Highlights
-
•
High protein diet is linked to various disorders such as cancer, obesity, diabetes or dysbiosis.
-
•
Undigested protein catabolism by microbiota results in metabolites considered as risk factors related to several gastrointestinal diseases.
-
•
Ammonia, amines, polyamines, hydrogen sulfide, indole and phenol are the main gut metabolites from undigested protein fermentation.
-
•
Indole and its derivatives have been positively associated with glycemic control and inflammation suppression.
-
•
Protein microbial metabolites are potential biomarkers to be used for several diseases’ diagnosis.
Keywords: Undigested protein, Gut microbiote, Amino acids catabolism, Noncomunicable diseases
Abstract
Gut microbiota is a complex ecosystem of symbiotic bacteria that contribute to human metabolism and supply intestinal metabolites, whose production is mainly influenced by the diet. Dietary patterns characterized by a high intake of protein promotes the growth of proteolytic bacteria’s, which produce metabolites from undigested protein fermentation. Microbioal protein metabolites can regulate immune, metabolic and neuronal responses in different target organs. Metabolic pathways of these compounds and their mechanisms of action on different pathologies can lead to the discovery of new diagnostic techniques, drugs and the potential use as functional ingredients in food. This review discusses the potential mechanisms by which amino acid catabolism is involved in microbial protein metabolites. In addition, results from several studies on the association of products from the intestinal metabolism of indigestible proteins and the state of health or disease of the host are revised.
Introduction
The human gut microbiota has been considered as an active metabolic organ due to its capacity to biotransform resistant components from food into microbial metabolites, which evidence has pointed out the beneficial or detrimental role they play in human health (Peredo-Lovillo, Romero-Luna, & Jiménez-Fernández, 2020). The large intestine contains about 500–1000 different species of bacteria (Canfora, Meex, Venema, & Blaak, 2019). In adults, Firmicutes, Actinobacteria, Proteobacteria and Verrucombria and one Archea (Euryarchaeota) are the five bacterial phyla that dominate the gut microbiota (Catalkaya et al., 2020). It has been estimated that the population of microorganisms from gut microbiota are 1.3 times higher than human cells (human colon harbors roughly 1012 microorganismś cells) (Gilbert et al., 2018). There is a vast diversity in the human gut microbiota, and it has been reported that indigestible dietary elements are one of the main factors that shape the composition of this complex ecosystem (Canfora et al., 2019).
The compounds that can resist the digestion and reach the colon are resistant carbohydrates, lipids, proteins and some bioactive compounds. Carbohydrates that are able to reach the colon during the digetion process are called Resistant Carbohydrates (RC) and are also known for being the preferred substrates for the gut microbiota (e.g., non-starch polysaccharides and resistant starch). These compounds have been extensively studied over the years due to the beneficial contribution of the metabolic products generated during fermentation and their positive effect in diseases related to large intestine (Yao, Muir, & Gibson, 2016).
After RC are depleted, the gut microbiota chooses protein fermentation (Canfora et al., 2019). Proteases are more active at neutral pH, and since luminal pH is more acid in the ascending colon, than in the distal (descending) colon, undigested protein fermentation mainly ferments in the distal colon (Canfora et al., 2019, Davila et al., 2013). It is estimated that around 12–18 g proteins reach the large intestine every day (2–3 g of nitrogen per day with 10–15% of urea, ammonia, nitrate and amino acids, 48–51% proteins and 34–42% peptides) (Davila et al., 2013). Currently, there is a trend in low-carbohydrate and high-protein diets which seek weight reduction by increasing the recommended protein intake. Thus, the amount of poorly absorbed or resistant protein may increase.
Nevertheless, there is still limited information available on the effects of increased ingestion of diet proteins in the microbial metabolites and gut microbiota composition, as well as, their effects in host health compared to malabsorbed carbohdyrates fermentation. Furthermore, protein fermentation leads to a more diverse range of metabolites compared to those produced from carbohydrates. It is has been reported that protein microbial metabolites could be available at significant physiological concentrations to impact in the gastrointestinal system. Even though protein microbial metabolites have been thought to increase the risk of several diseases, due to inconsistent evidence throughout the literature regarding the effects of several of these metabolites, it is still not possible to declare a direct relationship between these compounds and the overall effect in human health (Canfora et al., 2019, Davila et al., 2013, Liu et al., 2020).
Keeping this in view, the objective of this review is to summarize the evidence related to the protein-derived metabolites produced by gut microbiota, addressing their implications in human health and connection with cardiovascular, neurological and gastrointestinal diseases, obesity, cancer and diabetes.
Factors modulating protein fermentation during gastrointestinal digestion and colonic fermentation
Proteins that reach the colon derives from two sources. First, endogenous proteins such as glycoproteins, mucinous proteins and those contained in some pancreatic pathology processes. An increase of endogenous protein delivery could be associated by the exudation found in inflammatory and ulcerative condition. Meanwhile, the exogenous protein digestion process begins when proteins reach the stomach, starting the gastric digestion. The conditions in this stage are a pH ranging from 1 to 5 and a transit time of 30 min to 3 h, with the gastric juices promoting protein digestion. This causes the unfolding of proteins and guarantees the easier access of gastric enzymes (Giromini, Cheli, Rebucci, & Baldi, 2019). In the small intestine, trypsin (which cleaves the internal bond of a protein at lysine or arginine residues) and other enzymes such as carboxypeptidase, amino-oligopeptidase, aminopeptidase A, dipeptidase I, dipeptidase II, dipeptidyl aminopeptidase IV, carboxypeptidase P and gamma glutamyl transpeptidase contribute to the intestinal hydrolysis of proteins. These peptidases hydrolyze peptides ranging from 2 to 6 amino acids, and these peptides from protein hydrolysis can be absorbed into the bloodstream (Duerksen, 2020). The nutritional quality of protein is related to its digestibility according to amino acid uptake. Besides, the interactions among the components of the food matrix as lectins, phenolic compounds and phytic acid possibly contributed to limited digestibility of protein (Rovalino-Córdova et al., 2019, Yao et al., 2016).
In addition, protein source and food processing may play a role in altering its digestibility. Ileostomy studies have attempted to provide comparisons of the digestibility of individual protein supplements via the proportion of ingested protein recovered in the effluent. These studies verified that in a low-protein basal diet, purified protein from plants increases the protein content of the ileal effluent compared to animal proteins such as meat or milk derivatives (Sandström, Andersson, Kivistö, & Sandberg, 1986).
The digestion of dietary protein is highly efficient. Proteolysis begins in a gastric stage, which triggers a significant stage of enzymatic (proteolytic) activity in the duodenum and jejunum sections of the small intestine. Deficiency in some of these stages can be reflected in an increase in protein substrates for bacteria in the large intestine. Clinical conditions such as cystinuria, celiac and Crohn's disease affect the digestibility and absorption of proteins. Patients with these conditions tend to have inefficient protein absorption due to their mucosa-related pathologies, which puts them at risk for protein malnutrition. Protein digestion can be affected by a reduction of several digestive or proteolytic enzymes, like trypsin and pepsin, which are also associated with conical pancreatitis (Layer and Keller, 1999, Lewis and Cochrane, 2007, Yao et al., 2016). These unabsorbed polypeptides reach the large intestine where microbiota transforms them into several metabolites (Fan & Pedersen, 2021). Nevertheless, the dysbiosis of microbiota or the decline of several microbiota species relates to a rise in the prevalence of common diversity metabolic disorders as diabetes or obesity. Thus, the diet and lifestyle will permanently influence the final outcome of protein consumption and digestion in the human body.
Effects of protein fermentation in gut microbiota
The ability to metabolize amino acids from undigested protein is shared by a large number of endogenous bacteria in gut microbiota. However, biotransformation of these substrates modifies the profile of bacteria species, as the protein intake of the diet increases. It has been found that dietary patterns of high-protein intake changed the composition of gut microbiota as the abundance of beneficial bacteria (such as Bifidobacterium or Rothia) decreased (Salonen et al., 2014). Some studies have reported an increase in Clostridium perfrigens and a reduction in Bifidobacterium after 48 h of beef protein fermentation (Shen, Chen, & Tuohy, 2010). It also has been stated that the addition of casein in fecal inocula induces the growth of species of the genus Enterococcus, Shigella and Escherichia coli (Richardson, McKain, & Wallace, 2013). In addition to this, in vitro studies in animals have shown that the growth of Bacteroides is associated with the long-term intake of animal protein and some amino acids, leading to significant changes in the gut microbiota (Wu et al., 2011).
Diet intervention studies in healthy volunteers compared a high protein diet against a normal diet for 6 weeks without changes in the abundance of genus Clostridium, Bacteroides and sulfate reducing bacteria, but did report a decrease in Bifidobacteria and counts of total bacteria (Brinkworth, Noakes, Clifton, & Bird, 2009). However, in these studies, the fermentable carbohydrate content of high protein diets was also altered, so there may be a stronger influence on microbiota changes from carbohydrates availability than protein fermentation. This is observed in the study by Russell et al. (2011), where they studied the intake of a low-carbohydrate and high-protein diet and observed a significant decrease in the genus Roseburia and Eubacterium rectole, which are producers of butyrate.
The relevance of the proliferation of species of Enterobacteria from fermentation of undigested protein is related to the possible pathogenicity these microorganisms may have in the gastrointestinal system. This may have implications for the functional capacities of the colonic microbiota, leading to the development and progression of several diseases.
Metabolic pathways of undigested protein fermentation and its end products
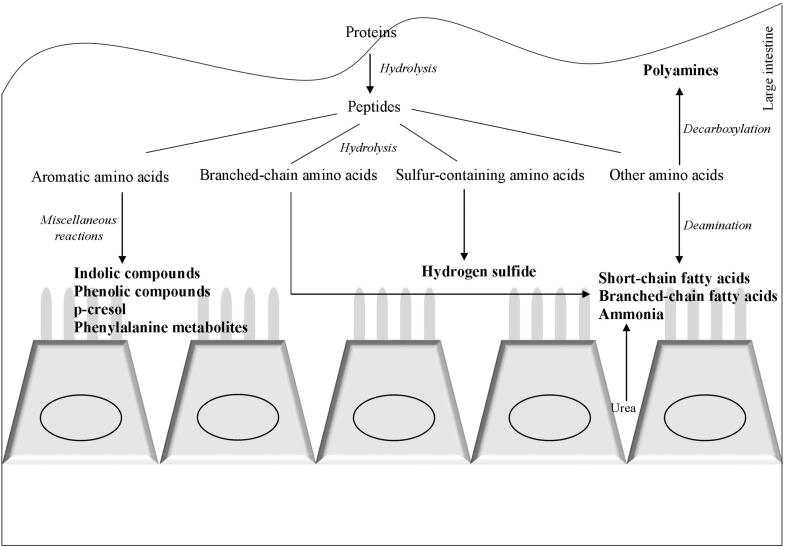
Gut microbiota posseses specialized enzymes for the catabolism of amino acids (Davila et al., 2013). The metabolic pathways of undigested protein fermentation are shown in Fig. 1 based on data published by Liu et al., 2020, Wu et al., 2020. Briefly, undigested proteins are hydrolyzed by extracellular proteases and peptidases into amino acids and peptides. Then, the amino acid catabolism starts with their transmination or deamination that can be oxidative, reductive or coupled. For instance, the Stickland reaction involves a pair of amino acids which one of them could be oxidized and decarboxylated and the other one being reduced. In this context, histidine, leucine, valine, isoleucine and alanine are the preferred H-donors and proline, arginine, tryptophane, glycine and ornithine are the preferred H-acceptors. These reactions yield keto acids or saturated fatty acids. Pyruvate is an important starting point that leads to the excretion of terminal H-acceptors (short chain fatty acids, organic acids, ethanol and gazes). The gazes generated can be metabolized by hydrogenotrophic microorganisms into methane, acetate and hydrogen sulfide. Also, amino acids can be transformed into amines and polyamines via decarboxylation but complex amino acids can be metabolized by fission, deamination, decarboxylation, oxidation and reduction into a several end products. Tyrosine microbial metabolism produces p-cresol, while tryptophan results into different indolic compounds including skatole. Sulfur-containing amino acids fermentation results in the release of sulfur, and then tranformed into hydrogen sulfide. On the other hand, deamination reactions and urea hydrolysis (by microbiota urease activities) leads to ammonia production (Davila et al., 2013).
Fig. 1.
Metabolic pathways of undigested protein fermentation based on data by Liu et al., 2020, Wu et al., 2020.
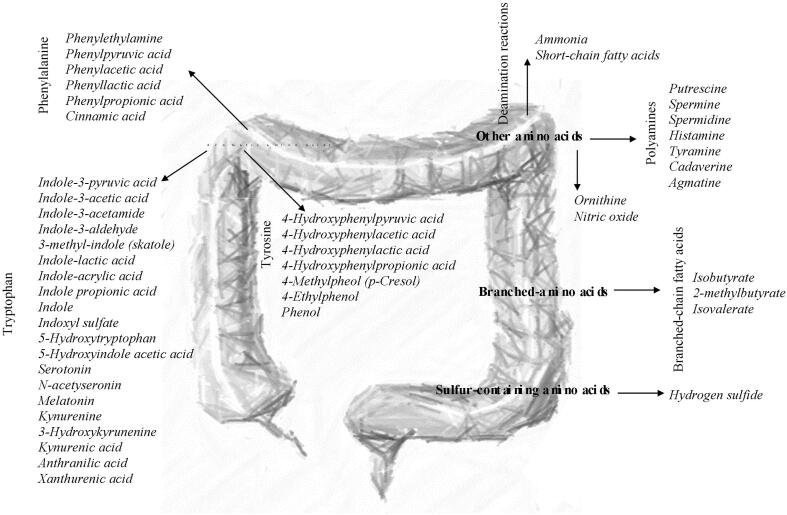
Undigested protein fermentation yields a wide range of metabolites as indolic and phenolic compounds, branched and short chain fatty acids, ammonia, amines, polyamines, and hydrogen sulfide. Fig. 2 shows the protein microbial metabolites and their precursors, classified into four amino acids groups: aromatic amino acids, branced-chain amino acids, sulfur containing amino acids and other amino acids.
Fig. 2.
Protein microbial metabolites.
Aromatic amino acids end products: Indolic and phenolic compounds
The most extensive variety of end products or microbial metabolites from undigested protein fermentation are produced from aromatic amino acids. For instance, at least 9 indolic compounds proceed from tryptophan metabolism (Fig. 2). Several indolic compounds may act as modulators of gastrointestinal function. The metabolic pathways followed by protein microbial metabolites involved in different diseases have been previously reviewed elsewhere (Fan and Pedersen, 2021, Liu et al., 2020, Roager and Licht, 2018) and they are summarized in Fig. 3, Fig. 4.
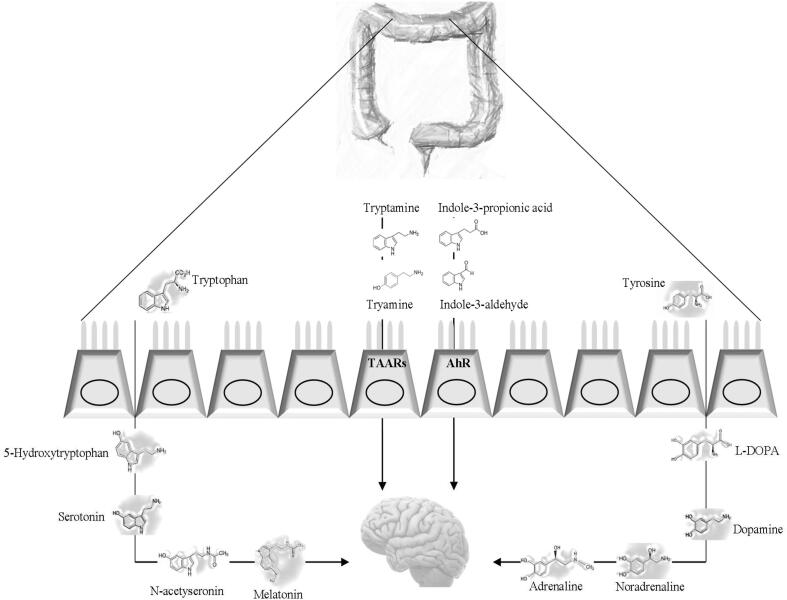
Fig. 3.
Mechanisms pathways of protein microbial metabolites involved on host physiology regarding gastrointestinal diseases and intestine-brain axis effects. Tryptophan and tyrosine are precursors of neurotransmissors. TAAR: Trace amine-associated receptor. Several indolic compounds act on AhR (aryl hydrocarbon receptor) found in intestinal immune cells.
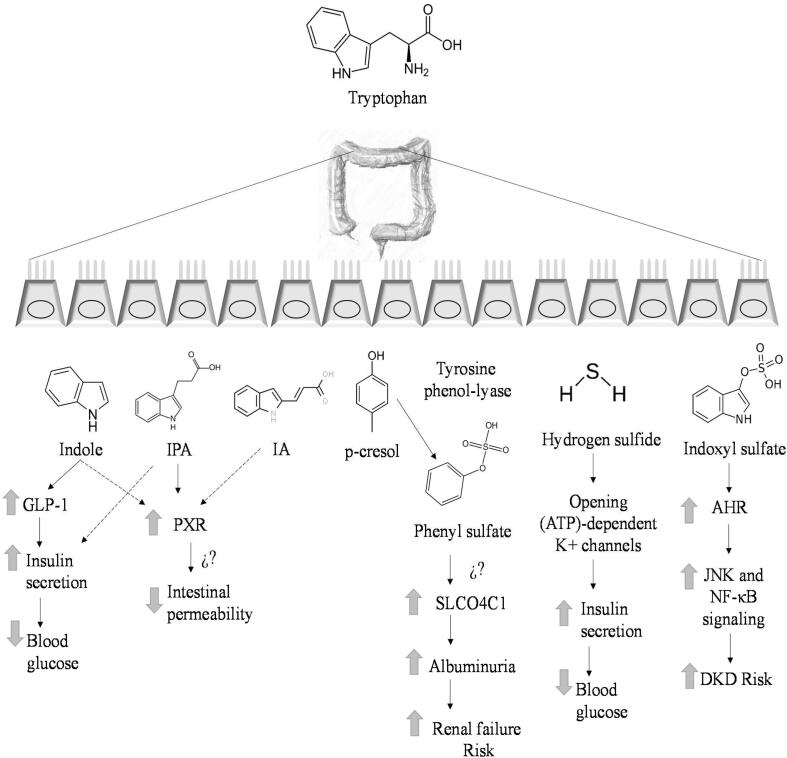
Fig. 4.
Tentative mechanisms pathways of protein microbial metabolites involved on type 2 diabetes. GLP-1, glucagon-like peptide-1; IPA, indole-3-propionic acid; indoleacrylic acid; AHR, aryl hydrocarbon receptor; DKD, Diabetic Kidney Disease; ⇑, increase; ⇓,
Fermentation of aromatic amino acids such as tyrosine, tryptophan and phenylamine are relevant as precursors for the synthesis of several important bioactive molecules. Tryptophan can be metabolized by endogenous bacteria (Clostridium, Bacteroides and Bifidobacterium) to produce indole and indole derivatives (indoleacetic acid, indole-3- acetaldehyde, indole-3-aldehyde and indoleacrylic acid) (Reigstad et al., 2015). Particularly, indole, indole propionic acid and indole-acrylic acid demonstrated to decrease intestinal permiabiality mediated by the pregnane X receptor (PXR) which affects mucosal homeostasis.
Indole also stimulates GLP1 receptor release, whose physiological function is to boost insulin production and decrease glucagon synthesis. This hormone suppress apetite and insulin secretion and it is also known for slowing gastric emptying. Moreover, indole metabolites can act as agonists for aryl hydrocarbon receptor (AHR), an important transcription factor responsible for numerous developmental and tissue-dependent influences on T cell immunity and exerting mainly protective and anti-inflammatory effects in the gut (Chimerel et al., 2014, Lavelle and Sokol, 2020). Indole and skatole produced from tryptophan as well as phenol produced upon fermentation of tyrosine in the large intestine can be absorbed, transformed and excreted mainly as p-cresol (Andriamihaja et al., 2010).
On the other hand, the tryptophan catabolite indole-3-aldehyde induces higher production of interleukin-22 (IL-22) via AHR, altering innate and adaptative immune responses in a ligand-specific fashion (i.e., accelerates the proliferation of epithelial cells from intestinal stem cells to balance tissue homeostasis) (Roager & Licht, 2018). Also, indole propionic acid is a endogenous ligand for PXR, which functions to strengthen the gut barrier by Toll-like receptor 4 (TLR4) or by inducing epithelial IL-10 receptor 1 (Liu et al., 2020).
Branched chain fatty acids
Branched-chain fatty acids are formed mainly from branched chain amino acids in a microbial metabolism produced by microorganisms of the genus Clostridium. Valine, isoleucine and leucine are the precursors of the branched chain fatty acids, isobutyrate, 2-methylbutyrate and isovalerate, respectively (Davila et al., 2013). Similarly, aliphatic amino acids such as alanine and glycine can also produce these fatty acids (Zhao et al., 2020).
Production of branched chain fatty acids can be rapidly increased by higher dietary protein level, as detected in an in vitro model of human colonic microbiota by Aguirre et al. (2016). As a result of the protein fermentation, branched-chain fatty acids increase (3.5 mmol for isovalerate and 1.5 mmol for isobutyrate) and short chain fatty acids decrease (82.3 mmol) compared to a high carbohydrate diet (137.3 mmol), which is associated with the increase in luminal pH from 5.8 to 6.3 at 72 h of fermentation (Aguirre et al., 2016). Such changes in the microbial environment and metabolites cause a leakage of pathogen-associated molecular patterns, including rise of lipopolysaccharides in the bloodstream as well as trigger systemic low-grade inflammation and insulin resistance (Cani et al., 2007).
Short chain fatty acids branched chain fatty acids
Undigested protein fermentation also yields short chain fatty acids. Nevertheless, it is important to note that, there is lack of evidence dose-related studies to establish the contribution of protein fermentation to the amount of short chain fatty acids produced in the distal colon and experiments using stable isotope-labelled proteins are required (Canfora et al., 2019). Acetate, propionate and butyrate are typìcal short chain fatty acids produced mainly by carbohydrate fermentation. However, these metabolites can also appear, althought at relative lower portion, as a result of amino acid fermentation by different species of Lactobacillus and Bifidobacterium. Butyrate can be produced by the gut microbiota from glutamate and lysine; acetate results mainly from fermentation of alanine, aspartate, glutamate, while serine and propionate from the fermentation of aspartate, alanine and methionine (Zhang et al., 2020).
Ammonia and polyamines
Ammonia is another metabolite of acidic amino acid fermentation; this compound diffuses through the intestinal barrier in large quantities and in the distal colon, as Pieper, Boudry, Bindelle, Vahjen, and Zentek (2014) described in the in vivo model study in pigs fed with a high protein diet.
A high ammonia concentration was found to have harmful effects on the colonic epithelium and promotion of inflammatory signals. Luminal ammonia in large intestine has been found from 3 mM to 44 mM and it could be absorbed (Davila et al., 2013). Notably, ammonia has been clasificated as an aberrant microbial metabolite due it can inhibit in a dose-dependent mechanism the mitochondrial oxygen consumption (Andriamihaja et al., 2010).
Some aromatic amino acids such as phenylalanine, tyrosine and tryptophan could be metabolized to polyamines via descarboxylases into phenylethylamine, tyramine and tryptamine, respectively. The responsible bacteriaś species for phenylethylamine, tyramine and tryptamine production are Morganella morganii, Ruminococcus gnavus and Staphylococcus pseudintermedius, respectively (Liu et al., 2020). Mechanisms of action of polyamines on host physiology are via enterochromaffin cells, where tryptamine induces the release of 5-Hydroxytryptamine (5-HT, serotonin) and stimulates gastrointestinal motility by acting on enteric nervous system neurons (Roager & Licht, 2018).
Hydrogen sulfide
Metabolism of the sulfur-containing amino acids by sulfate reducing bacteria results in the production of hydrogen sulfide (H2S) and methanethiol. Two substrates are essential for this bacterias to produce H2S, a sulfate and an electron donor for the sulfate reduction (Rey et al., 2013). Several anaerobic bacterial strains (E. coli, Salmonella enterica, Clostridia and Enterobacter aerogenes) convert cysteine to H2S, pyruvate and ammonia by cysteine desulfhtdrase (Awano et al., 2005). In addition, gut bacteria may produce H2S by sulfite reduction; sulfite reductase is present in many species such as E. coli, Salmonella, Enterobacter, Klebsiella, Bacillus, Staphylococcus, Corynebacterium, and Rhodococcus (Blachier et al., 2010). H2S has effects on both pro and antiinflammatory responses, smoothes muscle relaxation and pro and antinociception in the gastrointestinal system (Linden, 2014).
Microbial metabolites from undigested protein colonic fermentation: Their implication in different diseases
A growing body of evidence has concluded that is necessary to explore beyond the profile of microbial metabolites towards the evaluation of the activity of these metabolites in order to understand the impact of the gut microbiota on health (Roager & Dragsted, 2019). As previously, mentioned, gut microbiota is able to ferment indigestible/resistant compounds like proteins. Canfora et al. (2019) reported in human fecal samples mean concentrations of ammonia, p-cresol, total phenols (phenol and 4-ethylphenol), total polyamines, total indole-derived compounds, and branched chain fatty acids in healthy individuals as 160.98, 2.12, 2.39, 22.32, 1.56 and 18.87 mmol/kg dry matter, respectively.
As mentioned before, products from microbial protein fermentation have been often considered detrimental for health and linked to various disorders such as cancer, obesity or diabetes. However, discrepancy among the findings about health implications of microbial protein metabolites is persistent (Canfora et al., 2019). For instance, indole-derived compounds have been associated with detrimental effects (Zhao et al., 2018) whereas in contrast, other studies suggested that indole-derived compounds contributed to liver function, gut integrity and glucose homeostasis (Beaumont et al., 2018). Furthermore, it is important to note that despite p-cresol, hydrogen sulfide and branched-chain amino acids metabolites have been related to the development of insulin resistance, no human intervention studies have yet measured the contribution of different protein sources and protein microbial metabolites on host insulin resistance (Canfora et al., 2019). The recent studies around the evaluation of the effects of protein microbial metabolites are summarized in Table 1, Table 2. Some of the diseases related to protein metabolism are described in the following sections.
Table 1.
Protein microbial metabolites and their effects in obesity, cancer and intestine-brain axis system.
| Metabolite | Study design | Effects | Reference |
|---|---|---|---|
| Obesity | |||
| Spermidine | Animal model. Induced obesity mice (C57BL/6J) were treated with high-fat-diet and spermidine. | Spermidine decreased body weight, reversed the apparent hepatosteatosis and reduced serum triglyceride and total cholesterol. It decreased lipogenic genes expression through an AMPK-mediated mechanism. | Gao et al. (2018) |
| Cancer | |||
| p-cresol | In vitro: Batch culture protein fermentation using human fecal inocolum from 60 years volunteers. | p-cresol was found as a predictor of genotoxicity against colonocytes in protein fermentation supernatants. p-cresol induced DNA damage in dose-dependent manner against HT29 and Caco-2 cells | Al Hinai et al. (2019) |
| Indole propionic acid (IPA) | Animal model and in vitro. Experimental assay with eight C57BL/6 mice groups partially or totally irradiated and HIEC-6, MODE-K, HCT-8, and ME-180 cells. Intarget metabolomic LC-MS/MS analyses were performed. | IPA replenishment via oral route attenuated hematopoietic system and gastrointestinal tract (GI) injuries intertwined with radiation exposure without precipitating tumor growth. IPA treated mice represented a lower system inflammatory level, recuperative hematogenic organs, catabatic myelosuppression, improved GI function, and epithelial integrity following irradiation. | Xiao et al. (2020) |
| Intestine-brain axis | |||
| Indole | Animal model: cecuḿs rats injected with indole. | Rats overproducing indole displayed anxiety-like behavior. | Jaglin et al. (2018) |
| Indole-3-propionic acid and indole-3-aldehyde. | Animal model. mRNA expression in astrocytes from induced encephalomyelitis mice was analyzed. | Type I intereferons produced in the central nervous system function in combination with indolic compounds activated AHR signaling in astrocytes and supress central nervous system inflammation. | Rothhammer et al. (2016) |
Table 2.
Protein microbial metabolites and their effects in type 2 diabetes (T2D) and cardiovascular diseases.
| Metabolite | Study design | Effects | Reference |
|---|---|---|---|
| Diabetes | |||
| Indole | Animal models. Male Sprague–Dawley rats (8–12 weeks). A novel colonic-nerve electrophysiological technique was used to examine gut-to brain vagal signaling by bacterial products | GLP-1, secreted by indole, stimulated colonic vagal afferent activity. At a local level indole modified the sensitivity of submucosal neurons to GLP-1. | Buckley et al. (2020) |
| Indoxyl sulphate Indole-3-propionic acid (IPA) Indole-3- lactic acid (ILA) |
In vivo. Untargeted metabolomics analysis of plasma samples using LC-MS. A cross-sectional case–control study with 30 healthy controls, 30 patients with diabetes mellitus and normal renal function (DM-N), and 30 early diabetic nephropathy patients. | Suggest uremic solutes and oxidative stress markers as the compounds indicating early renal function decline in diabetes mellitus patients, including glutamine, (microbiome-associated) indoxyl sulfate, hippurate, and 3-methylhistidine. Elevated levels of IPA and ILA in the subjects with diabetes are likely the microbiome-mediated response to the treatments for improved glycemic control |
Tan et al. (2021) |
| Phenyl sulfate | Animal models. Diabetes was induced SLCO4C1-Tg rats and renal failure model C57BL/6N Jcl mice was used. Histological examination, uptake experiment, untargeted metabolomics in plasma, cell toxicity assay, mitocondrial function measurement, microbiome analysis was evaluated. In vivo. The Urinary biomarker for continuous and rapid progression of diabetic nephropathy (UCARE) study (n = 362 patients) |
In a diabetic patient cohort, phenyl sulfate levels significantly correlate with basal and predicted 2-year progression of albuminuria in patients with microalbuminuria. Inhibition of tyrosine phenol-lyase, reduces albuminuria in diabetic mice. | Kikuchi et al. (2019) |
| IPA | In vivo. Non-targeted metabolomics examining those who either early developed T2D (n = 96) or did not convert to T2D within the 15-year follow-up (n = 104) | IPA is a potential biomarker for the development of T2D that may mediate its protective effect by preservation of β-cell function | de Mello et al. (2017) |
| Cardiovascular diseases | |||
| Indolic compounds and branched-chain fatty acids. | In vivo. 7000 participants. Evaluation of metabolites in serum by H NMR spectroscopy. | Atherosclerosis was associated with disturbances of metabolism of branched-chain and aromatic amino acids. | Tzoulaki et al. (2019) |
| Indolic compounds | In vivo. 100 patients with advanced atherosclerosis. | Indolic compounds were associated with advanced human atherosclerosis and postoperative cardiac complications. | Cason et al. (2018) |
| Microbial metabolites from aromatic amino acids | Animal model. Plasma from rats treated for seven days with the non-absorbed antibiotic vancomycin or a mixture of streptomycin, neomycin, polymyxin B and bacitracin was analyzed by mass spectrometry-based metabolite profiling platforms. | Microbial metabolites from aromatic amino acids were linked to severity of myocardial infarction. | Lam et al. (2016) |
| Indole-lactic acid | Animal model. Composition of the gut microbiome was analyzed in faecal pellets of mices treated with high salt diet and normal salt diet. | Decrease ot L. murinus was related with decreased indole-lactic acid production which was linked with an increased TH17 cells response, which promotes hypertension. | Wilck et al. (2017) |
Obesity
As a consequence of the increased consumption of highly processed foods and adoption of sedentary lifestyles, obesity is a global concern disease and one of the major socio-economic problems in the 21st century. These obesity disturbances are related with the development of diabetes or liver diseases (Canfora et al., 2019). A large number of studies have been done around short chain fatty acids effects. Generally, they have been linked on the prevention of metabolic syndrome (Decreased triglycerides, blood pressure, insulin resistance, glucose, body mass index, and abdominal obesity) (Peredo-Lovillo et al., 2020). It has been reported that propionic and butyric acid prevented obesity and insulin resistance via induction of intestinal gluconeogenesis (Canfora et al., 2019). Of note, treatment of induced obese mice with 10 mg/kg spermidine (A polyamine that can be produced by the biotransformation of arginine by commensal intestinal bacteria such as the genus Bacteroides and Fusobacterium) decreased body weight, hepatic intracellular, visceral fat content and total cholesterol once a day for 4 weeks. The mechanism indicated that spermidine increased the phosphorylation of hepatic AMP-activated protein kinase (AMPK) decreasing lipogenic genes expression (Gao et al., 2018).
Irritable bowel syndrome (IBS) and colorectal cancer (CRC)
Cancer is a generalized term that involves several diseases that affect different systems and organs in the human body. This illness occurs when mutations that affect certain cell mechanisms, such as Programmed Cell Death (PCD) or apoptosis, damage the genetic information of the cells. The origins for every cancer are multifactorial, whether it may have a genetic background, environmental risk factors, or a combination of both. It is also known that these genetic and environmental factors have interactions that cause the conditioning of proliferation, prognosis, survival and differentiation of the disease in the organism. According to the World Health Organization (WHO), cancer is one of the main death causes around the globe. Just in 2020, 10 million people are estimated to have died from this disease (IARC, 2021). Among the several types of cancer, colorectal cancer (CRC) is the third most common cancer in the world, with an estimate of 1.93 million of cases in 2020.
It has been previously stated that gut microbiota profiles from CRC patients significantly differs from that of healthy individuals (Abu-Ghazaleh, Chua, & Gopalan, 2021) leading to the so-called “dysbiosis”. Some of these proteolytic bacteria (Fusobacterium nucleatum, Bacteroides fragilis, E. coli, Clostridium, Streptococcus and Staphylococcus) are capable of metabolizing the dietary protein elements into potentially genotoxic compounds, which are related to the inflammation state of the IBS, and subsequently, the generation of the development environment for CRC (Barrett et al., 2020, Grazioso et al., 2019, Meng et al., 2018).
Particularly, it has been recorded that certain compounds such as phenylalanine (and other amino acids), hydrogen sulfide and secondary bile acids (SBAs) are elevated in patients with IBS and CRC. Yachida et al. (2019) recently reported that phenylalanine metabolism can be used as a potential marker for detecting CRC. Also, aromatic amino acid derived metabolites are linked with CRC occurrence. Specifically, p-cresol was found as a predictor of genotoxicity against colonocytes in protein fermentation supernatants. Exogenous p-cresol increased DNA damage and independently p-cresol induced DNA damage in dose-dependent manner against HT29 and Caco-2 cells (Al Hinai et al., 2019).
Nitrosamines and heterocyclic amines (HCAs) are formed from the presence of amino acids and direct-heat when cooking meat products, causing an increased risk factor for CRC evidenced mainly by observational studies (Góngora et al., 2019, Ruiz-Saavedra et al., 2021). However, it varies according to dietary patterns and cooking preferences. The highest concentration of HCA is found in fried seafood (88.30 ng/g), baked seafood (88.08 ng/g), dried pork (66.82 ng/g), deep fried seafood (64.18 ng/g), broiled poultry (55.61 ng/g) and barbecued poultry (53.79 ng/g) (Pouzou, Costard, & Zagmutt, 2018). The harmful dose of HCA has been reported mainly for in vitro and animal models, this due to the ethical restrictions of human studies and depend on the specific HCA. Recently, concentrations of 8 to 800 ng/mL of 2-Amino-3-methylimidazole [4,5-f] quinoline (one of the most common HCA) have been associated with damage to hepatocytes and induction of Parkinson's disease gen protein using zebrafish (Danio rerio) (Li et al., 2021, Li et al., 2021).
The population‐based prospective cohort study conducted by Wada et al. (2017) in 13,957 men and 16,374 women from Japan, revealed significant positive associations of total and red meat consumption with CRC risk in men (114 g median intake of total meat and 64 g median intake of red meat). In addition, HCAs have been shown to form HCA-DNA adducts in human colorectal tissue providing a plausible mechanism of action by which these compounds may increase the risk of CRC development (Crowe, Elliott, & Green, 2019).
Bile Acids are normal components of the luminal contents of the gastrointestinal tract that act as a physiologic detergent (enable absorption of lipids, cholesterol, and fat-soluble vitamins) and regulator of intestinal epithelial homeostasis (Ajouz, Mukherji, & Shamseddine, 2014). Even when most of the bile acids are absorbed in the small intestine, those who arrive the colon are metabolized by gut microbiota as secondary bile salts (SBA) particularly to lithocholic and deoxycholic acids, which are potentially mutagens and also may induce DNA damage, as well as apoptosis resistance. Shabanzadeh, Sørensen, and Jørgensen (2017) reported in a cohort study with a population of 5,928 patients, that there is a CRC predilection of the right-side colon, suggested through a greater proximal colonic absorption of SBA, which have been considered carcinogenic for a long time. In addition, bile acids and gut bacteria interact with the nuclear farnesoid X receptor and the G protein–coupled membrane receptor 5 to regulate numerous metabolic pathways that support a potential role of bile acids in cancer development through microbiota-dependent metabolic mechanisms (Fiorucci, Biagioli, Zampella, & Distrutti, 2018).
Sulfur is another compound that is enriched in red and processed meat, which can be external, like sulfites used as food additives (e.g. sulfur dioxide E220, sodium sulfite E221, potassium sulfite E225, calcium sulfite E226), or contained in certain amino acids (e.g., cysteine and methionine), and its transformation to hydrogen sulfide by sulfidogenic bacteria can potentially influence colorectal carcinogenesis. It is hypothesized that it diffuses into intestinal epithelial cells and interferes with mitochondrial function, ultimately leading to hyperproliferation via the Ras/MAPK pathway. The Ras/MAPK pathway is a known mechanism of carcinogenesis in many malignancies, including CRC (Dahmus, Kotler, Kastenberg, & Kistler, 2018). Yazici et al. (2017) found a correlation between the presence of sulfidogenic bacteria and CRC, establishing that fat intake and daily servings of meat were significantly higher in African American compared with non-Hispanic whites (38.9 ± 6.9 and 39 ± 7 g respectively), and multiple dietary components correlated with a higher abundance of sulfidogenic bacteria, resulting in a higher incidence of CRC. However, according to Song, Garrett, and Chan (2015) sulfur-containing foods may have divergent effects on CRC development depending on the specific sulfur-associated compounds, since allyl sulfur components from garlic and sulfur-containing glycosides found in cruciferous vegetables may have a CRC reduction effect. However, there is lack of human evidence to recommend modification of sulfur intake for the prevention of CRC.
Intestine-Brain axis
It has been reported that microbial metabolites play an important role in the bidirectional gut-brain communication. Phenylalanine is a precursor to tyrosine (via phenylalanine hydroxylase in the liver). Tyrosine is further metabolized to dopamine, norepinephrine, adrenaline and melanin, which are excitatory neurotransmitters (Liu et al., 2020). In this context, tryptophan is a precursor to kynurenine or could be used for serotonin synthesis in the gut and brain (Cervenka, Agudelo, & Ruas, 2017). In addition, some polyamines as tyramine and tryptamine are high-affinity endogenous agonists at TAAR1 which is located in neuroendocrine cells of the intestine and B cells of the pancreas. It has been suggested that TAAR1 can mediates host-nutrition microbiota crosstalk in immune and behavioral control which must be validated with more studies (Liu et al., 2020).
Jaglin et al. (2018) reported that rats overproducing indole showed anxiety-like behavior through vagus nerve activation in the gut-brain axis. According to the in vivo assay, supplementation with indole-3-propionic acid and indole-3-aldehyde suppress central nervous system inflammation in induce encephalomyelitis mice via AhR and type I interferons in astrocytes, which modulate central nervous system and neuronal transmission (Rothhammer et al., 2016).
Cardiovascular diseases
Indole, indole-3-aldehyde and indole propionic acid are associated with advanced atherosclerosis (Cason et al., 2018, Tzoulaki et al., 2019). Recently, phenylacetic acid, phenyllactic acid, p-cresol and 4-Hydroxyphenylpropionic acid were associated to myocardial infarction (Lam et al., 2016). On the other hand, high salt consumption is also related to cardiovascular diseases. It has been observed that high salt consumption levels decrease Lactobacillus murinus proliferation. This species is capable to produce indole-lactic acid, indole-3-acetic acid and indole-3-aldehyde. The decrease in indole-lactic acid production was related with increased T helper (TH)17 cells response which promotes hypertension. Also, indole-lactic acid showed inhibition of the polarization of TH17 cells in vitro (Wilck et al., 2017).
Type 2 diabetes mellitus
Type 2 diabetes mellitus (T2DM) is an insulin-resistance condition (defective insulin signaling within glucose receptor tissues) or beta-cell dysfunction (inadequate glucose sensing to stimulate insulin secretion) and approximately 90% of patients with diabetes mellitus have T2DM, which can be a result of genetic predisposition, environmental factors, or a combination of these two. Worldwide, an estimated 462 million people are affected by type 2 diabetes corresponding to 6.28% of the world’s population (4.4% of those aged 15–49 years, 15% of those aged 50–69, and 22% of those aged 70 + ) and more than 1 million deaths were attributed to this condition in 2017 alone, ranking it as the ninth cause of mortality (Khan et al., 2020).
A strong positive association with diabetic nephropathy is observed between higher adherence to a dietary pattern rich in Western-style dietary protein sources such as high-fat dairy products, egg, as well as red and processed meats in women with T2D (Aziz et al., 2021). Plasma metabolites (e.g. butyrylcarnitine, N-oleoylserine, 1-palmitoyl-GPA, alpha-ketoglutarate, 5-oxoproline) affect a range of metabolic functions in humans, including insulin sensitivity. However, these metabolites were not directly linked to the gut microbiota composition, underscoring the intricate relation between plasma metabolites, the gut microbiota, and insulin sensitivity (Koopen et al., 2020). The findings demonstrate that the neuromodulatory molecule, glucagon-like peptide-1 (GLP-1), secreted by colonic enteroendocrine l-cells in response to indole, stimulated colonic vagal afferent activity. At a local level indole modified the sensitivity of submucosal neurons to GLP-1. These findings elucidate a cellular mechanism by which sensory l-cells act as cross-barrier signal transducers between microbial products in the gut lumen and the host peripheral nervous system (Buckley et al., 2020).
Diabetic kidney diseases affect about 40 % of patients with T2D. Recently, indoxyl sulfate, a liver metabolite of indole a gut bacteria-derived product of tryptophan has been associated with an early decline in kidney function in patients with T2D (Tan et al., 2021). Because of this, therapies have recently been developed that can mitigate the effects of indoxyl sulfate. Oral spherical carbon adsorbent reduced serum IS levels in moderate to severe chronic kidney disease patients (Kim et al., 2020). Meanwhile, Zhao et al. (2020) reported that the inhibitory effect of Tangshen Formula (is a traditional Chinese herbal medicine) on renal inflammation was associated with the inhibition of aryl hydrocarbon, a receptor of indoxyl sulfate, and TLR4, thereby inhibiting JNK and NF-κB signaling in the kidney. In addition, gut p-cresol is absorbed into the bloodstream and sulfated by tyrosine phenol-lyase, a bacterial enzyme responsible for the synthesis of phenol from dietary tyrosine before it is metabolized into phenyl sulfate in the liver. Phenyl sulfate increase with the progression of diabetes in rats overexpressing human uremic toxin transporter SLCO4C1 in the kidney, and are decreased in rats with limited proteinuria and Inhibition of tyrosine phenol-lyase reduces albuminuria in diabetic mice (Kikuchi et al., 2019).
For its part, H2S inhibits insulin secretion by opening of adenosine triphosphate (ATP)-dependent K + channels (KATP) channels via S-sulfhydration. Opening of KATP channels causes membrane hyperpolarization and therefore closing of voltage-dependent calcium channels (VDCC). H2S also inhibits VDCC directly via S-sulfhydration. H2S inhibits glucose-induced mitochondrial membrane hyperpolarization and ATP production (Gheibi, Samsonov, Gheibi, Vazquez, & Kashfi, 2020).
Nonetheless, indole propionic acid (IPA) is another deamination product of the amino acid tryptophan. de Mello et al. (2017) suggest that indolepropionic acid, a gut microbiota-produced metabolite, is a potential biomarker for the development of T2D that may mediate its protective effect by preservation of β-cell function. Higher IPA at 1-year study was inversely associated with the incidence of T2D and tended to be directly associated with insulin secretion during the mean 7-year follow-up. Moreover, IPA correlated positively with dietary fiber intake and negatively with hsCRP concentrations at both sampling and study follow-up. Therefore, it has been suggested that the putative effect of IPA on reducing the risk of T2D could be mediated by the interaction between dietary fiber intake and inflammation or by the direct effect of IPA on β and cell function (Tuomainen et al., 2018). It is clear that a potential effect of protein gut metabolites is observed, however, most investigations have been observational, animal models, and in vitro systems. It is necessary to test the effects in clinical trials with methodological rigor and that evaluate the effects over time
Conclusions
An extensive body of evidence has studied relationship between the gut microbiota and the host through microbial metabolites and how they influence human health. Nevertheless, detailed metabolic pathways and responsible bacteria origin of certain protein microbial metabolites remain incompletely understood and are becoming central questions for further studies. Protein microbial metabolites are potential biomarkers to be used for several diseases’ diagnosis, prognosis and prevention. Nevertheless, considering the discrepancy between the growing body of evidence around protein microbial metabolites, in vivo studies as well as meta-analyses that involve dietary patterns and other several factors regarding lifestyle of a robust population of patients are of great importance to help clarify the public health debate about the animal protein intake.
CRediT authorship contribution statement
José de Jesús Rodríguez-Romero: Conceptualization, Writing – original draft, Writing – review & editing. Alba Cecilia Durán-Castañeda: Writing – review & editing. Alicia Paulina Cárdenas-Castro: Visualization, Writing – review & editing. Jorge Alberto Sánchez-Burgos: Writing – review & editing. Víctor Manuel Zamora-Gasga: Supervision, Visualization, Writing – review & editing. Sonia Guadalupe Sáyago-Ayerdi: Conceptualization, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Durán-Castañeda AC acknowledge to CONACYT México for financial support (Registration number: 934864).
Contributor Information
José de Jesús Rodríguez-Romero, Email: jrguez344@gmail.com.
Alba Cecilia Durán-Castañeda, Email: alceduranca@ittepic.edu.mx.
Alicia Paulina Cárdenas-Castro, Email: alpacardenasca@ittepic.edu.mx.
Jorge Alberto Sánchez-Burgos, Email: jsanchezb@ittepic.edu.mx.
Victor Manuel Zamora-Gasga, Email: vzamora@ittepic.edu.mx.
Sonia Guadalupe Sáyago-Ayerdi, Email: ssayago@ittepic.edu.mx.
References
- Abu-Ghazaleh N., Chua W.J., Gopalan V. Intestinal microbiota and its association with colon cancer and red/processed meat consumption. Journal of Gastroenterology and Hepatology. 2021;36(1):75–88. doi: 10.1111/jgh.15042. [DOI] [PubMed] [Google Scholar]
- Aguirre M., Eck A., Koenen M.E., Savelkoul P.H., Budding A.E., Venema K. Diet drives quick changes in the metabolic activity and composition of human gut microbiota in a validated in vitro gut model. Research in Microbiology. 2016;167(2):114–125. doi: 10.1016/j.resmic.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Ajouz H., Mukherji D., Shamseddine A. Secondary bile acids: An underrecognized cause of colon cancer. World Journal of Surgical Oncology. 2014;12(1):1–5. doi: 10.1186/1477-7819-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hinai E.A., Kullamethee P., Rowland I.R., Swann J., Walton G.E., Commane D.M. Modelling the role of microbial p-cresol in colorectal genotoxicity. Gut Microbes. 2019;10(3):398–411. doi: 10.1080/19490976.2018.1534514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriamihaja M., Davila A.-M., Eklou-Lawson M., Petit N., Delpal S., Allek F.…Blachier F. Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;299(5):G1030–G1037. doi: 10.1152/ajpgi.00149.2010. [DOI] [PubMed] [Google Scholar]
- Aziz M., Jalilpiran Y., Nekouimehr M., Fattahi S., Mokhtari P., Jayedi A.…Mirzaei K. Dietary protein sources and risk of diabetic nephropathy in women: A case-control study. BMC Endocrine Disorders. 2021;21(1):1–9. doi: 10.1186/s12902-021-00841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett M., Hand C.K., Shanahan F., Murphy T., O’Toole P.W. Mutagenesis by microbe: The role of the microbiota in shaping the cancer genome. Trends in Cancer. 2020;6(4):277–287. doi: 10.1016/j.trecan.2020.01.019. [DOI] [PubMed] [Google Scholar]
- Beaumont M., Neyrinck A.M., Olivares M., Rodriguez J., de Rocca Serra A., Roumain M.…Muccioli G.G. The gut microbiota metabolite indole alleviates liver inflammation in mice. The FASEB Journal. 2018;32(12):6681–6693. doi: 10.1096/fj.201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F., Davila A.M., Mimoun S., Benetti P.H., Atanasiu C., Andriamihaja M.…Tomé D. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010;39(2):335–347. doi: 10.1007/s00726-009-0445-2. [DOI] [PubMed] [Google Scholar]
- Brinkworth G.D., Noakes M., Clifton P.M., Bird A.R. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. British Journal of Nutrition. 2009;101(10):1493–1502. doi: 10.1017/S0007114508094658. [DOI] [PubMed] [Google Scholar]
- Buckley M.M., O’Brien R., Brosnan E., Ross R.P., Stanton C., Buckley J.M., O’Malley D. Glucagon-like peptide-1 secreting L-cells coupled to sensory nerves translate microbial signals to the host rat nervous system. Frontiers in Cellular Neuroscience. 2020;14:95. doi: 10.3389/fncel.2020.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E.E., Meex R.C., Venema K., Blaak E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nature Reviews Endocrinology. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D.…Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- C.A. Cason K.T. Dolan G. Sharma M. Tao R. Kulkarni I.B. Helenowski … E.B. Chang Plasma microbiome-modulated indole-and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes Journal of Vascular Surgery 68 5 2018 1552–1562. e1557 10.1016/j.jvs.2017.09.029. [DOI] [PMC free article] [PubMed]
- Catalkaya G., Venema K., Lucini L., Rocchetti G., Delmas D., Daglia M.…Xiao J. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Frontiers. 2020;1(2):109–133. doi: 10.1002/fft2.25. [DOI] [Google Scholar]
- Cervenka I., Agudelo L.Z., Ruas J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349) doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- Chimerel C., Emery E., Summers D.K., Keyser U., Gribble F.M., Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Reports. 2014;9(4):1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe W., Elliott C.T., Green B.D. A review of the in vivo evidence investigating the role of nitrite exposure from processed meat consumption in the development of colorectal cancer. Nutrients. 2019;11(11) doi: 10.3390/nu11112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmus J.D., Kotler D.L., Kastenberg D.M., Kistler C.A. The gut microbiome and colorectal cancer: A review of bacterial pathogenesis. Journal of Gastrointestinal Oncology. 2018;9(4):769–777. doi: 10.21037/jgo.2018.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila A.-M., Blachier F., Gotteland M., Andriamihaja M., Benetti P.-H., Sanz Y., Tomé D. Re-print of “Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host”. Pharmacological Research. 2013;69(1):114–126. doi: 10.1016/j.phrs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Duerksen D. In: Encyclopedia of Gastroenterology. Second Edition. Kuipers E.J., editor. Academic Press; Oxford: 2020. Protein digestion and absorption; pp. 311–314. [Google Scholar]
- de Mello V.D., Paananen J., Lindström J., Lankinen M.A., Shi L., Kuusisto J.…Rolandsson O. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Scientific Reports. 2017;7(1):1–12. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nature Reviews Microbiology. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Fiorucci S., Biagioli M., Zampella A., Distrutti E. Bile Acids Activated Receptors Regulate Innate Immunity. Frontiers in Immunology. 2018;9:1853. doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Zhao W., Li C., Xie X., Li M., Bi Y.…Liu X. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochemical Biophysical Research Communications. 2018;505(1):93–98. doi: 10.1016/j.bbrc.2018.09.078. [DOI] [PubMed] [Google Scholar]
- Gheibi S., Samsonov A.P., Gheibi S., Vazquez A.B., Kashfi K. Regulation of carbohydrate metabolism by nitric oxide and hydrogen sulfide: Implications in diabetes. Biochemical Pharmacology. 2020;176 doi: 10.1016/j.bcp.2020.113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R. Current understanding of the human microbiome. Nature Medicine. 2018;24(4):392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giromini C., Cheli F., Rebucci R., Baldi A. Invited review: Dairy proteins and bioactive peptides: Modeling digestion and the intestinal barrier. Journal of Dairy Science. 2019;102(2):929–942. doi: 10.3168/jds.2018-15163. [DOI] [PubMed] [Google Scholar]
- Góngora V.M., Matthes K.L., Castaño P.R., Linseisen J., Rohrmann S.J.C.E., Biomarkers P. Dietary heterocyclic amine intake and colorectal adenoma risk: A systematic review and meta-analysis. Cancer Epidemiology Prevention Biomarkers. 2019;28(1):99–109. doi: 10.1158/1055-9965.EPI-17-1017. [DOI] [PubMed] [Google Scholar]
- Grazioso, T. P., Brandt, M., & Djouder, N. (2019). Diet, Microbiota, and Colorectal Cancer. iScience, 21, 168-187. https://doi.org/10.1016/j.isci.2019.10.011. [DOI] [PMC free article] [PubMed]
- IARC. (2021). Cancer Today. In, vol. 2021): World Health Organization.
- Jaglin M., Rhimi M., Philippe C., Pons N., Bruneau A., Goustard B.…Rabot S. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Frontiers in Neuroscience. 2018;12:216. doi: 10.3389/fnins.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. Journal of Epidemiology Global Health. 2020;10(1):107. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Saigusa D., Kanemitsu Y., Matsumoto Y., Thanai P., Suzuki N.…Asaji K. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nature Communications. 2019;10(1):1–17. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-H., Jhee J.H., Choi H.Y., Lee S.-H., Shin S.K., Lee S.-Y.…Jo Y.-I. New oral spherical carbon adsorbent effectively reduces serum indoxyl sulfate levels in moderate to advanced chronic kidney disease patients: A multicenter, prospective, open-label study. BMC Nephrology. 2020;21(1):1–10. doi: 10.1186/s12882-020-01971-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopen A.M., de Clercq N.C., Warmbrunn M.V., Herrema H., Davids M., de Groot P.F.…Groen A.K. Plasma metabolites related to peripheral and hepatic insulin sensitivity are not directly linked to gut microbiota composition. Nutrients. 2020;12(8):2308. doi: 10.3390/nu12082308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam V., Su J., Hsu A., Gross G.J., Salzman N.H., Baker J.E. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PloS one. 2016;11(8) doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle A., Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology. 2020;17(4):223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- Layer P., Keller J. Pancreatic enzymes: Secretion and luminal nutrient digestion in health and disease. Journal of Clinical Gastroenterology. 1999;28(1):3–10. doi: 10.1097/00004836-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Lewis S., Cochrane S. Alteration of sulfate and hydrogen metabolism in the human colon by changing intestinal transit rate. Official Journal of the American College of Gastroenterology ACG. 2007;102(3):624–633. doi: 10.1111/j.1572-0241.2006.01020.x. [DOI] [PubMed] [Google Scholar]
- Li D., Li Z., Zhang T., Peng B., Zhang Y., Sun H., Wang S. 2-Amino-3-methylimidazo [4, 5-f] quinoline Triggering Liver Damage by Inhibiting Autophagy and Inducing Endoplasmic Reticulum Stress in Zebrafish (Danio rerio) Toxins. 2021;13(11):826. doi: 10.3390/toxins13110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Cao P., Meng H., Li D., Zhang Y., Li Y., Wang S. Long-term exposure to 2-amino-3-methylimidazo [4, 5-f] quinoline can trigger a potential risk of Parkinson's disease. Journal of Hazardous Materials. 2021;412 doi: 10.1016/j.jhazmat.2021.125230. [DOI] [PubMed] [Google Scholar]
- Linden D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxidants Redox Signaling. 2014;20(5):818–830. doi: 10.1089/ars.2013.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Hou Y., Wang G., Zheng X., Hao H. Gut microbial metabolites of aromatic amino acids as signals in host–microbe interplay. Trends in Endocrinology & Metabolism. 2020;31(11):818–834. doi: 10.1016/j.tem.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Meng C., Bai C., Brown T.D., Hood L.E., Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics, Proteomics & Bioinformatics. 2018;16(1):33–49. doi: 10.1016/j.gpb.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peredo-Lovillo A., Romero-Luna H., Jiménez-Fernández M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Research International. 2020;136 doi: 10.1016/j.foodres.2020.109473. [DOI] [PubMed] [Google Scholar]
- Pieper R., Boudry C., Bindelle J., Vahjen W., Zentek J. Interaction between dietary protein content and the source of carbohydrates along the gastrointestinal tract of weaned piglets. Archives of Animal Nutrition. 2014;68(4):263–280. doi: 10.1080/1745039X.2014.932962. [DOI] [PubMed] [Google Scholar]
- Pouzou J.G., Costard S., Zagmutt F. Probabilistic estimates of heterocyclic amines and polycyclic aromatic hydrocarbons concentrations in meats and breads applicable to exposure assessments. Food Chemical Toxicology. 2018;114:346–360. doi: 10.1016/j.fct.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Reigstad C.S., Salmonson C.E.I., Rainey J.F., Szurszewski J.H., Linden D.R., Sonnenburg J.L.…Kashyap P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. The FASEB Journal. 2015;29(4):1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey F.E., Gonzalez M.D., Cheng J., Wu M., Ahern P.P., Gordon J.I. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proceedings of the National Academy of Sciences. 2013;110(33):13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A.J., McKain N., Wallace R.J. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiology. 2013;13(1):1–8. doi: 10.1186/1471-2180-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roager H., Dragsted L. Diet-derived microbial metabolites in health and disease. Nutrition Bulletin. 2019;44(3):216–227. doi: 10.1111/nbu.12396. [DOI] [Google Scholar]
- Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nature Communications. 2018;9(1):1–10. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V., Mascanfroni I.D., Bunse L., Takenaka M.C., Kenison J.E., Mayo L.…Blain M. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nature Medicine. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovalino-Córdova A.M., Fogliano V., Capuano E. The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans. Food Chemistry. 2019;286:557–566. doi: 10.1016/j.foodchem.2019.02.057. [DOI] [PubMed] [Google Scholar]
- Ruiz-Saavedra S., García-González H., Arboleya S., Salazar N., Emilio Labra-Gayo J., Díaz I.…de los Reyes-Gavilán C.G. Intestinal microbiota alterations by dietary exposure to chemicals from food cooking and processing. Application of data science for risk prediction. Computational and Structural Biotechnology Journal. 2021;19:1081–1091. doi: 10.1016/j.csbj.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W.R., Gratz S.W., Duncan S.H., Holtrop G., Ince J., Scobbie L.…Wallace R.J. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. The American Journal of Clinical Nutrition. 2011;93(5):1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- Salonen A., Lahti L., Salojärvi J., Holtrop G., Korpela K., Duncan S.H.…Lobley G.E. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. The ISME Journal. 2014;8(11):2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström B., Andersson H., Kivistö B., Sandberg A.-S. Apparent small intestinal absorption of nitrogen and minerals from soy and meat-protein-based diets. A study on human ileostomy subjects. The Journal of Nutrition. 1986;116(11):2209–2218. doi: 10.1093/jn/116.11.2209. [DOI] [PubMed] [Google Scholar]
- Shabanzadeh D.M., Sørensen L.T., Jørgensen T. Association between screen-detected gallstone disease and cancer in a cohort study. Gastroenterology. 2017;152(8):1965–1974.e1961. doi: 10.1053/j.gastro.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Shen Q., Chen Y.A., Tuohy K.M. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe. 2010;16(6):572–577. doi: 10.1016/j.anaerobe.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Song M., Garrett W.S., Chan A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–1260.e1216. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.M., Gao Y., Teo G., Koh H.W., Tai E.S., Khoo C.M.…Choi H. Plasma metabolome and lipidome associations with type 2 diabetes and diabetic nephropathy. Metabolites. 2021;11(4):228. doi: 10.3390/metabo11040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomainen M., Lindström J., Lehtonen M., Auriola S., Pihlajamäki J., Peltonen M.…Diabetes Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutrition Diabetes. 2018;8(1):1–5. doi: 10.1038/s41387-018-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzoulaki I., Castagne R., Boulange C.L., Karaman I., Chekmeneva E., Evangelou E.…Mosen D. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. European Heart Journal. 2019;40(34):2883–2896. doi: 10.1093/eurheartj/ehz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Oba S., Tsuji M., Tamura T., Konishi K., Goto Y.…Nagata C. Meat consumption and colorectal cancer risk in Japan: The Takayama study. Cancer Science. 2017;108(5):1065–1070. doi: 10.1111/cas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilck N., Matus M.G., Kearney S.M., Olesen S.W., Forslund K., Bartolomaeus H.…Markó L. Salt-responsive gut commensal modulates TH 17 axis and disease. Nature. 2017;551(7682):585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.-Y., Keilbaugh S.A.…Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Tang Z., Chen H., Ren Z., Ding Q., Liang K., Sun Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Animal Nutrition. 2020;7:11–16. doi: 10.1016/j.aninu.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H.-W., Cui M., Li Y., Dong J.-L., Zhang S.-Q., Zhu C.-C.…Wang H.-C. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome. 2020;8:1–17. doi: 10.1186/s40168-020-00845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S., Mizutani S., Shiroma H., Shiba S., Nakajima T., Sakamoto T.…Kubo M. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nature Medicine. 2019;25(6):968–976. doi: 10.1038/s41591-019-0458-7. [DOI] [PubMed] [Google Scholar]
- Yao C., Muir J., Gibson P. Insights into colonic protein fermentation, its modulation and potential health implications. Alimentary Pharmacology Therapeutics. 2016;43(2):181–196. doi: 10.1111/apt.13456. [DOI] [PubMed] [Google Scholar]
- Yazici C., Wolf P.G., Kim H., Cross T.-W.-L., Vermillion K., Carroll T.…Gaskins H.R. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66(11):1983–1994. doi: 10.1136/gutjnl-2016-313321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wielen N.V.D., Hee B.V.D., Wang J., Hendriks W., Gilbert M. Impact of fermentable protein, by feeding high protein diets, on microbial composition, microbial catabolic activity, gut health and beyond in pigs. Microorganisms. 2020;8(11):1735. doi: 10.3390/microorganisms8111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zhang F., Ding X., Wu G., Lam Y.Y., Wang X.…Ma J. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang H., Yin X., Zhao H., Ma L., Yan M.…Li P. Tangshen formula modulates gut Microbiota and reduces gut-derived toxins in diabetic nephropathy rats. Biomedicine Pharmacotherapy. 2020;129 doi: 10.1016/j.biopha.2020.110325. [DOI] [PubMed] [Google Scholar]