Abstract
Two patients with subacute sclerosing panencephalitis (SSPE) were treated safely and effectively with high doses of intravenous ribavirin combined with intraventricular alpha interferon. The ribavirin concentrations maintained in the serum and cerebrospinal fluid were higher than those which inhibit SSPE virus replication in vitro and in vivo.
Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal central nervous system disorder that results from a persistent measles (SSPE) virus infection. There is currently no specific treatment for SSPE. SSPE virus strains are genetically altered measles viruses, altered particularly in the viral genes coding for the structural proteins. The alteration appears to be important in the pathogenesis of the persistent central nervous system infection that yields the syndrome of SSPE. We examined a wide variety of antiviral compounds for their inhibitory effects on measles and SSPE virus strains in vitro and found that ribavirin inhibited the replication of SSPE virus strains more than other nucleoside and nonnucleoside compounds (6), including inosiplex and interferon (IFN), which are reported to prolong the lives of patients with SSPE. The 50% inhibitory concentration of ribavirin in vitro was calculated to be 8.0 μg/ml (6, 7). In the hamster SSPE model, ribavirin administered into the subarachnoid space inhibited the replication of SSPE virus in the brains and improved the survival rate (5). The minimum effective concentration of ribavirin in hamster brain is estimated to be 5 to 10 μg/g (8). Intravenous ribavirin therapy was effective for the treatment of measles pneumonia (1, 3, 4) and subacute measles encephalitis in immunocompromised hosts (10). Oral ribavirin therapy for patients with SSPE, however, was reported to be ineffective. The maximum concentrations achieved in the cerebrospinal fluid (CSF) of those patients (0.8 to 2.3 μg/ml) were below the concentration that inhibits the SSPE virus replication in vitro and in vivo (11). As ribavirin crosses the blood-brain barrier (2), we treated patients with SSPE using intravenous administration of high doses and examined whether the ribavirin concentration achieved an effective level in the CSF.
Two patients with SSPE were treated with intravenous ribavirin combined with intraventricular alpha IFN (IFN-α) therapy. The clinical information about the patients is only briefly mentioned here, as details of the clinical courses and the laboratory data for those patients will be reported elsewhere (14). Patient 1 experienced measles at the age of 9 months. At the age of 13, he presented with mental alteration, myoclonic seizures, and an unsteady gait. On the basis of the clinical symptoms and an elevated hemagglutination inhibition (HI) titer of measles virus antibodies in the CSF (1:32), he was diagnosed as having stage II SSPE under Jabbour's classification. Despite high-dose intraventricular IFN-α (300 × 104 IU three times a week) and oral inosiplex (5,600 mg/day) for 4 months his neurologic status deteriorated rapidly. He developed dementia, neurobladder incontinence, and dysphagia and developed stage III SSPE. Patient 2 experienced measles at the age of 4 years. At the age of 12, she had intellectual dysfunction and myoclonic seizures and was diagnosed as having stage II SSPE under Jabbour's classification on the basis of the clinical symptoms, electroencephalography abnormalities, and an elevated HI titer of measles virus antibodies in the CSF (1:128). She was treated with intraventricular IFN-α (300 × 104 IU three times a week) and oral inosiplex (5,600 mg/day). Her neurologic state, including myoclonic seizures and mental alteration, gradually deteriorated despite those treatments. Seven months after starting the therapy, magnetic resonance imaging indicated slowly progressive brain atrophy, and an audiogram revealed hearing loss on the right side.
Ribavirin at a dose of 10 mg/kg of body weight was administered intravenously as a 30-min infusion three times a day for 7 days, combined with intraventricular IFN-α therapy (300 × 104 IU three times a week). At 7-day intervals, we increased the dose to 20 mg/kg, and then to 30 mg/kg, as the maximum tolerated dose for human immunodeficiency virus-infected adults is suggested to be 2,400 mg (9), which corresponds to 30 mg/kg. Serum and CSF samples were collected simultaneously 3 h after the 15th administration. Because ribavirin can accumulate in red blood cells, serum samples were immediately separated. CSF samples were collected from lumbar taps. Serum and CSF samples were frozen and stored until the assay was run. To determine the pharmacokinetics of ribavirin, serum samples were obtained from patients who received ribavirin at a dose of 20 mg/kg 1, 3, and 6 h after the initial and 15th administrations. CSF samples were collected 3 h after the initial and 15th administrations.
Ribavirin concentrations in serum and CSF were measured using high-performance liquid chromatography (8). In brief, the sample was diluted with 2 volumes of phosphate-buffered saline, 60% HClO4 was added to a final concentration of 0.5 M, and the sample was kept on ice for 30 min. The treated sample was centrifuged at 1,600 × g for 10 min. The supernatant was collected, neutralized with KH2PO4 and KOH, and kept on ice for 5 min. The supernatant after centrifugation at 1,600 × g for 10 min was used as a sample for high-performance liquid chromatography assay. The sample (10 μl) was loaded onto a reverse-phase column (TSKgel ODS-120T column; Tosoh, Tokyo, Japan) and was eluted with the buffer at a flow rate of 1.0 ml/min. The A226 was measured with a UV detector. The ribavirin concentration in a sample was estimated from a standard curve of the optical density-ribavirin concentration. The correlation coefficient of optical density to ribavirin concentration was 0.99 in the range of 0 to 500 μg/ml. The lower limit of detection for the assay was 0.1 μg/ml. The data were averaged over two independent experiments.
Patient 1 received intravenous ribavirin therapy at doses of 10, 20, and then 30 mg/kg combined with intraventricular IFN-α therapy. At the highest dose for 7 days, he experienced adverse effects (i.e., moderate reversible anemia at a hemoglobin level of 9.4 g/dl and oral mucosal swelling) attributable to the ribavirin. We repeatd the intravenous ribavirin therapy for 7 days at 7-day intervals for more than 6 months. His hypertonicity, neurobladder incontinence, and dysphagia improved, although other neurologic symptoms did not change after using the combination treatment for 6 months. He remained in stage III SSPE under Jabbour's classification.
Patient 2 demonstrated remarkable clinical improvement after beginning ribavirin therapy at a dose of 20 mg/kg; therefore, this dose was continued. Intravenous ribavirin therapy for 7 days at 7-day intervals was repeated for more than 6 months. Her myoclonic seizures disappeared, hearing in her right ear improved, and the HI measles virus antibody titer in the CSF decreased to 1:4, with clinical improvement at 3 weeks, 1 month, and 5 months after starting the ribavirin therapy. The magnetic resonance imaging findings indicated no further progression of brain atrophy during the combination therapy, although slow progression was observed during intraventricular IFN-α therapy alone. She returned to stage I SSPE under Jabbour's classification.
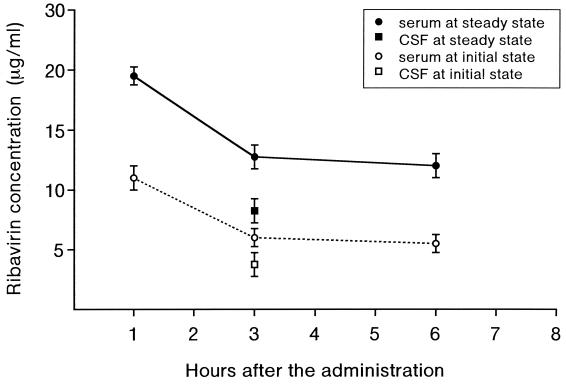
When the dose of ribavirin administered was increased from 10 to 30 mg/kg, the ribavirin concentration in the serum sample collected 3 h after the 15th administration increased from 1.3 to 20.9 μg/ml in a dose-dependent manner (Table 1). Ribavirin administered intravenously penetrates well into the CSF of patients with SSPE, achieving 74% (range, 50 to 89%) of the concentration in serum. The ribavirin concentration in CSF also increased from 1.1 to 17.4 μg/ml in a dose-dependent manner (Table 1). The ribavirin level in CSF reached a concentration of greater than 7.5 μg/ml by intravenous administration at a dose of 20 mg/kg. The pharmacokinetic study indicated mean ribavirin concentrations of 5.9 and 5.7 μg/ml in the serum samples collected at 3 and 6 h after the initial administration, respectively (Fig. 1). At steady state, those concentrations increased to 12.8 and 12.4 μg/ml, respectively. There was little decrease in the serum ribavirin concentration at 6 h compared with that at 3 h after the administration and a significant increase in the concentration at the steady state compared with that at the initial state, which can be attributed to the prolonged serum elimination half-life of ribavirin (9). Corresponding to the increase in the concentration in serum, the mean concentration in CSF increased from 3.9 μg/ml at the initial state to 8.1 μg/ml at the steady state (Fig. 1).
TABLE 1.
Serum and CSF ribavirin concentrations after high-dose intravenous administration
| Patient | Age (yr) | Gender | Onset | Clinical stage (Jabbour) | Dose (mg/kg)a | Ribavirin concn (μg/ml) inb:
|
CSF/plasma ribavirin concn ratio | |
|---|---|---|---|---|---|---|---|---|
| Serum | CSF | |||||||
| 1 | 14 | Male | August 1998 | III | 10 | 1.3 | 1.1 | 0.84 |
| 20 | 11.1 | 7.8 | 0.70 | |||||
| 20 | 10.2 | 9.0 | 0.88 | |||||
| 30 | 20.9 | 17.4 | 0.83 | |||||
| 30 | 16.7 | 14.8 | 0.89 | |||||
| 2 | 13 | Female | November 1998 | II | 10 | 3.0 | 1.5 | 0.50 |
| 20 | 10.4 | 8.2 | 0.79 | |||||
| 20 | 14.3 | 8.0 | 0.56 | |||||
| 20 | 11.5 | 7.5 | 0.65 | |||||
Ribavirin at doses of 10, 20, and then 30 mg/kg was administered every 8 h for 7 days at 7-day intervals.
Serum and CSF samples were collected simultaneously 3 h after the 15th administration. The data are averages from two independent experiments.
FIG. 1.
Pharmacokinetics of ribavirin. Serum samples were obtained from patients, who received ribavirin at a dose of 20 mg/kg three times a day, at 1, 3, and 6 h after the initial and 15th (steady-state) administrations. CSF samples were collected 3 h after the initial and 15th administrations.
High-dose intravenous ribavirin administration maintained the ribavirin level in CSF at a steady-state concentration of higher than 7.5 μg/ml, which was comparable to the concentration inhibitory to SSPE virus in vitro and in vivo (6, 8). Although transient reversible anemia and oral mucosal swelling were noted as adverse effects attributable to ribavirin, we repeated the intravenous ribavirin therapy for 7 days at 7-day intervals for more than 6 months. There seemed to be definite improvements in the neurologic states of both patients. As IFN-α enhanced the therapeutic effect of ribavirin on SSPE virus infection in vivo (13) and the combination therapy of ribavirin and IFN-α was effective for the treatment of patients with hepatitis C (12), the cooperative effect of ribavirin and IFN-α might result in a favorable clinical course for patients with SSPE. Worldwide placebo-controlled trials will be required, however, to reach the conclusion that intravenous ribavirin therapy is clinically effective.
Intravenous administration of high-dose ribavirin combined with intraventricular administration of IFN-α should be further pursued for its potential use in the therapy of patients with SSPE, while the ribavirin concentrations in serum and CSF should be monitored.
REFERENCES
- 1.Banks G, Fernandez H. Clinical use of ribavirin in measles: a summarized review. In: Smith R A, Knight V, Smith J A D, editors. Clinical applications of ribavirin. New York, N.Y: Academic Press; 1980. pp. 203–209. [Google Scholar]
- 2.Connor E, Morrison S, Lane J, Oleske J, Sonke R L, Connor J. Safety, tolerance, and pharmacokinetics of systemic rivabirin in children with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1993;37:532–539. doi: 10.1128/aac.37.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forni A L, Schluger N W, Roberts R B. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis. 1994;19:454–462. doi: 10.1093/clinids/19.3.454. [DOI] [PubMed] [Google Scholar]
- 4.Gururangan S, Stevens N W, Morris D J. Ribavirin response in measles pneumonia. J Infect Dis. 1990;20:219–221. doi: 10.1016/0163-4453(90)91094-t. [DOI] [PubMed] [Google Scholar]
- 5.Honda Y, Hosoya M, Ishii T, Shigeta S, Suzuki H. Effect of ribavirin on subacute sclerosing panencephalitis virus infections in hamsters. Antimicrob Agents Chemother. 1994;38:653–655. doi: 10.1128/aac.38.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosoya M, Shigeta S, Nakamura K, De Clercq E. Inhibitory effect of selected antiviral compounds on measles (SSPE) virus replication in vitro. Antiviral Res. 1989;12:87–98. doi: 10.1016/0166-3542(89)90072-7. [DOI] [PubMed] [Google Scholar]
- 7.Huffman J H, Sidwell R W, Khare G P, Witkowski J T, Allen L B, Robins R K. In vitro effect of 1-β-d-ribofuranosy1–1,2,4-triazole-3-carboxamide (Virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1973;3:235–241. doi: 10.1128/aac.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii T, Hosoya M, Mori S, Shigeta S, Suzuki H. Effective ribavirin concentration in hamster brain for antiviral chemotherapy for subacute sclerosing panencephalitis. Antimicrob Agents Chemother. 1996;40:241–243. doi: 10.1128/aac.40.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laskin O L, Longstreth J A, Hart C C, Scavuzzo D, Kalman C M, Connor J D, Roberts R B. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987;41:546–55. doi: 10.1038/clpt.1987.70. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa M M, Witman S D, Winick N J, Bellini W J, Timmons C F, Siegel J D. Subacute measles encephalitis in the young immunocompromised host: report of two cases diagnosed by polymerase chain reaction and treated with ribavirin and review of literature. Clin Infect Dis. 1993;16:654–660. doi: 10.1093/clind/16.5.654. [DOI] [PubMed] [Google Scholar]
- 11.Ogle J W, Toltzis P, Parker W D. Oral ribavirin therapy for subacute sclerosing panencephalitis. J Infect Dis. 1989;159:748–750. doi: 10.1093/infdis/159.4.748. [DOI] [PubMed] [Google Scholar]
- 12.Reichard O, Norkrans G, Fryden A, Braconier J-H, Sonnerborg A, Weiland O for the Swedish Study Group. Randomised, double-blind, placebo-controlled trial of interferon α-2b with and without ribavirin for chronic hepatitis C. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T, Hosoya M, Kimura K, Ohno K, Mori S, Takahashi K, Shigeta S. The cooperative effect of interferon-α and ribavirin on subacute sclerosing panencephalitis (SSPE) virus infections, in vitro and in vivo. Antiviral Res. 1998;37:29–35. doi: 10.1016/s0166-3542(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 14.Tomoda, A., S. Shiraishi, M. Hosoya, A. Hamada, and T. Miike. Combined treatment with interferon-α and ribavirin for SSPE. Pediatr. Neurol., in press. [DOI] [PubMed]