Abstract
Introduction
Socioeconomic status (SES) is recognized as an important determinant of kidney health. We aimed to evaluate the association of social deprivation with different indicators at kidney replacement therapy (KRT) initiation in the French pediatric metropolitan population.
Methods
All patients with end-stage kidney disease (ESKD) who started KRT before 20 years old in France between 2002 and 2015 were included. We investigated different indicators at KRT initiation, which are as follows: KRT modality (dialysis vs. pre-emptive transplantation), late referral to a nephrologist, and dialysis modality (hemodialysis [HD] vs. peritoneal dialysis [PD], urgent vs. planned start of dialysis, use of catheter vs. use of fistula for HD vascular access). An ecological index (European Deprivation Index [EDI]) was used as a proxy for social deprivation.
Results
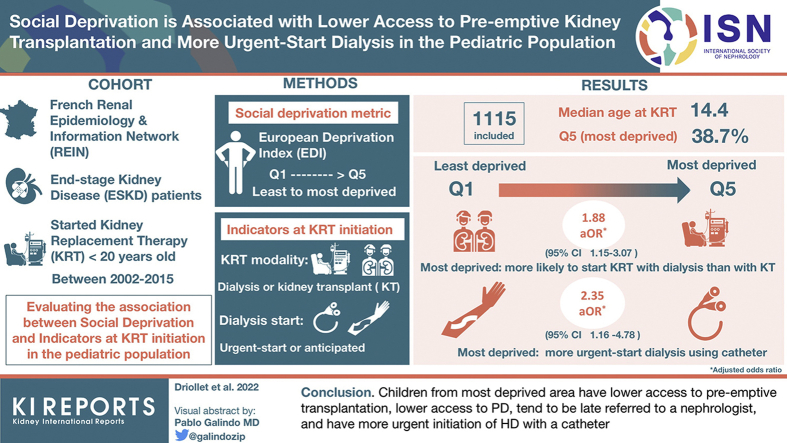
A total of 1115 patients were included (males 59%, median age at dialysis 14.4 years, glomerular/vascular diseases 36.8%). The most deprived group represented 38.7% of the patients, suggesting pediatric patients with ESKD come from a more socially deprived background. The most deprived group was more likely to initiate KRT with dialysis versus kidney transplantation. Among patients on HD, the odds of starting treatment in emergency with a catheter was >2-fold higher for the most deprived compared with the least deprived children (adjusted odds ratio [aOR] 2.35, 95% CI 1.16–4.78).
Conclusion
Children from the most deprived area have lower access to pre-emptive transplantation, have lower access to PD, tend to be late referred to a nephrologist, and have more urgent initiation of HD with a catheter.
Keywords: dialysis initiation, end stage kidney disease, French EDI, pediatric kidney replacement therapy, social deprivation
Graphical abstract
See Commentary on Page 671
The relationship between social deprivation and chronic kidney disease (CKD) has been increasingly investigated in the recent years. Various studies, mainly conducted in adult patients, have identified different social determinants of kidney health.1 Factors such as individual-level SES, neighborhood poverty, or belonging to disadvantaged populations may contribute to disparities in CKD, including provision of KRT.2 Indeed, low SES has been associated with decreased glomerular filtration rate, higher albuminuria level, and higher risk of kidney failure.3 Furthermore, social determinants are known to be associated with worse transplant outcomes in adults, such as lower access to transplantation and poorer kidney graft survival.4, 5, 6
Pediatric studies have mostly focused on kidney transplant outcomes. For example, SES of the family (evaluated by income, educational level, or a social deprivation index) has been found to be significantly associated with kidney graft survival in children in the United States7 and France.7,8 In the United States, a higher level of deprivation was also associated with a lower access to the transplant waiting list and a shorter kidney graft survival.9,10 Data on the role of social deprivation in earlier CKD stages are scarce in the pediatric population, although it would help for evaluating the needs of implementation of appropriate socioeducational measures. In Australia and New Zealand, lower SES was associated with poorer parent-rated clinical conditions of children in early stages of CKD but not in dialysis.11 In the United States and Canada, African American children had higher mortality and lower access to transplant within 1 year of dialysis initiation,12 and SES partly explained their observed faster progression to ESKD.13 In the United Kingdom, social deprivation was not associated with late referral, but it strongly associated with access to pre-emptive kidney transplantation.14
To our knowledge, very few studies investigated the role of social deprivation in children undergoing KRT initiation in a country with a universal health system such as in France. Indeed, in France, CKD is officially recognized as a long-term condition, and as such, all patients with CKD benefit from a care offer covered at 100% by the health insurance. This includes medications, equipment for PD, traveling to the hospital, psychosocial support, and school assistance, which implies not out-of-pocket expenses for patients. Although we found disparities in kidney graft survival in this specific context,8 it is questionable whether socioeconomic disparities also exist for access to pre-emptive kidney transplantation, or for the modality of dialysis initiation.
The objective of this study was to evaluate the association of social deprivation with different indicators at KRT initiation, such as KRT modality, dialysis modality, urgent start of dialysis, and late referral to a nephrologist, in the French pediatric population.
Methods
Study Design, Setting, and Population
Data were collected from the French Renal Epidemiology and Information Network (REIN), an exhaustive national KRT registry.15 The present cross-sectional study included all patients living in metropolitan France, who started KRT before the age of 20 years between January 1, 2002 and December 31, 2015. Patients with both (i) a kidney tumor or congenital nephrotic syndrome or bilateral nephrectomy and (ii) a missing or an estimated glomerular filtration rate higher than 30 ml/min per 1.73 m2 before KRT were excluded because they were not considered as having reached ESKD.
Outcomes
We studied different indicators at KRT initiation, which are as follows: KRT initiation with dialysis (vs. pre-emptive transplantation), KRT initiation after pre-emptive registration on the kidney transplant waiting list (vs. not), dialysis initiation with HD (vs. PD), urgent initiation of dialysis (vs. planned), urgent HD initiation with a catheter (vs. other HD initiation), HD initiation with a catheter (vs. an arteriovenous fistula), and late referral (vs. not). The development of the variable late referral has been described elsewhere.16 It was based on a linkage between the REIN registry and the French national health care administrative database, which have recorded all medical information since January 1, 2008.16,17 The date of referral was the earliest date found among the following 3 types of events: (i) the first nephrology-related prescription drug (any CKD-related drug); (ii) the first hospitalization with a nephrology diagnostic (CKD-compatible diagnosis, not acute kidney injury or pyelonephritis); or (iii) the first consultation with a nephrologist.16 A child was defined as having a late referral when the date of referral was within the 3 months preceding dialysis initiation. To ensure at least 1 year follow-up in the insurance database before dialysis initiation for all patients, we conducted this analysis on a subgroup of patients who started chronic dialysis between January 1, 2009 and December 31, 2015.
Social Deprivation Assessment
In the REIN registry, information on the individual SES is not collected. Hence, we used a validated ecological index of deprivation, the 2011 French version of the EDI,18 built from the 2011 French population census data and a 2006 European survey on social deprivation (European Union—Statistics on Income and Living).19 EDI is a continuous score taking into account individuals’ cultural and social environments, based on the linear combination of the following 10 variables representing the proportion, in the neighborhood, of: (i) overcrowded housing, (ii) housing with no access to a bath or a shower, (iii) household without access to a car, (iv) no executive nor intermediate occupation, (v) single-parent household, (vi) household with ≥6 persons, (vii) unemployed people, (viii) household nonowners, (ix) persons with foreign nationality, and (x) persons with less than the first stage of secondary educational level.18 Geographic delimitation of the neighborhood was defined by a geographic unit called Ilots Regroupés pour l’Information Statistiques (IRIS), which has been developed to divide the country into units of equal size of approximately 2000 residents, to prepare population census.20
For each child, we assigned the IRIS, and thus a quantitative value of EDI corresponding to his/her residential address collected at the time of registration on the transplant waiting list. Indeed, the exact addresses of patients at the time of dialysis initiation were collected in the REIN database from January 2016 only, thus after our inclusion period. Before 2016, only the postal code at dialysis initiation was available. For all children who were waitlisted between January 1, 2002 and December 31, 2016 (actually almost all children as described in the Results section), we thus used their address at registration and assumed that they did not move between the dialysis initiation and registration (median time of approximately 6 months). The children who had an address at registration not sufficiently precise were excluded from the main statistical analysis. Patients with a temporary address (corresponding to the hospital) were also excluded from the main statistical analysis. For some of the few patients never waitlisted, we were able to retrieve their IRIS at dialysis initiation from their postal code. We used the continuous version of the EDI and its quintiles to categorize each patient into each 5 groups of deprivation, from the least deprived (Q1) to the most deprived (Q5). The quintiles correspond to the IRIS of the 2011 French population census.
Potential Confounders and Effect Modifier
We used a causal diagram to identify the set of adjustment factors to investigate the association between deprivation and each indicator at KRT initiation (Supplementary Figure S1, only factors with available data are represented). As a result, we adjusted all analyses for age at KRT initiation (in years), context of living environment (urban vs. rural), and primary kidney disease (congenital anomalies of the kidney and urinary tract, hereditary nephropathy, glomerular or vascular disease, and other or unknown). We also investigated whether the association between social deprivation (EDI in quintile) and the different outcomes differed across sex and the type of center (pediatric vs. adult).
Statistical Analysis
First, we compared the characteristics of included and excluded patients. Then, we compared the characteristics of patients according to the quintiles of EDI. To evaluate the association between deprivation and the different binary indicators, we performed logistic regressions, adjusted for all potential confounders, using either the continuous score of EDI or its quintiles. To account for potential nonlinear effects of quantitative variables (continuous EDI and age at KRT initiation), we used natural cubic splines with a number of interior knots placed to minimize the Akaike Information Criterion.21,22 The nonlinear effect was tested using Wald test between the model including the nonlinear function versus the model excluding the variable. We did not take into account the correlation of observations between children living in the same IRIS, given the small number of children living in the same IRIS (<5%). We considered that there was an effect modification by sex and the type of center if the P value of the likelihood ratio test evaluating all interaction terms was <5%. In a sensitivity analysis, we imputed the missing quintiles of EDI and the context of rural environment variable using multiple imputation (R package MICE, R Foundation, Vienna, Austria).
Results
Population Characteristics
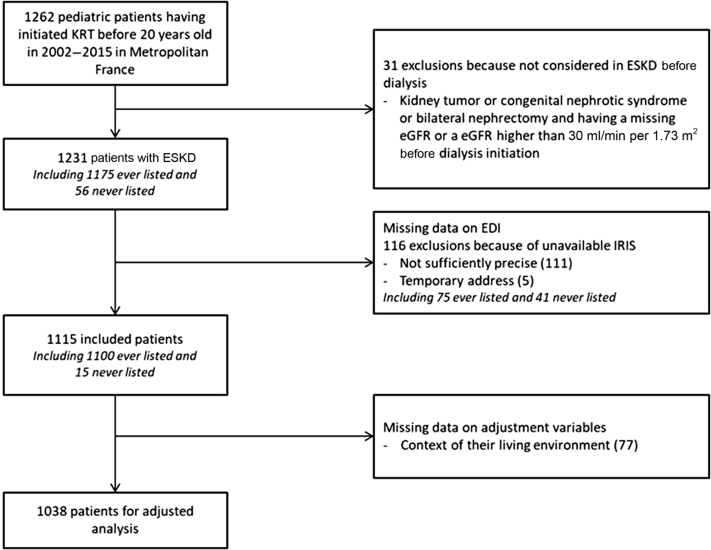
Among 1262 children and adolescents who started KRT between January 1, 2002 and December 31, 2015, and were living in metropolitan France, 31 were excluded because they were not considered having reached ESKD before KRT (Figure 1). Among the remaining 1231 children constituting our target population, 116 were further excluded from the main statistical analysis because of missing data on their reported residential address: 111 had an address not precise enough to identify the corresponding IRIS and 5 gave a temporary address at the hospital. A total of 1115 patients with ESKD were thus included in the main statistical analysis, including 1038 complete cases for adjusted analyses. Compared with the patients with ESKD living in metropolitan France (n = 1231), the 1115 patients included in the main analysis and the 1038 patients contributing to the adjusted analyses had similar characteristics (Supplementary Table S1).
Figure 1.
Selection of children from the French renal registry (REIN), 2002 to 2015. EDI, European Deprivation Index; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IRIS, Ilots Regroupés pour l’Information Statistiques; KRT, kidney replacement therapy; REIN, French kidney replacement therapy registry.
Characteristics of Included Patients at KRT Initiation
Among the 1115 patients included in the main statistical analysis, 59% were males, most lived in urban areas (79%), and median age at KRT initiation was 14.4 years. The most common cause of ESKD was glomerular or vascular disease (36.8%), and 63.1% of them initiated treatment in a pediatric care center (Table 1). Most patients started KRT with dialysis (approximately 80%), and 38.4% were registered pre-emptively on the transplant waiting list (Table 2). Between 12% and 20% of patients were distributed in each quintile 1 to 4 (Q1–Q4) of EDI, whereas the most deprived quintile (Q5) comprised nearly 39% of the patients. Patients in Q1 to Q3 of EDI were more often placed pre-emptively on the waiting list (approximately 48%) than those from quintiles Q4 and Q5 (approximately 33%), and patients in Q1 were younger at registration than in Q5 (13.7 years vs. 15.1 years). Moreover, patients in Q5 started dialysis more frequently with HD than those in Q1 (65.0% vs. 46.0%) and received less often a pre-emptive kidney transplantation (15.5% vs. 25.8%) (Table 2).
Table 1.
Characteristics of young patients with ESKD at KRT initiation according to EDI quintiles (quintile 5 most deprived). Data from REIN, metropolitan France, 2002 to 2015 (N = 1115)
| Variable | All children N = 1115 |
Quintile 1 n = 163 (14.6%) |
Quintile 2 n = 144 (12.9%) |
Quintile 3 n = 162 (14.5%) |
Quintile 4 n = 215 (19.3%) |
Quintile 5 n = 431 (38.7%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Median [IQ] | n (%) | Median [IQ] | n (%) | Median [IQ] | n (%) | Median [IQ] | n (%) | Median [IQ] | n (%) | Median [IQ] | |
| Male | 659 (59.0) | 101 (62.0) | 82 (56.9) | 96 (59.3) | 128 (59.9) | 252 (58.5) | ||||||
| Age at KRT initiation (yr) | 1115 | 14.4 [9.0–17.9] | 163 | 13.5 [7.7–17.3] | 144 | 14.2 [7.3–17.7] | 162 | 14.8 [9.0–17.9] | 215 | 15.0 [9.8–18.0] | 431 | 14.7 [9.2–18.0] |
| 0–4 | 181 (16.2) | 26 (16.0) | 27 (18.8) | 25 (15.4) | 29 (13.5) | 74 (17.2) | ||||||
| 5–12 | 276 (24.8) | 47 (28.8) | 33 (22.9) | 40 (24.7) | 59 (27.4) | 97 (22.5) | ||||||
| 13–17 | 394 (35.3) | 58 (35.6) | 53 (36.8) | 57 (35.2) | 73 (34.0) | 153 (35.5) | ||||||
| 18–20 | 264 (23.7) | 32 (19.6) | 31 (21.5) | 40 (24.7) | 54 (25.1) | 107 (24.8) | ||||||
| Age at registration on waiting list (yr) (missing = 15) | 1100 | 14.6 [9.1–18.0] | 158 | 13.7 [8.4–17.4] | 142 | 14.3 [8.0–17.7] | 161 | 15.2 [9.2–17.8] | 213 | 15.1 [9.5–18.0] | 426 | 15.1 [9.3–18.4] |
| Primary disease | ||||||||||||
| CAKUT | 313 (28.1) | 51 (31.3) | 41 (28.9) | 45 (28.0) | 63 (29.4) | 113 (26.3) | ||||||
| Hereditary nephropathy | 228 (20.4) | 32 (19.6) | 29 (20.4) | 42 (26.1) | 44 (20.6) | 81 (18.9) | ||||||
| Glomerular/vascular disease | 362 (32.5) | 46 (28.2) | 48 (33.8) | 47 (29.3) | 64 (29.9) | 157 (36.6) | ||||||
| Other/unknown | 212 (19.0) | 34 (20.9) | 24 (16.9) | 27 (16.8) | 43 (20.1) | 78 (18.2) | ||||||
| Context of rural environment (missing = 77) | 218 (21.0) | 46 (29.1) | 51 (36.4) | 59 (37.8) | 53 (26.8) | 9 (2.3) | ||||||
| Pediatric kidney care center | 704 (63.1) | 114 (69.9) | 89 (61.8) | 90 (55.6) | 131 (60.9) | 280 (65.0) | ||||||
| eGFR (ml/min per 1.73 m2)a (missing = 411) | 704 | 8.1 [5.9–10.8] | 101 | 7.7 [5.6–10.5] | 82 | 8.5 [6.0–10.7] | 95 | 8.4 [6.0–10.9] | 140 | 7.9 [6.2–10.8] | 286 | 8.2 [5.8–10.8] |
CAKUT, congenital anomalies of the kidney and urinary tract; EDI, European Deprivation Index; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; IQ, interquartile; KRT, kidney replacement therapy; REIN, French kidney replacement therapy registry.
Only for children having initiated KRT with dialysis, n = 885.
Table 2.
Care indicators of young patients with ESKD at KRT initiation according to EDI quintiles (quintile 5 most deprived). Data from REIN, metropolitan France, 2002 to 2015
| Indicator | All children N = 1115 |
Quintile 1 n = 163 (14.6%) |
Quintile 2 n = 144 (12.9%) |
Quintile 3 n = 162 (14.5%) |
Quintile 4 n = 215 (19.3%) |
Quintile 5 n = 431 (38.7%) |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Treatment at initiation | ||||||
| Pre-emptive transplantation | 230 (20.6) | 42 (25.8) | 38 (26.4) | 42 (25.9) | 41 (19.1) | 67 (15.5) |
| Dialysis | 885 (79.4) | 121 (74.2) | 106 (73.6) | 120 (74.1) | 174 (80.9) | 364 (84.5) |
| HD | 644 (57.8) | 75 (46.0) | 71 (49.3) | 85 (52.5) | 133 (61.8) | 280 (65.0) |
| Peritoneal dialysis | 241 (21.6) | 46 (28.2) | 35 (24.3) | 35 (21.6) | 41 (19.1) | 84 (19.5) |
| Pre-emptive registration (missing = 15) | 428 (38.4) | 77 (48.7) | 59 (41.5) | 78 (48.4) | 74 (34.7) | 140 (32.9) |
| Urgent dialysis initiation (missing = 59) | 271 (32.8) | 30 (27.8) | 44 (45.8) | 34 (30.6) | 60 (35.9) | 103 (29.9) |
| HD initiation with a catheter (missing = 22) | 376 (60.4) | 38 (51.4) | 52 (75.4) | 46 (55.4) | 80 (62.5) | 160 (59.7) |
| Late referrala (missing = 467) | 62 (11.4) | 3 (6.2) | 7 (13.2) | 7 (13.2) | 15 (16.1) | 30 (16.4) |
EDI, European Deprivation Index; ESKD, end-stage kidney disease; HD, hemodialysis; KRT, kidney replacement therapy; REIN, French kidney replacement therapy registry.
Only for children having initiated dialysis in 2009 to 2015, n = 545.
Association Between Social Deprivation and Care Indicators at KRT Initiation
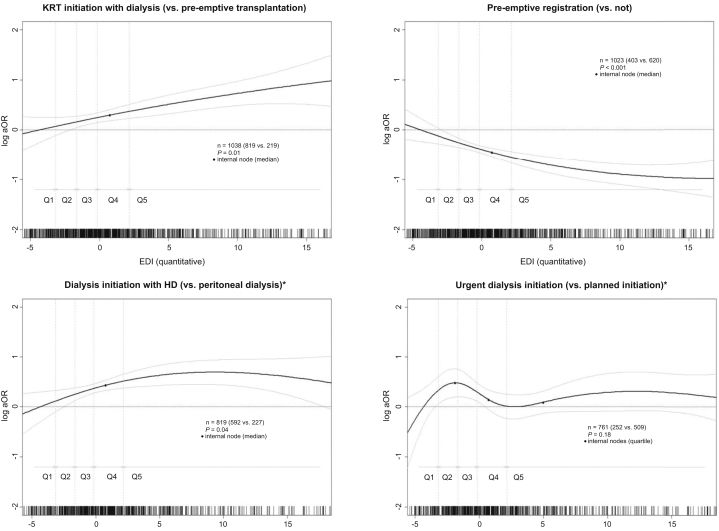
After adjustment for potential confounders, the odds of initiating KRT with dialysis (vs. pre-emptive transplantation) continuously increased with increasing deprivation index (Figure 2) and was almost twice higher for children from the most deprived than for the least deprived area (Q5 vs. Q1: aOR 1.88, 95% CI 1.15–3.07) (Table 3). The odds of pre-emptive registration continuously decreased with increasing deprivation index (Figure 2) and was 50% lower for children from Q5 than from Q1 (aOR 0.50, 95% CI 0.33–0.77) (Table 3).
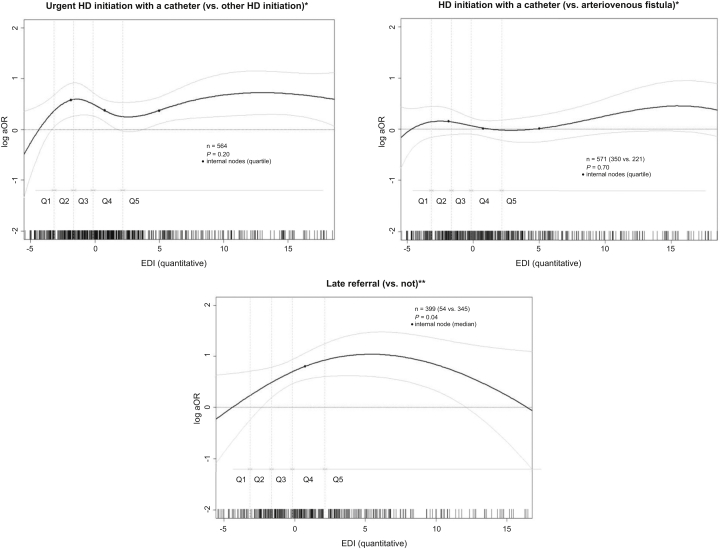
Figure 2.
Association between social deprivation measured by the EDI (in continuous) and care indicators at kidney replacement therapy initiation in young patients in Metropolitan France. Adjusted for age at KRT initiation (spline), context of rural environment (rural/urban), and primary kidney disease (4 categories). The reference value of EDI for the log OR indicated in the y-axis was arbitrarily chosen at the median value of Q1 (least deprived). Data from REIN, 2002 to 2015. ∗Only for children having initiated a dialysis. ∗∗Only for children having initiated KRT in 2009 to 2015. EDI, European Deprivation Index; ESKD, end-stage kidney disease; HD, hemodialysis; KRT, kidney replacement therapy; OR, odds ratio; Q, quintile; REIN, French kidney replacement therapy registry.
Table 3.
Association between social deprivation measured by the EDI (in quintile) and care indicators at KRT initiation in young patients with ESKD in metropolitan France. Data from REIN, 2002 to 2015
| Indicator | ORa | 95% CI | P value |
|---|---|---|---|
| All patients | |||
| KRT initiation with dialysis (vs. pre-emptive transplantation) (n = 1038) | |||
| EDI quintiles | 0.03 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 1.01 | 0.58–1.77 | |
| Q3 | 1.05 | 0.61–1.81 | |
| Q4 | 1.66 | 0.97–2.85 | |
| Q5 (most deprived) | 1.88 | 1.15–3.07 | |
| Pre-emptive registration (vs. not) (n = 1023) | |||
| EDI quintiles | 0.001 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 0.71 | 0.43–1.19 | |
| Q3 | 0.95 | 0.58–1.55 | |
| Q4 | 0.48 | 0.30–0.77 | |
| Q5 (most deprived) | 0.50 | 0.33–0.77 | |
| Patients initiating KRT with dialysis (n = 819) | |||
| Dialysis initiation with HD (vs. PD) | |||
| EDI quintiles | 0.22 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 1.37 | 0.72–2.61 | |
| Q3 | 1.58 | 0.83–2.98 | |
| Q4 | 1.88 | 1.04–3.42 | |
| Q5 (most deprived) | 1.77 | 1.03–3.02 | |
| Urgent dialysis initiation (vs. planned) (n = 761) | |||
| EDI quintiles | 0.08 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 2.18 | 1.16–4.10 | |
| Q3 | 1.20 | 0.64–2.24 | |
| Q4 | 1.70 | 0.96–3.01 | |
| Q5 (most deprived) | 1.28 | 0.74–2.19 | |
| Patients initiating dialysis with HD | |||
| HD initiation with a catheter (vs. AV fistula) | |||
| EDI quintiles (n = 571) | 0.07 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 2.90 | 1.33–6.31 | |
| Q3 | 1.14 | 0.56–2.31 | |
| Q4 | 1.46 | 0.77–2.78 | |
| Q5 (most deprived) | 1.66 | 0.92–2.98 | |
| Urgent HD initiation with a catheter (vs. other HD initiation) | |||
| EDI quintiles (n = 564) | 0.01 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 4.12 | 1.82–9.31 | |
| Q3 | 1.63 | 0.72–3.65 | |
| Q4 | 2.84 | 1.35–5.98 | |
| Q5 (most deprived) | 2.35 | 1.16–4.78 | |
| Patients initiating dialysis in 2009–2015 | |||
| Late referral (vs. not) | |||
| EDI quintiles (n = 399) | 0.61 | ||
| Q1 (least deprived) | Ref | ||
| Q2 | 2.44 | 0.56–10.54 | |
| Q3 | 2.19 | 0.51–9.37 | |
| Q4 | 2.78 | 0.71–10.83 | |
| Q5 (most deprived) | 2.94 | 0.78–11.09 |
AV fistula, arteriovenous fistula; EDI, European Deprivation Index; ESKD, end-stage kidney disease; HD, hemodialysis; KRT, kidney replacement therapy; OR, odds ratio; PD, peritoneal dialysis; Q, quintile; Ref, reference; REIN, French kidney replacement therapy registry.
Adjusted for age at KRT initiation (spline), context of rural environment (rural/urban), and primary kidney disease (4 categories).
Regarding children who initiated KRT with dialysis, the odds of starting with HD (vs. PD) also tended to continuously increase with increasing deprivation index (Figure 2) and was 77% higher for children from Q5 than from Q1 (aOR 1.77, 95% CI 1.03–3.02) (Table 3). The odds of other indicators (urgent dialysis initiation, HD initiation with a catheter, urgent HD initiation with a catheter, late referral) did not continuously increase with increasing EDI but tended to be systematically lower for the least deprived children (Q1) than for all others (Q2–Q5) (Figure 2). For example, the odds of urgent HD initiation with a catheter (vs. other HD initiation) was >2-fold higher for the most deprived than for the least deprived children (Q5 vs. Q1: aOR 2.35, 95% CI 1.16–4.78) (Table 3). In the subgroup of patients who initiated dialysis in 2009 to 2015, children from Q2 to Q5 tended to be late referred compared with children from the least deprived quintile Q1 (Table 3).
The association between EDI (quintile) and any outcome did not statistically differ between males and females (all P values for interaction > 0.05) and between pediatric and adult centers (all P values for interaction > 0.05).
After imputing the missing EDI quintiles and the missing context of rural environment variable, the odds ratios remained of the same magnitude in the full population of the 1231 children with ESKD living in metropolitan France (Supplementary Table S2).
Discussion
In this study, we evaluated, in a European country with universal coverage, the association between social deprivation assessed by the EDI and indicators at KRT initiation in pediatric patients. Our results suggested that pediatric patients initiating KRT were more deprived than the general population. Indeed, Q5 of EDI includes the 20% most deprived areas in metropolitan France, corresponding to approximately 30% of the overall child population, whereas it included approximately 39% of our pediatric patients. Patients from the most deprived areas had a lower likelihood to be pre-emptively registered, to initiate KRT with pre-emptive kidney transplantation, and to initiate dialysis with PD and a higher likelihood of being late referred, having unplanned dialysis initiation, or start HD treatment in emergency with a catheter, compared with children from the least deprived areas, after adjusting for age at KRT initiation, context of rural environment, and primary kidney disease.
These results are consistent with findings from few previous studies in the pediatric ESKD population. Although no association was found in Australia (contrary to remoteness),23 a study found such disparities in the United Kingdom for access to pre-emptive kidney transplantation but not for late referral.14 In the United States, a better access to pre-emptive transplantation was found for White children.9,24 Regarding modality of dialysis initiation, Schaefer et al.25 found that the use of nightly intermittent PD at a country level increased with growth national income contrary to continuous ambulatory PD. In the United States, poverty and low educational levels were arguments to rule out children from starting PD because they were associated with worse technique outcome.26 In a Lebanese study, among various factors, family structure and poor SES household were associated with poor adaptation to dialysis in children on chronic PD.27 Even if it has been found that there was no difference in survival between PD and HD,28,29 PD may offer a more “normal” social life (school, activities) in children and has been associated with a better quality of life.30 Schaefer et al.25 also found that pediatric patients in chronic dialysis from low-income countries had more often poor growth than patients from wealthier countries, achieved less targets for parathyroid hormone, calcium, and hemoglobin levels, and had a higher mortality. In studies on the impact of ethnicity, it was found that SES remained an independent determinant of progression toward first KRT.31 In a low-income country such as Nicaragua, a worse socioeconomic condition was a contributor to mortality in children with ESKD requiring KRT.32 In the French context, our results confirm the findings of studies in adults and suggest that social inequalities in the initiation of KRT begin in childhood in France. Chatelet et al.33 found that EDI was associated with an increased risk of death after transplantation but not with kidney graft failure in adults. It has been found that geographic clusters of municipalities at high risk of urgent start of dialysis in adults were actually less urbanized and had lower sociodemographic background than areas at lower risk.34 In 3 other studies also using EDI, social deprivation was associated with a lower PD uptake35 and a decreased access to pre-emptive kidney transplant36; the most deprived adults were less likely to be on self-care dialysis.37
One of our main findings is that children who were living in a more deprived area were more likely to initiate KRT with dialysis, with a clear social gradient. These results raise questions on the barriers leading to social inequality because pre-emptive kidney transplantation has been found to improve kidney transplant outcomes.38 Plumb et al.14 recently revealed in the United Kingdom that the association between social deprivation and access to pediatric kidney care could be mediated by the access to living donor transplant, but the reasons explaining this social gradient are certainly more complex (psychosocial decision, role of clinicians’ perspective …) and require further investigation.
Regarding modality of dialysis initiation, children from the most deprived areas had a lower likelihood to initiate dialysis with PD than those from the least deprived areas. One potential explanation is that PD is a home therapy and thus requires a good adherence to instructions by the parents and enough space in the house to store the dialysis machine and PD fluids. We may speculate that physicians might expect it to be easier for wealthier families to support this treatment burden, but this should be further investigated using mixed methods integrating both quantitative and qualitative data. Another explanation is the possibility of a center effect, which could be a potential effect modifier of the association between social deprivation and the different outcomes, especially in the choice of the dialysis modality. Because of the number of centers and their heterogeneity in terms of volume, we investigated this interaction using a binary indicator (pediatric vs. adult center), but no interaction was statistically significant. Our results also suggested that the most disadvantaged children tended to start KRT more urgently with a catheter than those from the least deprived areas. This urgent start of KRT of socially deprived patients may be a consequence of a lack of prenatal diagnosis of severe congenital anomalies of the kidney and urinary tract, lack of early detection of CKD, delayed referral to specialized care, and absence of follow-up by a pediatric nephrologist (or a reduced one) before reaching ESKD. The results of the subgroup analyses on late referral support this hypothesis, although the association did not reach statistical significance, maybe because of insufficient statistical power. As reported by others,39, 40, 41 our findings highlight the need to address the issue of early detection and regular follow-up of children with kidney diseases.
Our study has some strengths and limitations. A strength of our study is the exhaustiveness of cases of children initiating KRT in metropolitan France. Indeed, the REIN registry is a comprehensive nationwide registry of all adult and pediatric patients receiving KRT. Although the REIN registry includes patients living in French overseas departments, we decided to focus only on children living in the European part of France to have a more homogeneous context of living environment, and because EDI has not been validated for areas outside Europe. Nevertheless, further studies including this population are needed. Although clinical and process of care information on KRT was available, we did not take into account comorbidities because of the large amount of missing data. Indeed, in the REIN registry, comorbidities are available for a limited number of children only. Nevertheless, in our previous study investigating the association between EDI and kidney graft failure, adjusting for comorbidities did not affect the results. Another important point is that there was no information on individual SES, ethnicity, or language, which led us to use an ecological deprivation index rather than an individual social deprivation indicator. This use of an ecological index led us to exclude patients with insufficient precise address in the main statistical analysis. Nevertheless, our sensitivity analysis including all targeted children thanks to multiple imputation for EDI and urban/rural environment revealed similar results as in the main statistical analysis excluding children because of missing data on address. Despite important limitations, the EDI, which has been used in several other studies,33,42,43 has some advantages. First, it has been derived from the smallest administrative geographic area in France (IRIS, including on average 2000 inhabitants). Moreover, it is a multidimensional index, taking into account the subjective poverty (social dimension) and the objective poverty (material dimension) in the neighborhood. It also takes into account the proportion of foreigners in the neighborhood, knowing that most of foreign people belong to the most deprived quintile (INSEE data, not published). Moreover, it has also been found to have higher association with individual data on income and education than the Townsend index.18 Finally, the EDI is transposable in time and in various European countries, which facilitates comparisons between countries. Another strength of our study is the evaluation of several indicators at KRT initiation (not only dialysis vs. pre-emptive transplantation), allowing us a more in-depth understanding of the context at KRT initiation.
To conclude, this study suggests that KRT initiation indicators are associated with the level of deprivation in children with ESKD in France and that social inequalities in health at ESKD begin in childhood in the French context. The identification of social disparities at KRT initiation reveals that there is significant room for improvement in reducing the risk of urgent-start dialysis and optimizing access to kidney transplantation in socially deprived children. A broader comprehension of the social inequalities during KRT and before would help setting up specific interventions to reduce disparities, taking into account all persons involved in the patient’s disease: children, family and caregivers, and health professionals.44, 45, 46
Disclosure
All the authors declared no competing interests.
Acknowledgments
BD was supported by a doctoral award from the French Public Health Doctoral Network and a research fellowship from the Biomedicine Agency.
Footnotes
Table S1. Population characteristics among included and excluded patients of the study. Data from REIN, 2002–2015 (n = 1285).
Table S2. Association between social deprivation measured by the European Deprivation Index (EDI in quintile) and care indicators at KRT initiation in young ESKD patients in metropolitan France. Data from REIN, 2002–2015. Imputed data for EDI and context of rural environment (rural/urban).
Figure S1. Assumed causal relationships between social deprivation (measured by European Deprivation Index [EDI]) and indicators at KRT initiation and its potential confounders and effect modifiers.
STROBE Statement.
Contributor Information
Bénédicte Driollet, Email: benedicte.driollet@u-bordeaux.fr.
Jérôme Harambat, Email: jerome.harambat@chu-bordeaux.fr.
Supplementary Material
Table S1. Population characteristics among included and excluded patients of the study. Data from REIN, 2002–2015 (n = 1285).
Table S2. Association between social deprivation measured by the European Deprivation Index (EDI in quintile) and care indicators at KRT initiation in young ESKD patients in metropolitan France. Data from REIN, 2002–2015. Imputed data for EDI and context of rural environment (rural/urban).
Figure S1. Assumed causal relationships between social deprivation (measured by European Deprivation Index [EDI]) and indicators at KRT initiation and its potential confounders and effect modifiers.
STROBE Statement.
References
- 1.Brophy P.D., Shoham D.A., CKD Life Course Group, et al. Early-life course socioeconomic factors and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:16–23. doi: 10.1053/j.ackd.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Shoham D.A., Vupputuri S., Kshirsagar A.V. Chronic kidney disease and life course socioeconomic status: a review. Adv Chronic Kidney Dis. 2005;12:56–63. doi: 10.1053/j.ackd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Vart P., Gansevoort R.T., Joosten M.M., Bültmann U., Reijneveld S.A. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48:580–592. doi: 10.1016/j.amepre.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Stephens M.R., Evans M., Ilham M.A., Marsden A., Asderakis A. The influence of socioeconomic deprivation on outcomes following renal transplantation in the United Kingdom. Am J Transplant. 2010;10:1605–1612. doi: 10.1111/j.1600-6143.2010.03041.x. [DOI] [PubMed] [Google Scholar]
- 5.Fored C.M., Ejerblad E., Fryzek J.P., et al. Socio-economic status and chronic renal failure: a population-based case-control study in Sweden. Nephrol Dial Transplant. 2003;18:82–88. doi: 10.1093/ndt/18.1.82. [DOI] [PubMed] [Google Scholar]
- 6.Kihal-Talantikite W., Vigneau C., Deguen S., Siebert M., Couchoud C., Bayat S. Influence of socio-economic inequalities on access to renal transplantation and survival of patients with end-stage renal disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muneeruddin S., Chandar J., Abitbol C.L., et al. Two decades of pediatric kidney transplantation in a multi-ethnic cohort. Pediatr Transplant. 2010;14:667–674. doi: 10.1111/j.1399-3046.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 8.Driollet B., Bayer F., Chatelet V., et al. Social deprivation is associated with poor kidney transplantation outcome in children. Kidney Int. 2019;96:769–776. doi: 10.1016/j.kint.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Patzer R.E., Amaral S., Klein M., et al. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? Am J Transplant. 2012;12:369–378. doi: 10.1111/j.1600-6143.2011.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer R.E., Mohan S., Kutner N., McClellan W.M., Amaral S. Racial and ethnic disparities in pediatric renal allograft survival in the United States. Kidney Int. 2015;87:584–592. doi: 10.1038/ki.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didsbury M., van Zwieten A., Chen K., et al. The association between socioeconomic disadvantage and parent-rated health in children and adolescents with chronic kidney disease—the Kids with CKD (KCAD) study. Pediatr Nephrol. 2019;34:1237–1245. doi: 10.1007/s00467-019-04209-7. [DOI] [PubMed] [Google Scholar]
- 12.Laster M., Soohoo M., Hall C. Racial-ethnic disparities in mortality and kidney transplant outcomes among pediatric dialysis patients. Pediatr Nephrol. 2017;32:685–695. doi: 10.1007/s00467-016-3530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng D.K., Antiporta D.A., Matheson M.B., et al. Nonparametric assessment of differences between competing risk hazard ratios: application to racial differences in pediatric chronic kidney disease progression. Clin Epidemiol. 2020;12:83–93. doi: 10.2147/CLEP.S225763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plumb L.A., Sinha M.D., Casula A., et al. Associations between deprivation, geographic location, and access to pediatric kidney care in the United Kingdom. Clin J Am Soc Nephrol. 2021;16:194–203. doi: 10.2215/CJN.11020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couchoud C., Stengel B., Landais P., et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006;21:411–418. doi: 10.1093/ndt/gfi198. [DOI] [PubMed] [Google Scholar]
- 16.Parmentier C., Lassalle M., Berard E., et al. Setting reasonable objectives for improving preemptive kidney transplantation rates in children. Pediatr Nephrol. 2020;35:2353–2360. doi: 10.1007/s00467-020-04653-w. [DOI] [PubMed] [Google Scholar]
- 17.Raffray M., Bayat S., Lassalle M., Couchoud C. Linking disease registries and nationwide healthcare administrative databases: the French renal epidemiology and information network (REIN) insight. BMC Nephrol. 2020;21:25. doi: 10.1186/s12882-020-1692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pornet C., Delpierre C., Dejardin O., et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66:982–989. doi: 10.1136/jech-2011-200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora V.S., Karanikolos M., Clair A., Reeves A., Stuckler D., McKee M. Data resource profile: the European Union Statistics on Income and Living Conditions (EU-SILC) Int J Epidemiol. 2015;44:451–461. doi: 10.1093/ije/dyv069. [DOI] [PubMed] [Google Scholar]
- 20.INSEE IRIS: definition. https://www.insee.fr/en/metadonnees/definition/c1523 Published October 13, 2016.
- 21.Leffondré K., Jager K.J., Boucquemont J., Stel V.S., Heinze G. Representation of exposures in regression analysis and interpretation of regression coefficients: basic concepts and pitfalls. Nephrol Dial Transplant. 2014;29:1806–1814. doi: 10.1093/ndt/gft500. [DOI] [PubMed] [Google Scholar]
- 22.Eilers P.H.C., Marx B.D. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11:89–121. [Google Scholar]
- 23.Francis A., Didsbury M., Lim W.H., et al. The impact of socioeconomic status and geographic remoteness on access to pre-emptive kidney transplantation and transplant outcomes among children. Pediatr Nephrol. 2016;31:1011–1019. doi: 10.1007/s00467-015-3279-z. [DOI] [PubMed] [Google Scholar]
- 24.Amaral S., Patzer R. Disparities, race/ethnicity and access to pediatric kidney transplantation. Curr Opin Nephrol Hypertens. 2013;22:336–343. doi: 10.1097/MNH.0b013e32835fe55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer F., Borzych-Duzalka D., Azocar M., et al. Impact of global economic disparities on practices and outcomes of chronic peritoneal dialysis in children: insights from the International Pediatric Peritoneal Dialysis Network Registry. Perit Dial Int. 2012;32:399–409. doi: 10.3747/pdi.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin J., Kirchner K., Ray R., Bower J.D. Demographic factors associated with dialysis technique failures among patients undergoing continuous ambulatory peritoneal dialysis. Arch Intern Med. 1985;145:1041–1044. [PubMed] [Google Scholar]
- 27.Aoun B., Ulinski T., Sanjad S., et al. Chronic peritoneal dialysis in Lebanese children of families with low socioeconomic status. Perit Dial Int. 2015;35:93–96. doi: 10.3747/pdi.2013.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald S.P., Craig J.C. Australian and New Zealand Paediatric Nephrology Association.. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 29.Wong C.S., Hingorani S., Gillen D.L., et al. Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int. 2002;61:630–637. doi: 10.1046/j.1523-1755.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- 30.Dehennault M. [Paediatric features in childhood renal transplantation: quality of life, school, adherence, transfer to adult nephrologists] Nephrol Ther. 2011;7:599–603. doi: 10.1016/j.nephro.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Seear M., Amed S., Dionne J., et al. In support of point-of-care social needs screening: the effects of five social determinants on the health of children with chronic diseases in British Columbia. Paediatr Child Health. 2019;24:200–208. doi: 10.1093/pch/pxy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montini G., Edefonti A., Galan Y.S., et al. Non-medical risk factors as avoidable determinants of excess mortality in children with chronic kidney disease. A prospective cohort study in Nicaragua, a model low income country. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatelet V., Bayat-Makoei S., Vigneau C., Launoy G., Lobbedez T. Renal transplantation outcome and social deprivation in the French healthcare system: a cohort study using the European Deprivation Index. Transpl Int. 2018;31:1089–1098. doi: 10.1111/tri.13161. [DOI] [PubMed] [Google Scholar]
- 34.Padilla C.M., Raffray M., Pladys A., Vigneau C., Bayat S. Geographic variations in the risk of emergency first dialysis for patients with end stage renal disease in the Bretagne region, France. Int J Environ Res Public Health. 2018;16:18. doi: 10.3390/ijerph16010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beaumier M., Calvar E., Launay L., et al. Effect of social deprivation on peritoneal dialysis uptake: a mediation analysis with the data of the REIN registry. Perit Dial Int. https://doi.org/10.1177/08968608211023268 Published online July 1, 2021. [DOI] [PubMed]
- 36.Calvar E., Launay L., Boyer A., Launoy G., Lobbedez T., Châtelet V. Effects of social deprivation on the proportion of preemptive kidney transplantation: a mediation analysis. Transplant Direct. 2021;7 doi: 10.1097/TXD.0000000000001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beaumier M., Béchade C., Dejardin O., et al. Is self-care dialysis associated with social deprivation in a universal health care system? A cohort study with data from the Renal Epidemiology and Information Network Registry. Nephrol Dial Transplant. 2020;35:861–869. doi: 10.1093/ndt/gfz245. [DOI] [PubMed] [Google Scholar]
- 38.Prezelin-Reydit M., Madden I., Macher M.A., et al. Preemptive kidney transplantation is associated with transplantation outcomes in children: results from the French kidney replacement therapy registry. Transplantation. 2022;106:401–411. doi: 10.1097/TP.0000000000003757. [DOI] [PubMed] [Google Scholar]
- 39.Pruthi R., Casula A., Inward C., Roderick P., Sinha M.D., British Association for Paediatric Nephrology Early requirement for RRT in children at presentation in the United Kingdom: association with transplantation and survival. Clin J Am Soc Nephrol. 2016;11:795–802. doi: 10.2215/CJN.08190815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boehm M., Winkelmayer W.C., Arbeiter K., Mueller T., Aufricht C. Late referral to paediatric renal failure service impairs access to pre-emptive kidney transplantation in children. Arch Dis Child. 2010;95:634–638. doi: 10.1136/adc.2009.174581. [DOI] [PubMed] [Google Scholar]
- 41.Jander A., Nowicki M., Tkaczyk M., et al. Does a late referral to a nephrologist constitute a problem in children starting renal replacement therapy in Poland?—a nationwide study. Nephrol Dial Transplant. 2006;21:957–961. doi: 10.1093/ndt/gfi313. [DOI] [PubMed] [Google Scholar]
- 42.Poiseuil M., Coureau G., Payet C., et al. Deprivation and mass screening: survival of women diagnosed with breast cancer in France from 2008 to 2010. Cancer Epidemiol. 2019;60:149–155. doi: 10.1016/j.canep.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Petit M.-P., Bryere J., Maravic M., Pallaro F., Marcelli C. Hip fracture incidence and social deprivation: results from a French ecological study. Osteoporos Int. 2017;28:2045–2051. doi: 10.1007/s00198-017-3998-z. [DOI] [PubMed] [Google Scholar]
- 44.Friedman A.L. The broader burden of end-stage renal disease on children and their families. Kidney Int. 2006;70:1893–1894. doi: 10.1038/sj.ki.5001964. [DOI] [PubMed] [Google Scholar]
- 45.Favel K., Dionne J.M. Factors influencing the timing of initiation of renal replacement therapy and choice of modality in children with end-stage kidney disease. Pediatr Nephrol. 2020;35:145–151. doi: 10.1007/s00467-019-04391-8. [DOI] [PubMed] [Google Scholar]
- 46.Wightman A., Zimmerman C.T., Neul S., Lepere K., Cedars K., Opel D. Caregiver experience in pediatric dialysis. Pediatrics. 2019;143 doi: 10.1542/peds.2018-2102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.