Highlights
-
•
Postharvest hardening did not definitively affect bioactivity of bean peptides.
-
•
Peptides from hardened and non-hardened beans showed high vasorelaxant activity.
-
•
Thermal treatment positively affected the biological potential of hardened beans.
Keywords: Phaseolus vulgaris, Hard-to-cook, Easy-to-cook, Bioactive peptides, Nutraceutical, Vascular reactivity
Abstract
Aiming to understand the impact of hardening on the biological potential of bean protein and peptides, we evaluated the antioxidant and vasorelaxant properties of common beans after and before hardening. It was also evaluated the effect of extrusion and autoclaving in the biological potential of hardened beans. In general, hardening caused a reduction from 13.5 to 39.6% on the antioxidant activity of the peptide-rich fractions. On the other hand, hardening did not strongly interfere with the vascular reactivity in thoracic aorta rings, being observed maximal relation varying from 801% to 84.7%. The thermal treatment caused a general increase in the antioxidant and vasorelaxant potential of these fractions, being observed EC50 values ranging from 0.22 mg mL−1 to 0.26 mg mL−1. We can conclude that hardening did not seem to affect definitively the bioactivity of the obtained peptide-rich fractions. Finally, this study allows suggesting practical applications of extrusion as a thermal process in the production of functional food ingredients, and as ready-to-eat products presenting nutraceutical potential. In addition, autoclaving can be used as a pre-treatment of the hardened grains aiming to use them as whole grains with potentialized benefits for human health.
Introduction
Common beans (Phaseolus vulgaris) are leguminous widely consumed all over the world due to their high nutritional quality. In addition to the active components, common beans also present naturally-occurring and encrypted peptides with varied biological activities (Alves et al., 2021; Graziani, et al., 2021; Manzoor et al., 2022, Valencia-Mejía et al., 2019). However, there is no sufficient information about the effect of postharvest hardening on the antioxidant and vasorelaxant properties, chelating potential, and antihypertensive and antidiabetic activities. It is known that the nutritional quality of proteins from common beans can be affected depending on the postharvest conditions, being observed that storage in temperatures higher than 35 °C and moisture above 60% leads to the development of the hardening phenomenon, commonly known as hard-to-cook (HTC) effect (Batista and Fernandes, 2016, Siqueira et al., 2016). It is estimated that 3 million tons of beans per year are no longer used in human nutrition due to the hardening (Graziani, et al., 2021). Considering the current global scenario of increasing world population and reduction availability of new food sources, it is mandatory to find alternatives to reinclude these grains in human nutrition.
In the last decades, several studies have been aimed to develop alternative technologies of processing, such as extrusion and autoclaving (Pedrosa, Guillamón, & Arribas, 2021), that improves the nutritional quality, functional properties and technological features of HTC beans (Ratnaningsih et al., 2019; Siah, Wood, Agboola, Konczak, & Blanchard, 2014), being an interesting alternative to obtain good quality food ingredients, useful to human nutrition and health.
In addition, considering the currently reported potential of proteins and peptides from beans as antioxidant, antidiabetics and antihypertensive molecules, understanding the effect of postharvest hardening on the biological potential of these proteins and peptides will help to propose a more precise nutraceutical and/or functional use for HTC beans. Several studies have associated the development of chronic diseases (i.e., Alzheimer, Parkinson and cardiovascular diseases) to an imbalance between the production of free radicals and the endogenous antioxidant defense systems, which can cause oxidative damage to a wide range of biomolecules such as lipids, proteins and nucleic acids (Kaur et al., 2021, Sharma et al., 2021). In this scenario, dietary antioxidants are quite important because they can inhibit or reduce the initiation and propagation of free radical cascades, contributing to minimize the cellular damage induced by the oxidative effect of free radicals (González-Monotya, Hernández-Ledesma, Mora-Escobedo, & Martínez-Villaluenga, 2018).
Therefore, the aim of this study is (i) to determine the effect of postharvest hardening on the biological potential of proteins and peptides from common beans and (ii) to evaluate the effect of autoclaving and extrusion on the antioxidant potential and the vascular reactivity of proteins and naturally-occurring peptides from HTC beans. We hypothesized that despite the postharvest hardening interfering with the protein availability, it is possible that the biological potential of the peptide fractions remains unchanged or slightly altered. In addition, it is possible that thermal treatment by extrusion or autoclaving can recover or even improve the antioxidant and vasorelaxant properties of these peptide fractions.
Materials and methods
Materials
Pepsin, pancreatin, Phenylephrine (Phe), Acetylcholine (Ach), DPPH (1,1-diphenyl-2-picrylhydrazyl), HHL (hippuryl-l-histidyl-l-leucine), TT (2,4,6-trichloro-s-triazine), TPTZ (2,4,6-tris(2-pyridyl)-s-triazine), and Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) were purchased from Sigma Aldrich Chemical Co. (St. Louis, USA). Acetonitrile and formic acid were purchased from Vetec (Rio de Janeiro, Brazil). Qubit® Protein Assay Kit was obtained from ThermoFisher Scientific (Massachusetts, USA). Regenerated cellulose membranes (3 kDa-PLBC07610 and 10 kDa-PLGC07610) were purchased from Millipore® (Massachusetts, USA). All the reagents used in the experiments were of analytical grade.
Hardening process
Seeds from common beans (Phaseolus vulgaris, cv BRS Pontal) were kindly provided by EMBRAPA Rice and Beans (Santo Antônio de Goiás, Goiás, Brazil). After removing impurities, the beans were divided into two batches. The first batch (ease-to-cook (ETC) samples – non-hardened beans) was stored at 4 °C until further use to avoid the development of the hardening process. The second batch (HTC samples) was incubated at 40 °C (±2°C) and 75% of relative moisture for 120 days to induce hardening. This relative humidity was achieved by storing the beans above a saturated sodium chloride solution. After storage, the HTC beans were separated into three batches. The first batch was stored at 4 °C, the second was submitted to autoclaving process and the third was milled and extruded. Before analysis, all grains were milled and stored at −20 °C.
Autoclaving of hard-to-cook beans
100 g of HTC beans were placed in 250 mL glass containers for the autoclaving process without adding water. The containers were partially closed and autoclaved at 120 °C and 1.2 Kgf/cm2 for 15 min. After the autoclaving process, the beans were dried at room temperature (25 °C), dehulled and milled to produce flour with particle size of 500 μm.
Extrusion of hard-to-cook bean flours
Prior to the extrusion process, moisture content of the HTC flour was adjusted to 13% (in dry basis). The extrusion process was performed in a single-screw extruder (Cerealtec Intl. CT-L15) at a compression ratio of 3:1, matrix with 5 mm aperture and screw speed of 150 rpm (Batista & Fernandes, 2016). The extruded samples were dried at room temperature and milled to produce flour with particle size of 500 μm.
Protein extraction
In order to evaluate the effect of the solvent on the extraction of bioactive molecules, three different extraction solutions were tested. The total protein content was determined using the Qubit® Protein Assay Kit following the manufacturer instructions.
Method A: acetonitrile/water/formic acid
The extraction solution was prepared as described by Mahatmanto, Poth, Mylne, and Craik (2014) using as solvent a mixture of acetonitrile, water and formic acid (50:48:2). The protein extraction was carried out by mixing 50 mL of solvent and 10 g of flour, followed by incubation at 25 °C for 1 h, under magnetic stirring. After extraction, the mixture was centrifuged at 5.000 × g for 15 min at 25 °C and the supernatant was submitted to solvent evaporation under reduced pressure (Vacufuge® plus, Eppendorf, Hamburg, Germany). The produced protein extract was lyophilized for further analysis.
Method B: sodium acetate solution
For this method, a 20 mmol L-1 sodium acetate solution (pH 5.0) was used as extraction solvent (Mahatmanto, Poth, Mylne, & Craik, 2014). The protein extraction was carried out by mixing 10 g of flour with 50 mL of solvent, followed by incubation at 4 °C for 1 h, under magnetic stirring. After extraction, the mixture was centrifuged at 5.000 × g for 15 min at 4 °C and the supernatant was lyophilized for further analysis.
Method C: alkaline water
Protein extraction was performed according to Oseguera-Toledo, Mejia, Dia, and Amaya-Llano (2011), with minor modifications. A mixture of 10 g flour and 50 mL of alkaline water (pH 8.0, adjusted with 0.1 mol L-1 NaOH solution) was incubated at 35 °C for 1 h, under stirring. The mixture was centrifuged at 5.000 × g for 15 min at 25 °C and the supernatant was lyophilized for further analysis.
Protein fractionation by ultrafiltration
The protein extracts were fractionated through ultrafiltration, using membranes with different molecular weight cut-off (Millipore, Massachusetts, USA). The ultrafiltration process was carried out in a stirred dead-end cell with 400 mL using a 10 kDa regenerated cellulose membrane. The system was operated at 1.5 Kgf cm−2 and 4 °C. The retentate (F > 10 kDa) and permeate (F < 10 kDa) were collected separately and lyophilized for further analysis. The protein solutions extracted using method A were also submitted to ultrafiltration using a 3 kDa regenerated cellulose membrane, and the retentate (F3-10 kDa) and permeate (F < 3 kDa) were collected separately and lyophilized.
Bioactivities
Antioxidant activity
The ability of the protein and peptide fractions to scavenge the DPPH radical was determined using the method reported by Brand-Williams, Cuvelier, and Berset (1995). Briefly, 50 μL of sample were added to 200 μL of a 150 mmol L-1 DPPH solution and incubated at 25 °C for 15 min. The decrease in the absorbance of the resulting solution was monitored at 520 nm (Eppoch microplate spectrophotometer, Biotek Instruments, VT, USA). The antiradical activity of the samples was related to that of a Trolox standard, and the results were calculated as millimolar of Trolox Equivalents (TE) per milligram of sample (mmol L-1 TE mg-1 protein).
The ferric reducing antioxidant power (FRAP) assay was performed according to methodology described by Benzie and Strain (1996). The FRAP stock solution was prepared by mixing 2.5 mL of FeCl3 solution (0.02 mol L-1) with 25 mL of acetate buffer (0.01 mol L-1, pH 3.6) and 2.5 mL of 0.01 mol L-1 TPTZ solution (prepared in 0.04 mol L-1 HCl). Before using, the freshly prepared FRAP stock solution was incubated at 37 °C for 10 min. The reaction mixture was prepared by mixing 50 μL of sample with 150 μL of deionized water and 1.3 mL of FRAP solution. The system was incubated at 37 °C for 30 min before readings were taken at 595 nm (Eppoch microplate spectrophotometer, Biotek Instruments, VT, USA). The results from FRAP assays were related to that of a Trolox standard, and the results were calculated as millimolar of Trolox Equivalents (TE) per milligram of sample (mmol L-1 TE mg-1 protein).
Fe2 + -chelating activity was determined as described by Carter (1971). The mixture reaction was prepared by mixing 50 μL of sample with 180 μL of 0.1 mol L-1sodium acetate buffer (pH 4.9), and 60 μL of 0.01% FeCl2·4H2O aqueous solution. After incubation at 25 °C for 30 min, 10 μL of 0.4 mol L-1 ferrozine aqueous solution was added and the system was incubated at 25 °C for 10 min. The absorbance was measured at 562 nm using an Epoch microplate spectrophotometer (Biotek Instruments, VT, USA), and the results were expressed as percentage of chelating activity.
Cu2+-chelating activity was determined according to Saiga, Tanabe, and Nishimura (2003), by mixing 50 μL of sample with 200 μL of 0.05 mol L-1 sodium phosphate buffer (pH 6.0), containing 10 μg of CuSO4·5H2O After incubation at 25 °C for 10 min, 5 μL of pyrocatechol violet were added. The system was incubated for 5 min at 25 °C and the absorbance was measured at 632 nm (Eppoch microplate spectrophotometer, Biotek Instruments, VT, USA). Results were expressed as percentage of chelating activity.
Study of vascular reactivity
The experiments were conducted on healthy male Wistar rats, 10–12 weeks (200–300 g). All the procedure described herein was approved by Institutional Ethics in Research Committee at the Universidade Federal de Goiás, Goiás, Brazil (Protocol CEP/UFG 20/2013) and performed following to the Ethical Principles in Animal Experimentation, defined by the Brazilian College of Animal Experimentation (COBEA). Aiming to reduce the number of animals used in the experiments, only the fractions presenting the highest antioxidant activities were evaluated. The animals were maintained under a controlled temperature (22 ± 1 °C) with ad libitum access to food and water, on a 12 h light/dark cycle.
For the preparation of aortic rings, animals were euthanized by decapitation, followed by dissection of the thoracic aorta, which was cleaned in Krebs-Henseleit solution (Oliveira et al., 2016) and sectioned in circular transverse segments measuring 4 mm in length. The aortic rings were suspended horizontally in a tissue chamber containing 10 mL of Krebs-Henseleit solution at 37 °C, with bubbling of a gas mixture containing 95% O2 and 5% CO2. A resting tension of 1.5 g was imposed on each ring, and the rings were allowed to equilibrate for 1 h. The changes in basal tension were recorded by isometric transducers connected to a data acquisition system (AQCAD, AVS Projetos, São Carlos, Brazil). To validate the endothelium integrity, the aortic rings were pre-contracted using Phe (1 μmol L-1), followed by initiation of relaxation using Ach (10 μmol L-1). Aortic rings with relaxation lower than 90% were discarded. After assessing the presence of functional endothelium, vascular tissues were allowed to recuperate for at least 1 h before the protocols using the testing samples, with replacement of Krebs-Henseleit solution after 15 min (Oliveira et al., 2012).
For the evaluation of the vasorelaxant potential of proteins and naturally-occurring peptides from common beans, the aortic rings (n = 7 per sample) were precontracted with Phe (1 μmol L-1) and after reached a contractile plateau, cumulative concentrations (0.1 to 10 mg mL−1) of each fraction (prepared in distilled water) were added to evaluate the vascular effects. The vehicle was also tested and the results were expressed as percentage of relaxation (Neto, Oliveira, Ghedini, Vaz, & Gil, 2017).
Statistical analysis
All examinations in the in vitro tests were done at least in triplicate, and the values were reported as mean ± standard deviation. For the ex vivo tests, seven animals were used in each evaluation. The variance analysis (ANOVA) and Tukey’s test were used to define differences in mean values of the data using GraphPad Prism® 6.0 software (GraphPad Software, Inc.).
Results and discussion
Protein extraction
Considering that one of the major factors affecting protein solubility is related to the solvent polarity profile, three different extraction solutions were used aiming to evaluate the effect of postharvest hardening on the solubility and biological potential of extracted proteins and naturally-occurring peptides. In addition, HTC beans were submitted to autoclaving and extrusion in order to evaluate the effects of thermal processing on the bioactive potential of proteins and peptides extracted using different solvents. Previous works have pointed out that extrusion can reduce and/or eliminate the antinutritional activity of protein inhibitors and lectins, improving the water absorption and solubility, and the protein and starch digestibility of HTC beans (Batista and Fernandes, 2016, Batista et al., 2010, Lopes et al., 2012). In addition, autoclaving can reduce the content of enzyme inhibitors and resistant starch, increasing protein and starch digestibility without interfere with the functional properties of the HTC grains (Batista, Pereira, Moreira, Silva, & Fernandes, 2020). However, to the best of our knowledge, this is the first work evaluating the effect of extrusion and autoclaving on the biological activities of HTC beans.
As can be observed in Table 1, the extraction yield of proteins from both, ETC and HTC beans, varied according to the extraction method used, possibly due to the physicochemical differences of each solvent. As expected, extraction using alkaline water (method C) resulted in the highest protein yield, since the ionic state of the proteins and peptides in the alkaline extract is different from the isoelectric point, making them highly soluble in the aqueous medium (Carrasco-Castilla et al., 2012).
Table 1.
Protein content (mg g−1 flour) of the extracts obtained using different solvents.
| Extraction method | ETC beans1 | HTC beans | Autoclaved beans | Extruded beans |
|---|---|---|---|---|
| Method A (Acetonitrile/water/formic acid) | 28.9 ± 0.4a,C | 32.6 ± 3.7a,C | 22.7 ± 0.7b,C | 15.6 ± 1.7c,B |
| Method B (Sodium acetate solution) | 147.0 ± 1.7a,B | 118 ± 0.7b,B | 39.0 ± 0.7c,B | 14.6 ± 0.9d,B |
| Method C (Alkaline water) | 432.2 ± 4.4a,A | 367.3 ± 4.2b,A | 118.1 ± 3.6c,A | 41.3 ± 1.4d,A |
Results are means of three determinations ± standard deviation. Data followed by the same lowercase letters in the same line are not statistically different (p > 0.05). Data followed by different uppercase letters in the same column are statistically different (p < 0.05). ETC = easy-to-cook (non-hardened) beans; HTC = hard-to-cook (hardened) beans.
It is important to point out that the postharvest hardening did not interfere with the solubility of more hydrophobic proteins extracted using acetonitrile/water/formic acid (method A). However, it was observed a reduction of 19.7% on the protein yield from HTC beans extracted using sodium acetate solution (method B) and a reduction of 15% for those extracted using alkaline water (method C). This fact can be due to a possible complexation between hydrophilic proteins and other components of beans (i.e., carbohydrates and lipids) as function of the biochemical changes during the hardening process (Chigwedere, Flores, Panozzo, Loey, & Hendrickx, 2019). Probably, the more hydrophobic proteins are less reactive to complexation and thus, are equally extractable before and after the hardening.
In addition, although extraction using acetonitrile/water/formic acid (method A) presented the lowest protein content (Table 1), this method is referred as an effective methodology for extraction of peptides with potent biological activities. The medium to low polarity of this solvent is efficient on extracting proteins and peptides with different degrees of hydrophobicity (Kessel & Ben-Tal, 2018). In addition, the use of acetonitrile decreases the dielectric constant of the solvent solution, altering the polarity of the solution and, therefore increasing the solubility of molecules with lower polarity.
It was also verified that the content of extracted proteins from HTC beans was negatively affected by autoclaving and extrusion. The lowest protein yield was achieved in the extruded samples (Table 1), which can be related to (i) the process conditions that can modify the molecular structure of non-protein components, interfering with protein solubilization, and also (ii) by a decreased in the water absorption of the extrudates, which make difficult their rehydration and hence, reduces the protein extraction (Pietsch, Bühler, Karbstein, & Emin, 2019).
The results showed that the characteristics of the extractor solution and thermal processing are important factors that affect not only the extraction yield, but also would might interfere with the nature of the extracted biomolecules. The protein extracts were subsequently fractionated through ultrafiltration aiming to obtain (1) a protein-rich fraction (F > 10 kDa) and (2) a peptide-rich fraction (F < 10 kDa). This fractionating process was conducted in order to verify the potential of these biomolecules as bioactive compounds.
Antioxidant activity
Different plant proteins and peptides have been highlighted as potential antioxidant molecules with ability to prevent mechanisms of the oxidative stresses associated with numerous degenerative aging diseases. However, the effect of the postharvest hardening on this biological activity have been studied less often. It is known that there is no standard method to measure the antioxidant capacity of food components, even though there are several known methods. Therefore, it is recommended that the evaluation of antioxidant activity should be performed using different methods. Herein the antioxidant activity was assessed by DPPH radical scavenging, ferric reducing antioxidant power (FRAP), and metal-chelating potential. These methods have been widely used to evaluate the antioxidant activity of protein/peptides from food matrices (Agrawal, Joshi, & Gupta, 2019; Silva, Hernández-Ledesma, Amigo, Netto, & Miralles, 2017).
Radical scavenging activity
DPPH assay is widely used to evaluate the antioxidant potential of biomolecules because of its sensitivity and effectiveness, being a method that determines the ability of compounds to act as free radical scavengers or hydrogen donors (Wang et al., 2019). As can be seen in Table 2, fractions F < 10 kDa obtained from HTC beans extracted by using method A and method C were less efficient as radical scavengers than those from ECT beans, with a reduction of 13.5–18.7% in their antioxidant activity.
Table 2.
Antioxidant activity (mM TE/mg protein) of protein and peptide-rich fractions determined by FRAP and DPPH methods.
| Antioxidant assay1 |
Method A (Acetonitrile/water/formic acid) |
Method B (Sodium acetate solution) |
Method C (Alkaline water) |
||||
|---|---|---|---|---|---|---|---|
| F > 10 kDa | F < 10 kDa | F > 10 kDa | F < 10 kDa | F > 10 kDa | F < 10 kDa | ||
| DPPH | ETC | 30.1 ± 1.8b,A | 22.2 ± 0.1b,B | 1.7 ± 0.1b,D | 22.4 ± 1.3b,B | 18.4 ± 0.1c,C | 20.9 ± 1.2a,B |
| HTC | 16.9 ± 0.4d,B | 19.2 ± 0.2d,B | 1.9 ± 0.1a,C | 22.6 ± 1.0b,A | 16.6 ± 0.3d,B | 17.0 ± 0.1b,B | |
| Autoclaved | 39.8 ± 0.2a,A | 20.8 ± 0.1c,B | 0.9 ± 0.02c,D | 21.1 ± 1.0b,B | 21.8 ± 2.7b,B | 14.5 ± 0.2c,C | |
| Extruded | 28.1 ± 1.4b,B | 47.7 ± 0.5a,A | 2.5 ± 0.02a,D | 44.7 ± 1.5a,A | 26.1 ± 0.2a,B | 20.6 ± 1.3a,C | |
| FRAP | ETC | 3.7 ± 0.3c,D | 62.1 ± 1.6b,B | n.d.2 | 51.7 ± 1.3c,C | n.d. | 81.1 ± 1.9a,A |
| HTC | 3.4 ± 0.2c,C | 48.1 ± 3.2c,B | n.d. | 57.4 ± 1.9c,A | n.d. | 49.0 ± 0.7b,B | |
| Autoclaved | 8.6 ± 0.3b,D | 61.7 ± 1.9b,B | n.d. | 70.4 ± 3.9b,A | 1.6 ± 0.01a,E | 44.1 ± 1.6c,C | |
| Extruded | 15.5 ± 0.5a,D | 121.0 ± 3.1a,A | 3.6 ± 0.4E | 81.1 ± 3.9a,B | 0.9 ± 0.04b,F | 44.1 ± 0.2c,C | |
Results are means of three determinations ± standard deviation. Data followed by the same lowercase letters in the same column are not significantly different (p > 0.05). Data followed by different uppercase letters in the same line are statistically different (p < 0.05). 2n.d.: not detected. ETC = easy-to-cook (non-hardened) beans; HTC = hard-to-cook (hardened) beans.
It is known that antioxidant properties of proteins and peptides are due to complex interactions between their ability to inactivate reactive oxygen species, scavenge free radicals, chelate prooxidative transition metals, enzymatically eliminate specific oxidants, and alter the physical properties of food systems in a way that separates reactive species. In this sense, changes in the amino acid composition, length of the peptide chain and three-dimensional structure of proteins can interfere on their ability to act as antioxidants (Peng, Kong, Wang, Ai-lati, Ji, & Mao, 2021). It is possible that during hardening, the spatial structure of the amino acids in the protein/peptide sequence might be changed, interfering with their hydrogen donor ability and, consequently, reducing their DPPH• radical scavenging activity.
In addition, results evidenced that the antioxidant potential was enhanced in the protein and peptide-rich fractions obtained from extruded HTC beans, with the highest values being observed in the fractions F < 10 kDa, independently of the extraction method (Table 2). These results allow to suggest that extrusion process could be an interesting alternative to produce bean-based food components with enhanced antioxidant potential.
In general, the autoclaving process was also capable of increase the antioxidant potential of the peptide-rich fractions (F < 10 kDa); however, it was observed a distinct behavior depending on the extraction method used. For the fractions F < 10 kDa from extraction using acetonitrile/water/formic acid (method A), autoclaving was able to recover the antioxidant activity of the proteins from HTC beans to values similar to those from ETC beans. However, this thermal processing did not interfere with the DPPH• radical scavenging activity of the peptides extracted by method B, while decreased the antioxidant potential of those molecules extracted using method C. The combined effect of high temperature and pressure during autoclaving process can cause protein degradation, as well as chemical modifications, and formation of protein–phenolic complexes who interfered with the ability of these molecules to act as antioxidant agents. It is possible that the chemical modifications of the protein structure during autoclaving allowed the exposure of antioxidant amino acids on the hydrophobic proteins, while negatively interfered with the availability of antioxidant moieties in the hydrophilic proteins (Sanchiz et al., 2019).
Ferric reducing antioxidant power (FRAP)
The results from FRAP method evidenced that peptide-rich fractions (F < 10 kDa) were the main responsible by the antioxidant power of beans (Table 2). In addition, the ferric reducing power of the peptide-rich fractions was negatively affected by the hardening process, being observed antioxidant activities 22.5% lower in the HTC fraction F < 10 KDa extracted using acetonitrile/water/formic acid (method A) and 39.6% lower for the HTC fraction F < 10 kDa extracted with alkaline water (method C). However, except for fractions F < 10 kDa extracted by method C, autoclaving and extrusion processes were efficient in recover or even enhance the antioxidant activity by FRAP. As reported for DPPH assays, the best antioxidant performances by FRAP were achieved in the peptide-rich fractions (F < 10 kDa) obtained from extruded beans, evidencing that besides the improved nutritional and functional properties (Batista & Fernandes, 2016), extruded beans are also an interesting source of proteins and peptides with high antioxidant activity. Furthermore, the use of autoclaving can be a promising alternative of pretreatment to recover the nutritional and biological potential of HTC beans, allowing the re-inclusion of the whole grain in human nutrition.
Metal chelating activity
According to Jomova and Valko (2011), the redox active metals like iron and copper possess the ability to produce reactive radicals in biological systems. Excessive accumulation leads to oxidative stress due to an increased formation of reactive oxygen species, which are responsible for lipid peroxidation, DNA damage, protein modification and other effects which can result in numerous diseases, such as chronic inflammation, cancer, cardiovascular diseases, diabetes, atherosclerosis, neurological disorders and others. Considering that molecules interfering with the catalytic activity of transition metals could retard or prevent pro-oxidative processes, the determination of their chelating activity is important for the estimation of their antioxidant capacity (Agrawal, Joshi, & Gupta, 2019). In this study, the different protein and peptide-rich fractions were tested regarding their ability to chelate Fe2+ and Cu2+ (Fig. 1).
Fig. 1.
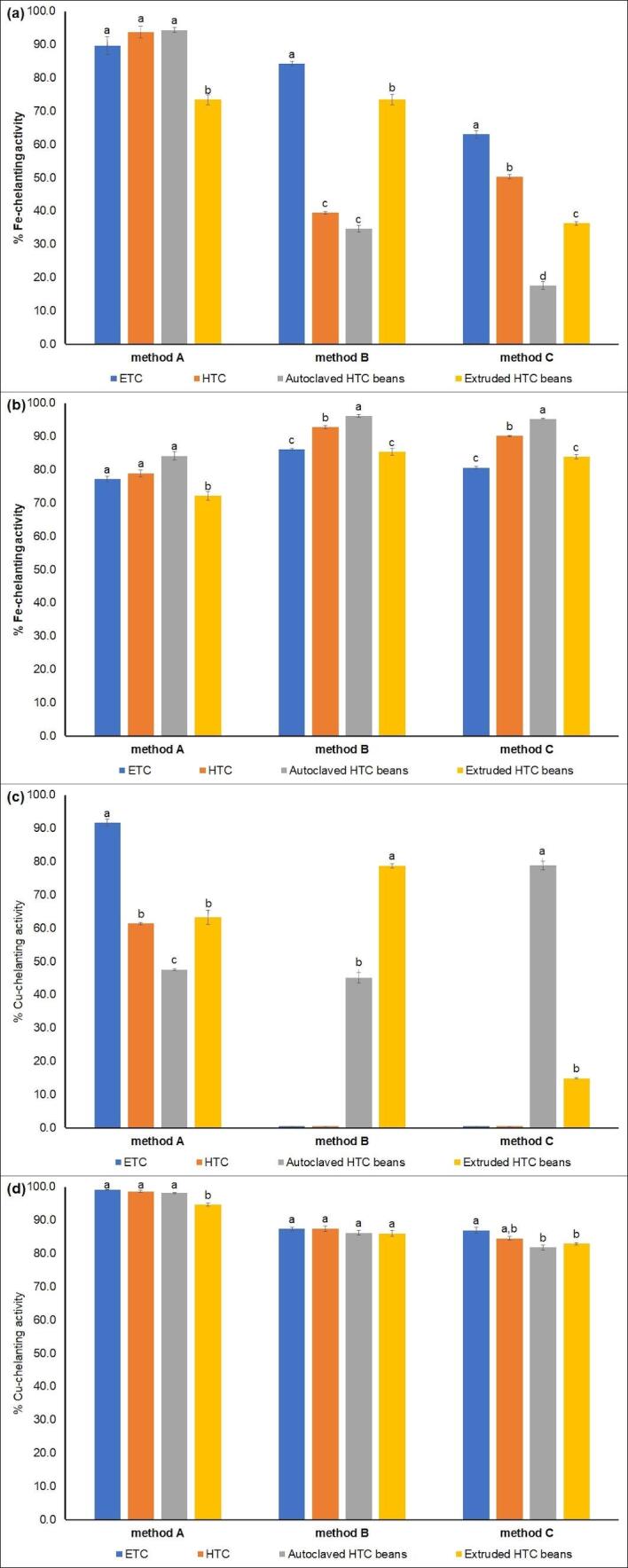
Metal-chelating activity of protein and peptide fractions from common beans. (a) Fe2+-chelating acitivity of protein-rich fractions (F > 10 kDa); (b) Fe2+-chelating activity of peptide-rich fractions (F < 10 kDa); (c) Cu2+-chelating activity of protein-rich fractions (F > 10 kDa), and (c) Cu2+-chelating activity of peptide-rich fractions (F < 10 kDa). Results are means of three determinations ± standard deviation. Data followed by the same superscript letter for the same extraction method are not significantly different (p > 0.05). Assays for protein-rich fractions were performed using a sample concentration of 2 mg mL−1. Assays for peptide-rich fractions were performed using a samples concentration of 0.2 mg mL−1. ETC = easy-to-cook (non-hardened) beans; HTC = hard-to-cook (hardened) beans.
Results have shown that protein and peptide-rich fractions were able to efficiently interact with iron. Nevertheless, chelating potential of the fractions F > 10 kDa and F < 10 kDa were quite different, being observed distinct profiles of chelation depending on the solvent used in the protein extraction and the development of postharvest hardening. The values of Fe2+-chelating activity for the protein fraction (F > 10 kDa) obtained from extraction with method A was maintained after hardening, being observed chelation values around 90% (Fig. 1a). However, the hardening process negatively affected the Fe2+-chelating properties of the fractions F > 10 kDa extracted with sodium acetate (method B) and alkaline water (method C), being observed reduction of 53% and 20% in their chelating activity, respectively.
Considering the peptide-rich fractions (F < 10 kDa), in the tests using sample concentration of 2 mg mL−1 the Fe2+-chelating activity reached 100% for all samples. In this case, chelating tests were also performed using a lower sample concentration (0.2 mg mL−1) and the results evidenced that the postharvest hardening did not affect the chelating activity of the fractions F < 10 kDa obtained by extraction with acetonitrile/water/formic acid (method A), while increased the chelating potential of those samples extracted using methods B and C (Fig. 1b). These results evidence that despite of the alterations in the physicochemical and functional properties occasioned by the postharvest hardening, the biological activities of these peptides remaining unchanged.
In addition, autoclaving was efficient in increase the Fe2+-chelating activity of all peptide-rich fractions (F < 10 kDa). This increase can be related to the exposure of amino acids unavailable to interact with iron in the native protein/peptide. Compared with autoclaving, the more drastic conditions of extrusion could result in protein fragmentation with consequent losses of reactive sites able to interact and chelate iron (Fig. 1b). However, except for the fractions F < 10 kDa obtained in the extraction using method B, the extruded samples presented Fe2+-chelating activity similar to those from ETC samples.
As shown for Fe2+-chelating activity, the peptide-rich fractions presented the highest Cu2+-chelating potential (Fig. 1d), being observed 100% of chelation when the tests were conducted using sample concentration of 2 mg mL−1. However, the extraction methods strongly interfered with the solubilization of proteins with ability to interact and chelate Cu2+ ions. In general, the more hydrophobic proteins obtained in the extraction method A had the best performances as Cu2+-chelating agents (Fig. 1c), being observed absence of activity in the fraction F > 10 kDa from those extracts obtained by method B and method C, for both ETC and HTC beans (Fig. 1c). In addition, the hardening process reduced the Cu2+-chelating activity of all fractions F > 10 kDa, although did not interfere with the chelating potential of the peptide-rich fractions (Fig. 1d).
The use of autoclaving or extrusion highly improved the Cu2+-chelating activity of the protein fractions F > 10 kDa containing hydrophilic proteins (Fig. 1c), while slight interfered with the chelating activity of fractions F < 10 kDa (Fig. 1d). These results reinforce the biological potential of HTC beans and confirms that the use of autoclaving and extrusion can be useful to restore or increase the biological potential of hardened beans either as component in foodstuffs or whole grain in human nutrition, contributing to the improvement of health conditions.
Study of vascular reactivity
In organism homeostasis, vascular smooth muscle tone is highly important since different pathologies related to the oxidative stress (i.e., vascular and neurodegenerative diseases) can cause changes in the muscle tone (Silva et al., 2018). Considering that the highest results of antioxidant activities were achieved in the samples with lower molecular weight, the fractions F < 10 kDa were selected for the evaluation of their vascular effect on endothelium-intact aortic rings precontracted with phenylephrine (Phe), and the results are presented in Fig. 2.
Fig. 2.
Vascular reactivity studies of protein and peptide fractions of common beans. Cumulative concentration–response curves for the peptide rich-fractions (F < 10 kDA) in rats aortic rings with intact endothelium precontracted with phenylephrine (Phe): (a) easy-to-cook (HTC) beans; (b) hard-to-cook (HTC) beans; (c) HTC beans submitted to autoclaving; and (d) HTC beans submitted to extrusion. Percentage of maximal relaxation (e) and EC50 values (e) for the peptide-rich fractions (F < 10 kDa) extracted with different solvents. The results show the mean ± SEM of 6–8 experiments. *p < 0.05. ETC = easy-to-cook (non-hardened) beans; HTC = hard-to-cook (hardened) beans; EC50 = concentration required to obtain a 50% relaxation.
In general, the peptide-rich fractions obtained from the extraction with acetonitrile/water/formic acid (method A) produced potent and similar relaxations (Fig. 2). In addition, postharvest hardening and thermal treatments improved the vasorelaxant potential of these samples, with values of EC50 in the range of 0.06 to 0.12 mg mL−1 (Fig. 2e). For the fractions F < 10 kDa obtained with sodium acetate (method B) and alkaline water (method C), the hardening process occasioned a reduction in the maximal percentage of relaxation (Fig. 2e) and increased the EC50 values of the samples (Fig. 2f), indicating that the physicochemical changes occurred during hardening decreased the relaxing power of the extracted molecules. These results corroborate the idea that hardening process differently interferes with the biological potential of hydrophobic and hydrophilic proteins and peptides.
The thermal treatments were able to restore the vasorelaxant power of the fractions F < 10 kDa obtained from method B (Fig. 2c and 2d), been observed EC50 values around 0.14 mg mL−1 (Fig. 2f). However, the maximum relaxation values were lower than those observed for the ETC beans (Fig. 2e). Considering the fraction F < 10 kDa extracted with alkaline water (method C), despite the values of maximum relaxation of the thermally-treated bean fractions were similar to those from HTC beans (Fig. 2e), the EC50 values were about 2-fold higher than those for the untreated HTC samples (Fig. 2f), evidencing a reduction on their vasorelaxant power.
It is important to point out that all fractions obtained, even those with vasorelaxant activity near to 50%, can be considered good vasorelaxant biomolecules and may be indicated and included in the testing routines to assess the role in the prevention and control of blood pressure. The results showed that the administration of fractions of peptides may induce the vasorelaxant effect and this effect is higher in the peptide-rich fractions (F < 10 kDa).
Additionally, as the best results of biological activity were achieved in the peptide-rich fractions extracted using method A, the F < 10 kDa fractions were submitted to another set of ultrafiltration using a membrane cut-off of 3 kDa. The obtained fractions (F3-10 kDa and F < 3 kDa) from untreated and thermally treated HTC beans were tested in aorta isolated from rat with preserved endothelium to verify if the vasorelaxant effect would be potentiated in any of these fractions. As can be observed in Fig. 3, the peptide fractions produced a concentration-dependent vasorelaxant effect (0.1–100 µmol L-1), with Emax of 93.4% in fraction F3-10 kDa from autoclaved beans and Emax of 84.7% in fraction F < 3 kDa from HTC beans (Table 3). These results confirmed that despite the physicochemical alterations occasioned by postharvest hardening, the proteins and peptides from HTC beans are of high quality if considered their potential benefits to human health.
Fig. 3.
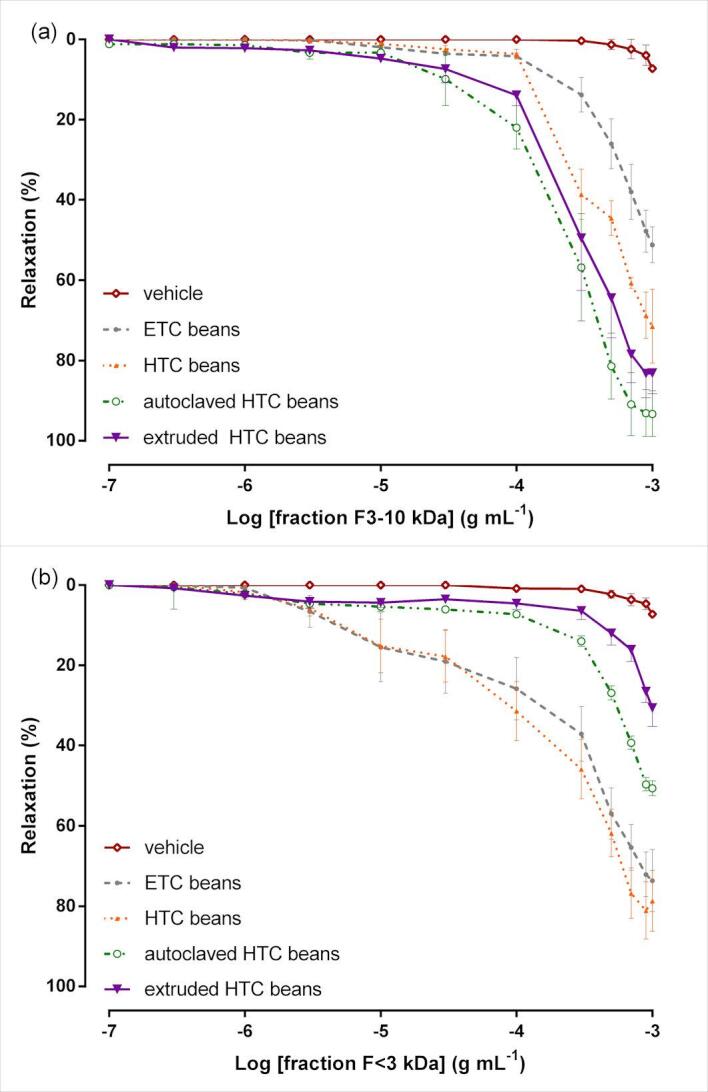
Cumulative concentration–response curves for the peptide rich-fractions in rats aortic rings with intact endothelium precontracted with phenylephrine (Phe): (a) fraction F3-10 kDa; (b) fraction F < 3 kDa. The results show the mean ± SEM of 6–8 experiments. ETC = easy-to-cook (non-hardened) beans; HTC = hard-to-cook (hardened) beans.
Table 3.
Percentage of Emax and EC50 values (mg mL−1) for the fractions F3-10 kDa and F < 3 kDa obtained from ETC and HTC beans with or without thermal treatment.
| Sample | Fractions |
||
|---|---|---|---|
| F3-10 kDa | F < 3 kDa | ||
| ETC beans1 | Emax1 | 51.2 ± 4.4 | 80.1 ± 8.9 |
| EC50 | 0.48 ± 0.03 | 0.20 ± 0.01 | |
| HTC beans | Emax | 71.5 ± 9.2 | 84.7 ± 8.6 |
| EC50 | 0.32 ± 0.05 | 0.16 ± 0.02 | |
| Autoclaved beans | Emax | 93.4 ± 10.5 | 50.6 ± 1.8 |
| EC50 | 0.22 ± 0.05 | 0.48 ± 0.02 | |
| Extruded beans | Emax | 83.1 ± 5.1 | 30.6 ± 4.7 |
| EC50 | 0.26 ± 0.04 | 0.64 ± 0.03 | |
Results show the means ± SEM of 6–8 experiments. ETC = easy-to-cook (non-hardened) beans; HTC = hard-to-cook (hardened) beans; Emax = maximal relaxation; EC50 = concentration required to obtain a 50% relaxation.
For fractions F3-10 kDa, autoclaving and extrusion presented a strong positive effect on the relaxation ability of the peptides (Fig. 3a), being observed EC50 values ranging from 0.22 mg mL−1 to 0.26 mg mL−1 for autoclaved and extruded beans, respectively (Table 3). These results can be explained by the probable changes in the three-dimensional structure of these medium-size peptides, with exposition of amino acids capable of interfere with the release of vasorelaxant molecules from the aorta endothelium.
Considering the lower-size peptides (F < 3 kDa), the thermal treatment had a negative effect on the vascular relaxation (Fig. 3b). It can be possible that during autoclaving and extrusion processes some small peptides could be fragmented in molecules unable to interact with endothelium cells and stimulate the release of the relaxant compounds. Nevertheless, the combined effect of both fractions of peptides still guarantees an excellent performance of these peptide fractions (F < 10 kDa) as vasorelaxant molecules by eliciting several pathways of endothelium-dependent relaxation.
Several mechanisms may be associated with vasorelaxant activity, such as: (a) prostacyclin pathway (PGI2), (b) nitric oxide (NO) production pathway by the enzyme eNOS and (c) the hyperpolarizing factor pathway derived from the endothelium. Depending on the type of vessel, all these pathways may be activated after stimulation of endothelial cell receptors in which signaling pathway increases the intracellular concentration of Ca2+. Acetylcholine (ACh), substance P (SP), bradykinin (BK), and adenosine triphosphate (ATP) are among the agonists. After release by endothelial cells, these mediators exert their actions on smooth muscle cells, through the production of second messengers such as cAMP or cGMP, or through hyperpolarization (Iqbal et al., 2017).
Although there are no indications in the literature of the use of peptides from beans to prevent cardiovascular diseases, the fractions herein obtained demonstrate the beneficial antioxidant effect and promising effect against cardiovascular diseases from both ETC and HTC. In addition, the thermal treatment can improve not only the technological and physicochemical properties of bean proteins but also increase their biological potential, especially regarding the antioxidant and vasorelaxant activities of their peptide fractions.
Conclusions
This study highlighted the vasorelaxant and antioxidant properties of different protein and peptide-rich fractions from ETC and HTC beans before and after thermal treatment. In general, a DPPH scavenging activity was observed ranging from 20.9 to 22.2 mM TE/mg protein for the fractions F < 10 kDa from ETC beans and values varying from 17 to 22.6 for the fractions from HTC beans. After thermal treatment of HTC beans, the values of antioxidant activity against DPPH• increased to values from 14.5 to 47.7 mM TE/mg protein, depending on the thermal process used. These results confirmed the occurrence of naturally-occurring peptides in seeds of common beans showing antioxidant activity and, therefore, presenting great potential as natural antioxidants. Additionally, peptide-rich fractions from both ETC and HTC beans presented high vasorelaxant activity, which making them potent molecules to be applied to prevent cardiovascular diseases. Before thermal treatment, a maximal relaxation of 84.7% was observed in the fraction F < 3 kDa from HTC beans. For the thermal-treated samples, a better value of maximal relaxation (93.4%) was achieved for the fraction F3-10 kDA from autoclaved beans. It was also possible to infer that the thermal processing affected the amount and nature of the extracted biomolecules, positively affecting their biological potential. Results also corroborate the idea that bioactivities are determined mainly by peptides and that the hardening process did not seem to definitively affect the bioactivity of the peptides. Ultimately, extrusion or autoclaving are promising alternatives for the re-inclusion of HTC beans in human nutrition as whole grains or ingredients for foodstuffs. Extrusion can have a practical application as thermal process in producing functional food ingredients and ready-to-eat products such as enriched snacks, cornflakes, biscuits and related foodstuffs that can be used for improve the human health. In addition, autoclaving can be used as pre-treatment of HTC grains aiming to potentialize their benefits to human health.
Funding sources
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq [grant number 426284/2016-0]; and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) [grant numbers 88882.156989/2017-01, 88881.134378/2016-01 and Finance Code 001].
CRediT authorship contribution statement
Ladyslene C. Paula: Methodology, Investigation, Formal analysis, Visualization, Writing – original draft. Ailton C. Lemes: Methodology, Investigation, Formal analysis, Writing – original draft. Erika Valencia-Mejía: Investigation, Methodology, Formal analysis, Validation. Bruna R. Moreira: Investigation, Methodology, Formal analysis, Validation. Thiago S. Oliveira: Investigation, Methodology, Formal analysis, Validation. Ivan T.N. Campos: Methodology, Formal analysis, Validation. Hiasmin F.S. Neri: Investigation, Methodology, Formal analysis, Validation. Claudio Brondani: Resources, Funding acquisition, Supervision. Paulo C. Ghedini: Conceptualization, Validation, Supervision. Karla A. Batista: Conceptualization, Validation, Visualization, Supervision, Writing – review & editing. F. Fernandes: Conceptualization, Resources, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Karla A. Batista, Email: karla.batista@ifg.edu.br.
Katia F. Fernandes, Email: kfernandes.lqp@gmail.com.
References
- Agrawal H., Joshi R., Gupta M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Research International. 2019;120:697–707. doi: 10.1016/j.foodres.2018.11.028. [DOI] [PubMed] [Google Scholar]
- Alves N.E.G., Gomes M.J., Vasconcelos C.M., Lima A.C., Lima S.L.S., Brito E.S.…Martino H.S.D. Six months under uncontrolled relative humidity and room temperature changes technological characteristics and maintains the physicochemical and functional properties of carioca beans (Phaseolus vulgaris L.) Food Chemistry. 2021;342 doi: 10.1016/j.foodchem.2020.128390. [DOI] [PubMed] [Google Scholar]
- Batista K.A., Fernandes K.F. Novas Edições Acadêmicas; 2016. Extrusão de farinha de feijão hard-to-cook: Características bioquímicas e propriedades funcionais. [Google Scholar]
- Batista K.A., Pereira W.J., Moreira B.R., Silva C.N.S., Fernandes K.F. Effect of autoclaving on the nutritional quality of hard-to-cook common beans (Phaseolus vulgaris) International Journal of Environment, Agriculture and Biotechnology. 2020;5:22–30. [Google Scholar]
- Batista K.A., Prudêncio S.H., Fernandes K.F. Changes in the functional properties and antinutritional factors of extruded hard-to-cook common beans. Journal of Food Science. 2010;75:286–290. doi: 10.1111/j.1750-3841.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reduncing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28:25–30. [Google Scholar]
- Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Jacinto-Hernández C., Alaiz M., Girón-Calle J.…Dávila-Ortiz G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chemistry. 2012;131:1157–1164. doi: 10.1016/j.foodchem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Analytical Biochemistry. 1971;40:450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Chigwedere C.M., Flores J.N.H., Panozzo A., Loey A.M.V., Hendrickx M.E. Instability of common beans during storage causes hardening: The role ofglass transition phenomena. Food Research International. 2019;121:506–513. doi: 10.1016/j.foodres.2018.12.006. [DOI] [PubMed] [Google Scholar]
- González-Monotya M., Hernández-Ledesma B., Mora-Escobedo R., Martínez-Villaluenga C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-iv, a-amylase, and a-glucosidase enzymes. International Journal of Molecular Sciences. 2018;19:1–14. doi: 10.3390/ijms19102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani D., Ribeiro J.V.V., Cruz V.S., Gomes R.M., Araújo E.G., Santos-Júnior A.C.M.…Xavier C.H. Oxidonitrergic and antioxidant effects of a low molecular weight peptide fraction from hardened bean (Phaseolus vulgaris) on endothelium. Brazilian Journal of Medical and Biological Research. 2021;54 doi: 10.1590/1414-431X202010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal Z., Bello I., Asmawi M.Z., Al-Mansoub M.A., Ahamad A., Jabeen Q., Fei Y.M. Vasorelaxant activities and the underlying pharmacological mechanisms of Gynura procumbens Merr. leaf extracts on rat thoracic aorta. Inflammopharmacology. 2017 doi: 10.1007/s10787-017-0422-4. [DOI] [PubMed] [Google Scholar]
- Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Kaur A., Kehinde B.A., Sharma P., Sharma D., Kaur S. Recently isolated food-derived antihypertensive hydrolysates and peptides: A review. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128719. [DOI] [PubMed] [Google Scholar]
- Kessel A., Ben-Tal N. Vol. 2. CRC Press; London: 2018. (Introduction to proteins: Structure, function, and motion). [Google Scholar]
- Lopes L.C.M., Batista K.A., Fernandes K.F., Santiago R.A.C. Functional, biochemical and pasting properties of extruded bean (Phaseolus vulgaris) cotyledons. International Journal of Food Science and Technology. 2012;47:1859–1865. [Google Scholar]
- Mahatmanto T., Poth A.G., Mylne J.S., Craik D.J. A comparative study of extraction methos reveals preferred solvents for cystine knot peptide isolation from Momordica cochinchinensis seeds. Fitoterapia. 2014;95:22–33. doi: 10.1016/j.fitote.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Manzoor M., Singh J., Gani A. Exploration of bioactive peptides from various origin as promising nutraceutical treasures: In vitro, in silico and in vivo studies. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131395. [DOI] [PubMed] [Google Scholar]
- Neto J.R.O., Oliveira T.S., Ghedini P.C., Vaz B.G., Gil E.S. Antioxidant and vasodilatory activity of commercial beers. Journal of Functional Foods. 2017;34:130–138. [Google Scholar]
- Oliveira L.M., Oliveira T.S., Costa R.M., Gil E.S., Costa E.A., Passaglia R.C.A.T.…Ghedini P.C. The vasorelaxant effect of gallic acid involves endothelium-dependent and -independent mechanisms. Vascular Pharmacology. 2016;81:69–74. doi: 10.1016/j.vph.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Oliveira L.M., Rodrigues A.G., Silva E.F., Cerqueira L.B., Castro C.H., Pedrino G.R.…Ghedini P.C. Endothelium-dependent vasorelaxant effect of butanolic fraction from Caryocar brasiliense Camb. leaves in rat thoracic aorta. Evidence-Based Complementary and Alternative Medicine. 2012;2012:1–10. doi: 10.1155/2012/934142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseguera-Toledo M.E., Mejia E.G., Dia V.P., Amaya-Llano S.L. Common bean (Phaseolus vulgaris L.) hydrolysates inhibit inflammation in LPS-induced macrophages through suppression of NF-jB pathways. Food Chemistry. 2011;127:1175–1185. doi: 10.1016/j.foodchem.2011.01.121. [DOI] [PubMed] [Google Scholar]
- Pedrosa M.M., Guillamón E., Arribas C. Autoclaved and Extruded Legumes as a Source of Bioactive Phytochemicals: A Review. Foods. 2021;10(2):379. doi: 10.3390/foods10020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Kong X., Wang Z., Ai-lati A., Ji Z., Mao J. Baijiu vinasse as a new source of bioactive peptides with antioxidant and anti-inflammatory activity. Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.128159. [DOI] [PubMed] [Google Scholar]
- Pietsch V.L., Bühler J.M., Karbstein H.P., Emin M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. Journal of Food Engineering. 2019;251:11–18. [Google Scholar]
- Ratnaningsih N., Suparmo H., Marsono E.Y. Physicochemical properties, in vitro starch digestibility, and estimated glycemic index of resistant starch from cowpea (Vigna unguiculata) starch by autoclaving-cooling cycles. International Journal of Biological Macromolecules. 2019 doi: 10.1016/j.ijbiomac.2019.09.092. [DOI] [PubMed] [Google Scholar]
- Saiga A., Tanabe S., Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Sanchiz A., Pedrosa M.M., Guillamón E., Arribas C., Cabellos B., Linacero R., Cuadrado C. Influence of boiling and autoclave processing on the phenolic content, antioxidant activity and functional properties of pistachio, cashew and chestnut flours. LWT. 2019;105:250–256. [Google Scholar]
- Sharma P., Kaur H., Kehinde B.A., Chhikara N., Sharma D., Panghal A. Food-Derived Anticancer Peptides: A Review. International Journal of Peptide Research and Therapeutics. 2021;27(1):55–70. [Google Scholar]
- Siah S., Wood J.A., Agboola S., Konczak I., Blanchard C.L. Effects of soaking, boiling and autoclaving on the phenolic contents and antioxidant activities of faba beans (Vicia faba L.) differing in seed coat colours. Food Chemistry. 2014;142:461–468. doi: 10.1016/j.foodchem.2013.07.068. [DOI] [PubMed] [Google Scholar]
- Silva F.G.D., Hernández-Ledesma B., Amigo L., Netto F.M., Miralles B. Identification of peptides released from flacseed (Linum usitatissimum) protein by Alcalase hydrolysis: Antioxidant activity. LWT - Food Science and Technology. 2017;76:140–146. [Google Scholar]
- Silva R.E.R., Morais L.P., Silva A.A., Bastos C.M.S., Pereira-Gonçalves A., Kerntopf M.R.…Barbosa R. Vasorelaxant effect of the Lippia alba essential oil and its major constituent, citral, on the contractility of isolated rat aorta. Biomedicine & Pharmacotherapy. 2018;108:792–798. doi: 10.1016/j.biopha.2018.09.073. [DOI] [PubMed] [Google Scholar]
- Siqueira B.S., Bassinello P.Z., Malgaresi G., Pereira W.J., Fernandes K.F. Analyses of technological and biochemical parameters related to the HTC phenomenon in carioca bean genotypes by the use of PCA. LWT - Food Science and Technology. 2016;65:936–945. [Google Scholar]
- Valencia-Mejía E., Batista K.A., Fernández J.J.A., Fernandes K.F. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.) Food Research International. 2019;121:238–246. doi: 10.1016/j.foodres.2019.03.043. [DOI] [PubMed] [Google Scholar]
- Wang L., Li T., Sun D., Tang M., Sun Z., Chen L.…Chen Z. Effect of electron beam irradiation on the functional properties and antioxidant activity of wheat germ protein hydrolysates. Innovative Food Science & Emerging Technologies. 2019;54:192–199. [Google Scholar]



