Highlights
-
•
Camellia japonica is an underexplored medicinal plant with associated bioactivities.
-
•
C. japonica is yet to be exploited industrially needing new strategies to this aim.
-
•
Novel approaches are suggested facing the large-scale application of C. japonica.
-
•
Camellia plantations represent a much-needed strategy to meet industrialization requirements.
-
•
Evidence on the use of C. japonica with food and cosmeceutical purposes is provided.
Keywords: Camellias, Natural bioactive compounds, Medicinal plants, Biological activities, Food science, Biochemical valorization
Abstract
In response to the increased popularity of medicinal plants, a number of conservation groups are recommending the investigation on poorly characterized and widely distributed species, as it is the case of camellias. In particular, Camellia japonica L. is a widespread species found in Galicia (NW Spain), where it has been largely exploited with ornamental purposes. Recent findings on its phytochemical characterization showed thousands of bioactive ingredients, mostly represented by phenolic compounds, together with terpenoids, and fatty acids. These molecules present associated biological activities, acting as antioxidant, antimicrobial, anti-inflammatory, and anticancer agents. This review is aimed at describing the main bioactive compounds of C. japonica, as well as the health-enhancing properties attributed to this medicinal plant. Novel strategies are needed to implement an efficient industrialization process for C. japonica, ranging from small-scale approaches to the establishment of large plantations, thus involving important sectors, such as the food, pharmaceutical and cosmetic industries.
Introduction
The genus Camellia (Theaceae) includes more than 250 plants with an intricate taxonomy (“Integrated Taxonomic Information System (ITIS)”, 2020), being Camellia sinensis L., Camellia oleifera Abel. and Camellia japonica L. the most outstanding species, traditionally used for multiple applications, including tea and essential oils production and ornamental purposes (Garcia-Jares, Sanchez-Nande, Lamas, & Lores, 2017). Its botanical origin is located in Eastern Asia, including the Himalayas, Japan, Southern China, and South-Eastern countries (Durrant, 1982), and it is currently distributed in tropical and subtropical regions worldwide.
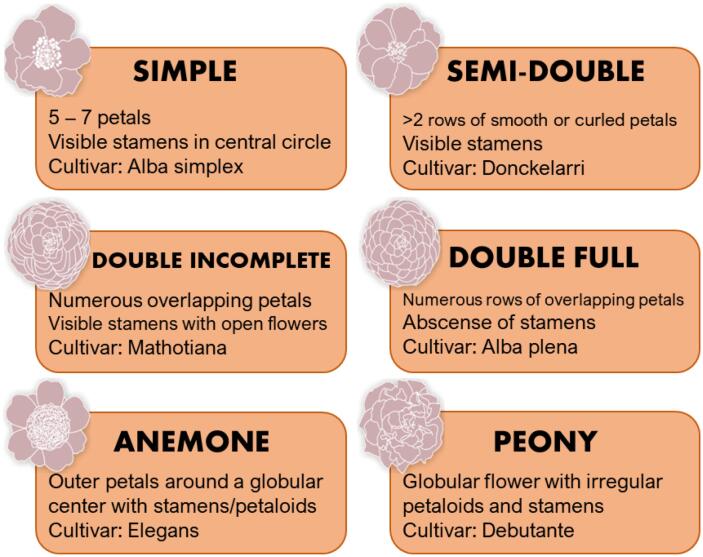
In particular, Galicia, a North-Western region from Spain, constitutes one of the international benchmarks regarding the cultivation and production of Camellia spp., presenting almost 8,000 different varieties (Xunta de Galicia, 2020), thanks to the edaphoclimatic conditions associated with this region, including humid climate, mild temperatures and fertile and acidic soils. However, the introduction of first camellias specimens to this region remains uncertain, although it is estimated to occur at late XVIII century (Salinero, Vela, De Ron, & Jesús, 2016). The most abundant species in Galicia is C. japonica, from which several types or varieties can be distinguished, due to the anatomical traits attributed to the flowers (Fig. 1), accounting for up to 3,000 different varieties, mostly known for their extended use in gardening (Kim, Cho, Yang, & Kim, 2017). In this sense, important Galician nurseries have gained specialization in the production of C. japonica and derived products, making heavy investments, especially for the mechanization and automation of production processes, obtaining a continuous production with stable quality of 1.5 million plants per year, for a value of around 40 million euros, which supposes about the 9% of the total Galician agricultural production (Salinero et al., 2016). In parallel, the importance of C. japonica as an industrially relevant plant was mostly reported in China, where more than 3,660,000 ha were dedicated to this crop, whose annual production reaches 645,000 tons of seeds, from which 164,000 tons of oil were obtained (Jiyin, Parks, & Yueqiang, 2005).
Fig. 1.
Classification of C. japonica according to flower shape (Deputación de Pontevedra, 1980, Salinero et al., 2016, Xunta de Galicia, 2020).
Despite of the popularity of C. japonica as an ornamental plant, little attention has been paid to the characterization of its health-enhancing properties, and its effectiveness as a source of bioactive compounds. In this sense, the first references about the use of C. japonica with medicinal purposes dates back to 1613, when the use of C. japonica seeds and flowers was reported by the doctor of oriental medicine Heo Jun (Song, Won, & Kim, 2016). Thanks to the production of bioactive compounds, such as triterpenes, flavonoids, and essential fatty acids, C. japonica is considered as a valuable medicinal plant (Majumder, Ghosh, & Bhattacharya, 2020). As a result, C. japonica and its derived products present several pharmacological properties, acting as gastrointestinal modulators, anticancer, antimicrobial, antioxidant, neuroprotective, hypolipidemic, anti-obesity, and anti-inflammatory agents (Guo, Tong, Ren, Tu, & Li, 2018). In this sense, different C. japonica-derived matrices have been extensively used as relevant sources of bioactive compounds, including seeds and their derived oil, flowers, fruits and leaves (Garcia-Jares et al., 2017). Keeping all this in mind, increasing interest is being observed in the last decades for the industrial exploitation of C. japonica. This review focuses on the phytochemistry of C. japonica, combining the description of its most prevalent bioactive compounds with their associated biological activities, with the aim of providing a current perspective about its potential as a medicinal plant to be exploited with nutraceutical purposes. This is a systematic review in which search engines with a multidisciplinary scope were employed, including PubMed, Web of Science, and Google Scholar. As well, the information regarding official data was directly obtained from original sources, as it was the case of the governmental sources Xunta de Galicia, Deputación de Pontevedra (both ascribed to Spanish government), and official organisms, such as the National Center for Biotechnology Information and the Integrated Taxonomy Information System (both ascribed to U.S. government). The inclusion and exclusion criteria were selected according to Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Bioactive compounds isolated from C. japonica
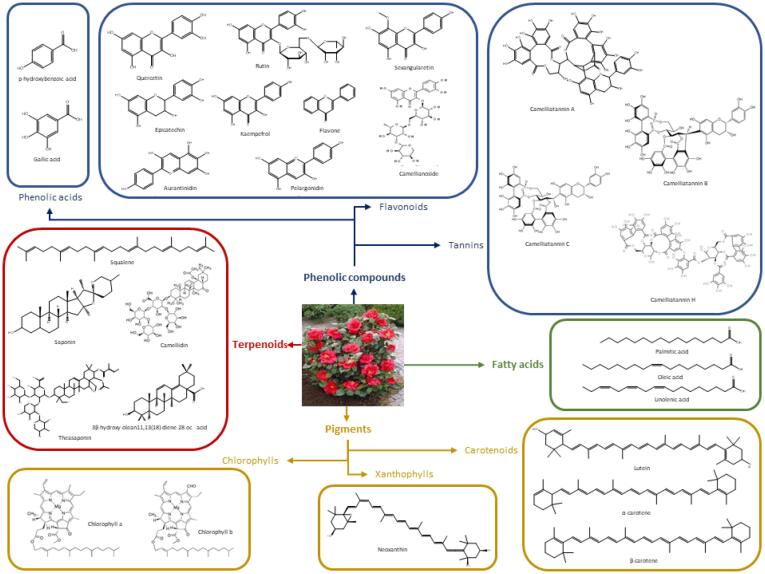
The great diversity of varieties of C. japonica implies that the study of its chemical composition is complex. In a generic way, its main compounds are phenolic compounds, terpenoids, fatty acids and a series of minor compounds that include pigments and biosugars (Meng et al., 2019). Such compounds are biosynthesized as the result of secondary metabolism, involved in the adaptative and defensive responses of plants against environmental conditions and threats (García-Pérez et al., 2021, Panghal et al., 2021). In this sense, the concentration of bioactive compounds are highly influenced by a set of both edaphoclimatic and physical factors, including geographical area, climatic conditions, soil quality, seasonal variations, and the exposure to several stresses, as it is the case of drought, heavy rains, or overexposure to ultraviolet light (Abe et al., 2021, Venthodika et al., 2021, Wendel and Parks, 1985). Furthermore, different components have been detected in an organ and tissue-dependent manner, as observed for different camellias, thus suggesting the high complexity of the phytochemical composition of these species. In this section, an overview of the most prevalent chemical constituents of C. japonica is provided (Fig. 2), with a special focus on those compounds with associated biological activities, the so-called bioactive compounds.
Fig. 2.
Chemical structures of the main molecules present in C. japonica.
Phenolic compounds
Phenolic compounds constitute the largest family of secondary metabolites, with tens of thousands different compounds isolated to date (García-Pérez, Lozano-Milo, Landin, & Gallego, 2020), ubiquitously found among all the species belonging to the plant kingdom, including camellias. The main types of phenolic compounds present in C. japonica are simple phenolic compounds, phenolic acids, flavonoids and tannins (Table 1), whose concentration depends on the part of the plant analyzed, being more abundant in the flowers and leaves (Stewart & Stewart, 2008). The concentration of phenolic compounds (Fig. 2) also displays a strong dependence on climatic factors, the state of maturity and seasonality. That is why the concentration of phenolic compounds in C. japonica is higher in young leaves, with a total phenol content of 74.62 mg per 100 g of dry weight, than in flower buds, with 65.02 mg per 100 g of dry weight, and flowers 62.42 mg per 100 g of dry weight (Lee et al., 2005).
Table 1.
Phenolic compounds found in C. japonica extracts, identification from different plant parts and associated bioactivities (Center, 2020).
| Compounds | Identification | Part | Associated bioactivities | Refs. |
|---|---|---|---|---|
| Phenolic acids | ||||
| Gallic acid | GC–MS | L, P, F | Antimicrobial, anti-inflammatory, antioxidant, anticancer | (Kanth et al., 2014, Lee et al., 2005, Lee et al., 2016, Moon and Kim, 2018) |
| p-hydroxybenzoic acid | HPLC | F | Antioxidant, antimicrobial | (Nakajima, Itokawa, & Ikuta, 1984) |
| 3,4,5-trihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, 2,3-digalloyl-O-α-d-glucopyranoside, and 2,3-digalloyl-O-β-d-glucopyranoside | NMR, HPLC | F | Antioxidant | (Lee, Cho, Moon, & Park, 2011) |
| Flavonoids | ||||
| Quercetin | GC–MS | L, F | Antimicrobial, anti-inflammatory, anticancer, antioxidant | (Kim, Jeong, & Shim, 2010, Lee et al., 2016, Moon and Kim, 2018, Piao et al., 2011) |
| Quercetin 3-O-β-d-galactopyranoside, quercetin 3-O-β-d-glucopyranoside | NMR, GC–MS | F, L | Antioxidant, anti-hyperuricemia | (Lee et al., 2011, Yoon et al., 2017) |
| Camellianoside | HPLC | L | Antioxidant, antiviral | (Onodera et al., 2006, Park et al., 2002) |
| Epicatechin | GC–MS | L, F | Antimicrobial, anti-inflammatory, anticancer, antioxidant | (Kim et al., 2010; Lee et al., 2016, Moon and Kim, 2018, Piao et al., 2011) |
| Kaempferol | HPLC | F | Antioxidant, anti-inflammatory, antimicrobial, anticancer, cardioprotective, neuroprotective, anti-diabetes | (Nakajima et al., 1984) |
| Kaempferol 3-O-β-d-galactopyranoside and kaempferol 3-O-β-d-glucopyranoside | NMR, GC–MS | F, L | Antioxidant, anti-hyperuricemia | (Lee et al., 2011, Yoon et al., 2017) |
| Kaempferol-3-O-β-d-xylopyranosyl(1 → 3)-α-l-rhamnopyranosyl(1 → 6)-O-β-d-galactopyranoside, kaempferol-3-O-α-l-rhamnopyranosyl-(1 → 6)-O-β-d-xylopyranosyl-(1 → 2)-O-β-d-galactopyranoside, kaempferol-3-O-β-d-xylopyranosyl-(1 → 3)-O-α-l-rhamnopyranosyl-(1 → 6)-O-β-d-glucopyranosyl-(1 → 2)-O-β-d-gluctopyranoside, tsubakiosides A-D, camelliaside B | HPLC-PDA | S | Antioxidant, anti-aging | (Ko et al., 2020) |
| Sexangularetin | HPLC | F | Antioxidant, anti-inflammatory, antimicrobial, anticancer | (Nakajima et al., 1984) |
| Anthocyanidins: cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-glucopyranoside, cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactopyranoside, cyanidin 3-O-(6-O-(E)-caffeoyl)-β-glucopyranoside, cyanidin 3-O-(6-O-(E)-caffeoyl)-β-galactopyranoside | HPLC | F | Antioxidant | (Li et al., 2009) |
| Tannins | ||||
| Camelliatannins A, B, C, H | NMR | L | Antiviral | (Yang et al., 2015) |
| Camelliatannins D, F, G | NMR | L | Antiviral | (Han et al., 1994, Hatano et al., 1991, Hatano et al., 1995) |
| Camelliins A and B | NMR | F | Antiviral | (Yoshida et al., 1990) |
Description. GC–MS: gas chromatography-mass spectrometry analysis; HPLC: high-performance liquid chromatography; NMR: nuclear magnetic resonance spectroscopy; TLC: thin-layer chromatography; HPLC-PDA: high-performance liquid chromatography coupled to photodiode array detector; // L: leaves; F: flowers; S: seeds; P: fruit peels.
The predominant phenolic acids of C. japonica are gallic acid and p-hydroxybenzoic acid, being both compounds present in the flowers. Gallic acid can also be isolated from leaves and fruits (Kanth et al., 2014, Kim et al., 2018). It is also possible to identify and quantify other phenolic compounds in lower concentrations. These molecules include 3,4,5-trihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, 2,3-digalloyl-O-α-d-glucopyranoside, and 2,3-digalloyl-O-β-d-glucopyranoside (Hee, Hye, Hyang, Ok, & Mi, 2010). The main flavonoids are quercetin, kaempferol, sexangularetin, camellianoside, epicathechin (Jeganathan et al., 2016, Onodera et al., 2006), quercetin 3-O-β-d-galactopyranoside, quercetin 3-O-β-d-glucopyranoside, kaempferol 3-O-β-d-galactopyranoside, and kaempferol 3-O-β-d-glucopyranoside (Hee et al., 2010), present in both leaves and flowers of C. japonica (Yoon et al., 2017) or quercetin-7-glucoside, which is the most important yellow pigment in Camellia chrysantha (Hwang, Yoshikawa, Miyajima, & Okubo, 1992). Many of these flavonoids are responsible for the coloration of the flowers. For example, red C. japonica extracts have flavonoid epicatechin (Zhang, Yin, Kong, & Jiang, 2011). These red and pink varieties are also rich in anthocyanins, being the most abundant cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-glucopyranoside, cyanidin 3-O-(6-O-(E)-p-coumaroyl)-β-galactopyranoside, cyanidin 3-O-(6-O-(E)-caffeoyl)-β-glucopyranoside, cyanidin 3-O-(6-O-(E)-caffeoyl)-β-galactopyranoside (Li, Hashimoto, Shimizu, & Sakata, 2009). This type of anthocyanins was not detected in C. japonica cultivars with white-petal flowers due to the absence of color (Li, Wang, Yin, Fan, & Li, 2019), as they present other pigments like leuco-anthocyanins (Endo, 1958). Other flavonoids have been described in seeds, including eight kaempferol oligosaccharides, namely tsubakiosides A-D, camelliaside B, and analogs (Ko, Rho, & Yoon, 2020). It is important to note that camellianoside constitutes an exclusive flavonoid of the genus Camellia, being a flavonol glucoside (quercetin-3-O-β-d-xylopyranosyl-(1 → 3)-O-α-l-rhamnopyranosyl-(1 → 6)-O-β-d-glucopyranoside) present in significant concentrations in the leaves. This compound is characterized by having an antioxidant capacity superior to that of reference antioxidants such as l-cysteine and l-ascorbic acid (Onodera et al., 2006).
Considering other subfamilies, tannins have been also isolated from C. japonica, represented by camelliatanins. The major molecules in this group are camelliatanins A, B, C, D, F, G and H (Han et al., 1994, Hatano et al., 1995, Hatano et al., 1991), all of them being constituted by an epicatechin core linked to a C-glucosyl ellagitannin moiety (Han et al., 1994). Furthermore, camelliins A and B represent another tannins from the genus Camellia, which have been detected in the flower buds of C. japonica (Yoshida, Chou, Maruyama, & Okuda, 1990).
Terpenoids
Together with phenolic compounds, terpenoids constitute another important family of secondary metabolites, closely related to plant adaptation and defense, with associated biological activities. The major terpenoids in C. japonica are saponins (Table 2), including camellidins as relevant saponin exclusive of the genus Camellia. Camellidins are composed of 3β-hydroxy-18β-acetoxy-28-norolean-12-en-16-one (camellidin I) or 3β, 8β-dihydroxy-28-norolean-12-en-16-one (camellidin II) as possible aglycon moieties, and d-glucuronic acid, d-glucose and two moles of d-galactose as the sugar moieties (Chakraborty, Chakraborty, & Saha, 2018). Other saponins present in C. japonica are camerosides, camelliasaponins (A1, A2, B1, B2, C1, C2) (Yoshikawa et al., 1996) and camellioside D (Cui et al., 2018, Rho et al., 2019, Yoshikawa et al., 2007); other terpenoids present in C. japonica include squalene, oleanane triterpenes (Uddin et al., 2014, Yang et al., 2015), 3-β-O-acetyl-16β-hydroxy-12-oxoolean, 3-β-O-acetyl-16β-hydroxy-11-oxoolean-12-ene, 3-β-O-acetyl-16β-hydroxyolean-12-ene, 3-α-hydroxy-1-oxofriedelan, friedelin, 3-β-friedelanol, canophyllol, 3-oxofriedelan-1(2)-ene, β-amyrin, camellenodiol, camelledionol (Thao et al., 2010), and two recently identified 3,4-dry-28-noroleanan-type triterpenoids (Cho et al., 2020).
Table 2.
Other bioactive compounds found in C. japonica extracts: identification from different plant parts and associated bioactivities (Center, 2020).
| Family | Compounds | Identification | Part | Associated bioactivities | Refs. |
|---|---|---|---|---|---|
| Terpenoids | |||||
| Tritepenoids | Squalene | GC–MS | L, B | Anti-inflammatory, anticancer, antioxidant | (Lee et al., 2016, Majumder et al., 2020, Moon and Kim, 2018, Thao et al., 2010) |
| Saponins | Camoreoside B | HCCC, RP-HPLC | S | Anti-obesity | (Ochiai et al., 2018) |
| Camoreoside C, G | HCCC, RP-HPLC | S | Anti-obesity | (Rho et al., 2019) | |
| Camelliasaponin C2 | HPLC, NMR | S | Anti-obesity | (Cui et al., 2018) | |
| Camelliasaponins A1, A2, B1, B2, C1, C2 | HPLC | S | Inhibition of alcohol absorption | (Yoshikawa et al., 1996) | |
| Theasaponin E1 | HPLC, GC–MS, LC-MS, NMR | S | Anti-obesity | (Cui et al., 2018) | |
| Camellidin | NMR | L | Antimicrobial | (Nagata et al., 1985) | |
| Oleanane triterpenes | Camelledionol, 3β-hydroxy-olean11,13(18)-diene-28-oic acid, 3β-acetoxyolean-12-ene-28-oic acid | HPLC, silica gel, RP-C18 | P, F | Anti-obesity, anti-diabetes, antiviral | (Uddin et al., 2014, Yang et al., 2015) |
| 3-β-O-acetyl-16β-hydroxy-12-oxoolean, 3-β-O-acetyl-16β-hydroxy-11-oxoolean-12-ene, 3-β-O-acetyl-16β-hydroxyolean-12-ene, 3-α-hydroxy-1-oxofriedelan, 3-oxofriedelan-1(2)-ene | NMR | S | Anticancer | (Thao et al., 2010) | |
| Camellioside D | NMR | F | Platelet aggregation control | (Yoshikawa et al., 2007) | |
| Fatty acids | |||||
| Saturated | Palmitic acid | GLC | O | Antiviral, antioxidant, anti-inflammatory | (Zeng & Endo, 2019) |
| Unsaturated | Oleic acid, linolenic acid | GC | O | Antihypertensive, antioxidant, anti-inflammatory | (Guo et al., 2018) |
| Pigments | |||||
| Carotenoids | Lutein, α-carotene, β-carotene | HPLC UV/VIS | L | Antimicrobial, antioxidant | (Taylor & McDowell, 1991) |
| Xanthophylls | Neoxanthin | TLC | F | Antioxidant | (Scogin, 1986) |
| Chlorophylls | Chlorophyll a, b | HPLC UV/VIS | L | – | (Taylor & McDowell, 1991) |
| Minor compounds | |||||
| Free sugars | Glucose, fructose, saccharose | HPLC RI | F | – | (Trinh et al., 2018) |
| Insoluble sugars | Arabinose, xylose | HPLC RI | F | – | (Trinh et al., 2018) |
| Vitamins | E | HPLC | L, S | – | (Saenjum et al., 2020) |
| Aminoacids | Aspartic acid, glutamic acid, histidine, alanine | FS | L | – | (Kim et al., 2005, Liu et al., 2020) |
| Minerals | Phosphorus, calcium, potassium, sodium, iron, manganese, zinc, aluminum, copper | FS | L | – | (Kim et al., 2005, Liu et al., 2020) |
Description. GC–MS: gas chromatography-mass spectrometry analysis; HCCC: high-performance countercurrent chromatography; RP-HPLC: reversed-phase high-performance liquid chromatography; HPLC: high-performance liquid chromatography; NMR: nuclear magnetic resonance spectroscopy; LC-MS: liquid chromatography–mass spectrometry; RP-C18: reverse phase-C18; GLC: gas liquid chromatography; GC: gas chromatography; HPLC UV/VIS: high-performance liquid chromatography equipped with a ultraviolet/visible detector; HPLC RI: high-performance liquid chromatography equipped with a refractive index detector; FS: flame spectroscopy; // L: leaves; B: bark; S: seeds; P: fruit peels; F: flowers; O: oil.
Fatty acids
Camellias have been traditionally used in the production of essential oils and edible oils, mainly containing oleic acid as the predominant fatty acid (Su, Shih, & Lin, 2014), which represents the 88.2% of the total lipids in C. japonica. Oleic acid, consequently, is the major fatty acid found as part of both glycolipids and phospholipids, being abundantly distributed in all positions within their structure in their esterified form (sn-1, 2 and 3) (Noh & Yoon, 2012). Other fatty acids found in minor concentrations are palmitic acid and linolenic acid (Table 2) (Zeng & Endo, 2019). Therefore, the essential oil of C. japonica has a similar composition to that of other edible oils, such as olive oil (Fang, Fei, Sun, & Jin, 2016), but with a lower content of saturated fatty acids (Salinero & Corral, 2008), thus suggesting a healthier profile motivated by the prevalence of polyunsaturated fatty acids, presenting meaningful beneficial properties to human health upon its consumption.
Minor compounds
Besides polyphenols, terpenoids, and fatty acids, C. japonica is considered a natural source of other compounds, as well, being reported at much lower concentrations in these species, as it is the case of pigments, major responsible for the photosynthetic activity and the colored nature of flowers together with anthocyanins, biosugars, vitamins and minerals. Most of the pigments of C. japonica are in the flowers, being one of the main characteristics to discriminate different varieties. These pigments vary from red to white, being possible to find yellow pigments. The interspecific hybrids of the variety 'Suzanne Withers' have as main pigments several carotenoids and two flavonoids (derived from kaempferol and quercetin). In some cases, it is possible to distinguish purple flowers in varieties of C. japonica of red flowers. This color modification may be due to the presence of aluminum ions, which were detected in concentrations 14–21 times higher than in red petals without modifications. Therefore, this color change may be due to the chelation of aluminum ions with anthocyanins (Tanikawa, Inoue, & Nakayama, 2016). In the case of leaves, the main pigments are chlorophylls (a and b) (Gogoi & Borua, 2017), as well as other chlorophyll-type compounds that were identified as chlorophyllides and pheoforbids (Taylor & McDowell, 1991).
C. japonica also has significant amounts of biosugars, mostly represented by hexoses and pentoses, being glucose, fructose, and sucrose the most prominent examples. This plant also presents insoluble sugars, as it is the case of xylose and arabinose (Trinh, Choi, & Bae, 2018). Other minor compounds present in C. japonica, principally in the leaves, include vitamin E, some amino acids, including essential amino acids, and other biologically active compounds related to hyperuricemia (Yoon et al., 2017). Vitamin E is present in various forms, i.e.: α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol, α-tocotrienol, γ-tocotrienol, and δ-tocotrienol, found in a great extent in the seeds of C. japonica (Saenjum, Pattananandecha, & Nakagawa, 2020). The most abundant amino acids in the whole plant are aspartic acid, glutamic acid, histidine and alanine, whose concentration decreases with the delay in the harvesting time. Camellias are also rich in minerals. The most abundant minerals are phosphorus, calcium, potassium, sodium, iron, manganese, zinc, aluminum and copper, which increase in concentration if the harvesting time is delayed or the plant is treated with aluminum solution (Kim et al., 2005, Liu et al., 2020).
Biological properties: C. japonica as an underexplored medicinal plant
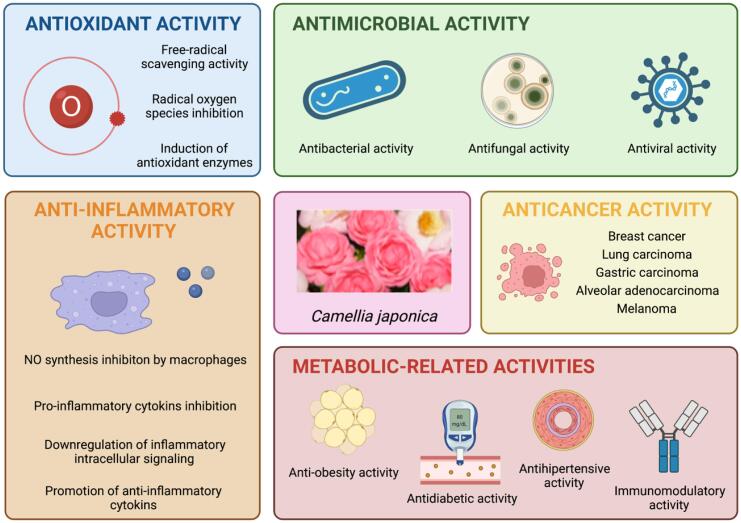
Although the study of its composition is to some extent limited, camellias have been traditionally used in the medicine and cosmetics of Eastern countries in a regular basis, motivated by the biologically active properties of the compounds present in this genus and their organoleptic characteristics. The most outstanding bioactivities associated with such compounds (Fig. 3) are antioxidant, antimicrobial, anti-inflammatory, anticancer, anti-obesity, antidiabetic, and antihypertensive, among others (Estevinho, Feás, Salinero, Mansilla, Vela, Saínz, Vázquez-Tato, & Seijas, 2012). Table 3 includes an overview of the most relevant mechanisms of action associated with the different bioactivities attributed to C. japonica extracts. Altogether, these bioactivities contribute to the consideration of this species as a medicinal plant, showing a promising potential for C. japonica facing its exploitation with industrial purposes, especially those related with the food, pharmaceutical and cosmetical sectors.
Fig. 3.
Bioactivities associated with C. japonica extracts.
Table 3.
Mechanisms of action attributed to the bioactivities associated with C. japonica extracts
| Bioactivities | Mechanisms of action | References |
|---|---|---|
| Antioxidant activity | Free-radical scavenging activity | (Kanth et al., 2014, Lee et al., 2011, Moon and Kim, 2018, Piao et al., 2011, Zhang et al., 2011) |
| Metal chelation | (Moon & Kim, 2018) | |
| Reducing power | (Moon & Kim, 2018) | |
| ROS quenching | (Moon and Kim, 2018, Piao et al., 2011) | |
| Induction of antioxidant enzymes: SOD, CAT, GPx | (Piao et al., 2011) | |
| Antimicrobial activity | Bacteriostatic activity against a wide range of foodborne bacteria and pathogens: Enterobacter spp., S. epidermidis, B. subtilis, K. pneumoniae, E. coli, S. aureus, P. aeruginosa, S. Typhimurium, L. monocytogenes | (Kharchenko, 2019, Kim et al., 2001, Lee et al., 2005, Moon and Kim, 2018) |
| Antiviral activity against porcine epidemic diarrhea virus and HIV-1 | (Park et al., 2002, Yang et al., 2015) | |
| Antifungal activity via inhibition of conidia germination | (Nagata et al., 1985) | |
| Anti-inflammatory activity | Reduction of oxidative stress | (Akanda & Park, 2017, Lee et al., 2016) |
| Reduction of LPS-induced NO production by RAW 264.7 macrophages | (Akanda & Park, 2017, Kim et al., 2012) | |
| Reduction of pro-inflammatory cytokines levels: PGE2, TNF-α, IL-1β, IκBα | (Kim et al., 2012, Nam et al., 2021) | |
| Reduction of pro-inflammatory enzymes: iNOS, COX-2 | (Kim et al., 2012, Nam et al., 2021) | |
| Anticancer activity | Cytotoxicity assessed against different in vitro-cultured cancer cell lines: B16 melanoma 4A5 cells, MCF-7 human breast adenocarcinoma cells, Calu-6 human lung adenocarcinoma cells, SNU-601 gastric adenocarcinoma cells, LLC1 Lewis lung carcinoma cells, HL-60 human promyelocytic leukemia cells | (Hwang et al., 2006; Kim et al., 2010; Nakamura et al., 2012, Thao et al., 2010) |
| Antidiabetic activity | Decrease of glucose blood levels | (Chereddy et al., 2011) |
| Inhibition of advanced glycation end-products synthesis | (Sato et al., 2017) | |
| Anti-obesity activity | Cholesterol-lowering effects: decrease of serum TC, TAG and LDL-C levels and increase of serum HDL-C levels | (Lee et al., 2016) |
| Decrease of body weight and regulation of hepatic lipid profiles | (Ochiai et al., 2018) | |
| Increase of fecal fat excretion | (Ochiai et al., 2018) |
Abbreviations: CAT, catalase; COX-2, cyclooxygenase; GPx, glutathione peroxidase; HDL-C: high density lipoproteins-cholesterol; HIV-1, human immunodeficiency virus 1; IL-1β, interleukin 1β; iNOS, inducible nitric oxide synthase; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha; LDL-C, low density lipoproteins-cholesterol LPS, lipopolysaccharide; NO, nitric oxide; PGE2, prostaglandin E2; SOD, superoxide dismutase; TAG, triglycerides; TC, total cholesterol; TNF-α: tumor necrosis factor alpha.
Antioxidant activity
Camellia species are unanimously considered a natural source of antioxidant compounds, whose content depends on several factors like cultivar and environmental conditions. This antioxidant activity may be due to the high concentrations of phenolic compounds found in their constitutive tissues, as reported by the determination of total phenolic content, reaching values up to 21.75 mg/g (Jeong et al., 2010). Concerning the mechanisms of action, the antioxidant activity of Camellia spp. has been reported by free-radical scavenging activity. Accordingly, young C. japonica leaves exhibited higher antioxidant activities than mature leaves in terms of radical oxygen species scavenging, especially hydrogen peroxide and hydroxyl radicals (Mizutani & Masaki, 2014). Regarding the influence of solvents on the extraction of antioxidant compounds from leaves, methanolic and acetonic extracts caused the highest rates of antioxidant activity, according to 2,2-diphenyl-1-picryl-hydrazyl free-radical scavenging method (DPPH), with inhibitory concentration 50 (IC50) values ranging from 246.56 μg/mL for methanolic extracts to 320.17 μg/mL for acetonic extracts, whereas the IC50 values obtained by the β-carotene-linoleic acid test were 258.19 μg/mL and 396.88 μg/mL, respectively (Wang, 2012).
Besides leaves, flowers from C. japonica present antioxidant properties, as well, as their extracts exhibited DPPH and reactive oxygen species (ROS) scavenging activity in human HaCaT keratinocytes (immortalized human keratinocytes), and increased the expression of cellular antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase (Piao et al., 2011). High concentrations of antioxidants prevent the damage caused by free radicals which is associated with the development of cancer. At this respect, the petals of several varieties of C. japonica had a maximum IC50 (evaluated with DPPH) value of 3.8 μg/mL in pink cultivars and 43.1 μg/mL in white cultivars, showing a direct correlation between antioxidant activity and phenolic content (Kanth et al., 2014). In the same way, C. japonica oil presents an antioxidant power comparable to gallic acid and α-tocopherol (Choi, Min, Oh, & Shin, 2013). All these antioxidant properties have prompted the use of camellias with cosmetical purposes due to their anti-aging and anti-pollution properties. However, the heterogeneity of phenomena associated with antioxidant activity should cover the development of different methodologies, in order to fully depict the total antioxidant capacity of camellia extracts, including in vivo models.
Antimicrobial activity
Extracts with antimicrobial properties from camellias can be obtained from leaves and flowers, being ethanol one of the best solvents that promote high rates of antimicrobial activity, in terms of antibacterial, antifungal and antiviral activity (Moon & Kim, 2018). A methanolic extract from petals of C. japonica has its best antimicrobial results in the acidic fraction, with an inhibitory zone of 14–19 mm diameter in a disk test against the pathogens Salmonella Typhimurium DT104, Escherichia coli O157:H7, Listeria monocytogenes and Staphylococcus aureus. This bioactivity is suggested to be due to the presence of fumaric acid. As a result of this associated activity, these extracts were applied with satisfactory results in milk fortification, promoting an increase in shelf life (Kim, Davidson, & Chung, 2001).
In parallel, the methanolic extracts of young leaves and stems from C. japonica have been evaluated in terms of antimicrobial activity, showing a high efficiency against S. aureus (Choi et al., 2013). Moreover, the ethanolic extract of fermented leaves of C. japonica showed great antimicrobial properties against Staphylococcus epidermidis, Bacillus subtilis, Klebsiella pneumonia, and Escherichia coli (Moon & Kim, 2018). These antimicrobial properties are common for different varieties of C. japonica, such as Kramer's Supreme, C.M. Wilson, La Pace, Mrs. Lyman Clarke, Benikarako, and Fanny Bolis. Among them, the extracts from the variety Mrs. Lyman Clarke caused the highest antimicrobial activity against Enterobacter cloacae (Kharchenko, 2019). Therefore, according to these previous results, it is suggested that C. japonica extracts show a greater effectiveness against Gram-positive bacteria, with only a slight activity reported for Gram-negative bacteria.
Furthermore, Sharma et al. recently reported that the antimicrobial activity of C. japonica leaf extracts can be improved through the use of gold nanoparticles, showing an increased antimicrobial activity against different microbial strains, such as B. subtilis, S. aureus, Streptococcus faecalis, K. pneumoniae, Pseudomonas aeruginosa, E. coli and the fungus Candida albicans (Sharma, Selvakumar, Hwa, Sami, & Kumaresan, 2019). Additionally, the antiviral activity of C. japonica was assessed in flower extracts against the porcine epidemic diarrhea virus (PEDV) due to the presence of oleanane triterpenes (Yang et al., 2015), as well as the acetonic leaf extracts were proven effective against human immunodeficiency virus (HIV), mostly due to camelliatannin H (Park et al., 2002). On these bases, a detailed study of such extracts is further needed to identify the secondary metabolites responsible for this activity and thus determining the potential of C. japonica as a source of natural antibiotics.
Anti-inflammatory activity
C. japonica oil has been proved to have an excellent anti-inflammatory activity, as reported by different methods. Regarding its mechanism of action, it was found that C. japonica oil inhibits lipopolysaccharide (LPS)-induced production of nitric oxide (NO) and reduced the levels of prostaglandin E (PGE), and tumor necrosis factor-α (TNF-α) in RAW264 macrophages, together with a decrease in the expression of pro-inflammatory enzymes, such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), activator protein 1 (AP-1), nuclear factor-kappa B (NF-κB), and a downregulation of the signaling pathways extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38) and c-Jun N-terminal kinase (JNK) (Kim et al., 2012).
Besides C. japonica oil, other derived extracts can also be used in the treatment of esophageal inflammation, as proved for the ethanolic extracts from C. japonica shoots via the reduction of ROS, NO, TNF-α, NF-κB, and interleukin-1β (IL-1β), among other pro-inflammatory signaling pathways. As a result, such extract reduced the rate of esophageal tissue damage by attenuating different histological changes (Nam, Nan, & Choo, 2021). This evidence provides a scientific insight on the promising attributes of C. japonica as a biological producer of anti-inflammatory agents, that should be assigned to individual compounds in further studies.
Anticancer activity
Leaf and flower extracts of C. japonica have been tested against different cancer cell lines, including MCF-7 (human breast adenocarcinoma pleural effusion), Calu-6 (human lung carcinoma) and SNU-601 (human gastric carcinoma) cells, with leaf extracts promoting a strong growth inhibitory concentration below 100 µg/mL (Hwang, Cha, Park, Lee, & Lee, 2006). Other cancer cell lines sensitive to C. japonica include A549 (adenocarcinoma human alveolar basal epithelial cells), LLC (Lewis lung carcinoma) and HL-60 (human promyelocytic leukemia cells), mostly due to the combination of different triterpenoid compounds (Thao et al., 2010). According to chromatographic studies, the triterpenoids found on C. japonica extracts are suggested to promote an intense antitumor activity. Flower extracts showed a high effectiveness against melanoma B16 (mouse melanoma) cell line, due to the presence of molecules such as camellioside (Nakamura et al., 2012) and triterpenic oligoglycosides (Fujimoto et al., 2012). Therefore, the development of extracts from camellia flowers could represent novel therapeutical targets against cancer, although there is an urgent need to perform the pertinent in vivo and interventional studies to satisfactorily assess the effectiveness of C. japonica extracts on human patients. Indeed, the reported antioxidant and anti-inflammatory activity of such extracts may assist on the development of anticancer activity of C. japonica since both processes are closely related to the onset of carcinogenesis (García-Pérez, Barreal, Rojo-De Dios, Cameselle-Teijeiro, & Gallego, 2019).
Anti-obesity activity
Besides inflammatory and cancerous diseases, the extracts of C. japonica have been widely evaluated in terms of the prevention of other chronic diseases, as it is the case of obesity. In this regard, seed extracts of C. japonica can be used to treat obesity, as suggested by the inhibitory activity on pancreatic lipase and accumulation of body fat promoted by saponins. In parallel, the same in vivo study carried out with mice fed a high-fat diet supplemented with seed extracts increased the fecal fat excretion, reduced the body weight and improved both the plasmatic and hepatic lipid profile (Ochiai, Nozaki, Kato, & Ishihara, 2018).
In the same way, fruit extracts were subjected to a murine in vivo model to assess their cholesterol-lowering properties, reflecting a reduction in serum total cholesterol, triglycerides, and low-density lipoproteins, accompanied by an increase in serum high-density lipoproteins (Lee et al., 2016). Consequently, C. japonica can be considered as a natural remedy to avoid hypercholesterolemia, as referred by different patents. In one of them, flower aqueous extracts were able to suppress CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPAR γ) expression, proving their anti-obesity properties (Hee et al., 2010). Overall, these observations suggest that the incorporation of C. japonica-derived products forms a promising solution to counter the physiological implications associated with the high prevalence of obesity worldwide.
Antidiabetic activity
As seen for obesity, other currently prevalent diseases have been addressed, from a preventive point of view, using C. japonica extracts. Thus, camellias can be used in the prevention and treatment of diabetes mellitus, as reported by different studies (Salehi et al., 2019). Thus, the methanolic leaf extract of C. japonica reduced the blood glucose levels of alloxane-induced diabetic rats after one only oral administration (Chereddy, Somasundaram, & Kandalam, 2011). This bioactivity has been suggested to be due to the presence of polyphenols, catechins and polysaccharides in the above-mentioned extracts, as observed in several interventional studies carried out with humans (Fu et al., 2017).
In the same way, C. japonica tea was shown to exert antidiabetic effects because of the high levels of theaflavins (Fu et al., 2017). In fact, different studies showed that regular tea consumption increases insulin activity up to 15 times in vitro in an epididymal fat cell assay. Chromatographic studies revealed that this property was due to the presence of epigallocatechin gallate, tannins, theaflavins and other undefined compounds (Anderson & Polansky, 2002). Nevertheless, despite these studies, further investigations should be focused on the effectiveness of C. japonica extracts as antidiabetic agents by the development of interventional studies.
Antihypertensive activity
Several authors valued the effectiveness of different parts of the camellia to reduce ACE angiotensin converting enzyme (ACE) activity. According to an optimization study, the extracts with the highest ACE activity are obtained from C. japonica seeds subjected to hydrolytic extraction at 50.98 °C and pH of 7.12, promoting a reduction in the diastolic blood pressure (Lim et al., 2017). Metabolomic studies have shown that this activity may be due to epigallocatechin 3-O-(3-O-methyl)gallate, which is characterized by having a high absorption rate and great stability in the blood (Kurita, Maeda-Yamamoto, Tachibana, & Kamei, 2010). As it was shown for the antidiabetic properties of C. japonica, specific studies are required in order to assess its antihypertensive and cardioprotective effects, through both in vivo and clinical studies.
Other biological activities
Some bioactivities from C. japonica have not been characterized and studied properly, to date. Many of these activities are closely related to the antioxidant capacity of the extracts obtained from this plant. For instance, C. japonica seeds can be used in the prevention and treatment of tooth decay, due to the presence of polyphenols, which have shown great potential in the prevention of oral diseases also motivated by their antimicrobial properties (Ferrazzano et al., 2011). The stems and fruits-derived extracts of C. japonica show promising wound healing effects, improving the generation of induced pluripotent stem cells (iPSC), as recorded on in vivo mouse wound models (Jeon et al., 2018). The gastroprotective effect of methanolic flower extracts from C. japonica exhibit potent inhibitory activities on ethanol-induced gastric mucosa lesions in rats, driven by the presence of triterpenic oligoglycosides and camelliosides A, B, C and D (Yoshikawa et al., 2007). Finally, the hypouricemic properties of C. japonica leaf extracts were reported through the inhibition of xanthine oxidase activity (Yoon et al., 2017).
Prospects on the industrialization of camellias
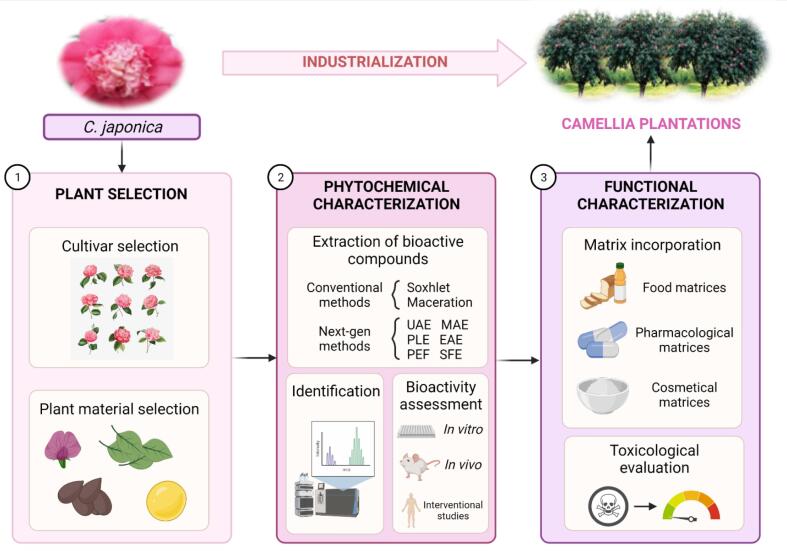
Up to date, only a limited number of C. japonica varieties have been used with food purposes, instead of other Camellia species in the food industry that have been widely exploited to that aim. Some examples include Camellia oleifera, which is used to produce edible and essential oils, and the leaves of Camellia sinensis for the production of different varieties of tea (Garcia-Jares et al., 2017). With respect to C. japonica, the seeds have been exploited for the production of the essential “tsubaki oil”, traditionally used in Eastern regions as an ingredient in cosmetic formulations for skin and hair protection and nutrition (Lim, 2014, Tanaka, 1976), as a result of its anti-inflammatory (Kim et al., 2012), anti-ageing and skin barrier stabilizer properties (Jung et al., 2007, Narayanaswamy and Ismail, 2015). In addition, C. japonica leaves show a potential application in tea production (Mizutani & Masaki, 2014), or as an edible vegetable in the food industry (Kunkel, 1984, Tanaka, 1976), together with the flowers (Way et al., 2009). Fig. 4 shows a proposed workflow about the large-scale production of C. japonica facing its industrialization with food, pharmaceutical and cosmetical purposes.
Fig. 4.
Proposed workflow for Camellia japonica industrialization.
The exploitation of C. japonica will depend on the major purpose of application, thus deciding the choice of a convenient plant part and an efficient extraction procedure. The part of the plant used, according to its chemical composition, will be decisive to establish the collection and storage conditions of the raw material. Thus, concerning suppliers' quality standards, the bark is essentially stored under controlled conditions in terms of air and humidity, whereas leaves are air-dried, and flowers are normally lyophilized and ground state (Zhao et al., 2019). Moreover, the application of the raw material will depend on the extraction and purification technique of the bioactive compounds, aiming at developing an efficient procedure to ensure their production, without interfering with their stability and function (Sasidharan et al., 2011, Zhao et al., 2019).
Once collected and pre-treated, plant materials are subjected to extraction. Among different extraction methods, conventional procedures have been classically performed in the case of camellias, for instance Soxhlet extraction, maceration, and distillation. Nevertheless, due to the low efficiency and sustainability of these techniques (Azmir et al., 2013), multiple novel methods have been established in recent decades e.g.: ultrasound-assisted extraction, enzyme-assisted extraction, microwave-assisted extraction, pulsed electric field-assisted extraction, supercritical fluid extraction or pressurized liquid extraction, etc. (Pereira et al., 2020). After bioactive compounds extraction, plant extracts are often subjected to the determination of their bioactivities through different in vitro, in vivo, and interventional studies (Fig. 3). Finally, once the bioactivity has been assessed, extracts are incorporated into different matrices ranging from food and cosmetic products to pharmaceutical preparations and further analyzed in terms of toxicology and risk assessment to ensure their efficacy and safety for human consumption.
Furthermore, the advanced stages of the industrialization process concern the cultivation of C. japonica, which requires the opening of new scenarios facing their large-scale production, based on the establishment of plantations. To boost camellia production on emerging areas, as it is the case of Galicia, the edaphoclimatic conditions of proposed locations should meet the optimal criteria to ensure a prominent growth of camellias, usually characterized by shady, humid climates with mild temperatures, acidic fertile soils, with preference to wind absence. In addition to geographical requirements, seasonality is another relevant factor facing the success of plantations, as sowing should avoid growing season (spring) and being preferentially performed in the shallow of convenient soils (Corral, Gesto, & Vázquez, 2000). In brief, a multifaceted approach from laboratory scale to open-field cultivation is required with the aim of promoting an efficient industrialization of C. japonica, contributing to its large-scale exploitation as a medicinal plant in different sectors, such as the food, cosmetic and pharmaceutical industries.
Conclusions
Although C. japonica is used as an ornamental plant, it is a source of bioactive compounds, including phenolic compounds, terpenoids, fatty acids and other minor compounds. The numerous studies compiled in this review confirm that the extracts obtained from different parts of this plant possess multiple associated bioactivities that play a beneficial effect on human health, acting as antioxidants, antimicrobial, anti-inflammatory, anticancer, and anti-aging agents, that would justify their exploitation at an industrial level as a medicinal plant. Therefore, more studies are essential to reveal the phytochemical composition and the mechanisms of action of camellia bioactive compounds. This information is key to the future development of different camellia-based preparations with commercial purposes, including drug design, food fortification, additives production, etc. Furthermore, the growing interest of the global population in consuming healthier foods with fewer artificial additives means that the development of this type of product has a potential good reception in the market, and the establishment of camellia plantations becomes a much-needed strategy to meet industrialization requirements, based on previous research conducted at a laboratory scale.
Funding
Funding for open access charge: Universidade de Vigo/CISUG. The research leading to these results was funded by Xunta de Galicia supporting the program EXCELENCIA-ED431F 2020/12; to Ibero-American Program on Science and Technology (CYTED—AQUA-CIBUS, P317RT0003) and to the Bio Based Industries Joint Undertaking (JU) under grant agreement No 888003 UP4HEALTH Project (H2020-BBI-JTI-2019). The JU receives support from the European Union’s Horizon 2020 research and innovation program and the Bio Based Industries Consortium.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research leading to these results was supported by MICINN supporting the Ramón y Cajal grant for M.A. Prieto (RYC-2017-22891) and and the Juan de la Cierva Incorporación Hui Cao (IJC2020-046055-I); by Xunta de Galicia for supporting the pre-doctoral grant of A.G. Pereira (ED481A-2019/0228); by European Union that supports the work of P. Garcia-Perez through the “Margarita Salas” grant from the “NextGenerationEU” program.
Contributor Information
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
Miguel A. Prieto, Email: mprieto@uvigo.es.
References
- Abe H., Miura H., Motonaga Y. Quantitative classification of Camellia japonica and Camellia rusticana (Theaceae) based on leaf and flower morphology. Plant Diversity. 2021;43(3):216–224. doi: 10.1016/j.pld.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanda M.R., Park B.-Y. Involvement of MAPK/NF-κB signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomedicine & Pharmacotherapy. 2017;95:1139–1146. doi: 10.1016/j.biopha.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Anderson R.A., Polansky M.M. Tea enhances insulin activity. Journal of Agricultural and Food Chemistry. 2002;50(24):7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F.…Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering. 2013;117(4):426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Chakraborty, B. N., Chakraborty, U., & Saha, A. (2018). Defense Strategies of Tea (Camellia sinensis) Against Fungal Pathogens. In Handbook of Phytoalexin Metabolism and Action (pp. 485–501). Routledge. 10.1201/9780203752647-21.
- Chereddy K.K., Somasundaram A., Kandalam S.K. Antidiabetic activity of methanolic extract of Camellia japonica leaves against alloxan induced diabetes in rats. International Jouurnal of Pharmacy and Biological Science. 2011;1(2) [Google Scholar]
- Cho H.M., Ha T.K.Q., Doan T.P., Dhodary B., An J.P., Lee B.W.…Oh W.K. Neuroprotective effects of triterpenoids from Camellia japonica against amyloid β-induced neuronal damage. Journal of Natural Products. 2020;83(7):2076–2086. doi: 10.1021/acs.jnatprod.9b00964. [DOI] [PubMed] [Google Scholar]
- Choi M.-H., Min M.-J., Oh D.-S., Shin H.-J. Antimicrobial and antioxidant activity of Camellia japonica extracts for cosmetic applications. KSBB Journal. 2013;28(2):99–105. doi: 10.7841/ksbbj.2013.28.2.99. [DOI] [Google Scholar]
- Corral C.S., Gesto M.J.L., Vázquez J.P.M. Manual para el cultivo de Camelia. Asociación Profesional Xóvenes. 2000 Agricultores. [Google Scholar]
- Cui C., Zong J., Sun Y., Zhang L., Ho C.T., Wan X., Hou R. Triterpenoid saponins from the genus Camellia: Structures, biological activities, and molecular simulation for structure-activity relationship. Food and Function. Royal Society of Chemistry. 2018 doi: 10.1039/c8fo00755a. [DOI] [PubMed] [Google Scholar]
- Durrant, T. (1982). The Camellia Story.
- Deputación de Pontevedra . Variedades y cultivo; Filatelia: 1980. La camelia. [Google Scholar]
- Endo T. Separation of anthocyanin and leucoanthocyanin in flowers of Camellia japonica. Nature. 1958;182(4638):801. doi: 10.1038/182801a0. [DOI] [PubMed] [Google Scholar]
- Estevinho, L. M., Feás, X., Salinero, C., Mansilla, J. P., Vela, P., Saínz, M. J., Vázquez-Tato, M. P., & Seijas, J. A. (2012). Antimicrobial properties of Camellia oleifera oil. In 16 ECSOC (pp. 4–6).
- Fang X., Fei X., Sun H., Jin Y. Aqueous enzymatic extraction and demulsification of camellia seed oil (Camellia oleifera Abel.) and the oil’s physicochemical properties. European Journal of Lipid Science and Technology. 2016;118(2):244–251. doi: 10.1002/ejlt.201400582. [DOI] [Google Scholar]
- Ferrazzano G.F., Amato I., Ingenito A., Zarrelli A., Pinto G., Pollio A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules. 2011;16(2):1486–1507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Q. Y., Li, Q. S., Lin, X. M., Qiao, R. Y., Yang, R., Li, X. M., Dong, Z.-B., Xiang, L.P., Zheng, X.-Q., Lu, J.-L., Yuan, C.-B., Ye, J.-H., & Liang, Y. R. Antidiabetic effects of tea. Molecules. MDPI AG. 10.3390/molecules22050849. [DOI] [PMC free article] [PubMed]
- Fujimoto K., Nakamura S., Nakashima S., Matsumoto T., Uno K., Ohta T.…Yoshikawa M. Medicinal flowers. XXXV. Nor-oleanane-type and acylated oleanane-type triterpene saponins from the flower buds of Chinese Camellia japonica and their inhibitory effects on melanogenesis. Chemical and Pharmaceutical Bulletin. 2012;60(9):1188–1194. doi: 10.1248/cpb.c12-00473. [DOI] [PubMed] [Google Scholar]
- Garcia-Jares C., Sanchez-Nande M., Lamas J.P., Lores M. Profiling the fatty acids content of ornamental camellia seeds cultivated in Galicia by an optimized matrix solid-phase dispersion extraction. Bioengineering. 2017;4(4) doi: 10.3390/bioengineering4040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez P., Barreal M.E., Rojo-De Dios L., Cameselle-Teijeiro J.F., Gallego P.P. In: Rahman A., editor. Vol. 61. Elsevier; Amsterdam (Netherlands): 2019. Bioactive natural products from the genus Kalanchoe as cancer chemopreventive agents: A review; pp. 49–84. (Studies in Natural Products Chemistry). [Google Scholar]
- García-Pérez P., Lozano-Milo E., Landin M., Gallego P.P. From ethnomedicine to plant biotechnology and machine learning: The valorization of the medicinal plant Bryophyllum sp. Pharmaceuticals. 2020 doi: 10.3390/ph13120444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez, P., Miras-Moreno, B., Lucini, L., & Gallego, P. P. (2021). The metabolomics reveals intraspecies variability of bioactive compounds in elicited suspension cell cultures of three Bryophyllum species. Industrial Crops and Products, 163(November 2020), 113322. 10.1016/j.indcrop.2021.113322.
- Gogoi A.S., Borua P.K. Profiling of total polyphenols and pigments in tea (Camellia sinensis (l.) O. Kuntze) in various seasons for manufacturing black tea and green tea. International Journal of Food and Nutritional Sciences. 2017;6(2):56. [Google Scholar]
- Guo N., Tong T., Ren N., Tu Y., Li B. Saponins from seeds of Genus Camellia: Phytochemistry and bioactivity. Phytochemistry. 2018;149:42–55. doi: 10.1016/j.phytochem.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Han, L., Hatano, T., Yoshida, T., & Okuda, T. (1994). Tannins of Theaceous Plants. V.1) Camelliatannins F, G and H, Three New Tannins from Camellia japonica L. Chemical and Pharmaceutical Bulletin, 42(7), 1399–1409. 10.1248/cpb.42.1399. [DOI] [PubMed]
- Hatano T., Han L., Taniguchi S., Okuda T., Yoshida T., Kiso Y., Tanaka T. Camelliatannin D, A new inhibitor of bone resorption, from Camellia japonica. Chemical and Pharmaceutical Bulletin. 1995;43(11):2033–2035. doi: 10.1248/cpb.43.2033. [DOI] [PubMed] [Google Scholar]
- Hatano T., Shida S., Han L., Okuda T. Tannins of Theaceous Plants. III. Camelliatannins A and B, Two New Complex Tannins from Camellia japonica L. Chemical and Pharmaceutical Bulletin. 1991;39(4):876–880. doi: 10.1248/cpb.39.876. [DOI] [PubMed] [Google Scholar]
- Hee, J. K., Hye, Y. E., Hyang, W. J., Ok, J. K., & Mi, S. H. (2010). Composition for anti-obesity and antioxidant comprising Camellia japonica flower extract as active ingredient. korea. Retrieved from https://www.surechembl.org/document/KR-20110127443-A.
- Hwang E.J., Cha Y.J., Park M.H., Lee J.W., Lee S.Y. Cytotoxicity and Chemosensitizing Effect of Camellia (Camellia japonica) Tea Extracts. Journal of The Korean Society of Food Science and Nutrition. 2006;33(3):487–493. [Google Scholar]
- Hwang Y.J., Yoshikawa K., Miyajima I., Okubo H. Flower colors and pigments in hybrids with Camellia chrysantha. Scientia Horticulturae. 1992;51(3–4):251–259. doi: 10.1016/0304-4238(92)90123-T. [DOI] [Google Scholar]
- Integrated Taxonomic Information System (ITIS) (2020). Retrieved November 11, 2020, from https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=506117#null.
- Jeganathan, B., Punyasiri, P. A. N., Kottawa-Arachchi, J. D., Ranatunga, M. A. B., Abeysinghe, I. S. B., Gunasekare, M. T. K., & Bandara, B. M. R. (2016). Genetic variation of flavonols quercetin, myricetin, and kaempferol in the Sri Lankan tea (Camellia sinensis L.) and their health-promoting aspects. International Journal of Food Science, 2016. 10.1155/2016/6057434. [DOI] [PMC free article] [PubMed]
- Jeon H., Kim J., Choi J., Han E., Song C.-L., Lee J., Cho Y. Effects of the Extracts from Fruit and Stem of Camellia japonica on Induced Pluripotency and Wound Healing. Journal of Clinical Medicine. 2018;7(11):449. doi: 10.3390/jcm7110449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C.H., Kim J.H., Choi G.N., Kwak J.H., Kim D.O., Heo H.J. Protective effects of extract with phenolics from camellia (Camellia japonica) leaf against oxidative stress-induced neurotoxicity. Food Science and Biotechnology. 2010;19(5):1347–1353. doi: 10.1007/s10068-010-0192-x. [DOI] [Google Scholar]
- Jiyin G., Parks C.R., Yueqiang D. Zhejiang Scientific & Technology; China: 2005. Collected species of the genus Camellia, an illustrated outline. [Google Scholar]
- Jung E., Lee J., Baek J., Jung K., Lee J., Huh S.…Park D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. Journal of Ethnopharmacology. 2007;112(1):127–131. doi: 10.1016/j.jep.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Kanth B.K., Lee K.Y., Lee G.J. Antioxidant and radical-scavenging activities of petal extracts of Camellia japonica ecotypes. Horticulture Environment and Biotechnology. 2014;55(4):335–341. doi: 10.1007/s13580-014-0024-7. [DOI] [Google Scholar]
- Kharchenko I. Evaluation of the In Vitro Antimicrobial Activity of Ethanolic Extracts Derived from Leaves Of Camellia japonica Cultivars (Theaceae) Against Enterobacter Cloacae Strain. Agrobiodiversity for Improving Nutrition, Health and Life Quality. 2019;333–347 doi: 10.15414/agrobiodiversity.2019.2585-8246.333-347. [DOI] [Google Scholar]
- Kim B.-S., Choi O.-J., Shim K.-H. Properties of Chemical Components of Camellia japonica L. loaves According to Picking Time. Journal of the Korean Society of Food Science and Nutrition. 2005;34(5):681–686. doi: 10.3746/jkfn.2005.34.5.681. [DOI] [Google Scholar]
- Kim E.A., Kim S.Y., Ye B.R., Kim J., Ko S.C., Lee W.W.…Heo S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. International Immunopharmacology. 2018;59:339–346. doi: 10.1016/j.intimp.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Kim J.-H., Jeong C.-H., Shim K.-H. Antioxidative and Anticancer Activities of Various Solvent Fractions from the Leaf of Camellia japonica L. The Korean Society of Food Preservation. 2010;17:267–274. [Google Scholar]
- Kim K.Y., Davidson P.M., Chung H.J. Antibacterial activity in extracts of Camellia japonica L. petals and its application to a model food system. Journal of Food Protection. 2001;64(8):1255–1260. doi: 10.4315/0362-028X-64.8.1255. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Cho C.H., Yang M., Kim S.C. The complete chloroplast genome sequence of the Japanese Camellia (Camellia japonica L.). Mitochondrial DNA Part B. Resources. 2017;2(2):583–584. doi: 10.1080/23802359.2017.1372719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jung E., Shin S., Kim M., Kim Y.S., Lee J., Park D. Anti-inflammatory activity of Camellia japonica oil. BMB Reports. 2012;45(3):177–182. doi: 10.5483/BMBRep.2012.45.3.177. [DOI] [PubMed] [Google Scholar]
- Ko J., Rho T., Yoon K.D. Kaempferol tri- and tetrasaccharides from Camellia japonica seed cake and their inhibitory activities against matrix metalloproteinase-1 secretion using human dermal fibroblasts. Carbohydrate Research. 2020;495 doi: 10.1016/j.carres.2020.108101. [DOI] [PubMed] [Google Scholar]
- Kunkel G. Koeltz Scientific Books; 1984. Plants for human consumption : An annotated checklist of the edible phanerogams and ferns. [Google Scholar]
- Kurita I., Maeda-Yamamoto M., Tachibana H., Kamei M. Antihypertensive effect of benifuuki tea containing O-methylated EGCG. Journal of Agricultural and Food Chemistry. 2010;58(3):1903–1908. doi: 10.1021/jf904335g. [DOI] [PubMed] [Google Scholar]
- Lee H.H., Cho J.Y., Moon J.H., Park K.H. Isolation and identification of antioxidative phenolic acids and flavonoid glycosides from Camellia japonica flowers. Horticulture Environment and Biotechnology. 2011;52(3):270–277. doi: 10.1007/s13580-011-0157-x. [DOI] [Google Scholar]
- Lee H.H., Paudel K.R., Jeong J., Wi A.J., Park W.S., Kim D.W., Oak M.H. Antiatherogenic effect of Camellia japonica fruit extract in high fat diet-fed rats. Evidence-Based Complementary and Alternative Medicine. 2016;2016 doi: 10.1155/2016/9679867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-Y., Hwang E.-J., Kim G.-H., Choi Y.-B., Lim C.-Y., Kim S.-M. Antifungal and antioxidant activities of extracts from leaves and flowers of Camellia japonica L. The Korean Journal of Medicinal Crop Science. 2005;13(3) [Google Scholar]
- Li J.B., Hashimoto F., Shimizu K., Sakata Y. A new acylated anthocyanin from the red flowers of Camellia hongkongensis and characterization of anthocyanins in the section Camellia species. Journal of Integrative Plant Biology. 2009;51(6):545–552. doi: 10.1111/j.1744-7909.2009.00828.x. [DOI] [PubMed] [Google Scholar]
- Li X.L., Wang J., Yin H.F., Fan Z.Q., Li J.Y. Variation of flower colors and their relationships with anthocyanins in cultivars of Camellia japonica. Journal of Ecology and Rural Environment. 2019;35(10):1307–1313. doi: 10.19741/j.issn.1673-4831.2019.0043. [DOI] [Google Scholar]
- Lim H.J., Kim M.S., Kim D.S., Kim Y.J., Lee J.H., Pan J.H.…Kim J.K. Blood pressure-lowering effects of alacalase-hydrolyzed camellia seed hull in vitro and in spontaneous hypertensive rats. Journal of Medicinal Food. 2017;20(7):720–723. doi: 10.1089/jmf.2016.0175. [DOI] [PubMed] [Google Scholar]
- Lim, T. K. (2014). Camellia japonica. In Edible Medicinal and Non Medicinal Plants (Vol. 8, pp. 764–776). doi: 10.1007/978-94-017-8748-2.
- Liu Y., Tao J., Cao J., Zeng Y., Li X., Ma J.…Sun L. The beneficial effects of aluminum on the plant growth in Camellia japonica. Journal of Soil Science and Plant Nutrition. 2020;20(4):1799–1809. doi: 10.1007/s42729-020-00251-9. [DOI] [Google Scholar]
- Majumder S., Ghosh A., Bhattacharya M. Natural anti-inflammatory terpenoids in Camellia japonica leaf and probable biosynthesis pathways of the metabolome. Bulletin of the National Research Centre. 2020;44(1):1–14. doi: 10.1186/s42269-020-00397-7. [DOI] [Google Scholar]
- Meng X.H., Li N., Zhu H.T., Wang D., Yang C.R., Zhang Y.J. Plant resources, chemical constituents, and bioactivities of tea plants from the genus camellia section thea. Journal of Agricultural and Food Chemistry American Chemical Society. 2019 doi: 10.1021/acs.jafc.8b05037. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Masaki H. Anti-photoaging capability of antioxidant extract from Camellia japonica leaf. Experimental Dermatology. 2014;23:23–26. doi: 10.1111/exd.12395. [DOI] [PubMed] [Google Scholar]
- Moon S.H., Kim M.Y. Phytochemical profile, antioxidant, antimicrobial and antipancreatic lipase activities of fermented Camellia japonica L. leaf extracts. Tropical Journal of Pharmaceutical Research. 2018;17(5):905–912. doi: 10.4314/tjpr.v17i5.22. [DOI] [Google Scholar]
- Nagata T., Tsushida T., Hamaya E., Enoki N., Manabe S., Nishino C. Camellidins, Antifungal Saponins Isolated from Camellia japonica. Agricultural and Biological Chemistry. 1985;49:1181–1186. doi: 10.1080/00021369.1985.10866833. [DOI] [Google Scholar]
- Nakajima H., Itokawa H., Ikuta A. Studies on the constituents of the flower of Camellia japonica. Yakugaku Zasshi. 1984;104:157–161. doi: 10.1248/yakushi1947.104.2_157. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Moriura T., Park S., Fujimoto K., Matsumoto T., Ohta T.…Yoshikawa M. Melanogenesis inhibitory and fibroblast proliferation accelerating effects of noroleanane- and oleanane-type triterpene oligoglycosides from the flower buds of Camellia japonica. Journal of Natural Products. 2012;75(8):1425–1430. doi: 10.1021/np3001078. [DOI] [PubMed] [Google Scholar]
- Nam H.H., Nan L., Choo B.K. Inhibitory effects of Camellia japonica on cell inflammation and acute rat reflux esophagitis. Chinese Medicine (United Kingdom) 2021;16(1) doi: 10.1186/s13020-020-00411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanaswamy R., Ismail I.S. Cosmetic potential of Southeast Asian herbs: An overview. Phytochemistry Reviews Springer. 2015 doi: 10.1007/s11101-015-9396-2. [DOI] [Google Scholar]
- National Center for Biotechnology Information. (2020). PubChem. Retrieved November 26, 2020, from https://pubchem.ncbi.nlm.nih.gov/.
- Noh S., Yoon S.H. Stereospecific positional distribution of fatty acids of camellia (Camellia japonica L.) Seed Oil. Journal of Food Science. 2012 doi: 10.1111/j.1750-3841.2012.02854.x. [DOI] [PubMed] [Google Scholar]
- Ochiai M., Nozaki T., Kato M., Ishihara K.O. Camellia japonica seeds extract suppresses lipid-induced hypertriglyceridemia and fat accumulation in mice. Journal of Oleo Science. Japan Oil Chemists Society. 2018 doi: 10.5650/jos.ess18138. [DOI] [PubMed] [Google Scholar]
- Onodera K.I., Hanashiro K., Yasumoto T. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica. Bioscience, Biotechnology and Biochemistry. 2006;70(8):1995–1998. doi: 10.1271/bbb.60112. [DOI] [PubMed] [Google Scholar]
- Panghal A., Shaji A.O., Nain K., Garg M.K., Chhikara N. Cnidoscolus aconitifolius: Nutritional, phytochemical composition and health benefits – A review. Bioactive Compounds in Health and Disease. 2021;4(11):260–286. doi: 10.31989/BCHD.V4I11.865. [DOI] [Google Scholar]
- Park J.C., Hur J.M., Park J.G., Hatano T., Yoshida T., Miyashiro H., Hattori M. Inhibitory effects of Korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytotherapy Research. 2002;16(5):422–426. doi: 10.1002/ptr.919. [DOI] [PubMed] [Google Scholar]
- Pereira A.G., Jimenez-Lopez C., Fraga M., Lourenço-Lopes C., García-Oliveira P., Lorenzo J.M.…Simal-Gandara J. Extraction, properties, and applications of bioactive compounds obtained from microalgae. Current Pharmaceutical Design. 2020;26(16):1929–1950. doi: 10.2174/1381612826666200403172206. [DOI] [PubMed] [Google Scholar]
- Piao M.J., Yoo E.S., Koh Y.S., Kang H.K., Kim J., Kim Y.J.…Hyun J.W. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. International Journal of Molecular Sciences. 2011;12(4):2618–2630. doi: 10.3390/ijms12042618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho T., Choi S.-J., Kil H.W., Ko J., Yoon K.D. Separation of nine novel triterpene saponins from Camellia japonica seeds using high-performance countercurrent chromatography and reversed-phase high-performance liquid chromatography. Phytochemical Analysis. 2019;30(2):226–236. doi: 10.1002/pca.2808. [DOI] [PubMed] [Google Scholar]
- Saenjum C., Pattananandecha T., Nakagawa K. Detection of antioxidant phytochemicals isolated from Camellia japonica seeds using HPLC and EPR imaging. Antioxidants. 2020;9(6):1–14. doi: 10.3390/antiox9060493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B., Ata A., Kumar N.V.A., Sharopov F., Ramírez-Alarcón K., Ruiz-Ortega A., Setzer W.N., Durazzo A., Lucarini M., Santini A., Capasso R., Ostrander E.A., Rahman A.-U., Choudhary M.I., Cho W.C., Sharifi-Rad J. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(551):1–121. doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinero, C., Vela, P., Ron, A. M. De, & Jesús, M. (2016). Camelia (pp. 463–480). Consejo Superior de Investigaciones Cientificas.
- Salinero P.M., Corral C.S. El aceite de camelia. Sociedad Española de La Camelia. 2008;13(1):1–5. [Google Scholar]
- Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. African Journal of Traditional, Complementary and Alternative Medicines. 2011;8(1):1–10. doi: 10.4314/ajtcam.v8i1.60483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Li W., Tsubaki M., Higai K., Takemoto M., Sasaki T.…Koike K. Flavonoid glycosides from Japanese Camellia oil cakes and their inhibitory activity against advanced glycation end-products formation. Journal of Functional Foods. 2017;35:159–165. doi: 10.1016/j.jff.2017.05.043. [DOI] [Google Scholar]
- Sharma T.S.K., Selvakumar K., Hwa K.Y., Sami P., Kumaresan M. Biogenic fabrication of gold nanoparticles using Camellia japonica L. leaf extract and its biological evaluation. Journal of Materials Research and Technology. 2019;8(1):1412–1418. doi: 10.1016/j.jmrt.2018.10.006. [DOI] [Google Scholar]
- Song B.K., Won J.H., Kim S. Historical medical value of donguibogam. Journal of Pharmacopuncture. 2016;19(1):16–20. doi: 10.3831/KPI.2016.19.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, A. J., & Stewart, R. F. (2008). Phenols. In Encyclopedia of Ecology, Five-Volume Set (pp. 2682–2689). Elsevier Inc. 10.1016/B978-008045405-4.00417-1.
- Su M.H., Shih M.C., Lin K.H. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chemistry. 2014;156:369–373. doi: 10.1016/j.foodchem.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Keigaku Publishing; Tokyo: 1976. Tanaka’s cyclopedia of edible plants of the world. [Google Scholar]
- Tanikawa N., Inoue H., Nakayama M. Aluminum ions are involved in purple flower coloration in Camellia japonica ‘sennen-fujimurasaki’. Horticulture Journal. 2016;85(4):331–339. doi: 10.2503/hortj.MI-114. [DOI] [Google Scholar]
- Taylor S.J., McDowell I.J. Rapid classification by HPLC of plant pigments in fresh tea (Camellia sinensis L.) leaf. Journal of the Science of Food and Agriculture. 1991;57(2):287–291. doi: 10.1002/jsfa.2740570212. [DOI] [Google Scholar]
- Thao N.T.P., Hung T.M., Lee M.K., Kim J.C., Min B.S., Bae K.H. Triterpenoids from Camellia japonica and their cytotoxic activity. Chemical and Pharmaceutical Bulletin. 2010;58(1):121–124. doi: 10.1248/cpb.58.121. [DOI] [PubMed] [Google Scholar]
- Trinh L.T.P., Choi Y.S., Bae H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Industrial Crops and Products. 2018;125:261–268. doi: 10.1016/j.indcrop.2018.09.008. [DOI] [Google Scholar]
- Uddin M.N., Sharma G., Yang J.L., Choi H.S., Lim S.I., Kang K.W., Oh W.K. Oleanane triterpenes as protein tyrosine phosphatase 1B (PTP1B) inhibitors from Camellia japonica. Phytochemistry. 2014;103:99–106. doi: 10.1016/j.phytochem.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Venthodika A., Chhikara N., Mann S., Garg M.K., Sofi S.A., Panghal A. Bioactive compounds of Aegle marmelos L., medicinal values and its food applications: A critical review. Phytotherapy Research. 2021;35(4):1887–1907. doi: 10.1002/PTR.6934. [DOI] [PubMed] [Google Scholar]
- Wang B. In vitro antioxidant activity of Camellia japonica L. Advanced Materials Research. 2012;518–523:5555–5558. doi: 10.4028/www.scientific.net/AMR.518-523.5555. [DOI] [Google Scholar]
- Way T.D., Lin H.Y., Hua K.T., Lee J.C., Li W.H., Lee M.R.…Lin J.K. Beneficial effects of different tea flowers against human breast cancer MCF-7 cells. Food Chemistry. 2009;114(4):1231–1236. doi: 10.1016/j.foodchem.2008.10.084. [DOI] [Google Scholar]
- Wendel J.F., Parks C.R. Genetic diversity and population structure in Camellia japonica L. (Theaceae) American Journal of Botany. 1985;72(1):52–65. doi: 10.2307/2443568. [DOI] [Google Scholar]
- Xunta de Galicia. (2020). Ruta de la camelia. Retrieved November 12, 2020, from https://www.turismo.gal/que-facer/ruta-da-camelia?langId=es_ES.
- Yang J.L., Ha T.K.Q., Dhodary B., Pyo E., Nguyen N.H., Cho H.…Oh W.K. Oleanane triterpenes from the flowers of Camellia japonica inhibit porcine epidemic diarrhea virus (PEDV) replication. Journal of Medicinal Chemistry. 2015;58(3):1268–1280. doi: 10.1021/jm501567f. [DOI] [PubMed] [Google Scholar]
- Yoon I.S., Park D.H., Kim J.E., Yoo J.C., Bae M.S., Oh D.S.…Cho S.S. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. International Journal of Molecular Medicine. 2017;39(6):1613–1620. doi: 10.3892/ijmm.2017.2973. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Chou T., Maruyama Y., Okuda T. Tannins of theaceous plants: II: Camelliins A and B, two new dimeric hydrolyzable tannins from flower buds of Camellia japonica L, and Camellia sasanqua THUNB. Chemical and Pharmaceutical Bulletin. 1990;38(10):2681–2686. doi: 10.1248/cpb.38.2681. [DOI] [Google Scholar]
- Yoshikawa M., Morikawa T., Asao Y., Fujiwara E., Nakamura S., Matsuda H. Medicinal flowers. XV. The structures of noroleanane- and oleanane-type triterpene oligoglycosides with gastroprotective and platelet aggregation activities from flower buds of Camellia japonica. Chemical and Pharmaceutical Bulletin. 2007;55(4):606–612. doi: 10.1248/cpb.55.606. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Murakami T., Yoshizumi S., Murakami N., Yamahara J., Matsuda H. Bioactive saponins and glycosides. V. Acylated polyhydroxyolean-12-ene triterpene oligoglycosides, camelliasaponins A1, A2, B1, B2, C1, and C2, from the seeds of Camellia japonica L.: Structures and inhibitory activity on alcohol absorption. Chemical and Pharmaceutical Bulletin. 1996;44(10):1899–1907. doi: 10.1248/cpb.44.1899. [DOI] [PubMed] [Google Scholar]
- Zeng W., Endo Y. Lipid characteristics of camellia seed oil. Journal of Oleo Science. 2019;68(7):649–658. doi: 10.5650/jos.ess18234. [DOI] [PubMed] [Google Scholar]
- Zhang Y.L., Yin C.P., Kong L.C., Jiang D.H. Extraction optimisation, purification and major antioxidant component of red pigments extracted from Camellia japonica. Food Chemistry. 2011;129(2):660–664. doi: 10.1016/j.foodchem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Zhao L., Fan H., Zhang M., Chitrakar B., Bhandari B., Wang B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Research International. 2019;126 doi: 10.1016/j.foodres.2019.108660. [DOI] [PubMed] [Google Scholar]