Highlights
-
•
Nanoemulsion with 18% carnauba wax maintained papaya quality by retarding firmness loss, color changes, and reducing respiration rates, resulting in delayed ripening.
-
•
Changes in flavor was not perceived by the panel in tasting samples.
-
•
Coatings protection level was dependent on the storage conditions.
-
•
GEO associated with carnauba nano-emulsions, showed a positive effect in reducing natural diseases.
Keywords: Carica papaya L., Edible coatings, Postharvest quality, Disease control, Volatile compounds
Abstract
Carnauba wax nano and micro-sized emulsions and hydroxypropyl methylcellulose coatings, alone or combined with ginger essential oils (GEO) were applied on papayas and evaluated under several storage conditions. In a first experiment, storage parameters were: 6 days at 22 °C, and 9 days at 13 °C followed by 5 days at 22 °C. In a second experiment, storage was: 5 days at 22 °C, and 10 days at 16 °C followed by 3 days at 22 °C. Coating effects were dependent on storage conditions. While fruits were in cold storage, there were few changes; however, at 22 °C, the differences between coatings became more evident. Nanoemulsions maintained papaya quality during storage by retarding firmness loss, color changes, and reducing respiration rates, resulting in delayed ripening. GEO exhibited some positive effect on fungal disease control. Nanoemulsion-based coatings improved shelf life by reducing weight loss, color development, and slowing ripening of papaya fruit.
1. Introduction
Papaya (Carica papaya L.) is a native fruit of tropical America and is disseminated throughout the tropics. India is the largest global producer of papaya (5.9 million tons), followed by Brazil with a production of 1.1 million tons (FAO, 2022). According to Secex (Brazilian’s Secretariat of Foreign Trade), the Brazilian exportation of papaya fruit has increased annually, having reached 43.6 thousand tons by 2019 (CONAB, 2021).
Papaya, however, is a fragile, highly perishable fruit with a postharvest life of up to four weeks (Pérez-Carrillo & Yahia, 2004). Due to its thin skin, papaya fruit is very susceptible to mechanical damage and postharvest injuries. Additionally, the high volume of water in the mesocarp renders the fruit susceptible to microorganism attack and other physiological disorders (Singh & Rao, 2011).
Estimations of fruit postharvest losses are approximately 30 to 60 percent in both developed and developing countries (FAO, 2019), and additionally, intense fruit handling may reduce the overall quality (Miranda et al 2015). The application of coatings has been considered a valuable strategy in providing additional protection to intact or fresh-cut fruit by forming a semipermeable barrier that lowers water vapor permeability and inhibits microbial adherence and growth. Several types of materials have been proposed as suitable to coat fruits, each having advantages and disadvantages in their applications (Marín et al., 2021).
Particularly, hydrophobic compounds such as carnauba wax, lipid-based formulations, and more hydrophilic biopolymers such as chitosan, starch and cellulose salt derivatives, or their combinations (composites), have been extensively evaluated as protective edible films and coatings (Formiga et al., 2019, Zambrano-Zaragoza et al., 2020, Arroyo et al., 2020, Pestana et al., 2021). Among the composite edible coatings, for example, chitosan and carnauba wax composite coating with oregano essential oil, was effective in reducing water loss and microorganism decay on cucumbers (Gutiérrez-Pacheco et al., 2020). Chitosan and beeswax-pollen grains coating applied on pears resulted in decreased weight loss, decay, and fruit softening during postharvest cold storage for 105 days, followed by 7 days at room temperature (Sultan et al., 2021). Coatings containing chitosan, sodium alginate, carboxymethyl, microcrystalline cellulose, and probiotic strains such as Bifidobacterium lactis, Lactobacillus acidophilus, and Lactobacillus casei improved soft cheese shelf life. Coatings including chitosan and sodium alginate preserved the product's spoilage and retained its stability (El-Sayed et al., 2021).
Essential oil emulsions and nano-emulsions have been studied because of their antioxidant and antimicrobial properties to preserve foods, reduction of numerous pathogens thus showing a promising strategy as an additive to food industry (El-Sayed and El-Sayed, 2021, Al-Tayyar et al., 2020). Several essential oils and their nanoemulsions have been tested and showed the potential to enhance shelf life of food, such as: thyme essential oil nanoemulsion in yogurt (El-Sayed and El-Sayed, 2021), laurel essential oil nanoemulsion on fish (Özogul et al., 2022), ginger oil, individual or combined with coatings, on papaya fruit (Miranda et al., 2021). There are also studies blending different oils in binary and ternary combinations, for example ginger-cinnamon-cardamom essential oil nanoemulsion (Jafarizadeh-Malmiri et al., 2022). The use of nanoemulsions increases bioactivity, physical stability and aime to decrease sensory taste changes (Al-Tayyar, et al. 2020).
Nano-emulsions of carnauba wax and chitosan nanoparticles edible coatings, which are particulate instead of conventional continuous coatings, have been tested on citrus (Miranda et al., 2020), papayas (Ohashi et al., 2015, Zucchini et al., 2021) and apples (Pilon et al., 2015), showing effective reduction of weight loss, retention of firmness and preservation of postharvest attributes. In addition to reducing gas exchange and retarding water loss and ripening, one advantage of coatings is the opportunity to incorporate active compounds in the formulation, such as antimicrobials, enhancing the prevention of microbial spoilage (Campos et al., 2011, Miranda et al., 2021). The incorporation of antimicrobial essential oils in nanoparticulate form seems, in principle, to be more efficient than the addition of fungicides in continuous phase-type coating formulations. It is due to the essential oil bioactivity reduction when challenging an oscillation environmental condition, that is a limitation of their application (Maurya et al., 2021).
Nanocomposites have been observed in materials science and incorporated into edible coatings, as they may achieve new proprieties or improve them. A Nanocomposite pectin-based coating, containing curcumin nanoparticles and ajowan (Carum copticum) essential oil nanoemulsion, combined with gamma-irradiation significantly extended shelf-life of lamb loins and affected microbial and physicochemical qualities of the product (Fallah et al., 2022). Carboxymethyl cellulose/polyvinyl alcohol combined with a copper oxide nanoparticle coating was employed to coat processed cheese, enhancing water vapor permeability, physical and antimicrobial coating properties, and extending the cheese shelf-life during storage (Youssef et al., 2020). In addition, a nanocomposite coating based on carnauba wax nanoemulsion (CWNE) and hydroxypropyl methylcellulose (HPMC), mono and bilayer of the individual components was tested on papaya, showing that CWNE by itself or in bilayer combination with HPMC (HPMC followed by CWNE) demonstrated preservation of fruit quality (Miranda et al., 2019).
Therefore, the aim of the present study was twofold: first, the development and evaluation of carnauba nano and micro-sized emulsions, suitable for coating applications; second, the incorporation of antimicrobial ginger essential oil nanoemulsion (GEO) in coating formulations to inhibit fungal proliferation on papaya fruit. The ginger essential oil was chosen because of its demonstrated antimicrobial activity against several foodborne pathogens (López et al., 2017, Noori et al., 2018). A polysaccharide coating consisting of hydroxypropyl methylcellulose (HPMC), with or without GEO and blended with carnauba wax nanoemulsion, was also evaluated for comparison. HPMC has demonstrated positive effects on papaya quality when combined with nano-sized carnauba, forming a nanocomposite (Miranda et al., 2019).
2. Materials and methods
2.1. Materials
‘Redland’ papayas (Carica papaya L.) were harvested in Homestead, Florida, USA, brought to the USDA Horticulture Research Laboratory (Fort Pierce, FL) and stored overnight at 13 °C until treatment application. Carnauba wax type I flakes was obtained from Strahl & Pitsch (West Babylon, NY, USA). Oleic acid, myristic acid, ammonium hydroxide and HPMC (molar weight ∼ 90,000 da) were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). Commercial food-grade ginger oil from Sigma-Aldrich, purity 97%, CAS Number: 8007-08-7, was used as an antimicrobial agent. Ultrapure water (18 MΩ·cm) was used throughout the experiments.
2.2. Preparation of coatings formulations
2.2.1. Carnauba wax nano and micro-sized emulsion coatings.
A carnauba wax nano and micro-sized emulsions were formulated according to Hagenmaier and Baker (1997) with slight modifications Miranda et al. (2020). Micro-sized emulsion was produced in an open reactor and nano-sized coating obtained by inversion phase of the water in oil (W/O) to oil in water (O/W) system in a closed reactor. The final emulsion was formed by nanosized oil droplets (40.1 nm ± 1.0) and contained micro-sized oil droplets (0.2 to 1.7 µm) dispersed in the water phase previously characterized (Miranda, 2015).
2.2.2. Hydroxypropyl methylcellulose coating (HPMC).
For HPMC coating, 1 g of HPMC was slowly dispersed in 100 mL of hot water at 80 °C and homogenized under magnetic agitation for 5 min. Then, the suspension was left to cool to room temperature and remained stirring overnight. No plasticizer was used in this formulation.
2.2.3. Ginger essential oil nano-emulsion preparation (GEO).
The GEO emulsion was prepared by gradually adding 3% (v/v) ginger oil and 0.6% (v/v) of Tween 80 in distilled water while mixing in an Ultra-Turrax at 16,000 rpm for 4 min for complete homogenization.
2.2.4. Coatings containing GEO.
To incorporate GEO into coatings, 3% (v/v) ginger oil and 0.6% (v/v) Tween 80 were gradually added to the coatings prepared as in 2.2.1 and 2.2.2 (nano or micro-sized emulsion, or HPMC), followed by mixing in an Ultra-Turrax at 16,000 rpm for 4 min to assure homogenized coating.
2.2.5. Composite coating formulation combining wax emulsion (Nano or Micro-sized) with HPMC.
For composite coatings, 1 g of HPMC powder was slowly added to 100 mL of each emulsion (Nano or Micro), under magnetic stirring, and left to homogenize overnight at room temperature. These formulations were named as Nano + HPMC and Micro + HPMC.
2.3. Surface wettability
Surface wettability of papaya peel (15 × 4 mm), coated and uncoated, were analyzed by contact angle measurements using the sessile deionized water drop method (volume ∼ 3 µL) at day 0. Strips of coated or uncoated peel were cut with a scalpel, carefully mounted on glass slides and contact angles automatically registered in a CAN101 Optical Contact Angle Meter (KSV Instruments, Helsinki, Finland). The recorded angles were the average of three measurements per sample, determined using an adaptation of the ASTM D5725-99. The times 1.0, 20.0, 40.0 and 60.0 s were selected as check points. All measurements were repeated five times.
2.4. Particle size and zeta potential
Particle size distribution and zeta potential were obtained using a suspension (1:100) with concentrated coating dispersed in deionized water at room temperature using a Zetasizer Nano ZS (Malvern Instruments Inc., Westborough, MA, USA). The data consist of 10 measurements with five runs each, with a 1 s delay between the runs. All samples were analyzed in four replicates.
2.5. Scanning electron microscopy
Papaya peel, coated and uncoated, were characterized using field emission gun scanning electron microscopy (MEV-SEM JEOL JSM-6701F). For surface micrographs, stripes of papaya peel (coated by dip method) were allowed to dry for 48 h, mounted on carbon slides and gold-coated. For fracture micrographs, samples were frozen in liquid nitrogen and fractured. In the interest of coating characterization, spontaneous air drying was allowed to maintain biological tissue integrity. After the fracture, the samples were dried for 24 h in a desiccator and then gold-coated using an SCD 050 sputter coater (Leica Microsystems, Wetzlar, Germany). The microscopy operated at an acceleration voltage of 10 kV.
2.6. Fruit processing and coating
‘Redland’ papayas (Carica papaya L.) were obtained from a commercial papaya plantation located in Homestead, Florida, USA. The fruit were harvested at the green to breaker maturity stage, sorted, washed, and sanitized by immersion in 100 ppm peroxide acetic acid, for 3 min and air-dried at room temperature in a previously sanitized cold room. Formulations of 9% and 18% carnauba wax emulsions (micro and nano-sized), HPMC and carnauba emulsions with 1% (w/v) HPMC were applied to papaya fruit. The incorporation of GEO, as an antimicrobial agent to some coatings was also evaluated as well as GEO alone. The prepared formulations and coatings solutions are described in Table 1.
Table 1.
Coating formulations applied on papaya fruits.
| Coatings Identification | Formulations |
|---|---|
| Control | Rinsed with distilled water. |
| HPMC | Aqueous solution of 1% HPMC. |
| Nano 9% | Carnauba wax nano-emulsion with 9% of solid phase in suspension. |
| Nano 18 % | Carnauba wax nano-emulsion with 18% of solid phase in suspension. |
| Nano 9 % + HPMC | 1% HPMC incorporated in Nano 9% |
| Micro 9 % | Carnauba wax micro-sized emulsion with 9% of solid phase in suspension. |
| Micro 9 % + HPMC | 1% HPMC incorporated in Micro 9 % coating |
| HPMC + GEO | 1% HPMC with 3% (v/v) ginger essential oil (GEO). |
| Nano 9% + GEO | Carnauba wax nano-emulsion with 9% of solid phase in suspension plus 3% (v/v) of GEO. |
| Nano 9%+HPMC + GEO | Carnauba wax nanoemulsion with 9% plus 1% HPMC with 3% of GEO addition. |
| GEO | Ginger essential oil nano-emulsion prepared at 3% (v/v). |
The coatings were applied manually by placing 2 mL of coating on latex-gloved hands and spreading onto the sanitized papayas. The coated fruit were dried at room temperature. The hand-coating procedure assures the use of a consistent amount of coating per sample with no cross-contamination between fruit (Sun et al., 2015). Two postharvest papaya experiments were conducted. Experiment 1 was comprised of seven treatments: uncoated control, HPMC, Nano 9%, Nano 18 %, Nano 9 % + HPMC, Micro 9 % and Micro 9 % + HPMC (Table 1), divided into two groups and stored at different temperature regimes: 70 fruits were kept at 22 °C for 6 days; and 98 fruits were kept at 13 °C for 9 days, then transferred to a simulated marketing condition of 5 days at room temperature (22 °C). A total of 168 fruits were evaluated, counting destructive and non-destructive analyses. Experiment 2 consisted of evaluating papaya coatings with the addition of GEO as an antimicrobial agent: uncoated control, HPMC, Nano 9%, Nano 18 %, Nano 9 % + HPMC, HPMC + GEO, Nano 9% + GEO, Nano 9% + HPMC + GEO and GEO alone (Table 1). For these, the samples were also divided into two groups as follows: 90 fruits, stored at 22 °C for 5 days; and 126 fruit stored at 16 °C for 10 days before transfer to simulated marketing condition (3 days at 22 °C). A total of 216 fruits were evaluated, counting destructive and non-destructive analyses.
2.7. Postharvest analyzes
2.7.1. Soluble solids, titratable acidity, pH, and ratio.
Before analyzes, papaya juice was prepared. Four papaya fruit per treatment were peeled, seeds discarded, and the pulp cut in cubed pieces of about 2 cm3. The pulp (50 g) was blended in 50 mL of deionized water for 30 s. Soluble solids content (SS) was determined by a refractive index with a digital refractometer (ATAGO PR-101, Tokyo, Japan). Titratable acidity (TA) and pH were calculated using a titration of 10 mL of juice with 0.1 mol/ L of NaOH to pH 8.1 endpoint in an auto titrator (Mettler Toledo DL50, Columbus, USA). The TA was expressed as grams of citric acid per 100 mL of juice. The ratio (SS/TA) was estimated using SS (%) and TA (%) and expressed in absolute values (Baldwin et al., 2012).
2.7.2. Weight loss percentage
Fruit weight was determined to the nearest 0.01 g using a digital scale balance (Metller PJ6000). Weight loss percentage for each fruit was calculated in relation to the initial weight. At the end of each storage segment (chilled or room temperature, after 6, 9, and 14 days for Experiment 1 and, 5, 10, and 13 days for Experiment 2). Six fruits per treatment were individually weighed.
2.7.3. Flesh firmness evaluation
Fruit flesh firmness was measured using a XT Plus Texture Analyzer (Stable Micro Systems, London, UK), equipped with a 50 N load cell and stainless-steel probe with 6 mm diameter. The penetration speed was 1 mm/s to a depth of 5 mm in the fruit equatorial region perpendicular to the probe with the peel removed. Four fruit per treatment with three penetrations per fruit were measured, for each stored sampling time. The firmness (force to penetrate the flesh to a 5 mm depth) results were expressed in Newtons (N).
2.7.4. Skin color determination
Color parameters were determined with a colorimeter Minolta® CR-400 Chroma Meter (Minolta Camera Co., Osaka, Japan), using the CIELAB L*a*b* system. Chroma (C*) and hue angle (ho) were computed from colorimetric unities as C* = (a*2 + b*2)1/2 and h* = arctg (b*/a*), respectively. The instrument was calibrated using a standard white reflector plate. Values were obtained at three different positions in each fruit, in a total of six fruits per treatment at each sampling time.
2.7.5. Internal ethylene, CO2, and O2
Internal gas (10 mL) was withdrawn with a syringe from the internal cavity of four fruit per treatment. Ethylene (C2H4) concentrations were analyzed using a gas chromatograph - GC (Hewlett Packard HP 5890A, Avondale, USA) equipped with a flame ionization detector and a GSQ column (Agilent, Santa Clara, USA) according to Sun et al. (2017). The gas flow rate for He, H2, and air were 10, 35, and 350 mL·min−1, respectively. Temperatures of the oven, injector, and detector were 90, 200, and 250 °C, respectively. The CO2 and O2 concentrations were quantified by GC equipped with a CTR column (Cole-Parmer, Vernon Hills, USA) and a thermal conductivity detector; for these, the temperatures of the oven, injector, and detector were 70, 250, and 250 °C, respectively. The gas flow rate for helium and air was 80 and 350 mL·min−1, respectively.
2.7.6. Aroma volatile analyses
Samples of 15 g of each fruit flesh were rapidly frozen in liquid nitrogen and then stored at − 80 °C. The samples were milled in a blender pre-cooled with liquid nitrogen, and 4.3 g of milled frozen papaya flesh were transferred to 20 mL crimp-capped vial (previously cooled at −25 °C) with the addition of 1.7 mL of saturated sodium chloride solution and stored at − 25 °C. Then samples were thawed at room temperature and loaded into the autosampler (Model MPS2; Gerstel Inc., Linthicum, MD) equipped with a cooled tray holder [a cooling plate (Laird Tech, Sweden) controlled by a Peltier thermostat (CTC Analytics AG, Switzerland)]. Samples were incubated for 30 min at 40 °C. A 2-cm solid phase microextraction (SPME) fiber (50/30 μm DVB/Carboxen/PDMS; Supelco, Bellefonte, PA) was then exposed to the headspace for 62 min at 40 °C. After exposure, the SPME fiber was inserted into the injector of a GC–MS (Model 6890; Agilent Technologies, Santa Clara, CA) to desorb the extract for 15 min at 250 °C, equipped with a DB-5 (60 m length, 0.25 mm i.d., 1.00 μm film thickness; J&W Scientific, Folsom, CA) column coupled with a 5973 N MS detector (Agilent Technologies). The column oven was programmed to increase at 4 °C·min−1 from the initial 40 °C to 230 °C, then ramped at 100 °C·min−1 to 260 °C and held for 11.70 min for a total run time of 60 min. Helium was used as carrier gas at flow rate of 1.5 mL·min−1. Inlet, ionizing source, and transfer line were kept at 250, 230, and 280 °C, respectively. Mass units were monitored from 30 to 250 m/z and ionized at 70 eV. Data were collected using the ChemStation G1701 AA data system (Hewlett-Packard, Palo Alto, CA). A mixture of C-5 to C-18n-alkanes was run at the beginning of each day to calculate retention indices (RIs). Volatile compounds were identified by comparison of their mass spectra with authentic volatile compound standards and/or comparing RIs and library entries (NIST/EPA/NIH Mass Spectral Library, Version 2.0; National Institute of Standards and Technology, Gaithersburg, MA).
2.7.7. Determination of natural disease occurrence and severity
The occurrence of spontaneous disease was evaluated by visual inspection. Each fruit was rated on a 6-point severity scale: 1 (no visible disease), 2 (1% to 20% of the fruit surface area affected), 3 (21% to 40%), 4 (41% to 60%), 5 (61% to 80%) and 6 (81% to 100% of the area affected), (Nunes et al., 2012, Romanazzi et al., 2013). For Experiment 1, samples were evaluated after 6 days at 22 °C- lot 1, and for lot 2- after 6 and 9 days at 13 °C, and those stored 9 days at 13 °C + 5 days at 22 °C. Analyses in Experiment 2 were performed after 5 days of storage at 22 °C (lot 1) and for lot 2 - after 5- and 10-days storage at 16 °C and 3 more days at 22 °C. The data were analyzed as nonparametric tests of significance.
2.7.8. Sensory analyses
Fruit from Experiment 2 were subjected to sensory evaluation. The analyzes were carried out at room temperature under fluorescent light for samples stored 5 days at room temperature, and after 13 days of simulated marketing condition. For flavor evaluation, papayas were peeled, seeds removed, and the pulp cut in cubes of approximately 2 cm3. Samples were presented with a three-digit randomized code each on a plate. A total of 37 panelists rated attributes including sweetness, sourness, papaya flavor, fermented and off-flavor using a 10-point intensity scale (1, where the attribute was barely perceived, to 10 where the attribute was intensely perceived). Fifteen and twenty-two panelists participated in the flavor evaluation of papaya fruit after 5 days at 22 °C and after 10 days at 16 °C + 3 days at 22 °C, respectively.
2.8. Statistical analyses
The univariate parametric analyzes of variance (ANOVA) and multiple comparisons Duncan or Tukey tests were carried out by using the IBM SPSS Statistics software (Inc., Chicago, IL). For sensory analyzes, the scores were compared by means of nonparametric ANOVA and Mann-Whitney U Wilcoxon test. For all tests the level of significance was 5%.
3. Results and discussion
3.1. Coating characterization
3.1.1. Surface wettability, particle size and zeta potential
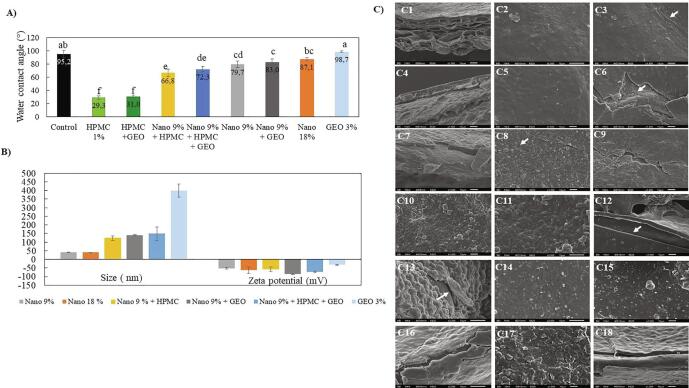
Surface hydrophobicity of coated papayas decreased compared to uncoated fruit, except when coated with GEO or Nano 18%, which had presented similar angles to that of control fruits. HPMC is a polysaccharide and possesses a hygroscopic nature due to the high density of polar functional groups, mainly hydroxypropoxy groups (OCH2CH(OH)CH3), and a higher hydrophilicity (lower contact angles) was fully expected (Fig. 1A). All coating/surface treatments had reduced hydrophobicity compared to the peel of papaya, which consists of the natural waxy cuticle, except for GEO (Fig. 1A). No differences were found among carnauba Nano 9%, Nano 9%+GEO, and Nano 18%. However, the incorporation of 1% (w/v) HMPC into the carnauba solution resulted in a reduction in the hydrophobicity of the coatings compared to those without HPMC (Fig. 1A), except when GEO was included. HPMC alone or with GEO had the lowest hydrophobicity. Nevertheless, adding an additional hydrophobic barrier helps to retard moisture loss, which enhances fruit shelf life. Carnauba Nano 18% and 9% formulations are nano-sized lipid droplet diameters of approximately 40 nm, and when combined with HPMC or GEO the diameter size of the particles in suspensions increased (Fig. 1B). The 3% GEO emulsion coating resulted in the biggest droplet size at around 400 nm. Different carnauba wax emulsion droplets-size (macro and nano) has shown distinct penetration on the cellulose pores sheets, resulting in different properties (de Campos et al., 2019), which perhaps results in different levels of blocking papaya peel stomata. Coatings can cover the fruit epicarp, block cracks and pores, and plug stomata and lenticels. Decreasing transpiration and respiration rate could be correlated with the capacity of coatings to clog stomata and change open to a closed stomata (Thakur et al., 2018, Ncama et al., 2018). All the coatings presented zeta potential values higher than |30| mV (Fig. 1B) indicating colloidal stability according to Attama et al. (2007).
Fig. 1.
A) Water contact angle on uncoated (control) skin papaya and coated papaya surfaces, (mean ± SD, n = 5). B) Particle size and zeta potential of coating formulations. C) Field emission gun scanning electron microscopy (FEG-SEM) of papaya skin uncoated (C1, C2, and C3) and coated with Nano 18% (C4, C5, and C6), Nano 9% (C7, C8, and C9), Nano 9% + GEO (C10 and C11), Nano 9% + HPMC (C12), HPMC (C13 and C14), Micro 9% (C15 and C16), and Micro 9% + HPMC (C17 and C18).
3.1.2. Scanning electron microscopy
The SEM micrographs of uncoated and coated papaya skin are presented in Fig. 1C: Fig. 1C1 shows a cross-section of an uncoated papaya peel sample, which contains some surface roughness (Fig. 1C1 and C2), and small cracks are demonstrated (Fig. 1C3).
Nano-emulsions made with 18% carnauba formed homogeneous layer on the peel surface (Fig. 1C5) with few gaps; however, cracks can be noted at some points, such as in Fig. 1C6. Coatings formed from 9% carnauba had a layer formed (Fig. 1C7), but with a rougher surface and holes (Fig. 1C8) and cracks (Fig. 1C9) when compared to 18% nanoemulsion (Fig. 1C5).
Fig. 1C10 and C11 show Nano 9% + GEO emulsion uniform film formation, and Fig. 1C12 exhibits a rupture from Nano 9% + HPMC emulsion. Furthermore, HPMC coating demonstrated detachments areas (Fig. 1C13) despite an efficient film former (Fig. 1C14). Micro-sized emulsion without HPMC (Fig. 1C16) or with HPMC (Fig. 1C18) still displays ruptures; although, the coating with HPMC produces a thicker film (Fig. 1C17) than without the polymer (Fig. 1C15). Therefore, emulsions formed from 18% carnauba (Fig. 1C5) showed a more compact matrix compared with 9% carnauba (Fig. 1C8) and Nano 9% + GEO (Fig. 1C10 and C11), where cracks can be evidenced on film (Fig. 1C6 and 1C9).
Coatings containing HPMC also demonstrated a consolidation of uniform matrix, confirming its film-forming capacity (Fig. 1C14 and C17); however, as demonstrated, detachments from papaya skin (Fig. 1C13) and coating ruptures (Fig. 1C12) were observed. Cracks and detachments interfere with the barrier property by allowing the increase of gas exchange (Baldwin et al., 1994). The closer microstructural observations reveal surface roughness, which is a consequence of the velocity of solvent evaporation.
3.2. Postharvest quality evaluation
3.2.1 Weight. loss
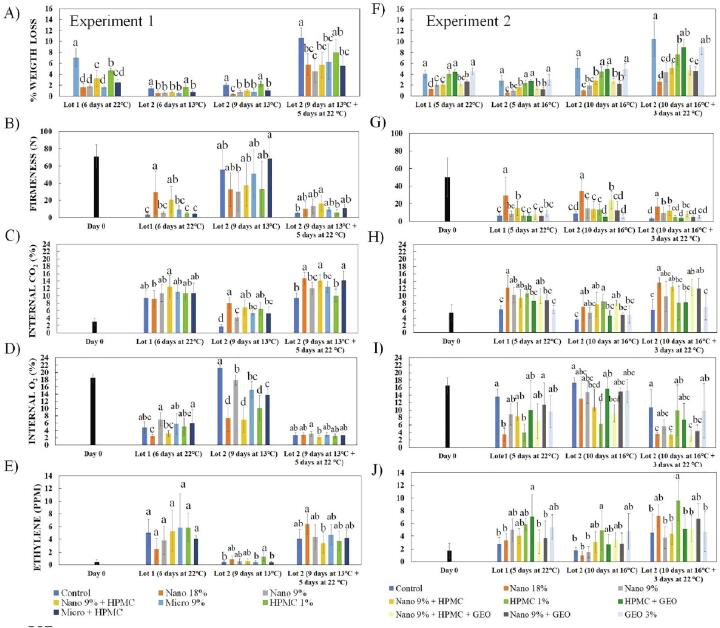
The measurement of papaya fruit weight/water loss from Experiment 1 (Fig. 2A) revealed that all coating formulations generally reduced weight loss. When the fruit was kept in cold storage, minor weight loss occurred. However, when fruit was moved to higher temperatures (22 °C or room temperature), more weight loss occurred. The carnauba-based coatings performed best in reducing weight loss, being more hydrophobic than HPMC- which was least effective (not different from control for fruit chilled then stored at room temperature). HPMC also reduced the effectiveness of carnauba emulsions when combined with them, due to its more hydrophilic nature, for fruit stored 6 days at 22 °C, as reported by Baldwin et al. (1997). Under refrigerated storage (13 °C) (Fig. 2 A), many physiological and biochemical processes are retarded, and as a consequence, the transport of moisture through the skin is reduced, resulting in slight differences in weight loss (below 1%). When the fruit was transferred to room temperature (22 °C), metabolism increased after 5 days of marketing simulation, leading to significant differences in weight loss. In the uncoated controls, an over 10% reduction of weight was measured. Overall, the 18 % carnauba nano-emulsion coatings were not different from 9% carnauba nano-emulsion, except for fruit stored 9 days at 13 °C, and carnauba nano 9% was not different from the carnauba micro 9%.
Fig. 2.
Weight loss (A, F), flesh firmness (B, G), internal carbon dioxide (C, H), oxygen (D, I) and ethylene (E, F) of ‘Redland’ papaya fruit with different coatings in experiment 1 and experiment 2, respectively. For each storage period, columns with different letters are significantly different by Duncan or Tukey test (p < 0.05), applied after ANOVA. Nano: carnauba wax nano-emulsion coating. Micro: carnauba wax micro-sized emulsion coating. HPMC: hydroxypropyl methylcellulose coating, and GEO: ginger essential oil nano-emulsion.
In a study evaluating the effect of carnauba wax nano-emulsion coating on ‘Golden’ papayas at 2.4% concentration stored for 9 days at 22 ± 1 °C and 60%–70% RH, the mass loss was reduced as compared to uncoated fruits (Ohashi et al. 2015). In another study, stomata sealing was observed before cold storage of ‘Pococí hybrid’ papaya treated by commercial wax or combination of hydrothermal (hot water 49 °C for 20 min) and commercial wax (Vargas, Jiménez and Calvo, 2020). A similar phenomenon is probably an additional reason for the decrease in water loss of coated papaya fruits in our study.
For Experiment 2 (Fig. 2F), a similar tendency was observed. Carnauba nano-emulsion coatings proved effective at weight loss control after 5 days at room temperature; the least weight loss occurred for the nano 18% coated papayas, which were not different from nano 9%. The pattern is similar for samples maintained under refrigeration. HPMC coatings were not as effective as the carnauba nano-emulsions, but when combined with the nano-emulsions, they were not different in weight loss inhibition than the nano-emulsion alone. GEO alone did not protect against weight loss, being similar to uncoated controls. There were no differences between carnauba nano 18% and nano 9% for weight loss.
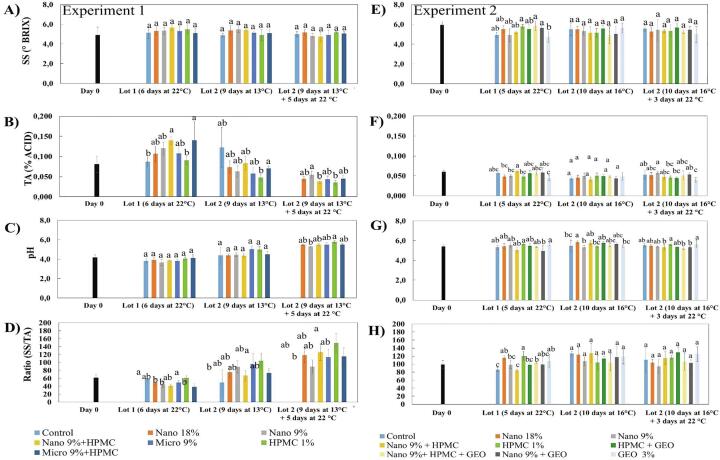
3.2.2. Soluble solids (SS), titratable acidity (TA), pH, and ratio SS/TA.
Experiment 1 resulted in no statistical differences between storage regimes for SS (Fig. 3A). This indicates that there was no significant variation in the sugar content due to the different treatments. Schweiggert et al. (2011) reported a slight increase in SS, from 10.5 to 10.8° Brix, in red-fleshed papaya at different postharvest ripening stages. Sucrose, glucose and fructose are the main sugars in papaya which gradually increase until the fruit is fully ripe (Selvaraj et al.1982). The increase in SS during ripening is hypothesized to be a mechanism of cell wall disassembly providing a source of carbon for sugar synthesis (Fabi et al., 2007, Schweiggert et al., 2011).
Fig. 3.
Papaya coated with different coatings to determine effects on sugar and acid measurements. (A, E) Soluble solids (SS), (B, F) titratable acidity (TA), (C, G) pH, and (D, H) SS/TA ratio values for Experiment 1 and 2, respectively, that were uncoated and coated with different coatings. Columns with different letters are significantly different by Tukey or Games Howell (according to homogeneity of variances) applied after ANOVA one way (p < 0.05) within the storage period.
For TA, nano 9% + HPMC and micro 9% + HPMC exhibited higher % TA than GEO, nano 18% and nano 9% for fruit stored 5 days at 22 °C (Fig. 3B). For fruit stored 9 days at 13 °C, HPMC had the lowest TA and micro 9% + HPMC had the highest. For fruit stored at 9 days at 13 °C + 5 days at 22 °C, nano 9% was greater than nano 9% + HPMC or HPMC alone. A decrease in titratable acidity is related to maturity development, so proportionally higher TA values are interpreted as a delay in the ripening process. The combination of carnauba and HPMC may have modified the internal atmosphere by decreasing O2 and increasing CO2 production, as pointed out by De Medeiros et al. (2012) except for the last storage regime that combined chilled and marketing storage. There were no differences for pH except for 9 days at 13 °C + days at 22 °C where HPMC was greater than nano 9% (Fig. 3C). Nevertheless, a tendency of increasing the pH values over time, is expected and indicates an ongoing ripening process, in which the citric and malic acids (the most common acids in papaya) are diminished (Selvaraj et al., 1982, Schweiggert et al., 2011).
The SS/TA ratio is an important indicator of fruit quality, indicating the balance of sweet/sour tastes. In all experimental conditions in Experiment 1 there was a large fluctuation of SS/TA with values increasing with time (Fig. 3D). For fruit stored 6 days at 22 °C, control and HPMC had higher SS/TA values than nano 9%, nano 9% + HPMC, and micro 9% + HPMC. For fruit stored 9 days at 13 °C, HPMC was greater than control. For fruit stored 9 days at 13 °C + 5 days at 22 °C, HPMC was greater than nano 9%. According to Schweiggert et al. (2011), the SS/TA found for red fleshed papaya at pre-harvest stage increased from 55 to 85, and to 105 during full ripening.
For Experiment 2, the SS assessment (Fig. 3E) after 5 days of room temperature storage, showed that fruits coated with HPMC had greater values than GEO, and nano 9% + HPMC + GEO. There were no differences for the other two storage regimes. For TA of fruit (Fig. 3F) stored 5 days at 22 °C, nano + HPMC was greater than nano 18%, nano 9% and GEO. There were no differences for TA of fruit stored 10 days at 16 °C, however, for fruit stored 10 days at 13 °C + 3 days at 22 °C, nano 9% was greater than HPMC, HPMC + GEO and GEO alone. For pH (Fig. 3G), fruit stored 5 days at 22 °C showed that HPMC + GEO was higher than nano 9% + GEO; for fruit stored for 10 days at 16 °C, nano 18% was greater than control, nano 9% and HPMC + GEO; and for fruit stored 10 days at 13 °C + 3 days at 22 °C, HPMC + GEO was higher than nano 9% + HPMC, nano 9% + HPMC + GEO and nano 9% + GEO.
For SS/TA of fruit (Fig. 3H) stored 5 days at 22 °C, HPMC had higher values compared to control, nano 9%, nano 9% + HPMC, HPMC + GEO and nano 9% + HPMC + GEO. Nano 18%, nano 9% + GEO and GEO alone were greater than control, nano 9% + HPMC and nano 9% + HPMC + GEO. For fruit stored 10 days at 16 °C or 10 days at 13 °C + 3 days at 22 °C, there were no differences. In general, the differences between treatments did not fit a particular pattern.
3.2.3. Flesh firmness
Flesh firmness of fruits was influenced by treatments (Fig. 2B and G) exhibiting a reduction in firmness from an initial 71 N to 30 N. For Experiment 1 (Fig. 2B), fruit stored 6 days at 22 °C, nano 18% was firmer than all other treatments except nano 9% + HPMC. Nano 9% + HPMC was firmer than control, HPMC and micro + HPMC. For fruit stored 9 days at 13 °C, micro + HPMC was firmer than nano 9%. For fruit stored 9 days at 13 °C + 5 days at 22 °C, nano 9% + HPMC was firmer than control and micro + HPMC.
For Experiment 2 (Fig. 2G) fruit stored 5 days at 22 °C, nano 18% was firmer than all other treatments. Nano 9% + HPMC was firmer than control, HPMC, HPMC + GEO, nano 9% + HPMC + GEO and nano 9% + GEO. For fruit stored 10 days at 16 °C, nano 18% again was firmer than all other treatments. Nano 9% + HPMC + GEO was firmer than all except nano 18%, and nano 9%, nano 9% + HPMC and HPMC were firmer than HPMC + GEO and GEO alone. For fruit stored 10 days at 16 °C + 3 days at 22 °C, nano 18% was again firmer than the rest. Nano 9% and nano 9% + HPMC were firmer than control, HPMC, HPMC + GEO, nano 9% + GEO and GEO alone.
Summarizing, nano 18% and nano 9% + HPMC generally seemed to retain fruit firmness while treatments with GEO or GEO alone tended to be less firm. GEO combined with other components somehow interferes withformation of film structure, reducing the prevention of firmness loss. GEO alone does not form a continuous film structure; such effect is observed in the presence of filmogenic matrices, such as HPMC. It’s interesting to note that when GEO was combined with HPMC itself, a similar tendency was observed, proving that GEO in some way affects coating arrangement on the papaya skin. Several enzymes, such as polygalacturonase, β-galactosidase, and pectinesterase are related to ripening and the cell wall degradation that occurs due to the pectin and hemicellulose hydrolysis. Such a process is assumed to be operating during papaya fruit softening during ripening (Fabi et al., 2007). The fruit firmness has a close relationship to temperature. When stored at 13 °C or even 16 °C, fruits were generally firmer than when they were exposed to 22 °C, where ripening and thus loss of firmness is accelerated.
3.2.4. Peel color
Changes in visual color reflect fruit ripening. In papaya, the color changes are characterized by an increase of chroma and hue angle (values increase as peel changes from green to yellow/orange). The color changes are due to synthesis of yellow/orange carotenoids and degradation of green chlorophyll as the fruit ripens (Schweiggert et al., 2011).
During fruit ripening the level of different types of carotenoids determines the peel color and its intensity. A relationship between the color of the peel and total carotenoids can be established through the Chroma quantification (Singh & Rao, 2011), as displayed in Table 2.
Table 2.
Chroma variation (C*) and Hue angle (h*) of Redland coated papaya fruits under different stored conditions for Experiment 1 and 2.
| Treatments | Lot 1(6 days at 22 °C)Average ± SD | Lot 2(6 days at 13 °C)Average ± SD | Lot 2z(9 days at 13 °C)Average ± SD | Lot 2(14 days*)Average ± SD |
|---|---|---|---|---|
| Experiment 1 | Chroma | |||
| Control | 53.4 ± 5.7 a | 34.2 ± 5.1 a | 33.6 ± 6.1 ab | 44.7 ± 2.0 ab |
| Nano 18% | 34.1 ± 4.6 d | 32.1 ± 2.5 a | 33.8 ± 4.0 ab | 39.2 ± 4.4 abc |
| Nano 9% | 47.7 ± 7.6 ab | 24.0 ± 2.2b | 28.2 ± 3.0c | 36.6 ± 5.5 bc |
| Nano 9% + HPMC | 39.7 ± 4.4 cd | 35.1 ± 4.3 a | 38.3 ± 4.4 a | 38.1 ± 8.8 abc |
| Micro 9% | 44.4 ± 7.9 bc | 30.1 ± 3.4 a | 28.9 ± 2.9 bc | 43.6 ± 8.4 ab |
| HPMC | 54.9 ± 8.1 a | 34.4 ± 5.0 a | 35.7 ± 3.7 a | 45.3 ± 8.3 a |
| Micro 9% + HPMC | 43.0 ± 4.6 bc | 30.4 ± 3.1a | 28.7 ± 4.0 bc | 32.2 ± 3.2c |
| Lot 1(5 days at 22 °C) | Lot 2(5 days at 16 °C) | Lot 2 z(10 days at 16 °C) | Lot 2(13 days**) | |
| Experiment 2 | Chroma | |||
| Control | 52.6 ± 5.6 a | 40.2 ± 7.6 a | 44.3 ± 5.7 ab | 49.6 ± 4.1 a |
| Nano 18% | 39.2 ± 5.5 bc | 35.2 ± 6.3 a | 34.9 ± 3.8b | 39.9 ± 9.0 bc |
| Nano 9% | 44.3 ± 7.6 abc | 41.1 ± 4.0 a | 41.0 ± 6.3 ab | 53.0 ± 3.7 a |
| Nano 9% + HPMC | 42.5 ± 2.0 bc | 36.7 ± 6.3 a | 41.6 ± 3.9 ab | 39.5 ± 6.1 bc |
| HPMC | 45.9 ± 3.7 abc | 35.3 ± 8.4 a | 43.5 ± 6.7 ab | 35.8 ± 4.7c |
| HPMC + GEO | 44.4 ± 5.8 abc | 40.7 ± 8.2 a | 49.1 ± 2.5 a | 45.7 ± 5.0 ab |
| Nano 9% + HPMC + GEO | 37.1 ± 9.3c | 34.9 ± 6.0 a | 36.6 ± 6.4b | 40.8 ± 6.4 bc |
| Nano 9% + GEO | 45.0 ± 7.0 abc | 38.2 ± 5.6 a | 44.1 ± 6.9 ab | 52.5 ± 3.2 a |
| GEO | 46.5 ± 9.5 ab | 34.9 ± 10.1 a | 49.9 ± 7.0 a | 41.6 ± 9.4 bc |
| Treatments | Lot 1(6 days at 22°C)Average ± SD | Lot 2(6 days at 13°C)Average ± SD | Lot 2(9 days at 13°C)Average ± SD | Lot 2(14 days*)Average ± SD |
|---|---|---|---|---|
| Experiment 1 | Hue | |||
| Control | 83.3 ± 5.5 d | 108.6 ± 11.3b | 111.8 ± 10.3 a | 88.6 ± 4.4c |
| Nano 18% | 111.2 ± 6.6 a | 114.8 ± 3.6 ab | 112.3 ± 6.5 a | 104.3 ± 4.3 ab |
| Nano 9% | 91.2 ± 6.8c | 120.2 ± 1.6 a | 118.1 ± 2.7 a | 104.2 ± 6.0 ab |
| Nano 9% + HPMC | 103.3 ± 5.1 ab | 113.5 ± 5.7 ab | 112.5 ± 4.5 a | 104.6 ± 10.6 ab |
| Micro 9% | 99.8 ± 9.5 bc | 115.5 ± 3.5 ab | 116.0 ± 3.8 a | 100.9 ± 7.0b |
| HPMC | 94.0 ± 6.6c | 114.2 ± 5.4 ab | 112.9 ± 4.9 a | 101.0 ± 7.5b |
| Micro 9% + HPMC | 93.5 ± 6.4c | 116.1 ± 3.7 ab | 116.9 ± 4.3 a | 110.6 ± 2.4 a |
| Lot 1(5 days at 22 °C) | Lot 2(5 days at 16 °C) | Lot 2(10 days at 16 °C) | Lot 2(13 days**) | |
| Experiment 2 | Hue | |||
| Control | 90.0 ± 5.2b | 106.0 ± 7.6 ab | 96.9 ± 6.2 bcd | 85.9 ± 6.7 d |
| Nano 18% | 108.5 ± 10.6 a | 114.9 ± 8.4 a | 110.9 ± 3.1 a | 100.4 ± 13.6 a |
| Nano 9% | 98.2 ± 8.3 ab | 102.8 ± 8.6 ab | 97.6 ± 8.7 bc | 83.2 ± 3.2 d |
| Nano 9% + HPMC | 104.0 ± 3.9 a | 109.1 ± 9.2 ab | 99.0 ± 4.0 bc | 101.6 ± 7.6 a |
| HPMC | 101.0 ± 7.2 ab | 112.2 ± 7.3 ab | 100.2 ± 6.9b | 104.0 ±5.5 a |
| HPMC + GEO | 97.1 ± 12.2 ab | 101.4 ± 14.8b | 91.0 ± 6.1cd | 90.4 ± 7.2 bcd |
| Nano 9% + HPMC + GEO | 110.2 ±13.3 a | 113.8 ±7.4 a | 108.7 ± 8.3 a | 97.8 ± 9.0 ab |
| Nano 9% + GEO | 97.4 ± 11.9 ab | 109.7 ± 8.0 ab | 99.2 ± 6.7 bc | 86.5 ± 3.7cd |
| GEO | 102.6 ± 10.9 ab | 112.6 ± 9.3 ab | 89.1 ± 7.0 d | 95.5 ± 5.4cd |
*9 days at 13 °C followed by 5 days at 22 °C (simulated marketing storage) ** 10 days at 16 °C followed by 3 days at 22 °C. Columns with different letters are significantly different by Duncan and Tukeyz test (p < 0.05) applied after ANOVA.
During fruit storage, both Experiment 1 and 2 showed the tendency of chroma values to increase for fruit stored at the higher temperature (22 °C). For Experiment 1, differences, again were greatest for fruit stored continuously at 22 °C with control showing higher chroma values compared to all but HPMC, and higher chroma being indicative of a more advanced stage of maturation. For fruit stored 9 days at 13 °C + 5 days at 22 °C, HPMC exhibited higher chroma values than nano 9% and micro 9% + HPMC but was not different from control among other treatments. For chilled storage, there were less meaningful differences. For fruit stored 6 days at 13 °C, nano 9% was lower in chroma than all other treatments. For fruit stored 9 days at 13 °C, Nano 9% + HPMC was higher in chroma than nano 9%, micro 9% and micro 9% + HPMC, but not different from control and other treatments.
For Experiment 2 (Table 2) fruit that experienced 22 °C continuously, control showed the highest chroma value, higher than nano 18%, nano 9% + HPMC and nano 9% + HPMC + GEO. For fruit stored at 10 days 16 °C + 3 days at 22 °C, control along with nano 9%, HPMC + GEO and nano 9% + GEO were higher than the other treatments.
By means of hue angle, it is possible to determine papaya peel color transformation from green to yellow. As papaya fruit ripen, in our case during storage, the peel hue angle decreases as green peel color becomes more yellow (Schweiggert et al., 2011). For experiment 1 (Table 2), at end 6 days at 22 °C, nano 18% was higher in Hue (more green/less ripe) than all other treatments but not different from nano 9% + HPMC. Meanwhile control had the lowest hue value (most yellow/ripe) (Fig. S1-supplementary material). For fruit stored 6 days at 13 °C, there were less differences, but nano 9% was higher in Hue than control. For fruit stored 9 days at 13 °C, there were no differences, showing that cold storage retards ripening and the accompanying change from green to yellow peel color (Fig. S2-supplementary material). For fruit stored 9 days at 13 °C + 5 days at 22 °C, micro 9% + HPMC was higher in hue value than micro 9%, HPMC and control. Control fruits were lowest in Hue (Fig. S3-supplementary material).
For Experiment 2 (Table 2), fruit stored 5 days at 22 °C showed that nano 18%, nano 9% + HPMC and nano 9% + HPMC + GEO fruit were higher in Hue values than control (Fig. S4-supplementary material). For fruit stored 5 days at 16 °C, those treated with nano 18% and nano 9% + HPMC + GEO were higher in Hue than control, similar to those stored at 22 °C. Fruit stored 5 or 10 days at 16 °C (Fig. S5-supplementary material) were similar. For fruit stored 10 days at 16 °C + 3 days at 22 °C, nano 18%, nano 9% + HPMC and HPMC were higher in hue than all but nano 9% + HPMC + GEO. Control and nano 9% were lower than all but HPMC + GEO, nano 9% + GEO and GEO (Fig. S6-supplementary material). Chilled storage at 16 °C in Experiment 2 did not delay ripening-induced color change as effectively at fruit stored at 13 °C in Experiment 1. For coating treatments, controls fruit generally exhibited lower Hue values than coated fruit. Carnauba nano-emulsions at 18% generally had higher Hue values followed by nano or micro-sized emulsions at 9% with HPMC, indicating that those coatings delayed ripening-related color change, and maintained more green color.
3.2.5. Internal CO2, O2 and ethylene contents
3.2.5.1. Internal CO2 and O2
For Experiment 1, the level of internal gases (%), shows that for fruit stored 6 days at 22 °C, there was an increase in CO2 with concomitant reduction in the O2 level compared to day 0 during storage (Fig. 2C and D). The CO2 levels for fruit stored 6 days at 22 °C, there were not a lot of differences, but nano 18% was lower than nano 9% + HPMC. For fruit stored 9 days at 13 °C, nano 18% was higher in CO2 than control, nano 9%, micro 9% and micro + HPMC. For fruit stored 9 days at 13 °C + 5 days at 22 °C, nano 18%, nano 9% + HPMC and Micro + HPMC were higher in CO2 levels than control, nano 9%, micro 9% and HPMC 1%. For O2 levels nano 18% had the lowest followed by nano 9% + HPMC. Nano 9% and micro + HPMC were highest. For fruit stored 9 days at 13 °C, control showed higher O2 levels than all other treatments. Nano 9% and micro 9% were higher in O2 than the other treatments except for micro 9% and micro + HPMC. For fruit stored 9 days at 13 °C + days at 22 °C, nano 9% was higher in O2 than nano 9% + HPMC, otherwise no differences.
For Experiment 2, there was also increased CO2 and decreased O2 compared to day 0 (Fig. 2H and I). In addition, control fruit were generally lower in CO2 and higher in O2 than coated fruit. For fruit stored 5 days at 22 °C, control was lowest in CO2, but not different from HPMC + GEO, nano 9% + GEO or GEO 3%. Nano 18% was higher than those treatments but not different from nano 9%, nano 9% + HPMC, HPMC 1% and nano 9% + HPMC. For fruit stored 10 days at 16 °C, control fruit were lowest in CO2, but not different from HPMC + GEO, nano 9% + GEO or GEO 3%. Conversely, HPMC 1% was highest in CO2 but not different from nano 18%, nano 9%, nano 9% + HPMC and nano 9% + HPMC + GEO. For O2, fruit stored 5 days at 22 °C, control fruit and nano 9% + GEO were highest, and nano 18% and HPMC 1% were lowest. For fruit stored 10 days at 16 °C, control fruit were highest in O2, higher than nano 9% + HPMC, nano 9% + HPMC + GEO and HPMC, which was lowest. For fruit stored 10 days at 16 °C + 3 days at 22 °C, control fruit were highest in O2, higher than nano 18%, nano 9% + HPMC, nano 9% + HPMC + GEO and nano 9% + GEO.
Generally, nano 18%, nano 9% + HPMC, nano 9% + HPMC + GEO were high in CO2 and low in O2. This means that these coating treatments altered the internal atmosphere by restricting gas exchange more than other treatments. Nano 18% has more carnauba material, while nano 9% needed HPMC or HPMC + GEO to significantly affect gas exchange. On the other hand, uncoated control was often low and high in CO2 and O2, respectively, compared to coating treatments. These same treatments generally had lower chroma and higher hue values, indicating restricted ripening as evidenced by peel color having more green color (Table 2). Refrigeration at 16 °C in Experiment 2 was less effective at lowering metabolism/gas exchange than those stored at 13 °C in Experiment 1 in terms of respiration driven CO2/O2 levels. Low levels of internal O2 are measured in parallel with high levels of CO2, mainly inside coated samples. Coatings with low permeability, which retain a large concentration of CO2 could favor an anaerobic/fermentative environment and increase in ethanol production (Baldwin et al., 1994). Nevertheless, edible coatings normally present different degrees of permeability, even for a similar formulation. That occurs due to inevitable formation of irregular structures and thickness during film consolidation, as observed and commented by McHugh and Krochta (1994).
Lipid-based coatings, such as carnauba wax, are more hydrophobic than HPMC, and more effective against moisture/water loss, as confirmed by the firmness measurements. Both materials may act as a barrier to gas exchange (Lin and Zhao, 2007, Ohashi et al., 2015, Miranda et al., 2020). Based on these analyses one can draw the conclusion that nano and micro-sized emulsion of carnauba, as a protective coating on papaya, reduced respiration rate delaying ripening in several conditions of storage.
3.2.5.2. Ethylene (C2H4)
Papaya is a climacteric fruit with endogenous C2H4 gas playing a fundamental role in the regulation of the ripening process. Generally, the ethylene production increases simultaneously with color development, and respiration rate up to the climacteric peak, after which C2H4 levels decrease (Singh & Rao, 2011).
As measured for Experiment 1 (Fig. 2E), C2H4 production was generally suppressed for fruit stored at low temperature (13 °C) and increased during storage after at 22 °C. After 6 days C2H4 levels increased from a baseline of 0.46 ppm on day 0, to an average concentration of 5 ppm for fruit stored 6 days at 22 °C. Due to high variation, the levels of C2H4 were not statically different for fruit stored at this temperature. For fruit stored 9 days at 13 °C, control, micro, and micro + HPMC had the lowest levels, HPMC the highest, and the other treatments intermediate levels of C2H4. For fruit stored 9 days at 13 °C + 5 days at 22 °C, nano 18% had the highest value and nano 9% + HPMC the lowest, otherwise there were no differences.
In Experiment 2 (Fig. 2J), again suppression of C2H4 levels was evident for fruit stored continuously at 16 °C, although the suppression was less than for those stored at continuously at 13 °C in Experiment 1. Also, as in Experiment 1, C2H4 levels increased from initial levels on day 0 for fruit stored 5 days at 22 °C with HPMC + GEO showing the highest level, higher than control, nano 18%, nano 9% + HPMC and nano 9% + GEO. For fruit stored 10 days at 16 °C, HPMC 1% was highest, higher than control, nano 18% and nano 9%. For fruit stored 10 days at 16 °C + 3 days at 22 °C, HPMC 1% was highest, not different from nano 18% or nano 9% + GEO. In general, HPMC 1% or HPMC + GEO coatings resulted in higher C2H4 levels. Nano 18% also showed higher ethylene levels in Experiment 1 for fruit stored 13 °C + 5 days at 22 °C. Control and coatings with nano 9% were often low in ethylene. It is well-known that C2H4 production is oxygen dependent, and low levels of internal O2 can cause a reduction in C2H4 production (Kader, 1983), but treatments that resulted in reduced O2, did not translate into reduced C2H4. It could be that interaction of the coatings on the papaya fruit surface induced some wound response C2H4.
Increases after storage at 22 °C for 5 days was also expected. The initial level of 1.69 reached 4.48 ppm after that period. In fruits coated with HPMC + GEO highest level of C2H4 were measured, which may be related to advanced maturity stages. The other coatings were positioned at intermediate levels. Cold temperature was essential to slow-down ethylene production to an average content of 2.8 ppm after 10 days at 16 °C and after simulated marketing conditions (additional 3 days at 22 °C), the levels increased again, reaching values of 5.86 ppm (Fig. 2J).
3.2.6. Aroma volatile analyses.
Seventy-two volatiles (data not shown) were detected in ´Redland́ papaya from Florida. Benzyl isothiocyanate was the major constituent for all treatments initially and after 6 days at 22 °C. This compound (benzyl isothiocyanate) followed by phenyl acetonitrile were leading constituents in a study with four cultivars from Indonesia and one from Brazil (Ulrich and Wijaya, 2010). Linalool was the major compound identified in ‘Golden’ papaya from Brazil (Gomes et al., 2016); whereas methyl butanoate and ethyl butanoate were the main compounds for ‘Red Maradol’ papaya from Cuba (Pino, 2014).
In Experiment 1, after 6 days at 22 °C, benzyl isothiocyanate, butanoic acid, benzaldehyde and oxime-methoxy-phenyl were the four most abundant compounds in the samples in this order of area response (data not shown). Benzyl isothiocyanate is a compound that has odor description and is well known as papaya. Butanoic acid is often described as buttery with a fruity nuance and reported by Ulrich and Wijaya (2010), known as a typical stinky and unpleasant odor of papaya.
Table 3 shows a list of flavor compounds recognized as papaya flavor contributors (Ulrich and Wijaya, 2010, Pino, 2014). It is a tentative to identify among the numerous volatile compounds present, which volatiles are causing an impact on papaya aroma. Nine of twenty-five compounds considered to contribute to papaya odor identified by SPME GC-O by Pino (2014) were detected in 'Redland' fruit from Florida. Benzyl isothiocyanate, ethyl butanoate, methyl benzoate, 1-hexen-3-one, and E-β-ionone were the most odor-active constituents reported by those authors. Interestingly, the first three compounds were found in our study. Those authors reported a total of 118 constituents by GC–MS in contrast to 72 compounds identified in 'Redland'.
Table 3.
List of aroma volatiles detected in ‘Redland’ papaya coated with different coatings and which have demonstrated odor-activity contributing to recognition of overall aroma papaya.
| Compoundb | Odour | Base line | Control | Nano 18% | Nano 9% | Nano 9% + HPMC | Micro 9% | HPMC 1% | Micro + HPMC |
|---|---|---|---|---|---|---|---|---|---|
| acetaldehydec | winey | 0.41 ± 0.12 a | 0.71 ± 0.15 a | 1.07 ± 0.53 a | 0.9 ± 0.23 a | 2.24 ± 1.62 a | 0.41 ± 0.09 a | 1.07 ± 0.18 a | 1.31 ± 0.08 a |
| 1-penten-3-onec | fruity | 0.51 ± 0.05 a | 0.76 ± 0.38 a | 0.77 ± 0.31 a | 0.36 ± 0.07 a | 0.31 ± 0.08 a | 0.31 ± 0.13 a | 0.71 ± 0.03 a | 0.65 ± 0.01 a |
| methyl butanoatec | intense fruity | 0.08 ± 0.01b | 0.66 ± 0.17 ab | 0.41 ± 0.08b | 0.29 ± 0.12b | 0.25 ± 0.06b | 1.10 ± 0.39 a | 1.18 ± 0.29 a | 0.08 ± 0.03b |
| 2-methyl-1 butanolc | winey | 0.17 ± 0.01b | 0.00b | 0.24 ± 0.12b | 0.00b | 0.74 ± 0.26 a | 0.00b | 0.00b | 0.00b |
| butanoic Acid d | stinky | 0.00c | 63.24 ± 20.33 bc | 48.10 ± 9.05 bc | 48.18 ± 38.82 bc | 68.42 ± 58.71 bc | 184.64 ± 70.2 a | 122.09 ± 33.24 ab | 7.68 ± 0.33 bc |
| ethyl butanoatec | intense fruity | 0.26 ± 0.01b | 1.71 ± 0.98b | 0.86 ± 0.35b | 0.00b | 18.45 ± 10.40a | 0.00b | 0.31 ± 0.01b | 0.00b |
| hexanal c, d | herbaceous | 30.25 ± 10.69 a | 2.11 ± 1.00b | 12.88 ± 8.64 ab | 3.12 ± 1.35b | 8.89 ± 7.37b | 1.92 ± 0.94b | 1.42 ± 0.35b | 3.31 ± 0.01b |
| γ-octalactone d | flowery | 0.41 ± 0.01 a | 0.37 ± 0.11 a | 0.37 ± 0.08 a | 0.33 ± 0.06 a | 0.31 ± 0.05 a | 0.29 ± 0.07 a | 0.35 ± 0.04 a | 0.29 ± 0.00a |
| limonenec | citrus | 1.14 ± 0.40 a | 0.51 ± 0.16 ab | 0.41 ± 0.02b | 0.23 ± 0.05b | 0.46 ± 0.20b | 0.15 ± 0.04b | 0.00b | 0.00b |
| cis-linalool oxide (furanoid) c | woody | 0.67 ± 0.01 a | 0.00b | 0.00b | 0.38 ± 0.31 a | 0.00b | 0.46 ± 0.01 a | 0.00b | 0.00b |
| nonanal d | herbaceous | 9.57 ± 2.06 a | 0.00c | 5.90 ± 0.01b | 0.00c | 3.98 ± 0.01b | 0.89 ± 0.01c | 0.00c | 0.00c |
| benzyl isothiocyanate c, d | papayac, smokeyd | 288.80 ± 26.10 ab | 93.84 ± 33.09de | 230.44 ± 71.93bc | 69.87 ± 10.44e | 100.31 ± 11.39de | 83.37 ± 18.25e | 375.06 ± 0.01a | 187.07 ± 0.09cd |
a RI – Retention index on DB-5 column.
Values expressed by GC–MS area response × 106 - average of 4 fruits per treatment. Identification by matching RI and/ or mass spectra from libraries NiST129 by equal ≥ 90. Different letters in the same row indicate significant differences by Duncan test (p < 0.05) applied after ANOVA. Odor-active compound identified by SPME GC-O for Pino (2014)c; Ulrich & Wijaya (2010)d
Five compounds of a total twelve compounds reported by Ulrich & Wijaya (2010) as character odorants to overall papaya aroma by GC-O were also identified in our study and their trends may help to understand a typical aroma bouquet of papaya that is a result of a combination of several aroma compounds. Hexanal, (Z)-2-pentenol, nonanal, (Z)-linalool oxide, linalool, butanoic acid, verbenone, phenyl methyl ester of butanoic acid, γ-octalactone and isothiocyanato benzene were the most important compounds to impact odorants among 49 volatiles compounds measured by GC-O in five varieties of papaya studied for Ulrich & Wijaya (2010). Identification of key aroma volatile are essential to describe and understand the fruit flavors and aroma perception (Zhou et al., 2021).
The highest amount of butanoic acid was shown for fruits coated with Micro 9% and HPMC 1% and could give undesirable characteristics, thus this compound is described as papaya's nasty and unpleasant odor. For uncoated fruits, the level of acetaldehyde increased, which was predictable during storage. However, fruits coated with Nano 18%, Nano 9 + HPMC, Micro + HPMC, and HPMC coatings exhibited high levels of these well-known off-flavor compounds. The lowest level was found for Nano 9% and Micro 9% coatings, which created a more suitable layer. Edible coatings can induce a modified atmosphere into fruits by decreasing O2 and increasing CO2 levels (El Hadi et al., 2013). On the other hand, some coatings can cause an anaerobic respiration condition, causing fruits to produce high levels of ethanol and acetaldehyde (off-flavor compounds), trapping off-flavors in the fruits (Baldwin et al., 1999).
Benzyl isothiocyanate (characteristic odor of papaya) showed reduced levels during storage compared to uncoated fruit at baseline and after 6 days of storage. Uncoated fruits and fruits coated with Nano 9%, Nano 9% + HPMC, and Micro 9% coatings exhibited low values, whereas HPMC coating followed by Nano 18% and Micro + HPMC coatings exhibited high levels.
Methyl butanoate and ethyl butanoate (intense fruity odours) are important esters that give the fruity top notes in oranges juice (Plotto et al., 2008), and they may play an important role in papaya flavor (Pino, 2014). Those components increased during storage for uncoated fruits and had higher values for fruits coated with Nano 9% + HPMC coating, followed by HPMC 1% and Micro 9% coatings than other treatments. On the other hand, hexanal compound responsible for herbaceous odor descriptor reduced intensely during storage. The highest level after 6 days at 22 °C was exhibited in fruits coated with Nano 18% coating, followed by Nano 9% + HPMC coating that remains higher levels than other lots of fruits. Nano 9% + HPMC coating, followed by Nano 18% showed higher amounts of 2-methyl-1-butanol compared to control (null production after 6 days of storage), which odor description is winey odor. Cis-linalool oxide (furanoid) and nonanal, related with herbaceous and woody notes, also had statistically significant production in fruits treated with coatings compared to null uncoated fruit production.
3.2.7. Sensory analyzes
The evaluation of fruits by panelists was conducted in samples from Experiment 2, after 5 days at 22 °C and after 10 days at 16 °C + 3 days at 22 °C. For each treatment the data was obtained for all measured attributes (sweetness, sourness, papaya flavor, fermented flavor, and off-flavor). There were no significant differences for any attributes, which indicates that no flavor differences were perceptible by panelists between treatments (Figs. S7 and S8-supplementary material), therefore, the effect of coatings in the fruit internal atmosphere (O2 and CO2) were not enough to cause detectable fermentation or other off-flavors.
3.3. Effect of coatings on disease development.
3.3.1. Natural diseases severity
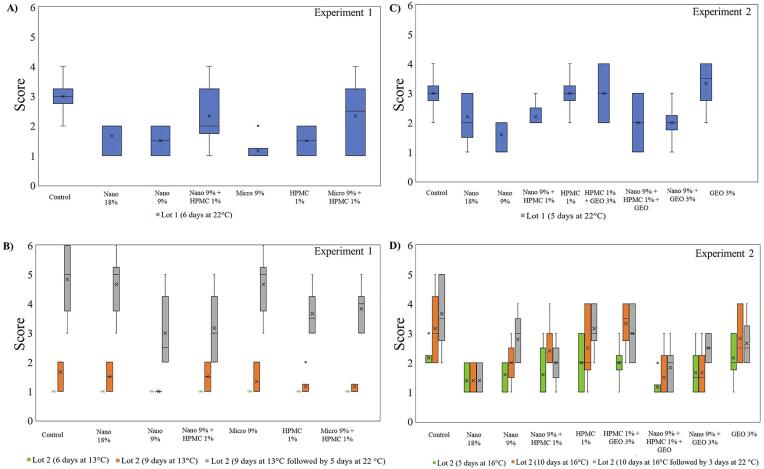
On a 6-point scale, a high severity of natural decay was recorded for samples from Experiment 1 during room temperature (Fig. 4 A and B). By storing at 22 °C (Fig. 4A), all samples undergo deterioration over time, however the uncoated fruits resulted in higher disease severity compared to other treatments (median 3). Although the reasons are not clear, they may be due to the structural heterogeneities formed during coating formation, the results indicate that combining HPMC with carnauba micro and nano-sized emulsions resulted in increased incidence of natural decay. Pure carnauba coatings (Nano 18%, 9%, and Micro) performed better in decay prevention, even at 22 °C (Fig. 4A). For lot 2, fruit stored for 6 and 9 days at 13 °C no visual difference in the level of disease severity was evident. When the fruit were transferred to simulated marketing conditions (5 days at 22 °C), the uncoated papayas developed the highest disease severity (Fig. 4B). Under these conditions, the Nano 9% followed by Nano 9 % + HPMC coatings resulted in the lowest disease severity (Fig. 4B) demonstrating a positive effect in protecting papaya peel from natural diseases.
Fig. 4.
Natural disease severity scores of ‘Redland’ papaya fruits with different coatings from Experiment 1 (A and B) and 2 (C and D). 6-point severity scale (1 is no visible disease to 6–81% to 100% of the area affected). Nano: carnauba wax nano-emulsion coating; Micro: carnauba wax micro-sized emulsion coating; HPMC: hydroxypropyl methyl cellulose coating.
The same trend was observed for Experiment 2, for fruit stored at either 5 days at 22 °C (Fig. 4C) or 10 days at 16 °C + 3 days at 22 °C (Fig. 4D). For fruit stored 5 days at 22 °C, fruits coated with HPMC, GEO or in their combination, presented higher disease incidence than that of uncoated fruits. Coatings containing carnauba wax nano-emulsion, with and without GEO, exhibited less diseases severity (Fig. 4C). Similar results were reported by Miranda et al. (2021) using carnauba wax nanoemulsion coating alone or in association with GEO at 3% or 9%, which showed fungal disease control on coated papaya fruit storage six days at 22 °C.
For lot 2, fruit stored 10 days at 16 °C or after simulated marketing conditions showed slight differences in disease severity (Fig. 4D). A tendency in higher disease incidence was observed for control samples (uncoated fruits), as well as for HPMC coatings with or without GEO, and GEO alone. It is interesting to note that the combination of HPMC and GEO did not result in a reduction in disease severity. The lowest disease severity, after simulated marketing conditions (22 °C), was observed for papayas coated with nano 18% and nano 9% + HPMC + GEO.
4. Conclusions
Fruit coatings promote extension of postharvest shelf life and quality maintenance. The most effective formulation for delaying the ripening process was the carnauba 18% nano-emulsion, which reduced over three times the loss of firmness compared to uncoated fruit, formed a gas barrier that slowed fruit respiration, both at 22 °C and under refrigeration. Coating with nano-emulsions (9% and 18%) also delayed around 20% chroma peel color development under the different storage conditions evaluated. HPMC combined with nano-emulsions also resulted in effective fruit protection while delaying papaya ripening, but the effect can be attributed to the presence of carnauba in the coatings. The carnauba emulsions also proved to be a good barrier to gas exchange, resulting in low internal O2 levels (∼2 to 10% versus 5 to 21% of O2 for uncoated papaya), which contributes to retardation of ripening. Changes in flavor was not perceived by the panel. Treatment with GEO did not contribute antimicrobial activity as was expected. When associated with carnauba nano-emulsions, a positive effect was observed in reducing natural diseases occurrence over time. In short, by considering the overall results, it is evident that the application of carnauba nanoemulsion-based coatings, improved shelf life and maintained papaya fruit quality, by reducing weight loss, color changes and slowing the ripening process.
CRediT authorship contribution statement
Marcela Miranda: Conceptualization, Methodology, Project administration, Validation, Formal analysis, Investigation, Data curation, Funding acquisition, Writing – original draft, Writing – review & editing, Visualization. Xiuxiu Sun: Methodology, Formal analysis. Ana Marín: Methodology, Formal analysis. Luana Cristina dos Santos: Formal analysis, Visualization. Anne Plotto: Methodology, Resources, Writing – review & editing. Jinhe Bai: Methodology, Resources, Writing – review & editing. Odílio Benedito Garrido Assis: Conceptualization, Methodology, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing, Visualization. Marcos David Ferreira: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing. Elizabeth Baldwin: Methodology, Validation, Resources, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Disclaimer: Mention of a trademark or proprietary product is for identification only and does not imply a guarantee or warranty of the product by the U.S. Department of Agriculture. The U.S. Department of Agriculture prohibits discrimination in all its programs and activities on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, and marital or family status.
Acknowledgments
Acknowledgments
The authors also would like to thank Milene C. Mitsuyuki, from Embrapa Instrumentation for statistical analyzes support; David Wood, Holly Sisson, Elena Branca and Nancy Owens from ARS Horticultural Research Laboratory- USDA for laboratory assistance and Guilherme da Cruz Silva from Federal University of São Carlos, Department of Biotechnology for his contact angle and particle size data acquisition. We also thank CAPES, FAPESP, Embrapa and USDA for their support, scholarships, and funding.
Funding sources
This study was financed by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001 and PDSE/201688881.132643/2016-01; São Paulo Research Foundation (FAPESP - grant 16/23419-5 and 18/10657-0), Brazilian National Council for Scientific and Technological Development - CNPq (grant# 407956/2016-6, Research Productivity Fellowship 310728/2019-3) and Empresa Brasileira de Pesquisa Agropecuária (Embrapa) from Brazil; and U. S. Department of Agriculture.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100249.
Contributor Information
Marcos David Ferreira, Email: marcos.david@embrapa.br.
Elizabeth Baldwin, Email: liz.baldwin@usda.gov.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Al-Tayyar N.A., Youssef A.M., Al-Hindi R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustainable Materials and Technologies. 2020;26:e00215. doi: 10.1016/j.susmat.2020.e00215. [DOI] [Google Scholar]
- Arroyo B.J., Bezerra A.C., Oliveira L.L., Arroyo S.J., Melo E.A.d., Santos A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.) Food Chemistry. 2020;309:125566. doi: 10.1016/j.foodchem.2019.125566. [DOI] [PubMed] [Google Scholar]
- Attama A.A., Schicke B.C., Paepenmüller T., Müller-Goymann C.C. Solid lipid nanodispersions containing mixed lipid core and a polar heterolipid: Characterization. European Journal of Pharmaceutics and Biopharmaceutics. 2007;67(1):48–57. doi: 10.1016/j.ejpb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Baldwin E.A., Bai J., Plotto A., Cameron R., Luzio G., Narciso J.…Ford B.L. Effect of extraction method on quality of orange juice: Hand-squeezed, commercial-fresh squeezed and processed. Journal of the Science of Food and Agriculture. 2012;92(10):2029–2042. doi: 10.1002/jsfa.v92.1010.1002/jsfa.5587. [DOI] [PubMed] [Google Scholar]
- Baldwin E.A., Burns J.K., Kazokas W., Brecht J.K., Hagenmaier R.D., Bender R.J., Pesis E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biology and Technology. 1999;17(3):215–226. doi: 10.1016/S0925-5214(99)00053-8. [DOI] [Google Scholar]
- Baldwin E.A., Hagenmaier R., Bai J. 2ed. Boca Raton:CRC Press; 1994. Edible Coatings and Films to Improve Food Quality; p. 392. [Google Scholar]
- Baldwin E.A., Nisperos M.O., Hagenmaier R.H., Baker R.A. Use of lipids in edible coatings for food products. Food Technology. 1997;51:56–62. [Google Scholar]
- Campos C.A., Gerschenson L.N., Flores S.K. Development of edible films and coatings with antimicrobial activity. Food and Bioprocess Technology. 2011;4(6):849–875. doi: 10.1007/s11947-010-0434-1. [DOI] [Google Scholar]
- CONAB – Companhia Nacional de Abastecimento. Boletim Hortigranjeiro v. 7, n.2., 2021. Available in: https://www.conab.gov.br/info-agro/hortigranjeiros-prohort/boletim-hortigranjeiro/item/download/35982_f6cb91e559a891d7d9b0ec4cab761905.
- de Campos, A., Claro, P. C., Luchesi, B. R., Miranda, M., Souza, F. V., Ferreira, M. D., & Marconcini, J. M. (2019). Curaua cellulose sheets dip coated with micro and nano carnauba wax emulsions. Cellulose, 26(13), 7983-7993. Doi: 10.1007/s10570-019-02637-0.
- El Hadi M.A.M., Zhang F.J., Wu F.F., Zhou C.H., Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18:8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed H.S., El-Sayed S.M., Mabrouk A.M., Nawwar G.A., Youssef A.M. Development of eco-friendly probiotic edible coatings based on chitosan, alginate and carboxymethyl cellulose for improving the shelf life of UF soft cheese. Journal of Polymers and the Environment. 2021;29(6):1941–1953. doi: 10.1007/s10924-020-02013-1. [DOI] [Google Scholar]
- El-Sayed S.M., El-Sayed H.S. Antimicrobial nanoemulsion formulation based on thyme (Thymus vulgaris) essential oil for UF labneh preservation. Journal of Materials Research and Technology. 2021;10:1029–1041. doi: 10.1016/j.jmrt.2020.12.073. [DOI] [Google Scholar]
- Fabi, J. P., Cordenunsi, B. R., de Mattos Barreto, G. P., Mercadante, A. Z., Lajolo, F. M., & Oliveira do Nascimento, J. R. (2007). Papaya fruit ripening: response to ethylene and 1-methylcyclopropene (1-MCP). Journal of Agricultural and Food chemistry, 55, 6118-6123. https://doi.org/ 10.1021/jf070903c. [DOI] [PubMed]
- Fallah A.A., Sarmast E., Habibian Dehkordi S., Isvand A., Dini H., Jafari T.…Mousavi Khaneghah A. Low-dose gamma irradiation and pectin biodegradable nanocomposite coating containing curcumin nanoparticles and ajowan (Carum copticum) essential oil nanoemulsion for storage of chilled lamb loins. Meat Science. 2022;184:108700. doi: 10.1016/j.meatsci.2021.108700. [DOI] [PubMed] [Google Scholar]
- FAO. (2019). The State of Food and Agriculture 2019. Moving forward on food loss and waste reduction. Rome. Licence: CC BY-NC-SA 3.0 IGO. http://www.fao.org/state-of-food-agriculture/2019/en/.
- FAO. (2022). Food and Agricultural Organization of the United Nations. 2022. Crops—Extent, causes and prevention. 05 Jan. 2022. https://www.fao.org/faostat/en/#data/QC.
- Formiga A.S., Pinsetta J.S., Pereira E.M., Cordeiro I.N.F., Mattiuz B.-H. Use of edible coatings based on hydroxypropyl methylcellulose and beeswax in the conservation of red guava ‘Pedro Sato’. Food Chemistry. 2019;290:144–151. doi: 10.1016/j.foodchem.2019.03.142. [DOI] [PubMed] [Google Scholar]
- Gomes B.L., Fabi J.P., Purgatto E. Cold storage affects the volatile profile and expression of a putative linalool synthase of papaya fruit. Food Research International. 2016;89:654–660. doi: 10.1016/j.foodres.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Pacheco M.M., Ortega-Ramírez L.A., Silva-Espinoza B.A., Cruz-Valenzuela M.R., González-Aguilar G.A., Lizardi-Mendoza J., Miranda R., Ayala-Zavala J.F. Individual and combined coatings of chitosan and carnauba wax with oregano essential oil to avoid water loss and microbial decay of fresh cucumber. Coatings. 2020;10(7):614. doi: 10.3390/coatings10070614. [DOI] [Google Scholar]
- Hagenmaier R.D., Baker R.A. Edible coatings from morpholine-free wax microemulsions. Journal of Agricultural and Food Chemistry. 1997;45(2):349–352. doi: 10.1021/jf9604551. [DOI] [Google Scholar]
- Jafarizadeh-Malmiri H., Anarjan N., Berenjian A. Developing three-component ginger-cinnamon-cardamom composite essential oil nanoemulsion as natural food preservatives. Environmental Research. 2022;204:112133. doi: 10.1016/j.envres.2021.112133. [DOI] [PubMed] [Google Scholar]
- Kader A.A. In: Lieberman M., editor. vol 46. Springer; Boston, MA: 1983. Postharvest Quality Maintenance of Fruits and Vegetables in Developing Countries. (Post-Harvest Physiology and Crop Preservation). [Google Scholar]
- Lin D., Zhao Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Comprehensive Reviews in Food Science and Food Safety. 2007;6(3):60–75. doi: 10.1111/crfs.2007.6.issue-310.1111/j.1541-4337.2007.00018.x. [DOI] [Google Scholar]
- López E.I.C., Balcázar M.F.H., Mendoza J.M.R., Ortiz A.D.R., Melo M.T.O., Parrales R.S., Delgado T.H. Antimicrobial activity of essential oil of Zingiber officinale Roscoe (Zingiberaceae) American Journal of Plant Sciences. 2017;8:1511–1524. doi: 10.4236/ajps.2017.87104. [DOI] [Google Scholar]
- Marín A., Baldwin E.A., Bai J., Wood D., Ference C., Sun X., Brecht J.K., Plotto A. Edible Coatings as Carriers of Antibrowning Compounds to Maintain Appealing Appearance of Fresh-cut Mango. HortTechnology. 2021;31(1):27–35. doi: 10.21273/HORTTECH04687-20. [DOI] [Google Scholar]
- Maurya A., Singh V.K., Das S., Prasad J., Kedia A., Upadhyay N., Dubey N.K., Dwivedy A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Frontiers in Microbiology. 2021;12:751062. doi: 10.3389/fmicb.2021.751062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh T.H., Krochta J.M. Milk-protein-based edible films and coatings. Food Technology. 1994;48:97–103. [Google Scholar]
- de S. Medeiros B.G., Pinheiro A.C., Carneiro-da-Cunha M.G., Vicente A.A. Development and characterization of a nanomultilayer coating of pectin and chitosan–Evaluation of its gas barrier properties and application on ‘Tommy Atkins’ mangoes. Journal of Food Engineering. 2012;110(3):457–464. doi: 10.1016/j.jfoodeng.2011.12.021. [DOI] [Google Scholar]
- Miranda, M. (2015). Revestimento nanoestruturado de cera de carnaúba na manutenção da qualidade pos-colheita de tomates. MS Thesis, Federal University of Sao Carlos, Sao Paulo, Brazil. 14 Oct. 2021. https://repositorio.ufscar.br/bitstream/handle/ufscar/8588/DissMM.pdf?sequence=1&isAllowed=.
- Miranda M., Spricigo P.C., Ferreira M.D. Mechanical damage during harvest and loading affect orange postharvest quality. Engenharia Agrícola. 2015;35(1):154–162. doi: 10.1590/1809-4430-Eng.Agric.v35n1p154-162/2015. [DOI] [Google Scholar]
- Miranda, M., Sun, X., Assis, O.B.G., Ference, C., Ferreira, M.D., & Baldwin, E.A. (2021). Antifungal activity of Zingiber officinale Roscoe (ginger) oil and extracts, associated with carnauba wax nanoemulsions, on fungal control of harvest papaya. Acta Horticulturae. 1325, 199-198 https://doi.org/10.17660/ActaHortic.2021.1325.28.
- Miranda M., Sun X., Ference C., Plotto A., Bai J., Wood D., Assis O.B.G., Ferreira M.D., Baldwin E. Nano-and Micro-Carnauba Wax Emulsions versus Shellac Protective Coatings on Postharvest Citrus Quality. Journal of the American Society for Horticultural Science. 2020;1:1–10. doi: 10.21273/JASHS04972-20. [DOI] [Google Scholar]
- Miranda, M.; Gozalbo, A.M.; Sun, X.; Plotto, A.; Bai, J.; de Assis, O.; Ferreira, M.; Baldwin, E. (2019). Effect of mono and bilayer of carnauba wax based nano-emulsion and HPMC coatings on post-harvest quality of ‘redtainung’ papaya. In Proceedings of the Embrapa Instrumentação-Artigo em anais de congresso (ALICE), São Carlos, Brazil, 3–5 December 2019. http://www.alice.cnptia.embrapa.br/alice/handle/doc/1116606.
- Ncama K., Magwaza L.S., Mditshwa A., Tesfay S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packaging and Shelf Life. 2018;16:157–167. doi: 10.1016/j.fpsl.2018.03.011. [DOI] [Google Scholar]
- Noori S., Zeynali F., Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. doi: 10.1016/j.foodcont.2017.08.015. [DOI] [Google Scholar]
- Nunes, M.C.N., Morais, A.M.M.B., Brecht, J.K., Sargent, S.A., Bartz, J.A., Allen, R.A., Lee, J.H., Pires, D.M., & Pittet-Moore, J. (2012). Occurrence of gray mold in stored strawberries as affected by ripeness, temperature, and atmosphere. Proceedings of the Florida State Horticultural Society. 125, 287–294.
- Ohashi, T. L., Pilon, L., Spricigo, P. C., Miranda, M., Corrêa, D. S., & Ferreira, M. D. (2015). Postharvest quality of ‘golden’ Papayas (Carica papaya. L.) coated with carnauba wax nanoemulsions. Revista Iberoamericana de Tecnología Postcosecha, 16, 199-209. Avaiable in: https://www.redalyc.org/pdf/813/81343176008.pdf.
- Özogul Y., El Abed N., Özogul F. Antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens and fish spoilage bacteria. Food Chemistry. 2022;368:130831. doi: 10.1016/j.foodchem.2021.130831. [DOI] [PubMed] [Google Scholar]
- Pérez-Carrillo, E.; & Yahia, E.M. (2004). Effect of postharvest hot air and fungicide treatments on the quality of ‘Maradol’ papaya (Carica papaya L.). Journal of Food Quality, 27,127-139. Avaiable in: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1745-4557.2004.tb00643.x.
- Pestana G.C., dos Santos K.A.F., Miranda M., Spricigo P.C., Mitsuyuki M.C., Bresolin J.D., Hubinger S.Z., Assis O.B.G., Ferreira M.D. Effects of carnauba wax and chitosan bilayer edible coating on shelf life of fresh-cut apple. Acta Horticulturae. 2021;1325:215–224. doi: 10.17660/ActaHortic.2021.1325.31. [DOI] [Google Scholar]
- Pilon L., Spricigo P.C., Miranda M., de Moura M.R., Assis O.B.G., Mattoso L.H.C., Ferreira M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. International Journal of Food Science and Technology. 2015;50(2):440–448. doi: 10.1111/ijfs.2015.50.issue-210.1111/ijfs.12616. [DOI] [Google Scholar]
- Pino J.A. Odour-active compounds in papaya fruit cv. Red Maradol. Food Chemistry. 2014;146:120–126. doi: 10.1016/j.foodchem.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Plotto A., Margaría C.A., Goodner K.L., Baldwin E.A. Odour and flavour thresholds for key aroma components in an orange juice matrix: Esters and miscellaneous compounds. Flavour and Fragrance Journal. 2008;23(6):398–406. doi: 10.1002/ffj.v23:610.1002/ffj.1888. [DOI] [Google Scholar]
- Romanazzi G., Feliziani E., Santini M., Landi L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Post-harvest Biology and Technology. 2013;75:24–27. doi: 10.1016/j.postharvbio.2012.07.007. [DOI] [Google Scholar]
- Schweiggert R.M., Steingass C.B., Mora E., Esquivel P., Carle R. Carotenogenesis and physico-chemical characteristics during maturation of red fleshed papaya fruit (Carica papaya L.) Food Research International. 2011;44(5):1373–1380. doi: 10.1016/j.foodres.2011.01.029. [DOI] [Google Scholar]
- Selvaraj, Y., Pal, D. K., Subramanyam, M. D., & Iyer, C. P. A. (1982). Changes in the chemical composition of four cultivars of papaya (Carica papaya L.) during growth and development. Journal of Horticultural Science, 57, 135-14, 1982. Doi: 10.1080/00221589.1982.11515033.
- Papaya (Carica papaya L.). Post-Harvest Biology and Technology of Tropical and Subtropical Fruits. 2011;vol. 4:86–124. [Google Scholar]
- Sultan M., Hafez O.M., Saleh M.A., Youssef A.M. Smart edible coating films based on chitosan and beeswax–pollen grains for the postharvest preservation of Le Conte pear. RSC Advances. 2021;11(16):9572–9585. doi: 10.1039/D0RA10671B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Baldwin E., Ritenour M., Hagenmaier R., Bai J. Formulating a natural colorant containing wax for a one-step color-add application for fresh citrus. HortScience. 2017;52:408–412. doi: 10.21273/HORTSCI11534-16. [DOI] [Google Scholar]
- Sun X., Baldwin E., Ritenour M., Plotto A., Bai J. Evaluation of natural colorants and their application on citrus fruit as alternatives to Citrus Red No. 2. HortScience. 2015;50:1353–1357. doi: 10.21273/HORTSCI.50.9.1353. [DOI] [Google Scholar]
- Thakur R., Pristijono P., Golding J.B., Stathopoulos C.E., Scarlett C.J., Bowyer M.…Vuong Q.V. Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina) Scientia Horticulturae. 2018;237:59–66. doi: 10.1016/j.scienta.2018.04.005. [DOI] [Google Scholar]
- Ulrich D., Wijaya C.H. Volatile patterns of different papaya (Carica papaya L.) varieties. Journal of Applied Botany and Food Quality. 2010;83:128–132. [Google Scholar]
- Vargas T., Jiménez M., Calvo P. Effect of hydrothermal postharvest treatment on skin ultrastructure of papaya (Carica papaya L.) Pococí hybrid. Acta Horticulture. 2020;1278:19–28. doi: 10.17660/ActaHortic.2020.1278.4. [DOI] [Google Scholar]
- Youssef A.M., Assem F.M., El-Sayed H.S., El-Sayed S.M., Elaaser M., Abd El-Salam M.H. Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese. RSC Advances. 2020;10(62):37857–37870. doi: 10.1039/D0RA07898K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano-Zaragoza M.L., Quintanar-Guerrero D., Del Real A., González-Reza R.M., Cornejo-Villegas M.A., Gutiérrez-Cortez E. Effect of nano-edible coating based on beeswax solid lipid nanoparticles on strawberry’s preservation. Coatings. 2020;10:253. doi: 10.3390/coatings10030253. [DOI] [Google Scholar]
- Zhou Z., Ford R., Bar I., Kanchana-Udomkan C. Papaya (Carica papaya L.) Flavour Profiling. Genes. 2021;12:1416. doi: 10.3390/genes12091416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchini, N.M., Florencio, C., Miranda, M., Borba, K.R., Oldoni, F.C.A., Oliveira Filho, J.G., Bonfim, N.S., Rodrigues, K.A., de Oliveira, R.M.D., Mitsuyuki, M.C., Hubinger, S.Z., Bresolin, J.D. & Ferreira, M.D. (2021). Effect of carnauba wax nanoemulsion coating on postharvest papaya quality. Acta Horticulturae. 1325, 199-206. Doi: 10.17660/ActaHortic.2021.1325.29.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.