Abstract
Background
Thyroid nodules (TN) are common in the adult population. Some physicians use suppressive levothyroxine (LT4) therapy to achieve a reduction in the number and volume of TN. In addition, minimally invasive treatments, such as percutaneous ethanol injection (PEI) sclerotherapy, laser photocoagulation (LP), and microwave (MW), radiofrequency (RF) and high‐intensity focused ultrasound (HIFU) ablation, have been proposed, especially for pressure symptoms and cosmetic complaints, as an alternative to surgery. However, the risk to benefit ratio of all treatments for benign TN is currently unknown.
Objectives
To assess the effects of LT4 or minimally invasive therapies (PEI, LP, and RF/HIFU/MW ablation) on benign TN.
Search methods
We identified studies from computerised searches of The Cochrane Library, MEDLINE, EMBASE and LILACS (all performed up to April 2014). We also searched trial registers, examined reference lists of included randomised controlled trials (RCTs) and systematic reviews, and contacted study authors.
Selection criteria
We included studies if they were RCTs of LT4, PEI, LP, RF, HIFU or MW therapy in participants with an established diagnosis of benign TN. We excluded trials investigating the prevention of recurrence of thyroid disease after surgery, irradiation or treatment with radioiodine.
Data collection and analysis
Two review authors independently extracted data, assessed studies for risk of bias and evaluated overall study quality utilising the GRADE instrument. We assessed the statistical heterogeneity of included studies by visually inspecting forest plots and quantifying the diversity using the I² statistic. We synthesised data using random‐effects model meta‐analysis or descriptive analysis, as appropriate.
Main results
Thirty‐one studies randomised 2952 outpatients to investigate the effects of different therapies on benign TN. Studies on LT4, PEI, LP and RF ablation therapy randomised 2083, 607, 192 and 70 participants, respectively. We found no RCTs of HIFU or MW ablation therapy in benign TN. The duration of treatment varied according to the applied therapies: up to five years for LT4 and one to three PEI ablations, one to three LP sessions and one or two RF sessions. Median follow‐up was 12 months for LT4 and six months for minimally invasive therapies. Evidence was of low‐to‐moderate quality, and risk of performance and detection bias for subjective outcomes was high in most trials.
No study evaluated all‐cause mortality or health‐related quality of life. Only one LT4 study provided some data on the development of thyroid cancer, reporting no abnormal cytological findings. One LP study provided limited information on costs of treatment.
LT4 compared with no treatment or placebo was associated with a nodule volume reduction of 50% or more in 16% compared with 10% of participants after 6 to 24 months of follow‐up (risk ratio (RR) 1.57 (95% confidence interval (CI) 1.04 to 2.38); P = 0.03; 958 participants; 10 studies; moderate‐quality evidence). Pressure symptoms or cosmetic complaints were not investigated in LT4 studies. LT4 therapy was generally well tolerated: three studies provided quantitative data on signs and symptoms of hyperthyroidism, which were observed in 25% of LT4‐treated versus 7% of placebo‐treated participants at 12 to 18 months of follow‐up (269 participants; 3 trials; low‐quality evidence).
PEI compared with cyst aspiration only was associated with a nodule volume reduction of 50% or more in 83% compared with 44% of participants after 1 to 24 months of follow‐up (RR 1.83 (95% CI 1.32 to 2.54); P = 0.0003; 105 participants; 3 studies; low‐quality evidence). Improvements in neck compression symptoms after 6 to 12 months of follow‐up were seen in 78% of participants receiving PEI versus 38% of those in comparator groups. No reliable summary effect estimate could be established, RR ranged from 1.0 to 3.06 in favour of PEI (370 participants; 3 trials; low‐quality evidence). In all trials, participants experienced periprocedural cervical tenderness and light‐to‐moderate pain usually lasting from minutes to several hours. As a result of the PEI procedure, 26% of participants reported slight‐to‐moderate pain compared with 12% of those receiving cyst aspiration only (RR 1.78 (95% CI 0.62 to 5.12); P = 0.28; 104 participants; 3 studies; low‐quality evidence).
One study comparing LP with LT4 showed a nodule volume reduction of 50% or more in favour of LP after 12 months of follow‐up in 33% of LP participants versus 0% of LT4 participants, respectively (62 participants; 1 trial; low‐quality evidence). A total of 82% of LP‐treated versus 0% of untreated participants showed improvements in pressure symptoms after 6 to 12 months of follow‐up (RR 26.65 (95% CI 5.47 to 129.72); P < 0.0001; 92 participants; 3 trials; low‐quality evidence). Around 20% of LP‐treated participants reported light‐to‐moderate cervical pain lasting 48 hours or more (97 participants; 3 trials; low‐quality evidence).
One trial with 40 participants, comparing RF with no treatment, resulted in a mean nodule volume reduction of 76% in the RF group compared with 0% of those in the no‐treatment group at six months of follow‐up (low‐quality evidence). These RF‐treated participants had fewer pressure symptoms and cosmetic complaints after 12 months of follow‐up compared with untreated participants (a 2.8 decrease versus a 1.1 increase on a six‐point scale, respectively, with higher values indicating more severe symptoms; low‐quality evidence). All participants complained of pain and discomfort during RF, which disappeared when the energy was reduced or turned off (low‐quality evidence).
Authors' conclusions
No study evaluated all‐cause mortality, health‐related quality of life or provided systematic data on the development of thyroid cancer. Longest follow‐up was five years and median follow‐up was 12 months. Nodule volume reductions were achieved by PEI, LP and RF, and to a lesser extent, by LT4. However, the clinical relevance of this outcome measure is doubtful. PEI, LP and RF led to improvements in pressure symptoms and cosmetic complaints. Adverse events such as light‐to‐moderate periprocedural pain were seen after PEI, LP and RF. Future studies should focus on patient‐important outcome measures, especially health‐related quality of life, and compare minimally invasive procedures with surgery. RCTs with follow‐up periods of several years and good‐quality observational studies are needed to provide evidence on the development of thyroid cancer, all‐cause mortality and long‐term adverse events.
Keywords: Humans, Catheter Ablation, Catheter Ablation/methods, Ethanol, Ethanol/therapeutic use, High‐Intensity Focused Ultrasound Ablation, High‐Intensity Focused Ultrasound Ablation/methods, Laser Therapy, Laser Therapy/methods, Microwaves, Microwaves/therapeutic use, Randomized Controlled Trials as Topic, Sclerotherapy, Sclerotherapy/methods, Thyroid Nodule, Thyroid Nodule/pathology, Thyroid Nodule/therapy, Thyroxine, Thyroxine/therapeutic use
Plain language summary
Thyroid hormone therapy or minimally invasive treatments for benign thyroid nodules
Review question
What are the effects of thyroid hormone treatment (levothyroxine) and minimally invasive procedures on benign thyroid nodules?
Background
Nodules (lumps) within the thyroid gland are common and usually benign. They are more frequent in women, the elderly and in iodine‐deficient areas. Thyroid nodules are often observed as an incidental finding in the course of ultrasonography of the thyroid, nodules of more than 1 cm in size are usually detected by palpation of the thyroid gland during a physical examination. Thyroid nodules may occur as a single nodule or as multiple nodules and may contain fluid (cyst). About 5 in 100 palpable thyroid nodules have a risk of becoming malignant (thyroid cancer). Thyroid nodules are often treated with thyroid hormones in order to reduce the size of the nodule. If thyroid nodules cause problems such as pressure symptoms or cosmetic complaints, surgery may be performed. Other therapies try to destroy the thyroid nodule by means of minimally invasive procedures (techniques which are less invasive than open surgery) and are usually performed on an outpatient basis.
Study characteristics
We identified 31 randomised controlled trials for this systematic review. Altogether 2952 participants were allocated to the various intervention and comparator groups. In total, 16 studies lasting six months to five years investigated the effects of levothyroxine therapy. Eight studies lasting 1 to 12 months investigated the efficacy of injections, mostly of ethanol, into thyroid nodules from which fluid had been slowly removed. Laser therapy (one or up to three sessions) was applied to nodules in five studies lasting 6 to 12 months. Two studies investigated the application of one or two radiofrequency (high‐frequency radiowaves) sessions over 6 to 12 months.
Key results
None of the interventions investigated death from any cause, the development of thyroid cancer or health‐related quality of life. Nodule volume reductions were achieved by all therapies; however, the clinical relevance of this outcome is doubtful. Minimally invasive treatments resulted in improvements in pressure symptoms and cosmetic complaints. Some side effects such as light‐to‐moderate pain were observed after minimally invasive procedures.
Quality of the evidence
Most study results were of overall low quality, mainly because only a few people were investigated, findings were imprecise or measurements were prone to bias. Future studies should investigate more patient‐important outcomes, such as health‐related quality of life, and should compare minimally invasive therapies with surgery. Studies with longer follow‐up periods are needed to provide evidence on the development of thyroid cancer, death from any cause and long‐term side effects of treatments.
Currentness of data
This evidence is up to date as of April 2014.
Summary of findings
Summary of findings for the main comparison. Summary of findings (levothyroxine treatment).
| Thyroid hormone treatment compared with placebo or no treatment for benign thyroid nodules | ||||||
|
Participant: participants with benign thyroid nodules Settings: outpatients Intervention: thyroid hormone treatment (levothyroxine (LT4)) Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riska | Corresponding risk | |||||
| Placebo or no treatment | Levothyroxine | |||||
| All‐cause mortality | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Thyroid cancer Follow‐up: 12 and 24 months | See comment | See comment | Not estimable | See comment | ⊕⊕⊝⊝ lowb |
One study confirmed benignity of some treated nodules through FNAB and cytological re‐evaluation in the non‐responder group, defined as participants with constant or increasing nodule volume (33/58 participants) |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
|
Adverse events Follow‐up: 12 to 18 months |
See comment | See comment | Not estimable | 269 (3) | ⊕⊕⊝⊝ lowc |
LT4 therapy was generally well tolerated. One of three studies reported more signs and symptoms of hyperthyroidism after LT4, a reliable effect estimate could not be established |
| Pressure symptoms / cosmetic complaints | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
|
Nodule volume reduction ≥ 50% Follow‐up: 6 to 24 months |
98 of 1000 | 154 of 1000 (102 to 233) |
RR 1.57 (1.04 to 2.38) | 958 (10) | ⊕⊕⊕⊝ moderated | ‐ |
| Socioeconomic effects | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median comparator group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FNAB: fine‐needle aspiration biopsy; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk was derived from the event rates in the comparator groups bDowngraded by two levels because of few participants and only one study investigating this outcome cDowngraded by two levels because of inconsistency, few participants and high risk of detection bias dDowngraded by one level because of indirectness (surrogate outcome parameter)
Summary of findings 2. Summary of findings (percutaneous ethanol injection sclerotherapy).
| Percutaneous ethanol injection compared with aspiration, levothyroxine or isotonic saline for benign thyroid nodules | ||||||
|
Participant: participants with benign thyroid nodules Settings: outpatients Intervention: percutaneous ethanol injection (PEI) Comparison: aspiration, levothyroxine, isotonic saline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riska | Corresponding risk | |||||
| Aspiration | PEI | |||||
| All‐cause mortality | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Thyroid cancer | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
|
Adverse events Follow‐up: 6 to 12 months |
118 of 1000 | 209 of 1000 (73 to 602) | RR 1.78 (0.62 to 5.12) | 104 (3) | ⊕⊕⊝⊝ lowb |
In all studies participants experienced periprocedural cervical tenderness and light‐to‐moderate pain lasting from minutes to several hours |
|
Pressure symptoms / cosmetic complaints Follow‐up: 6 to 12 months |
See comment | See comment | RR range 1.00 to 3.06 | 370 (3) | ⊕⊕⊝⊝ lowc |
No reliable effect estimate because of unexplained considerable heterogeneity |
|
Nodule volume reduction ≥ 50% Follow‐up: 1 to 12 months |
442 of 1000 | 809 of 1000 (584 to 1123) | RR 1.83 (1.32 to 2.54) | 105 (3) | ⊕⊕⊕⊝ moderated |
‐ |
|
Socioeconomic effects Follow‐up: 6 months |
See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median comparator group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk was derived from the event rates in the comparator groups bDowngraded by two levels because of imprecise results (CI includes null effect and appreciable benefit or harm) and high risk of detection bias cDowngraded by two levels because of inconsistency, high risk of performance bias and high or unclear risk of detection bias dDowngraded by one level because of few participants and indirectness (surrogate outcome parameter)
Summary of findings 3. Summary of findings (laser photocoagulation).
| Laser photocoagulation compared with no treatment or levothyroxine for benign thyroid nodules | ||||||
|
Participant: participants with benign thyroid nodules Settings: outpatients Intervention: laser photocoagulation (LP) Comparison: no treatment, levothyroxine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riska | Corresponding risk | |||||
| No treatment | Laser photocoagulation | |||||
| All‐cause mortality | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Thyroid cancer | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
|
Adverse events Follow‐up: 6 to 12 months |
See comment | See comment | See comment | 97 (3) | ⊕⊕⊝⊝ lowb |
Three studies reported that 10/49 (20%) participants treated by laser photocoagulation experienced light to moderate pain lasting 48 hours and more |
|
Pressure symptoms / cosmetic complaints Follow‐up: 6 to 12 months |
See comment | See comment | 26.65 (5.47 to 129.72) | 92 (3) | ⊕⊕⊝⊝ lowc |
No participant in the no‐treatment comparator group showed signs of improvement |
|
Nodule volume reduction ≥ 50% Follow‐up: 12 months |
See comment | See comment | Not estimable | 62 (1) | ⊕⊕⊝⊝ lowd |
One study investigated laser therapy versus LT4 or no treatment and showed that 7/21 (33%) treated participants compared with no participants (0/41) in either comparator groups achieved this outcome |
|
Socioeconomic effects Follow‐up: 12 months |
See comment | See comment | Not estimable | 62 (1) | ⊕⊕⊝⊝ lowe |
The costs of laser photocoagulation therapy including equipment, medical team, and disposable kits was about €450 (approx. US$550, September 2012 conversion) |
| *The basis for the assumed risk (e.g. the median comparator group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LT4: levothyroxine; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aAssumed risk was derived from the event rates in the comparator groups bDowngraded by two levels because of inconsistency, few participants and high risk of performance bias cDowngraded by two levels because of wide CIs, few participants and high risk of performance bias dDowngraded by two levels because of few participants, one study only, an unclear risk of detection bias and indirectness (surrogate outcome parameter) eDowngraded by two levels because of few participants, one study only and no formal cost‐benefit analysis
Summary of findings 4. Summary of findings (radiofrequency ablation).
| Radiofrequency ablation compared with no treatment | ||||||
|
Participants: participants with benign thyroid nodules Settings: outpatients Intervention: radiofrequency ablation (RF) Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Radiofrequency ablation | No treatment | |||||
| All‐cause mortality | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Thyroid cancer | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
|
Adverse events Follow‐up: 12 months |
See comment | See comment | Not estimable | 40 (1) | ⊕⊕⊝⊝ lowa |
All participants complained of pain and discomfort during radiofrequency ablation which disappeared when the energy was reduced or turned off |
|
Pressure symptoms / cosmetic complaints Follow‐up: 12 months Scale: sum of individual scores including pressure symptoms in the neck, difficulty in swallowing, aesthetic complaint (0: absent, 1: moderate, 2: severe; range 0 to 6) |
See comment | See comment | Not estimable | 40 (1) | ⊕⊕⊝⊝ lowb |
Intervention group: decline from 3.4 (SD 1.3) at baseline to 0.6 (SD 0.5) No‐treatment group: increase from 3.0 (SD 1.3) at baseline to 4.1 (SD 0.9) Difference between groups: P < 0.0001 |
|
Nodule volume reduction ≥ 50% Follow‐up: 12 months |
See comment | See comment | Not estimable | 40 (1) | ⊕⊕⊝⊝ lowc |
Statistically significant differences in favour of RF at 3, 6 and 12 months |
| Socioeconomic effects | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median comparator group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels because of few participants, one study only and high risk of performance bias bDowngraded by two levels because of few participants, one study only and high risk of performance bias cDowngraded by two levels because of few participants, one study only and an unclear risk of detection bias
Background
Description of the condition
Nodular thyroid disease is common. Thyroid nodules are more frequent in women, the elderly and in iodine‐deficient areas, becoming malignant possibly more often in men and especially in individuals aged over 70 years (Belfiore 1992). Palpable thyroid nodules were detected in 4% to 7% of individuals in the USA (Mazzaferri 1993) and in 0.8% of adult men and 5.3% of adult women in Northeast England (Tunbridge 1977). Thyroid nodules are even more common when detected using ultrasonography of the thyroid (Brander 1991), with prevalence rates varying from 20% to 60% (Galofré 2008). Many nodules are thyroid incidentalomas, which are discovered when neck structures are imaged for other reasons (Daniels 1996). In the Framingham study population, new nodules appeared in 0.1% of participants per year during a 15‐year follow‐up period (Vander 1968).
A clinically solitary thyroid nodule is a discrete swelling within an otherwise palpable normal thyroid gland. The overwhelming majority of these nodules are composed of irregularly enlarged follicles containing abundant colloid (benign adenomatous nodules). About half of individuals with clinically apparent solitary nodules are found to have multinodular goitres (MNGs) at surgery. The risk of cancer in people with true solitary nodules confirmed at surgery has been reported to be about the same as that in those with MNGs (McCall 1986). In contrast, however, a recent systematic review and meta‐analysis found MNGs to be associated with a lower risk of thyroid cancer than solitary nodules (odds ratio (OR) 0.8 (95% confidence interval (CI) 0.67 to 0.96); 44,288 participants; 14 longitudinal and cross‐sectional observational studies) (Brito 2013).
Thyroid nodules are often hypofunctioning, as determined by radionuclide scanning (termed 'cold' nodules), are incompletely encapsulated and sometimes poorly demarcated. Some authors consider a 'warm' TN as a distinct entity, although most distinguish nodules that are autonomously functioning as 'hot' from those that are 'cold'. Using this definition, virtually all thyroid cancers are cold. However, approximately 95% to 97% of cold nodules are benign (Daniels 1996). Benign thyroid nodules are commonly caused by thyroid adenomas, cysts and thyroiditis.
The discovery of a thyroid nodule leads to concerns that the nodule may develop into thyroid cancer. Factors that favour the development of thyroid cancer include a history of neck irradiation, rapid tumour growth, male sex, age younger than 20 years or older than 70 years, a family history of thyroid cancer or features suggestive of neoplasia. The incidence of cancer in individuals with clinical features suggestive of malignancy (e.g. firm, fixed nodule, enlarged cervical lymph nodes, recurrent laryngeal nerve palsy in the absence of previous surgery) is high, but most do not have these features (Hamming 1990). From a clinical viewpoint, fewer than 5% of palpable thyroid nodules are malignant. Nodule growth alone, however, does not predict malignancy. Alexander 2003 found that cystic nodules grew less than those with more solid components, and that malignity was proved after repeated fine‐needle aspiration (FNA) in 1 of 74 nodules.
Well‐differentiated thyroid carcinomas (papillary and follicular) comprise 80% of all thyroid cancers (Kaplan 1990). The annual incidence is approximately 4 in 100,000 persons (0.004%), with an estimated prevalence of 1 in 1000 persons (0.1%) (Daniels 1996). Many more people have clinically silent thyroid cancers: up to 35% of thyroid glands removed at autopsy (Mazzaferri 1988) or surgically (Pelizzo 1990) contain small (less than 1.0 cm), thought to be clinically insignificant, papillary carcinomas. Despite an increasing incidence in the detection of papillary carcinomas, mortality from thyroid cancer between 1973 and 2002 remained stable (Davies 2006). In the USA, approximately 37,200 cases of new thyroid cancers were estimated to be diagnosed in 2009, with about 1630 deaths resulting from the disease (Jemal 2009).
Recent developments, such as the use of FNA biopsy (FNAB), the application of high‐resolution ultrasonography and sensitive thyroid‐stimulating hormone (TSH) assays, have resulted in important advances in the diagnosis and management of thyroid nodules. Many publications have defined and classified nodules according to cytological features, described techniques for monitoring thyroid functional status in the course of TSH suppression and raised concerns about the potential complications of suppressive therapy.
Description of the intervention
It is unclear whether asymptomatic thyroid nodules should be treated because in most cases they are benign, small and can be managed by active surveillance (Gharib 2007). However, some thyroid nodules grow and can cause pressure and other symptoms as well as cosmetic complaints, and hence require treatment. Until recently, surgical approaches have been used for the management of nodules causing severe symptoms; however, the risk of complications persists and there may be a problem with the availability of experienced thyroid surgeons. Thyroid hormone suppression therapy with levothyroxine (LT4) is an alternative option for the treatment of thyroid nodules. In addition, a number of minimally invasive therapies, all guided by ultrasound imaging, are increasingly employed in the treatment of symptomatic thyroid nodules.
LT4 therapy
The use of thyroid hormone suppressive therapy in individuals with thyroid nodules and nodular goitre is based on the presumption that TSH (thyroid stimulating hormone also known as thyrotropin) is a growth factor for thyroid tissue (Burch 1995; Morita 1989). The rationale for TSH suppression (i.e. that thyroid nodules and nodular goitre are caused by TSH stimulation as the main stimulator of thyroid function or growth) has never been clearly proven (Cooper 1995). Despite considerable controversy among experts about its efficacy, suppressive therapy of the thyroid nodule with thyroxine, with the goal of suppressing TSH production and reducing the size of the nodule, has gained wide acceptance. The efficacy of thyroid hormone suppressive therapy for nodules and goitre is supported by extensive anecdotal clinical experience as well as numerous uncontrolled trials (Daniels 1996). Thyroid hormone suppression therapy for thyroid nodules resurfaced as a legitimate therapy with the publication of uncontrolled experiences in 1960 (Astwood 1960). Over the next decades, discordant reports about the efficacy of this therapy were published, possibly being associated with the aetiological heterogeneity of thyroid nodules and their unpredictable patterns of growth. With time, solitary nodules may enlarge, shrink or even disappear spontaneously (Kuma 1992), but most do not change appreciably (Vander 1968). Similarly, the possible presence of cystic nodules, which can either resolve or grow spontaneously, was not taken into consideration in some studies. Moreover, confounding variables, such as the lack of a comparator population, a short period of follow‐up, an inaccurate quantification of nodule size and the lack of proof of effective TSH suppression, did not allow conclusive results. By definition, LT4 suppressive therapy is a dose of levothyroxine sufficient to suppress pituitary TSH secretion to concentrations that are below the lower limits of normal (Gharib 1998). Although the optimal level of TSH suppression has not been clearly defined, complete suppression of serum TSH concentrations to less than 0.1 mIU/L is thought to be unnecessary in individuals with benign thyroid disease (Burch 1995).
Percutaneous injection sclerotherapy
Percutaneous ethanol injection (PEI) is an ultrasound‐guided minimally invasive therapeutic procedure suggested for the non‐surgical management of benign thyroid nodules in individuals with pressure symptoms or cosmetic complaints. PEI was first proposed in 1990 as a possible alternative to surgery and radioiodine therapy for the treatment of autonomously functioning thyroid nodules in outpatients (Bennedbaek 1997; Livraghi 1990; Papini 1995). The procedure is currently described as effective in the treatment of benign thyroid cysts and complex nodules with a dominant fluid component. The method should not be performed in solitary solid nodules, whether hyperfunctioning or not, or in MNGs (AACE/AME/ETA Guidelines 2010). The Latin American Thyroid Society (LATS) also does not recommend PEI for the routine treatment of thyroid nodules other than cysts in their recent guidelines (LATS 2009). However, some authors have described satisfactory results with PEI for the treatment of thyroid solid nodules in individuals with pressure symptoms or cosmetic complaints who refuse surgery or are at surgical risk, reporting an overall nodule volume reduction of 43% (Bennedbaek 1995). Mainly ethanol is injected into the thyroid cysts, some investigators however use other substances such as the antibiotic tetracycline.
Technique: The individual lies on his/her back with the neck hyperextended. The nodule is identified by ultrasound. After applying local anaesthesia (optional), the operator inserts a needle that is connected to a syringe into the cyst. The cyst fluid is smoothly and slowly aspirated and the contents are extracted totally. Sterile ethanol 95% is then injected carefully into the cyst to refills the cavity. The quantity of ethanol injected is usually equivalent to 50% to 70% of the cystic fluid extracted. The alcohol (deposited within the cyst) is gradually reabsorbed during the next 24 to 48 hours without major discomfort. Alcohol causes permanent tissue ablation by local necrosis and thrombosis of small intranodular vessels. Experience is imperative for the performance of neck ultrasound and ultrasound‐guided PEI because the manoeuvre is safe only in expert hands (PEI Valcavi 2004).
Another variation of the PEI technique was proposed by Bennedbaek et al, which involves subtotal cyst aspiration, washing with ethanol and subsequent complete fluid aspiration after two minutes (without removing the needle) under ultrasound control (PEI Bennedbaek 2003 ). The authors report treatment failure in 18% of participants. Such individuals subsequently underwent hemithyroidectomy; in one of them the surgeon mentioned that periglandular fibrosis resulting from the ethanol injection made the surgical procedure more difficult.
Ultrasound‐guided interstitial laser photocoagulation
Interstitial laser photocoagulation (LP), also called percutaneous laser ablation, is described as a rapid, minimally invasive technique, and proposed as an alternative to thyroidectomy for benign thyroid lesions causing compressive symptoms or cosmetic complaints. The procedure is highly effective for achieving volume reductions in thyroid lesions, and is usually performed in selected cases (individuals at high‐surgical risk) and in specialised centres (Filetti 2006). In most individuals with thyroid nodules, one to three sessions of LP induce a significant decrease in nodule volume and the amelioration of local symptoms (AACE/AME/ETA Guidelines 2010). Two new studies with three and five years of follow‐up observed comparable nodule volume reductions of about 50% and 75%, with an improvement in pressure symptoms (Dossing 2011). Because of potential complications, thermal ablation procedures should be performed only by experienced operators (AACE/AME/ETA Guidelines 2010).
Technique: Under sterile conditions the individual undergoes local anaesthesia and light sedation to avoid abrupt movements. Ultrasound‐guided, the laser fibre is positioned in the thyroid nodule through the lumen of one small or multiple (up to four) needles. The needle is then withdrawn 20 mm leaving the end of the fibre in direct contact with the tissue. After the penetration of the laser light, absorbed energy produces heat (temperatures of up to 180°C to 200°C), inducing tissue charring and necrosis with subsequent volume decrease. To avoid injuries from the thermal effects of LP, a safety distance of at least 15 mm from the neurovascular bundle is required (Pacella 2000). Before the procedure is terminated, three or four areas are treated.
A variation of this procedure has also been described as effective (LP Gambelunghe 2006): during the manoeuvre a small needle is moved from the initial position in steps of 2 to 5 mm, to a distance of 10 mm from the cranial portion of the capsule. The energy applied varies from 100 J to 400 J per step, based on the extent of the hyperechoic area produced by photocoagulation.
Ultrasound‐guided radiofrequency ablation therapy
Ultrasound‐guided radiofrequency (RF) ablation therapy has been investigated in elderly individuals with benign, compressive and large thyroid nodules (Spiezia 2009). RF ablation therapy, using small needles and internally cooled electrodes, enables the therapist to prevent scar formation without skin incision (Baek 2010). This procedure has previously been used for treating primary and secondary malignant neoplasms and liver tumours. RF energy is applied in 3.8 to 4 MHz quantities, and tissues are heated at temperatures between 60°C and 100°C resulting in subsequent cell death. The needles utilised are generally larger than those used for LP (Spiezia 2009) and this method is ordinarily performed under conscious sedation. Some authors have used single‐hook needles (Baek 2010) and others prefer multiple expandable hook needles (RF Faggiano 2012). Safety and efficacy in prospective randomised controlled trials (RCTs) have yet to be adequately investigated, so RF ablation is currently not recommended in the routine management of benign thyroid nodules (AACE/AME/ETA Guidelines 2010).
High‐intensity focused ultrasound ablation therapy
This procedure is employed in the ambulatory setting and has been used to treat localised prostate cancer. The technique has been shown to lower costs and shorten hospitalisation, and represents an interesting alternative for individuals in whom surgery is contraindicated (Esnault 2008). High‐intensity focused ultrasound (HIFU) ablation is a process of delivering a large amount of heat energy to a restricted space, where ultrasound produces necrosis with a minimum effect on surrounding structures. The first human feasibility study was an open‐label, non‐randomised and uncontrolled trial performed in 25 participants who were scheduled for thyroid surgery two weeks later. No serious adverse events were observed, especially those affecting the recurrent nerves or the trachea (Esnault 2011). Histological analysis provided some preliminary results about the efficacy of this method and studies are ongoing to asses the changes in nodules at longer follow‐up (Esnault 2011).
Ultrasound‐guided microwave ablation therapy
This procedure has been used to treat benign and malignant tumours of the liver, kidneys, adrenal glands, spleen and lungs (Feng 2012). The technique has been performed on an inpatient basis under continuous control of blood pressure, partial oxygen pressure and electrocardiography. Under local anaesthesia, a small incision (< 2 mm in length) was made to introduce the internally cooled needle antenna into the thyroid nodule. After placement of the antenna, the ultrasound‐guided microwave (MW) procedure was then performed under intravenous anaesthesia (Feng 2012). A power output of 20 W to 30 W was used during MW ablation. The penetration of the microwaves into the tissue is the consequence of a fast rotation of the molecules, growth of local energy and a rapid increase in temperature in the focused area (Gharib 2013). One small feasibility, non‐randomised trial, performed in 11 participants with compressive neck symptoms, 9 with pain due to nodular goitre and 2 with Hashimoto’s thyroiditis demonstrated a nodule volume decrease of more than 50% and an improvement in cosmetic complaints (Feng 2012). Currently, MW is currently considered an experimental procedure for the treatment of thyroid nodules (Gharib 2013).
Known adverse effects of the intervention
LT4
The majority of thyroid hormone studies were of short duration and severe adverse effects were not observed despite adequate TSH inhibition under LT4 suppressive therapy (Mainini 1995). Studies investigating cardiovascular and osteoporosis risks sparked several controversies about the possibilities of fractures with long‐term LT4 therapy, especially in postmenopausal women (Bauer 2001; Leese 2011; Stall 1990; Uzzan 1996). LT4 suppressive treatment is also reported to increase pulse rate, left ventricular mass and the frequency of atrial arrhythmias (Biondi 1993).
PEI
Adverse effects were mostly few and transient, and generally related to the percutaneous injection of ethanol into solid nodules rather than cysts (Bennedbaek 1997). Perinodular fibrosis due to ethanol injection into solid nodules may seriously hamper subsequent surgery (Bennedbaek 1997). In almost all studies, pain was of mild‐to‐moderate intensity lasting for one or two days. Other observed effects were: local burning sensation and transient dysphonia (Alcantara‐Jones 2006; Braga‐Brasaria 2002; Kanotra 2008; Kim 2005; Lima 2007; Zingrillo 1998). Severe complications, such as permanent dysphonia and infections, were not observed.
LP
Documented complications were mostly mild‐to‐moderate pain lasting for up to days (Dossing 2007; Papini 2004), sometimes requiring additional medication (Dossing 2002; Dossing 2011). Usually, no serious adverse effects, such as dysphonia, local infections, vocal cord paralysis or hypothyroidism, were noted.
RF
The most frequently described complications were pain of different intensities and durations, usually occurring during the procedure (Baek 2009; Baek 2010; Deandrea 2008; Jeong 2008; Kim 2006; Spiezia 2009). Haematoma and fever were also observed. Generally, complications resolved without sequela.
HIFU
Commonly reported adverse effects were local pain, skin burns, blisters and cough. It is hoped that safety can be improved by implementing technological improvements (Esnault 2011).
MW
Currently, the evidence base for MW ablation therapy is scarce. After MW ablation, 8 of 11 participants complained of a sensation of heat in the neck, slight pain, or both, at the ablated site. All participants could tolerate the symptoms and needed no analgesics (Feng 2012). One participant complained of coughing and choking when drinking and a small change in voice six hours after ablation. Laryngoscopic evaluation demonstrated ipsilateral vocal cord palsy. The participant's voice recovered within two months after corticosteroid therapy (Feng 2012).
Why it is important to do this review
Thyroid nodules are a frequent problem seen in a medical practice, and the primary objective of their management ‐ if not causing pressure symptoms or cosmetic complaints ‐ is to exclude malignancy. Uncertainties about aetiology, pathophysiology and prognosis complicate the choice of an efficient and safe treatment. In addition, there is considerable interest in finding therapeutic alternatives to surgery.
We identified several systematic reviews and meta‐analyses investigating the effects of thyroid hormone therapy for benign thyroid nodules (Castro 2002; Richter 2002; Sdano 2005; Yousef 2010; Zelmanovitz 1998). Since the publication of these reviews, new studies have been carried out making it necessary not only to re‐analyse data on thyroid hormone treatment, but also to establish evidence for all available treatment options for benign thyroid nodules.
Objectives
To assess the effects of LT4 or minimally invasive therapies (PEI, LP, and RF/HIFU/MW ablation) on benign thyroid nodules.
Methods
Criteria for considering studies for this review
Types of studies
RCTs. We excluded RCTs investigating the prevention of the recurrence of thyroid disease after surgery, irradiation or treatment with radioiodine.
Types of participants
Participants with an established diagnosis of benign thyroid nodule(s).
Diagnostic criteria
Benign thyroid nodules had to be identified by ultrasonography and FNAB with cytology. Additional investigations included physical examination, thyroid hormone measurements and scintigraphy.
Types of interventions
We looked for the following comparisons:
Interventions
(a) LT4.
(b) PEI.
(c) LP.
(d) RF ablation.
(e) HIFU ablation.
(f) MW ablation.
Comparator interventions
Placebo compared with (a) or (b).
Cyst aspiration only compared with (b).
No treatment compared with (a), (b), (c), (d), (e) or (f).
Any other treatment compared with (a), (b), (c), (d), (e) or (f).
Another treatment regimen for (a), (b), (c), (d), (e) or (f).
Types of outcome measures
Primary outcomes
Pressure symptoms, cosmetic complaints, or both.
Nodule volume reduction of 50% or more.
Adverse events.
Secondary outcomes
Compliance.
Tolerability.
Thyrotropin (TSH), thyroxine (T4) and tri‐iodothyronine (T3) serum levels.
Thyroid cancer.
All‐cause mortality.
Health‐related quality of life.
Socioeconomic effects.
Method and timing of outcome measurement
Pressure symptoms, cosmetic complaints, or both: as measured by questionnaires in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Nodule volume reduction of 50% or more: as measured by ultrasonography in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Adverse events (such as infection, severe cervical pain, bone loss and risk of fractures, atrial fibrillation, signs of hyperthyroidism): measured in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Compliance: as measured by questionnaires or pill count in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Tolerability of the procedure: as measured by questionnaires in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
TSH, T4 and T3 serum levels: laboratory measurements in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Thyroid cancer: as measured by clinical or register data in the long‐term (≥ 12 months).
All‐cause mortality: as measured by clinical or register data in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Health‐related quality of life (measured using a validated instrument) and indicators of well‐being: measured in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
Socioeconomic effects (e.g. hospital stay, sick leave days, avoidance of surgery, costs): as measured by clinical or register data in the short‐term (≤ 6 months), medium‐term (6 to 12 months) and long‐term (≥ 12 months).
'Summary of findings' table
The following outcomes are listed according to priority.
All‐cause mortality.
Thyroid cancer.
Health‐related quality of life.
Adverse events.
Pressure symptoms, cosmetic complaints or both.
Nodule volume reduction of 50% or more.
Socioeconomic effects.
Potential covariates, effect modifiers and confounders
Compliance/tolerability.
Disease status.
Search methods for identification of studies
Electronic searches
We used the following sources from inception until the date specified for the identification of trials.
The Cochrane Library (April 2014).
MEDLINE (April 2014).
EMBASE (April 2014).
LILACS (April 2014).
We also searched trial registers, including ClinicalTrials.gov (http://ClinicalTrials.gov/), metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/), the EU Clinical Trials register (https://www.clinicaltrialsregister.eu/) and the World Health Organization (WHO) International Clinical Trials Registry Platform Search Portal (http://apps.who.int/trialsearch/). For every included study we tried to find its protocol, either in databases of ongoing trials, in publications of study designs, or both.
For detailed search strategies, see Appendix 1. Searches were not older than one month at the moment the final review draft was checked into the Cochrane Information and Management System for editorial approval. We used PubMed's 'My NCBI' (National Center for Biotechnology Information) email alert service to identify newly published studies using a basic search strategy (see Appendix 1).
If additional key words of relevance had been detected during any of the electronic or other searches we had intended to modify electronic search strategies to incorporate these terms. However, it was not necessary to add additional key words. We included studies published in any language.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of the retrieved included trials, (systematic) reviews, meta‐analyses and health‐technology assessment reports.
Data collection and analysis
Selection of studies
Two review authors (EBE, BR) independently scanned the title, abstract and keywords of every record retrieved to determine which studies required further assessment. We investigated all potentially relevant articles as full text, and resolved any disagreements by discussion; reference to a third party (KB) was not required. We attach an adapted PRISMA (preferred reporting Items for systematic reviews and meta‐analyses) flow‐chart of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, two review authors (EBE, BR) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details, see Characteristics of included studies, Table 5; Table 6; Table 7; Table 8; Table 9; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16). We resolved any disagreements by discussion; reference to a third party (KB) was not required.
1. Overview of study populations (levothyroxine treatment).
| Intervention(s) and comparator(s) | Screened/eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | |
| 1. LT4 Bayani 2012 | LT4 | ‐ | 20 | 20 | ‐ | 20 | 100 |
| No treatment | 20 | 20 | ‐ | 20 | 100 | ||
| total: | ‐ | ||||||
| 2. LT4 Boguszewski 1998 | LT4 | ‐ | 25 | 25 | ‐ | 25 | 100 |
| Placebo | 23 | 23 | ‐ | 23 | 100 | ||
| total: | 48 | 48 | ‐ | 48 | 100 | ||
| 3. LT4 Cesareo 2010a | LT4 | 95 | 36 | 36 | ‐ | 21 | 58.3 |
| No treatment | 35 | 35 | ‐ | 20 | 57.1 | ||
| total: | 71 | 71 | ‐ | 41 | 57.7 | ||
| 4. LT4 Gharib 1987 | LT4 | 56 | 28 | 28 | ‐ | 28 | 100 |
| Placebo | 25 | 25 | ‐ | 25 | 100 | ||
| total: | 53 | 53 | ‐ | 53 | 100 | ||
| 5. LT4 Grineva 2003 | LT4 | ‐ | 59 | 59 | ‐ | 59 | 100 |
| Sodium iodide | 59 | 59 | ‐ | 59 | 100 | ||
| total: | 118 | 118 | ‐ | 118 | 100 | ||
| 6. LT4 Grussendorf 2011b | LT4 + iodide | 1245 | 250 | 191 | 191 | ‐ | N/A |
| LT4 | 260 | 206 | 206 | ‐ | N/A | ||
| Iodide | 256 | 198 | 198 | ‐ | N/A | ||
| Placebo | 254 | 199 | 199 | ‐ | N/A | ||
| total: | 1020 | 794 | 794 | 682 | 66.9 | ||
| 7. LT4 Koc 2002c | TSH high‐level suppression | 79 | 13 | 13 | ‐ | 11 | 84.6 |
| TSH low‐level suppression | 12 | 12 | ‐ | 10 | 83.3 | ||
| Placebo | 12 | 12 | ‐ | 9 | 75.0 | ||
| Placebo | 12 | 12 | ‐ | 10 | 83.3 | ||
| total: | 49 | 49 | ‐ | 40 | 81.6 | ||
| 8. LT4 La Rosa 1995d | LT4 | ‐ | 27 | 27 | ‐ | 23 | 85.2 |
| Potassium iodide | 28 | 28 | ‐ | 25 | 89.3 | ||
| No treatment | 25 | 25 | ‐ | 22 | 88.0 | ||
| total: | 80 | 80 | ‐ | 70 | 87.5 | ||
| 9. LT4 Larijani 2005 | LT4 | 62 | 31 | 31 | ‐ | 31 | 100 |
| Placebo | 27 | 27 | ‐ | 27 | 100 | ||
| total: | 58 | 58 | ‐ | 58 | 100 | ||
| 10. LT4 Ozkaya 2010 | LT4 | ‐ | 35 | ‐ | 35 | 100 | |
| No treatment | 27 | ‐ | 27 | 100 | |||
| total: | 62 | ‐ | 62 | 100 | |||
| 11. LT4 Papini 1993 | LT4 | 215 | 54 | 51 | ‐ | 51 | 94.4 |
| Placebo | 56 | 50 | ‐ | 50 | 89.3 | ||
| total: | 110 | 101 | 101 | 91.8 | |||
| 12. LT4 Papini 1998 | LT4 | 100 | 51 | 42 | ‐ | 42 | 82.4 |
| No treatment | 49 | 41 | ‐ | 41 | 83.7 | ||
| total: | 100 | 83 | ‐ | 83 | 83.0 | ||
| 13. LT4 Reverter 1992 | LT4 | ‐ | 20 | 20 | ‐ | 14 | 70.0 |
| No treatment | 20 | 20 | ‐ | 20 | 100 | ||
| total: | 40 | 40 | ‐ | 34 | 85.0 | ||
| 14. LT4 Tsai 2006 | LT4 | ‐ | 30 | 30 | ‐ | 30 | 100 |
| Placebo | 30 | 30 | ‐ | 30 | 100 | ||
| total: | 60 | 60 | ‐ | 60 | 100 | ||
| 15. LT4 Wemeau 2002 | LT4 | 135 | 64 | 64 | 64 | 58 | 90.6 |
| Placebo | 59 | 59 | 59 | 48 | 81.4 | ||
| total: | 123 | 123 | 123 | 106 | 86.2 | ||
| 16. LT4 Zelmanovitz 1998 | LT4 | ‐ | 24 | 21 | ‐ | 21 | 87.5 |
| Placebo | 27 | 24 | ‐ | 24 | 88.9 | ||
| total: | 51 | 45 | ‐ | 45 | 88.2 | ||
| Subtotals for levothyroxine treatmente | Levothyroxine groups | 789 | N/A | N/A | |||
| Comparator groups | 1294 | N/A | N/A | ||||
| All participants | 2083 | 1641 | 78.8 | ||||
"‐" denotes not reported
an = 41 ("were followed for 24 months and the obtained results prompted us to stop the observation period after 12 months for the remaining subjects") bn = 1020 ‐ 7 (did not receive medication) = 1013 (sensitivity analysis); total = 682 (86% from 794 finishing the study; information from authors' letter in JCEM 2011;96:2786‐95; post hoc analysis: n = 600) cCross‐over study without washout period dPredetermined total sample size n = 160; study was stopped with the results from 80 participants eCalculation of all subtotals was not possible due availability of total numbers finishing study only (LT4 Grussendorf 2011)
ITT: intention‐to‐treat; LT4: levothyroxine; N/A: not applicable; TSH: thyrotropin
2. Overview of study populations (percutaneous sclerotherapy).
| Intervention(s) and comparator(s) | Screened/eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | |
| 1. PEI Bennedbaek 1998 | PEI | 123 | 25 | 25 | 25 | 25 | 100 |
| LT4 | 25 | 25 | 25 | 25 | 100 | ||
| total: | 50 | 50 | 50 | 50 | 100 | ||
| 2. PEI Bennedbaek 1999a | PEI‐1 | 160 | 30 | 30 | 30 | 30 | 100 |
| PEI‐3 | 30 | 30 | 30 | 27 | 90.0 | ||
| total: | 60 | 60 | 60 | 57 | 95.0 | ||
| 3. PEI Bennedbaek 2003 | PEI | 68 | 33 | 33 | ‐ | 33 | 100 |
| NaCl | 33 | 33 | ‐ | 33 | 100 | ||
| total: | 66 | 66 | ‐ | 66 | 100 | ||
| 4. PEI Chu 2003 | PEI | ‐ | 10 | 10 | ‐ | 10 | 100 |
| PHI | 8 | 8 | ‐ | 8 | 100 | ||
| Aspiration | 9 | 9 | ‐ | 9 | 100 | ||
| total: | 27 | 27 | ‐ | 27 | 100 | ||
| 5. PEI Sung 2013b | PEI | 53 | 25 | 25 | 21 | 20 | 80 |
| RF | 25 | 25 | 21 | 19 | 76 | ||
| total: | 50 | 50 | 42 | 39 | 78 | ||
| 6. TETRA Hegedüs 1998 | Tetracycline | 60 | 23 | 23 | ‐ | 23 | 100 |
| NaCl | 30 | 30 | ‐ | 30 | 100 | ||
| total: | 53 | 53 | ‐ | 53 | 100 | ||
| 7. PEI Valcavi 2004 | PEI | ‐ | 143 | ‐ | 135 | 94.4 | |
| Aspiration | 138 | ‐ | 131 | 94.9 | |||
| total: | 281 | ‐ | 266 | 94.7 | |||
| 8. PEI Verde 1994 | PEI | ‐ | 10 | 10 | ‐ | 10 | 100 |
| Aspiration | 10 | 10 | ‐ | 10 | 100 | ||
| total: | 20 | 20 | ‐ | 20 | 100 | ||
| Subtotals for sclerotherapy | Sclerotherapy groups | 337 | 321 | 95.3 | |||
| Comparator groups | 270 | 257 | 95.2 | ||||
| All participants | 607 | 578 | 95.2 | ||||
"‐" denotes not reported
an = 160 screened ‐ 42 (operated) ‐ 58 (refused surgery/treatment) = 60 randomised bn = 4 in each group were lost to follow‐up after treatment
ITT: intention‐to‐treat; LT4: levothyroxine; NaCl: isotonic saline; PEI: percutaneous ethanol injection; PEI‐1: percutaneous ethanol injection ‐ one session; PEI‐3: percutaneous ethanol injection ‐ three sessions; PHI: percutaneous hydrochloric acid injection; RF: radiofrequency
3. Overview of study populations (laser photocoagulation).
| Intervention(s) and comparator(s) | Screened/eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | |
| 1. LP Dossing 2005 | LP | ‐ | 15 | 15 | ‐ | 15 | 100 |
| No treatment | 15 | 15 | ‐ | 15 | 100 | ||
| total: | 30 | 30 | ‐ | 30 | 100 | ||
| 2. LP Dossing 2006 | LP‐1 | ‐ | 15 | 15 | 15 | 15 | 100 |
| LP‐3 | 15 | 15 | 15 | 15 | 100 | ||
| total: | 30 | 30 | 30 | 30 | 100 | ||
| 3. LP Dossing 2013 | LP + ASP | 22 | 22 | ‐ | 22 | 100 | |
| ASP | 22 | 22 | ‐ | 22 | 100 | ||
| total: | 44 | 44 | ‐ | 44 | 100 | ||
| 4. LP Gambelunghe 2006 | LP | ‐ | 13 | 13 | ‐ | 13 | 100 |
| No treatment | 13 | 13 | ‐ | 13 | 100 | ||
| total: | 26 | 26 | ‐ | 26 | 100 | ||
| 5. LP Papini 2007 | LP | 86 | 21 | 21 | ‐ | 21 | 100 |
| LT4 | 21 | 21 | ‐ | 21 | 100 | ||
| No treatment | 20 | 20 | ‐ | 19 | 95.0 | ||
| total: | 62 | 62 | ‐ | 61 | 98.4 | ||
| Subtotals for laser photocoagulation | Laser photocoagulation groups | 101 | 101 | 100 | |||
| Comparator groups | 91 | 90 | 98.9 | ||||
| All participants | 192 | 191 | 99.5 | ||||
"‐" denotes not reported
ASP: aspiration; ITT: intention‐to‐treat; LP: ultrasound‐guided laser photocoagulation; LP‐1: ultrasound‐guided laser photocoagulation ‐ one session; LP‐3: ultrasound‐guided laser photocoagulation ‐ three sessions; LT4: levothyroxine
4. Overview of study populations (radiofrequency ablation).
| Intervention(s) and comparator(s) | Screened/eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | |
| 1. RF Faggiano 2012 | RF | 44 | 20 | 20 | ‐ | 20 | 100 |
| No treatment | 20 | 20 | ‐ | 20 | 100 | ||
| total: | 40 | 40 | ‐ | 40 | 100 | ||
| 2. RF Huh 2012 | RF‐1 | 142 | 15 | 15 | 15 | 15 | 100 |
| RF‐2 | 15 | 15 | 15 | 15 | 100 | ||
| total: | 30 | 30 | 30 | 30 | 100 | ||
| Subtotals for radiofrequency ablation | Radiofrequency ablation groups | 50 | 50 | 100 | |||
| Comparator groups | 20 | 20 | 100 | ||||
| All participants | 70 | 70 | 100 | ||||
"‐" denotes not reported
ITT: intention‐to‐treat; RF: radiofrequency ablation; RF‐1: radiofrequency ablation ‐ one session; RF‐2: radiofrequency ablation ‐ two sessions
5. Overview of study populations (all interventions and comparators).
| Intervention(s) and comparator(s) | Randomised [N] | Finishing study [N] | Randomised finishing study [%] | |
| Grand totala | All interventions | 1277 | N/A | N/A |
| All comparators | 1675 | N/A | N/A | |
| All interventions and comparators | 2952 | 2480 | 84 |
aNumbers do not exactly match for 'all interventions' versus 'all comparators' owing to provision for total numbers only in LT4 Grussendorf 2011
N/A: not applicable
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we maximised the yield of information by the simultaneous evaluation of all available data. We used recent publications to complement results from preliminary articles (LT4 Larijani 2005; LT4 Wemeau 2002).
Assessment of risk of bias in included studies
Two authors (EBE, BR) assessed each trial independently. We resolved any disagreements by discussion; reference to a third party (KB) was not required.
We assessed risk of bias using The Cochrane Collaboration tool (Higgins 2011a; Higgins 2011b). We used the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We used the criteria for individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We present a 'Risk of bias' figure and a 'Risk of bias summary' figure.
We assessed the impact of individual bias domains on study results at endpoint and study levels.
For blinding of participants and personnel (performance bias), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data), we evaluated risk of bias separately for subjective and objective outcomes (Hrobjartsson 2012; Hrobjartsson 2013). We investigated the impact of missing data on outcome measures.
We defined the following endpoints as subjective outcomes.
Pressure symptoms.
Cosmetic complaints.
Tolerability (indicator pain).
Adverse events.
Health‐related quality of life
We defined the following outcomes as semi‐objective outcomes.
Compliance (pill count and thyroid hormone measurements).
Nodule volume reduction of 50% or more (measured by ultrasonography).
We defined the following outcomes as objective outcomes.
All‐cause mortality.
Thyroid cancer.
Laboratory measurements of thyroid function.
Socioeconomic effects.
Measures of treatment effect
Dichotomous data
We expressed dichotomous data (e.g. improvement in or disappearance of pressure symptoms: yes or no) as risk ratios (RRs) with 95% CIs.
Continuous data
We expressed continuous data (e.g. nodule volumes measured in mL) as mean differences with 95% CIs.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. No study of such design was included in any meta‐analysis.
Dealing with missing data
Whenever possible, we obtained relevant missing data from authors. We carefully evaluated important numerical data, such as screened, randomised participants, as well as intention‐to‐treat (ITT), as‐treated and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals) and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, we did not report study results as meta‐analytically pooled effect estimates.
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity using the I² statistic, which quantifies inconsistency across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I² statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011a).
When we found heterogeneity, we attempted to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We planned to use funnel plots in when 10 studies or more were included for a given outcome, in order to assess small study effects. Owing to several possible explanations for funnel plot asymmetry we intended to interpret the results carefully (Stern 2011).
Data synthesis
We primarily summarised data with a low risk of bias by means of a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects (Higgins 2009) and performed statistical analyses according to the guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses and wanted to investigate interaction.
Duration of follow‐up.
Type of nodule.
Type of treatment.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect sizes.
Restricting the analysis to published studies.
Restricting the analysis taking into account risk of bias, as specified in the section Assessment of risk of bias in included studies.
Restricting the analysis to very long or large studies to establish how much they dominate the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RR, OR etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
We identified 4597 records including 46 (systematic) reviews/meta‐analyses or guidelines. From these, we recognised 51 potentially relevant publications including six systematic reviews (Castro 2002; Fuller 2014; Richter 2002; Sdano 2005; Yousef 2010; Zelmanovitz 1998) for full‐text examination. The other records were excluded on the basis of their abstracts, titles or both because they were not relevant to our question or clearly did not meet inclusion criteria. After screening the full text of the selected papers and excluding 10 studies, six systematic reviews and four potentially relevant ongoing studies, 31 completed RCTs (33 publications) fulfilled the inclusion criteria. We did not identify additional studies after scrutinising the full publications of the six identified systematic reviews. For details, see Figure 1 of the amended PRISMA (preferred reporting Items for systematic reviews and meta‐Analyses) flow diagram of study selection (Liberati 2009).
1.
Study flow diagram
Assessment of interrater agreement
Interrater agreement between the two authors (EBE, BR) who rated studies for selection (i.e. decided whether a study was included or potentially relevant) was 100%. Consultation with a third party (KB) was not required.
Included studies
Of the 31 included trials,16 studies investigated treatment with LT4 (LT4 Bayani 2012; LT4 Boguszewski 1998; LT4 Cesareo 2010; LT4 Gharib 1987; LT4 Grineva 2003; LT4 Grussendorf 2011; LT4 Koc 2002; LT4 Larijani 2005; LT4 La Rosa 1995; LT4 Ozkaya 2010; LT4 Papini 1993; LT4 Papini 1998; LT4 Reverter 1992; LT4 Tsai 2006; LT4 Wemeau 2002; LT4 Zelmanovitz 1998), eight studies analysed PEI sclerotherapy, seven using ethanol (PEI Bennedbaek 1998; PEI Bennedbaek 1999; PEI Bennedbaek 2003; PEI Chu 2003; PEI Sung 2013; PEI Valcavi 2004; PEI Verde 1994) and one using tetracycline hydrochloride (TETRA Hegedüs 1988). Five studies evaluated ultrasound‐guided interstitial or percutaneous LP (LP Dossing 2005; LP Dossing 2006; LP Dossing 2013; LP Gambelunghe 2006; LP Papini 2007). Two studies investigated the effects of RF ablation therapy by comparing one with two treatment sessions (RF Huh 2012) or no treatment (RF Faggiano 2012).
We identified no RCTs that investigated HIFU or MW ablation therapy.
We also detected one trial comparing potassium iodide with no treatment (LT4 Grineva 2003) and one trial comparing LT4 plus potassium iodide combination therapy with placebo, potassium iodide or LT4 (LT4 Grussendorf 2011).
We identified trial registrations for four of the included studies (LT4 Bayani 2012; LT4 Grussendorf 2011; PEI Sung 2013; RF Faggiano 2012).
For details about the included studies, see Characteristics of included studies; Table 5; Table 6; Table 7; Table 8; Table 9; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16.
Study design
LT4
We evaluated 16 RCTs with a duration from six months to five years. All trials were parallel RCTs except one cross‐over study without a washout period between treatment periods (LT4 Koc 2002). Studies were published in English and in peer‐reviewed journals, with the exception of one which was published in Russian (LT4 Grineva 2003). Six studies mentioned commercial or non‐commercial funding (LT4 Bayani 2012; LT4 Boguszewski 1998; LT4 La Rosa 1995; LT4 Larijani 2005; LT4 Wemeau 2002; LT4 Zelmanovitz 1998) and two trials were terminated early (LT4 Cesareo 2010; LT4 La Rosa 1995).
PEI
In eight RCTs one up to five PEI treatment sessions were applied. Follow‐up varied from 1 (PEI Verde 1994) to 12 months (PEI Bennedbaek 1998; PEI Valcavi 2004; TETRA Hegedüs 1988). All studies were published in English and in peer‐reviewed journals. Three trials reported funding (PEI Bennedbaek 1998; PEI Bennedbaek 1999; PEI Bennedbaek 2003); no study was terminated early. One trial directly compared PEI with RF (PEI Sung 2013).
LP
Five RCTs applied one up to three photocoagulation sessions monthly, with follow‐up ranging from 6 to 12 months; one trial comparing LP with LT4 lasted 12 months (LP Papini 2007). All trials were published English and in peer‐reviewed journals. Two trials reported funding (LP Dossing 2005; LP Dossing 2006) and no trial was terminated early.
RF
One RCT compared one versus two ablation sessions and had a follow‐up of six months (RF Huh 2012). One of the authors, who is patent holder for the unidirectional ablation electrode technique investigated in this study mentioned no direct financial activities related to this study. Another study investigated one session of RF versus no treatment and had a follow‐up of 12 months. Both studies were published in English in peer‐reviewed journals and were not terminated early. A third trial directly compared PEI with RF (PEI Sung 2013).
Participants
LT4
A total of 2083 participants were randomised, 789 to the intervention and 1294 to the comparator groups. Eight studies compared LT4 with placebo (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Koc 2002; LT4 Larijani 2005; LT4 Papini 1993; LT4 Tsai 2006; LT4 Wemeau 2002; LT4 Zelmanovitz 1998), six studies compared LT4 with no treatment (LT4 Bayani 2012; LT4 Cesareo 2010; LT4 La Rosa 1995; LT4 Ozkaya 2010; LT4 Papini 1998; LT4 Reverter 1992) and one study compared LT4 with potassium iodide (LT4 Grineva 2003). One trial examined a combination of LT4 plus iodine versus LT4, iodine or placebo alone (LT4 Grussendorf 2011). Participants were euthyroid, mostly female, 18 to 69 years old and had single palpable thyroid nodules without compressive symptoms. In total, 40% of trials were conducted in non‐endemic areas and 20% in iodine‐deficient regions (Appendix 12). Number of nodules, measurements and characteristics (solid, mixed or cystic nodules) were detected by ultrasound, benignity was confirmed by cytologic diagnosis from FNAB and thyroid nodule function was assessed by thyroid scanning. Participants with suspicious or positive FNAB results, Hashimoto's thyroiditis, osteoporosis, cardiovascular disease or pregnancy were excluded. Three studies reported no comorbidity among participants (LT4 La Rosa 1995; LT4 Ozkaya 2010; LT4 Papini 1993) and two trials mentioned that no participant had previously received any thyroid medication (LT4 Bayani 2012; LT4 Ozkaya 2010). Two studies reported that outcome data were analysed according to the ITT principle (LT4 Grussendorf 2011; LT4 Wemeau 2002).
PEI
Overall 607 participants were randomised, 337 to various interventions and 270 to comparator groups. Trials compared PEI with other doses of PEI (PEI Bennedbaek 1999), NaCl (PEI Bennedbaek 2003), LT4 (PEI Bennedbaek 1998), percutaneous hydrochloric acid injection (PEI Chu 2003), aspiration alone (PEI Chu 2003; PEI Valcavi 2004; PEI Verde 1994) and RF ablation (PEI Sung 2013). One study from Denmark compared tetracycline hydrochloride injection with NaCl (TETRA Hegedüs 1988). Participants were predominantly women complaining of local neck compression due to cystic nodules, who were euthyroid and between 18 to 85 years old. Two trials applied therapy to solid nodules (fluid content less than 10%) (PEI Bennedbaek 1998; PEI Bennedbaek 1999). Trialists identified nodule characteristics by ultrasound, confirmed benignity by cytologic diagnosis from FNAB and assessed thyroid nodule function by thyroid scan. Participants with suspicious or positive FNAB findings were excluded. TETRA Hegedüs 1988 excluded toxic or large multinodular goitres. No publication provided substantial information about comorbidities or comedications.
LP
A total of 192 participants were randomised, 101 to the intervention and 91 to the comparator groups. Three studies compared LP to no treatment (LP Dossing 2005; LP Gambelunghe 2006; LP Papini 2007). One study arm in LP Papini 2007 compared LP with LT4 therapy and another study compared one session of laser ablation with three sessions (LP Dossing 2006). One study compared LP plus cyst aspiration with cyst aspiration only (LP Dossing 2013). Participants were mostly women, euthyroid and between 28 to 58 years old. In one trial, half of the participants had subclinical hyperthyroidism and were between 63 and 92 years old (LP Gambelunghe 2006). Over 80% of women complained of neck compression symptoms, refused thyroidectomy or had a high surgical risk. Diagnostic criteria were based on ultrasound nodule findings, cytologic FNAB confirming benignity and thyroid scintigram for nodule function assessment. In case of MNGs only the dominant nodule was analysed. No publication provided substantial information about comedications or comorbidities.
RF
Overall, 70 participants from two studies were randomised, 50 to the intervention and 20 to the comparator groups. In one trial, comparing one session with two sessions of RF ablation, participants were euthyroid, around 37 years old and mostly women complaining of cosmetic or pressure symptoms (RF Huh 2012). The other trial analysed one session of RF versus no treatment and participants had toxic or non‐toxic thyroid nodules with compressive symptoms (RF Faggiano 2012). In both studies, participants refused or were ineligible for surgery or radioiodine therapy. Diagnostic criteria were based on ultrasound evaluation, on two FNABs with cytology confirming benignity and on thyroid scans showing nodule hypofunction. No information about comedications and comorbidities was provided.
Interventions and comparisons
For details, see Appendix 2.
LT4
The vast majority of trials were monocentric but four were multicentric (LT4 Grussendorf 2011; LT4 Papini 1993; LT4 Papini 1998; LT4 Wemeau 2002); they were conducted in outpatients, seven in Europe (one in France, four in Italy, one in Spain and one in Germany), six in Eurasia (two in Iran, one in Russia, two in Turkey and one in Taiwan), two in Brazil and one in the USA. In eight trials, participants were drug‐naive and in one, participants underwent previous suppressive therapy longer than one year before the start of the study (LT4 Zelmanovitz 1998). Oral doses varied from 1 μg/kg/day (LT4 La Rosa 1995) to 3 µg/kg/day (LT4 Gharib 1987), being adjusted to TSH suppression levels that ranged from less than 0.01 mIU/L to 0.2 to 0.8 mIU/L (reference value for TSH was mostly between 0.2 to 4.0 mIU/L).
PEI
The eight studies were monocentric and took place in Denmark (PEI Bennedbaek 1998; PEI Bennedbaek 1999; PEI Bennedbaek 2003; TETRA Hegedüs 1988), Italy (PEI Valcavi 2004; PEI Verde 1994) and Asia (South Korea (PEI Sung 2013) and Taiwan (PEI Chu 2003)). All trials were conducted in outpatients of hospitals referred from primary care physicians or from clinics specialising in thyroid diseases. Thyroid cysts were initially aspirated and afterwards filled with ethanol to produce cyst ablation in seven studies. The ethanol volume given varied from 21% (PEI Chu 2003) to 70% of the extracted cyst fluid (PEI Valcavi 2004). For trials with solid or predominantly solid nodules, the median injected volume of ethanol in one session varied from 21% to 25% of pretreatment cyst volume (PEI Bennedbaek 1998; PEI Bennedbaek 1999). Resistance during infusion or pain were reasons for procedure interruption. One study compared the use of tetracycline hydrochloride and NaCl in solitary thyroid cysts of at least 2 mL volume (TETRA Hegedüs 1988). Under ultrasound control the cyst fluid was first aspirated and either 2 mL tetracycline hydrochloride or 2 mL NaCl was injected and then re‐aspirated up to five times to achieve complete emptying.
LP
All five studies were monocentric, performed in Europe (three in Denmark and two in Italy) and in outpatients of hospitals. One trial noted that participants were untreated for thyroid disease before intervention (LP Papini 2007). Thyroid nodules were usually solid and photocoagulation was mostly performed in one session. The median energy deposition per mL of pretreatment volume varied from 224 J to 262 J (LP Dossing 2005; LP Dossing 2006). Another study chose a 'step by step' procedure: median energy given was 100 J to 400 J per retracting step (LP Gambelunghe 2006). All procedures were performed with one needle, except in one trial where trialists used four needles for nodule volumes greater than 20 mL (LP Papini 2007).
RF
Both studies were monocentric, performed in Italy and South Korea in outpatients treated in hospital (RF Faggiano 2012; RF Huh 2012). In RF Huh 2012, the mean energy deposited per mL of pretreatment volume was 4377 J compared with 6157 J in one versus two sessions, respectively. The mean total energy deposition was 51,930 J versus 69,160 J, respectively. The method was performed with one needle with an active tip internally cooled electrode. RF Faggiano 2012 utilised one needle with four expandable hooks. The exposure time during the procedure ranged from 5 to 7 minutes and the temperature reached was between 100°C and 105°C.
Outcome measures
Appendix 11 provides an overview on how many studies, comparisons and participants contributed data to the various comparisons.
Primary outcomes
Pressure symptoms, cosmetic complaints, or both
For details on methods of outcome measurements for local symptoms, cosmetic complaints, or both, see, Appendix 13.
LT4
Not investigated.
PEI
Five trials measured participants' cosmetic complaints and local discomfort using.
A questionnaire (PEI Valcavi 2004);
Direct questions and answers (yes/no) (PEI Bennedbaek 2003);
Graded answers (PEI Bennedbaek 1998; PEI Sung 2013);
A visual analogue scale (VAS) (PEI Bennedbaek 1999; PEI Sung 2013).
LP
The effects on participants' pressure symptoms and cosmetic complaints were evaluated using a VAS in four of five interventions (LP Dossing 2005; LP Dossing 2006; LP Dossing 2013; LP Gambelunghe 2006). In one trial, the participants' questionnaire was not validated (LP Papini 2007).
RF
Participants rated pressure symptoms using a VAS and physicians recorded a cosmetic nodule score (from 1 = no palpable mass to 4 = readily observable) at the start of the study and one, three and six months after the procedure (RF Huh 2012). Participants estimated their neck symptoms separately, from 0 (absent), 1 (moderate) and 2 (severe), before, and after 3, 6 and 12 months, creating a final sum score (SYS score) varying from 0 to 6 (RF Faggiano 2012).
Nodule volume reduction of 50% or more
LT4
Nodule volume reduction from baseline of 50% or more was investigated in 12 (75%) studies (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Grineva 2003; LT4 Grussendorf 2011; LT4 Koc 2002; LT4 La Rosa 1995; LT4 Larijani 2005; LT4 Papini 1993; LT4 Reverter 1992; LT4 Tsai 2006; LT4 Wemeau 2002; LT4 Zelmanovitz 1998).
PEI and sclerotherapy using other agents
All eight included trials investigated this outcome.
LP
All five included studies reported this endpoint and whether the decrease was related to the mean or median total energy deposition. One study reported that the number of previous aspirations was associated with reduced treatment success if the cyst volume was 1 mL or less (LP Dossing 2013).
RF
RF Huh 2012 defined therapeutic success as a decrease in nodule volume of 50% or more, and investigated whether this decrease was related to the mean total energy deposition.
Adverse events
LT4
In four studies, participants reported signs of hyperthyroidism, such as nervousness, palpitations, sweating or tremor (LT4 Koc 2002; LT4 Papini 1993; LT4 Papini 1998; LT4 Wemeau 2002).
PEI
All studies reported adverse events ranging from mild‐to‐moderate pain and a burning sensation. Two trials found that major side effects, such as dysphonia, persistent nerve paralysis and paranodular fibrosis (PEI Bennedbaek 1999) and transient laryngeal dysfunction lasting two months (PEI Valcavi 2004), were dependent on the administrated ethanol dose. Two participants experienced extreme pain right after injection of tetracycline that lasted nearly 24 hours (TETRA Hegedüs 1988).
LP
In some studies, participants suffered slight‐to‐moderate pain lasting three (LP Dossing 2005) up to eight days (LP Dossing 2006), which had to be treated with "mild" analgesics. Generally, the procedure was well tolerated and pain stopped as soon as the energy was turned off. None of the authors described serious complications such as dysphonia, infection, hematoma, vocal cord paralysis or thyrotoxicosis.
RF
All participants experienced some pain or discomfort during the ablation, which ceased once the energy was decreased or turned off (RF Huh 2012). Mild burning sensation was described without the need to interrupt the procedure (RF Faggiano 2012). RF ablation therapy was reported as well tolerated, and no serious complications, such as dysphonia, skin burn, infection, hematoma or oesophageal injury, were observed.
Secondary outcomes
LT4
Compliance was defined and analysed as the suppression of TSH in all studies. Some trialists checked suppression status by applying thyrotropin‐releasing hormone (TRH) injection (LT4 Boguszewski 1998; LT4 Gharib 1987) or a combination of TSH suppression measurements with pill counts at follow‐up visits (LT4 Grussendorf 2011; LT4 Tsai 2006). All studies measured thyroid hormones at baseline and throughout to demonstrate thyroid function during LT4 therapy. No study reported on thyroid cancer, all‐cause mortality, health‐related quality of life or socioeconomic effects.
PEI
The degree of pain reported by participants was an indicator of the tolerability of PEI. Use of local anaesthesia was not described in studies treating thyroid cysts (PEI Bennedbaek 2003; PEI Chu 2003; PEI Sung 2013; PEI Verde 1994; TETRA Hegedüs 1988) in contrast to studies in which solid nodules were injected with ethanol, which necessitated the use of local anaesthesia and analgesics(PEI Bennedbaek 1998; PEI Bennedbaek 1999). All studies except three (PEI Chu 2003; PEI Sung 2013; PEI Valcavi 2004) described thyroid hormone measurements periodically during follow‐up. PEI Bennedbaek 1999 mentioned cost‐effectiveness but did not provide data. Two studies stated the necessity and importance of confirming the absence of malignancy at long‐term follow‐up (PEI Chu 2003;PEI Valcavi 2004). No trial evaluated all‐cause mortality or health‐related quality of life.
LP
Investigators measured tolerability as the degree of pain or discomfort experienced by participants after the procedure by means of a VAS (LP Dossing 2005; LP Dossing 2006). In two trials, participants were asked if they would repeat the procedure or not (LP Gambelunghe 2006; LP Papini 2007). In all studies but one (LP Papini 2007), participants received local anaesthesia. In this one trial, participants received an intramuscular injection of betamethasone before LP was applied. In case of persisting cervical pain, participants received ketoprofen for two days. All trials measured thyroid hormones initially and throughout the study. No study reported on all‐cause mortality or health‐related quality of life. LP Papini 2007 reported the costs of the procedure.
RF
Thyroid hormones were measured at study start and during follow‐up. Authors did not evaluate all‐cause mortality, health‐related quality of life or socioeconomic effects.
Excluded studies
In total we excluded 10 studies after evaluation of the full publication. For more details about reasons for exclusion of studies, see the Characteristics of excluded studies. The main reason for exclusion was a non‐randomised study design.
Risk of bias in included studies
For details on study populations. such as numbers randomised, analysed, and the ITT and safety populations, see Table 5; Table 6; Table 7; Table 8; Table 9. For an overview of authors' judgements about each 'Risk of bias' item, see Characteristics of included studies, Figure 2 and Figure 3.
2.
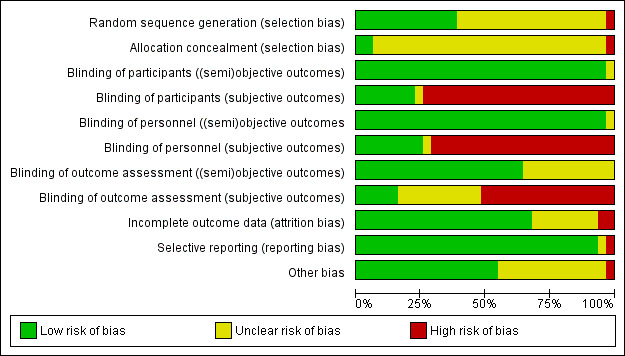
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
Outcomes were classified into: subjective (i.e. pressure symptoms, cosmetic complaints, tolerability, adverse events, health‐related quality of life); (semi)objective (i.e. compliance, nodule volume reduction ≥ 50%); objective (i.e. all‐cause mortality, thyroid cancer, laboratory measurements of thyroid function, socioeconomic effects)
3.
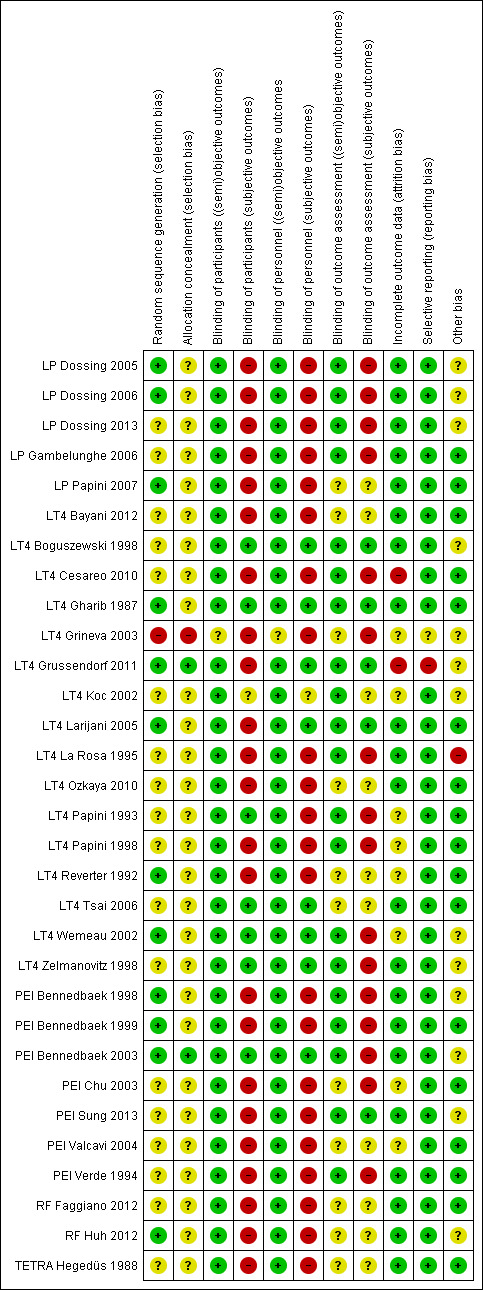
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Outcomes were classified into: subjective (i.e. pressure symptoms, cosmetic complaints, tolerability, adverse events, health‐related quality of life); (semi)objective (i.e. compliance, nodule volume reduction ≥ 50%); objective (i.e. all‐cause mortality, thyroid cancer, laboratory measurements of thyroid function, socioeconomic effects)
Allocation
LT4
All 16 studies were described as randomised trials, but we judged only five as having a low risk of bias for random sequence generation because these trials provided adequate details (LT4 Gharib 1987; LT4 Grussendorf 2011; LT4 Larijani 2005; LT4 Reverter 1992; LT4 Wemeau 2002). Only one study specifically reported how the allocation sequence was generated and concealed (LT4 Grussendorf 2011). We judged the remaining 10 trials as unclear and one study (LT4 Grineva 2003) as having a high risk of selection bias.
PEI
Three of eight studies using PEI sclerotherapy described the randomisation process in adequate detail (PEI Bennedbaek 1998; PEI Bennedbaek 1999; PEI Bennedbaek 2003) and we judged these studies to have a low risk of bias for random sequence generation. Only one study provided details of both generation of allocation sequence and concealment of allocation (PEI Bennedbaek 2003).
LP
Three of four studies described the randomisation process in adequate detail (LP Dossing 2005; LP Dossing 2006; LP Papini 2007), but none reported how allocation was concealed.
RF
One of two studies reported on the randomisation process (RF Huh 2012). No study provided adequate information about concealment of allocation.
Blinding
LT4
With regard to the blinding of participants for both (semi)objective and subjective outcomes, we judged 6 of 16 studies to have a low risk of bias (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Papini 1993; LT4 Tsai 2006; LT4 Wemeau 2002; LT4 Zelmanovitz 1998). For the blinding of personnel, we considered seven studies to have a low risk of bias for both (semi)objective and subjective outcomes (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Grussendorf 2011; LT4 Larijani 2005; LT4 Tsai 2006; LT4 Wemeau 2002; LT4 Zelmanovitz 1998). Considering both (semi)objective and subjective outcomes, we judged only four studies to have a low risk of bias for outcome assessors (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Grussendorf 2011; LT4 Larijani 2005). One study (all outcomes) was triple masked for participants, physicians and outcome assessors (LT4 Gharib 1987).
PEI
Considering the blinding of participants or personnel for both (semi)objective and subjective outcomes, we judged one of eight studies to have a low risk of bias (PEI Bennedbaek 2003). We considered only one study to have a low risk of bias concerning the blinding of outcome assessors for both (semi)objective and subjective outcomes (PEI Sung 2013).
LP
All four studies compared laser treatment with no therapy. For this procedure, participants and personnel were not masked, but in all trials an awareness of treatment allocation could have influenced the endpoints, especially subjective outcomes. We considered all but one study (LP Papini 2007) to have a low risk of bias concerning the blinding of outcome assessors for (semi)objective outcomes.
RF
We judged both studies to have a low risk of bias for the blinding of participants and personnel regarding (semi)objective endpoints and a high risk of bias for subjective outcomes (RF Faggiano 2012; RF Huh 2012). Blinding of outcome assessors was unclear in both trials.
Incomplete outcome data
LT4
We considered eight studies to have a low risk of bias, either because all participants were followed up until the end of the study or the reasons for dropouts or exclusion from the analyses were adequately specified and attrition rates did not differ considerably (LT4 Bayani 2012; LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Larijani 2005; LT4 La Rosa 1995; LT4 Ozkaya 2010; LT4 Tsai 2006; LT4 Zelmanovitz 1998). Two studies used ITT analyses (LT4 Grussendorf 2011; LT4 Wemeau 2002). In one study approximately 42% of participants in both intervention and comparator groups discontinued the study (LT4 Cesareo 2010) and in another trial 30% versus 0% of participants discontinued the study in the LT4 versus the no‐treatment comparator group, respectively (LT4 Reverter 1992).
PEI
We judged six studies to have a low risk of bias, since either all participants were followed up until the end of the study or the reasons for dropouts or exclusion from the analyses were specified and attrition rates did not differ considerably (PEI Bennedbaek 1998; PEI Bennedbaek 1999; PEI Bennedbaek 2003; PEI Sung 2013; PEI Verde 1994; TETRA Hegedüs 1988). In one study, 24% versus 20% of participants discontinued the study in the RF ablation versus the PEI therapy groups, respectively (PEI Sung 2013).
LP
We considered all five studies to have a low risk of bias, since either all participants were followed up until the end of the study or the reasons for dropouts or exclusion from the analyses were specified and attrition rates did not differ considerably. One trial analysed outcomes data according to the ITT principle (LP Dossing 2006)
RF
We judged both trials to have a low risk of bias for attrition bias, as all participants completed the study. One study reported analyses according to the ITT principle (RF Huh 2012).
Selective reporting
LT4
We judged 14 studies to have a low risk of selective outcome reporting, because all expected und prespecified outcomes were reported and analysed. One study had a high risk of reporting bias (LT4 Grussendorf 2011).
PEI
We considered all trials to have a low risk of bias, as all expected outcomes were reported and analysed, or similar endpoints were found in previous publications.
LP
We judged all trials to have a low risk of bias, as all expected outcomes were reported and analysed, or similar endpoints were found in previous publications.
RF
We considered both studies to have a low risk of bias, as all expected outcomes were reported and analysed.
Other potential sources of bias
LT4
We judged one study to have a high risk of bias because this trial was stopped early, probably for benefit (LT4 La Rosa 1995). Four trials mentioned commercial sponsoring (LT4 Boguszewski 1998; LT4 Grussendorf 2011; LT4 La Rosa 1995; LT4 Wemeau 2002), two studies had a combination of commercial and non‐commercial funding (LT4 Larijani 2005; LT4 Zelmanovitz 1998), three reported non‐commercial funding (LT4 Cesareo 2010; LT4 Gharib 1987; LT4 Koc 2002), and the others did not provide information about funding (LT4 Ozkaya 2010; LT4 Papini 1993; LT4 Papini 1998; LT4 Reverter 1992; LT4 Tsai 2006).
PEI
We considered all studies to have a low or unclear risk of bias.
LP
We judged studies to have either a low (LP Gambelunghe 2006; LP Papini 2007) or unclear risk of bias (LP Dossing 2005; LP Dossing 2006; LP Dossing 2013).
RF
We considered all studies to have either a low (RF Faggiano 2012) or unclear risk of bias (RF Huh 2012).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
LT4 versus no treatment or placebo
Two studies are mainly descriptively reported in the appendices: one cross‐over study with no wash‐out phase in 49 participants investigated LT4 therapy versus placebo over one year in low or high level TSH suppression subgroups (LT4 Koc 2002). Another study evaluated LT4 versus potassium iodide for six months in 108 participants but was at high risk of selection bias (LT4 Grineva 2003).
One study investigated the effects of a combination of LT4 and iodine versus placebo, LT4 only or iodine supplementation only (LT4 Grussendorf 2011). Participants had mild‐to‐moderate iodine deficiency (Appendix 12). In this review we report the findings of the comparisons of all LT4‐containing regimens versus placebo. For the outcome nodule volume reduction of 50% or more, the results for placebo, iodine, LT4 and LT4 plus iodine were 6.5%, 7.1%, 9.7% and 16.2%, respectively (LT4 Grussendorf 2011).
One study (LP Papini 2007) compared LP ablation with LT4 therapy and is described in the section on LP below. Another study investigated LT4 treatment versus PEI sclerotherapy (PEI Bennedbaek 1998) and is described in the section on PEI below.
Primary outcomes
Pressure symptoms/cosmetic complaint
This outcome was not investigated in any LT4 study.
Nodule volume reduction of 50% or more
No study investigated nodule volume reduction of 50% or more as a primary outcome, although all trials evaluated nodule volume changes following LT4 treatment. Considering the 10 of 16 studies investigating this outcome independent of study duration and follow‐up, and excluding the cross‐over study by LT4 Koc 2002, this endpoint was achieved by 80/489 (16%) participants in the LT4 treatment groups and by 46/469 (10%) participants in the comparator groups after 6 to 24 months of follow‐up (Figure 4). The RR was 1.57 (95% CI 1.04 to 2.38); P = 0.03; I² = 17%; 958 participants; 10 studies; Analysis 1.1) in favour of LT4. Overall, we considered this outcome to have a low risk of performance bias across all studies. We judged two studies to have an unclear risk of detection bias (LT4 Bayani 2012; LT4 Reverter 1992). Exclusion of these studies did not substantially change the effect estimate. We judged one study to have a high risk of attrition and reporting bias (LT4 Grussendorf 2011). Excluding this study did not substantially change the effect estimate.
4.
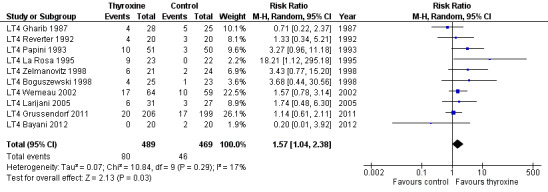
Forest plot of comparison: 1 Levothyroxine versus control (no treatment, placebo), outcome: 1.1 Nodule volume reduction ≥ 50%.
1.1. Analysis.
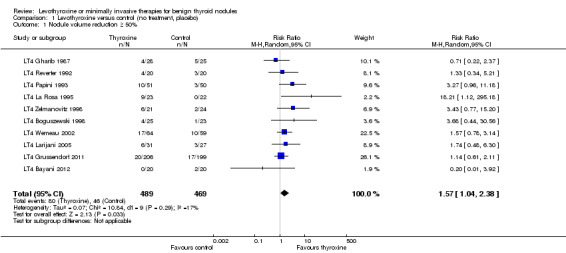
Comparison 1 Levothyroxine versus control (no treatment, placebo), Outcome 1 Nodule volume reduction ≥ 50%.
Adverse events
Study authors described LT4 therapy as generally well tolerated; for more details, see Appendix 14. Some studies observed no adverse events (LT4 Boguszewski 1998; LT4 La Rosa 1995; LT4 Papini 1993) and others did not report untoward effects of the medications (LT4 Gharib 1987; LT4 Grussendorf 2011; LT4 Larijani 2005; LT4 Ozkaya 2010; LT4 Reverter 1992; LT4 Tsai 2006).
Bone loss as measured by bone mineral density
One trial analysed the effect of suppressive doses of LT4 versus placebo on bone mineral density (BMD) in 16 pre‐ and postmenopausal (intervention group) and 19 pre‐ and postmenopausal (comparator group) women (LT4 Zelmanovitz 1998 ‐ Appendix 15). After one year, no statistically significant differences in BMD were found. BMD was measured at the lumbar spine and femur before and after one year of treatment.
Hyperthyroidism
In one study, one participant in the LT4 and one in the placebo group developed severe hyperthyroidism requiring withdrawal. Investigators diagnosed underlying Graves' disease in the participant in the placebo group (LT4 Wemeau 2002). Three studies provided quantitative data on signs and symptoms of hyperthyroidism, such as nervousness, tachycardia and tremor (LT4 Papini 1993; LT4 Papini 1998; LT4 Wemeau 2002). Untoward effects were observed in 35/138 (25%) LT4‐treated versus 9/131 (7%) placebo‐treated participants at 12 to 18 months of follow‐up. Random‐effects and fixed‐effect meta‐analyses of numbers of participants without signs of hyperthyroidism indicated either a statistically significant or non‐significant effect in favour of placebo. However, heterogeneity was considerable in both cases and we therefore do not report a pooled effect estimate (Analysis 1.2). All three studies had a high risk of detection bias for this outcome.
1.2. Analysis.
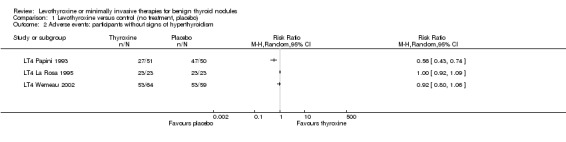
Comparison 1 Levothyroxine versus control (no treatment, placebo), Outcome 2 Adverse events: participants without signs of hyperthyroidism.
Nodule volume increase of more than 50%
Three studies reported the numbers of participants showing a thyroid nodule volume increase of more than 50% (LT4 Grussendorf 2011; LT4 Papini 1993; LT4 Zelmanovitz 1998). Analysis 1.3, showing the RR for participants without a nodule volume increase greater than 50% (to conserve forest plot orientation), reveals no statistically significant differences (RR 1.10 (95% CI 0.99 to 1.22); P = 0.09; I² = 0%; 551 participants; 3 trials). Risk of detection bias was low for this outcome.
1.3. Analysis.
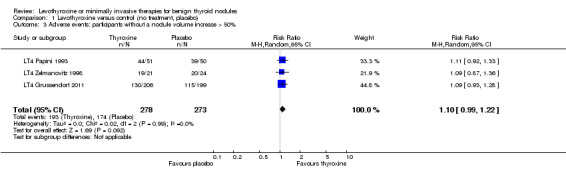
Comparison 1 Levothyroxine versus control (no treatment, placebo), Outcome 3 Adverse events: participants without a nodule volume increase > 50%.
Secondary outcomes
Compliance
Some studies defined compliance as the number of returned or taken pills throughout follow‐up visits, but no details were published (LT4 Grussendorf 2011; LT4 Tsai 2006; LT4 Wemeau 2002). Three trials also considered suppression of TSH after TRH injection (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Zelmanovitz 1998), the degree of suppression of TSH measured indicated compliance to treatment.
Tolerability
This outcome was not investigated in any LT4 study.
TSH and T4 serum levels
In most studies, thyroid hormones including thyroid autoantibodies were evaluated at the beginning and throughout the study. Baseline values were always documented. Eight trials (LT4 Bayani 2012; LT4 Boguszewski 1998; LT4 Cesareo 2010; LT4 Gharib 1987; LT4 Ozkaya 2010; LT4 Papini 1993; LT4 Papini 1998; LT4 Zelmanovitz 1998) showed ‐ with the exception of Ozkaya 2010 ‐ lower TSH values following LT4 therapy. However, due to otherwise unexplained considerable heterogeneity we do not report an effect estimate (Analysis 1.4). In five studies comparing LT4 with placebo, total T4 in 296 participants at the end of the trials showed a difference of 48.3 nmol/L (95% CI 35.1 to 61.4; P < 0.00001; 296 participants; 5 trials; I² = 66%; Analysis 1.5) in favour of LT4 (LT4 Boguszewski 1998; LT4 Gharib 1987; LT4 Papini 1993; LT4 Tsai 2006; LT4 Zelmanovitz 1998). We considered the risk of performance and detection bias to be low for these outcomes.
1.4. Analysis.
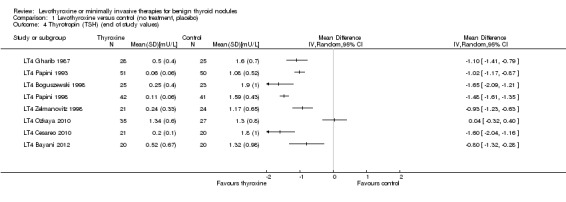
Comparison 1 Levothyroxine versus control (no treatment, placebo), Outcome 4 Thyrotropin (TSH) (end of study values).
1.5. Analysis.
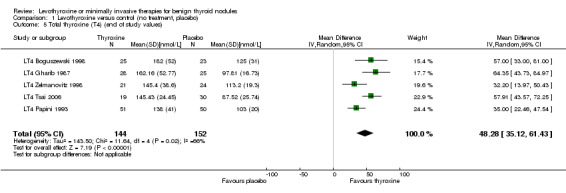
Comparison 1 Levothyroxine versus control (no treatment, placebo), Outcome 5 Total thyroxine (T4) (end of study values).
Thyroid cancer
One study confirmed the benignity of some treated nodules through FNAB and cytological re‐evaluation at 12 and 24 months in the non‐responder group, defined as participants (33/58) with constant or increasing nodule volume (LT4 Larijani 2005).
All‐cause mortality
This outcome was not investigated in any LT4 study.
Health‐related quality of life
This outcome was not investigated in any LT4 study.
Socioeconomic effects
This outcome was not investigated in any LT4 study.
PEI treatment and sclerotherapy with other agents versus cyst aspiration, isotonic saline, LT4 or RF
Primary outcomes
Pressure symptoms/cosmetic complaints
Signs of improvement of neck compression symptoms at end of study were demonstrated in three trials in 370 participants after 6 to 12 months of follow‐up (145/187 (78%) in the treatment groups and 70/183 (38%) in the various comparator groups). Heterogeneity between studies was considerable. Since we could not explain the heterogeneity we do not present an effect estimate (Analysis 2.1). RRs ranged from 1.0 to 3.06 in favour of PEI . Symptom and cosmetic scores did not show statistically significant differences in one trial comparing PEI with RF treatment (PEI Sung 2013). There was a high risk of performance bias for these outcomes across all three studies. All studies showed a high or unclear risk of detection bias.
2.1. Analysis.
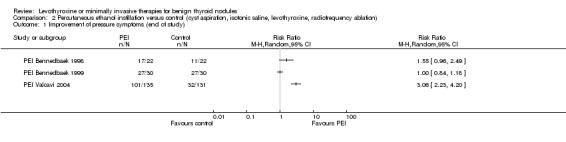
Comparison 2 Percutaneous ethanol instillation versus control (cyst aspiration, isotonic saline, levothyroxine, radiofrequency ablation), Outcome 1 Improvement of pressure symptoms (end of study).
Nodule volume reduction of 50% or more
Six of eight studies applying PEI provided data on this outcome. PEI versus cyst aspiration showed a statistically significant benefit in favour of PEI after 1 to 24 months of follow‐up, with 44 of 53 (83%) participants versus 23/52 (44%) showing a nodule volume reduction of 50% or more (RR 1.83 (95% CI 1.32 to 2.54; P = 0.0003; I² = 0%; 105 participants; 3 trials; Analysis 2.2.1). One study compared PEI with LT4 treatment and showed a nodule volume reduction of 50% or more in 19 of 25 (76%) PEI‐treated participants compared with 0 of 25 (0%) LT4‐treated participants (Analysis 2.2.2). One study compared PEI with RF ablation therapy and showed a nodule volume reduction of 50% or more in all participants in both the intervention (21/21) and comparator groups (21/21) (Analysis 2.2.3). TETRA Hegedüs 1988 compared tetracycline hydrochloride with isotonic saline injections for the treatment of thyroid cysts of at least 2 mL in volume. In the tetracycline group, the thyroid cyst volume declined more than 50% in 10 of 23 (43%) participants versus 14/30 (47%) in the saline group (difference not statistically significant). This outcome was associated with a low risk of performance bias across all studies. Three studies had an unclear risk of detection bias (PEI Chu 2003; PEI Valcavi 2004; TETRA Hegedüs 1988).
2.2. Analysis.
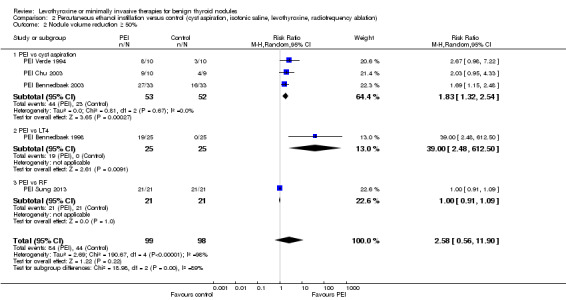
Comparison 2 Percutaneous ethanol instillation versus control (cyst aspiration, isotonic saline, levothyroxine, radiofrequency ablation), Outcome 2 Nodule volume reduction ≥ 50%.
Adverse events
The study authors described the adverse events profile of PEI therapy as acceptable; for details, see Appendix 16.
Cervical pain
In all trials, participants experienced periprocedural cervical tenderness and light‐to‐moderate pain lasting from minutes to several hours. The duration of pain correlated with the dose of ethanol in one study (PEI Bennedbaek 1999). The injections were applied to predominant solid nodules and were described as painful despite local anaesthesia and analgesics in two studies (PEI Bennedbaek 1998; PEI Bennedbaek 1999). One study comparing PEI with RF treatment reported that PEI was associated with almost no periprocedural pain whereas RF ablation showed a tendency for more pain (PEI Sung 2013). Three studies investigated PEI treatment compared with cyst aspiration: 26% of PEI participants reported slight‐to‐moderate pain compared with 12% of those receiving cyst aspiration only (RR 1.78 (95% CI 0.62 to 5.12; P = 0.28; 104 participants; 3 studies; Analysis 2.3). All studies had a high risk of detection bias (PEI Bennedbaek 2003; PEI Chu 2003; PEI Verde 1994) and two a high risk of performance bias (PEI Chu 2003; PEI Verde 1994) for this outcome.
2.3. Analysis.
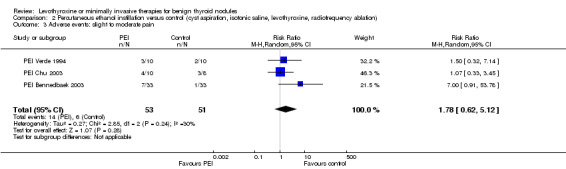
Comparison 2 Percutaneous ethanol instillation versus control (cyst aspiration, isotonic saline, levothyroxine, radiofrequency ablation), Outcome 3 Adverse events: slight to moderate pain.
Major adverse effects
In one study, participants who experienced major adverse effects,such as dysphonia, persistent nerve paralysis and paranodular fibrosis, were given larger ethanol doses (PEI Bennedbaek 1999). Iatrogenic thyrotoxicosis and hyperpyrexia were unrelated to PEI dose in one study (PEI Bennedbaek 1999). One participant suffered from permanent facial dysaesthesia and an increased flow of tears still persisting after one year (PEI Bennedbaek 1999). Two participants reported extreme pain lasting for two days (TETRA Hegedüs 1988).
Secondary outcomes
Compliance
This outcome was not investigated in any PEI study.
Tolerability
Most studies characterised the procedure as well tolerated and did not specify use of local anaesthesia. In one study, investigators reported that local anaesthesia was not necessary and no participant refused further ethanol injections (PEI Valcavi 2004). Local anaesthesia was usually applied for solid nodules (PEI Bennedbaek 1998; PEI Bennedbaek 1999).
TSH and T4 serum levels
In most studies, thyroid hormones including thyroid autoantibodies were evaluated at the beginning and throughout the study. Baseline values were always documented. Some studies described thyroid function as not altered with no significant changes in thyroid hormones levels after PEI treatment (PEI Bennedbaek 1999; PEI Verde 1994; TETRA Hegedüs 1988).
Thyroid cancer
This outcome was not investigated in any PEI study.
All‐cause mortality
This outcome was not investigated in any PEI study.
Health‐related quality of life
This outcome was not investigated in any PEI study.
Socioeconomic effects
This outcome was not investigated in any PEI study.
LP versus no treatment or comparing different LP sessions
Primary outcomes
Pressure symptoms/cosmetic complaints
Considering the three studies comparing LP with no treatment (LP Dossing 2005; LP Gambelunghe 2006; LP Papini 2007), 36 of 44 (82%) laser‐treated participants showed improvement/disappearance of initial pressure symptoms after 6 to 12 months of follow‐up. No participant in the no‐treatment comparator group showed signs of improvement. The RR for improvement/disappearance of pressure symptoms was 26.65 (95% CI 5.47 to 129.72; P < 0.0001; I² = 0%; 92 participants; 3 trials; Analysis 3.1) in favour of LP. We considered there to be a high risk of performance bias and a high or unclear risk of detection bias for this outcome across all three studies. Comparing one with three PL sessions did not reveal statistically significant differences (LP Dossing 2006).
3.1. Analysis.
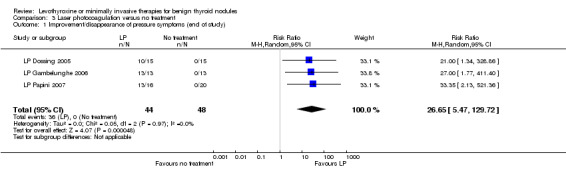
Comparison 3 Laser photocoagulation versus no treatment, Outcome 1 Improvement/disappearance of pressure symptoms (end of study).
Nodule volume reduction of 50% or more
Three of five studies reported this outcome at end of study: LP Dossing 2006 compared one with three laser treatment sessions showing an overall mean nodule reduction of 45% versus 58% at six months in favour of three sessions (P = 0.03; Analysis 4.1). LP Papini 2007, investigating laser therapy versus LT4 or no treatment after 12 months of follow‐up, found that a mean nodule volume decrease of more than 50% was achieved in 7/21 (33%) treated participants versus no participants (0 of 41) in either comparator groups. LP Dossing 2013, comparing laser plus aspiration versus aspiration for mixed thyroid nodules, showed a median nodule volume reduction of 73% versus 26% (P = 0.001; 44 participants) at six months of follow‐up. We associated this outcome with a low risk of performance bias across these three studies. We considered one study to have an unclear risk of detection bias (LP Papini 2007).
4.1. Analysis.
Comparison 4 Laser photocoagulation comparing various LP sessions, Outcome 1 Nodule volume reduction (baseline to end of follow‐up).
| Nodule volume reduction (baseline to end of follow‐up) | ||||
|---|---|---|---|---|
| Study | Comparator groups [N participants] | Baseline, mean thyroid nodule volume [ml (SD)] | End of follow‐up (6 months) [ml (SD)] | Mean difference between groups |
| LP Dossing 2006 | Intervention: 1 session (15) Comparator: 3 sessions (15) |
Intervention: 10.1 (4.3) Comparator: 10.7 (9.0) |
Intervention: 5.7 (3.2) Comparator: 4.6 (3.0) |
Intervention: ‐45% Comparator: ‐58% Difference: 13% (P = 0.03) |
A decrease in mean nodule volume was also observed in the two remaining studies. In one study comparing LP with no treatment, the overall mean nodule volume reduction was 44% after LP in contrast to a volume increase after no treatment (LP Dossing 2005). In the second study, the median nodule volume decrease was 44% in the LP group versus no volume change in the no‐treatment group (LP Gambelunghe 2006).
Adverse events
The study authors described LP therapy as generally well tolerated; for details, see Appendix 16.
Cervical pain
Three studies reported light‐to‐moderate cervical pain lasting 48 hours or more (Analysis 3.2), only occurring in the LP treatment group: events ranged between 0% (LP Gambelunghe 2006) and 47% (LP Dossing 2005). We associated this outcome with a high risk of performance bias across both studies. We considered two studies to have a high risk of detection bias for this outcome (LP Dossing 2005; LP Gambelunghe 2006). Altogether, 95% (LP Papini 2007) and 38% of LP treated participants (LP Gambelunghe 2006) experienced intraoperative mild burning cervical pain. Some participants (40% to 50%) reported pain lasting up to three days (LP Dossing 2005; LP Dossing 2006; LP Dossing 2013). In addition, 20% to 27% of participants complained of tenderness for up to one week (LP Dossing 2005; LP Dossing 2006; LP Papini 2007). One participant in one study described the LP procedure as extremely painful, whereas in the LT4‐comparator group, 38% of participants reported persistent tachycardia or nervousness (LP Papini 2007).
3.2. Analysis.
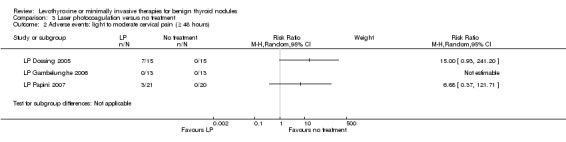
Comparison 3 Laser photocoagulation versus no treatment, Outcome 2 Adverse events: light to moderate cervical pain (≥ 48 hours).
Major adverse effects
No study reported serious adverse effects such as dysphonia, infection, haematoma, bleeding or vocal cord paralysis.
Secondary outcomes
Compliance
This outcome was not investigated in any LP study.
Tolerability
Tolerability was evaluated by asking the participants if they would repeat the treatment according to the degree of pain suffered. According to this definition, all participants in three studies tolerated the treatment well (LP Dossing 2005; LP Dossing 2006; LP Gambelunghe 2006). One participant refused a second LP session, describing the technique as extremely painful (LP Papini 2007).
TSH and T4 serum levels
In most studies, thyroid hormones including thyroid autoantibodies were evaluated at the beginning and throughout the study. Baseline values were always documented. Two studies including 60 participants and comparing LP with no treatment showed no statistically significant changes in thyroid hormone levels at follow‐up (LP Dossing 2005; LP Dossing 2006). Another study found that all participants had normal thyroid function at the end of follow‐up (LP Gambelunghe 2006). Finally, in one study two participants treated with LP had an increase in antithyroglobulin autoantibodies of more than 70 U/mL at end of study (LP Papini 2007); the other laboratory parameters were within the normal range and TSH was suppressed in the LT4 group only.
Thyroid cancer
This outcome was not investigated in any LP study.
All‐cause mortality
This outcome was not investigated in any LP study.
Health‐related quality of life
This outcome was not investigated in any LP study.
Socioeconomic effects
Only one trial reported costs (LP Papini 2007). The cost of LP therapy, including equipment, medical team and disposable kits, was about €450 (approximately US$550, September 2012 conversion).
RF ablation therapy versus no treatment or comparing various RF sessions
One study compared RF ablation with PEI therapy (PEI Sung 2013) and is described in the section on PEI above.
Primary outcomes
Pressure symptoms/cosmetic complaints
The two included studies reported a decrease in pressure symptom scores in both groups at the end of follow‐up.
One study, comparing one with two RF sessions after six months, showed a decrease in the symptom score of a 10 cm VAS, from 5.4 (standard deviation (SD) 1.7) at baseline to 2.0 (SD 1.3) after one session, and from 5.3 (SD 1.8) at baseline to 2.2 (SD 0.9) after two sessions (P = 0.25; 30 participants) (RF Huh 2012).
Another study comparing RF with no treatment in 40 participants at 12 months (RF Faggiano 2012) showed a decline in the sum of individual scores (ranging from 0 to 6) from 3.4 (SD 1.3) at baseline to 0.6 (SD 0.5), i.e. a 2.8 decrease, in the intervention group compared with an increase from 3.0 (SD 1.3) to 4.1 (SD 0.9), i.e. a 1.1 increase, in the no‐treatment group. The difference between the groups was statistically significant at 12 months (P < 0.0001).
We associated this outcome with a high risk of performance bias and an unclear risk of detection bias for both studies.
Nodule volume reduction of 50% or more
RF Huh 2012 described a mean nodule volume reduction of 70% (range 51% to 94%) for one session and a mean reduction of 78% (range 66% to 93%) after two sessions of RF ablation at six months follow‐up (8% difference; P = 0.078; 30 participants; Analysis 5.1).
5.1. Analysis.
Comparison 5 Radiofrequency versus no treatment or comparing various RF sessions, Outcome 1 Nodule volume reduction (baseline to end of follow‐up).
| Nodule volume reduction (baseline to end of follow‐up) | |||||
|---|---|---|---|---|---|
| Study | Comparator groups [N participants] | Mean thyroid nodule volume at baseline [mL, SD] | Mean thyroid nodule volume at 6 month [mL, SD] | Mean volume reduction [% (SD)] | Statistical significance |
| RF Faggiano 2012 | Intervention: 1 session (20) | 13.3 (8) | 3.2 (2.7) | 76 (12) | P < 0.001 |
| RF Faggiano 2012 | Comparator: no treatment (20) | 11.2 (6.7) | 11.4 (6.7) | ||
| RF Huh 2012 | Intervention: 1 session (15) | 13.3 (12.9) | 3.8 (4.4) | 70 (13.2) | P = 0.078 |
| RF Huh 2012 | Comparator: 2 sessions (15) | 13.0 (6.8) | 3.0 (2.2) | 78 (7.8) | |
RF Faggiano 2012 reported a mean nodule volume reduction of 76% for one RF session versus no reduction in the no‐treatment group at six months follow‐up (P < 0.001; 40 participants; Analysis 5.1); nodule volume reduction was 85% at nine months follow‐up.
We associated this outcome with a low risk of performance bias and an unclear risk of detection bias for both studies.
Adverse events
The study authors described RF therapy as generally well tolerated; for details, see Appendix 16. All participants complained of pain and discomfort during RF ablation, which disappeared when the energy was reduced or turned off (RF Huh 2012). All participants experienced a mild sensation of heat in the neck without the need to stop the procedure (RF Faggiano 2012). Neither of the two studies reported any serious adverse event. We associated this outcome with a high risk of performance bias and an unclear risk of detection bias for both studies.
Secondary outcomes
Compliance
This outcome was not investigated in any RF study.
Tolerability
This outcome was not investigated in any RF study.
TSH and T4 serum levels
In most studies, thyroid hormones including thyroid autoantibodies were evaluated at the beginning and throughout the study. Baseline values were always documented. Results of laboratory tests were within reference range at the end of the six month follow‐up (RF Huh 2012). All participants who were euthyroid in the treatment group had normal function at each follow‐up, whereas in the comparator group, TSH serum levels had fallen in two euthyroid participants (subclinical hyperthyroidism) (RF Faggiano 2012). In a subgroup of 10 participants with toxic nodules treated with RF, hyperthyroidism recovered in 40% (demonstrated after methimazole withdrawal) and improved in a further 40% (demonstrated after methimazole reduction); in a subset of eight participants with toxic nodules in the no‐treatment group, hyperthyroidism persisted and methimazole therapy was extended for the entire follow‐up period(RF Faggiano 2012).
Thyroid cancer
This outcome was not investigated in any RF study.
All‐cause mortality
This outcome was not investigated in any RF study.
Health‐related quality of life
This outcome was not investigated in any RF study.
Socioeconomic effects
This outcome was not investigated in any RF study.
Reporting bias
Only one outcome (nodule volume reduction of 50% or more) was investigated in 10 included studies of LT4, and hence provided sufficient data for assessing small study effects. Visual inspection of the funnel plot does not indicate reporting bias (Figure 5).
5.
Funnel plot of comparison: 1 Levothroxine versus comparator, outcome: 1.1 Nodule volume reduction ≥ 50%
Subgroup analyses
Not performed due to lack of data. Future updates of this review might provide adequate data to perform subgroup analyses.
Sensitivity analyses
We were able to perform only one sensitivity analysis with regard to risk of bias. Ten studies investigated the outcome nodule volume reduction of 50% or more and we judged these studies overall to have a low risk of performance bias. We considered two studies to have an unclear risk of detection bias (LT4 Bayani 2012; LT4 Reverter 1992). Exclusion of these studies did not substantially change the effect estimate. Another study we judged to have a high risk of attrition and reporting bias (LT4 Grussendorf 2011). Excluding this study also did not substantially change the effect estimate. Future updates of this review might provide adequate data to perform additional sensitivity analyses.
Ongoing studies
We identified four ongoing RCTs. LP Dossing 2001, a phase 3 open‐label study, is investigating one versus two or three LP sessions for the treatment of benign solitary cold thyroid nodules and also LP versus radioiodine for benign solitary autonomous thyroid nodules. LP Pacella 2008, a multicentre phase 4 open‐label study, is evaluating the long‐term effects of LP versus no active therapy on benign thyroid nodules, reporting re‐occurrence rates during three years of follow‐up, reproducibility of results in different environments and under different operators, and the presence of major or minor adverse effects. LT4 Shih 2007, a single‐centre cross‐over open‐label study, is investigating the effect of same dose of LT4 taken before and after breakfast. RF Baek 2013, a single‐blind comparison of RF versus ethanol ablation, is investigating the effects of treatments in participants with predominantly cystic thyroid nodules. For more details, see the Characteristics of ongoing studies table.
Discussion
Summary of main results
A total of 31 studies randomised 2952 participants to various treatments for benign thyroid nodules; LT4 trials represented the majority of studies (71%). We identified no RCTs of HIFU or MW ablation therapy for benign thyroid nodules. The duration of treatment varied according to the applied therapies: up to five years for LT4, one to three PEI ablations, one to three LP sessions and one or two RF sessions. Median follow‐up was 12 months for LT4 and six months for minimally invasive therapies. Evidence was of low‐to‐moderate quality, and risk of performance and detection bias for subjective outcomes was high in most trials. For an overview of the main findings see Table 1, Table 2, Table 3 and Table 4.
No study evaluated all‐cause mortality or health‐related quality of life. Only one LT4 study provided some data on the development of thyroid cancer, and reported no abnormal cytological findings. One LP study provided limited information on costs of treatment.
All treatments produced a nodule volume reduction of 50% or more in favour of the active intervention; however, the clinical relevance of this finding is doubtful, mainly because of the unclear relationship between nodule growth alone and malignant transformation of the nodule. Pressure symptoms or cosmetic complaints were not investigated in LT4 studies, but showed improvement following PEI, LP or RF treatments. Signs and symptoms of hyperthyroidism in LT4‐treated compared with placebo‐treated participants at 12 to 18 months of follow‐up were 25% versus 7%, respectively. All minimally invasive procedures induced some light‐to‐moderate pain and discomfort; serious adverse events were rarely reported.
Overall completeness and applicability of evidence
The overall evidence base for the treatment of benign thyroid nodules is rather incomplete, with the majority of trials evaluating the effects of LT4 suppression therapy. Though LT4 often results in a reduction of the volume of thyroid nodules, the effects on pressure symptoms were not investigated in these included studies. Current clinical practice guidelines do not recommend the use of LT4 for benign thyroid nodules (ATA 2009; Gharib 2010); however, clinical guidelines providing guidance for the management of thyroid nodules and cancer differ in methodological quality (Huang 2013).
In the included studies of this systematic review, minimally invasive therapies reduced pressure symptoms and cosmetic complaints, though risk of bias for these findings was high. With regard to ultrasound‐guided PEI, approximately 15% to 30% of thyroid nodules are reported to be cystic or predominantly cystic. Cyst aspiration often results in an improvement of pressure symptoms but the recurrence rate may be high. Ethanol seepage outside the treatment area may result in serious adverse events and hinder later surgery due to local fibrosis. However, depending on the availability of treatment alternatives in a given setting, ultrasound‐guided PEI is currently seen as the treatment of choice for recurrent, benign cystic thyroid nodules (Gharib 2013). With regard to LP, experienced operators are needed. The precision of this procedure, especially for small thyroid nodules, is high and tissue ablation is well controlled with minimal or no extranodular tissue damage. Currently, only five trials with 192 participants could be included in this systematic review, so the evidence base is rather low. With regard to RF, experienced operators are needed. The evidence base for this procedure is also very low, currently consisting of two included trials with 70 participants. We found no RCTs on HIFU or MW therapy for benign thyroid nodules. Unfortunately, many of our predefined patient‐important outcomes, such as health‐related quality of life, adverse events and the development of thyroid cancer, were not (adequately) investigated. Therefore, apart from therapies for thyroid nodules causing symptoms, the question of how to best approach asymptomatic thyroid nodules remains unsolved.
Quality of the evidence
The evidence base for outcomes was of low‐to‐moderate quality. Key methodological limitations were risk of performance and detection bias for subjective outcomes, indirectness, imprecision of results and few trials with few participants per evaluated intervention. The majority of patient‐important outcome measures were not addressed in the included trials.
Potential biases in the review process
Although we undertook a comprehensive literature search, there may be relevant unpublished studies or grey literature that we did not find. Outcome reporting bias could be addressed only partly because we had limited access to study protocols. The strength of our 'Risk of bias' evaluation is the separation between subjective and objective outcome measures, which revealed a high risk of performance and detection bias for subjective outcomes. In future updates of this review we plan to search for observational studies because it is likely that the occurrence of thyroid cancer will not be adequately addressed in RCTs. In addition, we will focus on patient‐important outcome measures in future updates of this review.
Agreements and disagreements with other studies or reviews
Several previously published systematic reviews evaluating the treatment of thyroid nodules confirm our findings with regard to the effects of LT4 therapy (Castro 2002; Fuller 2014; Richter 2002; Sdano 2005; Yousef 2010; Zelmanovitz 1998).
Zelmanovitz 1998 reported, by means of a cumulative meta‐analysis, a nodule volume decrease of 50% or more following LT4 therapy (risk difference 16.7% (95% CI 5.8 to 27.6%) but did not recommend offering this therapy to all individuals with thyroid nodules. The authors hypothesised that in participants experiencing a reduction in thyroid nodule volume, treatment could be prolonged with lower LT4 doses and TSH levels around the lower reference limit.
Castro 2002 analysed six RCTs investigating the effects of LT4 therapy given for six months or more. The overall treatment response (decreasing volume of solitary nodules by more than 50%) did not achieve statistical significance (RR 1.9 (95% CI 0.95 to 3.81)), depending on the statistical model used.
Richter 2002 reported that TSH suppression therapy inhibits solitary thyroid nodule growth and reduces nodule size. However, the authors noted that "uncertainly about predictors of response or impact on outcomes that are important to participants leaves considerable doubt about the wisdom of applying suppressive therapy."
Sdano 2005 confirmed that LT4 therapy may lead to thyroid nodule volume reduction but did not recommend the routine use of this therapy.
Yousef 2010 noted that significant volume reductions in benign solitary thyroid nodules can be achieved and postulated that, especially in younger participants, LT4 suppression could decrease the chance of malignancy through a reduction in nodule volume size.
Finally, Fuller 2014, in a recently published systematic review, analysed the effects of RF for the treatment of benign thyroid nodules. They included three RCTs and six observational trials. The authors noted a reduction in thyroid nodule size and improvements in symptoms and cosmetic scores. Authors were concerned about a lack of RCTs comparing RF with surgical and non‐surgical treatment modalities.
In summary, all systematic reviews showed general agreements with our findings. However, none of these other reviews tried to evaluate all the available RCT evidence for all currently existing interventions for benign thyroid nodules.
Authors' conclusions
Implications for practice.
It is unclear whether asymptomatic thyroid nodules should be treated because in most cases they are benign, small and can be managed by active surveillance (Gharib 2007). Thyroid nodules are common in the adult population and from a clinical viewpoint, fewer than 5% of palpable thyroid nodules are malignant. Several therapeutic approaches are available, such as suppressive LT4 therapy and minimally invasive treatments (currently PEI sclerotherapy, LP, and MW, RF and HIFU ablation treatment). Although nodule volume reduction is achievable by all these treatments, the clinical relevance of this outcome measure is doubtful and the evidence base is of moderate‐to‐low quality. Improvements in pressure symptoms and cosmetic complaints are possible using minimally invasive techniques such as PEI, LP and RF; however, the evidence base for these outcomes is of low quality. These techniques are associated with mild‐to‐moderate periprocedural pain. RCT evidence is currently not available for HIFU and MW. Included studies provided no information on all‐cause mortality, health‐related quality of life and the development of thyroid cancer. No firm evidence therefore exists to establish the optimal treatment strategy for thyroid nodules, with the possible exception of minimally invasive techniques utilised for thyroid nodules causing pressure symptoms, cosmetic complaints, or both, especially as an alternative to surgery and depending on the availability of experienced operators.
Implications for research.
RCTs with several years of follow‐up and good‐quality observational studies are needed to provide evidence on all‐cause mortality, the development of thyroid cancer and long‐term adverse events profiles. One ongoing trial might provide additional insights into the long‐term benefits and harms of LP compared with no treatment on benign thyroid nodules (LP Pacella 2008). Patient‐important outcome measures, such as health‐related quality of life, adverse effects, compliance and tolerance, and socioeconomic effects should be primary endpoints in future trials of thyroid nodule management.
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2014 | Amended | Contact address corrected. |
Acknowledgements
None.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Thyroid nodule explode all trees #2 MeSH descriptor Goiter, nodular explode all trees #3 (thyroi* in All Text near/6 nod*in All Text) #4 (thyroi* in All Text near/6 incidentalom*in All Text) #5 (thyroi* in All Text near/6 goiteri n All Text) #6 (#1 or #2 or #3 or #4 or #5) |
| MEDLINE |
| 1 exp Thyroid Nodule/ 2 exp Goiter, Nodular/ 3 (thyroi* adj6 (nod* or incidentalom* or goiter)).tw,ot. 4 1 or 3 or 2 5 randomized controlled trial.pt. 6 controlled clinical trial.pt. 7 randomi?ed.ab. 8 placebo.ab. 9 drug therapy.fs. 10 randomly.ab. 11 trial.ab. 12 groups.ab. 13 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14 exp Technology Assessment, Biomedical/ 15 hta.tw,ot. 16 (health technology adj6 assessment$).tw,ot. 17 (search or Cochrane or MEDLINE or EMBASE).tw. 18 (systematic adj3 review).tw. 19 meta‐analysis.pt. 20 or/14‐19 21 (comment or editorial or historical‐article).pt. 22 20 not 21 23 4 and (13 or 22) 24 limit 23 to yr="2009 ‐ 2011" 25 (animals not (animals and humans)).sh. 26 24 not 25 |
| EMBASE |
| 1 exp thyroid nodule/ 2 exp nodular goiter/ 3 (thyroi* adj6 (nod* or incidentalom* or goiter)).tw,ot. 4 1 or 2 or 3 5 randomized controlled trial/ 6 exp controlled clinical trial/ 7 randomi?ed.ab. 8 placebo.ab. 9 randomly.ab. 10 trial.ab. 11 groups.ab. 12 5 or 6 or 7 or 8 or 9 or 10 or 11 13 exp biomedical technology assessment/ 14 hta.tw,ot. 15 (health technology adj6 assessment*).tw,ot. 16 (search or Cochrane or Medline or Embase).tw,ot. 17 (systematic adj3 review).tw. 18 meta analysis/ 19 13 or 14 or 15 or 16 or 17 or 18 20 12 or 19 21 (comment or editorial or historical‐article).pt. 22 20 not 21 23 4 and 22 24 limit 23 to yr="2009 ‐ 2011" 25 limit 24 to human |
| LILACS |
| Descriptor: thyroid AND nodule$ |
| 'My NCBI' alert service |
| thyroid nodul* AND random (thyroid* AND nodul*) OR (nodul* AND goiter*) |
| Web of Science |
| TS=(thyroid nodul*) AND TS=(random*) |
Appendix 2. Description of interventions
| Intervention(s) [route, frequency, total dose/day] | Comparator(s) [route, frequency, total dose/day] | |
| Levothyroxine treatment | ||
| LT4 Bayani 2012 | LT4: initial dose 50 µg/day (dose adaptation to archive TSH levels < 0.5 mU/L) | No treatment |
| LT4 Boguszewski 1998 | LT4: participants < 70 kg: 200 μg/day (2 tablets); participants > 70 kg: 250 μg (2 and 3 tablets on alternate days) | Placebo |
| LT4 Cesareo 2010 | LT4: 2 μg/kg/day | No treatment |
| LT4 Gharib 1987 | LT4: 3 μg/kg/day | Placebo |
| LT4 Grineva 2003 | LT4: 75 to 150 μg/day, dose adjusted until TSH ≤ 0.5 mU/L | Potassium iodide 200 μg/day |
| LT4 Grussendorf 2011 | LT4 (75 μg/day; dose adjusted based on TSH between 0.2 to 0.8 mU/L) + potassium iodide (150 μg/day) | Comparator 1: LT4: 75 μg/day (dose adjusted based on TSH between 0.2 to 0.8 mU/L) Comparator 2: potassium iodide 150 μg/day Comparator 3: placebo |
| LT4 Koc 2002 | Intervention 1: LT4 (group 2; TSH high‐level suppression: < 0.01 mU/L; LT4 3 μg/kg/day) Intervention 2: LT4 (group 4; TSH low‐level suppression: 0.4 to 0.6 mU/L; LT4: 1.5 μg/kg/day) | Comparator 1: placebo (group 1) Comparator 2: placebo (group 3) |
| LT4 La Rosa 1995 | Intervention 1: LT4 1 μg/kg/day oral (1 dose) up 1.8 μg/kg/day oral after 15 days; dose adjusted until TSH < 0.3 mU/L (first 4 months); mean 1.94 (0.16) μg/kg/day Intervention 2: potassium iodide 1.5 mg every 2 weeks |
No treatment |
| LT4 Larijani 2005 | LT4: 1.5 to 2 μg/kg/day | Placebo |
| LT4 Ozkaya 2010 | LT4: 50 to 100 mg/day | No treatment |
| LT4 Papini 1993 | LT4: 2 μg/kg/day (initial dose: 50 μg before breakfast and increased by 25 to 50 μg/week to the full dose) | Placebo |
| LT4 Papini 1998 | LT4: 2 μg/kg/day | No treatment |
| LT4 Reverter 1992 | LT4: 100 μg/day for 2 weeks, then 200 μg | No treatment |
| LT4 Tsai 2006 | LT4: 100 μg/day | Placebo |
| LT4 Wemeau 2002 | LT4: 2.5 μg/kg/day, one dose in the morning, adjusted after the first 4 weeks until TSH < 0.3 mU/L | Placebo |
| LT4 Zelmanovitz 1998 | LT4: 2.5 to 3.0 μg/kg/day for 1.5 months; dose adjusted until TSH < 0.3 μU/mL or TSH (after TRH stimulation) < 2 μU/mL; mean dose: 2.73 ± 0.32 μg/kg/day | Placebo |
| Percutaneous sclerotherapy | ||
| PEI Bennedbaek 1998 | PEI (98% ethanol): 1 intranodular injection under US control; median ethanol dose given 21% (95% CI 18 to 25) of the pretreatment nodule volume | LT4: 1.5 µg/kg/day until 12 months (dose adjusted monthly until 6 months to lower serum TSH to < 0.40 mU/L) |
| PEI Bennedbaek 1999 |
1 injection PEI (98% ethanol): 1 intranodular injection (US control); median ethanol dose given: 2.4 ± 1.4 mL; total amount: 2.4 ± 1.4 mL, corresponding to 24.7% ± 7.5% of pretreatment volume |
3 injections PEI (98% ethanol): 3 intranodular injections/week (1 injection/session), under US control; median ethanol dose given (SD): session 1: 1.8 mL ± 1.1 mL session 2: 1.5 ± 0.9 mL session 3: 1.4 ± 0.8 mL; total amount: 4.4 ± 2.5 mL corresponding to 47.9% ± 21.3% of pretreatment volume |
| PEI Bennedbaek 2003 | PEI (99% ethanol): 1 session (in case of recurrence, repetition after 1 month to a maximum of 3 treatments) (US control); median ethanol dose given: 3.5 mL (quartiles 2 to 5) corresponding to 36% of the cyst volume | NaCl: 1 session (in case of recurrence, repetition after 1 month to a maximum of 3 treatments) (under US control); median NaCl dose given: 3.0 mL (quartiles 2 to 5), corresponding to 36% of the cyst volume |
| PEI Chu 2003 | PEI (95% ethanol): weekly until curea; percutaneous ethanol injected: 10% of the aspirated volume with a maximum of 2 mL | Comparator 1: percutaneous hydrochloric acid (pH 1.0) weekly until curea ; percutaneous hydrochloric acid injected: 10% of the aspirated volume with a maximum of 2 mL Comparator 2: cyst aspiration |
| PEI Sung 2013 | PEI (99% ethanol): percutaneous ethanol injected: usually 50% of the aspirated volume, 10 minutes of ethanol retention | RF ablation: 18‐gauge with 1 cm or 1.5 cm active‐tip internally cooled electrode; aspiration of internal fluid ablation; ablation power from 50W/15 W (1 cm /0.5 cm active tip) and increased in 5‐ to 10‐W increments up to 70 W |
| TETRA Hegedüs 1998 | 2 mL of tetracycline hydrochloride (50 mg/mL) + re‐aspiration 3 to 4 times (under US control) in one session (if no recurrence) | 2 mL of NaCl + re‐aspiration 3 to 4 times (under US control) in one session (if no recurrence) |
| PEI Valcavi 2004 | PEI (95% ethanol): US‐guided, 1 session (2 to 3, if fluid content > 2 mL); amount ethanol injected: 50% to 70% of the cystic fluid extracted | Cyst aspiration only |
| PEI Verde 1994 | PEI (95% ethanol): US guided; amount injected: between 1 to 10 mL, based on volume aspirated + alcohol distribution | Cyst aspiration only (US guided) |
| Laser photocoagulation | ||
| LPDossing 2005 | 1 session with median total energy deposition: 2007 J (quartiles 1750 to 2880); median energy given per mL of the pretreatment nodule volume 224 J (quartiles 182 to 331) | No treatment |
| LPDossing 2006 |
1 session Mean total energy deposition: 2284 ± 1160 J (duration: mean 744 ± 211 sec); mean total energy/mL of the pretreatment nodule volume given was 262 ± 205 J |
3 sessions 3 sessions in one month; mean total energy deposition: 4133 ± 1709 J (duration: mean 1539 ± 599 seconds) 1st session: mean energy deposition 1683 ± 448 J 2nd session: mean energy deposition 1420 ± 720 J 3rd session mean energy deposition 1512 ± 555 J |
| LPGambelunghe 2006 | 1 session with single 21‐gauge spinal needle (US guided) placed in the centre of the dominant nodule; laser 300‐µm quartz fibre inserted into the lumen of the needle; total median energy given 1900 J (700 to 2200); at each step: energy given (100 to 400 J) based on the hyperechoic area produced by photocoagulation | No treatment |
| LPPapini 2007 | 1 session with two 75 mm, 21‐gauge spinal needles inserted into the thyroid lesions (US monitoring; 4 needles and 2 illuminations, if volume > 20 mL); laser 300‐µm quartz fibre inserted into the lumen of the needle; laser energy output power 3 W and 10 minutes for illumination; total energy for entire session 3600 to 14400 J; total energy given/fibre/treatment 1800 J; mean energy given/mL was 1221 ± 679 J, median 1054 J/mL | Comparator 1: LT4: 1,5 μg/kg oral (LT4 dose ↑, if TSH > 0.30 μU/mL; LT4 dose ↓, if TSH undetectable with FT3 increase, persistent nervousness, tremor, or tachycardia) Comparator 2: no treatment |
| LP Dossing 2013 | Cyst aspiration session with an 18‐gauge needle (US‐guided) placed in the cystic part of the nodule; laser fibre inserted in the lumen of this steering needle; total median energy given 1272 J (quartiles 990 to 1500), corresponding to 83 J (quartiles 49; 224)/mL of nodule tissue | Cyst aspiration only |
| Radiofrequency ablation | ||
| RF Faggiano 2012 | 1 session with StarBurst® Talon with 14‐gauge and 10‐cm long needle with four expandable hooks; these opened to maximal 3.5 cm with exposure time between 5 and 7 minutes and temperature reached between 100°C and 105°C | No treatment |
| RFHuh 2012 |
1 session 18‐gauge with 1‐cm or 1.5‐cm active‐tip internally cooled electrode; trans‐isthmic approach (electrode placed along the short axis of the nodule); TN divided into multiple ablation units: unit‐by‐unit ablation by moving the electrode (moving shot technique); ablation power from 30 to 50 W up to 120 W in 10‐W increases, if transient hyperechoic zone did not form within 5 to 10 seconds; mean total energy deposition: 51,930 ± 47,080 J; energy/mL of pretreatment volume: 4377.3 ± 2199.5 J |
2 sessions 1st session: mean energy deposition 38,740 ± 15,454 J 2nd session: mean energy deposition: 30,420 ± 16,057 J Mean total energy deposition: 69,160 ± 27,808 J; energy/mL of pretreatment volume: 6156.7 ± 2661.3 J |
|
aCure: nodule disappearance or volume reduction < 0.5 mL, maximum 5 sessions ±: single standard deviation; CI: confidence interval; FT3: free tri‐iodothyronine; LP: laser photocoagulation; LT4: levothyroxine; NaCl: isotonic saline; PEI: percutaneous ethanol injection; RF: radiofrequency ablation; SD: standard deviation; TN: thyroid nodule; TRH: thyrotropin‐releasing hormone; TSH: thyrotropin; US: ultrasound | ||
Appendix 3. Baseline characteristics (levothyroxine treatment I)
| Intervention(s) and comparator(s) |
Participating population |
Sex [female %] | Age [mean years (SD)/(range)] | Nodule volume at baseline [mL (SD)/median (95% CI, range)] | US characteristics and/or nodule cytologya | |
| LT4 Bayani 2012 | I: LT4 C: no treatment |
Euthyroid participants with a single palpable thyroid nodule | I: 85 C: 85 |
I: 41.6 (9.4) C: 44.5 (10.9) |
I: longitudinal dimension 1.9 cm (1.1); transverse dimension 1.4 cm (0.9) C: longitudinal dimension 2.2 cm (1.3); transverse dimension 1.6 cm (1) |
‐ |
| LT4 Boguszewski 1998 | I: LT4 C: PLAC |
Euthyroid participants with a single palpable thyroid nodule | I: 100 C: 91 |
I: 41 (9) C: 40 (9) |
I: 14.1 (12.3) C:12.7 (12.1) |
Solid (> 50%), colloid goitre |
| LT4 Cesareo 2010 | I: LT4 C: no treatment |
Euthyroid premenopausal women from Latin America with thyroid multinodular disease (2 to 5 nodules) | I: 100 C: 100 |
I: 37.2 (10.3) C: 34.0 (9.1) |
I: 1.8 (2.1) C: 1.2 (0.9) |
Solid (≤ 30% fluid), colloid |
| LT4 Gharib 1987 | I: LT4 C: PLAC |
Participants with a single palpable thyroid nodule proved to be benign by FNAB |
I: 93 C: 88 |
I: 42.0 (15) C: 48.2 (17) |
I: 3.0 (2.6) C: 2.6 (1.7) |
Solid, cyst (mixed), colloid |
| LT4 Grineva 2003 | I: LT4 C: PLAC |
Participants with benign nodular thyroid lesions (colloid or colloid hypercellular by FNAB; cold or warm by scintigraphy) | I: 97 C: 97 |
I: 46 (1.4) C: 45.5 (1.5) |
‐ | Colloid |
| LT4 Grussendorf 2011 | I1: LT4 + PI C1: LT4 C2: PI C3: PLAC |
Euthyroid participants with a nodular goitre, in a region with sufficient iodine supply | I: 68 C1: 71 C2: 72 C3: 74 |
I1: 47.4 (46.1 to 48.8) C1: 47.1 (45.7 to 48.5) C2: 47 (45.6 to 48.4) C3: 46.1 (44.6 to 47.5) | I: 2 (1.7 to 2.3) C1: 1.7 (1.4 to 1.9) C2: 1.5 (1.3 to 1.7) C3: 1.7 (1.5 to 2) |
Solid (< 20% fluid), mixed |
| LT4 Koc 2002 | I1: LT4: 3 μg/kg/day I2: LT4: 1.5 μg/kg/day C1: PLAC high‐dose C2: PLAC low‐dose |
Euthyroid participants with nodular thyroid disease (solitary TN on palpation) | I1: 91 I2: 90 C1: 100 C2: 90 |
I1: 40.2 (9.7) I2: 47.9 (16.6) C1: 38 (8.3) C2: 47.7 (19.4) |
I1: 3.7 (5.9) I2: 3.4 (4.4) C1: 4.2 (3.8) C2: 3.6 (2.8) |
Solid |
| LT4 La Rosa 1995 | I1: LT4 I2: PI C: no treatment |
Euthyroid participants with a solitary TN | I1: 93 I2: 96 C: 100 |
I1: 35.7 (11.6) I2: 38 (10.3) C: 41 (12.9) |
I1: 5.9 (5.7) I2: 5.0 (6.1) C: 5.7 (5.8) |
Solid (< 10% fluid), parenchymatous, colloid |
| LT4 Larijani 2005 | I: LT4 C: PLAC |
Participants with one palpable benign TN (FNAB, cytology); endemic goitre area) | I: 81 C: 74 |
I: 34.4 (9.4) C: 37.1 (11.8) |
I: 12.8 (11.9) C: 13.0 (10.2) |
Solid |
| LT4 Ozkaya 2010 | I: LT4 C: no treatment |
Euthyroid participants with benign TN (FNAB, cytology) | I: ‐ C: ‐ |
I:b C:b |
I: 0.8 C: 0.4 |
Solid |
| LT4 Papini 1993 | I: LT4 C: PLAC |
Euthyroid participants with a single, palpable TN (non‐endemic area) | I: 90 C: 88 |
I: 43 (10) C: 42 (11) |
I: 6.20 (8.9) C: 6.25 (7.41) |
Solid (< 1 mL fluid), colloid |
| LT4 Papini 1998 | I: LT4 C: no treatment |
Participants with one palpable TN (non‐endemic area) | I: 88 C: 78 |
I: 41.4 C: 41.9 |
I: 1.5 C: 1.5 |
Solid, colloid |
| LT4 Reverter 1992 | I: LT4 C: no treatment |
Euthyroid women with solitary TN on palpation, cold and single by thyroid scanning and benign by FNAB (colloid goitre) | I: 100 C: 100 |
I: 40.1 (8.2) C: 39.5 (12.8) |
I: 10.3 (11.9) C: 9.2 (6.4) |
Solid, mixed, colloid goitre |
| LT4 Tsai 2006 | I: LT4 C: PLAC |
Euthyroid participants with solitary TN (US), benign (FNAB cytology) | I: 60 C: 67 |
I: 32 (7.2) C: 34 (10.1) |
I: 7.2 (5.1) C: 7.3 (4.8) |
Solid |
| LT4 Wemeau 2002 | I: LT4 C: PLAC |
Euthyroid participants (area thought to have a sufficient iodine supply) with a single palpable benign TN | I: 91 C: 90 |
I: 40.0 (9.0) C: 38.2 (9.2) |
I: 2.8 (2.5) C: 3.5 (3) |
Solid (≤ 20% fluid) |
| LT4 Zelmanovitz 1998 | I: LT4 C: PLAC |
Euthyroid participants with a single TN | I: 90 C1: 96 |
I: 44.8 (10.3) C: 41.3 (13.1) |
I: 16.4 (18.7) C: 13.6 (13.9) |
Solid (≤ 20% fluid), colloid goitre |
| "‐" denotes not reported Numbers in italic were calculated by review authors aAll TN were benign by FNAB cytology bNo statistical significant differences between I and C for age (P value = 0.11), BMI (P value = 0.17) and time from diagnosis (P value = 0.06) BMI: body mass index; C: comparator; CI: confidence interval; FNAB: fine needle aspiration biopsy; I: intervention; LT4: levothyroxine; PI: potassium iodine; PLAC: placebo; SD: standard deviation; TN: thyroid nodule(s); US: ultrasonography | ||||||
Appendix 4. Baseline characteristics (levothyroxine treatment II)
| Intervention(s) and comparator(s) | Country | Duration of disease/ months from diagnosis [mean/median (SD)] | Duration of intervention | Duration of follow up (n= number of participants) | Comorbidities | Comedications | |
| LT4 Bayani 2012 | I: LT4 C: no treatment |
Iran | ‐ | 6 mo | 6 mo | No serious cardiovascular, hepatic or renal disease | ‐ |
| LT4 Boguszewski 1998 | I: LT4 C: PLAC |
Brazil | I: 66 (62) C: 52 (71) |
12 mo | 12 mo | ‐ | ‐ |
| LT4 Cesareo 2010 | I: LT4 C: no treatment |
Italy | ‐ | 12 mo | 12 mo
(n = 71) 24 mo (n = 41) |
‐ | ‐ |
| LT4 Gharib 1987 | I: LT4 C: PLAC |
USA | I: 2 C: 1 |
6 mo | 6 mo | ‐ | ‐ |
| LT4 Grineva 2003 | I: LT4 C: PLAC |
Russia | I: 6.2 (1.0) C: 6.9 (1.2) |
6 mo | 6 mo | ‐ | ‐ |
| LT4 Grussendorf 2011a | I1: LT4 + PI C1: LT4 C2: PI C3: PLAC |
Germany | ‐ | 12 mo | 12 mo | I1: 63% C1: 62% C2: 70% C3: 60% |
‐ |
| LT4 Koc 2002 | I1: LT4: 3 μg/kg/day I2: LT4: 1.5 μg/kg/day C1: PLAC high‐dose C2: PLAC low‐dose |
Turkey | ‐ | 12 mo | 24 mo | ‐ | ‐ |
| LT4 La Rosa 1995 | I1: LT4 I2: PI C: no treatment |
Italy | ‐ | 12 mo | I: 12 mo + 4 mo C: 12 + 12 mo (received LT4 after 1 yr) |
No major concomitant disease | ‐ |
| LT4 Larijani 2005 | I: LT4 C: PLAC |
Iran | ‐ | 24 mo | 24 mo | ‐ | ‐ |
| LT4 Ozkaya 2010 | I: LT4 C: no treatment |
Turkey | ‐ | 12 mo | 12 mo | No cardiovascular‐, liver‐ or renal disease | No LT4 suppressive therapy; no other thyroid medication |
| LT4 Papini 1993 | I: LT4 C: PLAC |
Italy | I: 9.8 (7.1) C: 9.3 (6.2) |
12 mo | 12 mo | Absence of clinically relevant cardiovascular, hepatic, pulmonary or renal diseases |
‐ |
| LT4 Papini 1998 | I: LT4 C: no treatment |
Italy | ‐ | 5 yr | 5 yr | ‐ | ‐ |
| LT4 Reverter 1992 | I: LT4 C: no treatment |
Spain | ‐ | 11 mo | Ib: 6 to 12 mo C: 12 mo |
‐ | ‐ |
| LT4 Tsai 2006 | I: LT4 C: PLAC |
Taiwan | I: 6.2 (3.5) C: 6.4 (4.1) |
6 mo | 6 mo | ‐ | ‐ |
| LT4 Wemeau 2002 | I: LT4 C: PLAC |
France | < 12 | 18 mo | 18 mo | ‐ | None |
| LT4 Zelmanovitz 1998 | I: LT4 C: PLAC |
Brazil | I: 65 (84) C: 44 (60) |
12 mo | 12 mo | ‐ | ‐ |
| "‐" denotes not reported aAllowed and not allowed comedications were described and reviewed periodically; no information available about how many participants received comedications bn = 6 participants dropped out or abandoned the study at different times of follow up C: comparator; I: intervention; LT4: levothyroxine; mo: months; PI: potassium iodide; PLAC: placebo; SD: standard deviation; yr: year(s) | |||||||
Appendix 5. Baseline characteristics (minimally invasive treatments I)
| Intervention(s) and comparator(s) | Participants | Sex [female%] | Age [mean/median years (SD)/range/quartiles] | Months from diagnosis [mean (SD) or median (quartiles)] | Nodule volume [mean/median mL (SD)/95% CI, range)/quartiles] | US characteristics and/or nodule histology | |
| PEI Bennedbaek 1998 | I: PEI C: LT4 |
Participants who were 20 to 70 yr of age with a benign solitary solid cold palpable thyroid nodule causing local discomfort | I: 88 C: 96 |
Ia: 46 (41; 52) Ca: 41 (37; 45) |
Ia: 9 (7; 17) Ca: 8 (6; 12) |
I: 9.2 (7.2; 11.6) C: 7.1 (4.9; 10.8) |
Solid, colloid |
| PEI Bennedbaek 1999 | I: PEI (1 dose) C: PEI (3 doses) |
Participants who were 20 to 70 yr of age with a palpable and clinically solitary thyroid nodule causing local discomfort | I: 97 C: 100 |
I: 42.6 (10.6) C: 42.7 (10.0) |
I: 21.2 (28.3) C: 15.2 (15.2) |
I: 9.9 (5.7) C: 9.4 (4.2) |
Solid (< 10% fluid), colloid |
| PEI Bennedbaek 2003 | I: PEI C: NaCl |
Participants who were 20 to 70 yr of age with a benign, solitary cold, palpable thyroid nodule causing local discomfort and/or cosmetic complaints | I: 88 C: 79 |
I: 48
(33 to 57) C: 46 (40 to 53) |
Ib: 9 (4;12) Cb: 7 (4;13) |
I: 8 (5; 14) C: 8 (4; 15) |
Cystic > 90%, colloid |
| PEI Chu 2003 | I1: PEI C1: PHI C2: ASP |
Participants aged 24 to 70 yr with thyroid cystic nodules | I1: 60 C1: 50 C2: 44 |
I1: 50.4 (12.6) C1: 56.1 (11.9) C2: 57.2 (11.8) |
‐ | I: 17.3 (11.4) C1: 13 (9.7) C2: 19 (13.7) |
Cystic ≥ 90%, benign by US‐FNAB |
| PEI Sung 2013 | I: PEI C: RF |
Euthyroid participants with thyroid cystic nodules | I: 92 C: 88 |
I: 45 (10.9) C: 44.9 (10.6) |
‐ | I: 12.2 (11.0) C: 9.3 (11.7) |
Cystic > 90%, benign by US‐FNAB |
| TETRA Hegedüs 1998 | I: TETRA C: NaCl |
Euthyroid participants with a solitary nodule cyst of at least 2 mL and absence of any residual nodule following complete cyst aspiration | I: 83 C: 60 |
‐ | ‐ | I: 10 (2; 45) C: 8 (2; 50) |
Cystic > 2 mL, benign by US‐FNAB |
| PEI Valcavi 2004 | I: PEI C: ASP |
Participants with benign thyroid cystic nodules | T: 79 | Tc: 18 ‐ 85 | ‐ | I: 19 (19) C: 20 (13.4) |
Cystic ≥ 50%, benign by US‐FNAB |
| PEI Verde 1994 | I: PEI (group 2) C: ASP (group 1) |
Euthyroid participants with predominantly cystic thyroid nodules | T: 65 | T: 47.3 (9.8) | ‐ | I: 16.6 (10.5; 52.4) C: 25.8 (12.3; 50.3) |
Cystic > 70%, benign by US‐FNAB |
| LPDossing 2005 | I: LP C: no treatment |
Euthyroid participants with a solid, solitary, cold, benign thyroid nodule causing discomfort | I: 100 C: 100 |
Ib: 47 (43; 52) C1b: 46 (41; 51) |
I1b: 6 (3; 12) C1b: 6 (3; 7.5) |
I: 8.2 (6.1; 11.9) C: 7.4 (5.1; 13.8) |
Solid, colloid, benign by US‐FNAB |
| LPDossing 2006 | I: LP (1 session) C: LP (3 sessions) |
Euthyroid participants, with a solid, solitary, cold, benign thyroid nodule causing pressure symptoms | I: 93 C: 100 |
I: 46 (7) C: 45 (12) |
I: 8 (5) C: 10 (9) |
I: 10.1 (4.4) C: 10.7 (9.0) |
Solid, colloid, benign by US‐FNAB |
| LP Dossing 2013 | I: ASP + LP C: ASP |
Euthyroid participants with a recurrent solitary predominantly cystic cold thyroid nodule causing discomfort | I: 77 C: 59 |
Ib: 49 (39; 56) Cb: 49 (40; 56) |
Ib: 12 (6; 18) Cb: 7.5 (5; 12) |
Ib: 11.8 (5.8; 26.8) Cb: 10 (5.6; 22) |
Solid‐cystic, colloid, benign by US‐FNAB |
| LPGambelunghe 2006 | I: LP C: no treatment |
Participants with compressive symptoms due to multinodular goitre and a high surgical risk | T: 81 | I: 63
(52; 92) C: 70 (62; 81) |
‐ | I: 8.2 (2.8; 26.9) C: 8.1 (7; 12) |
Solid, mixed, benign by US‐FNAB |
| LPPapini 2007 | I1: LP C1: LT4 C2: no treatment |
Euthyroid participants with a cold thyroid nodule | I1: 86 C1: 90 C2: 90 |
I1: 44.9 (SD? 5.1) C1: 46.5 (SD? 8.2) C2: 47.1 (SD? 7.7) |
‐ | I: 11.7 (5.1) C1: 13.6 (6.3) C2: 12.1 (3.9) |
Solid (< 20% fluid), benign by US‐FNAB |
| RF Faggiano 2012 | I: RF C: no treatment |
Participants with benign, solid or predominantly solid, toxic and nontoxic thyroid nodules causing pressure symptoms | I: 80 C: 75 |
I: 58.3 (19.2) C: 62.1 (13.9) |
‐ | I: 13.3 (8.1) C: 11.2 (6.71) |
Solid, cystic < 30%, benign by US‐FNAB |
| RFHuh 2012 | I: RF (1 session) C: RF (2 sessions) |
Euthyroid participants refusing or ineligible for surgery with predominant solid, cold, benign thyroid nodules causing compressive symptoms | I: 87 C: 100 |
I: 37.5 (11.5) C: 37.7 (9.8) |
‐ | I: 13.3 (12.9) C: 13 (6.8) |
Solid portion > 50%, benign by US‐FNAB |
| "‐" denotes not reported Numbers in italic were calculated by review authors aValues are medians (95% CI) bValues are medians (with 25th and 75th centiles/quartiles) cRange (yr) ASP: aspiration; C: comparator; CI: confidence intervals; FNAB: fine‐needle aspiration biopsy; I: intervention; LP: laser photocoagulation; LT4: levothyroxine; NaCl: isotonic saline; PEI: percutaneous ethanol injection; PHI: percutaneous hydrochloric acid injection; RF: radiofrequency ablation; SD: standard deviation; SD?: unclear whether SD was correctly reported; T: total; TETRA: tetracycline hydrochloride; US: ultrasonography; yr: year(s) | |||||||
Appendix 6. Baseline characteristics (minimally invasive treatments II)
| Intervention(s) and comparator(s) | Country | Duration of intervention | Duration of follow up | Comorbidities | Comedications | |
| PEI Bennedbaek 1998 | I: PEI C: LT4 |
Denmark | I: 1 session C: until 12 mo |
12 mo | No major concomitant disease | No medication affecting thyroid function |
| PEI Bennedbaek 1999 | I: PEI (1 dose) C: PEI (3 doses) |
Denmark | I: 1 session (1 single injection) C: 3 sessions/wk (1 single injection/session) |
6 mo | No major concomitant disease | No medication affecting thyroid function |
| PEI Bennedbaek 2003 | I: PEI C: NaCl |
Denmark | 1 session (+ 1 session after 4 wk up to max. 3 sessions: 12 wk) | 6 mo | No major concomitant disease | No medication affecting thyroid function |
| PEI Chu 2003 | I1: PEI C1: PHI C2: ASP |
Taiwan | I: 1x/wk until cure (maximum 5 wk) | 12 mo | ‐ | None |
| PEI Sung 2013 | I: PEI C: RF |
Korea | PEI: 1 session RF: 1 session |
6 mo | ‐ | No medication or treatment for thyroid nodules |
| TETRA Hegedüs 1998 | I: TETRA C: NaCl |
Denmark | At least one session | 12 mo after cyst puncture |
‐ | ‐ |
| PEI Valcavi 2004 | I: PEI C: ASP |
Italy | 1 session (2 to 3, if fluid content > 2 mL) | 12 mo | ‐ | ‐ |
| PEI Verde 1994 | I: PEI (group 2) C: ASP (group 1) |
Italy | 1 session | 1 mo | ‐ | ‐ |
| LPDossing 2005 | I: LP C: no treatment |
Denmark | 1 session | 6 mo | ‐ | ‐ |
| LPDossing 2006 | I: LP (1 session) C: LP (3 sessions) |
Denmark | 1 session vs 3 sessions (in 1 mo) | 6 mo | ‐ | ‐ |
| LP Dossing 2013 | I: ASP + LP C: ASP |
Denmark | 1 session | 6 mo | ‐ | ‐ |
| LPGambelunghe 2006 | I: LP C: no treatment |
Italy | 1 session (for wide nodules the procedure was repeated) |
30 wk | ‐ | ‐ |
| LPPapini 2007 | I1: LP C1: LT4 C2: no treatment |
Italy | LP: 1 session LT4 treatment: 12 mo |
12 mo | ‐ | ‐ |
| RF Faggiano 2012 | I: RF C: no treatment |
Italy | US RF: 1 session | 12 mo | ‐ | ‐ |
| RFHuh 2012 | I: RF (1 session) C: RF (2 sessions) |
Korea | RF: 1 session RF: 2 sessions |
6 mo | ‐ | ‐ |
| "‐" denotes not reported ASP: aspiration; C: comparator; I: intervention; LT4: levothyroxine; LP: laser photocoagulation; mo: month(s); NaCl: isotonic saline; PEI: percutaneous ethanol injection; PHI: percutaneous hydrochloric acid injection; RF: radiofrequency ablation; TETRA: tetracycline hydrochloride; US: ultrasound; wk: week(s) | ||||||
Appendix 7. Matrix of study endpoints (levothyroxine treatment)
| Primary endpoint(s)a | Secondary endpoint(s)b | Otherc endpoint(s) | Time of endpoint measurement | |
| LT4 Bayani 2012 | ‐ | ‐ |
|
|
| LT4 Boguszewski 1998 | ‐ | ‐ |
|
|
| LT4 Cesareo 2010 | ‐ | ‐ |
|
All: 0, 6,12, 24 mo |
| LT4 Gharib 1987 | ‐ | ‐ |
|
|
| LT4 Grineva 2003 | ‐ | ‐ |
|
|
| LT4 Grussendorf 2011 | Change in total volume of all nodules | Change in goitre volume; number and echogenicity of nodules |
|
Primary and secondary outcomes:
0, 12 mo
|
| LT4 Koc 2002 | ‐ | ‐ |
|
|
| LT4 La Rosa 1995 | ‐ | ‐ |
|
|
| LT4 Larijani 2005 | ‐ | ‐ |
|
|
| LT4 Ozkaya 2010 | ‐ | ‐ |
|
All: 0, 12 mo |
| LT4 Papini 1993 | ‐ | ‐ |
|
|
| LT4 Papini 1998 | ‐ | ‐ |
|
|
| LT4 Reverter 1992 | ‐ | ‐ |
|
|
| LT4 Tsai 2006 | ‐ | ‐ |
|
|
| LT4 Wemeau 2002 | Nodule volume changes (US) | ‐ |
|
|
| LT4 Zelmanovitz 1998 | ‐ | ‐ |
|
|
| "‐" denotes not reported a,bAs stated in the publication cNot stated as primary or secondary endpoint(s) in the publication BMD: bone mineral density; FT3: free tri‐iodothyronine; FT4: free thyroxine; LT4: levothyroxine; mo: month(s): palp.: palpatory; T3: plasma (serum) tri‐iodothyronine; T4: plasma (serum) thyroxine; Tg: thyroglobulin; TgAb: antithyroglobulin autoantibody; TN: thyroid nodule; TPOAb: antiperoxidase autoantibody; TRH: thyrotropin‐releasing hormone; TSH: thyrotropin; US: ultrasound; wk: week(s) | ||||
Appendix 8. Matrix of study endpoints (minimally invasive treatments)
| Primary endpoint(s)a | Secondary endpoint(s)b | Otherc endpoint(s) | Time of endpoint measurement | |
| PEI Bennedbaek 1998 | ‐ | ‐ |
|
|
| PEI Bennedbaek 1999 | ‐ | ‐ |
|
|
| PEI Bennedbaek 2003 | ‐ | ‐ |
|
|
| PEI Chu 2003 | ‐ | ‐ |
|
|
| PEI Sung 2013 | Volume reduction ratio (%) |
|
‐ |
|
| TETRA Hegedüs 1998 | ‐ | ‐ |
|
|
| PEI Valcavi 2004 | ‐ | ‐ |
|
|
| PEI Verde 1994 | ‐ | ‐ |
|
|
| LPDossing 2005 | ‐ | ‐ |
|
|
| LPDossing 2006 | Nodule volume reduction | ‐ |
|
|
| LP Dossing 2013 | ‐ | ‐ |
|
|
| LPGambelunghe 2006 | ‐ | ‐ |
|
|
| LPPapini 2007 | ‐ | ‐ |
|
|
| RF Faggiano 2012 | ‐ | ‐ |
|
|
| RFHuh 2012 | ‐ | ‐ |
|
|
| "‐" denotes not reported a,bAs stated in the publication cNot stated as primary or secondary endpoint(s) in the publication dMentioned, but not evaluated in the trial eAssociated with tolerability fFNAB + cytology = suspicious and positive biopsies were similar between groups FNAB: fine‐needle aspiration biopsy; FT3: free tri‐iodothyronine; FT4: free thyroxine; LP: laser photocoagulation; mo: month(s); PEI: percutaneous ethanol injection; PLA: percutaneous laser ablation; RF: radiofrequency; SYS score: the sum of the single scores including pressure symptoms in the neck, difficulty in swallowing, aesthetic complaints; symptoms were scored separately with 0 (absent), 1 (moderate) and 2 (severe); T3: plasma (serum) tri‐iodothyronine; T4: plasma (serum) thyroxine; TETRA: tetracycline hydrochloride; T TN: thyroid nodule; TPOAb: antiperoxidase autoantibody; TSH: thyrotropin; US: ultrasonography; VAS: visual analogue scale; wks: weeks | ||||
Appendix 9. Definition of endpoint measurement (levothyroxine treatment)
| Compliance | Responder | Partial responder/non‐responder | New nodules | |
| LT4 Bayani 2012 | ‐ | Complete response (> 50% reduction in longitudinal and transverse dimensions of nodules) | Partial response (20% to 50% reduction in the longitudinal and transverse dimensions of nodules) | ‐ |
| LT4 Boguszewsky 1998 | TSH suppressiona + response to TRH injection |
‐ | ‐ | ‐ |
| LT4 Cesareo 2010 | ‐ | ‐ | ‐ | Number of nodules > 0.5 mL at follow up |
| LT4 Gharib 1987 | TSH suppressionb + TSH response to TRH injection |
‐ | ‐ | ‐ |
| LT4 Grussendorf 2011 | At least 80% of dose taken |
‐ | ‐ | Change in number of nodules |
| LT4 Larijani 2005 | ‐ | TN volume reduction ≥ 50% | Partial responder: TN volume reduction < 50% Non‐responder: TN volume increase or constant |
‐ |
| LT4 La Rosa 1995 | TSH suppression + self‐report |
‐ | ‐ | ‐ |
| LT4 Papini 1993 | TSH suppression | ‐ | ‐ | ‐ |
| LT4 Papini 1998 | ‐ | ‐ | ‐ | Lesions with diameter > 10 mm at follow up |
| LT4 Tsai | Pill count | TN volume reduction > 50% | Non‐responder: TN volume reduction ≤ 50% | ‐ |
| LT4 Wemeau 2002 | Pill count + self report | TN volume reduction ≥ 50% | Partial responder: TN volume reduction ≥ 20% to 50% Non‐responder: TN volume reduction < 20% |
Non palpable TN, additionally detected by US |
| LT4 Zelmanovitz 1998 | TSH suppression | ‐ | ‐ | ‐ |
| "‐" not reported a,bTSH suppression: from < 0.2 mU/L to 0.5 mU/L LT4: levothyroxine; TN: thyroid nodule; TRH: thyrotropin‐releasing hormone; TSH: thyrotropin; US: ultrasound | ||||
Appendix 10. Definition of endpoint measurement (minimally invasive treatments)
| Cure | Sucess rate/therapy success | Recurrence | Cosmetic/pressure complaint | Tolerability/compliance | |
| PEI Bennedbaek 1998 | ‐ | TN disappearance or 50% reduction in size | ‐ | Questionnaire (items none to severe) | ‐ |
| PEI Bennedbaek 1999 | ‐ | ‐ | ‐ | VAS (0 to 10 cm) | Degree of pain |
| PEI Bennedbaek 2003 | Cyst volume ≤ 1 mL | ‐ | Cyst volume > 1 mL | Interview (yes, no) | ‐ |
| PEI Chu 2003 | TC disappearance or volume reduction < 0.5 mL (maximum 5 sessions) | ‐ | Cyst volume > 1 mL | ‐ | ‐ |
| PEI Sung 2013 | ‐ | Percentage of participants who showed a volume reduction > 50% | ‐ | VAS (0 to 10 cm) for symptoms by participants Cosmetic score by physician: 1 = no palpable mass; 2 = no cosmetic problem but a palpable mass; 3 = cosmetic problem on swallowing only; 4 = readily detected cosmetic problem at all times |
Degree of procedure‐related pain: Ethanol ablation:
RF:
|
| TETRA Hegedüs 1988 | Absence of any residual nodule and TC < 1 mL (US) 12 months after last treatment | ‐ | ND | ‐ | ‐ |
| PEI Valcavi 2004 | Elimination of discomfort + cosmetic complaints | ‐ | ‐ | Questionnaire | Local burning sensation |
| PEI Verde 1994 | ‐ | ‐ | ND | ‐ | ‐ |
| LPDossing 2005 | ‐ | ‐ | ‐ | VAS (0 to 10 cm) | Degree of pain (VAS: 0 to 10 cm) |
| LPDossing 2006 | ‐ | ‐ | ‐ | VAS (0 to 10 cm) | Degree of pain (VAS: 0 to 10 cm) |
| LP Dossing 2013 | Cyst volume ≤ 1 mL (determined by US or aspiration) | Cyst volume ≤ 1 mL (determined by US or aspiration) | Cystic part of nodule > 1 mL (US or aspiration) | VAS (0 to 10 cm) | ‐ |
| LP Gambelunghe 2006 | ‐ | ‐ | ‐ | VAS (0 to 6 cm) | Asked for another ablation |
| LPPapini 2007 | ‐ | ‐ | ‐ | Questionnaire | Asked for a second ablation |
| RF Faggiano 2012 | ‐ | ‐ | ‐ | SYS scorea (range 0 to 6) | Mild sensation of heat in the neck |
| RFHuh 2012 | ‐ | TN volume reduction > 50% | ‐ | VAS (0 to 10 cm) | Acceptance of another therapy session |
| "‐" denotes not reported aSYS score: the sum of the single scores including pressure symptoms in the neck, difficulty in swallowing, aesthetic complaints; symptoms were scored separately with 0 (absent), 1 (moderate) and 2 (severe) LP: laser photocoagulation; ND: not defined; PEI: percutaneous ethanol injection; RF: radiofrequency ablation; TC: thyroid cyst; TETRA: tetracycline hydrochloride; TN: thyroid nodule; US: ultrasound; VAS: visual analogue scale | |||||
Appendix 11. Study(arms), comparisons and participants contributing data to comparisons
|
Interventions Comparators |
LP [studies (participants)] | LT4 [studies (participants)] | PEI [studies (participants)] | RF [studies (participants)] | TETRA [studies (participants)] |
| Aspiration | 1 (44) | ‐ | 3 (320) | ‐ | ‐ |
| LP | 1 (30) | 1 (42) | ‐ | ‐ | ‐ |
| NaCl | ‐ | ‐ | 1 (66) | ‐ | 1 (53) |
| No treatment | 3 (91) | 6 (365) | ‐ | 1 (40) | ‐ |
| PEI | ‐ | 1 (50) | 1 (160) | 1 (50) | ‐ |
| PHI | ‐ | ‐ | 1 (18) | ‐ | ‐ |
| Placebo | 9 (806) | ‐ | ‐ | ‐ | |
| RF | ‐ | ‐ | ‐ | 1 (30) | ‐ |
| "‐" denotes no comparison available LP: laser photocoagulation; LT4: levothyroxine; NaCl: isotonic saline; PEI: percutaneous ethanol injection; PHI: percutaneous hydrochloric acid injection; RF: radiofrequency ablation; TETRA: percutaneous tetracycline injection | |||||
Appendix 12. Iodine intake status and median urinary iodine concentration
| Median urinary iodine concentration [µg/L] | Corresponding approximate iodine intake [µg/day] | Iodine nutrition status |
| < 20 | < 30 | Severe deficiency |
| 20 to 49 | 30 to 74 | Moderate deficiency |
| 50 to 99 | 75 to 149 | Mild deficiency |
| 100 to 199 | 150 to 299 | Optimal |
| 200 to 299 | 300 to 449 | More than adequate |
| > 299 | > 449 | Possible excess |
| Reference values as used by WHO/ICCIDD/UNICEF to relate iodine nutrition levels to urinary iodine concentration ICCIDD: International Council for the Control of Iodine Deficiency Disorders; UNICEF: United Nations International Children’s Emergency Fund; WHO: World Health Organization | ||
Appendix 13. Methods of measurement of local symptoms/cosmetic complaints following minimally invasive thyroid nodule therapy
| Symptom score | Cosmetic score | |
| PEI Bennedbaek 1998 | Absent Mild Moderate Severe |
Absent Mild Moderate Severe |
| PEI Bennedbaek 1999 | 10 cm VAS (0 to 10 cm) | 10 cm VAS (0 to 10) |
| PEI Bennedbaek 2003 | Absent Present |
Absent Present |
| PEI Sung 2013 | 10 cm VAS (0 to 10 cm) | 1 = no palpable mass 2 = no cosmetic problem but a palpable mass 3 = cosmetic problem on swallowing only 4 = readily detected cosmetic problem at all times |
| PEI Valcavi 2004 | Disappearance of compressive or cosmetic symptoms | Disappearance of compressive or cosmetic symptoms |
| LP Dossing 2005 | 10 cm VAS (0 to 10 cm) | 10 cm VAS (0 to 10 cm) |
| LP Dossing 2006 | 10 cm VAS (0 to ‐10 cm) | 10 cm VAS (0 to 10 cm) |
| LP Dossing 2013 | 10 cm VAS (0 to 10 cm) | 10 cm VAS (0 to 10 cm) |
| LP Gambelunghe 2006 | VAS (1 to 6 cm) | VAS (1 to 6 cm) |
| LP Papini 2007 | 0 = absent 1 = presence of local symptoms (cervical constriction, dyspnoea, dysphagia) |
0 = absent 1 = cosmetic complaints |
| RF Faggiano 2012 | Pressure symptoms in the neck; difficulty in swallowing 0 = absent 1 = moderate 2 = severe SYS scorea : 0 to 6 |
‐ |
| RF Huh 2012 | 10 cm VAS (0 to 10 cm) | 1 = no palpable mass 2 = palpable mass + no cosmetic problem 3 = cosmetic problem only on swallowing 4 = light observable cosmetic problem |
|
aSYS score: the sum of the single scores including pressure symptoms in the neck, difficulty in swallowing, aesthetic complaints; symptoms were scored separately with 0 (absent), 1 (moderate) and 2 (severe) LP: laser photocoagulation; PEI: percutaneous ethanol injection; RF: radiofrequency ablation; VAS: visual analogue scale | ||
Appendix 14. Adverse events (levothyroxine treatment)
| Intervention(s) and comparator(s) | AEs [N (%)] | SAEs [N (%)] | Discontinued study due to an AE [N (%)] | Nodule regrowth > 50% [N (%)] | Surgery [N (%)] | Thyroid cancer [N (%)] | Deaths [N] | |
| LT4 Bayani 2012 | I: LT4 C: no treatment |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| LT4 Boguszewski 1998 | I: LT4 C: PLAC |
‐ | I: 0 C: ‐ |
I: 0 C: ‐ |
‐ | ‐ | ‐ | I: 0 C: ‐ |
| LT4 Cesareo 2010 | I: LT4 C: no treatment |
Ia: 3/71 (4) C: ‐ |
‐ | I: 3/71 (4) C: 0 |
‐ | I: 3/71 (4) C: 5/71 (7) |
‐ | I: 0 C: 0 |
| LT4 Gharib 1987 | I: LT4 C: PLAC |
‐ | ‐ | I: 0 C: 0 |
‐ | ‐ | I: 0 C: 0 |
|
| LT4 Grussendorf 2011b | I1: LT4 + PI C1: LT4 C2: PI C3: PLAC |
‐ | C3: 2/199 (1) | 38/794 (5) Serious adverse events (safety population) |
‐ | ‐ | ‐ | ‐ |
| LT4 Koc 2002 | I1: LT4: 3 μg/kg/day I2: LT4: 1.5 μg/kg/day C1: PLAC high‐dose C2: PLAC low‐dose |
I1a: 2/11 (18) I2: 0 C1a: 2/9 (22) C2: 0 |
‐ | ‐ | ‐ | ‐ | ‐ | I1: 0 I2: 0 C1: 0 C2: 0 |
| LT4 La Rosa 1995 | I1: LT4 I2: PI C: no treatment |
I1: 0 I2: 0 C: 0 |
I1: 0 I2: 0 C: 0 |
I1: 0 I2: 0 C: 0 |
I1: 0 I2: 0 C: 3 (14) |
I1: 1/27 (4) I2: 0 C: 0 |
I1c: 0 I2c: 0 Cc: 0 |
‐ |
| LT4 Larijani 2005 | I: LT4 C: PLAC |
‐ | ‐ | I: 0 C: 0 |
‐ | ‐ | I: 0 C: 0 |
I: 0 C: 0 |
| LT4 Ozkaya 2010 | I: LT4 C: no treatment |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| LT4 Papini 1993 | I: LT4 C: PLAC |
Ia: 10/51 (20) Ca: 3/50 (6) |
I: 0 C: 0 |
I: 0 C: 0 |
I: 7/51 (14) C: 11/50 (22) |
‐ | ‐ | ‐ |
| LT4 Papini 1998 | I: LT4 C: no treatment |
Ia: 7/51 (14) C: ‐ |
‐ | I: 3/51 (6) C: ‐ |
‐ | I: 1/51 (2) C: 2/49 (4) |
I: 0 C: 0 |
‐ |
| LT4 Reverter 1992 | I: LT4 C: no treatment |
‐ | ‐ | I: ‐ C: 0 |
‐ | I: 3/20 (15) C: 0 |
‐ | I: ‐ C: 0 |
| LT4 Tsai 2006 | I: LT4 C: PLAC |
‐ | ‐ | I: 0 C: 0 |
‐ | ‐ | ‐ | I: 0 C: 0 |
| LT4 Wemeau 2002 | I: LT4 C: PLAC |
Ie: 2/64 (3) Ce: 9/59 (15) |
‐ | Ie: 2/64 (3) Ce: 9/59 (15) |
‐ | If: 1/64 (2) Cf: 5/59 (8) |
I: ‐ Cd: 1/59 (2) |
‐ |
| LT4 Zelmanovitz 1998 | I: LT4 C: PLAC |
‐ | ‐ | I: 0 C: 0 |
I: 2/21 (10) C: 4/24 (17) |
‐ | ‐ | I: 0 C: 0 |
| "‐" denotes not reported aAEs: signs of hyperthyroidism (palpitations, sweating, tremor, nervousness, persistent tachycardia) bAEs: AEs and SAEs were defined and investigated during and at the end of the study, but the results were not presented cSecond FNAB confirmed benignity for nodules which were not reduced in size and for those which were surgically removed dPapillary carcinoma eAEs: I: iatrogenic thyrotoxicosis, nodule growth; C: suspect FNAB at 12 months requiring hemithyroidectomy, nodule pain, benign follicular adenoma, myeloproliferative syndrome, hyperthyroidism and Grave's disease fI: thyroidectomy; C: hemithyroidectomy AE: adverse events; C: comparator; FNAB: fine‐needle aspiration biopsy; I: intervention; LT4: levothyroxine; PI: potassium iodine; PLAC: placebo; SAE: serious adverse events | ||||||||
Appendix 15. Effects of levothyroxine treatment on bone mineral density
| LT4 Zelmanovitz 1998 | Location of measurements | Intervention (I) and comparator (C) | Baseline [g/cm3 (SD)] | 12 months [g/cm3 (SD)] |
| Spine L2 to L4 | I: LT4 | 1.133 (0.208)a | 1.116 (0.213)a | |
| C: PLAC | 1.093 (0.188)b | 1.066 (0.164)b | ||
| Femoral neck | I: LT4 | 0.910 (0.205)a | 0.950 (0.163)a | |
| C: PLAC | 0.881 (0.110)b | 0.869 (0.117)b | ||
| Femoral ward | I: LT4 | 0.793 (0.227)a | 0.848 (0.178)a | |
| C: PLAC | 0.750 (0.142)b | 0.744 (0.140)b | ||
| Femoral trochanter | I: LT4 | 0.765 (0.158)a | 0.793 (0.127)a | |
| C: PLAC | 0.714 (0.084)b | 0.708 (0.094)b | ||
|
aMean BMD of 16 women bMean BMD of 19 women BMD: bone mineral density; LT4: levothyroxine therapy; PLAC: placebo; SD: standard deviation | ||||
Appendix 16. Adverse events (minimally invasive treatments)
|
Intervention(s) and comparator(s) |
AEs [N (%)] | SAEs [N (%)] | Discontinued study due to an AE [N (%)] | Worsening / no improvement (VAS or other instrument) [N (%)] | Nodule regrowth > 50% [N (%)] | Surgery [N (%)] | Carcinoma development [N (%)] | Deaths [N] | |
| PEI Bennedbaek 1998 | I: PEI C: LT4 | Ia: 24/25 (96) Cb: 5/25 (20) |
Ia: 0 C: 0 |
I: 0 C: 0 |
I: 6/25 (24) C: 12/25 (48) |
I: 1/25 (4) C: ‐ |
I: 2/25 (8) C: ‐ |
I: 0 C: ‐ |
‐ |
| PEI Bennedbaek 1999 | I: PEI (1 dose) C: PEI (3 doses) |
Ic: 30/30 (100) Cc: 30/30 (100) |
Id: 14/30 (47) Cd: 8/30 (28) Cd: 28/30 (92) |
I: 0 C: 0 |
I: 3/30 (10) C: 3/30 (10) |
‐ | I: 1/30 (3) C: ‐ |
I: 0 C: ‐ |
‐ |
| PEI Bennedbaek 2003 | I: PEI C: NaCl |
Ic: 7/33 (21) Cc: 1/33 (3) |
I: 0 C: 0 |
I: 0 C: 0 |
Ie: 6/33 (18) Ce: 17/33 (52) |
‐ | I: 6/33 (18) C: 10/33 (30) |
‐ | I: 0 C: 0 |
| PEI Chu 2003 | I1: PEI C1: PHI C2: ASP |
I1c: 4/10 (40) C1c: 3/8 (38) C2: 0 |
I1: 0 C1: 0 C2: 0 |
I1: 0 C1: 0 C2: 0 |
‐ | ‐ | ‐ | ‐ | I1: 0 C1: 0 C2: 0 |
| PEI Sung 2013 | I: PEI C: RF |
I: 0/23 (0) C: 21/21 (100) |
‐ | ‐ | I: 1/25 (4) C: 2/25 (8) |
‐ | ‐ | ‐ | ‐ |
| TETRA Hegedüs 1998 | I: TETRA C: NaCl |
If: 2/23 (9) C: 0/30 (0) |
I: 0 C: 0 |
I: 0 C: 0 |
Ig,h: 7/23 (30) Cg,h: 14/30 (47) |
‐ | I: 3/23 (13) C: ‐ |
I: 0 C: ‐ |
I: 0 C: 0 |
| PEI Valcavi 2004 | I: PEI C: ASP |
Ic,i: (33) C: ‐ |
Ij: 1/143 (< 1) C: ‐ |
‐ | I: 34/135 (25) C: 99/131 (76) |
‐ | ‐ | Ik Ck |
‐ |
| PEI Verde 1994 | I: PEI C: ASP |
Ic: 3/10 (30) Cc: 2/10 (20) |
I: 0 C: 0 |
I: 0 C: 0 |
‐ | ‐ | ‐ | ‐ | I: 0 C: 0 |
| LPDossing 2005 | I: LP C: no treatment |
Ic: 10/15 (67) C: ‐ |
I: 0 C: 0 |
I: 0 C: 0 |
I: 3/15 (20) C: 15/15 (100) |
I: ‐ C: 0 |
I: 1/15 (7) C: ‐ |
I: 0 C: ‐ |
I: 0 C: 0 |
| LPDossing 2006 | I: LP (1 session) C: LP (3 sessions) |
Ic,l: 10/15 (67) Cc,l: 6/15 (40), 5/13(38), 3/11(27) |
I: 0 C: 0 |
I: C: 0 |
I: 8/15 (53) (VAS) C: 7/13 (54) (VAS) |
‐ | ‐ | ‐ | I: 0 C: 0 |
| LP Dossing 2013 | I: ASP + LP C: ASP |
Ic: 11/22 (50)l(2) C: ‐ |
I: 0 C: 0 |
I: 0 C: 0 |
I: 7/22 (32) C: 18/22 (82) |
‐ | I: 4/22 (18) C: 5/22 (23) |
I: 0 C: 0 |
I: 0 C: 0 |
| LPGambelunghe 2006 | I: LP C: no treatment |
Im: 8/13 (62) C: ‐ |
I: 0 C: 0 |
I: 0 C: 0 |
I: 0/13 (0) C: 13/13 (100) |
I: 0 Cn |
‐ | ‐ | I: 0 C: 0 |
| LPPapini 2007o | I1: LP C1: LT4 C2: no treatment |
I1p: 20/21 (95) C1q: 8/21 (38) C2: 0 |
I1p: 1/21 (5) C1: 0 C2: 0 |
I1: 0 C1: 0 C2: 1/20 (5) |
I1: 0/21 (0) C1: 1/21 (5) C2: 9/20 (45) |
I1: ‐ C1: ‐ C2: 1/20 (5) |
I1: 0 C1: 0 C2r: 1/20 (5) |
‐ | ‐ |
| RF Faggiano 2012 | I: RF C: no treatment |
Is: 20 (100) C: ‐ |
I: 0 C: 0 |
I: 0 C: 0 |
I: ‐ Ct: 20 ( 100) |
I: 0 C: ‐ |
I: C: |
‐ | I: 0 C: 0 |
| RFHuh 2012 | I: RF‐1 (1 session) C: RF‐2 (2 sessions) |
Iu: 15 (100) Cu: 15 (100) |
I: 0 C: 0 |
I: 0 C: 0 |
I: 0 C: 0 |
I: 0 C: 0 |
I: 0 C: 0 |
‐ | I: 0 C: 0 |
| "‐" denotes not reported Numbers in italic were calculated by review authors aAEs: slight‐to‐moderate pain lasting ± tenderness; SAE: fever, haematomas, dysphonia, dysthyroid symptoms bAEs: symptoms of hyperthyroidism (diarrhoea, palpitations, sweating, tremor) cAEs: slight‐to‐moderate pain ± tenderness d For participants who received larger ethanol doses: I: dysphonia (47%); C: persistent nerve paralysis (28%) and paranodular fibrosis (92%) eFailure: thyroid cyst volume after intervention > 1 mL fExtreme pain with injection of TETRA gRecurrence: cyst volume ≥ 1 mL, 12 months after last puncture for a median of three treatments hNon‐recurrent group, development of cold, solid nodules: I: n = 6/16 (38%); C: n = 2/16 (13%); I: n = 3/16 (19%) were operated on and histological examination revealed pronounced inflammatory changes iBurning sensation jTransient laryngeal dysfunction kFrequency of suspicious/positive biopsy findings (similar between groups) lParticipants receiving analgesics, duration of AEs: I:) mean 3 ± 3 days; C: 4 ± 4 days, 4 ± 4 days, 2 ± 4 days l(2)Slight‐to‐moderate pain with a median (interquartile range) duration of 2 (0; 5) days, necessitating mild analgesics in 8 participants mMild pain during the procedure + fever (12 hours): n = 5 + 3/13 (62%); transient hyperthyroidism until 6 weeks: n = 8/13 (62%) nNon‐significant increase oLong‐term AEs for LT4 therapy were not assessed (cardiac hypertrophy, atrial arrhythmia and increase in osteoporosis risk) pAEs: mild cervical pain (20/21), persistent cervical pain (3/21) for 48 hours; SAEs: extremely painful (1/21) qPersistent tachycardia and nervousness rBenign follicular adenoma sMild sensation of heat in the neck tC: SYS score (sum of the individual scores) worsened in group B from 3.0 (± 1.3) at baseline to 4.1 (± 0.9) uPain/discomfort during ablation AE: adverse events; ASP: aspiration; C: comparator; I: intervention; NaCl: isotonic saline; LP: laser photocoagulation; LT4: levothyroxine; PEI: percutaneous ethanol injection; PHI: percutaneous hydrochloric acid injection; RF: radiofrequency ablation; SAE: serious/severe adverse events; TETRA: tetracycline hydrochloride; VAS: visual analogue scale | |||||||||
Data and analyses
Comparison 1. Levothyroxine versus control (no treatment, placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nodule volume reduction ≥ 50% | 10 | 958 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.04, 2.38] |
| 2 Adverse events: participants without signs of hyperthyroidism | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Adverse events: participants without a nodule volume increase > 50% | 3 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.99, 1.22] |
| 4 Thyrotropin (TSH) (end of study values) | 8 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Total thyroxine (T4) (end of study values) | 5 | 296 | Mean Difference (IV, Random, 95% CI) | 48.28 [35.12, 61.43] |
Comparison 2. Percutaneous ethanol instillation versus control (cyst aspiration, isotonic saline, levothyroxine, radiofrequency ablation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement of pressure symptoms (end of study) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Nodule volume reduction ≥ 50% | 5 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [0.56, 11.90] |
| 2.1 PEI vs cyst aspiration | 3 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.32, 2.54] |
| 2.2 PEI vs LT4 | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 39.00 [2.48, 612.50] |
| 2.3 PEI vs RF | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.91, 1.09] |
| 3 Adverse events: slight to moderate pain | 3 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.62, 5.12] |
Comparison 3. Laser photocoagulation versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement/disappearance of pressure symptoms (end of study) | 3 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 26.65 [5.47, 129.72] |
| 2 Adverse events: light to moderate cervical pain (≥ 48 hours) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 4. Laser photocoagulation comparing various LP sessions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nodule volume reduction (baseline to end of follow‐up) | Other data | No numeric data |
Comparison 5. Radiofrequency versus no treatment or comparing various RF sessions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nodule volume reduction (baseline to end of follow‐up) | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
LP Dossing 2005.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: palpable thyroid nodule; solitary cold (scintigraphically); solid benign (US‐guided FNAB) thyroid nodule; cytology (colloid nodule); no suspicion of or a family history of thyroid cancer; no prior neck radiation Exclusion criteria: see inclusion criteria Diagnostic criteria: blood tests: TSH, serum T3, T4, FT3, FT4, TPOAb; thyroid scan: cold; US‐FNAB: benignity; US (solid); cytology (colloid) |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark/Odense Setting: outpatients referred from primary care physicians Treatment before study: partial thyroidectomy for benign nodular goitre (n = 4/30) |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume decrease/increase; thyroid volume changes; pressure and cosmetic complaints evaluated on a 10‐cm VAS; correlation between energy deposition and nodule volume reduction; thyroid function (routine assays) | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal Commercial funding from Novo Nordisk Foundation and non‐commercial funding from the Agnes and Knut Mørk Foundation, the AP Møller Relief Foundation and the AJ Andersen and Wife Foundation |
|
| Stated aim of study | Quote from publication: "To evaluate the efficacy of ultrasound (US)‐guided interstitial laser photocoagulation (ILP) on thyroid function, nodule size and patient satisfaction in benign solitary solid cold thyroid nodules by comparing one ILP session with no treatment in a prospective randomised study" | |
| Notes | LP: "Under sterile conditions and guided by US, the laser fibre (0.4 mm in diameter) was positioned in the thyroid nodule through the lumen of an 18 gauge (1.2 mm) needle and preceded by local anaesthesia with lidocaine (10 mg/ml). The needle was withdrawn 20 mm leaving the end of the fibre in direct contact with the tissue"; degree of pain/discomfort rated on a VAS as a surrogate marker for tolerability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Random allocation was achieved using a random number generator on a computer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "The measurements ... same investigator ... blinded ..." |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants in the laser photocoagulation group completed the study; probably also in the no‐treatment group |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias |
LP Dossing 2006.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: euthyroid participants, normal serum calcitonin, palpable thyroid nodule causing pressure symptoms; solitary cold (scintigraphically), solid benign (US‐guided FNAB) thyroid nodule; cytology (colloid nodule); no suspicion of or a family history of thyroid cancer; no prior neck radiation Exclusion criteria: see inclusion criteria Diagnostic criteria: blood tests: TSH, serum T3, T4, FT3, FT4, anti‐TPOAb; thyroid scan: cold; US‐FNAB: benignity; US (solid); cytology (colloid) |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark/Odense Setting: outpatients referred from primary care physicians Treatment before study: partial thyroidectomy for benign nodular goitre (n = 1/30); 131I years ago for autonomous nodule in the contralateral lobe (n = 1/30) |
|
| Outcomes | Outcomes reported in abstract of publication: thyroid nodule volume decrease (US); pressure symptoms and cosmetic complaints (VAS); correlation between energy deposition and nodule volume reduction (dose‐response relationship); participant satisfaction; side effects | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal Commercial funding from the Novo Nordisk Foundation and non‐commercial funding from the Agnes and Knut Mørk Foundation, the AP Møller Relief Foundation and the AJ Andersen and Wife Foundation |
|
| Stated aim of study | Quote from publication: "To evaluate the efficacy and dose‐response relationship, as well as the safety of US‐guided ILP, on the volume of benign solitary solid cold thyroid nodules. Additionally, we evaluated nodule related symptoms in this prospective randomized study comparing one ILP treatment with three treatment sessions" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Random allocation was achieved using a random number generator on a computer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "The measurements ... performed ... same investigator.. blinded as to previous results" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "The treatment was well‐tolerated as evidenced by the fact that none of the patients in either group requested termination of the procedure" Comment: all participants randomised to LP‐1 and for LP‐3 completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias |
LP Dossing 2013.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: euthyroid participants, normal serum calcitonin, palpable thyroid nodule causing pressure symptoms; neither suspicion of thyroid cancer nor a family history of it (clinical); benign cold solitary solid‐cystic nodule (US‐FNAB, scintigraphy) Exclusion criteria: not stated Diagnostic criteria: blood tests: serum TSH, T3, T4; thyroid scan: cold; US‐FNAB: benignity; US (solid); cytology (colloid) |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark/Odense Setting: outpatients referred from primary care physicians Treatment before study: cyst aspiration |
|
| Outcomes | Outcomes reported in abstract of publication: cyst volume decrease ≤ 1 mL ("successful outcome"); reduction of solid (cystic) part of the nodule; decrease of pressure symptoms (VAS); thyroid function; side effects | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal Commercial funding from the Novo Nordisk Foundation |
|
| Stated aim of study | Quote from publication: "The aim of this study was to follow up on our pilot study (11) and evaluate the remission rate in patients with a recurrent benign predominantly cystic thyroid nodule randomized to aspiration, with or without subsequent ILP" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The patients ... and randomized without stratification for nodule size" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "US measurements were performed by the same investigator (H.D.) with blinding toward the previous measurements" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants randomised for cyst aspiration only and for cyst aspiration and laser completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias |
LP Gambelunghe 2006.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: participants with compressive symptoms due to nodular goitre or single benign (FNA) nodules Exclusion criteria: not reported Diagnostic criteria: FNA (nodules > 1 mL): benignity; thyroid scan: cold or mild hyperfunctioning nodules (subclinical hyperthyroidism) |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume change; compressive symptoms/cosmetic complaints; tolerability; correlation between energy deposition and nodule volume decrease; thyroid function | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: " ... to test the efficacy and safety of percutaneous ultrasound (US)‐guided laser photocoagulation (PLP) for treatment of subjects with compressive symptoms due to benign thyroid nodules and/or at high surgical risk" | |
| Notes | Only the dominant nodule was treated in case of multinodular goitres | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "... randomly assigned to one session PLP ... or observation ..." Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "The volume of nodules ... measured ... same investigator, blinded for treatment ..." |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "All patients tolerated the treatment well ... answered ... could repeat it" Comment: all randomised participants were evaluated at study end |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LP Papini 2007.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: a) presence of a single or dominant palpable nodule, either solid or with a small fluid component (< 20% at US examination), with volume > 5 mL, and with at least one diameter > 30 mm; b) hypoactive appearance at 99mTc thyroid scintiscan; c) benign cytology at two consecutive US‐guided FNAB; d) free thyroid hormones, TSH, and antithyroid antibodies within normal range; e) age between 18 and 60 years; f) refusal of or ineligibility for surgery; g) untreated thyroid disease Exclusion criteria: autoimmune thyroid disease; previous thyroid surgery, radioiodine or LT4 treatment Diagnostic criteria: US (solid or small fluid component < 20%); volume > 5 mL, and with at least one diameter > 30 mm); 99mTc thyroid scintiscan (hypoactive appearance); 2 consecutive US‐guided FNAB (benignity); blood tests: serum TSH, FT3, FT4, Tg, thyroid antibodies |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatients Treatment before study: none |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume changes (decrease/growth); nodule volume reduction > 50%; local symptoms improvement; complications | |
| Study details |
Run‐in period: LT4 treatment at day 35, scheduled dose based on TSH levels Study terminated before regular end: no |
|
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: " ... to compare the 12‐month changes in nodule volume and local symptoms induced by a single PLA session with those induced by long‐term LT4 suppressive therapy in a series of benign large cold thyroid nodules and then to compare the findings in the two groups with the natural history of a series of thyroid nodules followed by means of clinical surveillance with no active treatment" | |
| Notes | "All the patients lived in greater Rome metropolitan area, a borderline iodine‐deficient area (median daily urinary excretion: 92 μg)" Immediately before PLA: betamethasone intramuscularly, if persistent pain: ketoprofen for 2 days (n = 3/21 (14%)); assessment of local symptoms after 12 months treatment or follow up: not validated questionnaires; total thyroid volume was not systematically assessed Cost of PLA (equipment + medical team + kits): approx. €450 (US$550) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "A computer‐based number generator was used to randomly assign each patient to one of the three groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the unblinded design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Quote from publication: "This fact, together with the unblinded design of the trial, makes it impossible to role out ... placebo effect skewing the analysis of the changes in subjective symptoms in the treated ... patients" Comment: the unblinded design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "One drop‐out (5%) was registered in the follow‐up group. This patient underwent surgical treatment because of the progressive growth of his nodule and the associated worsening of local symptoms" (Group 3 (C2)); "Eight out of 21 (38.1%) patients complained of persistent tachycardia or nervousness, but no one withdrew from the study" (Group 2 (C1)) Comment: reasons for dropouts explained |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Bayani 2012.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: participants with single palpable thyroid nodule with confirmed tumour benignity based on FNAB; to ensure existence of single nodule, sonography was performed Exclusion criteria: thyroid neoplastic lesions in participants or their family, history of neck radiation, hot nodules Diagnostic criteria: palpation; ultrasonography; FNAB; total T4 and TSH serum tests showed euthyroid status |
|
| Interventions |
Number of study centres: 1 Country/location: Iran/Babol Treatment before study: none |
|
| Outcomes | Outcomes reported in abstract of publication: serum levels of TSH; longitudinal and transverse dimensions of thyroid nodules before and after treatment | |
| Study details |
Study terminated before regular end: no Registered trial: IRCT 201103185692 N3 (WHO Trial Registration Data Set) Date of registration: 2011‐05‐26 Primary sponsor: Bobol University of Medical Sciences and Health Services Date of first enrolment: 2010‐04‐28 Target sample size: 40 Study design: parallel RCT; single blind Inclusion criteria: age lower than 60 years old; single thyroid nodule with fine needle aspiration; TSH in normal limits (0.5 to 4.5 mU/L) Exclusion criteria: age more than 60 years; history of hypo‐ or hyperthyroidism; neck radiation; history of any cancer; history of thyroid cancer in family; living in another region where the study is performed; history of levothyroxine or other thyroid related drugs; TSH lower than normal; pregnancy; other disease (cardiovascular or hepatic) Intervention 1: LT4 at an initial dose of 50 µg/day, levothyroxine dose was adapted according to TSH serum levels after 6 weeks of suppressive treatment in order to maintain TSH levels at less than 0.5 mU/L Intervention 2: comparator group; no intervention Primary outcome(s): size of benign thyroid nodule; time point: before and 6 months after intervention; method of measurement: sonography Secondary outcome(s): TSH before, 6 weeks and 6 months after the intervention |
|
| Publication details |
English language publication in a peer‐reviewed journal Non‐commercial funding by the Vice Chancellery For Research of Babol University of Medical Sciences |
|
| Stated aim of study | Quote: "... to investigate the effect of suppressive treatment with levothyroxine on the size of thyroid nodules" | |
| Notes | None of the participants was under suppressive treatment with LT4 or other thyroid‐associated drugs prior to the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The patients were randomly divided into two groups ..." Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: no detailed information; study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: no detailed information; study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Boguszewski 1998.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: non‐functional ("cold") or isofunctional ("warm" and LT4‐suppressible) thyroid nodule on thyroid scanning; cytology negative for malignancy (FNAB); solid or predominantly solid nodule (> 50% of the area) on US; single (palpation) in clinically euthyroid participants Exclusion criteria: pregnancy; any contraindication for thyroid suppressive therapy; autonomously functioning nodules Diagnostic criteria: functional diagnosis (thyroid scanning); solid or predominantly solid nodule (> 50% of the area) (US); malignancy or benignity (FNAB and cytology) |
|
| Interventions |
Number of study centres: 1 Country/location: Brazil Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume reduction (US); nodule size and number of nodules reduction (palpation); T3, T4, Tg, TgAb, TPOAb measurements; correlation between changes in nodule size and clinical, laboratory markers and scintigraphic characteristics | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal Commercial funding by Sanofi‐Winthrop + Laboratorios Aché (LT4 + placebo tablets) |
|
| Stated aim of study | Quote from publication: " ... to evaluate the effect of TSH‐suppressive therapy with levothyroxine (LT4) on the volume of clinically solitary thyroid nodules, assessing possible correlations between response to therapy and clinical and laboratory parameters" | |
| Notes | TRH test: 200 μg i.v. after 30 to 60 minutes, if TSH < 2 mU/L TSH response was considered as suppressed LT4 and PLAC tablets appeared identical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "A prospective, randomized and placebo controlled ... hypothesis" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Both allocation to treatment (LT4 or placebo) and US measurements were double‐blind with respect to patients and physicians"; "Both placebo and LT4 tablets were externally identical" |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "Both allocation to treatment (LT4 or placebo) and US measurements were double‐blind with respect to patients and physicians"; "Both placebo and LT4 tablets were externally identical" |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "All US examinations were done by the same radiologist"; "Both allocation to treatment (LT4 or placebo) and US measurements were double‐blind with respect to patients and physicians" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "All patients completed the study"; "None of the patients in the LT4 group had side effects requiring withdrawal ... or modification of the initial dose" |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Quote from publication: "LT4 and placebo tablets were a generous gift of Sanofi ... Laboratorios Aché" Comment: possible sponsor bias |
LT4 Cesareo 2010.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: ultrasonography characteristics of thyroid multinodular disease (2 to 5 nodules); cytology on dominant or suspicious nodule consistent with a colloid pattern by FNA; 99mTc scan consistent with hypofunctioning or non‐visualised nodules; no cystic or mixed nodules with fluid area higher than 30%; normal levels of serum TSH, FT4, FT3 and absence of TgAb and TPOAb antibodies; no previous treatment with thyroid hormones, iodine compounds or antithyroid drugs; no smoking history; no pregnancy in the past 12 months, body mass index between 18.5 and 30 kg/m2, no history of neck irradiation or surgery Exclusion criteria: see inclusion criteria Diagnostic criteria: clinical evaluation; laboratory measurements (TSH, FT4, FT3, Tg, TgAb, TPOAb, urinary iodine excretion); US (thyroid multinodular disease); thyroid scan with 99mTc (hypofunctioning) |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatients Treatment before study: none |
|
| Outcomes | Outcomes reported in abstract of publication: dominant nodule mean volume changes; thyroid volume changes; number of nodules > 0.5 mL; laboratory parameters for thyroid function (TSH, FT4, FT3) | |
| Study details | Study terminated before regular end: yes, probably for benefit (see notes) | |
| Publication details |
English language publication in a peer‐reviewed journal Non‐commercial funding from Italian Ministry of University and Research (Linea D1 “ex‐60%” 2008‐2009 Università Cattolica Sacro Cuore) |
|
| Stated aim of study | Quote from publication: "To evaluate the short term effects of levothyroxine treatment in never treated, pre‐menopausal women affected by thyroid multinodular disease" | |
| Notes | It is not clear why only one part of participants continued the study until 24 months: "Forty‐one patients were followed for 24 months and the obtained results prompted us to stop the observation period after 12 months for the remaining subjects" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The study was a prospective randomized clinical trial"; "Seventy‐one consecutive pre‐menopausal ... were randomly assigned to a L‐T4 (2 μg/kg body weight) treated group or to a non‐treated control group" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: no detailed information; study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "Clinical and hormonal evaluations were unblinded" Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Quote from publication: "Clinical and hormonal evaluations were unblinded" Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "... whereas US scans were blinded performed" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for US scans only |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: "Drop‐out patients: 8 subjects underwent surgical treatment (5 in the control and 3 in the L‐T4 treated group); 13 patients (5 in the L‐T4 treated group and 8 in the control group) abandoned the study and 3 patients of the L‐T4 treated group experienced side‐effects (nervousness, tachycardia and headache)"; "Forty‐one patients were followed for 24 months and the obtained results .... to stop the observation... after 12 months ... remaining subjects" Comment: 8/36 participants in the intervention group and 13/35 in the comparator group dropped out; only 58% (41/71) of total participants were evaluated at study end |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Gharib 1987.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: single palpable thyroid nodule < 3 cm; benignity (FNAB); functional, hypofunctional, cold (99mTc scan); volume, size, solid, cystic, mixed, no halo, no calcification (US‐characteristics); serologic data (T4, TSH) Exclusion criteria: more than one palpable nodule; nodule > 3 cm in any dimension; cytologic findings suggesting neoplastic process; pregnancy or CVD Diagnostic criteria: US (nodule volume, margin, composition; thyroid characteristics); FNAB (benignity); 99mTc thyroid scan (nodule function) |
|
| Interventions |
Number of study centres: 1 Country/location: USA Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: nodule diameter reduction; nodule volume reduction; thyroid function (TSH suppression confirmed by TRH test) | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal Non‐commercial funding from the Mayo Foundation (partial grant) |
|
| Stated aim of study | Quote from publication: "To compare prospectively ... the effect of thyroxine therapy with that of a placebo on the size of benign thyroid nodules that were solitary on palpation" | |
| Notes | Inclusion criteria: "single palpable nodule"; US identification: 27 participants had 1 nodule; 12 had 2 nodules; 11 had 3 nodules, 2 had 4 nodules and 1 participant had 6 nodules; only 25 participants had one nodule at follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were then randomly assigned to levothyroxine or placebo treatment groups with use of a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "our study was randomized, included a placebo group ... in a double‐blind fashion" "Both levothyroxine and placebo ... in externally identical capsules" |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "Neither the ultrasound examiner nor the clinician palpating the gland ... access to previous findings about the nodule or to the treatment code"; "Both levothyroxine and placebo... in externally identical capsules" |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Neither the ultrasound examiner nor the clinician palpating the gland ... access to previous findings about the nodule or to the treatment code" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants were evaluated at study end |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Grineva 2003.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: benign nodular thyroid lesions (colloid or colloid hypercellular by FNAB; cold or warm by scintigraphy) Exclusion criteria: hot nodules on scintigraphy, cyst > 1% of nodules, non‐euthyroid, pregnancy, ischaemic heart disease, other contraindications for thyroxine Diagnostic criteria: FNAB (benignity), scintigraphy (nodule function) |
|
| Interventions |
Number of study centres: 1 Country/location: Russia Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: dominant nodule size reduction ≥ 50%; other changes on dominant nodule size and number of nodules; thyroid gland size | |
| Study details | Study terminated before regular end: no | |
| Publication details |
Russian language publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "To study efficacy of thyroxine (TX) and potassium iodide (PI) in the treatment of benign nodular thyroid lesions (BNTL)" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: judgement in relation to translation/translator's remarks Comment: high risk for selection bias could influence all other domains |
| Allocation concealment (selection bias) | High risk | Comment: judgement in relation to translation/translator's remarks Comment: high risk for selection bias could influence all other domains |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: judgement in relation to translation/translator's remarks |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: judgement in relation to translation/translator's remarks |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Unclear risk | Comment: judgement in relation to translation/translator's remarks |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: judgement in relation to translation/translator's remarks |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: judgement in relation to translation/translator's remarks |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: judgement in relation to translation/translator's remarks |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: judgement in relation to translation/translator's remarks |
| Selective reporting (reporting bias) | Unclear risk | Comment: judgement in relation to translation/translator's remarks |
| Other bias | Unclear risk | Comment: judgement in relation to translation/translator's remarks |
LT4 Grussendorf 2011.
| Methods | Parallel RCT (superiority design) with randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: White; age 18 to 65 years; TSH normal (0.6 to 3.0 mU/L), TN normal size or enlarged thyroid; at least one TN solid (cyst component ≤ 20%), TN ≥ 1 cm, for TN > 1 cm, diagnosis according to guidelines for diagnostic standards of thyroid disorders to exclude malignancy Exclusion criteria: thyroid therapy within past 3 years; focal or diffuse autonomous thyroid structure; iodine contraindication; concomitant medication containing iodine (amiodarone); use of iodine‐containing contrast medium within past 6 weeks; TPO‐Ab 2 x above normal value; autoimmune thyropathy; symptomatic coronary disease; former radioiodine therapy or surgery; acute or chronic illness or allergy; pregnancy at screening; dermatitis herpetiformis; pathological laboratory values Diagnostic criteria: medical history, physical examination, clinical laboratory and TSH, anti‐TPO measurements, US nodule examination; nodules > 1 cm diagnosis according to guidelines for diagnostic standards to exclude malignancy |
|
| Interventions |
Number of study centres: 60 Country/location: Germany Setting: outpatients Treatment before study: not reported Titration period: after 3 months dose adjustment in the LT4 + I and LT4 groups based on TSH values (target range 0.2 to 0.8 mU/L) |
|
| Outcomes |
Study ID: NCT00277589 (ClinicalTrials.gov); LISA Study Outcomes reported in abstract of publication: percent volume reduction of all thyroid nodules measured by US; thyroid volume reduction |
|
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal Commercial funding from Sanofi‐Syntelabo GmbH, Henning Berlin |
|
| Stated aim of study | Quote from publication: "The measurement of the effect of a treatment with (non suppressive) LT4, iodine, or a combination of both compared with placebo on volume of thyroid nodules and thyroid" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Centerwise randomization sequences with variable block lengths ... by the study statistician and sent to the pharmacy ... blindness" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Centerwise randomization... and sent to the pharmacy that produced unlabeled coded medication packages for the total follow‐up period with sufficient medication for the titration ... guarantee concealment and blindness" |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "In the I and P arm, the medication was not changed, but an adaptation was simulated to keep investigators and patients blind"; "However, because only licensed drugs ... the patient but not the physician ... found out what group she/he is ... visiting pharmacy and comparing his pills with the available drugs" Comment: the possibility of unblinding probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: see above, the possibility of unblinding could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "In the I and P arm, the medication was not changed, but an adaptation was simulated to keep investigators and patients blind" Comment: at visit 3, if TSH was outside the target range (0.2 to 0.8 mU/L), the central laboratory sent new medication to the physician, who gave it blindly to the participants |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: there was probably no unblinding of personnel potentially introducing bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Ultrasonography ... by experienced and proficient examiner, who ... was masked to treatment assignment" Comment: ultrasonographer was masked to treatment assignment (supplementary appendix) |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Low risk | Comment: there was probably no unblinding of personnel potentially introducing bias for subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote from publication: " ... thus, the primary analysis ... remaining 794 patients. However, a sensitivity analysis in all 1013 patients ... performed ... whether... would change the results" Comment: the number of participants analysed (post‐hoc) after randomisation was similar between groups, but the reasons for incomplete follow up and dropouts were not explained (86% from n = 794 completed the study; 33% dropouts or missing data) |
| Selective reporting (reporting bias) | High risk | Quote from publication: "In 38 patients, who stopped medication because of serious adverse events (e.g. hospitalization ... accidents, gynecological operations, infections, etc.), no ... relationship to medication ... by the investigators" Comment: serious adverse events (assumed as not related to the medication) were mentioned under 'Methods', but it was not specified in which treatment groups they occurred; other adverse events were not described |
| Other bias | Unclear risk | Comment: the study was supported by Sanofi‐Aventis, Germany; possible sponsor bias |
LT4 Koc 2002.
| Methods | Cross‐over RCT with randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: cold (scan) solitary nodule with palpation (≤ 30 mm); benign (cytology); multiple nodules (US ≤ 10 mm) Exclusion criteria: Hashimoto's thyroiditis, antithyroid antibodies, previous neck surgery, previous radiation therapy; contraindication to LT4 suppressive therapy (pregnancy, cardiovascular disease), low TSH (< 0.4 mU/L) or high TSH (> 2.5 mU/L); > 45 years old; thyroid nodule diagnosis > 5 years; previously treated with LT4; palpable thyroid nodule > 30 mm or second nodule detected on US > 10 mm; cystic or degenerative nodules Diagnostic criteria: palpation, thyroid scan, FNAB, cytology, US |
|
| Interventions |
Number of study centres: 1 Country/location: Turkey/Istanbul Setting: outpatients Treatment before study: not reported Titration period: LT4 was adjusted every 3 weeks until desired TSH level (± 25 μg/day) |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume reduction or increase; percentage of participants with 50% or more nodule volume reduction; TSH levels | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal Non‐commercial funding from Health Science Research Support Grant no. SA‐29, Marmara University, Istanbul, Turkey |
|
| Stated aim of study | Quote from publication: "To determine the response of solitary thyroid nodules to low‐ or high‐level TSH suppression in a placebo‐controlled, randomized crossover trial" | |
| Notes | Istanbul residents: region with adequate iodine intake; there was no washout period between treatments; urinary iodine excretion was not measured | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were basically randomized to two main groups according to the level of TSH suppression ... " Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: no detailed information; study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: no detailed information; study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Ultrasonography was performed ... same operator ... no access to patients' clinical and laboratorial data" Comment: outcome assessor probably blinded |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: unblinding of outcome assessors for other outcomes than ultrasound measurements not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "Nine patients (three patients in group 1...), were excluded from the analysis ... noncompliance or inadequate TSH suppression" |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: there was no washout period between intervention periods |
LT4 La Rosa 1995.
| Methods | Parallel RCT with a randomisation ratio of 1:1:1 | |
| Participants |
Inclusion criteria: solitary nodule ≤ 3.5 cm, solid (< 10% cyst component) (US); other nodules (maximum diameter < 50% of maximum diameter from main nodule; newly diagnosed nodules (< 1 year prior to study start) Exclusion criteria: nodules > 3.5 cm (US); "hot" nodules (radioiodine scan 25 μCi); malignancy, follicular lesion, cyst haemorrhagic lesion, thyroiditis (FNAB cytology); thyroid hormone, TSH abnormal; serum thyroid antibodies; urinary iodine excretion < 8 μg/dL or > 27 μg/dL; CVD, liver diseases, pregnancy, osteoporosis Diagnostic criteria: radioiodine scanning (nodule function); US (nodule size and characteristics); FNAB cytology (malignancy, benignity, follicular lesion, cyst haemorrhagic lesion, thyroiditis |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatients Treatment before study: not reported Titration period: LT4 dose adjusted after the first 4 months until TSH < 0.3 mU/L |
|
| Outcomes | Outcomes reported in abstract of publication: mean nodule volume decrease or increase, percentage of participants with clinically relevant nodule volume reduction (50% or more) | |
| Study details | Study terminated before regular end: yes, probably for benefit | |
| Publication details |
English language publication in a peer‐reviewed journal Commercial funding from Cyanamid Italia SpA providing PI tablets |
|
| Stated aim of study | Quote from publication: "To determine the effectiveness of levothyroxine and potassium iodide in treating patients with benign solitary cold thyroid nodules" | |
| Notes | Area with sufficient iodine supply and goitre prevalence in schoolchildren < 1%; urinary iodine excretion in this area ranged from 80 to 300 µg/day and was measured (all participants at 4‐month intervals) to check compliance (participants receiving potassium iodide) and the absence of iodine contamination (participants receiving no treatment and receiving levothyroxine) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "We then randomly assigned patients ... to one of the three treatments (using randomized blocks with a coin slightly biased in favour of treatment groups ..." Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Quote from publication: "Compliance with therapy was individually controlled in patients receiving levothyroxine by carefully asking the patient ... 4‐month intervals" Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "...with allocation blinded only to the ultrasonography operator" Comment: outcome assessor blinded to treatment groups (ultrasound measurements only) |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasonography measurements only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "Of the 80 patients, 70 (87.5%) completed ... follow‐up. Three ... not receiving treatment dropped out (1 ... moved and 2 missed follow‐up), as did 4 ... levothyroxine ... and 3 ... iodine (2 ... further treatment and 1 missed follow‐up)" |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | High risk | Quote from publication: "The study was stopped because at the interim analysis, we obtained clinically important results for the first 80 patients who entered the study; we did not include 18 patients who were still being studied at the time of interim analysis" Comment: study probably stopped for benefit; total sample size including 10% lost to follow up was estimated to be n = 160 Comment: possible sponsor bias |
LT4 Larijani 2005.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: one benign palpable TN (FNAB, cytology) Exclusion criteria: suspicion of or malignancy (FNAB); LT4 consumption at least in the preceding year; abnormal T4, T3, TSH; > 1 palpable TN; pregnancy; CVD; age ≤ 15 years or ≥ 60 years Diagnostic criteria: serum T3, T4, TSH; US (solid or cystic, single or multiple); FNAB cytology (benign) |
|
| Interventions |
Number of study centres: 1 Country/location: Iran Setting: outpatients Treatment before study: not reported Titration period: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: nodule size reduction; mean nodule volume change | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal Commercial funding (?) from Iran Hormone Company and non‐commercial funding from Teheran University of Medical Sciences (educational grant) |
|
| Stated aim of study | Quote from publication: "This study addresses the problem in an iodine‐deficient area, evaluating the efficacy of levothyroxine suppression therapy on a 2‐year course" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were randomly assigned to either the levothyroxine ... placebo, with the use of a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "Both clinical and ultrasonographic studies were applied blindly"; "The attending physician and sonographer were blind to the treatment protocol" |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "A total of 58 patients of the primary enrolled ... completed the second year of study (31 cases and 27 controls). One of the four dropouts ... surgery by choice" |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected (unclear whether commercial funding took place) |
LT4 Ozkaya 2010.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: benign TN (FNAB, cytology) Exclusion criteria: TN > 2 cm; cystic nodules; pregnancy Diagnostic criteria: ultrasonography; FNAB (cytology) |
|
| Interventions |
Number of study centres: 1 Country/location: Turkey/Ankara Setting: outpatients Treatment before study: none |
|
| Outcomes | Outcomes reported in abstract of publication: dominant nodule volume change; thyroid right lobe and thyroid left lobe change | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "We studied the efficacy of thyroxine‐suppressive therapy in patients with euthyroid nodular disease" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The patients were divided randomly into 2 groups, one group ... receiving levothyroxine ... and in the other ... without medication" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Quote from publication: "The measurements of nodule diameter ... performed by the same person, using high‐resolution sonography" Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: nodule volume analysis after one year remained similar with 2 drop‐outs in each group. |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Papini 1993.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: "(a) single thyroid nodule diagnosed by an endocrinologist with expertise in thyroid disease; (b) cytology consistent with a colloid nodule by FNA; (c) ultrasonic characteristics of a solid or prevalently solid nodule; (d) thyroid scan showing a decreased or normal pertechnetate 99mTc uptake of the nodule; (e) normal 131I uptake at 6 and 24 hours; (f) normal titres of TgAb and TPOAb antibodies; (g) normal serum thyroid hormones and TSH concentrations; (h) diagnosis made no more than 2 years before enrolment; no treatment with thyroid hormones, iodine compounds or antithyroid drugs in the same period of time; no history of neck irradiation or surgery; (i) age between 18 and 60 years; (j) absence of clinically relevant cardiovascular, hepatic, pulmonary or renal diseases" Exclusion criteria: nodules containing a fluid volume ≥1 mL Diagnostic criteria: palpation (single nodule); cytology FNA with a 22 or 25‐gauge needle (colloid nodule); US (solid lesions < 3 mm size and with a theoretical axial resolution < 1 mm); normal values of serum TSH, FT3, FT4, TgAb, TPOAb; decreased or normal values for thyroid scan with 99mTc pertechnetate and 131I uptake at 6 and 24 hours |
|
| Interventions |
Number of study centres: 3 Country/location: Italy/Rome metropolitan area (non‐endemic for goitre) Setting: outpatients Treatment before study: none Titration period: initial dose 50 μg before breakfast and increased by 25 to 50 μg weekly to the full dose, which was thereafter adjusted to induce TSH suppression |
|
| Outcomes | Outcomes reported in abstract of publication: nodule size (palpation) and nodule volume changes (US); nodule size and thickness of thyroid lobe correlation (palpation and US); contralateral thyroid lobe thickness (US); number of nodules which decreased in size; clinical and laboratory parameters (FT4, FT3, T4, T3, TSH, Tg, TgAb, TPOAb) | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "... to test whether a 12‐month suppression of serum TSH below normal range, verified during the whole study duration with a ultra sensitive assay, would modify the clinical evolution of solitary thyroid nodules" | |
| Notes | Statistical analysis: repeated, excluding nodules which were "not cold" (LT4: n = 35% (18/51) vs placebo: n = 32% (16/50) ‐ results were unchanged | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The study was multicentre randomized ..."; "The patient population consisted ... consecutive patients seen ... 1991, randomly allocated to the treatment with standard doses of levothyroxine ... and placebo group" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Clinical evaluations were single‐blinded (patients) while ultrasound measurements were double‐blind (patients and examiners)" |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: see above (relating to ultrasound measurements) |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasonography measurements only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "There were six dropouts in the placebo ... and three ... treatment group" Comment: reasons for dropouts were not provided |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Papini 1998.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: single palpable nodule (greatest diameter between 10 to 30 mm; cytology consistent with a colloid nodule (FNAB); single solid nodule (US); thyroid volume within normal limits (< 14.3 mL); 99m Tc thyroid scan consistent with a hypofunctioning or non‐visualised nodule; 131I uptake within normal limits; serum TSH, FT3, FT4, Tg, TgAb, TPOAb; no previous treatment with thyroid hormones, iodine compounds, or antithyroid drugs; no history of neck irradiation or surgery. Exclusion criteria: concomitant nonpalpable nodules > 5 mm Diagnostic criteria: US (solid), FNAB cytology (benignity); normal levels of serum TSH, FT3, FT4, TgAb, TPOAb ; thyroid scan with 99mTc pertechnetate (hypofunctioning or non‐visualised nodule) and 131I uptake with gamma‐camera within normal limits |
|
| Interventions |
Number of study centres: multicentric Country/location: Italy/Rome Setting: outpatients Treatment before study: none |
|
| Outcomes | Outcomes reported in abstract of publication: nodule or thyroid volume changes; appearance of new nodules; serum TSH, clinical parameters | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "The present study evaluated over a 5‐yr period 1) changes in nodule size and thyroid volume in a homogeneous group of patients randomly assigned to L‐T4 suppressive therapy or to a control group; 2) enlargement of small concomitant lesions and appearance of new nodules; 3) correlations among baseline size, clinical and laboratory parameters, degree of TSH suppression, and observed thyroid changes; 4) rate of growth (or reduction) of thyroid nodules; and 5) reliability of cytological diagnosis" | |
| Notes | "FNA and scintiscans were performed at enrollment and after 5 yr, after 2 months of L‐T4 withdrawal" This study was a "similar study with a longer follow‐up", proposed by Papini 1993 in order to verify whether long‐term suppressive therapy prevents growth of new nodules and induces reduction of size in a subgroup of nodules |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "The study was ... randomized clinical trial" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "Clinical and hormonal evaluations were unblinded ..." Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: see above; the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: " whereas US scans were blindly performed"; "All US evaluations were performed ... same center ... three blinded examiners ..." Comment: relating to ultrasound measurements |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors were blinded to ultrasound scans only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "There were 14 dropouts ... 6 in the control group). In the L‐T4 group, 7 patients experienced side effects ... and 3 of them abandoned the study" Comment: no reasons stated for comparator group |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Reverter 1992.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: TN single on palpation; "cold" and single by 99mpertechnetate thyroid scan; benign (colloid goitre) by FNAB Exclusion criteria: multiples nodules by palpation or by scintigraphy; suggestion of neoplastic process (cytological findings); Hashimoto's thyroiditis; pregnancy and/or any contraindication for LT4 treatment Diagnostic criteria: palpation (TN single); thyroid scan with 99mpertechnetate (TN "cold" and single); FNAB, cytology (benign, colloid goitre); US |
|
| Interventions |
Number of study centres: 1 Country/location: Spain Setting: outpatients Treatment before study: not reported Titration period: LT4 dose adjusted until TSH suppression was achieved (TSH < 0.1 mU/L) |
|
| Outcomes | Outcomes reported in abstract of publication: nodule diameter changes (US); nodule volume changes; number of nodules with significantly volume reduction (> 50%); TSH, T4, FT4, T3 | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "To evaluate the effect of treatment with TSH suppressive dose of levothyroxine in patients with benign thyroid nodules" | |
| Notes | LT4 dose adjusted until TSH suppression was achieved (TSH < 0.1 mU/L): mean dose to obtain this effective TSH suppression without hyperthyroidism was 2.82 ± 0.6 μg/kg body weight/day; only female participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were randomly allocated in two groups... a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: participants were unblinded; the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: personnel was unblinded; the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: personnel was unblinded; the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "In this group, six patients dropped out ... (three patients desired the surgical treatment ... and three patients abandoned treatment ...)"; "All the group B patients completed the study" Comment: first group consisted of thyroxine treated patients, second group (group B) of patients with no treatment. Disparate attrition rates (30%); however, intention‐to‐treat analysis was performed for nodule volume reduction of more than 50% (n = 4/20 vs n = 3/20) |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Tsai 2006.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: TN single (US); cold (131I thyroid scan); benign (FNAB cytology); normal levels of T3, T4, FT4, TSH; absence of CVD or renal disease; no LT4 suppressive therapy or other thyroid medication before study Exclusion criteria: TN > 1 (US and scintiscan); cystic nodules, neoplastic lesion, hot nodules (scintiscan); pregnancy; serious CVD, renal or liver disease Diagnostic criteria: US, 131I thyroid scan, FNAB and cytology, normal levels of serum TSH, T4, T3, FT4 |
|
| Interventions |
Number of study centres: 1 Country/location: Taiwan Setting: outpatients Treatment before study: not reported Titration period: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume reduction > 50% (responders); nodule size reduction; serum Tg level | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "To study the efficacy of thyroxine‐suppressive therapy in patients with solitary non‐toxic thyroid nodules and its relation to serum thyroglobulin levels" | |
| Notes | TSH suppression: < 0.3 mU/L after LT4 therapy; compliance measurement: pill count | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "All patients ...were randomly divided into two groups ... levothyroxine ... and ... placebo" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "All investigators and patients did not know if the pill was placebo or levothyroxine" |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: see above |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Quote from publication: "The measurements of nodule diameter ... performed ... same person ... linear transducer" Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: see above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
LT4 Wemeau 2002.
| Methods | Parallel RCT with randomisation 1:1 | |
| Participants |
Inclusion criteria: single palpable nodule; benign (FNAB); nodule identified < 1 year before begin of study; age from 18 to 55 years Exclusion criteria: more than 1 palpable nodule; history of CVD, osteoporosis, previous thyroid surgery, neck irradiation and/or thyroiditis; abnormal serum thyroid hormone or TSH levels, circulating autoantibodies, nodules with cystic component > 20%, hot nodules and suppression of surrounding tissue at thyroid imaging; nodule > 3 cm in any dimension Diagnostic criteria: serum levels of FT3, FT4, TSH, TgAb, TPOAb, TSH Ab; nodule size, internal contents, peripheral halo or calcifications; additional nodules non palpables (US); benignity (US‐FNAB); activity in the nodule region: non‐functional, hypofunctional, functional (thyroid scan 99mTc pertechnetate) |
|
| Interventions |
Number of study centres: 25 Country/location: France Setting: outpatients Treatment before study: none Titration period: until TSH < 0.3 mU/L |
|
| Outcomes | Outcomes reported in abstract of publication: variations in nodule volume (US); nodule size changes (palpation); clinically relevant TN volume reduction (50% or more); proportion of participants with reduced number of additional nodules (US), serum TSH level | |
| Study details | Study terminated before regular end: yes (recruitment problems) | |
| Publication details |
English language of publication in a peer‐reviewed journal Commercial funding from Merck‐Lipha Santé France (computer, statistical support and LT4 drug supply) |
|
| Stated aim of study | Quote from publication: "To assess the efficacy of TSH‐suppressing L‐T4 therapy in reducing the volume of solitary benign thyroid nodules and in modifying perinodular thyroid tissue" | |
| Notes | Area suggesting a sufficient iodine supply: median urinary iodine excretion: 8 μg/100 mL; ß‐Blocker prescription allowed when tachycardia was present (bisoprolol, 5 to 10 mg/day); mean LT4 dose for effective TSH suppression (< 0.3 mU/L) without hyperthyroidism was 2.24 ± 0.45 μg/kg/day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were then randomly allocated to the LT4 treatment or placebo using a table of random numbers, without stratification according to site" Comment: no stratification by study centre |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Both LT4 and placebo were administered ... identical tablets" |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment:see above |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "Ultrasonography was repeated ... examiner had no access to previous findings, about the nodule, TSH ... treatment code" Comment: relating to ultrasound measurements |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasonography only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "Six of the 17 patients who did not complete the protocol spontaneously ... withdrew from the study. Of the remaining 11, 2 ... L‐T4 ... dropped‐out (one developed iatrogenic thyrotoxicosis ... other ... thyroidectomy ...). The 9 other patients, ... placebo group ... dropped out ... following reasons ... and Graves' disease" "The characteristics of the patients who dropped out and of their nodules were similar to those ...who completed the study (data not shown)" Comment: disparate attrition rates; however, analyses were performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Quote from publication: "Despite an expected total sample of 300 patients, patients recruitment was difficult, and it was stopped after 135 informed patients ... 25 centers" Comment: commercial funding from Merck‐Lipha Santé France (computer, statistical support and LT4 drug supply); possible sponsor bias |
LT4 Zelmanovitz 1998.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: normal range serum TSH (0.4 to 3.8 μU/mL), T3 (86 to 187 ng/dL), T4 (4.5 to 12.5 μg/dL); antimicrosomal, TgAb (< 1/100); Tg (0 to 52 ng/mL); single thyroid nodule (US); hypofunctioning (131I scintigraphy); benignity (cytology) Exclusion criteria: cystic or mixed nodules (cystic component > 20%); Hashimoto's thyroiditis (positive antithyroid antibodies or cytopathological findings); previous neck irradiation; cardiovascular disease, pregnancy; contraindication for the use of LT4 suppressive therapy Diagnostic criteria: US (internal contents); scintiscan (hypofunctional); cytology (benignity); thyroid hormone measurements |
|
| Interventions |
Number of study centres: 1 Country/location: Brazil Setting: outpatients Treatment before study: previous suppressive therapy > 1 year before entering the study: LT4: 19%; placebo: 21% Titration period: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: mean nodule volume change (US); nodule volume reduction (> 50%); nodule volume increase (> 50%); BMD | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal Commercial funding from Sanofi (LT4 and placebo tablets) and non‐commercial funding from Hospital de Clínicas de Porto Alegre and CAPES scholarship (partial grants) |
|
| Stated aim of study | Quote from publication: "To analyze the effect of suppressive doses of T4 on the volume of benign STN and BMD. Furthermore, meta‐analyses were performed to examine the quantitative synthesis of data from similar designed controlled trials" | |
| Notes | TSH suppression: TSH < 2 μU/mL measured at 20 minutes by TRH test (200 μg intravenous bolus) or TSH < 0.3 μU/mL after LT4 dose‐adjustment; female participants were analysed according to their menopausal status (LT4: n = 10 premenopausal and n = 6 postmenopausal women; placebo: n = 12 premenopausal and n = 7 postmenopausal women) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "This study was a randomized ... trial" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "The patients ... randomly allocated ... T4 or identical placebo pills" Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "All examinations were done by the same radiologist, ... no access ... patients' data or ... assignment" Comment: probably relating to ultrasound measurements |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: low risk of bias for outcomes measured by radiologist only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Quote from publication: "Laboratory Sanofi kindly provided T4 and placebo tablets" Comment: possible sponsor bias |
PEI Bennedbaek 1998.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: 1) 99mTc pertechnetate scintigraphy demonstrating a solitary cold nodule; 2) US demonstrating solitary solid nodule; 3) US‐guided FNAB compatible with a colloid nodule; 4) euthyroidism; 5) normal serum ionised calcium and calcitonin; 6) no major concomitant disease; 7) no medication affecting thyroid function; 8) no history of previous head or neck irradiation; and 9) normal indirect laryngoscopy. Exclusion criteria: not described Diagnostic criteria: US‐guided FNAB compatible with a colloid nodule and benign follicular cells; 99mTc pertechnetate scintigraphy demonstrating a cold nodule; US demonstrating a solitary nodule |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark/County of Funen Setting: outpatients (referred by their primary care physicians) Treatment before study: none Titration period: for LT4: up to 6 months dose adjusted to reduce serum TSH to subnormal levels (0.10 to 0.40 mU/L) |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume reduction (US); total thyroid volume (US); biochemical thyroid measurements; symptom scores (pressure and cosmetic) evaluated by questionnaire; median TN volume reduction; median perinodular thyroid volume reduction; percent of participants with clinical response (TN volume reduction = 50%); side effects | |
| Study details | Study terminated before regular end: no | |
| Publication details | English language of publication in a peer‐reviewed journal Non‐commercial funding from Agnes and Knut Mørk Foundation and the Clinical Institut of Research, Odense University | |
| Stated aim of study | Quote from publication: "To determine the effectiveness of a single small dose of sterile 98% ethanol injected into the nodule against that of suppressive LT4 (TSH < 0.4 mU/L)" | |
| Notes | PEI: n = 2/25 (8%) were operated at 6 months; 6 months follow up; success rate: nodule disappearance or > 50% reduction in size; compliance for LT4: "satisfactory" (two participants had only partial suppression of TSH) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Random allocation was achieved using a random number generator on a computer"; "However, randomization resulted in a comparatively higher frequency of smaller nodules in the LT4 group. Thus, 10 of 25 nodules (40%) were less than 5 mL, compared with 4 of 25 nodules (16%) in the PEIT group ... Nodule volume in both groups showed marked deviations from a normal distribution skewed toward smaller volumes in the LT4 group, thus favoring outcome in the LT4 group, as evidenced by previously published data (9)"; "... some refused ... treatment or control once a benign diagnosis established. Only nodules causing neck discomfort ... combined with a wish for treatment to achieve alleviation were considered for inclusion, ... randomization to no treatment could not be achieved" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: participants were unblinded; the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "For each patient, ultrasound... same operator ... blinding ..." |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasound measurements only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "One drop‐out in each group was anticipated"; "Six months after ... two patients in the PEIT... operated upon due to unaltered complaints, 6‐month evaluation was the end point ..." Comment: evaluation was based on intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: baseline imbalance for thyroid nodules < 5 mL, see above |
PEI Bennedbaek 1999.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: (1) 99mTc pertechnetate scintigraphy demonstrating a solitary no‐uptake or low‐uptake lesion; (2) US‐demonstrated solitary solid nodule, including those with minimal (< 10%) cystic component; (3) US‐guided FNAB compatible with a colloid nodule; (4) euthyroidism; (5) normal serum ionised calcium and calcitonin; (6) no major concomitant disease; (7) no medication affecting thyroid function; (8) no history of previous head or neck irradiation; and (9) normal indirect laryngoscopy. Exclusion criteria: none described Diagnostic criteria: US‐guided FNAB compatible with a colloid nodule, 99mTc pertechnetate scintigraphy demonstrating a cold nodule; US demonstrating a solitary nodule |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark/County of Funen Setting: outpatients (referred by their primary care physicians) Treatment before study: previous thyroidectomy: PEI‐1 = 6 participants; PEI‐3 = 3 participants; previous LT4 treatment: PEI‐1 = 8 participants; PEI‐3 = 7 participants |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume reduction; dose ethanol response relationship; pressure/cosmetic symptoms on a VAS; treatment tolerability | |
| Study details |
Run‐in period: for LT4 participants: 3‐month medication stop before randomization and PEI therapy Study terminated before regular end: no |
|
| Publication details |
English language of publication in a peer‐reviewed journal Non‐commercial funding from Agnes and Knut Mørk Foundation and the Clinical Institut of Research, Odense University |
|
| Stated aim of study | Quote from publication: " ... to evaluate the efficacy of percutaneous ethanol injection therapy (PEIT) with special reference to dose response and symptom score and to describe side effects" | |
| Notes | "All patients received 1 g of oral paracetamol or 1 g of oral acidum acetyl salicylicum and local anesthesia with 1 mL of subcutaneous lidocaine (10 mg/mL) prior to treatment" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Random allocation was achieved using a random number generator on a computer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: participants were unblinded; the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "US measurements ... the same operator ... blinding ..." |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasound measurements only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "Statistically analysis is based on intention‐to treat and no patients were excluded or changed ..." Comment: reasons why participants discontinued the predetermined therapy were mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
PEI Bennedbaek 2003.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: 1) 99mTc pertechnetate scintigraphy demonstrating a solitary cold nodule; 2) US‐demonstrated solitary or prominent (additional nodule(s) < 1 cm detected on US but not on the scintiscan) anechoic cystic lesion with no or < 10% solid component and cyst volume at least 2 mL; 3) recurrence of the cyst fluid more than 1 month after primary aspiration; 4) cytological samples, obtained by FNAB under sonographic guidance, of the cyst fluid, the cyst wall and, if present, a residual solid component, to rule out malignancy; 5) euthyroidism; 6) normal serum calcitonin; 7) no major concomitant disease; 8) no medication affecting thyroid function; 9) no history of previous head or neck irradiation; and 10) normal indirect laryngoscopy. Exclusion criteria: US‐guided FNAB ruled out malignancy Diagnostic criteria: US to demonstrate solitary nodule; US‐guided FNAB for cystic colloid goitre or colloid goitre; 99mTc pertechnetate scintigraphy to demonstrate a cold nodule. |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark/County of Funen Setting: outpatients (referred by their primary care physicians) Treatment before study: previous surgery/131I: PEI = 2 vs NaCl = 4; previous number of aspirations: PEI = 1 (1 to 2) vs NaCl = 1 (1 to 2) |
|
| Outcomes | Outcomes reported in abstract of publication: recurrence rate for reduction of benign recurrent thyroid cyst (recurrence: volume > 1 mL); cure (cyst volume ≤ 1 mL); thyroid cyst volume; chance of success; adverse events | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal Non‐commercial funding from Agnes and Knut Mørk Foundation, A. P. Møller Support Foundation and commercial funding from Novo Nordisk Foundation |
|
| Stated aim of study | Quote from publication: "To determine whether US‐guided ethanol injection reduces the recurrence rate of benign thyroid cysts" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Random allocation was achieved using a random number generator on a computer"; "The study was carried out with complete blinding of both investigators (F.N.B. and L.H.) and patients"; "Complete blinding was maintained throughout the whole study period until 6‐month evaluation of the last patient. Allocation of treatment was thus carried out in a unbiased way" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "The Pharmacy of Odense University Hospital (Centralapoteket OUH) was responsible for the production of absolute ethanol (800 mg/ml) and isotonic saline, for preparation of bottles labeled “project ethanol vs. saline,” and for providing sealed code lists. A pharmacist independent of the investigators provided the investigators with 68 sealed boxes (labeled patient no. 1, 2, etc.). Each box contained three sealed bottles with 10 ml of sterile fluid (34x3 with saline and 34x3 with ethanol), and each was labeled “project medicine." "The corresponding list with codes detailing the content of the bottles was stored in a sealed envelope at the pharmacy" |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "The study was carried out with complete blinding of both investigators (F.N.B. and L.H.) and patients" |
| Blinding of participants (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: see above |
| Blinding of personnel (subjective outcomes) Subjective outcomes | Low risk | Comment: see above |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "US measurements were performed ... same operator ... blinding toward previous measurements." |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasound measurements only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "Two of the 68 patients were excluded due to technical difficulties in one and due to pain during the instillation procedure and therefore discontinuation of the treatment in the other" |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias |
PEI Chu 2003.
| Methods | Parallel RCT with randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: 1) single palpable thyroid nodule; 2) ultrasonographic picture of a simple thyroid cyst (cystic component over 90% of total nodule volume without intracystic nodules or septa); 3) a thyroid cyst volume over 4 mL; 4) euthyroid on plasma thyroid function test; and 5) an absence of suspicious or malignant cytology Exclusion criteria: not reported Diagnostic criteria: US compatible with cystic component over 90% of total nodule volume |
|
| Interventions |
Number of study centres: 1 Country/location: Taiwan Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: cure rate; recurrence rate; treatment failure; cyst volume changes | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: " ... study was designed to determine whether sclerotherapy is a more effective treatment of TCN than aspiration alone"; "We therefore compared PEI with percutaneous hydrochloric acid injection at a pH of 1.0 ... in order to evaluate the role of pH in the efficacy of the sclerosant solution" | |
| Notes | Cure: nodule disappearance or volume reduction < 0.5 mL (maximum 5 sessions); recurrence: cystic volume > 1 mL PEI therapy: additional 14 participants were enrolled for the long‐term results of the treatment; no thyroxine therapy during the intervention and follow‐up period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "TCN patients were randomly assigned to 1 of 3 treatment groups ..." Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Quote from publication: "Thyroid ultrasonography was carried out ... same observer ..." Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote from publication: "Excluding those lost of follow‐up, 19 patients received follow‐up for 18 months and 8 patients for 24 months" Comment: no reasons for missing data were provided; 33% (8/24) of participants were followed‐up until 24 months |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: non detected |
PEI Sung 2013.
| Methods |
Parallel, non‐inferiority RCT with randomisation ratio 1:1 Primary endpoint was the mean difference in volume reduction ratio (%) 6 months after treatment: non‐inferiority margin was set as ‐8% (ethanol injection minus RF ablation) + subsequent superiority comparison after establishment of non‐inferiority (two‐sided 95% confidence interval of the outcome difference) |
|
| Participants |
Inclusion criteria: a) presence of a cystic thyroid nodule (cystic portion > 90%); b) reports of pressure symptoms or cosmetic problems; c) cytologic confirmation of benignancy in at least two separate US‐guided FNAC examinations (i.e. two biopsies performed with an interval of several months apart) for cystic fluid and/or a mural, solid component; (d) serum levels of thyroid hormone, thyrotropin, and calcitonin within normal limits Exclusion criteria: a) nodules showing malignant features (i.e. taller than wide, spiculated margin, markedly hypoechoic, micro‐ or macrocalcifications) at US; b) the participant was prescribed medication or underwent other treatments for thyroid nodules within 6 months before enrolment in this study Diagnostic criteria: US to demonstrate cystic portion > 90% in the TN, US‐guided FNAC (benignity according to the Bethesda classification system), and laboratory and clinical evaluation |
|
| Interventions |
Number of study centres: 1 Country/location: Korea/Seoul Setting: outpatients Treatment before study: no medication for thyroid or other thyroid treatments were allowed six months before study begin |
|
| Outcomes | Outcomes reported in abstract of publication: volume reduction ratio (percentage) at 6‐month follow up, therapeutic success rate, improvement of symptoms and cosmetic problems, and number of major complications | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language publication in a peer‐reviewed journal Commercial funding: "JHB is patent holder of unidirectional ablation electrode (but no money paid from the company yet)"; "SHP: Financial activities not related to the present article: institution received a research grant from Dongkook Pharmaceutical and from GE Healthcare". |
|
| Stated aim of study | Quote from publication: "The purpose of this study was to compare the volume reduction of single session EA and RF ablation for the treatment of cystic thyroid nodules" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "This study was a single‐institution, randomized, non‐inferiority trial" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "The outcome assessors (J.Y.S. and K.S.K.) were blinded to the treatment group allocation. US examination was performed in all patients at the time of the 1‐ and 6‐month follow‐up examinations" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Low risk | Comment: outcome assessors were probably blinded throughout the whole study period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from publication: "Therefore, we performed the per‐protocol analysis and showed that the results were consistent with the results of the intention‐to‐treat analysis" Comment: Three participants (n =1 in the EA group and n = 2 in the RF group) required additional interventions due to incomplete improvement of symptoms. These participants were included in the intention‐to‐treat analysis, but excluded from the per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: JHB is patent holder of unidirectional ablation electrode; commercial funding: institution received a research grant from Dongkook Pharmaceutical and from GE Healthcare |
PEI Valcavi 2004.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: local discomfort or cosmetic damage, volume exceeding 2 mL, 50% or more fluid component as assessed by US examination, benignity as demonstrated by cytologic assessment obtained by US‐guided FNAB; euthyroidism Exclusion criteria: inadequate, suspicious, or positive FNAB cytologic specimens, high serum calcitonin levels, and contralateral laryngeal cord palsy Diagnostic criteria: US demonstrating fluid component; US‐guided FNAB for benignity |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: cyst volume reduction; cure rate (after 12 months); compressive/cosmetic symptoms; side events | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "To provide am overview of ultrasound (US)‐guided percutaneous ethanol injection (PEI) therapy for thyroid cystic nodules and discuss the practical and technical details" | |
| Notes | Cure rate: elimination of discomfort and cosmetic complaint one year after PEI therapy; no local anaesthesia needed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were randomly assigned ..." Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no reasons for missing data provided |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
PEI Verde 1994.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: solitary cystic thyroid nodule; no malignancy (FNA); cold areas (scintiscan); no treatment with thyroid hormones, iodine or antithyroid drugs before or after enrolment Exclusion criteria: not reported Diagnostic criteria: thyroid cystic nodule: fluid volume > 70% of the total nodule volume (US evaluation); clinically and biochemically (TSH, FT3, FT4, Tg, TPOAb, TgAb) |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatient clinics for thyroid diseases Treatment before study: none |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume reduction; percent of participants with nodule volume reduction > 50%; cyst fluid recurrence (US); success rate; serum TSH, FT3, FT4, Tg, TPOAb, TgAb; cost | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "To test whether PEI was more effective than FNA alone in reducing the volume of cystic thyroid nodules" | |
| Notes | Group 3 (prospective study): after evaluation of the study results of group 1 and 2, "a prospective trial was carried out to test long‐term efficacy and safety of the procedure on clinical, ultrasonographic and hormonal grounds" with 12 months follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "20 patients with predominantly cystic thyroid nodules ... randomized in two groups ..." Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Quote from publication: "After the first visit, nodule volumes ... evaluated ... two consecutive ultrasound scans ... two blinded examiners" |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | High risk | Comment: outcome assessors blinded for ultrasound scans only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants finished the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
RF Faggiano 2012.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: age above 18 years; benign thyroid nodules; solid or predominantly solid ((cystic component 30%), large (4.0 ml) thyroid nodules); and refusal and/or inefficacy of surgery and/or radioiodine therapy; TN with pressure symptoms Exclusion criteria: pregnancy and malignant or suspicious thyroid nodules Diagnostic criteria: benignity confirmed by US‐guided FNAC in nodules > 1 cm (or less in the presence of US characteristics suspected for malignancy), blood tests for thyroid function (TSH, FT3, FT4, TgAb, TPOAb) |
|
| Interventions |
Number of study centres: 1 Country/location: Italy Setting: outpatients (in a hospital) Treatment before study: unsuccessful surgery (n = 2); unsuccessful radioactive iodine therapy (131I) (n = 2) |
|
| Outcomes | Outcomes reported in abstract of publication: change in TN volume and thyroid function; pressure symptoms (changes); thyroid function changes; clinically, biochemically evaluation; tolerability | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal Non‐commercial funding by the Department of Molecular and Clinical Endocrinology and Oncology, Frederico II University of Naples |
|
| Stated aim of study | Quote from publication: "To investigate the long‐term effectiveness of RTA in patients with TNs. Both toxic and nontoxic TNs will be evaluated" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients enrolled ... randomized as follows: 20 patients ... single RTA, ... 20 patients ... followed up (group B)" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants finished the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: none detected |
RF Huh 2012.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: 1) predominantly solid nodule (solid portion > 50%); 2) pressure symptoms or cosmetic problems; 3) largest diameter of TN > 2 cm; 4) cold nodule at 99mTc pertechnetate scintigraphy; 5) normal serum levels of thyroid hormone, TSH and calcitonin; 6) cytologic confirmation of benignity (at least two separate US‐guided FNAC examinations; 7) nodules showing no malignant features (taller than wide, spiculated margin, markedly hypoechoic, micro‐ or macrocalcifications) at US; 8) refusal of or ineligibility for surgery Exclusion criteria: solid portion of the nodule < 50%; TN size < 2 cm; autonomously functioning TN; recurrent thyroid cancers Diagnostic criteria: laboratory values in normal range (TSH, T3, FT4, TPOAb, serum calcitonin, blood coagulation tests), cytologic examination after at least two FNAB confirming benignity, thyroid scintiscan with 99mTc pertechnetate showing cold thyroid nodule, US investigation |
|
| Interventions |
Number of study centres: 1 Country/location: Korea/Seoul Setting: outpatient (in a hospital) Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: nodule volume changes; pressure symptoms / cosmetic complaints changes | |
| Study details | Study terminated before regular end: no | |
| Publication details | English language of publication in a peer‐reviewed journal Commercial funding: "JHB is patent holder of unidirectional ablation electrode (but no money given by the company yet)" | |
| Stated aim of study | Quote from publication: "To prospectively evaluate the efficacy of additional radiofrequency (RF) ablation by comparing the results of one and two sessions" | |
| Notes | "The patients were treated with 2% lidocaine at the puncture site for local anesthesia" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Finally, 30 patients were prospectively randomly ... by using a computer‐assisted random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Quote from publication: "The operator was aware of the group for the patient at the time of thyroid ablation" Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Unclear risk | Comment: possible sponsor bias Comment: four cross‐over cases throughout the study: group 1: n = 3/15 (20%) received two sessions of RF, due unsatisfactory results after the first ablation and group 2: n = 1/15 (7%) received only one session because of satisfactory results. |
TETRA Hegedüs 1988.
| Methods | Parallel RCT with randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: solitary nodule cyst of at least 2 mL and the absence of any residual following complete cyst aspiration Exclusion criteria: toxic goitres; large multinodular goitre Diagnostic criteria: US demonstrating solitary thyroid cyst |
|
| Interventions |
Number of study centres: 1 Country/location: Denmark Setting: outpatients Treatment before study: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: cure; recurrence/no recurrence; cyst volume changes | |
| Study details | Study terminated before regular end: no | |
| Publication details |
English language of publication in a peer‐reviewed journal No information on funding |
|
| Stated aim of study | Quote from publication: "To investigate, if tetracycline hydrochloride instillation seems promising in further reducing the number of patients who have to undergo surgery in a larger series of patients with solitary thyroid cysts" | |
| Notes | Cure: absence of any residual nodule and an ultrasonic cyst volume of less than 1 mL 12 months after last treatment; subgroups: hemorrhagic cyst fluid and clear yellow cyst fluid | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Patients were randomized to aspiration followed by flushing ... either ... tetracycline or ... isotonic saline" Comment: no detailed information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detailed information |
| Blinding of participants ((semi)objective outcomes) (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of participants (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of personnel ((semi)objective outcomes (Semi)objective outcomes | Low risk | Comment: the study design probably did not introduce bias for (semi)objective outcomes |
| Blinding of personnel (subjective outcomes) Subjective outcomes | High risk | Comment: the study design could have introduced bias for subjective outcomes |
| Blinding of outcome assessment ((semi)objective outcomes) (Semi)objective outcomes | Unclear risk | Comment: no detailed information |
| Blinding of outcome assessment (subjective outcomes) Subjective outcomes | Unclear risk | Comment: no detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all randomised participants finished the study |
| Selective reporting (reporting bias) | Low risk | Comment: none detected |
| Other bias | Low risk | Comment: gender baseline imbalance |
BMD: bone mineral density; CVD: cardiovascular disease; EA: ethanol ablation; FNA: fine‐needle aspiration; FNAB: fine‐needle aspiration biopsy; FNAC: fine needle aspiration cytology; FT3: free tri‐iodothyronine; FT4: free thyroxine; I: iodine; 131I: iodine 131; ILP: interstitial laser photocoagulation; i.v.: intravenously; LP: laser photocoagulation; LP‐1: laser photocoagulation – one session; LP‐3: laser photocoagulation ‐ three sessions; LT4/L‐T4: levothyroxine; NaCl: sodium chloride; PEI: percutaneous ethanol injection; PEI‐1: percutaneous ethanol injection ‐ one session; PEI‐3: percutaneous ethanol injection ‐ three sessions; PLA: percutaneous laser ablation; PLAC: placebo; RCT: randomised controlled trial; RF: radiofrequency; RTA: radiofrequency thermal ablation; T3: plasma (serum) tri‐iodothyronine; T4: plasma (serum) thyroxine; 99mTc: Technetium 99m; TCN: thyroid cyst nodule; Tg: thyroglobulin; TgAb: antithyroglobulin autoantibody; TN: thyroid nodule; TPOAb: antiperoxidase autoantibody; TRH: thyrotropin‐releasing hormone; TSH: thyrotropin; TSH Ab: anti‐thyrotropin receptor antibody; US: ultrasonography; VAS: visual analogue scale; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baek 2010 | Not a randomised controlled trial |
| Cheung 1989 | Palpation as a method for measurement of nodule size |
| Diacinti 1992 | Not a randomised controlled trial |
| Dossing 2002 | Not a randomised controlled trial |
| Erdem 1997 | Not a randomised controlled trial |
| Kanotra 2008 | Not a randomised controlled trial |
| Kim 2005 | Not a randomised controlled trial |
| Knight 2006 | Aim of treatment was goitre reduction |
| Lima 1997 | Not a randomised controlled trial |
| Mainini 1995 | Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
LP Dossing 2001.
| Trial name or title | NCT00150150 |
| Methods |
Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: benign solitary solid and cystic thyroid nodules Enrollment: 70 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): interstitial laser photocoagulation (ILP) (one session) Comparator(s): ILP (two or three sessions); 131I |
| Outcomes |
Primary outcome(s)
|
| Starting date |
Study start date: January 2001 Study completion date: March 2006 |
| Contact information | Responsible party/principal investigator: Helle Dossing, MD; Odense University Hospital, Denmark |
| Notes | "The recruitment status of this study is unknown because the information has not been verified recently" No study results posted on ClinicalTrials.gov |
LP Pacella 2008.
| Trial name or title | NCT00858104 |
| Methods |
Allocation: randomised, multicentre Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: benign thyroid nodules Enrollment: 200 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): PLA Comparator(s): no intervention (only follow up) |
| Outcomes |
Primary outcome(s): "Short‐ (1‐year) and long‐ (3‐year) term evolution of the thyroid nodules volume and symptoms after the treatment vs. simple clinical observation (... endpoint: % nodules with greater than 50% base volume reduction and % patients free of symptoms; time frame 3 years)" SECONDARY OUTCOME(S): "Assessment of short‐term and long‐term PLA safety, tolerability and reproducibility ... time frame 3 years)" |
| Starting date |
Study start date: November 2008 Study completion date: December 2012 |
| Contact information | Responsible party/principal investigator: Dr Claudio Maurizio Pacella |
| Notes | This study is ongoing, but not recruiting participants. No study results posted on ClinicalTrials.gov for this study. |
LT4 Shih 2007.
| Trial name or title | NCT00552253 |
| Methods |
Allocation: randomised, single centre Endpoint classification: safety/efficacy study Intervention model: cross‐over Masking: open label Primary purpose: treatment |
| Participants |
Condition: benign thyroid nodule Enrollment: 10 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): levothyroxine 100 µg/day (one hour before breakfast) for three months Comparator(s): levothyroxine (just after breakfast) for three months |
| Outcomes | Primary outcome(s): "The size of thyroid nodules (time frame: 3 months after levothyroxine treatment)" |
| Starting date |
Study start date: October 2007 Study completion date: July 2008 |
| Contact information | Responsible party/principal investigator: Shyang‐Rong Shih, Internal Medicine, National Taiwan University Hospital |
| Notes | No study results posted on ClinicalTrials.gov |
RF Baek 2013.
| Trial name or title | NCT01778400 |
| Methods |
Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: thyroid nodules Enrollment: 50 Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention(s): radiofrequency ablation Comparator(s): ethanol ablation |
| Outcomes |
Primary outcome(s): "Quantitative volume reduction ratio of a thyroid lesion at six months following compared with before the ablation treatment" Secondary outcomes(s): "Binary therapeutic success rate which was defined as the proportion of patients who showed volume reduction > 50%, improvement of symptomatic and cosmetic scores, and the number of major complications" |
| Starting date |
Study start date: February 2013 Study completion date: March 2014 |
| Contact information | Responsible party/principal investigator: Jung Hwan Baek, Asan Medical Center |
| Notes | This study is currently recruiting participants |
131I: iodine 131; PLA: percutaneous laser ablation; 99mTc: Technetium 99m; TSH: thyrotropin; US: ultrasound
Differences between protocol and review
Due to a significant time lag between publication of the protocol and first review draft almost all aspects of the review underwent significant changes due to improvements in methodology, reporting and standardisation. In particular, the contact person and team of authors changed. The scope of the review was enlarged from the evaluation of LT4 therapy only to encompass LT4 and all minimally invasive therapies. Consequently, the title was changed from 'Pharmacotherapy for thyroid nodules' to 'Levothyroxine or minimally invasive therapies for benign thyroid nodules'.
Contributions of authors
Elizabeth Bandeira‐Echtler (EBE): protocol development, first person for trial selection, data extraction, data analysis, data interpretation, review development and future review update.
Bernd Richter (BR): protocol development, second person for trial selection, checked on data extraction, checked on data analysis, checked on data interpretation, review development and future review update.
Karla Bergerhoff (KB): protocol development, acquiring trial reports, third person for trial selection and review development.
Sources of support
Internal sources
No sources of support supplied
External sources
Heinrich‐Heine‐University of Duesseldorf, Germany.
Declarations of interest
EBE: none known.
BR: none known.
KB: none known.
Edited (no change to conclusions)
References
References to studies included in this review
LP Dossing 2005 {published data only}
- Dossing H, Bennedbaek FN, Hegedus L. Effect of ultrasound‐guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules ‐ a randomised study. European Journal of Endocrinology 2005;152(3):341‐5. [DOI] [PubMed] [Google Scholar]
LP Dossing 2006 {published data only}
- Dossing H, Bennedbaek FN, Hegedus L. Effect of ultrasound‐guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules: one versus three treatments. Thyroid 2006;16(8):763‐8. [DOI] [PubMed] [Google Scholar]
LP Dossing 2013 {published data only}
- Dossing H, Bennedbaek FN, Hegedus L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules ‐ a prospective randomized trial. The Journal of Clinical Endocrinology and Metabolism 2013;98(7):E1213‐7. [DOI] [PubMed] [Google Scholar]
LP Gambelunghe 2006 {published data only}
- Gambelunghe G, Fatone C, Ranchelli A, Fanelli C, Lucidi P, Cavaliere A, et al. A randomized controlled trial to evaluate the efficacy of ultrasound‐guided laser photocoagulation for treatment of benign thyroid nodules. Journal of Endocrinological Investigation 2006;29(9):RC23‐6. [DOI] [PubMed] [Google Scholar]
LP Papini 2007 {published data only}
- Papini E, Guglielmi R, Bizzarri G, Graziano F, Bianchini A, Brufani C, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow‐up. Thyroid 2007;17(3):229‐35. [DOI] [PubMed] [Google Scholar]
LT4 Bayani 2012 {published data only}
- Bayani M, Amani M, Moazezi Z. Efficacy of levothyroxine on benign thyroid nodule. Caspian Journal of Internal Medicine 2012;3(1):359‐62. [PMC free article] [PubMed] [Google Scholar]
LT4 Boguszewski 1998 {published data only}
- Boguszewski CL, Pedrazzani M, Graf H. Assessment of levothyroxine suppressive therapy in patients with solitary thyroid nodules: a double‐blind, placebo‐controlled, clinical trial. Arquivos Brasileiros de Endocrinologia e Metabologia 1998;42(3):214‐21. [Google Scholar]
LT4 Cesareo 2010 {published data only}
- Cesareo R, Iozzino M, Isgro MA, Annunziata F, Stasio E. Short term effects of levothyroxine treatment in thyroid multinodular disease. Endocrine Journal 2010;57(9):803‐9. [DOI: 10.1507/endocrj.K10E-144] [DOI] [PubMed] [Google Scholar]
LT4 Gharib 1987 {published data only}
- Gharib H, James EM, Charboneau JW, Naessens JM, Offord KP, Gorman CA. Suppressive therapy with levothyroxine for solitary thyroid nodules. A double‐blind controlled clinical study. The New England Journal of Medicine 1987;317(2):70‐5. [DOI] [PubMed] [Google Scholar]
LT4 Grineva 2003 {published data only}
- Grineva EN, MalakhovaTV, Tsoi UA, Smirnov BI. Efficacy of thyroxine and potassium iodide in benign nodular lesions of the thyroid. Terapevticheskii Arkhiv 2003;75(8):72‐5. [PubMed] [Google Scholar]
LT4 Grussendorf 2011 {published data only}
- Grussendorf M, Reiners C, Paschke R, Wegscheider K, on behalf of the LISA investigators. Reduction of thyroid nodule volume by levothyroxine and iodine alone and in combination: a randomized, placebo‐controlled trial. The Journal of Clinical Endocrinology and Metabolism 2011;96:2786‐95. [DOI: 10.1210/jc.2011-0356] [DOI] [PMC free article] [PubMed] [Google Scholar]
LT4 Koc 2002 {published data only}
- Koc M, Ersoz HO, Akpinar I, Gogas‐Yavuz D, Deyneli O, Akalin S. Effect of low‐ and high‐dose levothyroxine on thyroid nodule volume: a crossover placebo‐controlled trial. Clinical Endocrinology 2002;57(5):621‐8. [DOI] [PubMed] [Google Scholar]
LT4 Larijani 2005 {published data only}
- Larijani B, Pajouhi M, Bastanhagh MH, Sadjadi A, Aghakhani S, Zare F, et al. Role of levothyroxine suppressive therapy for benign cold nodules of thyroid: a randomized, double‐blind, placebo‐controlled clinical trial. Therapy 2005;2(6):883‐8. [Google Scholar]
- Larijani B, Pajouhi M, Bastanhagh MH, Sadjadi A, Sedighi N, Eshraghian MR. Evaluation of suppressive therapy for cold thyroid nodules with levothyroxine: double‐blind placebo‐controlled clinical trial. Endocrine Practice 1999;5(5):251‐6. [DOI] [PubMed] [Google Scholar]
LT4 La Rosa 1995 {published data only}
- Rosa GL, Lupo L, Giuffrida D, Gullo D, Vigneri R, Belfiore A. Levothyroxine and potassium iodide are both effective in treating benign solitary solid cold nodules of the thyroid. Annals of Internal Medicine 1995;122(1):1‐8. [DOI] [PubMed] [Google Scholar]
LT4 Ozkaya 2010 {published data only}
- Ozkaya EC, Aydin Y, Ozkan B, Karaahmetoglu OS, Eskioglu E, Guler S. The effect of thyroxine‐suppressive therapy in patients with euthyroid nodular disease: A randomized controlled study. Endocrinologist 2010;20(4):182‐4. [Google Scholar]
LT4 Papini 1993 {published data only}
- Papini E, Bacci V, Panunzi C, Pacella CM, Fabbrini R, Bizzarri G, et al. A prospective randomized trial of levothyroxine suppressive therapy for solitary thyroid nodules. Clinical Endocrinology 1993;38(5):507‐13. [DOI] [PubMed] [Google Scholar]
LT4 Papini 1998 {published data only}
- Papini E, Petrucci L, Guglielmi R, Panunzi C, Rinaldi R, Bacci V, et al. Long‐term changes in nodular goiter: a 5‐year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. The Journal of Clinical Endocrinology and Metabolism 1998;83(3):780‐3. [DOI] [PubMed] [Google Scholar]
LT4 Reverter 1992 {published data only}
- Reverter JL, Lucas A, Salinas I, Audi L, Foz M, Sanmarti A. Suppressive therapy with levothyroxine for solitary thyroid nodules. Clinical Endocrinology 1992;36(1):25‐8. [DOI] [PubMed] [Google Scholar]
LT4 Tsai 2006 {published data only}
- Tsai CC, Pei D, Hung YJ, Wang TF, Tsai WC, Yao CY, et al. The effect of thyroxine‐suppressive therapy in patients with solitary non‐toxic thyroid nodules ‐ a randomised, double‐blind, placebo‐controlled study. International Journal of Clinical Practice 2006;60(1):23‐6. [DOI] [PubMed] [Google Scholar]
LT4 Wemeau 2002 {published data only}
- Wemeau JL, Caron P, Schvartz C, Schlienger JL, Orgiazzi J, Cousty C, et al. Effects of thyroid‐stimulating hormone suppression with levothyroxine in reducing the volume of solitary thyroid nodules and improving extranodular nonpalpable changes: a randomized, double‐blind, placebo‐controlled trial by the French Thyroid Research Group. The Journal of Clinical Endocrinology and Metabolism 2002;87(11):4928‐34. [DOI] [PubMed] [Google Scholar]
- Wemeau JL, Cousty C, Vlaeminck V. Suppressive hormone therapy for thyroid nodules. Prospective evaluation. Preliminary results [Hormonotherapie freinatrice pour nodule thyroidien. Evaluation prospective. Resultats preliminaires]. Annales d'Endocrinologie 2000;61(2):119‐24. [PubMed] [Google Scholar]
LT4 Zelmanovitz 1998 {published data only}
- Zelmanovitz F, Genro S, Gross JL. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double‐blind controlled clinical study and cumulative meta‐analyses. The Journal of Clinical Endocrinology and Metabolism 1998;83(11):3881‐5. [DOI] [PubMed] [Google Scholar]
PEI Bennedbaek 1998 {published data only}
- Bennedbaek FN, Nielsen LK, Hegedus L. Effect of percutaneous ethanol injection therapy versus suppressive doses of L‐thyroxine on benign solitary solid cold thyroid nodules: a randomized trial. The Journal of Clinical Endocrinology and Metabolism 1998;83(3):830‐5. [DOI] [PubMed] [Google Scholar]
PEI Bennedbaek 1999 {published data only}
- Bennedbaek FN, Hegedus L. Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: a randomized trial comparing one injection with three injections. Thyroid 1999;9(3):225‐33. [DOI] [PubMed] [Google Scholar]
PEI Bennedbaek 2003 {published data only}
- Bennedbaek FN, Hegedus L. Treatment of recurrent thyroid cysts with ethanol: a randomized double‐blind controlled trial. The Journal of Clinical Endocrinology and Metabolism 2003;88(12):5773‐7. [DOI] [PubMed] [Google Scholar]
PEI Chu 2003 {published data only}
- Chu CH, Chuang MJ, Wang MC, Lam HC, Lu CC, Lee JK. Sclerotherapy of thyroid cystic nodules. Journal of the Formosan Medical Association 2003;102(9):625‐30. [PubMed] [Google Scholar]
PEI Sung 2013 {published data only}
- Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single‐session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 2013;259(1):293‐300. [10.1148/radiol. 13122134] [DOI] [PubMed] [Google Scholar]
PEI Valcavi 2004 {published data only}
- Valcavi R, Frasoldati A. Ultrasound‐guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocrine Practice 2004;10(3):269‐75. [DOI] [PubMed] [Google Scholar]
PEI Verde 1994 {published data only}
- Verde G, Papini E, Pacella CM, Gallotti C, Delpiano S, Strada S, et al. Ultrasound guided percutaneous ethanol injection in the treatment of cystic thyroid nodules. Clinical Endocrinology 1994;41(6):719‐24. [DOI] [PubMed] [Google Scholar]
RF Faggiano 2012 {published data only}
- Faggiano A, Ramundo V, Assanti AP, Fonderico F, Macchia PE, Misso C, et al. Thyroid nodules treated with percutaneous radiofrequency thermal ablation: a comparative study. The Journal of Clinical Endocrinology and Metabolism 2012;97(12):4439‐45. [DOI: 10.1210/jc.2012-2251] [DOI] [PubMed] [Google Scholar]
RF Huh 2012 {published data only}
TETRA Hegedüs 1988 {published data only}
- Hegedus L, Hansen JM, Karstrup S, Torp‐Pedersen S, Juul, N. Tetracycline for sclerosis of thyroid cysts. Archives of Internal Medicine 1988;148(5):1116‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baek 2010 {published data only}
- Baek JH, Kim YS, Lee D, Huh JY, Lee JH. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. American Journal of Roentgenology 2010;194(4):1137‐42. [DOI] [PubMed] [Google Scholar]
Cheung 1989 {published data only}
- Cheung PS, Lee JM, Boey JH. Thyroxine suppressive therapy of benign solitary thyroid nodules: a prospective randomized study. World Journal of Surgery 1989;13(6):818‐21. [DOI] [PubMed] [Google Scholar]
Diacinti 1992 {published data only}
- Diacinti D, Salabe GB, Olivieri A, D'Erasmo E, Tomei E, Lotz‐Salabe H, et al. Efficacy of L‐thyroxine (L‐T4) therapy on the volume of the thyroid gland and nodules in patients with euthyroid nodular goiter (ENG). [Italian] [Efficacia della terapia con L‐tiroxina (L‐T4) sul volume della tiroide e dei noduli in pazienti con gozzo nodulare eutiroideo (GNE)]. Minerva Medica 1992;83(11):745‐51. [PubMed] [Google Scholar]
Dossing 2002 {published data only}
- Dossing H, Bennedbaek FN, Karstrup S, Hegedus L. Benign solitary solid cold thyroid nodules: US‐guided interstitial laser photocoagulation‐initial experience. Radiology 2002;225(1):53‐7. [DOI] [PubMed] [Google Scholar]
Erdem 1997 {published data only}
- Erdem E, Bostanci B, Ozden A, Sungurtekin U, Nessar M. The effectiveness of levothyroxine suppressive therapy in patients with multinodular goiter [Multinoduler guatrli hastalarda levotiroksinle supresyon tedavisinin etkinligi]. Turkish Journal of Surgery 1997;13(6):400‐4. [Google Scholar]
Kanotra 2008 {published data only}
- Kanotra S.P, Lateef M, Kirmani O. Non‐surgical management of benign thyroid cysts: use of ultrasound‐guided ethanol ablation. Postgraduate Medical Journal 2008;84(998):639‐43. [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim DW, Rho MH, Kim HJ, Kwon JS, Sung YS, Lee SW. Percutaneous ethanol injection for benign cystic thyroid nodules: is aspiration of ethanol‐mixed fluid advantageous?. American Journal of Neuroradiology 2005;26(8):2122‐7. [PMC free article] [PubMed] [Google Scholar]
Knight 2006 {published data only}
- Knight JS, Satchidanand RY, Yiangou C, Jackson A, Cummings MH. A double‐blind randomised trial to evaluate the effect of anastrozole for the treatment of non‐toxic multinodular goitre. International Journal of Clinical Practice 2006;60(8):911‐3. [DOI] [PubMed] [Google Scholar]
Lima 1997 {published data only}
- Lima N, Knobel M, Cavaliere H, Sztejnsznajd C, Tomimori E, Medeiros‐Neto G. Levothyroxine suppressive therapy is partially effective in treating patients with benign, solid thyroid nodules and multinodular goiters. Thyroid 1997;7(5):691‐7. [DOI] [PubMed] [Google Scholar]
Mainini 1995 {published data only}
- Mainini E, Martinelli I, Morandi G, Villa S, Stefani I, Mazzi C. Levothyroxine suppressive therapy for solitary thyroid nodule. Journal of Endocrinological Investigation 1995;18(10):796‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
LP Dossing 2001 {published data only}
- NCT00150150. Ultrasound guided interstitial laser photocoagulation on benign thyroid nodules. http://clinicaltrials.gov/ct2/show/NCT00150150 (accessed 29 July 2013).
LP Pacella 2008 {published data only}
- NCT00858104. Multicentric randomized controlled study of percutaneous laser ablation versus follow up in benign thyroid nodules. Long term results. http://clinicaltrials.gov/ct2/show/NCT00858104 (accessed 29 July 2013).
LT4 Shih 2007 {published data only}
- NCT00552253. Levothyroxine treatment in thyroid benign nodular goiter. http://clinicaltrials.gov/ct2/show/NCT00552253 (accessed 29 July 2013).
RF Baek 2013 {published data only}
- NCT01778400. Single session treatment of RFA versus EA for predominantly cystic thyroid nodules: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01778400 (accessed 29 July 2013).
Additional references
AACE/AME/ETA Guidelines 2010
- Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association: medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocrine Practice 2010;16 Suppl 1:1‐43. [DOI] [PubMed] [Google Scholar]
Alcantara‐Jones 2006
- Alcantara‐Jones DM, Araujo LM, Almeida MA, Jones DA, Cardoso LJG, Passos MC. Percutaneous ethanol injection for the treatment of thyroid nodules [Efeito da injecao percutânea de etanol na reducao de nódulos tireoideanos]. Arquivos Brasileiros de Endocrinologia & Metabologia 2006;50(1):97‐104. [DOI] [PubMed] [Google Scholar]
Alexander 2003
- Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet MP, et al. Natural history of benign solid cystic thyroid nodules. Annals of Internal Medicine 2003;138:315‐8. [DOI] [PubMed] [Google Scholar]
Astwood 1960
- Astwood EB, Cassidy C, Aurbach GD. Treatment of goiter and thyroid nodules with thyroid. JAMA 1960;174:459‐64. [DOI] [PubMed] [Google Scholar]
ATA 2009
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167‐1214. [DOI] [PubMed] [Google Scholar]
Baek 2009
- Baek JH, Moon WJ, Kim YS, Lee JH, Lee D. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World Journal of Surgery 2009;33(9):1971‐7. [DOI] [PubMed] [Google Scholar]
Bauer 2001
- Bauer DC, Ettinger B, Nevitt MC, Stone KL. Risk for fracture in women with low serum levels of thyroid‐stimulating hormone. Annals of Internal Medicine 2001;134(7):561‐8. [DOI] [PubMed] [Google Scholar]
Belfiore 1992
- Belfiore A, Rosa GL, Porta GA. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age and multinodularity. American Journal of Medicine 1992;93:363‐9. [DOI] [PubMed] [Google Scholar]
Bennedbaek 1995
- Bennedbaek FN, Hegedus L. Alcohol sclerotherapy for benign solitary solid cold thyroid nodules. Lancet 1995;346(8984):1227. [DOI] [PubMed] [Google Scholar]
Bennedbaek 1997
- Bennedbaek FN, Karstrup S, Hegedüs L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. European Journal of Endocrinology 1997;136:240‐50. [DOI] [PubMed] [Google Scholar]
Biondi 1993
- Biondi B, Fazio S, Carella C, Amato G, Cittadini A, Lupoli G, et al. Cardiac effects of long term thyrotropin‐suppressive therapy with levothyroxine. The Journal of Clinical Endocrinology and Metabolism 1993;77(2):334‐8. [DOI] [PubMed] [Google Scholar]
Braga‐Brasaria 2002
- Braga‐Basaria M, Trippia MA, Stolf AR, Mesa CJr, Graf H. Treatment of autonomous and cystic thyroid nodules with intranodular ethanol injection [Tratamento de nódulos autônomos e císticos da tireóide com injecao intranodular de etanol]. Revista da Associacao Médica Brasileira 2002;48(4):335‐40. [DOI] [PubMed] [Google Scholar]
Brander 1991
- Brander A, Viikinkoski P, Nickels J. Thyroid gland: OS screening in a random adult population. Radiology 1991;181:683‐7. [DOI] [PubMed] [Google Scholar]
Brito 2013
- Brito JP, Yarur AJ, Prokop LJ, McIver B, Murad MH, Montori VM. Prevalence of thyroid cancer in multinodular goiter versus single nodule: a systematic review and meta‐analysis. Thyroid 2013;23(4):449‐55. [DOI] [PubMed] [Google Scholar]
Burch 1995
- Burch HB. Evaluation and management of the solid thyroid nodule. Endocrinology and Metabolism Clinics of North America 1995;24:663‐710. [PubMed] [Google Scholar]
Castro 2002
- Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta‐analysis. The Journal of Clinical Endocrinology and Metabolism 2002;87(9):4154‐9. [DOI] [PubMed] [Google Scholar]
Cooper 1995
- Cooper DS. Clinical review 66: thyroxine suppression therapy for benign nodular disease. The Journal of Clinical Endocrinology and Metabolism 1995;80(2):331‐4. [DOI] [PubMed] [Google Scholar]
Daniels 1996
- Daniels GH. Thyroid nodules and nodular thyroids: a clinical overview. Comprehensive Therapy 1996;22:239‐50. [PubMed] [Google Scholar]
Davies 2006
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973‐2002. JAMA 2996;295(18):2164‐7. [DOI] [PubMed] [Google Scholar]
Deandrea 2008
- Deandrea M, Limone P, Basso E, Mormile A, Ragazzoni F, Gamarra E, et al. US‐guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound in Medicine & Biology 2008;34(5):784‐91. [DOI] [PubMed] [Google Scholar]
Dossing 2007
- Dossing H, Bennedbaek FN, Bonnema SJ, Grupe P, Hegedus L. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. European Journal of Endocrinology 2007;157(1):95‐100. [DOI] [PubMed] [Google Scholar]
Dossing 2011
- Dossing H, Bennedbaek FN, Hegedus L. Long‐term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. European Journal of Endocrinology 2011;165(1):123‐8. [DOI] [PubMed] [Google Scholar]
Esnault 2008
- Esnault O, Leenhardt L. High intensity focused ultrasound (HIFU) ablation therapy for thyroid nodules. In: Baskin HJ, Duick DS, Levine RA editor(s). Thyroid ultrasound and ultrasound‐guided FNA. 2nd Edition. New York: Springer, 2008:219‐36. [Google Scholar]
Esnault 2011
- Esnault O, Franc B, Ménégaux F, Rouxel A, Kerviler E, Bourrier P, et al. High‐Intensity focused ultrasound ablation of thyroid nodules: first human feasibility study. Thyroid 2011;21(9):965‐73. [DOI] [PubMed] [Google Scholar]
Feng 2012
- Feng B, Liang P, Cheng Z, Yu X, Yu J, Han Z, et al. Ultrasound‐guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. European Journal of Endocrinology / European Federation of Endocrine Societies 2012; Vol. 166, issue 6:1031‐7. [1479‐683X: (Electronic)] [DOI] [PubMed]
Filetti 2006
- Filetti S, Durante C, Torlontano M. Nonsurgical approaches to the management of thyroid nodules. Nature Clinical Practice Endocrinology & Metabolism 2006;2(7):384‐94. [DOI] [PubMed] [Google Scholar]
Fuller 2014
- Fuller CW, Nguyen SA, Lohia S, Gillespie MB. Radiofrequency ablation for treatment of benign thyroid nodules: systematic review. The Laryngoscope 2014;124:346‐53. [DOI] [PubMed] [Google Scholar]
Galofré 2008
- Galofré JC, Lomvardias S, Davies TF. Evaluation and treatment of thyroid nodules: a clinical guide. Mount Sinai Journal of Medicine 2008;75(3):299‐311. [DOI] [PubMed] [Google Scholar]
Gharib 1998
- Gharib H, Mazzaferri EL. Thyroxine suppressive therapy in patients with nodular thyroid disease. Annals of Internal Medicine 1998;128(5):386‐94. [DOI] [PubMed] [Google Scholar]
Gharib 2007
- Gharib H, Papine E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinology and Metabolism Clinics of North America 2007;36(3):707‐35. [DOI] [PubMed] [Google Scholar]
Gharib 2010
- Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Journal of Endocrinological Investigation 2010; Vol. 33, issue Suppl:51‐6. [PubMed]
Gharib 2013
Hamming 1990
- Hamming JF, Goslings BM, Steenis GJ, Ravenswaay Claasen H, Hermans J, et al. The value of fine needle aspiration biopsy in patients with nodular thyroid disease divided into groups of suspicion of malignant neoplasms on clinical grounds. Archives of Internal Medicine 1990;150(1):113‐6. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrobjartsson 2012
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [PUBMED: 22371859] [DOI] [PubMed] [Google Scholar]
Hrobjartsson 2013
- Hrobjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [PUBMED: 23359047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2013
- Huang TW, Lai JH, Wu MY, Chen SL, Wu CH, Tam KW. Systematic review of clinical practice guidelines in the diagnosis and management of thyroid nodules and cancer. BMC Medicine 2013;11:191. [DOI: 10.1186/1741-7015-11-191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jemal 2009
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians 2009;59(4):225‐49. [DOI] [PubMed] [Google Scholar]
Jeong 2008
- Jeong WK, Baek JR, Rhim H, Kim YS, Kwak MS, Jeong HJ, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow‐up in 236 patients. European Radiology 2008;18(6):1244‐50. [DOI] [PubMed] [Google Scholar]
Kaplan 1990
- Kaplan MM. Progress in thyroid cancer. Endocrinology and Metabolism Clinics of North America 1990;19(3):469‐78. [PubMed] [Google Scholar]
Kim 2006
- Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006;16(4):361‐7. [DOI] [PubMed] [Google Scholar]
Kuma 1992
- Kuma K, Matsuzuka F, Kobayashi A. Outcome of long standing solitary thyroid nodules. World Journal of Surgery 1992;16(4):583‐7. [DOI] [PubMed] [Google Scholar]
LATS 2009
- Camargo R, Corigliano S, Friguglietti C, Gauna A, Harach R, Munizaga F, et al. Latin American thyroid society recommendations for the management of thyroid nodules [Recomendacoes da Sociedade Latino‐Americana de Tireoide no manejo de nódulos tireoideos]. Arquivos Brasileiros de Endocrinologia e Metabologia 2009;53(9):1167‐75. [DOI] [PubMed] [Google Scholar]
Leese 2011
- Leese GP, Flynn RV. Levothyroxine dose and fractures in older adults. BMJ 2011;342:d2250. [DOI: 10.1136/bmj.d2250] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Med 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lima 2007
- Lima MA, Fagundes TA, Raffaelli CM, Ferreira BP, Resende EM, Fonseca EC, et al. Alcoholization in the treatment of thyroid nodule in colloid goiter endemic region [Alcoolizacao de nódulo tiroidiano em regiao endêmica de bócio colóide]. Arquivos Brasileiros de Endocrinologia e Metabologia 2007;51(6):1007‐12. [DOI] [PubMed] [Google Scholar]
Livraghi 1990
- Livraghi T, Paracchi A, Ferrari C, Bergonzi M, Garavaglia G, Raineri P, et al. Treatment of autonomous thyroid nodules with percutaneous ethanol injection: preliminary results. Work in progress. Radiology 1990;175(3):827‐9. [DOI] [PubMed] [Google Scholar]
Mazzaferri 1988
- Mazzaferri EL, Santos ET, Rofaghan‐Keyhani S. Solitary thyroid nodule: diagnosis and management. Medical Clinics of North America 1988;72(5):1177‐211. [DOI] [PubMed] [Google Scholar]
Mazzaferri 1993
- Mazzaferri EL. Management of a solitary thyroid nodule. The New England Journal of Medicine 1993;328(8):553‐9. [DOI] [PubMed] [Google Scholar]
McCall 1986
- McCall A, Jarosz H, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in solitary cold nodules and in multinodular goiters. Surgery 1986;100(6):1128‐32. [PubMed] [Google Scholar]
Morita 1989
- Morita T, Tamai H, Oshima A, Komaki G, Matsubayashi S, Kuma K, et al. Changes in serum thyroid hormone, thyrotropin and thyroglobulin concentrations during thyroxine therapy in patients with solitary thyroid nodules. The Journal of Clinical Endocrinology and Metabolism 1989;69(2):227‐30. [DOI] [PubMed] [Google Scholar]
Pacella 2000
- Pacella CM, Bizzarri G, Guglielmi R, Anelli V, Bianchini A, Crescenzi A, et al. Thyroid tissue: US‐guided percutaneous interstitial laser ablation ‐ a feasibility study. Radiology 2000;217(3):673‐7. [DOI] [PubMed] [Google Scholar]
Papini 1993
- Papini E, Bacci V, Panunzi C, Pacella CM, Fabbrini R, Bizzarri G, et al. A prospective randomized trial of levothyroxine suppressive therapy for solitary thyroid nodules. Clinical Endocrinology 1993;38(5):507‐13. [DOI] [PubMed] [Google Scholar]
Papini 1995
- Papini E, Pacella CM, Verde G. Percutaneous ethanol injection (PEI): what is its role in the treatment of benign thyroid nodules?. Thyroid 1995;5(2):147‐50. [DOI] [PubMed] [Google Scholar]
Papini 2004
- Papini E, Guglielmi R, Bizzarri G, Pacella CM. Ultrasound‐guided laser thermal ablation for treatment of benign thyroid nodules. Endocrine Practice 2004;10(3):276‐83. [DOI] [PubMed] [Google Scholar]
Pelizzo 1990
- Pelizzo MR, Piotto A, Rubello D, Casara D, Fassina A, Busnardo B. High prevalence of occult papillary thyroid carcinoma in a surgical series for benign thyroid disease. Tumori 1990;76(3):255‐7. [DOI] [PubMed] [Google Scholar]
Richter 2002
- Richter B, Neises G, Clar C. Pharmacotherapy for thyroid nodules. A systematic review and meta‐analysis. Endocrinology and Metabolism Clinics of North America 2002;31(3):699‐722. [DOI] [PubMed] [Google Scholar]
Sdano 2005
- Sdano MT, Falciglia M, Welge JA, Steward DL. Efficacy of thyroid hormone suppression for benign thyroid nodules: meta‐analysis of randomized trials. Otolaryngology ‐ Head and Neck Surgery 2005;133(3):391‐6. [DOI] [PubMed] [Google Scholar]
Spiezia 2009
- Spiezia S, Garberoglio R, Milone F, Ramundo V, Caiazzo C, Assanti AP, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 2009;19(3):219‐25. [DOI] [PubMed] [Google Scholar]
Stall 1990
- Stall GM, Harris S, Sokoll LJ, Dawson‐Hughes B. Accelerated bone loss in hypothyroid patients overtreated with L‐thyroxine. Annals of Internal Medicine 1990;113(4):265‐9. [DOI] [PubMed] [Google Scholar]
Stern 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Tunbridge 1977
- Tunbridge WMG, Evered DC, Hall R. The spectrum of thyroid disease in a community: The Wickham survey. Clinical Endocrinology 1977;7:481‐93. [DOI] [PubMed] [Google Scholar]
Uzzan 1996
- Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta‐analysis. The Journal of Clinical Endocrinology & Metabolism 1996;81(12):4278‐89. [DOI] [PubMed] [Google Scholar]
Vander 1968
- Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15‐year study on the incidence of thyroid malignancy. Annals of Internal Medicine 1968;69(3):537‐40. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousef 2010
- Yousef A, Clark J, Suhail AR. Thyroxine suppression therapy for benign, non‐functioning solitary thyroid nodules: a quality‐effects meta‐analysis. Clinical Medicine and Research 2010;8(3‐4):150‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelmanovitz 1998
- Zelmanovitz F, Genro S, Gross JL. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double‐blind controlled clinical study and cumulative meta‐analyses. The Journal of Clinical Endocrinology and Metabolism 1998;83(11):3881‐5. [DOI] [PubMed] [Google Scholar]
Zingrillo 1998
- Zingrillo M, Collura D, Ghiggi MR, Nirchio V, Trischitta V. Treatment of large cold benign thyroid nodules not eligible for surgery with percutaneous ethanol injection. The Journal of Clinical Endocrinology and Metabolism 1998;83(11):3905‐7. [DOI] [PubMed] [Google Scholar]


