Highlights
-
•
PLE using DES aqueous solutions is an eco-friendly method for anthocyanin recovery.
-
•
Pressurized DES solutions can be used to valorize the jaboticaba by-product.
-
•
DES (ChCl:Pro and ChCl:Ma) solutions had yields 50% higher than conventional solvents.
-
•
Recovered extracts showed antioxidant, anti-diabetic, and anti-obesity potential.
-
•
Pressurized ChCl:Ma solution maintained the color and bioactivity of the extracts.
Keywords: Green solvent, Pressurized liquid extraction, Natural colorant, Antioxidant, Anti-diabetic, Anti-obesity
Abstract
Deep eutectic solvents (DES) are emergent solvents with high extractability of bioactive compounds. Therefore, anthocyanin rich-fractions were recovered from jaboticaba peels by combining aqueous solutions of DES and pressurized liquid extraction (PLE). The extraction occurred at 10 MPa, 12 min, with conditions optimized through response surface methodology: 47% DES concentration, 90 °C, and 5.3 mL/min flow rate. PLE with different DES (choline chloride combined with propylene glycol or malic acid) solutions were compared to conventional solvents (water and acidified water) concerning yield, thermostability, antioxidant, anti-diabetic, and anti-obesity activities. DES solutions presented anthocyanin yields up to 50% higher than conventional solvents. ChCl:Ma, with the highest anthocyanin stability ( = 77.5 kJ.mol−1), was a promising solvent concerning color, anti-diabetic and anti-obesity potential. Environmental analysis by Green Certificate and EcoScale indicated PLE with DES solutions is a green and efficient approach to recover anthocyanin from jaboticaba peel, providing useful extracts.
Introduction
The relation between diet and human health is increasingly evident, which has been affecting the food choice of consumers. Additionally, the development of sustainable food systems is still an industrial challenge, associated with the necessity to decrease food wastage, and with better use of the underutilized resources. Therefore, natural food ingredients or additives have been evaluated as substitutes for synthetic ones due to their nutritional and technological effects associated with health benefits (Benvenutti et al., 2021, Martins et al., 2016). For that purpose, bioactive compounds obtained from plants are widely evaluated. Besides the extensively studied plant materials, the vegetable by-products such as peel, seeds, stalk, pomace, and others, have been also considered for studies because most biomasses contain a high concentration of bioactive compounds, and they are considered cheap and widely available sources of bioactive compounds, stimulating the biorefinery and circular economy concepts (del Garcia-Mendoza et al., 2017, Sorita et al., 2020).
Jaboticaba (Myrciaria cauliflora) is an underutilized Brazilian berry, unknown to the international trade market, although it presents a high nutritional value and an appreciated taste. Its cultivation is mainly by small-scale agriculture, or extractive form, however, formal data related to jaboticaba are rising, being registered commercialization of 2460 tons in 2017 only in the State of São Paulo, Brazil (Benvenutti et al., 2021). The high perishability of this fruit justifies the industrial obtention of juices, jams, syrups, liquors, fermented beverages, among others. The natural consumption and the industrial process generate the jaboticaba processing by-product, representing about 40% of the whole fruit. This processing by-product consists mainly of peel and seeds, where the peel is about 25% of the whole fruit, and the seeds reach 15%. The jaboticaba fruit and its peel show rich phytochemical composition including ascorbic acid (vitamin C), β-carotene, tocopherol, and phenolic compounds, mainly anthocyanins (Benvenutti et al., 2021, Inada et al., 2015).
Anthocyanins are water-soluble compounds belonging to the flavonoids class and are naturally present in a wide variety of flowers, leaves, vegetables, fruits, and grains, showing color from reddish to purplish (de Mejia, Zhang, Penta, Eroglu, & Lila, 2020). These compounds have application potential in food, cosmetic and pharmaceutical products as a natural colorant, but they also present antioxidant, anti-inflammatory, anti-obesity, anti-diabetic, cardiovascular protection, neuroprotection, and anticarcinogenic potential (de Mejia et al., 2020, Teixeira et al., 2021).
The anthocyanin and other phytochemicals can be recovered by Deep Eutectic Solvents (DES), a new generation of solvents with potential application in various industrial fields. These solvents are formed by one hydrogen bond acceptor (HBA) and one or more hydrogen bond donors (HBD), which form a mixture, through molecular interactions, with lower melting point than an ideal eutectic mixture, which mostly present high performance for bioactive compounds extraction (Benvenutti et al., 2019, Dai et al., 2016).
The above-mentioned molecular interactions form a supramolecular structure that increases the solubility of target compounds. Therefore, the components used in the HBA:HBD mixture, as well as their molar ratio, define the DES solvation capacity and their environmental characrteristics, such as toxicity and biodegradability (Ahmadi et al., 2018, Benvenutti et al., 2019, Dai et al., 2016). Also, when the formers HBA and HBD are natural components, the resulting DES have been widely evaluated as harmless and non-toxic solvents compared to conventional ones. Therefore, they are often called as Natural Deep Eutectic Solvent (NADES), for instance, when common HBA and HBD compounds are the natural choline chloride, sugars, organic acids, among others (Benvenutti et al., 2019). However, despite being GRAS (Generally recognized as safe) substances, with their mixtures presenting high biodegradability and low cytotoxicity (Ahmadi et al., 2018, Aslan Türker and Doğan, 2021, Radošević et al., 2016), they are mostly industrially manufactured, and therefore much caution is required with the use of the term natural.
Some DES present water in their composition, which precipitates the supramolecular structure of these solvents, decreasing their viscosity. As a result, the analytes dissolution rate increases, while the solvent costs decrease. However, excessive water concentration weakens the hydrogen bonds between HBA and HBD, reducing the solvation ability of this solvent (Bajkacz and Adamek, 2017, Liu et al., 2018). Nevertheless, the recovery of polar compounds from solid matrix is more efficient with DES-water solutions, compared with only DES, since the water can form hydrogen bonds with the target compound, contribution to its dissolution (Benvenutti et al., 2020, Shishov et al., 2021).
In order to associate DES-water systems with genuine green extractions, besides factors such as safe solvents, and efficient performance, it is also necessary to combined innovative and environmentally-friendly technology, aiming to intensify the process, reducing time, energy and solvent consumption (Chemat et al., 2019). Then, within the emergent technologies, the pressurized liquid extraction (PLE) applies high-pressure to improve the solvent diffusion, promoting high solvation power, besides facilitating the rupture of the cells from the solid matrix, which results in high yields at low processing time and solvent amount (Rodrigues et al., 2019, Zielinski et al., 2021). Concerning high-pressure methods, DES have already been reported as an enhancer to subcritical water extraction (SWE). For instance, 30% DES (alanine: citric acid) water solution, by SWE method, was highly efficient to recover xanthone from mangosteen pericarp (Machmudah et al., 2018). Similarly, SWE with 30% DES (choline chloride: urea) provided an efficient recovery of phenolic compounds from winemaking byproduct (Loarce, Oliver-Simancas, Marchante, Díaz-Maroto, & Alañón, 2020), while SWE with 30% DES (choline chloride: oxalic acid) extracted anthocyanin from grape pomace (Loarce, Oliver-Simancas, Marchante, Díaz-Maroto, & Alañón, 2021).
Therefore, the aims of this study were: (i) to optimize the recovery of anthocyanin-rich fractions from jaboticaba by-product using DES aqueous solutions associated with PLE method, (ii) to evaluate the effect of choline chloride-based DES on the thermostability of the recovered anthocyanins, as well as on the antioxidant, anti-diabetic, and anti-obesity potentials from the extracts and (iii) to investigate this approach as a green extraction alternative for the recovery of valuable bioactive compounds.
Material and methods
Materials
The jaboticaba (Myrciaria cauliflora) processing by-product, consisting of peel, seed, and remaining pulp (10 kg) was acquired from Sítio do Bello (Piraibuna, SP, Brazil). DES components such as malic acid were purchased from Êxodo Científica (Sumaré, SP), 2-Hydroxyethyltrimethylammonium chloride (Choline Chloride – ChCl) >98% purity was obtained from Sigma-Aldrich (Steinheim, Germany) and propylene glycol (>99.5) from Neon Commercial (Suzano, SP, Brazil). Solvents such as 99.5% ethanol, 98–100% formic acid, and ethyl acetate 99.5%, from Êxodo Científica (Sumaré, SP, Brazil). Other chemicals as Amberlite XAD-7HD resin, methanol suitable for HPLC (≥99,9% purity), TPTZ (2,4,6-tri(2-pyridyl)-s-triazine), ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid), pNPG (p-nitrophenyl-α-d-glucopyranoside), pNPB (p-nitrophenol-butyrate), DNS (3,5-dinitro salicylic acid), standards Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) (97% purity) and gallic acid (>97% purity), and enzymes α-amylase from porcine pancreas, α -glucosidase from Saccharomyces cerevisiae and lipase from human pancreas were acquired from Sigma Aldrich (Steinheim, Germany).
DES preparation
The DES constituents were weighed in analytical balance (Shimadzu, AUY220, SP, Brazil) to produce choline chloride and propylene glycol at 1:2 M ratio (ChCl:Pro), and chlorine chloride and malic acid (ChCl:Ma) at 1:1 M ratio. The hydrogen bonds between choline chloride and the hydroxyl groups of HBD must be adequate to form the eutectic mixtures, which are dependent of the molar ratios, selected according to Dai et al. (2013). Then, the mixtures were heated at 80 °C, at continuous stirring, by a type Dubnoff bath (Ethik technology, 304 TPA model, SP, Brazil), until homogeneous and transparent liquid solvents were observed. To decrease the ChCl:Pro and ChCl:Ma viscosities, enabling the handling by PLE unit and improving dissolution rates, the solvents were diluted in distilled water at concentrations from 5 to 55%.
Sample preparation
The Myrciaria cauliflora processing by-product was manually separated into seeds (JS) and peel containing remaining pulp (JP). In this study, just the JP fraction was used because it contains a higher anthocyanin concentration than seeds. Thus, the JP was dried to conserve the vegetal matrix and concentrate the target compound in an oven with air circulation at 50 °C (Lucadema, Model 82/27, SP, Brazil), ground in a knife mill (Marconi, Model MA340, SP, Brazil), and sieved to standardize particle size between 0.30 and 0.85 mm. The centesimal composition in terms of moisture, fixed mineral residue (ashes), total fat, protein, and crude fiber of the prepared sample (dried, ground, and sieved JP sample) was performed according to AOAC methodologies (AOAC, 2005) performed in triplicates. The content of non-fibrous carbohydrates was calculated by difference.
Pressurized liquid extraction (PLE)
The obtention of the anthocyanin-rich extract was performed by combining the use of aqueous solution of DES with pressurized liquid extraction (PLE). The process was performed in a self-assembled apparatus described by Rodrigues, Mazzutti, Vitali, Micke, & Ferreira (2019). The assays were made at continuous mode, with pressure fixed in 10 MPa and time of 12 min, which was defined through an extraction kinetic (performed with 5 g of sample, at 90 °C, 30% of DES solution as the solvent and flow rate of 4 mL/min) based in monomeric anthocyanin pigment (MAP) concentration, according to section 2.5.1. The kinetic data were fitted to a three straight lines model using a Statistica v.13.5 software (TIBCO Software Inc., Palo Alto, CA, USA).
For this study, the independent variables were temperature (60, 90, and 120° C), DES concentration in water (15, 30, and 45%), and flow rate (3, 4, and 5 mL/min). The PLE process was optimized using the variable conditions (factors) combined through a Central Composite Rotatable Design (CCRD), as shown at Table 1. The design method allows evaluating the main effects and their interactions, providing a nonlinear response function. To CCRD study was systematically organized by the Statistica v.13.5 software, and consisted of 23 factorial designs, 6 factorial points [±α = (2k)1/4, where k is the number of variables, and 3 central points, to evaluate the assays repeatability and the pure error of the nonlinear model (Rodrigues & Iemma, 2014).
Table 1.
Central Composite Rotatable Design (CCRD) for the independent variables considered for the PLE extraction with aqueous solutions of DES (ChCl:Pro) from JP samples at the pressure of 10 MPa, and the results obtained for the extraction assays in terms of yield and extract quality.
|
Independent Variables |
Responses |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Assays |
DES concentration |
Temperature |
Flow rate |
MAP |
PC |
AR |
ABTS |
FRAP |
| (%) | (°C) | (mL/min) | (mgC3GE/100 g dw) | (%) | (%) | (μmolTE/g dw) | (μmolTE/g dw) | |
| 1 | 15 (-1) | 60 (-1) | 3 (-1) | 99.17d ± 3.01 | 17.24j ± 1.29 | 39.74d ± 1.21 | 145.67l ± 0.14 | 403.80gh ± 32.96 |
| 2 | 15 (-1) | 120 (1) | 5 (1) | 26.43f ± 4.30 | 49.09c ± 1.28 | 10.59f ± 1.72 | 265.29d ± 0.28 | 333.84i ± 8.61 |
| 3 | 45 (1) | 60 (-1) | 5 (1) | 213.05a ± 14.37 | 31.70e ± 0.87 | 85.38a ± 5.76 | 277.06b ± 0.12 | 571.14bc ± 34.98 |
| 4 | 45 (1) | 120 (1) | 3 (-1) | 81.41e ± 4.77 | 33.28e ± 3.19 | 32.63e ± 1.91 | 159.01k ± 0.32 | 443.17f ± 53.46 |
| 5 | 30 (0) | 90 (0) | 4 (0) | 152.25c ± 8.83 | 26.65gh ± 1.99 | 61.02c ± 3.54 | 203.98i ± 0.17 | 532.46d ± 34.18 |
| 6 | 15 (-1) | 60 (-1) | 5 (1) | 181.72b ± 2.27 | 25.86h ± 2.11 | 72.82b ± 0.91 | 267.22c ± 0.11 | 534.53d ± 58.88 |
| 7 | 15 (-1) | 120 (1) | 3 (-1) | 10.65g ± 1.41 | 29.20f ± 1.00 | 4.27g ± 0.56 | 86.94n ± 0.09 | 243.69j ± 19.18 |
| 8 | 45 (1) | 60 (-1) | 3 (-1) | 149.19c ± 3.38 | 25.90h ± 1.61 | 59.79c ± 1.36 | 204.01i ± 0.15 | 395.60gh ± 1.29 |
| 9 | 45 (1) | 120 (1) | 5 (1) | 97.09d ± 2.87 | 55.74b ± 0.75 | 38.91d ± 1.15 | 277.08b ± 0.07 | 668.20a ± 1.32 |
| 10 | 30 (0) | 90 (0) | 4 (0) | 174.30b ± 9.30 | 26.77gh ± 1.07 | 69.85b ± 3.73 | 204.48h ± 0.13 | 541.16cd ± 19.07 |
| 11 | 5 (-1.67) | 90 (0) | 4 (0) | 148.24c ± 3.09 | 21.38i ± 0.36 | 59.41c ± 1.24 | 209.03f ± 0.11 | 373.17h ± 3.97 |
| 12 | 55 (+1,67) | 90 (0) | 4 (0) | 184.62b ± 17.03 | 28.76fg ± 1.39 | 73.99b ± 6.83 | 207.86g ± 0.36 | 480.51e ± 3.32 |
| 13 | 30 (0) | 40 (-1.67) | 4 (0) | 147.97c ± 1.43 | 18.93j ± 1.25 | 59.30c ± 0.57 | 204.51h ± 0.15 | 402.31gh ± 27.82 |
| 14 | 30 (0) | 140 (+1.67) | 4 (0) | 0.80g ± 0.01 | 58.36a ± 1.35 | 0.32g ± 0.01 | 203.02j ± 0.10 | 325.68i ± 37.63 |
| 15 | 30 (0) | 90 (0) | 2.30 (-1.67) | 96.97d ± 13.37 | 14.50k ± 0.64 | 38.86d ± 5.36 | 111.57m ± 0.08 | 424.87fg ± 17.54 |
| 16 | 30 (0) | 90 (0) | 5.70 (+1.67) | 152.10c ± 27.90 | 39.12d ± 2.02 | 60.95c ± 11.18 | 306.25a ± 0.69 | 596.95b ± 48.36 |
| 17 | 30 (0) | 90 (0) | 4 (0) | 175.86b ± 16.65 | 28.16fg ± 0.95 | 70.48b ± 6.67 | 213.17e ± 0.23 | 576.35b ± 9.60 |
Note: PLE – pressurized liquid extraction, DES – deep eutectic solvent, ChCl:Pro-DES composed by choline chloride and propylene glycol, MAP - Monomeric anthocyanin pigment, PC - polymeric color, AR – anthocyanin recovered, C3GE – cyanidin 3-O-glucoside equivalent, TE – Trolox equivalent, dw = dry weight, abc – different letters in the same column indicate significant difference among the assays by Fisher test (p < 0.05).
Chemical analysis of the anthocyanin-rich extracts
Monomeric anthocyanin pigment (MAP) and polymeric color (PC)
Monomeric anthocyanin pigment (MAP) and polymeric color (PC) of the extracts were quantified according to Giusti & Wrolstad (2001), with adaptation to microplate reader (Multileader Infinite M200 TECAN, ZH, Switzerland). MAP content was determined by pH differential method using potassium chloride buffer (pH 1.0) and 0.4 M sodium acetate buffer (pH 4.5). The concentrations of MAP were calculated using (1), (2):
| (1) |
| (2) |
where A520 and A700 are the absorbances at 520 and 700 nm, respectively, MW is the molecular weight of the cyanidin 3-O-glucoside (449.2 g/mol), DF diluted factor (20 µL of extract in 280 µL of the buffer, DF = 15), ɛ is extinction coefficient for cyanidin-3-O-glucoside (C3G) (26900) and l is the pathlength (cm). The final results obtained from triplicate were expressed in mg of C3G equivalent per gram of dry waste (mgC3GE/100 g dw), which were calculated from the final ratio solid-to-solvent in each assay.
For the polymeric color percentual (% PC), the extracts diluted in potassium chloride buffer (pH 1.0) were blanched using potassium metabisulfite (0.9 M). The color density (DC) and polymeric color (PC) of the extracts were calculated through Eq. (3) using the absorbances (A) of the control (without potassium metabisulfite) and blanched sample, respectively.
| (3) |
were A420, A520 and A700 are the absorbances at 420, 520, and 700 nm.
Finally, the % PC was determined by Eq. (4):
| (4) |
Anthocyanin recovery (AR)
The total anthocyanin content (TAC) from the JP sample was determined after five sequential extractions using 50 mL of 80% methanol solution acidified with 0.1 mol/L of HCl for 0.5 g of the sample, and under agitation at room temperature for 30 min, followed by five extractions using 50 mL of 70% acetone solution also under agitation at room temperature for 30 min (Gurak, De Bona, Tessaro, & Marczak, 2014). The percentage of anthocyanin recovery (% AR) was calculated by monomeric anthocyanin pigment (MAP) obtained for each assay concerning TAC, according to Eq. (5):
| (5) |
Antioxidant activity
The antioxidant potential of the anthocyanin-rich extracts was determined by ABTS radical scavenging, according to Re et al., (1999), and by ferric reducing antioxidant power (FRAP) assays proposed by Benzie and Strain (1996). Both methods were adapted to the microplate reader. Antioxidant activity was calculated concerning the Trolox curves (ABTS = ; R2 = 0.97, and FRAP = ; R2 = 0.99). The final results were calculated from a triplicate and expressed in μmol Trolox equivalent per gram of sample (μmolTE/g dw).
Optimization process
The independent factors of the PLE operating conditions, DES concentration in aqueous solution (%, x1), temperature (°C, x2), and solvent flow rate (mL/min, x3), conducted each of them at three levels, were analyzed by multiple regression analysis and response surface methodology (RSM) using Statistica v.13.5 software. For this, a generalized second-order polynomial equation was used to fit the experimental data, according to Eq. 6:
| (6) |
where, y is the predicted response, β0, βi, βii, and βij are the regression coefficients for linear, quadratic, and interaction terms, respectively, and xi e xj are the independent variables. The quality of each model fitted was verified by ANOVA, and the non-significant terms were removed from the mathematical models. Thus, the models were re-fitted using only the significant terms (p < 0.05). The fitting adequacy was verified by plack of fit, and the quality by regression coefficient (R2) and its adjusted R2. Then, the response surfaces were plotted.
After obtaining the mathematical models, the simultaneous optimization of all responses was performed employing the desirability function according to Derringer & Suich (1980). The aim was to maximize the responses MAP, AR, ABTS, and FRAP and minimize the PC (since it is related to anthocyanin degradation). Finally, external validation of the suggested optimal PLE conditions was performed to verify the repeatability of models, comparing the predicted values with the experimental data using relative error (RE) Eq. (7):
| (7) |
Comparing DES aqueous solutions with other solvents for the PLE method
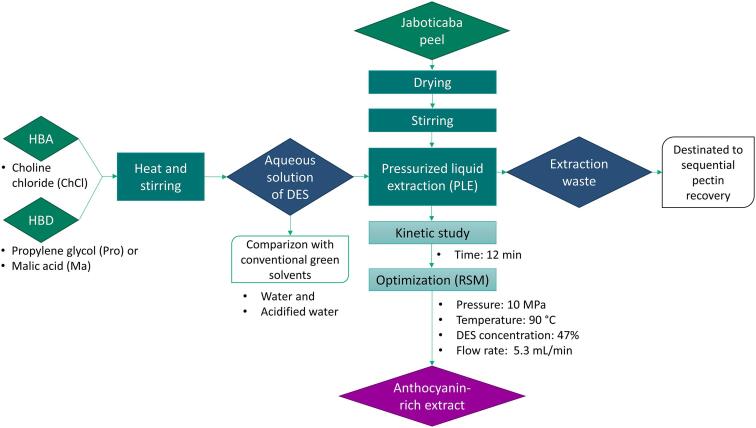
The effect of the type of solvent on PLE yield and extracts quality was evaluated using the PLE optimized conditions (Section 2.6) as illustrated in Fig. 1. Two choline chloride-based DES, ChCl:Pro and ChCl:Ma, were selected according to a previous study (Benvenutti et al., 2020), and compared with water and acidified water (pH 1.5) used as control solvents. The final residues of these extractions were dried and stored at −18° C, for further pectin recovery. Besides the extract quality attributes, defined by MAP, PC, AR, and the antioxidant activity by ABTS and FRAP (performed as described in section 2.5), the following analyses were also conducted to evaluate the quality of the extracts recovered by PLE using different solvents:
Fig. 1.
Schematic diagram illustrating the extraction process performed. Note: DES – deep eutectic solvent, PLE - pressurized liquid extraction, RSM – response surface methodology.
Total phenolic content (TPC)
The TPC values of the extracts obtained by PLE using different solvents (ChCl:Pro, ChCl:Ma, water, or acidified water) were quantified by the Folin-Ciocalteu method according to Singleton and Rossi (1965). The values were obtained from triplicate and expressed in mg of gallic acid equivalent per g of sample (mg GAE/g dw) using a standard curve ( previously prepared.
Anthocyanin profile by LC-MS
The anthocyanin profiles were identified and quantified according to Teixeira (2021) with adaptations to LC-MS. The four recovered extracts, obtained by PLE with different solvents (ChCl:Pro, ChCl:Ma, water, and acidified water) were semi-purified to obtain a concentrated fraction in monomeric anthocyanin using a glass column (1.0 cm × 30 cm) filled with 10 g of Amberlite XAD-7HD resin, as described by Benvenutti et al. (2020). The freeze-dried semi-purified extracts were resuspended in methanol and filtered through a 0.22 μm nylon syringe filter. The samples (10 μL) were injected in high-performance liquid chromatography (model LCMS-2020, Shimadzu, Kyoto, Japan), coupled with a quadrupole mass spectrometer with an electrospray ionization source in positive mode. The separation was carried out using a reverse C18 column (5 µm, 4.6 × 250 mm) (NST, Santos, Brazil) at 25 °C. The mobile phase flow was 1.2 mL/min and was composed of A (0.1% formic acid, v/v) and B (0.1% formic acid in methanol, v/v). The gradient elution system was programmed as: 0–16 min,14%-55% B, 16–27, 55–100% B, 27–30, 100–14% B, 30–32, 14%B. The temperature of the rotary spray spectrum interface was 350 °C, nebulizer gas flow 1.5 L/min, heat block 200 °C, and drying gas flow 15 L/min. The interface voltage was 4.5 kV and the RF-beam voltage was 60 V. The identification of cyanidin-3-O-glucoside was performed comparing its retention time with the reference standard and by its mass spectra based on the values of mass-to-charge ratio (m/z) and quantification was performed by an external standard curve of cyanidin-3-O-glucoside and the results expressed in mg per g of sample (mg/g dw).
Color measured of the anthocyanin-rich extracts
For color determination, aliquots of 300 µL of the extracts obtained by PLE using different solvents (ChCl:Pro, ChCl:Ma, water, and acidified water) were placed in a 96-well microplate and the absorbance was measurement, in the range of 400 to 700 nm, in multi-reader (Tecan, Model Infinite M200, ZH, Switzerland). After reading, the results were analyzed by ColorBySpectra software (Far & Giusti, 2017), which converts the absorbances in color values using the CIE (Commission Internationale de l’éclairage) L* a* b* and L* C* h° scales according to 1964 standard observatory, using Illuminant D65 spectral distribution and 10° view angle. Finally, the CIELAB values were converted into images using an online converter (https://www.nixsensor.com/free-color-converter/).
Anthocyanin-rich extract stability
The thermostability of the anthocyanin-rich extracts recovered by PLE with different solvents (ChCl:Pro, ChCl:Ma, water, and acidified water) was evaluated through a model system, by heating, according to Peron, Fraga, & Antelo (2017) with minor changes. The samples were placed in tubes, sealed, and immersed in a thermostatic bath (TECNAL, model TE-2005, SP, Brazil) containing water for temperatures of 60, 70, and 80° C and glycerin solution for temperatures of 90 and 100° C. In the heating times of 0, 30, 60, 90, 120, and 240 min, a tube was removed from the system and immediately cooled in ice both. Each tube was a degradation point quantified in terms of MAP content (section 2.5.1), and the isothermal degradation of the anthocyanins was fitted in a first-order kinetic model, Eqn 8 (Peron et al., 2017, Teixeira et al., 2021):
| (8) |
where is the MAP concentration with time t (mgC3G/100 g), is the MAP initial concentration (mgC3G/100 g), is the degradation rate (min−1) and t is the time (min).
Time of half-live ( of anthocyanin degradation was given by Eqn 9:
| (9) |
The activation energy () was determined through the Arrhenius model, according to Eqn 10:
| (10) |
where A is the frequency factor (min−1), R is the ideal gas constant (8.314 J/mol.K) and T is the temperature (K).
Additionally, the activation enthalpy (ΔH), the free energy of activation (ΔG), and the activation entropy (ΔS) were calculated by (11), (12), (13).
| (11) |
| (12) |
| (13) |
where is the activation energy for the degradation reaction (J/mol), R is the ideal gas constant (8.314 J/mol.K), T is the temperature (K), is the kinetic rate constant (s−1), is the Boltzmann constant (1.2806 10-23 J/K), and is Planck’s constant (6.6262 10-34 J/s).
Anti-diabetic and anti-obesity potential of PLE anthocyanin-rich extracts
α-glucosidase inhibition assay
The extracts recovered by PLE using different solvents (ChCl:Pro, ChCl:Ma, water, and acidified water) were diluted in phosphate buffer (0.1 mol/L, pH 6.8) forming a set of concentrations from 2 to 20 μg/mL for the semi-purified extracts (according to section 2.7.2), and from 0.01 to 0.1 μg C3GE/mL for crude extracts (before semi-purification process). The assays followed the method described by Barik et al. (2020) and were measured by absorbance at 405 nm in a microplate reader. The percentage of inhibition was calculated according to Eq. (14):
| (14) |
where the control is the reaction without sample and is the absorbance at 405 nm after being subtracted from the blank.
The results were expressed in mean inhibitory concentration (IC50) through inhibitory activity calculated from the curves obtained in triplicate from the set of concentrations of each sample.
α-amylase inhibition assay
The semi-purified and crude extracts obtained by PLE with different solvents were diluted in phosphate buffer, reaching concentrations from 0.5 to 10 mg/mL for the semi-purified extracts, and from 1 to 50 μg C3GE/mL for crude extracts. The assays were performed according to Ali, Houghton and Soumyanath (2006) with absorbances measured in a spectrophotometer (800 XI, Femto, Brazil) at 540 nm. The final results were expressed in IC50 according to described in section 2.8.2.
Porcine pancreatic lipase inhibition assay
The tests followed the procedure described by Zhang, Wu, Wei, & Qin (2020), and the inhibition activities were calculated according to Eq. (14). The IC50 was estimated with the results obtained for the set of dilutions from 100 to 1000 μg/mL for semi-purified extracts and from 0.06 to 15 μgC3GE/mL for crude extracts.
Green metric tools
The environmental analyses performed to the PLE approach were based on the Green Certified and the EcoScale, green metric tools described respectively by Espino et al. (2018) and by Van Aken, Strekowski, and Patiny (2006). Briefly, the methods consider Penalty Points (PP) to reduce from 100% environmentally safe process, according to various parameters, with tabulated PP values. The parameters used by the Green Certificate are: amount and environmental hazard of the solvents, energy, and generated waste. The EcoScale combines the above parameters with process yield, safety, economic and environmental aspects of the extraction. For PLE method, the analyses compared the environmental performance of the solvents: 47% DES aqueous solutions (ChCl:Pro and ChCl:Ma); the conventional solvents: water, acidified water (pH 1.5), and 47% ethanol aqueous solution; with also HBA (choline chloride) and HBD (propylene glycol and malic acid) solutions of approximately 25% in water.
Statistical analysis
The dataset was presented as mean and standard deviation. Firstly, the homogeneity of variance by Levene's (p ≥ 0.05) was verified, and the significant differences between samples were evaluated by one-way ANOVA (p ≤ 0.05), followed by Ficher's LSD test. The statistical significance of the models used was also determined by ANOVA and the quality and adequacy of the adjustments were assessed by the determination coefficient (R2), adjusted R2, and the root-mean-square error (RMSE). All statistical analysis was performed using Statistica v.13.5 software (TIBCO Software Inc., Palo Alto, CA, USA).
Results and discussion
Centesimal composition of the jaboticaba processing by-product
The centesimal composition of the dry separated by-product of jaboticaba processing (JP sample) presented the following values: 15.7 ± 0.4% of moisture, 2.0 ± 0.2% of fixed mineral residue (Ash), 4.2 ± 0.5% of total fat (Soxhlet), 5.5 ± 0.6% of protein (N × 6.25), 33.0 ± 0.1% of crude fiber, and 39.6% of non-fibrous carbohydrates. Inada et al. (2015) reported the chemical composition of different parts of jaboticaba fruit on a dry weight basis, including jaboticaba peel (JP). These data presented similar total carbohydrate content (86.9%), but higher protein (8.5%) and ash (0.6%) and lower lipid content (4%) than the present study. The range in compositions is mainly related to fruit cultivar since that author studied the M. jaboticaba while the M.cauliflora was used in the present work. Other factors as the ripening stage, climate conditions, and soil also influence the chemical composition of jaboticaba fruit (Benvenutti et al., 2021).
Optimization of PLE extraction conditions
A kinetics curve from PLE, conducted at 10 MPa, 90 °C, and using 30% ChCl:Pro aqueous solution as the solvent, with a flow rate of 4 mL/min, enabled the definition of the extraction time, and then, this time was applied for all solvents studied. The extraction kinetics provides the visualization of the mass transfer mechanisms present in the process, analogous to that defined for Supercritical Fluid Extraction (SFE) (Ferreira & Meireles, 2002). This analogy to SFE was applied by Ferro et al. (2020) for the PLE from Sida rhombifolia leaves. Then, the analysis of the kinetics behavior for PLE from JP samples identified the following mass-transfer mechanisms: constant extraction rate period (CER), falling extraction rate period (FER), and diffusion-controlled period (DC) (Fig. 1S, Supplementary material). Therewith, the extraction time was fixed at 12 min for all assays, because it represents the beginning of the DC period, where most part of soluble solute was already recovered by the solvent. Then, all PLE assays were performed according to CCRD plan and considering the fixed conditions of pressure, 10 MPa, and time, 12 min, for the recovery of extract samples rich in anthocyanins.
The recovered extracts were analyzed according to MAP, AR, PC, and antioxidant activity by ABTS and FRAP methods, and the results are presented in Table 1. The results showed significant differences (p < 0.05) among the results from all assays, performed following the CCRD (section 2.4). Therefore, this design detects the effect of DES concentration, temperature, and solvent flow rate on the anthocyanin content and antioxidant capacity of the extracts recovered from JP samples. Then, the parametric data were fitted by multiple regression analysis coupled to RSM, providing mathematical models represented by (15), (16), (17), (18), (19), which were significant (pmodel < 0.01) and did not show a lack of fit (plack of fit > 0.05). Furthermore, the regression coefficients varied from 0.88 to 0.97 with R2adj from 0.83 to 0.95, which explains at least 83% of the variations in responses (Table 1S, Supplementary material).
| (15) |
| (16) |
| (17) |
| (18) |
| (19) |
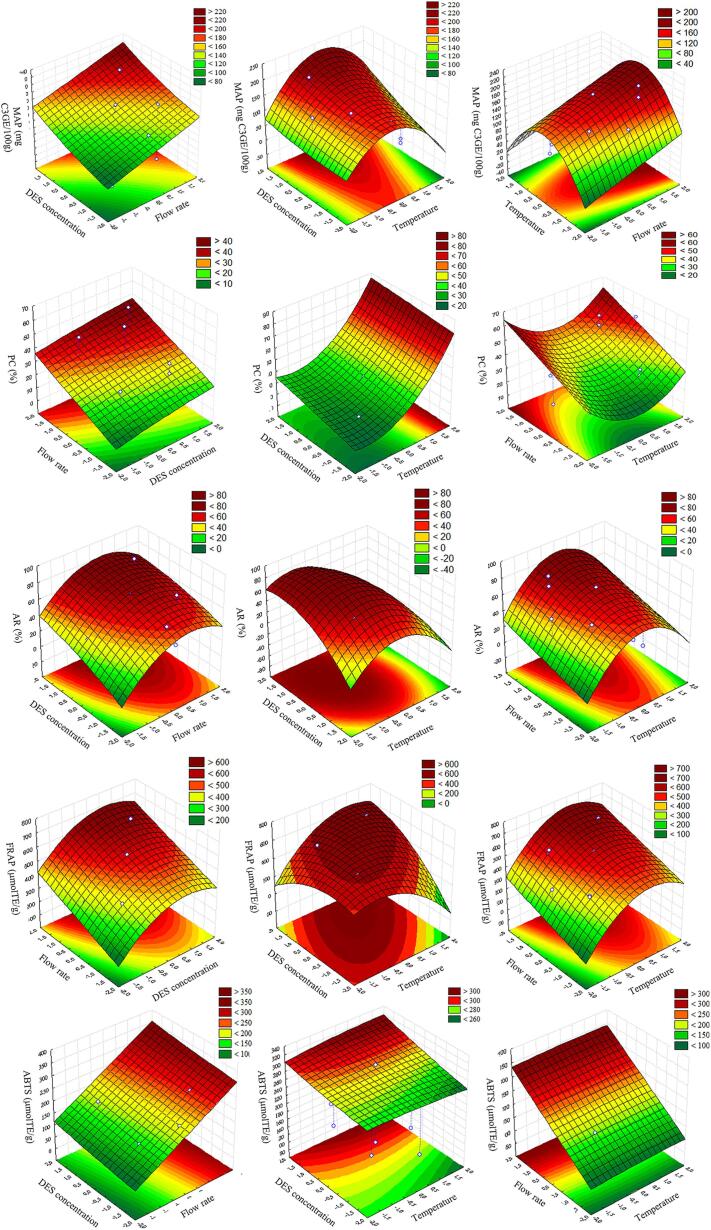
According to the proposed models and three-dimensional (3D) response Fig. 2, the DES concentration (x1) has a significant and positive effect on all responses. In general, the increase in DES concentration improved the extraction performance. However, PC values, a parameter related to anthocyanin degradation, also increased with the enhancement in DES concentration. This fact, combined with the negative quadratic effect of x1 from the FRAP model (Eq. (19)) and the negative synergic effect between x1 and x3 (flow rate) from the ABTS model (Eq. (18)) indicates that intermediate DES concentrations improve the antioxidant potential. The use of aqueous solutions of DES instead of pure DES, increases the extraction ability, mainly due to the decrease in solvent viscosity, facilitating the matrix penetration and the analyte dissolution. However, excessive water content impairs the hydrogen bond between HBA and HBD, reducing the DES solvation capability and the ability to stabilize the analyte, and consequently decreasing the extraction ability of the solvent (Benvenutti et al., 2019).
Fig. 2.
Three-dimensional (3D) response surfaces showing the effect of independent variables (temperature, DES concentration, and flow rate) on the dependent variables (MAP, PC, AR, FRAP, and ABTS) for anthocyanin-rich extraction by PLE using aqueous solution of DES (ChCl:Pro) as the solvent.
The temperature (x2) presented the highest effect on anthocyanin extraction performance, evaluated by MAP, PC, and AR values. The linear coefficients had a higher influence, compared to the quadratic ones, in these three responses, with negative values for MAP and AR, which indicates that the lowest temperatures evaluated resulted in higher anthocyanin yield. According to the results from Table 1, the temperatures of 60 and 90 °C provided the highest MAP and AR values. Besides, the positive effect of temperature on PC model Eq. (16) is probably related to the increase in anthocyanin degradation at high temperatures. This effect can be observed from assays conducted at the highest temperature: assays 2 (15% DES, 120 °C and 5 mL/min), 9 (45% DES, 120 °C and 5 mL/min), and 14 (30% DES, 140 °C and 4 mL/min), which provided high PC values of 49.09, 55.74 and 58.36%, respectively (Table 1). According to the literature, the increase in PLE temperature accelerates anthocyanin degradation by oxidation, hydrolysis, and polymerization reactions (del Garcia-Mendoza et al., 2017, Teixeira et al., 2021). Corroborating with our results, Santos, Veggi & Meireles (2012), presented a positive effect of temperature in anthocyanin recovery from jaboticaba peel by PLE method, in the range from 40 to 80 °C, but a negative effect in the range from 80 to 120 °C. Therefore, we could conclude that there is a limiting maximum temperature for the recovery of thermosensitive compounds like anthocyanins. This temperature limit depends on several factors, mainly the extraction time (Santos & Meireles, 2011).
The temperature (x2) also presented an interactive (linear and positive) effect with flow rate (x3) and with DES concentration (x1), for the antioxidant activity by ABTS and FRAP, respectively. This occurred probably because the increase in temperature improves the diffusion and the solubility of total analytes, disrupting solute-matrix bonds, and decreasing viscosity and surface tension of the solvent mixture, which improves their penetration in the solid matrix, increasing surface area (Rodrigues et al., 2019, Teixeira et al., 2021). This synergic effect can be observed from assays 1 and 2, with temperatures varying from 60 to 120 °C, which presented ABTS values of 145.67 μmolTE/g dw and 265.29 μmolTE/g dw, respectively. Also, assays 6 and 9 for FRAP values in the same temperature variation provided results of 534.53 μmolTE/g dw, and 668.20 μmolTE/g dw, respectively.
The solvent flow rate (x3) is an important process variable that affects the extraction yield, which maximum value is the equilibrium condition of solute content in the solvent phase and solid matrix. When PLE is performed at continuous mode, generally, the flow rate exerts a positive influence on the extraction rate, allowing higher solvent flux throughout the solid material (Essien et al., 2020, Teixeira et al., 2021). However, the flow rate influence depends on the extraction period, observed through the kinetics curve, i.e., the flow rate effect is not relevant at the diffusion-controlled (DC) period (Essien et al., 2020). All response models ((15), (16), (17), (18), (19)) had a positive linear effect on the flow rate, especially ABTS and FRAP models, where the flow rate was the main effect, which was also a positive effect for PC value.
A multiresponse optimization was provided by modeling all process variables and conducted using the desirability function (d) (Fig. 2S, Supplementary material). This optimization, performed considering all responses simultaneously, suggests the best extraction performance is achieved using 47% DES solution, 90 °C, and flow rate of 5.3 mL/min, with a d value of 0.80038. This value represents 80% of all desired responses, a satisfactory result since d value ranges from 0, completely undesirable, to 1, totally desirable (Derringer & Suich, 1980).
This optimization was externally validated by conducting a PLE assay at the optimized condition suggested by the desirability function (d). The observed and predicted values, with relative error (RE), for each variable, were:
-
(1)
MAP (mgC3GE/100 g dw): 172.70 ± 9.25 (observed), 183.33 (predicted), and RE = 5.80%;
-
(2)
PC (%): 15.29 ± 0.10 (observed), 15.021 (predicted), and RE = 13,97%;
-
(3)
AR (%): 69.08 ± 2.62 (observed), 73.98 (predicted) and RE = 9.61%;
-
(4)
ABTS (mmol/g ds): 289.27 ± 7.84 (observed), 279.37 (predicted) and RE = 3.35%;
-
(5)
FRAP (mmol/g ds): 685.99 ± 59.65 (observed), 640.59 (predicted), and RE = 6.94%.
All observed values are within the predicted interval of 95% confidence level. Therefore, the optimized conditions were validated, i.e., the proposed models can be used to predict the responses following the desirability function.
Solvent effect on PLE recovery of anthocyanin-rich extracts
Anthocyanin-rich extracts were obtained by PLE at optimum condition (previous section) using different solvents (water, acidified water, and ChCl:Pro and ChCl:Ma solutions). The solvent type affected the process yield and the quality of the extracts since significant differences (p < 0.05) were observed for TPC, MAP, antioxidant, and color parameters (Table 2).
Table 2.
Characterization of the extracts obtained by PLE using different solvents: water, acidified water or deep eutectic solvents (ChCl:Pro or ChCl:Ma).
| Analysis |
Solvents |
|||
|---|---|---|---|---|
| Water (pH 6.7) |
Acidified water (pH 1.5) |
ChCl:Pro (pH 4.5) |
ChCl:Ma (pH 1.5) |
|
| TPC (mg GAE/g dw) | 74.47b ± 7.15 | 72.97b ± 7.74 | 85.68a ± 8.29 | 78.99ab ± 2.59 |
| Anthocyanin content | ||||
| MAP (mgC3GE/g dw) | 1.10c ± 0.03 | 1.16c ± 0.01 | 1.7a ± 0.06 | 1.60b ± 0.09 |
| PC (%) | 13.75b ± 0.02 | 12.00c ± 0.25 | 15.29a ± 0.10 | 11.56d ± 0.27 |
| AR (%) | 44.06c ± 0.97 | 46.50c ± 0.41 | 69.08a ± 2.62 | 62.90b ± 3.85 |
| Individual anthocyanin (HPLC) | ||||
| Cyanidin-3-O-glucoside (mg/g dw) | 9.51d ± 0.09 | 13.89c ± 2.44 | 29.40a ± 8.94 | 25.78b ± 2.09 |
| Antioxidant Activity | ||||
| ABTS (μmol TE/g dw) | 219.72b ± 2.87 | 169.05c ± 1.71 | 286.47a ± 3.06 | 288.16a ± 4.88 |
| FRAP (μmol TE/g dw) | 695.56a ± 22.62 | 517.12b ± 13.18 | 685.99a ± 59.65 | 727.98a ± 42.82 |
| Color | ||||
| L* | 78.33b ± 0.09 | 86.17a ± 0.08 | 59.67d ± 0.86 | 64.49c ± 0.07 |
| a* | 13.04c ± 0.14 | 9.12d ± 0.25 | 17.49b ± 0.35 | 32.49a ± 0.02 |
| b* | 10.63c ± 0.14 | 6.29d ± 0.07 | 19.88a ± 0.19 | 17.22b ± 0.01 |
| C* | 16.82c ± 0.20 | 11.08d ± 0.25 | 26.48b ± 0.37 | 36.77a ± 0.01 |
| °h | 39.20b ± 0.06 | 34.60c ± 0.46 | 48.65a ± 0.30 | 27.93d ± 0.03 |
 |
 |
 |
 |
|
Note: PLE – pressurized liquid extraction, ChCl - chlorine chloride, Pro-propylene glycol, Ma - malic acid, TPC - total phenolic content, GAE - gallic acid equivalent, dw – dry waste, MAP - monomeric anthocyanin pigment content, C3GE – cyanidin-3-glucoside equivalent, PC – polymeric color, AR – anthocyanin recovery, TE - Trolox equivalent, abc – different letters in the same line indicate significative difference among the assays by Fisher test (p < 0.05).
The extraction yield, presented in terms of TPC values, ranged from 72.97 to 85.68 mg GAE/g dw, with the highest value obtained by ChCl:Pro solution, followed by ChCl:Ma solution. Santos, Veggi and Meireles (2012) optimized the extraction of the phenolic from jaboticaba peel by PLE method, reaching a maximum TPC of 18.7 mg GAE/g dw using ethanol 99% as solvent at 120 °C, 5 MPa for 15 min. However, Paludo et al. (2019) showed that TPC from jaboticaba varies according to cultivar and year of harvest, and for jaboticaba peel, the TPC ranged from 55.27 to 147.88 mg GAE/g dw for six different jaboticaba cultivars, harvested between 2014 and 2015.
Regarding the MAP content, the ChCl:Pro and ChCl:Ma solutions provided 57% and 42% higher values than obtained by water as the solvent, respectively. When compared to acidified water, the MAP values from DES aqueous solutions were 48% and 35% higher, respectively. The cyanidin-3-O-glucoside (C3G), the main anthocyanin from jaboticaba (Benvenutti et al., 2021, Inada et al., 2015), was the only individual anthocyanin detected from the different extracts, with concentrations varying from 9.51 to 29.40 mg/g dw (Table 2). Inada et al. (2015) evaluated the phenolic composition of different jaboticaba parts, with 12.61 mg/g dw of C3G detected for jaboticaba peel after sequential stirring extractions using methanol 50% and acetone: water: acetic acid (70:29.5:0.5, v/v/v), at ambient temperature. Therefore, PLE using aqueous solutions of DES provided up to 3-folds higher cyanidin concentration, compared to conventional solvents (water and acidified water), and of 2.33-folds higher than values obtained by Inada (2015).
The efficiency of pressurized DES aqueous solutions for the recovery of extracts rich in MAP and C3G, compared to control solvents, evidences the selectivity towards anthocyanin components. This valuable selectivity, previously evidenced by in silico and experimental studies, indicated ChCl:Pro and ChCl:Ma as promising eutectic solutions for anthocyanins recovery due to their high affinity with these natural colorants (Benvenutti et al., 2020).
The high extraction ability of DES can be explained by the hole or liquid crystal theory or the binding theory. The arrangement of HBA, HBD, and water, form a polymer-like matrix where the solute can dissolve into the space (or holes) of this molecular network. From the binding theory, significant intermolecular interactions, mostly hydrogen bonds among HBA, HBD, and target molecules make the solute part of the supramolecular structure of DES (Benvenutti et al., 2019, Dai et al., 2016, Liu et al., 2018). Despite that water weakens molecular interactions, probably the HBA and HBD (from DES) are partly dissolved in water, while the remaining amount form the supramolecular structure, as suggested by Liu et al. (2018). In general, polar compounds such as anthocyanin are better recovered by DES solutions, compared to pure DES, since water also form hydrogen bonds with these target compounds (Shishov et al., 2021).
However, the extract recovered by ChCl:Pro solution presented the highest percentage of polymeric color (PC), of 15.29 ± 0.10 %, suggesting lower maintenance of the anthocyanin integrity, while the extract recovered by ChCl:Ma solution presented the lower PC value (11.56 ± 0.27 %), probably due to low pH (1.5) of the malic acid as HBD. According to de Mejia et al., (2020), anthocyanins recovery and stability are favored in the acid medium due to the stabilization of favynium ion. The significant differences in the MAP and PC values, from samples recovered by ChCh:Ma and acidified water, both with pH 1.5, also suggest the influence of the acid chemical structure on anthocyanins recovery and stability.
High-pressure DES solutions recovered extracts with higher antioxidant activity compared to control solvents (Table 2). This behavior is probably associated with the DES selectivity towards bioactive compounds, as previously discussed. A high correlation between the two antioxidant methods, FRAP and ABTS was observed, with a correlation coefficient (r) of 0.81 (p < 0.05). However, only the antioxidant activity by ABTS assay presented correlation with TPC (r = 0.60, p < 0.05), MAP content (r = 0.87, p < 0.05) and C3G content (r = 0.83, p < 0.05). The antioxidant activity by ABTS presents a higher correlation with anthocyanin content (MAP and C3G), compared to TPC, because anthocyanins are the main phenolic compounds from the extracts, with high antioxidant ability. For instance, C3G presents eight hydroxyls groups, which are the main functional group related to antioxidant activity efficiency. Besides, anthocyanins are more potent antioxidants than proanthocyanidins and other flavonoids due to their chemical structure (de Mejia et al., 2020).
Concerning the color attributes, that affect consumers' acceptance and selection (Martins et al., 2016), significant differences (p < 0.05) were detected for color parameters among the anthocyanin-rich extracts (Table 2). Extracts obtained by DES solutions, compared to controls, presented higher redness (a*), yellowness (b*), and Chroma values (C*), and lower luminosities (L*). According to Mojica, Berhow, & Gonzalez de Mejia (2017), higher anthocyanins concentration, from anthocyanin-rich samples from black beans, increases the C*, a parameter related to color intensity, and decreases the hue angle value (h°), related to color tone. Therefore, the lowest h°, and highest C* and a* (red color intensity) from the sample by ChCl:Ma is probably due to its high MAP content and low solvent pH, which maintains the conformation of anthocyanin color, predominantly flavyliun cation (de Mejia et al., 2020, Giusti and Wrolstad, 2001, Mojica et al., 2017). Highest yellowness (b*) and lowest L*, from ChCl:Pro sample, can be related to high PC value, which indicates the anthocyanin degradation. The PC value involves the presence of colorless anthocyanins, according to pH, and the formation of melanoidin pigments at high process temperatures (Jiang et al., 2019).
Solvent effect on the thermostability of the anthocyanin-rich extracts
The stability of bioactive molecules from natural extracts is relevant to define its applications and storage conditions and aids the definition of the extractions conditions (Dai et al., 2016). Therefore, the influence of the solvent type (ChCl:Pro, ChCl:Ma, water, and acidified water) on anthocyanin recovery and their thermostability was investigated.
The thermostability of anthocyanin-rich extracts are represented by thermal degradation curves (Figure 3S, Supplementary material) and kinetics parameters (Table 3). The lowest anthocyanin degradation was observed at 60 °C (lower temperature) for the ChCl:Ma sample, followed by acidified water sample, solvents with pH close to 1.5. At pH close to 1, the flavylium cation is the only existent conformation. However, with pH increase, different conformations coexist in complex chemical equilibrium, forming a color hemiketal stable form, which is related to the color fading of anthocyanins (Giusti & Wrolstad, 2001). Besides, van der Waals and hydrogen bond molecular interactions between DES structure and anthocyanins improve their solubility, decreasing the molecule mobility, and the contact with oxygen at the air interface, reducing oxidative degradation, the main degradation mechanism at low temperatures (Dai et al., 2016).
Table 3.
Kinetics parameters of thermal stability of anthocyanin-rich extracts obtained by PLE using different solvents: water, acidified water or natural deep eutectic solvents (ChCl:Pro or ChCl:Ma).
| kd (min−1) | RSS | R2 | R2adj | t1/2 (h) | |
|---|---|---|---|---|---|
| 60 °C | |||||
| Water | 0.0025 | 0.0686 | 0.7746 | 0.7746 | 4.6582 |
| Acidified water | 0.0015 | 0.0261 | 0.8829 | 0.8829 | 7.6506 |
| ChCl:Pro | 0.0020 | 0.0603 | 0.7859 | 0.7859 | 5.6630 |
| ChCl:Ma | 0.0012 | 0.0525 | 0.7624 | 0.7624 | 9.7902 |
| 70 °C | |||||
| Water | 0.0031 | 0.0932 | 0.7953 | 0.7953 | 3.7630 |
| Acidified water | 0.0020 | 0.0393 | 0.9087 | 0.9087 | 5.8642 |
| ChCl:Pro | 0.0020 | 0.0451 | 0.8257 | 0.8257 | 5.6630 |
| ChCl:Ma | 0.0017 | 0.0565 | 0.8594 | 0.8594 | 6.7165 |
| 80 °C | |||||
| Water | 0.0064 | 0.0196 | 0.98258 | 0.98258 | 1.7939 |
| Acidified water | 0.0051 | 0.0703 | 0.93422 | 0.93422 | 2.2741 |
| ChCl:Pro | 0.0049 | 0.0218 | 0.97693 | 0.97693 | 2.3433 |
| ChCl:Ma | 0.0047 | 0.0328 | 0.97062 | 0.97062 | 2.4424 |
| 90 °C | |||||
| Water | 0.0121 | 0.0305 | 0.9784 | 0.9784 | 1.1097 |
| Acidified water | 0.0104 | 0.0292 | 0.9812 | 0.9812 | 0.9540 |
| ChCl:Pro | 0.0098 | 0.0366 | 0.9763 | 0.9763 | 1.1764 |
| ChCl:Ma | 0.0091 | 0.0354 | 0.9737 | 0.9737 | 1.2695 |
| 100 °C | |||||
| Water | 0.0260 | 0.0682 | 0.9647 | 0.9647 | 0.4440 |
| Acidified water | 0.0166 | 0.0210 | 0.9878 | 0.9878 | 0.6943 |
| ChCl:Pro | 0.0231 | 0.0030 | 0.9986 | 0.9986 | 0.5012 |
| ChCl:Ma | 0.0212 | 0.0057 | 0.9973 | 0.9973 | 0.5439 |
Note: PLE – pressurized liquid extraction, kd – degradation rate, RSS: residual sum of squares, R2 - determination coefficient, R2adj - adjusted determination coefficient, t1/2 - time of half-live, ChCl - choline chloride, Pro-propylene glycol, Ma - malic acid.
At 80 and 90 °C the samples recovered by both DES solutions presented lower degradation rates (Table 3). The anthocyanins' thermal degradation occurs through the hydrolysis of glycosidic bond, forming aglycone (cyanidin), which is more unstable and discolors faster than the glucoside form. Another type of degradation is the opening of the heterocyclic ring, forming chalcone, the colorless structure of anthocyanin (del Garcia-Mendoza et al., 2017). Therefore, the protective effect of DES solutions at these temperatures can also be related to the molecular interactions, which reduce the mobility and protect the anthocyanin from the nucleophilic attack of water molecules, decreasing its susceptibility to degradation reactions (Dai et al., 2016). The strength of these molecular interactions is mainly related to the number of hydroxyls and carboxyls groups, besides the size and spatial structure of the molecules (Benvenutti et al., 2019). Therefore, the better protective effect of ChCl:Ma compared to ChCl:Pro could be explained by the malic acid structure, with one hydroxyl and two carboxyl groups, resulting in more interactions with anthocyanins than propylene glycol (two hydroxyl groups). Besides, the acylation of the anthocyanin glycosyl by malic acid may have occurred at the extract recovered by ChCl:Ma because the –OH groups of the anthocyanin glycosyls are partially or fully esterified by organic acids. The acylated anthocyanin has higher color stability and higher resistance to physicochemical and biochemical factors (e.g. digestive enzymes, light, heat, pH, among others) than the nonacylated form (Zhao et al., 2017).
At 100 °C, the extract recovered by acidified water had the lowest degradation rate ( = 0.0166 min−1, t1/2 = 0.69 h). The degradation rate of anthocyanins increased with temperature for all solvents because high temperatures favor the hydrolysis reactions, converting chalcones (colorless structure of anthocyanin) into brown precipitates, commonly observed as the final product of anthocyanin degradation (del Garcia-Mendoza et al., 2017). From 90 to 100° C the increase in degradation rates was higher for the extracts recovered by DES solution, probably due to the extensive hydrogen bonds network among DES and anthocyanin molecules, favoring the formation of brown precipitates involving chalcones.
To evaluate the temperature dependence of anthocyanin degradation, the ln values against 1/T were fit by the Arrhenius equation, predicting the activation energy ( with good adust (R2 > 0.92 and R2adj > 0.90) (Table 4). is a thermodynamic parameter representing the energy needed to reach the transition state of the chemical reactions. Therefore, high represents low susceptibility to degradation. As expected, ChCl:Ma presents a higher , followed by acidified water, ChCl:Pro, and water.
Table 4.
Thermodynamic parameters and Arrhenius adjustment of the anthocyanin degradation in the extracts obtained by PLE using different solvents: water, acidified water or aqueous solution of DES (ChCl:Pro or ChCl:Ma).
| T (°C) | (kJ mol−1) | RSS |
R2 |
R2adj |
ΔH (kJ/mol) |
ΔG (J/mol.K) |
ΔS (J/mol) |
|---|---|---|---|---|---|---|---|
| Water | 62.4420 | 0.1358 | 0.9641 | 0.9522 | |||
| 60 | 59.6720 | 86.9727 | −81.9471 | ||||
| 70 | 59.5889 | 89.0588 | −85.8806 | ||||
| 80 | 59.5057 | 89.5631 | −85.1123 | ||||
| 90 | 59.4226 | 90.2768 | −84.9628 | ||||
| 100 | 59.3394 | 90.4741 | −83.4374 | ||||
| Acidified water | 66.7102 | 0.1124 | 0.9737 | 0.9650 | |||
| 60 | 63.9415 | 88.3060 | −73.1668 | ||||
| 70 | 63.8583 | 90.2838 | −77.0423 | ||||
| 80 | 63.7752 | 90.2201 | −74.9148 | ||||
| 90 | 63.6920 | 90.6948 | −74.3879 | ||||
| 100 | 63.6089 | 91.8229 | −75.6409 | ||||
| ChCl:Pro | 65.0670 | 0.3177 | 0.9258 | 0.9010 | |||
| 60 | 62.2983 | 87.4731 | −75.5999 | ||||
| 70 | 62.2152 | 90.1843 | −81.5426 | ||||
| 80 | 62.1320 | 90.3081 | −79.8188 | ||||
| 90 | 62.0489 | 91.1007 | −80.0327 | ||||
| 100 | 61.9657 | 90.8124 | −77.3368 | ||||
| ChCl:Ma | 77.5523 | 0.1045 | 0.9818 | 0.9757 | |||
| 60 | 74.7835 | 88.9888 | −42.6583 | ||||
| 70 | 74.7004 | 90.6709 | −46.5611 | ||||
| 80 | 74.6173 | 90.4296 | −44.7942 | ||||
| 90 | 74.5341 | 90.8709 | −45.0050 | ||||
| 100 | 74.4510 | 91.0660 | −44.5443 |
Note: PLE – pressurized liquid extraction, DES – deep eutectic solvent, ChCl - choline chlorine, Pro-propylene glycol, Ma - malic acid, - activation energy, ΔH -activation enthalpy, ΔG - free energy of activation, ΔS - activation entropy.
Activation enthalpy (ΔH), free energy of activation (ΔG), and activation entropy (ΔS) were also determined (Table 4). The ΔH is calculated from the and measures the energy barrier that can be overcome by reactant molecules, representing the strength of the bond that must be broken and remade during the reaction. Positive ΔH values indicate endothermic reaction, then, an increase in temperature enhances degradation. Additionally, positive ΔG values indicate that the degradation reaction is not spontaneous, besides, close ΔG values from different solvents are explained by the anthocyanins from the same source, with similar degradation reactions (Peron et al., 2017).
Finally, negative ΔS values represent that the transition molecules are more organized than that from the initial reaction. Higher negative ΔS values indicate higher distance from the equilibrium condition, resulting in a quick reaction to obtain the active complex (Peron et al., 2017). Therefore, these thermodynamic parameters indicate ChCl:Ma solution as the best solvent to slow the thermal degradation reaction for anthocyanin from JP samples.
Anti-diabetic and anti-obesity potentials of anthocyanin rich-extracts obtained by PLE with different solvents
The PLE extracts were semi-purified using Amberlite XAD-7HD resin to concentrate the C3G, the main anthocyanin from this study due to its bioactive and colorant potential. The crude extracts (before semi-purification) and the semi-purified extracts, obtained by PLE with different solvents, were evaluated for inhibition activity of the enzymes α -amylase, α -glucosidase, and pancreatic lipase (PL).
The α-amylase catalyzes the starch hydrolysis during human digestion while α-glucosidase catalyzes the hydrolysis of disaccharides and monosaccharides. Therefore, the inhibition of these enzymes maintains the glucose levels by reducing the rate of blood sugar absorption from the small intestine, decreasing the spread and progression of type 2 diabetes mellitus (T2DM) (Barik et al., 2020, Teixeira et al., 2021). The results from enzyme inhibition are presented in Table 5, which shows that all anthocyanin-rich extracts provided higher inhibition of α -glucosidase, compared to α -amylase. The α -glucosidase inhibition from semi-purified extracts (IC50 from 7.07 to 4.12 μg/mL) were better than reported inhibition of crude bilberry extract, IC50 = 13.2 μg/mL (Alnajjar et al., 2020), of semi-purified black bean extract, IC50 = 33 μg/mL (Teixeira et al., 2021), and also of the commercial inhibitor acarbose (1 mg/mL inhibit 47–59% of the α -glucosidase activity) (Alnajjar et al., 2020, Barik et al., 2020). However, the α -amylase inhibition of the semi-purified extracts (IC50 from 1.62 to 7.44 mg/mL) were less efficient than purified black bean extract, IC50 = 0.521 mg/mL (Teixeira et al., 2021), and the acarbose (0.645 mg/mL inhibit 66.8% of α -amylase activity) (Mojica et al., 2017).
Table 5.
Inhibitory activity of digestive enzymes of crude and semi-purified anthocyanin-rich extracts obtained by PLE using water, acidified water, or DES (ChCl:Pro or ChCl:Ma) as the solvent.
| Inhibitory activity of digestive enzymes | Crude extracts | Semi-purified extracts | ||||||
|---|---|---|---|---|---|---|---|---|
| Walter | Acidified water | ChCl: Pro | ChCl:Ma | Water | Acidified water | ChCl:Pro | ChCl:Ma | |
| α -Amylase (IC50 - mg/mL) | – | – | – | – | 7.13a ± 0.09 | 7.44a ± 0.18 | 6.36b ± 0.88 | 1.62c ± 0.39 |
| α -Glucosidase (IC50 - μg/mL) | – | – | – | – | 7.07a ± 0.69 | 6.78a ± 0.09 | 5.34b ± 0.085 | 4.12c ± 0.18 |
| Lipase (IC50 - μg/mL) | – | – | – | – | 614.29a ± 14.78 | 499.04b ± 43.80 | 171.88c ± 7.20 | 173.81c ± 23.41 |
| α -Amylase (IC50 - μg C3GE/mL) | 9.42de ± 0.08 | 11.92d ± 0.03 | 8.41de ± 0.25 | 2.51e ± 0.32 | 86.31b ± 1.11 | 92.06b ± 2.21 | 113.93a ± 15.44 | 23.7c ± 5.68 |
| α -Glucosidase (IC50 - μg C3GE/mL) | 0.04b ± 0.01 | 0.08d ± 0.01 | 0.02e ± 0.01 | 0.01f ± 0.01 | 0.08b ± 0.01 | 0.08b ± 0.01 | 0.10a ± 0.01 | 0.06c ± 0.01 |
| Lipase (IC50 - μg C3GE/mL) | 2.73c ± 0.23 | 0.85e ± 0.15 | 0.36f ± 0.01 | 0.12f ± 0.01 | 11.16a ± 0.27 | 6.17b ± 0.54 | 2.08d ± 0.08 | 2.54c ± 0.34 |
Note: PLE – pressurized liquid extraction, DES – deep eutectic solvent, IC50 - mean inhibitory concentration, mgC3G/g - milligrams of cyanidin 3-O-glucoside, ChCl: choline chlorine, Pro: propylene glycol, Ma: malic acid, abc: different letters in the same line indicate significant differences, p < 0.05.
According to Barik et al. (2020), the anthocyanins are most effective to inhibit the α-glucosidase than other polyphenols. This high effectivity probably is related to the anthocyanin molecular structure of flavan-3-ols, mainly of cyanidin, that presents high inhibitor potential due to the one bond in the B ring and two hydroxyl substitutions in positions 2 and 3 of the B-ring. Besides, Sui, Zhang, & Zhou (2016) reported that inhibition of α -amylase activity increased when cyanidin bounds to glucose in position 3, such as C3G. These molecular structure favors the anthocyanin occupation of the active site of α -amylase and α -glucosidase, inhibiting their activities and also, inhibiting the catalytic action of these enzymes by hydrogen bonding.
DES solutions provided PLE extracts with the best inhibitory effects, compared to other solvents, probably due to the higher anthocyanin levels due to their higher solubility in these solvents. DES solutions also improve the bioavailability of the bioactive components (Radošević et al., 2016). ChCl:Ma solution provided the highest inhibitor effects for these two enzymes, probably due to the higher anthocyanin integrity, expressed by lower PC value. In addition, the possible anthocyanin glycosyl acylation by malic acid can increase the in vitro and in vivo chemical stability, including stronger inhibition of digestive enzymes (Zhao et al., 2017).
Despite the high inhibitory effect of anthocyanin, their combination with other phenolic compounds from crude extracts resulted in higher inhibition compared to purified extracts, for all solvents. Flavonols, specially myricetin and quercetin, and hydrolyzable tannins are polyphenols present in jaboticaba peel which have been reported as inhibitory to carbohydrate-hydrolyzing enzymes (Lacroix & Li-Chan, 2014).
On the other hand, the inhibition of the pancreatic lipase (PL) activity is reported as a beneficial health effect associated with anthocyanins consumption, since it decreases the absorption of fat from the diet, and attenuates cases of obesity (Xie, Su, Sun, Zheng, & Chen, 2018). The IC50 values of semi-purified extracts, presented at Table 5 (from 171.88 to 614.29 μg/mL) are in accordance with that from pure cyanidin-3-O-glucoside (IC50 = 385 μg/mL), but less efficient than Oristatic® (IC50 = 64 μg/mL), a potent specific lipase inhibitor (Fabroni, Ballistreri, Amenta, Romeo, & Rapisarda, 2016). The semi-purified extracts from DES solutions presented higher PL inhibition probably due to the higher anthocyanin content, which agrees with the positive correlation between anthocyanin content and enzymatic inhibition capacity, reported in the literature (Fabroni et al., 2016). The galloyl moiety, a common structure from flavonoids, including anthocyanins, can be associated with PL inhibition capacity, probably due to a bond between the structure with the PL active site (Rahim, Takahashi, & Yamaki, 2015). However, as observed for carbohydrases, the PL inhibition capacity was higher from crude extracts compared to the semi-purified ones due to the synergic effect with other polyphenols (Fabroni et al., 2016).
Therefore, the DES solutions combined with high-pressure technology showed is a promising approach to obtain anthocyanin-rich extracts with possible biological potential. The ChCl:Ma was the more suitable solvent due to their protective effect on anthocyanins' stability, preserving their color and bioactivity.
Green metric tools
Concerning the security of DES components, choline chloride (ChCl), malic acid, and propylene glycol are GRAS substances, when following good manufacturing practices, and are approved by FDA for use in food formulations (https://www.ecfr.gov/). Besides, ChCl:Ma has high biodegradability (>80% after 28 days) and, according to Türker and Dogen (2021), can be considered a biodegradable green solvent. This DES also shows low cytotoxicity, with less than 50% inhibition of human cells growth (MCF – 7 and HeLe) at concentrations from 10 to 2000 mg/L (Radošević et al., 2016). The cytotoxicity of ChCl-based DES was evaluated by Ahmadi et al., (2018) using in vitro model with human HEK-293 cells. The results show that ChCl:Pro at 1:2 M ratio presented no cytotoxicity.
Considering the previous study (Benvenutti et al., 2020), the jaboticaba peel matrix, after anthocyanin recovery, still contains valuable substances, such as pectin, and can be submitted to further sequential extractions to value this by-product. Therefore, this step of anthocyanin recovery does not generate waste from the vegetal matrix. The proposed approach can be considered a green extraction method according to Chemat et al. (2019) since it uses renewable and low explored food crops, applies eco-friendly solvents at a high-pressure method that improves yield, reduces processing time, solvent consumption, and unit operations.
Because it is important to identify how green is the “green process”, the metric tools Green Certificate and EcoScale were applied. From the Green Certificate, , the PLE method performed with the 8 solvents [water, acidified water, ChCl:Pro solution and ChCl:Ma solution, 47% ethanol in aqueous solution, and solutions of HBA (choline chloride) and HBD (malic acid and propylene glycol)] was classified as effectively green (Table 2S, Supplementary material). The certificate values obtained were 99 for water, 97 for acidified water and individual solutions of HBA and HBD, and 95 for both DES solutions and ethanol solution on a 0–100 scale. Nevertheless, in spite that water is the most environmentally friendly solvent, its resulting yield was 57%, lower than reached by DES solutions, and this aspect was not considered by this analysis.
Therefore, due to the yield parameter, the PLE method also was evaluated byEcoScale tool with results in Table 3S (Supplementary material). Aqueous solution of DES presented the highest EcoScale values, 72.54 and 69.45, for ChCl:Pro and ChCl:Ma solutions respectively. Water presented the third better score, 65.03, followed by 47% ethanol solution, 60.75. The solutions of choline chloride, propylene glycol, and malic acid showed lower yields compared to DES solutions, with consequent lower EcoScale scores, 57.23, 56.59, and 54.29, respectively (Table 3S, Supplementary material). According to Van Aken (2006), EcoScale scores above 75 are considered excellent, above 50 are acceptable and below 50 are unacceptable. Therefore, PLE with DES solutions was considered a green method with high extraction yield, compensating the slightly higher solvent cost and severity to the environment, compared to water and the other conventional solvents evaluated.
After the environmental analysis, it is safe to suggest the use of PLE with DES solutions for the recovery of anthocyanin-rich extract. The resulting extract can be applied as a natural colorant with bioactive properties as antioxidant, anti-diabetic, and anti-obesity with the possibility of no need for solvent removal. The protective effect of DES on the solute corroborates with this approach. Due to the protective effect of DES on the solute, they have been evaluated for use as a delivery vehicle of bioactive compounds in pharmaceutical or nutraceutical applications (da Silva et al., 2020).
Conclusion
The optimum PLE conditions using aqueous solutions of DES as solvents were established for the valorization of a Brazilian berry by-product. The best processing conditions were 47% of aqueous solution at 90 °C and a flow rate of 5.3 mL/min. The ChCl:Ma solution was the most promising DES for anthocyanin recovery from jaboticaba peel, and also presented a high protective effect against the thermodegradation of the recovered extract, which showed good bioactivities, especially high anti-diabetic potential. This protective effect is mainly related to the low pH value of the DES solution, the molecular interactions, and possible anthocyanin glycosyl acylation by malic acid. Therefore, ChCl:Ma aqueous solution coupled to PLE method is an efficient and eco-friendly approach for the recovery of anthocyanin from an underutilized Brazilian berry by-product. According to the green metrics of Green Certificate and EcoScale, the PLE using DES solutions as solvent can be considered a green method. This approach provides high yields of a bioactive natural colorant with high potential for application in food, nutraceutical, and pharmaceutical formulations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to CNPq (Grant numbers 404347/2016-9, 423425/2018-8), CAPES/PROEX (Project 1624/2018), and CAPES/PRINT (Project 88887.310560/2018-00) for LB scholarship and research support, the partner laboratory LAMEB (Laboratório Multiusuário de Estudos em Biologia) and the Federal University of Santa Catarina, UFSC, Brazil, for the support and infrastructure.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100236.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmadi R., Hemmateenejad B., Safavi A., Shojaeifard Z. Chemosphere Assessment of cytotoxicity of choline chloride-based natural deep eutectic solvents against human HEK-293 cells: a QSAR analysis. Chemosphere. 2018;209:831–838. doi: 10.1016/j.chemosphere.2018.06.103. [DOI] [PubMed] [Google Scholar]
- Ali H., Houghton P.J., Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. Journal of Ethnopharmacology. 2006;107(3):449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Alnajjar, M., Kumar Barik, S., Bestwick, C., Campbell, F., Cruickshank, M., Farquharson, F., … Hoggard, N. (2020). Anthocyanin-enriched bilberry extract attenuates glycaemic response in overweight volunteers without changes in insulin. Journal of Functional Foods, 64(September 2019), 103597. 10.1016/j.jff.2019.103597.
- Aoac Official methods of analysis of the Association of Official Analytical Chemists International Arlingto. AOAC International. 2005 [Google Scholar]
- Aslan Türker D., Doğan M. Application of deep eutectic solvents as a green and biodegradable media for extraction of anthocyanin from black carrots. LWT – Food Science and Technology. 2021;138 doi: 10.1016/j.lwt.2020.110775. [DOI] [Google Scholar]
- Bajkacz S., Adamek J. Development of a method based on natural deep eutectic solvents for extraction of flavonoids from food samples. Food Analytical Methods. 2017:1330–1344. doi: 10.1007/s12161-017-1118-5. [DOI] [Google Scholar]
- Barik S.K., Russell W.R., Moar K.M., Cruickshank M., Scobbie L., Duncan G., Hoggard N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting α-glucosidase while other phenolics modulate salivary α-amylase, glucose uptake and sugar transporters. Journal of Nutritional Biochemistry. 2020;78 doi: 10.1016/j.jnutbio.2019.108325. [DOI] [PubMed] [Google Scholar]
- Benvenutti L., del Sanchez-Camargo A., Zielinski A.A.F., Ferreira S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. Journal of Molecular Liquids. 2020;315 doi: 10.1016/j.molliq.2020.113761. [DOI] [Google Scholar]
- Benvenutti L., Zielinski A.A.F., Ferreira S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends in Food Science and Technology. 2019;90 doi: 10.1016/j.tifs.2019.06.003. [DOI] [Google Scholar]
- Benvenutti L., Zielinski A.A.F., Ferreira S.R.S. Jaboticaba (Myrtaceae cauliflora) fruit and its by-products : Alternative sources for new foods and functional components. Trends in Food Science & Technology. 2021;112:118–136. doi: 10.1016/j.tifs.2021.03.044. [DOI] [Google Scholar]
- Benzie I.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1039/c6ay01739h. [DOI] [PubMed] [Google Scholar]
- Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green extraction of natural products. Origins, current status, and future challenges. TrAC - Trends in Analytical Chemistry. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- da Silva D.T., Rodrigues R.F., Machado N.M., Maurer L.H., Ferreira L.F., Somacal S.…Emanuelli T. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Research International. 2020;138 doi: 10.1016/j.foodres.2020.109718. [DOI] [PubMed] [Google Scholar]
- Dai Y., Rozema E., Verpoorte R., Choi Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. Journal of Chromatography A. 2016;1434:50–56. doi: 10.1016/j.chroma.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Dai Y., Spronsen J.V., Witkamp G., Verpoorte R., Choi Y.H. Natural Deep Eutectic Solvents as new potential media for green technology. Analytica Chimica Acta. 2013;766:61–68. doi: 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- de Mejia E.G., Zhang Q., Penta K., Eroglu A., Lila M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Annual Review of Food Science and Technology. 2020;11(1):145–182. doi: 10.1146/annurev-food-032519-051729. [DOI] [PubMed] [Google Scholar]
- Derringer G., Suich R. Simultaneous optimization of several response variables. Journal of Quality Technology. 1980;12(4):214–219. doi: 10.1080/00224065.1980.11980968. [DOI] [Google Scholar]
- Espino M., de Fernández M., Los Á., Gomez F.J.V., Boiteux J., Silva M.F. Green analytical chemistry metrics: Towards a sustainable phenolics extraction from medicinal plants. Microchemical Journal. 2018;141:438–443. doi: 10.1016/j.microc.2018.06.007. [DOI] [Google Scholar]
- Essien S.O., Young B., Baroutian S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends in Food Science and Technology. 2020;97:156–169. doi: 10.1016/j.tifs.2020.01.014. [DOI] [Google Scholar]
- Fabroni S., Ballistreri G., Amenta M., Romeo F.V., Rapisarda P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. Journal of the Science of Food and Agriculture. 2016;96(14):4713–4723. doi: 10.1002/jsfa.7708. [DOI] [PubMed] [Google Scholar]
- Ferreira S.R.S., Meireles M.A.A. Modeling the supercritical fluid extraction of black pepper (Piper nigrum L.) essential oil. Journal of Food Engineering. 2002;54(4):263–269. doi: 10.1016/S0260-8774(01)00212-6. [DOI] [Google Scholar]
- Ferro D.M., Mayer D.A., Müller C.M.O., Ferreira S.R.S. Scale-up simulation of PLE process applied to recover bio-based materials from Sida rhombifolia leaves. The Journal of Supercritical Fluids. 2020;166 doi: 10.1016/j.supflu.2020.105033. [DOI] [Google Scholar]
- del Garcia-Mendoza M.P., Espinosa-Pardo F.A., Baseggio A.M., Barbero G.F., Maróstica Junior M.R., Rostagno M.A., Martínez J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. Journal of Supercritical Fluids. 2017;119:9–16. doi: 10.1016/j.supflu.2016.08.014. [DOI] [Google Scholar]
- Giusti M.M., Wrolstad R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Handbook of Food Analytical Chemistry. 2001;2:19–31. doi: 10.1002/0471709085.ch18. [DOI] [Google Scholar]
- Gurak P.D., De Bona G.S., Tessaro I.C., Marczak L.D.F. Jaboticaba pomace powder obtained as a co-product of juice extraction: A comparative study of powder obtained from peel and whole fruit. Food Research International. 2014;62:786–792. doi: 10.1016/j.foodres.2014.04.042. [DOI] [Google Scholar]
- Inada K.O.P., Oliveira A.A., Revorêdo T.B., Martins A.B.N., Lacerda E.C.Q., Freire A.S.…Monteiro M.C. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. Journal of Functional Foods. 2015;17:422–433. doi: 10.1016/j.jff.2015.06.002. [DOI] [Google Scholar]
- Jiang T., Mao Y., Sui L., Yang N., Li S., Zhu Z.…He Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chemistry. 2019;274:460–470. doi: 10.1016/j.foodchem.2018.07.141. [DOI] [PubMed] [Google Scholar]
- Lacroix I.M.E., Li-Chan E.C.Y. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Molecular Nutrition and Food Research. 2014;58(1):61–78. doi: 10.1002/mnfr.201300223. [DOI] [PubMed] [Google Scholar]
- Liu Y., Friesen J.B., McAlpine J.B., Lankin D.C., Chen S.-N., Pauli G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. Journal of Natural Products. 2018;81(3):679–690. doi: 10.1021/acs.jnatprod.7b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loarce L., Oliver-Simancas R., Marchante L., Díaz-Maroto M.C., Alañón M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109728. [DOI] [PubMed] [Google Scholar]
- Loarce L., Oliver-Simancas R., Marchante L., Díaz-Maroto M.C., Alañón M.E. Modifiers based on natural deep eutectic mixtures to enhance anthocyanins isolation from grape pomace by pressurized hot water extraction. Lwt. 2021;149(June) doi: 10.1016/j.lwt.2021.111889. [DOI] [Google Scholar]
- Machmudah, S., Lestari, S. D., Widiyastuti, Wahyudiono, Kanda, H., Winardi, S., & Goto, M. (2018). Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. Journal of Supercritical Fluids, 133(June 2017), 615–624. 10.1016/j.supflu.2017.06.012.
- Martins N., Roriz C.L., Morales P., Barros L., Ferreira I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends in Food Science and Technology. 2016;52:1–15. doi: 10.1016/j.tifs.2016.03.009. [DOI] [Google Scholar]
- Mojica L., Berhow M., Gonzalez de Mejia E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chemistry. 2017;229:628–639. doi: 10.1016/j.foodchem.2017.02.124. [DOI] [PubMed] [Google Scholar]
- Paludo, M. C., Colombo, R. C., Filho, J. T., Hermosín‑Gutiérrez, I., Ballus, C. A., & Godoy, H. T. (2019). Optimizing the Extraction of Anthocyanins from the Skin and Phenolic Compounds from the Seed of Jabuticaba Fruits (Myrciaria jabuticaba o (Vell.) O. Berg) with ternary mixture experimental designs. Journal of the Brazilian Chemical Society, 30(7), 1506–1514. 10.21577/0103-5053.20190047.
- Peron D.V., Fraga S., Antelo F. Thermal degradation kinetics of anthocyanins extracted from juçara (Euterpe edulis Martius) and “Italia” grapes (Vitis vinifera L.), and the effect of heating on the antioxidant capacity. Food Chemistry. 2017;232:836–840. doi: 10.1016/j.foodchem.2017.04.088. [DOI] [PubMed] [Google Scholar]
- Radošević K., Ćurko N., Gaurina Srček V., Cvjetko Bubalo M., Tomašević M., Kovačević Ganić K., Radojčić Redovniković I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT - Food Science and Technology. 2016;73(73):45–51. doi: 10.1016/j.lwt.2016.05.037. [DOI] [Google Scholar]
- Rahim A.T.M.A., Takahashi Y., Yamaki K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Research International. 2015;75:289–294. doi: 10.1016/j.foodres.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegruini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues L.G.G., Mazzutti S., Vitali L., Micke G.A., Ferreira S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatalysis and Agricultural Biotechnology. 2019;22 doi: 10.1016/j.bcab.2019.101367. [DOI] [Google Scholar]
- Rodrigues, M. I., & Iemma, A. F. (2014). Experimental design and process optimization. (C. Press, Ed.).
- Santos D.T., Meireles M.A.A. Optimization of bioactive compounds extraction from jabuticaba (Myrciaria cauliflora) skins assisted by high pressure CO2. Innovative Food Science and Emerging Technologies. 2011;12(3):398–406. doi: 10.1016/j.ifset.2011.02.004. [DOI] [Google Scholar]
- Santos D.T., Veggi P.C., Meireles M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. Journal of Food Engineering. 2012;108(3):444–452. doi: 10.1016/j.jfoodeng.2011.08.022. [DOI] [Google Scholar]
- Shishov A., Dubrovsky I., Kirichenko S., Bulatov A. Behavior of quaternary ammonium salts and terpenoids-based deep eutectic solvents in aqueous phase. Journal of Molecular Liquids. 2021;xxxx doi: 10.1016/j.molliq.2021.117987. [DOI] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolic with phosphomolibdic acid reagent. American Journal of Enology and Viticulture. 1965;16(3):144–158. [Google Scholar]
- Sorita G.D., Leimann F.V., Ferreira S.R.S. Biorefinery approach: Is it an upgrade opportunity for peanut by-products ? Trends in Food Science & Technology. 2020;105:56–69. doi: 10.1016/j.tifs.2020.08.011. [DOI] [Google Scholar]
- Sui X., Zhang Y., Zhou W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic α-amylase. Journal of Functional Foods. 2016;21:50–57. doi: 10.1016/j.jff.2015.11.042. [DOI] [Google Scholar]
- Teixeira R.F., Benvenutti L., Burin V.M., Gomes T.M., Ferreira S.R.S., Zielinski A.A.F. An eco-friendly pressure liquid extraction method to recover anthocyanins from broken black bean hulls. Innovative Food Science and Emerging Technologies. 2021;67 doi: 10.1016/j.ifset.2020.102587. [DOI] [Google Scholar]
- Van Aken K., Strekowski L., Patiny L. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein Journal of Organic Chemistry. 2006;2:1–7. doi: 10.1186/1860-5397-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Su H., Sun C., Zheng X., Chen W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends in Food Science and Technology. 2018;72:13–24. doi: 10.1016/j.tifs.2017.12.002. [DOI] [Google Scholar]
- Zhang H., Long W., Xiao Q., Wei X., Qin, Ming X. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Bioscience. 2020;37 doi: 10.1016/j.fbio.2020.100682. [DOI] [Google Scholar]
- Zhao C.L., Yu Y.Q., Chen Z.J., Wen G.S., Wei F.G., Zheng Q.…Xiao X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chemistry. 2017;214:119–128. doi: 10.1016/j.foodchem.2016.07.073. [DOI] [PubMed] [Google Scholar]
- Zielinski A.A.F., del Sanchez-Camargo A.P., Benvenutti L., Ferro D.M., Dias J.L., Ferreira S.R.S. High-pressure fluid technologies: Recent approaches to the production of natural pigments for food and pharmaceutical applications. Trends in Food Science & Technology. 2021;118:850–869. doi: 10.1016/j.tifs.2021.11.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.