Highlights
-
•
Streptococcus thermophilus enriched polyphenols in fermented jujube puree.
-
•
Fermentation improved jujube puree DPPH scavenging capability by 26%.
-
•
12 phenolics were identified as differential metabolites.
-
•
Fermentation could be a promising approach to improve jujube phenolic quality.
Keywords: Jujube, Lactic acid bacteria fermentation, Streptococcus thermophilus, UPLC-MS/MS, Phenolic compound, Antioxidant activity
Abstract
To investigate the effect of lactic acid bacteria fermentation on jujube bioactivity, Streptococcus thermophilus was used to ferment jujube puree. The number of viable bacteria cells, physicochemical properties, phenolics profile and antioxidant capacity were analyzed, and their correlation were investigated. Streptococcus thermophilus exhibited a high growth capacity in jujube puree, and significantly (p < 0.05) increased the total phenolics content, 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and reducing power after 48 h fermentation, while 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) scavenging activity was decreased. 12 differentially metabolized polyphenols were identified in fermented jujube puree. Upregulated phenolics exhibited a positive correlation with DPPH radical-scavenging ability and reducing power. This work demonstrated that Streptococcus thermophilus fermentation can be an effective method with great practical application potential to improve the antioxidant activity in jujube puree by modifying the phenolic compositional quantity and quality.
1. Introduction
The use of lactic acid bacteria (LAB) has been found to be the most economical and valuable biotechnological method for maintaining and improving the safety and sensory and nutritional properties of foods (Kwaw et al., 2018). Fruits and vegetables are excellent matrices for lactic acid fermentation because of their high contents of carbohydrates, vitamins, minerals, and fibers. This has led to the development of a large number of new fruit and vegetable products via LAB fermentation (Kumar et al., 2015). After LAB fermentation, the content of polyphenols with potential health-promoting effects in fruits and vegetables increases. This occurs because the hydrolysis of cell walls releases bound phenols, which results in a significant increase in the content of polyphenols (De Souza et al., 2019). In general, the changes in polyphenols that occur after fermentation can also promote improvements in functional activity. Previous studies have reported that the antioxidant activities of tomato (Ricci et al., 2020), cabbage (Satora et al., 2021), and apple juices (Wu et al., 2020) were enhanced by LAB strains, such as Lacticaseibacillus casei, Pediococcus acidilactici, and Lactobacillus helveticus.
Studies of the fermentation of jujubes by LAB have recently been reported. The jujube (Ziziphus jujuba Mill.) was one of the earliest traditional fruits to be cultivated in China and has been grown for over 4000 years. This fruit is widely accepted by consumers because of its abundant nutritional compounds, including polysaccharides, polyphenols, dietary fiber, and triterpene acids (Liu et al., 2020). Mahmoudi et al. (2021) utilized Lactobacillus plantarum and Lactobacillus delbrueckii to ferment beverages from a jujube extract and found that the total phenolics concentration and free radical-scavenging ability significantly increased. Li et al. (2021) studied the correlation between the phenolics content and the antioxidant capacity of fermented jujube juices. They found that increased contents of caffeic acid and rutin in fermented jujube juices improved the antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl and ferric-reducing antioxidant power. However, no study has investigated the biotransformation pathways of polyphenols in fermentation. Therefore, in order to further improve the nutritional value of fermented jujube puree, it is necessary to reveal the changes in polyphenol profiles during fermentation and further explain the relationships between polyphenols and biological activities.
As shown in Fig. S1, the number of viable cells and the total polyphenol content after jujube puree was fermented by Streptococcus thermophilus were significantly higher than those observed after fermentation using Lacticaseibacillus rhamnosus, Pediococcus pentosaceus, and Leuconostoc mesenteroides.
Hence, the pH, sugars, organic acids, total phenols, and antioxidant capacity of jujube puree fermented by S. thermophilus were initially studied. Next, ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) analysis was used to construct profiles of phenols and differential metabolites. The biotransformation of differentially metabolized polyphenols was analyzed using metabolic pathways from the Kyoto Encyclopedia of Genes and Genomes. Finally, the relationship between phenolic compounds in fermented jujube puree and antioxidant activity was investigated. This study should provide valuable insights into the fermentation of jujube puree by S. thermophilus, as well as a practical reference for the development of jujube products via LAB fermentation.
2. Materials and methods
2.1. Raw materials, microorganisms, and reagents
Ziziphus jujuba cv. Jinsixiaozao was purchased from Laoling (Shandong Province, China). Streptococcus thermophilus CICC6220 was purchased from the China Center of Industrial Culture Collection (Beijing, China). This strain was in lyophilized form. The jujubes and LAB strain were stored at 4 °C prior to use. Chromatography-grade organic acid standards and phenolics standards were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China) and Solarbio Science and Technology Co., Ltd (Beijing, China), respectively. The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were of 96% purity and were purchased from Macklin (Shanghai, China). All other reagents used were of analytical grade and were purchased from Sinopharm Chemical Reagent Company (Shanghai, China).
2.2. Fermentation of jujube puree
The jujubes were washed, boiled for 20 min, and cooled to room temperature, and then the pits were removed. The jujubes were mixed with distilled water in a ratio of 3:5 (w/w) using a kitchen blender (JYL-C91T; Joyoung Co., Ltd, Hangzhou, China), followed by homogenization using a colloid mill (JTM-L65; Lixian Co., Ltd, Shanghai, China) for 10 min. The resulting puree was poured into 50 mL polyethylene terephthalate bottles and sterilized under a high hydrostatic pressure of 550 MPa at 25 °C for 10 min using a high hydrostatic pressure pressurization unit (HHP-750; Baotou Kefa Co., Inner Mongolia, China) (Bi et al., 2020).
According to the method described by Pan et al., 2022 with minor modifications, S. thermophilus was added to 100 mL MRS broth and incubated at 37 °C for 24 h. Using the streak plate method, the strain was subcultured onto MRS agar to form a single colony, which was incubated again at 37 °C for 24 h prior to use. Under aseptic conditions, 5% by volume of the strain as an inoculant was added to the treated jujube puree, which was then incubated at 37 °C for 48 h according to our preliminary experiment (Fig. S2). Samples were removed and analyzed after fermentation for 8, 16, 24, 32, 40, and 48 h. Unfermented jujube puree was used as a control.
2.3. Determination of viable cell counts and physicochemical properties
Viable LAB cell counts were determined by the standard method of decimal serial dilution. Aliquots of 1 mL dilutions in saline (0.9%, w/v) were plated onto MRS agar in triplicate. The plates were then incubated at 37 °C for 72 h in an incubator. The LAB cell counts of plates containing 30–300 colonies were measured and recorded as log colony-forming units (CFU)/mL.
The pH was measured by a digital pH meter (868; Thermo Orion Co., MA, USA). The total titratable acid content was determined by titration with 0.1 M NaOH and calculated using the conversion coefficient for lactic acid (Chinese National Standards, GB/T 12456–2008, 2008). The contents of reducing sugars and total sugars were determined by the 3,5-dinitrosalicylic acid method and the phenol–sulfuric acid method, respectively, and were expressed as glucose equivalents (Wood et al., 2012, Zhang et al., 2020). The content of organic acids was determined by the national standard method with slight modifications (Chinese National Standards, GB 5009.157–2016, 2016). A sample of 2 g fermented jujube puree was added to 10 mL ultrapure water, and the resulting mixture was stirred for 1 min. After ultrasonic extraction at 70 °C for 20 min, the mixture was centrifuged at 12,000 g for 10 min at 4 °C. The supernatant was collected, filtered through a 0.22 μm PTFE filter, and then stored at 4 °C prior to use. The high-performance liquid chromatography (HPLC) conditions were as follows: HPLC system (e2695; Waters, Milford, MA, USA) with a C18 column (ANPEL Laboratory Technologies, Inc., Shanghai, China; 4.6 × 250 mm, 5 μm) and a photodiode array detector. The mobile phase comprised 0.01 mol/L KH2PO4 (pH 2.88) and methanol (9:1, v/v), and the methanol content was adjusted to gradually decrease to 3% within 20 min. The flow rate was 0.6 mL/min.
2.4. Determination of total phenolics content and total flavonoids content
The total phenolics content was determined using the Folin–Ciocalteu method with slight modifications (Li et al., 2021). In brief, 2.5 mL Folin–Ciocalteu reagent was prepared and added to a 1 g sample. Then 1.5 mL sodium carbonate solution (75 g/L) was added, and the resulting mixture was vortexed for 1 min. After the mixture had been kept at room temperature in the dark for 40 min, the absorbance at 760 nm was read using a UV spectrophotometer (UV-1800; Shimadzu, Kyoto, Japan). Gallic acid was used as a control. The total phenolics content was expressed as the gallic acid equivalent (GAE).
The total flavonoids content was determined by the AlCl3 colorimetric method (Mokrani et al., 2019) with minor modifications. In brief, a 1 g sample was added to 10 mL of 70% ethanol. The resulting mixture was sonicated at 25 °C for 40 min and then centrifuged at 5000 g for 10 min, and the supernatant was collected. Next, 100 µL supernatant and 30 µL of 5% NaNO2 were mixed. The mixture was allowed to react for 5 min before 30 µL of 10% AlCl3 was added and was then vortexed and incubated for 6 min. Then 200 µL NaOH (1 mol/L) was added, and the mixture was allowed to react for 5 min. The absorbance at 510 nm was measured by a UV spectrophotometer (UV-1800; Shimadzu, Kyoto, Japan). The total flavonoids content was expressed as the rutin equivalent.
2.5. Determination of in vitro antioxidant activities
2.5.1. DPPH and ABTS radical-scavenging activity
The DPPH radical-scavenging activity was determined by a previously reported method with minor modifications (Liu et al., 2019). Specifically, a 1 mL sample of jujube puree was added to 29 mL ultrapure water, and the resulting mixture was vortexed for 1 min. A sample of 1 mL diluted fermented jujube puree was taken out and mixed with 1 mL of a methanolic solution of DPPH (50 μmol/L), and then the mixture was added to a 96-well microplate and incubated in the dark for 30 min. The absorbance at 517 nm was measured by a microplate reader (SpectraMax iD5; Molecular Devices, Sunnyvale, CA, USA). Vitamin C was used as a positive control.
The ABTS radical-scavenging activity was determined by a previously described method with some modifications (Tang et al., 2019). ABTS (10 mg) and 2.6 mL K2S2O8 (2.45 mmol/L) were mixed in the dark at room temperature for 16 h to produce ABTS+. The resulting ABTS+ solution was diluted with absolute ethanol to give an absorbance of 0.70 at 734 nm. Then, 1 mL fermented jujube puree was added to 29 mL distilled water and vortexed for 20 s. Subsequently, 0.1 mL of the diluted jujube puree was mixed with 1.9 mL of the ABTS+ solution and added to a 96-well plate. The absorbance at 734 nm was measured after the plate had been kept in the dark for 10 min by a microplate reader (SpectraMax iD5; Molecular Devices, Sunnyvale, CA, USA). Vitamin C was used as a positive control.
2.5.2. Reducing power assay
The reducing power was determined by a previously reported method with slight modifications (Chen et al., 2012). In brief, 0.5 mL phosphate buffer (0.2 mol/L, pH 6.6) and 0.5 mL potassium ferricyanide (1%) were added to 1 mL of the sample solution, and the mixture was allowed to react at 50 °C for 20 min. Next, 0.5 mL of a 10% trichloroacetic acid solution was added to the mixture, which was then stirred for 3 min, followed by centrifugation at 3000 g for 10 min. Then 1 mL of the supernatant was taken and mixed with 1 mL distilled water and 0.25 mL of 0.1% ferric chloride. The absorbance at 700 nm was read by a microplate reader (SpectraMax iD5; Molecular Devices, Sunnyvale, CA, USA) after the reaction had proceeded for 10 min. The reducing power was regarded as proportional to the absorbance of the mixture.
2.6. Metabolomics analysis of fermented jujube puree
A freeze-dried sample of jujube puree was crushed using a mixer mill (MM 400, Retsch, Haan, Germany) with a zirconia bead for 1.5 min at 30 Hz. A sample of 100 mg of the resulting lyophilized powder was dissolved in 1.2 mL of a 70% methanol solution and then vortexed for 4 min and placed in a refrigerator at 4 °C overnight. Following centrifugation at 12,000 g for 10 min, the extract was filtered through a 0.22 μm filter (SCAA-104; ANPEL, Shanghai, China) before analysis by ultra-performance liquid chromatography-mass spectrometry/mass spectrometry (UPLC-MS/MS) analysis.
Metabolomics assays of secondary metabolites were performed according to the method described by Li et al. (2021) with slight modifications using a UPLC–electrospray ionization–MS/MS system (UPLC: Nexera X2; Shimadzu, Kyoto, Japan; MS: 4500 Q TRAP; Applied Biosystems, Waltham, MA, USA). Chromatographic separation of fermented jujube puree was performed in an SB-C18 column (2.1 × 100 mm, 1.8 µm; Agilent, CA, USA). The mobile phase used for separation consisted of phase A (pure water with 0.1% formic acid) and phase B (acetonitrile with 0.1% formic acid). The gradient elution program was as follows: 0 min, 5% B; 0–9 min, increase to 95% B; and then held for 1 min. Subsequently, the composition was adjusted to 5% B within 1.5 min and then held for 2.5 min. The column was maintained at 40 °C with a flow rate of 0.35 mL/min, and the injection volume was 4 μL.
Linear ion trap and triple quadrupole scans were performed using a triple quadrupole–linear ion trap (Q TRAP) mass spectrometer (AB4500 Q TRAP UPLC/MS/MS System). Sequence analyses were carried out in positive ion mode (ion spray voltage of 5500 V) and negative ion mode (ion spray voltage of − 4500 V) with an ion source temperature of 550 °C. The pressures of the ion source gases I and II and the curtain gas were set at 345, 414, and 172 kPa, respectively. Instrument tuning and mass calibration were performed with 10 and 100 μmol/L polypropylene glycol solutions in triple quadrupole and linear ion trap modes, respectively. The triple quadrupole scans were performed as multiple reaction monitoring (MRM) experiments with the pressure of the collision gas (nitrogen) set to medium. The declustering potential and collision energy were selected for the individual MRM transitions and were then further optimized. A specific set of MRM transitions were monitored for each period according to the metabolites eluted within this period.
2.7. Statistical analysis
All the experiments were carried out in triplicate. The results were expressed as the mean ± standard deviation (n = 3). The data were compared by analysis of variance followed by Duncan’s multiple-range test using SPSS 20.0 software (IBM, Armonk, NY, USA), and a value of p < 0.05 was regarded as indicating a statistically significant difference.
The raw data signals were processed by Analyst 1.6.3 software (AB Sciex, Framingham, MA, USA). Principal component analysis (PCA), hierarchical cluster analysis, and orthogonal partial least-squares discriminant analysis were carried out by the R software package (www.r-project.org) to visualize the metabolic changes between the unfermented jujube puree and the fermented jujube puree. Differential metabolites between groups were identified by a variable importance in projection score of ≥ 1 and a fold change score of ≥ 2 or ≤ 0.5. Heatmap analysis and cluster analysis of secondary metabolites were performed using R on the basis of their signal abundances in the fermented jujube puree.
3. Results and discussion
3.1. Viable cell counts and physicochemical properties during fermentation
To determine the growth ability of the S. thermophilus strain in fermented jujube puree, the viable cell count was monitored during fermentation, as shown in Table 1. In comparison with the count before fermentation, the viable cell count of the strain increased significantly after fermentation for 48 h from 7.25 log CFU/mL to 8.50 log CFU/mL (p < 0.05). Suitable fermentation conditions could cause rapid growth of S. thermophilus. It should be noted that at the end of fermentation the viable cell count of the S. thermophilus strain was higher than 8 log CFU/mL, which has been proved to have probiotic functions and provide potential health benefits to the consumer (Li et al., 2021). Hence, jujube puree may be an excellent carrier for S. thermophilus strains.
Table 1.
Dynamic changes in viable cell count, physicochemical properties, total polyphenols content, total flavones content, and antioxidant activity in jujube puree fermented by Streptococcus thermophilus.
| 0 h | 8 h | 16 h | 24 h | 32 h | 40 h | 48 h | |
|---|---|---|---|---|---|---|---|
| Viable cell counts (Log cfu/mL) | 7.25 ± 0.03e | 7.24 ± 0.04e | 7.70 ± 0.02d | 8.19 ± 0.02c | 8.21 ± 0.01c | 8.31 ± 0.01b | 8.50 ± 0.08a |
| pH values | 4.52 ± 0.02 | 4.41 ± 0.06 | 4.07 ± 0.04 | 3.84 ± 0.02 | 3.62 ± 0.03 | 3.63 ± 0.03 | 3.59 ± 0.01 |
| Total acidity (Lactic acid g/kg) | 4.65 ± 0.03f | 4.82 ± 0.03e | 5.73 ± 0.02d | 6.12 ± 0.03c | 7.00 ± 0.14b | 7.09 ± 0.09ab | 7.14 ± 0.05a |
| Reducing sugar (g/100 g) | 12.16 ± 0.63a | 12.01 ± 0.49a | 11.97 ± 0.99a | 11.86 ± 0.18ab | 11.84 ± 0.18ab | 11.32 ± 0.25b | 11.30 ± 0.18b |
| Total sugar (g/100 g) | 15.29 ± 0.14a | 15.26 ± 0.20a | 15.13 ± 0.13ab | 14.98 ± 0.13ab | 14.87 ± 0.79bc | 14.65 ± 0.34 cd | 14.40 ± 0.77d |
| Organic acids | |||||||
| Oxalic acid (mg/g) | 0.03 ± 0.02e | 0.03 ± 0e | 0.07 ± 0d | 0.1 ± 0c | 0.14 ± 0.01b | 0.23 ± 0.02a | 0.25 ± 0.01a |
| Tartaric acid (mg/g) | 1.15 ± 0c | 1.05 ± 0.03d | 1.06 ± 0.04d | 1.08 ± 0.01d | 1.13 ± 0.02c | 1.26 ± 0.03b | 1.74 ± 0a |
| Malic acid (mg/g) | 6.06 ± 0.01a | 5.9 ± 0.01b | 3.83 ± 0.02c | 3.73 ± 0.03d | 3.1 ± 0e | 2.72 ± 0.02f | 2.5 ± 0 g |
| lactic acid (mg/g) | 3.91 ± 0.01 g | 5.33 ± 0.01f | 5.63 ± 0.02e | 9.11 ± 0.01d | 12.47 ± 0.01c | 18.06 ± 0.01b | 19.66 ± 0.02a |
| Citric acid (mg/g) | 12.03 ± 0.01a | 11.46 ± 0.02b | 10.51 ± 0.03c | 9.15 ± 0.01d | 7.63 ± 0.02e | 7.5 ± 0.01f | 6.35 ± 0.01 g |
| Total polyphenol (mg GAE/g DM) | 7.21 ± 0.09 cd | 6.26 ± 0.16e | 7.02 ± 0.17d | 7.37 ± 0.14c | 8.02 ± 0.09b | 8.47 ± 0.12a | 8.66 ± 0.16a |
| total flavones (mg RE/g DM) | 0.31 ± 0.01d | 0.43 ± 0.02c | 0.43 ± 0.01c | 0.45 ± 0.01c | 0.49 ± 0.02b | 0.54 ± 0.01a | 0.54 ± 0.03a |
| DPPH radical scavenging (%) | 62.06 ± 0.61f | 66.90 ± 0.20e | 70.45 ± 0.41d | 75.77 ± 0.20c | 77.07 ± 0.20b | 77.78 ± 0.20a | 78.37 ± 0.35a |
| ABTS radical scavenging (%) | 94.00 ± 0.00a | 93.94 ± 0.10a | 93.94 ± 0.10a | 90.43 ± 0.28b | 83.53 ± 0.28c | 78.50 ± 0.38d | 75.29 ± 0.36e |
| Reducing power | 0.38 ± 0.01d | 0.41 ± 0.01c | 0.41 ± 0.00c | 0.41 ± 0.01c | 0.42 ± 0.01c | 0.44 ± 0.00b | 0.47 ± 0.01a |
Data are presented as the mean ± SD (n = 3). Different letters (a-f) in the same row indicate significant difference (p < 0.05). Reducing power was expressed
as OD value.
As shown in Table 1, S. thermophilus exhibited improved acidification characteristics in fermented jujube puree. The total acid content significantly increased from 4.65 g/kg to 7.14 g/kg (p < 0.05), while the pH decreased from 4.52 to 3.59. The change in total acidity was negatively correlated with the variation in pH (Wu et al., 2020), which indicates that more acids were produced after fermentation for 48 h. This result can be attributed to the fact that LAB strains utilize sugars for conversion into organic acids (Wang et al.,2003). As a result of further research, five organic acids were detected in the present study, namely, oxalic acid, tartaric acid, malic acid, lactic acid, and citric acid. It was found that the main organic acid in the control group was citric acid (12.03 mg/g), whereas lactic acid was the main organic acid at the end of fermentation. The content of lactic acid after fermentation for 48 h increased fivefold in comparison with that in the control, while the content of malic acid significantly decreased (p < 0.05), because malic acid can be converted into lactic acid by LAB strains via homolactic fermentation (Sumby et al., 2014). Wu et al. (2021) found that, in comparison with Bifidobacterium, S. thermophilus exhibited higher biotransformation ability in the fermentation of blueberry and could produce more lactic acid. Lactic acid can enhance flavor, act as a preservative, help to stabilize microorganisms, and improve the nutritional value of food (Roberts et al., 2018). In addition, the content of citric acid in jujube puree significantly decreased to 6.35 mg/g at the end of fermentation (p < 0.05). A possible reason is that citric acid can be used as a precursor substance to generate unique aroma substances, such as diacetyl and acetaldehyde, which make a significant contribution to the quality of fermented foods (Hugenholtz, 1993). In a similar study, large changes in the consumption of citric acid and production of lactic acid in a fermented jujube extract were also demonstrated (Mahmoudi et al., 2021).
The sugar content of fermented jujube puree was determined in terms of reducing sugars and total sugars (Table 1). There were dramatic decreases in the contents of reducing sugars and total sugars after fermentation (p < 0.05), which decreased by 7% and 5%, respectively. As mentioned above, LAB fermentation is a process in which sugars are consumed and acids are produced. Because the content of sugars was reduced and that of organic acids increased in fermented jujube puree, the sugar/acid ratio of the product was altered, which will have affected the taste. Most importantly, low-calorie foods meet consumer demand for healthy foods.
3.2. Total phenolics content and total flavonoids content
The total phenolics content and total flavonoids content in fruits and vegetables are important indices because of their positive effects on human health (De Souza et al., 2019). As shown in Table 1, the total phenolics content decreased at first, then an increase was observed during the 16–48 h time period of the fermentation process, and the final total phenolics content was 8.66 mg GAE/g. Kwaw et al. (2018) demonstrated an increase in the total phenolics content in mulberry juice fermented by L. plantarum, Lactobacillus acidophilus, and Lacticaseibacillus paracasei. The first decrease in the total phenolics content may have been due to the low number of viable cells of the S. thermophilus strain in the early stage of fermentation and the decomposition of phenolic compounds, but as the number of viable cells increased glycosidases and phenol esterases produced by the strain, which can hydrolyze bound phenolic compounds to form free phenols, may have caused the increase in the total phenolics content (Rodriguez et al., 2009). As was expected, the total flavonoids content increased by 74% after fermentation (Table 1), which could have been due to enzymes that decomposed complex polyphenols into simpler flavonol compounds during fermentation (Kwaw et al., 2018). However, the total flavonoids content significantly decreased in jujube juice fermented by L. acidophilus and L. plantarum (Li et al., 2021). The individual adaptability of the strain and its ability to produce hydrolytic enzymes may have caused the difference in the total flavonoids content in jujube puree.
3.3. Changes in antioxidant activities
The dynamic changes in the antioxidant activity of jujube puree after fermentation by S. thermophilus were measured in terms of DPPH and ABTS radical-scavenging activities and ferric-reducing antioxidant power (Table 1). In comparison with the control, the DPPH radical-scavenging activity of fermented jujube puree after fermentation for 48 h significantly increased by 26% (p < 0.05). Our results were similar to other results previously reported for jujube juices (Li et al., 2021). The increase in DPPH free radical-scavenging activity showed that LAB fermentation might increase the availability of polyphenol compounds with proton-donating properties (Kwaw et al., 2018). Conversely, the ABTS radical-scavenging activity notably decreased with an increase in the fermentation time from 94% to 75% (p < 0.05). Similar changes were observed by Wu et al. (2020). This can be attributed to the difference in antioxidant mechanism: in the case of ABTS this occurs via hydrogen atom transfer, whereas in the case of DPPH it is based on electron transfer (Apak et al., 2016). Perhaps the reduction in ABTS free radical-scavenging activity was also related to synergy and redox interactions among different compounds (Wu et al., 2020). Moreover, the antioxidant mechanism is characteristic of specific bacterial strains (Hoffmann et al., 2021). Therefore, the reducing power was further investigated. The reducing power is an important indicator that is often used to determine phenolic-reducing ability (Vasyliev et al., 2020). The reducing power of the sample increased with a prolongation of the fermentation time and reached its maximum (0.47 ± 0.01) after fermentation for 48 h, which represented a significant difference in comparison with the value before fermentation (p < 0.05).
3.4. Metabolites identified in fermented jujube puree
According to previous studies, a widely untargeted metabolomics method was implemented to fully understand changes in the composition or content of phytochemicals (Antonio et al., 2020). With reference to the Metware database, the results showed that a total of 575 secondary metabolites were identified, namely, 140 phenolic acids, 178 flavonoids, 12 quinones, 37 lignans and coumarins, 12 tannins, 64 alkaloids, 109 terpenes, one steroid, and 22 other metabolites.
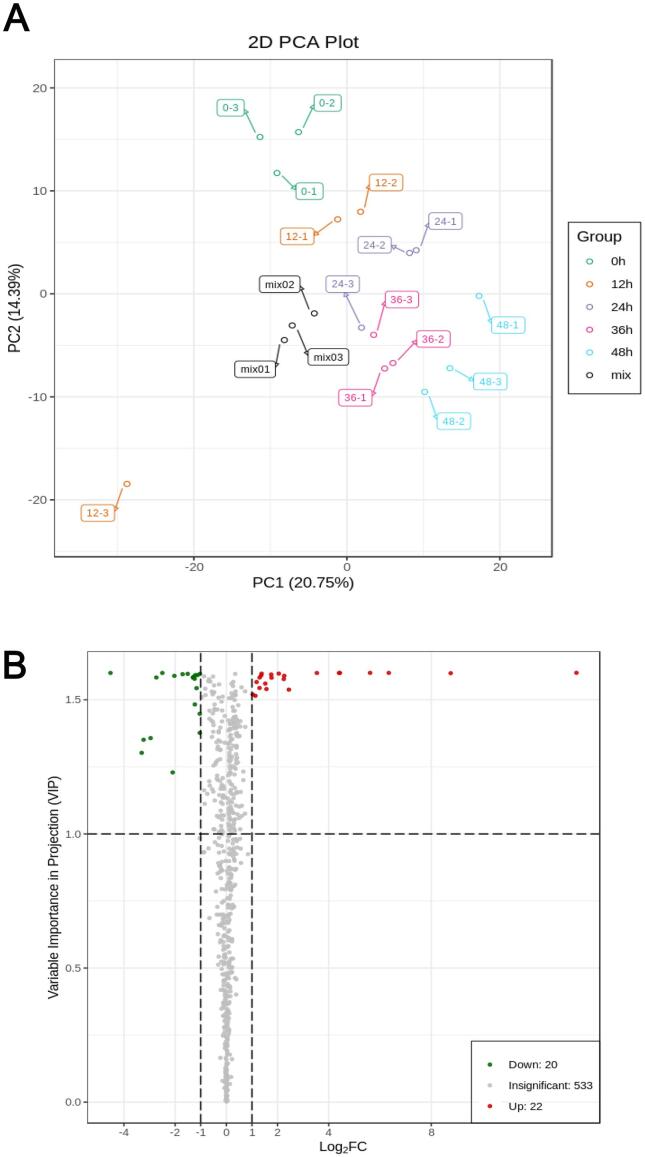
As shown in Fig. 1A, the mix samples (quality controls) were clustered in the center of the PCA score plot, which indicates that the instrument exhibited high stability during data acquisition. The two principal components can explain 35.14% of the variance. The samples with different fermentation times were clearly distinguished from each other, and the distance between the samples of fermented and unfermented jujube puree continually increased with the increase in the fermentation time, which indicates that the changes in metabolite abundance were associated with the fermentation time. Therefore, metabolites of which the abundance differed after fermentation for 48 h were selected for analysis, which is visually represented in Fig. 1B and Fig. 2. Several types of differential metabolite were more widely dispersed on the abscissa (Fig. 1B), which indicates that fermentation had endowed the jujube puree with more metabolic characteristics.
Fig. 1.
Overall assessment of dynamic changes in the abundance of all the metabolites detected in jujube puree. A: Score plot generated by principal component analysis of each group of samples and quality control samples (Mix). B: Volcano plot of differential metabolites detected between 0 h and 48 h in fermented jujube puree. Red dots represent differential metabolites of which the expression was upregulated; green dots represent differential metabolites of which the expression was downregulated; and gray dots represent metabolites with insignificant differences in expression. If the absolute value on the abscissa is large, the fold change was also large. If the absolute value on the ordinate is large, the differential metabolite was found to be more reliable. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
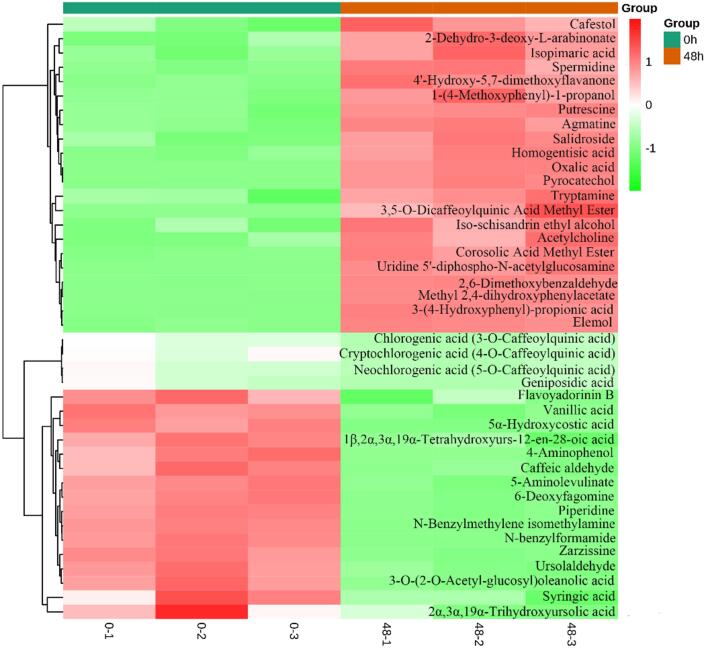
Fig. 2.
Hierarchical cluster analysis of metabolites detected between 0 h and 48 h during fermentation of jujube puree samples. Red represents a relatively high content of the metabolite, whereas green represents a relatively low content. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Differences in phenolics profiles after fermentation of jujube puree
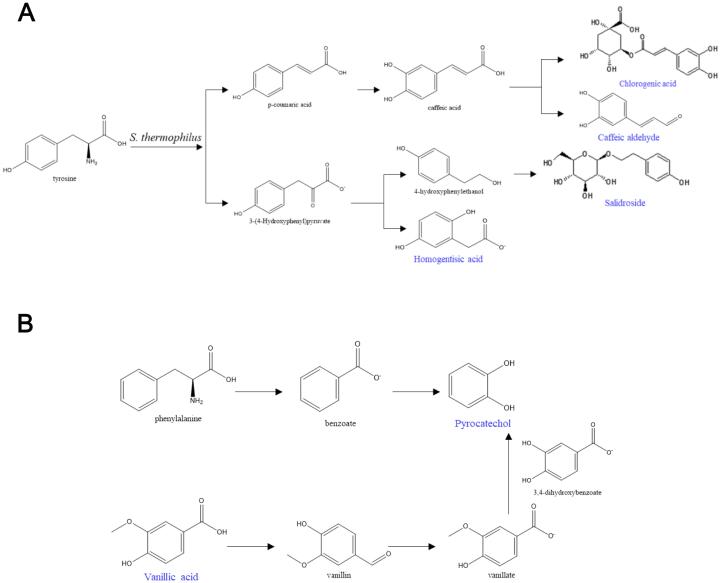
To track changes in the abundance of phenolic compounds in fermented jujube puree, compounds with significant differences in abundance in comparison with unfermented jujube puree were distinguished. In total, 12 phenolics were identified as key differential metabolites and are listed in Table 2. Of these, six compounds were upregulated and six compounds were downregulated. Specifically, the six upregulated compounds were homogentisic acid, methyl 2,4-dihydroxyphenylacetate, salidroside, pyrocatechol, 3-(4-hydroxyphenyl)propionic acid, and 3,5-di-O-caffeoylquinic acid methyl ester. According to our results, salidroside was found in jujube puree for the first time, and its content significantly increased fivefold after fermentation for 48 h (p < 0.05). Salidroside is an important active substance present in Rhodiola roots and has various physiological activities such as improving immunity and antiaging, antifatigue, anti-senile dementia, and antiradiation effects (Bai et al., 2014). The increase in salidroside content may have occurred because fermentation promoted the conversion of tyrosine, which was catalyzed by an aromatic aldehyde synthase to produce 4-hydroxyphenylacetic acid and then tyrosol (Fig. 3A). Tyrosol was then converted by a glycosyltransferase (specifically, T8GT) to produce salidroside (Torrens-Spence et al., 2018). As a compound that was upregulated after fermentation, pyrocatechol was an important phenolic compound in jujube puree (Dilek Tepe & Doyuk, 2020). The increase in pyrocatechol content may have been due to promotion of the degradation of phenylalanine by S. thermophilus (Fig. 3B). Moreover, the continuous production of 3-(4-hydroxyphenyl)propionic acid may have occurred because glycosylation and cyclization of hesperetin-7-O-rutinoside and naringenin-7-O-rutinoside were triggered by the LAB strain (Guo et al., 2021). 3-(4-Hydroxyphenyl)propionic acid can be used as an intermediate for drugs (such as cetraxate hydrochloride) or as a precursor for the synthesis of plant products such as myricanol and phloretin (Kawai et al., 2010).
Table 2.
Differential phenolic compounds identified in jujube puree before and after fermentation for 48 h.
| Rta (min) | Precursor ions (Da) | Product ions (Da) | CAS | Formula | Phenolic compound | VIPb | Fold change | Regulation (0 h vs 48 h) | Identification in referencesc |
|---|---|---|---|---|---|---|---|---|---|
| 2.26 | 167.1 | 123.04 | 451–13-8 | C8H8O4 | Homogentisic acid | 1.59 | 2.57 | up | |
| 2.58 | 353.09 | 191 | 906–33-2 | C16H18O9 | Neochlorogenic acid (5-O-Caffeoylquinic acid) | 1.35 | 0.11 | down | – |
| 2.59 | 181.05 | 135.04 | 67828–42-6 | C9H10O4 | Methyl 2,4-dihydroxyphenylacetate | 1.60 | 48.93 | up | – |
| 2.63 | 299.11 | 119 | 10338–51-9 | C14H20O7 | Salidroside | 1.54 | 5.43 | up | – |
| 2.74 | 353.09 | 191.01 | 327–97-9 | C16H18O9 | Chlorogenic acid (3-O-Caffeoylquinic acid) | 1.30 | 0.10 | down | Ivanišovád |
| 2.92 | 353.09 | 191.05 | 905–99-7 | C16H18O9 | Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | 1.36 | 0.13 | down | Shene |
| 3.23 | 109.03 | 81 | 120–80-9 | C6H6O2 | Pyrocatechol | 1.60 | 13033.70 | up | Dilek Tepe f |
| 3.29 | 167.03 | 108.02 | 121–34-6 | C8H8O4 | Vanillic acid | 1.59 | 0.41 | down | Hongg |
| 3.38 | 197.05 | 123 | 530–57-4 | C9H10O5 | Syringic acid | 1.45 | 0.49 | down | Najjaah |
| 3.84 | 165.06 | 119.05 | 501–97-3 | C9H10O3 | 3-(4-Hydroxyphenyl)-propionic acid | 1.60 | 21.13 | up | – |
| 4.58 | 531.15 | 177.06 | 159934–13-1 | C26H26O12 | 3,5-O-Dicaffeoylquinic Acid Methyl Ester | 1.60 | 433.58 | up | – |
| 5.99 | 165.1 | 95.2 | 141632–15-7 | C9H8O3 | Caffeic aldehyde | 1.58 | 0.42 | down | – |
Rt, retention time.
VIP, variable importance in projection.
The minus sign (–) indicated no related references.
Dilek Tepe et al. (2020).
Fig. 3.
Kyoto Encyclopedia of Genes and Genomes metabolic pathways of specific metabolized phenols in fermented jujube puree. A: Tyrosine pathway. B: Phenylalanine pathway. Blue indicates differential phenolic compounds. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
On the other hand, the six downregulated phenolics were chlorogenic acid, cryptochlorogenic acid, neochlorogenic acid, vanillic acid, syringic acid, and caffeic aldehyde. Chlorogenic acid and cryptochlorogenic acid are found in many fruits and vegetables, such as jujubes, apples, blueberries, and potatoes (Ivanišová et al., 2017, Shen et al., 2019, Herrmann and Nagel, 1989). According to our results, one possible reason for the decrease in the content of chlorogenic acid was the decrease in the content of caffeic acid, which is an important intermediate in the synthesis of chlorogenic acid. It was speculated that this may have been due to the fact that as the fermentation time increased tyrosine was not completely converted into caffeic acid but rather into 3-(4-hydroxyphenyl)pyruvic acid, which further produced salidroside and homogentisic acid (Fig. 3A). Fritsch et al. (2016) mentioned that the content of chlorogenic acid in sunflower flour decreased after fermentation by animal-derived LAB. The S. thermophilus strain used in our experiment is of animal origin, which may be another important reason for the decrease in chlorogenic acid content during fermentation. Previous studies have shown that vanillic acid and syringic acid were found in different jujube extracts, and an aqueous jujube extract rich in vanillic acid was also found to have protective activity against alcohol-induced oxidative stress in cells (Hong et al., 2020, Najjaa et al., 2020). However, the content of vanillic acid in our study significantly decreased after fermentation, and it may have been converted into pyrocatechol (Fig. 3B).
3.6. Correlation analysis of phenolic compounds and antioxidant activities before and after fermentation
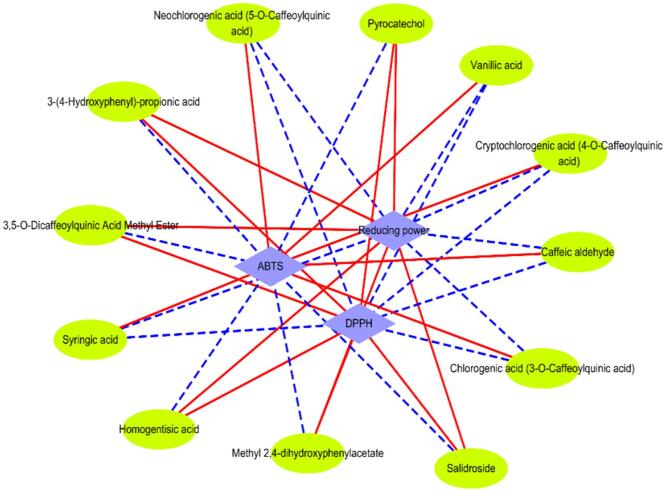
To determine the relationships between the contents of specific phenolic metabolites and antioxidant activities, the Pearson correlation test was used. The results showed that the DPPH radical-scavenging activity, ABTS radical-scavenging activity, and ferric-reducing antioxidant power were correlated with the contents of phenolic compounds (Fig. 4). The contents of salidroside and 3-(4-hydroxyphenyl)propionic acid were significantly positively correlated with the reducing power (p < 0.01). This means that salidroside and 3-(4-hydroxyphenyl)propionic acid have very strong antioxidant activities, despite the fact that their contents in fermented jujube puree were not the highest, and that they were the main antioxidant substances in fermented jujube puree. The pyrocatechol content was notably positively correlated with the DPPH radical-scavenging activity but notably negatively correlated with the ABTS radical-scavenging activity (p < 0.001), which indicates that pyrocatechol was the main contributor to the DPPH radical-scavenging capacity of fermented jujube puree. However, the DPPH radical-scavenging activity and reducing power were significantly negatively correlated with the content of syringic acid (p < 0.05). In addition, the vanillic acid content was negatively correlated with the DPPH radical-scavenging activity (p < 0.01). This indicates that different types of monomeric phenolic compound were selectively associated with different types of antioxidant activity.
Fig. 4.
Pearson correlation coefficients of differential metabolites and antioxidant activities. Green ellipses represent metabolites, and purple diamonds represent indicators of antioxidant activity. The blue dashed line represents a negative correlation, and the red solid line represents a positive correlation. The absolute value of the correlation coefficient greater than 0.8 was chosen for plotting. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Conclusions
In summary, this study showed that S. thermophilus exhibited excellent growth capacity, with a viable cell count of 8.50 log CFU/mL in jujube puree, which has been proved to provide health benefits. In addition, fermentation by S. thermophilus demonstrated a strong capacity for malolactic conversion. Most importantly, it has been confirmed that S. thermophilus can improve antioxidant capacity by altering the contents of pyrocatechol in fermented jujube puree via the metabolic conversion of phenylalanine. Our further research should focus on gastric and intestinal digestibility and absorption rates of fermented jujube puree to ascertain what impact fermented jujube puree has on human health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by Construction of Green Design Platform for Deep Processed healthy food of Jujube of HaoXiangNi Health Food Co., Ltd (No. 202005410610147).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100214.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Antonio A.D.S., Aguiar A.T.C., Santos G.R.C.D., Pereira H.M.G., Veiga-Junior V.F.d., Wiedemann L.S.M. Phytochemistry by design: A case study of the chemical composition of Ocotea guianensis optimized extracts focused on untargeted metabolomics analysis. RSC Advances. 2020;10(6):3459–3471. doi: 10.1039/C9RA10436D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apak R., Ozyurek M., Guclu K., Capanoglu E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. Journal of Agricultural and Food Chemistry. 2016;64(5):997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- Bai Y.F., Bi H.P., Zhuang Y.B., Liu C., Cai T., Liu X.N.…Ma Y.H. Production of salidroside in metabolically engineered Escherichia coli. Scientific Reports. 2014;4:8. doi: 10.1038/srep06640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S., Sun S., Lao F., Liao X., Wu J. Gas chromatography-mass spectrometry combined with multivariate data analysis as a tool for differentiating between processed orange juice samples on the basis of their volatile markers. Food Chemistry. 2020;311:125913. doi: 10.1016/j.foodchem.2019.125913. [DOI] [PubMed] [Google Scholar]
- Chen N., Yang H.M., Sun Y., Niu J., Liu S.Y. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides. 2012;38(2):344–349. doi: 10.1016/j.peptides.2012.09.017. [DOI] [PubMed] [Google Scholar]
- de Souza E.L., de Albuquerque T.M.R., dos Santos A.S., Massa N.M.L., Alves J.L.D. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities – A review. Critical Reviews in Food Science and Nutrition. 2019;59(10):1645–1659. doi: 10.1080/10408398.2018.1425285. [DOI] [PubMed] [Google Scholar]
- Dilek Tepe H., Doyuk F. Determination of phytochemical content by chromatographic methods and antioxidant capacity in methanolic extract of Jujube (Zizyphus jujuba Mill.) and oleaster (Elaeagnus angustifolia L.) International Journal of Fruit Science. 2020;20(sup3):S1876–S1890. doi: 10.1080/15538362.2020.1834900. [DOI] [Google Scholar]
- Fritsch C., Heinrich V., Vogel R.F., Toelstede S. Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol. 2016;57:178–186. doi: 10.1016/j.fm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Guo X., Guo A.L., Li E. Biotransformation of two citrus flavanones by lactic acid bacteria in chemical defined medium. Bioprocess and Biosystems Engineering. 2021;44(2):235–246. doi: 10.1007/s00449-020-02437-y. [DOI] [PubMed] [Google Scholar]
- Herrmann K., Nagel C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Critical Reviews in Food Science & Nutrition. 1989;28(4):315–347. doi: 10.1080/10408398909527504. [DOI] [PubMed] [Google Scholar]
- Hoffmann A., Kleniewska P., Pawliczak R. Antioxidative activity of probiotics. Archives of Medical Science. 2021;17(3):792–804. doi: 10.5114/aoms.2019.89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S., Kim, Y., Sung, J., Lee, H., Heo, H., Jeong, H. S., & Lee, J. (2020). Jujube (Ziziphus jujuba Mill.) protects hepatocytes against alcohol-induced damage through Nrf2 activation. Evidence-Based Complementary and Alternative Medicine, 2020, 6684331. 10.1155/2020/6684331. [DOI] [PMC free article] [PubMed]
- Hugenholtz J. Citrate metabolism in lactic-acid bacteria. Fems Microbiology Reviews. 1993;12(1–3):165–178. doi: 10.1111/j.1574-6976.1993.tb00017.x. [DOI] [Google Scholar]
- Ivanišová E., Grygorieva O., Abrahamová V., Schubertova Z., Terentjeva M., Brindza J. Characterization of morphological parameters and biological activity of jujube fruit (Ziziphus jujuba Mill.) Journal of Berry Research. 2017;7(4):249–260. doi: 10.3233/jbr-170162. [DOI] [Google Scholar]
- Kawai S., Nakata K., Ichizawa H., Nishida T. 3-(4-Hydroxyphenyl)propionic acid is involved in the biosynthesis of myricanol in Myrica rubra. Journal of Wood Science. 2010;56(2):148–153. doi: 10.1007/s10086-009-1082-9. [DOI] [Google Scholar]
- Kumar B.V., Vijayendra S.V.N., Reddy O.V.S. Trends in dairy and non-dairy probiotic products - a review. Journal of Food Science and Technology-Mysore. 2015;52(10):6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Wu M., Sackey A.S.…Tahir H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chemistry. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Li, T. L., Jiang, T., Liu, N., Wu, C. Y., Xu, H. D., & Lei, H. (2021). Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chemistry, 339, 127859. doi: 10.1016/j.foodchem.2020.127859. [DOI] [PubMed]
- Li W., Wen L.C., Chen Z.T., Zhang Z.L., Pang X.L., Deng Z.C.…Guo Y.F. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chemistry. 2021;357:129791. doi: 10.1016/j.foodchem.2021.129791. [DOI] [PubMed] [Google Scholar]
- Liu, S., Jia, M. Y., Chen, J. J., Wan, H. S., Dong, R. H., Nie, S. P., Xie, M. Y., & Yu, Q. (2019). Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocolloids, 93, 284-292. doi: 10.1016/j.foodhyd.2019.02.047.
- Liu X.X., Liu H.M., Yan Y.Y., Fan L.Y., Yang J.N., Wang X.D., Qin G.Y. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. Lwt-Food Science and Technology. 2020;117:108645. doi: 10.1016/j.lwt.2019.108645. [DOI] [Google Scholar]
- Mahmoudi B., Mousavi Z.E., Khodaiyan F. A functional non-dairy beverage produced from jujube extract using probiotic lactic acid bacteria. Journal of Agricultural Science and Technology. 2021;23(4):813–824. [Google Scholar]
- Mokrani A., Cluzet S., Madani K., Pakina E., Gadzhikurbanov A., Mesnil M.…Richard T. HPLC-DAD-MS/MS profiling of phenolics from different varieties of peach leaves and evaluation of their antioxidant activity: A comparative study. International Journal of Mass Spectrometry. 2019;445:116192. doi: 10.1016/j.ijms.2019.116192. [DOI] [Google Scholar]
- Najjaa H., Ben Arfa A., Elfalleh W., Zouari N., Neffati M. Jujube (Zizyphus lotus L.): Benefits and its effects on functional and sensory properties of sponge cake. Plos One. 2020;15(2) doi: 10.1371/journal.pone.0227996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X., Zhang, S., Xu, X., Lao, F., & Wu, J. H. (2022). Volatile and non-volatile profiles in jujube pulp co-fermented with lactic acid bacteria. Lwt, 154, 112772. doi: 10.1016/j.lwt.2021.112772.
- Ricci A., Marrella M., Hadj Saadoun J., Bernini V., Godani F., Dameno F.…Lazzi C. Development of lactic acid-fermented tomato products. Microorganisms. 2020;8(8):1192. doi: 10.3390/microorganisms8081192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D., Reyes V., Bonilla F., Dzandu B., Liu C., Chouljenko A., Sathivel S. Viability of lactobacillus plantarum NCIMB 8826 in fermented apple juice under simulated gastric and intestinal conditions. Lwt-Food Science and Technology. 2018;97:144–150. doi: 10.1016/j.lwt.2018.06.036. [DOI] [Google Scholar]
- Rodríguez H., Curiel J.A., Landete J.M., de las Rivas B., de Felipe F.L., Gómez-Cordovés C.…Muñoz R. Food phenolics and lactic acid bacteria. International Journal of Food Microbiology. 2009;132(2-3):79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Satora P., Skotniczny M., Strnad S., Piechowicz W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties (vol 136, 110325, 2021) Lwt-Food Science and Technology. 2021;141:1. doi: 10.1016/j.lwt.2020.110325. [DOI] [Google Scholar]
- Shen S., Wang J.B., Chen X., Liu T.T., Zhuo Q., Zhang S.Q. Evaluation of cellular antioxidant components of honeys using UPLC-MS/MS and HPLC-FLD based on the quantitative composition-activity relationship. Food Chemistry. 2019;293:169–177. doi: 10.1016/j.foodchem.2019.04.105. [DOI] [PubMed] [Google Scholar]
- Sumby K.M., Grbin P.R., Jiranek V. Implications of new research and technologies for malolactic fermentation in wine. Applied Microbiology and Biotechnology. 2014;98(19):8111–8132. doi: 10.1007/s00253-014-5976-0. [DOI] [PubMed] [Google Scholar]
- Tang J., Dunshea F.R., Suleria H.A.R. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods. 2019;9(1):7. doi: 10.3390/foods9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens-Spence M.P., Pluskal T., Li F.S., Carballo V., Weng J.K. Complete pathway elucidation and heterologous reconstitution of rhodiola salidroside biosynthesis. Molecular Plant. 2018;11(1):205–217. doi: 10.1016/j.molp.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Vasyliev G.S., Vorobyova V.I., Linyucheva O.V., Lvova L. Evaluation of reducing ability and antioxidant activity of fruit pomace extracts by spectrophotometric and electrochemical methods. Journal of Analytical Methods in Chemistry. 2020;2020:1–16. doi: 10.1155/2020/8869436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.C., Yu R.C., Yang H.Y., Chou C.C. Sugar and acid contents in soymilk fermented with lactic acid bacteria alone or simultaneously with bifidobacteria. Food Microbiology. 2003;20(3):333–338. doi: 10.1016/S0740-0020(02)00125-9. [DOI] [Google Scholar]
- Wood I.P., Elliston A., Ryden P., Bancroft I., Roberts I.N., Waldron K.W. Rapid quantification of reducing sugars in biomass hydrolysates: Improving the speed and precision of the dinitrosalicylic acid assay. Biomass and Bioenergy. 2012;44:117–121. doi: 10.1016/j.biombioe.2012.05.003. [DOI] [Google Scholar]
- Wu C., Li T., Qi J., Jiang T., Xu H., Lei H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. Lwt-Food Science and Technology. 2020;122:109064. doi: 10.1016/j.lwt.2020.109064. [DOI] [Google Scholar]
- Wu Y., Li S., Tao Y., Li D., Han Y., Show P.L.…Zhou J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chemistry. 2021;348:129083. doi: 10.1016/j.foodchem.2021.129083. [DOI] [PubMed] [Google Scholar]
- Zhang W.H., Wu J., Weng L., Zhang H., Zhang J., Wu A. An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydrate Polymers. 2020;227:115332. doi: 10.1016/j.carbpol.2019.115332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.