Graphical abstract
Abbreviations: ADI, Average daily intake; ANOVA, Analysis of variance; HQ, Hazard quotient; INF-1, First infusion; INF-2, Second infusion; INF-3, Third infusion; TPLBC, Tea pruning litter biochar; TV, Tocklai vegetative
Chemical compounds used in this article: Ammonium acetate (PubChem CID: 517165); Ammonium molybdate (PubChem CID: 21977535); Arsenic (PubChem CID: 5359596); Boric acid (PubChem CID: 7628); Cadmium (PubChem CID: 23973); Chromium (PubChem CID: 23976); Copper sulphate (PubChem CID: 24462); Hydrochloric acid (PubChem CID: 313); Nitric acid (PubChem CID: 944); Perchloric acid (PubChem CID: 24247); Potassium Dichromate (PubChem CID: 24502); Potassium iodide (PubChem CID: 4875); Potassium sulphate (PubChem CID: 24507); Sodium borohydrate (PubChem CID: 22959485); Sodium hydroxide (PubChem CID: 14798); Stannous chloride, dihydrate (PubChem CID: 10198436); Tin (PubChem CID: 5352426)
Keywords: Average daily intake, Hazard quotient, Health risk, Infusion, Tea pruning litter biochar, Trace elements, Transfer rate
Highlights
-
•
Tea pruning litter biochar was used for tea cultivation.
-
•
Application of 500 kg TPLBC ha−1 is beneficial for tea cultivation.
-
•
TPLBC was most effective in reducing As, Cd and Cr in made tea.
-
•
ADI and HQ of As, Cd and Cr were significantly (p ≤ 0.01) reduced.
-
•
Application of MANOVA resulted in significant differences among treatments.
Abstract
Effect of tea pruning litter biochar (TPLBC) on arsenic (As), cadmium (Cd) and chromium (Cr) content in made tea and successive tea infusions were investigated in a greenhouse experiment with two tea cultivars (TV23 and S.3A/3). Made tea prepared from TV23 and S.3A/3 clone, a decrease in the concentration of As, Cd, and Cr by 36.73%, 16.22%, 13.96%, and 36.63%, 27.78%, 10.54%, respectively over control on the application of the highest dose of TPLBC (500 kg TPLBC ha−1). Irrespective of treatments, studied element concentrations were significantly higher (p ≤ 0.05) in the first infusion and lower in the third. Considering Ten g made tea consumption per person per day, the maximum average daily intakes of As, Cd and Cr in a higher dose of TPLBC were far below the tolerable weekly intake prescribed by the World Health Organization. As hazard quotient values of selected elements were ≪ 1, no significant adverse health consequences are expected for tea consumers.
Introduction
Billion cups of tea infusion have been consumed daily throughout the world as a social drink not only for its non-alcoholic nature but also for its medicinal properties (Barman et al., 2020, Deka et al., 2021, Xu et al., 2018). The tea infusion is prepared from made tea which is processed from fresh and healthy young shoots (also known as two and a bud) of the tea plant (Camellia sinensis L.) grown in acidic soils under humid climatic conditions (13–32 °C) (Karak et al., 2017). Being an acidophilic crop, the tea plant has the potential to retain metals and metalloids from the growing medium, which subsequently is transferred from shoots to made tea and tea infusion. Several studies reported that soil acidification of tea plantations might contribute to elevated concentrations of available metals in soils because a decrease of pH gives rise to a reduction of the fraction of insoluble oxo-hydroxo species and of the stability of metal complexes with organic and inorganic ligands, which enhances the transport of these metals, and intensified accumulation of metals in tea plants (Qiang et al., 2015, Zhang et al., 2020; and references therein). Yaylalı-Abanuz and Tüysüz (2009) reported that tea plants cultivated in soils derived from mineralised rocks could exhibit high concentrations of toxic heavy metals. This may increase the human health risks due to the possible presence of heavy metals in tea leaves. Therefore, as one of the most popular beverages, heavy metals and metalloids in tea gradually attract attention (Tao et al., 2021). Askari et al (2020) reported that Cr in dry tea and tea infusions exceeded the concentration mentioned in the Iran’s Ministry of Health and WHO guidelines when tea samples were collected from wholesalers of Neishabour, the largest city in Khorasan Razavi province of Iran. It was also reported that the transfer rate of different metals like cadmium (Cd) and chromium (Cr) from made tea to tea infusion varies between 10% and 60%. Therefore, healthy management of tea cultivation is critical.
Among the metal and metalloids, arsenic (As), chromium (Cr), and cadmium (Cd) could be the most toxic ones that may be found in made tea, as reported by Zhang et al. (2020). Nagara, Sarkar, Luo, Biswas, & Datta, (2021) reported significantly higher (p < 0.05) amounts of As, Cd and Cr in black tea as compared to green tea analyzed from forty-five tea samples procured from local markets in China (n = 15), India (n = 15), and the USA (n = 15). They concluded that the accumulations of As, Cd and Cr in tea growing soil and their subsequent transfer into the tea infusion are of great concern. Recent studies reported that long time exposure and consumption of food containing As, Cd and Cr might cause several adverse health effects like cancer, cardiovascular disease, dermal changes, respiratory, pulmonary, gastrointestinal, hematological, hepatic, renal, neurological, developmental, immunologic, genotoxic, and mutagenic effects (Minari et al., 2020, Gholizadeh and Hu, 2021, Shekhawat et al., 2015, Yang et al., 2020). Fallah et al. (2020) reported that some metals like Cd and Pb, even in their small concentration, have damaging effects on human well-being such as Alzheimer’s disease (AD), dangerous poisoning, enzyme dysfunction, mental retardation, various cancers of the prostate, breast, etc.
Furthermore, uptake of As, Cd and Cr by tea plants from soils do not have any significant beneficial properties for tea plants and human health. Therefore, a holistic approach is suggested in order to prevent the uptake of As, Cd and Cr by tea plant through safe soil amendments (Khan, Reid, Li, & Zhu, 2014).
The common practice of tea consumption is to use a new set of made tea for making tea infusions (single infusion). In contrast, another practice is to use the same leaves to make several tea infusions by repeatedly steeping the same tea (repeated infusion) (Nagara et al., 2021). Mehra & Baker (2007) reported that the release of trace elements from made tea to tea infusion depends on infusion preparation steps. The release pattern of trace elements in the first infusion was the highest, followed by the second and the third infusions in decreasing order. Therefore, the resulting health risk assessments through the average daily intake (ADI) and hazard quotient (HQ, described in the “Materials and methods” section) of trace elements through consumption of tea infusion are pertinent (Khan et al., 2014, Nagara et al., 2021). If HQ is < 1, the doses are not likely to be associated with adverse health effects. Even though tea is the most popular beverage globally, few studies reported that carcinogenic risk for As through high consumption of tea is of concern (Nagara et al., 2021, Sofuoglu and Kavcar, 2008). In a recent study, Askari et al. (2020) highlighted that HQ value for Cr in black tea infusion was found higher than unit, which indicated adverse effects on tea consumers’ health. Karami et al. (2019) investigated fluoride concentrations in consumed tea for the Iranian population with different age groups (men and women belong to 21–72 years and children belongs to 0–11 years) and concluded that the consumption of tea as a popular drink could be a potential source of fluoride exposure to humans. Their results revealed that the HQ in age groups of women (21–72 years) and children (0–11 years) in Iran was within the safe zone (HQ < 1) which confirmed the non-carcinogenic risk associated with drinking tea within these groups. However, in one case of the men (21–72 years), the HQ greater than 1 shows a probable risk of fluorosis. Therefore, continuous monitoring is essential to determine the concentrations of toxic elements in tea samples.
In tea plantation systems, involves a specific pruning cycle for keeping the tea bushes at a manageable height, to reduce pest and disease incidence, for yield enhancement, and improvement of tea quality (Arafat et al., 2020). They reported that a large amount of tea pruned material returns to the soil surface, putting a high quantity of polyphenols into the soil. The accumulation of active allelochemicals in the tea soil may cause acidification and soil sickness, leads to the hindrance of plant growth and development, and low yield in long-term monoculture tea plantations. Therefore, alternative use of enormous quantity tea pruning litter as a raw material for biochar (black colour solid carbonaceous material prepared from waste biomass through thermochemical conversion of biomass in a limited or no supply of oxygen) production is thought to be the effective management of tea pruning litter in tea plantation systems (Borgohain et al., 2020). An excellent review article by Gholizadeh & Hu (2021) highlighted that the use of biochar in the soil could be a feasible method to reduce the uptake of heavy metals in different crops, viz. celery cabbage, corn, napa cabbage, potato, radish, rice, wheat etc. However, the application of tea pruning litter biochar (TPLBC) in tea growing soils to restrict the uptake of As, Cd and Cr by tea plant, which may be a candidate soil amendment, has not been conducted so far. Furthermore, it has been thought that the application of TPLBC could be an effective management strategy for tea pruning litter for sustainable tea plantations.
In this study, we aimed to reduce As, Cd and Cr concentrations from tea beverages concerning human health risk through the addition of ‘safe’ amendments to tea-growing soil. We have estimated the average daily intake (ADI) and hazard quotient (HQ) of these three potentially toxic trace elements to determine the potential human health risk after consumption of repeated tea infusion. Hence, the objectives of this study were to 1) quantify the concentrations of As, Cd and Cr in made tea influenced by different doses of TPLBC; and 2) assess the human health risks of As, Cd and Cr through intake of tea infusion. This study can provide the basis to understand and mitigate the potential risks of As, Cd and Cr in tea through TPLBC application as a soil amendment.
Materials and methods
Preparation of tea pruning litter biochar (TPLBC)
Tea pruning litter biochar (TPLBC) was prepared following the procedure described by Borgohain et al. (2020). In brief, dried mixed tea pruning litters were collected from tea growing region of upper Assam (94°33′46″ to 95°38′E longitude and 27°05′38″ to 27°48′N latitude) as feeding materials. Dried pruning litters were chopped to 1–1.5 in. and dried before the preparation of TPLBC. TPLBC was prepared in a retort reactor at 350 °C. The prepared TPLBC was collected after cooling to normal temperature. Prior to characterisation and application, the prepared TPLBC was crushed, passed through a < 0.5 mm nylon sieve, and kept in an air-tight plastic drum.
Soil sample used for the experiment
Top soil (0–15 cm) samples were collected from virgin land at Upper Assam Advisory Centre, Tea Research Association, Dikom, Dibrugarh, Assam (27°29′N and 94°55′E) (Fig. S1, Supplementary information) in August 2019 following the protocol described by Karak et al. (2016). In brief, 24 soil samples from 1 ha fallow land were selected to get a representative soil sample. Collected soil samples were natural dried in the field and mixed homogeneously to get a representative sample. The dried soil samples were taken to a greenhouse and dried again under shade followed by sieving through 2 mm mesh and kept in an airtight plastic drum at room temperature. This sieved and dried soil was used for present greenhouse experiments.
Greenhouse studies and experimental design
The experimental set-up of greenhouse study and experimental design are cited in the article by Karak et al. (2016). In brief, two popular tea clones, namely TV23 (Tocklai vegetative 23) and S.3/A with one and half year old plant (propagated in polyethylene sleeve) having identical collar diameters were used as test cultivars for the present experiment. Each pot was filled up with exactly 15 kg of soil. The soil in each pot was mixed thoroughly with 90 kg N ha−1 as urea,90 kg K2O ha−1 as muriate of potash (i.e. potassium chloride), and 50 g of P enriched vermicompost as basal dose. Altogether seven treatments, viz. T0: Control (without TPLBC); T1: 100 kg TPLBC ha−1; T2: 150 kg TPLBC ha−1; T3: 200 kg TPLBC ha−1; T4: 300 kg TPLBC ha−1; T5: 400 kg TPLBC ha−1, and T6: 500 kg TPLBC ha-1were imposed for present investigations after two months of plantation. Complete randomised design (CRD) with three replications was followed to arrange all pots. Plants were allowed to grow for eighteen months (16th October 2019 to 16th April 2021).
Characterisations of soil and TPLBC
The detailed procedures for the determination of selected physical and chemical properties of used soil and TPLBC are available in our previously reported articles (Karak et al., 2015, Borgohain et al., 2020). Selected physical and chemical properties including pH, electrical conductivity (EC), total organic C, total N, plant-available K, plant-available P, total As, Cd and Cr were determined for soil. Furthermore, TPLBC were analysed for pH, EC, total carbon (TC), total nitrogen (TN), total potassium (TK), total phosphorus (TP), and total As, Cd and Cr. pH, EC, total organic C, total N, plant-available K, plant-available P, total As, Cd and Cr in soil were 4.52 ± 0.01,0.05 ± 0.01 dSm−1, 1.26 ± 0.01%, 0.19 ± 0.002%, 123 ± 2.37 mg kg−1 as K2O, 16.52 ± 0.93 mg kg−1 as P2O5, 18.96 ± 0.32 mg kg−1, 0.36 ± 0.01 mg kg−1 and 2.39 ± 0.22 mg kg−1, respectively. Furthermore, pH, EC, TC, TN, TK, TP, and total As, Cd and Cr in TPLBC were 8.42 ± 0.02, 1.32 ± 0.10 dSm−1, 54.50 ± 2.39%, 1.85 ± 0.02%, 22.22 ± 1.02 g kg−1, 2.33 ± 0.001 g kg−1, 0.03 ± 0.001 mg kg−1, 0.32 ± 0.001 mg kg−1 and 2.17 ± 0.003 mg kg−1, respectively.
Preparation of made tea, repeated tea infusions and analysis of As, Cd and Cr
Young shoots comprising one bud and two leaves were plucked from three-year-old plants from each pot. Made tea samples were prepared separately from collected young shoot samples, and repeated infusions were prepared following the methods described by Karak et al. (2017). USEPA Method 3050B (EPA, 1996) was followed to estimate As, Cd and Cr content in made tea samples. 0.5 g made tea was used for this estimation. Standard infusion procedure commonly used in India was followed. The details of the repeated tea infusion protocol are available in our previous articles (Deka et al., 2021, Karak et al., 2017). In brief, 2 g made tea sample was infused by adding 50 mL boiled distilled water in a 100 mL porcelain beaker, and it was kept covered with the lid for 5 min with intermittent shaking for proper wetting. After 5 min, the solution was filtered using filter paper (Whatman No. 1) and decanted into a 50-mL volumetric flask and volume was adjusted to 50 mL using the same water as used for the tea infusion. Subsequently, the tea residue left after the first infusion was used for making the second and third infusions to mimic a common tea drinking habit in many regions (Nagara et al., 2021). The prepared samples were analysed for As, Cd and Cr using atomic absorption spectroscopy (AAS; model: AA240, Agilent, Malaysia).)coupled with vapour generation accessory (VGA, model: VGA-77, Agilent, USA)and graphite tube atomiser (GTA; model: GTA 120, Agilent, Singapore). Before aspiration of the samples, it was initially reduced with a solution mixture of 5% (w/v) potassium iodide and 5% (w/v) ascorbic acid. The treated solution was to the As-hydride device with another solution mixture with 10% HCl and 0.3% NaBH4 (in 0.05% NaOH) for As estimation. On the other hand, Cd and Cr were analysed by direct aspiration of the sample solutions into AAS.
Quality assurance
Two certified standard reference materials (SRMs), viz. tomato leaves (SRM-1573a) and Montana soil (SRM-2710a), were procured from the National Institute of Standards and Technology, Standard Reference Materials, Madison, USA and used for quality assurance for As, Cd and Cr determination in the analysed samples. The estimated values of analyzed As, Cd and Cr were in excellent agreement with the certified values (mg kg−1 unless otherwise stated) in SRM-1573a (certified:0.1226 and estimated:0.1226 ± 0.0001 for As; certified:1.517 and estimated:1.514 ± 0.001 for Cd; certified:1.988 and estimated:1.992 ± 0.003 for Cr) and SRM-2710a (certified:0.154% and estimated:0.154 ± 0.0002% for As; certified:12.3 and estimated:12.19 ± 1.02 for Cd; certified:23 and estimated: 22.98 ± 1.25 for Cr). All the samples were analysed with three replications.
Risk characterisation
Average daily intake (ADI) of As, Cd and Cr
Average daily intake (ADI; μg kg−1 body weight per day or μg kg−1 BW D-1) is the function of concentration of trace elements ( in mg kg−1), consumption of made tea ( ; 10.0 g per person per day), transfer rate of trace elements ( , %) from made tea sample into the tea infusion and body eight ( ; average body weight of men and women is 67.4 and 64.9 kg, respectively; Karak et al., 2017). The ADI was calculated using Eq 1. As described in Krstić et al. (2021)
| (1) |
Hazard quotient (HQ)
The non-carcinogenic health risks of As, Cd and Cr through consumption of tea infusions were assessed as the hazard quotient (HQ). HQ was estimated based on the following equation (Eq (2)) described by Karak et al. (2017):
| (2) |
where is the hazard quotient of the element, is the average daily intake of element, and is the reference dose of element. The values for As, Cd, and Cr are 3 × 10-4 mg kg−1 BW D-1, 5 × 10-4 mg kg−1 BW D-1 and 3 × 10-3 mg kg−1 BW D-1, respectively.
Statistical analysis
The experiments were conducted using a complete randomized design (CRD). There were total seven (7) treatments and each treatment was replicated three (3) times. Analysis of variance (ANOVA) has been used to find out the sum of squares due to treatment, replication and error for the two way classified data and ANOVA is obtained.
Following ANOVA, pairwise comparison of treatment means was carried out using Tukey's HSD (honestly significant difference) test. This test is a single-step multiple comparison procedure that used to find means that are significantly different from each other. The test is based on a studentised range distribution.
The univariate ANOVA does not produce multivariate results utilising information from all variables simultaneously. In addition, separate univariate tests are generally less powerful because they do not consider the inter-correlation of the dependent variables. Therefore, Multivariate ANOVA (MANOVA) was applied to check for overall significant difference among the different treatments considering all the responses simultaneously. MANOVA is a good option if two or more continuous dependent variables and one categorical predictor variable. It is a generalised form of ANOVA with several dependent variables. The purpose of MANOVA is to test whether the vectors of means for the two or more groups are sampled from the same sampling distribution.
Let, = ( is a p-variate vector of observations.
| y¯i.=1r∑j=1ryij ; y¯.j=1v∑i=1vyij and y¯..=1vr∑i=1v∑j=1ryij |
The observations can be represented by a two-way classified multivariate model
| yij=μ+ti+bj+eij i = 1, 2,…,v; j = 1, 2,…,b, … … … … (4) |
where μ = (μ1 μ2 … μk … μp)’ is the vector of general means, ti = (ti1ti2… tik… tip)’ are the effects of ith level of Factor A on p-characters, and bj=(bj1bj2… bjk… bjp)’ are the effects of jth level of Factor B on p-characters. eij = (eij1eij2 … eijk… eijp)’ is a p-variate random vector associated with yij and assumed to be distributed independently as p variate normal distribution . The equality of treatment effects is to be tested
| i.e. H0: (ti1ti2…tik…tip)’ = (t1t2…tk…tp)’ (say) ∀i=1,2,⋯,p |
against the alternative at least two of the Factor effects are unequal.
Under the null hypothesis, the model (4) reduced to
| (5) |
where .
The null hypothesis of equality of treatment mean vectors is rejected if the ratio of generalized variance (Wilk's lambda statistic) is very small.
where, R= , and
H =
Other criteria, viz. Pillai's Trace, Hotelling-Lawley Trace and Roy's Greatest root have also been used for the present investigation.
Moreover, hierarchical cluster analysis was applied to group the homogenous treatments based on all the responses. The agglomerative method of clustering has been used in the present paper. The agglomerative method is a “bottom-up” approach where each observation starts in its own cluster, and pairs of clusters are merged as one move up the hierarchy of clustering.
Result and discussion
As, Cd and Cr in made tea
The impact of different doses of TPLBC on As, Cd, and Cr concentrations in made tea is tabulated in Table 1. The addition of TPLBC significantly (p ≤ 0.05) decreased As concentration in made tea irrespective of clone used over control. Total As (mg kg−1) in made tea prepared from TV23 clone ranged from 0.33 ± 0.02 in T5 to 0.53 ± 0.02 in T0 treatments. However, it ranged from 0.36 ± 0.01 mg kg−1 in T6 to 0.57 ± 0.02 mg kg−1 in T0 treatments in made tea prepared from S.3A/3 clone. Across all treatments, As in made tea decreased by 38.17 and 36.64% for TV23 and S.3A/3 clone, respectively, when tea plants were grown in soils amended with TPLBC. All the made samples were within the permissible limit of As (5 mg kg−1) in raw dietary supplements prescribed by WHO (WHO, 2007). Khan et al. (2014) reported that the addition of sewage sludge biochar significantly (p = 0.01) decreased As (13.6–22.7%) over control in rice when the experiment was conducted in Miaoqian village known as China's “Cancer Village”. Chen et al. (2018) reported that As immobilisation by biochar was due to adsorption, ion exchange, and precipitation reactions. Rizwan et al. (2016) concluded that biochar amendments in soil change soil properties and promote As adsorption onto soil, reducing their plant availability.
Table 1.
Total arsenic (As), cadmium (Cd) and chromium (Cr) in made tea prepared from two popular clones (TV23 and S.3A/3) influenced by different doses of tea pruning litter biochar after one and half years of treatments. Data are presented as the means of three repetitions ± SE. Same symbol in a column indicates non-significant difference among the treatment whereas different symbol indicates significant differences among the treatments at 5% level of significance.
| Treatments* | Tea cultivars | |||||
|---|---|---|---|---|---|---|
| TV23 | S.3A/3 | |||||
| Concentration of trace elements (mg kg−1) | ||||||
| As | Cd | Cr | As | Cd | Cr | |
| T0 | 0.53a ± 0.02 | 0.037a ± 0.001 | 0.824a ± 0.002 | 0.57a ± 0.02 | 0.036a ± 0.001 | 0.901a ± 0.006 |
| T1 | 0.51a±-# | 0.036a,b ± 0.001 | 0.811b ± 0.003 | 0.55a ± 0.01 | 0.035a ± 0.001 | 0.879b ± 0.006 |
| T2 | 0.47b ± 0.02 | 0.034b,c ± 0.001 | 0.779c ± 0.006 | 0.43b ± 0.04 | 0.031b ± 0.001 | 0.860c ± 0.002 |
| T3 | 0.41c±- | 0.032c ± 0.001 | 0.726d ± 0.001 | 0.42b ± 0.01 | 0.032b ± 0.001 | 0.852c ± 0.005 |
| T4 | 0.41c±- | 0.032c ± 0.001 | 0.715e ± 0.001 | 0.41b ± 0.01 | 0.031b ± 0.001 | 0.790d ± 0.007 |
| T5 | 0.33d ± 0.02 | 0.030c ± 0.001 | 0.703e,f ± 0.001 | 0.41b±- | 0.025b ± 0.001 | 0.756e ± 0.001 |
| T6 | 0.33d ± 0.01 | 0.031c ± 0.002 | 0.709f ± 0.001 | 0.36c ± 0.01 | 0.026b ± 0.001 | 0.806f ± 0.003 |
* T0: control (no amendment); T1: 100 kg tea pruning litter biochar (TPLBC) ha−1; T2:150 kg TPLBC ha−1; T3: 200 kg TPLBC ha−1; T4: 300 kg TPLBC ha−1; T5: 400 kg TPLBC ha−1; T6: 500 kg TPLBC ha−1
# ‘-’indicates zero standard error.
Cadmium has no known biological function in tea plants and is considered one of the most toxic elements in the soil. Like As, Cd in made tea significantly decreased with increasing dose of TPLBC. However, made tea prepared from TV 23 clone contained a slightly higher amount of Cd (0.030 ± 0.001 to 0.037 ± 0.001 mg kg−1) than made tea prepared from S.3A/3 clone (0.025 ± 0.001 to 0.036 ± 0.001 mg kg−1) irrespective of treatment imposed. The permissible limit of Cd prescribed by WHO for any traditional herbal products is 0.3 mg kg−1 (WHO, 2007). Therefore, Cd concentration in all the made tea samples falls within this permissible limit and is around ten times lower than WHO’s permissible limit. Similar findings have been reported in black tea samples of Saudi Arabia (Ashraf & Mian, 2008), but the concentrations are much higher than results reported by Podwika et al. (2017) when 27 tea samples were purchased from a local market in southern Poland were analysed. Application of TPLBC @ 400 kg ha−1 reduced the Cd concentration in made tea 18.91 and 30.56% in TV23 and S.3A/3 clone respectively compared to plants growing on the same soil without biochar amendment, which is in good agreement with the findings of Zhu et al. (2020). They reported that biochar could reduce the transportation of Cd by surface complexation, precipitation, and ion exchange, thus reducing the accumulation of Cd in different organs of growing plants. The findings are also in agreement with Chen et al. (2018), who described that biochar application resulted in 32–40% reductions of plant Cd concentrations in acid, neutral, and alkaline soils. Medyńska-Juraszek, Rivier, Rasse, & Joner (2020) reported that decreasing trend of Cd uptake with increasing doses of biochar was attributed to immobilisation by root mucilage.
Total Cr in made tea prepared from TV23 clone ranged from 0.703 ± 0.001 mg kg−1 in T5 to 0.824 ± 0.002 mgkg−1. However, its concentration in made tea prepared from S.3A/3 was highest in T0 i.e.0.90 ± 0.016 mgkg-1and lowest in T5 i.e. 0.756 ± 0.001 mgkg−1.Similar results have also been reported by Zhang et al. (2020) when 22 tea samples were collected from the eastern region of Guizhou province (southwest China) were analysed. Khan et al. (2014) reported decreases in Cr Fallah et al., 2020, Karak et al., 2016bioaccumulation in rice cultivated in biochar amended soil and attributed this to lower Cr mobility in the presence of biochar. Ahmad et al. (2014) reported that non-carbonized fractions present in biochar interacted with soil Cr and formed Cr (III), which was strongly bounded by soil particles, thus becoming unavailable to tea plants. Furthermore, the extent of O-containing carboxyl, hydroxyl, and phenolic surface functional groups in biochar is supposed to effectively bind soil Cr. The permissible limit of Cr prescribed by WHO for any traditional herbal products is 2.0 mg kg−1 (WHO, 2007) and Cr concentration in all made samples was found to be within this permissible limit.
As, Cd and Cr in successive infusions
Application of TPLBC in tea growing soil significantly decreased the concentration of As in tea infusions irrespective of tea clone (Fig. 1a). Arsenic concentration in first, second and third infusions prepared from the TV23 clone ranged from1.04 ± 0.017 μg L-1 (in T5) to 1.61 ± 0.023 μg L-1 (in T0), 0.84 ± 0.0345 μg L-1 (in T5) to 1.01 ± 0.023μgL-1 (in T0), and 0.21 ± 0.011 μg L-1 (in T6) to 0.73 ± 0.046 μg L-1 (in T0), respectively (Table S1, See Supplementary material). On the other hand, As concentration in the first, second and third infusions ranges from 1.02 ± 0.057 μg L-1 (in T4) to 1.88 ± 0.08 μg L-1 (in T0), 0.84 ± 0.0288 μg L-1 (in T5) to 0.99 ± 0.023 μg L-1 (in T0), and 0.4 ± 0.017 μg L-1 (in T6) to 0.77 ± 0.023 μg L-1 (in T0), respectively when made tea prepared from S.3A/3 clone (Table S1, See Supplementary material). It is worth noting that TPLBC appears to be very effective not only to decrease the uptake of As by tea plant, but it also has a direct impact on As in tea infusion.
Fig. 1.
Concentration of (a) As, (b) Cd and (c) Cr in different tea infusions prepared from made tea of TV23 and S.3A/3 clones as a function of different doses on tea pruning litter biochar in soil. Each bar and point indicate the mean of three replications with standard error.
The contents of Cd in tea infusions influenced by different doses of TPLBC are shown in Fig. 1b. Cadmium concentrations in the first infusion range from 0.006 ± 0.001 μg L-1 for T5 to0.015 ± 0.001 μg L-1 for T0 in made tea prepared from TV23 clone and for S.3A/3, it ranges from 0.001 ± 0.001 μg L-1 for T5 to 0.011 ± 0.001 μg L-1for T0. Cd concentration in the second tea infusion was relatively lower than the first infusion, where it was observed to range from 0.001 ± 0.001 μg L-1 for T5 to 0.005 ± 0.001 μg L-1 for T0 of TV23 clone and for clone S.3A/3, it was ranged from 0.001 ± 0.001 μg L-1 for T5 to 0.005 ± 0.001 μg L-1 for T1 (Table S2, See Supplementary material). Rong et al. (2020) reported that available Cd in soils could be adsorbed by soil-applied biochar either through Cd+2-π bonding mechanisms or with soft ligands (e.g., ketonic group) in electron-rich domains on aromatic structures of biochar. Therefore, Cd adsorption onto TPLBC is responsible for the decrease in the uptake by tea plant and subsequent dissolution of Cd from made tea to tea infusion. Similar to the reported data by Rong et al. (2020), the results of this study are particularly agreeable concerning the application of TPLBC in soil as not only Cd, but also As and Cr concentrations (see next section) decreased in tea infusions.
The concentration of Cr released from made tea prepared from TV23 and S.3A/3 clone has been depicted in Fig. 1c. The concentration of Cr released from the first tea infusion from made tea prepared from the TV23 clone was observed to range from 0.241 ± 0.005 μg L-1to 0.311 ± 0.003 μg L-1and for S.3A/3 it ranged from 0.208 ± 0.002 μg L-1 to0.309 ± 0.002 μg L-1 (Table S3, See Supplementary material). Like, As and Cd, Cr released from both clones into the second infusion was relatively lower than into the first infusion. For TV23, it was observed from 0.094 ± 0.003 μg L-1 to 0.126 ± 0.002 μg L-1and for S.3A/3 it ranged between 0.109 ± 0.002 μg L-1to 0.141 ± 0.006 μg L-1. Among the three infusions, the lowest amount of Cr was released into the third infusion for both the clones and it ranged from 0.076 ± 0.002 μg L-1 to0.119 ± 0.047 μg L-1and 0.091 ± 0.003 μg L-1to 0.1 ± 0.003 μg L-1 in TV23 and S.3A/3 clone, respectively. Karak et al. (2017) reported that the content of metals in tea infusion depends on infusion time and the solubility of analysed metals. In the present study, it was observed that the amount of Cr present in the first infusion was significantly (p < 0.01) higher than in the second infusion and similarly significantly higher than in the third infusion (p < 0.01) which is similar to the findings of Karak et al. (2017).
Transfer rate of As, Cd and Cr from made tea into successive infusions
Per cent of As infused against total As in made tea was maximum in the first infusion followed by second and third infusions irrespective of clone (Fig. 2a). In the first infusion, the maximum amount of As (24.4 ± 0.07%) derived from the tea plant treated with 200 kg TPLBC ha-1and minimum (20.37 ± 0.03%) where 300 kg TPLBC ha−1 was applied as soil amendments for TV23 clone. However, it ranged from 18.61 ± 0.04% to 24.96 ± 0.06% for T4 and T0 treatment, respectively for the made tea prepared from S.3A/3 clone. In the second infusion, per cent of As transfer from the made tea of TV23clone was maximum for T6 (19.57 ± 0.25%) and minimum for T0 (14.37 ± 0.075%). For S.3A/3 clone it ranged from 18.01 ± 0.14% in T6 and a minimum of 12.87 ± 0.05% in T0 treatment. In the third infusion, the percentage of As transfer in TV23 clone was maximum for T6 (10.39 ± 0.035%) and minimum for T0 (4.72 ± 0.018%) treatments and for S.3A/3 clone maximum for T2 (10.59 ± 0.183%) and minimum for T4 (7.29 ± 0.022%) treatments. A similar trend was also observed for Cd and Cr, but it was significantly (p < 0.05) lower than for As (Fig. 2b and 2c).
Fig. 2.
Per cent transfer of trace elements[(a) As, (b) Cd and (c) Cr] from made tea to 1st, 2nd and 3rd tea infusions of made tea prepared from TV23 and S.3A/3 clones as a function of different doses of tea pruning litter biochar in soil. Each bar and point indicate the mean of three replications and vertical error bar indicates standard error.
The per cent of Cd and Cr transferred into second and third tea infusions influenced by different doses of TPLBC is given below:
Elemental transfer of Cd in the second infusion for TV23 clone:
First infusion: T0 > T1 > T2 > T4 > T6 > T3 > T5
Second infusion: T0 > T1 > T4 > T6 > T3 > T5 > T2
Third infusion: T1 > T0 > T2 > T3 > T4 > T5 > T6
Elemental transfer of Cd in second infusion for S.3A/3 clone:
First infusion: T0 > T1 > T6 > T2 > T3 > T4 > T5
Second infusion: T1 > T2 > T6 > T4 > T0 > T5 > T3
Third infusion: T2 > T3 > T0 > T1 > T5 > T6 > T4
Elemental transfer of Cr in third infusion for TV23 clone:
First infusion: T0 > T3 > T4 > T5 > T2 > T1 > T6
Second infusion: T2 > T0 > T1 > T4 > T3 > T6 > T5
Third infusion: T0 > T3 > T1 > T2 > T5 > T4 > T6
Elemental transfer of Cr in third infusion for S.3A/3 clone:
First infusion: T0 > T1 > T2 > T4 > T3 > T5 > T6
Second infusion: T0 > T1 > T2 > T4 > T3 > T5 > T6
Third infusion: T5 > T4 > T6 > T0 > T2 > T3 > T1
These results show that the percentage of elements transferred into the tea infusion varied widely for the different doses of TPLBC applied in the soil. This result was consistent with the previous report by Yuan et al. (2007), who concluded that repeated infusion decreased trace elements concentration in tea infusion. In contrast, Krstić et al. (2021) examined various tea samples and reported elevated Cd (0.72–0.97 mg kg−1) concentration in tea samples compared to the present tea samples. Cr in made tea samples greater than 5 mg kg−1 can harm the plant (Krstić et al., 2021). However, the average content of Cr in made tea ranges from 0.025 to 0.037 mg kg−1 in this study, indicating that TPLBC has a beneficial effect on tea cultivation. Keerthanan et al. (2020) also reported that the application of biochar in soil decrease lower percentage of As, Cd and Cr transfer into tea infusions. Mehra & Baker (2007) reported that the transfer rate of trace elements from made tea to tea infusion significantly differ due to biochemical components associated with trace metals, thus affecting the solubility of As, Cd and Cr from made tea prepared from TV23 and S.3A/3 clones influenced by TPLBC. Furthermore, made tea predominately contains polymeric polyphenols, which may form insoluble complexes with the different trace elements (Mehra & Baker, 2007).
Health risk assessment
Average daily intake (ADI) of As, Cr, and Cd from tea repeated tea infusions
The calculated average daily intakes (ADI; μg kg−1 BW D-1) of As, Cd and Cr by men and women through repeated tea infusion are presented in Fig. 3a to f. For this calculation, the average body weight of men and women were considered as 67.4 kg and 64.9 kg, respectively, and daily intake of tea beverage was considered as 600 mL D-1 from 10 g made tea. It should be noted that the ADI values for As, Cd and Cr differ significantly in made tea due to variation of the transfer rate of these elements, which depend on different doses of applied TPLBC, type of tea infusion and test cultivars. In general, ADI values decreased with increasing infusion steps, which is consistent with the reported results by Nagara et al. (2021). ADI values of As from tea infusions for men and women ranged from 0.23 × 10-3 to 1.86 × 10-3 μg kg−1 BW D-1 when infusions were prepared from made tea of TV23. On the other hand, it ranged between 0.45 × 10-3 and 2.17 × 10-3 μg kg−1 BW D-1 when infusions were prepared from made tea of S.3A/3. ADI values of Cd from tea infusions for men and women ranged from 0.001 × 10-3 to 0.017 × 10-3 μg kg−1 BW D-1 when infusions were prepared from made tea of TV23. However, it ranged between 0.001 × 10-3 and 0.013 × 10-3 μg kg−1 BW D-1 when infusions were prepared from made tea of S.3A/3. ADI values of As from tea infusions for men and women ranged from 0.086 × 10-3 to 0.359 × 10-3 μg kg−1 BW D-1 when infusions were prepared from made tea of TV23. However, it ranged between 0.101 × 10-3 and 0.357 × 10-3 μg kg−1 BW D-1 when infusions were prepared from made tea of S.3A/3. The highest ADI was found for As, followed by Cr, then Cd. Furthermore, the results revealed that increasing doses of TPLBC application in soil decrease ADI of analysed trace elements irrespective of clone used. This may be due to the impact of TPLBC on tea cultivation that hindered the uptake of As, Cd, and Cr by tea plant and consequently caused a decrease of As, Cd and Cr in made tea as well as a tea infusion. The results are in accordance with the findings of Krstić et al. (2021), who found a similar ADI trend of analysed trace elements in made tea collected from supermarkets in Belgrade, Republic of Serbia. The maximum tolerable daily intake (MTDI; the maximum amount of a contaminant which can be taken every day over a whole lifetime period without experiencing noticeable danger to health) for As is 2.1 μg kg−1 BW D-1, provisional tolerable weekly intake (PTWI) of Cd for a person is 6.25 μg kg−1 BW D-1, and MTDI of Cr is 33 μg kg−1 BW D-1 (WHO, 2007). Therefore, the ADI values of As, Cd and Cr contribute only 1.09 × 10-02 to 9.76 × 10-02, 3.34 × 10-05 to 5.01 × 10-04, and 8.46 × 10-03 to 3.46 × 10-04%, respectively of PTWI prescribed by the World Health Organization (WHO, 2007) through tea consumption. Hence, regular consumption of tea will not be harmful to tea consumers.
Fig. 3.
Average daily intake (ADI) of As (a: men; b: women), Cd (c: men; d: women), and Cr (e: men; f: women) through first, second, and third tea infusions prepared from the made tea of TV23 and S.3A/3 clones as a function of different doses on tea pruning litter biochar in soil [average body weight (BW) of men = 67.4 kg and for women = 64.9 kg; daily intake of tea is 10 g per person per day]. Each bar and point indicate the mean of three replications and vertical error bar indicates standard error.
Hazard quotient (HQ) of As, Cd and Cr
To qualitatively assess of potential non-carcinogenic effects on human health from As, Cd and Cr due to consumption of tea infusion produced from tea leaves of TV23 and S.3A/3 clones grown in soils amended with different doses of TPLBC, HQ was calculated and shown in Fig. 4a to f. The HQ values of As, Cr, and Cd gradually decrease with increasing doses of TPLBC application in soil irrespective of clone. As, Cd and Cr content does not contribute to carcinogenic risk as HQ values from each trace element were ≪1. Therefore, all made tea prepared from the test clones can be considered safe for human consumption. The results further confirm that the application of TPLBC as soil amendment provides a better avenue for tea cultivation as it decreases HQ values with increasing dose, thus reducing the human health risk from tested trace elements. Nagara et al. (2021) reported that the HQ values of As, Cd and Cr decrease with infusion steps in forty-five tea samples widely consumed in the USA. However, they concluded that carcinogenic risk for As was of concern and suggested avoiding drinking of the first infusion. On the contrary, the present study indicates that there is no need to discard any infusion as there is no any health risk towards tea consumers for As, Cd and Cr. The findings also suggest a better management strategy of tea pruning litters through TPLBC as a quality soil conditioner to reduce uptake of As, Cd and Cr.
Fig. 4.
Hazard quotient (HQ) of As (a: men; b: women), Cd (c: men; d: women), and Cr (e: men; f: women) through first, second, and third tea infusions prepared from made tea of TV23 and S.3A/3 clones as a function of different doses on tea pruning litter biochar in soil [the reference doses (RfD) in mg kg−1BWday−1forAs, Cd, and Cr are 3 × 10-4, 5 × 10-4 and 3 × 10-3, respectively]. Each bar and point indicate the mean of three replications and vertical error bar indicates standard error.
Statistical interpretation
Complete randomised design (CRD) has been carried out separately for TV23 and S.3A/3 to check for the significant differences among the different treatments concerning each of the responses, i.e. made tea (MT), first infusion (INF-1), second infusion (INF-2), third infusion (INF-3), men ADI from INF-1 (M−ADI−F), men ADI from INF-2 (M−ADI−S), men ADI from INF-3 (M−ADI−T), woman ADI from INF-1 (W-ADI-F), woman ADI from INF-2 (W-ADI-S), woman ADI from INF-3 (W-ADI-T), men HQ from INF-1 (M−HQ−F), men HQ from INF-2 (M−HQ−S), men HQ from INF-3 (M−HQ−T), women HQ from INF-1 (W-HQ-F), women HQ from INF-2 (W-HQ-S), women HQ from INF-3(W-HQ-T). The analysis has been carried out individually for As, Cr and Cd.
It is found that in all cases, the effects of the treatments have significant differences for each response. Subsequently, pairwise comparison of treatments was carried out to identify the best treatments by means of Tukey’s HSD test. It is found that treatment T5 has a significantly different effect as compared to all other treatments with respect to all 16 response variables.
Moreover, to have a collective analysis, multivariate analysis of variance (MANOVA) has been carried out, taking into consideration all the 16 response variables together to check for the difference in effect due to treatments. In order to keep sufficient degrees of freedom for error term, the response variables have been grouped as follows: Group 1: MT, INF-1-, INF-2-, INF-3-; Group 2: M−ADI−F, M−ADI−S, M−ADI−T, W-ADI-F, W-ADI-S, W-ADI-T; Group 3: M−HQ−F, M−HQ−S, M−HQ−T, W-HQ-F, W-HQ-S, W-HQ-T. MANOVA has been carried out separately for the above three groups of variables for TV23 and S.3A/3 considering As, Cr and Cd. It is observed that based on Wilks' Lambda, Hotelling-Lawley Trace and Roy's Greatest Root statistic, there are significant differences among the treatment effects in all cases, though Pillai's Trace statistic could not find significant differences among the treatment effect in many cases. The result is demonstrated in Tables S4 to S6 (See Supplementary materials).
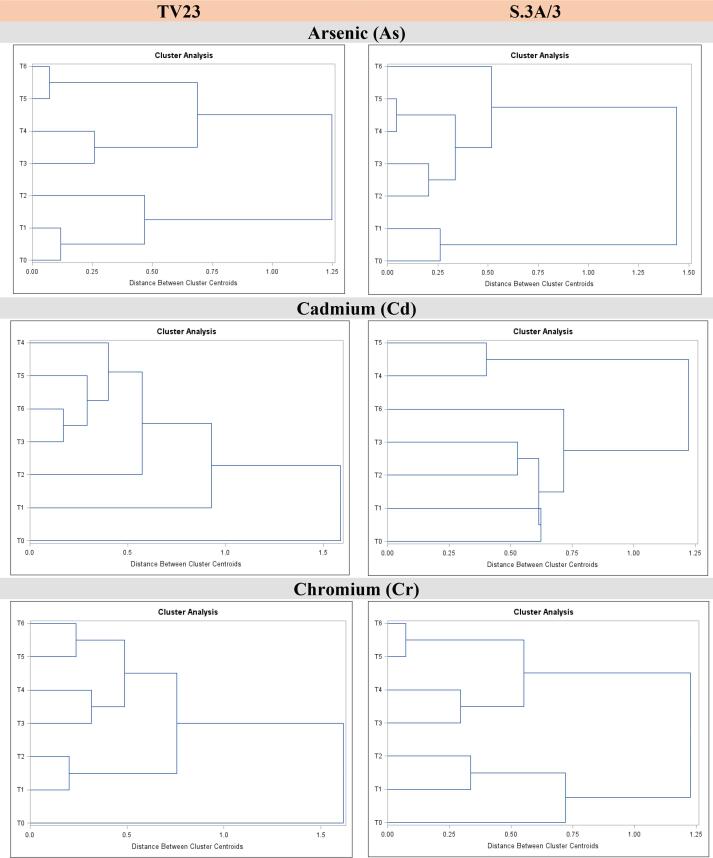
Finally, hierarchical clustering analysis has been carried out to form a homogenous group of treatments using the agglomerative method. The dendrogram is generated for TV23 and S.3A/3 considering As, Cr and Cd separately, and the same is represented in Fig. 5. In the case of As, for TV23, it is observed that three distinct groups have been formed T5, T6 formed a separate group, T3, T4 falls in the second group, and the remaining treatments formed another group; while in S.3A/3, T6 remains separate, T2-T5 formed a second group and remaining treatments come in another group. In Cr, for both TV23 and S.3A/3, T3 - T6 formed the first group, T1-T2 formed the second group, and T0 remained in the third group. In the case of Cd, for TV23, T2-T6 formed a homogenous group, T1 and T0 remain in two separate groups; whereas, for S.3A/3, T4-T5 formed the first group, T6 remains in a separate group, and the remaining treatments formed the third group.
Fig. 5.
Dendrogram for homogenous grouping of treatments.
Conclusion
To take care of the interrelation of the responses, MANOVA was applied, and it was found that treatment effect is significant with respect to all the responses considered simultaneously. It is clinched that TPLBC addition to tea-growing soil was operative in decreasing phytoavailable As, Cd and Cr. Results exposed that TPLBC amendments in tea growing acidic soil enabled As, Cd and Cr binding and decreased their uptake by tea plant. Increasing doses of TPLBC decreases total concentration of As, Cd and Cr. Application of 500 kg TPLBC ha−1 significantly decreases the content of As, Cd, and Cr in analyzed samples. The order of total concentration of analyzed trace elements in made tea was As > Cr > Cd. The trend of concentration for As, Cr and Cd in successive three tea infusions followed the order: first > second > third, which is also depicted in the values of ADI and HQ. The HQ values of As, Cr, and Cd gradually decrease with increasing doses of TPLBC application in soil irrespective of clone. It has also been observed that As, Cd and Cr content does not contribute to carcinogenic risk as HQ values from each trace elements were ≪1. Therefore, all made tea prepared from the test clones are safe for human consumption. Moreover, the application of hierarchical cluster analysis in TV23 and S.3A/3 for As, Cr and Cd resulted in three homogenous groups of treatments, though group members are not unique. While the described outcomes are reassuring, field research should be carried out to incorporate a higher dose of TPLBC to explore the potential of TPLBC as soil amendments. Therefore, these results can be beneficial for tea growers for sustainable tea production. Although, in all cases the level of analysed trace elements found was low, much effort is still needed to minimize further exposure of those elements to humans. Consequently, continuous monitoring of such trace elements and preventing them from entering the food chain, including planting, growing and harvesting, and also packing and Holding of Produce for Human Consumption, is essential.
CRediT authorship contribution statement
Arup Borgohain: Resources, Investigation, Formal analysis, Validation, Writing – original draft. Mridusmita Sarmah: Resources, Investigation, Formal analysis, Validation. Kaberijyoti Konwar: Resources, Investigation, Formal analysis, Validation. Rimjim Gogoi: Resources, Investigation, Formal analysis, Validation. Bidyot Bikash Gogoi: Resources, Investigation, Formal analysis, Validation. Puja Khar: Conceptualization, Methodology, Resources, Data curation, Validation, Writing – review & editing. Ranjit Kumar Paul: Formal analysis, Validation, Writing – original draft. Jyotirekha G. Handique: Resources, Data curation, Validation, Writing – original draft. Harisadhan Malakar: Resources, Data curation, Validation, Writing – original draft. Diganta Deka: Resources, Data curation, Validation, Writing – original draft. Jiban Saikia: Resources, Data curation, Validation, Writing – review & editing. Tanmoy Karak: Conceptualization, Methodology, Resources, Data curation, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work has been supported by the Department of Biotechnology (DBT), Govt. of India under the project “Unit of Excellence” (T.K.) (DBT’s Sanction Order No: BT/564/ NE/U-Excel/2016) and “Twinning Project” between Tea Research Association (T.K.) and CSIR-CIMAP (P.K.) (DBT’s Sanction Order No: BT/PR24706/NER/95/822/2017). Mr. Arup Borgohain and Mrs. Kaberijyoti Konwar expresses their thank and gratitude to DBT for providing their fellowship. We also gratefully acknowledge the encouragement received from Dr. A. K. Barooah, Director, TRA, and Mr. Joydeep Phukan, Secretary, TRA in pursuing the research work. Finally, we wish to record our thanks and gratitude to the two anonymous reviewers for their valuable suggestions that helped us a lot in improving this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100255.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmad M., Rajapaksha A.U., Lim J.E., Zhang M., Bolan N., Mohan D.…Ok Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Arafat Y., Ud Din I., Tayyab M., Jiang Y., Chen T., Cai Z.…Lin S. Soil sickness in aged tea plantation is associated with a shift in microbial communities as a result of plant polyphenol accumulation in the tea gardens. Frontiers in Plant Science. 2020;11 doi: 10.3389/fpls.2020.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf W., Mian A.A. Levels of selected heavy metals in black tea varieties consumed in Saudi Arabia. Bulletin of Environmental Contamination and Toxicology. 2008;81(1):101–104. doi: 10.1007/s00128-008-9402-0. [DOI] [PubMed] [Google Scholar]
- Askari , S. G., Oskoei , V., Abedi , F., Far , P. M., Naimabadi, A., & Javan, S. (2020) Evaluation of heavy metal concentrations in black tea and infusions in Neyshabur city and estimating health risk to consumers. International Journal of Environmental Analytical Chemistry, Doi: 10.1080/03067319.2020.1842388.
- Barman T., Barooah A.K., Goswami B.C., Sharma N., Panja S., Khare P., Karak T. Contents of chromium and arsenic in tea (Camellia sinensis L.): Extent of transfer into tea infusion and health consequence. Biological Trace Element Research. 2020;196(1):318–329. doi: 10.1007/s12011-019-01889-y. [DOI] [PubMed] [Google Scholar]
- Borgohain A., Konwar K., Buragohain D., Varghese S., Dutta A.K., Paul R.K.…Karak T. Temperature effect on biochar produced from tea (Camellia sinensis L.) pruning litters: A comprehensive treatise on physico-chemical and statistical approaches. Bioresource Technology. 2020;318 124023 doi: 10.1016/j.biortech.2020.12402. [DOI] [PubMed] [Google Scholar]
- Chen D.e., Liu X., Bian R., Cheng K., Zhang X., Zheng J.…Li L. Effects of biochar on availability and plant uptake of heavy metals – A meta-analysis. Journal of Environmental Management. 2018;222:76–85. doi: 10.1016/j.jenvman.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Deka H., Barman T., Sarmah P.P., Devi A., Tamuly P., Karak T. Impact of processing method on selected trace elements content of green tea: Does CTC green tea infusion possess risk towards human health? Food Chemistry: X. 2021;12:100173. doi: 10.1016/j.fochx.2021.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . Method 3050B: acid digestion of sediments, sludges, and soils. Environmental Protection Agency; Washington, DC: 1996. [Google Scholar]
- Fallah S.H., Bakaeian M., Parsian H., Amouei A., Asgharnia H., Ghanbarian M., Mousapour A., et al. Potentially harmful heavy metal contamination in Babolrood river: evaluation for risk assessment in the Mazandaran province, Iran. International Journal of Environmental Analytical Chemistry. 2020 doi: 10.1080/03067319.2020.1828386. [DOI] [Google Scholar]
- Gholizadeh, M., & Hu, X. (2021). Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. Journal of Environmental Chemical Engineering, 9(5), 105830. Doi: 10.1016/j.jece.2021.105830.
- Karak T., Paul R.K., Kutu F.R., Mehra A., Khare P., Dutta A.K.…Boruah R.K. Comparative assessment of copper, iron, and zinc contents in selected Indian (Assam) and South African (Thohoyandou) tea (Camellia sinensis L.) Samples and their infusion: A quest for health risks to consumer. Biological Trace Element Research. 2017;175(2):475–487. doi: 10.1007/s12011-016-0783-3. [DOI] [PubMed] [Google Scholar]
- Karak, T., Sonar, I., Paul, R. K., Frankowski, M., Boruah, R. K., Dutta, A. K., & Das, D. K. (2015). Aluminium dynamics from soil to tea plant (Camellia sinensis L.): Is it enhanced by municipal solid waste compost application? Chemosphere, 119, 917-926. Doi: 10.1016/j.chemosphere.2014.08.067. [DOI] [PubMed]
- Karami, M. A., Fakhri, Y., Rezania, S., Alinejad, A. A., Mohammadi, A. A., Yousefi, M., Ghaderpoori, M., Saghi , M. H., & Ahmadpour, M., (2019). Non-carcinogenic health risk assessment due to fluoride exposure from tea consumption in Iran using Monte Carlo simulation. International Journal of Environmental Research and Public Health, 16 (4261). https://doi.org/10.3390/ijerph16214261. [DOI] [PMC free article] [PubMed]
- Karak T., Paul R.K., Sonar I., Nath J.R., Baruah R.K., Dutta A.K. Nickel dynamics influenced by municipal solid waste compost application in tea (Camellia sinensis L.): a cup that cheers? International Journal of Environmental Science and Technology. 2016;13(2):663–678. doi: 10.1007/s13762-015-0900-4. [DOI] [Google Scholar]
- Keerthanan S., Bhatnagar A., Mahatantila K., Jayasinghe C., Ok Y.S., Vithanage M. Engineered tea-waste biochar for the removal of caffeine, a model compound in pharmaceuticals and personal care products (PPCPs), from aqueous media. Environmental Technology & Innovation. 2021;19:100847. doi: 10.1016/j.eti.2020.100847. [DOI] [Google Scholar]
- Khan S., Reid B.J., Li G., Zhu Y.-G. Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China. Environment International. 2014;68:154–161. doi: 10.1016/j.envint.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Krstić M., Stupar M., Đukić-Ćosić D., Baralić K., Mračević S.Đ. Health risk assessment of toxic metals and toxigenic fungi in commercial herbal tea samples from Belgrade, Serbia. Journal of Food Composition and Analysis. 2021;104:104159. doi: 10.1016/j.jfca.2021.104159. [DOI] [Google Scholar]
- Medyńska-Juraszek A., Rivier P.A., Rasse D., Joner E.J. Biochar Affects Heavy Metal Uptake in Plants through Interactions in the Rhizosphere. Applied Sciences. 2020;10(15):5105. doi: 10.3390/app10155105. [DOI] [Google Scholar]
- Mehra A., Baker C.L. Leaching and bioavailability of aluminium, copper and manganese from tea (Camellia sinensis L.) Food Chemistry. 2007;100(4):1456–1463. doi: 10.1016/j.foodchem.2005.11.03. [DOI] [Google Scholar]
- Minari G.D., Saran L.M., Constancio M.T.L., da Silva R.C., Rosalen D.L., de Melo W.J., Alves L.M.C. Bioremediation potential of new cadmium, chromium, and nickel-resistant bacteria isolated from tropical agricultural soil. Ecotoxicology and Environmental Safety. 2020;204 doi: 10.1016/j.ecoenv.2020.111038. [DOI] [PubMed] [Google Scholar]
- Nagara V., Sarkar D., Luo Q., Biswas J.K., Datta R. Health Risk Assessment of Exposure to Trace Elements from Drinking Black and Green Tea Marketed in Three Countries. Biological Trace Element Research. 2021 doi: 10.1007/s12011-021-02863-3. [DOI] [PubMed] [Google Scholar]
- Podwika W., Kleszcz K., Krośniak M., Zagrodzki P. Copper, manganese, zinc, and cadmium in tea leaves of different types and origin. Biological Trace Element Research. 2017;183(2):389–395. doi: 10.1007/s12011-017-1140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L., Jingshuang L., Qicun W., Yang W. Source identification and availability of heavy metals in peri-urban vegetable soils: A case study from China. Human and Ecological Risk Assessment: An International Journal. 2015;22(1):1–14. doi: 10.1080/10807039.2015.1044939. [DOI] [Google Scholar]
- Rizwan M., Ali S., Qayyum M.F., Ibrahim M., Zia-ur-Rehman M., Abbas T., Ok Y.S. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environmental Science and Pollution Research. 2016;23(3):2230–2248. doi: 10.1007/s11356-015-5697-7. [DOI] [PubMed] [Google Scholar]
- Rong C., Zhong K., Li F., Huang H., Li C., Xinyu Nong X., Zhang C. Combined effect of ferrous ion and biochar on cadmium and arsenic accumulation in rice. Applied Sciences. 2020;10:300. doi: 10.3390/app10010300. [DOI] [Google Scholar]
- Shekhawat K., Chatterjee S., Joshi B. Chromium toxicity and its health hazards. International Journal of Advanced Research. 2015;3(7):167–172. [Google Scholar]
- Sofuoglu S.C., Kavcar P. An exposure and risk assessment for fluoride and trace metals in black tea. Journal of Hazardous Materials. 2008;158(2–3):392–400. doi: 10.1016/j.jhazmat.2008.01.086. [DOI] [PubMed] [Google Scholar]
- Tao C., Song Y., Chen Z., Zhao W., Ji J., Shen N.…Frost R.L. Geological load and health risk of heavy metals uptake by tea from soil: What are the significant influencing factors? Catena. 2021;204:105419. doi: 10.1016/j.catena.2021.105419. [DOI] [Google Scholar]
- WHO World Health Organization, WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues, World Health Organization 2007 Geneva, Switzerland.
- Xu Y., Zhang Y., Chen J., Wang F., Du Q., Yin J. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chemistry. 2018;258:16–24. doi: 10.1016/j.foodchem.2018.03.042. [DOI] [PubMed] [Google Scholar]
- Yang Z., Liu X., Zhang M., Liu L., Xu X., Xian J., Cheng Z. Effect of temperature and duration of pyrolysis on spent tea leaves biochar: Physiochemical properties and Cd(II) adsorption capacity. Water Science and Technology. 2020;81(12):2533–2544. doi: 10.2166/wst.2020.309. [DOI] [PubMed] [Google Scholar]
- Yaylalı-Abanuz G., Tüysüz N. Heavy metal contamination of soils and tea plants in the eastern Black Sea region, NE Turkey. Environmental Earth Science. 2009;59(1):131–144. doi: 10.1007/s12665-009-0011-y. [DOI] [Google Scholar]
- Yuan C., Gao E., He B., Jiang G. Arsenic species and leaching characters in tea (Camellia sinensis) Food and Chemical Toxicology. 2007;45(12):2381–2389. doi: 10.1016/j.fct.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yang R., Li Y.C., Peng Y., Wen X., Ni X. Distribution, accumulation, and potential risks of heavy metals in soil and tea leaves from geologically different plantations. Ecotoxicology and Environmental Safety. 2020;195:110475. doi: 10.1016/j.ecoenv.2020.110475. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Wang H., Lv X., Zhang Y., Wang W. Effects of biochar and biofertilizer on cadmium-contaminated cotton growth and the antioxidative defense system. Scientific Reports. 2020;10(1) doi: 10.1038/s41598-020-77142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.