Highlights
-
•
Industrial hemp essential oil was successfully reinforced in formed nanoparticles.
-
•
Coating controlled the microbial growth of fish during storage.
-
•
The coated fishes retarded the increase of oxidation during storage.
-
•
Coating led to an extension in the shelf life of Rainbow trout fillets.
Keywords: Bioactive compound; Encapsulation; Active packaging, fish; Lipid oxidation; By-product; Natural preservative; Antimicrobial
Abstract
Essential oil of industrial hemp (Cannabis sativa L.) (IHEO) was reinforced in complexation of whey protein nanofibrils and mung bean protein nanoparticles (WPNF-MBP NPs) as a novel nano-carrier. A desirable retention rate range of 50.9–90.4% was confirmed for IHEO reinforced in WPNF-MBP NPs. Fourier transform infrared (FTIR) spectroscopy revealed that IHEO was successfully loaded within WPNF-MBP NPs without specific chemical interaction with the carrier matrix. The results indicated that incorporation of IHEO-reinforced WPNF-MBP NPs into active material coatings having acceptable inhibition activity against total viable and psychrotrophic bacteria. The coated fishes also retarded the increase of PV (peroxide value), TBA (thiobarbituric acid) and TVB-N (total volatile basic nitrogen) values during storage. The IHEO-reinforced WPNF-MBP NPs coating led to an extension in the shelf life of Rainbow trout fillets within 8–14 days of storage. Accordingly, IHEO-reinforced WPNF-MBP NPs can be suggested as a natural preservative for coating fishes.
1. Introduction
There is a growing interest in natural bioactive compounds both by producers and consumers in the food and pharmaceutical industries. Particularly, consumers are looking for foods without artificial and harmful preservatives that can promote their health (Garavand et al., 2021). Essential oils (EOs) as natural food preservatives are, generally, extracted from medicinal plants and herbs for their noticeable biological activities. EOs are complex combination of secondary metabolites, including terpenoid hydrocarbons, phenol derivatives and oxygenated terpenoids (Yousuf et al., 2021); however, due to having volatile components, their usage is limited; the volatile compounds can be degraded easily by adverse external conditions such as light, oxygen, temperature, pressure, and pH. In addition, controlled release of EOs is needed when used for specific purposes like food additives (Hadidi, Motamedzadegan, et al., 2021).
In recent years, the isolation and utilization of EOs from agro-industrial wastes and by-products have gained much research interest. Industrial hemp's (Cannabis sativa L.) inflorescences are usually discharged during the conventional hemp processing, resulting in an underused biomass for future uses (Ascrizzi et al., 2019). They are a rich source of EOs and contain mainly monoterpenes and sesquiterpene hydrocarbons like (E)-caryophyllene, α-pinene, myrcene and α-humulene, which exhibit important biological activities (Fiorini et al., 2019). Valorization of hemp by-products is a matter of interest for producers, allowing them to increase the market value of hemp cultivation (Ascrizzi et al., 2019). Some studies have recently been carried out on the functional and pharmacological applications/properties of industrial hemp essential oil due to their antioxidant, antimicrobial, and anti-inflammatory activities (Benelli et al., 2018, Tabari et al., 2020).
There is much interest in developing biodegradable nanoparticles (NPs) as an effective delivery system for delivering lipophilic food bioactives (Garavand et al., 2021). Proteins are attractive options for designing polymeric NPs as suitable wall materials thanks to their amphiphilic nature compatible with many active substances, as well as excellent functional properties (Tarhini et al., 2017). Whey protein, as a typical cheese processing by-product, is commonly used to foods due to its great nutritional value and techno-functional properties such as gelling, foaming and emulsifying (Hu et al., 2020). Protein fibrillation is used nowadays for fabrication of protein fibrils having novel functionalities and improved structure stability. Heating the protein above 80 °C for 5–24 h at low ionic strength and acidic conditions can result in production of WPI fibrils (Zhang et al., 2021), which are more suitable as delivery carriers comparing to native proteins, due to having multiple functional groups, and thus, promoting different interactions with numerous and drugs and nutrients (Hu et al., 2020). Protein complexation has recently been proposed as a promising and efficient way for improving the bioavailability, chemical stability and dispersion of bioactive compounds in an aqueous environment. Mung bean (Vigna radiate L.) protein (MBP) is a significant plant protein that demonstrates high potential as sustainable protein source for its availability, nutritional value, hypoallergenic, and desirable foaming, emulsifying, gelling, and film-forming capabilities (Hadidi, Jafarzadeh, et al., 2021).
The rainbow trout (Oncorhynchus mykiss) as an extremely perishable food belongs to the Salmonidae family. Recently, the demand for rainbow trout has increased remarkably, and this could be due to its desirable characteristics. Due to the demand for fresh refrigerated fish with extended shelf life, notable study has been directed toward prolonging the shelf life of this fish (Shadman et al., 2017). Although, there are scarce studies on the characterization and fabrication of IHEO encapsulated in complexation of WP nanofibrils and MBP NPs (WPNF-MBP NPs). The present study is to manufacture and characterize WPNF-MBP NPs as a novel wall material for loading IHEO. Furthermore, application of IHEO-reinforced WPNF-MBP NPs as a potential natural additive for increasing the shelf-life of Rainbow trout fillets during refrigerated storage is going to be evaluated.
2.1. Materials
Hemp (Cannabis sativa cv Felina 32) essential oil from aerial parts was purchased from Hempture (Dublin, Ireland) (Tetrahydrocannabinol at a concentration of 0.002%). Food grade whey protein (protein content 91.4%) and mung bean protein (protein content 88.2%) isolates were obtained from Hilmar Corp. (California, USA) and ET Protein (Suzhou, China). All other reagents and chemicals were of analytical grade from Panreac Química S.A. (Barcelona, Spain) and Sigma Chemicals Co. (Missouri, USA).
2.2. GC–MS analysis of IHEO
The IHEO analysis was carried out using GC–MS (Packard Hewlett (HP) 6890) with a fused capillary column DB-1 (60 m × 0.25 mm id, 0.25 μm film thickness). The oven temperature was kept at 50 °C for 3 min, increased to 260 °C by a ramp-up of 3 °C/min and then held for 5 min. The temperature of detector and injector s was set at 270 and 240 °C, respectively. Helium with a flow rate of 0.1 mL/min was employed as a carrier gas. The ionization voltage, mass range, solvent delay, and scan time were 70 eV, 30–600 m/z, 2 min, and 0.4 s, respectively. Analytical standards were compared together with the correspondence of retention indices and mass spectra with respect to those of Adams (2007).
2.3. Preparation of WPNF
The method of Hu et al. (2020) with minor modifications was used for preparation of WPNF. Whey protein isolate powder was dissolved in deionized water at a concentration of 4% (w/v) and adjusted to pH 2.0 with 6 M HCl; then the suspension was stored for 6 h at 4 °C to hydrate the protein. After filtration by a Hydrophilic PES 0.45 μm (Millipore Millex-HP), the solution was heated in a silicone oil bath at 80 °C for 18 h, and then freeze-dried.
2.4. Preparation of IHEO-reinforced WPNF-MBP NPs
The synthesis of IHEO-reinforced WPNF-MBP NPs was carried out using a nano-precipitation technique according to the method of Hadidi, Motamedzadegan, et al. (2021) with some modifications. To prepare WPNF-MBP stock solution, WPNF (2 g) and MBP (2 g) were dispersed in distillated water (100 mL) and solubilized for 60 min sonication (Skymen JP-008, China) at 40 °C. Undissolved WPNF and MBP were separated from the suspension using 1 μm pore size filter followed by adding Tween 80 (160 mg) into the suspension. Afterwards, different amounts of IHEO were added into the suspension and mixed at 25 °C for 30 min by a shaker (600 rpm) to achieve several WPNF-MBP/IHEO ratios (1:0, 1:0.2, 1:0.4, 1:0.6, 1:0.8, and 1:1). In order to promote the precipitation of particles, the absolute ethanol was added into the solution. The ratio of ethanol/solution was 5:1 (v/v). The solution was then mixed using a homogenizer (AD200L-H, Meditry Instrument Co., Ltd, Jiangyin, China) through ultra-dispersion for 10 min at 12,000 rpm. Next, it was centrifuged by a Cenhbn Centrifuge (600ML-4, Labtest Instruments, Maharashtra, India) at 600 × g for 10 min at 4 °C to separate the precipitate The achieved precipitate was then dried for 32 h at −55 °C using a freeze dryer (Telstar LyoQuest™, Terrassa, Spain).
2.5. Determination of retained IHEO in WPNF-MBP NPs
UV–Vis spectroscopy was used to measure the content of IHEO reinforced into WPNF-MBP NPs (Hesami et al., 2021). The obtained suspension (in 400 µL water) was added to 2 M aqueous hydrochloric acid solution (10 mL) and refluxed for 30 min at 95 °C. Then it was added to 96% v/v ethanol (2 mL) and centrifuged at room temperature at 10,000 rpm for 5 min. A Cary 60 UV–Vis spectrophotometer (Santa Clara, CA, United States) at 252 nm was employed to measure the amount of IHEO in the supernatant. Using the same procedure, WPNF-MBP NPs without IHEO were prepared as blanks. The calibration curve of free IHEO in ethanol (R2 = 0.992) was used to determine the amount of IHEO. The retention of IHEO in WPNF-MBP NPs was measured as follows:
| (1) |
2.6. Instrumental analysis of IHEO-reinforced WPNF-MBP NPs
The dried samples (1 mg) were diluted using distilled water (50 mL) and sonicated (200 W, 24 kHz with ultrasonic probe 3 mm) at 25 °C for 4 min. Next, 2 mL of the solution was poured separately into special cells and the poly-dispersity index (PDI), average particle size, and zeta potential were obtained by a ZEN 3600 Nano Zetasizer equipped with a laser operation of He–Ne at 4.0 mW and 633 nm (Malvern Ltd, UK).
FTIR was performed for WPNF-MBP, IHEO-reinforced WPNF-MBP NPs (with a WPNF-MBP/IHEO ratio of 1:0.8) and pure IHEO at the wavelength range of 400–4000 cm−1 using 16-scan, 4 cm−1 resolution with an FTIR spectrometer (Equinox55, Bruker, Germany). The dried samples were crushed by KBr and prepared in the form of disks by pressing.
A CM12 transmission electron microscope (Philips, Eindhoven, Netherlands) was used for TEM analysis. Images of the IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8 were then obtained at two magnifications.
2.7. Preparation of coating solutions and application to Rainbow trout fillets
To prepare WPNF-MBP stock solution incorporating IHEO-reinforced NPs, 0.1% v/v of Tween 80 and 0.5 g of glycerol/g WPNF-MBP were added into the solution via constant agitation for 60 min. Next, IHEO-reinforced NPs (1% v/v) were added to the stock solution and stirred for 1 h at 900 rpm at room temperature. The obtained solution was used to form the edible coating on the surface of Rainbow trout fillets. The Rainbow trout fillets were placed in the related coating solutions (WPNF-MBP or IHEO-reinforced WPNF-MBP NPs) for 30 s. After dripping excess solution, the Rainbow trout fillets were dried on stainless steel racks for 15 min at 25 °C. We repeated the coating process twice. Both the coated and uncoated (control) samples were placed in sterile plastic trays, enclosed with low-density polyethylene plastic films, and refrigerated for 14 days at 4 ± 1 °C. Three Rainbow trout fillets from each group were analyzed at days 0, 2, 4, 6, 8, 10, 12 and 14 of storage.
2.8. Bacteriological analyses of Rainbow trout fillets
Twenty five grams of samples were taken aseptically and homogenized with a stomacher (STOMACHER®, UK) along with 225 mL of 0.1% peptone water for 1 min. From this purpose, serial dilutions (1:10) were prepared in 0.1% peptone water solution and then 0.1 mL of each of them was spread on appropriate media. Plate count agar (Merck, Darmstadt, Germany) was used for determining total viable counts (48 h incubation at 37 °C). Psychrotrophic counts were carried out using King Agar (Merck, Darmstadt, Germany) by incubating the cultured plates at 21 °C for 48 h. The results were expressed as log CFU/g (Shokri et al., 2020).
2.9. Physicochemical analysis of Rainbow trout fillets
The method of Shadman et al. (2017) was used to analyze peroxide value (PV) in the total fat extracts, and the result was expressed as milliequivalents (meq) of peroxide oxygen per kilogram of fat. The thiobarbituric acid (TBA) value was evaluated colorimetrically based on the method of Natseba et al. (2005) and the outcome was expressed as mg of malondialdehyde (MDA) per kg of sample. The method of Shokri et al. (2020) was employed to calculate the amount of total volatile basic nitrogen (TVB-N) (mg N/100 g) following the micro-titration methodology (i.e. distillation step and titration with HCl).
2.10. Sensory analysis of Rainbow trout fillets
The attributes of sensory evaluation included appearance, texture, odor and color. A five-point descriptive hedonic scale (1 = dislike extremely and 5 = like extremely) was employed. Sensory evaluation of the coated and control fillets was performed by a 30 semi-trained panelists (18 females and 12 males) consisting of food hygiene students (21–32 years old).
2.11. Statistical analysis
Experimental data were analysed using the one-way analysis of variance (ANOVA). The significant difference among the mean values was examined by Duncan’s test (P ≤ 0.05) using the SPSS software (ver. 23.0).
3. Results and discussion
3.1. Chemical composition of IHEO
The chemical composition of IHEO analysed by GC-Mass is illustrated in Table 1. A total of 30 components identified in IHEO. As can be seen in Table 1, the composition of IHEO was dominated by monoterpene and sesquiterpene hydrocarbons as the major components, orderly. Overall, (E)-caryophyllene (27.1%) was the predominant compound of the EO, followed by myrcene (13.2%) and α-pinene (9.2%) Other compounds were α-humulene (8.2%), terpinolene (6.2%), and β-pinene (3.9%). Similar chemical composition was observed by previous studies for IHEO but in diverse quantities (Fiorini et al., 2019, Nafis et al., 2019, Tabari et al., 2020). The discrepancies in the chemical composition of terpenes in IHEO may be relevant to the source, harvesting, storage, and type of cultivation/extraction of the specimens (Ascrizzi et al., 2019).
Table 1.
Quantitative composition of industrial hemp essential oil (IHEO).
| Peak number | Compound | RI exp.a | RI lit.b | Area % |
|---|---|---|---|---|
| 1 | α-pinene | 937 | 933 | 9.2 |
| 2 | camphene | 952 | 953 | 0.6 |
| 3 | sabinene | 961 | 966 | 0.2 |
| 4 | β-pinene | 980 | 978 | 3.9 |
| 5 | myrcene | 995 | 991 | 13.2 |
| 6 | α-phellandrene | 1009 | 1007 | 0.1 |
| 7 | δ-3-carene | 1010 | 1009 | 0.4 |
| 8 | α-terpinene | 1020 | 1018 | 0.4 |
| 9 | para-cymene | 1028 | 1025 | 0.6 |
| 10 | limonene | 1030 | 1030 | 1.8 |
| 11 | eucalyptol | 1034 | 1032 | 0.1 |
| 12 | E-β-ocimene | 1045 | 1046 | 3.6 |
| 13 | γ-terpinene | 1065 | 1058 | 0.4 |
| 14 | terpinolene | 1096 | 1086 | 6.2 |
| 15 | n-nonanal | 1108 | 1100 | 0.2 |
| 16 | myrtenol | 1190 | 1194 | 0.2 |
| 17 | Z-caryophyllene | 1410 | 1413 | 0.4 |
| 18 | α-cis-bergamotene | 1418 | 1416 | 0.3 |
| 19 | E-caryophyllene | 1425 | 1424 | 27.1 |
| 20 | α-trans-bergamotene | 1433 | 1432 | 1.3 |
| 21 | α-humulene | 1465 | 1454 | 8.2 |
| 22 | 9-epi-caryophyllene | 1468 | 1464 | 0.6 |
| 23 | β-selinene | 1491 | 1492 | 2.0 |
| 24 | α-selinene | 1500 | 1501 | 0.6 |
| 25 | selina-4(15),7(11)-diene | 1542 | 1540 | 0.5 |
| 26 | selina-3,7(11)-diene | 1555 | 1546 | 0.6 |
| 27 | caryophyllene oxide | 1583 | 1587 | 4.5 |
| 28 | humulene epoxide | 1610 | 1613 | 2.1 |
| 29 | allo-aromadendrene epoxide | 1649 | 1644 | 0.6 |
| 30 | tetracosane | 2393 | 2400 | 2.7 |
| – | unknown compounds | – | – | 7.6 |
RI exp.: Experimentally determined retention index; RI lit: Retention index reported in the literature (Adams, 2007).
3.2. IHEO retention rate and physicochemical characterization of NPs
Free IHEO could undergo huge loss due to its volatility upon drying processes and subsequent storage (Ascrizzi et al., 2019). Table 2 shows the retention of IHEO within NPs at different IHEO/WPNF-MBP mass ratios. The results confirmed that with enhancement of IHEO ratio from 1:0.2 to 1:0.8, much higher retention of the oil in WPNF-MBP NPs was attained. No significant change was observed in the IHEO retention rate as the ratio of EO/WPNF-MBP further increased, probably because of the saturated EO level in the wall materials (Hadidi, Motamedzadegan, et al., 2021). This reflects that the engineered NPs helped promoting the uniform distribution and proper entrapment of EO within the carrier matrix (Arabpoor et al., 2021, Hernández-Nava et al., 2020).
Table 2.
Physicochemical characterization of IHEO-reinforced WPNF-MBP NPs.
| IHEO/WPNF-MBP ratio | Retention of IHEO (%) | Mean size (nm) | Zeta potential (mV) | Polydispersity index (PDI) |
|---|---|---|---|---|
| 1:0 | – | 218.2 ± 10.2e | −37.26 ± 1.2a | 0.355 ± 0.068a |
| 1:0.2 | 50.9 ± 3.1d | 254.3 ± 18.3d | −32.11 ± 3.2b | 0.278 ± 0.019b |
| 1:0.4 | 63.4 ± 2.8c | 300.8 ± 16.5c | −31.85 ± 2.5bc | 0.263 ± 0.020b |
| 1:0.6 | 80.3 ± 5.9b | 340.7 ± 23.7b | −29.23 ± 3.7c | 0.260 ± 0.038b |
| 1:0.8 | 90.4 ± 4.4a | 342.2 ± 30.5b | −28.36 ± 2.1c | 0.232 ± 0.046c |
| 1:1 | 88.7 ± 6.8a | 378.4 ± 13.7a | −25.10 ± 1.1e | 0.228 ± 0.031c |
*IHEO: Industrial hemp essential oil; WPNF-MBP: Whey protein nanofibrils-mung bean protein.
**Results (means ± SD, n = 3) within each column with the same letters are not significantly different (P > 0.05).
Average particle size was measured for all samples (Table 2) and a symmetric distribution was found for the IHEO-reinforced WPNF-MBP NPs, indicating a relatively uniform particle size distribution of the oil-containing nano-carriers. From Table 2, some conclusions can be drawn as follows. Firstly, mean diameter particle of the IHEO-free blank NPs was 218.2 nm, significantly different from the diameter particle of IHEO-reinforced WPNF-MBP NPs at the wall material to EO ratio of 1:0.2 (254.3 nm). Secondly, the average particle size of IHEO-reinforced WPNF-MBP NPs increased significantly (from 254.3 to 378.4 nm) as a function of EO addition at constant WPNF-MBP level, exhibiting that the inclusion of EO into WPNF-MBP NPs could remarkably influence the particle size of the nano-carriers. The most likely reason for this phenomenon could be insufficient level of protein molecules to properly cover the EO droplets at higher levels of IHEO; this can weaken the IHEO-wall material interactions, induce the formation of aggregate droplets, and thus, cause a notable increase in the particle diameter (Hadidi, Motamedzadegan, et al., 2021). Thirdly, the average particle size of all WPNF-MBP NPs, both blank and EO loading, was much smaller than 350 nm, indicating the uniformity and efficiency of the encapsulation method (Table 2). These findings are in agreement with results of Hesami et al., 2021, Rostamabadi et al., 2019.
Nevertheless, with further increasing of the IHEO ratio to 1:0.8, the loss of EO from WPNF-MBP NPs enhanced considerably (Table 2). As we proposed, by applying high levels of IHEO within WPNF-MBP NPs, the protein–protein and protein-oil interactions could be weakened and the majority of IHEO droplets could not be properly covered by the WPNF and MBP molecules, leading to a lower IHEO entrapment and aggregation of EO droplets. At smaller particle sizes, a greater space exists between the nano-capsules, hindering their agglomeration, increasing their stability, and thus, promoting their retention (Hernández-Nava et al., 2020). Another possible reason for this reduction might be the minimum zeta potential value of this formulation that reflects the lowest stability of NPs’ structure at 1:0.8 IHEO /WPNF-MBP ratio, in accordance with Chen et al. (2021). The same trend was also found by Prata & Grosso, (2015), who demonstrated that when lower concentrations of bioactive oil were loaded in the system, a higher oil retention was obtained due to the lower amount of payload within the core in the presence of an excess carrier agent.
All NPs presented negative zeta potential and all PDI indexes were<0.3 (Table 2). It is worth to mention that the less the PDI values, the more uniform the particle diameter distribution and the smaller the range of particle size (Rostamabadi et al., 2020). The zeta potential of WPNF-MBP NPs not reinforced with IHEO was about −37.26 mV, significantly different from that of WPNF-MBP NPs reinforced with IHEO. Generally, the zeta potential value of WPNF-MBP NPs quickly increased to –32.11 mV when the IHEO level was gradually elevated in system. As depicted in Table 2, an adequate repulsive force (-37.26 to −25.1 mV) was detected for the IHEO-reinforced WPNF-MBP at 1:0 to 1:1 IHEO/WPNF-MBP ratios, implying the proper physical colloidal stability of the prepared nano-carriers. It is to be noted that small zeta potentials can induce particle aggregation/flocculation as a result of Van der Waals attractive forces (Bamidele et al., 2019). However, non-zero zeta potentials can stabilize the colloidal system by sufficient imparting of electrostatic stability between particles (Fan et al., 2021). Accordingly, higher zeta potential of IHEO-reinforced WPNF-MBP than that of pure protein NPs can be due to the neutralization of the electric charge resulted from an interaction between the negatively charged WPNF-MBP and IHEO. Recent studies by Hesami et al. (2021), and Arabpoor et al. (2021) showed a significant reduction in zeta potential when a non-polar EO was loaded into biopolymer NPs. Concisely, IHEO-reinforced WPNF-MBPs designed in this study exhibited negative zeta potential, small PDI value, and thus, proper stability, implying their potential as practical nano-carriers in encapsulation of IHEO.
3.3. Fourier-transform infrared spectroscopy
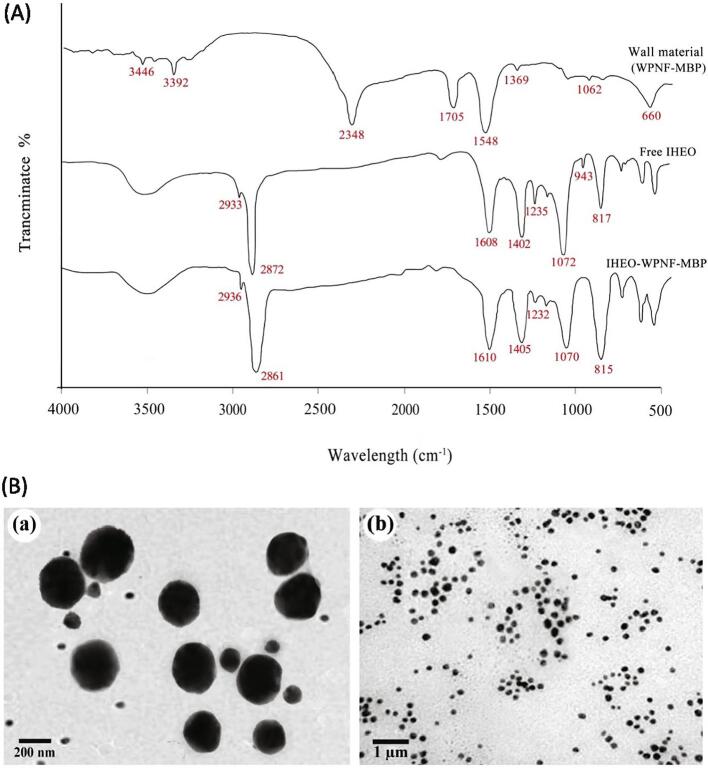
As shown in Fig. 1, free IHEO indicates signals at 3568, 1608, 1402, 1235, 1072, and 817 cm−1, illustrating O single bond H, N single bond H bending, CH2 bending, C single bond O single bond C stretching, C single bond O single bond C stretching, and C single bond H bending in the structure, respectively (Hasanpour Ardekani-Zadeh and Hosseini, 2019, Volić et al., 2018). Peaks 2933 and 2872 cm−1 were related to C single and bond H, which are common signals in EOs (Hernández-Nava et al., 2020). In the WPNF-MBP NPs, absorption peaks were observed at 1548 and 1705 cm−1, illustrating the symmetric stretching of N single bond C double bond O (amide II) and the stretching vibrations of C double bond O (amide I), respectively. A sharp peak at 3346 cm−1 associated to the –NH group stretching, and the band at 3392 cm−1 can be referred to the stretching vibrations of CH and NH2 (Fan et al., 2021, Hadidi et al., 2021). The bands at 1062 and 1369 cm−1 were assigned to the weak starching of C single bond O and the carboxylate group O—C—O, respectively (Ghobadi et al., 2021). The characteristic absorption peak of IHEO-reinforced WPNF-MBP NPs was diverse from those of pure IHEO and WPNF-MBP NPs. After IHEO was reinforced in the WPNF-MBP NPs, the characteristic peak of BC at 943 cm−1 disappeared, illustrating that the interaction of highly hydrophobic IHEO with WPNF-MBP’s hydrophobic amino acids (Zhang et al., 2021). However, there was no absorption peak suspected of other components except IHEO and protein molecules. Consequently, the FTIR results illustrated no significant chemical interaction between biopolymer WPNF-MBP and reinforced IHEO.
Fig. 1.
(A) FTIR spectra of free IHEO, WPNF-MBP NPs, and IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8; (B) TEM images of IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8. (IHEO: Industrial hemp essential oil; WPNF: Whey protein nanofibrils; MBP: Mung bean protein; NPs: Nanoparticles).
3.4. Morphology analysis
The morphology and particle size of the IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8 were studied by TEM (Fig. 1B). TEM images of the NPs indicate presence of spherical structure and regular distribution, which seem to be well stable throughout the fabrication process. All particle size of NPs was exhibited approximately < 500 nm, confirming the particle size measured by DLS analysis (Section 3.2 and Table 2).
3.5. Microbiological analysis of Rainbow trout fillets
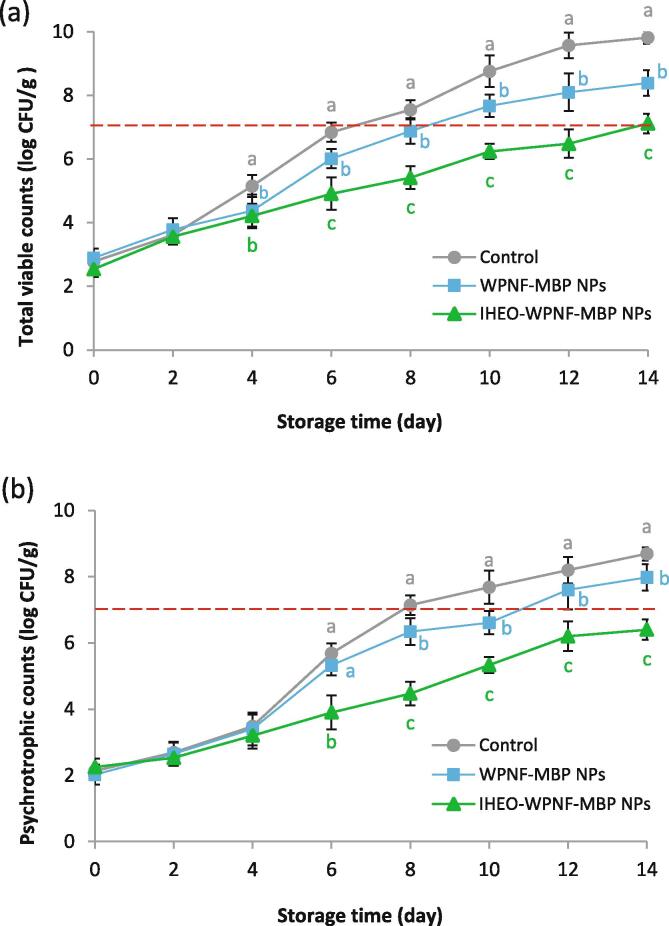
The effect of coating of Rainbow trout fillets with WPNF-MBP and IHEO-reinforced WPNF-MBP NPs on total viable (TVC) and psychrotrophic bacterial (PBC) counts during the refrigerated storage for 14 days is depicted in Fig. 2 a,b. Initial microbial counts in the control sample were 2.13 and 2.78 log CFU/g for TPC and TVC, respectively. In previous studies, the initial microbial counts of Rainbow trout were reported to be in the range of 2–3.5 log CFU/g, which were increased during the storage time (Meral et al., 2019, Ozogul et al., 2017). The values of both PBC and TVC gradually increased in both groups during the 14-day storage, however, with a lower increasing rate in the samples coated with IHEO-reinforced WPNF-MBP NPs. The value of TVC exceeded 7.0 log CFU/g in the control samples at the 8th day of storage, which is the maximum recommended limit of 7 log CFU/g (Sallam et al., 2007) for fresh fish (Fig. 2a). The samples coated with WPNF-MBP and IHEO-reinforced WPNF-MBP NPs had significantly (p < 0.05) lower TVC during 14 days of storage, when compared with the controls. TVC exceeded the limit value at the 14th day of storage for the samples coated with IHEO-reinforced WPNF-MBP NPs. The controls’ PBC value exceeded the maximum recommended limit of 7 log CFU/g in raw fish at the 8th day; this value lower than the acceptable limit in the Rainbow trout fillets coated with WPNF-MBP and IHEO-reinforced WPNF-MBP NPs until the 10th and 14th days of storage, respectively. In a similar study, Oğuzhan Yıldız and Yangılar, (2016) reported that the activity of the microorganisms of Rainbow trout fillets was inhibited by WP concentrate coating during the storage period. The antimicrobial potency observed in the IHEO-reinforced WPNF-MBP NPs may be related to the high content of α-humulene, caryophyllene oxide and (E)-caryophyllene in IHEO that can collapse the microbial cells interacting with the proteins existing in the cytoplasmic membrane, as well as the cell content leakage (Nafis et al., 2019). In addition, the very small size of IHEOs increases the surface area of unit volume, and thus, interact with the structural and biochemical attributes of microbes more effectively and cause cell death (Hadidi et al., 2020). Furthermore, encapsulated materials are able to enhance EOs’ dispersability and stability and transporting them to specific sites. Moreover, they work with nanomaterials synergistically, and increase the encapsulated EOs’ antimicrobial activity (Zhang et al., 2019). It has reported that nano-encapsulation may enhance the EOs’ antimicrobial activity through protecting them from degradation, and thus, increasing their solubility and stability (Hesami et al., 2021).
Fig. 2.
Effect of IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8 coating on the total viable counts (a) and psychrotrophic bacterial counts (b) of Rainbow trout fillets at refrigerated storage * Mean values with different letters in each day represent significant differences (p < 0.05). (IHEO: Industrial hemp essential oil; WPNF: Whey protein nanofibrils; MBP: Mung bean protein; NPs: Nanoparticles).
3.6. Chemical analysis of Rainbow trout fillets
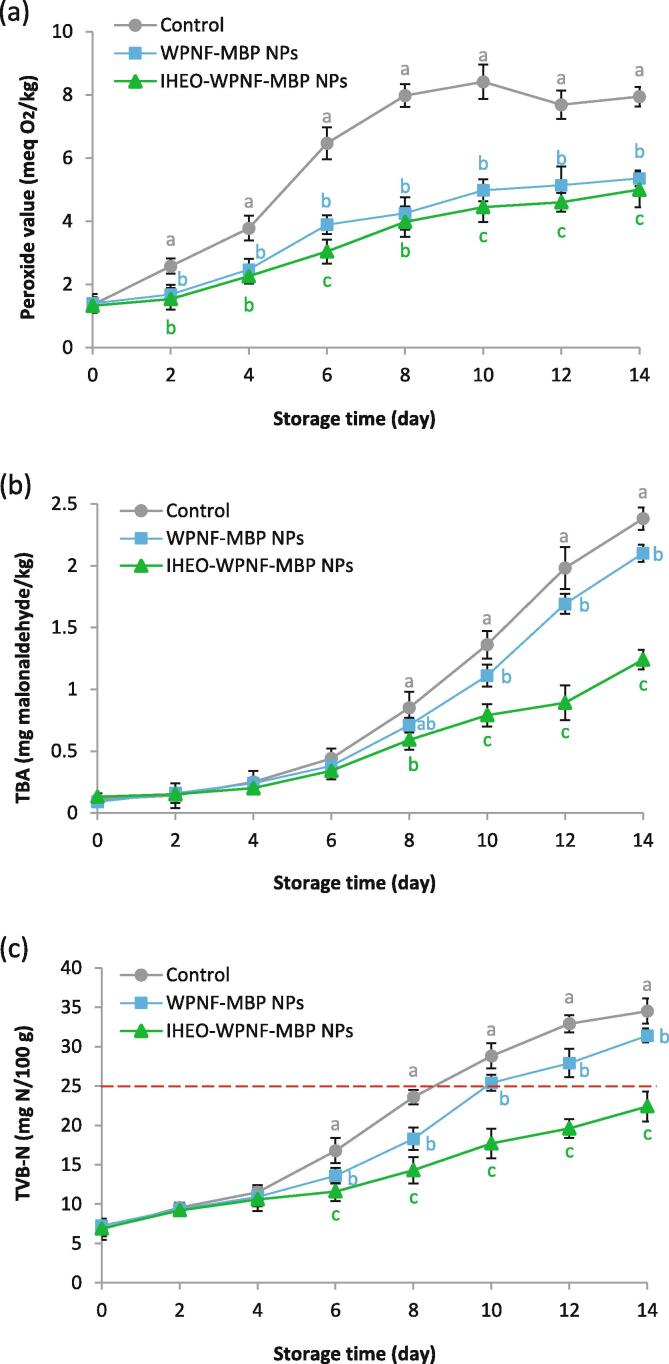
The chemical changes of Rainbow trout fillets were monitored through the measurement of TVB-N, TBA and PV values of the fish oil during storage. Hydroperoxides are the main products of lipid oxidation; thus, measurement of peroxide values is helpful for indicating oxidative rancidity (Zhang et al., 2019). As depicted in Fig. 3a, PV in the control samples increases from 1.33 to 8 meq O2/kg at day 10 and declines after this day, maybe due to the reaction of hydroperoxide with protein as well as the collapse of primary oxidation products into secondary oxidation products (Domínguez et al., 2019). PV level tends to increase toward the end of the storage period. A similar pattern of hydroperoxide content has also been reported in Rainbow trout fillets during storage (Ozogul et al., 2017, Rezaei et al., 2007). The samples coated with WPNF-MBP and IHEO-reinforced WPNF-MBP NPs showed significantly (p < 0.05) lower PV levels than the control during the storage period. The literature considers a PV value of 20 meq O2/kg oil is as the maximum limit for fish (Mexis et al., 2009). Our results indicated that the IHEO-reinforced WPNF-MBP NPs coating is significantly reduced the amount of primary lipid oxidation in fish during storage; this is in agreement with the results of other similar investigations (Farsanipour et al., 2020, Ozogul et al., 2017).
Fig. 3.
Effect of IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8 coating on the PV (a), TBA (b) and TVB-N (c) contents of Rainbow trout fillets at refrigerated storage * Mean values with different letters in each day represent significant differences (p < 0.05). (IHEO: Industrial hemp essential oil; WPNF: Whey protein nanofibrils; MBP: Mung bean protein; NPs: Nanoparticles).
TBA index is a common indicator for evaluating the lipid oxidation level by measuring such oxidation products as aldehydes like malondialdehyde (MDA). The recommended perceivable level of TBA in food as objectionable odour is about 1–2 mg MDA/kg (Dehghani et al., 2018); however, Raeisi et al. (2015) proposed the maximal acceptable level 5 mg MDA eq/kg in Rainbow trout with no negative effects on its safety and quality. As indicated in Fig. 3b, the TBA level remained below the maximal acceptable level during the 14-day storage. The TBA value of all treatments increased gradually up to the end of the storage time. However, in the uncoated samples, the TBA value was much higher than in the coated samples (p < 0.05); however, the initial value of TBA was in the range of 0.09–0.13 mg MDA/kg, consistent with reports of other researchers for fresh Rainbow trout (Dehghani et al., 2018, Shokri et al., 2020). The slightly lower oxidation rate in the IHEO-reinforced WPNF-MBP NPs coatings can be because of the oxygen barrier and antioxidant activity features of IHEO and WPNF. The antioxidant mechanism of IHEO can be due to its polyphenols, which show scavenging activity against free radicals through providing hydrogen atoms to free radicals, preventing radical chain initiation, and thus, preventing the formation of metal catalyzed free radicals (Fiorini et al., 2019). On the other hand, the WP coatings provided a great protection against oxidation. IHEO addition improved the WPNF-MBP NPs coatings’ antioxidant properties. WPs exhibit antioxidant activity through different ways: 1) formation of a coating, which is a good barrier for O2 permeability coated samples during storage, 2) enjoying a free radical scavenging capacity by some amino acids (tryptophan, cysteine and tyrosine) and metal chelation by bovine serum albumin and lactoferrin, 3) having sulfhydryl groups partially responsible for their antioxidant properties, and 4) containing β-lactoglobulins and α-lactalbumin with good antioxidant activity for having amino acid residues (Farsanipour et al., 2020). Furthermore, particle size reduction of IHEO and WPNF-MBP coatings after nano-encapsulation can increase these ingredients’ specific surface; thereby achieving an efficient amount of free radical absorption would be achieved.
TVB-N is produced from degradation of proteins and non-protein nitrogenous compounds, mainly as a result of microbial and enzymatic activities, as an indicator of meat and fish spoilage. The TVB-N level in the control samples was initially 6.68 mg N/100 g, and there was no significant difference (p < 0.05) among the different samples at the first day of storage (Fig. 3c). By increasing the bacterial counts, the TVB-N value increased gradually in all groups; however, in the samples coated with WPNF-MBP and IHEO-reinforced WPNF-MBP NPs, the TVB-N value was significantly lower than in the controls. Considering the maximal acceptable level of 25 mg N/100 g in fish flesh (Bamidele et al., 2019), the TVB-N limit was achieved at day 8 of storage in the controls, while in the WPNF-MBP coated samples, this limit was reached by the 10th days, and in the IHEO-reinforced WPNF-MBP NPs coated samples, it was lower than this limit during the whole period of storage. At the last day of storage, the TVB-N value for the IHEO-reinforced WPNF-MBP NPs coated samples was 12.1 and 9 mg N/100 g lower than the control and WPNF-MBP coated samples, respectively. We previously mentioned that TVB-N is produced mainly because of the bacterial degradation of the nitrogenous compounds of proteins and non-protein products, its low value in the coated samples can be due to the microbial inhibitory effects of treatments that decrease the formation of TVB-N. Similarly, Shokri et al. (2020) found that coating of Rainbow trout fillets with chitosan-Ferulago angulate EO nano-emulsion retarded the increasing rate of TVB-N index during storage at 4 °C. Ozogul et al. (2017) found that nano-emulsions based on plant EOs significantly inhibited the TVB-N formation in Rainbow trout fillets during ice storage. To the best of our knowledge, there are no studies on the effect of IHEO-reinforced WPNF-MBP NPs coating on the formation of TVB-N in Rainbow trout fillets; however, the lower TVB-N in the samples coated with IHEO-reinforced WPNF-MBP NPs in the present work could result from the antibacterial efficiency of WPNF-MBP coating facilitated by nano-encapsulation of IHEO.
3.7. Sensory analysis of Rainbow trout fillets
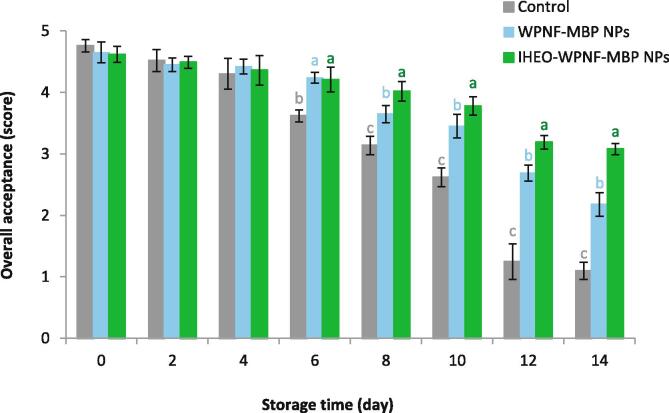
Fig. 4 illustrates the overall acceptable scores of the control and coated Rainbow trout fillets with WPNF-MBP and IHEO-reinforced WPNF-MBP NPs. In the present study, appearance, color, odor, and texture were taken into consideration in the overall acceptance scoring. The overall acceptance scores were in the range of 1–5. High preference levels represent high element scores. All samples exhibited high sensory and quality scores at the first day of analysis. An overall acceptance below 3 of fish is considered to be unacceptable for human consumption (Farsanipour et al., 2020). The overall acceptance scores of the coated and control fish samples showed a decreasing trend up to the end of storage time. The samples coated with IHEO-reinforced WPNF-MBP NPs exhibited a higher score comparing to the other samples during the refrigerated storage (14 days). The control samples’ sensory properties were ‘unacceptable’ by the 10th day. Also, at the day 12, the samples coated with WPNF-MBP did not achieve acceptable scores (2.69) due to their unpleasant appearance and sticky surface; however, the incorporation of IHEO reduced these defects. Hence, the samples coated with IHEO-reinforced WPNF-MBP NPs had better sensory scores than the others for control the lipid oxidation and bacterial population. These results are consistent with the findings of Oğuzhan Yıldız and Yangılar, 2016, Farsanipour et al., 2020 for Rainbow trout WP-based coated samples incorporated with EOs stored at refrigerator condition.
Fig. 4.
Effect of IHEO-reinforced WPNF-MBP NPs with IHEO/WPNF-MBP ratio of 1:0.8 coating on the overall acceptance score of Rainbow trout fillets at refrigerated storage * Mean values with different letters in each day represent significant differences (p < 0.05). (IHEO: Industrial hemp essential oil; WPNF: Whey protein nanofibrils; MBP: mung bean protein; NPs: nanoparticles).
4. Conclusion
As the application of EOs as food preservatives is limited due to having volatile components and their low solubility in water, encapsulation has been introduced over recent years as efficient techniques to boost their utilization in food system. Proteins are attractive options for designing polymeric NPs as suitable wall materials. The present research results confirmed clear discrepancies between the physicochemical properties of NPs prepared at different EO concentrations. Furthermore, the FTIR and TEM results corroborated that IHEO was successfully encapsulated within WPNF-MBP NPs in an amorphous form without specific chemical interaction with the carrier matrix. The present research results can be utilised as reference data for future encapsulation and formulation investigations on different health-promoting bioactive agents, in particular for liposoluble bioactives like EOs that are sensitive to in vitro/in vivo stresses and must be shielded into a proper vehicle matrix. Application of HEO-reinforced WPNF-MBP NPs retarded the bacterial growth of Rainbow trout fillets successfully at refrigeration storage. In addition, the increase of lipid oxidation and TVB-N were controlled notably in Rainbow trout fillets through this method. All these suggest that coating with HEO-reinforced WPNF-MBP NPs can be a natural active packaging for preserving fish fillets.
CRediT authorship contribution statement
Nava Majidiyan: Investigation, Methodology, Software, Data curation, Formal analysis, Writing – original draft. Milad Hadidi: Investigation, Data curation, Supervision, Visualization, Validation, Writing – original draft, Writing – review & editing. Dariush Azadikhah: Project administration, Resources, Funding acquisition, Writing – original draft. Andres Moreno: Supervision, Visualization, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams, R. (2007). Identification of essential oil components by gas chromatography/mass spectrometry.
- Arabpoor B., Yousefi S., Weisany W., Ghasemlou M. Multifunctional coating composed of Eryngium campestre L. essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry fruits. Food Hydrocolloids. 2021;111:106394. doi: 10.1016/j.foodhyd.2020.106394. [DOI] [Google Scholar]
- Ascrizzi R., Ceccarini L., Tavarini S., Flamini G., Angelini L.G. Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition. Industrial Crops and Products. 2019;139:111541. doi: 10.1016/j.indcrop.2019.111541. [DOI] [Google Scholar]
- Bamidele O.P., Duodu K.G., Emmambux M.N. Encapsulation and antioxidant activity of ascorbyl palmitate with normal and high amylose maize starch by spray drying. Food Hydrocolloids. 2019;86:124–133. doi: 10.1016/J.FOODHYD.2018.03.008. [DOI] [Google Scholar]
- Benelli G., Pavela R., Petrelli R., Cappellacci L., Santini G., Fiorini D.…Maggi F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Industrial Crops and Products. 2018;122:308–315. doi: 10.1016/j.indcrop.2018.05.032. [DOI] [Google Scholar]
- Chen F.-P., Kong N.-Q., Wang L., Luo Z., Yin J., Chen Y. Nanocomplexation between thymol and soy protein isolate and its improvements on stability and antibacterial properties of thymol. Food Chemistry. 2021;334:127594. doi: 10.1016/j.foodchem.2020.127594. [DOI] [PubMed] [Google Scholar]
- Dehghani P., Hosseini S.M.H., Golmakani M.T., Majdinasab M., Esteghlal S. Shelf-life extension of refrigerated rainbow trout fillets using total Farsi gum-based coatings containing clove and thyme essential oils emulsions. Food Hydrocolloids. 2018;77:677–688. doi: 10.1016/J.FOODHYD.2017.11.009. [DOI] [Google Scholar]
- R. Domínguez M. Pateiro M. Gagaoua F.J. Barba W. Zhang J.M. Lorenzo 8 10 429 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed]
- Fan L., Lu Y., Ouyang X., Kun, Ling J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. International Journal of Biological Macromolecules. 2021;169:194–205. doi: 10.1016/J.IJBIOMAC.2020.12.086. [DOI] [PubMed] [Google Scholar]
- Farsanipour A., Khodanazary A., Hosseini S.M. Effect of chitosan-whey protein isolated coatings incorporated with tarragon Artemisia dracunculus essential oil on the quality of Scomberoides commersonnianus fillets at refrigerated condition. International Journal of Biological Macromolecules. 2020;155:766–771. doi: 10.1016/J.IJBIOMAC.2020.03.228. [DOI] [PubMed] [Google Scholar]
- Fiorini D., Molle A., Nabissi M., Santini G., Benelli G., Maggi F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Industrial Crops and Products. 2019;128:581–589. doi: 10.1016/J.INDCROP.2018.10.045. [DOI] [Google Scholar]
- Garavand Farhad, Jalai-Jivan Mehdi, Assadpour Elham, Jafari Seid Mahdi. Encapsulation of phenolic compounds within nano/microemulsion systems: A review. Food Chemistry. 2021;364:130376. doi: 10.1016/j.foodchem.2021.130376. [DOI] [PubMed] [Google Scholar]
- Ghobadi Mohammad, Koocheki Arash, Varidi Mohammad Javad, Varidi Mehdi. Encapsulation of curcumin using Grass pea (Lathyrus sativus) protein isolate/Alyssum homolocarpum seed gum complex nanoparticles. Innovative Food Science & Emerging Technologies. 2021;72:102728. doi: 10.1016/j.ifset.2021.102728. [DOI] [Google Scholar]
- Hadidi Milad, Jafarzadeh Shima, Ibarz Albert. Modified mung bean protein: Optimization of microwave-assisted phosphorylation and its functional and structural characterizations. LWT. 2021;151:112119. doi: 10.1016/j.lwt.2021.112119. [DOI] [Google Scholar]
- Hadidi Milad, Motamedzadegan Ali, Jelyani Aniseh Zarei, Khashadeh Sanaz. Nanoencapsulation of hyssop essential oil in chitosan-pea protein isolate nano-complex. LWT. 2021;144:111254. doi: 10.1016/j.lwt.2021.111254. [DOI] [Google Scholar]
- Hadidi Milad, Pouramin Shiva, Adinepour Fateme, Haghani Shaghayegh, Jafari Seid Mahdi. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydrate Polymers. 2020;236:116075. doi: 10.1016/j.carbpol.2020.116075. [DOI] [PubMed] [Google Scholar]
- Hasanpour Ardekani-Zadeh Ali, Hosseini Seyed Fakhreddin. Electrospun essential oil-doped chitosan/poly(ε-caprolactone) hybrid nanofibrous mats for antimicrobial food biopackaging exploits. Carbohydrate Polymers. 2019;223:115108. doi: 10.1016/j.carbpol.2019.115108. [DOI] [PubMed] [Google Scholar]
- Hernández-Nava Ruth, López-Malo Aurelio, Palou Enrique, Ramírez-Corona Nelly, Jiménez-Munguía María Teresa. Encapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray drying. Food Hydrocolloids. 2020;109:106077. doi: 10.1016/j.foodhyd.2020.106077. [DOI] [Google Scholar]
- Hesami Golnaz, Darvishi Sholeh, Zarei Mohammad, Hadidi Milad. Fabrication of chitosan nanoparticles incorporated with Pistacia atlantica subsp. kurdica hulls’ essential oil as a potential antifungal preservative against strawberry grey mould. International Journal of Food Science & Technology. 2021;56(9):4215–4223. doi: 10.1111/ijfs.15110. [DOI] [Google Scholar]
- Hu Yu, He Chengxin, Jiang Chengjia, Liao Yang, Xiong Hua, Zhao Qiang. Complexation with whey protein fibrils and chitosan: A potential vehicle for curcumin with improved aqueous dispersion stability and enhanced antioxidant activity. Food Hydrocolloids. 2020;104:105729. doi: 10.1016/j.foodhyd.2020.105729. [DOI] [Google Scholar]
- Meral Raciye, Alav Aslıhan, Karakas CananYagmur, Dertli Enes, Yilmaz Mustafa Tahsin, Ceylan Zafer. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet. LWT. 2019;113:108292. doi: 10.1016/j.lwt.2019.108292. [DOI] [Google Scholar]
- Mexis S.F., Chouliara E., Kontominas M.G. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiology. 2009;26(6):598–605. doi: 10.1016/J.FM.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Nafis A., Kasrati A., Jamali C.A., Mezrioui N., Setzer W., Abbad A., Hassani L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Industrial Crops and Products. 2019;137:396–400. doi: 10.1016/J.INDCROP.2019.05.032. [DOI] [Google Scholar]
- Natseba A., Lwalinda I., Kakura E., Muyanja C.K., Muyonga J.H. Effect of pre-freezing icing duration on quality changes in frozen Nile perch (Lates niloticus) Food Research International. 2005;38(4):469–474. doi: 10.1016/J.FOODRES.2004.10.014. [DOI] [Google Scholar]
- Yıldız Pınar Oğuzhan, Yangılar Filiz. Effects of different whey protein concentrate coating on selected properties of rainbow trout (Oncorhynchus mykiss) during cold storage (4°C) International Journal of Food Properties. 2016;19(9):2007–2015. doi: 10.1080/10942912.2015.1092160. [DOI] [Google Scholar]
- Ozogul Y., Yuvka İ., Ucar Y., Durmus M., Kösker A.R., Öz M., Ozogul F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT. 2017;75:677–684. doi: 10.1016/J.LWT.2016.10.009. [DOI] [Google Scholar]
- Prata, A. S., & Grosso, C. R. F. (2015). Influence of the Oil Phase on the Microencapsulation by Complex Coacervation. Journal of the American Oil Chemists’ Society 2015 92:7, 92(7), 1063–1072. https://doi.org/10.1007/S11746-015-2670-Z.
- Raeisi M., Tajik H., Aliakbarlu J., Mirhosseini S.H., Hosseini S.M.H. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT - Food Science and Technology. 2015;64(2):898–904. doi: 10.1016/J.LWT.2015.06.010. [DOI] [Google Scholar]
- Rezaei M., Montazeri N., Langrudi H.E., Mokhayer B., Parviz M., Nazarinia A. The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss) stored in ice. Food Chemistry. 2007;103(1):150–154. doi: 10.1016/J.FOODCHEM.2006.05.066. [DOI] [Google Scholar]
- Rostamabadi Hadis, Falsafi Seid Reza, Assadpour Elham, Jafari Seid Mahdi. Evaluating the structural properties of bioactive-loaded nanocarriers with modern analytical tools. Comprehensive Reviews in Food Science and Food Safety. 2020;19(6):3266–3322. doi: 10.1111/1541-4337.12653. [DOI] [PubMed] [Google Scholar]
- Rostamabadi H., Sadeghi Mahoonak A., Allafchian A., Ghorbani M. Fabrication of β-carotene loaded glucuronoxylan-based nanostructures through electrohydrodynamic processing. International Journal of Biological Macromolecules. 2019;139:773–784. doi: 10.1016/J.IJBIOMAC.2019.07.182. [DOI] [PubMed] [Google Scholar]
- Sallam K.I., Ahmed A.M., Elgazzar M.M., Eldaly E.A. Chemical quality and sensory attributes of marinated Pacific saury (Cololabis saira) during vacuum-packaged storage at 4 °C. Food Chemistry. 2007;102(4):1061–1070. doi: 10.1016/J.FOODCHEM.2006.06.044. [DOI] [Google Scholar]
- Shadman S., Hosseini S.E., Langroudi H.E., Shabani S. Evaluation of the effect of a sunflower oil-based nanoemulsion with Zataria multiflora Boiss. essential oil on the physicochemical properties of rainbow trout (Oncorhynchus mykiss) fillets during cold storage. LWT - Food Science and Technology. 2017;79:511–517. doi: 10.1016/J.LWT.2016.01.073. [DOI] [Google Scholar]
- Shokri S., Parastouei K., Taghdir M., Abbaszadeh S. Application an edible active coating based on chitosan- Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. International Journal of Biological Macromolecules. 2020;153:846–854. doi: 10.1016/J.IJBIOMAC.2020.03.080. [DOI] [PubMed] [Google Scholar]
- Tabari Mohaddeseh Abouhosseini, Khodashenas Aref, Jafari Maryam, Petrelli Riccardo, Cappellacci Loredana, Nabissi Massimo.…Youssefi Mohammad Reza. Acaricidal properties of hemp (Cannabis sativa L.) essential oil against Dermanyssus gallinae and Hyalomma dromedarii. Industrial Crops and Products. 2020;147:112238. doi: 10.1016/j.indcrop.2020.112238. [DOI] [Google Scholar]
- Tarhini M., Greige-Gerges H., Elaissari A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. International Journal of Pharmaceutics. 2017;522(1–2):172–197. doi: 10.1016/J.IJPHARM.2017.01.067. [DOI] [PubMed] [Google Scholar]
- Volić Mina, Pajić-Lijaković Ivana, Djordjević Verica, Knežević-Jugović Zorica, Pećinar Ilinka, Stevanović-Dajić Zora.…Bugarski Branko. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydrate Polymers. 2018;200:15–24. doi: 10.1016/j.carbpol.2018.07.033. [DOI] [PubMed] [Google Scholar]
- Yousuf B., Wu S., Siddiqui M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends in Food Science & Technology. 2021;108:245–257. doi: 10.1016/J.TIFS.2021.01.016. [DOI] [Google Scholar]
- Zhang Chao, Fu Yuying, Li Zeya, Li Teng, Shi Yugang, Xie Hujun.…Li Zhenpeng. Application of whey protein isolate fibrils in encapsulation and protection of β-carotene. Food Chemistry. 2021;346:128963. doi: 10.1016/j.foodchem.2020.128963. [DOI] [PubMed] [Google Scholar]
- Zhang Huiyun, Li Xinling, Kang Huaibin. Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT. 2019;116:108580. doi: 10.1016/j.lwt.2019.108580. [DOI] [Google Scholar]