Highlights
-
•
Tea polysaccharides (TPS) and tea polyphenols (TPP) had synergic effect on colitis.
-
•
TPP + TPS was more effective on alleviating symptom severity.
-
•
TPP + TPS showed greater effects on promoting intestinal barrier function.
-
•
TPP + TPS increased the relative abundance of Lactobacillaceae and Lactobacillus.
Keywords: Tea polysaccharides, Tea polyphenols, Colitis, Intestinal barrier, Gut microbiota
Abstract
Both tea polysaccharides (TPS) and tea polyphenols (TPP) are promising in the treatment of inflammatory bowel disease (IBD). However, the effects of their combination against IBD are still unknown. In the present study, the therapeutic effects of TPS, TPP and TPS + TPP on dextran sodium sulfate-induced colitis in mice were investigated. Our results showed that administration of TPS + TPP achieved the best effects, followed by TPP and TPS, which were evidenced by the restoration of various physical signs (body weight, colon length and disease activity index) and the promoted intestinal barrier function (colon damage, mucin secretion and tight junction proteins expression). Furthermore, TPP and TPS decreased the relative abundance of Proteobacteria and Enterobacteriaceae, while TPP + TPS increased that of Lactobacillaceae and Lactobacillus. In conclusion, TPS together with TPP had greater effects on alleviating colitis and promoting intestinal barrier function. This result is interesting when developing functional foods against colitis.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a pathological condition of the gastrointestinal tract characterized by chronic and relapsing inflammation (Sugihara, Morhardt, & Kamada, 2019). It is widely prevalent in Western countries (Kaplan, 2015). The pathogenesis of IBD has not yet been fully elucidated, however, interactions between environmental and genetic factors, as well as intestinal barrier disruption and gut dysbiosis, are involved in the pathogenesis and development of IBD (Geremia et al., 2014, Zhang and Li, 2014). Current therapies for IBD mainly include the use of immunosuppressive drugs (e.g. mesalazine), biological agents (e.g. infliximab) and antibiotics (e.g. ciprofloxacin). Meanwhile, dietary intervention and microbial community-targeted intervention strategies are under development (Baumgart & Le Berre, 2021).
Intact and tightly connected epithelial cells form the intestinal barrier, the first line of host defense against exogenous invasions. The expression of tight junction (TJ) protein is changed in IBD patients, meanwhile, the disruption of intestinal barrier can lead to the deterioration of IBD (Chelakkot et al., 2018, Holmberg et al., 2018). The use of polysaccharides and polyphenol has been shown to improve intestinal TJ integrity (Fan et al., 2020, Shigeshiro et al., 2013), and their microbial end-products in the gut, i.e. short-chain fatty acids (SCFAs), can provide energy for colonic epithelial cells and contribute to IBD treatment (Catalkaya et al., 2020, Cui et al., 2021, Yajun et al., 2021).
Accumulated studies have revealed that gut dysbiosis is an important pathogenetic factor for IBD. Clinical and experimental data confirmed the change of gut microbiota composition in patients and animals with IBD (Nishida, Inoue, Inatomi, Bamba, Naito, & Andoh, 2018). Expansion of inflammation-related bacteria, such as Enterobacteriaceae, has been observed in IBD patients (Celiberto et al., 2018). Furthermore, microbial diversity of IBD patients was substantially reduced when compared to healthy individuals (Morgan et al., 2012). Considering the critical role of intestinal microbiome in IBD, gut microbiota is thus considered as a new territory for drug targeting in this field.
Current therapies for IBD could produce side effects such as diarrhea, abdominal pain, headache, nasopharyngitis and immune damage (Park, Ji, & Sung, 2012). Therefore, alternatives with fewer side effects are needed (Fan et al., 2020, Shigeshiro et al., 2013). As a beverage, green tea is consumed widely, especially in East Asia, with polysaccharides and polyphenol as the major bioactive components. The two bioactive compounds both have anti-inflammatory properties. Green tea polysaccharide has been shown to inhibit the progression of colitis-associated cancer (Liu et al., 2018), while green tea polyphenol and epigallocatechin gallate (EGCG) could decrease colon injury and histological scores for IBD (Oz, Chen, & de Villiers, 2013). Moreover, their combination has been shown to increase the production of SCFAs in the gut and enhance the anti-inflammatory properties (Xu et al., 2015, Zheng et al., 2017). Therefore, we hypothesize that tea polyphenol (TPP) in combined with tea polysaccharide (TPS) could possess a better protective effect on colitis than the individual compound. In the current study, we aimed to investigate the effects of TPP, TPS and their combination in colitis mice induced by DSS treatment, with focus on symptom severity, gut barrier integrity and gut microbiota.
Materials and methods
Materials
TPS was obtained from the China-Canada Joint Laboratory of Food Science and Technology, Nanchang University. TPP was commercial product from Wuyuan Hongda Tea Co., Ltd. (Jiangxi, China) and the composition was given in Table S1. DSS (Mw 40–50 kDa) was from MP Biomedicals (Santa Ana, California). AIN93G diet was customized from FBSH Biotechnology Co., Ltd (Shanghai, China). Anti-occludin and anti-claudin-1 antibodies were given by Abcam (Shanghai, China). All other reagents were of analytical grade.
Chemical composition of TPS
Neutral sugar was quantified at 490 nm according to a previous method (Dubois, Giles, Rebers, & Smith, 1955). Uronic acid was determined based on the sulfuric acid-carbazole method at 530 nm (Radhakrishnamurthy & Berenson, 1963). Protein was colorimetrically analyzed with Coomassie brilliant blue G-250 method at 590 nm, using bovine serum albumin adopted as a standard (Bradford, 1976). Monosaccharide compositions were analyzed by high-performance anion exchange chromatography (Zhang, Khan, Nunez, Chess, & Szabo, 2012).
Animal experimental design
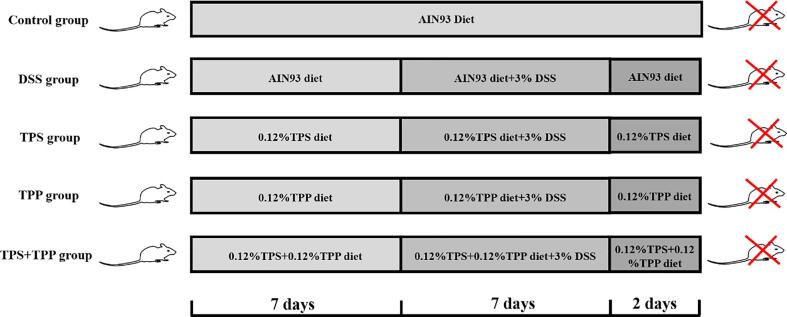
The current study was complied with the Guidelines for Experimental Animal Welfare and the protocol was approved by the Institutional Animal Care and Use Committee of Nanchang University [SYXK (Gan) 2015-0001]. Six-week-old C57BL/6J male mice (22 ± 2.0 g) were housed separately in cages with a constant temperature of 22 ± 2 °C and relative humidity of 50 ± 5% in a 12 h light/dark cycle. Since only male gender was included in the experiment, applications of the results may be limited. After one week acclimation, mice were randomly divided into 5 groups (10 mice per group), including the Control, DSS, DSS + TPS (TPS treatment), DSS + TPP (TPP treatment), and DSS + TPS + TPP groups (TPS + TPP treatment). The Control and DSS groups received AIN93 diet, while other groups were given customized feed containing 0.12% TPS, 0.12% TPP or both based on AIN93 diet formula, respectively, during the 16-day experiment (Kim et al., 2018) (Table S2). All groups were treated with 3% DSS from day 7 till day 14, except the Control group. Experimental design was shown in Fig. 1. On the last day, mice were sacrificed by cervical dislocation, colonic content was collected and immediately store at −80 °C temperature refrigerator for later analysis, and a portion of colon tissue was reserved in 4% paraformaldehyde for histological examination.
Fig. 1.
Animal study design.
Assessment of disease activity index (DAI)
Scores of stool consistency (0, normal; 2, soft but firm; and 4, diarrhea), fecal blood (0, none; 2, blood; and 4, abundant bleeding) and body weight loss (0, weight loss < 1%; 1, weight loss = 1–5% ; 2, weight loss = 5–10%; 3, weight loss = 10–20% loss; 4, weight loss>20%) of mice were daily measured from day 7 till the end of the experiment. DAI score for each animal was calculated with formula (1):
| (1) |
H&E staining and histological damage score
Formalin-fixed colon tissue was embedded in paraffin with a section thickness of 3 μm, and stained with hematoxylin and eosin (H&E). Colon tissue sections were observed under a microscope (Nikon Eclipse E100, Japan) and the images were recorded by an Imaging system (Nikon DS-U3, Japan). Histopathological score was determined according to a previously published method (Das, Batra, & Rachagani, 2017). Three parameters used for scoring were listed and graded as follows: crypt structure (0 = normal, 3 = severe crypt damage), inflammation (0 = normal, 3 = large amount of inflammatory infiltrate), muscle thickening (0 = bottom of crypt located in the muscularis mucosae, 3 = significant muscle thickening present). Histological damage score for each animal was calculated with the formula (2):
| (2) |
Measurement of mucin area
Mucin in colon tissues was stained by Alcian blue-Periodic acid Schiff (AB-PAS). Mucin area was then measured with Image Pro Plus software 6.0 (Media Cybernetics, Rockville, MD, USA) under biological microscopes (200 × magnification). Percentage of mucin area in total tissue area were calculated with the formula (3):
| (3) |
Immunofluorescence
Paraffin-embedded tissue was sectioned, treated with xylene and then rehydrated. Afterward, the colon sections were incubated overnight with anti-occludin and anti-claudin-1 at 4 ℃ followed by the incubation with goat anti-rabbit Cy3. Finally, the sections were counterstained with DAPI, all images were acquired by ortho-fluorescent microscopy (Nikon Eclipse Ti-SR, Nikon, Japan), and the mean fluorescence intensity was quantified using Image J ((NIH Image, Bethesda, MD, USA).
Gut microbiota analysis.
Total microbial DNA was isolated from colonic contents using TIANamp Stool DNA kit (Tiangen, Co., Ltd, China.). V4 regions of 16S rRNA gene were targeted for amplification by PCR using primers 515F 5′-barcode-GTGCCAGCMGCCGCGGTAA)-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-barcode-3′. Successful amplifications were confirmed by 2% agarose gels. PCR products were then purified with Common Agarose Gel DNA Recovery Kit (Tiangen, Co., Ltd, China). The purified products were then quantified by Qubit 3.0 (Life Invitrogen). DNA library was prepared according to Illumina‘s recommendation. The Agilent 2100 Bioanalyzer System (High Sensitive DNA Chip) was used to exam the quality of the library. Finally, the obtained DNA fragments were paired-end sequenced using MiSeq 500-cycle v2 kit on Illumina MiSeq platform (Illumina Inc, San Diego, CA, USA).
Microbial data were analyzed by Quantitative Insights into Microbial Ecology 2 (QIIME2) software pipeline. QIIME2-implemented dada2 plugin was used for quality control, filtering, trimming, merging paired reads and removing chimeras, and to obtain the characteristic table of the amplified sequence variant. Greengene database 13.5 for V4 region (515F/806R primers) of 16S rRNA gene was used as reference for taxonomy assignment. Principal component analysis (PCA) of microbiota data at family levels was performed by using the online tools (https://www.genescloud.cn). Discriminant microbial features between the study groups were determined with the use of linear discriminant analysis (LDA) effect size (LEfSE) on the Galaxy platform (http://huttenhower.sph.harvard.edu/galaxy/).
Statistical analysis
The data were analyzed by using IBM SPSS Statistics 23 software with one-way analysis of variance (ANOVA) followed by Tukey’s test. The normal distribution of data was confirmed by the Shapiro-Wilk test, and the results were expressed as the mean ± SEM or means ± SD.
Results
Chemical characteristics of tea polysaccharides
The chemical characteristics of TPS were shown in Table 1. The content of carbohydrate and protein in TPS was 90.47 ± 0.36% and 2.33 ± 0.20%, respectively, suggesting that carbohydrate was the dominant component in TPS. Meanwhile, the high uronic acid content (40.58 ± 0.89%) of TPS indicated that TPS was an acid polysaccharide. Monosaccharides in TPS were rhamnose, arabinose, galactose, glucose, ribose and galacturonic acid with the ratio of 2.15:6.64:10.29:6.70:2.41:20.74.
Table 1.
Chemical characteristics of TPS.
| Carbohydrate (%) | Uronic acid (%) | Protein (%) | Monosaccharide composition |
|---|---|---|---|
| 90.47 ± 0.36 | 40.58 ± 0.89 | 2.33 ± 0.20 | Rha:Ara:Gal:Glc:Rib:GalA = 2.15:6.64:10.29:6.70:2.41:20.74 |
Data are presented as mean ± SD (n = 3); Rha, rhamnose; Ara, arabinose; Gal, galactose; Glc, glucose; Rib, ribose; GalA, galacturonic acid.
Effect of TPS, TPP and TPS + TPP on body weight change and DAI in mice treated with DSS
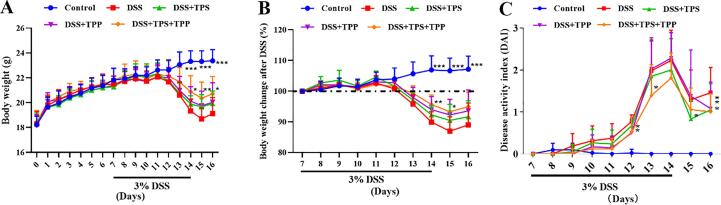
As shown in Fig. 2A and B, the body weight of colitis mice showed a decrease after 5-day of DSS treatment. Compared with the DSS group, the body weight of mice in the DSS + TPS + TPP group was increased significantly in the 15th day (p < 0.01), with no effect found for the TPS and TPP treatments (Fig. 2A). The DAI score is used to assess the severity of inflammation in mice with colitis. As shown in Fig. 2C, the DAI score of the DSS + TPS + TPP group was significantly lower than that of the DSS group in the 15th-16th days (p < 0.05). By the end of the experiment, the DAI score of mice in the TPS, TPP and TPS + TPP groups were lower than those of mice in the DSS group (p < 0.05).
Fig. 2.
Body weight and DAI of mice. (A) Body weight; (B) Body weight change after DSS; (C) Disease activity index (DAI) (n = 10). Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 were compared with the DSS group.
Effect of TPS, TPP and TPS + TPP on the colon length, histological changes and mucins expression in mice treated with DSS
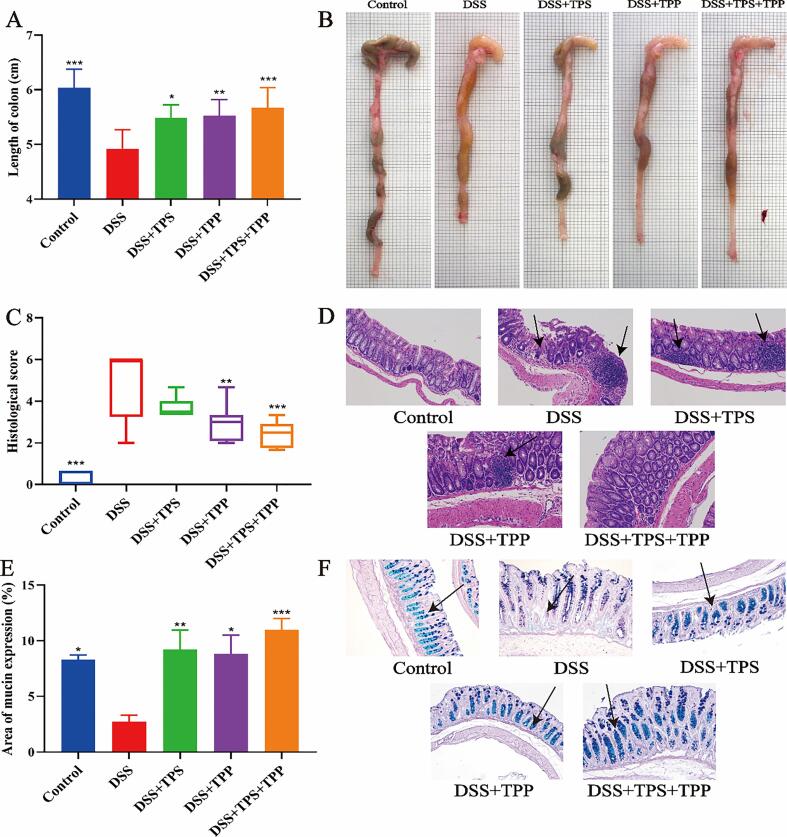
Colon length in the DSS group was decreased compared with the Control group (Fig. 3A). However, the average colon length of mice in the DSS + TPS + TPP (p < 0.001), DSS + TPP (p < 0.01) and DSS + TPS (p < 0.05) groups was significantly increased than that in the DSS group. Our results indicated that TPS, TPP and TPS + TPP treatments could improve the colon length in DSS-induced colitis in mice and TPS + TPP treatment had the best relief effect.
Fig. 3.
Pathological indicators. (A) Colon length; (B) Colon image; (C) Histological scores; (D) H&E staining of the colon tissues; (E) Area of mucus expression; (F) AB-PAS staining of the colon tissues (n = 8). Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 were compared with the DSS group.
H&E staining of the colonic segments from the DSS group showed severe colonic injuries, evidenced by severe colon ulcers, infiltration of inflammatory cells and damage of crypts (Fig. 4D). Compared with the DSS group, the DSS + TPP (p < 0.01) and DSS + TPS + TPP (p < 0.001) groups could significantly alleviate tissue damage (Fig. 4C). No difference was found between the DSS + TPS and the DSS groups. As indicated in Fig. 4F, the Control group revealed a high expression of mucin in the colon. On the contrary, in the DSS group, the expression of mucin was significantly lower compared with the Control group. Interestingly, all the treatments i.e. TPS (p < 0.01), TPP (p < 0.05) and TPS + TPP (p < 0.001), could significantly improve the production of mucin to different extend in the colon tissue of mice.
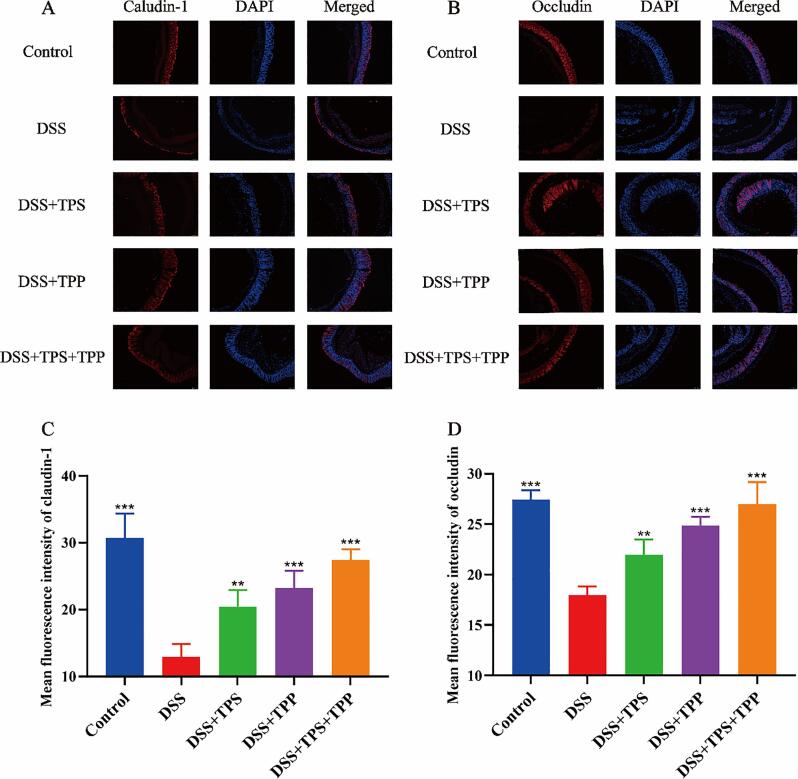
Fig. 4.
Immunofluorescence analysis for tight junction proteins. (A) Claudin-1 (red); (B) Occludin (red); (C) Mean fluorescence intensity of Claudin-1; (D) Mean fluorescence intensity of occludin; Nuclei were stained with DAPI (blue). Fluorescence images were captured by ortho-fluorescent microscopy (n = 5). Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 were compared with the DSS group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Effect of TPS, TPP and TPS + TPP on tight junction proteins in mice treated with DSS.
To ascertain whether or not TPS, TPP and TPS + TPP treatments can protect the integrity of intestinal barrier, we applied immunofluorescence to observe the expression of occludin and claudin-1. Ortho-fluorescent microscopic results of the colon in the control group revealed claudin-1 (Fig. 4A) and occludin (Fig. 4B) formed a continuous line in the epithelial cells. In the DSS group, the expressions of claudin-1 and occludin were reduced and could not constitute a continuous line, and there was only a weak and scattered fluorescence of claudin-1 and occludin in the colonic epithelial layer. The repair of claudin-1 (Fig. 4C) and occludin (Fig. 4D) in colonic epithelial cells was significantly improved after TPS (p < 0.01), TPP (p < 0.001) and TPS + TPP (p < 0.001) supplementation. In addition, the expression of claudin-1 and occludin in the DSS + TPS + TPP (p < 0.001) group was higher than that in the DSS + TPP (p < 0.001) and DSS + TPS (p < 0.01) group, indicating a better mucosal barrier repair.
Effect of TPS, TPP and TPS + TPP on gut microbiota in mice treated with DSS.
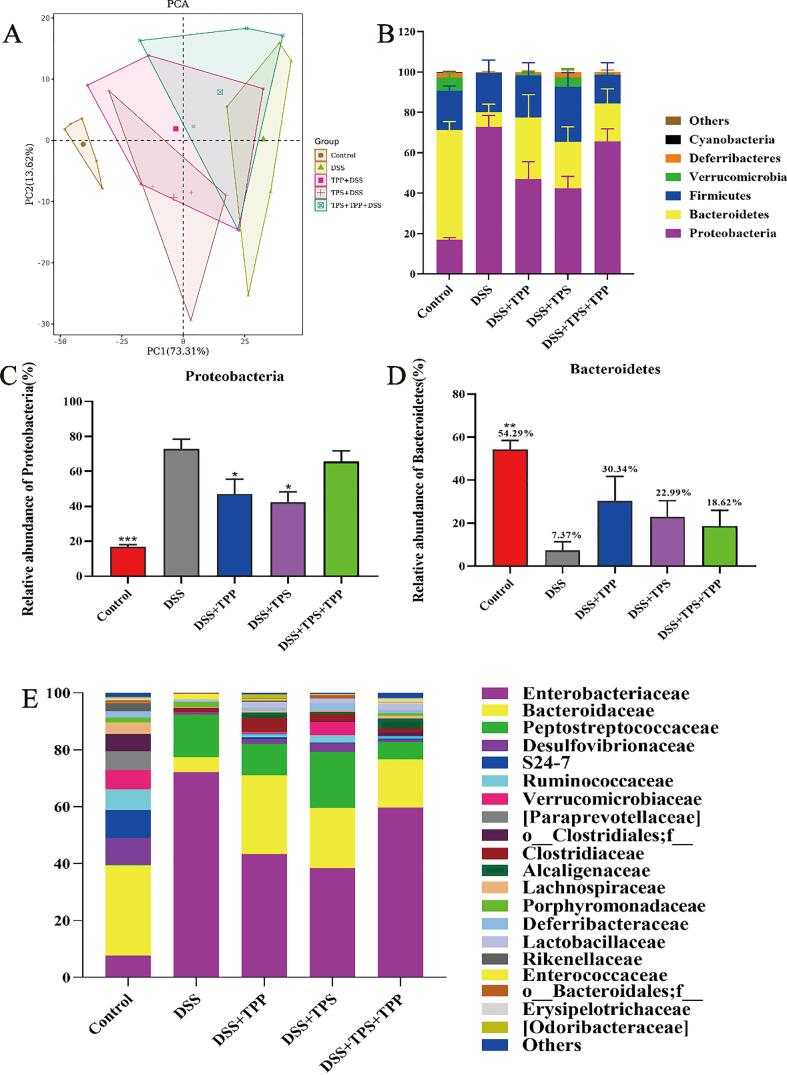
PCA was used to determine the phylogenetic similarity and difference within gut microbiota. As shown in Fig. 5A, there was an evident distance between the Control group and DSS group, and TPS, TPP and TPS + TPP administration can reverse this change and make the gut microbiota profile in colitis mice shift to the one in the normal mice from the first principal component (PC1), and the best effects was seen in the DSS + TPP group.
Fig. 5.
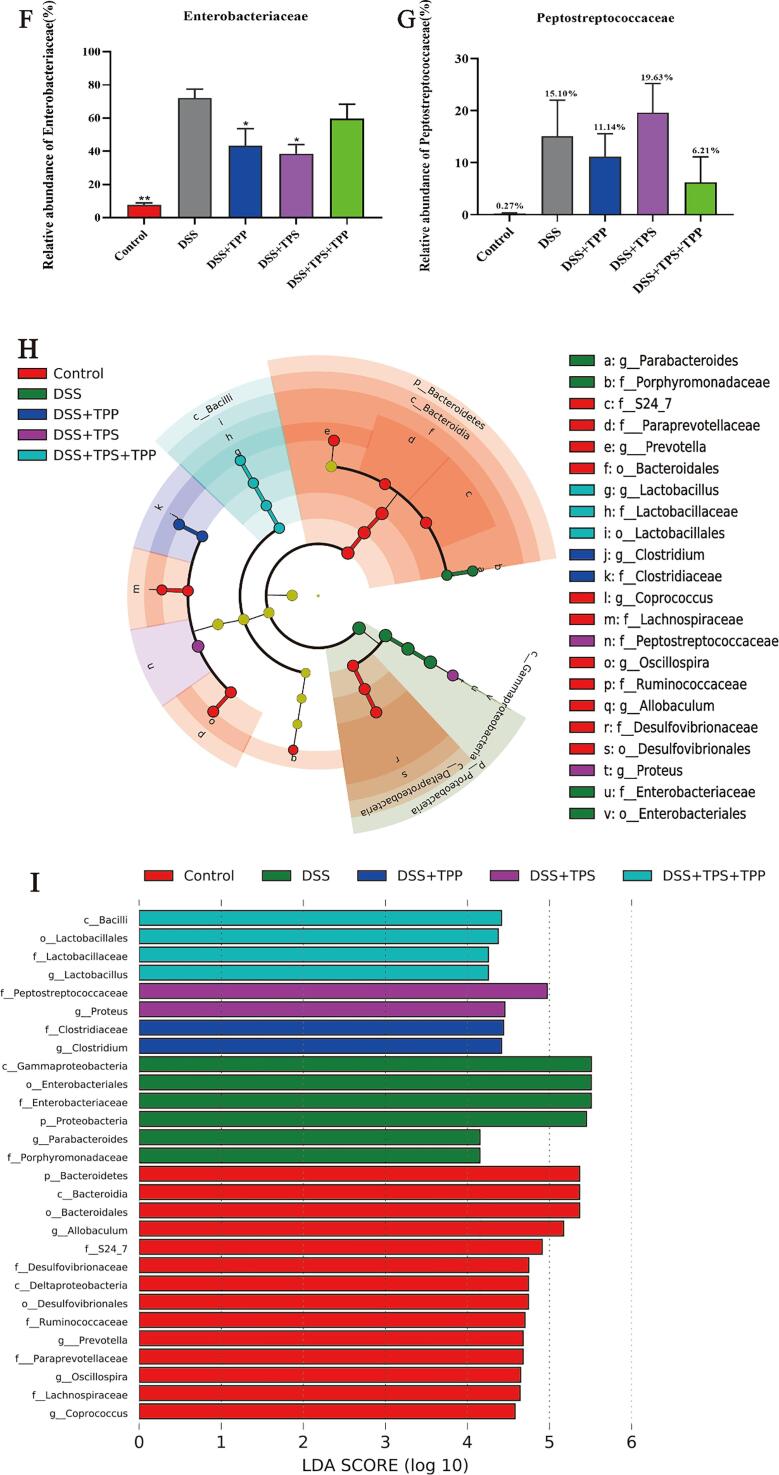
Beta diversity, composition and LEfSe of gut microbiota in different groups. (A) PCA analysis; (B) Gut microbial compositions at phylum level; (C) Relative abundances of Proteobacteria; (D) Relative abundances of Bacteroidetes; (E) Gut microbial compositions at family level; (F) Relative abundances of Enterobacteriaceae; (G) Relative abundances of Peptostreptococcaceae; (H) Taxonomic cladogram generated from default LEfSe analysis; (I) LDA scores of the differentially abundant taxa (with a LDA score of > 2 and a significance of α < 0.1) (n = 5). Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 were compared with the DSS group.
In order to study the further regulatory effects of TPS, TPP and TPS + TPP treatments, we evaluated bacterial composition in DSS-induced colitis mice. The relative abundance of Bacteroidetes was decreased while that of Proteobacteria was increased in the DSS group compared to the Control group (Fig. 5B). However, TPS, TPP and TPS + TPP treatments reversed the dysbiosis caused by DSS intake. TPS and TPP treatments could decrease the relative abundances of Proteobacteria significantly (p < 0.05), while TPS + TPP treatment had no such effect when compared with DSS treatment (Fig. 5C). The relative abundance of Bacteroidetes was increased by TPS, TPP and TPS + TPP treatments (DSS 7.37% vs. DSS + TPP 30.34% vs. DSS + TPS 22.99% vs. DSS + TPS + TPP 18.62%) (Fig. 5D). At the family level, there was a disparity in the relative abundance of Enterobacteriaceae and Peptostreptococcaceae between the Control and DSS groups (Fig. 5E). Compared with the DSS group, TPS and TPP administration decreased the relative abundances of Enterobacteriaceae significantly (p < 0.05) and the TPS + TPP treatment had no significant effect (Fig. 5F). Compared with the Control, Peptostreptococcaceae was increased by the DSS treatment. However, the TPP and TPS + TPP treatments decreased the relative abundances of Peptostreptococcaceae compared to the DSS groups (DSS 15.10% vs. DSS + TPP 11.4% vs. DSS + TPS + TPP 6.21%) (Fig. 5G). LEfSe analysis and linear discriminant analysis (LDA) also confirmed the change of Proteobacteria and Enterobacteriaceae abundance (Fig. 5H and I). Notably, the DSS + TPS + TPP group was enriched with Lactobacillaceae and Lactobacillus.
Discussion
Over the past decade, dietary intervention has emerged as a promising tool for the prevention and treatment of IBD (Lewis & Abreu, 2017). Green tea is a worldwide consumed beverage, containing anti-inflammatory components such as polysaccharides and polyphenols (Liu et al., 2018, Oz et al., 2013). In the present study, we investigated the effects of TPP, TPS and their combination on DSS-induced colitis in mice, with focus on disease severity, intestinal barrier integrity and gut microbiota. We found that all the treatments exerted certain effects against colitis, but TPP + TPS achieved a better effect than the single ingredients.
Accumulated studies have shown polysaccharides and polyphenols are effective in the prevention and treatment of colitis, with inflammation, gut barrier function and microbiota modulation as the most investigated (Fan et al., 2020, Kim et al., 2018, Wu et al., 2021). In the current study, colitis mice showed a chronic inflammation characterized by significant colon ulcers, infiltration of inflammatory cells and damage of crypts, but supplementation of TPS + TPP or TPP significantly attenuated the symptoms. Furthermore, the lowered expression of mucin and TJ proteins, including occludin and claudin-1 that regulate the selective permeability of intestinal epithelial barrier and prevent the pathogenic antigen into the mucosal lamina propria, in the DSS group was also reversed by supplementation of TPS, TPP or TPS + TPP. Therefore, improving intestinal barrier function may be one of the mechanisms of TPS and TPP against colitis. Interestingly, the best protective effects were seen in the TPS + TPP group. The enhanced effect induced by the combination of TPP and TPS has also been observed in obese mice regarding the anti-inflammatory activity, and also the combination of fructo-oligosaccharide and flavonoid in rats regarding the microbial production of SCFAs (Xu et al., 2015, Zheng et al., 2017). So far, the mechanism behind such an enhancement remains unknown, but how the single ingredient exerted the effects has been keeping revealed, which might provide a hint. It is generally accepted that microbial breakdown of these ingredients and the consequent products should take partial responsibility. Wu et al. (2021) have shown that butyrate-enriched gut microbial metabolite from mice receiving green tea polyphenol could alleviate colitis and colonic barrier damage (Wu et al., 2021). Butyrate, a type of SCFAs and also the main source of energy for colonocytes, could improve the barrier function and the expression of TJ protein, promote the immune response and the production of mucus, and thus is considered to confer benefits to UC patients (Maurer et al., 2019). Other type of microbial metabolites has also been found to play a role in attenuating colitis. Recently, Singh et al. (2019)have delicately demonstrated how urolithin A, a major microbial metabolite derived from polyphenolics of berries and pomegranate fruits, improved gut barrier integrity by upregulating epithelial TJ proteins via activation of AhR-Nrf2-dependent pathways (Singh et al., 2019). On the other hand, polysaccharides, the main substrate for gut microbiota, are also capable to produce certain beneficial metabolites such as SCFAs. In addition, both polyphenol and polysaccharides have great influence on microbial composition and activity. They can modulate TJ protein expression in vitro, suggesting a direct talk between epithelial cells and the bioactive ingredients (Bai et al., 2020, Bianchi et al., 2019). Taken together, inclusion of TPS in the TPP diet might positively influence intestinal barrier function by influencing the interaction between TPP and gut microbiota, probably also the direct contact with epithelial cells, to exert a better effect against colitis.
Gut dysbiosis is closely related to the development of ulcerative colitis. Some probiotics have been used in the treatment of colitis to regulate gut microbiota (Llewellyn et al., 2018, Wu et al., 2021). In our study, TPS, TPP and TPS + TPP treatments could reverse DSS induced change in microbial beta diversity to some extent and might play a positively regulatory role in colitis mice. Furthermore, the relative abundance of Bacteroidetes, the largest propionic acid producer in human gut, were increased upon TPP treatment, and the produced propionate could regulation of intestinal inflammation by intestinal γδ T cells to reduce IL-17 and IL-22 production (Dupraz et al., 2021). Multiple reports have shown microbial change at phylum level, e.g. reduction of Firmicutes and increment of Proteobacteria in IBD (Andoh et al., 2007, Ott et al., 2004). In addition, abnormal proliferation of Proteobacteria is considered to be a “microbial feature” of dysbiosis in the gut, and the abundance of Proteobacteria has been shown to be increased in colitis mice (Liu, Zhang, Qiu, Fan, Ding, & Liu, 2018). Consistent with these reports, our results showed a higher abundance of Proteobacteria in colitis mice, which was significantly reduced in the DSS + TPS and DSS + TPP groups. Interestingly, LEfSe analysis showed a higher abundance of Lactobacillaceae and Lactobacillus in the TPS + TPP group, which was not seen in the TPS or TPP group. Notably, Lactobacillus has been shown to negatively correlate with the IBD-promoting parameter IL-1β, and prevented epithelial barrier disruption induced by TNF-α (Hsieh, Osaka, Moriyama, Date, Kikuchi, & Tsuneda, 2015). According to these results, the TPP + TPS treatment may through regulated Lactobacillus to improve intestinal barrier system to alleviate colitis. However, 16S rRNA gene sequencing provides limited information and is generally down to the genus level, further analysis of gut microbiota, such as deeper sequencing and gut metabolomics, probably reveals the regulation of TPP + TPS on gut microbiota and its link to the therapeutic effects on colitis in details.
Conclusion
In conclusion, TPS, TPP and TPS + TPP had positive impacts on DSS-induced mice to different extents. TPS + TPP treatment remarkably relieved DSS-induced dysfunctions in body weight, DAI, colon length and colon tissue damage, while no effect of TPP and TPS on body weight nor TPS on tissue damage was seen. TPS, TPP and TPS + TPP treatments enhanced mucins and TJ proteins expression, with the best effect observed in the TPS + TPP group. Moreover, TPS and TPP reduced the relative abundance of Proteobacteria and Enterobacteriaceae, while TPS + TPP could enhance that of beneficial gut microbes such as Lactobacillaceae and Lactobacillus. Based on the current findings, TPP + TPS had the best effect on alleviating colitis and promoting intestinal barrier function. This laid the foundation for further investigations to elucidate the mixture of TPS and TPP.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial supports from National Natural Science Foundation of China (31801539) and Technological Innovation Guidance Plan of Jiangxi Province (20203AEI91007) are gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100190.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andoh A., Sakata S., Koizumi Y., Mitsuyama K., Fujiyama Y., Benno Y. Terminal restriction fragment length polymorphism analysis of the diversity of fecal microbiota in patients with ulcerative colitis. Inflammatory Bowel Diseases. 2007;13(8):955–962. doi: 10.1002/ibd.20151. [DOI] [PubMed] [Google Scholar]
- Bai Y., Huang F., Zhang R., Dong L., Jia X., Liu L.…Zhang M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydrate Polymers. 2020;229:115475. doi: 10.1016/j.carbpol.2019.115475. [DOI] [PubMed] [Google Scholar]
- Ingelfinger J.R., Baumgart D.C., Le Berre C. Newer biologic and small-molecule therapies for inflammatory bowel disease. New England Journal of Medicine. 2021;385(14):1302–1315. doi: 10.1056/NEJMra1907607. [DOI] [PubMed] [Google Scholar]
- Bianchi M.G., Chiu M., Taurino G., Brighenti F., Del Rio D., Mena P., Bussolati O. Catechin and Procyanidin B(2) modulate the expression of tight junction proteins but do not protect from inflammation-induced changes in permeability in human intestinal cell monolayers. Nutrients. 2019;11(10):2271. doi: 10.3390/nu11102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Catalkaya G., Venema K., Lucini L., Rocchetti G., Delmas D., Daglia M.…Capanoglu E. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Frontiers. 2020;1(2):109–133. doi: 10.1002/fft2.v1.210.1002/fft2.25. [DOI] [Google Scholar]
- Celiberto L.S., Graef F.A., Healey G.R., Bosman E.S., Jacobson K., Sly L.M., Vallance B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology. 2018;155(1):36–52. doi: 10.1111/imm.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & Molecular Medicine. 2018;50(8):1–9. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Guan X., Ding W., Luo Y., Wang W., Bu W.…Feng L. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. International Journal of Biological Macromolecules. 2021;166:1035–1045. doi: 10.1016/j.ijbiomac.2020.10.259. [DOI] [PubMed] [Google Scholar]
- Das, S., Batra, S., & Rachagani, S. (2017). Mouse Model of Dextran Sodium Sulfate (DSS)-induced Colitis. BIO-PROTOCOL, 7(16), e2515. 10.21769/BioProtoc.2515. [DOI] [PMC free article] [PubMed]
- Dubois M., Giles K., Rebers P., Smith F. Colorimetric method for determination of sugar and related substances. Analytical Chemistry. 1955;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Dupraz L., Magniez A., Rolhion N., Richard M.L., Da Costa G., Touch S.…Michel M.-L. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021;36(1):109332. doi: 10.1016/j.celrep.2021.109332. [DOI] [PubMed] [Google Scholar]
- Fan L., Zuo S., Tan H., Hu J., Cheng J., Wu Q., Nie S. Preventive effects of pectin with various degrees of esterification on ulcerative colitis in mice. Food & Function. 2020;11(4):2886–2897. doi: 10.1039/c9fo03068a. [DOI] [PubMed] [Google Scholar]
- Geremia A., Biancheri P., Allan P., Corazza G.R., Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity Reviews. 2014;13(1):3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Holmberg F.E.O., Pedersen J., Jørgensen P., Soendergaard C., Jensen K.B., Nielsen O.H. Intestinal barrier integrity and inflammatory bowel disease: Stem cell-based approaches to regenerate the barrier. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(4):923–935. doi: 10.1002/term.2506. [DOI] [PubMed] [Google Scholar]
- Hsieh C.-Y., Osaka T., Moriyama E., Date Y., Kikuchi J., Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiological Reports. 2015;3(3):e12327. doi: 10.14814/phy2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. The global burden of IBD: From 2015 to 2025. Nature Reviews. Gastroenterology & Hepatology. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- Kim H., Krenek K.A., Fang C., Minamoto Y., Markel M.E., Suchodolski J.S.…Mertens-Talcott S.U. Polyphenolic derivatives from mango (Mangifera Indica L.) modulate fecal microbiome, short-chain fatty acids production and the HDAC1/AMPK/LC3 axis in rats with DSS-induced colitis. Journal of Functional Foods. 2018;48:243–251. doi: 10.1016/j.jff.2018.07.011. [DOI] [Google Scholar]
- Lewis J.D., Abreu M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017;152(2):398–414.e396. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Liu L.Q., Nie S.P., Shen M.Y., Hu J.L., Yu Q., Gong D., Xie M.Y. Tea polysaccharides inhibit colitis-associated colorectal cancer via interleukin-6/STAT3 pathway. Journal of Agriculture and Food Chemistry. 2018;66(17):4384–4393. doi: 10.1021/acs.jafc.8b00710. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang Y., Qiu B., Fan S., Ding H., Liu Z. Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Scientific Reports. 2018;8(1):14916. doi: 10.1038/s41598-018-33092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn S.R., Britton G.J., Contijoch E.J., Vennaro O.H., Mortha A., Colombel J.-F.…Faith J.J. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology. 2018;154(4):1037–1046.e1032. doi: 10.1053/j.gastro.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer L.H., Cazarin C.B.B., Quatrin A., Minuzzi N.M., Costa E.L., Morari J.…Emanuelli T. Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: A major role for dietary fiber and fiber-bound polyphenols. Food Research International. 2019;123:425–439. doi: 10.1016/j.foodres.2019.04.068. [DOI] [PubMed] [Google Scholar]
- Morgan X.C., Tickle T.L., Sokol H., Gevers D., Devaney K.L., Ward D.V.…Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome biology. 2012;13(9):R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A., Inoue R., Inatomi O., Bamba S., Naito Y., Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical Journal of Gastroenterology. 2018;11(1):1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- Ott S.J., Musfeldt M., Wenderoth D.F., Hampe J., Brant O., Fölsch U.R.…Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53(5):685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz, H., Chen, T., & de Villiers, W. (2013). Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. 4(132). 10.3389/fimmu.2013.00132. [DOI] [PMC free article] [PubMed]
- Park M.Y., Ji G.E., Sung M.K. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Digestive Diseases and Sciences. 2012;57(2):355–363. doi: 10.1007/s10620-011-1883-8. [DOI] [PubMed] [Google Scholar]
- Radhakrishnamurthy B., Berenson G.S. Effect of temperature and time of heating on the carbazole reaction of uronic acids and acid mucopolysaccharides. Analytical Chemistry. 1963;35(9):1316–1318. doi: 10.1021/ac60202a067. [DOI] [Google Scholar]
- Shigeshiro M., Tanabe S., Suzuki T. Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis. Journal of Functional Foods. 2013;5(2):949–955. doi: 10.1016/j.jff.2013.02.008. [DOI] [Google Scholar]
- Singh R., Chandrashekharappa S., Bodduluri S.R., Baby B.V., Hegde B., Kotla N.G.…Jala V.R. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nature Communications. 2019;10(1) doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara, K., Morhardt, T. L., & Kamada, N. (2019). The role of dietary nutrients in inflammatory bowel disease. Frontiers in Immunology, 9, 3183-3183. 10.3389/fimmu.2018.03183. [DOI] [PMC free article] [PubMed]
- Wu Z., Huang S., Li T., Li N.a., Han D., Zhang B.…Wang J. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9(1) doi: 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang M., Wu T., Dai S., Xu J., Zhou Z. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food & Function. 2015;6(1):296–303. doi: 10.1039/C4FO00970C. [DOI] [PubMed] [Google Scholar]
- Yajun Z., Qingtong X., Lijun Y., Peter Chi-Keung C., Zhengang Z. Behavior of non-digestible polysaccharides in gastrointestinal tract: A mechanistic review of its anti-obesity effect. eFood. 2021;2(2):59–72. doi: 10.2991/efood.k.210310.001. [DOI] [Google Scholar]
- Zhang Y.Z., Li Y.Y. Inflammatory bowel disease: Pathogenesis. World Journal of Gastroenterology. 2014;20(1):91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Khan N.M., Nunez K.M., Chess E.K., Szabo C.M. Complete monosaccharide analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. Analytical Chemistry. 2012;84(9):4104–4110. doi: 10.1021/ac300176z. [DOI] [PubMed] [Google Scholar]
- Zheng C.-J., Liu R., Xue B., Luo J., Gao L., Wang Y.…Peng X. Impact and consequences of polyphenols and fructooligosaccharide interplay on gut microbiota in rats. Food & Function. 2017;8(5):1925–1932. doi: 10.1039/C6FO01783E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.