Introduction
Histiocytoid Sweet syndrome (H-SS) is a rare variant of acute febrile neutrophilic dermatosis. It is frequently associated with hematologic malignancies but has also been associated with certain inflammatory states. Here we report a case of concurrent anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis and H-SS diagnoses.
Case report
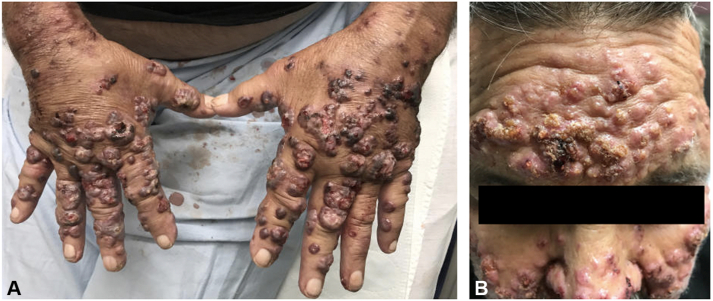
A 65-year-old man with a history of chronic obstructive pulmonary disease, chronic kidney disease, and mitral valve replacement presented with progressive skin lesions, acute kidney injury, and recent self-reported mild hemoptysis. He reported that the lesions first appeared 4 weeks prior to presentation as “small pimples” on his hands and rapidly progressed to involve a larger part of his upper extremities and face. He denied associated pain or itching, but the lesions had started to express purulent and sanguineous fluid per patient report. No iodine-containing imaging was performed prior to the emergence of the skin eruption. On examination, the patient had numerous violaceous and erythematous pseudovesicular papules and plaques on the dorsal aspects of his hands (Fig 1, A), some with hemorrhagic crust. Numerous pink indurated papules and plaques, also with hemorrhagic crust, were appreciated on his face (Fig 1, B). Laboratory test results were remarkable for anemia (hemoglobin, 9.4 g/dL), thrombocytopenia (platelet count, 81,000/mm3), and renal injury (creatinine, 3.2 mg/dL). Urinalysis showed both hematuria and proteinuria. ANCA proteinase-3 was positive at a titer of 1:160.
Fig 1.
A, Photograph of the dorsal aspects of the patient’s hands on admission showing vesicular papules and plaques. B, Photograph of the patient’s forehead on admission showing pink indurated papules and plaques.
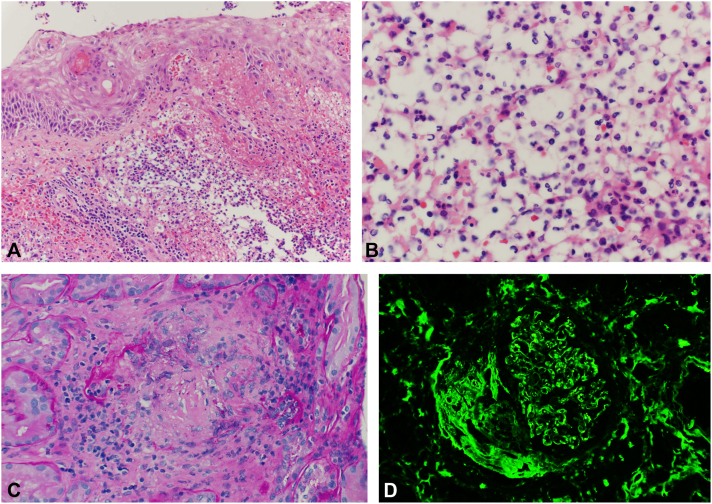
A skin biopsy was performed and showed partial dermoepidermal junction separation, spongiosis, and marked acute dermal inflammation as well as mononuclear cells with abundant cytoplasm, which stained positively with myeloperoxidase (Fig 2, A and B). Based on the clinical and microscopic data, a diagnosis of H-SS was made. Treatment with prednisone 60 mg daily was initiated. Flow cytometry and bone marrow biopsy were performed; however, no underlying malignancy was identified.
Fig 2.
A, Lesional skin biopsy demonstrating spongiotic epidermis overlying a dermis with marked inflammatory infiltrate consisting of mixed polymorphonuclear and mononuclear cells, as well as hemorrhage and focal dermal necrosis (hematoxylin-eosin stain). B, High-power view of a lesional skin biopsy demonstrating areas of mononuclear cells in the infiltrate (hematoxylin-eosin stain; original magnification: ×40). C, Renal biopsy demonstrating necrotizing granulomas involving the tubulointerstitium; loosely aggregated large epitheliod histiocytic cells and inflammatory lymphocytes, with tissue destruction and central necrosis (Periodic acid–Schiff stain). D, Fibrinogen direct immunofluorescence microscopy of the renal biopsy demonstrated crescent/fibrinoid necrosis as highlighted by positive fibrinogen staining.
A review of available records showed that the creatinine level had been normal 1 year previously but since that time had remained persistently elevated with periods of fluctuating improvement. Also, of note, the ANCA screen performed 10 months previously was negative. The patient underwent renal biopsy, which showed focally necrotizing/crescentic glomerulonephritis and tubulointerstitial inflammation with granulomatous lesions, consistent with granulomatosis with polyangiitis glomerulonephritis (Fig 2, C and D). Rituximab was added to the steroid treatment regimen. Skin lesions responded to treatment, but, unfortunately, kidney function did not improve. Admission was complicated by mechanical mitral valve failure and thrombus, volume overload, and progressive confusion. The patient and family elected to pursue comfort measures, and he passed away shortly thereafter.
Discussion
H-SS is a variant of Sweet syndrome that was first described in 2005. This variant is characterized by infiltration of the dermis with inflammatory histiocytoid mononuclear cells and is frequently associated with hematologic malignancies, especially myelodysplastic syndrome.1 Differential diagnoses for H-SS include erythema elevatum diutinum, leukemia cutis, neutrophilic eccrine hidradenitis, and halogenoderma. The clinical appearance and histologic patterns on the skin biopsy diminished the possibility of these differential diagnoses. Skin leukocytoclastic vasculitis occurs frequently in ANCA-associated vasculitis; however, only a few cases of comorbid neutrophilic dermatoses have been reported. Review of the literature shows that granulomatosis with polyangiitis is the most frequent ANCA-associated vasculitis associated with neutrophilic dermatoses, with the most common neutrophilic dermatosis being pyoderma gangrenosum.2, 3, 4, 5 Our patient had a very rare combination of H-SS skin lesions in the setting of ANCA proteinase-3 granulomatosis with polyangiitis glomerulonephritis. The pathogenesis of both disease entities is believed to involve neutrophil or neutrophil progenitor dysfunction;2,5 it is possible that they share some as-of-yet undefined dysregulatory immune mechanisms, and the determinants of tissue-tropism are also unclear.
This case demonstrates an uncommon skin presentation resulting in the diagnosis of 2 rare diseases in the same patient with a potentially similar pathoetiology.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Requena L., Kutzner H., Palmedo G., et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141(7):834–842. doi: 10.1001/archderm.141.7.834. [DOI] [PubMed] [Google Scholar]
- 2.Alegría-Landa V., Rodríguez-Pinilla S.M., Santos-Briz A., et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153(7):651–659. doi: 10.1001/jamadermatol.2016.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghoufi L., Ortonne N., Ingen-Housz-Oro S., et al. Histiocytoid Sweet syndrome is more frequently associated with myelodysplastic syndromes than the classical neutrophilic variant: a comparative series of 62 patients. Medicine. 2016;95(15) doi: 10.1097/MD.0000000000003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amouri M., Masmoudi A., Ammar M., et al. Sweet´s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55(9):1033–1039. doi: 10.1111/ijd.13232. [DOI] [PubMed] [Google Scholar]
- 5.de Boysson H., Martin Silva N., de Moreuil C., et al. Neutrophilic dermatoses in antineutrophil cytoplasmic antibody-associated vasculitis: a French multicenter study of 17 cases and literature review. Medicine (Baltimore) 2016;95(11) doi: 10.1097/MD.0000000000002957. [DOI] [PMC free article] [PubMed] [Google Scholar]