Abstract
Animals on islands typically depart from their mainland relatives in assorted aspects of their biology. Because they seem to occur in concert, and to some extent evolve convergently in disparate taxa, these changes are referred to as the ‘island syndrome’. While morphological, physiological and life-history components of the island syndrome have received considerable attention, much less is known about how insularity affects behaviour. In this paper, we argue why changes in personality traits and cognitive abilities can be expected to form part of the island syndrome. We provide an overview of studies that have compared personality traits and cognitive abilities between island and mainland populations, or among islands. Overall, the pickings are remarkably slim. There is evidence that animals on islands tend to be bolder than on the mainland, but effects on other personality traits go either way. The evidence for effects of insularity on cognitive abilities or style is highly circumstantial and very mixed. Finally, we consider the ecological drivers that may induce such changes, and the mechanisms through which they might occur. We conclude that our knowledge of the behavioural and cognitive responses to island environments remains limited, and we encourage behavioural biologists to make more use of these ‘natural laboratories for evolution’.
Keywords: animal behaviour, behavioural syndrome, cognition, island evolution, personality
1. Introduction
Animals and plants inhabiting islands tend to deviate from their mainland relatives in multiple aspects of their morphology, physiology, behaviour and life history; a pattern referred to as the ‘island syndrome’ [1–3]. A large body of literature on a variety of animal taxa documents how island populations stand out in body size and shape, colouration, locomotor abilities, diet, niche width, fecundity and lifespan, for instance (reviews in [2–7]). Curiously, far fewer studies have examined behavioural adaptations in island dwellers. Apart from the often documented phenomenon of ‘island tameness’ (i.e. insular prey species often fail to recognize or respond adequately to predators; [8,9]) and peculiarities of island birdsongs [10–12], whether and how insularity affects other behavioural aspects of animals has received far less attention. This is unfortunate, because behaviour is such an important part of the phenotype, and because many of the drivers of morphological, physiological and life-history evolution on islands are also likely to impinge on behavioural characteristics [13].
The island environment differs in many aspects from that of the mainland. Islands typically accommodate fewer species than mainland habitats of the same surface area [4,5,14]. The dearth of competitors, and especially of large predators, instigates ‘ecological release’ [15–17] which allows many insular prey species to reach much higher densities than on the mainland [18–20]. On the other hand, for many animals, dietary resources are often considered to be poor or more variable on islands compared to the mainland ([21–24], but see [25]). These environmental changes (i.e. poor or variable dietary resources, reduced predation pressure and interspecific competition, and increased intraspecific competition) are deemed responsible for many elements of the island syndrome described, although the mechanisms through which they exert their effects on animal phenotypes often remain elusive. Another line of research highlights the importance of environmental unpredictability on islands, and particularly on small islets, for the emergence of island syndromes [26,27]. We expect that the particularities of insular environments will likewise prompt adaptations in the behaviour of island dwellers. In this paper, we review the empirical evidence for a behavioural component of the island syndrome, focusing on two interrelated [28] behavioural domains: animal personality and cognition. We also consider the mechanisms and ecological drivers that could produce such changes.
Animal personality research has revealed, at least to some extent, repeatable and heritable inter-individual variation [29,30] in the way that animals behave in response to various external stimuli. Individual animals thus belong to one of several possible and coexisting ‘behavioural types’—groups that will respond in a distinctive, predictable way to challenges, even under different contexts. More specifically, individuals differ consistently in ‘personality traits’ such as boldness, aggression, activity, exploration and sociability (reviewed in [31]). Often, these personality traits intercorrelate, giving rise to a ‘behavioural syndrome’ [32]. Because island and mainland habitats differ in the opportunities and challenges they offer to their inhabitants, we can expect changes in the relative frequency of behavioural types or in the way that personality traits interconnect.
Cognition is the collective of neural processes responsible for the acquisition, retention and use of information [33] and, as such, a key determinant of fitness in vertebrate animals (e.g. [34–37]). Individuals differ in their cognitive abilities, but also in cognitive styles (e.g. learning speed versus accuracy [32]). Relatively little is known about the repeatability of cognitive testing in time, or across context [38]; or about how different cognitive scores interrelate [39]. In humans, scores on different cognitive tests tend to correlate positively, lending credibility to the concept of ‘general intelligence’ (the so-called ‘g-factor’ [40,41]). Similar covariation has been described for a handful of other mammals (e.g. [42]) and birds (e.g. [43]), but overall the evidence for a g-factor remains scarce [39,44]. Again, given how islands deviate from mainland habitats, different levels, types or sets of cognitive skills may be favoured in both types of environments.
2. The current evidence for a behavioural component to the island syndrome
Table 1 reviews the evidence that animals from islands differ from their mainland counterparts in aspects of their personality and cognition. Overall, few studies have thoroughly investigated the effect of insularity on personality. Careful analyses of animal temperament should consider whether individual behavioural scores (that are believed to reflect aspects of personality) are repeatable over time and over different contexts and whether and how these aspects covary to produce behavioural syndromes [32]. Inquiries into the evolution of insular personality will also require information on the heritability of such scores. Few of the studies in table 1 fulfil these conditions. Below we review behavioural studies that either measure personality traits (i.e. test for repeatability [105]) or behavioural changes (i.e. do not test for repeatability) that could indicate differences in personality.
Table 1.
Studies that have compared behavioural, personality or cognitive (including brain morphology studies) traits between island and mainland, or among-island populations/species. The ‘driver’ and ‘mechanism’ are those suggested (but not necessarily demonstrated) by the authors. FID stands for flight initiation distance. The ‘type of analysis’ also indicates whether the study includes repeated within-individual measurements (personality variation). References to these studies were found by feeding the Web of Science and Google Scholar search machines with a combination of behavioural keywords (e.g. ‘personality’, ‘cognition’, ‘bold*’, ‘aggression’, ‘explorati*’) with terms referring to island environments (e.g. island, insular*, archipel*). We also carefully checked the reference lists of all papers for additional sources.
| study species | trait | island | mainland | driver | mechanism | type of analysis | reference |
|---|---|---|---|---|---|---|---|
| personality | |||||||
| eastern chipmunk (Tamias striatus) | island chipmunks are less vigilant, but both island and mainland specimens adjust vigilance to microhabitat structure | Beaver Island, Michigan, USA | two sites on mainland Michigan, USA | lower predation pressure on island (checked with camera traps) | unknown | field experiments; personality variation measured | [45] |
| 14 species of macropodid marsupials | animals on islands are less wary and allocate more time to foraging | satellite islands of Australia | Australia | predation pressure | acknowledges possibilities of plasticity and selection | field observations; comparative analysis | [46] |
| reindeer (Rangifer tarandus) | island reindeer are more vigilant on the island, but response distances are the same | Edgeøya, Norway | four sites on Spitsbergen, Norway | possibly higher predation (by polar bears) on island | response distance believed to be ‘hard-wired' | field observations | [47] |
| bull-headed shrike (Lanius bucephalus) | longer FID in island shrikes | Kikaijima, Minami-daitojima and Nakanoshima Islands, Japan | three sites on main Japanse Islands | risk of predation (by rats) higher on islands | genetic change, plasticity and dispersal-related selection are considered | field observations | [48] |
| 11 species of Falkland Island birds | FID is lower on island than on mainland | Falkland Islands | mainland Argentina | absence of terrestrial predators | probably innate, evolutionarily acquired, but habituation also deemed possible | field observations; comparative analysis | [49] |
| California quail (Callipepla californica) | FID is similar on mainland and island, but starting distance is smaller on islands | Santa Catalina Island, USA | California, USA | some predators lacking on island | assumed genetic (antipredator genes) | field observations | [50] |
| orange-throated whiptail (Aspidoscelis hyperythra) | island lizards are more difficult to catch than mainland lizards | seven Gulf of California islands | Baja California | predation pressure, human collection for pet trade | reasoned to be genetically based, implying rapid evolution of antipredator behaviour | field observations | [51] |
| Galápagos marine iguana (Amblyrhynchus cristatus) | lizards on predator-free islet have shorter FIDs; although FID increases with experience, it remains insufficient to avoid predation | Caamaño Islet, Galápagos, Ecuador | St Cruz and San Cristobal Island, Galápagos, Ecuador | predation pressure | release from predation narrowed the reaction norm for FID | field observations | [52] |
| spiny-tailed iguana (Ctenosaura hemilopha) | island lizards behave less wary and have shorter FIDs than mainland lizards | Cerralvo Island, Gulf of California | Mexico | low predation pressure on the island | no hints | field observations | [53] |
| lava lizards (eight populations of three Tropidurus spp.) | lizards on islands with introduced cats have higher FID | eight islands of the Galapagos | no mainland population | presence of exotic predators (cats) | could be inherited, or learned | field observations; comparative analysis | [54] |
| Ibiza wall lizard (Podarcis pityusensis) | FID and distance fled is greater on islets with higher predation pressure | seven islets around Ibiza and Formentera, Spain | no mainland population | predation pressure as estimated by number and kind of predators, incl. humans | unknown, both phenotypic plasticity and evolutionary processes deemed possible | field observations; comparative analysis | [55] |
| Lilford's wall lizard (Podarcis lilfordi) | FID, distance fled, hiding time and probability to enter refuge are lower on islet with less predators | islets of Rei and Aire, Menorca, Spain | no mainland population | predation pressure | natural selection | field observations | [56] |
| Italian wall lizard (Podarcis siculus) | FID is shorter on island with lower predation pressure | comparison between two islands in the Adriatic Sea | no mainland population | predation pressure, through number of predators and habitat structure | unknown | field observations | [57] |
| 66 lizard species | FID decreases as distance to mainland increases | islands in the Atlantic and Pacific Oceans; Caribbean and Mediterranean Seas | five continents | predation pressure is lower on islands; reduced food availability may shorten FID to save energy | probably genetic changes, but tameness might be learned every generation | field observations; comparative analysis | [9] |
| Aegean wall lizard (Podarcis erhardii) | lizards from islets have shorter FID and act bolder than lizards from main island | four satellite islands of Naxos, Greece | 18 populations on Naxos, Greece | cat predation | phenotypic plasticity; which is also maintained on islets | field observations; laboratory experiments | [58] |
| 38 populations of Aegean wall lizard (Podarcis erhardii) | lizards from smaller and more isolated islets have shorter FIDs | 37 Cycladic islands, Greece | mainland Greece | predation | natural selection | field observations | [59] |
| Lilford's wall lizard (Podarcis lilfordi) | FID and distance fled is not correlated with predation pressure | nine islets around Menorca and Mallorca, Spain | no mainland population | predation pressure as estimated by number and kind of predators, incl. humans | unknown, both phenotypic plasticity and evolutionary processes deemed possible | field observations; comparative analysis | [55] |
| Dalmatian wall lizard (Podarcis melisellensis) | islet lizards are bolder, less wary but not less neophobic than island lizards | three small islets out of the coast of Vis, Croatia | Vis, Croatia | food availability, predation pressure smaller on islets | selection, plasticity or non-random gene flow are suggested | field experiments | [60] |
| northern quolls (Dasyurus hallucatus) | animals on island behave less wary than mainland animals | Astell Island, a satellite island of Australia | Australia | absence of predation on island | natural selection | laboratory experiments | [61] |
| common frog (Rana temporaria) | enhanced boldness and exploration in island tadpoles and froglets | four islands in the Gulf of Bothnia, Sweden | Sweden | dispersal propensity; unstable conditions on islands (pond drying); reduced predation on islands | founder effects caused by environmental filtering or differential natural selection | laboratory experiments with wild-caught specimen; personality variation and behavioural syndrome measured | [62] |
| house mice (Mus musculus domesticus) | enhanced boldness and exploration on island | Gough Island, Tristan da Cunha | Maryland, USA | novel food source (sea birds), loss of predatory danger, removal of human commensals, variable food availability | genetic change | common garden experiment with F1-offspring | [63] |
| 61 species of parrots (Psittacidae) | island species explore novel objects faster and longer than mainland species; island species are not less neophobic than mainland species | several, not specified | several sites, not specified | reduced predation pressure and higher risk of food shortage on islands | assumed genetic | comparative analysis; behavioural syndrome across species tested, but not at the individual level, or between island and mainland species | [64] |
| island scrub jay (Aphelocoma insularis) versus California scrub jay (A. californica) | island birds were more explorative than mainland birds | Santa Cruz Island, California, USA | mainland California, USA | reduced predation, more frequent food shortage on island | unknown | field observations/experiments | [65] |
| brown anole (Anolis sagrei) | lizards on islands with introduced predators are less explorative | eight small islands in the Caribbean, on four of which predatory lizards were introduced | no mainland population | presence of introduced predators (lizards) | natural selection | fitness gradient analysis; personality variation measured | [66] |
| red-backed vole (Myodes gapperi) | island voles are less aggressive than mainland voles; no difference in exploration | 10 islands in the Winnipeg River, Ontario, Canada | six sites on mainland Ontario | relaxed predation, higher population density | dispersal-related, ecological and evolutionary mechanisms all considered | laboratory experiment; comparative analysis | [67] |
| deer mouse (Peromyscus maniculatus) | wild-caught island mice are less aggressive, but difference disappears in subsequent generations | Saturna Island, Pender Island, Canada | British Columbia, Canada | population density thought to reduce aggressiveness | phenotypic plasticity | laboratory experiment with wild-caught specimen and their offspring; crossings | [68] |
| deer mouse (Peromyscus maniculatus) | island mice do not show aggressive behaviour towards juveniles; some mainland mice behave aggressively towards non-kin | Saturna Island, Canada | British Columbia, Canada | reduced intraspecific competition due to high food supply on island | unknown | laboratory test on P (interact with F1) | [69] |
| common shrew (Sorex araneus) | island and mainland shrews equally aggressive | four islands in the Baltic Sea | two sites on mainland Finland | island specimens often inbred | laboratory experiments with field-caught individuals | [70] | |
| Skyros wall lizard (Podarcis gaigeae) | islet lizards more likely to attack juveniles and behave more aggressively to other adults | islet Diavates, Skyros archipelago, Greece | Skyros main island, Greece | food scarcity and high population size prompt for cannibalism | Unknown | laboratory experiments with field-caught individuals | [71] |
| Italian wall lizard (Podarcis siculus) | island lizards are more aggressive than lizards on the mainland | Licosa Island, Italy | one mainland site (Punta Licosa), Italy | possibly low or fluctuating population density on island | unknown | field observations; laboratory experiments | [26] |
| tiger snake (Notechis scutatus) | adult snakes from predator-rich sites have more vigorous responses when handled, but neonatal behaviour is unrelated to predator species richness | eight islands around Australia | three sites, on mainland Australia and Tasmania | predation pressure, through number and type of predators | ontogenetic plasticity; experience, or genetically coded adjustment of behaviour to ontogenetically variable traits | observations on freshly caught individuals | [72,73] |
| common garter snake (Thamnophis sirtalis) | adult but not neonate snakes from the mainland behave more aggressively towards experimentor than island snakes | Beaver Archipelago, USA | Michigan, USA | fewer predators on island | both innate and environmental influences | laboratory behavioural observations | [74] |
| Pacific rattlesnake (Crotalus oreganus) | island snakes behave more aggressively towards humans | Santa Catalina Island, USA | mainland California, USA | island has fewer avian predators but perhaps more (introduced) mammalian predators | unknown | field observations | [75] |
| chuckwalla species (Sauromalus spp.) | island endemics are more sociable, less aggressive than mainland species | San Esteban Island and Angel Island, California, USA | mainland California, USA | dearth of predators, competitors, niche expansion, high but fluctuating food supply, high density on island | unknown | field observations | [76] |
| house mice (Mus musculus domesticus) | island mice do not show aggressive, defensive or cautious behaviour towards conspecifics | Isle of May, UK | Nottinghamshire, UK | interaction between resource distribution, habitat structure and predation risk | unknown | laboratory experiments on recently caught specimens | [77] |
| yellow-faced grass quit (Tiaris olivacea) | island birds are more territorial than mainland birds, which occur more often in flocks | Jamaica | Costa Rica | island density is lower | unknown | field observations | [78] |
| European earwig (Forficula auricularia) | island populations with high densities have larger proportion of macrolabic (fighter) morphs | 35 British islands | 11 mainland Britain sites | population density | intraspecific competition | laboratory experiments | [79] |
| human (Homo sapiens) | islanders exhibit greater animosity towards strangers and keep greater social distance | Croatian Islands | mainland Croatia | dangers associated with infectious disease | questionnaire | [80] | |
| human (Homo sapiens) | islanders had higher levels of consciousness, emotional stability and lower levels of extraversion and openness; no difference in agreeableness | Giglio, Ponza and Ventotene, and seven Aeolian islands, Italy | three sites on mainland Italy | island is harsh, restricted environment with limited social environment | assumed adaptive (changes in same direction); elimination of well less adapted through mortality, assortative mating or emigration | questionnaire | [81–83] |
| meadow vole (Microtus pennsylvanicus) versus beach vole (M. breweri) | island species is more sociable | Muskeget Island, Massachusetts, USA | mainland Massachusetts, USA | differential dispersal of intolerant specimens | field observations | [84] | |
| 46 species of birds | birds on islands tend to flock less than birds on the mainland | 22 different islands | 22 mainland sites to match islands | predation pressure | random drift or active selection are suggested | comparative analysis | [85] |
| long-tailed macaque (Macaca fascularis) | smaller group sizes on island | Simeulue, Indonesia | Sumatra, Indonesia | felid predation | unknown | field observations | [86] |
| tammar wallaby (Macropus eugenii) | time allocation is dependent on group size on mainland and islands with reduced number of predators, but not on predator-free island | Garden Island, Kangaroo Island, Australia; Kawau Island, New Zealand | Western Australia | absence of predators | maintained by natural selection, but ‘priming agents’ may be required to develop antipredator behaviour | field observations | [46] |
| oriental fire-bellied toad (Bombina orientalis) | island toads have lower levels of activity | Jeju Island, South Korea | two sites on mainland South Korea | predation is higher on island | local selection, founder effects also considered possible | laboratory observations | [87] |
| clouded anole (Anolis nebulosus) | island anoles are more active | San Agustín, Mexico | mainland Mexico | less variable environmental conditions on island may allow better thermoregulation; higher predation on mainland | field observations | [88] | |
| cognition | |||||||
| Minatogawa man (Homo sapiens) versus modern Japanese and Pleistocene/Holocene H. sapiens | Pleistocene island dwellers had relatively small endocrania | Okinawa Island, Japan | mainland Japan | undernutrition | phenotypic plasticity; genetic adaptation | comparative analysis of brain size | [89] |
| Human (Homo floresiensis versus Homo erectus) | relative brain size is lower in island species | Flores, Indonesia | biotic interactions and resource use | natural selection for smaller brains (in addition to selection for smaller body size) | quantitative genetic modelling | [90] | |
| mouse and dwarf lemurs (Cheirogaleidae) | disproportional reduction in brain size in this island clade | Madagascar | unpredictable food availability | natural selection | comparative analysis of brain size | [91] | |
| Malagasy dwarf hippo (Hippopotamus lemerlei, H. madagascariensis) versus hippopotamus (H. amphibius) | relative brain size is lower in island species | Madagascar | mainland Africa | poor dietary resources on islands | natural selection for smaller brains (in addition to selection for smaller body size) | ontogenetic modelling | [92] |
| Siculo-Maltese dwarf elephant (Palaeoloxodon falconeri) versus P. antiquus | relative brain size is lower in island species | Malta | mainland Africa | poor dietary resources on islands | natural selection for smaller brains (in addition to selection for smaller body size) | ontogenetic modelling | [92] |
| Siculo-Maltese dwarf elephant (Palaeoloxodon falconeri) versus P. antiquus | dwarfed insular species has a high encephalization quotient | Sicily, Italy | mainland Europe | need to maintain the minimal functional volume of the brain when the size of the skull was drastically reduced | allometric analysis | [93] | |
| Balearic Islands cave goat (Myotragus spp.) compared to 54 spp. of extant bovids | insular species have small brain and sense organs relative to body size | Balearic Islands, Spain | mainland Africa | absence of predators; overpopulation; limited energy availability | natural selection for smaller brains (in addition to selection for smaller body size). | scaling analysis | [94] |
| Cretan deer (Candiacervus), compared to extant deer (Cervidae) | insular dwarf deer have normal relative brain size | Crete, Greece | dearth of predators on islands | comparative analysis of brain size | [95] | ||
| Sardinian dhole (Cynotherium sardous), compared to two spp. of extant dog spp. (Canidae) | insular dwarf canid has normal relative brain size | Sardinia, Italy | comparative analysis of brain size | [96] | |||
| Minorcan giant rabbit (Nuralagus rex), compared to extant rabbit species (Leporidae) | Late Neogene insular giant had relatively small brain; especially sense-dependent areas are small | Minorca, Balearic Islands, Spain | absence of predators; limited energy availability | comparative analysis of brain size | [97] | ||
| 426 mammalian species | no effect of insularity on relative brain size | islands worldwide | mainland sites throughout the world | poor dietary resources on islands | natural selection for smaller brains | comparative analysis of brain size | [98] |
| dodo (Raphus cucullatus) compared to nine spp. of pigeons (Columbiformes) | endocranial volume not smaller than expected from pigeon allometry | Mauritius | allometric analysis | [99] | |||
| Rodrigues Island giant owl (Otus murivorus), compared to 10 extant spp. of owls (Strigidae) | reduction of brain volume in extinct island endemic | Rodrigues, Mauritius | diverse | absence of predators, reduction of interspecific competition | brain expansion cannot follow pace of body size increase (evolutionary pace dissociation) | comparative analysis of brain size | [100] |
| Haast's eagle (Harpagornis moorei), compared to 35 spp. of eagles (Accipitridae) | island endemic had low endocranial volume for its body mass | New Zealand | diverse | absence of predators, competitors on island | mismatch between neural and somatic growth | comparative analysis of brain size | [101] |
| 40 crow and raven species (Corvus) | brain size does not predict ability to colonize islands | islands worldwide | mainland sites throughout the world | islands are challenging environments, promoting enhanced cognition | comparative analysis of brain size | [102] | |
| 1900+ species of birds | insular species have larger brains | diverse | diverse | high environmental unpredictability across years | natural selection; high phenotypic plasticity may inhibit evolutionary change in some clades | comparative analysis of brain size | [103] |
| Deer mouse (Peromyscus maniculatus) | insular mice displayed shorter latencies to solve a Morris water-maze task | Moresby Island, Canada | British Columbia, Canada | differences in swimming abilities (rather than cognitive skills) between populations | unknown | laboratory observations | [104] |
(a) . Boldness
Boldness, i.e. risk-taking behaviour in the presence of a predator threat, is expected to change in a way that matches predation risk on islands. The few studies focusing on the effect of insularity on boldness have found island common frogs (Rana temporaria) [62] and house mice (Mus musculus domesticus) [63] to be bolder than their mainland counterparts. Perhaps inspired by early (mostly anecdotal) accounts of island tameness (e.g. [8,106,107]), most studies investigating the effect of insularity on animal behaviour have focused on vigilance and risk-taking, common proxies for boldness. The vast majority of these studies (table 1) confirmed that island animals, especially those living on remote, predator-poor islands, tend to be less vigilant than their relatives inhabiting the mainland or less remote, predator-ridden islands [9,108]. Reindeer (Rangifer tarandus) on Norwegian Edgeøya [47], bull-headed shrikes (Lanius bucephalus) on Japanese Nansei islands [48] and whiptail lizards (Aspidoscelis hyperythra) on islands in the Gulf of California [51] are exceptions, but their relative shyness is attributed to unusually high densities of predators (polar bears, rats and human collectors, respectively) in their habitat. However, it should be noted that most of these studies rely on field observations of the flight initiation distance (FID), a proxy of boldness that is not without difficulties. FID is known to depend on a variety of factors related to the internal status of the animal (e.g. satiation [109]; reproductive status [110]; body condition [111]), and its environment (e.g. temperature [112]; substrate type [113]; levels of human exposure [114]; social context [115]; distance to safety [116])—all of which may differ consistently between island and mainland sites. In addition, FID measures boldness as an animal's response in a single context (a human approaching), without testing whether inter-individual variation is consistent and repeatable across time and context (i.e. personality sensu stricto [31]). Therefore, future studies should test the boldness of island and mainland conspecifics in multiple, controlled contexts. This could be done for instance by observing the animals' behaviour (e.g. avoidance and inspection of predator models, startles, latency to resume feeding) when confronted with varied threat stimuli (e.g. predator cues, loud noise and novel objects) (see [31] for a review of techniques; and [60–63] for examples in island–mainland comparison).
Increased boldness on islands is typically attributed to reduced predation pressure, although quantitative or even qualitative estimates thereof are seldom presented. The (often implicit) rationale is that bold behaviour is less penalized in predator-poor environments and might even be favoured for its paybacks in a non-predatory context [117,118]. It should be noted that while many (oceanic) islands are indeed devoid of predators, others hold thriving predator populations (e.g. [119–121]). Bold anti-predatory behaviour could also evolve as a behavioural mechanism to deter some of these predators (see [122] for a curious example in New Zealand parrots). Future research on this topic should be careful to match-up behavioural traits of island animals and the predators they were exposed to through evolutionary time.
The mechanisms through which the differences in vigilance and boldness arise remain unclear. Most authors consider both phenotypic plasticity (learning) and genetic adaptation, usually with a slight preference for the former. In a rare study tackling the question directly, Stratton et al. [63] found that differences in boldness between Gough Island and mainland US mice (Mus musculus) persisted in the F1 generation, offering support for genetic change [63].
(b) . Explorativeness
We found only seven studies that investigated the effect of insularity on animal explorativeness (i.e. behavioural response to novelty), and these produced mixed results (table 1). Common frogs (Rana temporaria) on Bothnian islands [62] and giant house mice (M. musculus) on Gough Island [63] are more explorative than their conspecifics on the mainland; survival of brown anoles (Anolis sagrei) that were more inquisitive was lower on islets with predators than on predator-free islets [66], and among parrots (Psittacidae) [64] island species tend to be more neophilic and explorative. A study on the exploratory and neophilic behaviour of scrub jays (Aphelocoma coerulescens) [65] concords with these results. Most studies indicate that predation relaxation and unpredictable resource availability may be drivers of increased explorativeness on islands [123]. The mechanisms of change in explorativeness on the islands are less clear. Lapiedra et al. [66] provided evidence that the behavioural changes in brown anole (A. sagrei) populations following the introduction of a predator were due to natural selection [66]. Brodin et al. [62] argued how dispersal-related environmental filtering could be responsible for the higher explorativeness in island common frogs and tadpoles [62]. By contrast, deer mice (Peromyscus maniculatus) on islands in the Canadian Winnipeg River tended to be less explorative than mainland conspecifics, possibly reflecting differences between inland and oceanic island systems [67]. Camperio Ciani et al. showed that human inhabitants of the Egadi islands are less open to new experiences than their compatriots in mainland Italy, an observation they attribute to disproportionally high emigration of individuals with neophilic personalities [81,82]. They even identified a genetic polymorphism that could be associated with this ‘personality gene flow’ phenomenon [83].
(c) . Aggression
The empirical evidence on changes in aggression towards (but not necessarily limited to) conspecifics go either way. Reduced antagonism in island populations, as the result of high densities that make the monopolization of resources ineffective [124], was reported in five studies on a few rodent species (P. maniculatus [68,69], Myodes gapperi [67] and M. musculus [77]) and birds [11]. Interestingly, Baier & Hoekstra [68] found that the difference faded in the F1 generation, indicating an important role for phenotypic plasticity [68]. In reptiles, the results are less congruent. In tiger snakes (Notechis scutatus) [72,73] and common garter snakes (Thamnophis sirtalis) [74], adults (but not laboratory-raised juveniles) fit the pattern of reduced aggression on islands, suggesting that insular conditions induce a plastic response of soothing in these animals as well. However, Pacific rattlesnakes (Crotalus oreganus) from Santa Catalina Island behave more aggressively than mainland conspecifics; perhaps because of the high number of (recently introduced) terrestrial predators on the island [75]. Skyros wall lizards (Podarcis geigeae) on smaller islets attack conspecifics frequently, probably because food shortage forces them into cannibalism [71].
(d) . Sociability
The effects of insularity on sociability (i.e. the quality of being social) have hardly been studied. Older papers report that meadow voles (Microtus breweri) [84] and chuckwallas (Sauromalus spp.) [76] living on islands tend to be more sociable, than closely related species on the mainland. By contrast, yellow-faced grass quits (Tiaris olivacea) [78] and birds in general [85] are seen in flocks more often on the mainland than islands. Long-tailed macaque (Macaca fascicularis) group size tends to be larger on the mainland [86]. However, whether changes in group size actually reflect differences in sociability (a personality trait), requires further behavioural testing. Predation risk, population density, food availability and habitat structure are among the ecological factors suspected to influence sociality (i.e. the tendency to associate with other individuals) [85,86,125,126], but the mechanisms remain utterly unexplored. Work on humans suggests that islanders have lower levels of extraversion and openness, and exhibit greater animosity towards strangers, keeping for instance greater interpersonal distance [80–82].
(e) . Activity
We found very little information on differences in the level of activity (an animals' tendency to engage in physically demanding behaviours) displayed by island and mainland animals. Clouded anole lizards (Anolis nebulosus) are more active on islands [88], while insular oriental fire-bellied toads (Bombina orientalis) exhibit lower activity levels compared to mainland conspecifics [87]. Predation pressure, population density, resource availability and thermal conditions are considered to shape activity [87,88,127]. We did not find any studies concerning the mechanisms of activity level changes. Therefore, the dearth of information precludes any form of generalization. This is a pity, because there is currently great interest in the causes and consequences of variation in the amount of activity that animals display (see [128]), and mainland–island comparisons might have provided a nice insight into the matter.
(f) . Cognition (with brain size as surrogate)
Studies on a variety of mammals and birds report a reduction in brain size on islands. However, interpretation and generalization of these results is problematic. First, most of these studies have been performed on fossil species and are therefore fraught with practical difficulties concerning the estimation of brain size and body size (see e.g. the disparate results for the Siculo-Maltese dwarf elephant (Palaeoloxodon) [92,93]). Second, no evidence for insular brain size reduction was found for other species, such as the Cretan deer (Candiacervus spp.) [95], the Sardinian dhole (Cynotherium sardous) [96], or even the long-thought-dim dodo (Raphus cucullatus) [99]. Third, recent comparative studies on extant mammals [98] and crows and ravens [102] found no effect of insularity on relative brain size. A meta-analysis of over 1900 species of birds even suggests a tendency towards larger brains in insular species. The authors argue that island species require larger brains to cope with the difficulties of having to exploit novel dietary resources and deal with high environmental stochasticity [103]. Fourth, and most importantly, it has become increasingly clear that brain size must be considered a shaky proxy for cognitive capacity (e.g. [129,130]).
(g) . Cognition (with behaviour as surrogate)
More direct evidence comes from a few studies that have compared aspects of cognitive capacity between island and mainland populations. Deer mice (P. maniculatus) from Morseby Island solved a water-maze task faster than conspecifics from mainland British Columbia, but this was attributed to differences in swimming rather than cognitive skills [104]. White-faced capuchins (Cebus capucinus) from a single island in Coiba National Park, Panama, engage in innovative tool use, a behaviour never observed in mainland conspecifics [131]. Tool use is also remarkably often reported in island birds (e.g. New Caledonian crows (Corvus moneduloides) [132], Hawaiian crows (Corvus hawaiiensis) [133], woodpecker finches (Cactospiza pallida) [134], Goffin's cockatoos (Cacatua goffini) [135]), but no one seems to have checked for a general association between tool use and insularity. On the whole, the number of studies examining the evolutionary fate of cognition and behavioural flexibility on islands is very limited.
3. Routes towards insular behaviour
Below we review the multiple mechanisms that could produce such changes (also see figure 1). Because these routes are rarely documented in island–mainland comparisons, some of our examples come from other study systems. However, we try to explain why we think particular drivers and pathways seem likely to be especially relevant in an insular context.
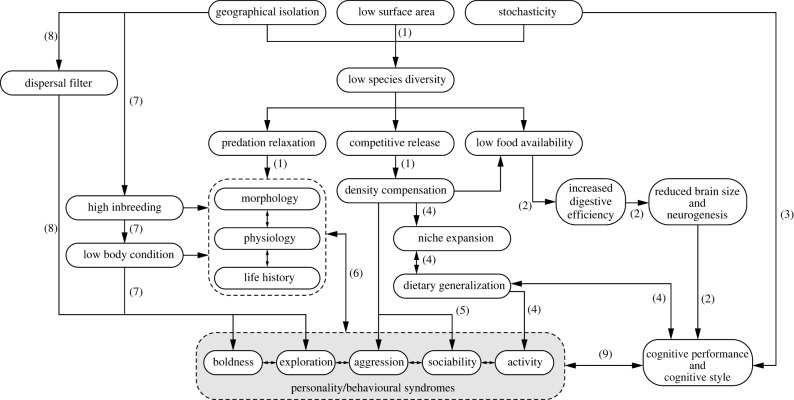
Figure 1.
Putative relationships between island conditions, personality and cognition. Arrows with (1) represent ‘traditional’ pathways leading to the island syndrome. Pathway (2) echoes the ETH [136], predicting lower cognitive capacity in island populations. By contrast, (3) depicts the possibility that the unpredictable nature of the island environment selects for behavioural flexibility, requiring high cognitive capacity [123]. Route (4) represents a possible connection between niche expansion, dietary specialization, and aspects of personality and cognition (as proposed by [137,138]). Arrows with (5) indicate that high densities could lead to reduced territoriality and changes in how animals interact behaviourally (e.g. [77]). Arrow (6) summarizes the multiple connections between ‘traditional’ elements of the island syndrome and personality, e.g. through pleiotropic effects [139] or correlated selection (e.g. pace of life syndrome [140]). Pathway (7) concerns non-adaptive consequences of inbreeding on personality and cognition (e.g. [141]). Route (8) describes a possible role for selection on dispersal-related personality traits (e.g. [142]). Finally, the arrows labelled (9) summarize ideas on how personality and cognition might interact (e.g. [143]).
(a) . Phenotypic changes
Personality traits can change within the lifetime of an individual, under the influence of environmental conditions experienced during (sometimes remarkably short) time windows during development [144–146]. For instance, smaller fathead minnows (Pimephales promelas) behave more boldly than larger conspecifics when raised in low-risk environments, but not when raised under high-perceived risk [147]. Jumping spiders (Marpissa muscosa) raised in socially enriched environments (i.e. raised in conspecific groups) demonstrated higher exploration later in life [148]. Hence, key components of insular environments such as reduced predation risk and increased population density could instigate changes in personality, even without genetic differentiation.
Cognitive capacity is notoriously plastic. In humans and traditional animal models, such as rats, pigs and guinea pigs, both intrauterine [149] and postnatal [150] undernutrition impedes neuronal and cognitive development. Effects have been observed in brain architecture, and on cognitive capacities such as spatial and reversal learning, memory and novelty seeking [149,150]. On the flip side, favourable developmental conditions are known to induce a ‘silver spoon’ effect, with positive effects on cognitive capacity. For instance, blue tits raised on taurine-enriched diets demonstrated improved memory and learning skills [151]. Islands tend to be relatively poor in dietary resources [21–24], and this may directly and negatively impact cognitive capacity. However, due to ecological release and niche enlargement, islands may sometimes provide more food per unit effort (e.g. [152]) and thus might constitute ‘silver spoon’ environments that boost cognitive development. Changes in foraging behaviour that accompany niche expansion may reverberate in personality [137] and cognitive [138] traits. Island dwellers may also experience different social contexts during ontogeny than their mainland relatives, as a consequence of high population density. In group-living animals, such as Australian magpies (Cracticus tibicen), growing up in a rich social environment boosts performance in a variety of cognitive tasks [37,153].
(b) . Genetic changes
Alternatively, since both personality and cognition traits display heritable variation [32,154], any evolutionary mechanism influencing allele frequencies may cause differences between island and mainland populations.
Genetic differences may arise by chance: through a founder effect (if allele frequencies of the colonizing propagule happen to differ from that of the mainland source population), or when random drift haphazardly alters the genetic constitution of the newly established island population [155,156]. Raffard et al. [157] found that the differentiation in boldness among 13 populations of a freshwater fish (the European minnow, Phoxinus phoxinus) could be attributed to random drift [157]. Other studies mention genetic drift as a potential cause of among-population variation in personality or cognitive traits, although often as a less glorious alternative to natural selection (e.g. [158]).
In the early stages after colonization, populations on oceanic islands may be prone to inbreeding [159], which typically results in increased homozygosity and decreased body condition [160,161]. If personality is condition-dependent (which seems plausible [162], but remains debated [141]), inbreeding may result in non-adaptive changes in average personality traits. In accordance, Verweij et al. [163] found negative associations between the level of inbreeding and personality traits such as novelty seeking and persistence in Finnish and Australian citizens [163]. In a rare study of the effects of inbreeding on animal personality, Müller & Juškauskas [164] found that inbred individuals of the leaf beetles (Phaedon cochleariae) behaved more boldly than outbred conspecifics [164]. Evidence that human cognitive abilities may suffer from inbreeding depression comes from genealogical studies on consanguineous marriages (e.g. [165]), including those of royal lineages (e.g. [166]). These traditional studies have recently been substantiated by genome-wide association studies describing negative associations between levels of inbreeding (homozygosity) and human intelligence [167–170], demonstrating that effects on cognition are not restricted to recent inbreeding events. Studies on the effect of inbreeding on animal cognitive capacity are extremely rare [171], but inbred lines of rats tend to exhibit cognitive deficits compared to outbred lines (e.g. [172]). In fruit flies (Drosophila melanogaster), Nepoux et al. [171] found a negative effect of severe inbreeding on aversive learning [171].
Differences in personality or cognitive traits between island and mainland populations could also arise through pleiotropic effects, i.e. when alleles that are selected because of their effect on unrelated morphological, physiological or life-history traits also happen to shape behaviour [139,173–175]. More concrete evidence for such piggyback riding of behavioural genes comes from artificial selection studies, in which selection for one trait has (often unexpected) effects on other characteristics. For instance, selection for high voluntary wheel-running activity in mice resulted in reduced aggressiveness towards conspecifics [176]. Selection for wheat digestibility in broiler chickens affected the birds' neophobia, sociability and explorativeness [177]. In Drosophila, selection for both nutritional stress resistance [178] and longevity [179] came at the cost of reduced learning ability. These studies have not worked with island populations, but high locomotory activity, increased digestive abilities, nutritional stress and longer lifespans have all been associated with insular conditions (e.g. [88,180,181]). As an example of how pleiotropic effects might come about at the molecular level, consider how the vertebrate melanocortin system affects a variety of traits, including colouration, immunity, energy expenditure and stress resistance, but also aggression and sexual activity [139]. In principle, altered background-matching requirements on islands might select for darker colouration by increasing the activity of melanocortin receptors, which would collaterally increase aggressiveness and sexual activity. Pleiotropic effects have been invoked to explain the multifaceted changes in island lizards [26], although in this study, the behavioural components (aggressiveness, voraciousness) were deemed the targets of selection, and the changes in colour a happy by-product.
Perhaps the most intuitive path towards genetic differentiation in personality or cognition between island and mainland populations is through natural selection. As recognized by studies of dispersal reduction ([182–184], but see [185,186]), island dwellers are likely to have been exposed to at least two successive bouts of selection during their evolutionary history: on their route to the island, and subsequently, when confronted with the new environment. The nature and even the direction of selection during these two stages may diverge strongly [187]. Trait values that facilitate dispersal to and colonization of islands may diverge from (or even oppose) those that benefit fitness once the population is established [188]. Which of the two selection bouts will be most reflected in the island population, will then depend on the time since colonization, and the plastic and/or evolutionary malleability of the trait concerned. Recent studies have documented instances of very rapid dispersal reduction in some insect and plant taxa (e.g. [185,189]), but there are also cases where dispersal capacity does not or only very slowly decreased post-colonization [190,191].
Dispersal barriers may act as a filter for or isolate island dwellers of certain personality and cognitive phenotypes. There is now ample evidence for personality-dependent dispersal (reviewed in [142,192]). Aggressiveness may influence dispersal either way. Antagonistic individuals may coerce more peaceful conspecifics to disperse [193,194], or move away themselves [195,196]. The relationship between sociality and dispersal tendency seems also taxon-specific (compare [197] with [198]) or density-dependent [199]. The relationship between dispersion and cognition has received little attention [200,201]. In theory, both positive and negative relationships could evolve: well-developed cognitive skills may help dispersers survive the perilous route towards new horizons; but individuals that invest heavily in cognition may be reluctant to disperse into unknown territories (e.g. [202]). Comparative research on wide a variety of animals suggests that cognitive abilities are a determinant of invasion success (reviewed in [203]), but these studies typically emphasize the role of cognition in coping with new challenges encountered in the invaded territories, rather than with the dispersal event itself.
Once arrived on an island, animals are likely to face-selective pressures that diverge from those experienced on the mainland in multiple aspects (see above §2), prompting adaptive, genetic changes in their personality and cognitive traits. Although studies on how personality traits evolve in response to environmental changes remain relatively rare [31], there is now evidence that changes in ecological factors such as food availability (e.g. [204]), predation pressure (e.g. [205]), parasite load (e.g. [206]) and habitat structure (e.g. [207]), all known to occur on islands, may drive personality evolution. In addition, personality traits seem a likely target of sexual selection (see [208,209] for an overview of ideas), whose strength and direction may vary among islands (e.g. [210–212]). Proof for the evolvability of personality comes from studies of the fitness gradient in wild populations, from artificial selection studies, and from analyses comparing populations or species (reviewed in [31,213]).
Probably because it is deemed key to the evolution of our own species, theories on why and when natural selection promotes high cognitive abilities abound (see e.g. [138] for a review). They can be pushed into two major schools. The ‘Social Intelligence Hypothesis’ postulates that cognition has evolved to meet the challenges of a complex social life, to be able to read the intentions of peers and manipulate their behaviour [214,215]. The ‘Ecological Intelligence Hypothesis' (EIH) states that other, non-social aspects of the environment have steered cognitive evolution: challenges associated with locating or manipulating food, finding shelter or avoiding predation, for instance [216]. Refining EIH, the widely cited ‘Cognitive Buffer Hypothesis’ [217] emphasizes the role of environmental variability and argues that cognition evolved as a means to buffer individuals against stochastic fluctuations in, for instance, food availability [123,218]. Instead, the ‘Expensive Tissue Hypothesis' (ETH) [136] argues how low or variable resource availability could select for reduced investment in costly brain tissue (and more performant gut tissue), which might come at the expense of cognitive ability. Artificial selection studies, selection gradient studies and comparative analyses have confirmed that cognition is indeed malleable through natural selection (see [138,219,220] for reviews). Interestingly, recent comparative genomic techniques found evidence for positive selection on genes associated with brain development in multiple lineages (e.g. dolphins: [221]; paper wasps: [222]; capuchin monkeys: [223], including our own [169,224]).
In short, insularization can affect personality and cognition for multiple reasons and through several pathways.
4. Challenges, opportunities and avenues for behavioural research on islands
If changes in personality and cognitive skills following insularization seem likely and important, then why have they not been studied more often? Clearly, behavioural traits tend to be highly plastic, do not fossilize well and are difficult to compare among species, all of which complicates evolutionary studies. However, these problems are not specific to island populations, and the recent spurt in animal cognition and personality research proves that they can be overcome. Actually, we believe that island–mainland or among-island comparisons constitute a very promising avenue for studying the micro-evolution of behaviour, just as they did for other traits and for the same reason: because they offer the opportunity to study recurrent phenotypic changes in relatively simple environments [5].
A possible explanation for the dearth of work on cognition and personality performed on insular systems could be a mismatch in study organisms. Studies of animal personality and cognition have traditionally used primates, other mammals, birds and fish as models [225,226]—species that are often not very abundant on islands—especially not on smaller, oceanic islands. Recently, however, techniques for measuring personality and cognitive capacity have been tailored to and successfully applied in other taxa, such as reptiles [227] and insects [228], that can be sampled in large numbers on even the smallest islands. With the right study organisms, it should be logistically possible to study how insularity affects personality and cognition.
Clearly, a number of quality criteria must be met. By definition, personality scores should be repeatable in time and across contexts, but this has rarely been assessed in island populations. Along the same line, cognitive scores should be carefully tested for repeatability within individuals, and consistency among individuals and across contexts. This requires recapturing the same individuals in sufficient numbers, which can be a major challenge in fast-moving animals. Obtaining robust behavioural measurements, preferably in a number of populations and species, is a necessary first step to establish whether there is, effectively, a behavioural component to the island syndrome.
Equipped with robust data on personality traits and cognitive abilities, hypotheses on how insularity incites changes in personality or cognition can then be put to the test (table 2). Several ideas on this matter can be formulated, but remain largely untested. For instance, inbreeding depression engenders individuals with maladaptive, under certain ecological contexts, personalities [141,161]. Predation intensity is considered a prime factor determining the relative fitness of different personalities (e.g. [205,229]), and an important driver of cognitive evolution (e.g. [230,231]). At least in some taxa, high population densities are expected to decrease aggressiveness and increase sociality in island dwellers (e.g. [232]), and in combination with low-resource availability, may constrain brain development and thus cognitive abilities [136]. By contrast, the unpredictable nature of island environments has been hypothesized to select for behavioural flexibility and, hence, superior cognitive abilities [103]. Inbreeding, predation relaxation, high population densities, increased intraspecific competition and environmental stochasticity are all examples of phenomena associated with, but not limited to, island environments. Therefore, studies on the behaviour of island dwellers will be of great value to our understanding of the evolution of personality and cognition, in general.
Table 2.
Outstanding questions on the evolution of personality and cognition on islands.
| The master data |
| Do island populations exhibit repeatable inter-individual differences in the way they behaviourally interact with their environment, and are these differences consistent across contexts? |
| Do populations (or communities) on islands exhibit the same range and relative frequencies of behavioural types as populations on the mainland, or on other islands? |
| Do animal populations on islands differ in cognitive skills from their counterparts on the mainland, or on other islands? |
| The mechanisms of change |
| What is the role of non-adaptive evolution (e.g. inbreeding, genetic drift, pleiotropy) in creating differences in cognition and personality between island and mainland populations? |
| What is the role of dispersal filtering in creating differences in cognition and personality between island and mainland populations? How long does this effect linger? |
| What is the role of phenotypic plasticity versus genetic adaptation in creating differences in cognition and personality between island and mainland populations? |
| The drivers of change |
| What is the effect of predator release on islands on personality traits? Are these effects general, or specific to a predatory context? |
| Does predator release on islands affect prey cognitive capacity? (How fast) do prey species lose their ability to recognize predators, to respond in adequate ways? Are these effects general, or specific to a predatory context? |
| How does reduced interspecific competition (and the possible resulting niche shift) on islands affect personality traits? Are these effects general, or specific to an interspecific context? |
| How do high population densities on islands affect personality? |
| How does low-resource availability or predictability affect personality traits, and cognitive capacity? |
| Covariation with other characteristics |
| Do changes in morphology (e.g. body size, shape and colour), physiology (e.g. brain size and digestive performance) or diet (type or breadth) observed in island populations concur with changes in personal or cognitive capacity? Are these changes adaptive or constrained? |
| Do changes in life history of the pace of life (fast to slow) on islands affect personality and cognition? |
| Generality and relevance |
| Are the magnitude and the direction of changes in personality and cognitive traits on islands consistent over taxonomic groups and island environments? If not, which factors are responsible? |
| How do personality and cognitive characteristics of island populations affect their vulnerability to alien species? How readily can island animals adjust personality and cognitive traits to cope with new challenges? |
A logical next step would be to assess whether and how personality traits and cognitive traits covary among themselves, and with the morphological, physiological and life-history traits traditionally implicated in the ‘island syndrome’. This would allow testing outstanding hypotheses on how personality differences are maintained over time [233], on the existence and consistency of cognitive syndromes and styles [44,234], on the role of behaviour in the pace-of-life theory [140], and on behavioural consequences of correlational selection on physiological or morphological traits (e.g. [235–237]).
The above questions primarily relate to the eventual outcome of evolutionary trajectories, but islands also offer unique opportunities to learn about the nature of the trajectories themselves. By studying populations of varying age (colonization history), one could assess the importance of adaptive landscapes [238], genetic covariance matrices [239] and the prevalence of ‘evolutionary paths of least resistance’ [240] in the evolution of behaviour. Such analyses could also reveal reversals in the direction of evolution, e.g. when distinct phenotypes facilitate dispersal and settlement [183]. Finally, studying islands with different colonization histories could reveal information on the rate at which behavioural changes occur. Such knowledge is of fundamental biological interest, but in addition may be valuable in the context of the conservation of island and other isolated populations.
Acknowledgements
We thank Kevin C. Burns and six anonymous reviewers for their helpful comments on an earlier version of the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
I.G.: conceptualization, investigation, writing—original draft and writing—review and editing; G.D.M.: investigation and writing—review and editing; R.V.D.: conceptualization, investigation, writing—original draft and writing—review and editing; S.B.: conceptualization, investigation and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
Simon Baeckens is on the editorial board of Biology Letters.
Funding
Financial support was given by the University of Antwerp (I.G., R.V.D.) and the Research Foundation-Flanders (FWO) (I.G.: 1105122N; G.D.M.: 1144118N; S.B.: 12I8822N).
References
- 1.Adler GH, Levins R. 1994. The island syndrome in rodent populations. Q. Rev. Biol. 69, 473-490. ( 10.1007/bf00329044) [DOI] [PubMed] [Google Scholar]
- 2.Baeckens S, Van Damme R. 2020. The island syndrome. Curr. Biol. 30, 338-339. ( 10.1016/j.cub.2020.03.029) [DOI] [PubMed] [Google Scholar]
- 3.Burns KC. 2019. Evolution in isolation: the search for an island syndrome in plants. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 4.Whittaker RJ, Fernández-Palacios JM. 2006. Island biogeography; ecology, evolution and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830-836. ( 10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 6.Covas R. 2012. Evolution of reproductive life histories in island birds worldwide. Proc. R. Soc. B 279, 1531-1537. ( 10.1098/rspb.2011.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doutrelant C, Paquet M, Renoult JP, Grégoire A, Crochet PA, Covas R. 2016. Worldwide patterns of bird colouration on islands. Ecol. Lett. 19, 537-545. ( 10.1111/ele.12588) [DOI] [PubMed] [Google Scholar]
- 8.Darwin C. 1839. Journal of researches into the geology and natural history of the various countries visited by H. M. S. Beagle, under the command of captain Fitzroy, R. N. from 1832–1836. London, UK: Henry Colburn. [Google Scholar]
- 9.Cooper WE Jr, Pyron RA, Garland T Jr. 2014. Island tameness: living on islands reduces flight initiation distance. Proc. R. Soc. B 281, 20133019. ( 10.1098/rspb.2013.3019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert A, Melo M, Lengagne T, Julien S, Gomez D, Doutrelant C. 2021. Patterns of bird song evolution on islands support the character release hypothesis in tropical but not in temperate latitudes. J. Evol. Biol. 34, 1580-1591. ( 10.1111/jeb.13928) [DOI] [PubMed] [Google Scholar]
- 11.Morinay J, Cardoso GC, Doutrelant C, Covas R. 2013. The evolution of birdsong on islands. Ecol. Evol. 3, 5127-5140. ( 10.1002/ece3.864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reudink MW, Pageau C, Fisher M, Mount N, Buckler N, Otter KA, Briskie JV. 2021. Evolution of song and color in island birds. Wilson J. Ornithol. 133, 1-10. ( 10.1676/19-00084) [DOI] [Google Scholar]
- 13.Montiglio PO, Dammhahn M, Dubuc Messier G, Réale D. 2018. The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow–fast continuum. Behav. Ecol. Sociobiol. 72, 1-9. ( 10.1007/s00265-018-2526-2) [DOI] [Google Scholar]
- 14.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 15.Lack D. 1969. The number of bird species on islands. Bird Study 16, 193-209. ( 10.1080/00063656909476244) [DOI] [Google Scholar]
- 16.Cox GW, Ricklefs RE. 1977. Species diversity, ecological release, and community structuring in Caribbean land bird faunas. Oikos 28, 113-122. ( 10.2307/3543330) [DOI] [Google Scholar]
- 17.Herrmann NC, Stroud JT, Losos JB. 2021. The evolution of ‘ecological release’ into the 21st century. Trends Ecol. Evol. 36, 206-215. ( 10.1016/j.tree.2020.10.019) [DOI] [PubMed] [Google Scholar]
- 18.MacArthur RH, Diamond JM, Karr JR. 1972. Density compensation in island faunas. Ecology 53, 330-342. ( 10.2307/1934090) [DOI] [Google Scholar]
- 19.Case TJ, Gilpin ME, Diamond JM. 1979. Overexploitation, interference competition, and excess density compensation in insular faunas. Am. Nat. 113, 843-854. ( 10.1086/283440) [DOI] [Google Scholar]
- 20.Buckley LB, Jetz W. 2007. Insularity and the determinants of lizard population density. Ecol. Lett. 10, 481-489. ( 10.1111/j.1461-0248.2007.01042.x) [DOI] [PubMed] [Google Scholar]
- 21.Janzen D. 1973. Sweep samples of tropical foliage insects: description of study sites, with data on species abundances and size distributions. Ecology 54, 659-686. ( 10.2307/1935358) [DOI] [Google Scholar]
- 22.Blanco G, Laiolo P, Fargallo JA. 2014. Linking environmental stress, feeding-shifts and the ‘island syndrome’: a nutritional challenge hypothesis. Popul. Ecol. 56, 203-216. ( 10.1007/s10144-013-0404-3) [DOI] [Google Scholar]
- 23.Andrews RM. 1979. Evolution of life histories: a comparison of Anolis lizards from matched island and mainland habitats. Brevoria 454, 1-51. ( 10.1111/j.1365-2699.2010.02466.x) [DOI] [Google Scholar]
- 24.Olesen JM, Valido A. 2003. Lizards as pollinators and seed dispersers: an island phenomenon. Trends Ecol. Evol. 18, 177-181. ( 10.1016/s0169-5347(03)00004-1) [DOI] [Google Scholar]
- 25.Sale MG, Arnould JPY. 2013. Inflated population density of island antechinus: a case of allochthonous marine inputs leading to increased food availability? Aust. J. Zool. 60, 343-351. ( 10.1071/ZO12073) [DOI] [Google Scholar]
- 26.Raia P, Guarino FM, Turano M, Polese G, Rippa D, Carotenuto F, Monti DM, Cardi M, Fulgione D. 2010. The blue lizard spandrel and the island syndrome. BMC Evol. Biol. 10, 289. ( 10.1186/1471-2148-10-289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monti DM, Raia P, Vroonen J, Maselli V, Van Damme R, Fulgione D. 2013. Physiological change in an insular lizard population confirms the reversed island syndrome. Biol. J. Linn. Soc. 108, 144-150. ( 10.1111/j.1095-8312.2012.02019.x) [DOI] [Google Scholar]
- 28.Dougherty Liam R, Guillette Lauren M.. 2018. Linking personality and cognition: a meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences 373(1756), 20170282. ( 10.1098/rstb.2017.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dochtermann NA, Schwab T, Sih A. 2015. The contribution of additive genetic variation to personality variation: heritability of personality. Proc. R. Soc. B 282, 20142201. ( 10.1098/rspb.2014.2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dochtermann NA, Schwab T, Anderson Berdal M, Dalos J, Royauté R. 2019. The heritability of behavior: a meta-analysis. J. Hered. 110, 403-410. ( 10.1093/jhered/esz023) [DOI] [PubMed] [Google Scholar]
- 31.Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291-318. ( 10.1111/j.1469-185x.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 32.Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241-277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 33.Dukas R. 2004. Evolutionary biology of animal cognition. Annu. Rev. Ecol. Evol. Syst. 35, 347-374. ( 10.1146/annurev.ecolsys.35.112202.130152) [DOI] [Google Scholar]
- 34.Huebner F, Fichtel C, Kappeler PM. 2018. Linking cognition with fitness in a wild primate: fitness correlates of problem-solving performance and spatial learning ability. Phil. Trans. R. Soc. B 373, 20170295. ( 10.1098/rstb.2017.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branch CL, Pitera AM, Kozlovsky DY, Bridge ES, Pravosudov VV. 2019. Smart is the new sexy: female mountain chickadees increase reproductive investment when mated to males with better spatial cognition. Ecol. Lett. 22, 897-903. ( 10.1111/ele.13249) [DOI] [PubMed] [Google Scholar]
- 36.Sonnenberg BR, Branch CL, Pitera AM, Bridge E, Pravosudov VV. 2019. Natural selection and spatial cognition in wild food-caching mountain chickadees. Curr. Biol. 29, 670-676. ( 10.1016/j.cub.2019.01.006) [DOI] [PubMed] [Google Scholar]
- 37.Ashton BJ, Ridley AR, Edwards EK, Thornton A. 2018. Cognitive performance is linked to group size and affects fitness in Australian magpies. Nature 554, 364-367. ( 10.1038/nature25503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cauchoix M, et al. 2018. The repeatability of cognitive performance: a meta-analysis. Proc. R. Soc. B 373, 20170281. ( 10.1098/rstb.2017.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin AS, Guillette LM, Healy SD. 2015. Cognition and personality: an analysis of an emerging field. Trends Ecol. Evol. 30, 207-214. ( 10.1016/j.tree.2015.01.012) [DOI] [PubMed] [Google Scholar]
- 40.Spearman C. 1904. General intelligence, objectively determined and measured. Am. J. Psychol. 15, 201-292. ( 10.1037/11491-006) [DOI] [Google Scholar]
- 41.Warne RT, Burningham C. 2019. Spearman's g found in 31 non-Western nations: strong evidence that g is a universal phenomenon. Psych. Bull. 145, 237-272. ( 10.31234/osf.io/uv673) [DOI] [PubMed] [Google Scholar]
- 42.Galsworthy MJ, Paya-Cano JL, Liu L, Monleón S, Gregoryan G, Fernandes C, Schalkwyk LC, Plomin R. 2005. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav. Genet. 35, 675-692. ( 10.1007/s10519-005-3423-9) [DOI] [PubMed] [Google Scholar]
- 43.Shaw RC, Boogert NJ, Clayton NS, Burns KC. 2015. Wild psychometrics: evidence for ‘general’ cognitive performance in wild New Zealand robins, Petroica longipes. Anim. Behav. 109, 101-111. ( 10.1016/j.anbehav.2015.08.001) [DOI] [Google Scholar]
- 44.Poirier MA, Kozlovsky DY, Morand-Ferron J, Careau V. 2020. How general is cognitive ability in non-human animals? A meta-analytical and multi-level reanalysis approach. Proc. R. Soc. B 287, 20201853. ( 10.1098/rspb.2020.1853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McWaters SR, Pangle WM. 2021. Heads up! Variation in the vigilance of foraging chipmunks in response to experimental manipulation of perceived risk. Ethology 127, 309-320. ( 10.1111/eth.13128) [DOI] [Google Scholar]
- 46.Blumstein DT, Daniel JC, Springett BP. 2004. A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology 110, 919-934. ( 10.1111/j.1439-0310.2004.01033.x) [DOI] [Google Scholar]
- 47.Reimers E, Lund S, Ergon T. 2011. Vigilance and fright behaviour in the insular Svalbard reindeer. Can. J. Zool. 89, 753-764. ( 10.1139/z11-040) [DOI] [Google Scholar]
- 48.Hamao S, Torikai H, Yoshikawa M, Yamamoto Y, Ijichi T. 2020. Risk-taking behavior of bull-headed shrikes that recently colonized islands. Curr. Zool. 67, 177-182. ( 10.1093/cz/zoaa036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphrey PS, Livezey BC, Siegel-Causey D. 1987. Tameness of birds of the Falkland Islands: an index and preliminary results. Bird Behav. 7, 67-72. ( 10.3727/015613887791918114) [DOI] [Google Scholar]
- 50.Rasheed AA, Hambley K, Chan G, de la Rosa CA, Larison B, Blumstein DT. 2017. Persistence of antipredator behavior in an island population of California quail. Ethology 124, 155-160. ( 10.1111/eth.12716) [DOI] [Google Scholar]
- 51.Delibes MC, Blázques MC, Soriano E, Revilla E, Godoy JA. 2011. High antipredatory efficiency of insular lizards: a warning signal of excessive specimen collection? PLoS ONE 6, e29312. ( 10.1371/journal.pone.0029312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rödl T, Berger S, Romero LM, Wikelski M. 2007. Tameness and stress physiology in a predator-naive island species confronted with novel predation threat. Proc. R. Soc. B 274, 577-582. ( 10.1098/rspb.2006.3755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blázquez MC, Rodríguez-Estrella R, Delibes M. 1997. Escape behavior and predation risk of mainland and island spiny-tailed iguanas (Ctenosaura hemilopha). Ethology 103, 990-998. ( 10.1111/j.1439-0310.1997.tb00141.x) [DOI] [Google Scholar]
- 54.Stone PA, Snell HL, Snell HM. 1994. Behavioral diversity as biological diversity: introduced cats and lava lizard wariness. Conserv. Biol. 8, 569-573. ( 10.1046/j.1523-1739.1994.08020569.x) [DOI] [Google Scholar]
- 55.Cooper WE Jr, Pérez-Mellado V. 2012. Historical influence of predation pressure on escape by Podarcis lizards in the Balearic Islands. Biol. J. Linn. Soc. 107, 254-268. ( 10.1111/j.1095-8312.2012.01933.x) [DOI] [Google Scholar]
- 56.Cooper WE Jr, Hawlena D, Pérez-Mellado V. 2009. Islet tameness: escape behavior and refuge use in populations of the Balearic lizard (Podarcis lilfordi) exposed to differing predation pressure. Can. J. Zool. 87, 912-919. ( 10.1139/z09-077) [DOI] [Google Scholar]
- 57.Vervust B, Grbac I, Van Damme R. 2007. Differences in morphology, performance and behaviour between recently diverged populations of Podarcis sicula mirror differences in predation pressure. Oikos 116, 1343-1352. ( 10.1111/j.0030-1299.2007.15989.x) [DOI] [Google Scholar]
- 58.Li BB, Belasen A, Pafilis P, Bednekoff P, Foufopoulos J. 2014. Effects of feral cats on the evolution of anti-predator behaviours in island reptiles: insights from an ancient introduction. Proc. R. Soc. B 281, 20140339. ( 10.1098/rspb.2014.0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brock KM, Bednekoff PA, Pafilis P, Foufopoulos J. 2015. Evolution of antipredator behavior in an island lizard species, Podarcis erhardii (Reptilia: Lacertidae): the sum of all fears? Evolution 69, 216-231. ( 10.1111/evo.12555) [DOI] [PubMed] [Google Scholar]
- 60.De Meester G, Lambreghts Y, Briesen B, Smeuninx T, Tadić Z, Van Damme R. 2018. Hunt or hide: how insularity and urbanization affect foraging decisions in lizards. Ethology 124, 227-235. ( 10.1111/eth.12722) [DOI] [Google Scholar]
- 61.Jolly CJ, Webb JK, Phillips BL. 2018. The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations. Biol. Lett. 14, 20180222. ( 10.1098/rsbl.2018.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brodin T, Lind MI, Wiberg MK, Johansson F. 2013. Personality trait differences between mainland and island populations in the common frog (Rana temporaria). Behav. Ecol. Sociobiol. 67, 135-143. ( 10.1007/s00265-012-1433-1) [DOI] [Google Scholar]
- 63.Stratton JA, Nolte MJ, Payseur BA. 2021. Evolution of boldness and exploratory behavior in giant mice from Gough Island. Behav. Ecol. Sociobiol. 75, 65. ( 10.1101/2020.09.10.292185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mettke-Hofmann C, Winkler H, Leisler B. 2002. The significance of ecological factors for exploration and neophobia in parrots. Ethology 108, 249-272. ( 10.1046/j.1439-0310.2002.00773.x) [DOI] [Google Scholar]
- 65.Haemig PD. 1988. A comparative experimental study of exploratory behaviour in Santa Cruz Island and mainland California scrub jays Aphelocoma coerulescens. Bird Behav. 8, 38-42. ( 10.3727/015613888791871296) [DOI] [Google Scholar]
- 66.Lapiedra O, Schoener TW, Leal M, Losos JB, Kolbe JJ. 2018. Predator-driven natural selection on risk-taking behavior in anole lizards. Science 360, 1017-1020. ( 10.1126/science.aap9289) [DOI] [PubMed] [Google Scholar]
- 67.Juette T, Garant D, Jameson JW, Réale D. 2020. The island syndrome hypothesis is only partially validated in two rodent species in an inland–island system. Oikos 129, 1739-1751. ( 10.1111/oik.07249) [DOI] [Google Scholar]
- 68.Baier F, Hoekstra HE. 2019. The genetics of morphological and behavioural island traits in deer mice. Proc. R. Soc. B 286, 20191697. ( 10.1101/443432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halpin ZT. 1981. Adult–young interactions in island and mainland populations of the deer mouse Peromyscus maniculatus. Oecologia 51, 419-425. ( 10.1007/bf00540916) [DOI] [PubMed] [Google Scholar]
- 70.Välimäki K, Hinten G, Hanski I. 2007. Inbreeding and competitive ability in the common shrew (Sorex araneus). Behav. Ecol. Sociobiol. 61, 997-1005. ( 10.1007/s00265-006-0332-8) [DOI] [Google Scholar]
- 71.Cooper WE Jr, Dimopoulos I, Pafilis P. 2014. Sex, age, and population density affect aggressive behaviors in island lizards promoting cannibalism. Ethology 121, 260-269. ( 10.1111/eth.12335) [DOI] [Google Scholar]
- 72.Bonnet X, Aubret F, Lourdais O, Ladyman M, Bradshaw SD, Maumelat S. 2005. Do ‘quiet’ places make animals placid? Island versus mainland tiger snakes. Ethology 111, 573-592. ( 10.1111/j.1439-0310.2005.01070.x) [DOI] [Google Scholar]
- 73.Aubret F, Michniewicz RJ, Shine R. 2011. Correlated geographic variation in predation risk and antipredator behaviour within a wide-ranging snake species (Notechis scutatus, Elapidae). Austral. Ecol. 36, 446-452. ( 10.1111/j.1442-9993.2010.02171.x) [DOI] [Google Scholar]
- 74.Placyck JS Jr. 2012. The role of innate and environmental influences in shaping antipredator behavior of mainland and insular garter snakes (Thamnophis sirtalis). J. Ethol. 30, 101-108. ( 10.1007/s10164-011-0302-0) [DOI] [Google Scholar]
- 75.Person CE, Fox GA, King J, Gren ECK, Briggs E, Hayes WK. 2006. Paradoxical exception to island tameness: increased defensiveness in an insular population of rattlesnakes. Toxicon 119, 375-376. ( 10.1016/j.toxicon.2016.06.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Case TJ. 1982. Ecology and evolution of the insular gigantic chuckawallas, Sauromalus hispidus and Sauromalus varius. In Iguanas of the world. Their behavior, ecology and conservation (eds Burghardt GM, Rand AS). Park Ridge, NJ: Noyes Publ. [Google Scholar]
- 77.Gray S, Hurst J. 1998. Competitive behaviour in an island population of house mice, Mus domesticus. Anim. Behav. 56, 1291-1299. ( 10.1006/anbe.1998.0890) [DOI] [PubMed] [Google Scholar]
- 78.Pulliam HR. 1973. Comparative feeding ecology of a tropical grassland finch (Tiaris olivacea). Ecology 54, 284-299. ( 10.2307/1934337) [DOI] [Google Scholar]
- 79.Tomkins J, Brown G. 2004. Population density drives the local evolution of a threshold dimorphism. Nature 431, 1099-1103. ( 10.1038/nature02918) [DOI] [PubMed] [Google Scholar]
- 80.Hromatko I, Grus A, Kolđeraj G. 2021. Do islanders have a more reactive behavioral immune system? Social cognitions and preferred interpersonal distances during the COVID-19 pandemic. Front. Psychol. 12, 2021.647586. ( 10.3389/fpsyg.2021.647586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Camperio Ciani AS, Capiluppi C. 2011. Gene flow by selective emigration as a possible cause for personality differences between small islands and mainland populations. Eur. J. Pers. 25, 53-64. ( 10.1002/per.774) [DOI] [Google Scholar]
- 82.Camperio Ciani AS, Capiluppi C, Veronese A, Sartori G. 2007. The adaptive value of personality differences revealed by small island population dynamics. Eur. J. Pers. 21, 3-22. ( 10.1002/per.595) [DOI] [Google Scholar]
- 83.Camperio Ciani AS, Edelman S, Ebstein RP. 2013. The dopamine D4 receptor (DRD4) exon 3 VNTR contributes to adaptive personality differences in an Italian small island population. Eur. J. Pers. 27, 593-604. ( 10.1002/per.1917) [DOI] [Google Scholar]
- 84.Reich LM, Tamarin RH. 1980. Trap use as an indicator of social behavior in mainland and island voles. Acta Theriol. 25, 295-307. ( 10.4098/at.arch.80-26) [DOI] [Google Scholar]
- 85.Beauchamp G. 2004. Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. B 271, 1039-1042. ( 10.1098/rspb.2004.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Schaik CP, van Noordwijk MA. 1985. Evolutionary effect of the absence of felids on the social organization of the macaques on the Island of Simeulue (Macaca fascicularis fusca, Miller 1903). Folia Primatol. 44, 138-147. ( 10.1159/000156208) [DOI] [Google Scholar]
- 87.Kang C, Sherratt TN, Kim YE, Shin Y, Moon J, Song U, Kang JY, Kim K, Jang Y. 2017. Differential predation drives the geographical divergence in multiple traits in aposematic frogs. Behav. Ecol. 28, 1122-1130. ( 10.1093/beheco/arx076) [DOI] [Google Scholar]
- 88.Siliceo-Cantero HH, García A. 2015. Actividad y uso del hábitat de una población insular y una continental de lagartijas Anolis nebulosus (Squamata: Polychrotidae) en un ambiente estacional. Rev. Mex. Biodiv. 86, 406-411. ( 10.1016/j.rmb.2015.04.011) [DOI] [Google Scholar]
- 89.Kubo D, Kono RT. 2011. Endocranial restoration and volume estimation of the Minatogawa IV cranium using micro-CT and 3D printer systems. Anthropol. Sci. 119, 201-209. ( 10.1537/ase.110228) [DOI] [Google Scholar]
- 90.Diniz-Filho JAF, Raia P. 2017. Island rule, quantitative genetics and brain–body size evolution in Homo floresiensis. Proc. R. Soc. B 284, 20171065. ( 10.1098/rspb.2017.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masters JC, Genin F, Silvestro D, Lister AM, DelPero M. 2014. The red island and the seven dwarfs: body size reduction in Cheirogaleidae. J. Biogeogr. 41, 1833-1847. ( 10.1111/jbi.12327) [DOI] [Google Scholar]
- 92.Weston EM, Lister AM. 2009. Insular dwarfism in hippos and a model for brain size reduction in Homo floresiensis. Nature 459, 85-88. ( 10.1038/nature07922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larramendi A, Palombo MR. 2015. Body size, biology and encephalization quotient of Palaeoloxodon ex gr. P. falconeri from Spinagallo cave (Hyblean Plateau, Sicily). Hystrix 26, 1-8. ( 10.4404/hystrix-26.2-11478) [DOI] [Google Scholar]
- 94.Köhler M, Moya-Soya S. 2004. Reduction of brain and sense organs in the fossil insular bovid Myotragus. Brain Behav. Evol. 63, 125-140. ( 10.1159/000076239) [DOI] [PubMed] [Google Scholar]
- 95.Palombo MR, Köhler M, Moya Sola S, Giovinazzo C. 2008. Brain versus body mass in endemic ruminant artiodactyls: a case studied of Myotragus balearicus and smallest Candiacervus species from Mediterranean Islands. Q. Internat. 182, 160-183. ( 10.1016/j.quaint.2007.08.037) [DOI] [Google Scholar]
- 96.Palombo MR, Giovanazzo C. 2004. Brain weight versus body mass in late Pleistocene Cynotherium sardous Studiati, 1847 from Dragonara Cave (north-western Sardinia). Giornate Paleontol. 4, 44. [Google Scholar]
- 97.Quintana J, Köhler M, Moya-Sola S. 2011. Nuralagus rex, gen. et sp. nov., an endemic insular giant rabbit from the Neogene of Minorca (Balearic Islands, Spain). J. Vertebr. Paleontol. 31, 231-240. ( 10.1080/02724634.2011.550367) [DOI] [Google Scholar]
- 98.Castiglione S, Serio C, Piccolo M, Mondanaro A, Melchionna M, Di Febbraro M, Sansalone G, Wroe S, Raia P. 2021. The influence of domestication, insularity and sociality on the tempo and mode of brain size evolution in mammals. Biol. J. Linn. Soc. 132, 221-231. ( 10.1093/biolinnean/blaa186) [DOI] [Google Scholar]
- 99.Gold MEL, Bourdon E, Norell MA. 2016. The first endocast of the extinct dodo (Raphus cucullatus) and an anatomical comparison amongst close relatives (Aves, Columbiformes). Zool. J. Linn. Soc. 177, 950-963. ( 10.1111/zoj.12388) [DOI] [Google Scholar]
- 100.Duhamel A, Hume JP, Guenser P, Salaviale C, Louchart A. 2020. Cranial evolution in the extinct Rodrigues Island owl Otus murivorus (Strigidae), associated with unexpected ecological adaptations. Sci. Rep. 10, 14019. ( 10.1038/s41598-020-69868-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scofield RP, Ashwell KWS. 2020. Rapid somatic expansion causes the brain to lag behind: the case of the brain and behavior of New Zealand's Haast's eagle (Harpagornis moorei). J. Vertebr. Paleontol. 29, 637-649. ( 10.1671/039.029.0325) [DOI] [Google Scholar]
- 102.Jønsson KA, Fabre PH, Irestedt M. 2013. Brains, tools, innovation and biogeography in crows and ravens. BMC Evol. Biol. 12, 72. ( 10.1186/1471-2148-12-72) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sayol F, Downing PA, Iwaniuk AN, Maspons J, Sol D. 2018. Predictable evolution towards larger brains in birds colonizing oceanic islands. Nat. Comm. 9, 2820. ( 10.1038/s41467-018-05280-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galea LAM, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. 1994. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 635, 18-26. ( 10.1016/0006-8993(94)91419-2) [DOI] [PubMed] [Google Scholar]
- 105.Dingemanse NJ, Wright J. 2020. Criteria for acceptable studies of animal personality and behavioural syndromes. Ethology 126, 865-869. ( 10.1111/eth.13082) [DOI] [Google Scholar]
- 106.Etheridge R. 1889. The general zoology of Lord Howe Island; containing also an account of the collections made by the Australian Museum Collecting Party, Aug–Sept 1887. Austral. Mus. Mem. 2, 1-42. ( 10.3853/j.0067-1967.2.1889.479) [DOI] [Google Scholar]
- 107.Rothschildt W, Hartert E. 1899. A review of the ornithology of the Galapagos islands. With notes on the Webster-Harris expedition. Novitates Zool. 6, 85-205. [Google Scholar]
- 108.Blumstein DT. 2002. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 29, 685-692. ( 10.1046/j.1365-2699.2002.00717.x) [DOI] [Google Scholar]
- 109.Zuberogoitia I, Martínez JE, Margalida A, Gómez I, Azkona A, Martínez JA. 2010. Reduced food availability induces behavioural changes in Griffon vulture Gyps fulvus. Ornis Fenn. 87, 52-60. [Google Scholar]
- 110.Bauwens D, Thoen C. 1981. Escape tactics and vulnerability to predation associated with reproduction in the lizard Lacerta vivipara. J. Anim. Ecol. 50, 733-743. ( 10.2307/4133) [DOI] [Google Scholar]
- 111.Seltmann MW, Öst M, Jaatinen K, Atkinson S, Mashburn K, Hollmén T. 2012. Stress responsiveness, age and body condition interactively affect flight initiation distance in breeding female eiders. Anim. Behav. 84, 889-896. ( 10.1016/j.anbehav.2012.07.012) [DOI] [Google Scholar]
- 112.Rocha C, Bergallo H. 1990. Thermal biology and flight distance of Tropidurus oreadicus (Sauria, Iguanidae) in an area of Amazonian Brazil. Ethol. Ecol. Evol. 2, 263-268. ( 10.1080/08927014.1990.9525411) [DOI] [Google Scholar]
- 113.Capizzi D, Luiselli L, Viguoli L. 2007. Flight initiation distance in relation to substratum type, sex, reproductive status and tail condition in two lacertids with contrasting habits. Amphibia-Reptilia 28, 403-407. ( 10.1163/156853807781374827) [DOI] [Google Scholar]
- 114.Engelhardt SC, Weladii RB. 2011. Effects of levels of human exposure on flight initiation distance and distance to refuge in foraging eastern gray squirrels (Sciurus carolinensis). Can. J. Zool. 89, 823-830. ( 10.1139/z11-054) [DOI] [Google Scholar]
- 115.Cooper WE Jr. 2009. Flight initiation distance during social activity in lizards (Sceloporus virgatus). Behav. Ecol. Sociobiol. 63, 1765-1771. ( 10.1007/s00265-009-0799-1) [DOI] [Google Scholar]
- 116.Dill LM, Houtman R. 1989. The influence of distance to refuge on flight initiation distance in the gray squirrel (Sciurus carolinensis). Can. J. Zool. 67, 233-235. ( 10.1139/z89-033) [DOI] [Google Scholar]
- 117.Wilson DS, Clark AB, Coleman K, Dearstyne T. 1994. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 9, 442-446. ( 10.1016/0169-5347(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 118.Dingemanse NJ, Reale D. 2005. Natural selection and animal personality. Behaviour 142, 1159-1184. ( 10.1163/156853905774539445) [DOI] [Google Scholar]
- 119.Polis GA, Hurd SD. 1995. Extraordinarily high spider densities on islands: flow of energy from the marine to terrestrial food webs and the absence of predation. Proc. Natl Acad. Sci. USA 92, 4382-4386. ( 10.2307/2367331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Novosolov M, Rodda GH, Feldman A, Kadison AE, Dor R, Meiri S. 2016. Power in numbers. Drivers of high population density in insular lizards. Global Ecol. Biogeogr. 25, 87-95. ( 10.1111/geb.12390) [DOI] [Google Scholar]
- 121.Bliard L, Paquet M, Robert A, Dufour P, Renoult JP, Grégoire A, Crochet PA, Covas R, Doutrelant C. 2020. Examining the link between relaxed predation and bird colouration on islands. Biol. Lett. 16, 20200002. ( 10.1098/rsbl.2020.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burns KC. 2021. An anecdotal observation of anti-predatory tool use in a New Zealand parrot. Behaviour 1, 1-7. ( 10.1163/1568539X-bja10137) [DOI] [Google Scholar]
- 123.Mettke-Hofmann C. 2014. Cognitive ecology: ecological factors, life-styles, and cognition. Wiley Interdiscip. Rev. Cogn. Sci. 5, 345-360. ( 10.1002/wcs.1289) [DOI] [PubMed] [Google Scholar]
- 124.Stamps JA, Buechner M. 1985. The territorial defense hypothesis and the ecology of insular vertebrates. Quart. Rev. Biol. 60, 155-181. ( 10.1086/414314) [DOI] [PubMed] [Google Scholar]
- 125.He P, Maldonado-Chaparro AA, Farine DR. 2019. The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behav. Ecol. Sociobiol. 73, 1-14. ( 10.1007/s00265-018-2602-7) [DOI] [Google Scholar]
- 126.Erlandsson R, Hasselgren M, Norén K, Macdonald D, Angerbjörn A. 2022. Resources and predation: drivers of sociality in a cyclic mesopredator. Oecologia 198, 381-392. ( 10.1007/s00442-022-05107-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gliwicz J, Dąbrowski MJ.. 2008. Ecological factors affecting the diel activity of voles in a multi-species community. In Annales zoologici fennici, vol. 45, pp. 242-247. Finnish; Zoological and Botanical Publishing Board, Helsinki, Finland. [Google Scholar]
- 128.Killen SS, Calsbeek R, Williams TD. 2017. The ecology of exercise: mechanisms underlying individual variation in behavior, activity, and performance. Integr. Comp. Biol. 57, 185-194. ( 10.1093/icb/icx083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Healy SD, Rowe C. 2007. A critique of comparative studies on brain size. Proc. R. Soc. B 274, 453-464. ( 10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Font E, García-Roa R, Pincheira-Donoso D, Carazo P. 2019. Rethinking the effects of body size on the study of brain size evolution. Brain Behav. Evol. 93, 182-195. ( 10.1159/000501161) [DOI] [PubMed] [Google Scholar]
- 131.Barrett BJ, Monteza-Moreno CM, Dogandžić T, Zwyns N, Ibáñez A, Crofoot MC. 2018. Habitual stone-tool-aided extractive foraging in white-faced capuchins, Cebus capucinus. R. Soc. Open Sci. 5, 181002. ( 10.1101/351619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rutz C, St Clair JJH. 2012. The evolutionary origins and ecological context of tool use in New Caledonian crows. Behav. Process. 89, 153-165. ( 10.1016/j.beproc.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 133.Rutz C, et al. 2016. Discovery of species-wide tool use in the Hawaiian crow. Nature 537, 403-407. ( 10.1038/nature19103) [DOI] [PubMed] [Google Scholar]
- 134.Tebbich S, Sterelny K, Teschke I. 2010. The tale of the finch: adaptive radiation and behavioural flexibility. Phil. Trans. R. Soc. B 365, 1099-1109. ( 10.1098/rstb.2009.0291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Auersperg AMI, Szabo B, von Bayern AMP, Kacelnik A. 2012. Spontaneous innovation in tool manufacture and use in a Goffin's cockatoo. Curr. Biol. 22, R903-R904. ( 10.1016/j.cub.2012.09.002) [DOI] [PubMed] [Google Scholar]
- 136.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199-221. ( 10.1086/204350) [DOI] [Google Scholar]
- 137.Toscano BJ, Gownaris NJ, Heerhartz SM, Monaco CJ. 2016. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia 182, 55-69. ( 10.1007/s00442-016-3648-8) [DOI] [PubMed] [Google Scholar]
- 138.Henke-von der Malsburg J, Kappeler PM, Fichtel C. 2020. Linking ecology and cognition: does ecological specialisation predict cognitive test performance? Behav. Ecol. Sociobiol. 74, 154. ( 10.1007/s00265-020-02923-z) [DOI] [Google Scholar]
- 139.Ducrest AL, Keller L, Roulin A. 2008. Pleiotropy in the melanocortin system, colouration and behavioural syndromes. Trends Ecol. Evol. 23, 502-510. ( 10.1016/j.tree.2008.06.001) [DOI] [PubMed] [Google Scholar]
- 140.Wolf M, Van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581-584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 141.Herdegen-Radwan M. 2019. Does inbreeding affect personality traits? Ecol. Evol. 9, 10 929-10 937. ( 10.1002/ece3.5487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cote J, Clobert J, Brodin T, Fogarty S, Sih A. 2010. Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil. Trans. R. Soc. B 365, 4065-4076. ( 10.1098/rstb.2010.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762-2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004-1011. ( 10.1016/j.anbehav.2012.12.031) [DOI] [Google Scholar]
- 145.Cabrera D, Nilsson JR, Griffen BD. 2021. The development of animal personality across ontogeny: a cross-species review. Anim. Behav. 173, 137-144. ( 10.1016/j.anbehav.2021.01.003) [DOI] [Google Scholar]
- 146.Kotrschal A, Taborsky B. 2010. Environmental change enhances cognitive abilities in fish. PLoS Biol. 8, e1000351. ( 10.1371/journal.pbio.1000351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meuthen D, Ferrari MCO, Lane T, Chivers DP. 2019. Plasticity of boldness: high perceived risk eliminates a relationship between boldness and body size in fathead minnows. Anim. Behav. 147, 25-32. ( 10.1016/j.anbehav.2018.11.003) [DOI] [Google Scholar]
- 148.Liedtke J, Redekop D, Schneider JM, Schuett W. 2015. Early environmental conditions shape personality types in a jumping spider. Front. Ecol. Evol. 3, 134. ( 10.3389/fevo.2015.00134) [DOI] [Google Scholar]
- 149.Hunter DS, Hazel SJ, Kind KL, Owens JA, Pitcher JB, Gatford KL. 2016. Programming the brain: common outcomes and gaps in knowledge from animal studies of IUGR. Physiol. Behav. 164, 233-248. ( 10.1016/j.physbeh.2016.06.005) [DOI] [PubMed] [Google Scholar]
- 150.Laus MF, Duarte Manhas Ferreira Vales L, Braga Costa TM, Sousa Almeida S. 2011. Early postnatal protein-calorie malnutrition and cognition: a review of human and animal studies. Int. J. Environm. Res. Publ. Health 8, 590-612. ( 10.3390/ijerph8020590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arnold KE, Ramsay SL, Donaldson C, Adam A. 2007. Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring. Proc. R. Soc. B 274, 2563-2569. ( 10.1098/rspb.2007.0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blondel J, Aronson J. 1999. Biology and wildlife of the Mediterranean region. Oxford, UK: Oxford University Press. [Google Scholar]
- 153.Ashton BJ, Thorton A, Ridley AR. 2018. An intraspecific appraisal of the social intelligence hypothesis. Phil. Trans. R. Soc. B 373, 20170288. ( 10.1098/rstb.2017.0288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Croston R, Branch CL, Kozlovsky DY, Dukas R, Pravosudov VV. 2015. Heritability and the evolution of cognitive traits. Behav. Ecol. 26, 1447-1459. ( 10.1093/beheco/arv088) [DOI] [Google Scholar]
- 155.Mayr E. 1942. Systematics and the origin of species. New York, NY: Columbia University Press. [Google Scholar]
- 156.Frankham R. 1997. Do island populations have less genetic variation than mainland populations? Heredity 78, 311-327. ( 10.1038/hdy.1997.46) [DOI] [PubMed] [Google Scholar]
- 157.Raffard A, Cucherousset J, Prunier JG, Loot G, Santoul F, Blanchet S. 2018. Variability of functional traits and their syndromes in a freshwater fish species (Phoxinus phoxinus): the role of adaptive and nonadaptive processes. Ecol. Evol. 9, 2833-2846. ( 10.1002/ece3.4961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gruber J, Brown G, Whiting MJ, Shine R. 2018. Behavioural divergence during biological invasions: a study of cane toads (Rhinella marina) from contrasting environments in Hawai'i. R. Open Sci. 5, 180197. ( 10.1098/rsos.180197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Frankham R. 1998. Inbreeding and extinction: island populations. Conserv. Biol. 12, 665-675. ( 10.1111/j.1523-1739.1998.96456.x) [DOI] [Google Scholar]
- 160.Charlesworth D. 2003. Effects of inbreeding on the genetic diversity of populations. Phil. Trans. R. Soc. Lond. B 358, 1051-1070. ( 10.1098/rstb.2003.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nat. Rev. Gen. 10, 783-796. ( 10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 162.Sih A, Mathot KJ, Moiron M, Montiglio PO, Wolf M, Dingemanse NJ. 2015. Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50-60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 163.Verweij KJH, et al. 2012. Maintenance of genetic variation in human personality: testing evolutionary models by estimating heritability due to common causal variants and investigating the effect of distant inbreeding. Evolution 66, 3238-3251. ( 10.1111/j.1558-5646.2012.01679.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Müller T, Juškauskas A. 2018. Inbreeding affects personality and fitness of a leaf beetle. Anim. Behav. 138, 29-37. ( 10.1016/j.anbehav.2018.02.002) [DOI] [Google Scholar]
- 165.Morton NE. 1978. Effect of inbreeding on mental retardation. Proc. Natl Acad. Sci. USA 75, 3906-3908. ( 10.1073/pnas.75.8.3906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alvarez G, Ceballos FC, Quintero C. 2009. The role of inbreeding in the extinction of a European royal dynasty. PLoS ONE 4, e5174. ( 10.1371/journal.pone.0005174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Howrigan DP, et al. 2016. Genome-wide autozygosity is associated with lower general cognitive ability. Mol. Psychiatry 21, 837-843. ( 10.1038/mp.2015.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gandin I, et al. 2015. Excess of runs of homozygosity is associated with severe cognitive impairment in intellectual disability. Genet. Med. 17, 396-399. ( 10.1038/gim.2014.118) [DOI] [PubMed] [Google Scholar]
- 169.Joshi PK, et al. 2015. Directional dominance on stature and cognition in diverse human populations. Nature 523, 459-462. ( 10.1038/nature14618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kornilov SA, Tan M, Aljughaiman A, Naumova OY, Grigorenko EL. 2019. Genome-wide homozygosity mapping reveals genes associated with cognitive ability in children from Saudi Arabia. Front. Genet. 10, 888. ( 10.3389/fgene.2019.00888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nepoux V, Haag CR, Kawecki TJ. 2010. Effects of inbreeding on aversive learning in Drosophila. J. Evol. Biol. 23, 2333-2345. ( 10.1111/j.1420-9101.2010.02094.x) [DOI] [PubMed] [Google Scholar]
- 172.Harker KT, Whishaw IQ. 2002. Place and matching-to-place spatial learning affected by rat inbreeding (Dark–Agouti, Fischer 344) and albinism (Wistar, Sprague–Dawley) but not domestication (wild rat vs. Long–Evans, Fischer–Norway). Behav. Brain Res. 134, 467-477. ( 10.1016/s0166-4328(02)00083-9) [DOI] [PubMed] [Google Scholar]
- 173.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361-368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 174.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051-4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Royauté R, Hedrick A, Dochtermann NA. 2020. Behavioural syndromes shape evolutionary trajectories via conserved genetic architecture. Proc. R. Soc. B 287, 20200183. ( 10.1098/rspb.2020.0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Klomberg KF, Garland T Jr, Swallow JG, Carter PA. 2002. Dominance, plasma testosterone levels, and testis size in house mice artificially selected for high activity levels. Physiol. Behav. 77, 27-38. ( 10.1016/s0031-9384(02)00767-9) [DOI] [PubMed] [Google Scholar]
- 177.Pelhaitre A, Mignon-Grasteau S, Bertin A. 2012. Selection for wheat digestibility affects emotionality and feeding behaviours in broiler chicks. Appl. Anim. Behav. Sci. 139, 114-122. ( 10.1016/j.applanim.2012.03.007) [DOI] [Google Scholar]
- 178.Kolss M, Kawecki TJ. 2012. Reduced learning ability as a consequence of evolutionary adaptation to nutritional stress in Drosophila melanogaster. Ecol. Entomol. 33, 583-588. ( 10.1111/j.1365-2311.2008.01007.x) [DOI] [Google Scholar]
- 179.Burger JM, Kolss M, Pont J, Kawecki TJ. 2008. Learning ability and longevity: a symmetrical evolutionary trade-off in Drosophila. Evolution 62, 1294-1304. ( 10.1111/j.1558-5646.2008.00376.x) [DOI] [PubMed] [Google Scholar]
- 180.Sagonas K, Pafilis P, Valakos E. 2015. Effects of insularity on digestion: living on islands induces shifts in physiological and morphological traits in island reptiles. Sci. Nat. 102, 1-7. ( 10.1007/s00114-015-1301-8) [DOI] [PubMed] [Google Scholar]
- 181.Wasser DE, Sherman PW. 2010. Avian longevities and their interpretation under evolutionary theories of senescence. J. Zool. 280, 103-155. ( 10.1111/j.1469-7998.2009.00671.x) [DOI] [Google Scholar]
- 182.Cody ML, Overton JM. 1996. Short-term evolution of reduced dispersal in island plant populations. J. Ecol. 84, 53-61. ( 10.2307/2261699) [DOI] [Google Scholar]
- 183.Freedman MG, Dingle H, Strauss SY, Ramírez SR. 2020. Two centuries of monarch butterfly collections reveal contrasting effects of range expansion and migration loss on wing traits. Proc. Natl Acad. Sci. USA 117, 28 887-28 893. ( 10.1073/pnas.2001283117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simberloff D, Wilson EO. 1969. Experimental zoogeography of islands: the colonization of empty islands. Ecology 50, 278-296. ( 10.2307/1934856) [DOI] [Google Scholar]
- 185.García-Verdugo C, Mairal M, Monroy P, Sajeva M, Caujapé-Castells J. 2017. The loss of dispersal on islands hypothesis revisited: implementing phylogeography to investigate evolution of dispersal traits in Periploca (Apocynaceae). J. Biogeogr. 44, 2595-2606. ( 10.1111/jbi.13050) [DOI] [Google Scholar]
- 186.Burns KC. 2019. Time to abandon the loss of dispersal ability hypothesis in island plants: a comment on Garcia-Verdugo, Mairal, Monroy, Sajeva and Caujape-Castells (2017). J. Biogeogr. 45, 1219-1222. ( 10.1111/jbi.13223) [DOI] [Google Scholar]
- 187.Chapple DG, Simmonds SM, Wong BB. 2012. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57-64. ( 10.1016/j.tree.2011.09.010) [DOI] [PubMed] [Google Scholar]
- 188.Price TD, Sol D. 2008. Introduction: genetics of colonizing species. Am. Nat. 172, S1-S3. ( 10.1086/588639) [DOI] [PubMed] [Google Scholar]
- 189.Foster BJ, McCulloch GA, Vogel MF, Ingram T, Waters JM. 2021. Anthropogenic evolution in an insect wing polymorphism following widespread deforestation. Biol. Lett. 17, 20210069. ( 10.1098/rsbl.2021.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McCulloch GA, Guhlin J, Dutoit L, Harrop TW, Dearden PK, Waters JM. 2021. Genomic signatures of parallel alpine adaptation in recently evolved flightless insects. Mol. Ecol. 30, 6677-6686. ( 10.1111/mec.16204) [DOI] [PubMed] [Google Scholar]
- 191.Donald D. 1985. The wing length of Sweltsa revelstoka (Plecoptera, Chloroperlidae). Can. Entomol. 117, 233-239. ( 10.4039/Ent117233-2) [DOI] [Google Scholar]
- 192.Wey TW, Spiegel O, Montiglio PO, Mabry KE. 2015. Natal dispersal in a social landscape: considering individual behavioral phenotypes and social environment in dispersal ecology. Curr. Zool. 61, 543-556. ( 10.1093/czoolo/61.3.543) [DOI] [Google Scholar]
- 193.Schradin C, Lamprecht J. 2002. Causes of female emigration in the group-living cichlid fish Neolamprologus multifasciatus. Ethology 108, 237-248. ( 10.1046/j.1439-0310.2002.00775.x) [DOI] [Google Scholar]
- 194.Qu J, Chen Q, Zhang Y. 2017. Behaviour and reproductive fitness of postdispersal in plateau pikas (Ochotona curzoniae) on the Tibetan Plateau. Mamm. Res. 63, 151-159. ( 10.1007/s13364-017-0344-y) [DOI] [Google Scholar]
- 195.Trefilov A, Berard J, Krawczak M, Schmidtke J. 2000. Natal dispersal in rhesus macaques is related to serotonin transporter gene promoter variation. Behav. Genet. 30, 295-301. ( 10.1023/a:1026597300525) [DOI] [PubMed] [Google Scholar]
- 196.Michelangeli M, Smith CR, Wong BBM, Chapple DG. 2017. Aggression mediates dispersal tendency in an invasive lizard. Anim. Behav. 133, 29-34. ( 10.1016/j.anbehav.2017.08.027) [DOI] [Google Scholar]
- 197.Hoset KS, Ferchaud AL, Dufour F, Mersch D, Cote J, Le Galliard JF. 2011. Natal dispersal correlates with behavioral traits that are not consistent across early life stages. Behav. Ecol. 22, 176-183. ( 10.1093/beheco/arq188) [DOI] [Google Scholar]
- 198.Blumstein DT, Wey TW, Tang K. 2009. A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc. R. Soc. B 276, 3007-3012. ( 10.1098/rspb.2009.0703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cote J, Clobert J. 2007. Social personalities influence natal dispersal in a lizard. Proc. R. Soc. B 274, 383-390. ( 10.1098/rspb.2006.3734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liedtke J, Fromhage L. 2021. The joint evolution of learning and dispersal maintains intraspecific diversity in metapopulations. Oikos 130, 808-818. ( 10.1111/oik.08208) [DOI] [Google Scholar]
- 201.Maille A, Schradin C. 2016. Survival is linked with reaction time and spatial memory in African striped mice. Biol. Lett. 12, 20160346. ( 10.1098/rsbl.2016.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vincze O. 2016. Light enough to travel or wise enough to stay? Brain size evolution and migratory behavior in birds. Evolution 70, 2123-2133. ( 10.1111/evo.13012) [DOI] [PubMed] [Google Scholar]
- 203.Szabo B, Damas-Moreira I, Whiting MJ. 2020. Can cognitive ability give invasive species the means to succeed? A review of the evidence. Front. Ecol. Evol. 8, 187. ( 10.3389/fevo.2020.00187) [DOI] [Google Scholar]
- 204.Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc. B 271, 847-852. ( 10.1098/rspb.2004.2680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bell AM, Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10, 828-834. ( 10.1111/j.1461-0248.2007.01081.x) [DOI] [PubMed] [Google Scholar]
- 206.Garamszegi LZ, Zagalska-Neubauer M, Canal D, Markó G, Szász E, Zsebők S, Szöllősi E, Herczeg G, Török J. 2015. Malaria parasites, immune challenge, MHC variability, and predator avoidance in a passerine bird. Behav. Ecol. 26, 1292-1302. ( 10.1093/beheco/arv077) [DOI] [Google Scholar]
- 207.Keiser CN, Ingley SJ, Toscano BJ, Scharf I, Pruitt JN. 2018. Habitat complexity dampens selection on prey activity level. Ethology 124, 25-32. ( 10.1111/eth.12700) [DOI] [Google Scholar]
- 208.Schuett W, Tregenza T, Dall SRX. 2010. Sexual selection and animal personality. Biol. Rev. 85, 217-246. ( 10.1111/j.1469-185x.2009.00101.x) [DOI] [PubMed] [Google Scholar]
- 209.Munson AA, Jones C, Schraft H, Sih A. 2020. You're just my type: mate choice and behavioral types. Trends Ecol. Evol. 35, 823-833. ( 10.1016/j.tree.2020.04.010) [DOI] [PubMed] [Google Scholar]
- 210.Anaya-Meraz ZA, Escobedo-Galván AH. 2020. Insular effect on sexual size dimorphism in the clouded anole Anolis nebulosus: when Rensch meets Van Valen. Curr. Zool. 66, 589-591. ( 10.1093/cz/zoaa034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roulin A, Salamin N. 2010. Insularity and the evolution of melanism, sexual dichromatism and body size in the worldwide-distributed barn owl. J. Evol. Biol. 23, 925-934. ( 10.1111/j.1420-9101.2010.01961.x) [DOI] [PubMed] [Google Scholar]
- 212.Griffith SC. 2000. High fidelity on islands: a comparative study of extrapair paternity in passerine birds. Behav. Ecol. 11, 265-273. ( 10.1093/beheco/11.3.265) [DOI] [Google Scholar]
- 213.Moiron M, Laskowski KL, Niemelä PT. 2020. Individual differences in behaviour explain variation in survival: a meta-analysis. Ecol. Lett. 23, 399-408. ( 10.1111/ele.13438) [DOI] [PubMed] [Google Scholar]
- 214.Humphrey N. 1976. The social function of intellect. In Growing points in ethology (eds Bateson PPG, Hinde RA), pp. 303-317. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 215.Ashton BJ, Kennedy P, Radford AN. 2020. Interactions with conspecific outsiders as drivers of cognitive evolution. Nat. Commun. 11, 1-9. ( 10.1038/s41467-020-18780-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Parker ST, Gibson KR. 1977. Object manipulation, tool use and sensorimotor intelligence as feeding adaptations in Cebus monkeys and great apes. J. Hum. Evol. 6, 623-641. ( 10.1016/s0047-2484(77)80135-8) [DOI] [Google Scholar]
- 217.Sol D. 2009. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 5, 130-133. ( 10.1098/rsbl.2008.0621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Allman JM. 2000. Evolving brains. New York, NY: Scientific American Library, Freeman and Company New York. [Google Scholar]
- 219.van Horik J, Emery NJ. 2011. Evolution of cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2, 621-633. ( 10.1002/wcs.144) [DOI] [PubMed] [Google Scholar]
- 220.Rochais C, Rymer T, Pillay N. 2022. Challenges in linking cognition and survival: a review. Front. Ecol. Evol. 10, 729546. ( 10.3389/fevo.2022.729546) [DOI] [Google Scholar]
- 221.McGowen MR, Grossman LI, Wildman DE. 2012. Dolphin genome provides evidence for adaptive evolution of nervous system genes and a molecular rate slowdown. Proc. R. Soc. B 279, 3643-3651. ( 10.1098/rspb.2012.0869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Miller SE, Legan AW, Henshaw MT, Ostevik KL, Samuk K, Uy FM, Sheehan MJ. 2020. Evolutionary dynamics of recent selection on cognitive abilities. Proc. Natl Acad. Sci. USA 117, 3045-3052. ( 10.1073/pnas.1918592117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Orkin JD, et al. 2021. The genomics of ecological flexibility, large brains, and long lives in capuchin monkeys revealed with fecalFACS. Proc. Natl Acad. Sci. USA 118, e2010632118. ( 10.1101/366112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Evans PD, Gilbert SL, Mekel-Bobrov N, Vallender EJ, Anderson JR, Vaez-Azizi LM, Tishkoff SA, Hudson RR, Lahn BT. 2005. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science 309, 1717-1720. ( 10.1126/science.1113722) [DOI] [PubMed] [Google Scholar]
- 225.Agrillo C, Bisazza A. 2018. Understanding the origin of number sense: a review of fish studies. Phil. Trans. R. Soc. B 373, 20160511. ( 10.1098/rstb.2016.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shettleworth SJ. 2009. The evolution of comparative cognition: is the snark still a boojum? Behav. Process. 80, 210-217. ( 10.1016/j.beproc.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 227.De Meester G, Baeckens S. 2021. Reinstating reptiles: from clueless creatures to esteemed models of cognitive biology. Behaviour 158, 1057-1076. ( 10.1163/1568539X-00003718) [DOI] [Google Scholar]
- 228.Perry CJ, Barron AB, Chittka L. 2017. The frontiers of insect cognition. Curr. Opin. Behav. Sci. 16, 111-118. ( 10.1016/j.cobeha.2017.05.011) [DOI] [Google Scholar]
- 229.Mitchell DJ, Beckmann C, Biro PA. 2020. Predation as a driver of behavioural variation and trait integration: effects on personality, plasticity, and predictability. EcoEvoRxiv. August 17. ( 10.32942/osf.io/jwd3c) [DOI]
- 230.Brown C, Braithwaite VA. 2005. Effects of predation pressure on the cognitive ability of the poeciliid Brachyraphis episcopi. Behav. Ecol. 16, 482-487. ( 10.1093/beheco/ari016) [DOI] [Google Scholar]
- 231.Jaatinen K, Møller A, Öst M. 2019. Annual variation in predation risk is related to the direction of selection for brain size in the wild. Sci. Rep. 9, 11847. ( 10.1038/s41598-019-48153-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mortelliti A, Brehm AM. 2020. Environmental heterogeneity and population density affect the functional diversity of personality traits in small mammal populations. Proc. R. Soc. B 287, 20201713. ( 10.1098/rspb.2020.1713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wolf M, Weissing FJ. 2010. An explanatory framework for adaptive personality differences. Phil. Trans. R. Soc. B 365, 3959-3968. ( 10.1098/rstb.2010.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liedtke J, Fromhage L. 2019. Modelling the evolution of cognitive styles. BMC Evol. Biol. 19, 234. ( 10.1186/s12862-019-1565-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Careau V, Garland T Jr. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiol. Biochem. Zool. 85, 543-571. ( 10.1086/666970) [DOI] [PubMed] [Google Scholar]
- 236.Roulin A, Ducrest AL. 2010. Association between melanism, physiology and behaviour: a role for the melanocortin system. Eur. J. Pharmacol. 660, 226-233. ( 10.1016/j.ejphar.2011.01.036) [DOI] [PubMed] [Google Scholar]
- 237.Goulet CT, Michelangeli M, Chung M, Riley JL, Wong B, Thompson MB, Chapple DG. 2018. Evaluating cognition and thermal physiology as components of the pace-of-life syndrome. Evol. Ecol. 32, 469-488. ( 10.1007/s10682-018-9948-1) [DOI] [Google Scholar]
- 238.Arnold SJ, Pfrender ME, Jones AG. 2001. The adaptive landscape as a conceptual bridge between micro- and macroevolution. In Microevolution rate, pattern, process. Contemporary issues in genetics and evolution, vol 8 (eds Hendry AP, Kinnison MT. ). Dordrecht, The Netherlands: Springer. [Google Scholar]
- 239.Arnold SJ, Bürger R, Hohenlohe PA, Ajie BC, Jones AG. 2008. Understanding the evolution and stability of the G-matrix. Evolution 62, 2451-2461. ( 10.1111/j.1558-5646.2008.00472.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Schluter D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766-1774. ( 10.1111/j.1558-5646.1996.tb03563.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.