Abstract
Background
We aimed to analyse the discrepancy in clinical features and prognosis between molecular subtypes in primary ductal carcinoma in situ (DCIS) patients with lumpectomy.
Methods
Primary DCIS patients were identified from the Surveillance, Epidemiology, and End Results registries database from 2010 to 2017. Based on immunohistochemistry markers of hormone receptor (HR) and human epidermal growth factor receptor-2 (HER2), enrolled DCIS cases were divided into four molecular subtypes, HR-HER2-, HR-HER2+, HR + HER2+, and HR + HER2-. Clinical features and prognosis were compared between molecular subtypes. Radiotherapy (RT) effects on prognosis were also analysed in each molecular subtype.
Results
A total of 5,628 DCIS cases were retrospectively enrolled in this study. HR-HER2-, HR-HER2+, HR+HER2+, and HR+HER2- are 299 (5.3%), 498 (8.8%), 1,086 (19.3%), and 3,745 (66.5%), respectively. HR + HER2- cases have smaller tumor size (72.6%, P < 0.001) and lower grade (23.5%, P < 0.001). Comedo necrosis is more frequent in HR-HER2- (24.4%, P < 0.001) and HR-HER2+ DCIS cases (24.3%, P < 0.001). In univariate analyses, HR-HER2+ cases have significantly higher ipsilateral breast event (IBE) recurrence than HR+HER2- cases (P = 0.010). HR-HER2- cases show higher disease-specific mortality than HR+HER2+ cases (P = 0.021). In high-risk DCIS cases, RT reduces the absolute 5-year IBE incidence by 1.3%, 0.7%, 1.9%, and 2.6%, respectively in HR-HER2-, HR-HER2+, HR+HER2+, and HR+HER2- cases, respectively.
Conclusion
In this population-based study, DCIS cases have diverse clinical and prognostic features for different molecular subtypes. Adjusting treatment strategies according to DCIS molecular subtypes is worth advancing.
Keywords: Ductal Carcinoma in situ, Molecular subtype, Lumpectomy, Recurrence
Highlights
-
•
HR-HER2+ DCIS shows the highest proportion of high-risk cases. HR + HER2- DCIS shows lowest proportion of high-risk cases.
-
•
HR-HER2+ DCIS shows high risk of IBE recurrence. The DSmortality has no differences among four molecular subtypes.
-
•
RT lowered the probability of IBE incidence in high-risk cases for each molecular subtype of DCIS.
1. Introduction
The gene expression profiling of invasive breast cancers (IBCs) reveals the heterogeneity of this disease [[1], [2], [3]]. There are four main intrinsic subtypes, luminal A, luminal B, human epidermal growth factor receptor-2 (HER2)-enriched, and basal-like, which are clinically classified by molecular profiling [4]. These intrinsic subtypes categorized by gene expression profiling have unique clinical and prognostic features [1,5,6]. Three immunohistochemistry (IHC) markers, estrogen receptor (ER), progesterone receptor (PR), and HER2, are commonly used as reliable surrogates for expression profiling-based subtyping. IHC-based subtyping has relatively high consistency with gene expression profiling-based subtyping in IBC and is recommended by the St Gallen guidelines for clinical diagnosis [7]. The treatment strategy, clinical features, and prognosis are diverse between the four subtypes classified by IHC-based subtyping [8,9].
Ductal carcinoma in situ (DCIS) is a preinvasive disease [10]. The prevalence of molecular subtypes is different between DCIS and IBC [11], and the clinical features and prognosis of distinct molecular subtypes of DCIS are unique from those of IBC. For example, the basal-like subtype has the poorest prognosis among the four subtypes in IBC [12]. However, it has less aggressive behaviors than the other subtypes in DCIS [13]. The molecular subtype of subsequent ipsilateral IBC is frequently inconsistent with primary DCIS [14]. Subclone evolution may cause this inconsistency [15,16]. All of these indicate that the molecular subtypes reflect the intrinsic features of DCIS implying its heterogeneity at molecular level.
Although the breast disease-related mortality for DCIS after surgery is extremely low [17], the possibility of ipsilateral breast event (IBE) incidence increases with time after lumpectomy [18]. In consideration of molecular subtypes in DCIS, specialized treatment options are applied in clinical trials to reduce IBE incidence. Hormone receptor-positive DCIS patients can be slightly benefit from endocrine therapy to reduce IBE incidence [19]. For HER2-positive DCIS patients, HER2-targeted therapy is an alternative option to reduce IBE incidence after lumpectomy [20]. Apart from the above treatments, the radiotherapy (RT) is a primary treatment for all DCIS patients after lumpectomy. The effect of RT on reducing IBE incidence is clear in DCIS with lumpectomy after stratification by different risk levels [18].
In this study, we aimed to use a population-based database to analyse the discrepancy in clinical features and prognosis between molecular subtypes in primary DCIS patients with lumpectomy.
2. Material and methods
2.1. Data sources and cohort selection
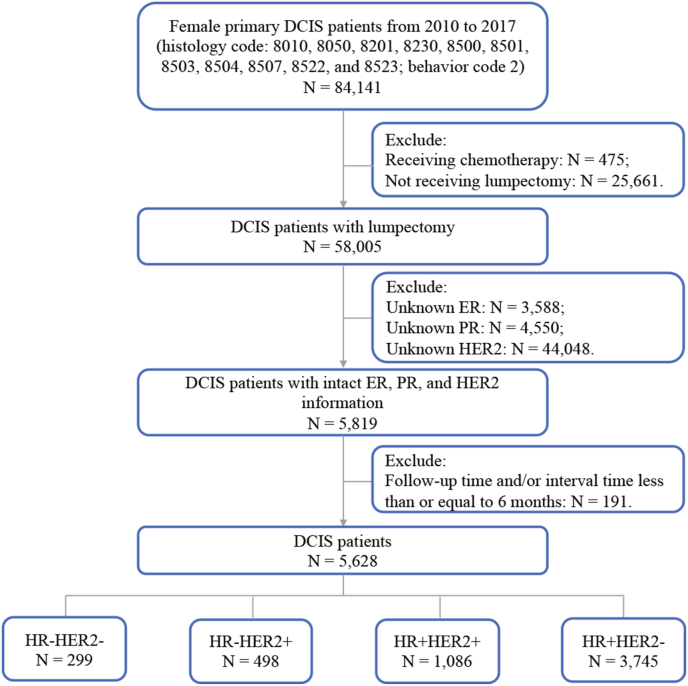
We identified primary DCIS cases from the Surveillance, Epidemiology, and End Results (SEER) registries database (November 2020 submission) [21]. SEER*Stat version 8.3.9 was used to conduct case listings. Since patient-level identifiers are not available to SEER users, this study was exempted from IRB review and approval. As the HER2 record was included for breast disease in the SEER database after 2010, we only included the primary DCIS cases from 2010 to 2017 in this study. Cases with behavior code 2 and the following histology codes were identified as DCIS: 8010, 8050, 8201, 8230, 8500, 8501, 8503, 8504, 8507, 8522, and 8523. Female patients with lumpectomy (surgery codes 20–24) and a follow-up time and/or interval time between primary DCIS and IBE of more than 6 months were included in this study. Patients who received chemotherapy were excluded. In total, 5,628 cases with intact ER, PR, and HER2 records were identified for further study. The flowchart shows the process of case selection in Fig. 1.
Fig. 1.
Flowchart in this study. (Abbreviations: DCIS, ductal carcinoma in situ; N, number; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor-2; HR, hormone receptor.)
2.2. Outcome variables
IBE and disease-specific (DS) mortality are the two main outcomes in this study. The IBE recurrence time is defined as the interval time between the primary disease and the first in situ and/or invasive recurrence. The DS mortality time is defined as the interval time between the primary disease and the death of breast disease. Moreover, the incidence of in situ IBE, invasive IBE, and non-DS mortality was also the secondary outcome in this study. Non-DS mortality is defined as death of other causes other than breast disease.
2.3. Statistical analyses
To address the missing data regarding race (n = 35, 0.6%), grade (n = 584, 10.4%), and size (n = 1,028, 18.3%), we imputed these missing data using the following variables in the model: year of diagnosis, age, race, histology subtype, grade, tumor size, hormone receptor (HR) status, HER2 status and RT. As ER and PR statuses have high consistency, we combined these two variables into HR status for analyses. After imputation ten times, the new data set with no missing data was used for further analyses.
According to HR and HER2 statuses, DCIS cases in this study were classified into four molecular subtypes, HR-HER2-, HR-HER2+, HR+HER2+, and HR+HER2-. The clinical features were compared among these four molecular subtypes by Chi-square test in SPSS software version 26 (IBM Corp., Armonk, NY, US). The modified prognostic score, which is a designed scheme with 6 total points by scoring age (>60 years, 40–60 years, and <40 years), tumor size (<16 mm, 16–40 mm, and >40 mm), and grade (low, intermediate, and high) from 0 to 2 for each factor, was used to stratify local recurrence risk for DCIS patients [18,22]. The cumulative incidence curves of IBE recurrence and mortality were compared between the four molecular subtypes by log-rank test. Multivariate analyses for prognosis between the four molecular subtypes were compared in non-weighted Cox proportional hazards and Fine & Gray competing risks regression models. In competing risk analyses, IBE recurrence and all-cause mortality, DS mortality and non-DS mortality, as well as invasive and in situ IBE recurrence were regarded as competing events in this study.
To evaluate the RT effects on prognosis in each subtype, in consideration of imbalanced clinical features between the RT and non-RT groups, we balanced variables between the two groups by inverse propensity score weighting (IPSW). The propensity score was calculated in SPSS software by using covariates (year of diagnosis, age, race, histology subtype, grade, and tumor size) through a binary logistic regression model. Cases in the RT and non-RT groups for each molecular subtype were weighted by inverse propensity score calculated as (1/[propensity score]) or (1/[1-propensity score]), respectively. The cumulative incidence of IBE and mortality for RT and non-RT groups in each molecular subtype were compared by weighted log-rank test. In multivariate analyses, the effects of RT on prognosis were computed in weighted and non-weighted Cox proportional hazards regression models, as well as Fine & Gray competing risks regression models.
The analyses in this study were conducted in RStudio software version 1.4.1103 (RStudio, Inc., Boston, MA, USA). The imputation process was conducted by mice package. The survival analyses were conducted by survival package. A two-tailed P value < 0.050 was considered statistically significant.
3. Results
3.1. Molecular subtypes have unique clinical characteristics
We identified 5,628 female patients who received lumpectomy from 2010 to 2017. By IHC-based molecular subtyping, 299 (5.3%) cases are HR-HER2-, 498 (8.8%) cases are HR-HER2+, 1,086 (19.3%) cases are HR+HER2+, and 3,745 (66.5%) cases are HR+HER2-. The diversity of clinical features among the four molecular subtypes is shown in Table 1. HR+HER2- cases have smaller tumor size (P < 0.001) and lower grade (P < 0.001). Comedo necrosis is more frequent in HR-HER2- (24.4%, P < 0.001) and HR-HER2+ cases (24.3%, P < 0.001). We then applied prognostic score [18] classification to each molecular subtype. Cases scored 0–2 were classified into low-risk group, and cases scored of 3–6 were classified into high-risk group. HR-HER2+ cases have the highest proportion of the high-risk group (65.7%, P < 0.001), whereas HR+HER2- cases have the lowest proportion of the high-risk group (27.9%, P < 0.001).
Table 1.
Clinical characteristics by molecular subtypes.
| Characteristics | N (%) | HR-HER2-(N (%)) | HR-HER2+ (N (%)) | HR+HER2+ (N (%)) | HR+HER2-(N (%)) | Chi square | P |
|---|---|---|---|---|---|---|---|
| Year of diagnosis | 13.242 | 0.004 | |||||
| 2010–2013 | 3,517 (62.5) | 214 (71.6) | 296 (59.4) | 687 (63.3) | 2,320 (61.9) | ||
| 2014–2017 | 2,111 (37.5) | 85 (28.4) | 202 (40.6) | 399 (36.7) | 1,425 (38.1) | ||
| Age, years | 37.427 | <0.001 | |||||
| ≤60 | 2,805 (49.8) | 124 (41.5) | 254 (51.0) | 623 (57.4) | 1,804 (48.2) | ||
| >60 | 2,823 (50.2) | 175 (58.5) | 244 (49.0) | 463 (42.6) | 1,941 (51.8) | ||
| Race | 26.536 | <0.001 | |||||
| African American | 789 (14.0) | 56 (18.7) | 41 (8.2) | 135 (12.4) | 557 (14.9) | ||
| Caucasian | 4,404 (78.3) | 220 (73.6) | 420 (84.3) | 876 (80.7) | 2,888 (77.1) | ||
| Other | 435 (7.7) | 23 (7.7) | 37 (7.4) | 75 (6.9) | 300 (8.0) | ||
| Tumor size, mm | 71.106 | <0.001 | |||||
| <16 | 3,918 (69.6) | 185 (61.9) | 281 (56.4) | 732 (67.4) | 2,720 (72.6) | ||
| 16–40 | 1,377 (24.5) | 98 (32.8) | 172 (34.5) | 284 (26.2) | 823 (22.0) | ||
| >41 | 333 (5.9) | 16 (5.4) | 45 (9.0) | 70 (6.4) | 202 (5.4) | ||
| Grade | 1,093.189 | <0.001 | |||||
| Low | 964 (17.1) | 11 (3.7) | 6 (1.2) | 67 (6.2) | 880 (23.5) | ||
| Intermediate | 2,304 (40.9) | 63 (21.1) | 54 (10.8) | 356 (32.8) | 1,831 (48.9) | ||
| High | 2,360 (41.9) | 225 (75.3) | 438 (88.0) | 663 (61.0) | 1,034 (27.6) | ||
| Histology Subtype | 322.407 | <0.001 | |||||
| Intraductal, solid | 2,240 (39.8) | 128 (42.8) | 221 (44.4) | 484 (44.6) | 1,407 (37.6) | ||
| Comedo necrosis | 599 (10.6) | 73 (24.4) | 121 (24.3) | 142 (13.1) | 263 (7.0) | ||
| Cribriform | 525 (9.3) | 9 (3.0) | 16 (3.2) | 58 (5.3) | 442 (11.8) | ||
| Other | 2,264 (40.2) | 89 (29.8) | 140 (28.1) | 402 (37.0) | 1,633 (43.6) | ||
| Radiotherapy | 47.361 | <0.001 | |||||
| Yes | 4,040 (71.8) | 243 (81.3) | 390 (78.3) | 824 (75.9) | 2,583 (69.0) | ||
| No | 1,588 (28.2) | 56 (18.7) | 108 (21.7) | 262 (24.1) | 1,162 (31.0) | ||
| Prognostic Score* | 426.311 | <0.001 | |||||
| Low risk | 3,549 (63.1) | 142 (47.5) | 171 (34.3) | 536 (49.4) | 2,700 (72.1) | ||
| High risk | 2,079 (36.9) | 157 (52.5) | 327 (65.7) | 550 (50.6) | 1,045 (27.9) | ||
Abbreviations: N, numbers; HR, hormone receptor; HER2, human epidermal growth factor receptor-2.
Note: * Prognostic score is a scoring method by age, tumor size, and grade to stratify DCIS patients into low-risk and high-risk group.
3.2. Molecular subtypes have unique prognoses
During the follow-up period (from 7 to 107 months), 121 cases of IBE recurrence, 37 cases of DS mortality, and 249 cases of mortality with other causes were recorded. In HR-HER2-, HR-HER2+, HR+HER2+, and HR+HER2-cases, the 5-year IBE incidence for each group is 1.9%, 3.7%, 2.3%, and 1.4%, respectively. The 5-year DS mortality for each group is 1.1%, 0.2%, 0.2%, and 0.5%, respectively; and the 5-year non-DS mortality is 2.7%, 4.9%, 3.0%, and 3.3%, respectively. For DCIS cases with invasive IBE records, 4 of 6 (66.7%) HR-HER2-, 8 of 11 (72.7%) HR-HER2+, 7 of 20 (35.0%) HR+HER2+, and 42 of 49 (85.7%) HR+HER2- DCIS cases share the same molecular subtype with invasive IBE (Supplementary Table 1).
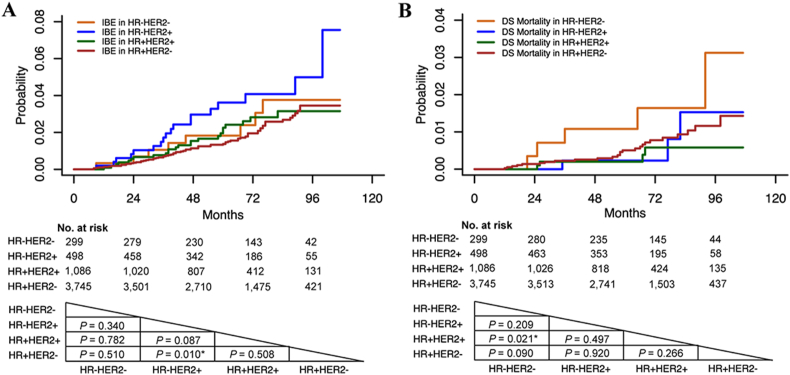
In univariate analyses, we compared prognosis between four molecular subtypes. HR-HER2+ cases have significantly higher IBE recurrence than HR+HER2-cases (P = 0.010, Fig. 2A). HR-HER2-cases show higher DS mortality than HR+HER2+ cases (P = 0.021, Fig. 2B). For non-DS mortality, there is no difference between the four molecular subtypes (P > 0.050).
Fig. 2.
Ipsilateral breast event (IBE) and disease-specific (DS) mortality in each molecular subtype. A. IBE; B. DS mortality. (Abbreviations: IBE, ipsilateral breast event; DS, disease specific; HR, hormone receptor; HER2, human epidermal growth factor receptor-2. Note: P value was calculated by log-rank test.)
In multivariate analyses (Table 2), HR-HER2+ cases also show a higher risk of IBE recurrence in both Cox proportional hazards (P = 0.027) and Fine & Gray competing risks (P = 0.032) regression models comparing with HR+HER2-cases. For DS mortality, HR-HER2-cases do not show a higher risk than HR+HER2+ cases in the two regression models (P > 0.050).
Table 2.
Comparing risk of ipsilateral breast event (IBE) and disease-specific (DS) mortality among four molecular subtypes in multivariate analyses.
| Molecular Subtype | Estimated 5-year incidence | Cox proportional hazards |
Fine & Gray competing risks |
|||
|---|---|---|---|---|---|---|
| *HR (95% CI) | P | *HR (95% CI) | P | |||
| IBE | HR-HER2- | 1.9% | 1.326 (0.608–2.890) | 0.479 | 1.319 (0.604–2.877) | 0.490 |
| HR-HER2+ | 3.7% | 1.945 (1.079–3.506) | 0.027 | 1.940 (1.057–3.430) | 0.032 | |
| HR+HER2+ | 2.3% | 1.140 (0.701–1.852) | 0.598 | 1.140 (0.702–1.852) | 0.670 | |
| HR+HER2- | 1.4% | 1 (referent) | – | 1 (referent) | – | |
| DS mortality | HR-HER2- | 1.1% | 3.410 (0.946–11.300) | 0.061 | 3.470 (0.967–12.500) | 0.056 |
| HR-HER2+ | 0.2% | 1.750 (0.374–8.192) | 0.477 | 1.770 (0.376–8.280) | 0.470 | |
| HR+HER2+ | 0.2% | 1 (referent) | – | 1 (referent) | – | |
| HR+HER2- | 0.5% | 1.440 (0.441–4.698) | 0.546 | 1.440 (0.443–4.710) | 0.540 | |
Abbreviations: *HR, hazard ratio; CI, confidence interval; IBE, ipsilateral breast event; DS, disease specific; HR, hormone receptor; HER2, human epidermal growth factor receptor-2.
Note: Multivariate analyses in Cox proportional hazards regression model and Fine & Gray competing risks regression model were adjusted by year of diagnosis, age, race, tumor size, grade, histology subtype, and radiotherapy.
3.3. Treatment effects on prognosis diverse in molecular subtypes
As RT is universally applied in the treatment of DCIS, we then analysed the RT effects on IBE recurrence and mortality in different molecular subtypes. In each subtype, the clinical features are different between RT and non-RT groups. To balance the clinical features between RT and non-RT groups, we conducted IPSW in each molecular subtype to balance the differences between the two groups.
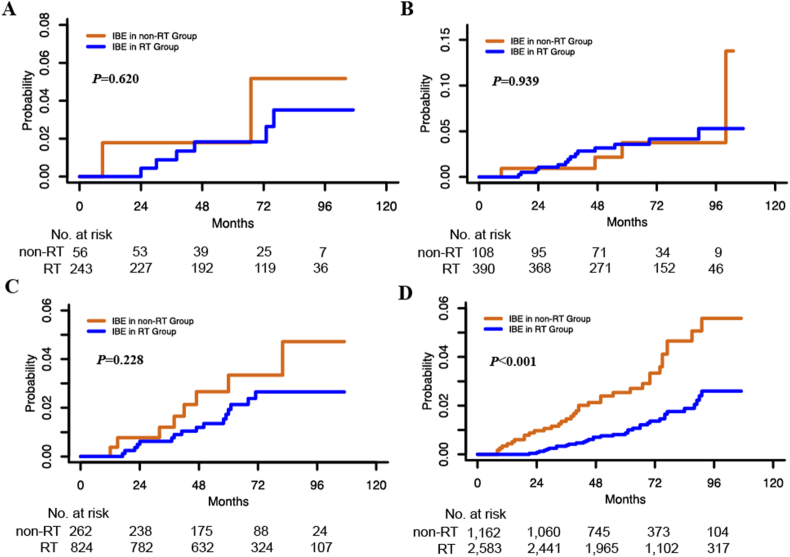
The 5-year IBE incidence is 0.9% in the RT group in HR+HER2- cases, which is significantly lower than that in the non-RT group (2.6%) for this subtype in univariate (P < 0.001, Fig. 3D) and multivariate analyses (P < 0.001, Table 3). Compared with cases without RT, the absolute 5-year IBE incidence is 0.5% lower in HR-HER2+ cases and 1.5% lower in HR+HER2+ cases with RT, whereas the differences are not statistically significant in neither univariate (P > 0.050, Fig. 3B and C) nor multivariate analyses (P > 0.050, Table 3). For DS mortality, there is no significant difference between the RT and non-RT groups for each subtype in neither univariate analyses (P > 0.050) nor multivariate analyses (P > 0.050).
Fig. 3.
Effects of radiotherapy on ipsilateral breast event (IBE) in each molecular subtype. IBE in HR-HER2- (A), HR-HER2+ (B), HR+HER2+ (C), and HR+HER2- (D). (Abbreviations: IBE, ipsilateral breast event; HR, hormone receptor; HER2, human epidermal growth factor receptor-2. Note: P value was calculated by log-rank test.)
Table 3.
Radiotherapy effects on ipsilateral breast event (IBE) in each molecular subtype.
| Molecular Subtype |
Events/No. of cases |
5-year IBE incidence (%) |
Weighted Cox proportional hazards |
Non-weighted Cox proportional hazards |
Fine & Gray competing risks |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| RT | Non-RT | RT | Non-RT | *HR (95% CI) | P | *HR (95% CI) | P | *HR (95% CI) | P | |
| HR-HER2- | 6/243 | 2/56 | 1.9 | 1.8 | 0.768 (0.196–3.027) | 0.708 | 0.548 (0.133–2.264) | 0.406 | 0.642 (0.146–2.820) | 0.560 |
| HR-HER2+ | 14/390 | 4/108 | 3.6 | 4.1 | 1.131 (0.304–4.202) | 0.855 | 0.903 (0.298–2.740) | 0.858 | 0.954 (0.321–2.840) | 0.930 |
| HR+HER2+ | 16/824 | 8/262 | 2.0 | 3.5 | 0.530 (0.210–1.342) | 0.181 | 0.597 (0.233–1.527) | 0.282 | 0.616 (0.242–1.570) | 0.310 |
| HR+HER2- | 35/2,583 | 36/1,162 | 0.9 | 2.6 | 0.334 (0.207–0.539) | <0.001 | 0.346 (0.213–0.561) | <0.001 | 0.355 (0.219–0.577) | <0.001 |
Abbreviations: RT, radiotherapy; HR, hazard ratio; CI, confidence interval; IBE, ipsilateral breast event; HR, hormone receptor; HER2, human epidermal growth factor receptor-2.
*Note: In weighted Cox proportional hazards regression model, cases were weighted by inverse propensity score. These three multivariate regression models were adjusted by year of diagnosis, age, race, tumor size, grade, and histology subtype.
After prognostic score stratification in each subtype, high-risk cases have a higher 5-year IBE incidence than low-risk cases in both RT and non-RT groups. In high-risk cases, RT reduces the absolute 5-year IBE incidence by 1.3%, 0.7%, 1.9%, and 2.6% in HR-HER2-, HR-HER2+, HR+HER2+, and HR+HER2- cases, respectively. In multivariate regression models, the IBE incidence reduction by RT is statistically significant in low-risk and high-risk HR+HER2- cases (P < 0.050).
4. Discussion
In this study, we analysed the differences in clinical outcomes between four molecular subtypes in primary DCIS. Each DCIS molecular subtype has unique clinical features. There are more high-risk cases in the HR-HER2+ subtype, but fewer in the HR+HER2- subtype. HR-HER2+ cases also have a significantly higher probability of IBE recurrence than HR+HER2- cases.
The different gene expression profiles between DCIS and IBC reflect the need to classify DCIS by its own features [23,24]. However, attempts on classify DCIS by genomic signatures still need more studies for verification [25]. Although IHC-based subtyping of DCIS is derived from IBC, this kind of subtyping also provides insights into the nature of DCIS.
From this study cohort, the HR-/+ HER2+ cases account for 28.1% of all enrolled DCIS cases, which is much higher than that in IBCs (15%–20%) [26]. In NSABP B-43, 33.6% of DCIS cases have HER2 positive expression [27]. Visser et al. [14] also reported that 36% of primary HER2+ DCIS cases developed an HER2- IBC. In our analyses (Supplementary Table 1), 7 of 31 (22.6%) HER2+ cases with invasive IBE recurrence have subsequent HER2- IBC. However, only 3 of 55 (5.5%) HER2- DCIS with invasive IBE recurrence have subsequent HER2+ IBC. The different genome evolution from DCIS to IBC may cause this phenomenon [28]. The commonly expressed HER2 protein in DCIS was reported to be related to higher grade and comedo necrosis [29], as well as a higher possibility of recurrence [30]. In our study, more HER2+ cases (69.5%) have a higher grade than HER2- cases (31.1%, P < 0.001). After stratification by HR status in HER2+ cases, HR-HER2+ cases display more aggressive behavior than HR+HER2+ cases. HR-HER2+ cases have more comedo necrosis, larger tumor size, and higher grade than HR+HER2+ cases. After prognostic score stratification, there is a higher proportion of the high-risk group in HR-HER2+ cases (65.7%) than HR+HER2+ cases (50.6%, P < 0.001). A similar phenomenon can also be found in IBCs, in which HR-HER2+ IBCs have more aggressive features than HR+HER2+ IBCs [31]. In our prognosis analyses, HR-HER2+ cases do not show a significant difference from HR+HER2+ cases on IBE recurrence (P = 0.087, Fig. 2A). However, when comparing IBE recurrence with HR+HER2- cases, only HR-HER2+ cases have a statistically higher IBE recurrence (P = 0.010), and HR+HER2+ cases do not show a higher risk of recurrence (P = 0.508, Fig. 2A). In further analyses, HR-HER2+ cases also show a relatively high invasive IBE recurrence compared with HR+HER2- cases (P = 0.009). The in situ IBE incidence is not different between HR-HER2+ and HR+HER2+ cases (P = 0.636). This implies that the HR-HER2+ subtype could predict a higher risk of invasive IBE recurrence, especially invasive IBE recurrence, in primary DCIS.
Triple-negative (HR-HER2-) IBC does not respond to hormone therapy and HER2-targeted therapy; and it has a worse prognosis than other IBC molecular subtypes [32]. However, HR-HER2- DCIS does not have the worst prognosis among these four molecular subtypes. It was reported to have less aggressive features than HR-HER2+ DCIS [33]. In our study, HR-HER2- cases have a higher grade and more comedo necrosis than HR+HER2+ cases (Table 1). However, they have a lower grade than HR-HER2+ cases (Table 1). For prognosis, HR-HER2- cases do not show higher IBE recurrence than the other three DCIS molecular subtypes. Regarding DS mortality, HR-HER2- cases have significantly higher DS mortality than HR+HER2+ cases in univariate analysis (P = 0.021, Fig. 2B). In multivariate analysis, HR-HER2- cases do not show a difference from HR+HER2+ cases in terms of DS mortality (P > 0.050, Table 2). In 299 HR-HER2- cases, 6 cases have invasive IBE recurrence. 4 of 6 (66.7%) invasive IBEs are HR-HER2-. Some studies have reported that HR-HER2- IBCs often lack their DCIS precursors [34] and have a lower prevalence of adjacent DCIS than other molecular subtypes [35]. Bergholtz et al. also reported genetic contrast between HR-HER2- DCIS cases and HR-HER2- IBCs, indicating that the HR-HER2- DCIS cases may not be precursors of HR-HER2- IBCs [13]. All these results imply that HR-HER2- DCIS cases have distinct features from the others in the same molecular subtype in IBCs.
In this study, the majority (66.5%) of DCIS cases are HR+HER2-. This molecular subtype is also the major subtype in IBC [11]. The HR + HER2- DCIS cases have a smaller tumor size, lower grade, less comedo necrosis, and lower RT acceptance than the other three subtypes (Table 1). When stratifying HR+HER2- DCIS cases by prognostic score, only 27.9% of HR+HER2- cases are high risk, which is the lowest among the four subtypes. These less aggressive features lead to indolent biological behaviors of HR+HER2- DCIS cases. The cumulative incidence curves also show relatively low IBE recurrence in the HR+HER2- subtype (Fig. 2A). During the follow-up period, 56 HR+HER2- DCIS cases are recorded to have invasive IBE recurrence. 49 invasive IBEs have records of HR and HER2 statuses, 42 of which (85.7%) have the same subtype as primary HR+HER2- DCIS cases (Supplementary Table 1).
In our previous study, we stratified primary DCIS cases by prognostic score and found that RT could reduce IBE incidence [18]. In general, the high-risk group has a significantly higher IBE incidence than the low-risk group [18]. Regarding the distinction of each DCIS molecular subtype, we analysed the RT effects on IBE recurrence and DS mortality in each molecular subtype. In HR+HER2- cases, RT significantly lower the probability of IBE recurrence (P < 0.001, Fig. 3D and Table 3). Both invasive (P = 0.061) and in situ (P < 0.001) IBE incidence is reduced after RT in HR+HER2- cases. In HR-HER2+ and HR+HER2+ cases, the absolute 5-year IBE incidence is reduced in the RT group without statistical significance. By prognostic score stratification, the RT group also shows a lower 5-year IBE incidence in high-risk cases for each subtype. This result suggested that RT still has roles in risk reduction in each molecular subtype, especially in high-risk DCIS cases.
As molecular subtypes identify relevant biology, some studies have explored new treatment strategies for different DCIS molecular subtypes. Combining adjuvant tamoxifen therapy with RT can significantly reduce the risk of IBE recurrence for DCIS cases in NSABP B-24 [36]. NSABP B-35 shows superior disease-free survival provided by anastrozole compared with tamoxifen therapy in HR+ DCIS cases, especially in patients younger than 60 years old [37]. The B-35 study also finds that endocrine therapy does not reduce the IBE incidence. However, endocrine therapy significantly reduces the risks of contralateral breast events (CBEs) [37]. Because of the extremely low probability of CBE over a long follow-up period and the potential adverse effects of endocrine therapy, few HR+ DCIS cases received endocrine therapy, even under recommendation [38]. For HER2+ DCIS cases with aggressive features, HER2 targeted therapy significantly reduced mortality in HR-HER2+ DCIS cases [39]. However, in NSABP B-43, trastuzumab plus RT treatment only achieves a statistically insignificant IBE reduction of 19% [20]. The controversies about HER2 targeted therapy in DCIS cases still exist. More trials aiming to improve disease-free survival and quality of life for DCIS cases are ongoing to reach an ideal treatment milestone.
In summary, we found that DCIS cases have diverse clinical and prognostic features for different molecular subtypes. More treatment strategies aimed at unique DCIS molecular subtypes may help to reach a more satisfactory outcome.
Funding
This study was supported by Post-Doctor Research Project, West China Hospital, Sichuan University (2021HXBH024), the 1.3.5 Project for disciplines of excellence (ZYGD18012), and Postdoctoral Science Foundation of China (2021M692267).
Declaration of competing interest
The authors declare that they have disclosed no potential conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.03.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sorlie T., Tibshirani R., Parker J., et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perou C.M., Sorlie T., Eisen M.B., et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T., Perou C.M., Tibshirani R., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker J.S., Mullins M., Cheang M.C., et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia S.K., Bramwell V.H., Tu D., et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18(16):4465–4472. doi: 10.1158/1078-0432.CCR-12-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis-Filho J.S., Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378(9805):1812–1823. doi: 10.1016/S0140-6736(11)61539-0. [DOI] [PubMed] [Google Scholar]
- 7.Goldhirsch A., Winer E.P., Coates A.S., et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugras S., Stempel M., Patil S., et al. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol. 2014;21(12):3780–3786. doi: 10.1245/s10434-014-3851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitale A., Mazzola P., Soldini D., et al. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009;20(4):628–635. doi: 10.1093/annonc/mdn675. [DOI] [PubMed] [Google Scholar]
- 10.Pinder S.E., Ellis I.O. The diagnosis and management of pre-invasive breast disease: ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)--current definitions and classification. Breast Cancer Res. 2003;5(5):254–257. doi: 10.1186/bcr623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamimi R.M., Baer H.J., Marotti J., et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 2008;10(4):R67. doi: 10.1186/bcr2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y.J., Kim J.S., Kim I.A. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol. 2018;144(9):1803–1816. doi: 10.1007/s00432-018-2697-2. [DOI] [PubMed] [Google Scholar]
- 13.Bergholtz H., Lien T.G., Swanson D.M., et al. Contrasting DCIS and invasive breast cancer by subtype suggests basal-like DCIS as distinct lesions. NPJ Breast Cancer. 2020;6:26. doi: 10.1038/s41523-020-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visser L.L., Elshof L.E., Van de Vijver K., et al. Discordant marker expression between invasive breast carcinoma and corresponding synchronous and preceding DCIS. Am J Surg Pathol. 2019;43(11):1574–1582. doi: 10.1097/PAS.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 15.Pareja F., Brown D.N., Lee J.Y., et al. Whole-exome sequencing analysis of the progression from non-low-grade ductal carcinoma in situ to invasive ductal carcinoma. Clin Cancer Res. 2020;26(14):3682–3693. doi: 10.1158/1078-0432.CCR-19-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowell C.F., Weigelt B., Sakr R.A., et al. Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol Oncol. 2013;7(5):859–869. doi: 10.1016/j.molonc.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narod S.A., Iqbal J., Giannakeas V., et al. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1(7):888–896. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 18.Yang L., Lu D., Lai Y., et al. Prognostic score-based stratification analysis reveals universal benefits of radiotherapy on lowering the risk of ipsilateral breast event for ductal carcinoma in situ patients with different risk levels. Ann Surg Oncol. 2021;28(2):975–984. doi: 10.1245/s10434-020-09003-6. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B., Dignam J., Wolmark N., et al. Tamoxifen in treatment of intraductal breast cancer: national Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 20.Cobleigh M.A., Anderson S.J., Siziopikou K.P., et al. Primary results of NRG Oncology/NSABP B-43: phase III trial comparing concurrent trastuzumab (T) and radiation therapy (RT) with RT alone for women with HER2-positive ductal carcinoma in situ (DCIS) after lumpectomy. J Clin Oncol. 2020;38(15_suppl):508. [Google Scholar]
- 21.National Cancer Institute: surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/.
- 22.Sagara Y., Freedman R.A., Vaz-Luis I., et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol. 2016;34(11):1190–1196. doi: 10.1200/JCO.2015.65.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kristensen V.N., Vaske C.J., Ursini-Siegel J., et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A. 2012;109(8):2802–2807. doi: 10.1073/pnas.1108781108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesurf R., Aure M.R., Mork H.H., et al. Molecular features of subtype-specific progression from ductal carcinoma in situ to invasive breast cancer. Cell Rep. 2016;16(4):1166–1179. doi: 10.1016/j.celrep.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Strand S.H., Rivero-Gutiérrez B., Houlahan K.E., et al. DCIS genomic signatures define biology and correlate with clinical outcome: a Human Tumor Atlas Network (HTAN) analysis of TBCRC 038 and RAHBT cohorts. bioRxiv. 2021 doi: 10.1101/2021.06.16.448585. [DOI] [Google Scholar]
- 26.Wolff A.C., Hammond M.E., Hicks D.G., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 27.Siziopikou K.P., Anderson S.J., Cobleigh M.A., et al. Preliminary results of centralized HER2 testing in ductal carcinoma in situ (DCIS): NSABP B-43. Breast Cancer Res Treat. 2013;142(2):415–421. doi: 10.1007/s10549-013-2755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casasent A.K., Edgerton M., Navin N.E. Genome evolution in ductal carcinoma in situ: invasion of the clones. J Pathol. 2017;241(2):208–218. doi: 10.1002/path.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Bockstal M., Lambein K., Denys H., et al. Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Arch. 2014;465(3):275–289. doi: 10.1007/s00428-014-1609-3. [DOI] [PubMed] [Google Scholar]
- 30.Borgquist S., Zhou W., Jirstrom K., et al. The prognostic role of HER2 expression in ductal breast carcinoma in situ (DCIS); a population-based cohort study. BMC Cancer. 2015;15:468. doi: 10.1186/s12885-015-1479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H.J., Park I.A., Park S.Y., et al. Two histopathologically different diseases: hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer. Breast Cancer Res Treat. 2014;145(3):615–623. doi: 10.1007/s10549-014-2983-x. [DOI] [PubMed] [Google Scholar]
- 32.Bauer K.R., Brown M., Cress R.D., et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S., Thike A.A., Koh V.C.Y., et al. Triple-negative and HER2 positive ductal carcinoma in situ of the breast: characteristics, behavior, and biomarker profile. Virchows Arch. 2018;473(3):275–283. doi: 10.1007/s00428-018-2416-z. [DOI] [PubMed] [Google Scholar]
- 34.Kurbel S. In search of triple-negative DCIS: tumor-type dependent model of breast cancer progression from DCIS to the invasive cancer. Tumour Biol. 2013;34(1):1–7. doi: 10.1007/s13277-012-0602-1. [DOI] [PubMed] [Google Scholar]
- 35.Doebar S.C., van den Broek E.C., Koppert L.B., et al. Extent of ductal carcinoma in situ according to breast cancer subtypes: a population-based cohort study. Breast Cancer Res Treat. 2016;158(1):179–187. doi: 10.1007/s10549-016-3862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allred D.C., Anderson S.J., Paik S., et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30(12):1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganz P.A., Cecchini R.S., Julian T.B., et al. Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387(10021):857–865. doi: 10.1016/S0140-6736(15)01169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow M. Refining the use of endocrine therapy for ductal carcinoma in situ. J Clin Oncol. 2012;30(12):1249–1251. doi: 10.1200/JCO.2011.40.5514. [DOI] [PubMed] [Google Scholar]
- 39.Lewis G.D., Haque W., Farach A., et al. Vol. 79. Cancer Res; San Antonio, TX: 2018. The impact of HER2-directed targeted therapy on HER2-positive DCIS of the breast [abstract] (Proceedings of the 2018 San Antonio breast cancer Symposium). 4 Suppl. Abstract nr P3-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.