Highlights
-
•
Orange juice by-products valorized by green PLE process.
-
•
Terpenoid-rich PLE extract with enhanced in-vitro neuroprotective activity.
-
•
Molecular docking between terpenoids and active sites of the target enzymes.
-
•
Selected PLE extracts show human cell-based neuroprotection capacity.
Keywords: Terpenoids, Neuroprotective, Anti-inflammatory, Green-extraction, GC-q-TOF-MS, Orange juice by-products, Pressurized liquid extraction
Abstract
Pressurized liquid extraction (PLE) conditions were optimized to improve the recovery of orange (Citrus sinensis) by-products terpenoids. The neuroprotective potential of the PLE extracts were tested against a set of in-vitro assay (antioxidant (ABTS), reactive oxygen/nitrogen species (ROS/RNS)) as well as enzymatic tests (acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and lipoxygenase (LOX)). Gas chromatography coupled to high-resolution mass spectrometry (GC-q-TOF-MS) analysis revealed a higher enrichment in mono- and sesquiterpenoids of the PLE extracts with the highest neuroprotection capacity. In-silico molecular docking analysis showed the specific interaction of representative terpenoids with enzymes active sites. The results demonstrate that the selected extract at 100 °C and 30 minutes possesses high antioxidant (ABTSIC50 = 13.5 μg mL−1; ROSIC50 = 4.4 μg mL−1), anti-cholinesterase (AChEIC50 = 137.1 vg L−1; BChEIC50 = 147.0 μg mL−1) and anti-inflammatory properties (against IL-6 and LOXIC50 = 76.1 μg mL−1), with low cytotoxicity and protection against L-glutamic acid in cell models.
Introduction
The agri-food industry plays a key role in many countries and economies, such as in Spain. Among the different sectors, Spain is the largest producer of citrus juice in the European Union (EU) and one of the largest in the world (Rodriguez et al., 2017). However, several environmental problems are associated to these activities, such as food waste generation. In 2013, the global citrus industry generated 24.3 million tons of waste, of which 1.3 million tons corresponded to Spain (mainly from the orange juice production) (Pacheco et al., 2019). Traditionally, food waste is incinerated or disposed of in landfills with the subsequent air/water pollution, and soil/food contamination. To decrease these problems, the EU has promoted the reduction of food wastes, and the revalorization of agricultural by-products has been suggested as a major opportunity to reduce the environmental impact and to improve the economical exploitation (Pacheco et al., 2019, Rodriguez et al., 2017). Several studies have presented orange juice by-products (pulp, peel and seeds) as a rich source of bioactive molecules (terpenoids, phenolics compounds or alkaloids) that can be directed to the production of new products such as supplements, nutraceuticals or functional foods with additional added-value (Sánchez-Martínez et al., 2021).
The recovery of bioactive compounds from plant material or agricultural waste through conventional techniques (such as maceration) involves several environmental constrains due to the important amount of organic solvents, large extraction times and high energy consumption requirements (Gallego et al., 2020). In this sense, the use of advanced environmentally friendly extraction techniques such as pressurized liquid extraction (PLE), which uses high temperatures (above the boiling point and below the critical point) and pressures enough to maintain the solvent in the liquid state, presents some advantages, like the reduction of organic solvents volume and extraction times, and the improvement of the extraction yield of bioactive compounds through the increase of mass transfer rate between the solvent and the biomass (Gallego et al., 2020). As a result of utilizing these particular conditions of pressure and temperature, a change in the solvent physicochemical properties occurs. For instance, mass transfer rates are enhanced, while at the same time, solvent surface tension and viscosity are decreased and solubility of analytes is increased. This allows the solvent to penetrate easier and deeper into the solid matrix being extracted. As a consequence, significantly higher extraction yields are obtained compared with conventional extractions (Alvarez-Rivera et al., 2020). Under these conditions, PLE has been postulated as a high efficient green extraction process, ideal to recover bioactive molecules, such as terpenoids, from orange by-products. These terpenoids have been described as potential neuroprotective molecules due to their anticholinesterase, antioxidant and anti-inflammatory capacity (Cutillas et al., 2018, González-Cofrade et al., 2019).
Neurodegenerative disorders are a group of pathological conditions characterized by chronical and progressive impairment of brain tissue. These disorders include Alzheimer’s disease (AD), schizophrenia, depression and Parkinson’s disease, among others (Auti & Kulkarni, 2017). AD is the main neurodegenerative disorder, that affected 47.47 million people in 2015; foresight for 2030 and 2050 are 75.63 and 135.46 million cases, showing the large social and economic impact of AD (Auti & Kulkarni, 2017). The main physiopathological hallmarks of AD are oxidative stress, progressive cognitive impairment, neuroinflammation, aggregation of amyloid-beta (Aβ) plaques, hyper-phosphorylation of tau proteins and their aggregation into neurofibrillary tangles (AlFadly et al., 2019). In addition, acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and lipoxygenases (LOX) enzymes have shown crucial roles in neurological disorders due to their cholinergic system control and neuroinflammatory response (AlFadly et al., 2019), but the etiology of AD is not completely understood. Neurons are also susceptible to oxidative stress caused by reactive oxygen species (ROS) and reactive nitrogen species (RNS) that can lead to mitochondrial alteration and neuronal cell death, or to glutamate excitotoxicity (Armstrong, 2016, Sabogal-Guáqueta et al., 2019). Currently, there is no effective cure for AD, but AChE and BChE inhibition have shown to improve the cholinergic function due to increase acetylcholine neurotransmitter in brain tissue in patients with AD (Mushtaq et al., 2014).
A previous work developed in our laboratory has showed that orange juice by-products are an important source of terpenoids with not only in-vitro neuroprotective capacity, but also high permeability to cross the blood brain barrier (BBB) (in-vitro, in a PAMPA-BBB model (Sánchez-Martínez et al., 2021)). Hence, the aim of the present work is to optimize the PLE conditions to obtain terpenoids enriched extracts with neuroprotective properties from an orange juice by-product and to characterize these extracts by using gas chromatography coupled to high-resolution mass spectrometry (GC-q-TOF-MS) in order to increase the recovery of these compounds. Moreover, the neuroprotective potential of the PLE orange extracts is explored and confirmed by a pool of in-vitro assays including anti-enzymatic (AChE, BChE and LOX) and antioxidant (ABTS, ROS and RNS) tests. Finally, in-vitro cytotoxicity is evaluated by using different human cell culture models (HK-2, THP-1 and SH-SY5Y cells), as well as the potential anti-inflammatory capacity and neuroprotective activity against Aβ1-42 and l-glutamic acid insults. Moreover, in-silico molecular docking is also applied to better understand the interaction between target mono- and sesquiterpenoids with acetylcholinesterase, butyrylcholinesterase and lipoxygenase active sites.
Material and methods
Plant material
Orange (Citrus sinensis variety Navel Late) residues were kindly supplied by J. García Carrión, S.L (Huelva, Spain). The biomass was treated as follows: seeds and other impurities were manually separated from peel and pulp; both peel and pulp were lyophilized in a freeze-drier during 7 days at −84.5 °C and 4800 Pa (Lyobeta 15 Telstar, Terrassa, Spain), ground with a laboratory-grade knife mill (Grindomix GM200-Retsch GmbH, Haan, Germany), and sieved to a particle size between 500 and 1000 μm (BA 200 N CISA, La Rioja, Spain). Finally, the resulting powder was vacuum-packed (C400 Multivac Wolfertschwenden, Germany) and stored at –18 °C.
Reagents and materials
HPLC-grade solvents ethyl acetate (ETAC) and ethanol (EtOH) were purchased from VWR Chemicals (Barcelona, Spain). Lipopolysaccharide (LPS) from E. coli O55:B5, acetylcholinesterase (AChE) Type VI-S from Electrophorus electricus, butyrylcholinesterase from equine serum (BChE), acetylthiocholine iodide (ATCI), linoleic acid (LA), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), Trizma hydrochloride (Tris-HCl), disodium phosphate (Na2HPO4), monopotassium phosphate (KH2PO4), sodium nitroprusside dehydrate (SNP), fluorescein sodium salt, sulphanilamide, naphthylethylene diamine dihydrochloride, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), gallic acid, ascorbic acid, quercetin, l-glutamic acid and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma-Aldrich (Madrid, Spain). Lipoxidase from glycine max (soybean), 4-(amino- 359 sulfonyl)-7-fluoro-2,1,3-benzoxadiazole (ABD-F), galantamine hydrobromide, 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH) were purchased from TCI Chemicals (Tokyo, Japan). Ultrapure water was obtained from a Millipore system (Billerica, MA, USA). Human proximal tubular epithelial cells (HK-2), human SH-SY5Y neuroblastoma cells and human THP-1 monocytes were purchased from ATCC®, Rockville, MD, USA. Cell culture medium Dulbecco's Modified Eagle Medium Nutrient Mixture (DMEM/Ham's F12), fetal bovine serum (FBS), Roswell Park Memorial Institute (RPMI 1640), PBS, l-glutamine, antibiotic solution (including penicillin and streptomycin), antibiotic–antimycotic solution (including penicillin, streptomycin and amphotericin B), β-mercaptoethanol and insulin-transferrine-selenium (ITS) were obtained from Thermo Fisher, Grand Island, NY, USA. Retinoic acid (RA) was acquired from Enzo Life Sciences GmbH, Germany. Human Brain-Derived Neurotrophic Factor (BDNF) was purchased from Bachem AG, Bubendorf, Switzerland. ELISA kits were provided from BD Biosciences, Aalst, Belgium. Amyloid-peptide β 1–42 (Aβ42) was obtained from HelloBio, United Kingdom. All the 96-well microplate assays were performed in a spectrophotometer and fluorescent reader (Synergy HT, BioTek Instruments, Winooski, VT, USA).
Terpenoids recovery from the orange juice by-product
Optimization of the pressurized liquid extraction (PLE) procedure
As previously reported by our research group, the conventional extracts of orange by-products showed in-vitro neuroprotective properties, mainly using ethyl acetate (ETAC) as extraction solvent (Sánchez-Martínez et al., 2021). Thus, ETAC was chosen as PLE solvent in the present study and the effect of the temperature and time was evaluated on the extraction yield and a set of in-vitro bioactivities involved in the neuroprotective potential. A Dionex accelerated solvent extractor (ASE 200, Sunnyvale, CA, USA), equipped with a 33 mL stainless steel extraction cell was used for PLE extraction. Two factors were considered at three levels: temperature (25, 62.5 and 100 °C) and time (10, 20 and 30 min). Besides temperature and time other factors were constant for all the extractions. The dried orange residue grinded was mixed with sea sand (1:2 w/w, 4 g of orange powder residue mixed with 8 g of sea sand) and placed into the extraction cell. All the extractions were performed at constant pressure (10 MPa) on static mode using 25 mL of ethyl acetate per extraction. And, finally, 1 min of nitrogen purging was applied to push any residual solvent from exhausted orange powder residue. Extracts were collected in 40 mL glass vials and stored in −20 °C in dark before drying.
To optimize the extraction of terpenoids with neuroprotective potential from the orange residues, a response surface methodology (RSM) was proposed, using a central composite design (CCD). Response variables studied were extraction yield, enzymatic inhibition activity (AChE, BChE and LOX) and antioxidant capacity (ROS, RNS and ABTS), as described below. The extraction yield was expressed as the percentage of extract weight per initial powder orange by-product weight. After extraction process with ETAC the liquid extracts were dried under nitrogen flow and, after completely dry, they were weighed. Therefore, the equation was determined by a relationship between the extracted mass (Em) and orange by-product mass used (Om), as observed in the following Equation (1).
| (1) |
The quadratic equation model for each variable (Yi) was (equation 2):
| Yi = β0 + β1t + β2T + β1,2 T × t + β1,1 t2 + + β2 ,2 T2 + error | (2) |
where t is extraction time, T is the temperature, β0 is the intercept, β1 and β2 are the linear coefficients, β1,2 is the linear-by-linear interaction coefficients, β1,1, β2,2 are the quadratic coefficients and error the error variable. Data analysis for experimental design and multi response
optimization was performed using Statgraphics Centurion XVI software (StatPoint Technologies, Inc., Warrenton, VA, USA). The analysis of variance (ANOVA), standardized Pareto charts, coefficient of determination (R2), response surfaces, p values for the model, interaction plot and lack-of-fit testing were analyzed, accepting the significances at p ≤ 0.05 (see Supplementary material: Supplementary Table 1-7; Supplementary Figs. 1 and 2).
Conventional extraction
Extracts were also obtained through a conventional maceration method described in our previous work (Sánchez-Martínez et al., 2021). In Brief, 5 g of orange by-product were mixed with 45 mL of ethyl acetate (ETAC) and placed in an orbital shaker (200 rpm for 24 h) at room temperature in the darkness (Compact digital mini rotator, Thermo Scientific, Massachusetts, USA). Extracts were filtered by 0.45 μm Nylon filter (Agilent Technologies, California, USA) and the solvent was evaporated under N2 gas to dryness (TurboVap® LV Biotage, Uppsala, Sweden). Finally, the extracts were weighted and stored at −20 °C until their analyses. Extraction was carried out in triplicate.
Quantification of terpenoids by gas chromatography-mass spectrometry (GC- MS)
Based on the phytochemical profiling of terpenoids from orange by-product reported in our previous work (Sánchez-Martínez et al., 2021), the content of terpenoids in the PLE samples was determined. Samples were dissolved in pure ethanol at a concentration of 7.5 mg mL−1 and analyzed in an Agilent 7890B GC system coupled to an Agilent 7200 quadrupole time-of-flight (q-TOF) MS, equipped with an electronic impact (EI) ionization source. All the GC – MS conditions were detailed also in (Sánchez-Martínez et al., 2021). Quantitative analysis was achieved using the Agilent MassHunter Quantitative analysis software for Q-TOF (version B.08.00). Compounds were quantified by using calibration curves with their corresponding reference standards, or semi-quantified using reference standards with structural similarity (Table 1). Terpenoids concentration was used to determine the capacity of the PLE conditions to extract the target terpenoids.
Table 1.
Concentration (ng/mL) and relative abundance (%) of tentatively identified terpenes in the different organic extracts.
| Concentration ng mL−1 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Conventional extraction |
PLE 25 °C 30 min |
PLE 62.5 °C 20 min |
PLE 100 °C 30 min |
||||||||||||||
| No | Ret. Time (min) | Tentative identification | Conc.(RSD, %) | (%) | Conc. (RSD, %) | (%) | Conc.(RSD, %) | (%) | Conc.(RSD, %) | (%) | |||||||
| Monoterpenes | |||||||||||||||||
| 1 | 5.856 | Limonene1 | 22.3c | (1.2) | 1.3 | 5.5d | (4.5) | 0.6 | 41.3b | (1.2) | 2.9 | 214.2a | (0.2) | 17.0 | |||
| 2 | 6.688 | 3-carene1 | 4.4d | (4.3) | 0.3 | 10.5c | (2.6) | 1.1 | 15.4b | (4.3) | 1.1 | 73.8a | (3.1) | 5.8 | |||
| 3 | 7.164 | (-)Myrtenol2 | 1.0c | (1.1) | 0.1 | 1.7c | (5.9) | 0.2 | 14.2a | (1.1) | 1.0 | 8.5b | (7.7) | 0.7 | |||
| 4 | 8.046 | L-α-Terpineol2 | 32.8c | (1.5) | 2.0 | 16.2d | (2.5) | 1.6 | 50.5a | (1.5) | 3.6 | 44.0b | (0.8) | 4.4 | |||
| 5 | 8.395 | Nerol3 | 6.0c | (7.7) | 0.4 | 0.8d | (2.9) | 0.1 | 15.7b | (7.7) | 1.2 | 23.8a | (9.9) | 1.9 | |||
| 6 | 10.075 | Limonene epoxide3 | 3.1c | (4.4) | 0.2 | 0.7d | (3.5) | 0.1 | 4.4b | (4.4) | 0.3 | 4.7a | (0.6) | 0.4 | |||
| ∑ Monoterpenes | 68.0c | (3.7) | 4.9 | 34.6d | (3.2) | 2.1 | 139.6b | (2.6) | 12.6 | 371.0a | (0.6) | 30.2 | |||||
| Sesquiterpenes | |||||||||||||||||
| 7 | 10.480 | α-copaene4 | 52.7c | (1.7) | 3.1 | 18.7d | (0.5) | 1.9 | 115.3b | (3.7) | 8.2 | 132.2a | (1.1) | 10.5 | |||
| 8 | 10.631 | β-Elemen4 | 4.1a | (1.0) | 0.2 | 0.6d | (6.6) | 0.1 | 2.5b | (2.6) | 0.2 | 1.2c | (6.0) | 0.1 | |||
| 9 | 11.074 | β-Caryophyllene-14 | 3.6a | (8.7) | 0.2 | 0.6d | (5.8) | 0.0 | 1.7c | (4.6) | 0.3 | 2.6b | (0.7) | 0.1 | |||
| 10 | 11.191 | Farnesene4 | 3.6a | (2.0) | 0.2 | 0.7c | (2.2) | 0.1 | 3.4a | (6.4) | 0.2 | 1.7b | (1.5) | 0.2 | |||
| 11 | 11.342 | 7-Prop4 | 1.8a | (1.8) | 0.1 | 0.5d | (5.5) | 0.1 | 0.8c | (1.7) | 0.1 | 1.4b | (0.6) | 0.1 | |||
| 12 | 11.419 | β-Caryophyllene-24 | 3.6a | (3.4) | 0.2 | 0.6d | (0.4) | 0.1 | 1.7c | (7.7) | 0.1 | 2.6b | (0.0) | 0.2 | |||
| 13 | 11.778 | β-panasinsene4 | 2.3a | (3.2) | 0.1 | 0.5c | (1.1) | 0.0 | 1.3b | (2.1) | 0.1 | 2.4a | (2.5) | 0.2 | |||
| 14 | 11.875 | (-)-Aristolene4 | 2.4a | (8.5) | 0.2 | 0.0c | (1.5) | 0.0 | 1.3b | (5.9) | 0.2 | 2.4a | (1.0) | 0.2 | |||
| 15 | 11.998 | Valencene4 | 43.7a | (1.2) | 2.6 | 14.3c | (1.4) | 1.4 | 40.0a | (0.6) | 2.8 | 33.3b | (0.2) | 2.6 | |||
| 16 | 12.033 | γ-selinene4 | 3.8c | (3.1) | 0.2 | 2.4d | (0.6) | 0.2 | 4.6b | (6.1) | 0.3 | 6.0a | (2.0) | 0.5 | |||
| 17 | 12.116 | δ-Cadinene4 | 1.8a | (3.8) | 0.1 | 0.2d | (1.4) | 0.0 | 1.4b | (0.1) | 0.1 | 0.7c | (5.7) | 0.1 | |||
| 18 | 12.267 | Isoledene4 | 2.0a | (9.5) | 0.1 | 0.2d | (3.6) | 0.0 | 0.6b | (3.2) | 0.0 | 0.4c | (0.2) | 0.0 | |||
| 19 | 12.324 | (-)-α-Panasinsen4 | 4.0a | (1.3) | 0.2 | 0.5c | (3.7) | 0.1 | 1.0b | (7.8) | 0.1 | 0.3c | (6.2) | 0.0 | |||
| 20 | 12.649 | Elemol4 | 4.9c | (0.5) | 0.3 | 1.6d | (6.1) | 0.2 | 11.1b | (2.9) | 0.8 | 14.5a | (0.7) | 1.2 | |||
| 21 | 13.598 | Guaiol4 | 0.6a | (7.6) | 0.0 | 0.2c | (1.3) | 0.0 | 0.4b | (1.9) | 0.0 | 0.2c | (0.8) | 0.0 | |||
| 22 | 14.068 | α-Gurjunenepoxide4 | 2.1a | (2.6) | 0.1 | 0.1d | (2.7) | 0.0 | 1.0b | (0.9) | 0.1 | 0.5c | (5.0) | 0.0 | |||
| 23 | 14.292 | β -Sinensal5 | 14.4a | (6.7) | 0.9 | 4.0c | (0.7) | 0.4 | 7.4b | (0.0) | 0.5 | 3.7c | (6.5) | 0.3 | |||
| 24 | 14.410 | β-oplopenone5 | 1.9a | (0.6) | 0.1 | 0.3c | (1.5) | 0.0 | 0.8b | (6.6) | 0.1 | 0.3c | (4.0) | 0.0 | |||
| 25 | 15.214 | Isololiolide5 | 2.0a | (0.8) | 0.1 | 0.6c | (1.2) | 0.1 | 0.9b | (2.8) | 0.1 | 0.4d | (6.5) | 0.0 | |||
| 26 | 15.617 | Nootkatone5 | 9.6a | (1.1) | 0.6 | 3.6c | (2.4) | 0.4 | 5.8b | (4.0) | 0.4 | 3.1c | (0.4) | 0.2 | |||
| 27 | 15.751 | Ylangenal5 | 1.8a | (1.9) | 0.1 | 0.6c | (5.8) | 0.1 | 1.1b | (3.4) | 0.1 | 0.5c | (0.3) | 0.0 | |||
| ∑ Sesquiterpene | 164.8b | (0.7) | 10.1 | 49.8c | (0.2) | 5.1 | 203.4a | (1.6) | 14.7 | 204.7a | (0.6) | 16.6 | |||||
| Triterpenes | |||||||||||||||||
| 28 | 24.102 | Squalene8 | 133.3a | (3.1) | 8.0 | 85.5bc | (0.1) | 8.6 | 92.7b | (1.4) | 6.6 | 79.1c | (0.6) | 6.3 | |||
| 29 | 25.759 | γ-Tocopherol6 | 27.7a | (8.5) | 1.7 | 11.3c | (0.1) | 1.1 | 14.4b | (3.2) | 1.0 | 7.6d | (1.6) | 0.6 | |||
| 30 | 26.272 | α-tocopherol7 | 641.5a | (0.1) | 38.3 | 402.6c | (0.7) | 40.6 | 478.9b | (0.9) | 33.9 | 280.0d | (0.1) | 22.2 | |||
| 31 | 26.992 | Campesterol8 | 48.8a | (9.9) | 2.9 | 25.1c | (2.4) | 2.5 | 32.2b | (5.9) | 2.3 | 16.8d | (2.1) | 1.3 | |||
| 32 | 27.157 | Stigmasterol9 | 34.1a | (2.1) | 2.0 | 17.1c | (5.7) | 1.7 | 22.1b | (1.3) | 1.6 | 11.3d | (3.6) | 0.9 | |||
| 33 | 27.548 | γ-Sitosterol10 | 434.1a | (4.1) | 25.9 | 286.3c | (6.2) | 28.9 | 348.7b | (4.4) | 24.7 | 216.0d | (0.8) | 17.1 | |||
| 34 | 27.639 | Fucosterol8 | 80.6a | (9.9) | 4.8 | 47.5b | (7.1) | 4.8 | 42.3b | (2.4) | 3.0 | 33.6c | (9.0) | 2.7 | |||
| 35 | 27.874 | Lupeoli9 | 33.1a | (4.3) | 2.0 | 26.4bc | (1.0) | 2.7 | 27.8b | (1.2) | 2.0 | 23.9c | (0.0) | 1.9 | |||
| 36 | 28.186 | β-Amyrin8 | 3.4a | (0.3) | 0.2 | 2.5b | (5.5) | 0.3 | 2.2b | (5.8) | 0.2 | 2.3b | (1.3) | 0.2 | |||
| ∑ Triterpene | 1402a | (1.6) | 85.8 | 882.4c | (1.1) | 91.3 | 1035b | (0.2) | 75.1 | 654d | (0.6) | 53.2 | |||||
Different letters in the same row show statistically differences (p < 0.05).
Quantified by limonene standard calibration curve parameter.
Quantified by L-α-Terpineol standard calibration curve parameter.
Quantified by nerol standard calibration curve parameter.
Quantified by valencene standard calibration curve parameter.
Quantified by nootkatone standard calibration curve parameter.
Quantified by γ-tocopherol standard calibration curve parameter.
Quantified by α-tocopherol standard calibration curve parameter.
Quantified by campesterol standard calibration curve parameter.
Quantified by stigmasterol standard calibration curve parameter.
Quantified by γ-sitosterol standard calibration curve parameter.
In-vitro neuroprotective assessment
Neuroprotective properties of PLE extracts were measured in a set of in-vitro assays. The 96-well microplate fluorescent method of AChE, BChE and LOX enzymatic inhibition activity by a plant extract was developed and described in a previous work done in our laboratory (Sánchez-Martínez et al., 2021). The antioxidant activity assays (ABTS•+, ROS and RNS scavenging capacity) were also described in a previous research (Sánchez-Martínez et al., 2021). Galantamine hydrobromide and quercetin were used as positive controls for cholinesterases (AChE and BChE) and LOX enzymes, respectively. Ascorbic acid was used as positive controls for antioxidant experiments.
Molecular docking
Molecular docking simulation was performed to evaluate the affinity of selected terpenoids for the active sites of the target enzymes: LOX (PDB: 1JNQ), AChE (PDB: 5HFA), and BChE (PDB: 6EQP). A single crystal structure of each enzyme from Protein Data Bank (PDB) was obtained (http://www.rscb.org/pdb) (Berman et al., 2000). The first step of the docking simulation process involved the preparation of proteins and ligands. Proteins were prepared with the Molegro Molecular Software Viewer 7.0.0 (MMV), and the ligands were prepared with Chimera software (version 1.14). The ligands valencene (CAS: 4630-07-3), alpha-terpineol (CAS:98–55-5), and limonene epoxide (CAS:203719-54-4), were obtained from PUBCHEM (https://pubchem.ncbi.nlm.nih.gov/), and downloaded in SDV format.
The autodocking process was validated for each enzyme and the RSMD values acquired were: 0.2248 for LOX 0.1767 for AChE and 0.3157 for BChE. Grid boxes were built in the active sites of the enzymes, showing the coordinates: X: 27.383, Y: 4.270, Z:15.298 for LOX; X: −2.686 Y: −49.014, Z: 30.162 for AChE, and X: 132.836733, Y: 116.056133, Z: 41.709600, for BChE. The results were expressed as binding energies. Therefore, structures with the highest binding affinity (free energy of binding) were selected and analyzed using Discovery Studio 4.5 (COMPASS, COMPASS-II, Forcite, Discover and Materials Studio software. BIOVIA2015, San Diego, CA) using the Lamarckian genetic algorithm. The results of the docking study for the positive control group were compared with the terpene structures that presented the best statistical correlation with the studied enzyme inhibitory activities.
Cell culture grow conditions and in-vitro cytotoxicity assay
Cell culture in-vitro cytotoxicity of the optimum PLE extract was evaluated on three different cell human lines: human proximal tubular epithelial cells (HK-2), human THP-1 monocytes and human neuroblastoma (SH-SY5Y). HK-2 and THP-1 cells were cultured and seeded as previously described by our group (Suárez Montenegro et al., 2021). In the case of HK-2 cells, toxicity evaluation was performed by adding increasing concentrations (7.5, 15, 30, 60 and 120 μg mL−1) of the PLE extract to the wells and incubating the cells for 24 h. In the case of THP-1, the highest concentrations (30, 60 and 120 μg mL−1) of the extract were added into the wells and cells were incubated for 24 h.
Human SH-SY5Y neuroblastoma cells were seeded and differentiated by the method previously described by Medeiros et al. in 2019 (Medeiros et al., 2019). Differentiated cells were seeded in 24-well plates at a density of 4.2 × 104 cells/cm2 for 24 h. To evaluate the neurotoxic effect, cells were incubated with 30, 60 and 120 μg mL−1 of the extract for 24 h. In all cases, the viability of HK-2 cells, THP-1 monocytes and SH-SY5Y neuroblastoma was determined by MTT assay (Mosmann, 1983). The toxicity of the different concentrations of the extract is shown as relative cell viability, which is expressed as the percentage of living cells compared to controls (EtOH-treated). In all cells experiments, EtOH was used for diluting the extract and did not exceed the concentration of 0.4% (v/v).
Cell culture anti-inflammatory evaluation
After macrophage differentiation, cells were washed with PBS and inflammation response was induced by addition of 0.05 μg mL−1 of LPS for 24 h in the presence of non-toxic concentrations (30, 60 and 120 μg mL−1) of the optimum PLE extract. Thereafter, supernatant was collected and stored at − 20 °C. Positive controls were performed inducing cells with LPS but without extract, and cells without LPS or extract stimulation were considered negative controls. All conditions were performed in triplicate. Production of pro-inflammatory cytokynes (interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and IL-1β) in the collected cell culture supernatants was measured by ELISA kits (BD Biosciences, Aalst, Belgium), following the manufacturer’s instructions. Then, generated color by pro-inflammatory cytokynes interaction was measured at 450 nm (with substrate correction at 570 nm) in a 96-well microplate reader.
Neuroprotective evaluation against Aβ1-42 and l-glutamic acid
Differentiated SH-SY5Y cells were seeded in 24-well plates at a density of 42.000 cells/cm2 for 24 h. Thereafter, cells were pre-treated with control medium (DMEM/F12 supplemented with 1% FBS, 100 U/mL penicillin, 100 µg mL−1 streptomycin, 250 ng mL−1 antimycotic and 0.4% of EtOH) with or without 30 µg mL−1 of the optimum PLE extract for 24 h. The next day, control and treated cells were incubated for another 24 h with control medium, medium with 30 μM of Aβ1-42, or medium with 23 mM of l-glutamic acid. The viability of the cells was then determined using the MTT assay.
Statistical analysis
Three independent assays were performed for enzymatic, cell culture and antioxidant methodologies. IC50 values in μg mL−1 (concentration of extract or positive control to achieve 50% of inhibition) were calculated by ID% for each sample at different concentrations to obtain concentration-dependent curves by linear regression. The determination coefficients obtained from the linear regression analysis (R2 > 0.99) indicate that our ni calibration points appropriately fit the proposed linear model (Microsoft excel 2010, Washington USA). All experimental results are shown as mean ± standard deviation (Mean ± SD). ANOVA analysis was conducted to compare means by Tukeýs HSD test (SPSS statics V15 IBM, New York, USA). Statistical significance (p < 0.05) was indicated by different alphabetical letters along means in tables at the same column. For multivariate data analysis, a data matrix was previously scaled using an auto-scaling approach; that is, the data were mean-centered and divided by the standard deviation of each variable. Principal component analysis (PCA) was conducted using the statistical software XLSTAT (Addinsoft, Paris, France). Heatmap was carried out using the online software http://www.heatmapper.ca/. A hierarchical clustering was applied using a complete linkage clustering method with Pearson distance measurement.
Results and discussion
In order to obtain terpenoids-rich extracts with enhanced neuroprotective potential, a PLE procedure was optimized according to the central composite design described in section 2.3.1. Response surfaces of each response variable and their corresponding standardized Pareto charts are depicted in Supplementary Fig. 1. Individual analysis of each response variable (AChE, BChE, LOX, ABTS, ROS, RNS and yield) showed that temperature is the most critical experimental factor, exhibiting a significant influence in almost all variables; except for RNS; as can be seen in Pareto charts (Supplementary Fig. 1). On the other hand, the effect of the extraction time in the bioactive potential of the extract was negligible (p ≥ 0.05), whereas the extraction yield was significantly affected by this factor. The highest extraction yield in the optimization model (2.1 %) was obtained at the highest temperature (100 °C) and the longest time (30 min) (Supplementary Fig. 1 and Table 2). These results can be explained considering that mass transfer is favored by temperature and time (Alvarez-Rivera et al., 2020). The influence of temperature in each bioactivity assay response variables is discussed in detail below (section 3.1.)
In-vitro neuroprotective potential optimization
Anticholinergic activity in-vitro assays
Orange PLE extracts were evaluated for their inhibitory enzymatic capacity of AChE and BChE.
Medium and high temperature (62.5 °C and 100 °C) extracts (30 min extraction time) showed the highest inhibition capacity of AChE (Supplementary Fig. 1). These conditions enhanced the AChE inhibition capacity, compared to our previous conventional extract (p < 0.05) (Table 2), whereas BChE inhibition capacity was not significantly improved (Table 1). Although AChE and BChE exhibit large structural similarities, they share near 55% of aminoacid sequence, differences in the aminoacidic sequence could cause variability in their catalytic properties and affect inhibition condition (Politeo et al., 2018). Galantamine as reference inhibitor has lower IC50 values for both enzymes (0.8 ± 0.0 μg mL−1; 2.5 ± 0.0 μg mL−1, respectively). Highest IC50 values were obtained by Ademosun and Oboh (2014) for AChE and BChE inhibition assay carried out with conventional aqueous extract of sweet orange (Ademosun & Oboh, 2014). The proposed PLE procedure demonstrate that the use of organic solvents combined with advanced extraction techniques improves the recovery of bioactive compounds with anticholinergic potential.
Table 2.
Yield in % and IC 50 values from in to vitro assays of different orange juice by-products extracts using AChE, BChE, LOX, ABTS, ROS and RNS assays.
| PLE Extract |
AChE |
BChE |
LOX |
ABTS |
ROS - ORAC |
RNS |
Yield |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (IC 50 µg/mL) | % | ||||||||||||||||||||||||||
| Temp (°C) | Time (min) | ||||||||||||||||||||||||||
| 100 | 10 | 181.3c | ± | 22.7 | 160.1abcd | ± | 9.2 | 75.3f | ± | 8.7 | 16.5e | ± | 0.4 | 4.0ef | ± | 0.4 | 872c | ± | 16 | 1.5cde | ± | 0.1 | |||||
| 62.5 | 30 | 133.0de | ± | 12.0 | 143.0cde | ± | 11.4 | 95.7def | ± | 6.8 | 24.0 cd | ± | 1.8 | 4.4def | ± | 0.2 | 480f | ± | 55 | 1.1def | ± | 0.1 | |||||
| 62.5 | 10 | 113.6e | ± | 9.7 | 139.9de | ± | 9.9 | 115.4 cd | ± | 12.9 | 27.5c | ± | 1.6 | 4.6de | ± | 0.1 | 671de | ± | 48 | 0.7df | ± | 0.1 | |||||
| 62.5* | 20* | 117.9de | ± | 12.6 | 127.6ef | ± | 9.1 | 101.1de | ± | 4.5 | 23.1cde | ± | 2.7 | 4.8d | ± | 0.4 | 453.3f | ± | 57 | 0.7df | ± | 0.1 | |||||
| 25 | 30 | 298.2a | ± | 3.2 | 190.2a | ± | 15.9 | 161.4a | ± | 7.4 | 72.9ab | ± | 3.1 | 7.3a | ± | 0.4 | 1060ab | ± | 103 | 0.4f | ± | 0.0 | |||||
| 100# | 30# | 137.1de | ± | 8.1 | 147.0cde | ± | 7.5 | 76.1f | ± | 10.4 | 13.5e | ± | 0.8 | 4.4def | ± | 0.4 | 1199a | ± | 98 | 2.1b | ± | 0.2 | |||||
| 100 | 20 | 150.0 cd | ± | 11.2 | 153.9bcde | ± | 15.8 | 78.0f | ± | 5.5 | 18.2cde | ± | 0.6 | 3.9f | ± | 0.3 | 841 cd | ± | 99 | 1.9 cd | ± | 0.1 | |||||
| 25 | 20 | 218.4b | ± | 21.4 | 181.9ab | ± | 23.7 | 152.6ab | ± | 12.9 | 71.4b | ± | 7.1 | 6.6b | ± | 0.2 | 877bc | ± | 119 | 0.4f | ± | 0.0 | |||||
| 25 | 10 | 225.0b | ± | 8.1 | 171.9abc | ± | 5.4 | 125.4c | ± | 9.8 | 75.5ab | ± | 7.4 | 6.0bc | ± | 0.0 | 1073a | ± | 91 | 0.4f | ± | 0.0 | |||||
| 120 | 30 | 80.1ef | ± | 8.1 | 2.8bc | ± | 0.4 | ||||||||||||||||||||
| 160 | 30 | 89.0ef | ± | 0.70 | 9.2a | ± | 1.0 | ||||||||||||||||||||
| Conventional | 179.2c | ± | 25.1 | 102.2f | ± | 4.5 | 130.7c | ± | 7.0 | 84.1a | ± | 7.7 | 5.5c | ± | 0.1 | 556.9ef | ± | 11 | 0.5f | ± | 0.0 | ||||||
| Positive control | |||||||||||||||||||||||||||
| Galantamine | 0.8f | ± | 0.0 | 2.5 g | ± | 0.0 | |||||||||||||||||||||
| Quercetin | 137.2bc | ± | 15.4 | ||||||||||||||||||||||||
| Ascorbic acid | 23.4cde | ± | 1.1 | 2.3 g | ± | 0.1 | 1100a | ± | 13 | ||||||||||||||||||
Different letters in the same column show statistically differences (p < 0.05).
* Central point.
# Optimal point.
LOX in-vitro assay
In-vitro neuro-inflammatory protection was evaluated through LOX enzymatic inhibition. High extraction temperatures (100 °C) yield the highest inhibitory capacity (Supplementary Fig. 1) even higher than quercetin, the positive control (p < 0.05) (Table 2). To evaluate the impact of higher temperature values on LOX enzymatic inhibition, extraction temperatures above 100 °C were tested (120 °C and 160 °C). However, higher IC50 values for LOX inhibition activity were obtained (Table 2), most probably due to the degradation of bioactive terpenoids at temperatures above 100 °C. Baylac and Racine (2003) reported the use of sweet orange in aromatherapy, as a large source of terpenoids with anti-inflamatory and LOX inhibitor capacity (Baylac & Racine, 2003). However others authors suggest that LOX inhibition potential is mainly due to phenolic compounds, underestimating the potential of terpenoids in the orange extract (Malterud & Rydland, 2000). Total phenolic and carotenoids are not considered in the present research, since no correlation with bioactivity assays was found in our previous work (Sánchez-Martínez et al., 2021).
Antioxidant in-vitro assays
Antioxidant capacity from PLE extracts in ABTS test was significantly improved in extracts obtained at 100 °C (p < 0.05) (Supplementary Fig. 1). In fact, no statistical differences between 100 °C extracts and ascorbic acid (positive control) was observed (Table 2). Regarding ROS-ORAC assay, all the PLE extracts show similar values, except those obtained at 25 °C that showed higher IC50 values. Ascorbic acid had lower IC50 in ORAC probe (Table 2). On the other hand, the best RNS scavenging capacity was observed in the central point of the optimization experiment (62.5 °C and 20 min) (Supplementary Fig. 1), showing significantly lower IC50 values than ascorbic acid (Table 2). Compared to our conventional extract obtained with ETAC (Sánchez-Martínez et al., 2021), PLE extracts at optimal conditions significantly improve the antioxidant capacity in ABTS and ORAC assays, whereas similar values were obtained for RNS assay. The antioxidant capacity of orange extracts has been traditionally attributed to phenolic compounds (Park et al., 2014). However, Mohammadian et al. (2011) did not find relationship between phenolic compounds content and antioxidant activity, in agreement with our previous results (Sánchez-Martínez et al., 2021). In our previous work, conventional extracts from orange by-product were performed, using organic solvents covering a wide range of polarities (heptane, ethyl acetate, acetone and ethanol). The extracts obtained with ethanol (high polarity) showed the higher phenolic content, whereas weak antioxidant results were observed, regarding ROS and RNS scavenging capacity. On the other hand, the conventional extract obtained with ethyl acetate showed lower phenolic compound content but higher antioxidant capacity than ethanolic extract. Thus, direct correlation was not found between phenolic compounds present in orange by-product and antioxidant capacity (Sánchez-Martínez et al., 2021). Most works reported in literature commonly attributed antioxidant capacity of orange extract to the presence of phenolic compound; however, the terpenoids content are not frequently considered. In this sense, it is important to remark that our starting material was mainly composed by peels and non-juice pulp. So the ratio terpenoids/phenolic compounds is not the typical of oranges. For this reason, our result are not in line with (Park et al., 2014). Other possible explanation for this results may be the large presence of ascorbic acid in orange sample, that could exert a synergic and confounding factor (Mohammadian et al., 2011). In fact, monoterpenoids such as limonene present in large amount in orange peel showed important antioxidant activity (Shah et al., 2019).
Global optimization
PLE extraction was optimized considering the above mentioned response variables. For this purpose, a desirability function was achieved combining IC50 values of AChE, BChE, LOX, ABTS, ROS and RNS; and yield (%). Considering that the extraction yield is an important factor in the development of nutraceuticals or food supplements based on plant extracts and the need for a large amount of extracts in order to conduct neuroprotective in-vivo experiments (Farr et al., 2016), extraction yield was foster in the present PLE optimization model. In this sense, the weight of the yield in the multi-response optimization was given double than the other response variables. Under these conditions, the final PLE optimal conditions were 96 °C and 30 min (Supplementary Fig. 2), similar to the experimental point obtained at 100 °C and 30 min (Supplementary Table 8 and 9). Therefore, this extract was considered the optimum and employed for further experiments.
Terpenes content in the organic extract.
Four orange by-products extracts were selected and comparatively evaluated in terms of terpenoids content, as determined by GC–qTOF–MS analysis: PLE62 (62.5 °C and 20 min) as PLE optimization central point; PLE25 (25 °C and 30 min) as extract showing weak results in bioactivity assays; PLE100 (100 °C and 30 min) as the optimum extract; and the extract obtained under conventional conditions (Conventional). Table 1 shows terpenoids quantification results in terms of concentration (mean ± relative standard deviation, ng mL−1). Terpenoids significant differences between extracts were indicated by different letters in the same row (p < 0.05). Terpenoids were classified into families, based on the number of isoprene units in the chemical structure: monoterpenoids (C10), sesquiterpenoids (C15) and triterpenoids (C30).
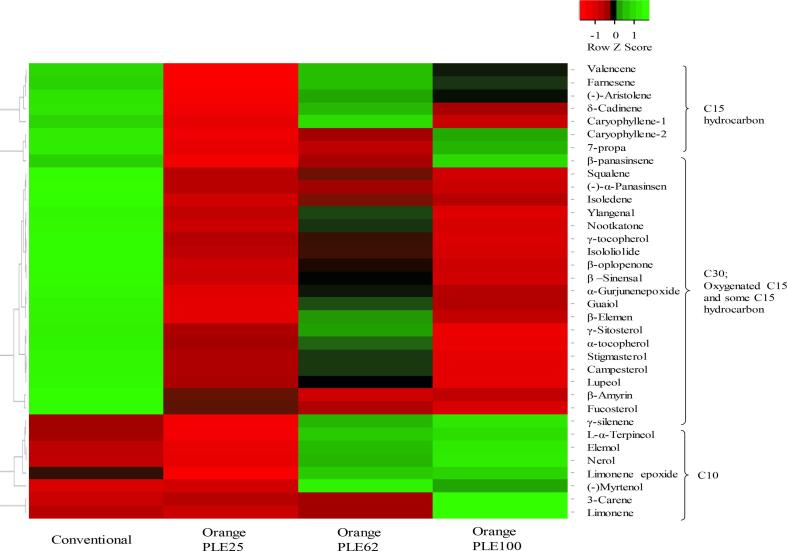
Remarkable differences between the selected extracts can be clearly observed in Fig. 1, where selected samples are grouped according to their terpenoids content as a result of a cluster analysis. The resulting heatmap shows a color code from lower (light red) to higher concentration levels (light green). Although α-tocopherol and γ-Sitosterol are the major triterpenoids in most of the studied extracts, the largest differential enrichment is observed for monoterpenes. Thus, PLE100 showed the highest enrichment in monoterpenes like limonene and 3-carene. A reduction in monoterpenes content can be observed as PLE temperature decreases (p < 0.05) (Table 1); which can be explained by the higher thermostability of C10 compounds that allow mass transfer improvement at high temperatures. With regard to sesquiterpenoids, there are no large differences between extracts, nevertheless PLE62 and Conventional extracts exhibit higher content in oxygenated sesquiterpenes such as nootkatone or β –sinensal. Meanwhile, conventional extract presents higher enrichment of triterpenoids like squalene or fucosterol; suggesting that low temperatures and long extraction times (24 h) improve the recovery of large molecules like triterpenoids. In fact, C30 terpenoids seems to be more sensitive to temperature and decreases under optimum conditions (100 °C). The weak results observed in in-vitro bioactivity test of PLE25 can be explained by their poor content in C10 and C15 terpenoids (Table 1).
Fig. 1.
Heat map from Orange PLE extract and their terpenoid composition.
Relationship between bioactivity and chemical composition
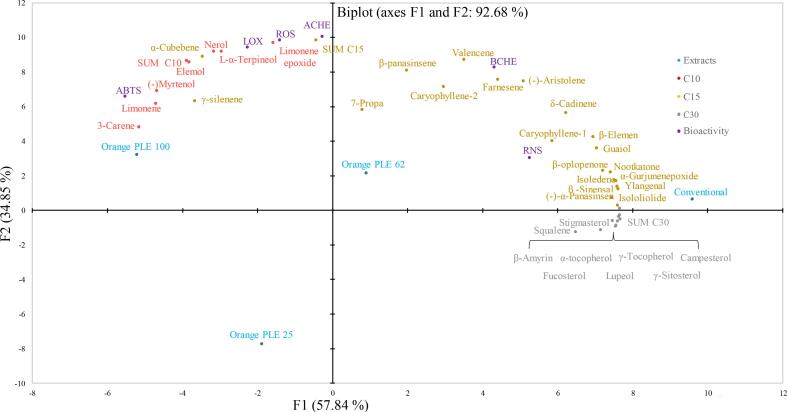
A principal component analysis (PCA) was conducted in order to establish relationships between terpenoid chemical structure of orange by-product extracts and in-vitro neuroprotection. PCA was built considering the terpenoid concentration in orange by-product extracts as compound variables, and IC50 results from the different in-vitro assay as bioactivity variables. The proposed unsupervised multivariate analysis allows the evaluation of the compositional variability of the data to obtain the correlation between variables. Associations between variables were established by proximity in the multivariate space.
According to the PCA biplot results displayed in Fig. 2, the first two principal components (PC1 and PC2) explain 92.68% of the variance, showing a large distribution of samples (extracts) and variables. The main principal component (PC1, 57% of variance) explains the capacity of the assayed PLE conditions to extract different types terpenoids. Thus, PLE100 show positive correlation with C10 terpenoids, including 3-carene, limonene, nerol and limonene epoxide; whereas PLE62 extract presents higher enrichment in some of C15 terpenods, such as farnesene, valencene, α-copaene and nootkatone. On the other hand, Conventional extraction was the preferred method to selectively extract C30 terpenoids such as squalene, campesterol, stigmasterol and γ-sitosterol; whereas PLE25 extract shows a poor terpenoids enrichment, compared to other extracts, without a clear correlation with any of the variables (Fig. 2).
Fig. 2.
PCA projection of PLE orange extracts and studied variables.
Regarding PC2 axis (34% of variance), PC2 explains general in-vitro neuroprotection. Thus, extracts distributed along the positive axis of PC2 shows lower IC50 value in in-vitro assays, whereas higher IC50 values in in-vitro assays were shown by those extracts distributed along the negative axis of PC2. In line with the in-vitro bioactivity results obtained in section 3.1, PCA shows that PLE100 extract exhibits the highest general in-vitro neuroprotection capacity, and C10 terpenoids are the main responsible compounds associated to the bioactive properties. In fact, Cutillas et al. (2018) highlighted monoterpenes such as limonene, α-terpineol, or 3-carene- present in our orange by-product PLE extract as compounds with large antienzymatic (AChE and LOX) and antioxidant (ABTS and ORAC assays) activities, in line with our results in-vitro (Cutillas et al., 2018). In agreement with the PCA results reported in our previous work (Sánchez-Martínez et al., 2021), AChE and BChE inhibitory activity seems to be associated to the presence of some C15 terpenoids, such as γ-selinene or δ-cadinene. The total contribution of C15 terpenoids (SUM C15) and some C10 like limonene epoxide were related to AChE enzymatic inhibition, whereas BChE enzymatic inhibition seems to be associated to C15 hydrocarbons such as valencene. In this line, researches made by Politeo et al. (2018) shows that hydrocarbon terpenoids exhibit more cholinesterase inhibition potential than oxygenated terpenes (Politeo et al., 2018). Results obtained in this work do not allow attributing RNS scavenging capacity to C10 terpenoids, as reported in our previous research. This might be explained by the large content in C30 of Conventional and PLE62 extract, that can act as potent RNS scavengers by synergic effect. In summary, tocopherols and phytosterols are large molecules with multiple hydroxyl group capable to cancel RNS through hydrogen donation (Graßmann, 2005).
Molecular docking
Molecular docking is a powerful tool for drug discovery, frequently used to understand the therapeutic effectiveness of a potential drug. In this study, a molecular docking simulation was performed to evaluate the binding affinity of different complexes between three potentially bioactive terpenoids (alpha-terpineol, limonene epoxide and valencene) and the target enzymes LOX, AChE, and BChE (Supplementary Fig. 3). As can be seen, satisfactory binding energy values (-6.04 kcal/mol) were obtained for alpha terpineol-Lipoxygenase complex, in comparison to the biding energy obtained for the complex with quercetin (control). Regarding binding energy, a lower Dock score represents a more stable binding, that may be attributed to the hydrogen bonds with the residual amino acids (HIS: 518) and (GLN: 716). This conformational behaviour demonstrates that alpha terpineol can be a strong inhibitor of LOX enzyme, acting in one of the main catalytic sites, as highlighted by Skrzypczak-Jankun et al. (2003) (Skrzypczak-Jankun et al., 2003). Regarding the structures of acetylcholinesterase and butyrylcholinesterase, there are few differences in the active sites of these cholinergic enzymes, mainly associated to the accommodation of bulkier molecules in BChE catalytic site. This is because there are aromatic residues in the active site of AChE (Tyr72, Tyr124, Tyr337, Phe295, Phe297) while in BuChE aliphatic residues are present (Asn72, Gln124, AlaA337, Leu286, Val288) (Brus et al., 2014). These enzymes are characterized by a narrow active site (known as deep gorge (20 Å)) where the peripheral anionic site (PAS) is located at the entrance, and the catalytic acylation site (CAS) at the lower point from the deep gorge. Within the CAS a catalytic triad (Ser, His, Glu) is responsible for important bonds between the ligand and the substrate (Sharma et al., 2019). As can be seen, for both AChE and BChE enzymes, the binding values are not far from the complexes obtained with the galantamine. We verified that for AChE, pi-alkyl interactions can occur between the epoxide limonene and the amino acid HIS447, and electrostatic forces with SER203. These interactions demonstrate that this structure is located close to the catalytic triad. Previous reports also found significant interactions between monoterpenoids and amino acids residues of AChE such as Ser203 and His447, among others (Wojtunik-Kulesza et al., 2021). Similarly, the valencene – BChE complex showed a pi-alkyl bond with HIS438 observed in the catalytic triad, and also Pi-sigma interactions with TRP86, a binding site with choline. Other essential oils from citrus extracts showed satisfactory docking simulations towards BChE inhibition (Boiangiu et al., 2020).
Cell culture in-vitro cytotoxicity of PLE100 extract
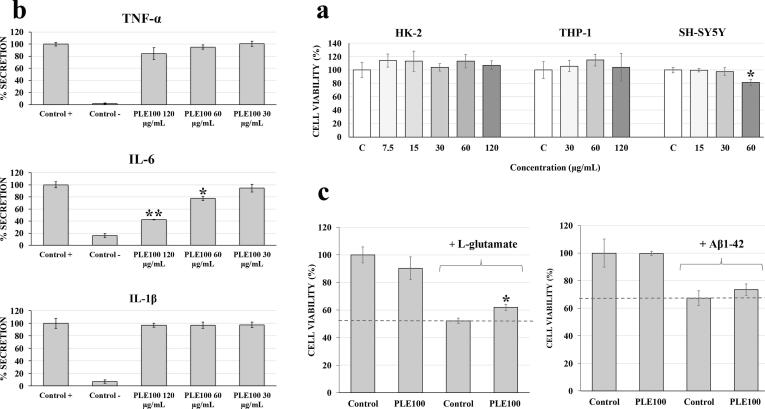
Based on the previous results, the possible in-vitro cytotoxicity of the most promising neuroprotective extract (PLE100) was evaluated. Three different cell culture models were used due to their different characteristics (Fig. 3a). HK-2 cell line was selected because it is considered as a suitable model to predict in-vitro toxicity in humans (Gunness et al., 2010). As can be observed in Fig. 3a, none of the tested concentrations (from 7.5 to 120 µg mL−1) of the extract exhibited any toxicity on HK-2 cells. On the other hand, the THP-1 cell line is extensively used to evaluate macrophage behavior and their production of pro-inflammatory cytokines (Chanput et al., 2014). In our experiments, the three highest concentrations of the PLE100 extract which showed no toxicity on HK-2 cells (30, 60 and 120 µg mL−1), were tested. As it can be observed in Fig. 3a, all the concentrations tested maintained the cell viability at maximum. Finally, the SH-SY5Y cell line was selected because it is consider a potential and validated cell line model for in-vitro neurodegenerative disorders studies like AD (de Medeiros et al., 2019). Based on our previous knowledge about cell culture susceptibility, lower concentrations of the extracts than those used with HK-2 cells were selected. As it can be observed in Fig. 3a, only the highest concentration tested (60 µg mL−1) was toxic, and therefore the concentration of 30 µg mL−1 was selected for the neuroprotective evaluation. Different studies have evaluated the citotoxicity of some of the main components in orange extracts. This is the case for limonene (Shah et al., 2019) and valencene (Yang et al., 2016) but also the whole orange extracts have been studied (Atolani et al., 2020). These reports have shown that the most abundant compounds as well as the whole extracts are safe for organisms which may support the future use of the obtained PLE100 extract.
Fig. 3.
PCA projection of PLE orange extracts and studied variablesFigure 3. a Effect of different concentrations of PLE100 extract on cell viability in HK-2, differentiated THP-1 and SHSY5Y cells. Fig. 3b. Secretion levels of TNF-α, IL-6 and IL-1β in differentiated THP-1 cells treated with and without LPS treatment (Control + and Control -, respectively), and treated with LPS in the presence of PLE100 extract 30, 60 and 120 μg mL−1. Each bar is the mean of three determinations ± SD. * Denotes statistical differences when compared PLE100-treated cells with positive control (*: p < 0.05; **: p < 0.01). Fig. 3c. Neuroprotective effect of pre-incubation of PLE100 extract against the neurotoxic agents l-glutamate (23 mM) and Aβ1-42 (30 µM) in differentiated SH-SY5Y cells. Non Aβ1-42-treated cells were used as Control, together with only PLE100-treated cells (PLE100) at 30 μg mL−1. The results are mean ± SD. * Denotes statistical differences between Control and PLE100 when neurotoxic agent is added (*: p < 0.05).
Anti-inflammatory Activity of PLE100 extract in Differentiated Human Macrophages
The TNF-α, IL-1β and IL-6 pro-inflammatory cytokines evaluated in the present work are considered as key mediators of neuroinflammation and they are the most studied cytokines in inflammatory hypothesis of neurodegenerative disorders (Armstrong, 2016). According to the cytotoxicity results obtained in the previous sections, differentiated THP-1 macrophages were induced to inflammation through LPS, and three non-toxic concentrations of the PLE100 extract (30, 60 and 120 μg mL−1) were evaluated. As it can be observed in Fig. 3b, the positive control (cells only treated with LPS) showed an important increase in all pro-inflammatory cytokines measured (TNF-α, IL-6 and IL-1β), compared to the negative control (non LPS-treated cells) after 24 h of incubation. Thus, LPS-induced cells were considered as the maximum cytokine secretion level (100 %). Comparing the secretion of the three cytokines, only IL-6 showed significant differences when the PLE100 extract was added. The inhibition of IL-6 secretion levels reached 57.6 % and 22.4 % when 60 and 120 μg mL−1 of PLE100 were used, respectively, in comparison with the levels obtained in the absence of extract and presence of LPS. The lowest concentration tested did not reach a significant secretion inhibition. Regarding TNF-α, only the highest concentration of PLE100 had an effect (15.7 % of inhibition of secretion), although it was not significant compared to the positive control. Previous studies have shown that some hydrocarbon terpenoids like limonene C10 and valencene C15 (present in the PLE100 extract) can reduce the pro-inflammatory cytokines IL-1β and IL-6 after LPS stimulated in RAW 264.7 Macrophages (Yoon et al., 2010). Moreover, some authors have reported interactions between LOX enzyme activity and pro-inflammatory cytokines (Pihlaja et al., 2017). In fact, cascade reactions from LOX activity can trigger the increase of enzymatic products like chemokines and leukotrienes, that can stimulate the generation of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β. Moreover, LOX inhibitors have shown the capacity to reduce the production of some inflammatory cytokines like IL-6 in astrocytes and glia model cells (Pihlaja et al., 2017). All these results might support the observed LOX and cytokine anti-inflammatory capacity of the PLE100 extract.
Neuroprotection effect of PLE100 extract against Aβ1-42 and l-glutamic acid using SH-SY5Y Cells
After the in-vitro toxicity evaluation of the PLE100 extract on differentiated SH-SY5Y cells, the highest non-toxic concentration (30 µg mL−1) was selected to evaluate its potential neuroprotective capacity. As it can be observed in Fig. 3c, the incubation of SH-SY5Y cells with Aβ1-42 or l-glutamic acid for 24 h reduced the cell viability to 60% and 50%, respectively, as compared to the control conditions (Fig. 3c). It can also be observed that the pre-treatment of the cells with the PLE100 extract slightly protects the cells against Aβ1-42 and l-glutamic acid, being significant in the case of l-glutamic acid (Fig. 3c). In this regard, two recently published studies from our laboratory have showed that a terpenoid-rich extract from olive leaves has neuroprotective effects against Aβ1-42 (Gallego et al., 2021a), and a carotenoid-rich extract from Dunaliella salina microalgae can protect against both Aβ1-42 and l-glutamic acid toxic effects (Gallego et al., 2021b). Moreover, the neuroprotective effect against l-glutamic acid was also observed by Fernandes et al. (2018), after using different edible seaweed (Ochrophyta) extracts (Fernandes et al., 2018). The neuroprotective mechanism proposed for PLE100 extract is the protection against the massive oxidative stress induced by l-glutamic acid, and one of the main bioactive compound of PLE100, limonene, has neuroprotection potential by its large antioxidant and anti-inflammatory properties in vivo. As commented above, one of the main components of the PLE100 extract is a C10 terpene (limonene), and some research articles have suggested C10 compounds like eucalyptol or linalool as potential neuroprotective compounds that can reduce cell death caused via Aβ plaques deposition or glutamic acid toxicity induction (Habtemariam, 2018). Previous reports have also showed that pure limonene (and whole orange extracts mainly composed by limonene) has neuroprotective capacity in-vivo (Bigdeli et al., 2019). Moreover, Bigdeli et al. (2019) described the principal mechanism of limonene neuroprotection, suggesting that antioxidant and anti-inflammatory activities are the principal makers (Bigdeli et al., 2019). This report supports our present antioxidant and anti-inflammatory results, and the possible protection against massive oxidative stress induced by glutamic acid. However, further in-vitro and in-vivo experiments are needed to verify these results.
Conclusions
In the present work, orange by-product extracts from pressurized liquid extraction (PLE) were compared in a neuroprotective in-vitro assessment. High temperature selected extract from PLE (PLE100) not only enhanced some terpenoid extraction yield but also antioxidant (ABTS and ORAC assays), anti-inflammatory (LOX and IL-6 pro-inflammatory mediator’s inhibition) and anti-acetylcholinesterase capacities. Terpenoid quantification and multivariate data analysis placed monoterpenoids like limonene and some sesquiterpenoids such as valencene as a potential bioactive compound against neurodegenerative hallmarks. Moreover, the in-silico molecular docking demonstrate that possible connections with limonene epoxide, valencene and alpha terpineol terpenoids occurred at low energies, providing different molecular interactions capable of providing enzymatic inhibition. Moreover, PLE100 extract shows low level of cytotoxicity in all cell lines evaluated and reveals protection in SH-SY5Y neuroblastoma cells after neurotoxicity induced by l-glutamic acid exposition. Finally, 100 °C provided the best extraction conditions in terms of neuroprotective capacity by their large content in C10 and C15 bioactive molecules. The results obtained in this work positioned the orange juice by-product as a wide and economical source of bioactive compounds, and represent a first step to develop extraction methodologies to purify monoterpenoids and sesquiterpenoids with low cytotoxicity and neuroprotective potential due to their important in-vitro BBB accessibility, antioxidant and anti-inflammatory capacity, that can be considered an important basement for future in vivo experiments that can reinforce their use as supplements or nutraceuticals for neurodegenerative disorders prevention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
J.D.S.-M. would like acknowledge The Ministry of Education and Vocational Training for a FPU predoctoral grant FPU17/01876. G.A.-R. and A.V. would like to acknowledge the Ministry of Science and Innovation (MICINN) for their “Juan de la Cierva-Incorporación” postdoctoral grants IJC2019-041482-I and IJC2018-037560-I, respectively. The authors also thank the support from the AGL2017-89417-R project (MINECO).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100242.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ademosun A.O., Oboh G. Anticholinesterase and antioxidative properties of water-extractable phytochemicals from some citrus peels. Journal of Basic and Clinical Physiology and Pharmacology. 2014;25(2):199–204. doi: 10.1515/jbcpp-2013-0027. [DOI] [PubMed] [Google Scholar]
- AlFadly E.D., Elzahhar P.A., Tramarin A., Elkazaz S., Shaltout H., Abu-Serie M.M.…Belal A.S.F. Tackling neuroinflammation and cholinergic deficit in Alzheimer’s disease: Multi-target inhibitors of cholinesterases, cyclooxygenase-2 and 15-lipoxygenase. European Journal of Medicinal Chemistry. 2019;167:161–186. doi: 10.1016/j.ejmech.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Alvarez-Rivera G., Bueno M., Ballesteros-Vivas D., Mendiola J.A., Ibañez E. Liquid-Phase Extraction. Elsevier; 2020. Pressurized Liquid Extraction; pp. 375–398. [DOI] [Google Scholar]
- Armstrong, S. R. D. (2016). Oxidative Stress and Antioxidant Protection.
- Atolani O., Adamu N., Oguntoye O.S., Zubair M.F., Fabiyi O.A., Oyegoke R.A.…Olatunji G.A. Chemical characterization, antioxidant, cytotoxicity, Anti-Toxoplasma gondii and antimicrobial potentials of the Citrus sinensis seed oil for sustainable cosmeceutical production. Heliyon. 2020;6(2):e03399. doi: 10.1016/j.heliyon.2020.e03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auti S.T., Kulkarni Y.A. A systematic review on the role of natural products in modulating the pathways in alzheimer’s disease. International Journal for Vitamin and Nutrition Research. 2017;87(1–2):99–116. doi: 10.1024/0300-9831/a000405. [DOI] [PubMed] [Google Scholar]
- Baylac S., Racine P. Inhibition of 5-lipoxygenase by essential oils and other natural fragment extracts. International Journal of Aromatherapy. 2003;13(2–3):138–142. doi: 10.1016/S0962-4562(03)00083-3. [DOI] [Google Scholar]
- Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H.…Bourne P.E. The Protein Data Bank. Nucleic Acids Research. 2000;28(1):235–242. doi: 10.1093/NAR/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigdeli Y., Asle-Rousta M., Rahnema M. Effects of Limonene on Chronic Restraint Stress-Induced Memory Impairment and Anxiety in Male Rats. Neurophysiology. 2019;51(2):107–113. doi: 10.1007/s11062-019-09800-0. [DOI] [Google Scholar]
- Boiangiu, R. S., Brinza, I., Hancianu, M., Orhan, I. E., Eren, G., Gündüz, E., Ertas, H., Hritcu, L., & Cioanca, O. (2020). Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches. Molecules 2020, Vol. 25, Page 1519, 25(7), 1519. https://doi.org/10.3390/MOLECULES25071519. [DOI] [PMC free article] [PubMed]
- Brus B., Košak U., Turk S., Pišlar A., Coquelle N., Kos J.…Gobec S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. Journal of Medicinal Chemistry. 2014;57(19):8167–8179. doi: 10.1021/jm501195e. [DOI] [PubMed] [Google Scholar]
- Chanput, W., Mes, J. J., & Wichers, H. J. (2014). THP-1 cell line: An in vitro cell model for immune modulation approach. In International Immunopharmacology (Vol. 23, Issue 1, pp. 37–45). Elsevier. https://doi.org/10.1016/j.intimp.2014.08.002. [DOI] [PubMed]
- Cutillas A.-B., Carrasco A., Martinez-Gutierrez R., Tomas V., Tudela J. Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant Biosystems - An International Journal Dealing with All Aspects of Plant Biology. 2018;152(6):1282–1292. doi: 10.1080/11263504.2018.1445129. [DOI] [Google Scholar]
- de Medeiros L.M., De Bastiani M.A., Rico E.P., Schonhofen P., Pfaffenseller B., Wollenhaupt-Aguiar B.…Klamt F. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Molecular Neurobiology. 2019;56(11):7355–7367. doi: 10.1007/s12035-019-1605-3. [DOI] [PubMed] [Google Scholar]
- Farr S.A., Niehoff M.L., Ceddia M.A., Herrlinger K.A., Lewis B.J., Feng S.…Morley J.E. Effect of botanical extracts containing carnosic acid or rosmarinic acid on learning and memory in SAMP8 mice. Physiology and Behavior. 2016;165:328–338. doi: 10.1016/j.physbeh.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Fernandes F., Barbosa M., Pereira D.M., Sousa-Pinto I., Valentão P., Azevedo I.C., Andrade P.B. Chemical profiling of edible seaweed (Ochrophyta) extracts and assessment of their in vitro effects on cell-free enzyme systems and on the viability of glutamate-injured SH-SY5Y cells. Food and Chemical Toxicology. 2018;116:196–206. doi: 10.1016/J.FCT.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Gallego R., Suárez-Montenegro Z.J., Ibáñez E., Herrero M., Valdés A., Cifuentes A. In vitro Neuroprotective Potential and Lipidomics Study of Olive Leaves Extracts Enriched in Triterpenoids. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.769218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R., Tardif C., Parreira C., Guerra T., Alves M.J., Ibáñez E., Herrero M. Simultaneous extraction and purification of fucoxanthin from Tisochrysis lutea microalgae using compressed fluids. Journal of Separation Science. 2020;43(9-10):1967–1977. doi: 10.1002/jssc.v43.9-1010.1002/jssc.202000021. [DOI] [PubMed] [Google Scholar]
- Gallego R., Valdés A., José David S.-M., Suárez-Montenegro Z.J., Ibáñez E., Cifuentes A., Herrero M. Analytical and Bioanalytical Chemistry; 2021. Study of the potential neuroprotective effect of Dunaliella salina extract in SH-SY5Y cell model. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Cofrade, L., De Las Heras, B., Apaza Ticona, L., & Palomino, O. M. (2019). Molecular Targets Involved in the Neuroprotection Mediated by Terpenoids. In Planta Medica (Vol. 85, Issue 17, pp. 1304–1315). Georg Thieme Verlag. https://doi.org/10.1055/a-0953-6738. [DOI] [PubMed]
- Graßmann J. Terpenoids as Plant Antioxidants. Vitamins and Hormones. 2005;72(05):505–535. doi: 10.1016/S0083-6729(05)72015-X. [DOI] [PubMed] [Google Scholar]
- Gunness P., Aleksa K., Kosuge K., Ito S., Koren G. Comparison of the novel HK-2 human renal proximal tubular cell line with the standard LLC-PK1 cell line in studying drug-induced nephrotoxicity. Canadian Journal of Physiology and Pharmacology. 2010;88(4):448–455. doi: 10.1139/Y10-023. [DOI] [PubMed] [Google Scholar]
- Habtemariam S. iridoids and other monoterpenes in the Alzheimer’s brain: Recent development and future prospects. Molecules. 2018;23(1):117. doi: 10.3390/molecules23010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malterud K.E., Rydland K.M. Inhibitors of 15-lipoxygenase from orange peel. Journal of Agricultural and Food Chemistry. 2000;48(11):5576–5580. doi: 10.1021/jf000613v. [DOI] [PubMed] [Google Scholar]
- Mohammadian M.A., Mobrami Z., Sajedi R.H. Bioactive compounds and antioxidant capacities in the flavedo tissue of two citrus cultivars under low temperature. Brazilian Journal of Plant Physiology. 2011;23(3):203–208. doi: 10.1590/S1677-04202011000300004. [DOI] [Google Scholar]
- Mushtaq G., Greig N., Khan J., Kamal M. Status of Acetylcholinesterase and Butyrylcholinesterase in Alzheimer's Disease and Type 2 Diabetes Mellitus. CNS & Neurological Disorders – Drug Targets. 2014;13(8):1432–1439. doi: 10.2174/1871527313666141023141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco M.T., Moreno F.J., Villamiel M. Chemical and physicochemical characterization of orange by-products derived from industry. Journal of the Science of Food and Agriculture. 2019;99(2):868–876. doi: 10.1002/jsfa.2019.99.issue-210.1002/jsfa.9257. [DOI] [PubMed] [Google Scholar]
- Park J.H., Lee M., Park E. Antioxidant activity of orange flesh and peel extracted with various solvents. Preventive Nutrition and Food Science. 2014;19(4):291–298. doi: 10.3746/pnf.2014.19.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlaja R., Haaparanta-Solin M., Rinne J. The Anti-Inflammatory Effects of Lipoxygenase and Cyclo-Oxygenase Inhibitors in Inflammation-Induced Human Fetal Glia Cells and the Aβ Degradation Capacity of Human Fetal Astrocytes in an Ex vivo Assay. Frontiers in Neuroscience. 2017;11(MAY) doi: 10.3389/FNINS.2017.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politeo O., Burcul F., Blazevic I., Radan M. Terpenes, Phenylpropanoids, Sulfur and Other Essential Oil Constituents as Inhibitors of Cholinesterases. Current Medicinal Chemistry. 2018;27(26):4297–4343. doi: 10.2174/0929867325666180330092607. [DOI] [PubMed] [Google Scholar]
- Rodriguez M.M., Nolasco S.M., Dimitrov K. Ingredients Extraction by Physico-Chemical Methods in Food. Elsevier Inc.; 2017. Extracting Valuable Bioactive Compounds Using Green Processes. [DOI] [Google Scholar]
- Sabogal-Guáqueta A.M., Hobbie F., Keerthi A., Oun A., Kortholt A., Boddeke E., Dolga A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomedicine & Pharmacotherapy. 2019;118:109295. doi: 10.1016/j.biopha.2019.109295. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martínez J.D., Bueno M., Alvarez-Rivera G., Tudela J., Ibañez E., Cifuentes A. In vitro neuroprotective potential of terpenes from industrial orange juice by-products. Food & Function. 2021;12(1):302–314. doi: 10.1039/d0fo02809f. [DOI] [PubMed] [Google Scholar]
- Shah B., Shaikh M.V., Chaudagar K., Nivsarkar M., Mehta A. D-limonene possesses cytotoxicity to tumor cells but not to hepatocytes. Polish Annals of Medicine. 2019;26(2):98–104. doi: 10.29089/2017.17.00047. [DOI] [Google Scholar]
- Sharma P., Srivastava P., Seth A., Tripathi P.N., Banerjee A.G., Shrivastava S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Progress in Neurobiology. 2019;174:53–89. doi: 10.1016/J.PNEUROBIO.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Skrzypczak-Jankun E., Zhou K., Jankun J. Inhibition of lipoxygenase by (-)-epigallocatechin gallate: X-ray analysis at 2.1 Å reveals degradation of EGCG and shows soybean LOX-3 complex with EGC instead. International Journal of Molecular Medicine. 2003;12(4):415–420. doi: 10.3892/IJMM.12.4.415. [DOI] [PubMed] [Google Scholar]
- Suárez Montenegro Z.J., Álvarez-Rivera G., Sánchez-Martínez J.D., Gallego R., Valdés A., Bueno M.…Ibáñez E. Neuroprotective Effect of Terpenoids Recovered from Olive Oil By-Products. Foods. 2021;10(7):1507. doi: 10.3390/foods10071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtunik-Kulesza, K., Rudkowska, M., Kasprzak-Drozd, K., Oniszczuk, A., & Borowicz-Reutt, K. (2021). Activity of Selected Group of Monoterpenes in Alzheimer’s Disease Symptoms in Experimental Model Studies—A Non-Systematic Review. International Journal of Molecular Sciences 2021, Vol. 22, Page 7366, 22(14), 7366. https://doi.org/10.3390/IJMS22147366. [DOI] [PMC free article] [PubMed]
- Yang I.J., Lee D.-U., Shin H.M. Inhibitory Effect of Valencene on the Development of Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. Evidence-Based Complementary and Alternative Medicine. 2016;2016:1–11. doi: 10.1155/2016/9370893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon W.-J., Lee N.H., Hyun C.-G. Limonene Suppresses Lipopolysaccharide-Induced Production of Nitric Oxide, Prostaglandin E2, and Pro-inflammatory Cytokines in RAW 264.7 Macrophages. Journal of Oleo. Science. 2010;59(8):415–421. doi: 10.5650/JOS.59.415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.