Abstract
There remains a critical need for improved staging of non-small-cell lung cancer, as recurrence and mortality due to undetectable metastases at the time of surgery remain high even after complete resection of tumors currently categorized as ‘early stage.’ A 14-gene quantitative PCR-based expression profile has been extensively validated to better identify patients at high-risk of 5-year mortality after surgical resection than conventional staging – mortality that almost always results from previously undetectable metastases. Furthermore, prospective studies now suggest a predictive benefit in disease-free survival when the assay is used to guide adjuvant chemotherapy decisions in early-stage non-small-cell lung cancer patients.
Keywords: : adjuvant therapy, molecular assay, molecular prognostic classifier, non-small-cell lung cancer, predictive, risk stratification, tumor genetic profile
Lay abstract
There is a need for improvement in the way early-stage non-small-cell lung cancers are staged and treated because many patients with ‘early-stage’ disease suffer high rates of cancer recurrence after surgery. In recent years, a specialized test has been developed to allow better characterization of a tumor's risk of recurrence based on the genes being expressed by tumor cells. Use of this test, in conjunction with standard staging methods, is better able to identify patients at high risk of cancer recurrence after surgery. Evidence suggests that giving chemotherapy to patients at high risk of recurrence after surgery reduces recurrence rates and improves long-term patient survival.
Lung cancer remains the leading cause of cancer-related mortality in the United States. It is estimated that 228,220 new cases of lung cancer are diagnosed nationally each year, leading to approximately 140,000 annual deaths [1]. Over the past several decades, even with improvements in our understanding, diagnosis and treatment of lung cancer, there has been only a modest improvement in the 5-year survival for the disease. In 1975, the 5-year survival rate for all lung cancers was 12%; today, it is estimated to be 19.9% [2].
The 5-year survival for lung cancer is dependent upon several variables, including anatomic factors – such as tumor size, local obstruction, nodal involvement and distant metastasis – and a variety of patient, immunologic and molecular factors [3]. From an anatomic standpoint, low rates of lung cancer survival reflect a substantial proportion of patients (57%) with known metastatic disease at the time of diagnosis, for whom the expected 5-year survival is only 5%. Earlier diagnosis through enhanced screening of patients at high risk of lung cancer therefore represents an opportunity for improvement via increased diagnosis and treatment before metastasis has occurred. However, even the diagnosis of smaller, earlier tumors would not completely solve the problem of high lung cancer mortality since an unusually high percentage of these small, ‘early-stage’ lung cancers already have the potential for metastasis [4–7]. As a result, only half of patients with disease that is believed to be localized at the time of diagnosis and surgical resection survive 5 years [1].
Clinically, the most widely utilized tool for predicting survival of patients with lung cancer is the tumor, node, metastasis (TNM) staging system [8]. These clinicopathological descriptors of tumors, including size, local invasion and presence of nodal or distant metastasis, were first integrated into the TNM staging system by Denoix in 1953 [9]. The eighth edition of the TNM staging system (hereinafter ‘eighth edition’) for non-small-cell lung cancer (NSCLC), the most common form of lung cancer, was released in 2018 and categorizes patients into one of 11 distinct clinical stages: IA1, IA2, IA3, IB, IIA, IIB, IIIA, IIIB, IIIC, IVA, IVB [10]. Despite the eighth edition's extensive training dataset, validation studies have demonstrated disappointing results in adequately risk-stratifying patients with stage IA–IIB disease. In these validation studies, the 5-year overall survival for patients with pathological stage I NSCLC remains as low as 55% [11,12]. Even after complete surgical resection of the earliest stage IA lung cancers, the 5-year survival is only approximately 70%, demonstrating that TNM staging alone does not adequately identify patients at high risk of undetected metastasis and therefore does not predict patient outcome [13,14].
Surgical resection is the primary treatment option for patients with stage I and II disease and represents the best opportunity for cure [15,16]. In patients with ‘completely resected’ stage I lung cancer (i.e., without evidence of distant or regional spread), recurrence typically occurs as a result of the presence of undetectable, often microscopic metastatic disease that is present at the time of surgery [17]. The 5-year recurrence rate of resected stage I NSCLC has been reported to be as high as 35–40%, demonstrating that the presence of micrometastases is a common phenomenon [18]. This is particularly staggering when compared with 5-year recurrence statistics for other types of stage I cancers, including breast cancer (3.1%) [19] and colon cancer (4.6%) [20]. In a randomized prospective trial of patients with stage I lung cancer undergoing lobectomy versus segment or wedge resection, it was shown that patients who had lobectomy developed less locoregional recurrence [21]. It is thought that this change is related to more complete excision of occult micrometastases. It has become paramount, therefore, to accurately predict which patients with early-stage disease are more likely to have micrometastases that are missed by current imaging and pathological modalities.
Over the past 15 years, substantial effort has been applied toward identifying prognostic indicators that signify aggressive NSCLC tumor biology on a molecular level. Early efforts in this arena focused on profiling oncoproteins and other cellular products that were being transcribed at a cellular level to ascertain the risk of a particular tumor [22]. More contemporary efforts have focused on genomic and gene expression models to provide additional prognostic information for risk stratification beyond that provided by the TNM staging system alone. Although multiple models have been proposed and developed, this review will focus on the only genetic model that is currently in clinical use today.
A 14-gene prognostic & predictive molecular assay for early-stage NSCLC
Tumorigenesis is thought to be promoted by both genetic and epigenetic changes within a precancerous cell and changes in the tumor microenvironment [23–25]. For example, it is clearly established that alteration in the level of expression of certain genes is causative of cancer [26]. Several groups previously developed gene expression analysis tools that predicted patients with higher than expected mortality after resection of early-stage lung cancer [17,27–35]. Many of these early gene signatures were based on a microarray platform that utilized frozen tissue samples, making their use impractical in clinical settings because of difficult reproducibility, excessive cost and lack of widespread availability [36]. This early research underscored the need for a more practical, available and reliable molecular assay to fill the need for risk stratification in patients who have early-stage disease by conventional TNM criteria but who may have a high risk of treatment failure following resection.
Development & technical validation of the 14-gene lung cancer assay
The 14-gene lung cancer assay utilizes quantitative reverse transcription PCR (qRT-PCR) for analysis of readily available and highly stable formalin-fixed, paraffin-embedded (FFPE) tissue samples [37]. The assay was designed at the University of California San Francisco (UCSF) using a training cohort of samples from 361 patients with nonsquamous NSCLC. Notably, patients with squamous cell lung carcinoma were excluded from assay development, as prior investigations have suggested fundamental differences in the molecular makeup between squamous cell and nonsquamous cell lung carcinoma [38]. Four target genes were selected from over 200 cancer-related gene candidates using L1-penalized Cox proportional hazards modeling in an initial pilot study [37]. For each sample, RNA was extracted from FFPE tissue blocks, and the relative expression of individual genes was measured using qRT-PCR. In later studies, L2-penalized Cox proportional hazards modeling was used to develop a more elaborate scoring system based on the expression of 11 active genes [39]. Gene expression profiles were translated into risk scores that reflected the biological aggression of a tumor. The resultant risk scores from the training cohort of patient samples were divided into thirds to stratify patients at low, intermediate and high risk of mortality after surgical resection of lung cancer. After the assay was completely specified, technical validation was completed in line with Clinical Laboratory Improvement Amendments (CLIA) guidelines [40].
The final assay includes 11 cancer-related target genes and three reference genes (Table 1). Several of the genes in the 14-gene lung cancer assay, including BAG1, BRCA1, CDC6, ERBB3 and WNT3A, are known participants in established oncogenic pathways. The 11 target genes are all implicated in molecular lung cancer pathways, including KRAS, BRAF, EGRF, HER2, ALK and p53. Five of the genes overlap with prognostic gene signatures previously identified in NSCLC (CDC6 [27,33,34], ERBB3 [29], FUT3 [27,33,35], LCK [29] and RND3 [33]).
Table 1. . Algorithm genes utilized in the University of California San Francisco molecular assay.
| Gene | Name | Chromosome | Reference sequence | Protein location | Relevant biological functions and pathways | Role in algorithm |
|---|---|---|---|---|---|---|
| BAG1 | BCL2-associated athanogene | 9p12 | NM_004323 | Cytoplasm | Apoptosis; cell surface receptor linked signaling | Prognosis |
| BRCA1 | Breast cancer 1, early onset | 17q21–q24 | NM_007294 | Nucleus | Induction of apoptosis; protein ubiquitination; regulation of G2/M transition DNA damage checkpoints; regulation of DNA repair | Prognosis |
| CDC6 | Cell division cycle 6 homolog | 17q21.3 | NM_001254 | Nucleus | Regulation of transcription involved in G1/S phase of mitotic cell cycle; regulation of cell proliferation | Prognosis |
| CDK2AP1 | Cyclin-dependent kinase 2-associated protein 1 | 12q24 | NM_004642 | Nucleus | Regulation of S phase of mitotic cell cycle; DNA-dependent DNA replication | Prognosis |
| ERBB3 | V-erb-b2 erythroblastic leukemia viral oncogene homolog 3 |
12q13 | NM_001982 | Plasma membrane | Regulation of cell adhesion; transmembrane receptor protein tyrosine kinase signaling; regulation of phosphoinositide 3-kinase cascade; regulation of cell proliferation | Prognosis |
| FUT3 | Fucosyltransferase 3 | 19p13.3 | NM_000149 | Cytoplasm | Carbohydrate metabolism; protein amino acid glycosylation | Prognosis |
| IL11 | Interleukin 11 | 19q13.3–q13.4 | NM_000641 | Extracellular | Regulation of cell proliferation; B-cell differentiation; megakaryocyte differentiation; regulation of peptidyl-serine phosphorylation; regulation of MAPKKK cascade | Prognosis |
| LCK | Lymphocyte-specific protein tyrosine kinase | 1p34.3 | NM_001042771 | Cytoplasm | Induction of apoptosis; activation of caspase activity; regulation of T-cell receptor signaling; leukocyte migration | Prognosis |
| RND3 | Rho family GTPase 3 | 2q23.3 | NM_005168 | Cytoplasm | Cell adhesion; small GTPase mediated signal transduction; actin cytoskeleton organization | Prognosis |
| SH3BGR | SH3 domain-binding glutamic acid-rich protein | 21q22.3 | NM_007341 | Cytoplasm | Protein complex assembly | Prognosis |
| WNT3A | Wingless-type MMTV integration site family, member 3A | 1q42 | NM_033131 | Extracellular | Canonical Wnt receptor signaling; multicellular organismal development; regulation of cell proliferation and differentiation; regulation of catenin protein nuclear translocation | Prognosis |
| ESD | Esterase D | 13q14.1–q14.2 | NM_001984 | Cytoplasm | Recycling of sialic acids; serine hydrolysis | Reference |
| TBP | TATA box binding protein | 6q27 | NM_003194 | Nucleus | Transcription initiation from RNA polymerase II promoter; RNA elongation from RNA polymerase II promoter; transcription from RNA polymerase III promoter | Reference |
| YAP1 | Yes-associated protein 1 | 11q13 | NM_006106 | Nucleus | Hippo signaling cascade; regulation of transcription; contact inhibition | Reference |
Reproduced with permission from [37] © Elsevier (2012).
One weakness of previously reported genetic prognostic models in lung cancer was the lack of external blinded validation in large independent cohorts [36]. In fact, the only study that included a blinded independent validation was published by a consortium of major academic institutions in response to a National Cancer Institute director's challenge. In this study, the best performing genomic classifier failed blinded validation without the use of clinical covariates [34]. By contrast, the 14-gene lung cancer assay was externally validated by two large-scale, multicenter, blinded studies with entirely independent cohorts and included international patients. The first external validation was completed by the Kaiser Permanente Division of Research in a cohort of 433 patients with stage I nonsquamous NSCLC. The results from the molecular assay were compared with the known patient outcomes by Kaiser Permanente Division of Research, and the 5-year lung cancer-specific survival was 84.6% (95% CI: 74.4–91.0) in the low-risk group, 70.3% (95% CI: 60.6–78.0) in the intermediate-risk group and 63.3% (95% CI: 55.8–69.8) in the high-risk group. After multivariate analysis (adjusting for patient age, sex, smoking history, tumor cell histology and tumor size), it was demonstrated that both high-risk and intermediate-risk groups were statistically significant predictors of patient mortality. A second validation involved an international, large-scale trial of the molecular assay with a cohort of 1006 Chinese patients who had undergone resection of early-stage disease at one of several participating institutions in the China Clinical Trials Consortium. Similarly, it was shown on multivariate analysis that high-risk and intermediate-risk designation remained statistically and clinically significant predictors of survival [37].
The 14-gene lung cancer assay is unique compared with its predecessors based on a variety of metrics. Although previously developed gene signatures prognostic of lung cancer survival were based on analysis of snap-frozen specimens [17,36], the 14-gene lung cancer assay utilizes FFPE tissues for analysis. Although snap-frozen tissues yield extremely high-quality RNA, they are not routinely collected, are difficult to handle and are expensive to transport. Conversely, the use of FFPE tissue samples, which are widely available, makes this assay relevant to the routine management of patients with lung cancer in a global community setting. Additionally, the assay utilizes qRT-PCR-based signatures, whereas older models used primarily microarray-based molecular profiles; qRT-PCR has a substantial advantage over microarray-based assays, including widespread availability, reduced cost, model simplicity, significantly greater reproducibility and the ability to use stored paraffin-embedded versus snap-frozen tissues [41–43].
Importantly, the 14-gene lung cancer assay is an analytically precise and reproducible tool, as validated in a CLIA-certified laboratory [40]. The quantitative PCR primer and probe data showed a linear input RNA range extending between 210- and 215-fold, which is paramount, as it enables analysis of tumors from the FFPE specimens with a wide range of RNA yields. The assay has high PCR efficiency, with a median efficiency of 91.2%, which is on par with previously published reports of FFPE qPCR-based primer and probe sets [44]. The reproducibility, tested by repeating the assay on 15 samples representative of a wide range of risk scores for five independent experiments, was also exceptionally high [40]. This demonstrated reproducibility and reliability of the UCSF molecular assay, which is a critical component for validation of the clinical utility of the test. Blinded, large-scale, independent clinical validation; the ability to run the assay on FFPE tissue specimens; the use of well-established quantitative PCR; and the high reliability and reproducibility of the assay are the major reasons the 14-gene lung cancer assay remains the only prognostic and predictive lung cancer assay in clinical use today.
Staging refinement using the 14-gene lung cancer assay
It has been previously established that biological molecular signatures can refine prognosis in patients with other types of malignancies [45,46], though such molecular predictors have yet to be formally incorporated into the established TNM staging system for lung cancer [17,36]. The utilization of the 14-gene lung cancer assay as a complement to conventional staging has been evaluated, and it has been shown to improve survival predictions [47,48].
In the initial study, a novel staging system that incorporated the 14-gene lung cancer assay was developed and validated [47]. Within the UCSF training cohort, each tumor was analyzed with the molecular assay to determine the phenotype of its biological risk profile [37]. Through a supervised reclassification staging system that integrated the outcome of the prognostic assay with the eighth edition, the patient's stage was downgraded by one stage, for low risk; upgraded by one stage, for high risk; or unchanged, for intermediate risk [47]. This newly developed staging system, renamed TNM biology (TNMB) (Table 2), was subsequently applied to the independent Kaiser Permanente Division of Research and China Clinical Trials Consortium validation cohorts (n = 1373).
Table 2. . Tumor, Node, Metastasis eighth edition staging system versus Tumor, Node, Metastasis, Biology staging system.
| TNM eighth edition stage and molecular prognostic classifier | TNMB stage |
|---|---|
| Stage IA1 | |
| Low risk | Stage IA1 |
| Intermediate risk | Stage IA2 |
| High risk | Stage IA3 |
| Stage IA2 | |
| Low risk | Stage IA1 |
| Intermediate risk | Stage IA2 |
| High risk | Stage IA3 |
| Stage IA3 | |
| Low risk | Stage IA2 |
| Intermediate risk | Stage IA3 |
| High risk | Stage IB |
| Stage IB | |
| Low risk | Stage IA3 |
| Intermediate risk | Stage IB |
| High risk | Stage IIA |
| Stage IIA | |
| Low risk | Stage IB |
| Intermediate risk | Stage IIA |
| High risk | Stage IIB |
| Stage IIB | |
| Low risk | Stage IIA |
| Intermediate risk | Stage IIB |
| High risk | Stage IIIA |
| Stage IIIA | |
| Low risk | Stage IIB |
| Intermediate risk | Stage IIIA |
| High risk | Stage IIIB |
| Stage IIIB | |
| Low risk | Stage IIIA |
| Intermediate risk | Stage IIIB |
| High risk | Stage IIIC |
| Stage IIIC | |
| Low risk | Stage IIIB |
| Intermediate risk | Stage IIIC |
| High risk | Stage IIIC |
The TNMB staging system maintains the order of the eighth edition of the TNM staging system but allows reclassification of patients based on molecular assay results. It upgrades patients by one stage for high-risk disease, downgrades patients by one stage for low-risk disease and does not change stage for intermediate-risk disease.
TNM: Tumor, node, metastasis; TNMB: Tumor, node, metastasis, biology.
Data taken from [48].
In a Kaplan–Meier analysis of overall survival, the survival curve separation for TNMB staging was superior to conventional staging by either the seventh or eighth edition (Figure 1) [47]. Numerically, this is represented by the chi-square statistic of the log-rank test, which was 159 according to the seventh edition versus 155 according to the eighth edition. By contrast, the chi-square statistic improved to 194 for the TNMB staging system, which indicates improved survival discrimination with the TNMB staging system.
Figure 1. . Overall survival of patients with non-small-cell lung cancer after surgical resection by TNM seventh edition, TNM eighth edition and TNMB staging in the validation cohort (n = 1373).
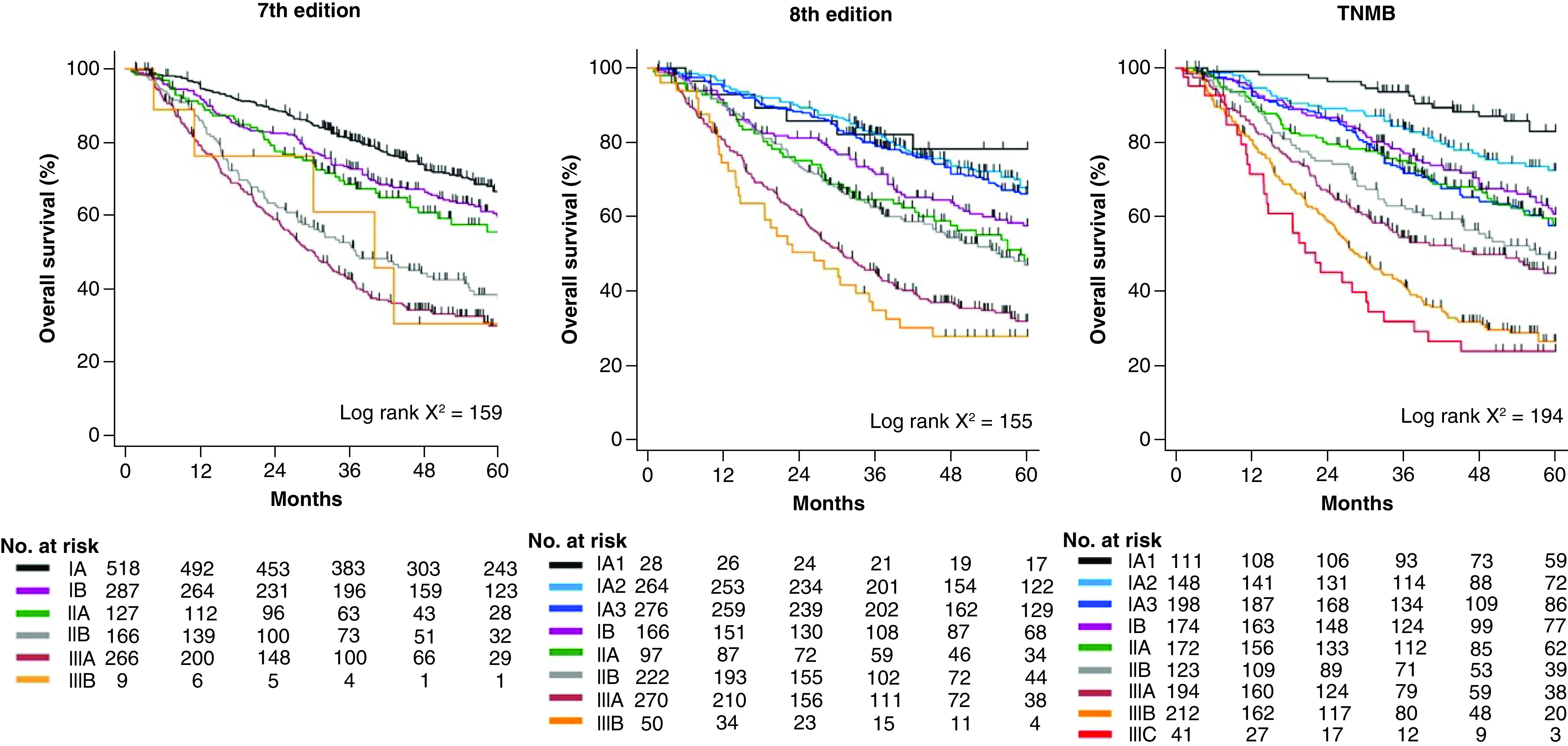
The proposed TNMB system is a novel staging system that integrates the results of the 14-gene molecular prognostic classifier into the eighth edition TNM staging. TNMB staging has distinct separation of survival by stage and a larger range of survival between early and late stages compared with conventional staging.
No.: Number; TNM: Tumor, node, metastasis; TNMB: Tumor, node, metastasis, biology.
Reproduced with permission from [47] © Elsevier (2019).
More recently, other, more sophisticated reclassification metrics have been utilized to better assess improvements in prognostic systems. Net reclassification improvement (NRI) is an index used to quantify how well a new model correctly reclassifies subjects in comparison with an established model [49]. NRI ranges from -1 to 1, where -1 indicates that the new model is perfectly worse than the old one and 1 indicates the new model is perfectly better [50]. In this case, NRI showed improvement by 0.33 (95% CI: 0.24–0.41) for the TNMB system over the eighth edition [47]. The relative integrated discrimination improvement, an index that quantifies the relative improvement in the capability of a new staging system to distinguish between subjects who survive [51], showed that the TNMB system improved by 22.1% (95% CI: 8.8–35.3%) compared with the eighth edition [47]. Strikingly, in comparing the change in performance between the seventh and eighth editions, there was no improvement at all in either NRI (0.03 [95% CI: -0.01 to 0.06]) or relative integrated discrimination improvement (-2.5% [95% CI: -17.6 to 12.4%]). These findings demonstrate improved prognostic ability of the TNMB staging system in a way that has been difficult to attain with reorganization of conventional nonmolecular approaches alone.
Following retrospective analysis, a prospective validation was performed [48]. In a prospective cohort study, 238 patients with stage I–IIIC nonsquamous NSCLC underwent surgical resection and prognostic assay evaluation at the time of cancer treatment at UCSF. All patients underwent complete R0 surgical resection of early-stage disease, including sublobar or lobar resections, with mediastinal lymph node dissection following best practice guidelines. Patients who did not undergo a residual tumor resection were excluded from the study. Patients were restaged in accordance with the seventh edition, eighth edition and TNMB staging system. Compared with the seventh edition, staging with the eighth edition did not show any improvement in classification, with NRI of 0.02 (95% CI: -0.18 to 0.21). However, with adoption of the TNMB staging system, NRI improved significantly to 0.28 (95% CI: 0.08–0.46). This prospective cohort study demonstrated that the TNMB staging system improved prognostication compared with conventional TNM staging.
Clinical utility of the 14-gene lung cancer assay in clinical practice
Several large-scale, prospective trials have previously demonstrated that adjuvant chemotherapy can decrease recurrence and increase overall survival in patients with stage II and higher NSCLC [52–55]. In these same studies, however, adjuvant chemotherapy failed to provide a benefit to patients with stage I disease even though 30–50% of these patients will die within 5 years because of disease recurrence from occult micrometastases at the time of initial resection.
Current guidelines from the National Comprehensive Cancer Network (NCCN) suggest that patients with stage IB and IIA disease who are believed by their clinicians to be at high risk of recurrence and death should receive adjuvant chemotherapy. However, these same guidelines recognize that there are no clinical or histopathological criteria that have been validated to provide adequate guidance for the determination of ‘high risk’ [56,57].
Prospective clinical investigation using the 14-gene lung cancer assay
In a recent prospective study, 100 consecutive patients with stage IA, IB or IIA nonsquamous NSCLC resected at UCSF were evaluated with the 14-gene lung cancer assay [58]. Based on assay results, patients with intermediate- or high-risk scores, and therefore at elevated risk of recurrence, were referred to a thoracic oncologist for discussion of adjuvant chemotherapy. The decision of whether to pursue adjuvant chemotherapy was made on an individual basis between the patient and the primary oncologist. Patients were followed to evaluate for disease recurrence, which closely mirrors 5-year survival.
Results from the molecular assay stratified 52 (52%) patients as clinically low risk and 48 (48%) patients as high risk (intermediate risk: n = 22; high risk: n = 26). Based on NCCN guidelines, 36 (36%) patients were identified as eligible for adjuvant chemotherapy, and 13 (13%) patients actually received adjuvant chemotherapy. In all, NCCN and molecular assay recommendations for adjuvant chemotherapy were discordant in 34 (34%) patients. Ultimately, 13 (13%) patients received adjuvant chemotherapy, all of whom were molecular high risk; most cases of refusal of adjuvant chemotherapy were related to patient preference.
At 23-month median follow-up, recurrence had occurred in 11 (11%) patients. Ten (91%) of the recurrences occurred in the molecular high-risk group, with a 21% recurrence rate for high-risk patients. This was significantly higher than the 2% rate of recurrence for the low-risk group (Fisher's exact test p = 0.003) [58]. The Kaplan–Meier estimate of 5-year disease-free survival was 93.8% among molecular low-risk patients, which was significantly higher than that for molecular high-risk patients (58.8%; log-rank p = 0.006). Conversely, NCCN stratification was not as successful at predicting patient prognosis, as recurrence did not differ significantly between patients with NCCN high-risk clinicopathological features (5-year disease-free survival: 73.1%) and patients without high-risk features (75.7%; log-rank p = 0.112). On multivariate regression analysis controlling for other variables, including NCCN risk category and clinical stage, molecular high-risk status was the only significant predictor of disease recurrence (p = 0.046).
Importantly, the analysis showed that among patients who were part of the molecular high-risk group, those who received adjuvant chemotherapy had significantly better 5-year disease-free survival (91.7%) compared with those who did not (48.7%; log-rank p = 0.004). These data suggest that selective administration of adjuvant chemotherapy to early-stage patients designated as molecular high risk reduces recurrence to rates similar to those experienced by the low-risk patients who were observed.
Results from an expanded cohort of 250 patients were recently presented at the International Association for the Study of Lung Cancer 2020 North America Conference on Lung Cancer [59]. In the expanded cohort, molecular low-risk patients had freedom from recurrence (FFR) of 94.6% at median follow-up of 29 months compared with FFR of 72.4% in the high-risk group that did not receive adjuvant chemotherapy. Importantly, for the high-risk cohort that did receive adjuvant chemotherapy, FFR increased to 97.0%. This trend held true for the specific subset of 168 patients with stage IA disease, with FFR of 97.4% for low-risk, 73.2% for untreated high-risk and 100% for high-risk patients treated with adjuvant chemotherapy.
At this time, an international randomized clinical trial is underway, designed to measure the magnitude of benefit that may be derived from adjuvant chemotherapy in molecular high-risk patients [60]. Based on the technical rigor and reproducibility of the assay results coupled with a demonstration of clinical utility in a prospective study, the 14-gene lung cancer assay is now reimbursed by Medicare and many private insurers as an important component of decision-making for early-stage patients with resected NSCLC.
Opportunity for a new standard of care with higher survival for early-stage NSCLC
Patient outcomes following a diagnosis of early-stage NSCLC remain poor compared with other solid tumor types despite advances in our understanding of the molecular mechanisms of disease. Although several randomized controlled trials [52–54,61–63] have previously demonstrated improved survival in patients with stage II–IIIA NSCLC, a meta-analysis of these studies [55] failed to show definitive benefit in patients with stage I disease. Similarly, a randomized controlled trial of paclitaxel plus carboplatin in patients with stage IB lung cancer [64] demonstrated no survival benefit in comparison with observation alone.
In the majority of patients who die after resection of stage I disease, the inciting factor is distant disease recurrence [65]. This suggests that many patients are incorrectly identified as having localized disease by conventional eighth edition criteria, when in reality they harbor occult, likely micrometastatic disease at the time of the attempt at curative surgery. It is probable that the inability of prior studies to demonstrate benefit in these patients with early-stage disease is due to the inability to select patients most likely to harbor these micrometastases, leading to a dilution of treatment effect from overtreating patients with favorable tumor biology (i.e., likely cured in the operating room). This possibility is supported by results of a retrospective review [66] that assessed several tumor gene profiles and found that patients at high risk of treatment failure would have benefited from adjuvant treatment.
High recurrence rates after surgery for NSCLC underscore the need for a practical molecular assay to more reliably identify early-stage patients with increased risk of micrometastases and recurrence after surgery. The 14-gene lung cancer assay is practical on a large scale, in that it utilizes widely available FFPE tissue blocks and highly reliable qRT-PCR to measure expression of a small number of important cancer-related genes. The assay has been rigorously validated in two large, international patient cohorts using a blinded study design [37,40]. Compared with conventional staging alone, this robust assay successfully improves our ability to identify patients at increased risk of death.
The TNMB staging system has proved more robust in distinguishing between patients with and without disease recurrence compared with conventional TNM staging [47]. This observation supports the notion that a substantial and clinically relevant improvement over conventional staging can be attained through the incorporation of molecular classifiers. The incorporation of this fourth category representing tumor biology is easy for clinicians to understand and apply and allows for the flexibility to integrate future refinements from novel characterizations of tumor biology.
Conclusion
A rigorously validated molecular assay has been shown to significantly improve identification of patients at highest risk of disease recurrence and mortality after resection of early-stage nonsquamous NSCLC. This risk classifier can be incorporated into conventional TNM staging in a clinically intuitive way and significantly improves risk discrimination beyond conventional staging. The assay has been shown to predict benefit from adjuvant chemotherapy in a prospective cohort of patients. An international randomized controlled trial investigating the magnitude of benefit of adjuvant chemotherapy in high-risk patients is underway.
Future perspective
A large, multicenter, international, randomized trial is underway to measure the magnitude of benefit derived from adjuvant chemotherapy in patients with molecular high-risk early-stage lung cancer. Adoption of the TNMB staging system may meaningfully identify patients who would benefit from additional therapy to target micrometastatic disease. Overall, clinical use of this assay may lead to substantive improvement in survival for patients with early-stage NSCLC.
Executive summary.
Despite unacceptably high rates of recurrence and mortality in patients diagnosed with early-stage non-small-cell lung cancer, prior studies have failed to demonstrate benefit from adjuvant chemotherapy in patients with stage I disease after surgery.
Development & technical validation
A Clinical Laboratory Improvement Amendments-certified, commercially available and reimbursed 14-gene molecular prognostic assay has been developed and validated in two large international patient cohorts.
The prognostic assay utilizes quantitative reverse transcription PCR-based analysis of readily available and formalin-fixed, paraffin-embedded tissue blocks rather than snap-frozen tissue samples, as used in prior-generation assays.
Staging refinement using the 14-gene lung cancer assay
The molecular assay reliably identifies patients at low, intermediate and high risk of mortality after surgery for early-stage nonsquamous non-small-cell lung cancer.
The molecular assay improves prognostic accuracy beyond conventional tumor, node, metastasis staging.
Prospective clinical investigation using the 14-gene lung cancer assay
The molecular assay predicts benefit from adjuvant chemotherapy in patients with molecular high-risk disease.
Patients with high-risk disease have significantly lower freedom from recurrence than low-risk patients, and high-risk patients treated with adjuvant chemotherapy have significantly improved freedom from recurrence, on par with patients with low-risk disease.
An international randomized clinical trial is underway, designed to measure the magnitude of benefit that may be derived from adjuvant chemotherapy in molecular high-risk patients.
Footnotes
Author contributions
AR Gupta, GA Woodard, DM Jablons, MJ Mann and JR Kratz participated in the literature review and study interpretation. The manuscript was written by AR Gupta and was edited by all authors, who have approved the final version.
Financial & competing interests disclosure
G A Woodard reports having a consulting relationship with Oncocyte Corporation, the company that licenses the molecular assay based on University of California San Francisco technology. J R Kratz reports having a consulting relationship with Oncocyte Corporation, the company that licenses the molecular assay, and Razor Genomics, Inc., the company that established a Clinical Laboratory Improvement Amendments-certified laboratory and developed the molecular assay based on University of California San Francisco technology. J R Kratz also reports being an inventor of related technology owned by the University of California, for which the university submitted patent applications that have been licensed to Razor Genomics, Inc. M J Mann and D M Jablons report having a consulting relationship with Oncocyte Corporation, the company that licenses the molecular assay, and an owner interest and consulting relationship with Razor Genomics, Inc., the company that established a Clinical Laboratory Improvement Amendments-certified laboratory and developed the molecular assay based on University of California San Francisco technology. M J Mann and D M Jablons also report being inventors of related technology owned by the University of California, for which the university submitted patent applications that have been licensed to Razor Genomics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 70(1), 7–30 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results. SEER cancer statistics review (CSR) 1975-2015. https://seer.cancer.gov/archive/csr/1975_2015/index.html
- 3.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 122(3), 1037–1057 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Aberle DR, Adams AM, Berg CD et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 365(5), 395–409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastorino U, Silva M, Sestini S et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann. Oncol. 30(7), 1162–1169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huo J, Shen C, Volk RJ, Shih YCT. Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: intended and unintended uptake. JAMA Intern. Med. 177(3), 439–441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards TB, Doria-Rose VP, Soman A et al. Lung cancer screening inconsistent with U.S. Preventive Services Task Force recommendations. Am. J. Prev. Med. 56(1), 66–73 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin MB, Greene FL, Edge SB et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67(2), 93–99 (2017). [DOI] [PubMed] [Google Scholar]; • Serves as commentary on the eighth edition of the American Joint Committee on Cancer (AJCC) staging manual, including organizational and structural changes to the system from the seventh edition.
- 9.Burke HB. Outcome prediction and the future of the TNM staging system. J. Natl Cancer Inst. 96(19), 1408–1409 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 151(1), 193–203 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Wang S, Zhou Y et al. Evaluation of the 7th and 8th editions of the AJCC/UICC TNM staging systems for lung cancer in a large North American cohort. Oncotarget 8(40), 66784–66795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chansky K, Detterbeck FC, Nicholson AG et al. The IASLC Lung Cancer Staging Project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 12(7), 1109–1121 (2017). [DOI] [PubMed] [Google Scholar]; • External validation of the revisions to the eighth edition of the AJCC staging system for lung cancer utilizing the National Cancer Database of the American College of Surgeons.
- 13.Pfannschmidt J, Muley T, Bülzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer 55(3), 371–377 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Fang D, Zhang D, Huang G, Zhang R, Wang L, Zhang D. Results of surgical resection of patients with primary lung cancer: a retrospective analysis of 1,905 cases. Ann. Thorac. Surg. 72(4), 1155–1159 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N. Engl. J. Med. 350(4), 379–392 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Spiro SG, Silvestri GA. One hundred years of lung cancer. Am. J. Respir. Crit. Care Med. 172(5), 523–529 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Kratz JR, Jablons DM. Genomic prognostic models in early-stage lung cancer. Clin. Lung Cancer 10(3), 151–157 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Consonni D, Pierobon M, Gail MH et al. Lung cancer prognosis before and after recurrence in a population-based setting. J. Natl Cancer Inst. 107(6), djv059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvold ND, Taghian AG, Niemierko A et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J. Clin. Oncol. 29(29), 3885–3891 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keum MA, Lim SB, Kim SA et al. Clinicopathologic factors affecting recurrence after curative surgery for stage I colorectal cancer. J. Korean Soc. Coloproctol. 28(1), 49–55 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann. Thorac. Surg. 60(3), 615–622 (1995). [DOI] [PubMed] [Google Scholar]
- 22.D'Amico TA. Molecular biologic staging of lung cancer. Ann. Thorac. Surg. 85(2), S737–S742 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Coussens LM, Werb Z. Inflammation and cancer. Nature 420(6917), 860–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 454(7203), 436–444 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Jones PA, Baylin SB. The epigenomics of cancer. Cell 128(4), 683–692 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin L, Gray JW. Translating insights from the cancer genome into clinical practice. Nature 452(7187), 553–563 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beer DG, Kardia SLR, Huang CC et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat. Med. 8(8), 816–824 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Boutros PC, Lau SK, Pintilie M et al. Prognostic gene signatures for non-small-cell lung cancer. Proc. Natl Acad. Sci. U. S. A. 106(8), 2824–2828 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen HY, Yu SL, Chen CH et al. A five-gene signature and clinical outcome in non–small-cell lung cancer. N. Engl. J. Med. 356(1), 11–20 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Guo L, Ma Y, Ward R, Castranova V, Shi X, Qian Y. Constructing molecular classifiers for the accurate prognosis of lung adenocarcinoma. Clin. Cancer Res. 12(11 Pt 1), 3344–3354 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Roepman P, Jassem J, Smit EF et al. An immune response enriched 72-gene prognostic profile for early-stage non–small-cell lung cancer. Clin. Cancer Res. 15(1), 284–290 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Tomida S, Koshikawa K, Yatabe Y et al. Gene expression-based, individualized outcome prediction for surgically treated lung cancer patients. Oncogene 23(31), 5360–5370 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Raponi M, Zhang Y, Yu J et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 66(15), 7466–7472 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Shedden K, Taylor JMG, Enkemann SA et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat. Med. 14(8), 822–827 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Z, Wigle DA, Yang P. Non-overlapping and non-cell-type-specific gene expression signatures predict lung cancer survival. J. Clin. Oncol. 26(6), 877–883 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Subramanian J, Simon R. Gene expression-based prognostic signatures in lung cancer: ready for clinical use? J. Natl Cancer. Inst. 102(7), 464–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kratz JR, He J, Van Den Eeden SK et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 379(9818), 823–832 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Details the development of the 14-gene lung cancer assay that is utilized for the purposes of this review, as well as the external validation of the assay, including two large-scale, multicenter, blinded studies with independent patient cohorts, including international patients.
- 38.Herbst RS, Heymach JV, Lippman SM. Lung cancer (2009). www.nejm.org/doi/10.1056/NEJMra0802714 [DOI] [PMC free article] [PubMed]
- 39.Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox's proportional hazards model via coordinate descent. J. Stat. Softw. 39(5), 1–13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kratz JR, Tham PT, Mulvihill MS et al. Analytical validation of a practical molecular assay prognostic of survival in nonsquamous non–small cell lung cancer. Diagn. Mol. Pathol. 22(2), 65–69 (2013). [DOI] [PubMed] [Google Scholar]; • Details the technical validation, including analytical precision and reproducibility, of the 14-gene lung cancer assay in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory environment.
- 41.Ramaswamy S. Translating cancer genomics into clinical oncology (2009). www.nejm.org/doi/10.1056/NEJMp048059 [DOI] [PubMed]
- 42.Paik S, Kim C, Song Y, Kim W. Technology insight: application of molecular techniques to formalin-fixed paraffin-embedded tissues from breast cancer. Nat. Clin. Pract. Oncol. 2(5), 246–254 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Xu J, Wong C. Hunting for robust gene signature from cancer profiling data: sources of variability, different interpretations, and recent methodological developments. Cancer Lett. 296(1), 9–16 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Cronin M, Sangli C, Liu ML et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin. Chem. 53(6), 1084–1091 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Bao T, Davidson NE. Gene expression profiling of breast cancer. Adv. Surg. 42, 249–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Güler EN. Gene expression profiling in breast cancer and its effect on therapy selection in early-stage breast cancer. Eur. J. Breast Health 13(4), 168–174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kratz JR, Haro GJ, Cook NR et al. Incorporation of a molecular prognostic classifier improves conventional non–small cell lung cancer staging. J. Thorac. Oncol. 14(7), 1223–1232 (2019). [DOI] [PubMed] [Google Scholar]; •• Describes how incorporating the 14-gene lung cancer assay into tumor, node, metastasis (TNM) staging (termed ‘tumor, node, metastasis, biology,’ or TNMB) leads to significant improvement in the identification of high-risk patients and survival predictions in comparison with traditional TNM staging alone.
- 48.Haro GJ, Sheu B, Cook NR, Woodard GA, Mann MJ, Kratz JR. Comparison of conventional TNM and novel TNMB staging systems for non–small cell lung cancer. JAMA Netw. Open 2(12), e1917062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Shows improvement in risk discrimination with utilization of the TNMB staging system in a prospective cohort of patients.
- 49.Pencina MJ, Steyerberg EW, D'Agostino RB. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 30(1), 11–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jewell ES, Maile MD, Engoren M, Elliott M. Net reclassification improvement. Anesth. Analg. 122(3), 818–824 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: extension to survival analysis. Stat. Med. 30(1), 22–38 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Winton T, Livingston R, Johnson D et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N. Engl. J. Med. 352(25), 2589–2597 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Douillard JY, Rosell R, De Lena M et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 7(9), 719–727 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Arriagada R, Bergman B, Dunant A et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N. Engl. J. Med. 350(4), 351–360 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Pignon JP, Tribodet H, Scagliotti GV et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 26(21), 3552–3559 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi N, Toyooka S, Soh J et al. Risk factors for recurrence and unfavorable prognosis in patients with stage I non-small cell lung cancer and a tumor diameter of 20 mm or less. J. Thorac. Oncol. 2(9), 808–812 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Hsu CP, Hsia JY, Chang GC et al. Surgical-pathologic factors affect long-term outcomes in stage IB (pT2 N0 M0) non-small cell lung cancer: a heterogeneous disease. J. Thorac. Cardiovasc. Surg. 138(2), 426–433 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Woodard GA, Wang SX, Kratz JR et al. Adjuvant chemotherapy guided by molecular profiling and improved outcomes in early stage, non-small-cell lung cancer. Clin. Lung Cancer 19(1), 58–64 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Woodard GA, Kratz JR, Haro GJ et al. Molecular risk stratifications independent of EGFR mutation status in identifying early stage non-squamous non-small cell lung cancer patients at risk for recurrence and likely to benefit from adjuvant chemotherapy. Presented at: 2020 North America Conference on Lung Cancer. 16–17 October 2020. [DOI] [PubMed]; •• Details patient outcomes when the 14-gene lung cancer assay is utilized to drive decision-making for adjuvant chemotherapy in patients with early-stage lung cancer.
- 60.Kratz JR, Mann MJ, Jablons DM. International trial of adjuvant therapy in high risk stage I non-squamous cell carcinoma identified by a 14-gene prognostic signature. Transl. Lung Cancer Res. 2(3), 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arriagada R, Dunant A, Pignon J et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 28(1), 35–42 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Scagliotti GV, Fossati R, Torri V et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J. Natl Cancer Inst. 95(19), 1453–1461 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Waller D, Peake MD, Stephens RJ et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur. J. Cardiothorac. Surg. 26(1), 173–182 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Strauss GM, Herndon JE, Maddaus MA et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J. Clin. Oncol. 26(31), 5043–5051 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelsey CR, Marks LB, Hollis D et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 115(22), 5218–5227 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Zhu CQ, Ding K, Strumpf D et al. Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer. J. Clin. Oncol. 28(29), 4417–4424 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


