Introduction:
The rising incidence of renal cell carcinoma (RCC) in the last two decades12 has coincided with increasing use of noninvasive imaging.3 This has been associated with diagnosis of a larger proportion of localized and small renal tumors.4,5 The resultant stage migration has necessitated a shift in treatment paradigms6–9, and led to the emphasis of patient-specific risk assessment in an effort to balance morbidity, survival and renal functional risks.10,11 While there are several preoperative nomograms that can aid clinical decision-making, predictive accuracy is generally low12,13, and renal mass biopsy (RMB) remains an important component of risk stratification and treatment planning. The American Urological Association (AUA) guidelines recommend RMB on a “utility-based approach,”1 that is, when results are expected to impact management, but there is considerable variation in practice patterns among urologists, and data suggests that greater than 20% of resected masses still ultimately prove to be benign.14 Indeed, the decision to pursue RMB is complex and depends on multiple patient and tumor factors, the likelihood of impact on management, and the expected diagnostic success based on tumor size and anatomic complexity. Prior studies have found that larger tumor size and exophytic location15 are associated with improved biopsy outcomes, while lower diagnostic rates were associated with smaller tumors, lower enhancement, larger skin-to-tumor distance, and cystic renal masses.16 Concerns about RMB feasibility and accuracy, particularly in the setting of anatomically complex renal tumors, have contributed to low adoption rates nationally17 despite reported improvements in diagnostic performance.15,18 Here we assess the feasibility of core biopsies in anatomically complex renal masses, and, compare their diagnostic adequacy and concordance with surgical pathology to those of non-complex lesions.
Materials & Methods:
We queried our institutional prospectively maintained database after Institutional Review Board (IRB) approval. All patients with a solid renal mass who underwent a computed tomography (CT)-guided core renal mass biopsy between June 2005 and September 2019 and had an associated nephrometry score within the database were included. We excluded: patients with Bosniak 2F and 3 cysts, urothelial cell carcinoma pathology, familial genetic syndromes, regional or distal metastatic disease on imaging.
Biopsies were performed by dedicated attending body radiologists (RP and BM), both with over 20 years in practice, using 18–20 Gauge core biopsy needles under CT guidance. A cytopathologist was present during all RMB procedures and fine needle aspiration with real-time interpretation used to assist in confirming adequate targeting of the lesion. Biopsy samples were read by dedicated genitourinary pathologists.
Definitions
For purposes of this study, complex renal lesions were defined under one of four conditions that may adversely affect percutaneous access, feasibility or outcome. Complex lesions were (1) small (<2 cm), or (2) entirely endophytic (nephrometry E=3), or (3) hilar(h) or (4) partially endophytic (E=2) and on the anterior surface of the kidney (Figure 1). Complexity was therefore defined based on the risks of inability to adequately access the lesion or obtain sufficient tissue for a tissue diagnosis. Lesions without these criteria were deemed non-complex.
Figure 1.
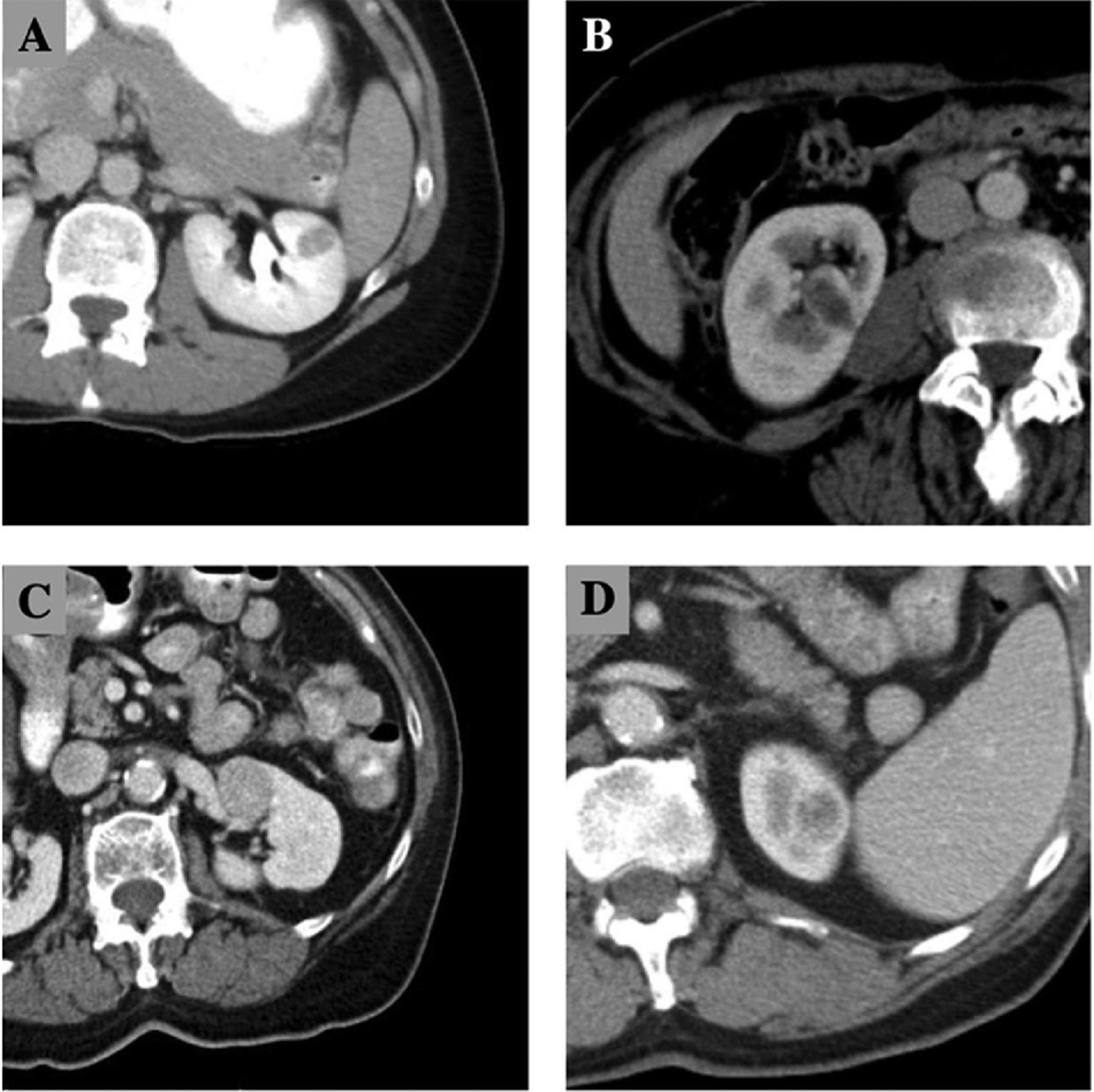
Computed tomography examples of a) small renal mass < 2 cm, b) endophytic mass (E=3), c) hilar mass, d) partially endophytic (E=2) and anterior.
Adequacy was assigned if the RMB successfully targeted the lesion resulting in a diagnosis. Biopsies with renal parenchyma or perirenal fat, for example, were deemed inadequate.
Biopsy concordance was determined by comparing results of the RMB to the “gold standard” surgical pathology obtained at the time of an extirpative procedure. Concordance was evaluated by oncologic, histologic, and grade components. This cohort of patients included only those who underwent an extirpative procedure such as nephrectomy or partial nephrectomy where final surgical pathology was available for comparison. Patients who underwent ablative procedures were excluded. Oncologic concordance was assigned if the RMB sample correctly identified a lesion as malignant or benign compared to the final surgical pathology. Histologic concordance was assigned if the RMB histology matched the final histology. Grade scores were pooled into low (Fuhrman grade 1 or 2) and high (3 or 4) categories, and grade concordance was assigned if the RMB grade matched the final grade category.
If a lesion was reported as a benign tumor on RMB and the surgical pathology was benign, then this would be an example of an adequate and oncologically concordant biopsy. If a lesion was a benign tumor on RMB but malignant tumor on surgical pathology, then this would be an example of an adequate but discordant biopsy.
Statistical analysis
Demographic characteristics including age at biopsy, sex, year of biopsy, RENAL nephrometry score, body mass index (BMI), and race were summarized with descriptive statistics for the complex and non-complex groups. Lesion complexity by nephrometry complexity definition components 1 through 4 were summarized and compared. Continuous variables were reported as medians with interquartile ranges (IQRs), categorical variables were reported as proportions. Baseline characteristics for patients with complex and non-complex lesions were compared using the Wilcoxon rank-sum test and the chi-square or Fisher exact test for continuous and categorical variables, respectively.
Adequacy rates and concordance rates divided into oncologic, histologic and grade components were compared for complex and non-complex lesions using logistic regressions. The odds ratios (ORs) are presented along with the 95% confidence intervals (CIs).
A 2×2 contingency table was constructed using biopsy and surgical pathology results. Using the definition for oncologic concordance, true positive (TP), false positive (FP), true negative (TN), and false negative (FN) values were obtained and sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated. Surgical pathology results were considered the gold standard for these calculations. RMBs were considered “benign” if their histopathologic diagnosis was benign tissue, oncocytic lesion or oncocytoma; lesions were considered “malignant” if their diagnosis was clear cell, papillary, chromophobe, collecting duct, sarcomatoid, poorly differentiated or necrotic carcinoma.
Logistic regression was performed to determine whether the diagnostic rate of RMB improved over time in terms of adequacy and concordance. Logistic regression was used to compare changes in diagnostic rates over time for complex and non-complex lesions. Variables included in these models were chosen based on clinical judgement of plausible relevance. Statistical analyses were performed using STATA (StataCorp, College Station, Texas), and statistical significance was set to p < 0.05.
Results:
A total of 460 renal mass biopsies were performed at our institution during the study period. After excluding lesions biopsied by fine needle aspiration and missing nephrometry scores, 306 renal masses were evaluated, of which 179 (58%) were classified as complex, and 127 (42%) as non-complex reflecting our tertiary referral patterns. A total of 199 (65%) lesions were ultimately excised and concordance analyses were performed. Lesions were deemed complex with regards to RMB in 29 (16%) because they were < 2 cm in size, 77 (43%) because they were entirely endophytic (E3), 58 (32%) because they were hilar (h), and 62 (35%) because they were partially endophytic (E2) and anterior. Notably, these were not mutually exclusive categories. Size and hilar location data were missing for 1 and 3 lesions respectively in the non-complex group. Table 1 compares the baseline characteristics of the complex and non-complex groups. The nephrometry score for complex lesions was significantly higher (median = 9 (IQR 7–10)) than for non-complex lesions (median=7 (IQR 6–9)), p<0.001. Patients with complex lesions were slightly younger (median 68 vs 72 p = 0.03) and had lower body mass index (BMI) (median 28 vs 31, p = 0.003) (Table 1).
Table 1.
Baseline patient and renal mass characteristics by biopsy complexity
| Patient characteristic | Description | Non-complex | Complex | p value |
|---|---|---|---|---|
| Total, n (%) | Number, proportion | 127 (42%) | 179 (58%) | |
| Complexity by component, n (%) | Number, proportion | None |
|
|
| Age at biopsy, median (IQR) | Years | 72 (63–79) | 68 (61–75) | 0.03 |
| Gender, n (%) | 0.90 | |||
| Male | 87 (69) | 121 (68) | ||
| Nephrometry score, median (IQR) | Scale 1–10 | 7 (6–9) | 9 (7–10) | <0.001 |
| BMI, median (IQR)* | Score | 31 (28–36) | 28 (26–34) | 0.003 |
| Race, n (%) | 0.84 | |||
| Other | 2 (2) | 2 (1) | ||
| Charlson Comorbidity Index, median (IQR) | Score 0–24 | 5 (4–7) | 5 (4–6) | 0.055 |
| Management type, n (%) | 0.051 | |||
| Radical Nx | 29 (23) | 58 (32) |
IQR = interquartile range; Nx = nephrectomy
When evaluating all patients who underwent renal biopsy, diagnostic adequacy was excellent in both complex and non-complex lesions (89% vs 96%) but lower for complex lesions(p=0.03) (Table 2). When evaluating patients who underwent renal biopsy and an extirpative procedure with final pathology, oncologic concordance was lower for complex lesions as well (89% vs 97%, p=0.03). There were no statistically significant differences in histologic or grade concordance rates (Table 2). Biopsy performance characteristics for masses meeting individual criteria for complexity are shown in Supplemental table 1. These were generally similar to the overall cohort of complex masses, with slightly worse numerical performance generally seen in masses <2cm (e.g. adequacy 83%, oncologic concordance 69%, histologic concordance 62%). Only two patients suffered complications, both Clavien grade 1 perinephric hematomas.
Table 2.
Biopsy adequacy by cancer diagnosis, histology, and grade in all patients who underwent RMB, and concordance by cancer diagnosis, histology, and grade in patients who underwent biopsy and had an extirpative procedure with available final pathology
| Component | Non-complex | Complex | P value | |
|---|---|---|---|---|
| Biopsy adequacy, n (%) | n = 306 | 122 (96) | 159 (89) | .03 |
| Biopsy concordance, n (%) | Oncologic (n = 199) | 72 (97) | 111 (89) | .03 |
| Histologic (n = 199) | 64 (86) | 95 (76) | .10 | |
| Grade (n = 199) | 38 (51) | 60 (48) | .66 |
On univariable analysis, a statistically significant association was observed between biopsy adequacy and male sex (OR 2.86, 95% CI 1.16–7.06, p=0.02); the association remained on multivariable analysis (OR 3.31, 95% CI 1.28–8.55, p=0.01) when age, BMI, nephrometry score, year of biopsy, and lesion complexity were used as covariates (Supplemental Table 2).
Univariable analyses showed no statistically significant associations between oncologic, histologic or grade concordance and our study covariates. Multivariable analyses showed a negative association between oncologic concordance and mass complexity (OR 0.19, 95% CI 0.04–0.93, p=0.04) (Supplemental Table 3).
Using biopsy and surgical pathology results for oncologic outcomes, sensitivity in our cohort was 93%, specificity 93%, PPV 99%, NPV 52%, and accuracy 93% (Supplemental Table 4).
Because percutaneous biopsy has become increasingly utilized and the techniques have evolved and improved over time, we evaluated these data to assess longitudinal improvements in diagnostic accuracy over time. We were unable to identify any significant difference over time in biopsy adequacy or concordance.
Comment:
A growing effort to reduce over-treatment of small or benign renal masses with invasive measures has led to the emergence of alternative management options such as active surveillance and ablation in appropriately selected patients.6–9 For localized renal masses, current guidelines emphasize consideration of all management options, appropriate counseling, and patient-specific risk assessment10, but patient-specific risk assessment is nuanced and depends heavily on clinical judgement. Current guidelines recommend consideration of biopsy when non-RCC pathology is suspected (e.g. metastases from another primary, lymphoma, abscess), prior to planned cryoablation, and when pathology would change management.1 While nomograms can assist in clinical decision-making, treatment planning and counseling are done in the setting of considerable uncertainty unless a biopsy of the mass is performed.
Over the last 4 decades, renal mass biopsy has been obtained infrequently during the evaluation of renal tumors. Several reasons may exist for this historic standard of care to include (1) the risks of bleeding and AVF, (2) the risks of tumor seeding, (3) the lack of systemic alternatives to surgical therapy for RCC, (4) the underappreciated treatment and renal functional risks associated with surgical removal and (5) the risk of inadequate sampling or incorrect oncologic diagnoses. With recent improvements in imaging modalities and biopsy technique, significant biopsy complication rates are low (<5%) while diagnostic rates are relatively high (>90%).18 Moreover, coaxial needles have significantly reduced the risk of tumor seeding such that it is an extremely rare event.
Despite these reassuring outcomes, RMB remains underutilized. In 2018, Patel et al published survey results detailing practice patterns of urologists within the United States. They reported that 32% of urologists would “never” perform biopsy of a renal mass ≤ 4 cm. The primary reason for forgoing biopsy in 68% of those surveyed was that biopsy results “would not change their management of the renal mass”.19 Other concerns with RMB relate to the ability of the radiologist to adequately sample the lesion given its size, location and/or complexity. These perceived impediments perpetuate the possibility of overtreatment of small and benign or indolent renal masses. Previous studies have shown that smaller lesions, endophytic location,15 cystic nature, necrosis, hypoenhancement, long skin-to-tumor distance,16 anterior and upper pole location20 are associated with non-diagnostic outcomes on RMB. In the present study we evaluated whether lesion complexity, as determined by size, endophycity and renal location, affects biopsy adequacy and concordance by oncologic, histologic, and grade components.
Overall, we found high diagnostic and oncologic concordance rates in our cohort regardless of tumor location and complexity. Adequacy was 89% for complex lesions and 96% for non-complex lesions (p=0.03) and oncologic concordance was 89% for complex lesions and 97% for non-complex lesions (p=0.03). While differences between complex and non-complex rates have statistical significance, from a clinical perspective, biopsies of complex lesions are still highly adequate and oncologically concordant. These rates demonstrate RMB value even in the setting of complex renal lesions.
Our results are consistent with an extensive systematic review and meta-analysis by Marconi et al who reviewed data from 57 studies involving 5,228 patients. They report an overall median diagnostic yield of 92% (IQR 81–97%) for identification of malignancy in core biopsy and fine-needle aspiration samples, and overall median concordance rates of 90% (IQR 84–94%) when compared to nephrectomy in the overall cohort. Importantly, none of the studies included in their pooled analysis assessed differences in diagnostic yield based on tumor characteristics such as size, endophycity or location. In fact, fewer than half of the studies they identified reported a mean or median tumor size in their biopsy cohort. Most studies of RMB report results with a median tumor sizes >4cm and only one reported biopsy outcomes for tumors on average <2cm. In that report by Park et al,21 the authors reported on complications but not on diagnostic yield of biopsy on n=59 tumors undergoing an ultrasound-guided core biopsy. Our data therefore represent the first time that renal mass characteristics such as size, depth and location were incorporated into analysis on RMB outcomes demonstrating the feasibility of RMB in these anatomically complex circumstances.
Similar to prior studies, histologic and grade concordance rates in the present study were lower than oncologic concordance rates. While the differences here were not statistically significant, we found lower rates of histologic concordance in complex versus non-complex lesions (76% vs 86%, p=0.10). In the Marconi study, the median histologic concordance rate was 90% (IQR 84–94%), similar to our non-complex lesion results.
Grade concordance rates were relatively low in our study, although nearly identical for complex (48%) and non-complex (51%) lesions (p=0.66). For the 10 studies evaluating core biopsies only in the Marconi review, grade concordance rates were between 43–93%. Some of these discrepancies are likely due to insufficient sample or histologic misclassification, but some portion is likely due to innate tumor heterogeneity. Marconi et al also showed a higher histologic concordance (96%, IQR 90–100%) in the 6 studies that included only small renal masses ≤ 4 cm. While our study did not evaluate number or location of cores within the mass, it is possible that a fewer number of cores were taken in our cohort than in studies included in the meta-analysis, which may explain the discrepancy in results. Other differences in technique also cannot be excluded. Finally, our biopsy sensitivity (93%) and specificity (93%) are comparable to those published by Marconi et al, who reported sensitivity of 84% (95% CI 33.8–98.1) for cystic lesions and 99.7 (95% CI 81.5–100) for small lesions, and specificity of 98% (95% CI 80.9–99.8) to 98.2% (95% CI 83.3–99.8) for the same parameters.
We found that biopsy adequacy rates were higher in male patients. This may be due to well-recognized anatomic differences in para- and peri-renal fat distribution, although an association due to chance alone cannot be excluded in the context of multiple statistical testing. We are not aware of prior studies which have reported similar findings.
Our study is not without limitations. Its retrospective design and exclusion of patients without available nephrometry scores makes it susceptible to selection bias. We did not capture the indication for RMB or how RMB ultimately affected clinical decision making. Use of surgical resection as the gold standard for oncologic, histologic, and grade concordance also introduces an important selection bias, since patients who had benign biopsies would not undergo resection unless they had some other indication for surgery or some indication that the biopsy was a false negative (e.g. growth over time on imaging). This almost certainly artificially increased our false negative rate, which explains the poor negative predictive value (52%) and depressed the apparent sensitivity of biopsy, which remained very good (93%) despite this bias. While we did not find a significant association between BMI and diagnostic accuracy, we did not evaluate other anatomic patient factors such as skin-to-mass distance. Another limitation is that we did not assess effects of needle size, number/location of cores, imaging modality (CT versus ultrasound), specific technical maneuvers performed during biopsy, radiologists’ experience, or pathologists’ experience in this study even though these factors are very likely to contribute to biopsy success. Some of these factors, particularly relating to location of cores within the tumor and operator experience, will require further study.
Nonetheless, our results are overall reassuring in that core RMB from anatomically small, hilar, endophytic and anterior lesions yield high accuracy for identification of malignancy. For physicians and patients considering alternatives to extirpative surgery, information on histology and grade could change clinical management as histologic subtypes have been shown to affect prognosis.22 Given our findings, we recommend RMB in discussion with radiology colleagues even in cases of high lesion complexity; indeed, a multidisciplinary approach may lead to improved RMB utilization. The role of sestamibi scans in the characterization of renal masses is evolving, but the ability to differentiate between oncocytoma and chromophobe RCC remains limited, with 89% sensitivity and 67% specificity in a recent meta-analysis.23 Rather than obviate the need for RMB, sestamibi scans may serve as an adjunct to further increase diagnostic confidence. RMB has diverse roles in clinical management of renal masses including in risk stratification of patients considering surveillance, evaluation of masses suspicious for extrarenal metastasis, inflammation, or infection, and prior to surgery for patients at higher risk for dialysis such as those with bilateral masses, solitary or transplant kidneys. It can potentially impact decisions to perform radical or partial nephrectomy for lesions suspected to be aggressive based on imaging. Most importantly, it is a safe procedure that can help patients avoid unnecessary surgery and its related morbidity.
Conclusions:
Diagnostic adequacy and oncologic concordance of RMB for complex renal masses remain excellent despite being slightly lower than those of RMB for non-complex masses. Histologic and grade concordance are imperfect and may be affected by sample adequacy, pathologic diagnosis, and inherent tumor heterogeneity but do not differ based on lesion complexity. The small decreases in performance characteristics of RMB when performed on anatomically complex lesions should rarely alter the decision to perform a RMB when additional data can improve choice of management. Appropriate patient counseling and management of expectations remain essential given the potential need for subsequent treatment plan adjustments.
Supplementary Material
References
- 1.Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline Part I. J Urol. Published online June 11, 2021. doi: 10.1097/ju.0000000000001911 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9). doi: 10.1093/jnci/djx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MAS. The Natural History of Incidentally Detected Small Renal Masses. Cancer. 2004;100(4):738–745. doi: 10.1002/cncr.20025 [DOI] [PubMed] [Google Scholar]
- 4.Chow WH, Devesa SS, Warren JL, Fraumeni JF. Rising incidence of renal cell cancer in the United States. J Am Med Assoc. 1999;281(17):1628–1631. doi: 10.1001/jama.281.17.1628 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MM, Gill IS, Ellison LM. The Evolving Presentation of Renal Carcinoma in the United States: Trends From the Surveillance, Epidemiology, and End Results Program. J Urol. 2006;176(6):2397–2400. doi: 10.1016/j.juro.2006.07.144 [DOI] [PubMed] [Google Scholar]
- 6.Volpe A, Cadeddu JA, Cestari A, et al. Contemporary management of small renal masses. Eur Urol. 2011;60(3):501–515. doi: 10.1016/j.eururo.2011.05.044 [DOI] [PubMed] [Google Scholar]
- 7.Ristau BT, Correa AF, Uzzo RG, Smaldone MC. Active Surveillance for the Small Renal Mass: Growth Kinetics and Oncologic Outcomes. Urol Clin North Am. 2017;44(2):213–222. doi: 10.1016/j.ucl.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 8.Ristau BT, Kutikov A, Uzzo RG, Smaldone MC. Active Surveillance for Small Renal Masses: When Less is More. Eur Urol Focus. 2016;2(6):660–668. doi: 10.1016/j.euf.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 9.Kunkle DA, Egleston BL, Uzzo RG. Excise, Ablate or Observe: The Small Renal Mass Dilemma-A Meta-Analysis and Review. J Urol. 2008;179(4):1227–1234. doi: 10.1016/j.juro.2007.11.047 [DOI] [PubMed] [Google Scholar]
- 10.Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol. 2017;198(3):520–529. doi: 10.1016/j.juro.2017.04.100 [DOI] [PubMed] [Google Scholar]
- 11.Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28(2):311–317. doi: 10.1200/JCO.2009.22.4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane BR, Babineau D, Kattan MW, et al. A Preoperative Prognostic Nomogram for Solid Enhancing Renal Tumors 7 cm or Less Amenable to Partial Nephrectomy. J Urol. 2007;178(2):429–434. doi: 10.1016/j.juro.2007.03.106 [DOI] [PubMed] [Google Scholar]
- 13.Jeldres C, Sun M, Liberman D, et al. Can Renal Mass Biopsy Assessment of Tumor Grade be Safely Substituted for by a Predictive Model? J Urol. 2009;182(6):2585–2589. doi: 10.1016/j.juro.2009.08.053 [DOI] [PubMed] [Google Scholar]
- 14.Johnson DC, Vukina J, Smith AB, et al. Preoperatively Misclassified, Surgically Removed Benign Renal Masses: A Systematic Review of Surgical Series and United States Population Level Burden Estimate. In: Journal of Urology. Vol 193. Elsevier Inc.; 2015:30–35. doi: 10.1016/j.juro.2014.07.102 [DOI] [PubMed] [Google Scholar]
- 15.Richard PO, Jewett MAS, Bhatt JR, et al. Renal Tumor Biopsy for Small Renal Masses: A Single-center 13-year Experience. Eur Urol. 2015;68(6):1007–1013. doi: 10.1016/j.eururo.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Prince J, Bultman E, Hinshaw L, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol. 2015;193(6):1899–1904. doi: 10.1016/j.juro.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barwari K, De La Rosette JJ, Laguna MP. The penetration of renal mass biopsy in daily practice: A survey among urologists. J Endourol. 2012;26(6):737–747. doi: 10.1089/end.2011.0407 [DOI] [PubMed] [Google Scholar]
- 18.Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016;69(4):660–673. doi: 10.1016/j.eururo.2015.07.072 [DOI] [PubMed] [Google Scholar]
- 19.Patel RM, Safiullah S, Okhunov Z, et al. Pretreatment Diagnosis of the Small Renal Mass: Status of Renal Biopsy in the United States of America. In: Journal of Endourology. Vol 32. Mary Ann Liebert Inc.; 2018:884–890. doi: 10.1089/end.2018.0175 [DOI] [PubMed] [Google Scholar]
- 20.Seager MJ, Patel U, Anderson CJ, Gonsalves M. Image-guided biopsy of small (≤4 cm) renal masses: The effect of size and anatomical location on biopsy success rate and complications. Br J Radiol. 2018;91(1085). doi: 10.1259/bjr.20170666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SY, Park BK, Kim CK, Kwon GY. Ultrasound-guided core biopsy of small renal masses: Diagnostic rate and limitations. J Vasc Interv Radiol. 2013;24(1):90–96. doi: 10.1016/j.jvir.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 22.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27(5):612–624. doi: 10.1097/00000478-200305000-00005 [DOI] [PubMed] [Google Scholar]
- 23.Wilson MP, Katlariwala P, Murad MH, Abele J, McInnes MDF, Low G. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: a systematic review and meta-analysis. Abdom Radiol. 2020;45(8):2532–2541. doi: 10.1007/s00261-020-02469-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


