Highlights
-
•
AEW delayed pulp softening of longans via suppressing cell wall disassembly.
-
•
AEW down-regulated expression levels of longan pulp cell wall degrading-related genes.
-
•
AEW decreased activities of cell wall degrading enzymes in pulp of harvested longans.
-
•
AEW retained higher levels of longan pulp CWM, CSP, ISP, cellulose, and hemicellulose.
Keywords: Acidic electrolyzed water, Longan fruit, Pulp firmness, Cell wall polysaccharides, Cell wall degrading enzymes, Gene expression
Abbreviations: PG, polygalacturonase; PE, pectinesterase; XET, xyloglucan endotransglycosylase; β-Gal, β-galactosidase; CEL, cellulase; CWM, cell wall materials; ISP, ionic-soluble pectin; WSP, water-soluble pectin; CSP, covalent-soluble pectin; CWP, cell wall polysaccharides; CWDEs, cell wall degrading enzymes; NFT, near freezing temperature; AEW, acidic electrolyzed water; 1-MCP, 1-methylcyclopropene
Abstract
Effects of acidic electrolyzed water (AEW) treatment (pH = 2.5, ACC = 80 mg L−1, 10 min) on pulp firmness, amounts of CWM and CWP, activities and expression of relevant genes of CWDEs in pulp of Fuyan longan during storage at 25 °C were evaluated. Compared to control samples, during storage, AEW-treated fruit retained a higher pulp firmness, prevented WSP formation, reduced the degradation of CSP, cellulose and hemicellulose, and lowered CWDEs activities and their corresponding gene expression. When stored for 5 d, pulp firmness (113.6 g mm−1), CWM (13.9 g kg−1), and CSP (1.4 g kg−1) in AEW-treated fruit displayed the clearly higher contents than those in control samples. These data suggest that AEW treatment can slow down the pulp softening and retain higher pulp CWP levels in postharvest fresh longans, which was because AEW lowered activities of CWDEs and its gene expression levels, and maintained the cell wall structure's integrity.
1. Introduction
Longan (Dimocarpus longan Lour.) pulp containing high levels of polysaccharides, polyphenols, total soluble sugar and vitamins and have been reported to possess health benefits such as antioxidant, antitumoral, heart protection and immune regulation effects (Bai et al., 2020, Chen et al., 2020, Huang et al., 2019, Lin et al., 2021, Liu et al., 2021). Longan production and consumption have increased dramatically in recent decades. However, the rapid softening of longan pulp during storage at room temperature severely limits its quality, storability, and marketing potential (Chen et al., 2020, Chen et al., 2021, Lin et al., 2019, Lin et al., 2020, Yi et al., 2012). Recent research has linked fruit pulp softening to the destruction of cell wall materials (CWM), cell wall polysaccharides (CWP) and the enhancement of activities and expression of relevant genes of cell wall degrading enzymes (CWDEs), mainly characterized by declining in pectin, cellulose, and hemicelluloses, increasing in activities and gene expression of polygalacturonase (PG), pectinesterase (PE), xyloglucan endotransglycosylase (XET), β-galactosidase (β-Gal), and cellulase (CEL) (Chen et al., 2021, Li et al., 2019, Lin et al., 2019, Lin et al., 2020, Posé et al., 2019, Shi et al., 2019, Zhao et al., 2019, Zhong et al., 2008). Previous studies established the applications of paper-based 1-methylcyclopropene (1-MCP) (Chen et al., 2017a, Lin et al., 2018, Wang et al., 2020), chitosan (Lin et al., 2019), graft copolymer of salicylic acid and chitosan (Shi et al., 2019), nitric oxide fumigation (Zhao et al., 2019) or yeast mannan (Xie et al., 2017) in harvested fruits to inhibit different CWDEs and prevent degradation of CWP, and delay the fruit softening process. Therefore, it is critical to investigate the mechanisms of postharvest longan fruit softening and the degradation of CWP, as well as to identify the effective methods to regulate the pulp softening and retain higher pulp CWP levels in fresh longans during postharvest storage.
The commercial postharvest handling like refrigeration can maintain the nutrition, freshness and edible safety of fresh fruits and vegetables, extend the shelf life and obtain the greatest economic benefits. However, cold-chain system is expensive and may not be practical for all parts of the world. Acidic electrolyzed water (AEW) is a novel, safe, environmentally friendly and effective post-harvest commercial handling method. In 2002, it was recognized as a food additive by Japan. At present, the use of AEW on fruits and vegetables has been officially approved by China, Japan, and USA (Huang et al., 2008, Rahman et al., 2016, Shiroodi and Ovissipour, 2018). Compare with conventional preservation methods, the advantages of AEW include low cost, high efficiency, and convenient application (Sun et al., 2022). AEW has been applied for controlling postharvest diseases in Valencia late oranges (Youssef & Hussien, 2020), Phu Lae pineapples (Khayankarn, Uthaibutra, Setha, & Whangchai, 2013), Fuyan longans (Tang et al., 2021) and to improve the quality and storability of harvested longan fruit (Chen et al., 2020). In addition, the treatment of AEW effectively inhibited fruit softening and maintain pulp firmness of treated Brightwell blueberries (12.5 N) than distilled water-treated samples (7.8 N) after 15 days storage at 4 °C (Chen, Hung, Chen & Lin, 2017b). Recently, Chen et al. (2020) also evaluated AEW at various available chlorine concentrations (ACC) and found 80 mg L−1 ACC could efficiently decrease the respiration rate, prevent leakage of the cellular membrane, maintain color (e.g. chromaticity values of L*, a* and b* of the fruit surface) and nutritive values (e.g. pulp total soluble solid, total soluble sugars, sucrose and vitamin C), and retard pulp softening of harvested longans during storage at 25 °C. However, the mechanism of AEW for retarding longans pulp softening process has not been elucidated clearly. The purpose of this study was to investigate the changes in pulp firmness of AEW-treated Fuyan longans during postharvest storage. Changes in CWM and CWP, as well as activities and gene expression of related CWDWs in response to AEW treatment, were determined to gain insights into the pulp softening delaying process of longans during storage.
2. Materials and methods
2.1. Materials and treatments
AEW was generated using a HRW-1500 EO machine (Huo-Ren-Jing-Chuang Medical Equipment Co., Ltd., Beijing, China). The physical and chemical characteristics of AEW were measured according to Tang et al. (2021).
Fruit of longan cv. Fuyan at commercial maturity around 120 days after full-bloom stage were hand-harvested on September 4, 2018 from an orchard located in Nan’an, China, and immediately transported to the laboratory and selected for uniformity and without any mechanical damage. On day 0, three hundred longan fruit were chosen for analysis. The rest of the fruit were distributed into two groups, the first of which was (1 500 fruit) soaked in AEW (pH: 2.5, ACC: 80 mg L−1, 10 min) according to our previous work (Chen et al., 2020, Tang et al., 2021), the other 1 500 fruit were immersed in distilled water as the control group. After treatment, fruit were air-dried and packaged (50 fruit per bag) before being stored at 25 °C for five days. Six bags (a total of 300 fruit) were sampled daily from each group. Twenty longans were selected from each bag to measure pulp firmness, and the remaining 30 fruit were frozen at − 80 °C until used.
2.2. Determination of pulp firmness
Pulp firmness was measured using a fruit firmness tester (FitmTech 7, BioWorks, Inc., Kansas, United States). Twenty longan fruit (without pericarp) were placed on the test platform, and punctured using a test probe at the longan pulp equator using the method described in Lin et al. (2020), using the compression rate of 13 mm s−1, the compression force increasing from 20 g to 300 g, and the curve maximum slope was used. The pulp firmness was expressed as g mm−1.
2.3. Determination of CWM amount
Fifty grams of pulp from 50 longan fruit were applied to assay CWM amount based on the method of Lin et al. (2019); Supplementary material: The assay of CWM). The amount of CWM was expressed as g kg−1, which was based on the fresh weight of longan pulp.
2.4. Determination of CWP contents
Polysaccharides in fruit cell walls include the fraction of pectin (i.e. covalent-soluble pectin, CSP; water-soluble pectin, WSP; ionic-soluble pectin; ISP), hemicelluloses, and cellulose, were separated and measured according to our prior work (Lin et al. 2019; Supplementary material: The assay of CWP). The contents of CWP were expressed as g kg−1, which were based on the fresh weight of longan pulp.
2.5. Determination of CWDEs activities
Ten grams of pulp obtained from 20 longan fruit were ground in liquid nitrogen, and then extracted the supernatants with 100 mmol L−1 NaCl solution (with 5 % polyvinypyrrolidone solution, 2 % mercaptoethanol, and 40 mmol L−1 sodium acetate solution), and activities of PE, XET, PG, CEL and β-Gal were measured according to the methods described in Lin et al. (2019; Supplementary material: The assay of CWDEs activities). The protein content was measured as described in the method of Bradford (1976). The activities of CWDEs were expressed as U mg−1 protein.
2.6. Isolation and analysis of related genes
In this work, isolation of full-length cDNA sequences genes (DlXET, DlPE, DlPG, Dlβ-Gal and DlCEL) were achieved via applying transcriptome sequencing from longans. These transcriptome sequencing gene numbers were identified with the numbers on longan genome (Lin et al., 2017). According to Lin et al. (2020), the protocol of TransZol Up Plus RNA Kit was applied to extract the RNA from the longan pulp directly. The related RNA was reverse transcribed into cDNA using the TaKaRa PrimeScriptTM RT reagent Kit. The gene-specific primers were designed by using Primer Premier 5 software, and the supplementary Table S1 displayed the gene-specific primers in this work. The RT-qPCR was performed using TaKaRa’s TB Green™ Premix Ex Taq™ kit (TaKaRa, RR420A) and QuantStudio5 Applied Biosystems instrument according to manufacturer’s protocol. The RT-qPCR detection program was designed referring to the method of Lin et al. (2020). The reactions were normalized by the cycle threshold (Ct) values, which was compared to the reference gene DlACTIN.
2.7. Statistical analyses
In this study, all the measurements were performed three times, and the SPSS Statistics Version 22 (International Business Machines Corp., New York, United States) was used for statistical analysis with a significance level (* p < 0.05 and ** p < 0.01).
3. Results and discussion
3.1. Changes in pulp firmness and CWM
Pulp firmness is an important property parameter and directly affecting fruit quality and storage shelf life (Chen et al., 2015, Ren et al., 2020, Zhao et al., 2019). The decrease in pulp firmness led to the depolymerization of middle lamella and cell wall (Chen et al., 2017a, Chen et al., 2017b, Zhao et al., 2019). The cell wall is a complex network of polysaccharides and proteins secreted by cells into the spaces between them (Broxterman and Schols, 2018, Posé et al., 2019). The cell wall plays important roles in wound repair, cell–cell signaling, cell adhesion and other tissue functions. Usually, maintaining pulp firmness and CWM content during storage indicate high integrity of the cell wall structures (Ren et al., 2020, Wang et al., 2021).
As shown in Fig. 1A, the pulp firmness of harvested longan fruit decreased continuously throughout the storage period. Control fruit pulp firmness decreased quickly from day 0 (156 g mm−1) to day 5 (103 g mm−1) during storage at 25 °C. For the AEW-treated longan fruit, the pulp firmness decreased slowly from 0 to 3 d, followed by a rapid decline (114 g mm−1 at day 5). From day 3 to 5, AEW-treated fruit showed significantly higher (p < 0.01) pulp firmness than control fruit, suggesting that AEW treatment could reduce the rate of pulp softening of harvested longans. This finding was consistent with our past report that AEW treatment for 5 min kept higher firmness of Camellia and Brightwell blueberries during storage (Chen et al., 2017b). Moreover, Hayta and Aday (2015) reported higher firmness values for cherries treated with electrolyzed water at different ACC (25, 50, and 100 mg L−1) as compared to control samples.
Fig. 1.
Effect of AEW treatment on pulp firmness (A), CWM content (B), contents of CSP (C), ISP (D), WSP (E), cellulose (F) and hemicellulose (G) in pulp of harvested longans. Value presented in figure equals mean ± standard error of triplicate analyses, vertical bars express the standard error of mean (n = 3). Significant differences between control (○) and AEW treated fruit (●) are represented by * (p < 0.05) and ** (p < 0.01).
The pulp CWM content of control longans declined rapidly as storage time increased (Fig. 1B). Correlation analysis indicated that, the pulp CWM content (x) was significantly (p < 0.01) positively correlated with the pulp firmness (y) (correlation coefficient r = 0.942, Table 1). Therefore, this could be explained by the fact that the continuous decline in pulp CWM was the main factor in the softening in longan pulp. The content of CWM for the pulp from AEW-treated longans was higher than control longans during storage with an extremely significant (p < 0.01) difference from day 3 to day 5. In addition, the reduction AEW-treated pulp CWM content (x) was significantly (p < 0.01) positively correlated with the decrease of pulp firmness (y) (r = 0.926, Table 2). These findings demonstrated that AEW treatment can efficiently delay CWM degradation and inhibit longan pulp softening. This is in line with a previous work by Lin et al. (2019), who found that maintaining a higher pulp CWM content in chitosan-treated longans slowed the incidence of pulp softening.
Table 1.
Correlation analysis of pulp firmness of control longan fruit. The correlation coefficients were calculated on the basis of the mean values obtained from three biological replicates.
| Firmness | CWM | CSP | ISP | WSP | Cellulose | Hemicellulose | XET | PE | PG | β-Gal | CEL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Firmness | 1 | |||||||||||
| CWM | 0.942** | 1 | ||||||||||
| CSP | 0.915* | 0.842* | 1 | |||||||||
| ISP | – | – | – | 1 | ||||||||
| WSP | −0.893* | −0.951** | −0.863* | – | 1 | |||||||
| Cellulose | 0.938** | 0.930** | 0.951** | – | – | 1 | ||||||
| Hemicellulose | 0.932** | 0.971** | 0.894* | – | – | – | 1 | |||||
| XET | −0.993** | −0.921** | −0.875* | – | – | −0.908* | −0.909* | 1 | ||||
| PE | −0.870* | −0.920** | −0.870* | – | 0.989** | – | – | – | 1 | |||
| PG | −0.894* | −0.919** | −0.884* | – | 0.985** | – | – | – | – | 1 | ||
| β-Gal | −0.942** | −0.938** | −0.951** | – | 0.975** | – | – | – | – | – | 1 | |
| CEL | −0.995** | −0.931** | −0.903* | – | – | −0.940** | −0.925** | – | – | – | – | 1 |
Note: “−” means the correlation analysis is not done.
Table 2.
Correlation analysis of pulp firmness of AEW-treated longan fruit. The correlation coefficients were calculated on the basis of the mean values obtained from three biological replicates.
| Firmness | CWM | CSP | ISP | WSP | Cellulose | Hemicellulose | XET | PE | PG | β-Gal | CEL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Firmness | 1 | |||||||||||
| CWM | 0.926** | 1 | ||||||||||
| CSP | 0.934** | 0.970** | 1 | |||||||||
| ISP | – | – | – | 1 | ||||||||
| WSP | −0.872* | −0.950** | −0.978** | – | 1 | |||||||
| Cellulose | 0.950** | 0.986** | – | – | – | 1 | ||||||
| Hemicellulose | 0.978** | 0.974** | – | – | – | – | 1 | |||||
| XET | −0.996** | −0.908* | – | – | – | −0.939** | −0.969** | 1 | ||||
| PE | −0.975** | −0.965** | −0.973** | – | 0.951** | – | – | – | 1 | |||
| PG | −0.858* | −0.943** | −0.959** | – | 0.988** | – | – | – | – | 1 | ||
| β-Gal | −0.907* | −0.941** | −0.974** | – | 0.981** | – | – | – | – | – | 1 | |
| CEL | −0.983** | −0.930** | – | – | – | −0.954** | −0.971** | – | – | – | – | 1 |
Note: “−” means the correlation analysis is not done.
3.2. Changes in CWP
Pectin, hemicellulose and cellulose are the predominant CWP (Broxterman and Schols, 2018, Shi et al., 2019, Wang et al., 2013, Zhao et al., 2019) and decline significantly in the cell wall during storage and believe are associated with cell structure degradation during storage (Fan et al., 2018, Fan et al., 2019, Nguyen et al., 2021, Wang et al., 2021).
3.2.1. Pectin
The middle lamella is a pectin-rich area of the cell wall and serves as mucilage to help keep neighboring cells together (Broxterman and Schols, 2018, Wang et al., 2018, Wang et al., 2021). Pectin was fractionated into CSP, ISP, and WSP, which exerts a central role in middle lamella’s structure and function (Chen et al., 2018, Chen et al., 2021, Posé et al., 2019, Wang et al., 2013, Wang et al., 2021, Wang et al., 2018).
The pulp CSP content of the control group decreased rapidly on the first day, then decreased marginally from day 1 to day 4, before dropping dramatically (Fig. 1C). A sharp decrease of pulp CSP content in control longan from day 4 to day 5 (Fig. 1C) was associated with the quick softening and the rapid decline of pulp firmness (Fig. 1A). The pulp ISP content only changed slightly during storage (Fig. 1D), while WSP content increased sharply from day 0 to day 2, followed by a slow rising (Fig. 1E). The increased WSP could be attributed primarily to the CSP conversion into WSP. Correlation analysis confirmed that the decreased pulp CSP content (Fig. 1C) was negatively correlated (r = −0.863, p < 0.05, Table 1) with the increased pulp content of WSP (Fig. 1E) during storage. Furthermore, the decreased pulp firmness (Fig. 1A) was significantly positively correlated (r = 0.915, p < 0.05, Table 1) with the pulp CSP content (Fig. 1C), and was significantly negatively correlated (r = −0.893, p < 0.05, Table 1) with the pulp WSP content (Fig. 1E) of control fruit. The correlation analysis showed that the pulp softening process in longan fruit was linked to CSP degradation.
Furthermore, the pulp CSP content (Fig. 1C) of AEW treated-longans was significantly (p < 0.05) higher than the control longans for all 5 days storage period, and the pulp WSP content (Fig. 1E) increased at a slower rate than control longans. Correlation analysis confirmed that, for AEW treated-longans, the pulp firmness was also significantly negatively correlated (r = −0.872, p < 0.05, Table 2) with the pulp WSP content, and extremely notable positively correlated (r = 0.934, p < 0.01, Table 2) with the pulp CSP content. This further support the slow down on rising of WSP caused by CSP degradation enhanced the stability of pectin polysaccharides of cell wall and hence reduced the pulp softening development in postharvest longans. Applications of AEW (Chen et al., 2017b), electron beam radiation (Nguyen et al., 2021), paper containing 1-MCP (Chen et al., 2017a, Fan et al., 2018, Lin et al., 2018, Wang et al., 2020), novel chitosan (Lin et al., 2019), and near freezing temperature (NFT) storage (Fan et al., 2019) have all been suggested as capable of slowing reduction of pectin polysaccharides depolymerisation, and then maintained the pulp firmness of horticultural products.
3.2.2. Cellulose and hemicellulose network
Cellulose is the most abundant organic molecule providing the fibrous component of the cell wall, is constituted by a 500–7500 linear chain of β-(1–4) linked d-glucose monomers (Broxterman and Schols, 2018, Chen et al., 2018, Posé et al., 2019). Xylans, xyloglucans, and mannans are some of the common hemicelluloses. Xyloglucan is the most abundant hemicellulosic polysaccharide in fruit and vegetable primary plant cell walls (Broxterman & Schols, 2018). Hemicellulose binds to the surfaces of cellulose microfibrils, forming a strong cell wall structural network (Chen et al., 2018, Posé et al., 2019).
According to the current study, the amount of cellulose and hemicellulose in the pulp of longans decreased as storage time increased (Fig. 1F and 1G). Correlation analysis indicated the positive correlation of pulp firmness with the cellulose content (r = 0.938, p < 0.01, Table 1) and hemicellulose content (r = 0.932, p < 0.01, Table 1) in pulp of control fruit. Table 2 also showed significant correlation between pulp firmness and cellulose content (r = 0.95, p < 0.01) and hemicellulose content (r = 0.978, p < 0.01) in pulp of AEW-treated fruit. From this we inferred that the decrease of hemicellulose and cellulose contents in longan pulp is another major factor that accelerated the pulp softening process of longan fruit.
When compared to control longan, AEW-treated fruit maintained higher level of cellulose and hemicellulose (Fig. 1F and 1G) than control fruit. This supports the process of pulp softening of postharvest longans maybe related to the degradation of cellulose and hemicellulose. Zhao et al. (2019) found that contents of cellulose and hemicellulose in nitric oxide fumigation winter jujube were significantly higher than control fruit and attributed the limited cellulose-hemicellulose network degradation to the maintaining of pulp firmness. Nguyen et al. (2021) also reported that electron beam radiation treated mango fruit possess higher hemicellulose and cellulose content than untreated fruit, and hence retarded the disassembly of CWP.
3.3. Changes in CWDEs activities
Softening is mainly due to the degradation of CWP in the fruit, which leads to the separation and structural changes of the cell wall. In addition, under the action of enzymes involved in the cell wall hydrolysis such as PE, XET, PG, CEL and β-Gal, the structure of the cell wall and the polysaccharides are gradually decomposed and destroyed (Chen et al., 2017a, Chen et al., 2017b, Chen et al., 2018, Chen et al., 2021, Li et al., 2019, Posé et al., 2019, Zhao et al., 2019, Zhong et al., 2008).
PE hydrolyzed the pectin substance, resulting in demethylated pectin that is more easily hydrolyzed by PG (Chen et al., 2018, Shi et al., 2019). Another pectin-debranching enzyme, β-Gal, is capable to degrade pectin at the same time (Chen et al., 2018, Xie et al., 2017, Zhao et al., 2019). In general, CEL is likely to be involved in the degradation of cellulose, and XET is responsible for xyloglucan modifications. CEL and XET have been shown to degrade xyloglucan and cellulose, two main constituents of the cellulose-hemicellulose network linked to fruit cell wall integrity (Zhao et al., 2019, Zhong et al., 2008).
In the current study, PE, PG, and β-Gal activities in the pulp of the control longans increased with increasing storage time (Fig. 2A, 2B and 2C). Correlation analysis revealed that decreased CSP content was negatively related to the increase of PE (r = −0.87, p < 0.05), PG (r = −0.884, p < 0.05), and β-Gal (r = −0.951, p < 0.01) activities (Table 1). Longan pulp firmness was also negatively related to the increase of PE (r = −0.87, p < 0.05), PG (r = −0.894, p < 0.05), and β-Gal (r = −0.942, p < 0.01) activities (Table 1). However, WSP content was positively related to the increase of PE (r = 0.989, p < 0.01), PG (r = 0.985, p < 0.01), and β-Gal (r = 0.975, p < 0.01) activities (Table 1). These findings showed that increased PE, PG, and β-Gal activities accelerated the depolymerization of CSP to WSP and thus the pulp softening process in harvested longans. In the pulp of AEW-treated longans, however, weaker PE, β-Gal, and PG activities, higher pulp firmness, and stronger CSP and ISP levels than control longan pulp were observed, suggesting that AEW treatment could lower PE, PG, and β-Gal activities, slow down CSP and ISP degradation, and prevent longan fruit pulp softening. Reports in the literature found application of nitric oxide fumigation (Zhao et al., 2019) or yeast mannan (Xie et al., 2017) resulted in lower activities of PE, PG, and β-Gal, as well as retarding CWP degradation resulting in improved storability of winter jujube (Zhao et al., 2019) or tomato fruit (Xie et al., 2017).
Fig. 2.
Effect of AEW treatment on activities of PE (A), PG (B), β-Gal (C), XET (D), and CEL (E) in pulp of harvested longans. Value presented in figure equals mean ± standard error of triplicate analyses, vertical bars express the standard error of mean (n = 3). Significant differences between control (○) and AEW treated fruit (●) are represented by * (p < 0.05) and ** (p < 0.01).
Furthermore, decreased cellulose content was negatively related to the increase of XET (r = −0.908, p < 0.05) and CEL (r = −0.94, p < 0.01) activities (Table 1). Hemicellulose content was also negatively related to the increase of XET (r = −0.909, p < 0.05) and CEL (r = −0.925, p < 0.01) activities (Table 1). Longan pulp firmness was negatively related to the increase of XET (r = −0.993, p < 0.01) and CEL (r = −0.995, p < 0.01) activities (Table 1). These findings suggest that CEL and XET catalyze the degradation of cellulose and hemicelluloses, which then speed up the pulp softening phase in longans. However, lower CEL and XET activities (Fig. 2D and 2E), higher levels of pulp firmness (Fig. 1A), higher cellulose and hemicelluloses contents (Fig. 1F and 1G) were observed in AEW-treated longan pulp than the control, signifying that AEW treatment can lower CEL and XET activities, reduce the degradation process of hemicelluloses and cellulose, and hence prevent pulp softening of longans. These were also supported by the reports that the reduced CEL and XET activities, caused by novel chitosan or 1-MCP, led to the retarded process of pulp softening of longans (Lin et al., 2019), persimmons (Wang et al., 2020) or Huanghua pears (Chen et al., 2017a). Moreover, it is known that AEW could reduce the microbial load and has preservation effect on harvested fruits and vegetables (Huang et al., 2008, Rahman et al., 2016, Shiroodi and Ovissipour, 2018, Sun et al., 2022), which may induce less fruit damage and ethylene production. Further investigations are needed to clarify whether these inhibitory effects on activity of CWDEs in harvested longans during storage were related with reduced ethylene production by AEW treatment.
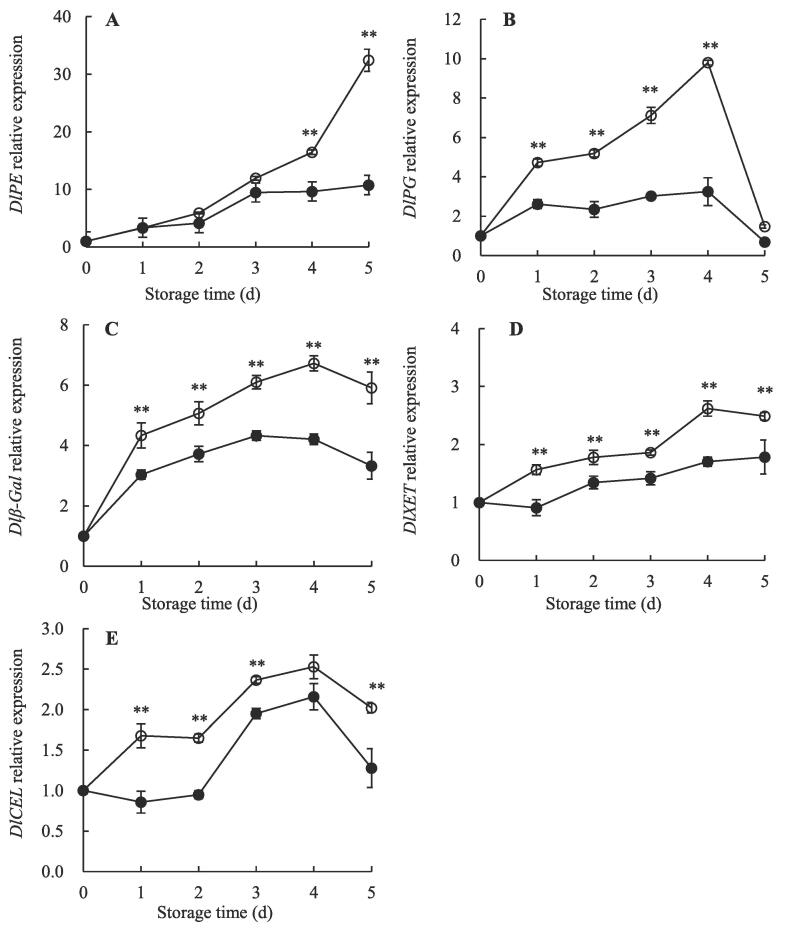
3.4. Changes in CWDEs gene expression
To further explain the disassembly of CWP during storage, changes in CWDEs gene expression were measured. This study found that lower levels of DlPE, DlXET, DlPG, Dl-Gal, and DlCEL gene expression (Fig. 3) in AEW-treated longans during storage related to a delay in CWP degradation. This is agreed with the results of a previous report that 1-MCP treatment strongly suppressed levels of pectin-related gene expression including PE, β-Gal, and PG, and hence delayed pectin degradation and softening process of apricot (Fan et al., 2018). Similarly, Shi et al., 2019, Fan et al., 2019 found that treatment of graft copolymer or NFT storage inhibited the gene expression of CWDEs such as PE, XET, PG, CEL, and β-Gal, suppressed the solubilization of CWP and maintained high firmness, and hence kept the integrity of the structure of cell wall.
Fig. 3.
Effect of AEW treatment on relative genes expression of DlPE (A), DlPG (B), Dlβ-Gal (C), DlXET (D), and DlCEL (E) in pulp of harvested longans. Value presented in figure equals mean ± standard error of triplicate analyses, vertical bars express the standard error of mean (n = 3). Significant differences between control (○) and AEW treated fruit (●) are represented by ** (p < 0.01).
4. Conclusion
AEW treatment inhibited pulp softening of longans was due to AEW slowing down activities and their corresponding gene expression of CWDEs, which could prevent the degradation of CWP and the destruction of structure in the cell wall of longan pulp, which inhibits pulp softening in harvested longans (Fig. 4). These results indicate that AEW could potentially provide a practical technology in suppressing CWP degradation and controlling pulp softening of postharvest longan fruit, and thus extend storage life of longan fruit after harvest.
Fig. 4.
The possible mechanism of AEW inhibited pulp softening of longans by regulating the metabolism of cell wall polysaccharides.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 32072272 and U21A20275), and the International Cooperation and Exchange Program on Science and Technology at Fujian Agriculture and Forestry University of China (Grant No. KXGH17006).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100265.
Contributor Information
Yihui Chen, Email: harris2197395@163.com.
Hetong Lin, Email: hetonglin@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bai Y.J., Huang F., Zhang R.F., Dong L.H., Jia X.C., Liu L., et al. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydrate Polymers. 2020;229 doi: 10.1016/j.carbpol.2019.115475. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broxterman S.E., Schols H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydrate Polymers. 2018;192:263–272. doi: 10.1016/j.carbpol.2018.03.070. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Hung Y.C., Chen M.Y., Lin H.T. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT – Food Science and Technology. 2017;84:650–657. doi: 10.1016/j.lwt.2017.06.011. [DOI] [Google Scholar]
- Chen Y.H., Lin H.T., Shi J., Zhang S., Lin Y.F., Lin T. Effects of a feasible 1-methylcyclopropene postharvest treatment on senescence and quality maintenance of harvested Huanghua pears during storage at ambient temperature. LWT - Food Science and Technology. 2015;64:6–13. doi: 10.1016/j.lwt.2015.05.021. [DOI] [Google Scholar]
- Chen Y.H., Sun J.Z., Lin H.T., Hung Y.C., Zhang S., Lin Y.F., et al. Paper based 1-MCP treatment suppresses cell wall metabolism and delays softening of Huanghua pears during storage. Journal of the Science of Food and Agriculture. 2017;97:2547–2552. doi: 10.1002/jsfa.8072. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Xie H.L., Tang J.Y., Lin M.S., Hung Y.C., Lin H.T. Effects of acidic electrolyzed water treatment on storability, quality attributes and nutritive properties of longan fruit during storage. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126641. [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Zhang S., Lin H.T., Sun J.Z., Lin Y.F., Wang H., et al. Phomopsis longanae Chi-induced changes in activities of cell wall-degrading enzymes and contents of cell wall components in pericarp of harvested longan fruit and its relation to disease development. Frontiers in Microbiology. 2018;9:1051. doi: 10.3389/fmicb.2018.01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.Z., Zhang S., Lin H.T., Lu W.J., Wang H., Chen Y.H., et al. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chemistry. 2021;351 doi: 10.1016/j.foodchem.2021.129294. [DOI] [PubMed] [Google Scholar]
- Fan X.G., Jiang W.B., Gong H.S., Yang Y.Q., Zhang A.D., Liu H., et al. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chemistry. 2019;300 doi: 10.1016/j.foodchem.2019.125194. [DOI] [PubMed] [Google Scholar]
- Fan X.G., Shu C., Zhao K., Wang X.M., Cao J.K., Jiang W.B. Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Scientia Horticulturae. 2018;232:63–70. doi: 10.1016/j.scienta.2017.12.061. [DOI] [Google Scholar]
- Hayta E., Aday M.S. The effect of different electrolyzed water treatments on the quality and sensory attributes of sweet cherry during passive atmosphere packaging storage. Postharvest Biology and Technology. 2015;102:32–41. doi: 10.1016/j.postharvbio.2015.02.009. [DOI] [Google Scholar]
- Huang F., Liu H.J., Zhang R.F., Dong L.H., Liu L., Ma Y.X., et al. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydrate Polymers. 2019;206:344–351. doi: 10.1016/j.carbpol.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Huang Y.R., Hung Y.C., Hsu S.Y., Huang Y.W., Hwang D.F. Application of electrolyzed water in the food industry. Food Control. 2008;19:329–345. doi: 10.1016/j.foodcont.2007.08.012. [DOI] [Google Scholar]
- Khayankarn S., Uthaibutra J., Setha S., Whangchai K. Using electrolyzed oxidizing water combined with an ultrasonic wave on the postharvest diseases control of pineapple fruit cv. ‘Phu Lae’. Crop Protection. 2013;54:43–47. doi: 10.1016/j.cropro.2013.07.004. [DOI] [Google Scholar]
- Li T.T., Shi D.D., Wu Q.X., Yin C.X., Li F.J., Shan Y.X., et al. Mechanism of cell wall polysaccharides modification in harvested ‘Shatangju’ Mandarin (Citrus reticulate Blanco) fruit caused by Penicillium italicum. Biomolecules. 2019;9:160. doi: 10.3390/biom9040160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.F., Lin Y.X., Lin H.T., Lin M.S., Li H., Yuan F., et al. Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chemistry. 2018;264:1–8. doi: 10.1016/j.foodchem.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Lin Y.F., Lin Y.Z., Lin Y.X., Lin M.S., Chen Y.H., Wang H., et al. A novel chitosan alleviates pulp breakdown of harvested longan fruit by suppressing disassembly of cell wall polysaccharides. Carbohydrate Polymers. 2019;217:126–134. doi: 10.1016/j.carbpol.2019.04.053. [DOI] [PubMed] [Google Scholar]
- Lin Y.L., Min J.M., Lai R.L., Wu Z.Y., Chen Y.K., Yu L.L., et al. Genome-wide sequencing of longan (Dimocarpus longan Lour.) provides insights into molecular basis of its polyphenol-rich characteristics. GigaScience. 2017;6(5):1–14. doi: 10.1093/gigascience/gix023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.X., Lin H.T., Wang H., Lin M.S., Chen Y.H., Fan Z., et al. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydrate Polymers. 2020;242 doi: 10.1016/j.carbpol.2020.116427. [DOI] [PubMed] [Google Scholar]
- Lin Y.X., Lin Y.F., Lin M.S., Fan Z.Q., Lin H.T. Influence of hydrogen peroxide on the ROS metabolism and its relationship to pulp breakdown of fresh longan during storage. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Y., Lin Y.F., Lin H.T., Lin M.S., Fan Z.Q. Impacts of exogenous ROS scavenger ascorbic acid on the storability and quality attributes of fresh longan fruit. Food Chemistry: X. 2021;12 doi: 10.1016/j.fochx.2021.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.T., Kato M., Ma G., Zhang L.C., Uthairatanakij A., Srilaong V., et al. Electron beam radiation delayed the disassembly of cell wall polysaccharides in harvested mangoes. Postharvest Biology and Technology. 2021;178 doi: 10.1016/j.postharvbio.2021.111544. [DOI] [Google Scholar]
- Posé S., Paniagua C., Matas A.J., Gunning A.P., Morris V.J., Quesada M.A., et al. A nanostructural view of the cell wall disassembly process during fruit ripening and postharvest storage by atomic force microscopy. Trends in Food Science and Technology. 2019;87:47–58. doi: 10.1016/j.tifs.2018.02.011. [DOI] [Google Scholar]
- Rahman S.M.E., Khan I., Oh D.H. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Comprehensive Reviews in Food Science and Food Safety. 2016;15:471–490. doi: 10.1111/1541-4337.12200. [DOI] [PubMed] [Google Scholar]
- Ren Y.Y., Sun P.P., Wang X.X., Zhu Z.Y. Degradation of cell wall polysaccharides and change of related enzyme activities with fruit softening in Annona squamosa during storage. Postharvest Biology and Technology. 2020;166 doi: 10.1016/j.postharvbio.2020.111203. [DOI] [Google Scholar]
- Shi Z.J., Yang H.Y., Jiao J.Y., Wang F., Lu Y.Y., Deng J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell wall degradation in grapefruit during storage. Food Chemistry. 2019;283:92–100. doi: 10.1016/j.foodchem.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Shiroodi S.G., Ovissipour M. In: Postharvest disinfection of fruits and vegetables. Siddiqui M.W., editor. Academic Press; London, United Kingdom: 2018. Electrolyzed water application in fresh produce sanitation; pp. 67–89. [DOI] [Google Scholar]
- Sun J.Z., Jiang X.J., Chen Y.H., Lin M.S., Tang J.Y., Lin Q., et al. Recent trends and applications of electrolyzed oxidizing water in fresh foodstuff preservation and safety control. Food Chemistry. 2022;369 doi: 10.1016/j.foodchem.2021.130873. [DOI] [PubMed] [Google Scholar]
- Tang J.Y., Chen H.B., Lin H.T., Hung Y.C., Xie H.L., Chen Y.H. Acidic electrolyzed water treatment delayed fruit disease development of harvested longans through inducing the disease resistance and maintaining the ROS metabolism systems. Postharvest Biology and Technology. 2021;171 doi: 10.1016/j.postharvbio.2020.111349. [DOI] [Google Scholar]
- Wang D.D., Zhang H.Y., Wu F.W., Li T.T., Liang Y.X., Duan X.W. Modification of pectin and hemicellulose polysaccharides in relation to aril breakdown of harvested longan fruit. International Journal of Molecular Sciences. 2013;14:23356–23368. doi: 10.3390/ijms141223356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen Y.H., Lin H.T., Lin M.S., Chen Y.H., Lin Y.F. 1-Methylcyclopropene containing-papers suppress the disassembly of cell wall polysaccharides in Anxi persimmon fruit during storage. International Journal of Biological Macromolecules. 2020;151:723–729. doi: 10.1016/j.ijbiomac.2020.02.146. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang J., Mujumdar A.S., Jin X.W., Liu Z.L., Zhang Y., et al. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa) Food Hydrocolloids. 2021;118 doi: 10.1016/j.foodhyd.2021.106808. [DOI] [Google Scholar]
- Wang J., Mujumdar A.S., Deng L.Z., Gao Z.J., Xiao H.W., Raghavan G.S.V. High-humidity hot air impingement blanching alters texture, cell-wall polysaccharides, water status and distribution of seedless grape. Carbohydrate Polymers. 2018;194:9–17. doi: 10.1016/j.carbpol.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Xie F., Yuan S.Z., Pan H.X., Wang R., Cao J.K., Jiang W.B. Effect of yeast mannan treatments on ripening progress and modification of cell wall polysaccharides in tomato fruit. Food Chemistry. 2017;218:509–517. doi: 10.1016/j.foodchem.2016.09.086. [DOI] [PubMed] [Google Scholar]
- Yi Y., Zhang M.W., Liao S.T., Zhang R.F., Deng Y.Y., Wei Z.C., et al. Structural features and immunomodulatory activities of polysaccharides of longan pulp. Carbohydrate Polymers. 2012;87:636–643. doi: 10.1016/j.carbpol.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Youssef K., Hussien A. Electrolysed water and salt solutions can reduce green and blue molds while maintain the quality properties of ‘Valencia’ late oranges. Postharvest Biology and Technology. 2020;159 doi: 10.1016/j.postharvbio.2019.111025. [DOI] [Google Scholar]
- Zhao Y.T., Zhu X., Hou Y.Y., Wang X.Y., Li X.H. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Biology and Technology. 2019;156 doi: 10.1016/j.postharvbio.2019.110954. [DOI] [Google Scholar]
- Zhong Y.X., Chen J.Y., Feng H.L., Kuang J.F., Xiao R., Ou M., et al. Expansin and XET genes are differentially expressed during aril breakdown in harvested longan fruit. Journal of the American Society for Horticultural Science. 2008;133:462–467. doi: 10.21273/JASHS.133.3.462. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.