Abstract
Premise
Ferns differ from seed plants in possessing a life cycle that includes a small, free‐living, seemingly vulnerable gametophyte stage in which sexual reproduction occurs. Most research on the response of fern gametophytes to environmental stress has been conducted on gametophytes grown in culture or harvested from natural habitats and subsequently manipulated and tested in laboratory experiments. We present a fixed‐distance photographic methodology for monitoring longevity of gametophytes and their response to environmental stress in natural, undisturbed habitats over their life spans.
Methods
We present methodology for non‐invasive monitoring of growth and development in response to environmental factors, using programmed, fixed‐distance photography, coupled with computer analyses allowing qualitative and quantitative comparisons. We tracked growth rates and stress responses of individual gametophyte plants to seasonal changes in a temperate climate.
Results
Gametophytes and young sporophytes survived freezing and drought in temperate habitats, as we document through photographs and growth measurements. Gametophyte growth was suspended during the cold season and resumed the following spring. Individual gametophytes survived for up to nearly three years with retention of the ability to produce sporophytes.
Discussion
Life histories of fern gametophytes in temperate habitats are more similar to those in tropical habitats than previous research has suggested. They survive and maintain reproductive capacity over several growing seasons, allowing extended opportunity for outbreeding. The application of photographic monitoring of additional species and habitats has great potential for a more complete understanding of the ecology of reproduction in homosporous vascular plants.
Keywords: fern gametophytes, photographic monitoring, reproductive strategies, winter stress
Ferns differ from seed plants in displaying two free‐living stages in their life cycle: a large, long‐lived, diploid, and spore‐producing “sporophyte” plant, and a small, relatively short‐lived, haploid, gamete‐producing “gametophyte” plant that is produced from the sporophyte's dispersed spores and that produces a new sporophyte plant through the union of sperm and egg. The seemingly delicate and vulnerable gametophyte stage has often been considered a “weak link” in the life cycle of ferns, even though it is responsible for all fern establishment via spore dispersal. Our goal in this study is to use field monitoring to examine the response of individual fern gametophytes to environmental stress over their lifetime in a temperate climate.
Although a substantial number of studies of the ecology of fern gametophytes have now been published (for reference see Lindsay, 1992; Watkins et al., 2007a, b; Farrar et al., 2008), most of these studies have focused on tropical zones, and thus information on growth and survival strategies of gametophytes and young sporophytes in temperate zones is still rather limited. In temperate zones, safe sites available for colonization by gametophytes and young sporophytes are restricted by dynamic seasonal processes such as cover by leaf litter, direct impact by rain and snow in the absence of a leaf canopy, drought, and the destabilizing effects of freeze–thaw cycles (Harper et al., 1961; Cousens et al., 1988; Fowler, 1988; Walker and Sharpe, 2010). Studying 13 fern species in temperate forests of central Iowa, Peck et al. (1990) reported substantial overwinter survival (up to 80%) of gametophytes in protected habitats, but dramatic loss of gametophyte populations that were exposed to drought and erosion during the fall and winter of their first year. These authors concluded that summer establishment conditions must be followed by a wet fall and adequate winter snow cover to prevent dehydration. Such protected gametophytes survived winter temperatures well below freezing and resumed growth the following spring.
Pioneering investigations by Pickett (1914) revealed tolerance of prothalli of Camptosorus rhizophyllus (L.) Link and Asplenium platyneuron (L.) Britton, Sterns & Poggenb. (Aspleniaceae) to freezing, desiccation, and differing temperatures and light conditions, both under cultivation and in nature. He showed that under natural conditions gametophytes survived temperatures as cold as −23°C and were resistant to drought. Detailed investigations of Kappen (1964, 1965), using artificial experiments, showed that native, temperate fern species in Germany survived the cold temperatures found in nature, and resisted drought. Farrar and Gooch (1975) also found that gametophytes of North American temperate species survive subfreezing winter conditions, reporting that 75% of gametophytes still alive at the end of November resumed growth and sporophyte production in March. For gametophytes of ferns native to northern Japan, experiments by Sato and Sakai (1980) and Sato (1982) revealed freeze resistance of most of the collected gametophyte species to −40°C, although the freeze resistance of young sporophytes was found to be much less at between −10°C to −20°C.
The ability of individual gametophytes of many fern species to produce both male and female gametangia and to produce sporophytes by gametophytic selfing has been well demonstrated (Sessa et al., 2016), as is the fact that naturally occurring populations of sporophytes of most fern species demonstrate heterozygosity that can be produced only through gametophytic crossing (Ranker and Geiger, 2008). Peck et al. (1990) documented that the ability of isolated single gametophytes of different species to produce viable sporophytes through gametophytic selfing ranged from 100% in weedy species such as Woodsia obtusa (Spreng.) Torr., to 2% in the rare Iowa species Cryptogramma stelleri (S. G. Gmel.) Prantl. They also reported production of abnormal leaves in 58% of sporophytes of Adiantum pedatum L. produced through gametophytic selfing, but no abnormal leaves produced by the same species through gametophytic crossing. Similar results were obtained for Athyrium filix‐femina (L.) Roth by Schneller (1979), with discussion in a broader study by Schneller (2008). These observations support the importance of gametophytic crossing, as well as a possible role of gametophytic longevity in promoting this mating behavior.
Possible relationships of gametophyte longevity to gametophyte breeding systems have been reviewed by Haufler et al. (2016). Studying tropical species with gametophytes demonstrating indefinite persistence through vegetative reproduction, Dassler and Farrar (2001) found such species to have a greater probability of occurring on distant islands than species without similarly persistent gametophytes, possibly because of extended opportunity for interaction with genetically different genotypes of later‐arriving spores. Farrar (1976) studied spore retention and release from fertile sporophyte leaves throughout the winter following the season of their production. In March of the following spring, 11 of 13 species studied in central Iowa were still capable of releasing viable spores that could theoretically promote outcrossed fertilization of long‐lived, overwintering gametophytes as described by Farrar and Gooch (1975).
METHODS
Our photographic monitoring of fern gametophytes in natural environments utilized a digital Olympus E‐10 camera (Olympus, Tokyo, Japan) equipped with Kooka extension tubes (Kooka Optronics Co. Ltd., Shenzhen, China) and a spacer ring adjusted to a fixed‐distance focus. At the selected distance for a given plant, a metric scale was photographed, and successive photos for each monitored plant were photographed from the same marked distance and orientation. The fixed focus and high speed of the digital camera allowed hand‐held photography and thus required no set up of equipment and minimal disturbance of the site. Individual plants were chosen from approximately 40 different populations and monitored throughout their lifespan up to production of first sporophyte leaves. Photographs were taken weekly (i.e., in early fall and late spring) to monthly (i.e., during winter months, when gametophytes and young sporophytes become dormant) to reflect growth rates and responses to climatic events. Gametophyte responses were analyzed through the open processing program ImageJ (Rasband, 1997−2016), measuring the width of gametophytes and the length of the first sporophyte leaves in order to calculate growth between monitoring periods.
The site of this investigation was a temperate forest near the city of Kusnacht, Switzerland (global latitude: 47°18′50″ to 47°19′22″, longitude 8°36′9″ to 8°36′57″; Swiss coordinates: 688/689, 241/242; altitude ca. 2000 ft.). The field work was conducted from March 2007 to March 2016, with replications using different populations over different times within the study period. The principal habitat of the populations monitored was rotting wood of downed logs and tree stumps, safe sites that were generally exposed above winter snow cover and were not easily eroded by heavy rain. Care was taken throughout the study to avoid disturbance to the plants and their natural habitats.
RESULTS
Gametophyte occurrence
Sporophytes of Athyrium filix‐femina (Woodsiaceae), Dryopteris dilatata (Hoffm.) A. Gray, and D. filix‐mas (L.) Schott (Dryopteridaceae) dominated the fern flora of the study area. Sporophytes on dead stumps, the primary sites of gametophytes monitored in this study, were dominated by D. dilatata, and glandular hairs present on larger gametophytes were consistent with this identity. All monitored populations consisted initially of many gametophytes and, in most cases, older gametophytes producing young sporophytes (Figure 1). As reported in earlier studies (Farrar and Gooch, 1975; Sato and Sakai, 1980), gametophytes and young sporophytes remained green throughout winter conditions and were observable throughout the seasons. We monitored individual plants from approximately 40 populations over a decade of study. Representative samples are presented in Figures 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and comparative growth rates are presented in Table 1.
Figure 1.

Natural populations of fern gametophytes showing (A) individuals of various sizes, and (B) older gametophytes producing their first sporophyte leaves.
Figure 2.
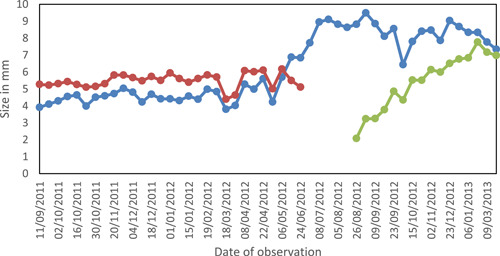
Line graph visualizing the growth of three gametophytes, represented by different colors, monitored over an 18‐month period (from September 2011 to March 2013).
Figure 3.
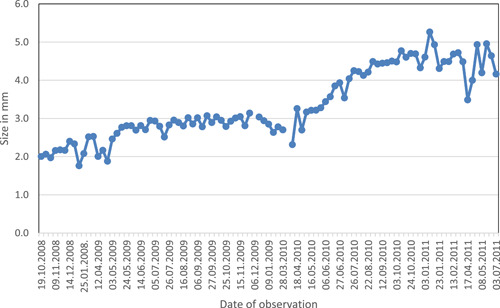
Line graph visualizing the growth of one gametophyte over a 33‐month period (from October 2008 to July 2011).
Figure 4.

The same gametophyte pictured before and after freezing temperatures and drought: (A) fall growth, (B) under midwinter ice, (C) dehydration, and (D) spring growth.
Figure 5.

The same gametophyte pictured before (A), during (B), and after (C) drought.
Figure 6.

The same plant producing a sporophyte after severe dehydration: (A) dehydrated plant, (B) revived plant, and (C) plant producing sporophyte.
Figure 7.

Death of gametophytes and young sporophytes after severe drought.
Figure 8.

Regeneration of a large gametophyte following dehydration.
Figure 9.
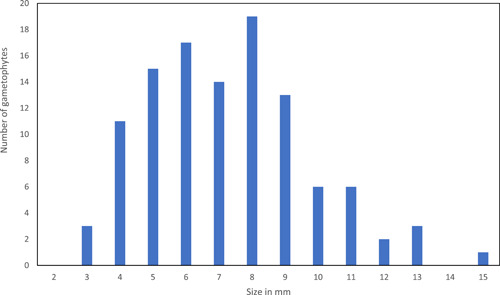
Bar graph visualizing the sizes of gametophytes at the time of sporophyte production.
Figure 10.
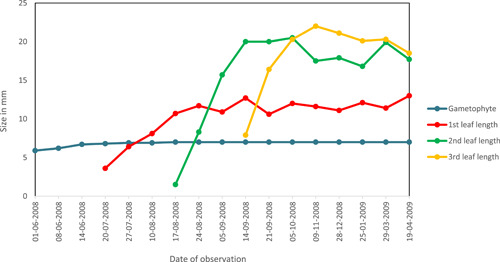
Line graph visualizing the growth of a gametophyte and its first sporophyte leaves over an 11‐month period (from June 2008 to April 2009).
Table 1.
Comparative growth rates of 10 different gametophytes and the first leaves of 10 different sporophytes produced during the growing seasons.
| Mean increase (mm/day) | ||
|---|---|---|
| Plant no. | Gametophytes | Sporophytes |
| 1 | 0.018 | 0.04 |
| 2 | 0.019 | 0.20 |
| 3 | 0.019 | 0.11 |
| 4 | 0.053 | 0.18 |
| 5 | 0.050 | 0.17 |
| 6 | 0.040 | 0.18 |
| 7 | 0.057 | 0.07 |
| 8 | 0.041 | 0.05 |
| 9 | 0.090 | 0.12 |
| 10 | 0.020 | 0.42 |
| Mean | 0.0857 | 0.154 |
Seasonal growth and persistence of gametophytes
All sites chosen for the study initially displayed many gametophytes in different developmental sizes, ranging from individuals less than 1 mm in width to fully grown individuals producing sporophytes (Figure 1). Our monitoring of chosen gametophytes showed continued presence over timespans up to nearly three years (Figures 2 and 3). As investigated by Kappen (1965), Sato and Sakai (1980), and Sato (1982), gametophytes in temperate climates are characterized by a plastic reaction to drought and freezing. In our study, the observed effect of drought and freezing during winter was an apparent reduction in the size of a gametophyte due to loss of its water content (Figures 4, 5, 6). Some sites experiencing longer periods of drought reached a lethal degree of dehydration for gametophytes and young sporophytes (Figure 7). The most severe dehydration seemed to be reached primarily in dry summer periods in sites that were more exposed to sun.
Gametophytes grew and developed during the warm season and remained in a dormant stage during most of the cold season (Figure 3). The daily growth rate of gametophytes during the growing season varied with local conditions of the microsite but averaged 0.09 mm in width per day (Table 1). The growing season of overwintering gametophytes in this study began around early April and ended in October, corresponding with a mean temperature of 5°C or less between October and April in the lowlands of Central Europe (Kappen, 1965). The size of the gametophytes remained approximately the same during the cold season. When sporophytes were produced, the parent gametophytes ceased to grow, only occasionally showing a small increase in size, and after a few weeks began to die. Only a few gametophytes displayed vegetative regeneration (Figure 8). The size of gametophytes initiating sporophyte production was between 3 and 15 mm, with the majority between 5 and 9 mm (Figure 9).
Seasonal growth of young sporophytes
New sporophytes were formed during the growing season, and like gametophytes, they remained dormant from October to the end of March. Only one sporophyte per gametophyte was observed. The growth rate of first leaves, measured in leaf length, was generally greater than that of the gametophytes that produced them (Table 1). Young sporophytes produced in the fall survived the winter season and continued leaf growth the following spring (Figure 10); three or four leaves developed over one season. Like gametophytes, they resisted drought and freezing without damage, but some died under extreme drought conditions (Figure 7). We did not continue to monitor the maturation of the young sporophytes observed in this study after the 10‐year study period, but assume that the probability of establishment of mature sporophytes does not differ between those formed in the first versus subsequent years. Continued monitoring of isolated gametophytes and their sporophytes described by Peck et al. (1990) documented the establishment of a population of Dryopteris goldiana (Hook. ex Goldie) A. Gray in newly available rockfall habitat (Farrar, personal observation).
DISCUSSION
Our photographic monitoring of individual fern gametophytes across all seasons in a temperate forest for more than one year allowed quantitative measurement of growth, persistence, and response to environmental stress, without artificial disturbance to the plants or their habitats. It confirmed the in situ observations of earlier studies (Kappen, 1965; Farrar and Gooch, 1975; Peck et al., 1990) and supports the conclusions of earlier experiments (e.g., Sato, 1982; Lindsay, 1992; Watkins et al., 2007a, b; Farrar et al., 2008) relative to gametophyte survival of subfreezing temperatures.
Although gametophytes in a temperate zone can survive freezing, cold winter conditions in temperate zones do reduce or stop growth until spring warming (Figure 2). At this study site, this period of growth dormancy generally occurred from the beginning of October through March, with growth resuming in April (Figure 3).
In addition to freeze resistance, gametophytes and young sporophytes in this study were also resistant to drought (Figure 4). Success of this resistance depended on the duration of the dry period and the degree of desiccation. Extended periods of extreme drought resulted in the death of some gametophytes and young sporophytes (Figure 7).
Gametophytes monitored in this study survived up to nearly three years (Figures 1 and 2). They survived winter conditions with no apparent damage, and resumed growth the following spring. Previous research has proposed that gametophyte longevity beyond one year offers a key benefit in providing an important opportunity for interaction with gametophytes from spores arriving in a second season, especially if these later‐arriving spores carry genotypes different from the original source (Peck et al., 1990; Dassler and Farrar, 2001; Haufler et al., 2016). A reproductive advantage of extended gametophyte longevity is clearly plausible in tropical ecosystems with continuous growing conditions, and our study supports its plausibility in temperate climates as well. Although our protocol of non‐disturbance did not allow for harvesting of gametophytes to determine their sexual expression or the degree of heterozygosity in the sporophytes produced, such analyses could be profitable additions to future applications of photographic monitoring.
Our study is presented as an example of insights to be gained through photographic monitoring of individual gametophyte plants over their natural lifespans. Similar monitoring of multiple species in temperate and tropical habitats can contribute enormously to a more complete understanding of fern reproductive biology. Such studies require only local access to a naturally occurring population of ferns.
AUTHOR CONTRIBUTIONS
J.J.S. conceived and executed the field study in Switzerland, contributed all photographs and figures, and wrote the initial draft of the manuscript. D.R.F. and J.J.S. collaborated on the final draft. D.R.F. provided additional data from similar studies in Iowa. Both authors approved the final version of the manuscript.
Schneller, J. J. , Farrar D. R.. 2022. Photographic analysis of field‐monitored fern gametophyte development and response to environmental stress. Applications in Plant Sciences 10(2): e11470. 10.1002/aps3.11470
This article is part of the special issue “Methodologies in Gametophyte Biology.”
REFERENCES
- Cousens, M. I. , Lacey D. G., and Scheller J. M.. 1988. Safe sites and the ecological history of Lorinseria areolata . American Journal of Botany 75: 797−807. [Google Scholar]
- Dassler, C. L. , and Farrar D. R.. 2001. Significance of gametophyte form in long‐distance colonization of tropical, epiphytic ferns. Brittonia 53: 352−369. [Google Scholar]
- Farrar, D. R. 1976. Spore retention and release from overwintering fern fronds. American Fern Journal 66: 49−52. [Google Scholar]
- Farrar, D. R. , and Gooch R. D.. 1975. Fern reproduction in Woodman Hollow, central Iowa: Preliminary observations and a consideration of the feasibility of conducting studies on fern reproductive biology in nature. Proceedings of the Iowa Academy of Science 82: 119−122. [Google Scholar]
- Farrar, D. R. , Dassler C. L., Watkins J. E., Jr., and Skelton C.. 2008. Gametophyte ecology. In Ranker T. A. and Haufler C. H. [eds.], Biology and evolution of ferns and lycophytes, 222–256. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Fowler, N. L. 1988. What is a safe site? Neighbour, litter, germination date, and patch effects. Ecology 69: 947−961. [Google Scholar]
- Harper, J. L. , Clatworthy J. N., McNaughton H., and Sagar G. R.. 1961. The evolution of closely related species living in the same area. Evolution 15: 209−227. [Google Scholar]
- Haufler, C. H. , K. M, Pryer , Schuettpelz E., Sessa E., Farrar D. R., Moran R., Schneller J. J., et al. 2016. Sex and the single gametophyte: Revising the homosporous vascular plant life cycle in light of contemporary research. BioScience 66: 928–937. [Google Scholar]
- Kappen, L. 1964. Untersuchungen über den Jahresverlauf der Frost‐, Hitze‐, und Austrocknungs‐resistenz von Sporophyten einheimischer Polypodiaceae (Filicinae). Flora 155: 123−166. [Google Scholar]
- Kappen, L. 1965. Untersuchungen über die Widerstandsfähigkeit der Gametophyten einheimischer Polypodiaceen gegenüber Frost, Hitze und Trockenheit. Flora 156: 101−115. [Google Scholar]
- Lindsay, S. 1992. Field experiments on the development of fern gametophytes. PhD thesis, University of Edinburgh, Edinburgh, United Kingdom. [Google Scholar]
- Peck, J. H. , Peck C. J., and Farrar D. R.. 1990. Comparative life history studies and the distribution of pteridophyte populations. American Fern Journal 80: 126−142. [Google Scholar]
- Pickett, F. L. 1914. Some ecological adaptations of certain fern prothallia Camptosorus rhizophyllus Link., Asplenium platyneuron Oakes. American Journal of Botany 1: 477−498. [Google Scholar]
- Ranker, T. A. , and Geiger J. M. O.. 2008. Population genetics. In Ranker T. A. and Haufler C. H. [eds.], Biology and evolution of ferns and lycophytes, 107–133. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Rasband, W. S. 1997–2016. ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA, Website: https://imagej.nih.gov/ij/ [accessed 10 March 2022].
- Sato, T. 1982. Phenology and wintering capacity of sporophytes and gametophytes of ferns native to Northern Japan. Oecologia 55: 53−61. [DOI] [PubMed] [Google Scholar]
- Sato, T. , and Sakai A.. 1980. Freezing resistance of the temperate fern, Polystichum retroso‐paleaceum . Canadian Journal of Botany 58: 1144−1148. [Google Scholar]
- Schneller, J. J. 1979. Biosystematic investigations on the lady fern (Athyrium filix‐femina) . Plant Systematics and Evolution 132: 255−277. [Google Scholar]
- Schneller, J. J. 2008. Antheridiogens. In Ranker T. A. and Haufler C. H. [eds.], Biology and evolution of ferns and lycophytes, 134–158. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Sessa, E. B. , Testo W. L., and Watkins J. E., Jr. 2016. On widespread capacity for, and functional significance of, extreme inbreeding in ferns. New Phytologist 211: 1108−1119. [DOI] [PubMed] [Google Scholar]
- Walker, L. R. , and Sharpe J. M.. 2010. Ferns, disturbance and succession. In Mehltreter K., Walker L. R, and Sharpe J. M. [eds.], Fern ecology, 177–219. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Watkins, J. E. , Michelle C., Mack M. C., Sinclair T. R., and Mulkey S. S.. 2007a. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist 176: 708–717. [DOI] [PubMed] [Google Scholar]
- Watkins, J. E. , Michelle C., Mack M. C., and Mulkey S. S.. 2007b. Gametophyte ecology and demography of epiphytic and terrestrial tropical ferns. American Journal of Botany 94: 701−708. [DOI] [PubMed] [Google Scholar]


