Abstract
Introduction
IgA nephropathy (IgAN) is the most common primary glomerulonephritis (GN) worldwide. The disease course fluctuates, and the most important challenge is the considerable variation in the time lag between diagnosis and the development of a hard clinical end point, such as end-stage kidney disease (ESKD). The reaction of renal tissue to damage resembles the common wound-healing response. One part of this repair in IgAN is the expansion of lymphatic vessels known as lymphangiogenesis. The aim of this work was to establish the prognostic value of the density of lymphatic vessels in the renal biopsy at the time of diagnosis, for predicting the risk of ESKD in a Spanish cohort of patients with IgAN.
Methods
We performed a retrospective multicenter study of 76 patients with IgAN. The end point of the study was progression to ESKD. The morphometric analysis of lymphatic vessels was performed on tissue sections stained with antipodoplanin antibody.
Results
Density of lymphatic vessels was significantly higher in patients with IgAN with mesangial hypercellularity >50%, segmental sclerosis, higher degrees of interstitial fibrosis, and tubular atrophy. Patients with more lymphatic vessels had significantly higher values of proteinuria and lower estimated glomerular filtration rate (eGFR). A density of lymphatic vessels ≥8 per mm2 was associated with a significantly higher rate of progression to ESKD at 3 years from biopsy. After adjustment for the International IgAN prediction score, at the multivariate logistic regression, high density of lymphatic vessels (≥8 per mm2) remained significantly associated with a higher rate of early progression to ESKD.
Conclusion
This study contributes to the understanding of the natural history of the progression to ESKD in patients with IgAN revealing the density of lymphatics vessels may optimize the prognostic value of the International IgA predicting tool to calculate the risk of ESKD, favoring the evaluation of new targeted therapies.
Keywords: end-stage kidney disease, IgAN, lymphatics, proteinuria
Graphical abstract
See Commentary on Page 667
IgAN is the most common primary GN worldwide. The incidence rate has been estimated at 2 to 10 per 100,000 person-years. A renal biopsy with mesangial deposition of IgA immune complex is required for diagnosis.1 The disease course is very variable because of its clinical, pathologic, and pathogenic heterogeneity; and it ranges from asymptomatic nonprogressive to aggressive, with up to 1 in 4 patients experiencing from ESKD within 20 years from diagnosis.2
Several retrospective studies have identified hypertension, renal function at diagnosis, and the magnitude of proteinuria during follow-up as the most important clinical predictors of ESKD. Of them, a sustained protein excretion >1 g/d is considered as the most determinant predictor of progression, and treatment recommendations are based on the magnitude of proteinuria.3 More recently, some studies have revealed that histologic lesions included in the Oxford Classification are independently associated with renal outcomes and significantly improve the prediction of disease progression to ESKD.4
The final pathway to chronic kidney disease (CKD) and kidney failure is always the loss of normal tissue and matrix deposition with fibrosis in every kidney compartment. The extent of renal fibrosis predicts the loss of function and progression of damage in the kidney. Thus, renal biopsy is not only useful for diagnosis and prognostic categorization but can also quantify fibrosis and elucidate new therapeutic targets that could prevent the progression of IgAN.5
The reaction of renal tissue to damage resembles the common wound-healing response that occurs in other tissues. Nevertheless, the repair and recovery of renal function do not always happen. One part of this repair in IgAN is the expansion of lymphatic vessels known as lymphangiogenesis; besides functional changes in lymphatic vessel morphology, lymphangiogenesis occurs in response to numerous noxious stimuli, including interstitial fluid overload and inflammation. Thus, determination of how to target lymphatic vessels may have impact in the treatment and prevention of CKD.6
Adequate categorization of patients in individual risk classification for ESKD in IgAN is an essential aspect when making therapeutic decisions and designing clinical trials. The most important challenge is the considerable variation in the time lag between diagnosis and the development of a hard clinical end point, such as ESKD.7
Previous immunohistochemical studies of lymphatics with antipodoplanin antibody have elucidated their relation to the overall anatomical structure of the normal human kidney and in ESKD, cortical infarction, acute tubular necrosis, and hydronephrosis; the use of podoplanin antibody for the detection of renal lymphatics is well characterized under both normal and pathologic conditions, and the distribution patterns of lymphatics are similar with those previously reported in animals.8
The aim of this work was to establish the prognostic value of the density of lymphatic vessels in the renal biopsy at the time of diagnosis to predict the risk of ESKD in a Spanish multicentric cohort of patients with IgAN that was followed for a prolonged period with periodic visits, in which all recognized clinical and laboratory risk factors for disease progression were systematically recorded.
In this way, we were able to consistently analyze the correlation of lymphangiogenesis with the appearance of renal fibrosis in the biopsy and its correlation with disease progression.
Methods
Study Population
This study was designed to include all consecutive patients with biopsy proven IgAN and followed up from January 2012 to December 2018 in 5 nephrology departments belonging to the Catalan Group for the Study of Glomerular Diseases (GLOMCAT). Patients with other underlying autoimmune diseases or hepatitis B or C infection were excluded from further consideration.
The diagnosis of IgAN was based on the Oxford classification criteria on native kidneys.9 Biopsies with fewer than 10 glomeruli were excluded to assure a representative sample. All data were retrospectively collected from the time of IgAN diagnosis to December 2020. All patients gave written informed consent. The study was approved by the institutional review board of the Hospital Clinic of Barcelona and was conducted in accordance with the Declaration of Helsinki.
Histologic Characterization
All biopsies were processed for light and immunofluorescence microscopy using standard techniques, examined by an experienced nephropathologist, and interpreted according to the Oxford Classification of IgAN.
The morphometric analysis of lymphatic vessels was performed on formalin-fixed, paraffin-embedded tissue sections using BenchMark ULTRA (Ventana) and commercially available antibody (podoplanin: D2-40, mouse monoclonal antibody, ready to use, Cell Marque). The D2-40 antibody was used after standard antigen retrieval (CC1 solution Ventana; 36 minutes, 95 °C).
Podoplanin is a ∼38 kD O-linked transmembrane sialoglycoprotein expressed on the endothelium of lymphatic capillaries, but not in the blood vasculature. Clone D2-40 recognizes podoplanin in normal and neoplastic tissues, and it has been used for the identification of lymphatic invasion in a variety of cancers.10,11
Lymphatic vessels were counted only in the cortex, and their areas were measured, using an Olympus Cell B Basic Imaging Software and the Olympus SC50 camera attached to an BX51 microscope. Lymphatic areas were defined as vascular areas delineated by D2-40–positive staining. Evaluated parameters included microvessel areas, the total area of the biopsy analyzed, the density of lymphatic vessels (number per mm2 biopsy area), and the proportion of the examined area occupied by lymphatic vessels.
Clinical Data
The following data were retrieved from the medical records of the patients: demographic data and concomitant medical conditions, both at presentation and follow-up (3, 6, 12, 24, 60, and 120 months or last outpatient clinic visit); time to last visit; time to the main follow-up landmarks (remission, progression to ESKD, death); information on treatment (corticosteroid, other immunosuppressive drugs, renin-angiotensin system blockers). The following laboratory tests at presentation and during follow-up were also retrieved: blood cell counts, serum creatinine, eGFR measured with CKD epidemiology collaboration, serum albumin, 24-hour proteinuria, urinary sediment, and albumin.
International IgAN Prediction Tool
The International IgAN Prediction Tool in adults was used to calculate the risk of 50% decline in eGFR or progression to end-stage renal disease at 5 years after renal biopsy. The risk equations were derived in a multiethnic international cohort of 2781 patients with biopsy proven idiopathic IgAN.4
Renal Survival
The main end point of the study was progression to ESKD, defined as an eGFR <15 ml/min per 1.73 m2, the need for dialysis or pre-emptive kidney transplantation.
Statistical Analysis
Continuous variables were summarized as mean and SD or median and interquartile range (IQR). Categorical variables were described by proportions and compared using the χ2 test or the Fisher exact test when applicable. Comparisons between groups were made using the t test for 2 independent samples and the Mann–Whitney U test for non-normal samples. Kaplan–Meier estimates were used for renal survival by interstitial fibrosis and tubular atrophy. The association between progression to ESKD at the 3-year landmark and either density of lymphatic vessels or the Oxford MEST score was evaluated by multivariable logistic regression and expressed as odd ratio (OR) and its 95% CI. Statistical analyses were performed with Stata software, version 11 (www.stata.com), and GraphPad Prism, version 7.0 (GraphPad Software, San Diego, CA). The significance level was fixed at 0.05.
Results
Baseline Clinical Characteristics
There were 125 patients with IgAN who were retrieved from our records, of whom 49 cases were excluded owing to nonfulfillment of inclusion criteria or because of missing data (criteria were very strict to ensure the quality of the data of this proof concept study). A total of 76 patients were finally included in the analysis. The mean age was 41 years (30–49 years), 68 were Caucasians and 8 Asiatic, and 48 were males (74%). At presentation, 25 patients (33%) had arterial hypertension, the mean proteinuria was 1.3 g/d, 68 (89%) were on treatment with renin–angiotensin system blockade agents, and 16 (21%) were receiving corticosteroids.
Correlation Between the Density of Lymphatic Vessels and Other Histopathologic Features
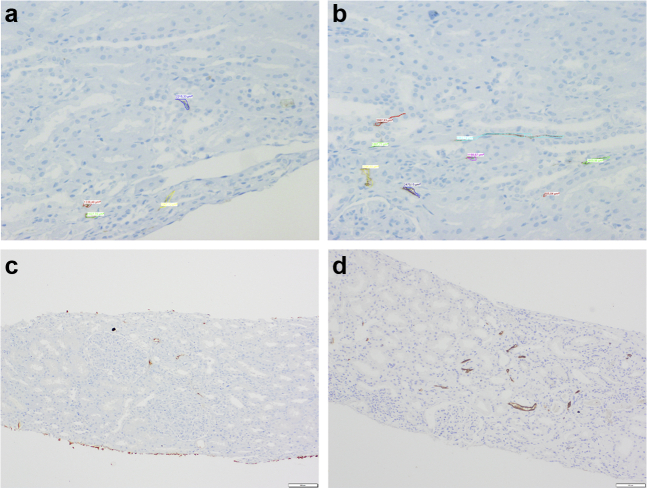
The median density of lymphatic vessels per mm2 of biopsy was 2.8 (IQR: 1.0–5.5) and occupied a median of 0.4% (IQR: 0.2–0.7) of the examined area. There was a strong correlation between density and area of lymphatic vessels (R2 = 0.87, P < 0.001; Supplementary Figure S1). Figure 1a–d reveals photomicrographs of renal biopsies stained with D2-40 (lymphatic vessels).
Figure 1.
Photomicrographs of renal biopsies stained with D2-40 (lymphatic vessels). Superior panels reveal measurements of areas of the lymphatic vessels (a and b). Inferior panels reveal examples of <8 lymphatics and >8 lymphatics (c and d, respectively).
Table 1 summarizes the correlation between density of lymphatic vessels and other histopathologic features. The density of lymphatic vessels was significantly higher in patients with mesangial hypercellularity >50%, segmental sclerosis, and higher degrees of interstitial fibrosis/tubular atrophy.
Table 1.
Association between density of lymphatic vessels and other histopathologic features in 76 patients with IgA nephropathy
| Histopathologic feature | No. of patients | Density of lymphatic vessels (number per mm2) | P |
|---|---|---|---|
| Mesangial hypercellularity | |||
| <50% | 40 | 2.0 (0.7–4.6) | 0.03 |
| ≥50% | 36 | 3.4 (1.8–6.2) | |
| Segmental sclerosis | |||
| No | 56 | 2.3 (1.1–4.3) | 0.01 |
| Yes | 20 | 4.6 (2.3–8.7) | |
| Endocapillary hypercellularity | |||
| No | 31 | 2.2 (1.1–4.4) | 0.08 |
| Yes | 45 | 3.4 (1.2–8.0) | |
| Interstitial fibrosis—tubular atrophy | |||
| <25% | 40 | 1.9 (0.7–4.3) | 0.007 |
| 25%–50% | 19 | 3.1 (1.3–7.5) | |
| >50% | 17 | 4.8 (2.2–9.3) |
To further investigate the clinical and prognostic features associated with the density of lymphatic vessels, we categorized density as ≥8 vessels per mm2 (n = 11) or lower (n = 65). We relied on receiver operating characteristics curve analysis to select the optimum density value at approximately the 0.75 percentile (Supplementary Figure S2).
Clinical Characteristics at the Time of Renal Biopsy According to the Density of Lymphatic Vessels
Table 2 summarizes the main clinical characteristics at the time of renal biopsy according to the density of lymphatic vessels. Patients with more lymphatic vessels had significantly higher values of proteinuria and lower eGFR. There were no significant differences between both groups in the incidence of comorbidities or microscopic hematuria.
Table 2.
Clinical features at the time of renal biopsy according to the density of lymphatic vessels in 76 patients with IgA nephropathy
| Feature | Total | Density of lymphatic vessels <8 per mm2 | ≥8 per mm2 | P |
|---|---|---|---|---|
| Age, yra | 41 (30–49) | 41 (30–52) | 32 (27–48) | 0.36 |
| Sex, males/females | 48 (74%)/17 | 42 (78%)/12 | 6(55%)/5 | 0.11 |
| Comorbidities | ||||
| Hypertension | 25 (33%) | 21 (32%) | 4 (36%) | 0.79 |
| Diabetes | 3 (4%) | 3 (5%) | 0 | 0.47 |
| Hepatic cirrhosis | 6 (8%) | 6 (9%) | 0 | 0.29 |
| Other | 19 (25%) | 15 (23%) | 4 (36%) | 0.55 |
| Hematuria | 62 (85%) | 52 (84%) | 10 (91%) | 0.55 |
| Proteinuria, g/24 ha | 1.3 (0.5–2.2) | 1.2 (0.4–1.8) | 3.5 (2–4) | <0.001 |
| Proteinuria > 3.5 g/24 h | 9 (12%) | 3 (5%) | 6 (55%) | <0.001 |
| Creatinine, mg/dla | 1.2 (0.9–2.0) | 1.1 (0.9–1.6) | 2.8 (1.0–4.0) | 0.01 |
| eGFR, ml/min | 60 (33–87) | 64 (42–90) | 26 (17–59) | 0.008 |
| RAS inhibition | 68 (89%) | 58 (94%) | 10 (91%) | 0.5 |
| Steroids | 16 (21%) | 12 (19%) | 4 (36%) | 0.5 |
eGFR, estimated glomerular filtration rate; RAS, renin-angiotensin system.
Median (interquartile range).
Clinical Outcomes According to the Density of Lymphatic Vessels
After a median follow-up of 2.5 years (IQR: 1.1–3.3), 12 patients had progressed to ESKD and 3 patients had died (2 in ESKD). Median survival was not achieved, and the estimated survival at 6 years from the biopsy was 90% (95% CI: 73–97).
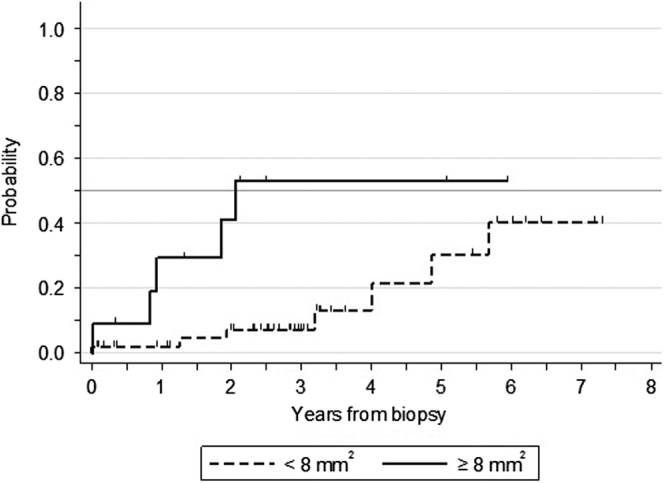
A density of lymphatic vessels ≥ 8 per mm2 was associated with a significantly higher rate of progression to ESKD (log-rank test P = 0.01; Figure 2). At 3 years from biopsy, the progression rate to ESKD was 7% in patients with density values <8 per mm2 versus 53% in patients with values ≥8 per mm2.
Figure 2.
International score according to density of lymphatic vessels in renal biopsy of patients with IgA nephropathy.
Correlation Between Density of Lymphatic Vessels and the International IgAN Prediction Score
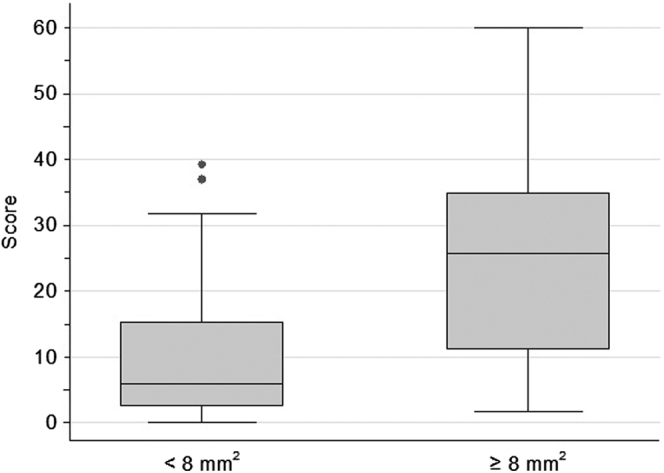
In the whole series of 76 patients with IgAN, the prediction score ranged from 0.18 to 60.0 with a median of 8.8. Patients with a high density of lymphatic vessels (≥8 per mm2) scored significantly higher (median: 25.7, IQR: 11.2–34.9) than patients with lower density (median: 6.0, IQR: 2.6–15.3, P = 0.003; Figure 3).
Figure 3.
Risk of progression to ESKD according to the density of lymphatic vessels in the renal biopsy of patients with IgA nephropathy. ESKD, end-stage kidney disease.
The Density of Lymphatic Vessels and International IgAN Prediction Score as Predictors of Progression to ESKD
On the basis of the profile of progression to ESKD found in Figure 2, we investigated whether a high density of lymphatic vessels can refine the prognostic significance of the international score to predict early progression to ESKD. After excluding patients who did not progress and were followed by <3 years, both the score (OR: 1.12, 95% CI: 1.03–1.22, P = 0.008) and density of lymphatics ≥ 8 per mm2 (OR: 26, 95% CI: 3.4–195, P = 0.002) were individually associated with a higher risk of progression within the first 3 years of follow-up. At the multivariable logistic regression, high density of lymphatic vessels (≥8 per mm2) remained significantly associated with a higher rate of early progression to ESKD (OR: 10.5, 95% CI: 1.1–96.6, P = 0.03), even after adjustment for the score (OR: 1.0, 95% CI: 0.9–1.2; P = 0.05).
Discussion
Here, we describe the clinical characteristics, outcomes, and the relation of renal lymphangiogenesis with the progression of the disease of an observational cohort of patients with IgAN. There are several major findings in this study. First, the density of lymphatic vessels was significantly higher in patients with IgAN with mesangial hypercellularity >50%, segmental sclerosis, higher degrees of interstitial fibrosis, and tubular atrophy. Second, patients with more lymphatic vessels had significantly greater values of proteinuria and lower eGFR. Third, the density of lymphatic vessels ≥8 per mm2 was associated with a significantly higher rate of progression to ESKD at 3 years from biopsy. Finally, after adjustment for the International IgAN prediction score, at the multivariate logistic regression, high density of lymphatic vessels (≥8 per mm2) remained significantly associated with a higher rate of early progression to ESKD.
It is not known with certainty whether there is a point of no return in IgAN, but most studies suggest that progression to ESKD is inexorable once the eGFR falls <20 to 30 ml/min per 1.73 m2 in the presence of advanced chronic changes in the renal biopsy.12,13 Establishing a reliable chronicity index that stratifies the appearance of interstitial fibrosis and tubular atrophy from preliminary stages is an urgent need in this complex glomerular disease.3 The field of risk stratification in IgAN has been optimized by the recent validation of a new international risk-prediction tool in large, multiethnic cohorts from the International IgA Network. This tool can predict the kidney-specific end points of a 50% decline in kidney function or ESKD with a quite reasonable accuracy in large cohorts, but the utility at the individual patient level has yet to be confirmed in the long term.14
Structural changes to the vasculature are a prominent feature of CKD. Although peritubular capillaries undergo rarefaction, potentially triggering interstitial hypoxia and generating a profibrogenic environment within diseased kidney15; renal lymphatics proliferate and sprout, giving rise to new vessels in a process termed lymphangiogenesis.16 It has been previously described in murine models of CKD and in clinical cases of IgAN, focal glomerulosclerosis, lupus nephritis, antineutrophil cytoplasmic autoantibody–related GN, diabetic kidney disease, and chronic interstitial nephritis, with disease ranging from moderate CKD to ESKD, that the cross-sectional area of lymphatics was significantly greater than in nondiseased kidneys.17, 18, 19
The results reported here not only confirmed these preliminary observations in a whole cohort of patients with IgAN if not, they represent, to the best of our knowledge, the first study that identifies a cutoff point of 8 lymphatic vessels/mm2 as a risk factor for rapid progression to ESKD, indicating the density of lymphatic vessels in the renal biopsy of patients with IgAN may become a new surrogate marker to optimize the personalized stratification of chronic damage and the risk of progression to ESKD. Moreover, using the international risk score, with the predictive values of the Oxford subgrouping and lymphatic density, we found a higher risk of progression at 3 years, being an additional tool for making therapeutic decisions at the time of diagnosis of IgAN.
From our point of view, conducting this proof-of-concept study in IgAN was a priority because it is the most common primary glomerular disease. Nevertheless, soon, it may be used in the study of CKD associated with other nephropathies because higher lymphatic density may be a common pathway, found in kidney fibrosis.
Unfortunately, we still do not have any specific treatment for patients diagnosed with having IgAN, after deposition of IgA1-containing circulating immune complexes, there is an inflammatory response characterized by the infiltration of immune cells with the objective of controlling the damage and recovering tissue functionality. Nevertheless, as nephron endowment is limited, and kidneys are unable to generate new nephrons, ongoing inflammation or repeated episodes of kidney injury will lead to further nephron loss that is associated with the development of cell senescence and kidney fibrosis.20, 21
Currently, evidence has confirmed that the clinical parameters of poor prognosis in IgAN have a lower weight when histologic lesions are well characterized.22 The density of lymphatic vessels may also be evaluated in the near future in follow-up biopsies to establish its potential role as a marker of treatment response, evaluating the ability to halt the progression of renal fibrosis of classic drugs, such as blockers of the renin-angiotensin-aldosterone axis, and of new molecules, such as sodium-glucose cotransporter-2 inhibitors. Owing to the recent published prespecified analysis of the DAPA-CKD trial, which revealed the effects of dapagliflozin on major adverse kidney events in patients with IgAN, it will also be especially attractive to analyze the role of sodium-glucose cotransporter-2 inhibitors owing to its multiple renal effects, not only avoiding proteinuria because of hemodynamic effect (control of glomerular hypertension) but also affecting metabolic stress and inflammation, thus breaking the vicious circle of inflammation, fibrosis, and lymphangiogenesis.23
Recently, the TESTING study revealed that patients with IgAN and proteinuria >1 g/d can benefit from long-term use of corticosteroids in nuanced doses. Adding new predictive markers for ESKD, such as density of lymphatics vessels, is of great relevance to better characterize the group of patients in whom the risk of exposure to steroids is well balanced with respect to the clinical benefit.24
This highlights the importance of renal biopsy not only for diagnosis but also to enable individualized treatment to be administered to patients with IgAN.
From another point of view, the kidney contains a network of lymphatic vessels that clear fluid, small molecules, and cells from the renal interstitium and are implicated in renal disease, but their function in inflammatory kidney diseases, such as IgAN, is poorly defined. Studies of lymphangiogenesis in development25 or pathology have identified a plethora of growth factors that promote or inhibit lymphatic vessel growth. Of those studied in the kidney, vascular endothelial growth factor (VEGF)-C and VEGF-D are central for lymphangiogenesis in renal disease. These growth factors predominantly trigger lymphangiogenesis by activation of VEGF receptor-3, but VEGF-C can also act by VEGF receptor-2 to stimulate lymphatic endothelial cells and blood vessel proliferation and migration.26 VEGF-C is highly expressed by macrophages in the rat remnant kidney,27 murine unilateral ureteral obstruction, human biopsies of IgAN, DKD36, and chronic allograft rejection.28
Thus, to determine how to target lymphatic vessels may have an impact in the treatment and prevention of CKD in IgAN. Structural changes to renal lymphatics are not evident in murine or human AKI. Nevertheless, the possibility that renal lymphatics are altered at the molecular or functional level in acute nephropathy, and whether these can be exploited to prevent the transition from AKI to CKD, warrants further study. The mechanisms of action of prolymphangiogenic or antilymphangiogenic factors to prevent fibrotic remodeling in preclinical models of CKD, whether through clearing inflammatory cells, modulating factors secreted by lymphatic endothelial cell, effects on the blood vasculature through VEGF receptor-2 activation, or consequences on other cells in the fibrotic environments are yet to be elucidated. Kasinath et al.29 have evaluated the role of the kidney-draining lymph node in experimental GN, including the role of fibroblastic reticular cells within the lymph nodes. Removing the kidney-draining lymph nodes before the induction of GN attenuated disease, as did interventions that affected the function of lymph nodes in general.
In IgAN, tubular atrophy and interstitial fibrosis may be induced by filtration of proteins to the tubular lumen and enhanced tubular reabsorption of proteins induces synthesis of proinflammatory and profibrotic factors, which drive kidney damage. Lymphatics in these areas might function to drain the misdirected filtrated fluid into the tubulointerstitial area, and in animal models, the distribution of lymphatic vessels was restricted around the affected nephron. Moreover, misdirected tubular fluid filtration into the periglomerular space appears to enhance periglomerular fibrosis and lymphangiogenesis.18,21
Some limitations should be acknowledged. First, owing to the observational and retrospective nature of the study, no causal relationships can be established. Second, treatment-related adverse events were not collected. Third, there is absence of a replication cohort. Despite these limitations, this study further contributes to the understanding of the natural history of the progression to CKD in patients with IgAN revealing the density of lymphatic vessels may optimize the prognostic value of the international IgA predicting tool to calculate the risk of ESKD at 3 years. Prospective studies, which ideally include control biopsy, are warranted to confirm these findings.
In conclusion, patients with a higher density of lymphatics (≥8 per mm2) progress faster to ESKD, and this effect of early progression is maintained after adjusting for the IgAN international score. This study identifies new surrogate measures of kidney outcomes and opens new ways for the improvement of ESKD prediction of patients with IgAN. This may decrease the cost and complexity of clinical trials in IgAN, thereby favoring the evaluation of new therapies.
Disclosure
LFQ reports receiving fees from GlaxoSmithKline, Akcea, and Alexion outside the submitted work. MB reports receiving fees from Alexion outside of the submitted work. All the other authors declared no competing interests.
Acknowledgments
The authors acknowledge the CERCA Programme/Generalitat de Catalunya. This work was supported by “Instituto de Salud Car-los III (ISCII)” and “Fondo Europeo de Desarrollo Regional (FEDER)” (PI17/00080). They have role in digital pathology and nuclear magnetic resonance in the early detection of interstitial fibrosis in CKD.
Footnotes
Figure S1. Correlation between density and area of lymphatic vessels in renal biopsy of patients with IgA nephropathy. Area occupied by lymphatic vessels is expressed as percentage of the total biopsy area examined.
Figure S2. Receiver-operator characteristics (ROC) curves for several cutpoints of lymphatic vessels density as predictors of progression to end-stage kidney disease (ESKD). Cutpoints were selected around the 0.75 percentile, and progression to ESKD was evaluated at the median follow-up for the whole series (2.5 years).
Supplementary Material
Figure S1. Correlation between density and area of lymphatic vessels in renal biopsy of patients with IgA nephropathy. Area occupied by lymphatic vessels is expressed as percentage of the total biopsy area examined.
Figure S2. Receiver-operator characteristics (ROC) curves for several cutpoints of lymphatic vessels density as predictors of progression to end-stage kidney disease (ESKD). Cutpoints were selected around the 0.75 percentile, and progression to ESKD was evaluated at the median follow-up for the whole series (2.5 years).
References
- 1.Donadio J.V., Grande J.P. IgA nephropathy. N Engl J Med. 2002;347:738–748. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- 2.Lai K.N., Tang S.C.W., Schena F.P., et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. doi: 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 3.Moran S.M., Cattran D.C. Recent advances in risk prediction, therapeutics, and pathogenesis of IgA nephropathy. Minerva Med. 2019;110:439–449. doi: 10.23736/S0026-4806.19.06165-2. [DOI] [PubMed] [Google Scholar]
- 4.Barbour S.J., Coppo R., Zhang H., et al. Evaluating a new international risk-prediction tool in IgA nephropathy [published correction appears in JAMA Intern Med. 2019;179:1007] JAMA Intern Med. 2019;179:942–1952. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nam K.H., Joo Y.S., Lee C., et al. Predictive value of mesangial C3 and C4d deposition in IgA nephropathy [published correction appears in Clin Immunol. 2020;213:108386] Clin Immunol. 2020;211:108331. doi: 10.1016/j.clim.2019.108331. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto I., Ito Y., Mizuno M., et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 2009;75:828–838. doi: 10.1038/ki.2008.661. [DOI] [PubMed] [Google Scholar]
- 7.Chen T., Li X., Li Y., et al. Prediction and risk stratification of kidney outcomes in IgA nephropathy. Am J Kidney Dis. 2019;74:300–309. doi: 10.1053/j.ajkd.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Jafree D.J., Long D.A. Beyond a passive conduit: implications of lymphatic biology for kidney diseases. J Am Soc Nephrol. 2020;31:1178–1190. doi: 10.1681/ASN.2019121320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimarchi H., Barratt J., Cattran D.C., et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Okajima E., Tomizawa M., Shimada K., Negishi T., Nishiyama N., Kitamura H. D2-40/podoplanin expression in cancer stroma by immunohistochemical staining is associated with poor prognosis in bladder cancer patients after radical cystectomy. Urol Oncol. 2020;38 doi: 10.1016/j.urolonc.2020.05.020. 10.1016/j.urolonc.2020.05.020 797.e7-797.e13. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Wang X., Carvalho V., et al. Prognostic value of podoplanin in various tumors. Technol Cancer Res Treat. 2021;20 doi: 10.1177/15330338211038142. 10.1177/15330338211038142 15330338211038142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu H., Fujimoto S., Sato Y., et al. “Point of no return (PNR)” in progressive IgA nephropathy: significance of blood pressure and proteinuria management up to PNR. J Nephrol. 2005;18:690–695. [PubMed] [Google Scholar]
- 13.Pozzi C., Del Vecchio L., Locatelli F. Can immunosuppressive therapy be useful in IgA nephropathy when the ‘point of no return’ has already been exceeded? Nephron. 2002;92:699–701. doi: 10.1159/000064080. [DOI] [PubMed] [Google Scholar]
- 14.Floege J. A new tool to predict the risk of progression in IgA nephropathy. Kidney Int. 2019;96:808–809. doi: 10.1016/j.kint.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Long D.A., Norman J.T., Fine L.G. Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- 16.Tammela T., Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Heller F., Lindenmeyer M.T., Cohen C.D., et al. The contribution of B cells to renal interstitial inflammation. Am J Pathol. 2007;170:457–468. doi: 10.2353/ajpath.2007.060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A.S., Lee J.E., Jung Y.J., et al. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction [published correction appears in Kidney Int. 2017;92:1018] Kidney Int. 2013;83:50–1062. doi: 10.1038/ki.2012.312. [DOI] [PubMed] [Google Scholar]
- 19.Zarjou A., Black L.M., Bolisetty S., et al. Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease. Lab Investig. 2019;99:1376–1388. doi: 10.1038/s41374-019-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rayego-Mateos S., Valdivielso J.M. New therapeutic targets in chronic kidney disease progression and renal fibrosis. Expert Opin Ther Targets. 2020;24:655–670. doi: 10.1080/14728222.2020.1762173. [DOI] [PubMed] [Google Scholar]
- 21.McWilliam S.J., Wright R.D., Welsh G.I., et al. The complex interplay between kidney injury and inflammation. Clin Kidney J. 2020;14:780–788. doi: 10.1093/ckj/sfaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler D.C., Toto R.D., Stefánsson B.V., et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215–224. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Lv J., Zhang H., Wong M.G., et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318:432–442. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobart S.A., Alexander M.P., Shawwa K., et al. The association of microhematuria with mesangial hypercellularity, endocapillary hypercellularity, crescent score, and renal outcomes in immunoglobulin A nephropathy. Nephrol Dial Transplant. 2021;36:840–847. doi: 10.1093/ndt/gfz267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Kataru R.P., Koh G.Y. Inflammation associated lymphangiogenesis: a double edged sword? J Clin Invest. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman J., Rutkowski J.M., Shields J.D., et al. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis [published correction appears in FASEB J. 2007;21:1942] FASEB J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 27.Matsui K., Nagy-Bojarsky K., Laakkonen P., et al. Lymphatic microvessels in the rat remnant kidney model of renal fibrosis: aminopeptidase P and podoplanin are discriminatory markers for endothelial cells of blood and lymphatic vessels [published correction appears in J Am Soc Nephrol. 2003;14] J Am Soc Nephrol. 2003;14:1981–1989. doi: 10.1097/01.asn.0000076078.50889.43. [DOI] [PubMed] [Google Scholar]
- 28.Kerjaschki D., Regele H.M., Moosberger I., et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 29.Kasinath V., Yilmam O.A., Uehara M., et al. Activation of fibroblastic reticular cells in kidney lymph node during crescentic glomerulonephritis. Kidney Int.Int. 2019;95:310–320. doi: 10.1016/j.kint.2018.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.