Highlight
-
•
Freeze-drying could notably decrease the purine release from lentinus edodes.
-
•
Roast-drying reduced guanine and adenine levels in lentinus edodes.
-
•
Roast-drying raised the level of strong uricogenic purine hypoxanthine in lentinus edodes.
-
•
The total purine content was higher than that of raw LE after moist heating.
Keywords: Lentinus edodes, Purine, Acidolysis, Drying technique, Moist heat
Abstract
Lentinus edodes (LE) is very popular in the world and also considered as high purine food. However, few focuses on purine types and its change during food processing. Here, we first compared 3 drying techniques, including roast-drying, freeze-drying, sun-drying on purine contents of LE by using acidolysis and HPLC. It showed that adenine decreased significantly after roast-drying (120 °C), which may be caused by thermal damage of DNA. Total purine decreased significantly after freeze-drying, while roast-dried and sun-dried LE remained unchanged. The effect of moist heat (boiling) on LE purine were also evaluated. Total purine increased due to xanthine increasement (331.72 ± 50.07%). And purine contents transferred into boiled liquid was higher than that in boiled solid. Compared with sun-dry and roast-dry processing, freeze-drying could notably affect the purine release from LE and decrease purine contents. Therefore, freeze-drying is recommended for process techniques for hyperuricemia and gouts populations.
1. Introduction
Uric acid (UA) is the final product of exogenous and endogenous purines metabolism (Maiuolo, Oppedisano, Gratteri, Muscoli, & Mollace, 2016). Exogenous purine mainly refers to purine from food. Food with high total purine contents can cause the elevation of serum uric acid (SUA) (Inazawa et al., 2014). However, most of the research simply focused on detecting total purine contents of food, neglecting the exact radio of each purine (adenine, guanine, hypoxanthine and xanthine, etc.) and their contents change during food process.
The detectable amounts and types of purines in foods vary depending on food processing techniques. The purine content changes as effected by food processing have been previously studied in high purine foods including red meat, seafood, and soybean products, etc. For examples, dry heat processes like roasting could reduce the purine contents in lamb than microwaving followed by broiling (Badiani et al., 2003). Also, moist heat processes, including boiling and steaming, could reduce the contents of adenine and hypoxanthine in tilapia (Lou, Chen, Hsu, & Chang, 2005). In addition to this, edible fungi which is very popular in Asia and even in the world, are also considered as high purine food, such as lentinus edodes. So far, few researches have been conducted in terms of the change of each purine contents affected by different food processing. In order to better understanding how purine changes in edible fungi during food processing, a precise method for purine detection is necessary.
Previous study showed that the combination of acidolysis followed by high performance liquid chromatography (HPLC) detection was a suitable method for detecting purines in food materials (Yamaoka et al., 2010). The first acidolysis step is very necessary since acid could break down the glycosidic bonds and phosphoester bonds inside nucleic acid molecule via nucleophilic reaction, generating free purines for further HPLC assay. For examples, perchloric acid, sulfuric acid, as well as the mix of trifluoroacetic acid and formic acid (TFA-FA), were commonly used for the acidolysis of food materials (Li et al., 2015, Qu et al., 2017, Yamaoka et al., 2010). As for edible fungi, lentinus edodes, it still remains unclear which kind of acid works better for acidolysis before free purine contents detection by HPLC.
In this study, the purine releasement of lentinus edodes as affected by different food processes, including sun-drying, roast-drying, freeze-drying and boiling treatments, were studied. Since we aimed to precisely measure the purine contents of lentinus edodes after different food processes, we optimized the acidolysis conditions as well as the HPLC procedures. In short, this study might set an example for investigating the purine contents and its release change by food processing techniques in edible fungi, and give suggestion for a better choice for lentinus edodes processing.
2. Materials and methods
2.1. Reagents and materials
Raw lentinus edodes and dried lentinus edodes were collected from Logistics Service Center of South China University of Technology and stored at 4 °C. Purine standards (guanine, adenine, hypoxanthine and xanthine, purity ≥ 99%) were obtained from Sigma-Aldrich Co. Ltd (St. Louis, MO, USA) and stored at 4 °C. HPLC-grade acetonitrile and methanol were obtained from Tianjin Damao Chemical Reagent Factory Co., Ltd. (Tianjin, China). Analytical Reagents (AR) monopotassium phosphate, sodium 1-pentanesulfonate, phosphoric acid, formic acid and trifluoroacetic acid were purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). PCA was gained from Cangzhou xinyuanquan Chemical Co., Ltd (Cangzhou, China). Ultrapure water was obtained using a Milli-Q Direct system (Millipore, USA).
2.2. Sample preparation
2.2.1. Pretreatment of lentinus edodes simulating industrial drying process
An appropriate amount of raw lentinus edodes were shearing to small pieces, then the pieces close to 3 mm × 3 mm × 3 mm were collected by 6 mesh and 8 mesh sieves. The raw lentinus edodes pieces were then roast-dried for 35 min at 120 °C in an oven, freeze-dried for 48 h at −80 °C in a lyophilizer, sun-dried for 12 h at about 30 °C or boiled in water for 25 min at 100 °C, separately. The preparation conditions are described in Table 1. After the pretreatments, raw lentinus edodes, roast-dried lentinus edodes, freeze-dried lentinus edodes, sun-dried lentinus edodes, boiled solid (0.20 g of each) were lysed using TFA-FA as mentioned in 2.2.2. Boiled liquid was centrifuged at 8445 × g for 10 min at 4 °C. The supernatant (1 mL) was filtered through a 0.22 μm membrane filter and stored at 4 °C for HPLC detection.
Table 1.
Food processes techniques for lentinus edodes supplied with dry or moist heat.
| Food process | Temperature (℃) | Time (min) | |
|---|---|---|---|
| Dry heat | Roast-drying | 120 | 35 |
| Sun-drying | 30 | 720 | |
| Freeze-drying | −80 | 1440 | |
| Moist heat | Boiling in water | 100 | 25 |
2.2.2. Acidolysis of lentinus edodes
Lentinus edodes were lysed using two methods of previous articles (Fukuuchi et al., 2018, Li et al., 2019, Qu et al., 2017) with slight modification. To be brief, dried lentinus edodes (0.20 g) was added to 50 mL centrifugal tube containing 11 mL TFA-FA-water (5:5:1, v/v/v) and then hydrolyzed 12 min at 90 °C. After chilling in cold water, the hydrolysates were transferred to a 100 mL flask and dried using a rotary vacuum evaporator at 55 °C. Mobile phase A (10 mL solution that consist of 0.20 mol/L monopotassium phosphate and 0.52 mmol/L sodium 1-pentane sulfonate, set to pH 3.8) was added to dissolve the purine bases (assisted by ultrasonic treatment). After dissolution, the solution was centrifuged at 8445×g for 10 min at 4 °C. The supernatant was filtered through a 0.22 μm membrane filter and then stored at 4 °C for HPLC detection.
Dried lentinus edodes (0.20 g) was hydrolyzed in 5 mL of 70% PCA for 55 min at 80 °C. Then the hydrolysate was diluted to pH 2, and the diluent was centrifuged at 8445×g for 10 min at 4 °C. The supernatant was filtered through a 0.22 μm membrane filter and stored at 4 °C for HPLC detection.
2.2.3. Preparation of purine standard solution
The stock solution of 1000 mg/L purine standard was prepared by dissolving 5 mg of adenine, hypoxanthine and xanthine in 5 mL ultrapure water containing 0.6 mL 1 mol/L sodium hydroxide (NaOH). The stock solution was diluted to prepare purine standard solutions with different concentrations of 250 mg/L, 125 mg/L, 50 mg/L, 10 mg/L and 1 mg/L, and stored at 4 °C for detection by HPLC. In addition, guanine standard solution was also prepared as the method mention above.
2.3. HPLC analysis procedure
The purine contents were determined by LC-20A HPLC system (Shimadzu, Kyoto, Japan) equipped with a variable-wavelength ultra-violet (UV) detector and an automatic injector according to the previous method (Hou et al., 2019), with some modifications. In short, the HPLC separations of samples were conducted on a Microsorb-MV C18 column (150 mm × 4.60 mm,5 μm) at 254 nm with keeping 35 °C of the column. The injection volume was 20 μL and the flow rate was 1.0 mL/min. Mobile phase A consist of 0.20 mol/L monopotassium phosphate and 0.52 mmol/L sodium 1-pentane sulfonate, set to pH 3.8 by adding phosphoric acid (HPLC-grade). Mobile phase B was made up of the same final concentrations as mobile phase A, apart from the addition of 10% acetonitrile (v/v). The gradient elution conditions of mobile phase were as follows, (1) 0–6.00 min, 100% mobile phase A isometric elution, (2) 6.00–14.00 min, 100% mobile phase A decrease to 30 % mobile phase A and 70% mobile phase B, gradient elution, (3) 14.00–17.40 min, 30% mobile phase A and 70% mobile phase B were eluted, (4)17.40–17.50 min,30% mobile phase A increase to 100% mobile phase A and 70% mobile phase B decrease to 0 % mobile phase B, gradient elution, (5)17.50–35 min,100% mobile phase A, isometric elution. In order to reduce the residue of lentinus edodes compounds in chromatographic column and affect the determination of subsequent samples, the elution time of mobile phase A was appropriately prolonged according to the samples.
2.4. Moisture determination
The Moisture content was determined according to National Standard of the People's Republic of China (GB5009.3—2016, 2016, 2016).
2.5. Determination of purine contents in the samples
The purine contents of the samples were calculated based on their peak areas and the standard curve. And then the purine contents of processed samples under dry weight condition was determined using the following equation:
2.6. Statistical analysis
All data were presented as mean ± standard deviation (SD) from triplicate analyses. Statistical analysis was carried out using the GraphPad 6.0 Prism (GraphPad Software, Inc., La Jolla, CA, USA). Results were statistically analyzed among groups using t-test and one-way analysis of variance (ANOVA) to analyze the difference between two or more groups. P-values < 0.05 were regarded as statistically significant.
3. Results and discussion
3.1. Optimization of acidolysis method
In the present study, HPLC was applied to investigate purines in food, for its precision and reproducibility. TFA with FA and PCA had long been use for the acidolysis of food materials. Therefore, TFA-FA and PCA were used to lyse the dried lentinus edodes for purine detection by HPLC. However, in our experiment, we found that PCA was not suitable for lysing lentinus edodes. In Fig. 1B, the chromatogram of the PCA acidolyzed sample showed poor resolution, while the TFA-FA acidolyzed sample showed more than 7 peaks during 3–15 min. This might because of the PCA remained in the test samples. Then, purine internal standards were added into the TFA-FA acidolyzed samples to verify the identity of the peaks. As shown in Fig. 1C, the area of four peaks were higher in the spiked sample (red) than in the control lentinus edodes sample (blue), the retention time (hypoxanthine: 3.86 min, xanthine: 4.22 min, guanine: 5.65 min, adenine: 10.70 min) of these purine peaks are similar with the pure standards in black line (hypoxanthine: 3.82 min, xanthine: 4.19 min, guanine: 5.88 min, adenine: 11.87 min). Therefore, TFA-FA acidolysis method were chose to lysed the lentinus edodes samples in the further experiments.
Fig. 1.
Comparison of the Lentinus edodes (LE) acidolysis with perchloric acid (PCA) or trifluoroacetic acid (TFA)-formic acid (FA). (A) Structure of PCA, TFA and FA. (B) Chromatogram of LE acidolyzed with PCA and TFA-FA. (C) Chromatogram of purine standard (black), LE acidolyzed with TFA-FA and its spiked sample. All samples are detected under A254nm.
3.2. Determination of purine by HPLC
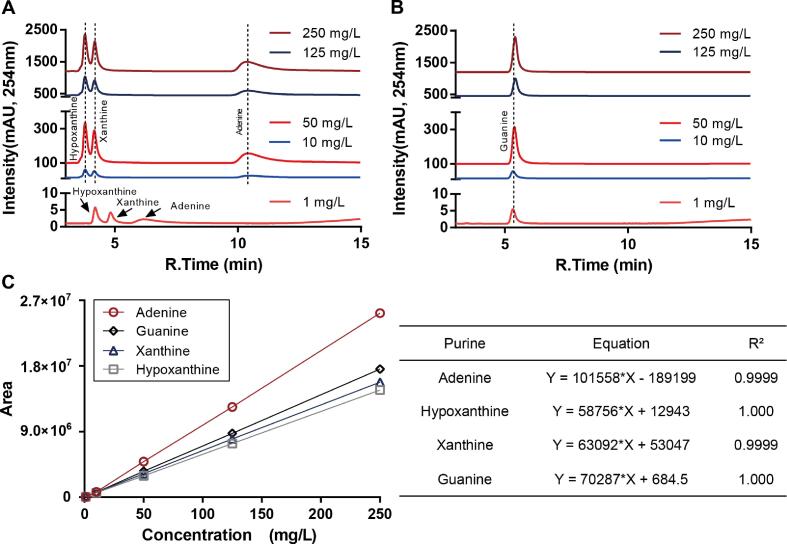
Gradient concentrations of four purine standards were detected to obtain the standard curves. Because the retention time of adenine under 1 mg/L is similar to guanine, three purine (adenine, hypoxanthine, xanthine) standards and guanine standard were detected separately. The chromatograms of different concentrations of purine standards are shown in Fig. 2A an B. But interestingly, the retention time of adenine stabilized at 10.70 min, and can be separated as Fig. 1C in samples. Fig. 2C is the standard curve of four purines, and the four standard curve equations of purines are listed in the table for the subsequent calculation of purine contents in samples.
Fig. 2.
Chromatograms of four purine standard curves. (A) HPLC chromatograms of hypoxanthine, xanthine, and adenine. (B) HPLC chromatograms of guanine. All samples are detected under A254 nm. (C) Standard curves of four standard curves. The equations of the standard curves and their R2 are listed in the table on the right of the curves.
3.3. Purine contents of lentinus edodes after drying process with various heat supply
Lentinus edodes was processed as the procedure as Fig. 3A, and the purine contents of the samples were evaluated by HPLC method. In order to maintain the repeatability of the retention times, the purine standards were added into the sample according to the purine contents detected, and the spiked samples were analyzed right after. The chromatogram (Fig. 3B–E) of raw, freeze-dried, roast-dried and sun-dried lentinus edodes samples and their spiked samples showed fine resolution, and the peak intensity of the corresponding purine increases significantly after the addition of standard, which further indicates that this method has well feasibility and accuracy.
Fig. 3.
Effects of three drying processes with various heat supply on the purine contents of Lentinus edodes (LE). (A) Flow chart of the drying processes and the detection of LE purine contents. (B) The chromatograms of raw LE and their spiked samples. (C) The chromatograms of sun-dried LE and their spiked samples. (D) The chromatograms of freeze-dried LE and their spiked samples. (E) The chromatograms of roast-dried LE and their spiked samples. (F) Moisture of LE after drying processes. (G) Purine contents of LE after three kinds of drying processes. *P < 0.05, **P < 0.01, ***P < 0.001.
To compare the total purine of the samples, we normalized the purine contents according to the moisture content after each process showed in Fig. 3F, which is 86.80 ± 1.16%, 22.53 ± 0.11%, 7.90 ± 2.6% and 11.30 ± 1.68% in raw, roast-dried, freeze-dried and sun-dried lentinus edodes separately. In comparison with raw lentinus edodes, adenine was significantly decreased (P < 0.0001) and hypoxanthine was notably increased (P < 0.0001) after roast-drying, while the xanthine content was significantly increased (P < 0.01) and hypoxanthine was significantly decreased (P < 0.01) after sun-drying and freeze-drying. And the content of total purine was decreased after freeze-drying. A significant reduction of adenine (and a slight reduction of guanine) occurs after roast-drying. Adenine and guanine are important components of macromolecules such as DNA and RNA (Lodish & Zipursky, 2001), which can be hydrolyzed to become guanine and adenine. In addition, the previous study reported that mushroom DNA was significantly degraded when dried at temperatures above 80 °C (Wang, Liu, & Xu, 2017). Moreover, a study showed that DNA loses remarkably at 120 °C or higher (Paunescu, Fuhrer, & Grass, 2013), accompanied with the oxidation and deamination of purine bases. The polycyclic aromatic hydrocarbons or heterocyclic amines produced by roasting can bind to the C8, N6, N2 and N7 of purine bases (Lukin & de los Santos, 2006), thus reducing the detectable content of guanine and adenine after acidolysis.
On the other hand, freeze-drying was carried out at low temperature, while lentinus edodes dried by roast-drying and sun-drying process went through varying degrees of high temperature. Low temperature in freeze-drying kept cells dormant and making their walls insusceptible to destruction (Qiu et al., 2021), which might result in fewer purine releasement. Therefore, freeze-drying process, reducing the purine content, is better than roast-drying and sun-drying processes. Besides, according to previous reports, hypoxanthine is a strong uricogenic purine (Clifford, Riumallo, Young, & Scrimshaw, 1976), and thus freeze-drying process, which reduced the total purine contents and especially the hypoxanthine content, is advisable for lentinus edodes drying and roasted-drying should be avoided.
3.4. Purine contents of lentinus edodes after moist heating
Lentinus edodes was treated with moist heat by boiling in water, and the procedure is indicated as Fig. 4A. The chromatogram of the boiled liquid was showed in Fig. 4B and boiled solid after acidolysis in Fig. 4C. As a result, the total purine contents increased significantly after moist heating because of the acute increase of xanthine. As showed in upper panel of Fig. 4E, both the boiled liquid and the boiled solid were rich in purine, and interestingly, the purine contents in boiled liquid were higher than the boiled solid, suggesting that lentinus edodes purine can easily transfer into water when boiling. Therefore, moist heat might increase the purine contents in lentinus edodes, and the liquid of boiling lentinus edodes was responsible for the larger part and should be controlled in daily consumption.
Fig. 4.
Influence of moist heat on the purine contents of Lentinus edodes (LE). (A) Flow chart of the moist heating process and the detection of LE purine. (B) The chromatograms of boiled solid and its spiked samples. (C) The chromatograms of boiled liquid and its spiked samples. (D) Moisture of LE after boiling in water. (E) Purine contents of LE after boiling. *P < 0.05, **P < 0.01, ***P < 0.001.
4. Conclusion
In this study, purine contents of lentinus edodes undergone various food processing were measured and analyzed. It showed that roast-drying reduced guanine and adenine levels, which may be related to oxidation, deamination or damage of purine bases necessary component for DNA and RNA. However, it raised hypoxanthine content, which is a kind of strong uricogenic purine. While sun-drying and freeze-drying were opposite, and freeze-drying even decreased the total purine contents of lentinus edodes. Moreover, after moist heating, the total purine contents were higher than that of raw lentinus edodes, and the purine contents in boiled liquid was higher than the boiled solid. In conclusion, we proved that freeze-drying is the best drying technique among the 3 tested drying techniques, and it is strongly recommended food processing for hyperuricemia and gout management.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the Natural Science Foundation of Guangdong Province of China (2019A1515012230) and the National Natural Science Foundation of China (No. 32072207).
References
- Badiani A., Montellato L., Maranesi M., Bochicchio D., Parazza P., Anfossi P. Contents and retentions of free and total purine bases in lamb meat cooked by several household methods. Italian Journal of Animal Science. 2003;2(sup1):572–574. [Google Scholar]
- Clifford A., Riumallo J., Young V., Scrimshaw N. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. The Journal of Nutrition. 1976;106(3):428–434. [Google Scholar]
- Fukuuchi T., Iyama N., Yamaoka N., Kaneko K. Simultaneous quantification by HPLC of purines in umami soup stock and evaluation of their effects on extracellular and intracellular purine metabolism. Nucleosides, Nucleotides and Nucleic Acids. 2018;37(5):273–279. doi: 10.1080/15257770.2018.1453074. [DOI] [PubMed] [Google Scholar]
- GB5009.3—2016 . China, Standards Press of China; Beijing: 2016. Determination of moisture in foods. [Google Scholar]
- Hou C., Liu D., Wang M., Gong C., Li Y., Yang L.…Ren J. Novel xanthine oxidase-based cell model using HK-2 cell for screening antihyperuricemic functional compounds. Free Radical Biology and Medicine. 2019;136:135–145. doi: 10.1016/j.freeradbiomed.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Inazawa K., Sato A., Kato Y., Yamaoka N., Fukuuchi T., Yasuda M.…Kaneko K. Determination and profiling of purines in foods by using HPLC and LC-MS. Nucleosides, Nucleotides and Nucleic Acids. 2014;33(4-6):439–444. doi: 10.1080/15257770.2013.865744. [DOI] [PubMed] [Google Scholar]
- Li, T., Ren, L., Wang, D., Song, M., Li, Q., & Li, J. (2019). Optimization of extraction conditions and determination of purine content in marine fish during boiling. PeerJ, 7, e6690. [DOI] [PMC free article] [PubMed]
- Li G., Liu Fang, Hao Jianqin, Liu Changshu. Determination of purines in beer by HPLC using a simple and rapid sample pretreatment. Journal of the American Society of Brewing Chemists. 2015;73(2):137–142. [Google Scholar]
- Lodish, H., & Zipursky, S. L. J. B. M. B. E. (2001). Molecular cell biology. 29, 126-133.
- Lou Shyi-Neng, Chen Hui-Huang, Hsu Po-Yang, Chang Da-Hsuan. Changes in purine content of tilapia surimi products during processing. Fisheries science. 2005;71(4):889–895. [Google Scholar]
- Lukin Mark, de los Santos Carlos. NMR structures of damaged DNA. Chemical reviews. 2006;106(2):607–686. doi: 10.1021/cr0404646. [DOI] [PubMed] [Google Scholar]
- Maiuolo Jessica, Oppedisano Francesca, Gratteri Santo, Muscoli Carolina, Mollace Vincenzo. Regulation of uric acid metabolism and excretion. International Journal of Cardiology. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- Paunescu Daniela, Fuhrer Roland, Grass Robert N. Protection and Deprotection of DNA—high-temperature stability of nucleic acid barcodes for polymer labeling. Angewandte Chemie International Edition. 2013;52(15):4269–4272. doi: 10.1002/anie.201208135. [DOI] [PubMed] [Google Scholar]
- Qiu Yang, Bi Jinfeng, Jin Xin, Hu Lina, Lyu Jian, Wu Xinye. An understanding of the changes in water holding capacity of rehydrated shiitake mushroom (Lentinula edodes) from cell wall, cell membrane and protein. Food Chemistry. 2021;351:129230. doi: 10.1016/j.foodchem.2021.129230. [DOI] [PubMed] [Google Scholar]
- Qu Xin, Sui Jianxin, Mi Nasha, Lin Hong. Determination of four different purines and their content change in seafood by high-performance liquid chromatography. Journal of the Science of Food and Agriculture. 2017;97(2):520–525. doi: 10.1002/jsfa.7755. [DOI] [PubMed] [Google Scholar]
- Wang S., Liu Y., Xu J. Comparison of different drying methods for recovery of mushroom DNA. Scientific Reports. 2017;7(1):1–10. doi: 10.1038/s41598-017-03570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka N., Kaneko K., Kudo Y., Aoki M., Yasuda M., Mawatari K.…Yamamoto T. Analysis of purine in purine-rich cauliflower. Nucleosides, Nucleotides and Nucleic Acids. 2010;29(4-6):518–521. doi: 10.1080/15257771003741372. [DOI] [PubMed] [Google Scholar]