of these basal stem cells in OE response to injury is complex and not fully understood.
Yes-associated protein (YAP) and its paralog, the transcriptional co-activator with PDZ-binding motif (TAZ), which is also known as WW-domain-containing transcription regulator 1 (WWTR1), act as transcriptional co-activators and well-known downstream effectors of the highly conserved Hippo pathway, are expressed in the stem/progenitor cells of vertebrate tissues, and control cell proliferation, tissue homeostasis, and organ size (Andl et al., 2017; Elbediwy and Thompson, 2018; Moya and Halder, 2019; Rognoni and Walko, 2019). Activation of YAP signaling has been shown to promote the proliferation of epidermal stem cells and progenitor cells (Rognoni and Walko, 2019; Schlegelmilch et al., 2011), whereas YAP deletion in K14-expressing mice led to fewer stem cells within the epidermis, fewer hair follicles, and faster death due to failure of the epidermal barrier, indicating the essential role of YAP in skin stem cell regeneration (Elbediwy and Thompson, 2018; Rognoni and Walko, 2019; Schlegelmilch et al., 2011). Moreover, YAP is highly expressed and required for the self-renewal of cultured embryonic stem cells as well as the suppression of differentiation (Lian et al., 2010). However, existing evidence also shows that knockdown of YAP and TAZ in the skin of mice by small interfering RNA does not affect the development and function of the skin (Lee et al., 2014). In addition, YAP knockout in neural stem cells (NSCs) is not required for their proliferation (Huang et al., 2016). Interestingly, the S1PR1/RhoA/YAP pathway promotes the proliferation of olfactory ensheathing cells, and is involved in development of the olfactory nerve layer, and growth and axonal projection of olfactory neurons into the olfactory bulb (OB) (Bao et al., 2020). However, it is still largely unclear whether YAP signaling is involved in OE regeneration after injury.
In the present study, we found that YAP deletion in HBCs impaired OE regeneration and olfactory functional recovery in the methimazole-induced OE injury model. Moreover, sphingosine 1-phosphate (S1P) activated YAP and promoted the proliferation of HBCs and OE regeneration after injury. Finally, activation of YAP signaling enhanced the proliferation of HBCs and improved functional recovery of the olfactory system after injury or in AD model mice. Therefore, this study provides novel insights into the mechanism of OE regeneration, providing a promising therapeutic strategy for OE injury.
Results
YAP is upregulated and activated in the HBCs of OE after acute injury
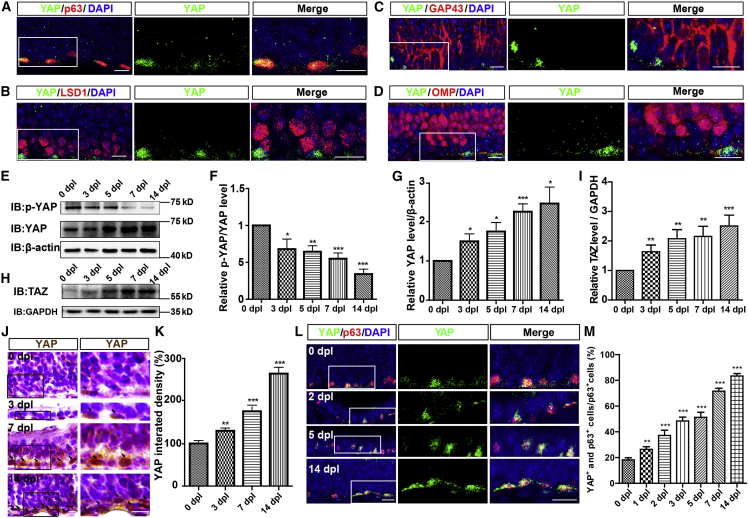
To explore the potential effects of YAP on OE regeneration after injury, we performed double immunostaining, which showed that YAP was highly expressed in p63+ (a marker of HBCs) cells of adult OE (Figure 1A), but not or very lowly expressed in LSD1+ cells (a marker of GBCs) (Figure 1B), GAP43+ cells (a marker of immature olfactory sensory neurons [iOSNs]) (Figure 1C), and OMP+ cells (a marker of mature olfactory sensory neurons [mOSNs]) (Figure 1D). These results suggest that YAP is mainly expressed in HBCs.
Figure 1.
YAP is upregulated and activated in HBCs after acute OE injury
(A–D) Double immunostaining of YAP (green) and p63 (red) (A), YAP (green) and LSD1 (red) (B), YAP (green) and GAP43 (red) (C), and YAP (green) and OMP (red) (D) in the OE of 2-month-old C57BL/6 mice.
(E) Western blot detected the expression of p-YAP and YAP in the OE of 2-month-old C57BL/6 mice at 0, 3, 5, 7, and 14 dpl.
(F and G) Quantitative analysis of the relative p-YAP/YAP (F) and YAP (G) levels as shown in (E) (n = 8 blots from 4 mice per group, normalized to the 0 dpl group).
(H) Western blot detected the expression of TAZ in the OE of 2-month-old C57BL/6 mice at 0, 3, 5, 7, and 14 dpl.
(I) Quantitative analysis of the relative TAZ level as shown in (H) (n = 15 blots from 5 mice per group, normalized to the 0 dpl group).
(J) Immunohistochemistry detected the expression of YAP in the OE of 2-month-old C57BL/6 mice at 0, 3, 7, and 14 dpl.
(K) Quantitative analysis of the relative YAP density as shown in (J) (n = 10 sections from 5 mice per group).
(L) Double immunostaining of YAP (green) and p63 (red) in the OE of 2-month-old C57BL/6 mice at 0, 2, 5, and 14 dpl.
(M) Quantitative analysis of the percentage of both YAP+ and p63+ cells over p63+ cells as shown in (L) (n = 9 sections from 3 mice per group).
Images of selected regions are shown at higher magnification. Data are mean ± SEM, one-way ANOVA with Bonferroni’s post tests, compared with 0 dpl group; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 20 μm.
A methimazole-induced OE injury model was used to test the function of YAP in HBCs. Consistent with a previous study (Ogawa et al., 2014), administration of methimazole caused the death of OE cells within 24 h and OE was gradually recovered within 4 weeks after injury (Figures S1A and S1B). Moreover, the buried pellet behavioral assay revealed that methimazole indeed impaired the olfactory function (Figures S1C and S1E).
Based on this model, western blot results showed that YAP was significantly upregulated from 3 days post lesion (dpl) (Figures 1E and 1G), while the p-YAP/YAP ratio was decreased from 3 dpl (Figures 1E and 1F). Interestingly, TAZ/WWTR1 was significantly upregulated after OE injury from 3 dpl (Figures 1H and 1I), indicating that TAZ might also be activated in OE after injury. Immunohistochemistry also showed that YAP was significantly increased in a time-dependent manner from 0 to 14 dpl (Figures 1J and 1K). Additionally, co-immunostaining analysis found that YAP was significantly increased and displayed the nuclear location in HBCs from 1 dpl (Figures 1L and 1M). Taken together, these results suggest that YAP is upregulated and activated in HBCs after acute OE injury.
Normal development of OE and olfactory function in YAPK5-CKO mice
YAPK5-CKO mice were generated by crossing the YAPf/f with K5-Cre transgenic mice (Figures S2A and S2B). YAP was indeed efficiently knocked out in p63+ and K5+ (a marker of HBCs) cells of YAPK5-CKO mice (Figures S2C and S2D). However, there was no significant difference in body weight between YAPf/f and YAPK5-CKO mice during development (Figure S2E). Furthermore, YAP deletion in K5+ cells also did not affect the development of skin (Figures S2F and S2G). Consequently, H&E staining showed that the morphology and thickness of OE were comparable between the YAPf/f and YAPK5-CKO mice (Figure S3A). The buried pellet test revealed that there was no significant difference between YAPf/f and YAPK5-CKO mice in the percentage of mice that found food in allotted time (Figure S3B) and the time taken to find food (Figure S3C). Moreover, YAP deletion in HBCs also did not affect the distribution and number of HBCs (Figures S3D and S3E), GAP43+ iOSNs (Figure S3F), OMP+ mOSNs (Figure S3G), and Sox9+ cells (a marker of Bowman’s gland duct cells) (Figure S3H). These results suggest that YAP deletion in HBCs does not affect the development of OE and olfactory function.
YAP deletion in HBCs impairs OE regeneration and functional recovery of the olfactory system after injury
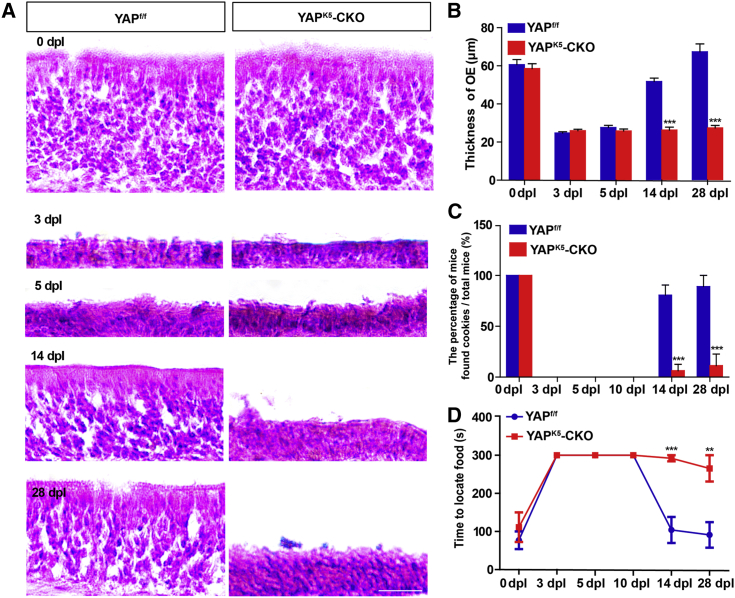
As shown in Figures 2A and 2B, OE gradually regenerated in YAPf/f mice after injury and almost recovered at 28 dpl. However, OE failed to completely recover in YAPK5-CKO mice within 28 days after injury. Moreover, the buried pellet test showed that the percentage of mice that found food was significantly decreased (Figure 2C), and the time consumed to find food was markedly prolonged in YAPK5-CKO mice from 14 to 28 dpl (Figure 2D). These results suggest that YAP deletion in HBCs impairs OE regeneration and functional recovery of the olfactory system after injury.
Figure 2.
YAP deletion in HBCs impairs OE regeneration and functional recovery of the olfactory system after injury
(A) H&E staining of the OE obtained from 2-month-old YAPf/f and YAPK5-CKO mice at 0, 3, 5, 14, and 28 dpl.
(B) Quantitative analysis of the thickness of OE as shown in (A) (n = 10 sections from 5 mice per group).
(C) Percentage of mice that found cookies over total observed 2-month-old YAPf/f (n = 9 mice) or YAPK5-CKO mice (n = 10 mice) in the buried pellet test at 0, 3, 5, 10, 14, and 28 dpl.
(D) Quantitative analysis of the time to locate food of 2-month-old YAPf/f (n = 9 mice) and YAPK5-CKO mice (n = 10 mice) at 0, 3, 5, 10, 14, and 28 dpl in the buried pellet test.
Data are mean ± SEM, two-way ANOVA with Bonferroni’s post tests, compared with YAPf/f group; ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 20 μm.
YAP deletion in HBCs reduces their proliferation and the regeneration of OSNs and inhibits axonal projection of OSNs into OB after injury
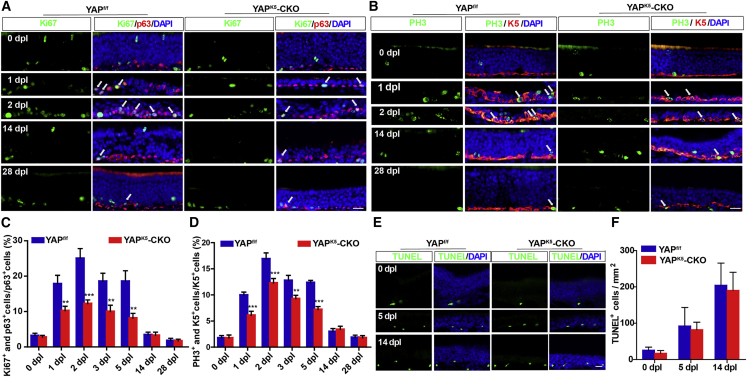
Given that YAP is involved in self-renewal of stem cells (Yao et al., 2014), the proliferation of HBCs was examined after injury in both YAPf/f and YAPK5-CKO mice. As expected, double immunostaining showed that the percentage of Ki67+ or PH3+ (markers of cell proliferation) cells was significantly decreased in HBCs of YAPK5-CKO mice from 1 dpl to 5 dpl (Figures 3A and 3D). However, TUNEL staining showed that there was no significant difference in the apoptosis of HBCs after injury between YAPf/f and YAPK5-CKO mice (Figures 3E and 3F). These results indicate that YAP deletion in HBCs reduces their proliferation after injury.
Figure 3.
YAP deletion in HBCs reduces the proliferation of HBCs in OE after injury
(A and B) Double immunostaining of Ki67 (green) and p63 (red) (A) or PH3 (green) and K5 (red) (B) in the OE of 2-month-old YAPf/f and YAPK5-CKO mice at 0, 1, 2, 14, and 28 dpl.
(C and D) Quantitative analysis of the percentage of Ki67+ and p63+ cells over total p63+ cells (C), or PH3+ and K5+ cells over total K5+ cells (D) in the OE of 2-month-old YAPf/f and YAPK5-CKO mice at 0, 1, 2, 3, 5, 14, and 28 dpl as shown in (A) and (B) (n = 10 sections from 5 mice per group).
(E) Cell apoptosis detected by TUNEL staining in the OE of 2-month-old YAPf/f and YAPK5-CKO mice at 0, 5, and 14 dpl.
(F) Quantitative analysis of the density of TUNEL+ cells as shown in (E) (n = 15 sections from 5 mice per group).
Data are mean ± SEM, two-way ANOVA with Bonferroni’s post tests, compared with YAPf/f group; ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 20 μm.
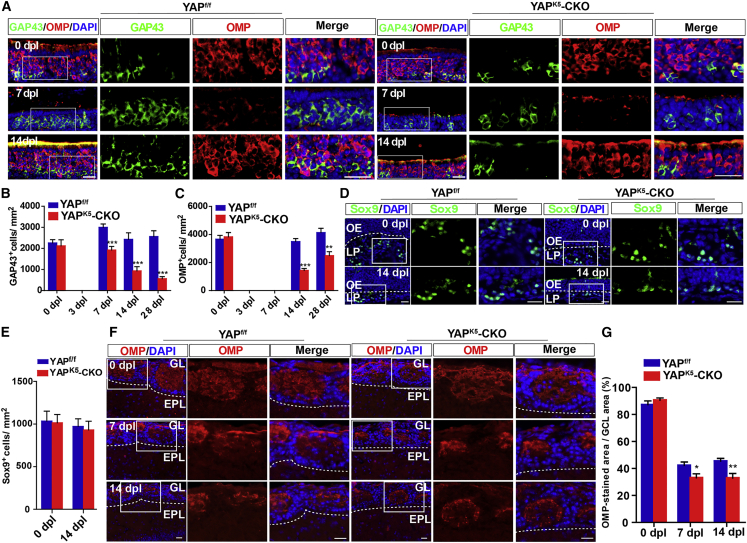
To further investigate whether YAP deletion in HBCs affected their differentiation after OE injury, we conducted immunostaining, which revealed a significant decrease in the number of GAP43+ iOSNs in the YAPK5-CKO mice from 7 dpl to 28 dpl (Figures 4A and 4B). Moreover, the number of OMP+ mOSNs was also significantly reduced in YAPK5-CKO mice from 14 dpl to 28 dpl (Figures 4A and 4C). However, the number of Sox9+ Bowman’s gland duct cells was not affected in the YAPK5-CKO mice at 14 dpl (Figures 4D and 4E). These results suggest that YAP deletion in HBCs reduces the number of OSNs after OE injury, probably due to reduction of HBC proliferation.
Figure 4.
YAP deletion in HBCs reduces the regeneration of OSNs after OE injury
(A) Double immunostaining of GAP43 (green) and OMP (red) in the OE of 2-month-old YAPf/f and YAPK5-CKO mice at 0, 7, and 14 dpl.
(B and C) Quantitative analysis of the density of GAP43+ (B) or OMP+ (C) cells in the OE of 2-month-old YAPf/f and YAPK5-CKO mice at 0, 3, 7, 14, and 28 dpl (n = 9 sections from 5 mice per group).
(D) Immunostaining of Sox9 (green) in the OE of 2-month-old YAPf/f and YAPK5-CKO mice at 0 and 14 dpl.
(E) Quantitative analysis of the density of Sox9+ cells as shown in (D) (n = 11 sections from 5 mice per group).
(F) Immunostaining of OMP (red) in the OB of 2-month-old YAPf/f and YAPK5-CKO mice at 0, 7, and 14 dpl. EPL, external plexiform layer; GL, glomerular layer.
(G) Quantitative analysis of the percentage of area that showed OMP+ area over granule cell layer (GCL) area as shown in (F) (n = 11 sections from 5 mice per group).
Images of selected regions are shown at higher magnification. Data are mean ± SEM, two-way ANOVA with Bonferroni’s post tests, compared with YAPf/f group; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 20 μm.
To examine the effects of YAP in HBCs on the axonal projections to the glomeruli of newly generated OSNs, we measured the OMP-positive areas within individual glomeruli of OB at 0, 7, and 14 dpl. Previous studies have shown that the OMP-positive area within the individual glomeruli in OB decreases at 7 and 14 dpl, probably due to the clearance of the degenerative olfactory axons after injury (Kikuta et al., 2015). Consistently, the positive area of OMP signal in OB decreased at 7 and 14 days after injury (Figures 4F and 4G), and the decrease was more significantly in YAPK5-CKO mice (Figures 4F and 4G). These results therefore suggest that YAP deletion in HBCs results in less projection of axons from newly generated OSNs.
The upstream signaling molecule S1PR2 is expressed in HBCs and activates YAP in OE after injury
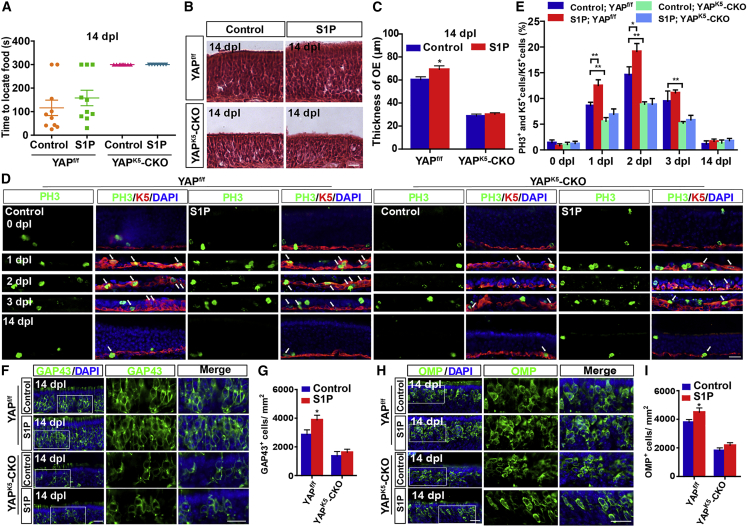
Given that S1P, being upstream of YAP, plays a prominent role in cell proliferation (Cheng et al., 2018; Liu et al., 2018; Yu et al., 2016), to explore whether S1P/YAP signaling enhances OE regeneration, S1P was applied to the methimazole-treated YAPf/f and YAPK5-CKO mice. Although S1P treatment did not improve functional recovery of the olfactory system (Figure 5A), it indeed accelerated OE regeneration of YAPf/f mice; however, it failed to promote OE regeneration of YAPK5-CKO mice (Figures 5B and 5C). Furthermore, S1P treatment promoted the proliferation of HBCs in YAPf/f mice but not in YAPK5-CKO mice at 1 dpl and 2 dpl (Figures 5D and 5E). Additionally, there was a significant increase in GAP43+ iOSNs and OMP+ mOSNs after S1P treatment at 14 dpl in YAPf/f mice but not in YAPK5-CKO mice (Figures 5F–5I).
Figure 5.
S1P promotes OE regeneration after injury through YAP signaling
(A) Quantitative analysis of the time to locate food in the buried pellet test in control-treated YAPf/f (n = 10 mice), S1P-treated YAPf/f (n = 10 mice), control-treated YAPK5-CKO (n = 10 mice), and S1P-treated YAPK5-CKO 2-month-old mice (n = 7 mice).
(B) H&E staining of the OE of control-treated 2-month-old YAPf/f and YAPK5-CKO mice and S1P-treated 2-month-old YAPf/f and YAPK5-CKO mice.
(C) Quantitative analysis of the thickness of OE as shown in (B) (n = 10 sections from 5 mice per group).
(D) Double immunostaining of PH3 (green) and K5 (red) in the OE of control-treated 2-month-old YAPf/f and YAPK5-CKO mice and S1P-treated 2-month-old YAPf/f and YAPK5-CKO mice at 0, 1, 2, 3, and 14 dpl.
(E) Quantitative analysis of the percentage of both PH3+ and K5+ cells over K5+ HBCs as shown in (D) (n = 10 sections from 5 mice per group).
(F and H) Immunostaining of GAP43 (green) (F) or OMP (green) (H) in the OE of control-treated 2-month-old YAPf/f and YAPK5-CKO mice and S1P-treated 2-month-old YAPf/f and YAPK5-CKO mice at 14 dpl.
(G and I) Quantitative analysis of the density of GAP43+ cells (G) or OMP+ cells (I) per mm2 as shown in (F) and (H) (n = 8 sections from 5 mice per group).
Images of selected regions are shown at higher magnification. Data are mean ± SEM, two-way ANOVA with Bonferroni’s post tests, compared with control group; ∗p < 0.05, ∗∗p < 0.01. Scale bars, 20 μm.
Double immunostaining further showed that S1PR1 was not expressed in HBCs but in lamina propria (LP) (Figure S4A); however, S1PR2 was mainly expressed in HBCs (Figure S4B). Additionally, the expression of S1PR2 in HBCs was significantly increased from 3 dpl to 14 dpl (Figures S4C and S4D), and the expression pattern of S1PR2 in HBCs after injury was similar to that of YAP, indicating that S1PR2 might be an upstream molecule of YAP signaling (Figure S4E).
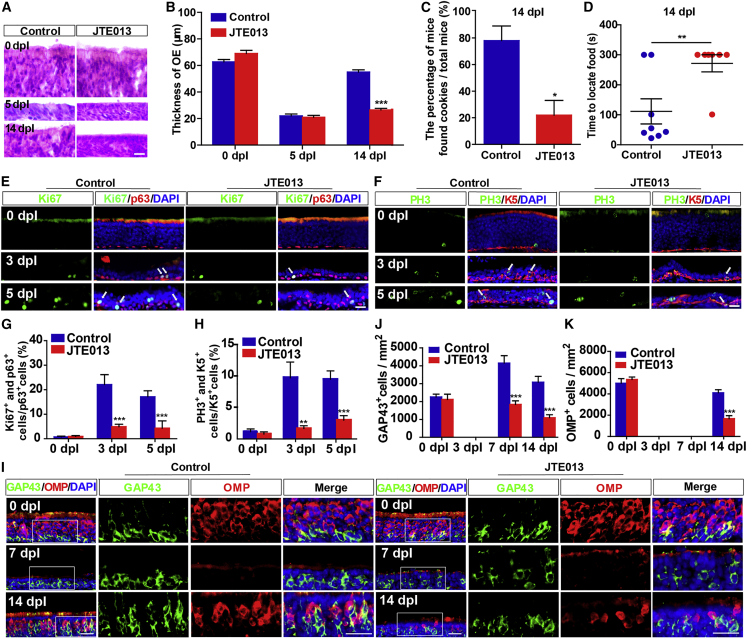
Moreover, the levels of YAP in HBCs as well as YAP+ HBCs were reduced after JTE013 (an antagonist of S1PR2) treatment from 5 dpl to 14 dpl (Figures S4F–S4I). In addition, OE became significantly thinner after treatment with JTE013 at 14 dpl (Figures 6A and 6B). The buried pellet test showed that JTE013 treatment significantly increased the possibility of failure (Figure 6C) and prolonged the time taken to find food at 14 dpl (Figure 6D). Furthermore, the percentage of Ki67+ or PH3+ cells in HBCs was reduced after JTE013 treatment at 3 dpl and 5 dpl (Figures 6E–6H). There was a significant reduction in the number of GAP43+ iOSNs after JTE013 treatment at 7 dpl to 14 dpl (Figures 6I and 6J). Moreover, the number of OMP+ mOSNs was also significantly reduced in the JTE013 group at 14 dpl (Figures 6I and 6K). These results suggest that S1P/S1PR2/YAP signaling promotes HBC proliferation and OE regeneration after injury.
Figure 6.
Inhibition of S1PR2 impairs OE regeneration and functional recovery of the olfactory system after injury
(A) H&E staining of the OE obtained from control- and JTE013-treated 2-month-old C57BL/6 mice at 0, 5, and 14 dpl.
(B) Quantitative analysis of the thickness of OE as shown in (A) (n = 7 sections from 4 mice per group).
(C) Percentage of mice that found cookies over total observed control-treated (n = 8 mice) or JTE013-treated 2-month-old C57BL/6 mice (n = 7 mice) in the buried pellet test at 14 dpl (Student’s t test).
(D) Quantitative analysis of the time to locate food in control-treated (n = 8 mice) or JTE013-treated 2-month-old C57BL/6 mice (n = 7 mice) at 14 dpl in the buried pellet test (Student’s t test).
(E and F) Double immunostaining of Ki67 (green) and p63 (red) (E) or PH3 (green) and K5 (red) (F) in the OE of control- and JTE013-treated 2-month-old C57BL/6 mice at 0, 3, and 5 dpl.
(G and H) Quantitative analysis of the percentage of both Ki67+ and p63+ cells (G) or PH3+ and K5+ (H) cells over p63+ or K5+ HBCs as shown in (E) and (F) (n = 10 sections from 4 mice per group).
(I) Double immunostaining of GAP43 (green) and OMP (red) in the OE of control- and JTE013-treated 2-month-old C57BL/6 mice at 0, 7, and 14 dpl.
(J and K) Quantitative analysis of the density of GAP43+ (J) or OMP+ (K) cells at 0, 3, 7, and 14 dpl (n = 6 sections from 4 mice per group).
Images of selected regions are shown at higher magnification. Data are mean ± SEM, two-way ANOVA with Bonferroni’s post tests unless otherwise indicated, compared with control group; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 20 μm.
Activation of YAP promotes HBC proliferation, OE regeneration, and functional recovery of the olfactory system after acute injury or in AD model mice
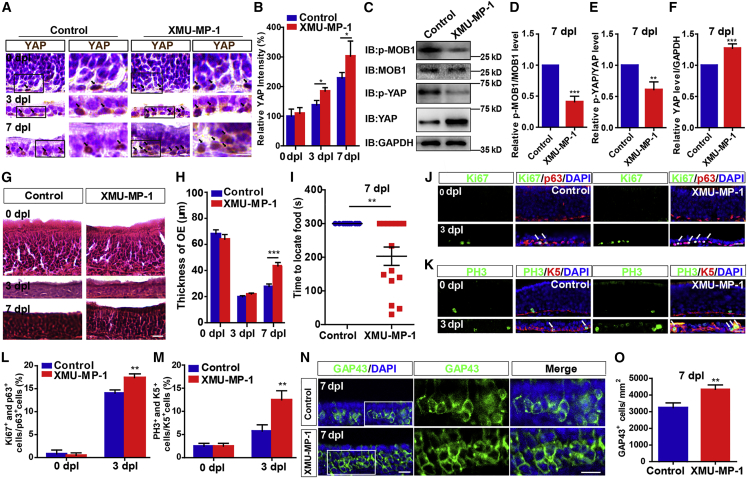
To test whether activation of YAP signaling promotes OE regeneration after injury, we applied XMU-MP-1, an inhibitor of MST1 and MST2. As expected, XMU-MP-1 treatment indeed significantly activated YAP in HBCs at 3 dpl and 7 dpl (Figures 7A–7C and 7F), and decreased the p-MOB1/MOB1 and p-YAP/YAP ratio (Figures 7C–7E). Interestingly, XMU-MP-1 treatment increased the thickness of OE (Figures 7G and 7H) and promoted functional recovery of the olfactory system at 7 dpl (Figure 7I). Furthermore, the proliferation of HBCs was increased at 3 dpl by XMU-MP-1 treatment (Figures 7J–7M). The number of GAP43+ OSNs was significantly increased at 7 dpl by XMU-MP-1 treatment (Figures 7N and 7O). These results suggest that YAP activation promotes OE regeneration and functional recovery of the olfactory system after acute injury.
Figure 7.
Activation of YAP signaling by XMU-MP-1 promotes OE regeneration and functional recovery of the olfactory system after injury
(A) Immunohistochemistry detected the expression of YAP in the OE of control- and XMU-MP-1-treated 2-month-old C57BL/6 mice at 0, 3, and 7 dpl.
(B) Quantitative analysis of the relative YAP density as shown in (A) (n = 6 sections from 6 mice per group).
(C) Western blot detected the expression of p-MOB1, MOB1, p-YAP, and YAP in OE of control- and XMU-MP-1-treated 2-month-old C57BL/6 mice at 7 dpl.
(D and F) Quantitative analysis of the relative p-MOB1/MOB1 (D), p-YAP/YAP (E), and YAP (F) levels as shown in (C) (normalized with control group, n = 8 blots from 4 mice per group, Student’s t test).
(G) H&E staining of the OE obtained from control- and XMU-MP-1-treated 2-month-old C57BL/6 mice at 0, 3, and 7 dpl.
(H) Quantitative analysis of the thickness of OE as shown in (G) (n = 12 sections from 6 mice per group).
(I) Quantitative analysis of the time to locate food of control-treated (n = 10 mice) and XMU-MP-1-treated 2-month-old C57BL/6 mice (n = 13 mice) in the buried pellet test at 7 dpl (Student’s t test).
(J and K) Double immunostaining of Ki67 (green) and p63 (red) (J) or PH3 (green) and K5 (red) (K) in the OE of control- and XMU-MP-1-treated 2-month-old C57BL/6 mice at 0 and 3 dpl.
(L and M) Quantitative analysis of the percentage of both Ki67+ and p63+ (L) or PH3+ and K5+ (M) cells over p63+ or K5+ HBCs as shown in (J) and (K) (n = 13 sections from 6 mice per group).
(N) Immunostaining of GAP43 (green) in the OE of control- and XMU-MP-1-treated 2-month-old C57BL/6 mice at 7 dpl.
(O) Quantitative analysis of the density of GAP43+ cells as shown in (N) (n = 12 sections from 6 mice per group, Student’s t test).
Images of selected regions are shown at higher magnification. Data are mean ± SEM, two-way ANOVA with Bonferroni’s post tests unless otherwise indicated, compared with control group; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bars, 20 μm.
Recent studies have shown that Aβ appears in OE after 1–2 months of age, coinciding with the degeneration of OSNs in AD model mice and patients as well as rapid decrease in the OE thickness (Bahar-Fuchs et al., 2010; Wu et al., 2013; Yoo et al., 2017). To explore the therapeutic effect of YAP on olfactory dysfunction in AD, we used APP/PS1 model mice. Thioflavin-S staining revealed a widespread distribution of Aβ plaques in cortex and hippocampus of AD model mice (Figure S5A). Interestingly, there was a significant decrease in YAP expression of AD model mice (Figures S5B and S5C). The buried pellet test also showed that, to a certain extent, the olfactory function of these mice was improved (Figure S5D). Furthermore, XMU-MP-1 treatment promoted the proliferation of HBCs (Figures S5E and S5F) and the regeneration of GAP43+ iOSNs and OMP+ mOSNs (Figures S5G–S5I) in AD model mice. These results suggest that YAP activation is sufficient to induce the proliferation of HBCs, promote OE regeneration, and improve functional recovery of the olfactory system in AD model mice.
Discussion
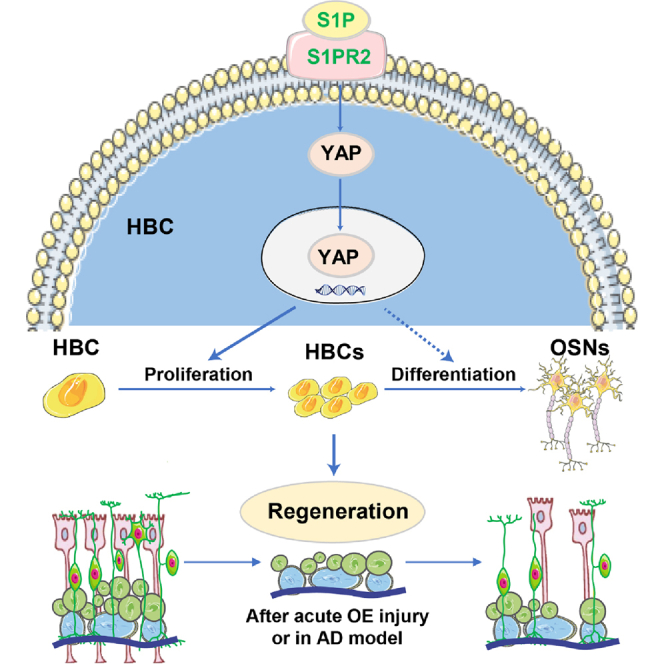
In this study, our findings reveal that YAP in HBCs is not required for development of the olfactory system. However, after OE injury, the S1P/S1PR2/YAP signaling triggers OE regeneration and improves functional recovery of the olfactory system by promoting the proliferation and differentiation of HBCs (Figure S6).
YAP signaling controls the regeneration of tissues, especially those characterized by rapid regeneration and renewal capability (Mendoza-Reinoso and Beverdam, 2018), such as the epidermis. In addition, previous studies showed that the distribution of YAP in epidermal tissue is similar to that of keratin, suggesting that YAP is mainly distributed in the basal cells of the epidermis (Akladios et al., 2017). Similarly, the present study showed that YAP was highly expressed in the HBCs of OE but not in OSNs and GBCs (Figure 1). Additionally, YAP knockout in the HBCs had no effect on the development of OE and olfactory functions (Figure S3). These results were consistent with those from previous studies, which have shown that YAP knockout in stem cells did not affect the proliferation of NSCs (Huang et al., 2016), pancreatic stem cells (Zhang et al., 2014), and small intestinal stem cells (Azzolin et al., 2014). However, several other studies have shown that YAP is necessary for the regeneration of epithelial stem cells after injury (Hageman et al., 2020). In mouse epidermal basal cells, YAP has been shown to play a key role in the formation of a positive feedback loop with the epidermal growth factor receptor to promote the proliferation of basal stem cells after injury (Elbediwy et al., 2016). Additionally, YAP in the stem cells of the small intestine has been reported to be involved in intestinal regeneration after drug- or irradiation-induced injury (Gregorieff et al., 2015; Taniguchi et al., 2015). Furthermore, previous studies have shown that TAZ/WWTR1 is also important for stem/progenitor cell functions, and loss of either YAP or TAZ individually had no visible phenotype, confirming that the two proteins act in a redundant fashion in this tissue (Elbediwy et al., 2016). Consistently, our studies also showed that YAP and TAZ were both significantly upregulated in OE after injury (Figure 1), indicating that YAP and TAZ might be important in OE after injury. Additionally, we found that the deletion of YAP in HBCs reduced their proliferation (Figure 3) and impaired the recovery of olfactory function (Figure 2) in the methimazole-induced OE injury model. These results indicate that YAP signaling in HBCs is required for OE regeneration after injury.
S1P is a sphingolipid metabolite with a wide range of biological activities and mainly secretes lipid mediators in the extracellular environment (Yu et al., 2012). S1PRs/YAP signaling plays an important role in various physiological and pathological conditions (Liu et al., 2018; Yu et al., 2012). In this study, S1PR1 was shown to be expressed in the LP but not in OE. S1PR2 was mainly expressed in HBCs and significantly upregulated in HBCs after injury (Figure S4). Moreover, treatment with JTE013 significantly inhibited the expression of YAP in HBCs (Figure S4), and attenuated the proliferation of HBCs and the regeneration of OSNs, thereby delaying regeneration of OE and the recovery (Figure 6). Notably, S1P was also shown to be generated in both blood and endothelial cells (Tukijan et al., 2018), which can release S1P into the circulation. Therefore, it is possible that after OE injury, the injured sites and/or the surrounding tissues such as blood vessels release S1P. Interestingly, although the exogenous application of S1P did not significantly improve the olfactory function recovery after injury (Figure 5), it indeed accelerated the regeneration of OE in the YAPf/f group; however, S1P failed to promote regeneration of OE in YAPK5-CKO mice (Figure 5). Taken together, our results show that S1P/S1PR2/YAP signaling promotes the OE regeneration.
Previous studies including those from Schwob’s lab found that Notch-mediated downregulation of p63 is necessary and sufficient for the activation of HBCs and promotion of OE regeneration (Herrick et al., 2017, 2018; Packard et al., 2011). Interestingly, some studies about crosstalk between p63 and the Hippo/YAP pathway were recently published (Saladi et al., 2017). In different subsets of head and neck squamous cell carcinomas, YAP has been shown to be alternatively dysregulated by Akt phosphorylation and cytoplasmic sequestration or through transcriptional repression by ΔNp63. Dysregulation of YAP, in turn, promoted cell proliferation and migration (Ehsanian et al., 2010). On the other hand, YAP can also positively regulate p63 in airway epithelial cells to tune airway epithelial size and architecture (Zhao et al., 2014). Interestingly, some studies have reported that YAP represses ΔNp63 through ZEB2 to inhibit squamous cells trans-differentiation in Lkb1-deficient lung adenocarcinoma (Gao et al., 2014). These previous results indicate that YAP might act as a negative upstream regulator of p63, leading to the activation of HBCs, thereby enhancing functional recovery in OE. Nonetheless, the specific regulatory mechanisms need to be explored further in future.
This study also sought to elucidate the therapeutic effect of YAP on the functional recovery of the olfactory system. We found that activation of YAP enhanced the proliferation of HBCs and promoted OE regeneration after injury (Figure 7) or in AD model mice (Figure S5). Recent studies have shown that Aβ appears in OE at 1–2 months of age, coinciding with the rapid decrease in OE thickness and degeneration of OSNs in both AD model mice and patients (Kovács et al., 1999; Yoo et al., 2017). Therefore, these data indicate that AD model mice suffer from chronic OE injury that leads to olfactory dysfunction and might be considered as a special kind of disease model for chronic OE injury. In summary, YAP may be a potential novel therapeutic target for the management of olfactory dysfunction caused by acute or chronic OE injury.
Experimental procedures
Mouse breeding and genotyping
YAPK5-CKO mice were obtained by crossing the floxed YAP allele (YAPf/f) with K5-Cre transgenic mice (gifted from Prof. Xiao Yang’s lab, Chinese Academy of Military Science, Beijing, China) (Yan et al., 2015). AD model mice (APP/PS1 mice) were purchased from Shanghai Model Organisms Center. Animal tests were approved by the Animal Bioethics Committee of Wenzhou Medical University and Hangzhou Normal University.
The methimazole-induced OE injury model
In brief, methimazole (#301507, Sigma) was dissolved in PBS (10 mg/mL in stock solution) and administered to 2-month-old mice at a dose of 75 mg/kg by intraperitoneal injection. Post-injection care was then provided.
Injection of drugs and reagents
After methimazole injection, mice were treated with JTE013 (#10009458, Cayman), XMU-MP-1 (HY-100526, MCE), or S1P (HY-108496, MCE). JTE013 was dissolved in PBS containing DMSO (5%) and BSA (3%) before being injected intraperitoneally at a dose of 3 mg/kg per day after injury (Japtok et al., 2015). XMU-MP-1 was dissolved in DMSO and injected intraperitoneally at a dose of 1 mg/kg, given every 2 days after injury (Xie et al., 2020). S1P was dissolved in 0.001% BSA and injected subcutaneously at a dose of 1 mg/mL, given every 7 days after injury (Roviezzo et al., 2015).
Behavioral test and analysis
In brief, cookies were put in cages 3 days before testing. In addition, mice were subjected to a fast for 18–20 h with water as the only provision, and transferred into the cages followed by acclimation for 5 min in a quiet and gloomy environment. A piece of cookie was buried 2.3–2.5 cm below the surface of a 3-cm-deep layer of clean bedding material, and the location of the food pellet was changed regularly in a random pattern. The mice were considered to have successfully completed the task after grasping the buried food with their forepaws and/or teeth. The time of completion was defined as the duration within which a mouse found the food after being transferred into the cage. Subjects that failed to find the food particles within 5 min were considered to have abnormal olfactory function.
Western blotting
OE tissues were lysed in lysis buffer for 30 min on ice. The lysis buffer consisted of 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% Triton X-100, 5 mM sodium fluoride, 1 mM EDTA, 1 mM phenylmethylsulfonylfluoride, 2 mM sodium orthovanadate, and a protease inhibitor cocktail (P8340, Sigma). The lysates were then centrifuged at 12,000 rpm for 30 min to obtain the suspended proteins. The proteins were extracted after adding 5× loading buffer and denatured at 100°C for 10 min before being separated by SDS-PAGE (8%–12%). The proteins were then transferred into nitrocellulose membranes (Life Sciences) and immersed in 5% skim milk for blocking at room temperature for 1 h, followed by an overnight incubation with primary antibodies at 4°C, then rinsed with Tris-buffered saline with Tween 20 and incubated for 1 h at room temperature after adding the appropriate secondary antibodies (1:5,000, Bio-Rad). Primary antibodies included anti-p-YAP (Ser127, 1:1,000, #4911, CST), anti-YAP (1:1,000, ab205270, Abcam), anti-TAZ (1:1,000, DF4653, Affinity), anti-MOB1 (1:1,000, #13730, CST), and anti-p-MOB1 (Thr35, 1:1,000, #8699, CST). Anti-GAPDH (1:5,000, #2118, CST) and anti-β-actin (1:10,000, A5316, Sigma) were used as loading controls. Protein bands were detected through ECL (Bio-Rad), and analyzed by Quantity One software (Bio-Rad).
Immunostaining
After perfusion with 0.1 M PBS followed by 4% PFA, the OE tissues were fixed with fresh 4% PFA after which they were decalcified using 10% EDTA in PBS at 4°C for 5 days. All tissues were also dehydrated using 30% sucrose in PBS until they sank. The OE tissues were then sectioned in 20-μm-thick sections. Sections were washed three times with PBS and fixed with fresh 4% PFA for 30 min. The antigens were repaired for 30 min at 90°C using sodium citrate antigen retrieval solution (Solarbio). The tissue sections were immersed in 0.3% Triton X-100 and 5% BSA for blocking at room temperature and processed by incubation with primary antibodies at 4°C overnight (Ho et al., 2019; Inokuchi et al., 2017; Ito-Ishida et al., 2018; Xie et al., 2020). Primary antibodies included anti-YAP (1:200, WH0010413M1, Sigma), anti-YAP (1:200, ab205270, Abcam), anti-p63 (1:200, CM 163A, Biocare Medical), anti-p63 (1:200, sc-25268, Santa Cruz Biotechnology), anti-K5 (1:200, ab52635, Abcam), anti-GAP43 (1:500, ab16053, Abcam), anti-OMP (1:500, #544-10001, Wako), anti-S1PR1 (1:200, ab23695, Abcam), anti-S1PR2 (1:200, 21180-AP, Proteintech), anti-Ki67 (1:300, AB9260, Millipore), anti-Ki67 (1:200, ab16667, Abcam), anti-PH3 (1:500, ab14955, Abcam), anti-Sox9 (1:500, ab185966, Abcam), and anti-LSD1 (1:200, ab17721, Abcam). The sections were washed three times with PBS and incubated with the corresponding secondary antibodies (1:1,000, Invitrogen) for 1 h. Images were acquired by a SLIDEVIEW VS200 microscope (Olympus, Germany) and confocal microscopes (Nikon, Japan), and analyzed by Image J or Adobe Photoshop CC 14.0 software.
Statistical analysis
All the data presented represent results from at least three independent experiments. GraphPad Prism software was used for statistical analysis, and Student’s t test, one-way ANOVA, or two-way ANOVA were utilized. Statistical significance was defined as p < 0.05.
Author contributions
Z.H., Y.W., and X.X. contributed to the conception and design of the study. Q.W. and X.M. carried out most of the western blot and immunofluorescent staining experiments. Q.W., X.B., W.W., L.X., X.S., X.W., D.Y., S.D., and J.Z. contributed to the acquisition and interpretation of the data and organized the pictures. Y.F., S.X., Z.C., X.L., Y.L., F.L., and S.L. helped to breed mice and did genotyping. Q.W. wrote the manuscript. X.X., Y.W., H.T., and Z.H. contributed to revising the original text and provided the funding. All authors approved the final version of the manuscript.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (LR18C090001, LQ21C090009), National Natural Science Foundation (92049104, 81771348, 81971172), Planted Talent Plan Projects for College Students in Zhejiang Province (2019R413078 and 2020R413077), National College Students’ Innovation and Entrepreneurship Training Program (201910343027), Basic Scientific Research Project of Wenzhou (Y2020241).
Published: February 10, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.01.007.
Contributor Information
Ying Wang, Email: nancywangying@163.com.
Honglin Teng, Email: honlinten@163.com.
Zhihui Huang, Email: huang0069@hznu.edu.cn.
Supplemental information
References
- Akladios B., Mendoza-Reinoso V., Samuel M.S., Hardeman E.C., Khosrotehrani K., Key B., Beverdam A. Epidermal YAP2-5SA-ΔC drives β-catenin activation to promote keratinocyte proliferation in mouse skin in vivo. J. Invest. Dermatol. 2017;137:716–726. doi: 10.1016/j.jid.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Andl T., Zhou L., Yang K., Kadekaro A.L., Zhang Y. YAP and WWTR1: new targets for skin cancer treatment. Cancer Lett. 2017;396:30–41. doi: 10.1016/j.canlet.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Attems J., Walker L., Jellinger K.A. Olfaction and aging: a mini-review. Gerontology. 2015;61:485–490. doi: 10.1159/000381619. [DOI] [PubMed] [Google Scholar]
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Bahar-Fuchs A., Chételat G., Villemagne V.L., Moss S., Pike K., Masters C.L., Rowe C., Savage G. Olfactory deficits and amyloid-β burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: a PiB PET study. J. Alzheimers Dis. 2010;22:1081–1087. doi: 10.3233/jad-2010-100696. [DOI] [PubMed] [Google Scholar]
- Bao X., Xu X., Wu Q., Zhang J., Feng W., Yang D., Li F., Lu S., Liu H., Shen X., et al. Sphingosine 1-phosphate promotes the proliferation of olfactory ensheathing cells through YAP signaling and participates in the formation of olfactory nerve layer. Glia. 2020;68:1757–1774. doi: 10.1002/glia.23803. [DOI] [PubMed] [Google Scholar]
- Carter L.A., MacDonald J.L., Roskams A.J. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J. Neurosci. 2004;24:5670–5683. doi: 10.1523/jneurosci.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Reed R.R., Lane A.P. Acute inflammation regulates neuroregeneration through the NF-κB pathway in olfactory epithelium. Proc. Natl. Acad. Sci. U S A. 2017;114:8089–8094. doi: 10.1073/pnas.1620664114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Reed R.R., Lane A.P. Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. 2019;25:501–513.e5. doi: 10.1016/j.stem.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Tian S., Yang X., Lane A.P., Reed R.R., Liu H. Wnt-responsive Lgr5⁺ globose basal cells function as multipotent olfactory epithelium progenitor cells. J. Neurosci. 2014;34:8268–8276. doi: 10.1523/jneurosci.0240-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.C., Wang E.Y., Yi Y., Thakur A., Tsai S.H., Hoodless P.A. S1P stimulates proliferation by upregulating CTGF expression through S1PR2-mediated YAP activation. Mol. Cancer Res. 2018;16:1543–1555. doi: 10.1158/1541-7786.mcr-17-0681. [DOI] [PubMed] [Google Scholar]
- Choi R., Goldstein B.J. Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018;3:35–42. doi: 10.1002/lio2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R.L. Age-related deficits in taste and smell. Otolaryngol. Clin. North Am. 2018;51:815–825. doi: 10.1016/j.otc.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Ehsanian R., Brown M., Lu H., Yang X.P., Pattatheyil A., Yan B., Duggal P., Chuang R., Doondeea J., Feller S., et al. YAP dysregulation by phosphorylation or ΔNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. 2010;29:6160–6171. doi: 10.1038/onc.2010.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A., Thompson B.J. Evolution of mechanotransduction via YAP/TAZ in animal epithelia. Curr. Opin. Cell Biol. 2018;51:117–123. doi: 10.1016/j.ceb.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Elbediwy A., Vincent-Mistiaen Z.I., Spencer-Dene B., Stone R.K., Boeing S., Wculek S.K., Cordero J., Tan E.H., Ridgway R., Brunton V.G., et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R.B., Das D., Gadye L., Street K.N., Baudhuin A., Wagner A., Cole M.B., Flores Q., Choi Y.G., Yosef N., et al. Deconstructing olfactory stem cell trajectories at single-cell resolution. Cell Stem Cell. 2017;20:817–830.e8. doi: 10.1016/j.stem.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher R.B., Prasol M.S., Estrada J., Baudhuin A., Vranizan K., Choi Y.G., Ngai J. p63 regulates olfactory stem cell self-renewal and differentiation. Neuron. 2011;72:748–759. doi: 10.1016/j.neuron.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhang W., Han X., Li F., Wang X., Wang R., Fang Z., Tong X., Yao S., Li F., et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat. Commun. 2014;5:4629. doi: 10.1038/ncomms5629. [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Liu Y., Inanlou M.R., Khomchuk Y., Wrana J.L. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- Hageman J.H., Heinz M.C., Kretzschmar K., van der Vaart J., Clevers H., Snippert H.J.G. Intestinal regeneration: regulation by the microenvironment. Dev. Cell. 2020;54:435–446. doi: 10.1016/j.devcel.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Herrick D.B., Guo Z., Jang W., Schnittke N., Schwob J.E. Canonical notch signaling directs the fate of differentiating neurocompetent progenitors in the mammalian olfactory epithelium. J. Neurosci. 2018;38:5022–5037. doi: 10.1523/jneurosci.0484-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick D.B., Lin B., Peterson J., Schnittke N., Schwob J.E. Notch1 maintains dormancy of olfactory horizontal basal cells, a reserve neural stem cell. Proc. Natl. Acad. Sci. U S A. 2017;114:E5589–E5598. doi: 10.1073/pnas.1701333114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I.H.T., Liu X., Zou Y., Liu T., Hu W., Chan H., Tian Y., Zhang Y., Li Q., Kou S., et al. A novel peptide interfering with proBDNF-sortilin interaction alleviates chronic inflammatory pain. Theranostics. 2019;9:1651–1665. doi: 10.7150/thno.29703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook E.H., Szumowski K.E., Schwob J.E. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J. Comp. Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Huang Z., Hu J., Pan J., Wang Y., Hu G., Zhou J., Mei L., Xiong W.C. YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development. 2016;143:2398–2409. doi: 10.1242/dev.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J.M., Schwob J.E. Cell cycle of globose basal cells in rat olfactory epithelium. Dev. Dyn. 1995;203:17–26. doi: 10.1002/aja.1002030103. [DOI] [PubMed] [Google Scholar]
- Inokuchi K., Imamura F., Takeuchi H., Kim R., Okuno H., Nishizumi H., Bito H., Kikusui T., Sakano H. Nrp2 is sufficient to instruct circuit formation of mitral-cells to mediate odour-induced attractive social responses. Nat. Commun. 2017;8:15977. doi: 10.1038/ncomms15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito-Ishida A., Yamalanchili H.K., Shao Y., Baker S.A., Heckman L.D., Lavery L.A., Kim J.Y., Lombardi L.M., Sun Y., Liu Z., et al. Genome-wide distribution of linker histone H1.0 is independent of MeCP2. Nat. Neurosci. 2018;21:794–798. doi: 10.1038/s41593-018-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai N., Zhou Z., Roop D.R., Behringer R.R. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japtok L., Schmitz E.I., Fayyaz S., Krämer S., Hsu L.J., Kleuser B. Sphingosine 1-phosphate counteracts insulin signaling in pancreatic β-cells via the sphingosine 1-phosphate receptor subtype 2. Faseb J. 2015;29:3357–3369. doi: 10.1096/fj.14-263194. [DOI] [PubMed] [Google Scholar]
- Joiner A.M., Green W.W., McIntyre J.C., Allen B.L., Schwob J.E., Martens J.R. Primary cilia on horizontal basal cells regulate regeneration of the olfactory epithelium. J. Neurosci. 2015;35:13761–13772. doi: 10.1523/jneurosci.1708-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S., Sakamoto T., Nagayama S., Kanaya K., Kinoshita M., Kondo K., Tsunoda K., Mori K., Yamasoba T. Sensory deprivation disrupts homeostatic regeneration of newly generated olfactory sensory neurons after injury in adult mice. J. Neurosci. 2015;35:2657–2673. doi: 10.1523/jneurosci.2484-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács T., Cairns N.J., Lantos P.L. beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol. Appl. Neurobiol. 1999;25:481–491. doi: 10.1046/j.1365-2990.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- Lee M.J., Byun M.R., Furutani-Seiki M., Hong J.H., Jung H.S. YAP and TAZ regulate skin wound healing. J. Invest. Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- Leung C.T., Coulombe P.A., Reed R.R. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat. Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Lian I., Kim J., Okazawa H., Zhao J., Zhao B., Yu J., Chinnaiyan A., Israel M.A., Goldstein L.S., Abujarour R., et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zhai C., Pan Y., Zhu Y., Shi W., Wang J., Yan X., Su X., Song Y., Gao L., et al. Sphingosine-1-phosphate induces airway smooth muscle cell proliferation, migration, and contraction by modulating Hippo signaling effector YAP. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;315:L609–L621. doi: 10.1152/ajplung.00554.2017. [DOI] [PubMed] [Google Scholar]
- Mendoza-Reinoso V., Beverdam A. Epidermal YAP activity drives canonical WNT16/β-catenin signaling to promote keratinocyte proliferation in vitro and in the murine skin. Stem Cell Res. 2018;29:15–23. doi: 10.1016/j.scr.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Moya I.M., Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019;20:211–226. doi: 10.1038/s41580-018-0086-y. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Takezawa K., Shimizu S., Shimizu T. Valproic acid promotes neural regeneration of olfactory epithelium in adult mice after methimazole-induced damage. Am. J. Rhinol. Allergy. 2014;28:e95–e99. doi: 10.2500/ajra.2014.28.4027. [DOI] [PubMed] [Google Scholar]
- Packard A., Schnittke N., Romano R.A., Sinha S., Schwob J.E. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J. Neurosci. 2011;31:8748–8759. doi: 10.1523/jneurosci.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J., Lin B., Barrios-Camacho C.M., Herrick D.B., Holbrook E.H., Jang W., Coleman J.H., Schwob J.E. Activating a reserve neural stem cell population in vitro enables engraftment and multipotency after transplantation. Stem Cell Rep. 2019;12:680–695. doi: 10.1016/j.stemcr.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognoni E., Walko G. The roles of YAP/TAZ and the Hippo pathway in healthy and diseased skin. Cells. 2019;8:411. doi: 10.3390/cells8050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roviezzo F., Sorrentino R., Bertolino A., De Gruttola L., Terlizzi M., Pinto A., Napolitano M., Castello G., D'Agostino B., Ianaro A., et al. S1P-induced airway smooth muscle hyperresponsiveness and lung inflammation in vivo: molecular and cellular mechanisms. Br. J. Pharmacol. 2015;172:1882–1893. doi: 10.1111/bph.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladi S.V., Ross K., Karaayvaz M., Tata P.R., Mou H., Rajagopal J., Ramaswamy S., Ellisen L.W. ACTL6A is co-amplified with p63 in squamous cell carcinoma to drive YAP activation, regenerative proliferation, and poor prognosis. Cancer Cell. 2017;31:35–49. doi: 10.1016/j.ccell.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J.R., Zhou D., Kreger B.T., Vasioukhin V., Avruch J., Brummelkamp T.R., et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittke N., Herrick D.B., Lin B., Peterson J., Coleman J.H., Packard A.I., Jang W., Schwob J.E. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc. Natl. Acad. Sci. U S A. 2015;112:E5068–E5077. doi: 10.1073/pnas.1512272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob J.E., Jang W., Holbrook E.H., Lin B., Herrick D.B., Peterson J.N., Hewitt Coleman J. Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J. Comp. Neurol. 2017;525:1034–1054. doi: 10.1002/cne.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob J.E., Youngentob S.L., Mezza R.C. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J. Comp. Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Wu L.W., Grivennikov S.I., de Jong P.R., Lian I., Yu F.X., Wang K., Ho S.B., Boland B.S., Chang J.T., et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukijan F., Chandrakanthan M., Nguyen L.N. The signalling roles of sphingosine-1-phosphate derived from red blood cells and platelets. Br. J. Pharmacol. 2018;175:3741–3746. doi: 10.1111/bph.14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N., Rao X., Gao Y., Wang J., Xu F. Amyloid-β deposition and olfactory dysfunction in an Alzheimer's disease model. J. Alzheimers Dis. 2013;37:699–712. doi: 10.3233/jad-122443. [DOI] [PubMed] [Google Scholar]
- Xie C., Shen X., Xu X., Liu H., Li F., Lu S., Gao Z., Zhang J., Wu Q., Yang D., et al. Astrocytic YAP promotes the formation of glia scars and neural regeneration after spinal cord injury. J. Neurosci. 2020;40:2644–2662. doi: 10.1523/jneurosci.2229-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Xu Z., Lou F., Zhang L., Ke F., Bai J., Liu Z., Liu J., Wang H., Zhu H., et al. NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat. Commun. 2015;6:7652. doi: 10.1038/ncomms8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Wang Y., Zhang P., Chen H., Xu Z., Jiao J., Yuan Z. BMP2-SMAD signaling represses the proliferation of embryonic neural stem cells through YAP. J. Neurosci. 2014;34:12039–12048. doi: 10.1523/jneurosci.0486-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.J., Lee J.H., Kim S.Y., Son G., Kim J.Y., Cho B., Yu S.W., Chang K.A., Suh Y.H., Moon C. Differential spatial expression of peripheral olfactory neuron-derived BACE1 induces olfactory impairment by region-specific accumulation of β-amyloid oligomer. Cell Death Dis. 2017;8:e2977. doi: 10.1038/cddis.2017.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J., Yuan H., Tumaneng K., Li H., et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu O.M., Miyamoto S., Brown J.H. Myocardin-related transcription factor A and yes-associated protein exert dual control in G protein-coupled receptor- and RhoA-mediated transcriptional regulation and cell proliferation. Mol. Cell Biol. 2016;36:39–49. doi: 10.1128/mcb.00772-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A.J., Lee A.C., Chu H., Chan J.F., Fan Z., Li C., Liu F., Chen Y., Yuan S., Poon V.K., et al. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 2021;73:e503–e512. doi: 10.1093/cid/ciaa995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Nandakumar N., Shi Y., Manzano M., Smith A., Graham G., Gupta S., Vietsch E.E., Laughlin S.Z., Wadhwa M., et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci. Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Fallon T.R., Saladi S.V., Pardo-Saganta A., Villoria J., Mou H., Vinarsky V., Gonzalez-Celeiro M., Nunna N., Hariri L.P., et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell. 2014;30:151–165. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.