Abstract
Background
While initial dietary management immediately after formal diagnosis is an 'accepted' cornerstone of treatment of type 2 diabetes mellitus, a formal and systematic overview of its efficacy and method of delivery is not currently available.
Objectives
To assess the effects of type and frequency of different types of dietary advice for adults with type 2 diabetes.
Search methods
We carried out a comprehensive search of The Cochrane Library, MEDLINE, EMBASE, CINAHL, AMED, bibliographies and contacted relevant experts.
Selection criteria
All randomised controlled trials, of six months or longer, in which dietary advice was the main intervention.
Data collection and analysis
The lead investigator performed all data extraction and quality scoring with duplication being carried out by one of the other six investigators independently with discrepancies resolved by discussion and consensus. Authors were contacted for missing data.
Main results
Thirty‐six articles reporting a total of eighteen trials following 1467 participants were included. Dietary approaches assessed in this review were low‐fat/high‐carbohydrate diets, high‐fat/low‐carbohydrate diets, low‐calorie (1000 kcal per day) and very‐low‐calorie (500 kcal per day) diets and modified fat diets. Two trials compared the American Diabetes Association exchange diet with a standard reduced fat diet and five studies assessed low‐fat diets versus moderate fat or low‐carbohydrate diets. Two studies assessed the effect of a very‐low‐calorie diet versus a low‐calorie diet. Six studies compared dietary advice with dietary advice plus exercise and three other studies assessed dietary advice versus dietary advice plus behavioural approaches. The studies all measured weight and measures of glycaemic control although not all studies reported these in the articles published. Other outcomes which were measured in these studies included mortality, blood pressure, serum cholesterol (including LDL and HDL cholesterol), serum triglycerides, maximal exercise capacity and compliance. The results suggest that adoption of regular exercise is a good way to promote better glycaemic control in type 2 diabetic patients, however all of these studies were at high risk of bias.
Authors' conclusions
There are no high quality data on the efficacy of the dietary treatment of type 2 diabetes, however the data available indicate that the adoption of exercise appears to improve glycated haemoglobin at six and twelve months in people with type 2 diabetes. There is an urgent need for well‐designed studies which examine a range of interventions, at various points during follow‐up, although there is a promising study currently underway.
Plain language summary
Dietary advice for treatment of type 2 diabetes mellitus in adults
No high quality data on the efficacy of diet alone exists for treatment of type 2 diabetes mellitus. This systematic review assesses the effects of studies that examined dietary advice with or without the addition of exercise or behavioural approaches. Eighteen studies were included. No data were found on micro‐ or macrovascular diabetic complications, mortality or quality of life. It is difficult to draw reliable conclusions from the limited data that are presented in this review, however, the addition of exercise to dietary advice showed improvement of metabolic control after six‐ and twelve‐month follow‐up.
Background
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (i.e. elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group on the Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups'). For an explanation of methodological terms, see the main Glossary on the Cochrane Library. There is now unequivocal evidence that type 2 diabetes can be prevented or at least delayed by dietary effort, generally resulting in weight loss and increased physical activity in those at high risk of progressing to diabetes. Risk of such progression was decreased by over half in the two major trials to date (Tuomilehto 2001; DPP 2002). The United States Diabetes Prevention Program reported a 58% reduction in the incidence of diabetes when participants were treated with the lifestyle intervention compared with a 31% reduction of incidence of diabetes for the metformin‐treated participants, (DPP 2002). However the role and type of dietary advice following diagnosis of type 2 diabetes, how often thereafter and its efficacy during longer‐term follow‐up over subsequent years is much less clear. It may be that using different methods of helping people to learn the principles behind these diets, and different patterns of support and follow‐up, could have effects on long term adherence. This Cochrane review aims to establish the evidence that exists on what kind of dietary advice is effective in treating type 2 diabetes. There are an estimated 2.35m people with diabetes in England, with up to another 750,000 undiagnosed cases and this is predicted to grow to more than 2.5m by 2010 (DOH website; Diabetes UK website). Type 2 diabetes mellitus is the more common of the two main types and accounts for between 85 ‐ 95% of all diabetic patients (Diabetes UK website). In the United Kingdom alone, treatment of type 2 diabetes costs around £1.8 billion per annum (Moore 2000). It has been recommended for many years that people diagnosed with type 2 diabetes should be treated with diet, and in those overweight to achieve weight loss and, independently, to lower blood glucose (glycaemia). On average, there is a 9% relative increase in the risk of type 2 diabetes, for every 1kg weight gain (Mokdad 2000). Eighty to ninety percent of type 2 diabetics are obese (BMI >30kg/m2) (Wing 2000) and obesity worsens the metabolic and physiologic abnormalities associated with diabetes, particularly hyperglycemia, hyperlipidemia, and hypertension (Maggio 1997; Wing 2000). Initiation of medication becomes necessary in addition to diet and exercise when the latter alone fail to control glycaemia adequately, (Nathan 1999). While short‐term benefits are assumed, attempting to treat type 2 diabetes using diet alone is not a particularly successful long‐term intervention, as illustrated by the United Kingdom Prospective Diabetes Study, (UKPDS 1998; UKPDS 1998b). This study showed that after three years of regularly reinforcing dietary advice in those randomised to the 'diet alone' arm, only 20% of patients, and after nine years only 8%, maintained the target fasting plasma glucose of less than 7.8 mmol/L, (Turner 1999). Part of the uncertainty in this field is whether the dietary and other non‐pharmacological measures actually 'fail' or whether patients themselves fail to continue implementing them adequately. Despite this, diet is usually the first treatment implemented on diagnosis of type 2 diabetes and is maintained throughout the addition of other interventions. Some observational studies have shown that exercise may have an additional benefit for the treatment of type 2 diabetes, possibly through lowering body fat and reducing blood pressure (Wallberg‐H. 1998), and several studies have produced some compelling evidence to show that regular physical exercise can serve to protect and decrease severity of type 2 diabetes (Frisch 1986; Dowse 1991; Manson 1992a). The review by Thomas et al, 2006, found that exercise improves blood glucose control and that this effect is evident even without weight loss (Thomas 2006).
Diets commonly used to control blood glucose levels include low fat and high unrefined carbohydrate, (those which take around 25‐30% of energy from fat and around 50% of the total energy from unrefined carbohydrate), or low glycaemic index diets (foods that have a low glycaemic index include pasta products, oats, beans and some fruits and vegetables) usually both in combination with weight reducing advice. Recently, research has also looked into the effect of individual macro‐ and micro‐nutrients such as fibre, chromium, and so called 'functional foods', but much of this evidence seems to be incomplete, with relatively few intervention trials carried out long‐term (Riccardi 2005). Similar diets may be of value in those with metabolic syndrome in preventing or delaying progression to frank diabetes (Riccardi 2000). Given the prevalence of type 2 diabetes and the potentially serious outcomes of the disease, it is important to establish which type of diet, either alone or in combination with other interventions (the addition of exercise, behavioural approaches and alternative treatments), is most effective.
This Review This review is a 2007 update of the initial (Moore 2004) review. It aimed to provide an update of evidence from studies which have employed a study design which sought to examine the effect of dietary advice and interventions to treat type 2 diabetes mellitus in the adult population. Although no additional included studies were identified, the review referenced further relevant background research and excluded studies, and has generated a QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection.
Objectives
The aim of this systematic review was to assess the effects of type and frequency of dietary advice to all adults (overweight and normal weight) with type 2 diabetes on morbidity, quality of life, total mortality, weight and measures of diabetic control, using all available randomised clinical trials and meta‐analytic techniques where appropriate. The primary objectives were to assess:
a) the effects of different types of dietary advice through weight change, and development of micro‐ and macro‐ vascular complications in people with type 2 diabetes, and b) the effects of dietary advice plus other lifestyle interventions (i.e. the addition of exercise, behavioural approaches and alternative therapies to dietary advice) through weight change, and development of micro‐ and macro‐vascular complications in people with type 2 diabetes.
Management of hits We prepared a QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection to describe how we processed the references identified through our searches. The hits identified from the searches of the electronic databases [Medline (492), AMED (2), CINAHL (87) , Cochrane Library (16) and EMBASE (1,110)] were combined (n = 1,707) and de‐duplicated (n = 1,413). These hit lists were then de‐duplicated against the hits of the previous version of this review. The reduced list of hits was then screened on titles and abstracts (LN).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled clinical trials of interventions that involved patients for at least six months were included. Randomisation of individuals or clusters of individuals were accepted.
Types of participants
All studies had to include adult participants (those who were 18 years or older), who were diagnosed with type 2 diabetes. To be consistent with changes that have occurred over time in the classification and diagnostic criteria of type 2 diabetes mellitus, the diagnosis of diabetes should have been established using the standard criteria valid at the time of the beginning of the trial. Ideally, the diagnostic criterion was described in the article. Diagnostic criteria used included those described by the National Diabetes Data Group standards (NDDG 1979), the World Health Organisation standards, (WHO 1980; WHO 1985; WHO 1998), and the American Diabetes Association Standards, (ADA 1997; ADA 1999). Studies performed on participants suffering from impaired glucose tolerance were not included in this review. Where a study reported combined results for participants with type 2 diabetes and participants with impaired glucose tolerance, then efforts were made to contact the authors of the study to obtain individual patient data. Where this was unsuccessful, the trial was excluded. In the protocol for this review we omitted to state how a study looking at a mixture of type 1 and type 2 diabetic participants would be dealt with. We decided that unless a majority of the participants (i.e. 90% or more) were classified as having type 2 diabetes, or the author of the paper could provide the data for the type 2 diabetic participants only, the trial was excluded. Participants could be of either sex, but those who were acutely ill or pregnant were excluded.
Types of interventions
Studies where the intervention was dietary advice with an aim of reducing weight and the severity of type 2 diabetes were included in the review. Dietary advice is taken to mean advice given with the intention of improving dietary habits (i.e. to either produce weight loss or to change diet composition). Studies were not included if they included medication that was provided differently in the control and intervention groups. Studies evaluating the effects of fish oils (omega‐3) advice or supplementation on type 2 diabetes mellitus were excluded as this had previously been addressed in a recent Cochrane review (Farmer 2001). Studies looking at the effects of Chinese medical herbs on type 2 diabetes mellitus were also excluded as these are addressed by another Cochrane review (Liu 2002).
Types of outcome measures
Primary outcomes
weight (where the main aim of the study was weight loss);
development of micro and macrovascular diabetic complications (including neuropathies, retinopathy, nephropathy and cardiovascular diseases).
Secondary outcomes
quality of life (ideally, measured using a validated instrument);
change in anti‐diabetic medication use (as an indicator of improving or worsening diabetic control);
overall cardiovascular disease risk assessment (using any of the scales which include at least three risk factors);
mortality;
glycated haemoglobin;
serum cholesterol (LDL and/or HDL) and serum triglycerides;
maximal exercise capacity (VO2 max);
blood pressure;
compliance.
Timing of outcome assessment
Randomised controlled trials of interventions that involved participants for a minimum of six months were included in the review. Outcome measures were extracted and assessed at baseline, six months, one year, two year and at one year intervals from that point where available.
Search methods for identification of studies
Electronic searches
The following sources were searched to identify relevant literature for the original review (Moore 2004):
The Cochrane Library, which includes the Cochrane Central Register of Controlled Trials, the Database of Systematic Reviews and the Database of Abstracts and Reviews of Effectiveness
The Cochrane Library, (Issue 3, 2003);
MEDLINE (1966 to October Week 1 2003);
EMBASE (1980 to Week 40 2003);
CINAHL (1982 to October Week 1 2003);
AMED (1985 to October 2003), bibliographies and experts.
For this update review, the following searches were completed :
The Cochrane Library, which includes the Cochrane Central Register of Controlled Trials, the Database of Systematic Reviews and the Database of Abstracts and Reviews of Effectiveness
The Cochrane Library, (October, 2006);
MEDLINE (October Week 1 2003 to October 2006);
EMBASE (Week 40 2003 to October 2006);
CINAHL (October Week 1 2003 to October 2006);
AMED (October 2003 to October 2006).
There were no language restrictions for either searching or trial inclusion. The search strategy below was used, adapted to suit the individual databases.
NOTE: Unless otherwise stated, search terms are free text terms;
For details of the search strategy see under Appendix 1.
The MEDLINE search was run as above, with the randomised controlled trials and systematic reviews searches added on separately. The CINAHL search was run as above, with randomised controlled trials search added on separately. The AMED search was run as above, with randomised controlled trials search added on separately. The EMBASE search was adapted from the original search above.
Once relevant papers were retrieved, their references were analysed and any relevant referenced papers were also sourced and added into the review.
Data collection and analysis
Selection of studies
Two reviewers undertook assessment of results data independently. Results data were assessed by the lead reviewer (LN) and duplicated by one of the co‐reviewers (HM, CS, VW, LH, KC and AV). Information on a number of measures of methodological quality of the included studies was assessed independently by two reviewers; study design, method of allocation concealment, blinding of outcome assessment and drop out rates. Where there was uncertainty, authors were contacted to clarify aspects of study design. Differences between reviewers' extraction results were resolved by discussion. Multiple publications were collated and assessed as one study.
In the first instance, relevant studies were determined from the initial search of electronic databases, and then through screening by the lead reviewer (LN). Articles were rejected during this initial screening if the reviewer could determine from the title and/or abstract that it did not meet the inclusion criteria, if rejection was not possible, full text copies were retrieved. For the 2007 update, full text copies of 68 papers were retrieved and assessed independently by two reviewers (LN plus one other from HM, CS, VW, LH, KC and AV) using an inclusion/exclusion form. Inter‐rater agreement (using Cohen's kappa (Cohen 1960)) was good with all of the reviewers achieving kappa scores of 0.7 or greater in the first review (Moore 2004), Inter‐rater agreement of this review achieved a value of 1.0 (using Cohen's kappa). Thirty‐six of the original 287 retrieved papers were included in this review, which reported a total of eighteen studies. In the update, none of the sixty‐eight papers were thought to fit the inclusion criteria, so no new papers were added. Where full text copies of articles were retrieved, but then were judged to not meet the inclusion criteria, the reasons were reported in the excluded studies table.
Data extraction and management
Two reviewers (the lead reviewer (LN) plus one other from HM, CS, VW, LH, KC and AV) independently extracted data from each study using a data extraction form based on the one provided by the Metabolism and Endocrine Disorders Review Group. Differences between reviewers' extraction results were resolved by discussion, and where necessary, in consultation with a third reviewer. Data concerning participants, interventions, and outcomes, as described above in the selection criteria section, were extracted. Trial quality characteristics, including method of randomisation, allocation concealment, blinding of outcome assessors and losses to follow‐up, were extracted onto this form. In addition, data were collected on potential effect modifiers including age, presence of diabetic micro or macrovascular disease, blood pressure, lipids, bodyweight, mortality and current use of diabetic medication.
Assessment of risk of bias in included studies
The quality of each trial was assessed based largely on the quality criteria specified by Jadad and Schulz (Schulz 1995; Jadad 1996). In particular the following factors were assessed: 1. Minimisation of selection bias ‐ score: 0 or 1 or 2. One point was given if the study could be described as randomized (which included the use of words such as 'random', 'randomly' and 'randomisation'). One additional point was given if the study described the method of randomisation and was an appropriate method. One point was taken away if the study described the method of randomisation, but was an inappropriate method. 2. Minimisation of detection bias ‐ score: 0 or 1 or 2. One point was given if the study could be described as blinded (in any capacity). One additional point was given if the method of blinding was described and was appropriate. One point was taken away if the method was described as blinding but was inappropriate. 3. Minimisation of attrition bias ‐ score: 0 or 1. One point was given if the withdrawals and dropouts from the study were described. We did not score on performance bias. Based on these criteria, studies were broadly subdivided into the following three categories (see Cochrane Handbook): A ‐ all quality criteria met: low risk of bias. (Studies with scores of four and five points (and with at least one point allocated from each section) were allocated to this category.) B ‐ one or more of the quality criteria only partly met: moderate risk of bias. (Studies with scores of three points (and with at least one point in each section) were allocated to this category.) C ‐ one or more criteria not met: high risk of bias. (Studies with scores of zero, one, two and three points were allocated to this category. (i.e. studies with no points in at least one section)) These subdivisions of the studies were not used as the basis of a sensitivity analysis, as the included studies received similar quality scores, (between one and three points). These quality scores and categories are reported in the 'characteristics of included studies' table.
Measures of treatment effect
Continuous data
Endpoint versus change data: Where possible, endpoint data were presented, as change standard deviations were not available for many studies. If both endpoint and change data were available for the same outcomes, only the former were reported in this review. If endpoint data were not available, but change data were, we reported the change data in the tables and text of the review. However, for inclusion of a study reporting change data in the meta‐analysis, we calculated the endpoint mean from the change data given and assumed that the endpoint standard deviation to be equal to the baseline standard deviation.
Summary statistic
For continuous outcomes a weighted mean difference (WMD) between groups was estimated.
Assessment of heterogeneity
We tested for heterogeneity by using the standard chi2 test and the I2 test (Higgins 2002), as well as by visually inspecting the graphs, and when there was little heterogeneity between trial results, data were summarised statistically by a fixed effect model for continuous data. A significance level less than 0.10 was interpreted as evidence of heterogeneity. If heterogeneity was found, the data were re‐analysed using a random effects model to see if this made a substantial difference.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned a priori but were not undertaken as they were not applicable to the included studies. These subgroup analyses were planned to assess whether particular groups of people with type 2 diabetes could obtain more benefit from a particular intervention than other groups could. Efficacy of different combinations of types of diets would have also been considered in the subgroup analyses. The main analyses carried out were as follows:
dietary advice versus different dietary advice;
dietary advice versus same dietary advice plus exercise;
dietary advice versus same dietary advice plus behavioural approaches.
Analyses for dietary advice versus same dietary advice plus alternative therapies and for dietary advice versus the same dietary advice delivered at a different frequency were planned, but as no studies were found these analyses could not be carried out. Subgroup analyses for each of these comparisons would have included: with or without weight loss advice (outcome measures: development of (further) microvascular disease only) presence/absence of microvascular disease (outcome measures: development of (further) microvascular disease only).
Sensitivity analysis
No sensitivity analyses were performed.
Results
Description of studies
Trials identified
From the initial searches of electronic databases, 8675 (775 from the Cochrane Library, 4278 from MEDLINE, 3310 from EMBASE, 269 from CINAHL and 43 from AMED) abstracts were screened after de‐duplication (HM). For this update review, 1413 articles were screened (16 from the Cochrane Library, 492 from MEDLINE, 1110 from EMBASE, 87 from CINAHL and 2 from AMED) (Figure 1). Articles were rejected on initial screening if the reviewer could determine from the title and/or abstract that it did not meet the inclusion criteria. For the 2007 update review, full text copies of 66 papers were retrieved and assessed independently by two reviewers (LN plus one other from HM, CS, LH, KC, VW and AV), none of which were eligible for inclusion in the study. However, 36 of the original 287 papers, which reported 18 studies, were included in this review. All articles for which hard copies were retrieved but which failed to meet the inclusion criteria were reported in the excluded studies table. Attempts were made to obtain full‐text translations and/or evaluations of all relevant non‐English articles.
1.
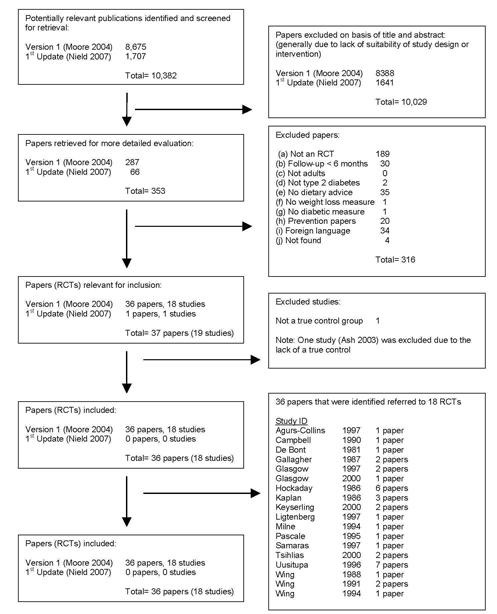
Adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
Interrater agreement
Full text copies of 68 papers were retrieved and assessed independently by two reviewers (LN plus one other from HM, CS, LH, KC, VW and AV) using an inclusion/exclusion form. Interrater agreement (using Cohen's kappa (Cohen 1960)) was good with all of the reviewers achieving kappa scores of 0.7 or greater.
Missing data
All authors were contacted by the lead reviewer in the event of data which has been reported as recorded but not reported in the text of published articles. Replies were received from Dr Hockaday, (Hockaday 1986), Dr Wolever, (Tsihlias 2000), and Dr Samaras, (Samaras 1997).
Included studies
Eighteen studies met the inclusion criteria. Nine studies focused on looking at the effects of two types of diabetic dietary advice that did not differ in intent to lose weight, (de Bont 1981; Hockaday 1986; Gallagher 1987; Campbell 1990; Wing 1991; Milne 1994; Wing 1994; Pascale 1995; Tsihlias 2000), three studies focused on looking at dietary advice versus dietary advice plus behavioural approaches, (Glasgow 1997; Glasgow 2000; Keyserling 2000), and six studies concentrated on dietary advice versus dietary advice plus exercise, (Kaplan 1986; Wing 1988; Uusitupa 1996; Agurs‐Collins 1997; Ligtenberg 1997; Samaras 1997).
Of the nine studies that compared two types of diabetic diet that did not differ in intent to lose weight, four took place in the United States of America, (Gallagher 1987; Pascale 1995; Wing 1991; Wing 1994), two were based in the United Kingdom, (de Bont 1981 and Hockaday 1986), one in Canada, (Tsihlias 2000), one in New Zealand, (Milne 1994) and one study was based in Australia, (Campbell 1990).
There were three studies that assessed dietary advice versus dietary advice plus behavioural approaches; all of these were conducted in the United States, (Glasgow 1997; Glasgow 2000; Keyserling 2000).
From a total of six dietary advice versus dietary advice plus exercise studies, one took place in Finland, (Uusitupa 1996), one in The Netherlands, (Ligtenberg 1997), one study was conducted in Australia, (Samaras 1997), and three took place in the United States of America, (Kaplan 1986; Wing 1988; Agurs‐Collins 1997).
Studies
Wide ranges of dietary approaches were examined in this review. Within the studies that compared two types of diabetic dietary advice that did not differ in intent to lose weight, there were three distinct groups; those studies that used exchange diets versus those that did not use exchange diets, (Gallagher 1987; Campbell 1990), those studies that used low‐fat versus moderate fat or low‐carbohydrate diets, (de Bont 1981; Hockaday 1986; Milne 1994; Pascale 1995; Tsihlias 2000), or very‐low‐calorie diets versus low‐calorie diets, (Wing 1991; Wing 1994).
From the studies that assessed dietary advice versus dietary advice plus exercise, it was seen that the types of exercise examined in the studies that are contributing to the review included various forms of aerobic exercise, (in general participants chose their exercise from suggestions that included walking, jogging, swimming, ball games or skiing).
There were similar interventions included within the grouping for dietary advice versus dietary advice plus behavioural approaches. Generally, the mode of imparting the dietary advice was what was changed in these interventions; either through a touch‐screen method, (Glasgow 1997), or through community resources, (Glasgow 2000; Keyserling 2000).
Participants
Thirty‐six articles reporting a total of eighteen trials following 1467 participants were included. There were 724 participants in the control groups, and 743 participants in the intervention groups included in this review. The samples used in the trials were generally representative of the overall diabetic population. Diagnostic criteria for the trials in this review included physician confirmed diabetic status, type 2 diabetes classified according to the National Diabetes Data Group, (NDDG 1979), and diabetes mellitus as defined by the World Health Organisation, (WHO 1985). Generally, the diagnostic criteria were not disclosed in the articles.
Interventions
The participants in the trial run by Campbell, (Campbell 1990), were randomised to one of two groups, to a typical portion exchange diet or to a reduced fat (focusing on reducing fat) diet. de Bont conducted a trial of a low‐fat diet versus a low‐carbohydrate diet, (de Bont 1981). The Gallagher study, (Gallagher 1987), evaluated the usual American Diabetic Association exchange diet (40% carbohydrate, 40% fat and 20% protein) compared to a reduced fat diet, which was based upon the four basic food groups, (see Results section for more details). Hockaday conducted a trial where participants were randomised to either a low‐carbohydrate or a low‐fat diet, (Hockaday 1986). Milne randomised the participants into groups that either followed a low‐carbohydrate or a low‐fat diet, (Milne 1994). Pascale described a trial that randomised the participants to follow either a calorie restricted diet or a calorie restricted and low‐fat diet (less than 20% of energy to come from fat), (Pascale 1995). The participants in the Tsihlias trial, (Tsihlias 2000), were randomised to receive either a low‐fat or a monounsaturated fat diet.
The participants in the trial described by Wing were randomised to either a balanced low‐calorie diet (1000‐1200 kcal per day) or a balanced low‐calorie diet with two periods of twelve weeks of a very‐low‐calorie diet (of 400‐500 kcal per day), (Wing 1994). Another trial by Wing, (Wing 1991), randomised the participants to either a low‐calorie yet balanced diet (with a calorie goal of ˜1000 to 1500 kcal per day) or to a low‐calorie (balanced) diet with a period of very‐low‐calorie diet (˜500 kcal per day) added for eight weeks.
The study conducted by Agurs‐Collins randomised participants to either a 'usual care' group or a 'usual diet with exercise' group, (Agurs‐Collins 1997). In the study conducted by Uusitupa and colleagues, (Uusitupa 1996), participants were randomised to either a standard dietary treatment group or a diet and exercise group. The trial by Ligtenberg randomised the participants to either continue with their usual diet or to receive their usual diet plus physical training instructions, (Ligtenberg 1997). Samaras randomly assigned participants to receive either their usual diet or to receive their usual diet plus a structured exercise programme, (Samaras 1997). The participants in the Kaplan, (Kaplan 1986), trial were randomly allocated to either weekly sessions of diet education, which focused on promoting a low‐fat (30% of total energy), high carbohydrate (55% of total energy) or to weekly sessions of diet and exercise education. Wing conducted a trial which randomised participants to either a diet only group or a diet plus exercise group, (Wing 1988).
Glasgow conducted a trial that randomised the participants to either receive usual care or usual care plus the brief intervention added in, (Glasgow 1997). Another trial by Glasgow, (Glasgow 2000), randomly assigned its participants to receive either a basic intervention (received information about low‐fat eating) or the community resources intervention. The trial conducted by Keyserling randomised the participants to receive either a clinic‐based intervention or a clinic‐based intervention plus community intervention, (Keyserling 2000).
Outcome measures
The primary outcomes that we intended to assess in this review were:
weight;
development of micro and macrovascular diabetic complications (including neuropathies, retinopathy, nephropathy and cardiovascular diseases).
Weight was generally well reported, whereas development of micro and macrovascular diabetic complications was only reported in one trial, (Hockaday 1986), and even here, this was only a brief description.
The secondary outcome measures that we intended to look at were:
1. Quality of life (ideally, measured using a validated instrument), 2. Change in anti‐diabetic medication use (as an indicator of improving or worsening diabetic control), 3. Overall cardiovascular disease risk assessment (using any of the scales which include at least three risk factors), 4. Mortality, 5. Glycated haemoglobin, 6. Serum cholesterol (LDL and/or HDL) and serum triglycerides, 7. Maximal exercise capacity (VO2 max), 8. Blood pressure, 9. Compliance.
These additional outcome measures were reported with varying degrees of comprehensiveness. Measures of quality of life and cardiovascular disease risk assessment were not reported in any studies. Change in anti‐diabetic medication and maximal exercise capacity were measured and reported in a few trials, although they used different outcome measures. Mortality was briefly reported in a few trials (de Bont 1981; Milne 1994) although details of cause of death were not often disclosed. Serum cholesterol and triglycerides and blood pressure were reported in some trials, although generally only the means (and not their associated standard deviations or standard errors) were reported. Glycated haemoglobin was recorded by nearly all trials and compliance was recorded by some trials, usually in the form of three day food diaries, and although generally not reported in any way, a few studies did report that 'compliance was generally good' (Kaplan 1986; Wing 1988; Samaras 1997).
Risk of bias in included studies
Further details of the methodological quality of the included studies can be found in the table of characteristics of included studies.
Minimisation of selection bias
Since randomisation was the first inclusion criterion that studies had to meet, all included studies started with a score of 1. Only two articles described the method of randomisation used, (Hockaday 1986; Glasgow 1997) and therefore were awarded another quality point. Where studies described the method of randomisation, it was appropriate.
Minimisation of detection bias
One point was assigned for the presence of assessor blinding in only one trial, (de Bont 1981). None of the included articles made any attempt to describe the method of blinding. In dietary trials, it is not possible to blind participants or the study personnel to the intervention or advice they receive (or are imparting).
Minimisation of attrition bias
More than half of the studies had withdrawals that were not fully described. However, there were seven studies that did describe the drop‐outs and withdrawals to an acceptable degree, (de Bont 1981; Hockaday 1986; Wing 1988; Wing 1991; Milne 1994; Pascale 1995; Keyserling 2000) and these studies all received a point to add to their quality scores. Eleven studies reported follow‐up data of more than 80% of the baseline sample, (de Bont 1981; Hockaday 1986; Wing 1988; Campbell 1990; Wing 1991; Milne 1994; Pascale 1995; Ligtenberg 1997; Samaras 1997; Glasgow 2000; Keyserling 2000). A number of studies reported higher drop‐out rates; 30% at twelve months (Pascale 1995), 32% at six months (Tsihlias 2000), 40% at six months and 44% at twelve months (Keyserling 2000). The study reported by Hockaday (Hockaday 1986) assessed subsections of the initial study population, so analysis of attrition bias was not appropriate. Two other studies did not describe attrition at all (Gallagher 1987; Glasgow 1997).
Allocation concealment
Allocation concealment to intervention or control was unclear in all but one, (Hockaday 1986), of the eighteen studies. In the published article, allocation concealment was unclear, but as a result of a personal communication from Dr Hockaday, this was then revised to 'adequate'.
The source of funding for more than half of the included studies (12 out of 18) was stated in the published papers. Generally, the funding came from national sources so conflicts of interests were avoided (i.e. operators/providers of treatment/intervention) were not paying for the study to be carried out.
Seventeen studies, (Hockaday 1986; Kaplan 1986; Gallagher 1987; Wing 1988; Campbell 1990; Wing 1991; Milne 1994; Wing 1994; Pascale 1995; Uusitupa 1996; Agurs‐Collins 1997; Glasgow 1997; Ligtenberg 1997; Samaras 1997; Glasgow 2000; Keyserling 2000; Tsihlias 2000), were judged to be at high risk of bias; one study, (de Bont 1981), was judged to be at medium risk of bias and no studies were judged to be at a low risk of bias.
Effects of interventions
All of the primary publications for the included studies were indexed by both MEDLINE and the Cochrane Central Register of Controlled Trials. Five of the primary references were indexed within CINAHL and twelve of the primary publications were indexed within EMBASE. None of the primary references of trials included in this review were indexed by AMED.
Heterogeneity
Three tests for heterogeneity were applied for the data in comparison 3 (Dietary Advice versus Dietary Advice plus Physical Activity). These were for
Comparison 3.02: Weight at twelve months
Comparison 3.03: Glycated haemoglobin at six months
Comparison 3.04: Glycated haemoglobin at twelve months
A fixed effects model was used to analyse these data. Significant heterogeneity was found within the studies included in category 3.02 (I2 value of 56.8%, a chi2 value of 4.63 with 2 degrees of freedom and a P‐value of 0.10, (when analysed using a random effects model, these values remained the same)). Although in usual circumstances we would take this P‐value to be an indicator of significance, we believe that this meta‐analysis may have been affected by the large difference between the endpoint data for the two groups in the Samaras trial, (Samaras 1997). Although the participants in this trial were randomly allocated to one of two groups, at baseline the mean weight of the control group was 15kg more than the intervention group, and therefore when we looked at endpoint data, even though the participants in both groups had actually gained a small amount of weight, there was still a large difference between the control, (mean weight at twelve months was 99.0kg) and intervention groups, (mean weight at twelve months was 83.1kg), which suggested that the intervention was particularly successful, when indeed this could not be concluded from the change data provided. The studies included within category 3.03 were not heterogeneous (I2 value of 34.1% and a P‐value of 0.21), and showed a statistically significant change in glycated haemoglobin in favour of the addition of exercise to dietary advice. The studies included within category 3.04 were not heterogeneous (I2 value of 0% and chi2 value of 0.16 with 2 degrees of freedom and had a P‐value of 0.92) and showed a statistically significant change in glycated haemoglobin in favour of the addition of exercise to dietary advice.
Effects of the interventions
Comparison 01: Two types of diabetic dietary advice that did not differ in intent to lose weight
Nine studies assessed two types of diabetic dietary advice that did not differ in intent to lose weight (dietary advice versus another form of dietary advice).
Campbell randomised 70 participants to one of two groups, usual diet or a reduced fat diet, (Campbell 1990). Usual diet was a typical portion exchange diet where caloric restriction was discussed. The intervention diet focused only on reducing fat. Change in weight was not reported in the paper using kilograms but through Body Mass Index. Body Mass Index decreased in the intervention group from 30.4 ± 4.8kg/m2 at baseline to 29.6 ± 4.6kg/m2 at six months compared to 32.0 ± 5.5kg/m2 at baseline to 31.1 ± 5.1kg/m2 at the six month follow‐up. Glycated haemoglobin was not reported in the paper.
de Bont conducted a trial of a low‐fat diet versus a low‐carbohydrate diet, (de Bont 1981). One hundred and forty eight type 2 diabetic participants were randomised and 136 were followed up six months later. The primary outcome of the study was body weight, although this was reported separately for obese and non‐obese participants. Weight in the obese participants decreased from 84.2kg at baseline to 81.5kg at six months, and in the non‐obese participants weight decreased from 60.1kg to 59.7kg, which represents a decrease of 2.7kg for the obese participants and of 0.4kg for the non‐obese participants in the low‐fat diet. This was compared to the group following the low‐carbohydrate diet where the subset of obese participants went from a baseline weight of 84.8kg to 83.9kg at six months, which was a decrease of 0.9kg; the non‐obese participants began the trial with a mean weight of 59.0kg which by the end of six months had risen to 59.1kg, a rise of 0.1kg. Glycated haemoglobin was reported for all participants, and at baseline was 10.0% and decreased to 9.3% in the low‐fat diet group at six months, whereas glycated haemoglobin was 10.1% at baseline and decreased to 9.5% in the low‐carbohydrate group at six months. (Although mean changes were reported, the associated standard deviations/errors were not.)
The papers describing the Gallagher study, (Gallagher 1987), evaluated the American Diabetic Association exchange diet (40% carbohydrate, 40% fat and 20% protein) compared to a reduced fat diet, which was based upon the four basic food groups (which are: 1) fruits and vegetables, 2) whole grains, cereals, and bread, 3) dairy products, 4) meats, fish, poultry, eggs, dried beans, and nuts). Fifty‐one male type 2 diabetic participants were randomised to one of the two groups. Weight and glycated haemoglobin was measured but not reported in the published articles. Attempts at personal communication with the authors proved to be unsuccessful.
Hockaday conducted a trial of 250 diabetic participants who were randomised to either a low‐carbohydrate or a low‐fat diet, (Hockaday 1986). However only subgroups of this trial have been reported in press. In the Hockaday study, the biggest subgroup of the trial was reported which was made up of 93 participants; 39 participants in the low‐carbohydrate group and 54 participants in the low‐fat group. Between baseline and one year, participants in the low‐carbohydrate group had a mean weight loss of 3.8kg compared to a mean weight loss of 4.6kg in the low‐fat diet group. (Although mean weight change was reported, the associated standard deviations/errors were not.) Changes in glycated haemoglobin were measured but not reported, although 'changes in glycated haemoglobin were not significant'.
Milne randomised 44 participants into either a low‐carbohydrate or a low‐fat diet, (Milne 1994). At baseline, participants had a mean weight of 83.1 ± 16.9kg and 80.8 ± 7.8kg for the low‐carbohydrate and the low‐fat diet groups respectively. At follow‐up six months later, mean weight of the low‐carbohydrate group had decreased to 82.1 ± 15.0kg (a decrease of 1.0kg) and the low‐fat group mean weight had decreased to 80.7 ± 7.8kg (a decrease of 0.1kg). At the 18 month follow‐up the mean weight of the low‐carbohydrate group had remained stable at 82.1 ± 15.0kg and the low‐fat group mean weight had also remained stable at 80.7 ± 13.8kg. Glycated haemoglobin decreased in the low‐fat group from a baseline value of 9.8 ± 3.1% to 9.5 ± 2.6% at six months and then rose to 9.7 ± 2.6% at 18 months (an overall decrease of 0.1%) and a decrease in the low‐carbohydrate group from a baseline measurement of 8.7 ± 2.3% to 8.5 ± 2.0% at six months and then remained stable at 8.5 ± 2% at the 18 month follow‐up (an overall decrease of 0.2%).
Pascale described a trial that randomised 44 participants to follow either a calorie restricted diet (1000‐1500 kcal per day) or a calorie restricted and low‐fat diet (1000‐1500 kcal per day, but less than 20% of energy to come from fat), (Pascale 1995). Thirty‐one participants were followed up (16 in the calorie restricted group and 15 in the calorie restricted and low‐fat diet group) one year from baseline. Participants in the calorie restricted diet group decreased their mean weight one year later by 0.96 ± 3.7kg from a baseline measurement of 93.1 ± 13.0kg, compared to the calorie restricted and low‐fat diet who decreased their mean weight one year later by 5.2 ± 7.3kg from the baseline measurement of 94.4 ± 9.5kg. At the twelve month follow‐up, glycated haemoglobin levels rose in the calorie restricted diet group by 0.2 ± 1.7% (from 10.9% ± 2.7% at baseline). In contrast, at twelve months in the calorie restricted and low‐fat diet group, the mean glycated haemoglobin levels remained the same (10.4 ± 1.9% at baseline with a change of 0.0 ± 1.9% at the one year follow‐up).
In the study reported by Tsihlias, (Tsihlias 2000), 61 participants were randomised to receive either the low‐fat (high‐carbohydrate) or the monounsaturated fat diet; six months later 41 participants were followed up. The participants in the monounsaturated fat diet had an overall weight gain of 0.1kg over the six month period (from 78.8 ± 12.5kg at baseline to 78.9 ± 13.0kg at six months), compared with the participants in the low‐fat diet group who lost an overall weight of 1.2kg over the six month period (from 77.4 ± 14.7kg at baseline to 76.2 ± 13.8kg at six months). There were 'no significant changes in glycaemic control' during the study period. Glycated haemoglobin data were obtained through a personal communication from Dr Wolever, and at baseline the monounsaturated fat diet group had a measurement of 7.7 ± 1.1%, which rose to 8.1 ± 1.5% at follow‐up six months later. The participants in the low‐fat (high carbohydrate) diet group at baseline had a blood glycated haemoglobin concentration of 8.1 ± 1.2%, which rose to 8.3 ± 1.5% at the six months follow‐up.
As mentioned previously, there are three distinct groups that the above studies can be assigned to, (two groups are assessed here, the third (very‐low‐calorie diets versus low‐calorie diets) is discussed in the next section). Clinical similarity, the decision to pool or not, study quality and an overview of the primary and secondary results will be discussed for each.
(a) Studies that assessed exchange diets versus not using an exchange diet
In both of these two studies, (Gallagher 1987; Campbell 1990), the American Diabetes Association exchange diet was compared with a standard reduced fat diet. The two trials recruited similar participants, (the only difference being that the Gallagher trial recruited solely male participants and the Campbell trial had mixed participation). The quality of the these two studies were assessed to be at high risk of being biased. Weight change was measured in the Gallagher study, where 9% of the intervention group lost weight compared to 25% of the control group losing weight, although no hard data were reported. Development of micro and macrovascular diabetic complications were not reported. Body Mass Index (as a measure of weight) was reported in the Campbell study, and the intervention group lost an average 0.8kg/m2 compared to 0.9kg/m2. Both studies measured, but did not report change in glycated haemoglobin.
These studies were clinically similar to permit pooling, but with only two studies in this category, pooling of the data was not possible. There were a total of 121 participants in this comparison grouping, where the quality of the trials was judged to be at high risk of being biased. No firm conclusions could be drawn from this comparison.
(b) Studies that assessed low‐fat diets versus moderate fat or low‐carbohydrate diets
In these five studies, (de Bont 1981; Hockaday 1986; Milne 1994; Pascale 1995; Tsihlias 2000), a low‐fat diet was compared with either a moderate fat or a low‐carbohydrate (although not necessarily mutually exclusive) diet. The trials recruited similar participants (with regards to age and sex). The quality of the trials were assessed, and five out of the six trials were assessed to be at a high risk of bias, and the other trial was assessed at a moderate risk of bias. Weight change was measured in all of the studies and in general more weight was lost in those groups that were following a low‐fat diet. Development of micro and macrovascular diabetic complications were reported in one trial, (Hockaday 1986), although not in great detail, and these outcome measures were not reported in the other five trials. Glycated haemoglobin was reported, but there were only marginal changes in the trials, so no firm conclusions could be drawn from this.
All of these studies were clinically similar enough to permit pooling, but there were only two studies at six months which had data that could be pooled, (two of the other trials reported means without their associated standard deviations, (de Bont 1981; Hockaday 1986)), and one reported change data, (Pascale 1995) therefore it was not possible to look at the statistical heterogeneity in this comparison. There were a total of 378 participants in this comparison grouping, where generally the quality of the trials was judged to be at high risk of being biased. Conclusions could not be drawn from this comparison.
Comparison 02: very‐low‐calorie Dietary Advice versus Another Form of Dietary Advice
Two studies assessed very‐low‐calorie dietary advice versus another form of dietary advice (low‐calorie diet).
Wing described a study in which 93 participants were randomised to either a balanced low‐calorie diet (1000‐1200 kcal per day) or a balanced low‐calorie diet with two periods of twelve weeks of a very‐low‐calorie diet (of 400‐500 kcal per day), (Wing 1994). Glycated haemoglobin was reported at 6 and 12 months, whereas weight was only reported at 12 months. Glycated haemoglobin for the low‐calorie diet group was 10.5 ± 2.0% at baseline, decreasing to 8.8 ± 1.8% at six months, which rose to 9.2 ± 2.0% at twelve months and then rose again to 10.7 ± 2.4% at twenty‐four months, compared to 10.4 ± 2.0% at baseline, a decrease to 8.4 ± 2.2% at six months, and then a slight rise to 8.9 ± 2.5% at twelve months and a greater rise to 10.4 ± 2.2% at twenty‐four months for the very‐low‐calorie diet group. Mean body weight for the low‐calorie diet group at baseline was 107.7 ± 18.7kg, which rose to 118.2 ± 11.6kg at twelve months, whereas participants in the very‐low‐calorie diet group had a mean baseline weight of 105.8 ± 19.4kg, which decreased to 91.6 ± 10.3kg at twelve months.
Wing randomised 36 people to either a low‐calorie yet balanced diet (with a calorie goal of ˜1000 to 1500 kcal per day) or to a low‐calorie (balanced) diet with a period of very‐low‐calorie diet (˜500 kcal per day) added for eight weeks, (Wing 1991). At baseline, the low‐calorie group had a mean weight of 104.5 ± 21.5kg and at follow‐up twelve months later their mean weight had dropped to 97.7 ± 17.4kg, compared with the very‐low‐calorie diet group who at baseline had a mean weight of 102.1 ± 11.7kg and twelve months later had a mean weight of 93.5 ± 10.4kg. Both groups lost a significant amount of weight and weight loss was not improved by use of a very‐low‐calorie diet. Glycated haemoglobin was 10.4 ± 2.0% at baseline, which rose to 11.8 ± 2.7% at twelve months for the low‐calorie group compared with a baseline glycated haemoglobin concentration of 10.4 ± 2.2%, which decreased to 9.2 ± 2.1% at the twelve month follow‐up for the very‐low‐calorie group. The changes in glycated haemoglobin were statistically significant in both groups.
Both studies, (Wing 1991; Wing 1994), assessed the effects of a very‐low‐calorie diet against the effects of a low‐calorie diet. The quality of these trials was judged to be at high risk of bias. Weight change was measured and reported and more weight was lost in the very‐low‐calorie diet groups at twelve months, although in the first trial, (Wing 1994), at twenty four months more weight loss was recorded in the low‐calorie diet group. Development of micro and macrovascular complications were not reported in these studies. Glycated haemoglobin was recorded and reported, at twelve months there was a greater decrease in glycated haemoglobin in the very‐low‐calorie dietary advice group, compared with the very‐low‐calorie dietary advice group, however there was very little change from baseline to twenty‐four months in either group.
The two studies were clinically similar enough to permit pooling, but as there were only two studies, it was not statistically possible to carry out an analysis on the heterogeneity of the data. One hundred and twenty‐nine participants took part in these two trials and overall the trial quality was assessed to be at high risk of bias. The evidence suggests that longer term (twenty‐four months) weight loss and glycaemic control is best achieved by low‐calorie dietary advice, compared with the very‐low‐calorie dietary advice, although once again, the high potential for bias should be considered, and no firm conclusions could be drawn from this data.
Comparison 03: Dietary Advice versus Dietary Advice plus Exercise
Six studies compared interventions that examined the effect of dietary advice alone or dietary advice plus exercise on participants with type 2 diabetes.
Agurs‐Collins randomised 64 participants to either a 'usual care' group or a 'usual diet with exercise' group, (Agurs‐Collins 1997). Published details of the usual care control were brief; participants in this group attended a class that discussed methods of glycaemic control and they also received two mailings of nutrition information. Participants in the intervention arm (the diet plus exercise group) were advised to adhere to a diet that had ˜55% of kcal from carbohydrate, 20% from protein and less than 30% from fat. The exercise component consisted of a five minute warm up, 20 minutes of low‐impact aerobic activity and 5 minutes of cool‐down exercises and was carried out three times a week. Participants were also encouraged to exercise on their own twice more per week. At the end of the trial period, (i.e. six months later) participants in the usual care group had gained a mean weight of 2.0kg, (from 94.9 ± 20.1kg at baseline to 96.9 ± 21.6kg at the six month follow‐up) compared to the participants in the diet plus exercise group who lost a mean weight of 2.6kg (from a baseline measurement of 93.3 ± 18.6kg to the six month follow‐up value of 90.7 ± 20.1kg). Concentration of glycated haemoglobin in the usual care group rose over the six month period from a mean of 10.0 ± 1.9% to 11.5 ± 4.4%, compared to the diet plus exercise group whose mean glycated haemoglobin concentration decreased from 11.0 ± 1.7% to 9.9 ± 2.0% over the six month period.
In the study conducted by Uusitupa and colleagues, (Uusitupa 1996), 86 participants were randomised to either a standard dietary treatment group or a diet and exercise group. Standard dietary treatment consisted of reduced energy intake with emphasis on reducing intake of total fat and cholesterol. In the diet only group the mean weight changed from 92.2 ± 14.7kg at baseline to a measurement of 90.2 ± 14.3kg at the follow‐up appointment at twelve months (which equates to a loss of 2.0 ± 11.4kg), compared to a weight change of 91.6 ± 14.5kg at baseline to a measurement of 86.5 ± 13.7kg at follow‐up at twelve months (a loss of 5.1 ± 11.1kg) in the diet plus exercise group. Over the twelve months between baseline and follow‐up, blood concentration of glycated haemoglobin decreased by 0.3 ± 1.5% in the diet only group (from 7.8 ± 2.0% at baseline to 7.5 ± 1.7% at twelve months) and by 0.5 ± 1.3% in the diet plus exercise group, (from 7.1 ± 1.8% at baseline to 6.6 ± 1.6% at twelve months).
Ligtenberg describes a study that randomised 58 participants to either continue with their usual diet or to receive physical training instructions for six weeks and then to continue training (with encouragement) at home for a following six weeks with a further fourteen weeks non‐encouraged exercise at home, (Ligtenberg 1997). Weight was measured for both groups at the start and the endpoint of the trial, although no data were reported in the article, but the authors did state 'body weight did not change in either group during the whole study period'. Glycated haemoglobin was measured and reported at baseline and follow‐up. In the diet only group, glycated haemoglobin rose by 0.2 ± 1.2% from baseline to follow‐up at six months (from 8.8 ± 1.5% to 9.0 ± 1.6%) compared to a decrease of 0.2 ± 0.8% from baseline to follow‐up for participants in the diet plus exercise group at six months (from 8.9 ± 1.0% to 8.7 ± 1.1%).
Samaras randomly assigned 26 non‐exercising participants to receive either their usual diet or to receive their usual diet plus a structured exercise programme that ran for one hour, once a month for six months, (Samaras 1997). This was a moderately paced aerobic exercise session run by an exercise physiologist. This paper reported baseline and change data. Baseline measurement of weight for the diet only group was 98.2 ± 12.3kg which rose by 1.0 ± 2.9kg after six months and then rose (compared to the baseline measurement) at the twelve month follow‐up by 0.8 ± 3.9kg compared to the diet plus exercise group where the baseline measurement was 83.0 ± 13.0kg, which decreased by 0.1 ± 2.7kg at the six month follow‐up and then rose (compared to the baseline measurement) by 0.1 ± 3.9kg at the twelve month follow‐up. Glycated haemoglobin concentrations in both groups of participants rose over the six month period of the trial, by 0.6 ± 0.9% in the control group (from 6.8 ± 2.2% at baseline) and by 0.1 ± 1.1% in the diet and exercise group (from 5.6 ± 1.1% at baseline). Measures of glycated haemoglobin also rose from baseline to the twelve month follow‐up in both control and intervention groups, rising by 0.9 ± 1.0% in the diet only group (from 6.8 ± 2.2% at baseline) and by 0.9 ± 1.0% in the diet and exercise group (from 5.6 ± 1.1% at baseline).
In Kaplan's trial, (Kaplan 1986), 76 participants were randomly allocated to either ten weekly sessions of diet education, which focused on promoting a low‐fat (30% of total energy), high carbohydrate (55% of total energy) or to ten weekly sessions of diet and exercise education. Five of these weekly sessions were spent on diet education (as for the control group) and the following five were spent on supervised exercise in an adult fitness programme that included stretching and walking/jogging. Some data on weight change were measured and reported in the text of the article; the mean weight loss over six months for the diet only group was 3.5kg compared to a loss of 0.2kg for the diet plus exercise group from baseline to six months. (Although mean weight change was reported, the associated standard deviations/errors were not.) Glycated haemoglobin was measured but not reported except to say that there was 'no significant difference between the groups at baseline and six month follow up'.
Wing conducted a trial that had 30 participants randomised to either a diet only group or a diet plus exercise group, (Wing 1988). The diet only group had an individualised daily calorie goal and participants were taught to try and increase their complex carbohydrate intake and to decrease their intake of fat. Participants in the diet plus exercise group had the same dietary advice imparted but also walked for three miles a day three times a week and were instructed to exercise (on their own) for another session per week. At the twelve month follow‐up, participants in the diet only group had lost a mean weight of 3.8 ± 15.4kg (from 102 ± 19.4kg to 98.2 ± 20.0kg) compared to a mean weight loss of 7.9 ± 18.3kg in the diet and exercise group at twelve months (from 104.1 ± 23.2kg to 96.2 ± 23.4kg). Glycated haemoglobin decreased in a similar fashion, in the diet only group, the concentration decreased by 0.8 ± 1.4% from baseline to follow‐up at twelve months, (from 10.9 ± 1.9% to 10.1 ± 1.6%), compared to a decrease of 1.4 ± 1.5% in the diet plus exercise group (from 10.6 ± 1.9% to 9.2 ± 1.8%) over the same time period.
In these six studies, (Kaplan 1986; Wing 1988; Uusitupa 1996; Agurs‐Collins 1997; Ligtenberg 1997; Samaras 1997), dietary advice was compared with dietary advice plus exercise. The trials recruited participants with similar characteristics (age and sex) and the quality of the trials were assessed to be at high risk of bias. Weight change was assessed in all trials, although not reported in two trials, (Ligtenberg 1997 and Kaplan 1986), and more weight was lost on average in the diet and exercise groups. The development of micro and macrovascular diabetic complications were not reported. Glycated haemoglobin was reported in most trials and decreased more in the participants in the dietary advice and exercise groups than those in the dietary advice only groups.
The six studies were clinically similar enough to permit pooling, (although the participants in the Samaras intervention group (Samaras 1997) were generally less heavy than the participants in the other trials in this category) and as there were three or more studies (with data that could be entered into the tables) in all but one category, (weight at six months), it was statistically possible to carry out an analysis on the heterogeneity of the data. For weight at twelve months the fixed effect model test for heterogeneity was significant. The test for heterogeneity was not significant for change in glycated haemoglobin at six and twelve months. At six months, dietary advice plus exercise was associated with a statistically significant mean (pooled weighted mean difference) decrease in glycated haemoglobin of 0.9% (with 95% confidence intervals of 0.4 to 1.3), and at twelve months, dietary advice plus exercise was associated with a statistically significant mean (pooled weighted mean difference) decrease in glycated haemoglobin of 1.0% (with 95% confidence intervals of 0.4 to 1.5). Three hundred and forty participants took part in these trials and overall the trial quality was assessed to be at high risk of bias. The evidence suggests that dietary advice plus exercise has the potential to have an impact on weight and glycaemic control, although the high potential for bias should be considered when interpreting the evidence.
Comparison 04: Dietary Advice versus Dietary Advice plus Behavioural Approaches
Three studies assessed dietary advice versus dietary advice plus behavioural approaches.
Glasgow conducted a trial that evaluated the efficacy of a personalised medical office‐based intervention, (Glasgow 1997). Two hundred and six participants in this study were randomised to either receive usual care or usual care plus the brief intervention added in. All participants went half an hour early to their physician appointment and completed the baseline assessment, which included a touch screen assessment. Usual care participants received a high quality quarterly medical care intervention including regular assessment and follow‐up of micro and macrovascular risk factors in addition to the touch screen computer assessment, although there was no focus on behavioural or psychosocial issues. The participants randomised to the 'brief' intervention group completed another five to ten minute touch screen dietary barriers assessment which immediately generated two printed feedback forms (one for the participant and one for the physician). No results were available in the articles, and attempts at personal communication proved to be unsuccessful.
Glasgow randomly assigned 160 participants to receive either the basic intervention or the community resources intervention, (Glasgow 2000). Participants assigned to the basic intervention group received information about low‐fat eating. Participants assigned to the community resources group received the same information about low‐fat eating as the basic group but in addition they had access to 'community resources' which was comprised of three‐ring binder of indexed community resources, four newsletters identifying opportunities for participants to obtain support, a food frequency was mailed and tailored feedback was sent with advice to decrease intake of dietary fat. The participants in the community resources group decreased their mean weight from 96.2 ± 22.2kg at baseline to a follow‐up value of 95.3 ± 20.9kg at six months, compared to the participants in the basic care group who decreased their mean weight from 90.3 ± 16.3kg at baseline to a follow‐up value of 89.4 ± 16.8kg at six months. The concentration of glycated haemoglobin remained stable in the community resources group (a value of 7.3 ± 1.5% at baseline to a value of 7.3 ± 1.4% at the six month follow‐up) compared with the basic intervention group who decreased their mean glycated haemoglobin from 7.6 ± 1.2% at baseline to 7.4 ± 1.2% at the six month follow‐up.
Keyserling describes a trial that had 133 participants randomised to receive a clinic‐based intervention or a clinic‐based intervention plus community intervention, (Keyserling 2000). All participants were given a single loose‐leaf notebook from which assessment and monitoring pages could be removed and filed. The clinic‐based intervention consisted of four individual visits to a counsellor (sessions lasting for 195 minutes in total) where counsellors would negotiate with the participant on the selection of two or three goals from each assessment area within the provided notebook. The additional community intervention included phone calls to participants and also three group sessions (two sessions between zero and six months and one between six and twelve months of the trial duration) which specifically addressed issues relating to cultural translation. Measurements were made at baseline, six and twelve months. In the clinic‐based arm of the study, mean weight decreased from a baseline of 92.5 ± 22.1kg to 91.6 ± 21.7kg at the six month follow‐up (a weight loss of by 0.9kg). Weight then increased by 1.9kg (92.5 ± 22.1kg at baseline to 94.4 ± 23.2kg) at the twelve month follow‐up. In the clinic and community resources group, the mean weight remained the same from baseline measurement (93.9 ± 19.3kg) to six months (96.2 ± 19.3kg) and then increased by 2.3kg from baseline (93.9 ± 19.3kg) to the twelve month follow‐up measurement (96.2 ± 19.3kg). In the clinic‐based group, concentration of glycated haemoglobin increased from a baseline measurement of 11.0 ± 3.2% to the six month follow‐up value of 11.1 ± 3.1%; from baseline measurement to the twelve month follow‐up, there was a decrease from 11.0 ± 3.2% (baseline) to 10.9 ± 3.8% (twelve month follow‐up). In comparison, concentration of glycated haemoglobin in participants in the clinic‐based and community resources arm of the study remained stable at the six month follow‐up (baseline measurement of 10.7 ± 2.3% compared to a six‐month measurement of 10.7 ± 3.1%). At the twelve month follow‐up, the glycated haemoglobin had risen from 10.7 ± 2.3% to 10.8 ± 2.9%.
In the three studies, (Glasgow 1997; Glasgow 2000; Keyserling 2000), dietary advice was compared with dietary advice plus a behavioural approach. The trials recruited participants with similar characteristics (age and sex) and the quality of the trials were assessed to be at high risk of bias. Weight change was assessed in all trials, although not reported at all in one of the trials, (Glasgow 1997), and although more weight was lost on average in the usual care (dietary advice only) groups, the amount of weight lost was not substantial. The development of micro and macrovascular diabetic complications was not reported in any of the studies. Glycated haemoglobin was reported in all but one trial, (Glasgow 1997), and generally more improvement in glycaemic control was seen in the usual care groups, although once more the changes were not significant.
Although the three studies were clinically similar enough to permit pooling; data were only available for two of these studies, therefore it was not possible for an analysis for heterogeneity to be carried out. There were a total of 499 participants in these three trials and overall the trial quality was assessed to be at high risk of bias. Firm conclusions could not be drawn from this comparison.
Discussion
Summary of main results
This systematic review assessed eighteen randomised controlled trials of dietary advice studying a total of 1467 participants with type 2 diabetes mellitus. Only a minority of the trials examined hard clinical endpoints (such as death or development of macrovascular or microvascular diabetic complications), and those that did offered no details; most articles concerned themselves with the reporting and discussion of the participants' weight and blood glucose control.
Meta‐analyses could not be carried out for the data within the dietary advice versus another (different) form of dietary advice, the very‐low‐calorie dietary advice versus low‐calorie dietary advice category and the dietary advice versus dietary advice plus behavioural approaches categories, as there were not sufficient data to allow this.
Within the dietary advice versus dietary advice plus exercise category, there were small, yet significant changes seen in mean glycated haemoglobin at six months and twelve months in the four (Uusitupa 1996; Agurs‐Collins 1997; Ligtenberg 1997; Samaras 1997) and three studies (Wing 1988; Uusitupa 1996; Samaras 1997) that contributed data to these analyses. At six months, dietary advice plus exercise was associated with a statistically significant mean (pooled weighted mean difference) decrease in glycated haemoglobin of 0.9% (with 95% confidence intervals of 0.4 to 1.3), and at twelve months, dietary advice plus exercise was associated with a statistically significant mean (pooled weighted mean difference) decrease in glycated haemoglobin of 1.0% (with 95% confidence intervals of 0.4 to 1.5).
A recent systematic review published in the Cochrane Library by Pirozzo, (Pirozzo 2002), suggested that there was no real difference between a low‐fat diet and other weight reducing diets (when looking at long term weight loss) in overweight or obese people. The reviewers found that generally there were small, non‐significant differences in weight loss between the participants in the control and intervention groups, however this difference was so small it was clinically insignificant. In this review, there were insufficient data to permit a meta‐analysis, so conclusions on the effects of low‐fat or other weight reducing diets were limited. However, clinically meaningful differences in glucose profile were not achieved. Recently, some small‐scale studies discussed the importance of dietary composition and low‐carbohydrate diets in the management of type 2 diabetes mellitus (Boden 2005; Nielsen 2005). Low carbohydrate diets appeared to have a significant effect on decreasing HbA1c and weight reduction. However, more research is required on larger populations and with a strict control group.
This update review yielded no further studies which fit the criteria for inclusion and exclusion, and thus, no further trials have been added to the meta‐analyses. However, one study by Ash (Ash 2003), investigated the effectiveness of three isocaloric dietary intervention groups (intermittent energy restriction, pre‐portioned meals, and self‐selected meals) for a duration of 12 weeks, with a follow‐up after 18 months. Despite this study being a randomised control trial, of male adults with type 2 diabetes, it was excluded from any further analyses due to a dubious control arm to the study. However, the study concluded that a moderate energy restriction of 1400‐1700kcal/day was effective in achieving a 6% weight loss and an improvement in glycaemic control. The method of implementation was found to be insignificant, whilst the weekly contact with health care professionals was suggested as the facilitator of the successful outcomes. This review is relevant to physicians treating patients with type 2 diabetes, the results suggest that the addition of exercise alongside a reduced energy diet is the best way to promote better glycaemic control in type 2 diabetic patients, with this achieving consistent success in the trials reported in this review. We found no significant results in relation to weight. In terms of the relevance of this review to future researchers, it is important to use these findings as a basis from which to generate hypotheses. As this review is high quality, we believe it can form a firm foundation for research project proposals and identifying gaps in areas of further research.
Limitations of the review
Diabetes mellitus is a major and growing health problem, and it has been predicted that the number of people with diabetes will double over the next 10 years (WHO/FAO 2003). The adoption of a more affluent and westernised lifestyle (i.e. a lifestyle where energy consumed is not matched or exceeded by energy expended) by some non‐Western populations is also contributing to an increase in the diabetic population (Roman 1997). For all diabetic patients, achieving good glycaemic control is central to their well‐being. Eighty to ninety percent of type 2 diabetic patients are overweight. With a decrease in weight, an improvement in glycaemic control is often observed.
There were not enough data in the studies that assessed one dietary advice versus another (different) type of dietary advice to enable us to reach any satisfactory conclusions. The data included in the trials in this review which assessed dietary advice plus behavioural approaches did not have the data to allow us to reach any satisfactory substantial conclusions. The studies which examined dietary advice versus dietary advice plus physical activity do suggest benefit from adoption of increasing physical activity levels alongside a reduced energy diet. We found no randomised controlled trials that examined dietary advice versus dietary advice plus alternative therapies.
Despite the frequency and severity of this condition, there are comparatively few trials and participants which have studied the impact of dietary advice and interventions. This may be partly due to type 2 diabetes being diagnosed relatively late by which time islet cell decompensation is reasonably advanced. As a consequence, even impressive degrees of weight loss can result in a rise rather than a fall in glycated haemoglobin. This is not an indication that the dietary intervention has failed but that the patient requires an oral anti‐diabetic agent.
There is a need for far more research into effects of dietary change (with and without the addition of physical activity) on micro and macrovascular diabetic complications, weight and glycaemic control. Many of the outcomes we initially wished to look at in this review were not investigated in the included studies. Work carried out in the future should take care to record and publish mortality, change (or delay) in onset of anti‐diabetic medication and also quality of life as these are outcomes of importance to people with type 2 diabetes. It would be desirable if measures of compliance would also be recorded and reported in published works.
The new long term study (known as 'look AHEAD' (Action for Health in Diabetes, (Kelley 2002)) sponsored by the National Institutes of Health, Bethesda, Maryland, USA, began in 2002 and aims to look at the long‐term health effects of weight loss in men and women who are overweight and have type 2 diabetes who are 45 to 75 years of age. It is envisaged that participants will be assessed up to eleven and a half years after enrolling in the programme, which will reveal the long‐term health effects of the trial.
Long‐term, high‐quality research in this area is necessary as the increase of type 2 diabetes is currently mirroring the recent rise of obesity that has been concerning health care professionals for the last ten years. Diabetes has now reached epidemic proportions and further immediate work is required in an attempt to halt it.
Authors' conclusions
Implications for practice.
Using exercise as an adjunct to dietary advice, compared with dietary advice alone, appears to improve glycated haemoglobin at six and twelve months in people with type 2 diabetes. There were small, yet significant changes in glycated haemoglobin in the four (Uusitupa 1996; Agurs‐Collins 1997; Ligtenberg 1997; Samaras 1997) and three (Wing 1988; Uusitupa 1996; Samaras 1997) studies that contributed data to these analyses. Dietary advice plus exercise was associated with a statistically significant mean (pooled weighted mean difference) decrease in glycated haemoglobin of 0.9% (with 95% confidence intervals of 0.4 to 1.3) at six months and of 1.0% (with 95% confidence intervals of 0.4 to 1.5) at twelve months (it should be noted that there is insufficient evidence to suggest what the effects of the dietary advice would be on weight or diabetic micro‐ and macrovascular diseases).
Implications for research.
The current available evidence assembled in this review points towards the benefit of increasing exercise in people with type 2 diabetes. Further high quality research to examine the addition of exercise alongside a reduced energy diet would be of great importance to either corroborate or contradict these findings. High quality research is needed to identify what types (low‐fat/high‐carbohydrate diet, modified‐fat diet, restricted protein diet etc.), frequency and style (addition of behaviour modification or not) of dietary advice are most efficacious in the long‐term for use by people with type 2 diabetes.
This review has also highlighted the lack of outcome studies of effects of dietary change on micro‐ and macrovascular diabetic complications. Many of the outcomes we wished to look at were not investigated in the included studies and therefore we recommend that any research carried out in the future should take care to note and report mortality, change (or delay) in onset of anti‐diabetic medication and also quality of life as these are outcomes of importance to people with type 2 diabetes. Measures of compliance should also be recorded and reported in published works.
Conclusions
There are no high quality data on the efficacy of the dietary treatment of type 2 diabetes programmes, however the data we do have indicate that the adoption of exercise appears to improve glycated haemoglobin at twelve months in people with type 2 diabetes, although the data presented are at high risk of bias. There is a need for well‐designed studies which examine a range of interventions, although there is a promising study currently underway.
What's new
| Date | Event | Description |
|---|---|---|
| 29 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 23 May 2007 | New search has been performed | No new studies have been added. The bulk of the background remains the same but some new references have been included and some sections reworded. The main thrust of the review remains the same. No changes have been made to the methodology. The search strategies used were the same, and the dates over which the databases were searched were simply updated. The results have not changed since the first publication as there were no further studies added. The discussion has been amended slightly, with the discussion of a few more recent studies added (e.g. Ash 2003). The conclusions remain the same ‐ more high quality evidence for the treatment of type 2 diabetes mellitus using dietary advice are required before further conclusions can be made. |
Notes
Currencies converted on 29th October 2003 Exchange rate: 1 US dollar = 0.855505 EUR ( $1 = €0.855505) www.x‐rates.com used to convert
lbs to kgs converted using http://annica.in‐cyberspace.net/en/lbs_kg.html
Acknowledgements
Dr Hockaday, Oxford, UK. Dr Wolever, Toronto, Canada. Dr Samaras, Sydney, Australia.
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. TYPE 2 DIABETES MELLITUS 1. See Cochrane Metabolic and Endocrine Disorders Group search strategy. DIET INTERVENTIONS 2. explode Diet Therapy/ [MeSH, all subheadings] 3. (diet$ adj5 diabet$).ab,ti. 4. (diet$ adj5 carbohydrat$).ab,ti. 5. (diet$ adj5 fat$).ab,ti. 6. (diet$ adj5 weigh$).ab,ti. 7. (diet$ adj5 sugar$).ab,ti. 8. (diet$ adj5 glyc?em$).ab,ti. 9. 2 or 3 or 4 or 5 or 6 or 7 or 8 EXERCISE INTERVENTIONS 10. explode Exercise Therapy/ [MeSH, all subheadings] 11. (walk$ or jog$ or swim$).ab,ti. 12. (exerci$ or (physic$ and activ$) or exert$ or (physic$ and fit$) or sports).ab,ti. 13. ((weight and lift$) or (strength and train$) or (resistance and train$) or (circuit and weight and train$) or (aerob$ and train$)).ab,ti. 14. Exercise/ [MeSH, all subheadings] 15. explode Exertion/ [MeSH, all subheadings] 16. explode "Physical Education and Training"/ [MeSH, all subheadings] 17. Physical Fitness/ [MeSH, all subheadings] 18. explode Sports/ [MeSH, all subheadings] 19. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 BEHAVIOURAL APPROACHES 20. explode Behavio?r Therapy/ [MeSH, all subheadings] 21. Cognitive Therapy/ [MeSH, all subheadings] 22. explode Hypnosis/ [MeSH, all subheadings] 23. explode Psychotherapy/ [MeSH, all subheadings] 24. behavio?r therap$.ab,ti. 25. cognitive therap$.ab,ti. 26. hypno$.ab,ti. 27. psychotherap$.ab,ti. 28. 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 RANDOMISED CONTROLLED TRIALS 29. See randomised controlled trials search strategy ‐ based on the Cochrane Pregnancy and Childbirth Review Group strategy. SYSTEMATIC REVIEWS/META‐ANALYSIS 30. See systematic review and meta‐analysis search strategy ‐ based on the Metabolism and Endocrine Disorders Review Group strategy. ALL INTERVENTIONS 31. 9 or 19 or 28 TYPE 2 DIABETES AND ALL INTERVENTIONS 32. 1 and 31 TYPE 2 DIABETES AND ALL INTERVENTIONS AND RANDOMISED CONTROLLED TRIAL 32. 32 and 29 TYPE 2 DIABETES AND ALL INTERVENTIONS AND SYSTEMATIC REVIEW/META‐ANALYSES 33. 32 and 30 |
Data and analyses
Comparison 3. Dietary Advice versus Dietary Advice plus Exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight at 12 months | 3 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐6.74 [‐11.72, ‐1.76] |
| 2 HbA1c at 6 months | 4 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.33, ‐0.38] |
| 3 HbA1c at 12 months | 3 | 132 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.53, ‐0.39] |
3.1. Analysis.
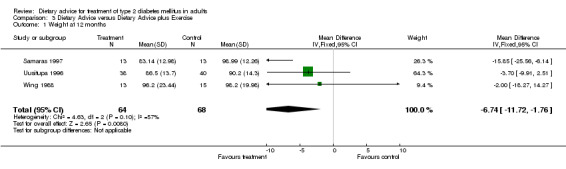
Comparison 3 Dietary Advice versus Dietary Advice plus Exercise, Outcome 1 Weight at 12 months.
3.2. Analysis.
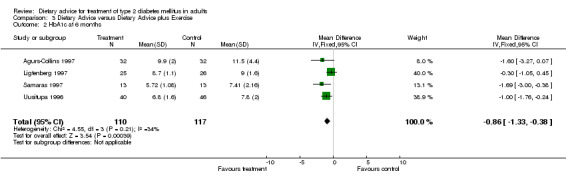
Comparison 3 Dietary Advice versus Dietary Advice plus Exercise, Outcome 2 HbA1c at 6 months.
3.3. Analysis.
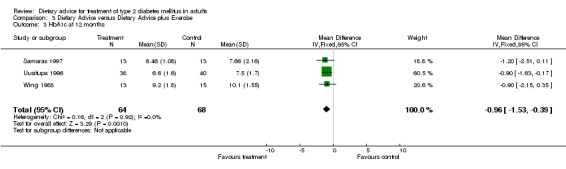
Comparison 3 Dietary Advice versus Dietary Advice plus Exercise, Outcome 3 HbA1c at 12 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agurs‐Collins 1997.
| Methods | Trial design:
RCT Randomisation procedure: "Assigned randomly (1:1 ratio within the medication strata). Randomisation was supervised by the study statistician." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Urban Hospital Number: 64 participants. (32 in IG, 32 in CG) Age: CG: 61.0 ± 5.7 years IG: 62.4 ± 5.9 years. Sex: CG: 12% male; 88% female IG: 34% male; 66% female Diagnostic Details: "Diagnosis of NIDDM by medical history" Inclusion Criteria: Should be 120% or greater than Metropolitan Weight Standards. Have HbA1c greater than 8% No medical contraindications Other characteristics: 1. Smoking CG: 6% smoke IG: 18.8% smoke |
|
| Interventions | Trial intervention(s):
Intervention was promotion of adherence to diet. A weight loss of (around 4.5kgs) 10lbs was the aim. During the first three months, participants attended a single individual session (diet counselling) and twelve weekly group sessions comprising 60 minutes nutrition education plus 30 minutes exercising. For the following three months, participants attended six bi‐weekly group sessions. Comparison intervention(s): Comparison was a "usual care" control. The participants in this group attended one class on methods of glycaemic control. They also received two nutrition information mailings. |
|
| Outcomes | Participants in the usual care group went from a baseline mean weight of 94.9 ± 20.1kg to 96.9 ± 21.6kg at the six month follow‐up
Participants in the diet plus exercise group saw their mean weight change from a baseline measurement of 93.3 ± 18.6kg to the six month follow‐up value of 90.7 ± 20.1kg. The mean concentration of glycated haemoglobin in the usual care group started at a baseline mean of 10.0 ± 1.9% and ended at a value of 11.5 ± 4.4% at the six month follow‐up. In the diet plus exercise group, the mean glycated haemoglobin concentration decreased from a baseline of 11.0 ± 1.7% to 9.9 ± 2% over the six month period. 1. Weight 2. BMI 3. HbA1c 4. Lipids 5. Blood Pressure were all measured at six months. |
|
| Notes | Source of funding of trial: Dissertation research grant AG10361 from the National Institute on Ageing of the NIH. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Campbell 1990.
| Methods | Trial design:
RCT Randomisation procedure: "subjects were allocated at random in groups of 8‐10" Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: Australia Setting: Diabetes Centre Number: 70 participants. (32 in CG, 38 in IG). Follow‐up on 62 participants. Age: CG: 59 ± 9 years IG: 58 ± 9 years Sex: CG: 59% males and 41% females IG: 55% males and 45% females. Inclusion criteria: 1. Age of diabetes onset greater than 30 years old 2. Duration of diabetes more than three months 3. Duration of current treatment more than a year 4. No attendance at an education program in the previous six months 5. English speaking No diagnostic details explicitly mentioned; just "NIDDM patients". |
|
| Interventions | Trial intervention(s):
An intensive programme which was based on the cognitive motivational theory by Heckhausen and Kuhl. Participants received simplified dietary instructions ‐ they were told to decrease all fats and to increase consumption of all legumes. Caloric restriction was not discussed. Dosage was a total of 22 hours delivered over 11 weeks. Comparison intervention(s): A conventional program of diabetes education delivered over three days. This was comprised of simple explanations of food composition that emphasised a calorie‐restricted‐carbohydrate portion exchange diet with reduced fat intake and increased complex carbohydrate and fibre intake. |
|
| Outcomes | In the intervention group, BMI decreased from 30.4 ± 4.8kg/m2 at baseline to 29.6 ± 4.6kg/m2 at six months
In the control group, BMI decreased from 32.0 ± 5.5kg/m2 at baseline to 31.1 ± 5.1kg/m2 at six months. 1. BMI 2. Lipids 3. Fasting Blood Glucose were all measured at six months. |
|
| Notes | Source of funding of trial: Health Services Research and Development Project Grant from the Australian Commonwealth Department of Health. Eight participants dropped out, but no indication was given as to which group they were in. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
de Bont 1981.
| Methods | Trial design:
RCT Randomisation procedure: "They were randomly allocated to receive...." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Yes Quality Assessment Category: B (three points awarded) ‐ moderate risk of bias. |
|
| Participants | Country: UK Setting: Outpatient Clinic Number: 148 randomised. (data presented for 136 participants: CG: 65 and IG: 71) Age: CG: 54 ± 8 years IG: 56 ± 7 years Sex: Both groups: 100% female Inclusion criteria: None mentioned. Exclusion criteria: None mentioned. Diagnostic criteria: None mentioned. Other characteristics: CG: 37% smoke IG: 44% smoke |
|
| Interventions | Trial intervention(s):
Advice was given which attempted to reduce the contribution of fat towards 30% of the prescribed energy intake (low fat diet). Comparison intervention(s): Usual care (low carbohydrate diet) ‐ had their current diets reviewed and advice was given to encourage a carbohydrate intake not exceeding 40% of the prescribed energy intake. Dose: Participants in both groups had one meeting with a dietitian, followed by three home visits from a nutritionist (to encourage continued adaptation of diet towards dietary targets). |
|
| Outcomes | Weight was reported for obese and non‐obese participants separately; glycated haemoglobin was reported for just the control and intervention groups. Although mean weight change was reported, the associated standard deviations/errors were not. In obese participants the mean weight of participants in the low fat diet group went from 84.2kg at baseline to 81.5kg at six months, and for the participants in the low carbohydrate group the mean weight changed from a baseline weight of 84.8kg to 83.9kg at six months. In the non‐obese participants in the low fat diet group saw their mean weight decrease from 60.1kg to 59.7kg, compared to the group following the low carbohydrate diet who began the trial with a mean weight of 59.0kg which by the end of six months had risen to 59.1kg. In the low fat diet group, the participants had a baseline glycated haemoglobin of 10% which decreased to 9.3% at six months. The low carbohydrate group had a baseline glycated haemoglobin measurement of 10.1% which decreased to 9.5% at six months. 1. Weight 2. HbA1c 3. Lipids 4. Fasting Blood Glucose were all measured at six months. |
|
| Notes | Source of funding of trial:
None mentioned. Eleven participants dropped out. No mention of which group they had been assigned to. Reasons for dropping out were: a) Six participants had been withdrawn by their physicians. b) Four participants withdrew themselves. c) One patient died. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gallagher 1987.
| Methods | Trial design:
RCT Randomisation procedure: "... were randomly assigned..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Outpatient Clinic Number: 51 participants. 23 randomised to the CG and 28 randomised to the IG. Age: CG: 44.5 ± 12.7 years IG: 47.8 ±16.2 years Sex: 100% male in both groups. Diagnostic criteria: "... due to such factors as adult age at onset, absence of ketoacidotic episodes, history of treatment by oral agents or by diet alone prior to insulin treatment..." Inclusion criteria: 1. Male 2. No more than 5% over ideal body weight in the past five years 3. Without a history of excess weight over 15% of ideal body weight 4. No complicating diagnoses affecting glucose or lipid levels |
|
| Interventions | Trial intervention(s):
Unmeasured diet ‐ based on the four basic food groups with advice on consistency of caloric intake, avoidance of refined sugars and limitation of saturated fats and cholesterol. Participants were seen by medical residents as necessary but at least every six months. Comparison intervention(s): ADA exchange diet ‐ dietary composition advised was 40% carbohydrate, 40% fat and 20% protein. Simple sugars were restricted. Daily calorie levels were calculated considering the participants ideal body weight and level of activity. Participants were seen by medical residents as necessary but at least every three months. |
|
| Outcomes | Weight and glycated haemoglobin was measured but not reported in the published articles.
Attempts at personal communication with the authors proved to be unsuccessful. 1. Weight 2. Fasting Blood Glucose were all measured at four years. |
|
| Notes | Source of funding of trial:
None mentioned. There was one type 1 diabetic in each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Glasgow 1997.
| Methods | Trial design:
RCT Randomisation procedure: Used a table of random numbers. Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Not mentioned, probably not Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Outpatient Clinic Number: 206 participants. (98 in CG and 108 in IG) Age: CG: 63.1 ± 10.5 years IG: 61.7 ± 12.1 years Sex: CG: 40% males, 60% females IG: 37% males, 63% females. Diagnostic criteria: None mentioned Inclusion criteria: 1. Having type 1 or type 2 diabetes 2. Must be aged 40 or older |
|
| Interventions | Trial intervention(s):
A brief intervention in addition to their usual care ‐ participants completed a five to ten minutes touch screen dietary barrier assessment, which generated two copies of a feedback form (one for the patient and one for the physician). Also had twenty minutes patient centered goal setting with interventionist. Comparison intervention(s): Usual care involved receiving a high quality quarterly medical care intervention. |
|
| Outcomes | No results were available in the articles, and attempts at personal communication proved to be unsuccessful. 1. BMI 2. HbA1c were all measured at 12 months |
|
| Notes | Source of funding of trial: Grant #3DK‐R01‐35524 from the National Institutes of Diabetes, Digestive, and Kidney Diseases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Glasgow 2000.
| Methods | Trial design:
2 x 2 randomised factorial design Randomisation procedure: "...Participants were randomly assigned to one of four conditions..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: Unclear Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Centre for Healthy Living Number: 320 participants randomised across the four arms. 80 in the CG (67 present at 6 month follow‐up) and 80 in the IG (75 present at 6 month follow‐up) Age: CG: 60.6 ± 9.5 years IG: 60.5 ± 8.6 years Sex: CG: 33.7% male, 66.3% female. IG: 52.6% male, 47.4% female. Diagnostic criteria: Welborn criteria for type 2 diabetes Inclusion criteria: 1. Type 2 diabetes 2. Having a telephone 3. Not planning to move out of the area of a year Exclusion criteria: 1. Type 1 diabetes 2. Planning to relocate out of area within one year |
|
| Interventions | Trial intervention(s): Community Resources Intervention. Comprised of a general pamphlet about low‐fat eating and access to community resources which were a three‐ring binder full of indexed community resources, four newsletters identifying opportunities for participants to obtain support. A food frequency questionnaire was mailed and tailored feedback was sent with advice to decrease dietary fat intake. Comparison intervention(s): A general pamphlet about low fat eating was handed out to the control group participants. |
|
| Outcomes | Participants in the community resources group decreased their mean weight from 96.2 ± 22.2kg at baseline to a follow‐up value of 95.3 ± 20.9kg at six months.
Participants in the basic care group decreased their mean weight from 90.3 ± 16.3kg at baseline to a follow‐up value of 89.4 ± 16.8kg at six months. In the community resources group concentration of glycated haemoglobin remained stable (7.3 ± 1.5% at baseline to 7.3 ± 1.4% at the six month follow‐up). In the basic care group, the concentration of glycated haemoglobin decreased from 7.6 ± 1.2% at baseline to 7.4 ± 1.2% at the six month follow‐up. 1. HbA1c 2. Weight 3. Dietary patterns 4. Lipids 5. Fat intake 6. Patient satisfaction measures 7. Quality of life were all measured at 6 months. |
|
| Notes | Source of funding of trial: Supported by grant RO1‐DK 35524‐13 from the National Institutes of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hockaday 1986.
| Methods | Trial design:
RCT Randomisation procedure: Using random number sequence Allocation concealment: Adequate Blinding: Recipients: No Providers of Care: (a) Dietitians ‐ No; (b) Doctors ‐ Unclear. Outcome Assessors: Unclear Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: UK Setting: Eye hospital Number: At baseline: 250 participants were randomised at the start of the trial to either the low or high carbohydrate diet. At 1 year: 93 followed up (54 in the low‐carbohydrate diet group, 39 in the high‐carbohydrate/modified‐fat diet group) Age: At baseline: No mention is given. At 1 year: Mean age of participants in the CG was 53 years (range was 22 to 65) and the mean age of the participants in the IG was 50 years (range was 24 to 65) Sex: At baseline: no mention of proportions of gender. At 1 year: 51% male, 49% female. Diagnostic criteria: "Attendance at a diabetes mellitus clinic" Inclusion criteria: 1. Between the recruitment period of 1973 and 1976, the participants had to be younger than 66 years old. 2. Participants must have untreated diabetes. Exclusion criteria: 1. Imminent life‐threatening condition. 2. Presence or history of endocrine disease, angina pectoris or neurological deficit. 3. Presence or past history of liver disease. |
|
| Interventions | Trial intervention(s):
High‐carbohydrate/modified‐fat diet. Originally designed to be a lipid lowering diet, therefore participants were advised that fats should provide no more than 30% of total energy (with a polyunsaturated fatty acids:saturated fatty acids ratio of ˜0.9). 20% of energy should come from protein and carbohydrate should provide 50% of total energy. Cholesterol was restricted to a maximum of 2.1grams per week. The advice to participants was given once only at the time of diagnosis. Comparison intervention(s): Standard low‐carbohydrate diet. The advice given to participants was to restrict carbohydrate up to a maximum of 40% of total energy. Protein should provide up to 20% and fat to provide about 40% (with a ratio of polyunsaturated fatty acids:saturated fatty acids of ˜0.3) The advice to participants was given once only at the time of diagnosis. |
|
| Outcomes | Although mean weight change was reported, the associated standard deviations/errors were not. Between baseline and one year, participants in the low carbohydrate group had a mean weight loss of 3.8kg compared to a mean weight loss of 4.6kg in the low fat diet. Changes in glycated haemoglobin were measured but not reported, although "changes in glycated haemoglobin were not significant". 1. Weight 2. BMI 3. Fasting plasma glucose were all measured at twelve months. |
|
| Notes | Source of funding of trial: financial support received from the British Diabetic Association and from the International Sugar Research Foundation Inc. NB: 1. It is important to note that different subsets of participants were reported on in the various papers that comprise this study. It has been very difficult to try and track these participants through. 2. The data which was reported was quite fragmented. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kaplan 1986.
| Methods | Trial design:
RCT Randomisation procedure: "...were randomly assigned..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Out‐patient clinic Number: 76 participants randomised to one of four groups (No indication of how many people assigned to each group was given) 70 participants were assessed at the 18 month follow‐up. Age: CG: 54.87 ± 12.32 years IG: 56.96 ± 8.95 years Sex: Overall, 42% male, 58% women. No indication of the male:female split in the individual groups. Diagnostic criteria: 1. Physicians had to complete a referral questionnaire providing confirmation of NIDDM status. Inclusion criteria: 1. Physician confirmed NIDDM. 2. Adult. Exclusion criteria: 1. Heart problems. |
|
| Interventions | Trial intervention(s):
Participants assigned to this group followed the exchange diet recommended by the American Dietetic Association (around 1200 kcalories per day) and an exercise prescription based on the results of the graded exercise test. Over the ten week programme, participants received five (two hour) sessions on diet and five (two hour) sessions on exercise. Comparison intervention(s): Participants assigned to this group followed the exchange diet recommended by the American Dietetic Association (around 1200 kcalories per day). Participants attended ten (two hour) weekly sessions. |
|
| Outcomes | The mean weight loss over six months for the diet only group was 3.5kg.
The mean weight loss over six months for the diet plus exercise group was 0.2kg. Although mean weight change was reported, the associated standard deviations/errors were not.) Glycated haemoglobin was measured but not reported except to say that there was "no significant difference between the groups at baseline and six month follow up". 1. HbA1c 2. Body Composition 3. Lipids were all measured at the six month follow‐up. |
|
| Notes | Source of funding of trial: Supported by grants R01 AM27901 and K04 908098 from National Institutes of Health. Financial Incentives: A deposit of $40 was requested, some of which was returned as a result of attendance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Keyserling 2000.
| Methods | Trial design:
RCT Randomisation procedure: "were randomised" "randomisation of individuals to treatment groups was stratified by practice site" Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Clinic and community based Number: 200 participants were randomised in total (IG = 67; CG = 66) At six month follow‐up, 60 participants were followed‐up in IG, 60 in CG. At 1 year follow‐up, 54 participants were followed‐up in IG, 59 in CG. Age: IG: 58.5 years CG: 59.8 years (Means only given ‐ no SD/SE given for this data) Sex: 100% female in all groups Diagnostic criteria: 1. Type 2 diabetes, defined as diagnosis of type 2 diabetes at age 20 or older with no history of ketoacidosis. Inclusion criteria: 1. African‐American women 2. Aged 40 years or older 3. Type 2 diabetes |
|
| Interventions | Trial intervention(s):
IG: A clinic and community based intervention designed to reduce total dietary fat. The clinic based intervention included individual counselling visits at month 1 (60 minutes), 2 (45 minutes), 3 (45 minutes) and 4 (45 minutes). All counselling and educational materials were included in a single loose‐leaf notebook from which assessment and monitoring pages could be removed for filing and subsequent follow‐up. Counsellors were encouraged to negotiate with the participant on the selection of two or three goals from each assessment area to accomplish before the next visit. The goal sheet was designed so it could be placed on the refrigerator where it would be seen frequently. The community intervention included phone calls to participants and group sessions. Alongside the four individual meetings IG participants had during the first six months, participants also had two group sessions of 90 minutes each and monthly telephone calls from the Community Diabetes Advisor. During the second six months, participants met for one group session of 90 minutes and maintaining monthly telephone contact with their Community Diabetes Advisor. Comparison intervention(s): CG: A clinic based intervention designed to reduce total dietary fat consisting of individual counselling visits at months 1 (60 minutes), 2 (45 minutes), 3 (45 minutes) and 4 (45 minutes). (These details are the same as for IG). |
|
| Outcomes | In the control (clinic) arm of the study, mean weight decreased from a baseline of 92.5 ± 22.1kg to 91.6 ± 21.7kg at the six month follow‐up (a weight loss of by 0.9kg). Weight then increased by 1.9kg (92.5 ± 22.1kg at baseline to 94.4 ± 23.2kg) at the twelve month follow‐up.
In the intervention (clinic and community resources) group, the mean weight remained the same from baseline measurement (93.9 ± 19.3kg) to six months (96.2 ± 19.3kg) and then increased by 2.3kg from baseline (93.9 ± 19.3kg) to the twelve month follow‐up measurement (96.2 ± 19.3kg). Concentration of glycated haemoglobin increased in the clinic‐based group, from a baseline measurement of 11.0 ± 3.2% to the six month follow‐up value of 11.1 ± 3.1%; and from baseline measurement to the twelve month follow‐up, there was a decrease from 11.0 ± 3.2% (baseline) to 10.9 ± 3.8% (twelve month follow‐up). Concentration of glycated haemoglobin in participants in the clinic‐based and community resources arm of the study remained stable at the six month follow‐up (baseline measurement of 10.7 ± 2.3% compared to a six‐month measurement of 10.7 ± 3.1%). At the twelve month follow‐up, the glycated haemoglobin had risen from 10.7 ± 2.3% to 10.8 ± 2.9%. 1. Weight 2. HbA1c 3. Physical activity 4. Lipids 5. Psychosocial assessment were all measured at six months and twelve months from baseline. |
|
| Notes | Source of funding of trial: Supported in part by a cooperative agreement (U48/CCU409660) with the Centers for Disease Control and Prevention. Drop‐outs: At six months, seven dropped out of the IG: two participants withdrew, three participants had medical illnesses, one did not return for the follow‐up and one dropped out for other reasons. At six months, six participants dropped out of the CG: two participants withdrew, one moved and three did not return for the follow‐up. At one year, thirteen participants had dropped out of the IG, three participants withdrew, three had medical illnesses, six participants did not return and one dropped out for other reasons. Seven had dropped out of the CG, three withdrew, two participants moved, one did not return for the follow‐up and one had medical illness. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ligtenberg 1997.
| Methods | Trial design:
RCT Randomisation procedure: "...patients were randomised to either.." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: The Netherlands Setting: Unclear Number: 58 participants randomised (28 in CG, 30 in IG). 51 participants completed the trial. Age: CG: 61 ± 5 years IG: 63 ± 5 years Diagnostic criteria: Classified according to NDDG Inclusion criteria: 1. Diabetes for at least a year 2. BMI greater than 25 3. Diabetes treated with drugs/insulin 4. Older than 55 years old Exclusion criteria: 1. Angina pectoris grades II‐IV 2. Silent ischaemia 3. Autonomic neuropathy 4. Moderate/severe intermittent claudication 5. Impaired renal function 6. Presence of other major diseases Sex: CG: 36% male, 64% female IG: 33% male, 67% female Other characteristics: All women were post‐menopausal. Smoking Status: 14% of participants in CG smoked and 30% of participants in IG smoked. |
|
| Interventions | Trial intervention(s):
A physical training programme, consisting of six weeks of supervised physical training, six weeks of physical training according to personalised training advice (encouragement given every two weeks) and fourteen weeks of training at home (without encouragement). Comparison intervention(s): Participants received a concise education programme, but no exercise advice. |
|
| Outcomes | Weight was measured but not reported in the article; the authors stated instead "body weight did not change in either group during the whole study period". In the diet only group, glycated haemoglobin rose from 8.8 ± 1.5% at baseline to 9.0 ± 1.6% at follow‐up at six months. In the diet plus exercise group , glycated haemoglobin decreased from 8.9 ± 1.0% at the baseline measurement to 8.7 ± 1.1% at the six month follow‐up. 1. HbA1c 2. Weight 3. VO2 Max were recorded at six months. |
|
| Notes | Source of funding of trial: Supported financially by the Dutch Diabetes Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Milne 1994.
| Methods | Trial design:
3‐armed RCT of which we are looking at both interventions (high carbohydrate diet as intervention and modified lipid diet as control) Randomisation procedure: "Randomly assigned" "After stratification for age, sex, BMI and diabetes duration, participants were randomly allocated to one of three groups" Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: New Zealand Setting: Diabetes Clinic Number: 70 participants randomised. (Unclear as to which groups they were assigned to) 44 participants began the trial. 43 participants completed the trial; IG: 22, CG: 21 Age: IG: 59 ± 9.4 years CG: 60 ± 9.2 years Sex: IG: 45% male, 55% female CG: 48% male, 53% female Diagnostic criteria: None given. Inclusion criteria: Participants must have NIDDM No other major illnesses |
|
| Interventions | Trial intervention(s):
IG: High carbohydrate/fibre diet which aimed to achieve/maintain a BMI less than or equal to 25, to derive 15% of energy from protein, 30% of energy from fat and 55% of energy from carbohydrate. There was also an aim to consume 10 or more grams of fibre per day and to increase the consumption of soluble fibre. Comparison intervention(s): Modified lipid diet which aimed to achieve/maintain a BMI less than or equal to 25, to derive 19% of energy from protein, 36% energy from fat. A saturated fatty acids:polyunsaturated fatty acids:monounsaturated fatty acids ratio of 1 and to derive 45% of energy from carbohydrate. For all three arms of the study, for participants with a BMI greater than 25, the diets had an energy deficit of 500 kcalories. |
|
| Outcomes | In the low carbohydrate group the participants had a baseline mean weight of 83.1 ± 16.9kg and at follow‐up six months later, the mean weight of this group had decreased to 82.1 ± 15.0kg. At the 18 month follow‐up the mean weight of the low carbohydrate group had remained stable at 82.1 ± 15.0kg. In the low fat group, the participants had a baseline mean weight of 80.8 ± 7.8kg and at follow‐up six months later, the mean weight of this group had decreased to 80.7 ± 7.8kg. At the 18 month follow‐up the mean weight had remained stable at 80.7 ± 13.8kg. Glycated haemoglobin decreased in the low carbohydrate group from a baseline measurement of 8.7 ± 2.3% to 8.5 ± 2.0% at six months and then remained stable at 8.5 ± 2.0% at the 18 month follow‐up. Glycated haemoglobin decreased in the low fat group from a baseline value of 9.8 ± 3.1% to 9.5 ± 2.6% at six months and then rose to 9.7 ± 2.6% at 18 months. 1. HbA1c 2. Lipids 3. Weight were all measured at 18 months. |
|
| Notes | Source of funding of trial: Supported by a grant from the Health Research Council of New Zealand. Drop‐outs: Six participants dropped out during the trial. (Two moved from the region, two started insulin treatment, one participant was diagnosed with cancer and one died.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pascale 1995.
| Methods | Trial design:
RCT Randomisation procedure: "...subjects were randomly assigned..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: Unclear Outcome Assessors: Unclear Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Unclear Number: 44 participants randomised (22 to CG; 22 to IG) 31 participants completed the trial (16 in CG; 15 in IG) Age: Overall, 56.5 ± 8.4 years Sex: 100% women in both groups. Diagnostic criteria: 1. Participants had to meet the criteria specified by the NDDG. 2. Diabetes controlled only by diet or oral medications. Inclusion criteria: 1. Must be 20% or more above their ideal body weight. 2. Must be type 2 diabetic. |
|
| Interventions | Trial intervention(s):
Calorie and fat restriction ‐ participants aimed to consume between 1000 and 1500 kcalories per day, (calorie goal was set depending on current weight). This diet has the same goals as the control group, but aimed to take less than 20% of calories from fat. Comparison intervention(s): A calorie‐restricted diet, of 1000‐1500 kcalories per day goal, (calorie goal was set depending on current weight). Emphasis was on staying below the calorie goal prescribed, although participants were encouraged to keep fat intake at less than 30% of total calories per day. Both groups had sixteen weekly group meetings, where information about healthy eating was disseminated. Follow‐up meetings were held at one, two, four and six months after treatment. |
|
| Outcomes | Participants in the calorie restricted diet group decreased their mean weight by 0.96 ± 3.7kg at twelve months from a baseline of 93.1 ± 13.0kg.
Participants in the calorie restricted and low fat diet decreased their mean weight by 5.2 ± 7.3kg (at the one year follow‐up) from their baseline of 94.4 ± 9.5kg. At one year, glycated haemoglobin levels rose in the calorie restricted diet group by 0.2 ± 1.7% from 10.9% ± 2.7% at baseline. At one year, glycated haemoglobin levels remained the same in the calorie restricted and low fat diet group, (a change of 0.0 ± 1.9% from 10.4 ± 1.9% at baseline). 1. Weight loss 2. Lipids 3. Glucose were all measured at twelve months. |
|
| Notes | Source of funding of trial: Not mentioned Drop‐outs: CG: Six participants dropped out. IG: Seven participants dropped out. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Samaras 1997.
| Methods | Trial design:
RCT Randomisation procedure: Simple randomisation without stratification Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: Australia Setting: Council run leisure centre Number: 26 participants started the trial (13 in the CG, 13 in the IG) No mention of dropouts, so assume all 26 participants completed the trial. Age: CG: 60.5 ± 7.6 years IG: 60.5 ± 7.8 years Sex: CG: 46% male; 54% female IG: 31% male; 69% female Diagnostic criteria: "...all patients with NIDDM..." No other details given. Inclusion criteria: 1. Participants must have NIDDM 2. Participants must be aged between 40 and 70 years Exclusion criteria: 1. A history, symptoms or signs of ischaemic heart disease 2. Current smoking 3. Poor comprehension of English Other characteristics: 1. Smoking ‐ all smokers were excluded from the study |
|
| Interventions | Trial intervention(s):
Participants followed their usual diet plus the Exercise Support Group Programme, which was conducted at the local leisure centre run by the local council. Staff involved included a nurse educator, exercise physiologist, dietitian, group facilitator and physician. Monthly sessions were of an hour with the group facilitator and one other team member dealing with various issues including safe exercise, exercise specific education to improve confidence, coping with diabetes and exercise and self‐esteem issues. A moderately paced aerobic exercise group session followed with emphasis placed on exercising up to 50% of VO2max. The intervention phase lasted for six months, with the exercise sessions remaining available to intervention group participants after this time. Comparison intervention(s): Participants followed their usual diet, but received no additional encouragement to exercise beyond that which was routinely given at clinic appointments. The participants made visits to the clinic at baseline, six and twelve months for assessment. |
|
| Outcomes | Participants in the diet only group had a baseline weight of 98.2 ± 12.3kg which rose by 1.0 ± 2.9kg after six months and then rose (compared to the baseline measurement) at the twelve month follow‐up by 0.8 ± 3.9kg.
Participants in the diet plus exercise group had a baseline weight of 83.0 ± 13.0kg, which decreased by 0.1 ± 2.7kg at the six month follow‐up and then rose (compared to the baseline measurement) by 0.1 ± 3.9kg at the twelve month follow‐up. In the diet only group, the participants' glycated haemoglobin concentrations rose over the six month period of the trial, by 0.6 ± 0.9% from a baseline value of 6.8 ± 2.2%, and then rose again from the baseline value by 0.9 ± 1.0% at the twelve month follow‐up. In the diet and exercise group glycated haemoglobin rose by 0.1 ± 1.1% over the six month period of the trial, from 5.6 ± 1.1% at baseline and then at the twelve month follow‐up rose from the baseline value by 0.9 ± 1.0%. 1. Weight 2. BMI 3. HbA1c 4. Lipids 5. SF‐36 questionnaire 6. Activity levels were all reported at six and twelve months. |
|
| Notes | Source of funding of trial: None mentioned. Note: The intervention used a model for changing behaviour (precede‐proceed health promotion model). It has been shown that interventions based on such models can be more effective. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tsihlias 2000.
| Methods | Trial design:
RCT Randomisation procedure: "...randomly assigned..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: Unclear Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: Canada Setting: Outpatient clinic Number: 91 participants randomised (32 to CG; 29 to IG). 62 participants were followed up at six months (20 in CG; 21 in IG) Age: CG: 63.0 ± 6.7 years IG: 62.9 ± 7.33 years Sex: At baseline: CG: 53% male; 47% female IG: 59% male; 41% female At 6 months: CG: 62% male; 38% female IG: 45% male; 55% female Diagnostic criteria: None specifically mentioned just "subjects with type 2 diabetes" Inclusion criteria: 1. Not pregnant. 2. Aged 40 to 80 years. 3. Have had diabetes for more than six months. 4. No biochemical evidence of impaired renal or hepatic function. 5. BMI greater than 36. 6. HbA1c greater than 6.5% and less than 10.1%. 7. Serum triglyceride concentration less than 10mmol/L. Exclusion criteria: 1. Insulin or acarbose treatment. |
|
| Interventions | Trial intervention(s):
IG: High GI breakfast cereal (Corn Flakes, Puffed Rice, or Crispy Rice) was consumed for breakfast every day for six months. No other breakfast food was permitted. Comparison intervention(s): CG: High monounsaturated fatty acids intake without breakfast cereal. Not permitted to eat breakfast cereals and were given non‐hydrogenated salted, or un‐salted, soft‐tub margarine or olive oil or both. |
|
| Outcomes | Participants in the monounsaturated fat diet saw their mean weight change from 78.8 ± 12.5kg at baseline to 78.9 ± 13.0kg at six months.
The mean weight for the participants in the low fat diet group decreased from 77.4 ± 14.7kg at baseline to 76.2 ± 13.8kg at six months. At baseline the monounsaturated fat diet group had a glycated haemoglobin measurement of 7.7 ± 1.1%, which rose to 8.1 ± 1.5% at follow‐up six months later. The participants in the low fat diet group at baseline had a glycated haemoglobin concentration of 8.1 ± 1.2%, which rose to 8.3 ± 1.5% at the six months follow‐up. 1. Body weight 2. HbA1c 3. Lipids were all measured at six months. |
|
| Notes | Source of funding of trial: None mentioned. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Uusitupa 1996.
| Methods | Trial design:
RCT Randomisation procedure: "...patients were randomised into one of two groups..." "...were randomly placed into one of two groups..." "...patients were randomised into the intervention or conventional groups..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: Finland Setting: Out‐patient Clinic Number: 86 participants were initially randomised. (40 participants in IG, 46 participants in CG) Age: (Reported separately as male and female ages) CG: 54.0 ± 6.6 years (male); 54.4 ± 6.4 years (female) IG: 50.7 ± 6.7 years (male); 53.7 ± 6.3 years (female) Sex: CG: 61% male, 39% female IG: 53% male, 47% female Diagnostic criteria: "Newly diagnosed NIDDM" Diabetes mellitus as defined by WHO 1985 Inclusion criteria: 1. 40‐64 years old 2. Having NIDDM as defined by WHO 1985 Exclusion criteria: 1. Unwilling to participate Other characteristics: 18% of all participants were current smokers. |
|
| Interventions | Trial intervention(s):
After a common three month basic education programme, the intensified treatment participants were treated at the outpatient clinic. They visited it six times over the year (at two month intervals) where they were seen by a physician, dietitian and a nurse. Participants received printed and oral instructions for effective exercise training. Physical activity was measured by daily exercise records. The diet was implemented through individually planned energy‐restriction. Comparison intervention(s): After a common three month basic education programme, the conventional "standard" treatment participants were treated at community health centres (that had originally referred the patients to the trial) for twelve months. They were advised to visit the health centres at two monthly intervals. Participants received basic information regarding the potential benefits of physical activity and also dietary instructions on how to reduce intake of total fat, total energy and dietary cholesterol. |
|
| Outcomes | Mean weight for the participants in the diet only group changed from 92.2 ± 14.7kg at baseline to a measurement of 90.2 ± 14.3kg at the twelve month follow‐up.
Mean weight change for the participants in the diet plus exercise group was from 91.6 ± 14.5kg at baseline to a measurement of 86.5 ± 13.7kg at the twelve month follow‐up. Over the twelve months between baseline and follow‐up, the mean blood concentration of glycated haemoglobin decreased by 0.3 ± 1.5% in the diet only group (from 7.8 ± 2% at baseline to 7.5 ± 1.7% at twelve months) and by 0.5 ± 1.3% in the diet plus exercise group, (from 7.1 ± 1.8% at baseline to 6.6 ± 1.6% at twelve months). 1. Weight 2. HbA1c 3. VO2 Max 4. Lipids were all measured at twelve months. |
|
| Notes | Source of funding of trial: Supported financially by grants from the Juho Vainio Foundation, the Yrjo Jahnsson Foundation and in part by the Academy of Finland. Drop‐outs: At 1 year 8 patients had dropped out (40 remained in conventional group, 38 remained in intervention group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1988.
| Methods | Trial design:
RCT Randomisation procedure: "...randomly assigned..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Yes Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: School of Medicine Number: 30 participants were randomised to each arm of the study (15 participants to the CG and 15 participants to the IG). Age: CG: 55.1 ± 7.2 years IG: 56.1 ± 6.4 years Sex: Overall, 70% of participants were female and 30% of participants were male. Diagnostic criteria: Participants had to be diagnosed according to the criteria laid down by the NDDG. Inclusion criteria: 1. Participants had to be more than 20% above ideal body weight. 2. Aged between 30 and 65. Exclusion criteria: 1. Known history of CHD. 2. Taking medication that would interfere with weight loss or measuring heart rates. 3. Orthopaedic problems that would limit walking. |
|
| Interventions | Trial intervention(s):
A diet and exercise programme. The diet component of the intervention remained the same as diet for the control group (i.e. low fat high/carbohydrate diet). The exercise component was made up of walking a three mile route with therapists three times a week and were instructed to exercise once more (on their own) during the week. Comparison intervention(s): Participants were taught to increase their intake of complex carbohydrates, and to decrease their intake of fat in keeping with the revised American Diabetes Association dietary guidelines. A calorie goal was prescribed, calculated by taking the patients pre‐intervention weight, multiplying by 26 and subtracting 1000 calories. Participants in both groups attended three meetings a week, for ten weeks, where they were shown demonstrations and films of low calorie cooking techniques, and how to practise behaviours such as portion size estimation and learning through role‐play. |
|
| Outcomes | Participants in the diet only group had a mean weight of 102 ± 19.4kg at baseline and a mean weight of 98.2 ± 20kg twelve months later.
Participants in the diet and exercise group had a mean weight of 104.1 ± 23.2kg at baseline and a mean weight of 96.2 ± 23.4kg at the twelve month follow‐up. Glycated haemoglobin decreased in the diet only group, from a baseline value of 10.9 ± 1.9% to a follow‐up value (at twelve months) of 10.1 ± 1.6%. In the diet plus exercise group, glycated haemoglobin also decreased from a baseline value of 10.6 ± 1.9% to a twelve month follow‐up value of 9.2 ± 1.8%. 1. Weight 2. Blood pressure 3. Lipids 4. HbA1c were all measured at 12 months. |
|
| Notes | Source of funding of trial: Trial was supported by grant AM 29757‐07 from the National Institutes of Health. Drop‐outs: Two participants dropped out of the intervention group, although no reasons were given for these drop‐outs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1991.
| Methods | Trial design:
RCT Randomisation procedure: "...subjects were randomly assigned to either..." Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (two points awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Unclear ‐ possibly university based. Number: 36 participants were randomised, 19 to CG and 17 to IG. (Note that the data that is presented is for 33 participants, 16 in CG and 17 in IG.) Age: CG: 51.9 ± 9.9 years IG: 50.6 ± 7.7 years Sex: CG: 25% male, 75% female. IG: 24% male, 76% female. Diagnostic criteria: Participants had type 2 diabetes as defined by the NDDG criteria. Inclusion criteria: 1. Participants must be between 35 and 70 years old. 2. Must be 30% or more above ideal body weight. 3. Having type 2 diabetes as defined by the NDDG criteria. 4. Having no evidence of liver, renal or heart disease that would contraindicate the use of VLCD's. |
|
| Interventions | Trial intervention(s):
Participants were given a calorie goal of 4200 to 6300 joules per day (depending on their initial body weight) . Information was presented regarding the different caloric content of protein, fat and carbohydrate. Subjects were encouraged to increase their complex carbohydrate intake especially fibre, while decreasing dietary fat. Participants allocated to the IG followed this diet for one month, and then switched to a very‐low‐calorie diet (VCLD) for the second and third months of this program. While following the VCLD, participants were instructed to consume 1680 joules per day of lean fish, meat or fowl and had the option of using a liquid meal for occasional meals. After the two months, other foods were gradually re‐introduced so that by week 17 all subjects were back onto a 4200 to 6300 joules per day diet. Comparison intervention(s): Participants were given a calorie goal of 4200 to 6300 joules per day (depending on their initial body weight) . Information was presented regarding the different caloric content of protein, fat and carbohydrate. Subjects were encouraged to increase their complex carbohydrate intake especially fibre, while decreasing dietary fat. The control and intervention lasted for twenty weeks in total. |
|
| Outcomes | Mean body weight for the participants in the low calorie diet group at baseline was 107.7 ± 18.7kg, which rose to 118.2 ± 11.6kg at twelve months.
Participants in the very low calorie diet group had a mean baseline weight of 105.8 ± 19.4kg, which decreased to 91.6 ± 10.3kg at twelve months. Glycated haemoglobin for the low calorie diet group was 10.5 ± 2.0% at baseline, decreasing to 8.8 ± 1.8% at six months, which rose to 9.2 ± 2.0% at twelve months and then rose again to 10.7 ± 2.4% at twenty‐four months. Glycated haemoglobin in the very low calorie diet group, was measured at 10.4 ± 2.0% at baseline, decreased to 8.4 ± 2.2% at six months, and then slightly rose to 8.9 ± 2.5% at twelve months and a greater rise to 10.4 ± 2.2% at twenty‐four months for the very low calorie diet group. 1. Weight 2. BMI 3. Fasting plasma glucose 4. Fasting insulin 5. Lipids were all measured at one year. |
|
| Notes | Source of funding of trial: Supported by grants from Western Pennsylvania Affiliate of the American Diabetes Association and the National Institutes of Health (NIDDK 29757), Drop‐outs: Three participants dropped out of the CG. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1994.
| Methods | Trial design:
RCT Randomisation procedure: "...randomly assigned.." No further detail given. Allocation concealment: Unclear Blinding: Recipients: No Providers of Care: No Outcome Assessors: Unclear Quality Assessment Category: C (one point awarded) ‐ high risk of bias. |
|
| Participants | Country: USA Setting: Out‐patient clinic Number: 93 participants in total were randomised (48 to IG and 45 to CG) at the start of the trial. At six months: No data reported. At one year: 38 participants in IG and 41 participants in CG were followed up. At two years: 36 participants in IG and 37 participants in CG were followed up. Age: At baseline: IG: 52.3 ± 10.7 years CG: 51.3 ± 8.7 years Sex: At baseline: CG: 38% male; 62% female. IG: 33% male; 67% female. Diagnostic criteria: 1. All participants must meet NDDG criteria for type 2 diabetes mellitus. Inclusion criteria: 1. More than 30% or 18kg above their ideal body weight (based on Metropolitan Life Insurance norms). 2. Aged 30 to 70 years. 3. No health problems that would preclude use of a VCLD. |
|
| Interventions | Trial intervention(s):
Low calorie diet (LCD) with two x twelve week periods of very low calorie diet (VCLD). Participants were prescribed a diet of 400 to 500 kcalories per day for two periods of twelve weeks (weeks 1 to 12 and weeks 24 to 36) interspersed with a low calorie diet (LCD: 1000 to 1200 kcalories per day as in the control diet). During the VLCD period of the study, participants could either consume up to 500 kcalories per day in liquid formula or from lean meat or fish. The LCD was slowly reintroduced after the VCLD period had finished, slowly increasing the calories over four weeks. Comparison intervention(s): Low calorie diet of 1000 to 1200 kcalories per day. Participants were able to select the food items they wished but they were encouraged to spread their calories over the day and to limit dietary fat to less than 30% of calories. |
|
| Outcomes | The low calorie group had a baseline mean weight of 104.5 ± 21.5kg and a follow‐up mean weight of 97.7 ± 17.4kg twelve months later.
The very low calorie diet group had a baseline mean weight of 102.1 ± 11.7kg and twelve months later had a mean weight of 93.5 ± 10.4kg. The mean blood concentration of glycated haemoglobin in the low calorie group was 10.4 ± 2.0% at baseline, and 11.8 ± 2.7% at follow‐up twelve months later. The mean glycated haemoglobin concentration in the participants of the very low calorie group was 10.4 ± 2.2% at baseline and 9.2 ± 2.1% at the twelve month follow‐up. 1. Weight 2. Glycaemic control 3. Blood pressure 4. Lipids were all measured at six, twelve and twenty‐four months. |
|
| Notes | Source of funding of trial: None mentioned A deposit of US$150 per participant was required at the start of the study, but was refunded in full for reaching behavioural goals and attending assessment sessions. Drop‐outs: At one year, ten participants from the IG and four from the CG had dropped out. At two years, twelve participants from the IG and eight from the CG had dropped out. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
HbA1c = Glycated Haemoglobin BMI = Body Mass Index CG = Control Group IG = Intervention Group NDDG = National Diabetes Data Group WHO = World Health Organisation Unless specified, ## ± ## is mean ± SD
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADA 2000 | (a) Was not a randomised controlled trial |
| ADA 2002 | (a) Was not a randomised controlled trial |
| Aizawa 2001 | (a) Was not a randomised controlled trial |
| Andersen 1987 | (a) Was not a randomised controlled trial |
| Anderson 2003 | (a) Was not a randomised controlled trial |
| Anonymous 1988 | (a) Was not a randomised controlled trial |
| Anonymous 1990 | (a) Was not a randomised controlled trial |
| Arky 1983 | (a) Was not a randomised controlled trial |
| Arora 2005 | (a) Was not a randomised controlled trial |
| Ash 2003 | (a) (b) (c) (d) (e) (f) (g) (h) (i) Was not a true control |
| Barnard 1982 | (a) (b) Was not a six month intervention |
| Barnard 1983 | (a) Was not a randomised controlled trial |
| Barnard 1992 | (a) (b) Was not a six month intervention |
| Barnard 1994 | (a) (b) Was not a six month intervention |
| Barnard 1997 | (a) (b) Was not a six month intervention |
| Barnett 2001 | (a) Was not a randomised controlled trial |
| Bassand 2006 | (a) Was not a randomised controlled trial |
| Bennett 1997 | (a) Was not a randomised controlled trial |
| Berg 2003 | (a) Was not a randomised controlled trial |
| Bergenstal 1999 | (a) Was not a randomised controlled trial |
| Bhaskarabhatla 2004 | (a) Was not a randomised controlled trial |
| Bjorntorp 1992 | (a) Was not a randomised controlled trial |
| Blake 1992 | (a) Was not a randomised controlled trial |
| Blonk 1994 | (a) Was not a randomised controlled trial |
| Bloomgarden 2000 | (a) Was not a randomised controlled trial |
| Bloomgarden 2004 | (a) Was not a randomised controlled trial |
| Bloomgarden 2005 | (a) Was not a randomised controlled trial |
| Boden 2005 | (a) Was not a randomised controlled trial |
| Bonanome 1991 | (a) (b) Was not a six month intervention |
| Bonnefont‐Rousselot | (a) Was not a randomised controlled trial |
| Borghouts 2000 | (a) Was not a randomised controlled trial |
| Boule 2001 | (a) Was not a randomised controlled trial |
| Boule 2002 | (a) Was not a randomised controlled trial |
| Bourn 1996a | (a) Was not a randomised controlled trial |
| Bourn 1996b | (a) Was not a randomised controlled trial |
| Brand‐Miller 2003 | (a) Was not a randomised controlled trial |
| Brown 1996 | (a) Was a randomised controlled trial (b) (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Brown 2002 | (a) Was a randomised controlled trial (b) (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Brownell 1998 | (a) Was not a randomised controlled trial |
| Calle‐Pascual 1992 | (a) Was not a randomised controlled trial |
| Campbell 2001 | (a) Was not a randomised controlled trial |
| Capstick 1997 | (a) (b) Was not a six month intervention |
| Carr 2005 | (a) (b) (c) (d) Type 2 diabetes not involved‐ Impaired Glucose Tolerance |
| Cefalu 2005 | (a) Was not a randomised controlled trial |
| Chakravarthy 2002 | (a) Was not a randomised controlled trial |
| Charatan 2001 | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Chen 1987 | (a) (b) (c) (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Chen 2001 | (a) Was a randomised controlled trial (b) (c) (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Chipkin 2001 | (a) Was not a randomised controlled trial |
| Christensen 1998 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control not measured |
| Ciliska 1995 | (a) Was not a randomised controlled trial |
| Clark 2001 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Coleman 2003 | (a) Was not a randomised controlled trial |
| Collins 1995 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice (was a pre‐prepared meal plan) |
| Colman 1995 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Comi 1995 | (a) (b) Was not a six month intervention |
| CRD 2002 | (a) Was not a randomised controlled trial |
| Creviston 2001 | (a) Was not a randomised controlled trial |
| de Fine Olivarius 06 | (a) Was not a randomised controlled trial |
| de Sonnaville 1997 | (a) Was not a randomised controlled trial |
| Delahanty 1995 | (a) Was not a randomised controlled trial |
| Dengel 1998 | (a) (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Devlin 1986 | (a) Was not a randomised controlled trial |
| Dey 2002 | (a) Was not a randomised controlled trial |
| Dhindsa 2003 | (a) Was not a randomised controlled trial |
| Dunstan 2002 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Elson 1998 | (a) Was not a randomised controlled trial |
| Eriksson 1991 | (a) Was a randomised controlled trial, but was a cross‐over trial which were not included |
| Eriksson 1997 | (a) Was not a randomised controlled trial |
| Eriksson 1998 | (a) Was not a randomised controlled trial |
| Eriksson 1999a | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Eriksson 1999b | (a) Was not a randomised controlled trial |
| Ernst 2001 | (a) Was not a randomised controlled trial |
| Evans 1995 | (a) Was not a randomised controlled trial |
| Evans 1997 | (a) Was not a randomised controlled trial |
| Evans 2002 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Fletcher 2002 | (a) Was not a randomised controlled trial |
| Franz 1995 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Frati 1990 | (a) (b) (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Fujinuma 1999 | (a) (b) Was not a six month intervention |
| Funnell 2004 | (a) Was not a randomised controlled trial |
| Gaede 2001 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not only dietary advice (was a multifactorial intervention) |
| Gaede 2006 | (a) Was not a randomised controlled trial |
| Gannon 2006 | (a) Was not a randomised controlled trial |
| Garg 1990 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Garg 1993 | (a) Was not a randomised controlled trial |
| Garg 1998 | (a) Was not a randomised controlled trial |
| Gautier 1995 | (a) Was not a randomised controlled trial |
| Genuth 2003 | (a) Was not a randomised controlled trial |
| Glasgow 1989 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Goldberg 1998 | (a) Was not a randomised controlled trial |
| Gonzelez 1994 | (a) Was not a randomised controlled trial |
| Grundy 1991 | (a) Was not a randomised controlled trial |
| Grundy 1999b | (a) Was not a randomised controlled trial |
| Guerrero‐Romero 2005 | (a) Was not a randomised controlled trial |
| Gumbiner 1998 | (a) (b) Was not a six month intervention |
| Gumbiner 1999 | (a) Was not a randomised controlled trial |
| Hadden 1982 | (a) Was not a randomised controlled trial |
| Hamdy 2001 | (a) Was not a randomised controlled trial |
| Hamilton 1992 | (a) Was not a randomised controlled trial |
| Hanefeld 1989 | (a) (b) Was not a six month intervention |
| Hart 2006 | (a) Was not a randomised controlled trial |
| Heath 1987 | (a) Was not a randomised controlled trial |
| Heath 1991 | (a) Was not a randomised controlled trial |
| Heine 1989 | (a) Was a randomised controlled trial, but was a cross‐over trial which were not included |
| Held 1991 | (a) Was not a randomised controlled trial |
| Helmrich 1994 | (a) Was not a randomised controlled trial |
| Henry 1991 | (a) Was not a randomised controlled trial |
| Hensrud 2001 | (a) Was not a randomised controlled trial |
| Holmes 1987 | (a) Was not a randomised controlled trial |
| Horrocks 1987 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Hu 1999 | (a) Was not a randomised controlled trial |
| Hu 2001a | (a) Was not a randomised controlled trial |
| Hu 2001b | (a) Was not a randomised controlled trial |
| Huh 1996 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Hutton 2004 | (a) Was not a randomised controlled trial |
| Ismail 2004 | (a) Was not a randomised controlled trial |
| Ito 2001 | (a) Was not a randomised controlled trial |
| Ivy 1997 | (a) Was not a randomised controlled trial |
| James 1998 | (a) Was not a randomised controlled trial |
| Janssen 2002 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Jenkins 2003 | (a) Was not a randomised controlled trial |
| Jenkins 2004 | (a) Was not a randomised controlled trial |
| Jeppesen 1997 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Kao 2000 | (a) Was not a randomised controlled trial |
| Karlstrom 1989 | (a) Was not a randomised controlled trial |
| Kelley 1995 | (a) Was not a randomised controlled trial |
| Kelley 1999 | (a) Was not a randomised controlled trial |
| Kelley 2001 | (a) Was not a randomised controlled trial |
| Kelly 2000 | (a) Was not a randomised controlled trial |
| Kennedy 1982 | (a) Was not a randomised controlled trial |
| Kirk 2003 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Kirkman 1994 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Kligler 2003 | (a) Was not a randomised controlled trial |
| Knowler 1994 | (a) Was not a randomised controlled trial |
| Kraus 2001 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Kriska 2000 | (a) Was not a randomised controlled trial |
| Kriska 2002 | (a) Was a randomised controlled trial (b) (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Kulkarni 2006 | (a) Was not a randomised controlled trial |
| Lehman 2005 | (a) Was not a randomised controlled trial |
| Lehmann 1998 | (a) Was not a randomised controlled trial |
| Liao 2003 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Ligtenberg 1998 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Lindstrom 2003 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Ling 1994 | (a) Was not a randomised controlled trial |
| Little 1996 | (a) Was not a randomised controlled trial |
| Lodha 2000 | (a) Was not a randomised controlled trial |
| Lomasky 1990 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Maggio 1997 | (a) Was not a randomised controlled trial |
| Manson 1992b | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Manson 1994 | (a) Was not a randomised controlled trial |
| Mazzeo 2001 | (a) Was not a randomised controlled trial |
| McCarty 1997 | (a) Was not a randomised controlled trial |
| Meinders 1992 | (a) Was not a randomised controlled trial |
| Melander 1996 | (a) Was not a randomised controlled trial |
| Mensink 2003a | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice, but the intervention and control groups did not received different advice |
| Mensink 2003b | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice, but the intervention and control groups did not received different advice |
| Metz 2000 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice (was a pre‐prepared meal plan) |
| Miles 2000 | (a) Was not a randomised controlled trial |
| Miller 1996 | (a) Was not a randomised controlled trial |
| Monteiro 2005 | (a) Was not a randomised controlled trial |
| Montori 2001 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Morelli 2000 | (a) Was not a randomised controlled trial |
| Mustajoki 2001 | (a) Was not a randomised controlled trial |
| Myers 2001 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Namdul 2001 | (a) Was a randomised controlled trial (b) (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Narayan 1998 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Narayan 2001 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Neff 2003 | (a) Was not a randomised controlled trial |
| Nicollerat 2000 | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Nielsen 2005 | (a) Was not a randomised controlled trial |
| Nilsson 1992 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Norris 2004 | (a) Was not a randomised controlled trial |
| Odegaard 2006 | (a) Was not a randomised controlled trial |
| Oli 1984 | (a) Was not a randomised controlled trial |
| Paffenbarger 1997 | (a) Was not a randomised controlled trial |
| Paisey 1995 | (a) Was not a randomised controlled trial |
| Paisey 1998 | (a) Was not a randomised controlled trial |
| Paisey 2002 | (a) Was not a randomised controlled trial |
| Pan 1997 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Parfitt 1994 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Pascale 1992 | (a) Was not a randomised controlled trial |
| Pejic 2006 | (a) Was not a randomised controlled trial |
| Perez‐Martin 2001 | (a) Was not a randomised controlled trial |
| Perri 1993 | (a) Was not a randomised controlled trial |
| Pfohl 2001 | (a) Was not a randomised controlled trial |
| Phillips 2006 | (a) Was not a randomised controlled trial |
| Pigman 2002 | (a) Was not a randomised controlled trial |
| Pischke 2006 | (a) Was not a randomised controlled trial |
| Poppitt 2002 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Pratley 2000 | (a) (b) (c) Participants were over 18 years old and human (d) (e) Main intervention was not dietary advice |
| Pritchard 1999 | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Rabkin 1983 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Racette 2001 | (a) Was not a randomised controlled trial |
| Ratner 2006 | (a) Was not a randomised controlled trial |
| Raz 1994 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Reaven 1995 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Rendell 2006 | (a) Was not a randomised controlled trial |
| Riccardi 2005 | (a) Was not a randomised controlled trial |
| Ronnemaa 1986 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Rosell 1999 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) (e) Main intervention was dietary advice (f) Weight loss not measured |
| Rowley 2000 | (a) Was a randomised controlled trial |
| Rubin 2002 | (a) Was not a randomised controlled trial (b) (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Rukgauer 2006 | (a) Was not a randomised controlled trial |
| Ryan 2003 | (a) Was not a randomised controlled trial |
| Rybka 1987 | (a) Was not a randomised controlled trial |
| Sato 2000a | (a) (b) Was not a six month intervention |
| Scheen 2000 | (a) Was not a randomised controlled trial |
| Schwartz 2006 | (a) Was not a randomised controlled trial |
| Shahid 2000 | (a) Was not a randomised controlled trial |
| Sherwin 2003 | (a) Was not a randomised controlled trial |
| Shintani 2001 | (a) Was not a randomised controlled trial |
| Siddiqui 2004 | (a) Was not a randomised controlled trial |
| Sigal 2004 | (a) Was not a randomised controlled trial |
| Simmons 1997 | (a) Was not a randomised controlled trial |
| Smith 1993 | (a) Was not a randomised controlled trial |
| Solano 2006 | (a) Was not a randomised controlled trial |
| Solano 2006b | (a) Was not a randomised controlled trial |
| Sone 2002 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Song 2006 | (a) Was not a randomised controlled trial |
| Spelsberg 1995 | (a) Was not a randomised controlled trial |
| Srimanunthiphol 2000 | (a) Was not a randomised controlled trial |
| Starke 1994 | (a) Was not a randomised controlled trial |
| Steyn 2004 | (a) Was not a randomised controlled trial |
| Stone 2001 | (a) Was not a randomised controlled trial |
| Strandberg 2000 | (a) Was not a randomised controlled trial |
| Sutherland 2004 | (a) Was not a randomised controlled trial |
| Svendsen 1996 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was not involved in study |
| Swinburn 2001 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Takekoshi 1987 | (a) (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Tamler 2006 | (a) Was not a randomised controlled trial |
| Taniguchi 2000 | (a) Was not a randomised controlled trial |
| Tariq 2001 | (a) (b) Was not a six month intervention |
| Thomson 2001 | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Toeller 1993 | (a) Was not a randomised controlled trial |
| Toeller 2005 | (a) Was not a randomised controlled trial |
| Torjesen 1997 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Trento 2002 | (a) (b) (c) (d) (e) Dietary advice was not main intervention‐ general lifestyle advice |
| Tsujiuchi 2002 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Tudor‐Locke 2000 | (a) Was not a randomised controlled trial |
| Tuomilehto 1992 | (a) Was not a randomised controlled trial |
| Tuomilehto 2001b | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Turner 1990 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) (d) (e) Main intervention was not dietary advice |
| Turner 1995 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was not dietary advice |
| Turner 1996a | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Turner 1996b | (a) (b) (c) Participants were over 18 years old and human (d) (e) Main intervention was not dietary advice |
| UKPDS 1993 | (a) Was a randomised controlled trial (b) (c) (d) (e) Main intervention was not dietary advice |
| Ullom‐Minnich 2004 | (a) Was not a randomised controlled trial |
| Uusitupa 1989 | (a) Was not a randomised controlled trial |
| Uusitupa 2000 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Valensi 2006 | (a) Was not a randomised controlled trial |
| Vuksan 2001 | (a) Was not a randomised controlled trial |
| Vuori 2001 | (a) Was not a randomised controlled trial |
| Walker 1996 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Walker 2001 | (a) Was not a randomised controlled trial |
| Wall 1973 | (a) Was not a randomised controlled trial |
| Wallberg‐H 1998 | (a) Was not a randomised controlled trial |
| Warnken 2005 | (a) Was not a randomised controlled trial |
| Wei 2000 | (a) Was not a randomised controlled trial |
| Wein 1999 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Weinstock 1998 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Welch 2006 | (a) Was not a randomised controlled trial |
| Wild 2004 | (a) Was not a randomised controlled trial |
| Williams 2000 | (a) Was not a randomised controlled trial |
| Williamson 2000 | (a) (b) Was a six month intervention (c) Participants were over 18 years old and human (d) (e) Main intervention was not dietary advice |
| Wing 1993 | (a) Was not a randomised controlled trial |
| Wing 1995 | (a) Was not a randomised controlled trial |
| Wing 1998 | (a) Was a randomised controlled trial (b) Was a six month intervention (c) Participants were over 18 years old and human (d) Type 2 diabetes was involved in study (e) Main intervention was dietary advice (f) Weight loss measured (g) Diabetic control measured (h) Prevention study |
| Wing 2001 | (a) Was not a randomised controlled trial |
| Wolever 2002 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Wolever 2003 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Wolffenbuttel 1989 | (a) Was not a randomised controlled trial |
| Wylie‐Rosett 2006 | (a) Was not a randomised controlled trial |
| Yamamoto 2001 | (a) Was a randomised controlled trial (b) Was not a six month intervention |
| Yamaoka 2005 | (a) Was not a randomised controlled trial |
| Yeh 2003 | (a) Was not a randomised controlled trial |
| Yoo 2005 | (a) Was not a randomised controlled trial |
| Yoshioka 1989 | (a) (b) Was not a six month intervention |
| Zhi‐cheng 1994 | (a) Was not a randomised controlled trial |
NB1. Any "no" disqualifies study from inclusion. NB2. If excluded because "prevention" these will be included in a second prevention review.
Characteristics of ongoing studies [ordered by study ID]
Kelley 2002.
| Trial name or title | Look AHEAD (Action in Health in Diabetes) |
| Methods | |
| Participants | Men and women who are overweight and have type 2 diabetes. Participants must be aged between 45 and 75 years of age. |
| Interventions | Participants will receive either a diabetes support and education program or a long‐term lifestyle change program for weight loss and weight maintenance. Both programs are offered at no cost to the participants. |
| Outcomes | "Long term effects of weight loss" |
| Starting date | During 2002, exact date unspecified. |
| Contact information | |
| Notes | The study is sponsored by the National Institutes of Health, Bethesda, Maryland, USA, It is envisaged that participants will be assessed up to eleven and a half years after enrolling in the programme, which will reveal the long‐term health effects of the trial. |
Contributions of authors
LUCIE NIELD: co‐ordinating the 2007 update review, data collection for the review, undertaking searches, organising retrieval of papers, screening retrieved papers against inclusion criteria, abstracting data from papers, data management of the review, entering data into RevMan, analysis of data, writing the review.
HELEN MOORE: designing the original review, co‐ordinating the review, data collection for the review, developing search strategy, undertaking searches, organising retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, writing to authors of papers for additional information, providing additional data about papers, obtaining and screening data on unpublished studies, data management for the review, entering data into RevMan, analysis of data, writing the review.
CAROLYN SUMMERBELL: conceiving the review, designing the review, screening search results, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, obtaining and screening data on unpublished studies, interpretation of data, providing general advice on the review, securing funding for the review, performing previous work that was the foundation of current study.
LEE HOOPER: conceiving the review, screening of retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, providing a clinical perspective, performing previous work that was the foundation of the current study, providing general advice on the review.
KENNEDY CRUICKSHANK: conceiving the review, screening of retrieved papers against inclusion criteria, abstracting data from papers, appraising quality of papers, providing a methodological perspective, providing a clinical perspective, performing work that was the foundation of the current study.
AVNI VYAS: conceiving the review, screening of retrieved papers against inclusion criteria, abstracting data from papers, appraising quality of papers, providing a clinical perspective, performing previous work that was the foundation of the current study.
VICKI WHITTAKER: screening retrieved papers against the inclusion criteria, data management for the review, entering data into RevMan, analysis of data, interpretation of data.
Sources of support
Internal sources
University of Manchester, UK.
University of Teesside, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Agurs‐Collins 1997 {published data only}
- Agurs‐Collins TD, Kumanyika SK, Have TR, Adams‐Campbell LL. A randomized controlled trial of weight reduction and exercise for diabetes management in older African‐American subjects. see comments. Diabetes Care 1997;20(10):1503‐11. [DOI] [PubMed] [Google Scholar]
Campbell 1990 {published data only}
- Campbell LV, Barth R, Gosper JK, Jupp JJ, Simons LA, Chisholm DJ. Impact of intensive educational approach to dietary change in NIDDM. Diabetes Care 1990;13(8):841‐7. [DOI] [PubMed] [Google Scholar]
de Bont 1981 {published data only}
- Bont AJ, Baker IA, Leger AS, Sweetnam PM, Wragg KG, Stephens SM, Hayes TM. A randomised controlled trial of the effect of low fat diet advice on dietary response in insulin independent diabetic women. Diabetologia 1981;21(6):529‐33. [DOI] [PubMed] [Google Scholar]
Gallagher 1987 {published data only}
- Gallagher A, Henderson W, Abraira C. Dietary patterns and metabolic control in diabetic diets: a prospective study of 51 outpatient men on unmeasured and exchange diets. Journal of the American College of Nutrition 1987;6(6):525‐32. [DOI] [PubMed] [Google Scholar]
- Gallagher AM, Abraira C, Henderson WG. A four‐year prospective trial of unmeasured diet in lean diabetic adults. Diabetes Care 1984;7(6):557‐65. [DOI] [PubMed] [Google Scholar]
Glasgow 1997 {published data only}
- Glasgow RE, Chance PA, Toobert DJ, Brown J, Hampson SE, Riddle MC. Long term effects and costs of brief behavioural dietary intervention for patients with diabetes delivered from the medical office. Patient Education and Counseling 1997;32(3):175‐184. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Toobert DJ, Hampson SE. Effects of a Brief Office‐Based Intervention to Facilitate Diabetes Dietary Self‐Management. Diabetes Care 1996;19(8):835‐42. [DOI] [PubMed] [Google Scholar]
Glasgow 2000 {published data only}
- Glasgow RE, Toobert DJ. Brief, computer‐assisted diabetes dietary self‐management counseling: effects on behavior, physiologic outcomes, and quality of life. see comments. Medical Care 2000;38(11):1062‐73. [DOI] [PubMed] [Google Scholar]
Hockaday 1986 {published data only}
- Hockaday TDR, Hockaday JM, Mann JI, Turner RC. Prospective comparison of modified‐fat‐high‐carbohydrate with standard low‐carbohydrate dietary advice in the treatment of diabetes: a one year follow‐up study. British Journal of Nutrition 1978;39:357‐62. [DOI] [PubMed] [Google Scholar]
- Hockaday TDR, Pandher KS, Bron A, et al. Progression of established retinopathy is unrelated to glycosylated hemoglobin in non‐insulin‐dependent diabetes. Transplantation Proceedings 1986;18(6):1574‐1575. [Google Scholar]
- Howard‐Williams J, Hillson RM, Bron A, Awdry P, Mann JI, Hockaday TDR. Retinopathy is associated with higher glycaemia in maturity‐onset type diabetes. Diabetologia 1984;27:198‐202. [DOI] [PubMed] [Google Scholar]
- Howard‐Williams J, Patel P, Jelfs R, Carter RD, Awdry P, Bron A, et al. Polyunsaturated fatty acids and diabetic retinopathy. British Journal of Ophthalmology 1985;69(1):15‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Espinoza I, Howard‐Williams J, Mann JI, Carter RD, Hockaday TD. Fatty acid composition of platelet phospholipids in non‐insulin‐dependent diabetics randomized for dietary advice. British Journal of Nutrition 1984;52(1):41‐7. [DOI] [PubMed] [Google Scholar]
- Mann JI, Hockaday TD, Hockaday JM, Turner RC. A prospective study of modified‐fat and low‐carbohydrate dietary advice in the treatment of maturity‐onset diabetes [proceedings]. Proceedings of the Nutrition Society 1976;35(2):72A‐73A. [PubMed] [Google Scholar]
Kaplan 1986 {published data only}
- Hartwell SL, Kaplan RM, Wallace JP. Comparison of behavioral interventions for control of type II diabetes mellitus. Behavior Therapy 1986;17(4):447‐461. [Google Scholar]
- Kaplan RM, Atkins CJ, Wilson DK. The cost‐utility of diet and exercise interventions in non‐insulin‐dependent diabetes mellitus. Health Promotion 1987;2(4):331‐340. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non‐insulin‐dependent diabetes mellitus. Journal of General Internal Medicine 1987;2(4):220‐8. [DOI] [PubMed] [Google Scholar]
Keyserling 2000 {published data only}
- Keyserling TC, Ammerman AS, Samuel‐Hodge CD, Ingram AF, Skelly AH, Elasy TA, et al. A diabetes management program for African American women with type 2 diabetes. Diabetes Educator 2000;26(5):796‐805. [DOI] [PubMed] [Google Scholar]
- Keyserling TC, Samuel‐Hodge CD, Ammerman AS, Henriquez‐Roldan CF, Elasy TA, Skelly AH, Johnston LF, Bangdiwala SI. A Randomised Trial of an Intervention to Improve Self‐Care Behaviors of African‐American Women With Type 2 Diabetes. Diabetes Care 2002;25(9):1576‐83. [DOI] [PubMed] [Google Scholar]
Ligtenberg 1997 {published data only}
- Ligtenberg PC, Hoekstra JB, Bol E, Zonderland ML, Erkelens DW. Effects of physical training on metabolic control in elderly type 2 diabetes mellitus patients. Clinical Science 1997;93(2):127‐35. [DOI] [PubMed] [Google Scholar]
Milne 1994 {published data only}
- Milne RM, Mann JI, Chisholm AW, Williams SM. Long‐term comparison of three dietary prescriptions in the treatment of NIDDM. Diabetes Care 1994;17(1):74‐80. [DOI] [PubMed] [Google Scholar]
Pascale 1995 {published data only}
- Pascale RW, Wing RR, Butler BA, Mullen M, Bononi P. Effects of a behavioral weight loss program stressing calorie restriction versus calorie plus fat restriction in obese individuals with NIDDM or a family history of diabetes. Diabetes Care 1995;18(9):1241‐8. [DOI] [PubMed] [Google Scholar]
Samaras 1997 {published data only}
- Samaras K, Ashwell S, Mackintosh AM, Fleury AC, Campbell LV, Chisholm DJ. Will older sedentary people with non‐insulin‐dependent diabetes mellitus start exercising? A health promotion model. Diabetes Research & Clinical Practice 1997;37(2):121‐8. [DOI] [PubMed] [Google Scholar]
Tsihlias 2000 {published data only}
- Tsihlias EB, Gibbs AL, McBurney MI, Wolever TMS. Comparison of high‐ and low‐glycemic‐index breakfast cereals with monounsaturated fat in the long‐term dietary management of type 2 diabetes. American Journal of Clinical Nutrition 2000;72(2):439‐49. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Schrade KB, Vogt JA, Tsihlias EB, McBurney MI. Do colonic short‐chain fatty acids contribute to the long‐term adaptation of blood lipids in subjects with type 2 diabetes consuming a high‐fiber diet?. American Journal of Clinical Nutrition 2002;75(6):1023‐30. [DOI] [PubMed] [Google Scholar]
Uusitupa 1996 {published data only}
- Laitinen J, Uusitupa M, Ahola I, Laakso M, Siitonen O. Metabolic and dietary variables associated with glycaemic control in patients with recently diagnosed Type II diabetes mellitus. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 1994;7(2):77‐87. [Google Scholar]
- Laitinen J, Uusitupa M, Ahola I, Siitonen O. Metabolic and dietary determinants of serum lipids in obese patients with recently diagnosed non‐insulin‐dependent diabetes. Annals of Medicine 1994;26(2):119‐24. [DOI] [PubMed] [Google Scholar]
- Uusitupa M, Laitinen J, Siitonen O, Vanninen E, Pyorala K. The maintenance of improved metabolic control after intensified diet therapy in recent type 2 diabetes. Diabetes Research & Clinical Practice 1993;19(3):227‐38. [DOI] [PubMed] [Google Scholar]
- Uusitupa MI. Early lifestyle intervention in patients with non‐insulin‐dependent diabetes mellitus and impaired glucose tolerance. Annals of Medicine 1996;28(5):445‐9. [DOI] [PubMed] [Google Scholar]
- Vanninen E, Laitinen J, Uusitupa M. Physical activity and fibrinogen concentration in newly diagnosed NIDDM. Diabetes Care 1994;17(9):1031‐8. [DOI] [PubMed] [Google Scholar]
- Vanninen E, Uusitupa M, Lansimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed type 2 diabetes. Diabetic Medicine 1993;10(1):66‐73. [DOI] [PubMed] [Google Scholar]
- Vanninen E, Uusitupa M, Siitonen O, Laitinen J, Lansimies E. Habitual physical activity, aerobic capacity and metaboic control in patients with newly‐diagnosed Type 2 (non‐insulin‐dependent) diabetes mellitus: Effect of 1‐year diet and exercise intervention. Diabetologia 1992;35(4):340‐346. [DOI] [PubMed] [Google Scholar]
Wing 1988 {published data only}
- Wing RR, Epstein LH, Paternostro‐Bayles M, Kriska A, Nowalk MP, Gooding W. Exercise in a behavioural weight control programme for obese patients with Type 2 (non‐insulin‐dependent) diabetes. Diabetologia 1988;31(12):902‐9. [DOI] [PubMed] [Google Scholar]
Wing 1991 {published data only}
- Marcus MD, Wing RR, Guare J, Blair EH, Jawad A. Lifetime prevalence of major depression and its effect on treatment outcome in obese type II diabetic patients. Diabetes Care 1992;15(2):253‐5. [DOI] [PubMed] [Google Scholar]
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very‐low‐calorie diet on long‐term glycemic control in obese type 2 diabetic subjects. see comments. Archives of Internal Medicine 1991;151(7):1334‐40. [PubMed] [Google Scholar]
Wing 1994 {published data only}
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year‐long weight loss treatment for obese patients with type II diabetes: does including an intermittent very‐low‐calorie diet improve outcome?. American Journal of Medicine 1994;97(4):354‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ADA 2000 {published data only}
- American Diabetes Association. Diabetes mellitus and exercise. Diabetes Care 2000;23(1). [Google Scholar]
ADA 2002 {published data only}
- American Diabetes Association. Evidence‐based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2002;25(1):202‐12. [DOI] [PubMed] [Google Scholar]
Aizawa 2001 {published data only}
- Aizawa T. Treatment of type 2 diabetes: The sooner, the better [1]. Journal of Internal Medicine 2001;250(3):255‐257. [DOI] [PubMed] [Google Scholar]
Andersen 1987 {published data only}
- Andersen E, Hellstrom P, Kindstedt K, Hellstrom K. Effects of a high‐protein and low‐fat diet vs a low‐protein and high‐fat diet on blood glucose, serum lipoproteins, and cholesterol metabolism in noninsulin‐dependent diabetics. American Journal of Clinical Nutrition 1987;45(2):406‐13. [DOI] [PubMed] [Google Scholar]
Anderson 2003 {published data only}
- Anderson JW, Kendall CW, Jenkins DJA. Importance of weight management in type 2 diabetes: review with meta‐analysis of clinical studies. Journal of the American College of Nutrition 2003;22(5):331‐339. [DOI] [PubMed] [Google Scholar]
Anonymous 1988 {published data only}
- Anonymous. High‐fiber diet in poorly controlled Type II diabetics. Nurses Drug Alert 1988;12(12):94‐5. [Google Scholar]
Anonymous 1990 {published data only}
- Anonymous. Exercise and NIDDM. Diabetes Care 1990;13(7):785‐9. [DOI] [PubMed] [Google Scholar]
Arky 1983 {published data only}
- Arky RA. Prevention and therapy of diabetes mellitus. Nutrition Reviews 1983;41(6):165‐73. [DOI] [PubMed] [Google Scholar]
Arora 2005 {published data only}
- Arora SK, McFarlane SI. The case for low carbohydrate diets in diabetes management. Nutrition & Metabolism 2005;2(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
Ash 2003 {published data only}
- Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with type II diabetes: a randomised trial. International Journal of Obesity 2003;27:797‐802. [DOI] [PubMed] [Google Scholar]
Barnard 1982 {published data only}
- Barnard RJ, Lattimore L, Holly RG, Cherny S, Pritikin N. Response of non‐insulin‐dependent diabetic patients to an intensive program of diet and exercise. Diabetes Care 1982;5(4):370‐4. [DOI] [PubMed] [Google Scholar]
Barnard 1983 {published data only}
- Barnard RJ, Massey MR, Cherny S, O'Brien LT, Pritikin N. Long‐term use of a high‐complex‐carbohydrate, high‐fiber, low‐fat diet and exercise in the treatment of NIDDM patients. Diabetes Care 1983;6(3):268‐73. [DOI] [PubMed] [Google Scholar]
Barnard 1992 {published data only}
- Barnard RJ, Ugianskis EJ, Martin DA. The effects of an intensive diet and exercise program on patients with non‐insulin‐dependent diabetes mellitus and hypertension. Journal of Cardiopulmonary Rehabilitation 1992;12(3):194‐201. [Google Scholar]
Barnard 1994 {published data only}
- Barnard RJ, Jung T, Inkeles SB. Diet and exercise in the treatment of NIDDM. The need for early emphasis. Diabetes Care 1994;17(12):1469‐72. [DOI] [PubMed] [Google Scholar]
Barnard 1997 {published data only}
- Barnard RJ, DiLauro SC, Inkeles SB. Effects of intensive diet and exercise intervention in patients taking cholesterol‐lowering drugs. American Journal of Cardiology 1997;79(8):1112‐1114. [DOI] [PubMed] [Google Scholar]
Barnett 2001 {published data only}
- Barnett AH. Maximising outcomes in type 2 diabetes through weight management. British Journal of Cardiology 2001;8(2):101‐102+104‐105. [Google Scholar]
Bassand 2006 {published data only}
- Bassand JP. Managing cardiovascular risk in patients with metabolic syndrome. Clinical Cornerstone 2006;8(S1):S7‐S14. [DOI] [PubMed] [Google Scholar]
Bennett 1997 {published data only}
- Bennett PH. Primary prevention of NIDDM a practical reality. Diabetes‐Metabolism Reviews 1997;13(2):105‐111. [DOI] [PubMed] [Google Scholar]
Berg 2003 {published data only}
- Berg AO. Behavioural counselling in primary care to promote a healthy diet: recommendations and rationale. American Journal of Preventive Medicine 2003;24(1):93‐100. [DOI] [PubMed] [Google Scholar]
Bergenstal 1999 {published data only}
- Bergenstal RM. Management of type 2 diabetes mellitus. Postgraduate Medicine 1999;105(1):121‐136. [PubMed] [Google Scholar]
Bhaskarabhatla 2004 {published data only}
- Bhaskarabhatla KV, Birrer R. Physical activity and type 2 diabetes: tailoring exercise to optimise fitness and glycemic control. Physician & Sportsmedicine 2004;32(1):13‐17. [DOI] [PubMed] [Google Scholar]
Bjorntorp 1992 {published data only}
- Bjorntorp PA. Efficacy of training in obese diabetic patients. Diabetes Care 1992;15(11):1783‐6. [DOI] [PubMed] [Google Scholar]
Blake 1992 {published data only}
- Blake GH. Control of type II diabetes. Reaping the rewards of exercise and weight loss. Postgraduate Medicine 1992;92(6):129‐32, 137. [DOI] [PubMed] [Google Scholar]
Blonk 1994 {published data only}
- Blonk MC, Jacobs M, Biesheuvel EHE, Weeda‐Mannak WL, Heine RJ. Influence on weight loss in Type 2 diabetic patients: Little long‐term benefit from group behaviour therapy and exercise training. Diabetic Medicine 1994;11(5):449‐457. [DOI] [PubMed] [Google Scholar]
Bloomgarden 2000 {published data only}
- Bloomgarden ZT. Obesity and diabetes. Diabetes Care 2000;23(10):1584‐90. [DOI] [PubMed] [Google Scholar]
Bloomgarden 2004 {published data only}
- Bloomgarden ZT. Glycemic control: control of glycemia. Diabetes care 2004;27(5):1227‐1234. [DOI] [PubMed] [Google Scholar]
Bloomgarden 2005 {published data only}
- Bloomgarden ZT. Diabetic nephropathy. Diabetes care 2005;28(3):745‐751. [DOI] [PubMed] [Google Scholar]
Boden 2005 {published data only}
- Boden G, Sargrad K, Homko C, Mozzoli M. TPS: effect of a low carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Annual of Internal Medicine 2005;142(6):403‐411. [DOI] [PubMed] [Google Scholar]
Bonanome 1991 {published data only}
- Bonanome A, Visona A, Lusiani L, Beltramello G, Confortin L, Biffanti S, et al. Carbohydrate and lipid metabolism in patients with non‐insulin‐dependent diabetes mellitus: effects of a low‐fat, high‐carbohydrate diet vs a diet high in monounsaturated fatty acids. American Journal of Clinical Nutrition 1991;54(3):586‐90. [DOI] [PubMed] [Google Scholar]
Bonnefont‐Rousselot {published data only}
- Bonnefont‐Rousselot D. The role of antioxidant micronutrients in the prevention of diabetic complications. Treatments in Endocrinology 2004;3(1):41‐52. [DOI] [PubMed] [Google Scholar]
Borghouts 2000 {published data only}
- Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. International Journal of Sports Medicine 2000;21(1):1‐12. [DOI] [PubMed] [Google Scholar]
Boule 2001 {published data only}
- Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. Jama 2001;286(10):1218‐27. [DOI] [PubMed] [Google Scholar]
Boule 2002 {published data only}
- Boule, Haddad, Sigal, Kenny. Exercise for type 2 diabetes mellitus. 2.
Bourn 1996a {published data only}
- Bourn DM. The potential for lifestyle change to influence the progression of impaired glucose tolerance to non‐insulin‐dependent diabetes mellitus. Diabetic Medicine 1996;13(11):938‐45. [DOI] [PubMed] [Google Scholar]
Bourn 1996b {published data only}
- Bourn DM, Mann JI. The 3‐yr follow‐up of subjects with impaired glucose tolerance or non‐insulin dependent diabetes mellitus in a diet and exercise intervention programme. Diabetes, Nutrition & Metabolism ‐ Clinical & Experimental 1996;9(5):240‐246. [Google Scholar]
Brand‐Miller 2003 {published data only}
- Brand‐Miller J, Hayne S, Petocz P, Colagiuri S. Low‐glycemic index diets in the management of diabetes: a meta‐analysis of randomized controlled trials. Diabetes care 2003;26(8):2261‐2267. [DOI] [PubMed] [Google Scholar]
Brown 1996 {published data only}
- Brown SA, Upchurch S, Anding R, Winter M, Ramirez G. Promoting weight loss in type II diabetes. Diabetes Care 1996;19(6):613‐24. [DOI] [PubMed] [Google Scholar]
Brown 2002 {published data only}
- Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self‐management education for Mexican Americans: the Starr County border health initiative. Diabetes Care 2002;25(2):259‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brownell 1998 {published data only}
- Brownell KD. Diet, exercise and behavioural intervention: the nonpharmacological approach. European Journal of Clinical Investigation 1998;28(Suppl 2):19‐21:19‐21; discussion 22. [DOI] [PubMed] [Google Scholar]
Calle‐Pascual 1992 {published data only}
- Calle‐Pascual AL, Rodriguez C, Camacho F, Sanchez R, Martin‐Alvarez PJ, Yuste E, et al. Behaviour modification in obese subjects with type 2 diabetes mellitus. Diabetes Research and Clinical Practice 1992;15(2):157‐162. [DOI] [PubMed] [Google Scholar]
Campbell 2001 {published data only}
- Campbell L, Rossner S. Management of obesity in patients with Type 2 diabetes. Diabetic Medicine 2001;18(5):345‐54. [DOI] [PubMed] [Google Scholar]
Capstick 1997 {published data only}
- Capstick F, Brooks BA, Burns CM, Zilkens RR, Steinbeck KS, Yue DK. Very low calorie diet (VLCD): A useful alternative in the treatment of the obese NIDDM patient. Diabetes Research & Clinical Practice 1997;36(2):105‐111. [DOI] [PubMed] [Google Scholar]
Carr 2005 {published data only}
- Carr DB, Utzschneider KM, Boyko EJ, Asberry PJ, Hull RL, Kodama K, Callahan HS, Matthys CC, Leonetti DL, Schwartz RS, Kahn SE, Fujimoto WY. A reduced‐fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra‐abdominal fat and improves insulin sensitivity but not beta‐cell function. Diabetes 2005;54(2):340‐347. [DOI] [PubMed] [Google Scholar]
Cefalu 2005 {published data only}
- Cefalu CA, Cefalu WT. Controlling hypoglycemia in type 2 diabetes: which agent for which patient. Journal of Family Practice 2005;54(10):855‐862. [PubMed] [Google Scholar]
Chakravarthy 2002 {published data only}
- Chakravarthy MV, Joyner MJ, Booth FW. An obligation for primary care physicians to prescribe physical activity to sedentary patients to reduce the risk of chronic health conditions. Mayo Clinic Proceedings 2002;77(2):165‐73. [DOI] [PubMed] [Google Scholar]
Charatan 2001 {published data only}
- Charatan F. Exercise and diet reduce risk of diabetes, US study shows. BMJ 2001;323(7309):359. [PMC free article] [PubMed] [Google Scholar]
Chen 1987 {published data only}
- Chen JF. A hemorrheological study on the effect of acupuncture in treating diabetes mellitus. Journal of Traditional Chinese Medicine 1987;7(2):95‐100. [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen JLCDPMY. Effect of acupuncture on plasmic levels of insulin, glucagon and hypercoagulability in NIDDM complicated by acute cerebral infarction. Journal of Traditional Chinese Medicine 2001;21(4):267‐9. [PubMed] [Google Scholar]
Chipkin 2001 {published data only}
- Chipkin SR, Klugh SA, Chasan‐Taber L. Exercise and diabetes. Cardiology Clinics 2001;19(3):489‐505. [DOI] [PubMed] [Google Scholar]
Christensen 1998 {published data only}
- Christensen JO, Svendsen OL, Hassager C, Christiansen C. Leptin in overweight postmenopausal women: no relationship with metabolic syndrome X or effect of exercise in addition to diet. International Journal of Obesity & Related Metabolic Disorders 1998;22(3):195‐9. [DOI] [PubMed] [Google Scholar]
Ciliska 1995 {published data only}
- Ciliska D, Kelly C, Petrov N, Chalmers J. A Review of Weight Loss Interventions for Obese People wth Non‐Insulin‐Dependent Diabetes Mellitus. Canadian Journal of Diabetes Care 1995;19(2):10‐5. [Google Scholar]
Clark 2001 {published data only}
- Clark M, Hampson SE. Implementing a psychological intervention to improve lifestyle self‐management in patients with type 2 diabetes. Patient Education & Counseling 2001;42(3):247‐56. [DOI] [PubMed] [Google Scholar]
Coleman 2003 {published data only}
- Coleman CI, Hebert JH, Reddy P. The effects of panax ginseng on quality of life. Journal of Clinical Pharmacy & Therapeutics 2003;28(1):5‐15. [DOI] [PubMed] [Google Scholar]
Collins 1995 {published data only}
- Collins RW, Anderson JW. Medication cost savings associated with weight loss for obese non‐insulin‐dependent diabetic men and women. Preventive Medicine 1995;24(4):369‐74. [DOI] [PubMed] [Google Scholar]
Colman 1995 {published data only}
- Colman E, Katzel LI, Rogus E, Coon P, Muller D, Goldberg AP. Weight loss reduces abdominal fat and improves insulin action in middle‐aged and older men with impaired glucose tolerance. Metabolism: Clinical & Experimental 1995;44(11):1502‐8. [DOI] [PubMed] [Google Scholar]
Comi 1995 {published data only}
- Comi D, Brugnani M, Gianino A. Metabolic effects of hypocaloric high‐carbohydrate/high‐fibre diet in non‐insulin dependent diabetic patients. European Journal of Clinical Nutrition 1995;49(3). [PubMed] [Google Scholar]
CRD 2002 {published data only}
- Centre for Reviews Dissemination, Reviewers. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. Database of Abstracts of Reviews of Effectiveness June 2002;2:2. [Google Scholar]
Creviston 2001 {published data only}
- Creviston T, Quinn L. Exercise and physical activity in the treatment of type 2 diabetes. Nursing Clinics of North America 2001;36(2):243‐71, vi. [PubMed] [Google Scholar]
de Fine Olivarius 06 {published data only}
- Fine Olivarius N, Andreasen H, Siersma V, Richelsen B, Beck‐Nielsen H. Changes in patient weight and the impact of antidiabetic therapy during the first 5 years after diagnosis of diabetes mellitus. Diabetologia 2006;49(9):2058‐2067. [DOI] [PubMed] [Google Scholar]
de Sonnaville 1997 {published data only}
- Sonnaville JJ, Bouma M, Colly LP, Deville W, Wijkel D, Heine RJ. Sustained good glycaemic control in NIDDM patients by implementation of structured care in general practice: 2‐year follow‐up study. Diabetologia 1997;40(11):1334‐40. [DOI] [PubMed] [Google Scholar]
Delahanty 1995 {published data only}
- Delahanty LM. Impact of intensified dietary therapy on energy and nutrient intakes and fatty acid composition of serum lipids in patients with recently diagnosed non‐insulin‐dependent diabetes mellitus... summary and commentary. Diabetes Spectrum 1995;8(2):102‐3. [DOI] [PubMed] [Google Scholar]
Dengel 1998 {published data only}
- Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. American Journal of Hypertension 1998;11(12):1405‐12. [DOI] [PubMed] [Google Scholar]
Devlin 1986 {published data only}
- Devlin JT. Effects of exercise in diabetes mellitus. Journal of the Florida Medical Association 1986;73(8):602‐3. [PubMed] [Google Scholar]
Dey 2002 {published data only}
- Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Alternative Medicine Review 2002;7(1):45‐58. [PubMed] [Google Scholar]
Dhindsa 2003 {published data only}
- Dhindsa P, Scott AR, Donnelly R. Metabolic and cardiovascular effects of very‐low‐calorie diet therapy in obese patients with Type 2 diabetes in secondary failure: Outcomes after 1 year. Diabetic Medicine 2003;20(4):319‐24. [DOI] [PubMed] [Google Scholar]
Dunstan 2002 {published data only}
- Dunstan DW, Daly RM, Owen N, Jolley D, Courten M, Shaw J, et al. High‐intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care 2002;25(10):1729‐36. [DOI] [PubMed] [Google Scholar]
Elson 1998 {published data only}
- Elson DF, Meredith M. Therapy for type 2 diabetes mellitus. Wisconsin Medical Journal 1998;97(3):49‐54. [PubMed] [Google Scholar]
Eriksson 1991 {published data only}
- Eriksson KF, Lindgarde F. Prevention of type 2 (non‐insulin‐dependent) diabetes mellitus by diet and physical exercise. The 6‐year Malmo feasibility study. Diabetologia 1991;34(12):891‐8. [DOI] [PubMed] [Google Scholar]
Eriksson 1997 {published data only}
- Eriksson J, Taimela S, Koivisto VA. Exercise and the metabolic syndrome. Diabetologia 1997;40(2):125‐35. [DOI] [PubMed] [Google Scholar]
Eriksson 1998 {published data only}
- Eriksson KF, Lindgarde F. No excess 12‐year mortality in men with impaired glucose tolerance who participated in the Malmo Preventive Trial with diet and exercise. Diabetologia 1998;41(9):1010‐6. [DOI] [PubMed] [Google Scholar]
Eriksson 1999a {published data only}
- Eriksson J, Lindstrom J, Valle T, Aunola S, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design and 1‐year interim report on the feasibility of the lifestyle intervention programme. Diabetologia 1999;42(7):793‐801. [DOI] [PubMed] [Google Scholar]
Eriksson 1999b {published data only}
- Eriksson JG. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Medicine 1999;27(6):381‐91. [DOI] [PubMed] [Google Scholar]
Ernst 2001 {published data only}
- Ernst E. Encouraging findings for Tibetan medicines in type 2 diabetes. Focus on Alternative & Complementary Therapies 2001;6(2):125. [Google Scholar]
Evans 1995 {published data only}
- Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. Of: ; Review Of: 23 refs. Journals of Gerontology. Series A, Biological Sciences & Medical Sciences 1995;50(pp):147‐50. [DOI] [PubMed] [Google Scholar]
Evans 1997 {published data only}
Evans 2002 {published data only}
- Evans MF. Can we prevent high‐risk patients from getting type 2 diabetes?. Canadian Family Physician 2002;48:279‐81. [PMC free article] [PubMed] [Google Scholar]
Fletcher 2002 {published data only}
- Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. Journal of Cardiovascular Nursing 2002;16(2):17‐23. [DOI] [PubMed] [Google Scholar]
Franz 1995 {published data only}
- Franz MJ, Monk A, Barry B, McClain K, Weaver T, Cooper N, et al. Effectiveness of medical nutrition therapy provided by dietitians in the management of non‐insulin‐dependent diabetes mellitus: a randomized, controlled clinical trial. Journal of the American Dietetic Association 1995;95(9):1009‐17. [DOI] [PubMed] [Google Scholar]
Frati 1990 {published data only}
- Frati AC, Jimenez E, Raul Ariza C. Hypoglycemic effect of Opuntia ficus indica in non insulin‐dependent diabetes mellitus patients. Phytotherapy Research 1990;4(5):195‐197. [Google Scholar]
Fujinuma 1999 {published data only}
- Fujinuma H, Abe R, Yamazaki T, Seino H, Kikuchi H, Hoshino T, et al. Effect of exercise training on doses of oral agents and insulin. letter; comment. Diabetes Care 1999;22(10):1754‐5. [DOI] [PubMed] [Google Scholar]
Funnell 2004 {published data only}
- Funnell MM, Kruger DF. Type 2 diabetes: treat to target. Nurse Practitioner 2004;29(1):11‐15. [DOI] [PubMed] [Google Scholar]
Gaede 2001 {published data only}
- Gaede P, Beck M, Vedel P, Pedersen O. Limited impact of lifestyle education in patients with Type 2 diabetes mellitus and microalbuminuria: results from a randomized intervention study. Diabetic Medicine 2001;18(2):104‐8. [DOI] [PubMed] [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen G V H, Parving H, Pedersen O. Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. The New England Journal of Medicine 2003;348(5):383‐93. [DOI] [PubMed] [Google Scholar]
Gaede 2006 {published data only}
- Gaede PH. Intensified multofactorial intervention in patients with type 2 diabetes and microalbuminuria: rationale and effect on late‐diabetic complications. Danish Medical Bulletin 2006;53(3):258‐284. [PubMed] [Google Scholar]
Gannon 2006 {published data only}
- Gannon MC, Nuttall FQ. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutrition & Metabolism 2006;3(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 1990 {published data only}
- Garg A, Bonanome A, Grundy SM, Unger RH, Breslau NA, Pak CY. Effects of dietary carbohydrates on metabolism of calcium and other minerals in normal subjects and patients with noninsulin‐dependent diabetes mellitus. Journal of Clinical Endocrinology & Metabolism 1990;70(4):1007‐13. [DOI] [PubMed] [Google Scholar]
Garg 1993 {published data only}
- Garg A. Dietary monounsaturated fatty acids for patients with diabetes mellitus. Annals of the New York Academy of Sciences 1993;683:199‐206. [DOI] [PubMed] [Google Scholar]
Garg 1998 {published data only}
- Garg A. High‐monounsaturated‐fat diets for patients with diabetes mellitus: a meta‐analysis. American Journal of Clinical Nutrition 1998;67(3 Suppl):577S‐582S. [DOI] [PubMed] [Google Scholar]
Gautier 1995 {published data only}
- Gautier JF, Scheen A, Lefebvre PJ. Exercise in the management of non‐insulin‐dependent (type 2) diabetes mellitus. International Journal of Obesity & Related Metabolic Disorders 1995;19(Suppl 4):S58‐61. [PubMed] [Google Scholar]
Genuth 2003 {published data only}
- Genuth S, Eastman R, Kahn R, Klein R, Lachin J, Lebovitz H, Nathan D, Vinicor F. Implications of the united kingdon prospective diabetes study. Diabetes Care 2003;26(Supplement 1):S28‐S32. [DOI] [PubMed] [Google Scholar]
Glasgow 1989 {published data only}
- Glasgow RE, Toobert DJ, Mitchell DL, Donnelly JE, Calder D. Nutrition education and social learning interventions for type II diabetes. Diabetes Care 1989;12(2):150‐2. [DOI] [PubMed] [Google Scholar]
Goldberg 1998 {published data only}
- Goldberg RB. Prevention of type 2 diabetes. Medical Clinics of North America 1998;82(4):805‐21. [DOI] [PubMed] [Google Scholar]
Gonzelez 1994 {published data only}
- Gonzelez C, Stern MP, Mitchell BD, Valdez RA, Haffner SM, Perez BA. Clinical characteristics of type II diabetic subjects consuming high versus low carbohydrate diets in Mexico City and San Antonio, Texas. Diabetes Care 1994;17(5):397‐404. [DOI] [PubMed] [Google Scholar]
Grundy 1991 {published data only}
- Grundy SM. Dietary therapy in diabetes mellitus. Is there a single best diet?. Diabetes Care 1991;14(9):796‐801. [DOI] [PubMed] [Google Scholar]
Grundy 1999b {published data only}
- Grundy SM, Blackburn G, Higgins M, Lauer R, Perri MG, Ryan D. Physical activity in the prevention and treatment of obesity and its comorbidities. Medicine & Science in Sports & Exercise 1999;31(11 Suppl):S502‐8. [DOI] [PubMed] [Google Scholar]
Guerrero‐Romero 2005 {published data only}
- Guerrero‐Romero F, Rodriguez‐Moran M. Complementary therapies for diabetes: the case for chromium, magnesium and antioxidants. Archives of Medical Research 2005;36(3):250‐257. [DOI] [PubMed] [Google Scholar]
Gumbiner 1998 {published data only}
- Gumbiner B, Low CC, Reaven PD. Effects of a monounsaturated fatty acid‐enriched hypocaloric diet on cardiovascular risk factors in obese patients with type 2 diabetes. Diabetes Care 1998;21(1):9‐15. [DOI] [PubMed] [Google Scholar]
Gumbiner 1999 {published data only}
- Gumbiner B. The treatment of obesity in type 2 diabetes mellitus. Primary Care; Clinics in Office Practice 1999;26(4):869‐83. [DOI] [PubMed] [Google Scholar]
Hadden 1982 {published data only}
- Hadden DR. Food and diabetes: the dietary treatment of insulin‐dependent and non‐insulin‐dependent diabetes. Clinics in Endocrinology & Metabolism 1982;11(2):503‐24. [DOI] [PubMed] [Google Scholar]
Hamdy 2001 {published data only}
- Hamdy O, Goodyear LJ, Horton ES. Diet and exercise in type 2 diabetes mellitus. Endocrinology & Metabolism Clinics of North America 2001;30(4):883‐907. [DOI] [PubMed] [Google Scholar]
Hamilton 1992 {published data only}
- Hamilton CC, Geil PB, Anderson JW. Management of obesity in diabetes mellitus. Diabetes Educator 1992;18(5):407‐10. [DOI] [PubMed] [Google Scholar]
Hanefeld 1989 {published data only}
- Hanefeld M, Weck M. Very low calorie diet therapy in obese non‐insulin dependent diabetes patients. International Journal of Obesity & Related Metabolic Disorders 1989;13(Suppl 2):33‐7. [PubMed] [Google Scholar]
Hart 2006 {published data only}
- Hart GR, Furniss JL, Laurie D, Durham SK. Measurement of vitamin D status: background, clinical use, and methodologies. Clinical Laboratory 2006;52(7‐8):335‐343. [PubMed] [Google Scholar]
Heath 1987 {published data only}
- Heath GW, Leonard BE, Wilson RH, Kendrick JS, Powell KE. Community‐based exercise intervention: Zuni Diabetes Project. Diabetes Care 1987;10(5):579‐83. [DOI] [PubMed] [Google Scholar]
Heath 1991 {published data only}
- Heath GW, Wilson RH, Smith J, Leonard BE. Community‐based exercise and weight control: diabetes risk reduction and glycemic control in Zuni Indians. American Journal of Clinical Nutrition 1991;53(6 Suppl):1642S‐1646S. [DOI] [PubMed] [Google Scholar]
Heine 1989 {published data only}
- Heine RJ, Mulder C, Popp‐Snijders C, Van DMJ, Van DVEA. Linoleic‐acid‐enriched diet: Long‐term effects on serum lipoprotein and apolipoprotein concentrations in insulin sensitivity in noninsulin‐dependent diabetic patients. American Journal of Clinical Nutrition 1989;49(3):448‐456. [DOI] [PubMed] [Google Scholar]
Held 1991 {published data only}
- Held NA. Weight loss strategies in diabetes. Connecticut Medicine 1991;55(11):647‐51. [PubMed] [Google Scholar]
Helmrich 1994 {published data only}
- Helmrich SP, Ragland DR, Paffenbarger RS, Jr. Prevention of non‐insulin‐dependent diabetes mellitus with physical activity. Medicine & Science in Sports & Exercise 1994;26(7):824‐30. [PubMed] [Google Scholar]
Henry 1991 {published data only}
- Henry RR, Gumbiner B. Benefits and limitations of very‐low‐calorie diet therapy in obese NIDDM. Diabetes Care 1991;14(9):802‐23. [DOI] [PubMed] [Google Scholar]
Hensrud 2001 {published data only}
- Hensrud DD. Dietary treatment and long‐term weight loss and maintenance in type 2 diabetes. Obesity Research 2001;9(4):348S‐53S. [DOI] [PubMed] [Google Scholar]
Holmes 1987 {published data only}
- Holmes J, Hadden DR, Atkinson AB, Kennedy AL, Wilson EA. Assessment of diet adherence in relation to long‐term follow up of non‐insulin‐dependent diabetics. Practical Diabetes 1987;4(6):276‐278. [Google Scholar]
Horrocks 1987 {published data only}
- Horrocks PM, Blackmore R, Wright AD. A long‐term follow‐up of dietary advice in maturity onset diabetes: the experience of one centre in the UK prospective study. Diabetic Medicine 1987;4(3):241‐4. [DOI] [PubMed] [Google Scholar]
Hu 1999 {published data only}
- Hu FB, Sigal RJ, Rich‐Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999;282(15):1433‐9. [DOI] [PubMed] [Google Scholar]
Hu 2001a {published data only}
- Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA, Speizer FE, et al. Physical activity and risk for cardiovascular events in diabetic women. Annals of Internal Medicine 2001;134(2):96‐105. [DOI] [PubMed] [Google Scholar]
Hu 2001b {published data only}
- Hu FB, Dam RM, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44(7):805‐17. [DOI] [PubMed] [Google Scholar]
Huh 1996 {published data only}
- Huh KB, Lee HC, Cho SY, Lee JH, Song YD. The role of insulin resistance in Korean patients with coronary atherosclerosis. Diabetes 1996;45(3). [DOI] [PubMed] [Google Scholar]
Hutton 2004 {published data only}
- Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. American Journal of Clinical Nutrition 2004;80(6):1461‐1468. [DOI] [PubMed] [Google Scholar]
Ismail 2004 {published data only}
- Ismail K, Winkley K, Rabe‐Hesketh S. Systematic review and meta‐analysis of randomised controlled trials of psychological interventions to improve glycemic ocntrol on patients with type 2 diabetes. Lancet 2004;363(9421):1589‐1597. [DOI] [PubMed] [Google Scholar]
Ito 2001 {published data only}
- Ito H, Ohshima A, Tsuzuki M, Ohto N, Yanagawa M, Maruyama T, et al. Effects of increased physical activity and mild calorie restriction on heart rate variability in obese women. Japanese Heart Journal 2001;42(4):459‐69. [DOI] [PubMed] [Google Scholar]
Ivy 1997 {published data only}
- Ivy JL. Role of exercise training in the prevention and treatment of insulin resistance and non‐insulin‐dependent diabetes mellitus. Sports Medicine 1997;24(5):321‐36. [DOI] [PubMed] [Google Scholar]
James 1998 {published data only}
- James SA, Jamjoum L, Raghunathan TE, Strogatz DS, Furth ED, Khazanie PG. Physical activity and NIDDM in African‐Americans: the Pitt County Study. Diabetes Care 1998;21(4):555‐62. [DOI] [PubMed] [Google Scholar]
Janssen 2002 {published data only}
- Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy‐restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 2002;25(3):431‐8. [DOI] [PubMed] [Google Scholar]
Jenkins 2003 {published data only}
- Jenkins DJA, Kendall CWC, Marchie A, Jenkins AL, Augustin LSA, Ludwig DS, Barnard ND, Anderson JW. Type 2 diabetes and the vegetarian diet. American Journal of Clinical Nutrition 2003;78(Supplement 3):610S‐616S. [DOI] [PubMed] [Google Scholar]
Jenkins 2004 {published data only}
- Jenkins DJA. Psychological, physiological and drug interventions for t ype 2 diabetes. Lancet 2004;363(9421):1569‐1570. [DOI] [PubMed] [Google Scholar]
Jeppesen 1997 {published data only}
- Jeppesen J, Schaaf P, Jones C, Zhou M, Chen YI, Reaven GM. Effects of low‐fat, high‐carbohydrate diets on risk factors for ischemic heart disease in postmenopausal women [corrected] [published erratum appears in AM J CLIN NUTR 1997 Aug; 66(2): 437]. American Journal of Clinical Nutrition 1997;65(4):1027‐33. [DOI] [PubMed] [Google Scholar]
Kao 2000 {published data only}
- Kao PC, Wu TJ, Ho LL, Li XJ. Current trends and new approaches in the management of diabetes mellitus. Annals of Clinical & Laboratory Science 2000;30(4):339‐45. [PubMed] [Google Scholar]
Karlstrom 1989 {published data only}
- Karlstrom B, Nydahl M, Vessby B. Dietary habits and effects of dietary advice in patients with type 2 diabetes. Results from a one‐year intervention study. European Journal of Clinical Nutrition 1989;43(1):59‐68. [PubMed] [Google Scholar]
Kelley 1995 {published data only}
- Kelley DE. Effects of weight loss on glucose homeostasis in NIDDM. Diabetes Reviews 1995;3(3):366‐377. [Google Scholar]
Kelley 1999 {published data only}
- Kelley DE, Goodpaster BH. Effects of physical activity on insulin action and glucose tolerance in obesity. Medicine & Science in Sports & Exercise 1999;31(11 Suppl):S619‐23. [DOI] [PubMed] [Google Scholar]
Kelley 2001 {published data only}
- Kelley DE, Goodpaster BH. Effects of exercise on glucose homeostasis in Type 2 diabetes mellitus. Medicine & Science in Sports & Exercise 2001;33(6 Suppl):S495‐501:S495‐501; discussion S528‐9. [DOI] [PubMed] [Google Scholar]
Kelly 2000 {published data only}
- Kelly GS. Insulin resistance: lifestyle and nutritional interventions. Alternative Medicine Review 2000;5(2):109‐32. [PubMed] [Google Scholar]
Kennedy 1982 {published data only}
- Kennedy L, Walshe K, Hadden DR, Weaver JA, Buchanan KD. The effect of intensive dietary therapy on serum high density lipoprotein cholesterol in patients with Type 2 (non‐insulin‐dependent) diabetes mellitus: a prospective study. Diabetologia 1982;23(1):24‐7. [DOI] [PubMed] [Google Scholar]
Kirk 2003 {published data only}
- Kirk A, Mutrie N, MacIntyre P, Fisher M. Increasing physical activity in people with type 2 diabetes. Diabetes Care 2003;26(4):1186‐92. [DOI] [PubMed] [Google Scholar]
Kirkman 1994 {published data only}
- Kirkman MS, Weinberger M, Landsman PB, Samsa GP, Shortliffe EA, Simel DL, et al. A telephone‐delivered intervention for patients with NIDDM. Effect on coronary risk factors. Diabetes Care 1994;17(8):840‐6. [DOI] [PubMed] [Google Scholar]
Kligler 2003 {published data only}
- Kligler B, Lynch D. An intergrative approach to the management of type 2 diabetes mellitus. Alternative Therapies in Health & Medicine 2003;9(6):24‐32. [PubMed] [Google Scholar]
Knowler 1994 {published data only}
- Knowler WC, Narayan KMV. Prevention of non‐insulin‐dependent diabetes mellitus. Preventive Medicine 1994;23(5):701‐703. [DOI] [PubMed] [Google Scholar]
Kraus 2001 {published data only}
- Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, et al. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Medicine & Science in Sports & Exercise 1774;33(10):1774‐84. [DOI] [PubMed] [Google Scholar]
Kriska 2000 {published data only}
- Kriska A. Physical activity and the prevention of type 2 diabetes mellitus: how much for how long?. Sports Medicine 2000;29(3):147‐51. [DOI] [PubMed] [Google Scholar]
Kriska 2002 {published data only}
- Kriska A. Striving for a more active community. Lessons from the diabetes prevention program and beyond. American Journal of Preventive Medicine 2002;22(4 Suppl):6‐7. [DOI] [PubMed] [Google Scholar]
Kulkarni 2006 {published data only}
- Kulkarni K. Diets do not fail: the success of medical nutrition therapy in patients with diabetes. Endocrine Practice 2006;12(Supplement 1):121‐123. [DOI] [PubMed] [Google Scholar]
Lehman 2005 {published data only}
- Lehman R. Evidently. Evidence Based Medicine 2005;10(4):105. [Google Scholar]
Lehmann 1998 {published data only}
- Lehmann R. The effects of exercise on cardiovascular risk factors in Type 2 diabetes mellitus. Practical Diabetes International 1998;15(5):151‐6. [Google Scholar]
Liao 2003 {published data only}
- Liao D, Asberry PJ, Shofer JB, Callahan H, Matthys C, Boyko EJ, et al. Improvement of BMI, body composition, and body fat distribution with lifestyle modification in Japanese Americans with impaired glucose tolerance. Diabetes Care 2002;25(9):1504‐10. [DOI] [PubMed] [Google Scholar]
Ligtenberg 1998 {published data only}
- Ligtenberg PC, Godaert GL, Hillenaar EF, Hoekstra JB. Influence of a physical training program on psychological well‐being in elderly type 2 diabetes patients. Psychological well‐being, physical training, and type 2 diabetes. Diabetes Care 1998;21(12):2196‐7. [DOI] [PubMed] [Google Scholar]
Lindstrom 2003 {published data only}
- Lindstrom J, Eriksson JG, Valle TT, Aunola S, Cepaitis Z, Hakumaki M, et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the finnish diabetes prevention study: Results from a randomized clinical trial. Journal of the American Society of Nephrology 2003;14(SUPPL 2):S108‐13. [DOI] [PubMed] [Google Scholar]
Ling 1994 {published data only}
- Ling Z. Acupuncture treatment of Type II diabetes mellitus (NIDDM): a clinical study of 21 cases. International Journal of Clinical Acupuncture 1994;5(3):261‐5. [Google Scholar]
Little 1996 {published data only}
- Little P, Margetts B. The importance of diet and physical activity in the treatment of conditions managed in general practice. Of: ; Review Of:. British Journal of General Practice 1996;46(404):187‐192. [PMC free article] [PubMed] [Google Scholar]
Lodha 2000 {published data only}
- Lodha R, Bagga A. Traditional Indian systems of medicine. Annals of the Academy of Medicine, Singapore 2000;29(1):37‐41. [PubMed] [Google Scholar]
Lomasky 1990 {published data only}
- Lomasky SJ, D'Eramo G, Shamoon H, Fleischer N. Relationship of insulin secretion and glycemic response to dietary intervention in non‐insulin‐dependent diabetes. Archives of Internal Medicine 1990;150(1):169‐72. [PubMed] [Google Scholar]
Maggio 1997 {published data only}
- Maggio CA, Pi‐Sunyer FX. The prevention and treatment of obesity: application to type 2 diabetes. Diabetes Care 1997;20(11):1744‐66. [DOI] [PubMed] [Google Scholar]
Manson 1992b {published data only}
- Manson JE, Nathan DM, Krolewski AS. A prospective study of exercise and incidence of diabetes among US male physicians.. JAMA 1992;268(63‐7). [PubMed] [Google Scholar]
Manson 1994 {published data only}
- Manson JE, Spelsberg A. Primary prevention of non‐insulin‐dependent diabetes mellitus. American Journal of Preventive Medicine 1994;10(3):172‐84. [PubMed] [Google Scholar]
Mazzeo 2001 {published data only}
- Mazzeo RS, Tanaka H. Exercise prescription for the elderly: current recommendations. Sports Medicine 2001;31(11):809‐18. [DOI] [PubMed] [Google Scholar]
McCarty 1997 {published data only}
- McCarty MF. Exploiting complementary therapeutic strategies for the treatment of type II diabetes and prevention of its complications. Medical Hypotheses 1997;49(2):143‐52. [DOI] [PubMed] [Google Scholar]
Meinders 1992 {published data only}
- Meinders AE, Pijl H. Very low calorie diets and recently developed anti‐obesity drugs for treating overweight in non‐insulin dependent diabetics. International Journal of Obesity & Related Metabolic Disorders 1992;16(Suppl 4):S35‐9. [PubMed] [Google Scholar]
Melander 1996 {published data only}
- Melander A. Review of previous impaired glucose tolerance intervention studies. Diabetic Medicine 1996;13(3 Suppl 2):S20‐2. [PubMed] [Google Scholar]
Mensink 2003a {published data only}
- Mensink M, Corpeleijn E, Feskens EJM, Kruijshoop M, Saris WHM, Bruin TWA, et al. Study on lifestyle‐intervention and impaired glucose tolerance Maastricht (SLIM): Design and screening results. Diabetes Research & Clinical Practice 61(1):49‐58. [DOI] [PubMed] [Google Scholar]
Mensink 2003b {published data only}
- Mensink M, Feskens EJM, Saris WHM, Bruin TWA, Blaak EE. Study on lifestyle intervention and impaired glucose tolerance Maastricht (SLIM): Preliminary results after one year. International Journal of Obesity 2003;27(3):377‐84. [DOI] [PubMed] [Google Scholar]
Metz 2000 {published data only}
- Metz JA, Stern JS, Kris‐Etherton P, Reusser ME, Morris CD, Hatton DC, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Archives of Internal Medicine 2000;160(14):2150‐8. [DOI] [PubMed] [Google Scholar]
Miles 2000 {published data only}
- Miles P, Kerr D. Very low calorie diets in diabetes: the Bournemouth experience. Journal of Diabetes Nursing 2000;4(4):108‐11. [Google Scholar]
Miller 1996 {published data only}
- Miller M. Type II diabetes: a treatment approach for the older patient. Geriatrics 1996;51(8):43‐4, 47‐9:43‐4, 47‐9; quiz 50. [PubMed] [Google Scholar]
Monteiro 2005 {published data only}
- Monteiro P, Goncalves L, Providencia LA. Diabetes and cardiovascular disease: the road to cardioprotection. Heart 2005;91(12):1621‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montori 2001 {published data only}
- Montori V. Changes in diet and physical activity prevented type 2 diabetes mellitus in persons with impaired glucose tolerance. ACP Journal Club 2001;135(3):101. [Google Scholar]
Morelli 2000 {published data only}
- Morelli V, Zoorob RJ. Alternative therapies: Part I. Depression, diabetes, obesity. American Family Physician 2000;62(5):1051‐60. [PubMed] [Google Scholar]
Mustajoki 2001 {published data only}
- Mustajoki P, Pekkarinen T. Very low energy diets in the treatment of obesity. Obesity Reviews 2001;2(1):61‐72. [DOI] [PubMed] [Google Scholar]
Myers 2001 {published data only}
- Myers KA. Lifestyle changes can prevent the development of diabetes mellitus. CMAJ (Canadian Medical Association Journal) 2001;164(13):1885. [PMC free article] [PubMed] [Google Scholar]
Namdul 2001 {published data only}
- Namdul T, Sood A, Ramakrishnan L, Pandey RM, Moorthy D. Efficacy of Tibetan medicine as an adjunct in the treatment of type 2 diabetes. Diabetes Care 2001;24(1):175‐6. [DOI] [PubMed] [Google Scholar]
Narayan 1998 {published data only}
- Narayan KM, Hoskin M, Kozak D, Kriska AM, Hanson RL, Pettitt DJ, et al. Randomized clinical trial of lifestyle interventions in Pima Indians: a pilot study. Diabetic Medicine 1998;15(1):66‐72. [DOI] [PubMed] [Google Scholar]
Narayan 2001 {published data only}
- Narayan KM, Bowman BA, Engelgau ME. Prevention of type 2 diabetes. Bmj 2001;323(7304):63‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neff 2003 {published data only}
- Neff LM. Evidence‐based dietary recommendations for patients with type 2 diabetes mellitus. Nutrition in Clinical Care 2003;6(2):51‐61. [PubMed] [Google Scholar]
Nicollerat 2000 {published data only}
- Nicollerat JA. Implications of the United Kingdom Prospective Diabetes Study (UKPDS) results on patient management. Diabetes Educator 2000;26:8‐10. [PubMed] [Google Scholar]
Nielsen 2005 {published data only}
- Nielsen JV, Jonsson E, Nilsson AK. Lasting improvement of hyperglycaemia and bodyweight: low‐carbohydrate diet in type 2 diabetes. Upsala Journal of Medical Science 2005;110:69‐73. [DOI] [PubMed] [Google Scholar]
Nilsson 1992 {published data only}
- Nilsson PM, Lindholm LH, Schersten BF. Life style changes improve insulin resistance in hyperinsulinaemic subjects: a one‐year intervention study of hypertensives and normotensives in Dalby. Journal of Hypertension 1992;10(9):1071‐8. [PubMed] [Google Scholar]
Norris 2004 {published data only}
- Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Serdula M, Brown TJ, Schmid CH, Lau J. Long‐term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta‐analysis. American Journal of Medicine 2004;117(10):762‐774. [DOI] [PubMed] [Google Scholar]
Odegaard 2006 {published data only}
- Odegaard AO, Pereira MA. Trans fatty acids, insulin resistance and type 2 diabetes. Nutrition Reviews 2006;64(8):364‐372. [DOI] [PubMed] [Google Scholar]
Oli 1984 {published data only}
- Oli JM, Ikeakor IP. High carbohydrate diet in the management of non‐obese non‐insulin‐dependent Nigerian diabetics. Human Nutrition ‐ Applied Nutrition 1984;38(6):479‐86. [PubMed] [Google Scholar]
Paffenbarger 1997 {published data only}
- Paffenbarger RS, Jr. , Lee IM, Kampert JB. Physical activity in the prevention of non‐insulin‐dependent diabetes mellitus. World Review of Nutrition & Dietetics 1997;82:210‐8. [DOI] [PubMed] [Google Scholar]
Paisey 1995 {published data only}
- Paisey RB, Harvey PR. Short‐term results of an open trial of very low calorie diet or intensive conventional diet in type 2 diabetes. Practical Diabetes International. 1995; Vol. 12, issue 6:263‐267.
Paisey 1998 {published data only}
- Paisey RB, Harvey P, Rice S, Belka I, Bower L, Dunn M, et al. An intensive weight loss programme in established type 2 diabetes and controls: effects on weight and atherosclerosis risk factors at 1 year. Diabetic Medicine 1998;15(1):73‐9. [DOI] [PubMed] [Google Scholar]
Paisey 2002 {published data only}
- Paisey RB, Frost J, Harvey P, Paisey A, Bower L, Paisey RM, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. Journal of Human Nutrition & Dietetics 2002;15(2):121‐127. [DOI] [PubMed] [Google Scholar]
Pan 1997 {published data only}
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20(4):537‐44. [DOI] [PubMed] [Google Scholar]
Parfitt 1994 {published data only}
- Parfitt VJ, Desomeaux K, Bolton CH, Hartog M. Effects of high monounsaturated and polyunsaturated fat diets on plasma lipoproteins and lipid peroxidation in type 2 diabetes mellitus. Diabetic Medicine 1994;11(1):85‐91. [DOI] [PubMed] [Google Scholar]
Pascale 1992 {published data only}
- Pascale RW, Wing RR, Blair EH, Harvey JR, Guare JC. The effect of weight loss on change in waist‐to‐hip ratio in patients with type II diabetes. International Journal of Obesity & Related Metabolic Disorders 1992;16(1):59‐65. [PubMed] [Google Scholar]
Pejic 2006 {published data only}
- Pejic RN, Lee DT. Hypertrigylceridemia. Journal of the American Board of Family Medicine 2006;19(3):310‐316. [DOI] [PubMed] [Google Scholar]
Perez‐Martin 2001 {published data only}
- Perez‐Martin A, Raynaud E, Mercier J. Insulin resistance and associated metabolic abnormalities in muscle: effects of exercise. Obesity Reviews 2001;2(1):47‐59. [DOI] [PubMed] [Google Scholar]
Perri 1993 {published data only}
- Perri MG, Sears SF, Jr. , Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care 1993;16(1):200‐9. [DOI] [PubMed] [Google Scholar]
Pfohl 2001 {published data only}
- Pfohl M, Schatz H. Strategies for the prevention of type 2 diabetes. Experimental & Clinical Endocrinology & Diabetes 2001;109(2). [DOI] [PubMed] [Google Scholar]
Phillips 2006 {published data only}
- Phillips PJ. Type 2 diabetes: not just a touch of sugar. Medicine Today 2006;7(7):39‐42. [Google Scholar]
Pigman 2002 {published data only}
- Pigman HT, Gan DX, Krousel‐Wood MA. Role of exercise for type 2 diabetic patient management. Southern Medical Journal 2002;95(1):72‐7. [PubMed] [Google Scholar]
Pischke 2006 {published data only}
- Pischke CR, Marlin RO, Weidner G, Chi C, Ornish D. The role of lifestyle in secondary prevention of coronary heart disease in patients with type 2 diabetes. Canadian Journal of Diabetes 2006;30(2):176‐182. [Google Scholar]
Poppitt 2002 {published data only}
- Poppitt SD, Keogh GF, Prentice AM, Williams DE, Sonnemans HM, Valk EE, et al. Long‐term effects of ad libitum low‐fat, high‐carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. American Journal of Clinical Nutrition 2002;75(1):11‐20. [DOI] [PubMed] [Google Scholar]
Pratley 2000 {published data only}
- Pratley RE, Hagberg JM, Dengel DR, Rogus EM, Muller DC, Goldberg AP. Aerobic exercise training‐induced reductions in abdominal fat and glucose‐stimulated insulin responses in middle‐aged and older men. Journal of the American Geriatrics Society 2000;48(9):1055‐61. [DOI] [PubMed] [Google Scholar]
Pritchard 1999 {published data only}
- Pritchard DA, Hyndman J, Taba F. Nutritional counselling in general practice: a cost effective analysis. Journal of Epidemiology & Community Health 1999;53(5):311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabkin 1983 {published data only}
- Rabkin SW, Boyko E, Wilson A, Streja DA. A randomized clinical trial comparing behavior modification and individual counseling in the nutritional therapy of non‐insulin‐dependent diabetes mellitus: comparison of the effect on blood sugar, body weight, and serum lipids. Diabetes Care 1983;6(1):50‐6. [DOI] [PubMed] [Google Scholar]
Racette 2001 {published data only}
- Racette SB, Weiss EP, Obert KA, Kohrt WM, Holloszy JO. Modest lifestyle intervention and glucose tolerance in obese African Americans. Obesity Research 2001;9(6):348‐55. [DOI] [PubMed] [Google Scholar]
Ratner 2006 {published data only}
- Ratner RE. An update on the diabetes prevention program. Endocrine Practice 2006;12(Supplement 1):20‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raz 1994 {published data only}
- Raz I, Hauser E, Bursztyn M. Moderate exercise improves glucose metabolism in uncontrolled elderly patients with non‐insulin‐dependent diabetes mellitus. Israel Journal of Medical Sciences 1994;30(10):766‐70. [PubMed] [Google Scholar]
Reaven 1995 {published data only}
- Reaven P. Dietary and pharmacologic regimens to reduce lipid peroxidation in non‐insulin‐dependent diabetes mellitus. American Journal of Clinical Nutrition 1995;62(6 Suppl):1483S‐1489S. [DOI] [PubMed] [Google Scholar]
Rendell 2006 {published data only}
- Rendell MS, Jovanovic L. Targeting postprandial hyperglycemia. Metabolism: Clinical & Experimental 2006;55(9):1263‐1281. [DOI] [PubMed] [Google Scholar]
Riccardi 2005 {published data only}
- Riccardi G, Capaldo B, Vaccaro O. Functional foods in the management of obesity and type 2 diabetes. Current Opinion in Clinical Nutrition and Metabolic Care 2005;8(6):630‐635. [DOI] [PubMed] [Google Scholar]
Ronnemaa 1986 {published data only}
- Ronnemaa TMKLAKV. A controlled randomized study on the effect of long‐term physical exercise on the metabolic control in type 2 diabetic patients. Acta Medica Scandinavica 1986;220(3):219‐24. [DOI] [PubMed] [Google Scholar]
Rosell 1999 {published data only}
- Rosell M, Regnstrom J, Kallner A, Hellenius ML. Serum urate determines antioxidant capacity in middle‐aged men ‐ a controlled, randomized diet and exercise intervention study. Journal of Internal Medicine 1999;246(2):219‐26. [DOI] [PubMed] [Google Scholar]
Rowley 2000 {published data only}
- Rowley KG, Daniel M, Skinner K, Skinner M, White GA, O'Dea K. Effectiveness of a community‐directed 'healthy lifestyle' program in a remote Australian aboriginal community. Australian & New Zealand Journal of Public Health 2000;24(2):136‐44. [DOI] [PubMed] [Google Scholar]
Rubin 2002 {published data only}
- Rubin RR, Fujimoto WY, Marrero DG, Brenneman T, Charleston JB, Edelstein SL, et al. The Diabetes Prevention Program: recruitment methods and results. Controlled Clinical Trials 2002;23(2):157‐71. [DOI] [PubMed] [Google Scholar]
Rukgauer 2006 {published data only}
- Rukgauer M, Schmitt Y, Zeyfang A. Importance of chromium, copper, selinium and zinc in diabetes mellitus, type 1 and type 2 with late disease. Laboratoriums Medizin 2006;30(4):192‐200. [Google Scholar]
Ryan 2003 {published data only}
- Ryan DH. Diet and exercise in the prevention of diabetes. International Journal of Clinical Practice 2003;Supplement(134):28‐35. [PubMed] [Google Scholar]
Rybka 1987 {published data only}
- Rybka J. Diabetes mellitus and exercise. Acta Universitatis Carolinae ‐ Medica ‐ Monographia 1987;118:1‐133. [PubMed] [Google Scholar]
Sato 2000a {published data only}
- Sato Y. Diabetes and life‐styles: role of physical exercise for primary prevention. British Journal of Nutrition 2000;84(Suppl 2):S187‐90. [DOI] [PubMed] [Google Scholar]
Scheen 2000 {published data only}
- Scheen AJ. Treatment of diabetes in patients with severe obesity. Biomedicine & Pharmacotherapy 2000;54(2):74‐9. [DOI] [PubMed] [Google Scholar]
Schwartz 2006 {published data only}
- Schwartz SL. Diabetes and dyslipidaemia. Diabetes, Obesity & Metabolism 2006;8(4):355‐364. [DOI] [PubMed] [Google Scholar]
Shahid 2000 {published data only}
- Shahid SK, Schneider SH. Effects of exercise on insulin resistance syndrome. Coronary Artery Disease 2000;11(2):103‐9. [DOI] [PubMed] [Google Scholar]
Sherwin 2003 {published data only}
- Sherwin RS, Anderson RM, Buse JB, Chin MH, Eddy D, Fradkin J, et al. The prevention or delay of type 2 diabetes. Diabetes Care 2003;26(SUPPL 1):S62‐9. [DOI] [PubMed] [Google Scholar]
Shintani 2001 {published data only}
- Shintani TT. Integrative medicine approach to obesity and diabetes. Hawaii Medical Journal 2001;60(10):262‐3. [PubMed] [Google Scholar]
Siddiqui 2004 {published data only}
- Siddiqui MA, Ahmad J, Das G, Hameed B. The impact of diabetes on the cardiovascular system and management strategies. JK Practitioner 2004;11(4):233‐241. [Google Scholar]
Sigal 2004 {published data only}
- Sigal RJ, Kenny GP, Wasserman DH, Castaneda‐Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care 2004;27(10):2518‐2539. [DOI] [PubMed] [Google Scholar]
Simmons 1997 {published data only}
- Simmons D, Voyle J, Swinburn B, O'Dea K. Community‐based approaches for the primary prevention of non‐insulin‐dependent diabetes mellitus. Diabetic Medicine 1997;14(7):519‐26. [DOI] [PubMed] [Google Scholar]
Smith 1993 {published data only}
- Smith RB. Noninsulin dependent diabetes mellitus. New Zealand Medical Journal 1993;106(949):34‐5. [PubMed] [Google Scholar]
Solano 2006 {published data only}
- Solano MP, Goldberg RB. Lipid management in type 2 diabetes. Clinical Diabetes 2006;24(1):27‐32. [Google Scholar]
Solano 2006b {published data only}
- Solano MP, Goldberg RB. Management of dyslipidemia in diabetes. Cardiology in Review 2006;14(3):125‐135. [DOI] [PubMed] [Google Scholar]
Sone 2002 {published data only}
- Sone H, Katagiri A, Ishibashi S, Abe R, Saito Y, Murase T, et al. Effects of lifestyle modifications on patients with type 2 diabetes: The Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three year‐interim report. Hormone & Metabolic Research 2002;34(9):509‐15. [DOI] [PubMed] [Google Scholar]
Song 2006 {published data only}
- Song Y, Hes K, Levitan EB, Manson JE, Liu S. Effects or oral magnesium supplements on glycaemic control in type 2 diabetes: a meta‐analysis of randomized double‐blind controlled trials. Diabetic Medicine 2006;23(10):1050‐1056. [DOI] [PubMed] [Google Scholar]
Spelsberg 1995 {published data only}
- Spelsberg A, Manson JE. Physical activity in the treatment and prevention of diabetes. Comprehensive Therapy 1995;21(10):559‐62. [PubMed] [Google Scholar]
Srimanunthiphol 2000 {published data only}
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Medical Journal 2000;59(7):295‐8, 313. [PubMed] [Google Scholar]
Starke 1994 {published data only}
- Starke AA. The influence of diet and physical activity on insulin sensitivity. Wiener Klinische Wochenschrift 1994;106(24):768‐73. [PubMed] [Google Scholar]
Steyn 2004 {published data only}
- Steyn NP, Mann J, Bennett PH, Temple N, Zimmet P, Tuomilehto J, Lindstrom J, Louheranta A. Diet, nutrition and the prevention of type 2 diabetes. Public Health Nutrition 2004;7(1A):147‐165. [DOI] [PubMed] [Google Scholar]
Stone 2001 {published data only}
- Stone NJ. The optimal dietary strategy to manage risk associated with various dyslipidemias. Current Cardiology Reports 2001;3(5):391‐400. [DOI] [PubMed] [Google Scholar]
Strandberg 2000 {published data only}
- Strandberg TE, Salomaa V. Factors related to the development of diabetes during a 20‐year follow‐up. A prospective study in a homogeneous group of middle‐aged men. Nutrition Metabolism & Cardiovascular Diseases 2000;10(5):239‐46. [PubMed] [Google Scholar]
Sutherland 2004 {published data only}
- Sutherland JE, Hoehns JD. Treating type 2 diabetes: targeting the causative factors. Journal of Family Practice 2004;53(5):376‐388. [PubMed] [Google Scholar]
Svendsen 1996 {published data only}
- Svendsen OL, Hassager C, Christiansen C, Nielsen JD, Winther K. Plasminogen activator inhibitor‐1, tissue‐type plasminogen activator, and fibrinogen: Effect of dieting with or without exercise in overweight postmenopausal women. Arteriosclerosis Thrombosis & Vascular Biology 1996;16(3):381‐5. [DOI] [PubMed] [Google Scholar]
Swinburn 2001 {published data only}
- Swinburn BA, Metcalf PA, Ley SJ. Long‐term (5‐year) effects of a reduced‐fat diet intervention in individuals with glucose intolerance. see comments. Diabetes Care 2001;2001 Apr;24(4):619‐24 24(4) 619‐24. [DOI] [PubMed] [Google Scholar]
Takekoshi 1987 {published data only}
- Takekoshi H, Matsuoka K, Suzuki Y, Atsumi Y, Kubo A, Hayashi K, et al. A ten year follow up study in NIDDM with or without exercise. Journal of the Medical Association of Thailand 1987;70(Suppl 2):149‐52. [PubMed] [Google Scholar]
Tamler 2006 {published data only}
- Tamler R, Mechanick JI. Dietary Supplements and nutraceuticals in the management of endocrine disorders. Current Opinion in Endocrinology & Diabetes 2006;6(4):407‐423. [Google Scholar]
Taniguchi 2000 {published data only}
- Taniguchi A, Fukushima M, Sakai M, Nagasaka S, Doi K, Nagata I, et al. Effect of physical training on insulin sensitivity in Japanese type 2 diabetic patients: role of serum triglyceride levels. Diabetes Care 2000;23(6):857‐8. [DOI] [PubMed] [Google Scholar]
Tariq 2001 {published data only}
- Tariq SH, Karcic E, Thomas DR, Thomson K, Philpot C, Chapel DL, et al. The use of a no‐concentrated‐sweets diet in the management of type 2 diabetes in nursing homes. Journal of the American Dietetic Association 2001;101(12):1463‐6. [DOI] [PubMed] [Google Scholar]
Thomson 2001 {published data only}
- Thomson GA. The implications of the UKPDS to elderly patients with type 2 diabetes: A time for action?. CME Journal Geriatric Medicine 2001;3(1):3‐6. [Google Scholar]
Toeller 1993 {published data only}
- Toeller M. Diet and diabetes. Diabetes‐Metabolism Reviews 1993;9(2):93‐108. [DOI] [PubMed] [Google Scholar]
Toeller 2005 {published data only}
- Toeller M. Evidence‐based recommendations to nutrition therapy and the prevention of diabetes mellitus. Ernahrungs‐Umschau 2005;52(6):214, 216‐219. [Google Scholar]
Torjesen 1997 {published data only}
- Torjesen PA, Birkeland KI, Anderssen SA, Hjermann I, Holme I, Urdal P. Lifestyle changes may reverse development of the insulin resistance syndrome. The Oslo Diet and Exercise Study: a randomized trial. Diabetes Care 1997;20(1):26‐31. [DOI] [PubMed] [Google Scholar]
Trento 2002 {published data only}
- Trento M, Passera P, Bajardi M, Tomalino M, Grassi G, Borgo E, Donnola C, Cavallo F, Bondonio P, Porta M. Lifestyle intervention by group care prevents deterioration of type II diabetes: a 4‐year randomized controlled clinical trial. Diabetologia 2002;45:1231‐1239. [DOI] [PubMed] [Google Scholar]
Tsujiuchi 2002 {published data only}
- Tsujiuchi T, Kumano H, Yoshiuchi K, He D, Tsujiuchi Y, Kuboki T, et al. The effect of Qi‐gong relaxation exercise on the control of type 2 diabetes mellitus: a randomized controlled trial. Diabetes Care 2002;25(1):241‐2. [DOI] [PubMed] [Google Scholar]
Tudor‐Locke 2000 {published data only}
- Tudor‐Locke CE, Bell RC, Meyers AM. Revisiting the role of physical activity and exercise in the treatment of type 2 diabetes. Canadian Journal of Applied Physiology 2000;25(6):466‐92. [DOI] [PubMed] [Google Scholar]
Tuomilehto 1992 {published data only}
- Tuomilehto J, Knowler WC, Zimmet P. Primary prevention of non‐insulin‐dependent diabetes mellitus. Diabetes‐Metabolism Reviews 1992;8(4):339‐53. [DOI] [PubMed] [Google Scholar]
Tuomilehto 2001b {published data only}
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐1350. [DOI] [PubMed] [Google Scholar]
Turner 1990 {published data only}
- Turner RC, Holman RR, Matthews DR, Howard‐Williams JR, Oakes S, Coster R, et al. UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients. Metabolism: Clinical & Experimental 1990;39(9):905‐912. [PubMed] [Google Scholar]
Turner 1995 {published data only}
- Turner RC, Holman RR. Lessons from UK prospective diabetes study. Of: ; Review Of: 32 refs. Diabetes Research & Clinical Practice 1995:32 refs. Diabetes Research & Clinical Practice 1995;28(7). [DOI] [PubMed] [Google Scholar]
Turner 1996a {published data only}
- Turner R, Cull C, Holman R. United Kingdom prospective diabetes study 17: A 9‐year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non‐insulin‐dependent diabetes mellitus. Annals of Internal Medicine 1996;124(1 II):136‐145. [DOI] [PubMed] [Google Scholar]
Turner 1996b {published data only}
- Turner R, Rachman J, Holman R. UK Prospective Diabetes Study.. Diabetes und Stoffwechsel 1996;5(3 SUPPL.):77‐80. [Google Scholar]
UKPDS 1993 {published data only}
- UK Prospective Diabetes Study (UKPDS). X. Urinary albumin excretion over 3 years in diet‐treated type 2, (non‐insulin‐dependent) diabetic patients, and association with hypertension, hyperglycaemia and hypertriglyceridaemia. Diabetologia 1993;36(10):1021‐1029. [PubMed] [Google Scholar]
Ullom‐Minnich 2004 {published data only}
- Ullom‐Minnich P. Strategies to reduce complications of type 2 diabetes. Journal of Family Practice 2004;53(5):366‐374. [PubMed] [Google Scholar]
Uusitupa 1989 {published data only}
- Uusitupa M, Laakso M, Sarlund H, Majander H, Takala J, Penttila I. Long term effects of a very low calorie diet on metabolic control and cardiovascular risk factors in the treatment of obese non‐insulin‐dependent diabetics. International Journal of Obesity & Related Metabolic Disorders 1989;13(Suppl 2):163‐4. [PubMed] [Google Scholar]
Uusitupa 2000 {published data only}
- Uusitupa M, Louheranta A, Lindstrom J, et al. The Finnish diabetes prevention study. British Journal of Nutrition 2000;83:S137‐42. [DOI] [PubMed] [Google Scholar]
Valensi 2006 {published data only}
- Valensi P, Chanu B, Cosson E. Obesity, metabolic sydrome, diabetes and arterial hypertension. Immunology, Endocrine & Metabolic Agents in Medicinal Chemistry 2006;6(4):407‐423. [Google Scholar]
Vuksan 2001 {published data only}
- Vuksan V, Sievenpiper JL, Xu Z, Wong EY, Jenkins AL, Beljan‐Zdravkovic U, et al. Konjac‐Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. Journal of the American College of Nutrition 2001:emerging alternative therapies for type 2 diabetes mellitus. Journal of the American College of Nutrition 2001;20(5 Suppl). [DOI] [PubMed] [Google Scholar]
Vuori 2001 {published data only}
- Vuori IM. Health benefits of physical activity with special reference to interaction with diet. Public Health Nutrition 2001;4(2B):517‐28. [DOI] [PubMed] [Google Scholar]
Walker 1996 {published data only}
- Walker KZ, O'Dea K, Johnson L, Sinclair AJ, Piers LS, Nicholson GC, et al. Body fat distribution and non‐insulin‐dependent diabetes: comparison of a fiber‐rich, high‐carbohydrate, low‐fat (23%) diet and a 35% fat diet high in monounsaturated fat. American Journal of Clinical Nutrition 1996;63(2):254‐60. [DOI] [PubMed] [Google Scholar]
Walker 2001 {published data only}
- Walker KZ, O'Dea K. Is a low fat diet the optimal way to cut energy intake over the long‐term in overweight people?. Nutrition Metabolism & Cardiovascular Diseases 2001;11(4):244‐8. [PubMed] [Google Scholar]
Wall 1973 {published data only}
- Wall JR, Pyke DA, Oakley WG. Effect of carbohydrate restriction in obese diabetics: relationship of control to weight loss. Bmj 1973;1(5853):577‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallberg‐H 1998 {published data only}
- Wallberg‐Henriksson H, Rincon J, Zierath JR. Exercise in the management of non‐insulin‐dependent diabetes mellitus. Sports Medicine 1998;25(1):25‐35. [DOI] [PubMed] [Google Scholar]
Warnken 2005 {published data only}
- Warnken W, Kelsberg G, Bryant S. Can type 2 diabetes be prevented through diet and exercise?. Journal of Family Practice 2005;54(1):78‐80. [PubMed] [Google Scholar]
Wei 2000 {published data only}
- Wei M, Schwertner HA, Blair SN. The association between physical activity, physical fitness, and type 2 diabetes mellitus. Comprehensive Therapy 2000;26(3):176‐82. [DOI] [PubMed] [Google Scholar]
Wein 1999 {published data only}
- Wein P, Beischer N, Harris C, Permezel M. A trial of simple versus intensified dietary modification for prevention of progression to diabetes mellitus in women with impaired glucose tolerance. Australian & New Zealand Journal of Obstetrics & Gynaecology 1999;39(2):162‐6. [DOI] [PubMed] [Google Scholar]
Weinstock 1998 {published data only}
- Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Archives of Internal Medicine 1998;158(22):2477‐83. [DOI] [PubMed] [Google Scholar]
Welch 2006 {published data only}
- Welch G, Shayne R. Interactive behavioral technologies and diabetes self‐management support: recent research findings from clinical trials. Current Diabetes Reports 2006;6(2):130‐136. [DOI] [PubMed] [Google Scholar]
Wild 2004 {published data only}
- Wild S, Byrne CD. The role of treatment to increase HDL‐cholesterol abd decrease triglyceride concentrations in prevention of coronary heart disease in type 2 diabetes. Diabetic Medicine Supplement 2004;21(4):8‐11. [DOI] [PubMed] [Google Scholar]
Williams 2000 {published data only}
- Williams KV, Kelley DE. Metabolic consequences of weight loss on glucose metabolism and insulin action in type 2 diabetes. Diabetes, Obesity & Metabolism 2000;2(3):121‐9. [DOI] [PubMed] [Google Scholar]
Williamson 2000 {published data only}
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23(10):1499‐504. [DOI] [PubMed] [Google Scholar]
Wing 1993 {published data only}
- Wing RR. Behavioral treatment of obesity. Its application to type II diabetes. Diabetes Care 1993;16(1):193‐9. [DOI] [PubMed] [Google Scholar]
Wing 1995 {published data only}
- Wing RR. Use of very‐low‐calorie diets in the treatment of obese persons with non‐insulin‐dependent diabetes mellitus. Journal of the American Dietetic Association 1995;95(5):569‐72; quiz 573‐4. [DOI] [PubMed] [Google Scholar]
Wing 1998 {published data only}
- Wing R R, Venditti E, Jakicic J M, Polley B A, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21(3):p. 350‐9. [DOI] [PubMed] [Google Scholar]
Wing 2001 {published data only}
- Wing RR, Goldstein MG, Acton KJ, Birch LL, Jakicic JM, Sallis JF, Jr. , et al. Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care 2001;24(1):117‐23. [DOI] [PubMed] [Google Scholar]
Wolever 2002 {published data only}
- Wolever TMS, Mehling C. High‐carbohydrate‐low‐glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. British Journal of Nutrition 2002;87(5):477‐87. [DOI] [PubMed] [Google Scholar]
Wolever 2003 {published data only}
- Wolever TMS, Mehling, C. Long‐term effect of varying the source or amount of dietary carbohydrate on postprandial plasma glucose, insulin, triacylglycerol, and free fatty acid concentrations in subjects with impaired glucose tolerance. American Journal of Clinical Nutrition 2003;77(3):612‐21. [DOI] [PubMed] [Google Scholar]
Wolffenbuttel 1989 {published data only}
- Wolffenbuttel BH, Weber RF, Koetsveld PM, Verschoor L. Limitations of diet therapy in patients with non‐insulin‐dependent diabetes mellitus. International Journal of Obesity & Related Metabolic Disorders 1989;13(2):173‐82. [PubMed] [Google Scholar]
Wylie‐Rosett 2006 {published data only}
- Wylie‐Rosett J, Herman WH, Goldberg RB. Lifestyle interventions to prevent diabetes: intensive and cost‐effective. Current Opinion in Lipidology 2006;17(1):37‐44. [DOI] [PubMed] [Google Scholar]
Yamamoto 2001 {published data only}
- Yamamoto K, Asakawa H, Tokunaga K, Watanabe H, Matsuo N, Tokimitsu I, et al. Long‐term ingestion of dietary diacylglycerol lowers serum triacylglycerol in type II diabetic patients with hypertriglyceridemia. Journal of Nutrition 2001;131(12):3204‐7. [DOI] [PubMed] [Google Scholar]
Yamaoka 2005 {published data only}
- Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta‐analysis of randomized controlled trials. Diabetes Care 2005;28(11):2780‐2786. [DOI] [PubMed] [Google Scholar]
Yeh 2003 {published data only}
- Yeh. Diabetes Care 2003;26(4). [Google Scholar]
Yoo 2005 {published data only}
- Yoo JS, Lee SJ. A meta‐analysis of the effects of exercise programs on glucose and lipid metabolism and cardiac function in patients with type II diabetes mellitis. Daehan Ganho Haghoeji 2005;35(3):546‐554. [DOI] [PubMed] [Google Scholar]
Yoshioka 1989 {published data only}
- Yoshioka N, Kuzuya T, Matsuda A, Iwamoto Y. Effects of dietary treatment on serum insulin and proinsulin response in newly diagnosed NIDDM. Diabetes 1989;38(2):262‐6. [DOI] [PubMed] [Google Scholar]
Zhi‐cheng 1994 {published data only}
- Zhi‐cheng LF‐mS. Acupuncture treatment of non‐insulin‐dependent diabetes mellitus: a clinical study. International Journal of Clinical Acupuncture 1994;5(3):249‐59. [Google Scholar]
References to studies awaiting assessment
Anderssen 2000 {published data only}
- Anderssen SA, Hjermann I. [Physical activity‐‐a crucial factor in the prevention of cardiovascular diseases]. Tidsskrift for Den Norske Laegeforening 2000;120(26):3168‐72. [PubMed] [Google Scholar]
Baan 2001 {published data only}
- Baan CA, Feskens EJ. [Prevention of diabetes mellitus type 2]. Nederlands Tijdschrift voor Geneeskunde 2001;145(35):1677‐80. [PubMed] [Google Scholar]
Berg 2000 {published data only}
- Berg TJ. [Can type 2 diabetes be prevented?]. Tidsskrift for Den Norske Laegeforening 2000;120(20):2430‐3. [PubMed] [Google Scholar]
Birkeland 2000 {published data only}
- Birkeland KI, Claudi T, Hansteen V, Hanssen KF, Hjermann I, Jenssen T, et al. [Prevention of cardiovascular disease in type 2 diabetes]. Tidsskrift for Den Norske Laegeforening 2000;120(21):2554‐9. [PubMed] [Google Scholar]
Charbonnel 1994 {published data only}
- Charbonnel B, Laurent C. Prevention of macroangiopathy of type 2 diabetes in clinical practice . Of: ; Review Of: French. Diabete et Metabolisme 1994;20(3 Pt 2):366‐74. [PubMed] [Google Scholar]
de Luis Roman 2001 {published data only}
- Luis Roman D, Izaola O, Aller R. [Assessment of the compliance of a 1,500 calorie diet in a population of overweight type‐2 diabetics]. Nutricion Hospitalaria 2001;16(4):122‐5. [PubMed] [Google Scholar]
Dela 2002 {published data only}
- Dela F. [Physical training in the treatment of metabolic syndrome]. Ugeskrift for Laeger 2002;164(16):2147‐52. [PubMed] [Google Scholar]
Florez 1997 {published data only}
- Florez H. Steps toward the primary prevention of type II diabetes mellitus. Various epidemiological considerations . Of: ; Review Of: 27 refs Spanish. Investigacion Clinica 1997;38(1):39‐52. [PubMed] [Google Scholar]
Gao 1998 {published data only}
- Gao Y, Lu R, Wang X, Geng J, Ren K, Wang Y, et al. A clinical trial of tang shen ning for treatment of diabetic nephropathy. Journal of Traditional Chinese Medicine 1998;18(4):247‐52. [PubMed] [Google Scholar]
Haisch 1996 {published data only}
- Haisch J, Braun S, Bohm BO, Stock D. Effects of patient education in type II diabetic patients after clinic admission. Results of a 3 month catamnesis after new patient‐centered education . German. Psychotherapie, Psychosomatik, Medizinische Psychologie 1996;46(11):400‐4. [PubMed] [Google Scholar]
Haisch 2000 {published data only}
- Haisch J, Remmele W. Effectiveness and efficiency of ambulatory diabetes education programs. A comparison of specialty practice and general practice . German. Deutsche Medizinische Wochenschrift 2000;125(7):171‐6. [DOI] [PubMed] [Google Scholar]
Kasuga 1997 {published data only}
- Kasuga M. [Prediction and prevention of NIDDM]. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 1997;55(Suppl):345‐8. [PubMed] [Google Scholar]
Kikhtiak 1997 {published data only}
- Kikhtiak OP. [Phytotherapy as a reliable natural means of treating patients with type‐2 diabetes mellitus]. 173‐5. [PubMed]
Krashenitsa 1994 {published data only}
- Krashenitsa GM, Botvineva LA, Mogila AV. [Effectiveness of increased contents of dietary fiber in early stages of non‐insulin‐dependent diabetes mellitus]. 35‐7. [PubMed]
Meloni 2002 {published data only}
- Meloni C, Morosetti M, Suraci C, Pennafina MG, Tozzo C, Taccone‐Gallucci M, et al. Severe dietary protein restriction in overt diabetic nephropathy: benefits or risks?. Journal of Renal Nutrition 2002;12(2):96‐101. [DOI] [PubMed] [Google Scholar]
Monnier 2000 {published data only}
- Monnier L, Colette C, Percheron C, Boniface H. [Very‐low‐calorie‐diets: is there a place for them in the management of the obese diabetic?]. Diabetes & Metabolism 2000;26(Suppl 3):46‐51. [PubMed] [Google Scholar]
Nagy 2000 {published data only}
- Nagy J, Wittmann I. [Nephropathy in non‐insulin‐dependent (type‐2) diabetes mellitus]. Orvosi Hetilap 2000;141(12):609‐14. [PubMed] [Google Scholar]
Nilsson 1998 {published data only}
- Nilsson P, Attvall S, Eliasson M. [More active therapy in diabetes type 2 is justified by findings in a break‐through study. 20‐year follow‐up of over 5000 patients]. Lakartidningen 1998;95(45):4983‐4, 4987. [PubMed] [Google Scholar]
Okajima 1997 {published data only}
- Okajima T. [Very‐low‐calorie diet therapy]. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 1997;55(Suppl):58‐62. [PubMed] [Google Scholar]
Oshida 2000 {published data only}
- Oshida Y. [Practice of exercise therapy for diabetes mellitus]. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 2000;58(Suppl):391‐6. [PubMed] [Google Scholar]
Pan 1995 {published data only}
- Pan X, Li G, Hu Y. Effect of dietary and/or exercise intervention on incidence of diabetes in 530 subjects with impaired glucose tolerance from 1986‐1992 . Chinese. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 1995;34(2):108‐12. [PubMed] [Google Scholar]
Pissarek 1980 {published data only}
- Pissarek D, Panzram G, Lundershausen R, et al. Intensified therapy of newly detected maturity onset diabetes. Endokrinologie 1980;75(1):105‐115. [PubMed] [Google Scholar]
Rasmussen 1995 {published data only}
- Rasmussen OW, Thomsen CH, Hansen KW, Vesterlund M, Winther E, Hermansen K. Favourable effect of olive oil in patients with non‐insulin‐dependent diabetes. The effect on blood pressure, blood glucose and lipid levels of a high‐fat diet rich in monounsaturated fat compared with a carbohydrate‐rich diet . see comments . Danish. Ugeskrift for Laeger 1995;157(8):1028‐32. [PubMed] [Google Scholar]
Ratzmann 1982 {published data only}
- Ratzmann KP, Zander E, Witt S, Schulz B. Effect of a combined diet‐training program on the insulin sensitivity of obese persons with normal or disordered glucose tolerance . German. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1982;37(10):304‐8. [PubMed] [Google Scholar]
Samaha {published data only}
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low‐carbohydrate as compared with a low‐fat diet in severe obesity. New England Journal of Medicine 2003;348(21):2074‐81. [DOI] [PubMed] [Google Scholar]
Sato 1996 {published data only}
- Sato Y, Sato J, Tokudome S. [Management of impaired glucose tolerance: physical training therapy]. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 1996;54(10):2745‐9. [PubMed] [Google Scholar]
Sato 2000b {published data only}
- Sato Y. [Effects of physical activities on the type 2 diabetes]. Nippon Rinsho ‐ Japanese Journal of Clinical Medicine 2000;58(Suppl):385‐90. [PubMed] [Google Scholar]
Sauer 1976 {published data only}
- Sauer H, Nassauer L. [Diet therapy of diabetes mellitus (without reference to juvenile diabetes)]. Internist 1976;17(10):502‐10. [PubMed] [Google Scholar]
Scheen 2001 {published data only}
- Scheen AJ. [Clinical study of the month. Prevention of type 2 diabetes in overweight patients with impaired glucose tolerance: efficiency of lifestyle changes]. Revue Medicale de Liege 2001;56(6):463‐5. [PubMed] [Google Scholar]
ten Hove 2000 {published data only}
- Hove WR, Meijer PH, Meinders AE. [Very‐low‐calorie diet in treatment of morbidly obese patient with diabetes mellitus type 2]. Nederlands Tijdschrift voor Geneeskunde 2000;144(23):1089‐92. [PubMed] [Google Scholar]
Villa‐Caballero 2000 {published data only}
- Villa‐Caballero L, Frati‐Munari A, Ponce‐Monter H, Hernandez Rodriquez‐de Leon SM, Becerra‐Perez AR. [Prescription of exercise in the diabetic patient]. Gaceta Medica de Mexico 2000;136(6):629‐37. [PubMed] [Google Scholar]
Wang 1993 {published data only}
- Wang XM. [Treating type II diabetes mellitus with foot reflexotherapy]. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1993;13(9):536‐8, 517. [PubMed] [Google Scholar]
References to ongoing studies
Kelley 2002 {published and unpublished data}
- Kelley D E. Action for health in diabetes: the look AHEAD clinical trial. Current Diabetes Reports 2002;2(3):207‐9. [DOI] [PubMed] [Google Scholar]
Additional references
ADA 1997
- American Diabetic Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.. Diabetes Care 1997;20:1183‐97. [DOI] [PubMed] [Google Scholar]
ADA 1999
- American Diabetic Association. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1999;22(Suppl 1):S1‐114. [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Diabetes UK website [Computer program]
- Diabetes UK. [www.diabetes.org.uk]. Accessed: 09.02.07.
DOH website [Computer program]
- Department of Health. www.dh.gov.uk. Accessed 09.02.07.
Dowse 1991
- Dowse GK, Zimmer PZ, Gareeboo H. Abdominal obesity and physical activity are risk factors for NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians.. Diabetes Care 1991;14:271‐82. [DOI] [PubMed] [Google Scholar]
DPP 2002
- The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farmer 2001
- Farmer, A. Montori, V. Dinneen, S. Clar, C. Fish oil in people with type 2 diabetes (Cochrane Review). Issue 1, 2002.
Frisch 1986
- Frisch RE, Wyshak G, Krolewski AS. Lower prevelance of diabetes in female former college athletes compared with nonathletes.. Diabetes 1986;35:1101‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ. Assessing the Quality of Reports of Randomised Clinical Trials: Is Blinding Necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Liu 2002
- Liu JP, Zhang M, Wang WY, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manson 1992a
- Manson JE, Nathan DM, Krolewski AS. A prospective study of exercise and incidence of diabetes among US male physicians.. JAMA 1992;268(63‐7). [PubMed] [Google Scholar]
Mokdad 2000
- Mokdad AH, Ford ES, Bowman BA. Diabetes trends in the US: 1990‐1998. Diabetes Care 2000;23:1278‐1283. [DOI] [PubMed] [Google Scholar]
Moore 2000
- Moore P. Type 2 diabetes is a major drain on resources. British Medical Journal 2000;320:732. [PMC free article] [PubMed] [Google Scholar]
Moore 2004
- Moore H, Summerbell C, Hooper L, Cruickshank K, Vyas A, Johnstone P, Ashton V, Kopelman P. Dietary Advice for treatment of type 2 diabetes mellitus in adults. The Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI] [PubMed] [Google Scholar]
Nathan 1999
- Nathan DM. Treating type 2 diabetes with respect. Annals of Internal Medicine 1999;130:440‐1. [DOI] [PubMed] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
Pirozzo 2002
- Pirozzo S, Summerbell C, Cameron C, Glasziou P. Advice on low‐fat diets for obesity (Cochrane Review). The Cochrane Library 2003, Issue 4. [DOI] [PubMed] [Google Scholar]
Riccardi 2000
- Riccardi G, Rivellese AA. Dietary treatment of the metabolic syndrome ‐ the optimal diet. British Journal of Nutrition 2000;83:S143‐8. [DOI] [PubMed] [Google Scholar]
Roman 1997
- Roman SH, Harris MI. Management of diabetes mellitus from a public health perspective. Endocrinology & Metabolism Clinics of North America 1997;26(3):443‐74. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Jama 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Thomas 2006
- Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews 2006, Issue 3. [CD003642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuomilehto 2001
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, Keinanen‐Kiukaanniemi S, Laaskso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Aunola S, Cepaitis Z, Moltchanov V, Hakumaki M, Mannelin M, Martikkala V, Sundvall J. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. The New England Journal of Medicine 2001;344(18):1343‐50. [DOI] [PubMed] [Google Scholar]
Turner 1999
- Turner RC, Cull CA, Frighi V, Holman RR. Glycaemic control with diet, sulfonylurea, metformin or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). Journal of the American Medical Association 1999;281:2005‐12. [DOI] [PubMed] [Google Scholar]
UKPDS 1998
- UKPDS Group. Intensive blood‐glucose control with sulfonyureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
UKPDS 1998b
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317(703‐13). [PMC free article] [PubMed] [Google Scholar]
Wallberg‐H. 1998
- Wallberg‐Henriksson H, Rincon J, Zierath JR. Exercise in the Management of Non‐Insulin‐Dependent Diabetes Mellitus. Sports Medicine 1998;25(1):25‐35. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. World Health Organisation, 1980. [646] [PubMed]
WHO 1985
- World Health Organisation. Diabetes Mellitus: Report of a WHO Study Group. Technical Report Series No. 727. World Health Organisation, 1985. [727] [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation.. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
WHO/FAO 2003
- WHO (Joint WHO/FAO Expert Consultation). DIET, NUTRITION AND THE PREVENTION OF CHRONIC DISEASES. Geneva 2003.
Wing 2000
- Wing RR. Weight loss in the management of type 2 diabetes. Long ‐term non‐pharmacological weight loss interventions for adults with type 2 diabetes 2005, Issue 2. [Google Scholar]


