Highlights
-
•
Nontargeted and targeted metabolomics studies of JHKC tea germplasm were performed.
-
•
61 differential metabolites were identified in wild JHKC accessions compared to CK.
-
•
Theacrine and 1,3,7-trimethyluric acid accumulated in JHKC but were absent in CK.
-
•
JHKC germplasm has > 10-fold non-epi-form and methylated flavanol contents.
-
•
JHKC is promising for breeding tea cultivars with high theacrine and methylated flavanols.
Keywords: Jianghua Kucha, Metabolomics, Nonvolatile metabolites, Theacrine, LC-MS
Abstract
Jianghua Kucha (JHKC) is a special tea germplasm with high bitterness growing in China; however, the chemical characteristics of JHKC are not completely understood. In this study, 61 differential metabolites were identified between 11 wild JHKC individuals and 3 control cultivars of Fudingdabai, Yunkang 10, and Zhuyeqi using comprehensive nontargeted and targeted metabolomics approach. The JHKC accessions mainly possessed significantly higher levels of purine alkaloids of theacrine (12.06 ± 5.23 mg/g) and 1,3,7-trimethyluric acid, non-epi-form flavanols (catechin, gallocatechin, catechin gallate, and gallocatechin gallate), and methylated flavanols of epigallocatechin-3-O-(3″-O-methyl)-gallate (4.79 ± 1.45 mg/g) and epicatechin-3-O-(3″-O-methyl)-gallate (1.02 ± 0.34 mg/g), as well as significantly lower levels of flavonol glycosides, which indicated that caffeine metabolism, flavonoid biosynthesis, and flavonol and flavone biosynthesis are mostly differential metabolic pathways. Our study demonstrated that JHKC germplasm is a promising resource for breeding novel tea cultivars with high contents of theacrine, non-epi-form flavanols, and methylated flavanols.
1. Introduction
Tea (Camellia sinensis) is one of the most popular and oldest non-alcoholic beverage crop species worldwide, and its health benefits have attracted widespread attention. China is likely the place of origin of tea plants and there are>3000 tea accessions and 900 bred cultivars (Ma, Yao, & Chen, 2015).
Kucha (Camellia sinensis) is a unique tea plant variety that tastes much more bitter than common tea cultivars widely planted in China and is primarily distributed in Yunnan, Hunan, Guangdong, Jiangxi and Guizhou provinces. Although Kucha has a strong bitter taste, locals enjoy drinking it to promote health. Previous studies have demonstrated that Kucha tea extract has relatively high antioxidative activity (Li, et al., 2012) and significantly suppressed the accumulation of lipid droplets (Li, et al., 2016). Jianghua Kucha [JHKC, Camellia sinensis var. assamica (CSA) cv. Jianghua] is an ancient and specific tea germplasm resource (Li, & Luo, 2011) that grows in the Nanling Mountain region in Hunan Province, Southeastern China. JHKC plants exist as semi-arbor or small-arbor trees with an upright shape, and their height varies from 2.5 to 6 m. The morphological characteristics of JHKC differ among population accessions; for example, white-leaf Kucha, golden-leaf Kucha, cyan-leaf Kucha, bamboo-leaf Kucha, and salix-leaf Kucha, each of whose leaves are morphologically distinct (Liu, 2006). Unique germplasms constitute the foundation for the breeding of new varieties, and an increasing number of tea tree breeders are focusing on the amounts of functional ingredients of germplasms, e.g., low amount of caffeine, no caffeine, high amount of theanine, high amount of flavanols, high amount of methylated catechin, and high amount of theacrine (Chen, Apostolides & Chen, 2013; Yu et al., 2020, Qin et al., 2020). At present, three cultivars have been bred from JHKC by the use of traditional breeding method (individual selection), namely, Jianghua Kucha 21–1 (JHKC-21–1), Jianghua Kucha 21–3 (JHKC-21–3) and Jianghua Kucha 37–2 (JHKC-37–2), all of which are suitable for the production of black tea (Li, Huang, Zhong, & Su, 2018).
JHKC is highly diverse, and there is an abundance of different populations of JHKC accessions. However, most researchers have analyzed the contents of only common nonvolatile compounds (for instance, water extracts, flavanols, amino acids, caffeine, and theobromine, etc.) in fresh leaves of JHKC and bred cultivars from JHKC (JHKC-B) (Li, 2012, Yang, 2013). Both a comprehensive investigation of nonvolatile metabolites and identification of new components in JHKC are needed. Nontargeted metabolomics combined with chemometric analysis enables the comprehensive detection of nonvolatile metabolites and has been widely used in tea plant studies (Dai et al., 2018, Li et al., 2019).
To comprehensively understand the chemical constitutions of JHKC germplasms and to utilize JHKC germplasms effectively for breeding, in this work, we investigated the differential accumulation of nonvolatile metabolites of tea varieties among representative JHKC plants, common tea cultivars and JHKC-B cultivars, based on nontargeted and targeted metabolomics analysis with ultra-high-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC-QTOF/MS) and ultra-performance liquid chromatography triple quadrupole mass spectrometry (UPLC-QqQ). This study provides novel insight into the metabolite profile and intrinsic metabolic mechanisms of JHKC germplasms.
2. Materials and methods
2.1. Chemicals
Formic acid and methanol (LC-MS grade) were purchased from TIC (Tokyo, Japan) and Merck (Darmstadt, Germany), respectively. Deionized ultrapure water was obtained from Millipore (Bedford, MA, USA). Flavanols of epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), epicatechin (EC), gallocatechin gallate (GCG), gallocatechin (GC), catechin gallate (CG), and catechin (C), 1,3,7-trimethyluric acid, kaempferol-3-glucoside, quercetin-3-glucoside, quercetin-3-galactoside, myricetin 3-galactoside, naringenin, valine, tryptophan, arginine, proline, theogallin, chlorogenic acid and 5-methoxysalicylic acid were obtained from Sigma (St. Louis, MO, USA). Theacrine, theobromine and caffeine were purchased from Yuanye (Shanghai, China). Procyanidin B1, procyanidin B2 and procyanidin B3 were gained from ChemFaces (Wuhan, Hubei, China). Epiafzelechin and epiafzelechin 3-gallate were obtained from Mreda (Beijing, China) and Asiabio (Basel, Switzerland), respectively. Epigallocatechin-3-O-(3″-O-methyl)-gallate (EGCG3″Me) and epicatechin-3-O-(3″-O-methyl)-gallate (ECG3″Me) were purchased from Nagara Science Co., Ltd. (Gifu, Japan). Phenylethyl primeveroside and benzyl primeveroside were synthesized by staff at the National Glycoengineering Research Center, Shandong University, Jinan, China (Li et al., 2019).
2.2. Materials
Three experimental tea groups were included in this study (Fig. 1). The JHKC group comprised 11 JHKC [Camellia sinensis var. assamica (CSA) cv. Jianghua] individuals of local accessions whose morphology was representative. The control (CK) group comprised three common tea cultivars: Fudingdabai [FDDB, Camellia sinensis var. sinensis (CSS)], Yunkang 10 (YK10, CSA), and Zhuyeqi (ZYQ, CSS). FDDB originates from Fujian Province of Southeastern China, YK10 originates from Yunnan Province of Southwestern China, and ZYQ originates from Hunan Province of Southcentral China. The JHKC-B group comprised three cultivars bred from local JHKC varieties, namely, JHKC-21–1 (CSA), JHKC-21–3 (CSA) and JHKC-37–2 (CSA). These three JHKC-bred cultivars were registered in 2019 under the following registration numbers: GPD tea plant (2019)430028, GPD tea plant (2019)430031, and GPD tea plant (2019)430029.
Fig. 1.
Workflow of samples collection and metabolomics investigation of JHKC accessions in this study: (1) the metabolomes of 11 wild JHKC individuals (JHKC group) and of three CK cultivars (FDDB, YK10, and ZYQ) were compared to investigate the most differential metabolites and differential metabolic pathways in JHKC; (2) three JHKC-bred cultivars were surveyed to further study the changes in differential metabolites after artificial breeding and cultivation.
The individuals in the JHKC group were collected from a mountainous region at elevations ranging from 400 to 600 m in Jianghua County, Hunan Province, while the CK and JHKC-B groups were collected from tea plantations of the Tea Research Institute, Hunan Academy of Agricultural Sciences, Changsha, Hunan, China. One bud with two leaves was removed from the tea plants on April 11–16, 2020, in triplicate, frozen in liquid nitrogen, and then freeze dried. The freeze-dried tea leaves were subsequently ground into a powder using a mill (IKA; Staufen, Germany) and then stored at −20 °C until use.
2.3. Tea sample preparation
Tea compounds were extracted according to the methods of our previous study, with minor adjustments (Dai, et al., 2020). Fifteen mL of a prewarmed 70% (v/v) methanol solution was added to 100 mg of tea powder, after which the mixture was placed in a water bath at 70 °C for 0.5 h. The solution was subsequently centrifuged at 8000 × g for 15 min (Centrifuge 5810R, Eppendorf, Hamburg, Germany), after which the supernatant was filtered through a 0.22 μm membrane for metabolomics analysis and absolute quantification of the compounds. Each tea sample was prepared in triplicate. Quality control (QC) samples were prepared by mixing 100 μL aliquots of each tea infusion.
2.4. Nontargeted metabolomics analysis
Nontargeted metabolomics analysis was conducted according to our previous methods (Li et al., 2018, Dai et al., 2020). Briefly, an UHPLC system (Infinity 1290, Agilent Technologies, Santa Clara, CA, USA) connected to a Q-TOF/MS instrument (6545, Agilent Technologies, Santa Clara, CA, USA) was used for the metabolomics analysis in ESI + mode. The compounds in the tea samples were separated with a Zorbax Eclipse Plus C18 column (150 × 3.0 mm, 1.8 μm, Agilent Technologies, Little Falls, DE, USA), and the column temperature was maintained at 40 °C; phase A consisted of 0.1% (v/v) formic acid-deionized water, phase B consisted of methanol at a flow rate of 0.4 mL/min, and the injection volume was 3 μL. The linear gradient elution procedure was as follows: 10% B (0–3 min), 10–15% B (3–4 min), 15–30% B (4–7 min), 30–50% B (7–16 min), 50–95% B (16–20 min), 95% B (20–22 min), 95–5% B (22–22.05 min), and 5% B (22.05–26 min). The MS parameters were set as follows: the temperature and the flow rate of the drying gas were 325 °C and 10 L/min, respectively; the temperature and the flow rate of the sheath gas were maintained at 325 °C and 11 L/min, respectively; the capillary voltage was 4 kV; the nebulizer pressure was 40 psi; and the mass scan range was m/z 100–1000. QC samples were injected after every nine samples to monitor the reproducibility of the metabolomic analysis results.
The raw metabolomics data acquired from UHPLC − QTOF/MS were first treated for peak picking using MassHunter Qualitative Analysis software (B.07.00 SP1, Agilent Technologies, Santa Clara, CA, USA) and for peak alignment using Mass Profiler Professional (version 13.0, Agilent Technologies, Santa Clara, CA, USA). The signal-to-noise (S/N) threshold for peak detection was set to 3. The retention time tolerance and mass tolerance for the peak alignment was set to 0.2 min and 0.01 Da, respectively. The obtained compound ion features with relative standard deviations (RSD) of mass intensities<30% in QC samples were used for multivariate and univariate analysis. Compounds were structurally identified based on authentic standards, MS2 spectra, mass accuracy, metabolomics databases (Metlin and HMDB), and the previous works (Dai et al., 2015, Dai et al., 2017, Yang et al., 2018, Chen et al., 2020).
2.5. Absolute quantifications of flavanols, methylated catechins, and alkaloids
The absolute amounts of eight flavanols (EGCG, EGC, ECG, EC, GCG, GC, CG, and C), two methylated flavanols (ECG3′'Me and EGCG3′'Me), and three alkaloids (theobromine, caffeine and theacrine) were quantified by an UPLC-PDA-TQS system (Waters, Manchester, UK) in accordance with an external standard method according to our previous work (Dai, Hu, Xie, Tan, & Lin, 2020).
2.6. Statistical and data analysis
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed using SIMCA 14.1 software (Umetrics, Umeå, Sweden). Statistical analyses were performed using SPSS 21 software (IBM, Chicago, IL, USA) via one-way ANOVA and Student’s t-tests. A heat map was generated by MultiExperiment Viewer 4.9.0 (Oracle, Redwood, CA, USA), and pathway analysis was performed by MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/).
3. Results and discussion
3.1. Differential metabolites between JHKC accessions and CK cultivars
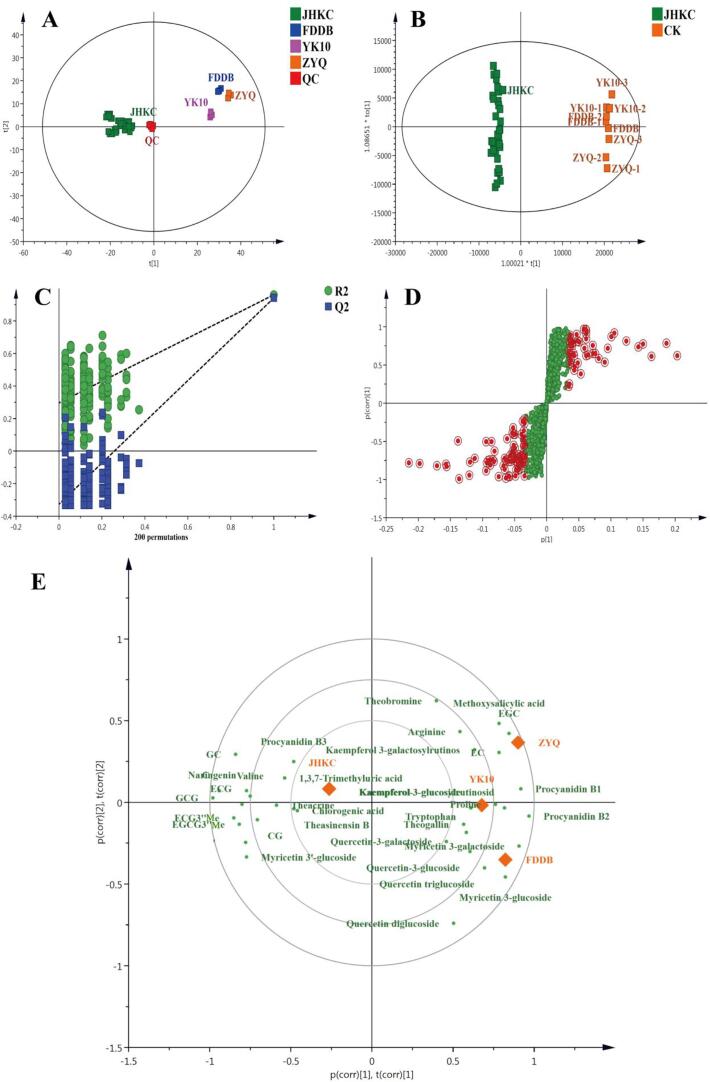
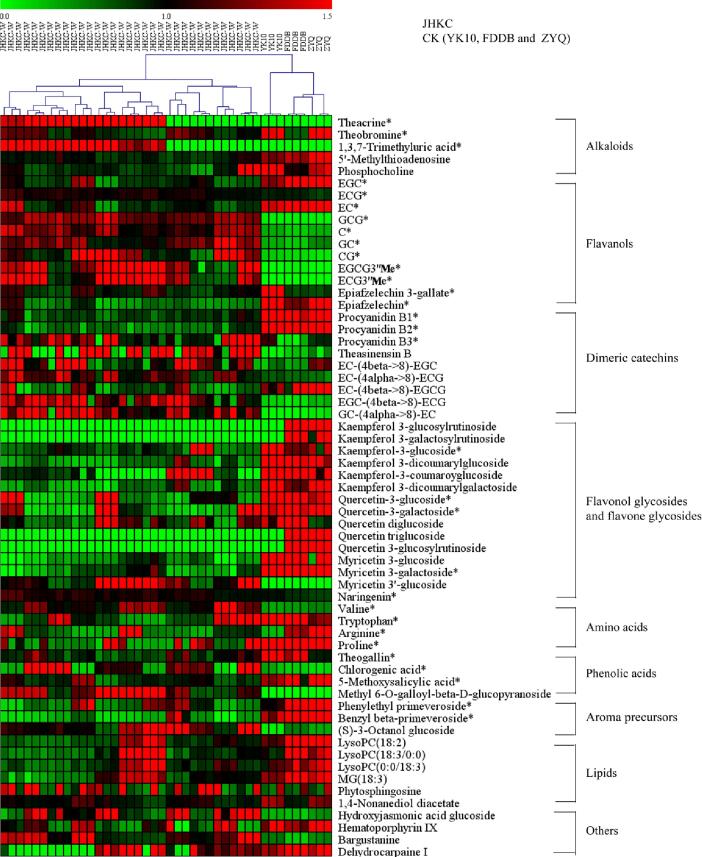
To comprehensively investigate the differential metabolites between wild JHKC (11 JHKC individuals) and CK (FDDB, YK10 and ZYQ) cultivars, the compounds in tea samples were measured via nontargeted metabolomics analysis by the use of UHPLC-QTOF/MS. A total of 3226 metabolite ion features were acquired after peak extraction and alignment. Among them, the RSD of 2258 metabolite ion features were <30% in QC samples; these were used for further statistical analysis. The QC samples clustered together at the center of the PCA score plot (Fig. 2A), indicating good reproducibility of the metabolomic analysis. In addition, the JHKC and CK samples were clearly divided into two groups via PCA and PLS-DA (Fig. 2A-B). The JHKC accessions clustered on the left of the PCA and PLS-DA score plots, while the CK group accessions (FDDB, YK10 and ZYQ) clustered on the right. These results indicated that the metabolite patterns of the JHKC accessions and CK cultivars are significantly different. The R2 and Q2 intercepts were 0.296 and −0.327, respectively, indicating that the PLS-DA model was not overfitted (Fig. 2C). A total of 146 compounds (Table S1) were ultimately structurally identified or tentatively identified, among them, 61 compounds, including five alkaloids, 11 flavanols, nine dimeric flavanols, 15 flavonol/flavone glycosides, four amino acids, four phenolic acids, three aroma precursors, six lipids and four other compounds, were screened out as the main differential metabolites between the JHKC and CK groups (shown as red dots in the S-plot, Fig. 2D). A PCA biplot (in which tea samples are shown as orange diamonds and in which metabolites are shown as green dots) was constructed to display the correlations between the tea samples and the 61 main differential compounds (Fig. 2E). Metabolites with relatively high content in the tea samples are located in the same quadrant. In addition, a heatmap was created to visualize the variations of the 61 differential metabolites in the JHKC and CK groups (Fig. 3). The tea samples clustered into two groups, a JHKC group and a CK group, which was consistent with the PCA and PLS-DA results (Fig. 2A-B). In the heatmap, a red color indicates that the content of a metabolite is higher than the average level among the samples, while a green color indicates that the content of a metabolite is at a lower level. The results clearly showed that, compared with CK group (FDDB, YK10, and ZYQ), the JHKC group mainly contains higher contents of non-epiform flavanols of C, GC, CG, and GCG, purine alkaloids of theacrine and 1,3,7-trimethyluric acid, methylated flavanols of EGCG3″Me and ECG3″Me, myricetin-3′-glucoside, ECG, procyanidin B3, and valine. In contrast, the levels of most flavonol glycosides (kaempferol, quercetin and myricetin glycosides), EC, EGC, procyanidin B1, procyanidin B2, theobromine, theogallin, arginine, proline, and tryptophan were lower in the JHKC group than in the CK group (Fig. 2E and Fig. 3). Notably, purine alkaloids of theacrine and 1,3,7-trimethyluric acid, methylated flavanols of EGCG3″Me and ECG3″Me, non-epi-form flavanols of CG and GCG were 10-fold higher contents in the JHKC group than in the CK group; these compounds could therefore be considered characteristic compounds of JHKC germplasms. On the other hand, the contents of most flavonol glycosides decreased, even the flavonol glycosides with trisaccharide moieties, such as kaempferol 3-glucosylrutinoside, kaempferol 3-galactosylrutinoside, quercetin triglucoside, and quercetin 3-glucosylrutinoside, were absent in the JHKC group.
Fig. 2.
Multivariate statistical analysis of JHKC accessions and CK cultivars (FDDB, YK10, and ZYQ): (A) PCA score plot; (B) PLS-DA score plot, R2X = 79.1%, R2Y = 99.6%, Q2 = 98.7%; (C) cross-validation plot of PLS-DA model with 200 permutation tests; (D) S-plot of PLS-DA (the red dots represent the most differential compounds); (E) PCA biplot of the levels of differential metabolites in JHKC accessions and CK cultivars (FDDB, YK10, and ZYQ). The orange diamonds represent tea samples, and the green dots represent metabolites. Positive correlations are present between any two variables located in the same quadrant.
Fig. 3.
Heat map of levels of differential metabolites between JHKC accessions and CK cultivars (FDDB, YK10, and ZYQ). Note: *, compounds verified by authentic standards. The data represent the ratios of the individual content to the average content.
3.2. Differences in metabolic pathways between JHKC accessions and CK cultivars
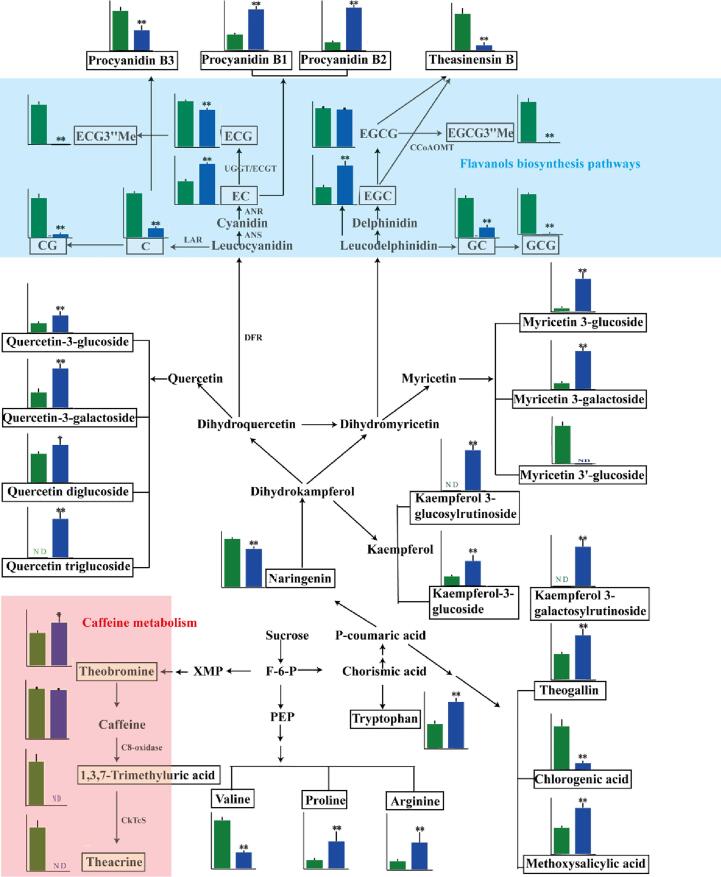
To understand the differences in the metabolic pathways between the JHKC accessions and CK cultivars, metabolic pathway analysis of the 61 differential metabolites was performed by MetaboAnalyst 4.0 (Fig. S1). The differential metabolites were involved mainly in the metabolic pathways associated with flavonoid biosynthesis, caffeine metabolism, flavone and flavonol biosynthesis, arginine biosynthesis, arginine and proline metabolism, valine-leucine and isoleucine biosynthesis, aminoacyl-tRNA biosynthesis, glycerophospholipid metabolism, and pantothenate and CoA biosynthesis. These results suggested that the flavonoid biosynthesis, caffeine metabolism, and flavone and flavonol biosynthesis pathways are the most critical metabolic pathways that differ between JHKC accessions and CK tea cultivars. Hence, the changes of critical metabolites involved in these pathways were further mapped, the results of which are shown in Fig. 4.
Fig. 4.
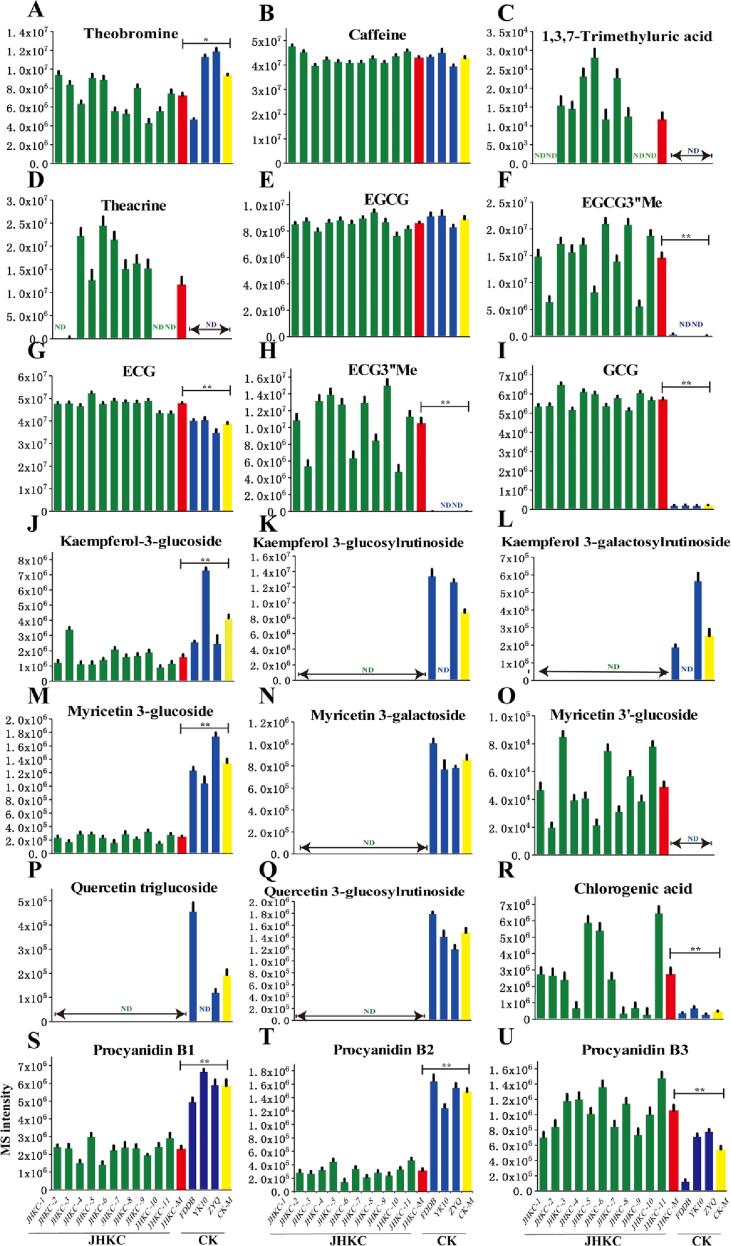
Metabolic pathways of differential metabolites between JHKC accessions and CK cultivars (FDDB, YK10, and ZYQ). The Y axis represents the MS intensity of the compound detected via LC-MS. The data represent the mean ± SD. The green and blue columns represent JHKC accessions and CK cultivars, respectively. *, P < 0.05; **, P < 0.01.
Flavonoids are metabolic products of flavonoid biosynthesis, flavone and flavonol biosynthesis pathways, mainly including flavanols, dimeric flavanols and flavonol/flavone glycosides. As shown in Fig. 4 and Table 1, as JHKC belonging to CSA accessions, the composition and content of flavanols in JHKC was partly consistent with the findings of previous studies (Jin et al., 2014, Yu et al., 2020). For instance, Jin, et al. (2014) reported that CSA accessions have relatively high contents of C, EC and ECG and that CSS accessions have a relatively high content of EGC; Yu, et al. (2020) demonstrated that C, EC, ECG and GC accumulated to high levels in CSA accessions or hybrid accessions with a CSA genetic background. Interestingly, our results showed that EC accumulated at lower levels in the JHKC accessions than in the CK cultivars [YK10 (CSA accession), FDDB (CSS accession) and ZYQ (CSS accession)]. Furthermore, GCG was present at a high level (17.73 ± 1.29 mg/g, ranging from 15.75 to 19.65 mg/g) in the JHKC, while was absent in the CK cultivars (FDDB, YK10, and ZYQ) (Table 1). In general, EGCG and EGC were present at the highest levels in the tea plants, followed by ECG, EC, and C (Yu, et al., 2020). However, EGCG (64.09 ± 5.17 mg/g) and GCG (17.73 ± 1.29 mg/g) were the most abundant flavanols in the JHKC accessions. Taken together, these results suggested that JHKC is a unique tea germplasm resource.
Table 1.
Quantitative analysis of alkaloids, flavanols and methylated flavanols in tea samples by UPLC-PDA-TQS (x ± SD, n = 3, mg/g).
| mg/g |
JHKC |
CK |
JHKC-B |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 JHKC individuals | Average | FDDB | YK10 | ZYQ | Average | JHKC-21–1 | JHKC-21–3 | JHKC-37–2 | Average | |
| TB | 6.38–13.11 | 9.52 ± 2.18* | 6.82 ± 0.25 | 14.72 ± 1.04 | 16.13 ± 0.78 | 12.55 ± 5.01 | 10.20 ± 1.62 | 20.18 ± 1.69 | 10.12 ± 1.24 | 13.50 ± 5.78 |
| CAF | 35.28–49.06 | 41.83 ± 4.49 | 37.13 ± 1.38 | 40.72 ± 2.01 | 41.48 ± 1.87 | 39.78 ± 2.32 | 42.41 ± 2.45 | 45.88 ± 2.61 | 38.18 ± 1.42 | 42.15 ± 3.85 |
| TC | ND-26.33 | 12.06 ± 5.23** | ND | ND | ND | – | ND | ND | 19.21 ± 1.12 | 6.40 ± 11.09** |
| EC | 1.01–3.71 | 2.12 ± 0.65** | 5.43 ± 0.08 | 4.32 ± 0.09 | 4.86 ± 0.11 | 4.87 ± 0.55 | 2.50 ± 0.46 | 4.06 ± 0.31 | 3.22 ± 0.23 | 3.26 ± 0.78* |
| ECG | 11.62–15.41 | 13.68 ± 1.29** | 9.62 ± 0.23 | 11.51 ± 0.43 | 8.16 ± 0.36 | 9.76 ± 1.67 | 9.35 ± 0.65 | 10.80 ± 0.71 | 12.13 ± 0.53 | 10.76 ± 1.39 |
| ECG3′'Me | 0.44–1.50 | 1.02 ± 0.34** | 0.01 ± 0.00 | ND | ND | 0.00 ± 0.01 | 0.01 ± 0.00 | 0.17 ± 0.01 | 0.03 ± 0.00 | 0.07 ± 0.08 |
| EGC | 2.46–8.35 | 5.33 ± 1.9** | 10.80 ± 0.26 | 7.31 ± 0.53 | 14.47 ± 0.62 | 10.86 ± 3.58 | 9.26 ± 0.70 | 10.66 ± 0.67 | 11.21 ± 0.82 | 10.37 ± 1.00 |
| EGCG | 54.16–72.03 | 64.09 ± 5.17 | 57.15 ± 2.65 | 61.56 ± 2.64 | 59.68 ± 3.01 | 59.46 ± 2.21 | 68.46 ± 3.21 | 66.01 ± 2.94 | 57.7 ± 3.13 | 64.05 ± 5.63 |
| EGCG3′'Me | 2.05–7.00 | 4.79 ± 1.45** | 0.04 ± 0.01 | ND | ND | 0.01 ± 0.02 | 0.13 ± 0.01 | 1.08 ± 0.12 | 0.18 ± 0.01 | 0.46 ± 0.53 |
| C | 8.43–13.17 | 10.88 ± 1.68** | 0.45 ± 0.01 | 1.61 ± 0.16 | 1.27 ± 0.54 | 1.11 ± 0.59 | 3.16 ± 0.56 | 0.48 ± 0.02 | 9.19 ± 1.10 | 4.27 ± 4.46 |
| CG | 0.09–1.54 | 0.58 ± 0.4** | ND | ND | ND | – | ND | ND | 0.06 ± 0.01 | 0.03 ± 0.03 |
| GC | 5.87–11.01 | 8.20 ± 1.39** | 1.45 ± 0.07 | 1.87 ± 0.05 | 2.98 ± 0.36 | 2.10 ± 0.79 | 9.08 ± 1.01 | 1.57 ± 0.48 | 2.85 ± 0.05 | 4.50 ± 4.01 |
| GCG | 15.75–19.65 | 17.73 ± 1.29** | ND | ND | ND | – | 9.68 ± 0.95 | ND | 10.25 ± 0.49 | 6.64 ± 5.76** |
Note: The data represent the mean from three replicates. TB, theobromine; CAF, caffeine; TC, theacrine; ND, not detected. Compared to CK, *P < 0.05, **P < 0.01.
It is well known that leucoanthocyanidin reductase (LAR), anthocyanidin synthase (ANS), anthocyanin reductase (ANR), epicatechin: 1-O-galloyl-β-d-glucose O-galloyltransferase (ECGT) and UDP-glucose: galloyl-1-O-β-d-glucosyltransferase (UGGT) are critical enzymes involved in the flavonoid biosynthesis pathway and contribute to the differential accumulations of flavanol components in different teas. LAR catalyzes the conversion of leucocyanidin/leucodelphindin to C/GC, ANS catalyzes the conversion of leucocyanidin/leucodelphindin to cyanidin/delphinidin, and ANR catalyzes the conversion of cyanidin/delphinidin to EC/EGC (Yu, et al., 2020). In addition, ECGT and UGGT are involved in the galloylated flavanols (CG, GCG, ECG, and EGCG) biosynthesis pathway (Liu, et al., 2012). As described above, the high accumulations of C and GC in the JHKC accessions may be due to high LAR activity; in contrast, the low accumulations of EC and EGC in the JHKC accessions may be due to low ANR activity. The concentrations change of galloylated flavanols exhibited higher galloylation levels in the JHKC accessions than in the CK cultivars. For example, the content of EC and EGC was 0.5-fold lower in the JHKC group than in the CK group, respectively, while the contents of ECG and EGCG were slightly higher in the JHKC group (Table 1), indicating that the UGGT and ECGT enzyme activity may be higher in the group JHKC than in the CK group.
Numerous studies have demonstrated that, compared with ECG and EGCG, ECG3′'Me and EGCG3′'Me methylated flavanols exhibit stronger antiallergic and metabolic syndrome-treating effects, respectively (Maeda-Yamamoto et al., 2012, Yang et al., 2015, Márquez-Campos et al., 2020). Moreover, it was strongly suggested that the EGCG3″Me content in tea leaves was positively correlated with the expression of the genes encoding leucocyanidin reductase (LAR), dihydroflavonol-4-reductase (DFR) and caffeoyl-CoA 3-O-methyltransferase (CCoAOMT); however, some tea varieties seemed to lack these related enzymes (Luo, et al., 2018). In the present study, compared with the CK cultivars, the JHKC accessions had significantly higher contents of ECG3′'Me and EGCG3′'Me (Fig. 4, Fig. 5F, H and Table 1). The EGCG3′'Me level in the JHKC accessions ranged from 2.05 to 7.00 mg/g (4.79 ± 1.45 mg/g), and the ECG3′'Me level ranged from 0.44 to 1.50 mg/g (1.02 ± 0.34 mg/g). Surprisingly, among the cultivars composing CK group, only the FDDB cultivar presented a small amount of EGCG3′'Me (0.04 ± 0.01 mg/g) and ECG3′'Me (0.01 ± 0.00 mg/g). A previous study (Maeda-Yamamoto, et al., 2012) showed that ECG3′'Me and EGCG3′'Me coexisted in different tea varieties and that their contents were strongly positively correlated, which was consistent with our data (Table 1). The content of EGCG did not obviously differ between the JHKC and CK groups; however, the methylated flavanol (EGCG3′'Me) content significantly differed in this study, indicating that methylation levels of flavanols were higher in the JHKC accessions than in CK cultivars. It demonstrated that the JHKC accessions are good materials for breeding plants with high amounts of bioactive components of methylated flavanols (EGCG3′'Me and ECG3′'Me), such as the Benifuuki cultivar in Japan (Maeda-Yamamoto, et al., 2012).
Fig. 5.
Variations in differential metabolites in the JHKC (JHKC-1, JHKC-2, JHKC-3, JHKC-4, JHKC-5, JHKC-6, JHKC-7, JHKC-8, JHKC-9, JHKC-10 and JHKC-11) and CK (FDDB, YK10, and ZYQ) groups. JHKC-M represents the average level of metabolites across 11 JHKC individuals; CK-M represents the average level of metabolites across FDDB, YK10, and ZYQ. The Y axis represents the MS intensity of the compound detected by LC-MS. The data represent the mean ± SD. The green, blue, red and yellow columns represent JHKC, CK, JHKC-M and CK-M values, respectively. ND, not detected; *, P < 0.05; **, P < 0.01.
The metabolic pathway of proanthocyanidins (PAs) was also mapped, as shown in Fig. 4. Procyanidin B1 [EC-(4b,8)-C] and procyanidin B2 [EC-(4b,8)-EC] are EC-based PAs; procyanidin B3 [C-(4b,8)-C] and procyanidin B4 [C-(4b,8)-EC] are C-based PAs (Wang, et al., 2020). Procyanidin B3 abundantly accumulated in the JHKC accessions, while procyanidin B1 and procyanidin B2 highly accumulated in the CK cultivars, as C was highly enriched in the JHKC accessions, while EC highly accumulated in the CK cultivars.
The glycoside forms of quercetin, myricetin and kaempferol are downstream metabolic products of naringenin (Fig. 4). In this study, with the exception of myricetin 3′-glucoside, glycosides of quercetin, myricetin and kaempferol were present at significantly lower levels in the JHKC accessions than in the CK cultivars. These results indicated more vigorous biosynthesis of quercetin, myricetin and kaempferol glycosides in the CK cultivars than in the JHKC accessions. Considering that the JHKC accessions contained higher levels of flavanols, we deduced that the flavonoid biosynthesis pathway tended to synthesize flavanols rather than flavonols in the JHKC accessions.
Caffeine metabolism was one of the most differential pathways between the JHKC and CK groups (Fig. 4). This study showed that caffeine, theacrine and theobromine were the main purine alkaloid components in the JHKC accessions (Table 1). Caffeine (41.83 ± 4.49 mg/g) was the most abundant purine alkaloid, followed by theacrine (12.06 ± 5.23 mg/g) and theobromine (9.52 ± 2.18 mg/g). A recent study indicated that theacrine had beneficial effects on sedation, anti-inflammatory, anti-depression, sleep improvement, lipid metabolism improvement, anti-influenza, and so on (Sheng, et al., 2020). Among different tea plant species, theacrine was first detected in and isolated from Yunnan Kucha tea [Camellia kucha (Chang et Wang) Chang] (Ye, et al., 1999) and has subsequently been reported to exist in Puan tea (Camellia sinensis var. puanensis Kurihara) (Li, et al., 2017), Dayaoshan tea (Camellia gymnogyna Chang) (Teng, et al., 2020), Baiyacha (Camellia gymnogyna Chang) (Jin, Jiang, Yao, & Chen, 2020), and Niedu Kucha (Camellia sinensis) (Wang, et al., 2020). In terms of its synthesis, theacrine is biosynthesized first through the conversion of caffeine to a 1,3,7-trimethyluric acid intermediate by a C8-oxidase, after which the intermediate is converted to theacrine by the N9-methyltransferase (CkTcS) (Zheng et al., 2002, Wang et al., 2020, Zhang et al., 2020). Among the 11 JHKC individuals evaluated in this study, 7 individuals contained theacrine, and the theacrine contents of the JHKC individuals ranged from 12.27 to 26.33 mg/g (Fig. 5C-D and Table 1), and 6 JHKC individuals contained theacrine levels > 15.0 mg/g. In contrast, 1,3,7-trimethyluric acid and theacrine were not detected in the CK cultivars, which may be due to the lack of C8-oxidase. Caffeine was the dominant purine alkaloid in the JHKC individuals, which was consistent with findings concerning Niedu Kucha (Camellia sinensis) (Wang, et al., 2020) but in contrast with findings concerning Yunnan Kucha [Camellia kucha (Chang et Wang) Chang], whose predominant purine alkaloid was theacrine (Yang et al., 2007, Qin et al., 2020).
In addition, our study showed that the levels of caffeine and EGCG of the different tea groups were not significantly different (P > 0.05, Fig. 4B, E and Table 1). These results were consistent with those of a previous study (Yu, et al., 2020) that the concentration of a few metabolites (such as caffeine and EGCG) seemed to be stable in different tea cultivars. It needs to note that picking time and plant region have an influence on compound contents in fresh tea leaves (Ahmed et al., 2019). For an example, methylated flavanols were found to have > 2-fold contents in tea leaves collected in summer and autumn than in spring in our previous study (Dai, et al., 2015). In this study, 11 JHKC individuals were collected from a mountainous region at elevations ranging from 400 to 600 m in Jianghua County of Hunan Province in April. The aforementioned contents of characteristic compounds (e.g., theacrine, 1,3,7-trimethyluric acid, non-epi-form flavanols, and methylated flavanols.) may vary in JHKC individuals collected from different seasons and plant regions.
3.3. Changes of differential metabolites in JHKC-B cultivars
To date, three cultivars have been bred from JHKC via agronomic selection, namely, JHKC-21–1 (CSA), JHKC-21–3 (CSA) and JHKC-37–2 (CSA). To study the metabolite changes in JHKC accessions after artificial breeding and cultivation, we analyzed the metabolites in the three JHKC-bred cultivars. As shown in Fig. S2, the PCA results suggested that the metabolite profiles of the JHKC-B cultivars were more similar to those of the CK cultivars than to those of the JHKC accessions. The changes in the accumulation of metabolites in the JHKC, CK (FDDB, YK10, and ZYQ) and JHKC-B (JHKC-21–1, JHKC-21–3 and JHKC-37–2) groups are shown in Fig. S3 and Table 1. As described above, compared with the CK cultivars, the JHKC accessions had significantly higher levels of mainly of 1,3,7-trimethyluric acid and theacrine purine alkaloids, non-epi-form flavanols (C, CG, GC, and GCG), and methylated flavanols (EGCG3′'Me and ECG3′'Me) and lower levels of flavonol glycosides (kaempferol, quercetin and myricetin glycosides).
After artificial breeding and cultivation, the levels of caffeine and EGCG in the JHKC-B cultivars did not significantly differ from those in the JHKC cultivars (P > 0.05, Fig. S3-B, E and Table 1). Among the three JHKC-bred cultivars, 1,3,7-trimethyluric acid and theacrine metabolites were detected in the JHKC-37–2 cultivar, and the theacrine level was 19.21 ± 1.12 mg/g (Fig. S3-C, D and Table 1). It is possible that the mother plants of the JHKC-21–1 and JHKC-21–3 varieties may lack C8-oxidase or that C8-oxidase was no longer expressed after artificial breeding and cultivation, ultimately leading to naturally free of 1,3,7-trimethyluric acid and theacrine. EGCG3′'Me and ECG3′'Me methylated flavanols were detected in JHKC-21–1, JHKC-21–3 and JHKC-37–2; however, their abundance was significantly lower than that in the JHKC group (P < 0.05, Fig. S3-F, H and Table 1). Similarly, non-epi-form flavanols of C (4.27 ± 4.46 mg/g), GC (0.03 ± 0.03 mg/g), CG (4.50 ± 4.01 mg/g), and GCG (6.64 ± 5.76 mg/g) also accumulated at low levels in the JHKC-B cultivars, as their contents decreased after artificial breeding and cultivation. With respect to flavonol glycosides, JHKC-21–1, JHKC-21–3 and JHKC-37–2 contained kaempferol 3-glucosylrutinoside, kaempferol 3-galactosylrutinoside and myricetin 3-galactoside, whereas these compounds were not detected in the JHKC group (Fig. S3-K, L and N). Moreover, JHKC-21–1 and JHKC-21–3 contained quercetin triglucoside (Fig. S3-P), and HKC-21–1 and JHKC-37–2 contained quercetin 3-glucosylrutinoside (Fig. S3-Q); however, these compounds were absent in the JHKC group. In addition, three dimeric flavanols (procyanidin B1, procyanidin B2 and procyanidin B3) accumulated to relatively high levels in JHKC-37–2 but low levels in JHKC-21–1 and JHKC-21–3 (Fig. S3-R, S and T). These results may have occurred because environmental factors can strongly influence the accumulation of metabolites in tea plant (Yu, et al., 2020).
4. Conclusions
This study qualitatively and quantitatively compared the metabolite profiles of wild JHKC germplasm with those of CK and JHKC-B cultivars via metabolomic analysis combined with chemometric analysis. The JHKC germplasm has a unique metabolite profile, and a total of 61 metabolites were demonstrated to differentially accumulated between JHKC and CK cultivars; these metabolites were mainly enriched in three critical differential metabolic pathways: caffeine metabolism, flavonoid biosynthesis, and flavone and flavonol biosynthesis. High accumulations of theacrine and 1,3,7-trimethyluric acid purine alkaloids, non-epi-form flavanols (C, GC, CG, and GCG), and EGCG3′'Me and ECG3′'Me methylated flavanols and low expression of flavonol glycosides (kaempferol, quercetin and myricetin glycosides) were the most significant metabolite characteristics of JHKC germplasms. Taken together, these results indicated that JHKC germplasm is a promising resource for the breeding of novel tea cultivars that have high contents of theacrine, non-epi-form flavanols, and methylated flavanols.
CRediT authorship contribution statement
Wenliang Wu: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Funding acquisition. Meiling Lu: Formal analysis. Jiakun Peng: Investigation. Haipeng Lv: Formal analysis. Jiang Shi: Formal analysis. Shuguang Zhang: Investigation. Zhen Liu: Investigation. Jihua Duan: Investigation. Dan Chen: Resources. Weidong Dai: Conceptualization, Formal analysis, Resources, Writing – review & editing, Funding acquisition. Zhi Lin: Conceptualization, Resources, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Hunan Provincial Natural Science Foundation of China (2021JK1008), the Hunan Agricultural Science and Technology Innovation Funds of China (2020CX36), the National Natural Science Foundation of China (31972467), the Earmarked Fund for China Agricultural Research System (CARS-19), the Hunan Provincial Natural Science Foundation of China (2020JJ5277), the Foundation of Public Projects of Zhejiang Province (LGN21C160012) and the National Key R&D Program of China (2018YFD0700500).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100270.
Contributor Information
Weidong Dai, Email: daiweidong@tricaas.com.
Zhi Lin, Email: linzhi@caas.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmed S., Griffin T.S., Kraner D., Schaffner M.K., Sharma D., Hazel M.…Cash S.B. Environmental factors variably impact tea secondary metabolites in the context of climate change. Frontiers in Plant Science. 2019;10 doi: 10.3389/fpls.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Shi J., Mu B., Chen Z., Dai W., Lin Z. Metabolomics combined with proteomics provides a novel interpretation of the changes in nonvolatile compounds during white tea processing. Food Chemistry. 2020;332:127412. doi: 10.1016/j.foodchem.2020.127412. [DOI] [PubMed] [Google Scholar]
- Dai W., Hu Z., Xie D., Tan J., Lin Z. A novel spatial-resolution targeted metabolomics method in a single leaf of the tea plant (Camellia sinensis) Food Chemistry. 2020;311:126007. doi: 10.1016/j.foodchem.2019.126007. [DOI] [PubMed] [Google Scholar]
- Dai W., Lou N.i., Xie D., Hu Z., Song H., Lu M.…Lin Z. N-ethyl-2-pyrrolidinone-substituted flavan-3-ols with anti-inflammatory activity in lipopolysaccharide-stimulated macrophages are storage-related marker compounds for green tea. Journal of Agricultural and Food Chemistry. 2020;68(43):12164–12172. doi: 10.1021/acs.jafc.0c03952. [DOI] [PubMed] [Google Scholar]
- Dai W., Qi D., Yang T., Lv H., Guo L.i., Zhang Y.…Lin Z. Nontargeted analysis using ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry uncovers the effects of harvest season on the metabolites and taste quality of tea (Camellia sinensis L.) Journal of Agricultural and Food Chemistry. 2015;63(44):9869–9878. doi: 10.1021/acs.jafc.5b03967. [DOI] [PubMed] [Google Scholar]
- Dai W., Tan J., Lu M., Zhu Y., Li P., Peng Q.…Lin Z. Metabolomics investigation reveals that 8-CN-ethyl-2-pyrrolidinone-substituted flavan-3-ols are potential marker compounds of stored white teas. Journal of Agricultural and Food Chemistry. 2018;66(27):7209–7218. doi: 10.1021/acs.jafc.8b02038. [DOI] [PubMed] [Google Scholar]
- Dai W., Xie D., Lu M., Li P., Lv H., Yang C.…Lin Z. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Research International. 2017;96:40–45. doi: 10.1016/j.foodres.2017.03.028. [DOI] [PubMed] [Google Scholar]
- Jin J., Jiang C., Yao M., Chen L. Baiyacha, a wild tea plant naturally occurring high contents of theacrine and 3''-methyl-epigallocatechin gallate from Fujian. China. Scientific Reports. 2020;10(1):1–9. doi: 10.1038/s41598-020-66808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Ma J., Ma C., Yao M., Chen L. Determination of catechin content in representative Chinese tea germplasms. Journal of Agricultural and Food Chemistry. 2014;62(39):9436–9441. doi: 10.1021/jf5024559. [DOI] [PubMed] [Google Scholar]
- Li, D. (2012). Evaluate of tea germplasm resources of Camellia sinensis var assamica cv. Jianghua. Master's thesis, Hunan Agricultural University, China. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201301&filename=1012441565.nh.
- Li D., Luo J. Relationship of thea assamica cv. Jianghua germplasm resources and its status in the process of tea evolution. Hunan agricultural science. 2011;10(1A):10–12. http://dx.chinadoi.cn/10.3969/j.issn.1006-060X.2011.01.004 [Google Scholar]
- Li K.K., Wong H.L., Hu T., Zhang C., Han X.Q., Ye C.X.…Ko C.H. Impacts of Camellia kucha and its main chemical components on the lipid accumulation in 3T3-L1 adipocytes. International Journal of Food Science & Technology. 2016;51(12):2546–2555. doi: 10.1111/ijfs.2016.51.issue-1210.1111/ijfs.13236. [DOI] [Google Scholar]
- Li K., Shi X., Yang X., Wang Y., Ye C., Yang Z. Antioxidative activities and the chemical constituents of two Chinese teas, Camellia kucha and C. ptilophylla. International journal of food science & technology. 2012;47(5):1063–1071. doi: 10.1111/j.1365-2621.2012.02942.x. [DOI] [Google Scholar]
- Li N., Huang H., Zhong X., Su B. Research advance on Hunan local tea tree resources Jianghua bitter tea. Tea Communication. 2018;45(3):3–7. http://dx.chinadoi.cn/10.3969/j.issn.1009-525X.2018.03.001 [Google Scholar]
- Li P., Dai W., Lu M., Xie D., Tan J., Yang C.…Lin Z. Metabolomic analysis reveals the composition differences in 13 Chinese tea cultivars of different manufacturing suitabilities. Journal of the Science of Food and Agriculture. 2018;98(3):1153–1161. doi: 10.1002/jsfa.2018.98.issue-310.1002/jsfa.8566. [DOI] [PubMed] [Google Scholar]
- Li P., Zhu Y., Lu M., Yang C., Xie D., Tan J.…Lin Z. Variation patterns in the content of glycosides during green tea manufacturing by a modification-specific metabolomics approach: Enzymatic reaction promoting an increase in the glycosidically bound volatiles at the pan firing stage. Food Chemistry. 2019;279:80–87. doi: 10.1016/j.foodchem.2018.11.148. [DOI] [PubMed] [Google Scholar]
- Li Y.-F., Ouyang S.-H., Chang Y.-Q., Wang T.-M., Li W.-X., Tian H.-Y.…He R.-R. A comparative analysis of chemical compositions in Camellia sinensis var. puanensis Kurihara, a novel Chinese tea, by HPLC and UFLC-Q-TOF-MS/MS. Food Chemistry. 2017;216:282–288. doi: 10.1016/j.foodchem.2016.08.017. [DOI] [PubMed] [Google Scholar]
- Liu X. Studies on the traits of Jianghua Kucha tea. Tea Communication. 2006;33(3):4–7. http://dx.chinadoi.cn/10.3969/j.issn.1009-525X.2006.03.007 [Google Scholar]
- Liu Y., Gao L., Liu L.i., Yang Q., Lu Z., Nie Z.…Xia T. Purification and characterization of a novel galloyltransferase involved in catechin galloylation in the tea plant (Camellia sinensis) Journal of Biological Chemistry. 2012;287(53):44406–44417. doi: 10.1074/jbc.M112.403071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Yu S., Li J., Li Q., Wang K., Huang J., Liu Z. Molecular characterization of WRKY transcription factors that act as negative regulators of o-methylated catechin biosynthesis in tea plants (Camellia sinensis L.) Journal of Agricultural and Food Chemistry. 2018;66(43):11234–11243. doi: 10.1021/acs.jafc.8b02175. [DOI] [PubMed] [Google Scholar]
- Ma J., Yao M., Chen L. Research progress on germplasms of tea plant (Camellia sinensis) Journal of Tea Science. 2015;35(01):11–16. http://dx.chinadoi.cn/10.3969/j.issn.1000-369X.2015.01.004 [Google Scholar]
- Maeda-Yamamoto M., Ema K., Monobe M., Tokuda Y., Tachibana H. Epicatechin-3-O-(3″-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. Journal of Agricultural and Food Chemistry. 2012;60(9):2165–2170. doi: 10.1021/jf204497b. [DOI] [PubMed] [Google Scholar]
- Márquez-Campos E., Jakobs L., Simon M. Antidiabetic effects of flavan-3-ols and their microbial metabolites. Nutrients. 2020;12(6):1592. doi: 10.3390/nu12061592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D., Wang Q., Li H., Jiang X., Fang K., Wang Q.…Wu H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia kucha (Chang et Wang) Chang] Food Research International. 2020;138:109789. doi: 10.1016/j.foodres.2020.109789. [DOI] [PubMed] [Google Scholar]
- Sheng Y.-Y., Xiang J., Wang Z.-S., Jin J., Wang Y.-Q., Li Q.-S.…Zheng X.-Q. Theacrine from Camellia Kucha and its health beneficial effects. Frontiers in Nutrition. 2020;7 doi: 10.3389/fnut.2020.596823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J., Yan C., Zeng W., Zhang Y., Zeng Z., Huang Y. Purification and characterization of theobromine synthase in a theobromine-enriched wild tea plant (Camellia gymnogyna Chang) from Dayao Mountain, China. Food Chemistry. 2020;311:125875. doi: 10.1016/j.foodchem.2019.125875. [DOI] [PubMed] [Google Scholar]
- Wang P., Zhang L., Zhao L., Zhang X., Zhang H., Han Y.…Xia T. Comprehensive analysis of metabolic fluxes from leucoanthocyanins to anthocyanins and proanthocyanidins (PAs) Journal of Agricultural and Food Chemistry. 2020;68(51):15142–15153. doi: 10.1021/acs.jafc.0c05048. [DOI] [PubMed] [Google Scholar]
- Yang, C. (2013). The biochemical composition analysis and excellent individuals screening with Camellia sinensis var assamica cv. Jianghua. Master's thesis, Hunan Agricultural University, China. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201402&filename=1014182209.nh.
- Yang C., Hu Z., Lu M., Li P., Tan J., Chen M.…Lin Z. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Research International. 2018;106:909–919. doi: 10.1016/j.foodres.2018.01.069. [DOI] [PubMed] [Google Scholar]
- Yang X.R., Ye C.X., Xu J.K., Jiang Y.M. Simultaneous analysis of purine alkaloids and catechins in Camellia sinensis, Camellia ptilophylla and Camellia assamica var. kucha by HPLC. Food Chemistry. 2007;100(3):1132–1136. doi: 10.1016/j.foodchem.2005.11.021. [DOI] [Google Scholar]
- Yang Y., Qiao L., Zhang X., Wu Z., Weng P. Effect of methylated tea catechins from Chinese oolong tea on the proliferation and differentiation of 3T3-L1 preadipocyte. Fitoterapia. 2015;104:45–49. doi: 10.1016/j.fitote.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Ye C., Lin Y., Su J., Song X., Zhang H. Purine alkaloids in Camellia assamica var. kucha Chang et Wang. Acta scientiarum Naturalium Universitatis Sunyatseni. 1999;38:82–86. http://xuebao.sysu.edu.cn/Jweb_zrb/CN/Y1999/V38/I5/82 [Google Scholar]
- Yu X., Xiao J., Chen S.i., Yu Y., Ma J., Lin Y.…Liu R. Metabolite signatures of diverse Camellia sinensis tea populations. Nature Communications. 2020;11(1) doi: 10.1038/s41467-020-19441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-H., Li Y.-F., Wang Y., Tan L.i., Cao Z.-Q., Xie C.…He R.-R. Identification and characterization of N 9-methyltransferase involved in converting caffeine into non-stimulatory theacrine in tea. Nature Communications. 2020;11(1) doi: 10.1038/s41467-020-15324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X., Ye, C., Kato, M., Crozier, A., & Ashihara, H. (2002). Theacrine (1, 3, 7, 9-tetramethyluric acid) synthesis in leaves of a Chinese tea, kucha (Camellia assamica var. kucha). Phytochemistry, 60(2), 129-134. https://doi.org/10.1016/S0031-9422(02)00086-9. [DOI] [PubMed]
Further reading
- Qin D., Wang Q., Li H., Fang K., Jiang X., Pan C.…Wu H. Research progresses on Camellia Kucha (Chang et Wang) Chang and theacrine. Food Science. 2020:1–10. doi: 10.1016/j.foodres.2020.109789. https://kns.cnki.net/kcms/detail/11.2206.TS.20201110.0910.002.html [DOI] [PubMed] [Google Scholar]
- Wang S., Chen J., Ma J., Jin J., Chen L., Yao M. novel insight into theacrine metabolism revealed by transcriptome analysis in bitter tea (Kucha, Camellia sinensis) Scientific Reports. 2020;10(1):1–11. doi: 10.1038/s41598-020-62859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.