Highlights
-
•
Carotenoid’s supplementation can control weight gain even in the HFD model.
-
•
Carotenoids extracted with ionic liquids displayed antioxidant activity on the kidney.
-
•
Carotenoids extracted with ionic liquids display an anti-inflammatory effect.
-
•
Carotenoids extracted with acetone increase pro-inflammatory cytokines on the kidney.
-
•
Carotenoids extracted with acetone display oxidative stress on the kidney.
Keywords: Alternative solvents, β-Carotene, Lycopene, Kidney, Inflammation
Abstract
Sustainable extraction processes based on alternative solvents to recover bioactive compounds of different raw materials have been highlighted as excellent alternatives to supply the needs of society towards a bioeconomy strategy. Little is known about the safety and biological effect of compounds extracted by these processes. In this work, carotenoids from Bactris gasipaes wastes obtained by an IL-based process were investigated in terms of safety, anti-inflammatory and, antioxidant activity in a high-fat-diet animal model on the kidney. Wistar rats were supplemented or not by carotenoids extracted with IL or VOS. The animals supplemented with carotenoids had lower weight than control and high-fat diets. In the animals supplemented with carotenoids, the group IL improved anti-inflammatory and antioxidant activity compared with carotenoids obtained by VOS. Also, the group HFD-VOS showed moderate-severe injuries on the kidney. Then, ILs could represent a novel tool for natural pigments safely applied to food industry.
Introduction
It is common sense that health and environmental care are essential factors for maintaining a good quality of life. In this sense, innovative proposals, allying healthy promoting strategies, and ecological consciousness are current trends (Yin et al., 2020). The booming economy and rapid urbanization promoted a massive development of food industries post-war, which became an essential part of contemporary society's food habits (Popkin et al., 2012). However, the food industry primarily seeks high palatability and long shelf-life products at a low cost. These features usually do not advocate for good nutritional values or a sustainable manufacturing process. That is why the food industry turns out to be a converging point of public health, economic and environmental concern (Huang et al., 2020).
Nowadays, most food industry still follows an outdated linear economy model-based production, culminating in a high waste production rate. Moreover, increasing agricultural residues become an issue for economic and environmental preservation as fast as the population grows (Ferronato & Torretta, 2019). Thus, establishing new sustainable strategies is critical to changing the current status quo (Huang et al., 2020). In this sense, the proposal of a novel economy design, namely, the circular economy, was a striking innovation. Furthermore, integrative platforms using food waste to create new and high-valuable products represent a clever solution for waste with economic advantages for the industry and must be an incentive for a future green industry (Clark et al., 2016). In this sense, Bactris gasipaes fruits waste was used in this work as the source of carotenoids. Popularly known as peach palm (or pupunha), it is a tropical biomass, rich in all isomers of β-carotene, γ-carotene, and lycopene, besides minor cis-isomers, high-value pigments applicable in the food, cosmetic, and pharmaceutical industries. Peach palm fruits are native to the Amazonian, considered an agricultural waste (or sub-product) from the palm heart production, a Brazilian delicatessen with high-added economical value, used in several Brazilian recipes and dishes (Shanley, 2011). However, peach palm wastes have substantial economic potential due to the high amount of other natural compounds of interest like flavonoids, fibers, and fatty acids, which display substantial health benefits to the consumers (Radice et al., 2014). However, despite the peach palm's substantial nutritional and economical importance, the fruits are only commercialized in the local Market by the Amazonian population, being underexplored in the National and Worldwide scenario (de Souza Mesquita, Neves, Pisani & de Rosso, 2020).
Most research using food industry wastes to develop new products/processes is based on bioactive compounds extraction using volatile organic solvents (VOS) (Clark, 2019). However, VOS use remains a problem since it has a high toxic potential and can be absorbed through mucosal contact, including breathing and the gastrointestinal tract (Brauner et al., 2020). Thus, without a precise safety examination, industrial products generated through VOS-based processes cannot be redirected to human consumption. As an alternative to the use of VOS, studies have currently been carried out with non-volatile alternative solvents, mostly the ionic liquids (ILs) and more recently deep eutectic solvents, which are already proved to be efficient alternative solvents replacing VOS-based extractions, in addition to representing a more sustainable option, since it can be recovered, reused and recycled in new (or even different) processes (de Souza Mesquita, Martins, Pisani, Ventura & de Rosso, 2021). However, despite the novelty, the applications of ILs remain poorly explored regarding biological effects, with little evidence of its safe application in vivo models (de Souza Mesquita, Casagrande et al., 2021d).
The replacement of synthetic food ingredients by natural-based ones is currently highly recommended. Natural pigments are recognized for their several benefits, such as increasing customer acceptance, adding nutritional value to industrialized food, reducing synthetic dyes that have several side effects, including food allergies, respiratory and cardiovascular diseases, and reducing the industry biomass waste. Although all the advantages, the use of natural pigments must be cautious due to the possible presence of contaminants obtained through the extraction process (Valluzzi et al., 2019). Recent data from our research group has established a new sustainable method for Bactris gasipaes carotenoids extraction applying an ethanolic solution of 1-butyl-3-methylimidazolium tetrafluoroborate – [C4C1im][BF4] utterly free of any residual level of VOS and ILs by the end of the process (de Souza Mesquita et al., 2019). The resulting extract was rich in carotenoids and gave rise to promising results regarding the safety intake and the preservation of bioactive molecules with high antioxidant activity and anti-inflammatory action, suggesting a potential safety for the food industry application (de Souza Mesquita, Casagrande et al., 2021d).
Alongside, the increasing worldwide intake of fast foods and highly industrialized food products is closely related to obesity prevalence, pathological changes in metabolism and immune system, favoring the development of chronic non-communicable diseases (NCD) (Calder et al., 2011). Also, highly processed foods' poor nutritional value leads to micronutrient deficiency, especially vitamins, despite the high caloric intake (Popkin, Corvalan & Grummer-Strawn, 2020). In this sense, the kidney system plays an essential role in homeostasis maintenance. However, several stimuli can induce kidney damage and renal failure. The leading extrinsic causes of kidney diseases include cardiovascular disease, obesity, and diabetes, all directly related to dietary patterns and food choices (Câmara, Iseki, Kramer, liu & Sharma, 2017). Furthermore, the Kidney also plays a prominent role in mediating the toxicity of numerous drugs, environmental pollutants, and natural substances (Barnett & Cummings, 2018). Considering this, it is crucial to investigate the effects of any nutritional intervention or new food additives such as natural pigments extract on kidney tissue function.
Thus, the purpose of this study was to evaluate whether the carotenoids extract obtained by an environmentally friendly manufacturing process could be safe and beneficial from a nutritional point of view, contributing to the development of a new natural pigment for the health promotion from industrial waste, thus combining vital and complementary interests of contemporary society and contributing for a responsible consumption and production (Sustainable development goal – SDG #12), trough industry innovation (SDG #9) to meet the needs of the population for good health and well-being (SDG #3).
1. Material and methods
2.1. Ethics
Animal procedures were approved by the Federal University of São Paulo Experimental Research Committee (CEUA UNIFESP – n° 1193300317), following the standards of the Brazilian guidelines for care and use of animals for scientific purposes and teaching by the national council of animal experimentation control. Based on these guidelines, the sample size was chosen, ensuring the number of animals for statistical analysis strength and consistency.
The animals were maintained in collective cages (four animals per cage) with controlled temperature (23 ± 2 °C), humidity (60 ± 5%), lighting (12-h light/dark), and received ad libitum water and chow. By the end of the seven days of supplementation, the animals were anesthetized with isoflurane and euthanized by beheading in the morning after 10 h of fasting (between 8:00 a.m. and 10:00 a.m.). Tissue samples were collected for analysis, immediately weighed, and stored at −80 °C.
2.2. Experimental procedures
Forty-eight outbred male Wistar rats 90-day-old were used after two weeks of acclimatization. The animals were randomly divided into six experimental groups: (n = 8 per group): control oil (C—O – fed with standard chow and gavage with sunflower oil), high-fat-diet oil (HFD-O – fed with obesogenic chow, and gavage with sunflower oil), control-VOS (C-VOS – fed with standard chow and supplemented by gavage with carotenoids obtained after extraction with VOS), control-IL (C-IL – fed with standard chow and supplemented by gavage with carotenoids obtained after extraction with IL), high-fat-diet-VOS (HFD-VOS – fed with obesogenic chow and supplemented by carotenoids obtained after extraction with VOS), and high-fat-diet-IL (HFD-IL – fed with obesogenic chow and supplemented with carotenoids obtained after extraction with IL). The exposure to the HFD and the tested supplementation were concomitant during the experimental period of seven days. This experimental procedure was chosen due to its efficiency in assessing the effects of supplementation on acute inflammation and oxidative stress indicators, as already performed by (Santamarina et al., 2018, Santamarina et al., 2019, de Souza Mesquita et al., 2021).
2.3. Diet and carotenoids
The control diet groups were fed standard rodent commercial chow (Nuvilab®). The experimental high-fat diet composition was previously described by Dornellas et al. (Dornellas et al., 2015), as shown in Table 1. The animals were weighed, and the diet consumption was measured every day during the experimental period of seven days. All experimental diets were stored at −20 °C and protected from light until further used.
Table 1.
Control and High-Fat-Diet (HFD) centesimal composition .
| Control diet * | High-fat diet (HFD) | |
|---|---|---|
| Energy (kcal/g) | 2.7 | 4.1 |
| Protein (g/100 g) | 22.4 | 23.6 |
| Carbohydrates (g/100 g) | 39.1 | 26.8 |
| Alimentary fiber (g/100 g) | 11.4 | 15.1 |
| Mineral residues (g/100 g) | 11.9 | 9 |
| Total fat | 4.8 | 22 |
* The standard rat commercial chow used was Nuvilab CR1 (Nuvital, Brazil).
adapted from Dornellas et al., 2015
A gavage protocol was used to supplement the carotenoids' extract. Each animal's daily water-gavage was applied during the acclimatization period to habituate them with this procedure. Bactris gasipaes fruits donated from an agricultural cooperative from Bahia State (Cooperativa Cabruca – Bahia, Brazil) were used to source carotenoids. The biomass was sanitized in running tap water to remove dirtiness, lyophilized, and stored under refrigeration (−40 °C) until use. Since the main objective of this work is to evaluate whether the extraction solvent display some influence on the metabolic results, two different extracts were made, namely (i) using an ethanolic solution of 1-butyl-3-methylimidazolium tetrafluoroborate [C4C1mim[BF4] herein named an ionic liquid (IL) or (ii) using mixtures of acetone and ether herein called volatile organic solvents (VOS), respectively. The extracts were prepared in a batch regime, and the same concentration of carotenoids was stored in individual flasks until use. Both extracts (IL and VOS) comprise 35% all-trans-β-carotene, 18% all-trans-γ-carotene, 9% all-trans-lycopene, and 38% other minor compounds (cis-isomers). Therefore, the carotenoids' dose supplementation was set up as 1 mgcarotenoids extract.kg−1body mass. It is essential to highlight that all the IL was efficiently withdrawn from the extract by thermal precipitation under −80 °C, as well as reported in (de Souza Mesquita, Murador et al., 2021). Furthermore, the organic solvents were removed from the extract by evaporation under a vacuum in the VOS extract. The detailed information of the extract preparation was previously shown by de Souza Mesquita et al. (2019).
2.4. Tissue cytokine levels
The 100 µg of right kidney samples (n = 7–8 per group) were homogenized in a specific buffer, containing: 100 mM Tris-HCl (pH 7.5), 1% Triton X-100, 10% sodium dodecyl sulfate (SDS), 100 mM EDTA, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 10 mM sodium orthovanadate, 2 mM phenyl-methyl-sulphonyl fluoride, and 0.1 mg.mL−1 aprotinin from bovine lung. The homogenate obtained was centrifugated at 20,800g for 40 min at 4 °C, and the supernatant was collected and stored at −80 °C for further analysis. According to the manufacturer's recommendation, the cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-10 (IL-10) were quantified with DuoSet ELISA kits (DuoSet ELISA, R&D System, Minneapolis, MN, USA). The Bradford's assay was performed to access the sample's protein concentrations.
2.5. Oxidative stress markers in kidney
All the oxidative stress assays were performed following the original methodology described in (Buege and Aust, 1978, Góth, 1991, Habig et al., 1974, Levine et al., 1990, Madesh and Balasubramanian, 1998). To evaluate the antioxidant enzymes, a specific homogenate using 100 mg of suitable proper kidney sample was homogenized in a phosphate buffer saline (PBS) solution with controlled temperature (4 ± 1 °C) and centrifugated at 3500g (4 °C) for 15 min. The supernatant was taken to analyze superoxide dismutase (SOD), and catalase activities. SOD activity was evaluated by the pyrogallol method based on the ability of this enzyme to catalyze the reaction of superoxide (O2−) and hydrogen peroxide (H2O2). CAT activity was estimated by measuring the rate of H2O2 decomposition for 3 min, using an 8-point calibration curve of H2O2 (R2 = 0.9999), and the amount of CAT was expressed as UCAT.min−1. Malondialdehyde (MDA) and protein carbonyl (PC) were estimated to evaluate the pro-oxidative markers. Lipid peroxidation was assessed by applying the same homogenate used to determine antioxidant markers. Still, this time was incubated with thiobarbituric acid to evaluate the levels of thiobarbituric acid-reactive substances, representing the amount of MDA in the Kidney. Protein oxidation was estimated by applying the PC methodology using the debris of the Kidney after the centrifugation step to prepare the homogenate used in the other analysis. The quantification of PC in kidney tissue was assessed using the 2.4-dinitrophenylhydrazine (DNPH) method, expressed as pmol.mL−1. Finally, all the results were normalized using the protein level quantified by Bradford’s method.
2.6. Renal histopathological analysis
The kidney samples were fixed in 10% buffered formalin for 24 h, then gradually dehydrated in alcohol, cleared in xylol, and subsequently embedded in paraffin blocks. The tissues were cut into 3 µm thick sections, then stained with hematoxylin-eosin (HE) for histopathological analysis. The evaluation of the renal tissue followed the criteria adopted by Jia and collaborators (Jia et al., 2013), taking into account the severity of damage to the renal cortex using a semi-quantitative scale. In addition, the following criteria were considered: inflammatory process, degenerations, and areas with necrosis. These findings were classified into scores, considering the severity of injuries to the renal cortex, as follows: score 0 (no injury); score 1 (mild injuries); score 2 (moderate injuries); score 3 (severe injuries); and score 4 (very serious injuries).
2.7. Immunohistochemistry for 8OHdG
For the immunohistochemical analysis, anti-8-hydroxy-20-deoxyguanosine (8OHdG, Santa Cruz Biotechnologies Inc.™, MO, USA) was used at a concentration of 1:100 for 24 h. After that, the sections were treated with universal biotin-conjugated secondary antibody (Starr Trek Universal HRP Detection, Biocare Medical, USA) at a ratio 1:100 for 30 min, followed by the application of DAB (3,3-diaminobenzidine) at 0.05% (DAKO® North America, Inc®, California, EUA) and counterstained with hematoxylin. The method was based on increasing scores, according to Claudio and co-authors (Claudio et al., 2019), based on the absence or presence of immunological cells associated with the extent of the stained sections, as follows: no staining (0), weak staining (1), moderate staining (2), and strong staining (3).
2.8. Statistical analysis
The data was processed in Microsoft Excel (2010) (Microsoft, Albuquerque, NM, USA), and Grubb's test was performed to remove significant outliers. All statistical analysis was performed using GraphPad Prism 8.0.2 (GraphPad Software Inc., San Diego, CA, USA). Parametrical data were submitted to two-way ANOVA followed by Tukey post-hoc test, and non-parametrical data were under Kruskal-Wallis test with post-Dunn's multiple comparisons, with a significance level fixed at 95% (p < 0.05). All the results were expressed as mean ± standard error. To investigate possible correlations between pairs of significant variables, the Spearman test was performed standing r-value < 0.5 for a strong correlation and considering a p < 0.05.
2. Results
3.1. Energy Intake, body, and kidney mass
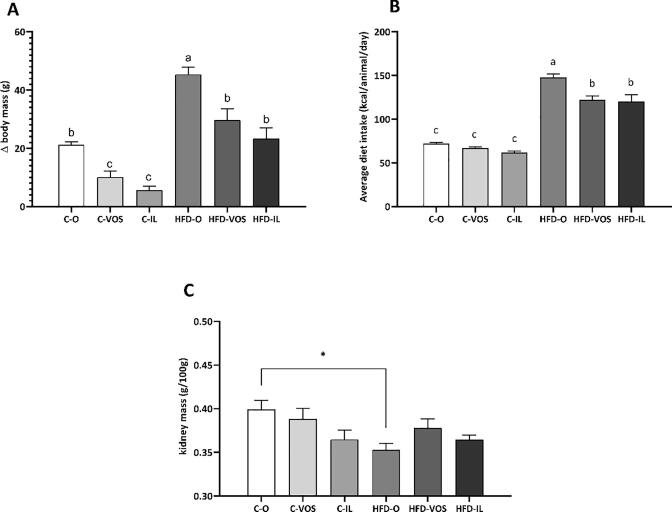
The higher body mass gain (Δ) displayed in Fig. 1A demonstrates that the HFD-O group had the highest weight gain among the experimental groups (C—O, C-VOS, C-IL, HFD-VOS, HFD-IL) during the supplementation period. Tough, there were no statistical differences among C—O, HFD-VOS, and HFD-IL body mass gain. Also, the C—O group had higher weight gain than the C-VOS and C-IL groups.
Fig. 1.
A) Rats body mass gain (Δ) during the supplementation period of seven days; B) Average daily energy intake during the supplementation; C) Relative kidney tissue mass corrected by body weight mass. * represents a reduction in the relative kidney mass from the HFD-O compared to C—O group. Different letters depicted on the bars represent significant differences in the post-hoc test considering the interaction between the diet and supplementation, i.e. statistically different values by two-way ANOVA followed by Tukey post-hoc (p < 0.05; n = 8 – 7 per group). Groups: C—O – fed with standard chow and gavage with sunflower oil, C-VOS – fed with standard chow and supplemented by gavage with carotenoids extracted with VOS; C-IL – fed with standard chow and supplemented by gavage with carotenoids extracted with IL; HFD-O – fed with obesogenic chow and gavage with sunflower oil; HFD-VOS: fed with obesogenic chow and supplemented with carotenoids extracted with VOS; HFD-IL: fed with obesogenic chow and supplemented with carotenoids extracted with IL.
As expected, the groups fed with an HFD (HFD-O, HFD-VOS, and HFD-IL) have shown higher energy intake compared to those receiving the control diet (C—O, C-VOS, and C-IL), and specially HFD-O had higher caloric consumption than all other experimental groups (C—O, C-VOS, C-IL, HFD-VOS, HFD-IL). However, there were no differences in diet intake between HFD-VOS and HFD-IL nor among control diet groups (C—O, C-VOS, C-IL), as seen in Fig. 1B. Additionally, the relative kidney mass (Fig. 1C) was reduced exclusively in the HFD-O group compared to C—O, without significant difference among the other groups.
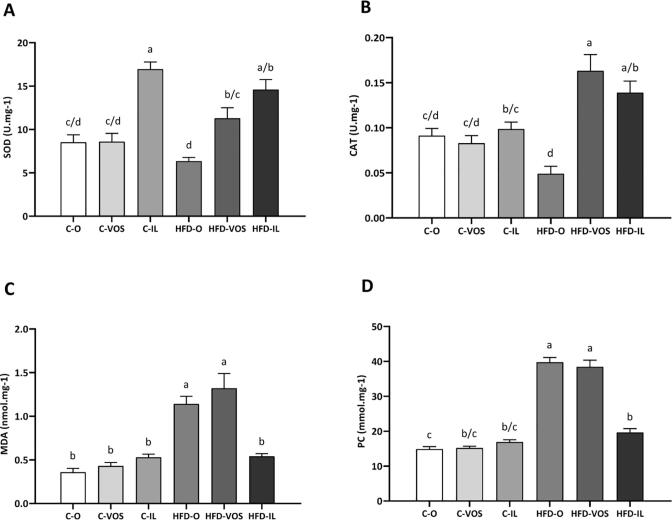
3.2. Oxidative stress markers in kidney
Accessing the concentrations of antioxidant and pro-oxidative molecules in the kidney samples, it is noteworthy that superoxide dismutase – SOD (Fig. 2A) activity in the C-IL group is remarkably increased compared to all experimental groups. Also, HFD-IL demonstrated higher levels of SOD compared to C—O, C-VOS, and HFD-O groups. In addition, the HFD-VOS group displayed an increase in SOD compared to HFD-O. Analyzing the catalase (CAT) enzyme data, the HFD-VOS showed higher levels than the HFD-O and control diet-fed groups (C—O, C-VOS, and C-IL). Moreover, HFD-IL had increased CAT versus C—O, C-VOS, and HFD-O groups. Also, HFD-O had lower CAT levels than the control diet groups (C—O, C-VOS, and C-IL), as seen in Fig. 2B. Otherwise, malondialdehyde (Fig. 2C) increased in HFD-O and HFD-VOS compared to the other control diet-fed groups (C—O, C-VOS, C-IL) and the HFD-IL group. Regarding protein carbonyl (Fig. 2D), HFD-O and HFD-VOS also have shown higher levels than all the other groups. However, HFD-IL was increased only versus the C—O group, and there were no differences compared to C-VOS or C-IL. The control diet-fed groups did not differ among themselves, as shown in Fig. 1D.
Fig. 2.
Oxidative stress markers activity was analyzed in kidney samples (n = 6–8 per group). A) SOD: superoxide dismutase; B) CAT: catalase; C) MDA: malondialdehyde; D) PC: protein carbonyl. Different letters represent significant differences among the groups. Different letters depicted on the bars represent significant differences in the post-hoc test considering the interaction between the diet and supplementation, i.e. statistically different values by two-way ANOVA followed by Tukey post-hoc (p < 0.05; n = 8 – 7 per group). Groups: C—O – fed with standard chow and gavage with sunflower oil, C-VOS – fed with standard chow and supplemented by gavage with carotenoids extracted with VOS; C-IL – fed with standard chow and supplemented by gavage with carotenoids extracted with IL; HFD-O – fed with obesogenic chow and gavage with sunflower oil; HFD-VOS: fed with obesogenic chow and supplemented with carotenoids extracted with VOS; HFD-IL: fed with obesogenic chow and supplemented with carotenoids extracted with IL.
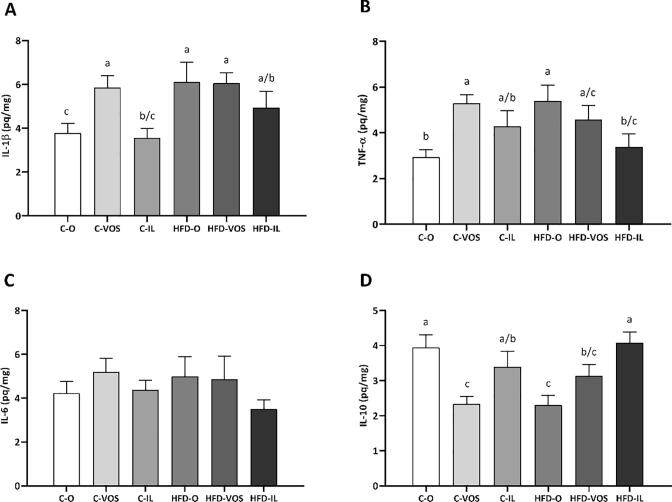
3.3. Tissue cytokine levels in kidney
Regarding inflammatory cytokines data, the IL-1β in C-VOS, HFD-O, and HFD-VOS were increased compared to C—O and C-IL. The HFD-IL concentrations were higher than C—O, but they did not differ from the other experimental groups (C-IL, C-VOS, HFD-O, HFD-VOS), as shown in Fig. 3A. The TNF-α levels were higher in C-VOS and HFD-O than C—O and HFD-IL. Also, HFD-VOS increased expression compared to the C—O group, and C-IL did not differ (Fig. 3B). Likewise, the IL-6 concentration did not change among the group, as shown in Fig. 3C.
Fig. 3.
Cytokines levels in kidney sample A) interleukin 1 β – IL-1β; B) tumor necrosis factor α – TNF-α; C) interleukin 6 – IL-6 and D) interleukin 10 – IL-10. Different letters represent significant differences among the groups. Different letters depicted on the bars represent significant differences in the post-hoc test considering the interaction between the diet and supplementation, i.e. statistically different values by two-way ANOVA followed by Tukey post-hoc (p < 0.05; n = 8 – 7 per group). Groups: C—O – fed with standard chow and gavage with sunflower oil, C-VOS – fed with standard chow and supplemented by gavage with carotenoids extracted with VOS; C-IL – fed with standard chow and supplemented by gavage with carotenoids extracted with IL; HFD-O – fed with obesogenic chow and gavage with sunflower oil; HFD-VOS: fed with obesogenic chow and supplemented with carotenoids extracted with VOS; HFD-IL: fed with obesogenic chow and supplemented with carotenoids extracted with IL.
Concerning the anti-inflammatory cytokine IL-10, the C—O and the HFD-IL groups had higher levels than C-VOS, HFD-O, and HFD-VOS. Nevertheless, C-IL had more significant IL-10 than C-VOS and HFD-O groups and did not differ from C—O and HFD-IL, as demonstrated in Fig. 3D.
3.4. Correlation results
Aiming to analyze the interface among variables, correlation tests were performed. Initially, the data was analyzed considering the interaction between all experimental groups (C—O, C-VOS, C-IL, HFD-O, HFD-VOS, and HFD-IL) as shown in Table S1 (supplementary material). It is noteworthy that oxidative stress markers link with the mass body gain since MDA and PC's pro-oxidative markers were positively correlated. At the same time, antioxidant enzyme SOD was negatively related to mass body gain. However, despite the significant p values, the r values were not considered strong enough for a deep discussion nor display carotenoids effect. So, the next set of correlation tests was performed considering the supplementation groups (VOS and IL) as a disentangling point for the carotenoids effect analysis.
The results based on supplementation groups (VOS and IL) show the divergent effect of VOS and IL-carotenoid extracts with robust correlation findings (Table 2). The groups receiving the extract of carotenoids extracted by VOS (C-VOS and HFD-VOS) showed a strong positive correlation among body mass, diet intake, and oxidative stress markers, namely CAT, MDA, and CP. On the other hand, the groups supplemented with carotenoids obtained after extraction with IL (C-IL- and HFD-IL) demonstrated the protective effects of carotenoids through a negative correlation between the catalase enzyme with mass body gain and diet intake. Also, IL-based groups display a negative correlation between PC and diet intake, while there is no correlation with MDA.
Table 2.
Results for the correlation coefficient (r spearman) were obtained on body mass gain (Δ) and oxidative stress markers considering the different supplementation groups (VOS and IL).
| Parameters |
VOS groups (n = 16) |
IL groups (n = 16) |
|||
|---|---|---|---|---|---|
| Body mass (Δ) | Diet intake | Body mass (Δ) | Diet intake | ||
| CAT | r | 0.577 | 0.738 | −0.601 | −0.838 |
| p | 0.021* | 0.002* | 0.015* | 0.0001* | |
| MDA | r | 0.635 | 0.742 | −0.115 | 0.119 |
| p | 0.010* | 0.001* | 0.670 | 0.657 | |
| CP | r | 0.576 | 0.745 | 0.264 | −0.544 |
| p | 0.021* | 0.001* | 0.321 | 0.031* | |
* Represents r > 0.5 and p < 0.05. CAT: catalase; MDA: malondialdehyde; CP: carbonyl protein.
3.5. Histopathological findings in kidney
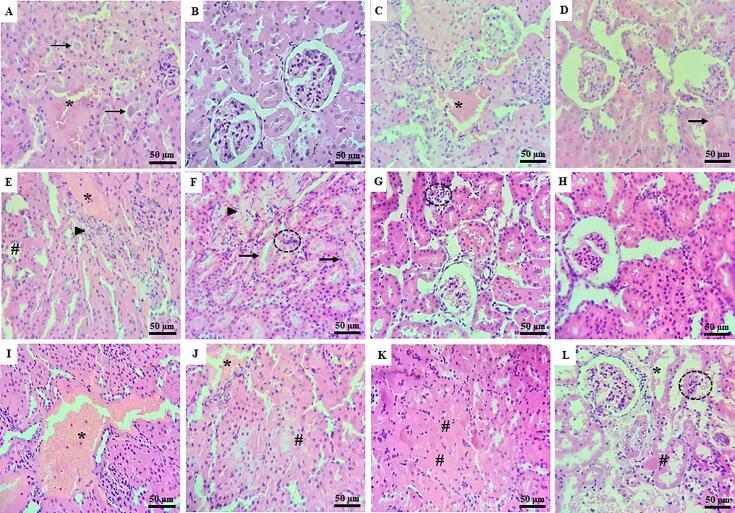
Small areas of interstitial hemorrhages and protein cylinders in the tubular lumen were observed in the C—O group samples. The other supplementation groups (HFD-O, C-VOS, C-IL, HFD-VOS, and HFD-IL) presented edema, hemorrhagic areas, and congested vessels. As well as the presence of protein cylinders in the tubular lumen, small foci of necrosis, and glomerular atrophy. One specimen presented fibrosis only. Being animals from the group HFD-O and HFD-VOS showed the worst classification. The severity of histopathological alterations was classified by scores shown in Table S2 (supplementary material). Such findings are illustrated in Fig. 4. Immunohistochemical expression of 8OHdG was detected in the cytoplasm of cells. The results showed that all the groups had immunoexpressing for 8OHdG. However, there were not any significant statistical differences (p > 0.05) among the groups, being the results shown in Fig. S1 (supplementary material).
Fig. 4.
Photomicrographs of kidney tissue samples under Hematoxylin-Eosin (HE) staining. A-B) C—O group: hemorrhagic areas (*) and protein cylinders in the tubular lumen (arrows); C-D) HFD-O group: hemorrhagic areas (*). Protein cylinders in the tubular lumen (arrows) and glomerular atrophy; E-F) C-VOS group: hemorrhagic areas (*). Fibrosis (arrowhead). Protein cylinders in the tubular lumen (arrows). Necrosis foci (#) and inflammatory cells (dotted circles). G-H) C-IL group: inflammatory cells (dotted circles) and glomerular atrophy; I-J) HFD-VOS group: hemorrhagic areas (*) and necrosis foci (#); K-L) HFD-IL group: inflammatory cells (dotted circles). Hemorrhage (*) and areas of necrosis (#). The sample size analyzed was n = 5 per group.
3. Discussion
This study elucidates the differential effect of carotenoid-rich extracts supplementation obtained with VOS or ILs associated with an obesogenic diet in the kidney tissue. The beneficial effects attributed to carotenoid intake are well-known and extensively described in the literature as a precursor molecule of Vitamin A, preventing hypovitaminosis-associated pathologies (Beydoun et al., 2019). Also, carotenoids are usually described as potent antioxidant molecules exerting protective effects against oxidative stress by mitigating reactive oxygen species (ROS) and promoting anti-inflammatory outcomes (Blaner, 2019). Moreover, it has been already proved by previous research the ecological and economic advantages of the carotenoids extraction by ILs instead of VOS for the environmental impact in a circular economy approach, besides the excellent use of these biomolecules as natural antioxidant pigments recovered from food industry waste (de Souza Mesquita et al., 2019). However, there is a lack of knowledge about these extracts' consumption safety and biological effects for potential applications in the food industry. In this context, we will discuss the influence of the extraction method – VOS or ILs mediated – on different carotenoid extract supplementation biological effects.
Notably, the high-fat diet (HFD) model efficiently induced body gain mass during the experimental period associated with hyperphagia and increased caloric intake (de Souza Mesquita, Casagrande et al., 2021d). The fast body mass gain reproduces an obesity induction model, which promotes the development of other non-communicable diseases such as cardiovascular diseases, diabetes, and kidney failure (Kiortsis and Christou, 2012, da Silva Junior et al., 2017). Obesity per se, independent of its association with hypertension and diabetes, has been suggested to exert a crucial role in developing chronic kidney disease, promoting the progression of underlying renal diseases and even leading to end-stage renal disease (Thakur, Morse & Reisin, 2006). The short-term consequences of an obesogenic diet can be noticed by the reduced kidney mass, which suggests damage with tissue loss on HFD intake in our results. The slow and progressive kidney mass loss is also strongly related to cardiovascular diseases development in long-term obesity, besides the increasing incidence of locus diseases such as kidney lithiasis, chronic kidney disease, nephritis, and others (Kiortsis & Christou, 2012).
As well known, the expansion of adipose tissue in obesity leads to an unbalance of adipokines with an increase in pro-inflammatory mediators and components of the renin-angiotensin-aldosterone system. In this sense, leptin and adiponectin seem to be closely related to kidney oxidative stress and inflammation processes. Leptin has been suggested to lead to endothelial cell proliferation, mesangial cell hypertrophy, vascular inflammation, increased transforming growth factor (TGF-β1), and oxidative stress. Additionally, the low adiponectin levels have been reported to stimulate the endothelial cyclooxygenase 2 (COX-2) and are related to renal dysfunction and proteinuria (Tesauro, Mascali, Franzese, Cipriani, Cardillo & Di Daniele, 2012). In our previous work (de Souza Mesquita, Casagrande et al., 2021d), we demonstrated that the adiponectin level corroborates this work's findings since animals supplemented with VOS-carotenoids have a lower level compared to those supplemented with IL-carotenoids, independent of the diet (Control or HFD). It states that obesity itself might be a trigger factor to kidney diseases and a molecular inflammatory derangement.
Additionally, the renal effects of obesity can be divided into structural and functional damage. The structural changes usually observed are the obesity-related glomerulopathy, characterized by glomerulosclerosis, glomerulomegaly, mesangial hyperplasia, and weak adhesion of podocytes to the glomerular basement membrane (Thakur et al., 2006). Our histological findings have demonstrated that HFD-O and HFD-VOS groups already developed kidney injury with edema, congested vessel, and small necrosis areas, even in an acute protocol of obesity induction, highlighting the critical role of Kidney on metabolic disorders.
The characteristic metabolic state in obesity can induce systemic oxidative stress through several biochemical mechanisms, such as the generation of superoxide from NADPH oxidases, oxidative phosphorylation, oxidation of glyceraldehyde, and activation of protein C kinase. Other factors contributing to oxidative stress in obesity include hyperleptinemia, low antioxidant defense, chronic inflammation, and generation of ROS (Tan et al., 2018). The ROS class – which includes superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•) – play a key role in pathological damage on cellular lipid peroxidation (producing F2-isoprostanes, 4-hydroxy-2-nonenal, and malondialdehyde), proteins carbonylation, and degradation of both nuclear and mitochondrial DNA (Arulselvan et al., 2016). It reflects our finding on kidney carbonylated protein (PC) and malondialdehyde (MDA), significantly increased by HFD testifying the oxidative stress installation. We have also shown a positive correlation of mass body gain with PC and MDA. It allows us to suggest that the HFD diet enhanced ROS accumulation and increased mitochondrial β-oxidation of free fatty acids. This subsequently leads to an electron overflow using cytochrome-c oxidase during NADH and FADH2 into NAD+ and FAD, elevating the superoxide anion and consequently the accumulation of ROS, in agreement with the literature (Curtis et al., 2010). Likewise, an HFD seems to reduce antioxidant enzymes activity such as – superoxide dismutase SOD and catalase shown here. It goes in agreement with the literature data demonstrating the decrease in glutathione peroxidase and glutathione reductase – alongside the increased accumulation of ROS and improved expression of NADPH oxidase (Valenzuela et al., 2017). The negative correlation found between body mass gain and SOD confirms the HFD effect on antioxidant enzymes in addition to the CAT and SOD lower levels in kidney samples.
In addition to oxidative stress, HFD triggers NADPH oxidase activation, which deregulates the production of redox-sensitive transcription mRNAs such as NFκB, interleukin-6 (IL-6) adiponectin, and TNF-α in the kidney (Ruggiero, Ehrenshaft, Cleland & Stadler, 2011). Therefore, the present data highlight that HFD is closely related to oxidative stress and might lead to other metabolic disorders. The pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) constitutes another causal factor of obesity-induced kidney diseases through macrophages infiltration, apoptosis, and up-regulation of NFκB and TGF-β1 (Gai & Kullak-Ublick, 2017). Establishing this inflammatory process can occur through the simultaneous activation of several intracellular mechanisms, like the TLR transmembrane receptor pathway, which culminates in the imbalance between pro-inflammatory molecules, such as TNF-α, IL-6, interleukin-1β (IL-1β) and anti-inflammatory molecules such as interleukin-10 (IL-10) and adiponectin. These biomarkers are intimately involved in the regulation of chemotaxis, cell migration, and proliferation (Lee et al., 2017). Although inflammation participates in homeostasis, pathological imbalances lead to the establishment of the deleterious process of chronic subclinical inflammation (Tahergorabi & Khazaei, 2013). Our present data has shown that even short-term exposure to HFD unleashes increased pro-inflammatory cytokines as expected. Interestingly, the IL-1β and TNF-α levels in the C-VOS group were similar to the HFD-O despite a pro-inflammatory diet, which is not observed in the other C groups (C—O and C-IL). It brings to light that VOS might exert a harmful pro-inflammatory effect in the Kidney even associated with a balanced diet differently from C-IL, indicating that VOS may be unsuitable as an extraction vehicle.
It is worth citing that the carotenoids are natural lipophilic pigments present in all photosynthetic organisms acting as photoprotective and antioxidant compounds. They can be metabolized through multiple pathways that contribute to their effects on adiposity. Besides, some carotenoids are also pro-Vitamin A molecules (Langi, Kiokias, Varzakas & Proestos, 2018). Despite the hydrophobic nature of carotenoids, they are easily absorbed through dietary lipids and add nutritional value to diet fats (de Souza Mesquita, Neves, & Pisani, 2020). On the other hand, synthetic pigments applied in the food industry have usually been produced from petroleum-derived organic solvents (Carocho, Barreiro, Morales & Ferreira, 2014). It impacts several side effects for the population, namely allergies, respiratory conditions, vitamin A deficiency, non-alcoholic fatty liver disease, and cardiovascular diseases, highlighting the need for the food industry to adopt more natural dyes (Valluzzi et al., 2019). The carotenoids' biological benefits were recently explored to manage obesity-related inflammation and oxidative stress (Tan and Norhaizan, 2019a, Yao et al., 2021).
The HFD pro-inflammatory and pro-oxidative challenge disturb the homeostasis, which might be targeted by carotenoids opposing the general harmful scenario (Tan & Norhaizan, 2019b). Therefore, it can be noticed that carotenoids supplementation exerted a protective effect, preventing the mass body gain through the experimental period (Tan & Norhaizan, 2019a). Both ILs and VOS displayed the same effect pattern proving that carotenoids have anti-obesogenic effects. Furthermore, the carotenoids also prevented kidney mass loss, indicating an absence of toxicity and even a protective effect against the HFD injury. It agrees with previous results presented by our group and other researchers regarding the use of carotenoids as a tool for obesity and body weight gain prevention since carotenoids can act on mitochondria, increasing the energy expenditure through thermogenesis and lipolysis mechanisms activation (de Souza Mesquita et al., 2021, Wu et al., 2016).
However, the differential effect between the supplementation of extracts obtained from VOS- or IL-mediated processes is evident from this point onwards. Complementary results of oxidative stress markers – MDA and PC – and antioxidant enzymes agree to demonstrate IL's antioxidant effect, especially on the HFD-IL group. The carotenoids extracted with IL exerted such a strong effect to bring HFD-IL to C—O levels of MDA, mitigating the lipid peroxidation promoted by the HFD. Therefore, the CAT and SOD levels were increased in kidney samples showing a high activity of antioxidant enzymes by carotenoids supplementation. The powerful reduction of oxidative stress byproducts can be in part related to the high lipid content of the diet, which facilitates the intestinal absorption of carotenoids lipophilic molecules, which are highly effective singlet oxygen quenchers and potent scavengers of ROS (Bohn, 2019). Indeed, there is a strong negative correlation of mass body gain and diet intake with CAT and PC in the groups receiving extracts obtained by ILs, which was not found for the VOS extract. Thus, with a possible effect of the supplementation promoted by the carotenoids extract obtained with the IL-mediated process and their relationship with the NFκB signaling pathways and extragenomic actions such as ROS scavenging.
The carotenoid intake has been linked to down-regulating intracellular inflammatory signaling cascades, thereby modulating gene expression and protein translation. Specifically, carotenoids can interact with the NFκB pathway, thus inhibiting the downstream production of pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α (Kaulmann & Bohn, 2014). As observed in our results, the ILs groups had the pro-inflammatory cytokines decreased, and the anti-inflammatory IL-10 increased, even on HFD groups demonstrating the substantial anti-inflammatory effect of carotenoids obtained through the IL-mediated process, preventing the installation of low-grade inflammation promoted by the obesity and HFD. Nonetheless, the groups under VOS extract supplementation did not show the same beneficial effect, on the contrary, showing high levels of TNF-α and IL-1β, even in the C-VOS group representing a harmful effect for kidney homeostasis. This evidence has been proved to happen also in adipose tissue and liver, suggesting a systemic level negative effect of VOS-mediated extract (de Souza Mesquita, Casagrande et al., 2021d). It indicates that VOS-mediated extraction is toxic at some level, activating the systemic inflammatory pathway.
So far, we propose that during inflammation, immune system cells, such as macrophages and leucocytes, are recruited and infiltrated on the tissues, starting the production of cytokines. At the same time, the increased cell infiltration results in a local overload of respiratory cell function, which leads to an overproduction of ROS, triggering cellular to oxidative stress. In turn, oxidative stress induces apoptotic pathways, which feedback the inflammatory process, making this a vicious cycle (Kaulmann & Bohn, 2014). Our results suggest that carotenoids, specifically the extract obtained by the IL-mediated process, could break through the pro-inflammatory and pro-oxidative processes promoted by the HFD. As a pioneering study, there is still room for in-depth discussions on the topic; we hope to bring thought-provoking data to develop further research in this area.
4. Conclusions
Summing up, the carotenoids from Bactris gasipaes extract obtained with ILs have proven to be superior to the extract obtained through the VOS-mediated process regarding anti-inflammatory and antioxidant effects in the Kidney. The carotenoids obtained with ILs displayed protective properties against the harmful effects of a HFD intake, not only on body mass gain but also on a cellular level. Furthermore, it exerted a robust antioxidant role reducing oxidative stress byproducts, increasing antioxidant enzymes, and promoting an anti-inflammatory milieu in the Kidney. Besides the proven biological effect, the carotenoids obtained with ILs are safe for consumption in vivo, unlike those obtained by the VOS-mediated extraction. Here, we bring sparks of pieces of evidence that natural pigments obtained specifically through this IL- mediated process represent an innovative and safe opportunity for the food industry with high added nutritional value. Nevertheless, these are initial data, and more research with long-term supplementation protocols and deeper molecular investigation are still needed.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Aline B. Santamarinaa, Leonardo M. de Souza Mesquita reports financial support was provided by Fundação de Amparo a Pesquisa do Estado de São Paulo. Luciana Pellegrini Pisani reports financial support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Acknowledgments
This work was supported by “Fundação de Amparo à Pesquisa do Estado de São Paulo- FAPESP” through fellowships (2016/18910; 2016/23242-8, 2020/04448-0, and 2018/10705-5), and to CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico, fellowship 305725/2020-3. This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020, financed by national funds through the Portuguese Foundation for Science and Technology/MCTES.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100245.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of antioxidants and natural products in inflammation. Oxidative Medicine and Cellular Longevity. 2016;2016:1–15. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett L.M.A., Cummings B.S. Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicological Sciences. 2018;164(2):379–390. doi: 10.1093/toxsci/kfy159. [DOI] [PubMed] [Google Scholar]
- Beydoun M.A., Chen X., Jha K., Beydoun H.A., Zonderman A.B., Canas J.A. Carotenoids, vitamin A, and their association with the metabolic syndrome: A systematic review and meta-analysis. Nutrition Reviews. 2019;77(1):32–45. doi: 10.1093/nutrit/nuy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacology & Therapeutics. 2019;197(1):153–178. doi: 10.1016/j.pharmthera.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants. 2019;8(6):1–44. doi: 10.3390/antiox8060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner C., Joveleviths D., Álvares-da-Silva M.R., Marroni N., Bona S., Schemitt E., Nardi R. Exposure to organic solvents and hepatotoxicity. Journal of Environmental Science and Health – Part A Toxic/Hazardous Substances and Environmental Engineering. 2020;55(10):1173–1178. doi: 10.1080/10934529.2020.1779532. [DOI] [PubMed] [Google Scholar]
- Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation Methods Enzymol 52: 302–310. Find This Article Online. [DOI] [PubMed]
- Calder P.C., Ahluwalia N., Brouns F., Buetler T., Clement K., Cunningham K.…Winklhofer-Roob B.M. Dietary factors and low-grade inflammation in relation to overweight and obesity. British Journal of Nutrition. 2011;106(S3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- Câmara N.O.S., Iseki K., Kramer H., Liu Z.H., Sharma K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nature Reviews Nephrology. 2017;13(3):181–190. doi: 10.1038/nrneph.2016.191. [DOI] [PubMed] [Google Scholar]
- Carocho M., Barreiro M.F., Morales P., Ferreira I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Comprehensive Reviews in Food Science and Food Safety. 2014;13(4):377–399. doi: 10.1111/1541-4337.12065. [DOI] [PubMed] [Google Scholar]
- Clark J.H. Green biorefinery technologies based on waste biomass. Green Chemistry. 2019;21(6):1168–1170. doi: 10.1039/C9GC90021G. [DOI] [Google Scholar]
- Clark J.H., Farmer T.J., Herrero-Davila L., Sherwood J. Circular economy design considerations for research and process development in the chemical sciences. Green Chemistry. 2016;18(14):3914–3934. doi: 10.1039/c6gc00501b. [DOI] [Google Scholar]
- Claudio S.R., Pidone Ribeiro F.A., De Lima E.C., Santamarina A.B., Pisani L.P., Pereira C.S.D.…Ribeiro D.A. The protective effect of grape skin or purple carrot extracts against cadmium intoxication in Kidney of rats. Pathophysiology. 2019;26(3-4):263–269. doi: 10.1016/j.pathophys.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Curtis J.M., Grimsrud P.A., Wright W.S., Xu X., Foncea R.E., Graham D.W.…Bernlohr D.A. Downregulation of adipose glutathione S-tansferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Mesquita L.M., Martins M., Pisani L.P., Ventura S.P.M., Rosso V.V. Insights on the use of alternative solvents and technologies to recover bio-based food pigments. Comprehensive Reviews in Food Science and Food Safety. 2021;20(1):787–818. doi: 10.1111/1541-4337.12685. [DOI] [PubMed] [Google Scholar]
- de Souza Mesquita L.M., Murador D.C., Neves B.V., Braga A.R.C., Pisani L.P., de Rosso V.V. Bioaccessibility and cellular uptake of carotenoids extracted from bactris gasipaes fruit: Differences between conventional and ionic liquid-mediated extraction. Molecules. 2021;26(13):3989. doi: 10.3390/molecules26133989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Mesquita L.M., Casagrande B.P., Santamarina A.B., Sertorio M.N., de Sousa D.V., Mennitti L.…Pellegrini Pisani L. Carotenoids obtained by an ionic liquid mediated process display anti-inflammatory response in adipose tissue-liver axis. Food & Function. 2021 doi: 10.1039/d1fo01429c. [DOI] [PubMed] [Google Scholar]
- de Souza Mesquita, L. M., Neves, B. V., Pisani, L. P., & de Rosso, V. V. (2020). Mayonnaise as a model food for improving the bioaccessibility of carotenoids from Bactris gasipaes fruits. Lwt, 122(October 2019), 109022. https://doi.org/10.1016/j.lwt.2020.109022.
- de Souza Mesquita L.M., Ventura S.P.M., Braga A.R.C., Pisani L.P., Dias A.C.R.V., De Rosso V.V. Ionic liquid-high performance extractive approach to recover carotenoids from: Bactris gasipaes fruits. Green Chemistry. 2019;21(9):2380–2391. doi: 10.1039/c8gc03283a. [DOI] [Google Scholar]
- Dornellas A.P.S., Watanabe R.L.H., Pimentel G.D., Boldarine V.T., Nascimento C.M.O., Oyama L.M.…Ribeiro E.B. Deleterious effects of lard-enriched diet on tissues fatty acids composition and hypothalamic insulin actions. Prostaglandins Leukotrienes and Essential Fatty Acids. 2015;102–103:21–29. doi: 10.1016/j.plefa.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Ferronato N., Torretta V. Waste mismanagement in developing countries: A review of global issues. International Journal of Environmental Research and Public Health. 2019;16(6):1060. doi: 10.3390/ijerph16061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta. 1991;196(2-3):143–151. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- Huang C.H., Liu S.M., Hsu N.Y. Understanding global food surplus and food waste to tackle economic and environmental sustainability. Sustainability (Switzerland) 2020;12(7):1–18. doi: 10.3390/su12072892. [DOI] [Google Scholar]
- Jia P., Teng J., Zou J., Fang Y.i., Jiang S., Yu X.…Burdmann E.A. Intermittent exposure to xenon protects against gentamicin-induced nephrotoxicity. PLoS ONE. 2013;8(5):e64329. doi: 10.1371/journal.pone.0064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutrition Research. 2014;34(11):907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Kiortsis D.N., Christou M.A. Management of obesity-induced kidney disease: A critical review of the literature. Obesity Facts. 2012;5(6):821–832. doi: 10.1159/000345919. [DOI] [PubMed] [Google Scholar]
- Langi P., Kiokias S., Varzakas T., Proestos C. Carotenoids: From plants to food and feed industries. Methods in Molecular Biology. 2018;1852:57–71. doi: 10.1007/978-1-4939-8742-9_3. [DOI] [PubMed] [Google Scholar]
- Lee Y.-M., Yoon Y., Yoon H., Park H.-M., Song S., Yeum K.-J. Dietary anthocyanins against obesity and inflammation. Nutrients. 2017;9(10):1089. doi: 10.3390/nu9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.-G.…Stadtman E.R. [49] Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Madesh M., Balasubramanian K.A. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian Journal of Biochemistry & Biophysics. 1998;35(3):184–188. [PubMed] [Google Scholar]
- Popkin B.M., Adair L.S., Ng S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutrition Reviews. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin B.M., Corvalan C., Grummer-Strawn L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. The Lancet. 2020;395(10217):65–74. doi: 10.1016/S0140-6736(19)32497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice M., Viafara D., Neill D., Asanza M., Sacchetti G., Guerrini A., Maietti S. Chemical characterization and antioxidant activity of Amazonian (Ecuador) Caryodendron orinocense Karst. and Bactris gasipaes Kunth seed oils. Journal of Oleo Science. 2014;63(12):1243–1250. doi: 10.5650/jos.ess14007. [DOI] [PubMed] [Google Scholar]
- Ruggiero C., Ehrenshaft M., Cleland E., Stadler K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the Kidney despite evident oxidative stress and mitochondrial ROS production. American Journal of Physiology – Endocrinology and Metabolism. 2011;300(6):E1047–E1058. doi: 10.1152/ajpendo.00666.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamarina A.B., Jamar G., Mennitti L.V., de Rosso V.V., Cesar H.C., Oyama L.M., Pisani L.P. The use of Jucara (Euterpe edulis Mart.) supplementation for suppression of NF-B pathway in the hypothalamus after high-fat diet in wistar rats. Molecules. 2018;23(7) doi: 10.3390/molecules23071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamarina A.B., Jamar G., Mennitti Laís.V., Ribeiro D.A., Cardoso C.M., de Rosso V.V.…Pisani L.P. Polyphenols-rich fruit (Euterpe edulis Mart.) prevents peripheral inflammatory pathway activation by the short-term high-fat diet. Molecules. 2019;24(9):1655. doi: 10.3390/molecules24091655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley P. Food and Agriculture Organization of the United Nations (FAO) 2011. Fruit trees and useful plants in Amazonian life. [Google Scholar]
- da Silva Junior G.B., Bentes A.C.S.N., Daher E.D.F., de Matos S.M.A. Obesity and kidney disease. Jornal Brasileiro de Nefrologia: ’orgao Oficial de Sociedades Brasileira e Latino-Americana de Nefrologia. 2017;39(1):65–69. doi: 10.5935/0101-2800.20170011. [DOI] [PubMed] [Google Scholar]
- Tahergorabi Z., Khazaei M. The relationship between inflammatory markers, angiogenesis, and obesity. ARYA Atherosclerosis. 2013;9(4):247–253. http://www.ncbi.nlm.nih.gov/pubmed/23970920 [PMC free article] [PubMed] [Google Scholar]
- Tan B.L., Norhaizan M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules. 2019;24(9):1801. doi: 10.3390/molecules24091801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.L., Norhaizan M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. 2019;11(11):2579. doi: 10.3390/nu11112579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.L., Norhaizan M.E., Liew W.-P.-P. Nutrients and oxidative stress: Friend or foe? Oxidative Medicine and Cellular Longevity. 2018;2018:1–24. doi: 10.1155/2018/9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesauro M., Mascali A., Franzese O., Cipriani S., Cardillo C., Di Daniele N. Chronic kidney disease, obesity, and hypertension: The role of leptin and adiponectin. International Journal of Hypertension. 2012;2012:1–7. doi: 10.1155/2012/943605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V., Morse S., Reisin E. Functional and structural renal changes in the early stages of obesity. Contributions to Nephrology. 2006;151:135–150. doi: 10.1159/000095325. [DOI] [PubMed] [Google Scholar]
- Valenzuela R., Echeverria F., Ortiz M., Rincón-Cervera M.Á., Espinosa A., Hernandez-Rodas M.C.…Videla L.A. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids in Health and Disease. 2017;16(1) doi: 10.1186/s12944-017-0450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valluzzi R.L., Fierro V., Arasi S., Mennini M., Pecora V., Fiocchi A. Allergy to food additives. Current Opinion in Allergy and Clinical Immunology. 2019;19(3):256–262. doi: 10.1097/ACI.0000000000000528. [DOI] [PubMed] [Google Scholar]
- Wu L., Guo X., Wang W., Medeiros D.M., Clarke S.L., Lucas E.A.…Lin D. Molecular aspects of β, β-carotene-9′, 10′-oxygenase 2 in carotenoid metabolism and diseases. Experimental Biology and Medicine. 2016;241(17):1879–1887. doi: 10.1177/1535370216657900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N., Yan S., Guo Y., Wang H., Li X., Wang L.…Cui W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food and Function. 2021;12(11):4768–4782. doi: 10.1039/D1FO00004G. [DOI] [PubMed] [Google Scholar]
- Yin J., Yang D., Zhang X., Zhang Y., Cai T., Hao Y. Diet shift: Considering environment, health and food culture. Science of the Total Environment. 2020;719 doi: 10.1016/j.scitotenv.2020.137484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.