Abstract
Introduction
Minimal change disease (MCD) is considered a podocyte disorder triggered by unknown circulating factors. Here, we hypothesized that the endothelial cell (EC) is also involved in MCD.
Methods
We studied 45 children with idiopathic nephrotic syndrome (44 had steroid sensitive nephrotic syndrome [SSNS], and 12 had biopsy-proven MCD), 21 adults with MCD, and 38 healthy controls (30 children, 8 adults). In circulation, we measured products of endothelial glycocalyx (EG) degradation (syndecan-1, heparan sulfate [HS] fragments), HS proteoglycan cleaving enzymes (matrix metalloprotease-2 [MMP-2], heparanase activity), and markers of endothelial activation (von Willebrand factor [vWF], thrombomodulin) by enzyme-linked immunosorbent assay (ELISA) and mass spectrometry. In human kidney tissue, we assessed glomerular EC (GEnC) activation by immunofluorescence of caveolin-1 (n = 11 MCD, n = 5 controls). In vitro, we cultured immortalized human GEnC with sera from control subjects and patients with MCD/SSNS sera in relapse (n = 5 per group) and performed Western blotting of thrombomodulin of cell lysates as surrogate marker of endothelial activation.
Results
In circulation, median concentrations of all endothelial markers were higher in patients with active disease compared with controls and remained high in some patients during remission. In the MCD glomerulus, caveolin-1 expression was higher, in an endothelial-specific pattern, compared with controls. In cultured human GEnC, sera from children with MCD/SSNS in relapse increased thrombomodulin expression compared with control sera.
Conclusion
Our data show that alterations involving the systemic and glomerular endothelium are nearly universal in patients with MCD and SSNS, and that GEnC can be directly activated by circulating factors present in the MCD/SSNS sera during relapse.
Keywords: endothelial activation, endothelial glycocalyx, glomerular endothelial cell, minimal change disease, podocyte, steroid sensitive nephrotic syndrome
Graphical abstract
See Commentary on Page 675
MCD is the most common type of nephrotic syndrome in children, and it is considered a podocyte disorder.1,2 The absence of infiltrating cells and immune complexes in the glomerulus has also suggested that MCD might be mediated by the direct injury of the podocyte by some circulating factor.3,4 Hence, research efforts have focused on the study of immune cells and podocytes. However, the pathogenesis of the disease remains poorly understood.
Although podocytes have a critical role in preventing plasma proteins from crossing into the urinary space,5 other components of the glomerular filtration barrier, the endothelium with its glycocalyx and the glomerular basement membrane, are also necessary to maintain glomerular filtration barrier integrity.6,7 The possibility that endothelium may also be sustaining injury in MCD has not been rigorously investigated, unlike in other conditions associated with proteinuria.6,8,9 However, injury to the endothelium and the EG can be subtle and may be missed with standard microscopy techniques. Recently, Royal et al.10 identified morphologic changes involving GEnCs in some patients with MCD. Furthermore, scattered reports showed endothelial dysfunction in MCD, but these observations have been generally underappreciated.11,12
Accordingly, in this study, we integrated endothelial biomarkers in human biosamples by different methods (ELISA, mass spectrometry, and immunofluorescence) and cell culture studies to test the hypothesis that MCD involves injury to the systemic and glomerular endothelium.
Methods
Definitions
We used standard definitions for SSNS, steroid resistant nephrotic syndrome, MCD, and disease activity.13 Relapse was defined as the presence of proteinuria (urinary protein-to-creatinine ratio >2.0 mg/mg or 3+ or greater by urine dipstick or >3.5 g of protein per day for the adult population) along with edema. Complete remission was defined as negative or trace proteinuria by dipstick or urinary protein-to-creatinine ratio <0.2 mg/mg.1,13 A total of 5 patients with urinary protein-to-creatinine ratio >0.2 or 1+ protein by dipstick were considered in remission based on previous and subsequent clinical course determined by the primary pediatric nephrologist. Likewise, 1 patient with urinary protein-to-creatinine ratio <2 was considered in relapse based on subsequent clinical course.
Participants
The study was performed according to the Declaration of Helsinki and was approved by the Colorado Multiple Institutional Review Board (#13-2700 and #16-1752) and respective collaborative institutions: Rocky Mountain Kidney Center (Denver, CO) (Rocky Mountain Kidney Center #13-2700), Hospital Universitario Central de Asturias (Oviedo, Spain) (Hospital Universitario Central de Asturias #221/19), Hospital Niño Jesus (Madrid, Spain) (Hospital Niño Jesus #R-0011/20), and Hospital Vall d’Hebron (Barcelona, Spain) (Hospital Vall d’Hebron #ID-RTF065). Written informed consents and assents, if appropriate, were obtained from participants and parents/guardian.
Pediatric Population
The participants were 44 children with SSNS and 1 with steroid resistant nephrotic syndrome (24 males, 21 females). Twelve patients had biopsy-proven MCD. On immunofluorescence of kidney tissue, 1 patient had mesangial IgA deposits, and another had IgM deposition, but both presented clinically as MCD. We included children with SSNS because a kidney biopsy is not routinely performed in these patients, but SSNS is usually associated with MCD.1 A total of 10 children were studied at onset of disease, 28 during relapse and 26 during remission. A total of 9 patients were studied during relapse and remission. Patients were recruited at 4 different institutions: Children’s Hospital Colorado (Aurora, CO), Rocky Mountain Kidney Center, Hospital Universitario Central de Asturias, and Hospital Niño Jesus. Samples from children without history of glomerular disease (except for 1 patient with remote history of postinfectious glomerulonephritis) served as control (n = 30, 19 females, 11 males) and were collected at the Children’s Hospital Colorado and Hospital Niño Jesus (n = 21) and acquired from Precision for Medicine (n = 9) (precisionformedicine.org).
Adult Population
We included 21 adults with biopsy-proven MCD during relapse (14 females, 8 males). Patients were recruited at the Hospital Vall d’Hebron. A total of 8 adults (6 males, 2 females) without known history of kidney or glomerular disorders served as controls, and samples were obtained from Precision for Medicine.
Blood Measurements
Blood samples were processed using standard protocols, and serum and plasma were stored at −80 °C until used for testing. Because of constrains on patients’ samples, the number of measurements per group varied among targets as reflected in each figure. All measurements were performed in serum samples, except for syndecan-1 and MMP-2 quantification in some control subjects in whom only plasma was available (12 of 16 samples and 13 of 15 samples were plasma, respectively). Because we had paired serum and plasma samples from some patients and controls, we compared syndecan-1 and MMP-2 levels and found no statistical differences between paired serum and plasma concentrations (Supplementary Figure S1A and B, respectively). Measurements of circulating levels of endothelial markers were performed at the University of Colorado using the following commercially available ELISA kits: syndecan-1 (Abcam, Cambridge, United Kingdom, catalog [cat] #ab46506, dilution 1:8), heparan degrading enzyme (Takara, Shiga, Japan, cat #MK412, dilution 1:50), MMP-2 (R&D Systems, Minneapolis, MN, cat #MMP200, dilution 1:20), thrombomodulin (Abcam, cat #ab46508, dilution 1:5), vWF (Assaypro, Saint Charles, MO, cat #EV2030-1, dilution 1:1000). All samples were run in duplicate. Quantification of circulating HS fragments was performed by mass spectrometry as previously described.14
Immunofluorescence of Human Kidney Tissue Sections
De-identified paraffin-embedded human kidney samples from adults with MCD in relapse (n = 11) and adults without history of glomerular disease (n = 5) were obtained from the Histology Subspecialties Laboratory at the University of Colorado (Dr. Lucia) and from Hospital Vall d’Hebron (Dr. Segarra). The average number of glomeruli (mean ± SD) in kidney tissue samples was 10 ± 5 and 14 ± 7 for MCD and controls, respectively. Tissues samples were deparaffinized and rehydrated according to the standard protocols. Slides were then immersed in 0.1% Sudan Black in 70% ethanol for 20 minutes at room temperature to reduce autofluorescence. After 3 washes with tris-buffered saline (TBST), antigen retrieval was performed with citrate buffer at 96 °C for 45 minutes. Once slides were completely cooled, they were washed for 10 minutes in 0.1 M glycine/TBST and then permeabilized with 0.1% Triton X-100 for 10 minutes. This was followed by 3 TBST washes. Slides were then placed in 10 mg/ml sodium borohydride/Hanks balanced salt solution in ice cold for 40 minutes. After 2 TBST washes, slides were blocked with a solution 1:1 of superblock and 5% bovine serum albumin in TBST for 1 hour followed by overnight incubation with rabbit anticaveolin 1 (Cell Signaling Technology, Danvers, MA, cat #3267, dilution 1:400) and with lectin rhodamine (Vector Laboratories, Burlingame, CA, ulex europaeus agglutinin I, RL-1062-2, dilution 1:1000). This was followed by 3 TBST washes and incubation with the goat antirabbit antibody (dilution 1:400) for 2 hours at room temperature. For the negative control group, the primary antibody was substituted by TBST to assess autofluorescence and nonspecific interactions. Slides were then washed and mounted with Prolong Gold antifade mounting medium (Invitrogen, Waltham, MA). Images were captured using Keyence BZ-X810 fluorescence microscope (Keyence Corporation of America, Itasca, IL).
Cell Cultures and Western Blotting
Human immortalized GEnC (hiGEnC) were kindly donated by Dr. Satchell (University of Bristol, United Kingdom). hiGEnC were cultured in EGM2 media with the EGM2-MV Bulletkit (Lonza, Basel, Switzerland, CC-3202) and were grown at 33 °C until reaching 80% confluence. Then, cells were transferred to 37 °C for 7 to 14 days until reaching differentiation. Fully differentiated hiGEnC were treated with 10% unpooled serum samples from children with MCD/SSNS in relapse (n = 1/n = 4 respectively) and control subjects (n = 5). Sera were added to fetal bovine serum–free media. After 24 hours, media containing huma sera were removed, and cultured cells were thoroughly rinsed with 5 PBS washes, and fresh media were added back to the cultures for 24 hours. The hiGEnC cultures were scraped and proteins collected using a mixture of mammalian protein extraction reagent (Thermo Fischer Scientific, Waltham, MA), protease inhibitor (Roche, Basel, Switzerland), and phosphatase inhibitor (Roche). Protein quantification was performed using a Bradford assay (Bio-Rad, Hercules, CA). Sample proteins were separated on 4% to 12% SDS polyacrylamide gels and transferred to Immobilon-FL polyvinylidene fluoride membranes (Merck MilliporeSigma Ltd., Burlington, MA). The membranes were blocked using Super Block (Thermo Fischer Scientific) for 1 hour at room temperature and incubated with primary antibodies diluted in Super Block overnight at 4 °C. The membranes were washed with TBST [10 mM Tris-hydrochloric acid (pH 7.4), 140 mM sodium chloride] containing 0.1% TBST and incubated with Horseradish peroxidase –bound secondary antibodies at room temperature for 1 hour. The bound proteins were visualized using Clarity ECL (Bio-Rad). The primary antibodies used for the blots were rabbit antithrombomodulin (Abcam, cat #109189, dilution 1:2000) and rabbit anti–glyceraldehyde 3-phosphate dehydrogenase (Cell Signaling Technology, cat #2118, dilution 1:7000). The secondary antibody was goat antirabbit- Horseradish peroxidase (Cell Signaling Technology, cat #7074, dilution 1:6000).
Glomerular Transcriptome
We searched for endothelial-specific markers of cell activation in the open-source database Nephroseq.org (The Regents of The University of Michigan, Ann Arbor, MI). We obtained glomerular expression data (Ju CKD Glom cohort) for thrombomodulin and NOS3 from patients with MCD in relapse and healthy controls (n = 14 and 21, respectively).15
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 9, GraphPad Software, San Diego, CA). We used D’Agostino-Pearson test to assess normality. Measurements of circulating markers in patients with SSNS/MCD and densitometry analysis of cell lysates followed a nonnormal distribution. Therefore, we examined differences among 2 groups using the unpaired two-tailed Mann–Whitney U test and associations using Spearman correlation. Paired serum and plasma samples followed a normal distribution, and analysis was performed with paired t test. No outliers were excluded for analysis, and the number of outliers is shown in the corresponding figure legends. Quantification of circulating markers was expressed as median ± interquartile range, and numerical variables from demographics as mean ± SD. Quantification of fluorescence in human kidney tissue was performed using ImageJ (Windows version 64-bit Java 1.8.0_172; Bio-Rad). A P < 0.05 was considered statistically significant.
Results
Study Population
Demographic characteristics of the pediatric population are shown in Tables 1, 2, and 3, and those of adult population are shown in Tables 4 and 5. Children with MCD/SSNS were younger and had a lower serum creatinine than pediatric controls (age: 7.6 ± 3.9 vs. 12.2 ± 5.1 years, P < 0.0001; serum creatinine: remission vs. control, 0.4 ± 0.1 mg/dl vs. 0.6 ± 0.2 mg/dl, P = 0.003, and relapse vs. control 0.2 ± 0.1 mg/dl vs. 0.6 ± 0.2 mg/dl, P < 0.0001). Adults with MCD were younger than controls (26.6 ± 9.5 vs. 36.3 ± 10.2 years, P = 0.01).
Table 1.
Demographic characteristics of children with idiopathic nephrotic syndrome during remission
| ID | Age (yr) | Sex/race | Histology | Pattern | S Alb (g/dl) | Dipstick/UPC (mg/mg) | S Cr (mg/dl) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 | F/W | MCD | SDNS | NA | NEG | NA | STD, FK |
| 2 | 13 | M/W | MCD | SSNS | NA | NEG | NA | U |
| 3 | 8 | F/Ua | NO | SDNS | NA | NEG | NA | FK |
| 4 | 7 | M/W | NO | SSNS | NA | NEG | NA | STD |
| 5 | 7 | F/Ua | NO | SDNS | NA | NEG | NA | FK |
| 6 | 7 | F/W | NO | SDNS | NA | TRA | NA | STD, MMF |
| 7 | 8 | F/W | NO | SDNS | NA | TRA | NA | STD |
| 8 | 12 | M/W | NO | SSNS | NA | NEG | NA | STD |
| 9 | 3 | M/W | NO | SSNS | NA | NEG | NA | STD |
| 10 | 3 | M/Ua | NO | NA | NA | NEG | NA | U |
| 11 | 4 | F/W | NO | NA | NA | NEG | NA | U |
| 12 | 6 | M/Wa | NO | SSNS | NA | NEG | NA | STD |
| 13 | 11 | M/W | MCD | SDNS | 4.3 | 0.09 | 0.5 | STD, FK |
| 14 | 9 | F/W | MCD | SDNS | 4.3 | 0.03 | 0.5 | STD, FK, RTX |
| 15 | 17 | F/W | NO | SDNS | 3.4 | 0.04 | 0.6 | FK |
| 16 | 10 | M/W | NO | SDNS | 4.1 | 0.11 | 0.3 | MMF |
| 17 | 10 | M/W | U | SRNS | 4.2 | 0.3 | 0.6 | FK |
| 18 | 9 | M/W | MCD | SDNS | 4 | 0.23 | 0.5 | STD, MMF |
| 19 | 6 | M/W | NO | SSNS | 2.2 | 0.15 | 0.3 | STD |
| 20 | 12 | M/W | NO | SSNS | 2.2 | 0.48 | 0.4 | STD |
| 21 | 7 | F/W | NO | SDNS | 4.1 | 0.09 | 0.3 | STD |
| 22 | 18 | M/W | NO | SDNS | 3.5 | 0.4 | 0.7 | STD |
| 23 | 2 | F/W | NO | SSNS | 2 | + | 0.1 | STD |
| 24 | 9 | M/W | NO | SSNS | NA | NEG | NA | STD |
| 25 | 15 | F/W | NO | SDNS | 3.9 | NEG | 0.5 | NONE |
| 26 | 10 | F/W | NO | SDNS | 4.1 | NEG | 0.5 | CSA |
| Mean ± SD | 9 ± 4 | 14M/12F | 5MCD | 3.5 ± 0.8 | 0.1 ± 0.1 | 0.4 ± 0.1 |
+, protein detected by urine dipstick; Alb, albumin; B, Black; Cr, creatinine; CSA, cyclosporine A; F, female; FK, tacrolimus; ID, identification; M, male; MCD, minimal change disease; MMF, mycophenolate mofetil; NA, not available; NEG, negative, ONSET, new onset nephrotic syndrome; RTX, rituximab; S, serum; SDNS, steroid dependent nephrotic syndrome; SRNS, steroid resistant nephrotic syndrome; SSNS, steroid sensitive nephrotic syndrome; STD, steroids; TRA, trace; U, unknown; UPC, urine protein-to-creatinine ratio; W, White.
Patients with idiopathic nephrotic syndrome during remission. Available quantitative data were expressed as mean ± SD.
Hispanic.
Table 2.
Demographic characteristics of children with idiopathic nephrotic syndrome at disease onset or during relapse
| ID | Age (yr) | Sex/race | Histology | Pattern | S Alb (g/dl) | Dipstick/UPC (mg/mg) | S Cr (mg/dl) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 27 | 6 | M/W | NO | SDNS | NA | ++++ | NA | NONE |
| 1 | 11 | F/W | MCD | SDNS | NA | ++++ | NA | STD, FK |
| 28 | 7 | F/W | MCD | SDNS | NA | ++++ | NA | STD, FK |
| 3 | 8 | F/Ua | NO | SDNS | NA | ++ | NA | FK |
| 4 | 7 | M/W | NO | SSNS | NA | ++++ | NA | STD |
| 29 | 9 | F/W | NO | SSNS | NA | ++++ | NA | NA |
| 7 | 8 | F/W | NO | SDNS | NA | ++++ | NA | STD |
| 8 | 12 | M/W | NO | SSNS | NA | ++++ | NA | STD |
| 9 | 3 | M/W | NO | ONSET | NA | ++++ | NA | STD |
| 30 | 4 | M/W | NO | U | NA | ++++ | NA | U |
| 11 | 4 | F/W | NO | U | NA | ++++ | NA | U |
| 31 | 4 | F/B | NO | ONSET | NA | ++++ | NA | STD |
| 32 | 5 | M/W | MCD | SDNS | 1.8 | ++++ | NA | STD |
| 33 | 16 | F/A | NO | ONSET | 1.8 | ++++ | NA | NONE |
| 34 | 5 | M/W | MCD | SSNS | 3.1 | 6.2 | NA | NONE |
| 35 | 10 | F/W | MCD | SSNS | 1.4 | 12.6 | NA | U |
| 36 | 7 | M/Wa | NO | SSNS | 3.2 | 1.7 | NA | STD |
| 37 | 3 | M/W | NO | ONSET | 1.9 | +++ | 0.2 | NONE |
| 38 | 5 | M/W | NO | ONSET | 1.6 | ++++ | 0.3 | NONE |
| 39 | 2 | M/W | NO | ONSET | 1.6 | ++++ | 0.2 | NONE |
| 40 | 4 | F/W | MCD/IgM | SRNS | 3.1 | 6.7 | 0.1 | STD, FK |
| 19 | 6 | M/W | NO | ONSET | 1.8 | 3.4 | 0.2 | STD |
| 20 | 12 | M/W | NO | ONSET | 2 | 6.5 | 0.4 | NONE |
| 41 | 8 | M/W | NO | SDNS | 1.9 | ++++ | 0.4 | STD, FK |
| 42 | 3 | F/W | NO | ONSET | 2.3 | 24.3 | 0.2 | NONE |
| 43 | 5 | M/W | MCD/IgA | SSNS | 3.2 | 8.6 | 0.3 | NONE |
| 44 | 6 | F/W | MCD | SDNS | 2 | 12.9 | 0.4 | STD, FK |
| 45 | 3 | F/W | NO | ONSET | 2.6 | 11.7 | 0.2 | NONE |
| Mean ± SD | 6.5 ± 3.3 | 15M/13F | 8MCD | 2.2 ± 0.6 | 9.4 ± 6.4 | 0.2 ± 0.1 |
+, protein detected by urine dipstick; Alb, albumin; B, Black; Cr, creatinine; F, female; FK, tacrolimus; ID, identification; M, male; MCD, minimal change disease; NA, not available; NEG, negative; ONSET, new onset nephrotic syndrome; S, serum; SDNS, steroid dependent nephrotic syndrome; SRNS, steroid resistant nephrotic syndrome; SSNS, steroid sensitive nephrotic syndrome; STD, steroids; U, unknown; UPC, urine protein-to-creatinine ratio; W, White.
Patients with idiopathic nephrotic syndrome during disease onset/relapse. Available quantitative data were expressed as mean ± SD.
Hispanic.
Table 3.
Demographic characteristics of pediatric control subjects
| ID | Age (yr) | Sex/race | S Alb (g/dl) | UPC | S Cr (mg/dl) | Diagnosis | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 20 | M/Wa | 4.7 | TRA | 0.8 | Stage 1 CKD, renal scars | Vitamin D |
| 2 | 15 | F/W | 4.1 | NEG | 0.8 | Nephrolithiasis | None |
| 3 | 12 | M/Wa | 4.6 | 0.02 | 0.4 | Nephrolithiasis | None |
| 4 | 13 | F/Wa | 4.7 | NEG | 0.76 | Resolved UTI/CAH | Fludrocortisone |
| 5 | 3 | F/Wb | 4.4 | NEG | 0.3 | Renal cyst | None |
| 6 | 9 | F/Wb | 4.5 | 0.4 | 0.46 | Follow-up PIGN | None |
| 7 | 15 | M/Wa | 4.7 | 0.06 | 0.9 | High creatinine Normal eGFR | None |
| 8 | 8 | F/W | NA | 0.11 | NA | Mastoiditis | Ceftriaxone |
| 9 | 5 | M/W | 4.4 | NEG | NA | Acute emesis | None |
| 10 | 10 | F/W | NA | NA | NA | Healthy donor | None |
| 11 | 7 | F/Wa | NA | NA | NA | Healthy donor | None |
| 12 | 6 | M/Wa | NA | NA | NA | Healthy donor | None |
| 13 | 7 | M/Wa | NA | NA | NA | Healthy donor | None |
| 14 | 10 | F/Wa | NA | NA | NA | ADD | None |
| 15 | 7 | M/Wa | NA | NA | NA | URI | Cough syrup |
| 16 | 10 | F/B | NA | NA | NA | Asthma | Albuterol |
| 17 | 7 | M/Wa | NA | NA | NA | None | None |
| 18 | 4 | F/Wa | NA | NA | NA | None | None |
| 19 | 19 | F/W | NA | NEG | 0.9 | Depression | Spironolactone, fluoxetine, rizatriptan |
| 20 | 15 | F/B | NA | NEG | 0.7 | Asthma | Albuterol |
| 21 | 13 | F/W | NA | NEG | 0.6 | Healthy donor | None |
| 22 | 16 | M/W | NA | NEG | 0.7 | Healthy donor | None |
| 23 | 20 | M/W | NA | NEG | 0.9 | Healthy donor | None |
| 24 | 20 | F/W | NA | NEG | 0.7 | Healthy donor | Oral contraceptive, doxycycline |
| 25 | 15 | F/Wa | NA | NEG | 0.5 | Healthy donor | None |
| 26 | 15 | F/W | NA | NEG | 0.8 | Healthy donor | Oral contraceptive |
| 27 | 18 | M/W | NA | NEG | 1.1 | Healthy donor | None |
| 28 | 19 | F/Ua | NA | NEG | 0.7 | Healthy donor | None |
| 29 | 16 | F/Ua | NA | NEG | 0.7 | Healthy donor | None |
| 30 | 12 | F/Wa | NA | NEG | 0.3 | Healthy donor | None |
| Mean ± SD | 12.2 ± 5.1 | 19F/11M | 4.5 ± 0.2 | 0.1 ± 0.1 | 0.6 ± 0.2 |
ADD, attention deficit disorder; Alb, albumin; B, Black; CAH, congenital adrenal hyperplasia; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; F, female; ID, identification; M, male; MCD, minimal change disease; NA, not available; NEG, negative, PIGN, history of postinfectious-infectious glomerulonephritis; S, serum; U, unknown; UPC, urine protein-to-creatinine ratio; UTI, urinary tract infection; W, White.
Pediatric control subjects. Available quantitative data were expressed as mean ± SD.
Hispanic.
Biracial.
Table 4.
Demographic characteristics of adults with MCD during relapse
| ID | Age | Sex/race | S Alb (g/dl) | Proteinuria (g/d) | S Cr (mg/dl) | Treatment |
|---|---|---|---|---|---|---|
| 1 | 23 | F/W | 2.6 | 14 | 0.7 | None |
| 2 | 32 | F/W | 2.9 | 12.6 | 0.84 | None |
| 3 | 45 | F/W | 2.6 | 14.4 | 0.9 | None |
| 4 | 18 | M/W | 2.8 | 8.5 | 0.69 | None |
| 5 | 24 | F/W | 2.3 | 13.8 | 0.76 | None |
| 6 | 30 | F/W | 2.4 | 10.4 | 0.85 | None |
| 7 | 21 | M/W | 2.4 | 14.5 | 0.9 | None |
| 8 | 56 | F/W | 2.8 | 6.6 | 0.8 | None |
| 9 | 18 | M/W | 2.1 | 14.3 | 0.7 | None |
| 10 | 19 | F/W | 1.8 | 8 | 0.9 | None |
| 11 | 22 | M/W | 2.1 | 10.4 | 0.86 | None |
| 12 | 20 | F/W | 2.1 | 15 | 1.02 | None |
| 13 | 27 | M/W | 2.2 | 13.4 | 0.63 | None |
| 14 | 19 | F/W | 2.4 | 7.2 | 0.5 | None |
| 15 | 25 | F/W | 2.2 | 14.4 | 0.68 | None |
| 16 | 22 | M/W | 2.8 | 13.5 | 0.48 | None |
| 17 | 26 | F/W | 2.7 | 13 | 0.7 | None |
| 18 | 37 | M/W | 2.7 | 6.6 | 0.56 | None |
| 19 | 31 | F/W | 2.8 | 7.2 | 0.9 | None |
| 20 | 19 | F/W | 2.3 | 15.5 | 0.87 | None |
| 21 | 25 | F/W | 2.7 | 14.8 | 0.7 | None |
| Mean ± SD | 26.6 ± 9.5 | 14F/8M | 2.4 ± 0.3 | 11.8 ± 3.1 | 0.7 ± 0.1 |
Alb, albumin; Cr, creatinine; F, female; ID, identification; M, male; MCD, minimal change disease; S, serum; W, White.
Available quantitative data were expressed as mean ± SD.
Table 5.
Demographic characteristics of healthy adults
| ID | Age (yr) | Sex | Disease | Treatment |
|---|---|---|---|---|
| 1 | 28 | M | Healthy donor | None |
| 2 | 30 | M | Healthy donor | None |
| 3 | 44 | M | Healthy donor | None |
| 4 | 46 | F | Healthy donor | None |
| 5 | 23 | M | Healthy donor | None |
| 6 | 48 | F | Healthy donor | None |
| 7 | 45 | M | Healthy donor | None |
| 8 | 27 | M | Healthy donor | None |
| Mean ± SD | 36.3 ± 10.2 | 6M/2F |
F, female; ID, identification; M, male.
Available quantitative data expressed as mean ± SD.
Markers of EG Degradation Are Elevated in MCD and SSNS
The EG is composed of core proteoglycans, such as syndecan-1, and glycosaminoglycans, predominantly HS, which is bound to syndecan-1. On injury, these components are enzymatically cleaved into circulation serving as markers of EG degradation.6 To assess for EG degradation in children with MCD/SSNS, we measured circulating syndecan-1 (ELISA), HS fragments (mass spectrometry), and cleaving enzymes including MMP-2 and heparanase activity (ELISA). Syndecan-1 was significantly higher in children with MCD/SSNS in relapse compared with controls and to patients in remission (Figure 1a, P < 0.0001 and P = 0.004, respectively), and levels remained higher in remission than in controls (Figure 1a, P = 0.01). HS levels were significantly increased in children with active MCD/SSNS compared with controls (Figure 1b, P = 0.0003), and there was a trend toward lower levels in remission compared with relapse (Figure 1b, P = 0.05), and between patients in remission and control subjects (Figure 1b, P = 0.07). In children with MCD/SSNS, we found a correlation between circulating syndecan-1 and HS (Figure 1c, r = 0.37, P = 0.03, n = 32). MMP-2 was significantly increased in children with active MCD/SSNS compared with controls and patients in remission (Figure 1d, P < 0.0001 and P = 0.001, respectively), and levels remained higher in remission compared with controls (Figure 1d, P < 0.0001). MMP-2 correlated with syndecan-1 (Figure 1e, r = 0.39, P = 0.006, n = 48) but not with HS (Supplementary Figure S2A). Heparanase activity was also higher in children with MCD/SSNS, during relapse and remission, compared with controls (Figure 1f, P = 0.0009 and P = 0.003, respectively) and also correlated with syndecan-1 (Figure 1g, r = 0.42, P = 0.003, n = 46) but not with HS (Supplementary Figure S2B). To test whether EG degradation is also involved in adults, we measured circulating syndecan-1 and HS in adults with MCD during relapse and in adults without history of kidney disease. Both markers were significantly higher in MCD compared with controls (Figure 2a and b, P = 0.02 and P < 0.0001, respectively), and we found a strong correlation between circulating syndecan-1 and HS (Figure 2c, r = 0.68, P = 0.001, n = 19).
Figure 1.
Endothelial glycocalyx degradation in children with MCD and SSNS. Datapoints indicate the average of duplicate measurements per subject of the following markers: (a) circulating syndecan-1; (b) circulating HS fragments; (c) correlation between syndecan-1 and HS fragments; (d) circulating MMP-2; (e) correlation between MMP-2 and syndecan-1; (f) heparanase activity in circulation; (g) correlation between heparanase activity and syndecan-1. Data presented as median ± interquartile range. Outside values were included in analyses and were a total of 3 for syndecan-1 (1 RL, 1 RM, 1 C), 1 for HS (relapse), 1 for MMP-2 (RM), and 2 for heparanase (RL). C, control; HS, heparan sulfate; MCD, minimal change disease; MMP-2, matrix metalloprotease-2; RL, relapse; RM, remission; SSNS, steroid sensitive nephrotic syndrome.
Figure 2.
Endothelial glycocalyx degradation in adults with MCD. Datapoints indicate the average of duplicate measurements per subject of the following markers: (a) circulating syndecan-1; (b) circulating HS; (c) correlation between circulating syndecan-1 and HS. Data presented as median ± interquartile range. Outside values were included in analyses and were a total of 1 for syndecan-1 and heparan sulfate (RL, for both groups). C, control; HS, heparan sulfate; MCD, minimal change disease; RL, relapse.
MCD and SSNS Are Associated With Systemic and Glomerular Endothelial Activation
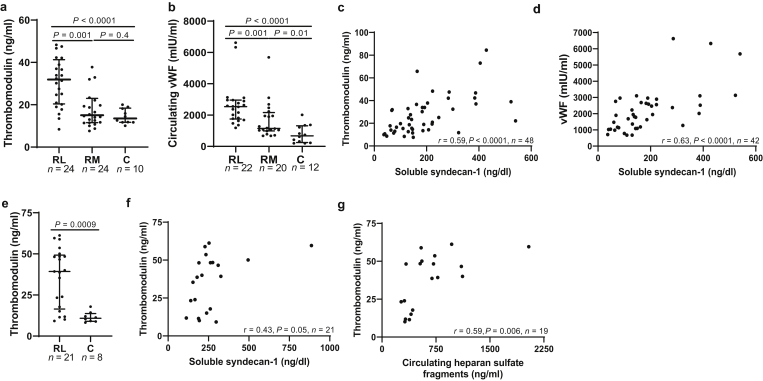
In response to vascular injury, ECs release thrombomodulin and vWF into the circulation.16,17 In children with MCD/SSNS, we found an increase in circulating thrombomodulin and vWF (Figure 3a and b, respectively) during active disease compared with controls (P < 0.0001, for both markers) and with children with MCD/SSNS in remission (P = 0.001, for both markers). Compared with controls, children with MCD/SSNS in remission had higher vWF levels (Figure 3b, P = 0.01), but we found no differences in thrombomodulin concentrations among both groups (Figure 3a, P = 0.4). Thrombomodulin and vWF showed a strong correlation with syndecan-1 in children with MCD/SSNS (Figure 3c and d, r = 0.59, P < 0.0001, n = 48; and r = 0.63, P < 0.0001, n = 42, respectively) but not with HS (Supplementary Figure S3A and B, respectively). In adults with MCD in relapse, thrombomodulin was significantly higher during relapse compared with controls (Figure 3e, P = 0.0009). Thrombomodulin levels showed a trend toward positive correlation with syndecan-1 (Figure 3f, r = 0.43, P = 0.05, n = 21) and correlated with circulating HS fragments (Figure 3g, r = 0.59, P = 0.006, n = 19).
Figure 3.
MCD is associated with systemic endothelial activation. Datapoints indicate the average of duplicate measurements per subject of the following markers: (a) circulating thrombomodulin in children; (b) circulating vWF in children; (c) correlation between thrombomodulin and syndecan-1 in children; (d) correlation between vWF and syndecan-1 in children; (e) circulating thrombomodulin in adults; (f) correlation between syndecan-1 and thrombomodulin; (g) correlation between HS and thrombomodulin in adults. Data presented as median ± interquartile range. Outside values were included in analyses and were a total of 2 for thrombomodulin (2 RM) and 3 for vWF (2 RL, 1 RM) from the pediatric cohort. C, control; HS, heparan sulfate; MCD, minimal change disease; RL, relapse; RM, remission; vWF, von Willebrand factor.
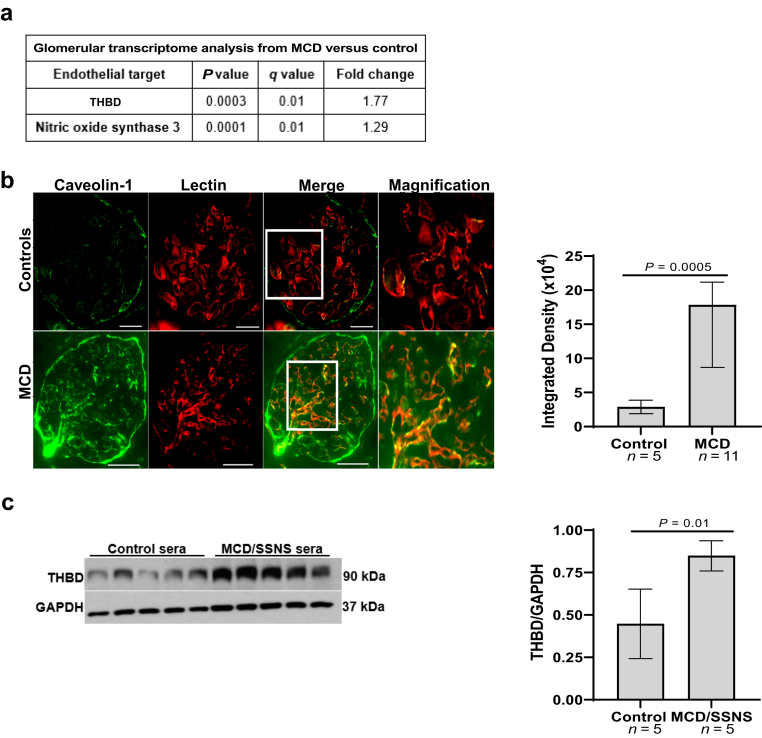
To examine whether endothelial activation also involves the MCD glomerulus, we obtained transcriptomic data from the open-source database Nephroseq (nephroseq.org, “Ju CKD Glom”).15 Expression of 2 endothelial markers of activation, thrombomodulin and nitric oxide synthase 3 (NOS3),16,18 was increased in glomeruli from patients with MCD in relapse (n = 14) compared with healthy controls (n = 21) (P = 0.0003 and 0.0001, respectively; and false discovery rate–adjusted P = 0.01 for both markers)15 (Figure 4a). Next, we performed immunofluorescence of human kidney tissue using caveolin-1 as marker of cell activation.19 Caveolin-1 expression was increased in adults with MCD during relapse compared with controls (Figure 4b, n = 11 and 5, respectively, P = 0.0005), and it colocalized with the lectin ulex europaeus agglutinin (Figure 4b, red), demonstrating its endothelial-specific expression.
Figure 4.
Glomerular endothelial activation in MCD and SSNS. (a) Glomerular transcriptome analysis from patients with MCD in relapse versus controls obtained from Nephroseq.org (“Ju CKD Glom cohort”); (b) immunofluorescence of human kidney showed up-regulation of caveolin-1 (green), colocalizing with rhodamine lectin (red), in adults with MCD in relapse compared with controls (n = 11 vs. n = 5), scale bars = 100 μm. Right graph represents immunofluorescence analysis of caveolin-1 expression in human kidney tissue, P = 0.0005; (c) Western blots of THBD and GAPDH from hiGEnC exposed for 24 hours to 10% sera from children with MCD/SSNS in relapse (n = 1/n = 4, respectively) versus control subjects (n = 5). Right graph represents densitometry analysis of Western blots from hiGEnC lysates, P = 0.01. Data presented as median ± interquartile range. GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; hiGEnC, human immortalized glomerular endothelial cell; MCD, minimal change disease; SSNS, steroid sensitive nephrotic syndrome; THBD, thrombomodulin.
To test whether GEnC activation is triggered directly by a circulating factor(s) present in the MCD sera, we cultured hiGEnC with 10% sera from MCD/SSNS during relapse and control subjects. Sera from children with MCD/SSNS dramatically increased thrombomodulin expression in hiGEnC compared with controls (Figure 4c, n = 5 subjects per group, P = 0.01).
Discussion
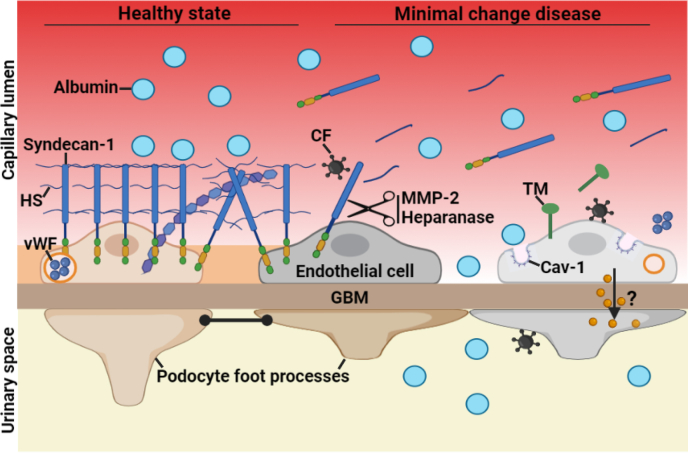
MCD is considered a podocyte disorder mediated by a circulating factor(s) that directly attacks podocytes.1, 2, 3, 4 For decades, most research efforts have focused on the role of immune cells and podocytes in MCD,2,5,20,21 but the pathogenesis remains poorly understood. In this study, we combined rigorous human and cell culture studies to test the hypothesis that EC activation is also involved in MCD. The most striking findings of our study were to demonstrate that MCD involves alterations of the systemic and glomerular endothelium and glycocalyx in nearly all patients, and that MCD sera directly activate GEnC, suggesting that the endothelium may play a role in the disease (Figure 5).
Figure 5.
Schematic showing endothelial alterations in MCD. In healthy state, the EG, which predominantly consists of syndecan-1 and HS, covers the luminal surface of the glomerular endothelial cells and their fenestrations. In MCD, the CF directly targets the endothelium, resulting in EG degradation, as noted by the shedding of syndecan-1 and HS into circulation, and GEnC activation involving the release of TM and vWF into circulation, and the up-regulation of Cav-1. We postulate that EG degradation may favor the passage of serum albumin through endothelial fenestration and may facilitate that circulating factors encounter and activate podocytes. Furthermore, activated GEnC may release factors locally that could contribute to podocyte injury. Cartoon created with BioRender.com. Cav-1, caveolin-1; CF, circulating factor; EG, endothelial glycocalyx; GBM, glomerular basement membrane; GEnC, glomerular endothelial cell; HS, heparan sulfate; MCD, minimal change disease; MMP-2, matrix metalloprotease-2; TM, thrombomodulin; vWF, von Willebrand factor.
We demonstrated that EG degradation is underrecognized in patients with MCD/SSNS in relapse. We showed that markers of EG degradation and cleaving enzymes are consistently elevated in most patients, children and adults, during relapse. Although EG degradation is usually associated with severe or chronic illnesses such sepsis, preeclampsia, or diabetes,6,22,23 here we showed that EG injury is also common in children with new onset nephrotic syndrome and normal kidney function. The source of these circulating EG products is likely the systemic endothelium, given the strong correlation between circulating syndecan-1 with thrombomodulin and vWF in children and HS and thrombomodulin in adults. Interestingly, HS did not correlate with degrading enzymes or markers of endothelial activation in children. Some EG products have a relatively low molecular weight and can be excreted into urine in nephrotic states,24 so it is possible that they may have different excretion rates into urine, and this could impact their serum concentrations differently.
Notably, markers of EG degradation and systemic endothelial activation in our population were as high as those reported in patients with severe systemic endotheliopathy including sepsis and COVID-19.25,26 Hence, our study provides evidence to support that MCD involves substantial alterations of the whole vascular endothelium and that MCD is not simply a podocyte disorder. Consistent with this, a recent study showed that MCD sera increased permeability of human umbilical vein ECs.27 These findings are important because EG degradation is associated with albuminuria in other diseases such diabetic nephropathy, preeclampsia, and sepsis.6,28 It is possible that EG degradation may also contribute to proteinuria in MCD and/or that it facilitates the passage and exposure of “toxic” circulating factors to podocytes. There is some evidence that the EG may generate an electrical field preventing the passage of negatively charged plasma proteins through endothelial fenestrae29,30; hence, further studies are needed to investigate if EG degradation plays a role in MCD.
We next provided evidence that endothelial alterations can persist during remission of proteinuria in some patients to a lesser extent that during relapse. It is possible that endothelial alterations may take longer than podocyte injury to recover but also could reflect the continued presence of the factor that drives MCD, despite the resolution of proteinuria in response to steroids. This seems clinically relevant because sustained endothelial injury is usually associated with poor clinical outcomes and progression of kidney disease.9,10,31 It is possible that patients with MCD/SSNS and sustained endothelial alterations (as noted by high circulating syndecan-1, for instance) may be at higher risk for relapse (on immunosuppression taper or after respiratory infections), poor response to therapies, progression to focal segmental glomerulosclerosis, or decline in kidney function. In support to this, circulating MMP-2, a mediator of EG degradation,32 is associated with poor response to steroids in children with idiopathic nephrotic syndrome.33 Notably, we found that 10 of 24 children with MCD/SSNS in remission had MMP-2 levels above the 95th percentile of healthy controls and that circulating MMP-2 correlated with syndecan-1. Therefore, our findings suggest that circulating syndecan-1 levels likely reflect ongoing EG degradation, and this may have a prognostic role in the disease. Likewise, MCD is associated with ultrastructural changes in GEnC, and these correlate with poor clinical outcomes.10 Future studies are needed to investigate whether serologic evidence of endothelial alterations may predict clinical outcomes, disease relapse, and/or precede morphologic changes in the glomerular endothelium.
We showed that endothelial activation in MCD also involves the glomerular endothelium, as noted by the up-regulation of caveolin-1 and thrombomodulin. This is consistent with previous studies by others and by our group.34,35 While podocyte damage can trigger endothelial injury,36 here we provided evidence that MCD sera can directly trigger GEnC activation. This raises the possibility that GEnC are a primary target of the presumed circulating factor(s) in MCD and that on activation, GEnC may release factors that could promote local inflammation, and if uncontrolled, it may lead to fibrosis and focal segmental glomerulosclerosis.9 In fact, an increase in endothelin receptor A expression and oxidative stress in GEnC has been reported in patients with focal segmental glomerulosclerosis and diabetic nephropathy.37, 38, 39 Therefore, sustained endothelial alterations may identify patients with MCD/SSNS at risk for progression to focal segmental glomerulosclerosis or advanced stages of chronic kidney disease.
Our study has limitations and strengths worth mentioning, including a relatively small cohort of children (blood markers) with MCD/SSNS and adults (unmatched blood samples and kidney tissue) with MCD, which may constrain the generalizability. Furthermore, our study does not address whether endothelial alterations have a prognostic and/or pathogenic role in MCD, but it does identify the vascular endothelium as a potential target and contributor to the disease. We acknowledge that endothelial alterations are also present in other kidney diseases,9 and whether MCD and SSNS have a distinct endothelial signature is yet to be determined. Strengths of this study include the consistency of several markers of EG degradation and endothelial activation in most patients. In addition, we leveraged different techniques to rigorously examine endothelial alterations as well as a diverse population.
In summary, MCD and SSNS are associated with alterations of the systemic and glomerular endothelium. Understanding the role of endothelium in the pathogenesis of MCD may contribute to the identification of prognostic tools (biomarkers) and therapeutic targets (such as agents able to restore the EG)40 and provide insight into the potential role of the endothelial-podocyte crosstalk in MCD.
Disclosure
JMT receives royalties from Alexion Pharmaceuticals, Inc., and is a consultant for Q32 Bio, Inc., a company developing complement inhibitors. He also holds stock and will receive royalty income from Q32 Bio, Inc. PB has acted as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Sanofi, Novo Nordisk, and Horizon Pharma. PB serves on the advisory boards for AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX. All the other authors declared no competing interests.
Acknowledgments
The authors thank Mr. Randy Thompson for his manuscript revision and comments. Financial support for this work was in part provided by the Asociación Española de Nefrología Pediátrica (AENP) to CLC; by the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL149422) to EPS, and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK116720) to PB. PB receives salary and research support from National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK129211, R21 DK129720, K23 DK116720, UC DK114886, and P30 DK116073), JDRF (2-SRA-2019-845-S-B, 3-SRA-2017-424-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Ludeman Family Center for Women’s Health Research at University of Colorado, the Department of Pediatrics, Section of Endocrinology, and Barbara Davis Center for Diabetes at University of Colorado School of Medicine.
Footnotes
Figure S1. Syndecan-1 and MMP-2 levels in paired serum and plasma samples.
Figure S2. Circulating HS fragments and cleaving enzymes in children with MCD and SSNS.
Figure S3. Circulating HS fragments and markers of endothelial activation in children with MCD and SSNS.
Supplementary Material
Figure S1. Syndecan-1 and MMP-2 levels in paired serum and plasma samples.
Figure S2. Circulating HS fragments and cleaving enzymes in children with MCD and SSNS.
Figure S3. Circulating HS fragments and markers of endothelial activation in children with MCD and SSNS.
References
- 1.Vivarelli M., Massella L., Ruggiero B., Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopp J.B., Anders H.J., Susztak K., et al. Podocytopathies. Nat Rev Dis Primers. 2020;6:68. doi: 10.1038/s41572-020-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colucci M., Corpetti G., Emma F., Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33:573–584. doi: 10.1007/s00467-017-3677-5. [DOI] [PubMed] [Google Scholar]
- 4.Shalhoub R.J. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 5.Grahammer F., Schell C., Huber T.B. The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol. 2013;9:587–598. doi: 10.1038/nrneph.2013.169. [DOI] [PubMed] [Google Scholar]
- 6.Butler M.J., Down C.J., Foster R.R., Satchell S.C. The pathological relevance of increased endothelial glycocalyx permeability. Am J Pathol. 2020;190:742–751. doi: 10.1016/j.ajpath.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naylor R.W., Morais M.R.P.T., Lennon R. Complexities of the glomerular basement membrane. Nat Rev Nephrol. 2021;17:112–127. doi: 10.1038/s41581-020-0329-y. [DOI] [PubMed] [Google Scholar]
- 8.Salmon A.H., Satchell S.C. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226:562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 9.Sol M., Kamps J., van den Born J., et al. Glomerular endothelial cells as instigators of glomerular sclerotic diseases. Front Pharmacol. 2020;11:573557. doi: 10.3389/fphar.2020.573557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royal V., Zee J., Liu Q., et al. Ultrastructural characterization of proteinuric patients predicts clinical outcomes. J Am Soc Nephrol. 2020;31:841–854. doi: 10.1681/ASN.2019080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tkaczyk M., Czupryniak A., Owczarek D., Lukamowicz J., Nowicki M. Markers of endothelial dysfunction in children with idiopathic nephrotic syndrome. Am J Nephrol. 2008;28:197–202. doi: 10.1159/000110088. [DOI] [PubMed] [Google Scholar]
- 12.Sharma B., Saha A., Dubey N.K., et al. Endothelial dysfuntion in children with idiopathic nephrotic syndrome. Atherosclerosis. 2014;233:704–706. doi: 10.1016/j.atherosclerosis.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 13.Noone D.G., Iijima K., Parekh R. Idiopathic nephrotic syndrome in children [published correction appears in Lancet. 2018;392:282] Lancet. 2018;392:61–74. doi: 10.1016/S0140-6736(18)30536-1. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt E.P., Li G., Li L., et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289:8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju W., Greene C.S., Eichinger F., et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. 2013;23:1862–1873. doi: 10.1101/gr.155697.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boehme M.W., Galle P., Stremmel W. Kinetics of thrombomodulin release and endothelial cell injury by neutrophil-derived proteases and oxygen radicals. Immunology. 2002;107:340–349. doi: 10.1046/j.1365-2567.2002.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowenstein C.J., Morrell C.N., Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med. 2005;15:302–308. doi: 10.1016/j.tcm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Lowry J.L., Brovkovych V., Zhang Y., Skidgel R.A. Endothelial nitric-oxide synthase activation generates an inducible nitric-oxide synthase-like output of nitric oxide in inflamed endothelium. J Biol Chem. 2013;288:4174–4193. doi: 10.1074/jbc.M112.436022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlides S., Gutierrez-Pajares J.L., Iturrieta J., Lisanti M.P., Frank P.G. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014;356:147–157. doi: 10.1007/s00441-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araya C.E., Wasserfall C.H., Brusko T.M., et al. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2006;21:603–610. doi: 10.1007/s00467-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 21.Lennon R., Singh A., Welsh G.I., et al. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol. 2008;19:2140–2149. doi: 10.1681/ASN.2007080940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Han X., Xia K., et al. Circulating heparin oligosaccharides rapidly target the hippocampus in sepsis, potentially impacting cognitive functions. Proc Natl Acad Sci U S A. 2019;116:9208–9213. doi: 10.1073/pnas.1902227116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissgerber T.L., Garcia-Valencia O., Milic N.M., et al. Early onset preeclampsia is associated with glycocalyx degradation and reduced microvascular perfusion. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuhashi H., Tsukada Y., Ono K., Yano S., Naruse T. Urine glycosaminoglycans and heparan sulfate excretions in adult patients with glomerular diseases. Clin Nephrol. 1993;39:231–238. [PubMed] [Google Scholar]
- 25.Hippensteel J.A., Anderson B.J., Orfila J.E., et al. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J Clin Invest. 2019;129:1779–1784. doi: 10.1172/JCI124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daviet F., Blin M.G., Fallague K., et al. Sera from patients with minimal change disease increase endothelial permeability to sodium. Kidney Int Rep. 2020;5:1071–1075. doi: 10.1016/j.ekir.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieuwdorp M., Mooij H.L., Kroon J., et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 29.Moeller M.J., Tenten V. Renal albumin filtration: alternative models to the standard physical barriers. Nat Rev Nephrol. 2013;9:266–277. doi: 10.1038/nrneph.2013.58. [DOI] [PubMed] [Google Scholar]
- 30.Moeller M.J., Kuppe C. Point: Proposing the electrokinetic model. Perit Dial Int. 2015;35:5–8. doi: 10.3747/pdi.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inkinen N., Pettilä V., Lakkisto P., et al. Association of endothelial and glycocalyx injury biomarkers with fluid administration, development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care. 2019;9:103. doi: 10.1186/s13613-019-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali M.M., Mahmoud A.M., Le Master E., Levitan I., Phillips S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am J Physiol Heart Circ Physiol. 2019;316:H647–H663. doi: 10.1152/ajpheart.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal S., Merchant M.L., Kino J., et al. Predicting and defining steroid resistance in pediatric nephrotic syndrome using plasma proteomics. Kidney Int Rep. 2019;5:66–80. doi: 10.1016/j.ekir.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriyama T., Tsuruta Y., Shimizu A., et al. The significance of caveolae in the glomeruli in glomerular disease. J Clin Pathol. 2011;64:504–509. doi: 10.1136/jcp.2010.087023. [DOI] [PubMed] [Google Scholar]
- 35.Cara-Fuentes G., Venkatareddy M., Verma R., et al. Glomerular endothelial cells and podocytes can express CD80 in patients with minimal change disease during relapse. Pediatr Nephrol. 2020;35:1887–1896. doi: 10.1007/s00467-020-04541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eremina V., Sood M., Haigh J., et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi H., Casalena G., Shi S., et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes. 2017;66:763–778. doi: 10.2337/db16-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daehn I., Casalena G., Zhang T., et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Lest N.A., Bakker A.E., Dijkstra K.L., et al. Endothelial endothelin receptor A expression is associated with podocyte injury and oxidative stress in patients with focal segmental glomerulosclerosis. Kidney Int Rep. 2021;6:1939–1948. doi: 10.1016/j.ekir.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gbadegesin R.A., Hernandez L.P.H., Brophy P.D. Case report: novel dietary supplementation associated with kidney recovery and reduction in proteinuria in a dialysis dependent patient secondary to steroid resistant minimal change disease. Front Pediatr. 2021;9:614948. doi: 10.3389/fped.2021.614948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.