Abstract
Maternal low-protein and postnatal high-fat (HF) diets program offspring obesity and type 2 diabetes mellitus (T2DM) risk by epigenetically reducing beige adipocytes (BAs) via increased G9a protein expression (Histone3 Lysine9 dimethyl transferase), an inhibitor of the BA marker fibroblast growth factor 21 (FGF21). Conversely, offspring exercise reduces fat mass and white adipocytes, but the mechanisms are not yet understood. This work investigated whether exercise reduces offspring obesity and T2DM risk caused by a maternal HF diet via regulation of G9a and FGF21 expression that would convert white to BA. Two-month-old female C57Bl/6J mice (F0) were fed a 16% (normal fat; NF) or a 45% HF diet for 3 months prior to breeding, and subsequent gestation and lactation. Male offspring (F1) were fed the same NF and HF diets and further divided into either sedentary (S) or voluntary wheel running (Ex) groups for an additional 3 months yielding eight groups: NF (maternal treatment condition)-NF-S (postweaning treatment conditions), NF-HF-S, NF-NF-Ex, NF-HF-Ex, HF-NF-S, HF-HF-S, HF-NF-Ex, and HF-HF-Ex. Subcutaneous adipose tissue was collected for protein and mRNA analysis of FGF21, peroxisome proliferator-activated receptor-gamma coactivator (PGC-1 alpha, inducer of FGF21), G9a, E4BP4 (G9a coactivator), and protein expression of H3K9 demethylases (KDM4C). Postnatal HF diet decreased FGF21 positive BA numbers regardless of maternal diets and postnatal exercise. Under sedentary conditions, postnatal HF diet increased protein expression of FGF21 transcription inhibitors G9a and E4BP4 compared to NF diet resulting in decreased FGF21 expression. In contrast, postnatal HF diet and exercise decreased G9a and E4BP4 protein expression while decreasing FGF21 expression compared to NF diet. Under exercised condition, postnatal HF diet-induced KDM4C protein expression while no changes in KDM4C protein expression were induced by postnatal HF diet under sedentary conditions. These findings suggest that the postnatal diet exerts a greater impact on offspring adiposity and BA numbers than maternal diets. These data also suggest that offspring exercise induces KDM4C to counter the increase in G9a that was triggered by maternal and postnatal HF diets. Future studies need to determine whether KDM4C induces methylation status of G9a to alter thermogenic function of BA.
Keywords: Exercise, High-fat diet, Beige adipocyte, Subcutaneous adipose tissue, Histone lysin methylase, Fibroblastic growth factor 21
1. Introduction
Obesity and obesity risk are perpetuated across generations. Understanding maternal factors that promote obesogenic changes is vital to developing maternal health habits that will reduce obesity risk across generations. Maternal obesity at conception results in fetal programming that promotes obesity in offspring [1].
Thermogenic activation of beige adipocytes (BAs) found in white adipose tissue may help to limit body adiposity [2,3]. BAs arise from white adipocyte precursor myogenic factor 5 negative (Myf5-) cells [4] upon activation of PR domain containing 16 (PRDM16) [4,5]. Activation of PRDM16-mediated pathways results in BA formation, and this process is increased in the subcutaneous compared to visceral adipose tissue in mouse [6]. In human adipose tissue, mRNA levels of uncoupling protein-1 (UCP1) and PRDM16 were increased in the visceral compared to subcutaneous adipose tissue (ScAT) suggesting that caution needs to be exercised when inferring mouse data to understand human obesity [7]. Understanding how nutritional determinants influence the number and the function of BAs at birth and their maintenance into adulthood is likely important for sustaining a healthy body weight, given the greater capacity of BAs to oxidize cellular energy stores [8,9]. Previously, we demonstrated that maternal low protein followed by postnatal high-fat (HF) diets resulted in a rapid increase in ScAT mass in the offspring contributing to development of obesity and insulin resistance [10] and caused reduction in transcription factor PRDM16 and fibroblast growth factor 21 (FGF21) expression resulting in decreased mitochondria copy number, oxygen consumption rate, FGF21, PRDM16, and BA numbers [11]. We further demonstrated that reduction in FGF21 mRNA expression was due to increased protein expression of an inhibitor of FGF21 transcription (histone methyltransferase, G9a) [11]. As a BA maker, FGF21 is secreted from a variety of tissues including liver and white adipose tissue (WAT) [12]. FGF21 also regulates glucose uptake and lipid metabolism [13] by binding to FGF receptors 1, 2 and 3 [14]. FGF21 expression is induced by fasting and energy restriction [15] or consumption of a HF ketogenic diet [16,17]. FGF21 is associated with regulation of adiposity by modulating energy expenditure and lipid metabolism [18–20], including greater thermogenesis and beiging of white adipocytes [21].
Using an animal model of maternal diet-induced offspring obesity, we have demonstrated that maternal low-protein diet-induced offspring obesity and type 2 diabetes mellitus (T2DM) risk [22] is partly due to reduced ScAT BA numbers [11]. We further demonstrated that this reduction in ScAT BA was due to epigenetic regulation of FGF21 gene expression via increased G9a (histone 3 lysine 9 dimethyl transferase or H3K9me2) protein expression [11].
Interestingly, the methylation status of H3K9 (e.g., mono- (me1), di- (me2), or trimethyl (me3)) is maintained by balancing activation of methyl transferase (G9a) and histone lysin demethylases (KDM). Of KDMs, KDM4C binds to the promoter region of activating transcription factor 4 (ATF4) to decrease H3K9 methylation [23]. In addition, exercise induces activation of the ATF4 transcription to stimulate FGF21 production in skeletal muscle [24]. Further, our recent studies demonstrated that exercise reduces inflammation and T2DM risk by enhancing insulin signaling pathway [25,26] possibly by reducing adiposity [27]. In rats, voluntary running increased WAT mitochondrial number and protein content (e.g., cytochrome c, COXIV-subunit I, and citrate synthase activity) [28] and increased mRNA concentrations of mitochondria synthesis regulators including PGC1-alpha, PPAR-delta, and NRF-1 in skeletal muscle and BAT [29].
Thus, for the current study, we aimed to determine whether maternal and offspring HF diet and offspring exercise influenced offspring obesity and T2DM risk by regulating FGF21 expressing BA numbers. We hypothesized that maternal HF diet increases offspring obesity and T2DM risk by decreasing ScAT FGF21 via upregulating G9a and down regulating KDM4C expression levels. We further hypothesized offspring exercise reduces offspring obesity and T2DM by decreasing maternal HF diet-induced reduction in BA and FGF21 expression.
2. Materials and methods
2.1. Study design and animals
Two-month-old female C57BL/6 mice (Envigo, Indianapolis, IN, USA) were fed diets containing either 16% (normal fat, NF) or 45% fat (HF) for 3 months prior to breeding while male breeders were fed NF diet (Table 1). Dams were maintained on their respective diets throughout pregnancy and lactation. At weaning, offspring male mice were divided into 2 postnatal diet groups and placed either on a diet containing 16% fat (NF diet) or 45% fat (HF diet) for 12 weeks [30,31] and further divided into either sedentary (S) or voluntary wheel running (Ex) groups for an additional 3 months yielding eight groups: NF (maternal treatment condition)-NF-S (postweaning treatment conditions), NF-HF-S, NF-NF-Ex, NF-HF-Ex, HF-NF-S, HF-HF-S, HF-NF-Ex, and HF-HF-Ex. Mice were injected with xylazine (Rompon, Moboay Inc., Shawnee, KS, USA) and ketamine (Ketaset, Aveco Inc., Fort Dodge, IA, USA) and killed by exsanguination according to animal use and care protocol approved by the USDA Agricultural Research Service Animal Care and Use Committee guidelines. Food intake, body weight, and body composition were measured weekly from weaning to 4 weeks postweaning period followed by biweekly measurement thereafter until 12 weeks postweaning.
Table 1.
Nutrient composition of normal- and high-fat diet
| NF | HF | |||
|---|---|---|---|---|
| Ingredient | g | kcal | g | kcal |
| High-protein casein | 200 | 800 | 200 | 800 |
| Dextrinized corn starch | 132 | 528 | 132 | 528 |
| Corn starch | 408 | 1632 | 110 | 440 |
| Sucrose | 100 | 400 | 100 | 400 |
| Corn oil | 44 | 396 | 44 | 396 |
| Soybean oil | 26 | 234 | 26 | 234 |
| Lard | 0 | 0 | 129 | 1161 |
| Cellulose | 41 | 0 | 41 | 0 |
| Mineral mix (AIN-93G) | 35 | 0 | 35 | 0 |
| Vitamin mix (AIN-93) | 10 | 49 | 10 | 49 |
| L-Cysteine | 3 | 0 | 3 | 0 |
| Choline bitartrate | 2.5 | 0 | 2.5 | 0 |
| Total | 1001.5 | 4039 | 832.5 | 4008 |
| kcal/g | 4.03 | 4.81 | ||
| Macronutrient composition (% kcal) | ||||
| Macronutrient | NF | HF | ||
| Protein | 19.8 | 19.8 | ||
| Carbohydrate | 63.4 | 34.1 | ||
| Fat | 15.6 | 44.7 | ||
2.2. Voluntary wheel running exercise
Postweaning, F1 mice assigned to voluntary wheel running were placed in cages containing a wheel with a Scurry Mouse Brake with Activity Counter model 86120 (Lafayette Instrument, Lafayette, IN, USA) that was interfaced with Scurry Activity Monitory software model 86165 to record the number of meters the mouse ran in a single day. For the first week postweaning mice were blocked from access to wheel to avoid injury due to size. The wheel software would activate the wheel brake if it met 10,000 m/d for 11 weeks.
2.3. EchoMRI measurements of body composition
Whole body composition (fat mass, lean mass, and total body water) was determined using an EchoMRI700 instrument (Echo Medical Systems, Houston, TX, USA) according to manufacturer’s recommended protocols as previously demonstrated [25,26].
2.4. Glucose tolerance test
At the end of the 12-week postnatal diet feeding, mice were fasted overnight and injected intraperitoneally with 2 g/kg body weight of 20% d-glucose. Approximately 5 μL of tail blood was used to measure the blood glucose concentrations using the Accu Check Aviva glucometer at 0, 15, 30, 60, and 120 min after glucose injection.
2.5. Measurement of plasma insulin
Mice were fasted overnight and plasma samples were obtained to analyze insulin (Mice Adipokine Multiplex Kit, Millipore, St. Charles, MO, USA) concentrations using the Bio-Rad Luminex system (Hercules, CA, USA) and Millipore kits (St. Charles, MO, USA) according to manufacturer’s protocols. The homeostatic model assessment of insulin resistance was calculated as glucose concentration (mmol/L)×insulin concentration (mU/L)/22.5 [32].
2.6. Real-time RT-PCR
Total RNA was extracted from ScAT tissue using a RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA, USA) and used to measure H3K9 demethylases (KDM4C) mRNA expression by real-time RT-PCR method. PTN primers (forward, 5′-GACCAGAAGAGGGAAGACAAAG, reverse, 5′-ACTCCAGAAGCCTGGTTTATTAC, and probe, /56-FAM/ATACCAAG/ZEN/AAGCACCCAGCCACC/3IABkFQ/) were purchased from Integrated DNA Technology (IDT, Coralville, IA, USA). The Taqman assay reagents and endogenous control (18S rRNA) were purchased from Applied Biosystems (Foster City, CA, USA) and were used to measure Ct values using the ABI Prism 7500 PCR system (Applied Biosystems, Foster City, CA, USA). Normalizations of the target gene expression were done using the 18S rRNA as a reference.
2.7. Immunohistochemistry
Subcutaneous white adipose tissue was fixed in neutral buffered 10% formalin. The tissue was embedded in paraffin blocks after processing and was cut into 5-μm-thick slices using an 820 Spencer Microtome (American Optical Corporation). Tissue slices were mounted onto saline-treated glass adhesion slides (StatLab, McKinney, TX, USA) to ensure optimal adhesion. Anti-mouse FGF21 (Biorbyt Berkeley, CA, USA) antibody were used to visualize BA using a Nikon Eclipse E400 microscope with 400X magnification. The photomicrographs were taken using a Nikon DS-Qi2 camera and NIS Elements Imaging Software (Nikon) was used for florescence intensity measurements. Three fields per section from each treatment group were randomly selected and the total number of nuclei and the number of nuclei of FGF21-positive cells were counted for each field. The percentage of FGF21-positive cells for each sample was calculated as the total number of FGF21-positive nuclei over the total number of nuclei in the three randomly selected fields. Cross-sectional areas of adipocytes were expressed in unit of μm2 according to methods described in the study by Chen et al. [33]. Fluorescence intensity was measured for FITC stained FGF21 within the same area, with three values taken per sample.
2.8. Western blot analysis
Twenty-five micrograms of protein from ScAT were suspended in 6.3 μL of sample buffer and 2.5 μL 1 M DTT to run electrophoresis using 10% bis-tris gel (Life Technologies, Rockville, MD, USA). Proteins were transferred to a polyvinylidene difluoride membrane, washed with tris-buffered saline and hybridized with primary anti-EHMT2/G9A antibody ab185050 (Abcam, Cambridge, MA, USA), anti-E4BP4 antibody sc-74415 (Santa Cruz, Dallas, TX, USA), anti-KDM4C NBP1–49600 (Novus Biologicals LLC, Centennial, CO, USA) and normalized to antibeta-actin ab8226 (Abcam, Cambridge, MA, USA) or anti-GAPDH antibody ab181602 (Abcam, Cambridge, MA, USA) 4 °C overnight. The membrane was washed with 1 × PBS containing 0.1% Tween and hybridized with a secondary antibody (goat antimouse 1:10,000 and goat antirabbit 1:10,000) for 1 h in buffer containing 0.1% Tween and 0.01% SDS. Images were developed using an Odyssey Infrared Imager (Li-Cor Biosciences Inc., Lincoln, NE, USA).
2.9. Statistical analysis
Data are reported as fold change of the means±standard error of the mean or error propagation of standard error of the mean. JMP 14.2.0 and GraphPad PRISM 7 software were used to test group differences using three-way ANOVA. Statistical significance was set at P<.05. Statistical significance is denoted as F0 (maternal) or F1 (offspring) and diet or exercise for main ANOVA interactions. When an interaction was significant (P < .05), Tukey contrasts were used to perform pairwise comparisons, which are reported as follows: *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001.
3. Results
3.1. Maternal and postweaning HF diet increase fat mass and insulin resistance
To determine whether maternal HF diet alters offspring obesity and associated T2DM risk by reducing BA numbers, dams were fed control 16% or 45% fat diets for 3 months and mated with control diet fed male mice. The same diet was fed to dams during gestation and lactation periods. Male offspring were fed 3 months of the control 16% or 45% diets with and without voluntary wheel running exercise as shown in Figure 1. F1 HF diet resulted in greater body weight and fat mass compared to offspring fed NF diet with was attenuated with EX (Fig. 2A and B). There were no differences in lean mass among the groups (Fig. 2C). Offspring food intake decreased with F1 HF diet and increased with Ex regardless of F0 diet (Fig. 2D).
Fig. 1.
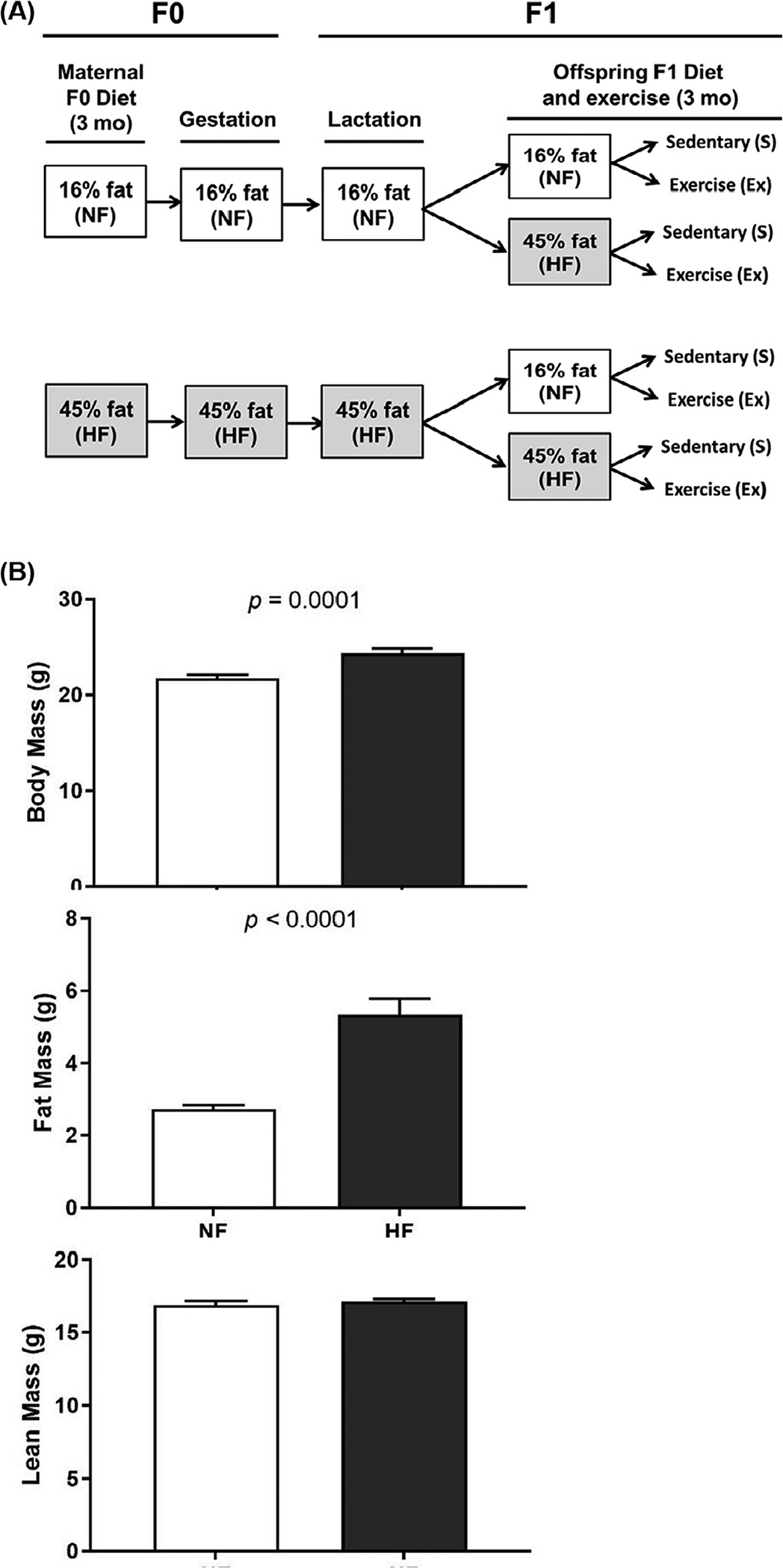
Experimental design and maternal body composition. (A) Two-month-old C57Bl/6 female mice were assigned randomly into two experimental treatments: a NF diet or a HF diet. The mice were maintained on the experimental diet for 12 weeks followed by breeding and lactation maintaining diet. Offspring were then placed on either NF diet or a HF diet with a sedentary or exercise component. (B) Effect of HF diet on F1 male mice body composition.
Fig. 2.
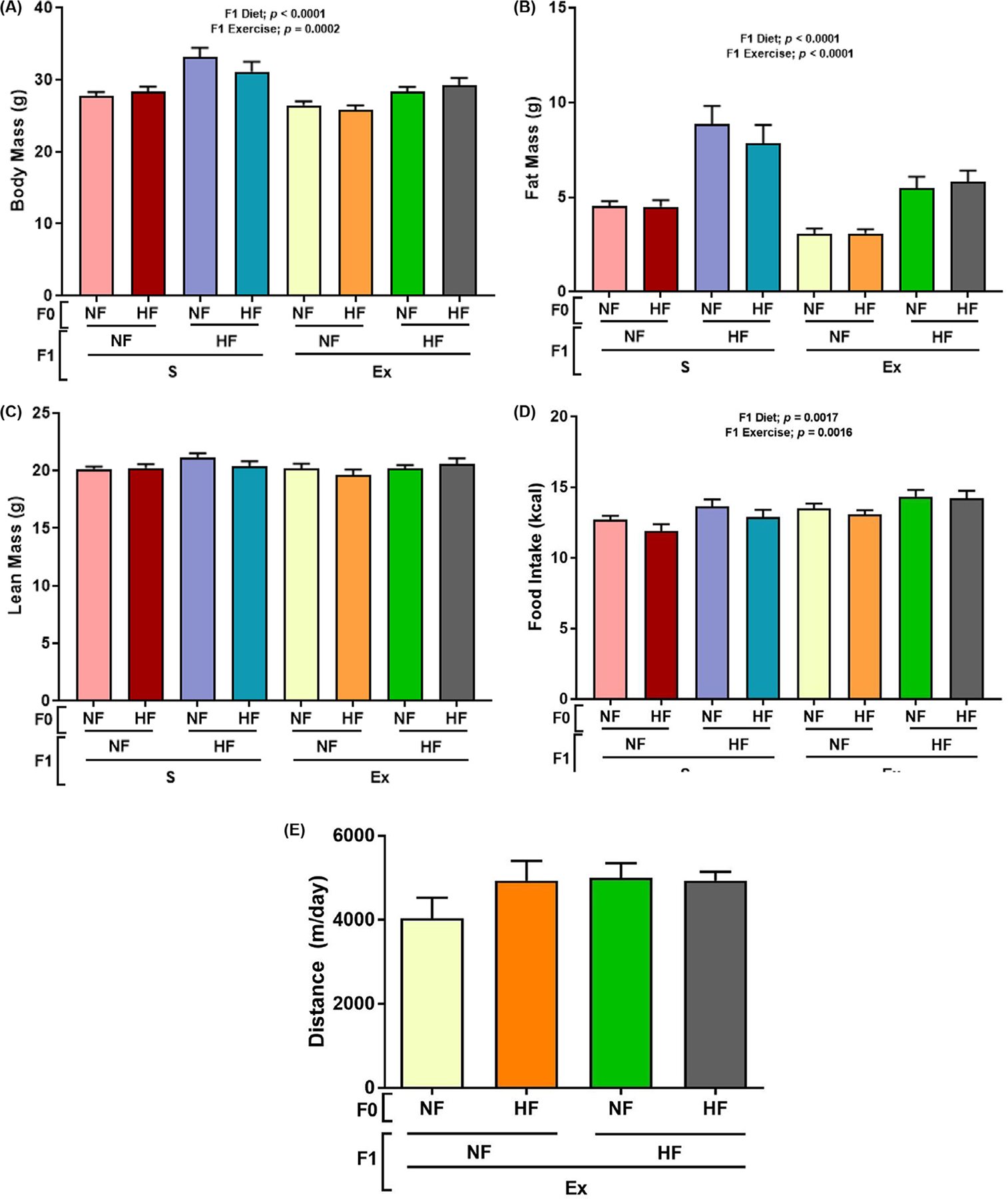
Effect of HF diet on F1 male mice body composition and food intake. Male offspring were placed onto experimental diets for 12 weeks with measurements of body weight (panel A), fat mass (panel B), lean mass (panel C), average daily food intake (panel D), and the distance traveled in the EX group (panel E) were measured. Data are present in mean±SEM n = 8–9. Significant (P < .05) effects from three-way ANOVA are indicated in figure.
3.2. Maternal and postweaning conditions regulate T2DM risk
As shown in Figure 3A, area under the curve calculated from the glucose tolerance test was increased by F1 HF diet regardless of F0 diet and F1 exercise conditions. The insulin resistance measured by homeostatic model assessment of insulin resistance showed increased insulin resistance in the F1 HF diet and F0 HF diet groups regardless of F1 exercise conditions (Fig. 3B). Immunohistochemistry results imaged in Figure 4A and B indicate F1 HF diet increased adipocyte cell size (Fig. 5C). Interestingly, F1 exercise resulted in smaller adipocytes under F0 and F1 HF diet conditions (Fig. 5C).
Fig. 3.
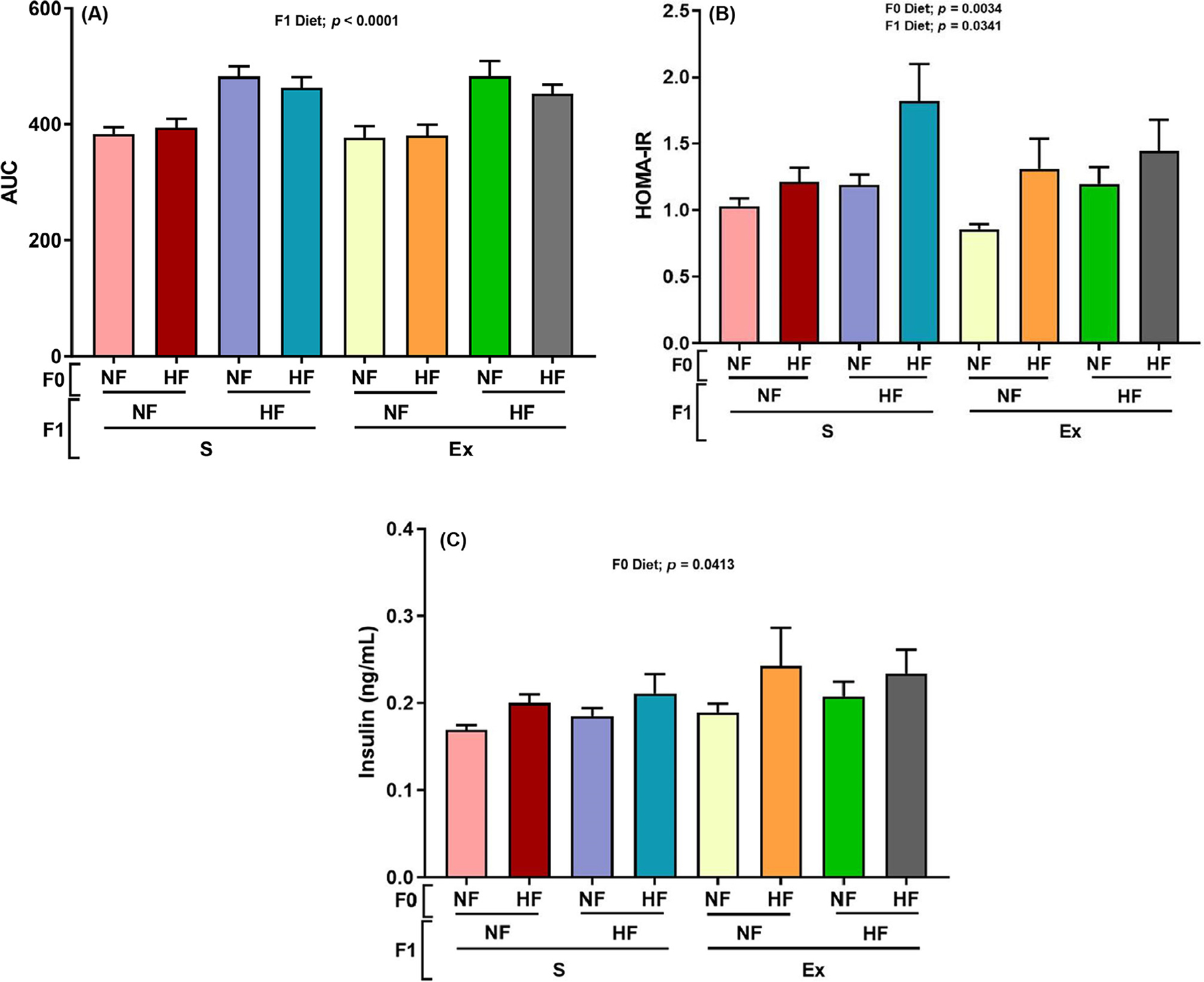
Effect of maternal HF diet on F1 male offspring HF diet and exercise glucose tolerance and AUC. The area under the curve (AUC) (panel A), HOMA-IR (panel B), and insulin concentration (panel C) were measured. Panel A and B data are present the mean±SEM, n = 4–8. Significant (P < .05) effects from three-way ANOVA are indicated in figure.
Fig. 4.
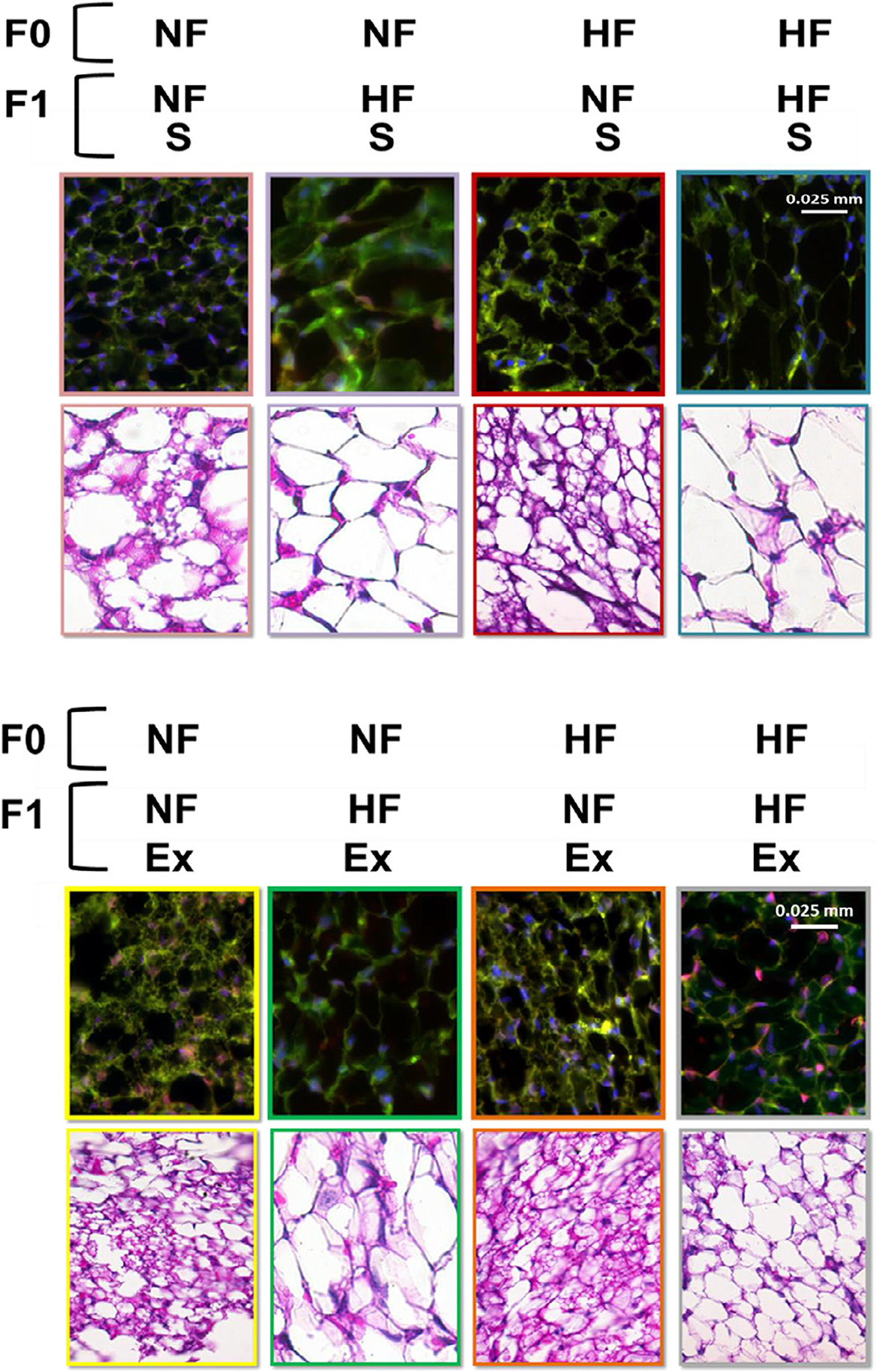
Effects of maternal HF diet on beige markers. The number of beige adipocytes were measured by taking three randomly selected areas from each mouse and averaging the numbers counted then normalizing the number of beige cells by the total mass of adipose tissue in sedentary animals (panel A) and exercised animals (panel B).
Fig. 5.
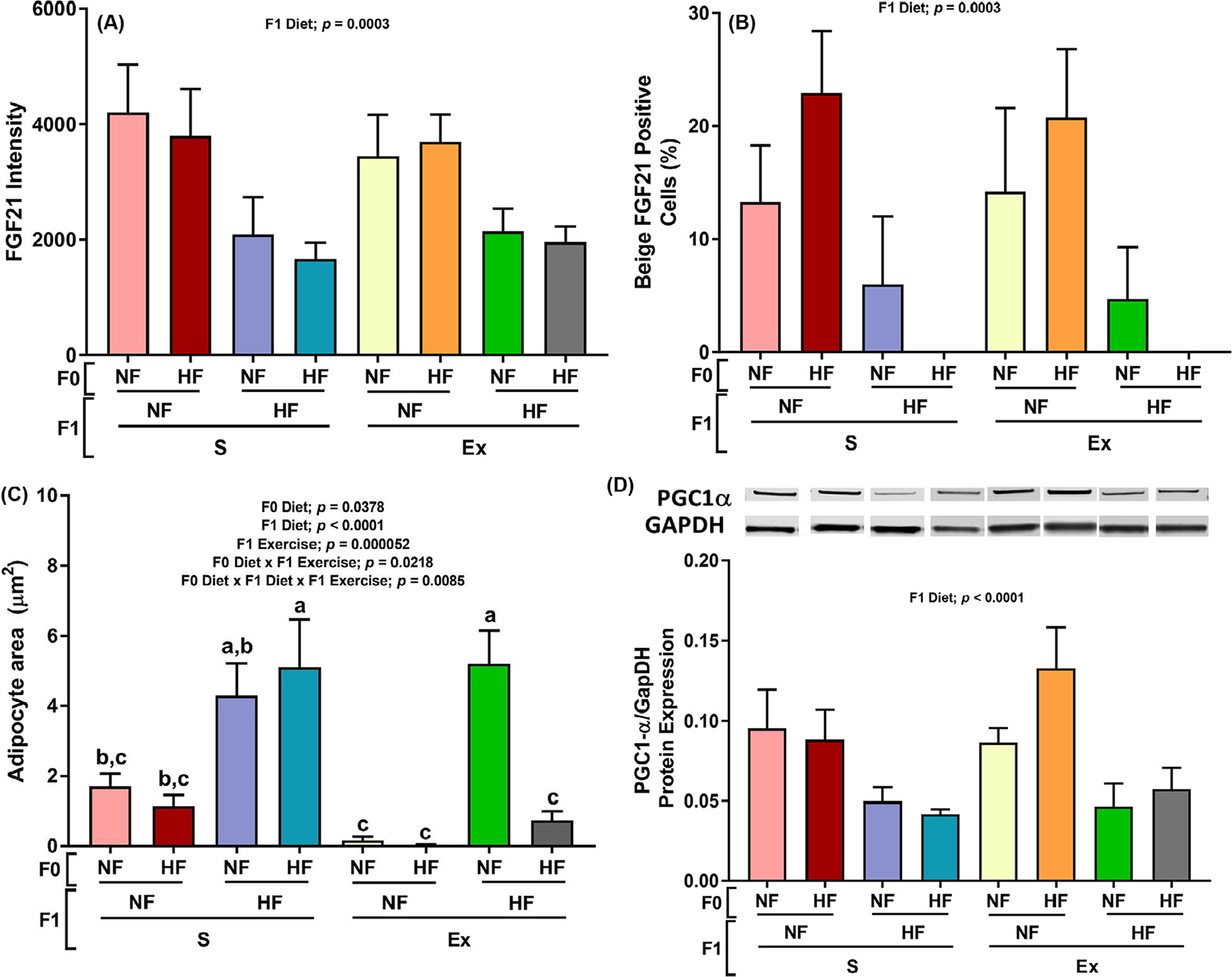
Effects of maternal HF and offspring HF and exercise on male offspring subcutaneous adipose tissue beige adipocyte expression. The FGF21 protein intensity was measured by IHC staining from taking three randomly selected areas for each mouse of adipose tissue placing the average in a bar graph (panel A), and the number of beige adipocytes were measured by taking three randomly selected areas from each mouse and averaging the numbers counted then normalizing the number of beige cells by the total mass of adipose tissue (panel B). The size of adipocytes was measured and calculated as described in the method section (section C). The protein expression of PGC1α (panel D) was measured. Data presented as mean±SEM n = 5–8. Significant (P < .05) effects from three-way ANOVA are indicated in figure.
3.3. Offspring HF diet decreases beige adipocyte numbers
F1 HF diet decreased the FGF21 fluorescent signal intensity, % BAs numbers (FGF21 positive/all adipocytes × 100), and protein expression of transcription coactivators Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha (inducer of FGF21 gene expression) [34].
We did not detect any visceral region BAs in all samples tested.
3.4. FGF21 expression is modulated by offspring HF diet and exercise
As shown in Figure 6A, E box binding factor E4BP4 and corepressor G9a transcription bind to repress FGF21. Both inhibitors of FGF21 expression, E4BP4 and G9a, were increased by F1 HF diet under sedentary conditions while decreases were observed under exercised conditions (Fig. 6B and C). These observations are contrary to expectation as FGF21 intensity (Fig. 5A), BA percent (Fig. 5B), and FGF21 activator PGC1 alpha (Fig. 5D) all had the same pattern of decrease by F1 HF diet in both sedentary and exercised conditions. Increases in adipocyte cell sizes (Fig. 5C) may have attributed by reduction in FGF21 intensity (Fig. 5A) and BA percent (Fig. 5B). Significant interactions were observed between F1 diet and exercise in Figure 6A and B. However, exercise did not affect FGF21 expression nor BA numbers (Fig. 5A and B) possibly by offsetting effects of FGF21 inhibitor G9a.
Fig. 6.
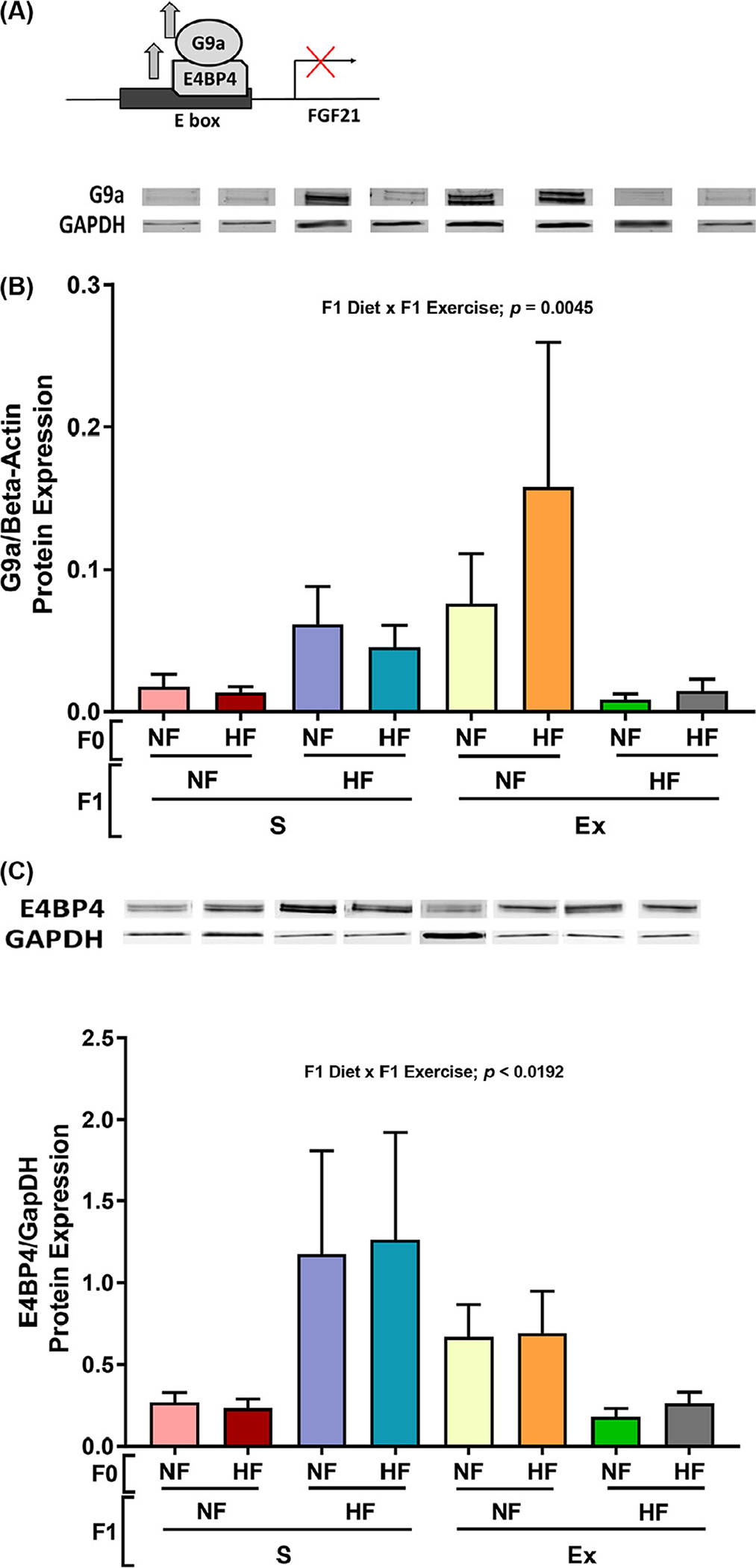
Effects of maternal HF and offspring HF and exercise on male offspring subcutaneous adipose tissue metabolic protein expression. The mechanism of regulators of FGF21 (panel A). The protein expression of G9a (panel B) and E4BP4 (panel C) were measured. Data presented as mean±SEM n = 8–9. Significant (P < .05) effects from three-way ANOVA are indicated in figure.
3.5. Offspring HF diet-induced increase in G9a and E4BP4 countered by increased G9a demethylation by offspring exercise
We tested G9a demethylation as a potential mechanism for the opposite G9a and E4BP4 changes by F1 exercise. As shown in Figure 7, H3K9 demethylase 4C (KDM4C) protein expression was increased by F1 exercise. F1 HF diet also moderately increased KDM4C protein expression under exercised conditions while F1 HF diet had no effect on KDM4C protein expression under sedentary conditions (Fig. 7).
Fig. 7.
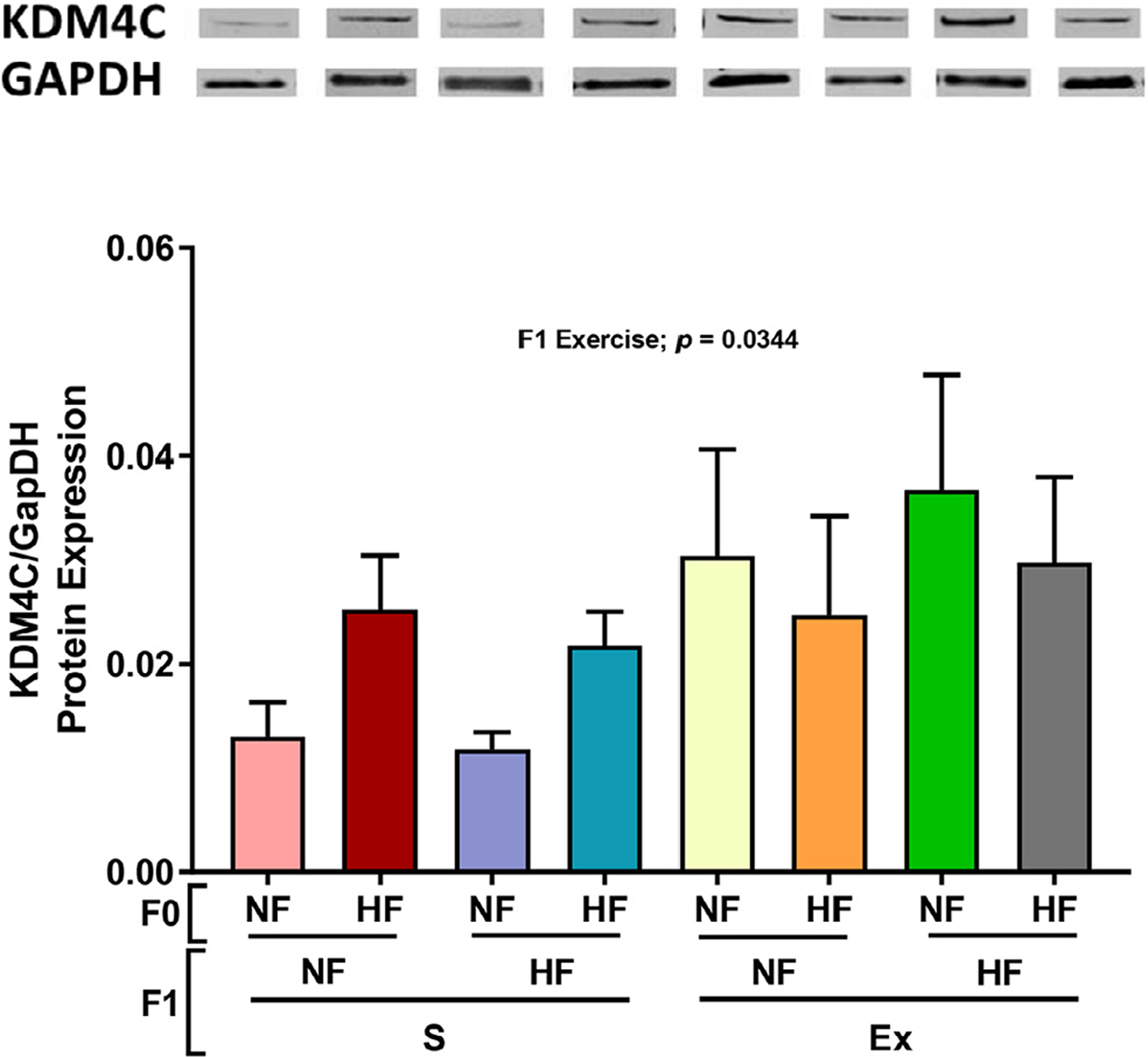
Effects of maternal HF diet on male offspring subcutaneous adipose tissue metabolic protein expression. The protein expression of KDM4C4 (panel A) was measured. Data presented as mean±SEM n = 8–9. Significant (P < .05) effects from three-way ANOVA are indicated in figure.
4. Discussion
The current study extends our understanding of how a maternal HF diet might modulate the physiological functions or number of BAs [35–37]. Furthermore, no studies have addressed whether a HF diet consumed by the F1 offspring and exercise by F1 offspring interact with a maternal HF diet to epigenetically modulate offspring obesity and T2DM risk. One of the key findings from the current study is that F1 diet and exercise conditions influence each other to regulate FGF21 expression. Specifically, under exercised condition, FGF21 inhibitors G9a and E4BP4 were not increased by F1 HF diet but decreased. This decrease in G9a and E4BP4 should result in increase in FGF21 protein expression with increased BA numbers. However, exercise under F1 HF diet condition did not affect FGF21 expression nor BA numbers (Fig. 5A and B) possibly by offsetting effects of FGF21 inhibitor G9a. Our data indicate that this effect may be regulated by KDM4C. Overall, the results from the current study demonstrated that postnatal HF diet exerts a greater impact than the maternal diet on offspring adiposity and decreased BA numbers in offspring ScAT, that postnatal HF diets contribute to increased insulin resistance, and that exercise offers a protective effect on maintaining BA numbers by inducing KDM4C protein expression in offspring fed a HF diet. Whether KDM4C complexes directly with other cofactors to modulate FGF21 gene expression and whether G9a and KDM homeostatic equilibrium/balance results in increased mono methylation of H3K9 to modulate BA should be investigated in future studies (Fig. 8).
Fig. 8.
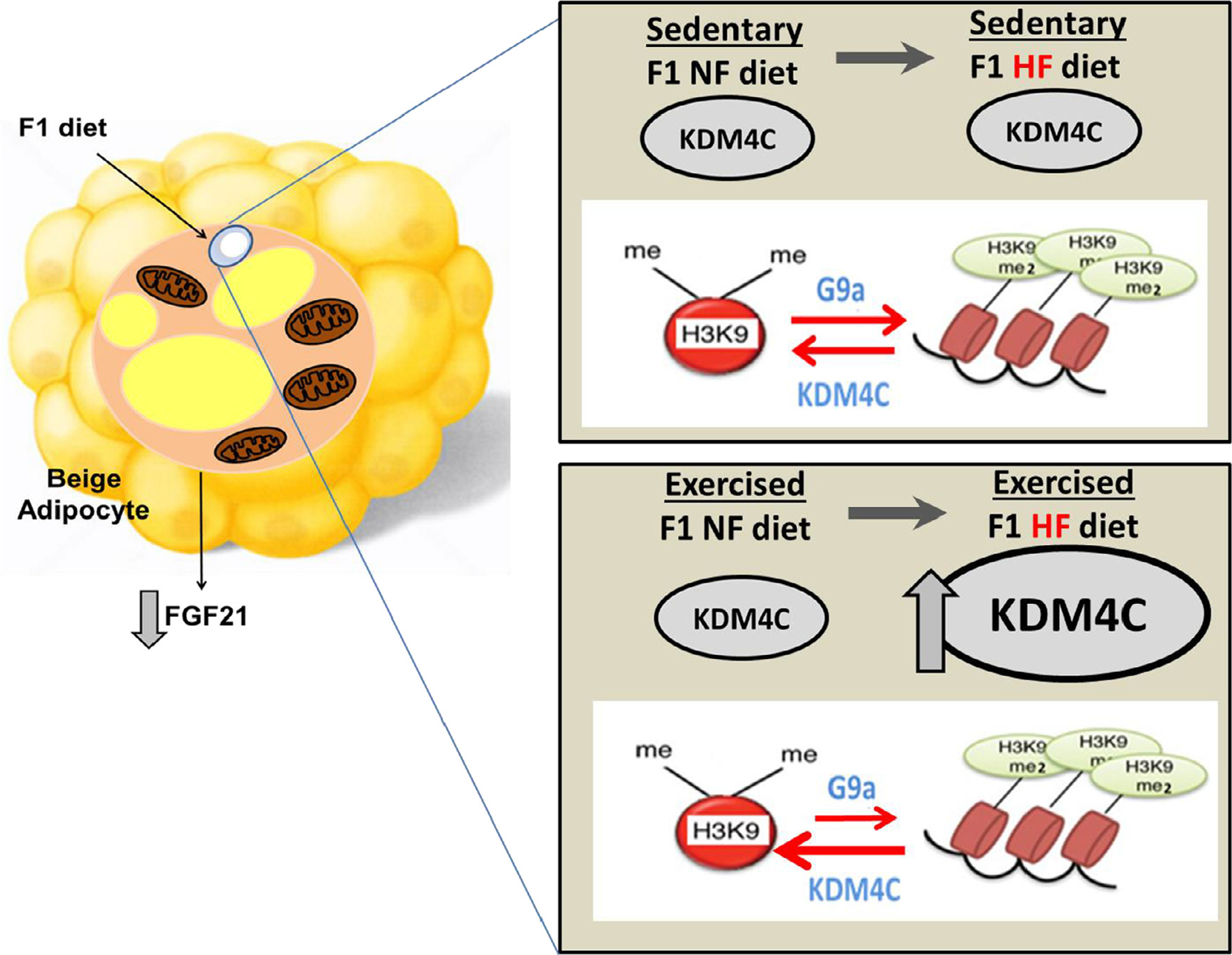
Proposed mechanism of protective mechanism underlying F1 HF diet-induced decrease in FGF21 via regulation of KDM4C. F1 HF diet-induced reduction in beige adipocyte and FGF21 is minimized by reducing G9a by F1 exercise by increasing KDM4C expression which reduces methylation of H9K9 thereby reducing G9a, an inhibitor of FGF21.
Other KDMs including KDM3A (also known as Jmjd1a) regulates beige adipogenesis by inducing KDM3A protein binding to the PPAR responsive element (PPRE) of the thermogenic Ucp1 gene to cause decreased H3K9 methylation at the PPRE site of UCP-1, but also by stimulating recruitment and assembly of coactivating transcription factors (e.g., PGC1-alpha and CBP/p300 proteins) [38]. In addition, the absence of KDM3A gene results in obesity and hyperlipidemia in mice [38]; however, whether expression of KDM3A is regulated by exercise is not yet known. The current study showed that offspring exercise does not have much effect on FGF21 and PGC1-α. Thus, it is plausible that some other beige markers such as UCP1, cell death-inducing DFFA-like effector A, and cytochrome coxidase subunit VIIIb [39], may be regulated by offspring exercise. Accordingly, future studies need to address whether KDM4C mediated H3K9 methylation changes alter expression of UCP1, cell death-inducing DFFA-like effector A, and cytochrome c oxidase subunit VIIIb.
Other histone lysine demethylases have been implicated in the regulation of adiposity. For example, KDM2B a scaffolding protein associated with polycomb complex is involved in adipose tissue in a diet-induced model of obesity by regulating white adipocyte adipogenesis [40]. Moreover, the lysine specific demethylases family of proteins such as KDM2B and KDM4C may have opposing effects [41]; KDM2B represses rRNA transcription [42] while KDM4C activates rRNA transcription [43]. Whether ScAT of mice fed maternal offspring HF diet and provided voluntary exercised express KDM2B to oppose KDM4C function is not yet known and should be investigated.
A recent study suggested that beiging of white adipose tissue occurs at a greater amount in female compared to male mice [44]. However, whether maternal and postnatal HF diets and postnatal exercise influence histone protein modifications via sex-dependent manner has not been addressed. Interestingly, our recent study of paternal HF diet had gender specific responses in placental and fetal tissue growth, placental tissue inflammation and nutrient transport gene expression via regulation of sperm histone 3 lysine 9 (H3K9) protein expression [25]. Our on-going studies address offspring gender-specific effects of maternal and offspring HF diets and exercise on H3K9 methylation, which influences BA numbers and thermogenic functions. Moreover, although BAs were detectable, their morphology under sedentary conditions appeared compromised or scarred. Future studies will determine whether BAs have internal structural or functional differences that contribute to the observed morphological abnormalities.
Overall, the results from our study demonstrated that offspring exercise offers a protective effect on maternal and postnatal HF diet-induced offspring obesity and T2DM risk. The data presented in this study indicate that this protective effect of offspring exercise is associated with H3K9 methylation changes. These findings offer histone epigenetic alteration as a potential antiobesogenic target to reduce body adiposity and T2DM risk.
Funding
This work was supported by USDA Agricultural Research Service (Project #3062-51000-054-00D). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Declaration of competing interests
The authors declare that there are no conflicts of interest.
References
- [1].Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 2008;51:383–92. [DOI] [PubMed] [Google Scholar]
- [2].Cereijo R, Giralt M, Villarroya F. Thermogenic brown and beige/brite adipogenesis in humans. Ann Med 2015;47:169–77. [DOI] [PubMed] [Google Scholar]
- [3].Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol 2015;55:207–27. [DOI] [PubMed] [Google Scholar]
- [4].Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zuriaga MA, Fuster JJ, Gokce N, Walsh K. Humans and mice display opposing patterns of “Browning” gene expression in visceral and subcutaneous white adipose tissue depots. Front Cardiovasc Med 2017;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Okla M, Ha JH, Temel RE, Chung S. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids 2015;50:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bi P, Shan T, Liu W, Yue F, Yang X, Liang XR, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med 2014;20:911–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Claycombe KJ, Uthus EO, Roemmich JN, Johnson LK, Johnson WT. Prenatal low-protein and postnatal high-fat diets induce rapid adipose tissue growth by inducing Igf2 expression in Sprague Dawley rat offspring. J Nutr 2013;143:1533–9. [DOI] [PubMed] [Google Scholar]
- [11].Claycombe KJ, Vomhof-DeKrey EE, Garcia R, Johnson WT, Uthus E, Roemmich JN. Decreased beige adipocyte number and mitochondrial respiration coincide with increased histone methyl transferase (G9a) and reduced FGF21 gene expression in Sprague-Dawley rats fed prenatal low protein and postnatal high-fat diets. J Nutr Biochem 2016;31:113–21. [DOI] [PubMed] [Google Scholar]
- [12].Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 2009;8:235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohta H, Konishi M, Itoh N. FGF10 and FGF21 as regulators in adipocyte development and metabolism. Endocr Metab Immune Disord Drug Targets 2011;11:302–9. [DOI] [PubMed] [Google Scholar]
- [14].Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007;282:26687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu S, Grunwald T, Kharitonenkov A, Dam J, Jockers R, De Luca F. Increased expression of fibroblast growth factor 21 (FGF21) during chronic undernutrition causes growth hormone insensitivity in chondrocytes by inducing leptin receptor overlapping transcript (LEPROT) and leptin receptor overlapping transcript-like 1 (LEPROTL1) expression. J Biol Chem 2013;288:27375–83. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [16].Murata Y, Nishio K, Mochiyama T, Konishi M, Shimada M, Ohta H, et al. Fgf21 impairs adipocyte insulin sensitivity in mice fed a low-carbohydrate, high-fat ketogenic diet. PLoS One 2013;8:e69330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Camporez JP, Asrih M, Zhang D, Kahn M, Samuel VT, Jurczak MJ, et al. Hepatic insulin resistance and increased hepatic glucose production in mice lacking Fgf21. J Endocrinol 2015;226:207–17. [DOI] [PubMed] [Google Scholar]
- [18].Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–25. [DOI] [PubMed] [Google Scholar]
- [19].Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–27. [DOI] [PubMed] [Google Scholar]
- [20].Murphy M, Samms R, Warner A, Bolborea M, Barrett P, Fowler MJ, et al. Increased responses to the actions of fibroblast growth factor 21 on energy balance and body weight in a seasonal model of adiposity. J Neuroendocrinol 2013;25:180–9. [DOI] [PubMed] [Google Scholar]
- [21].Keipert S, Ost M, Johann K, Imber F, Jastroch M, van Schothorst EM, et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab 2014;306:E469–82. [DOI] [PubMed] [Google Scholar]
- [22].Vomhof-DeKrey E, Darland D, Ghribi O, Bundy A, Roemmich J, Claycombe K. Maternal low protein diet leads to placental angiogenic compensation via dys-regulated M1/M2 macrophages and TNFalpha expression in Sprague-Dawley rats. J Reprod Immunol 2016;118:9–17. [DOI] [PubMed] [Google Scholar]
- [23].Zhao E, Ding J, Xia Y, Liu M, Ye B, Choi JH, et al. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep 2016;14:506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xu X, Krumm C, So JS, Bare CJ, Holman C, Gromada J, et al. Preemptive activation of the integrated stress response protects mice from diet-induced obesity and insulin resistance by fibroblast growth factor 21 induction. Hepatology 2018;68:2167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Claycombe-Larson KG, Bundy AN, Roemmich JN. Paternal high-fat diet and exercise regulate sperm miRNA and histone methylation to modify placental inflammation, nutrient transporter mRNA expression and fetal weight in a sex-dependent manner. J Nutr Biochem 2020;81:108373. [DOI] [PubMed] [Google Scholar]
- [26].Krout D, Roemmich JN, Bundy A, Garcia RA, Yan L, Claycombe-Larson KJ. Paternal exercise protects mouse offspring from high-fat-diet-induced type 2 diabetes risk by increasing skeletal muscle insulin signaling. J Nutr Biochem 2018;57:35–44. [DOI] [PubMed] [Google Scholar]
- [27].Krout D, Schaar A, Sun Y, Sukumaran P, Roemmich JN, Singh BB, et al. The TRPC1 Ca(2+)-permeable channel inhibits exercise-induced protection against high-fat diet-induced obesity and type II diabetes. J Biol Chem 2017;292:20799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, et al. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 2009;587:3729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seebacher F, Glanville EJ. Low levels of physical activity increase metabolic responsiveness to cold in a rat (Rattus fuscipes). PLoS One 2010;5:e13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Claycombe KJ, Uthus EO, Roemmich JN, Johnson LK, Johnson WT. Prenatal low-protein and postnatal high-fat diets induce rapid adipose tissue growth by inducing Igf2 expression in Sprague Dawley rat offspring. J Nutr 2013;143:1533–9. [DOI] [PubMed] [Google Scholar]
- [31].Claycombe KJ, Roemmich JN, Johnson L, Vomhof-DeKrey EE, Johnson WT. Skeletal muscle Sirt3 expression and mitochondrial respiration are regulated by a prenatal low-protein diet. J Nutr Biochem 2015;26:184–9. [DOI] [PubMed] [Google Scholar]
- [32].Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab 2008;294:E261–70. [DOI] [PubMed] [Google Scholar]
- [33].Chen HC, Farese RV Jr. Determination of adipocyte size by computer image analysis. J Lipid Res 2002;43:986–9. [PubMed] [Google Scholar]
- [34].Bargut TCL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig 2017;31(1):20160051. [DOI] [PubMed] [Google Scholar]
- [35].Li T, Gong H, Yuan Q, Du M, Ren F, Mao X. Supplementation of polar lipids-enriched milk fat globule membrane in high-fat diet-fed rats during pregnancy and lactation promotes brown/beige adipocyte development and prevents obesity in male offspring. FASEB J 2020;34:4619–34. [DOI] [PubMed] [Google Scholar]
- [36].Wang B, Fu X, Liang X, Wang Z, Yang Q, Zou T, et al. Maternal retinoids increase PDGFRalpha(+) progenitor population and beige adipogenesis in progeny by stimulating vascular development. EBioMedicine 2017;18:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, et al. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol 2017;595:1547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature 2009;458:757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garcia RA, Roemmich JN, Claycombe KJ. Evaluation of markers of beige adipocytes in white adipose tissue of the mouse. Nutr Metab (Lond) 2016;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Inagaki T, Iwasaki S, Matsumura Y, Kawamura T, Tanaka T, Abe Y, et al. The FBXL10/KDM2B scaffolding protein associates with novel polycomb repressive complex-1 to regulate adipogenesis. J Biol Chem 2015;290:4163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Oie S, Matsuzaki K, Yokoyama W, Tokunaga S, Waku T, Han SI, et al. Hepatic rRNA transcription regulates high-fat-diet-induced obesity. Cell Rep 2014;7:807–20. [DOI] [PubMed] [Google Scholar]
- [42].Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature 2007;450:309–13. [DOI] [PubMed] [Google Scholar]
- [43].Feng W, Yonezawa M, Ye J, Jenuwein T, Grummt I. PHF8 activates transcription of rRNA genes through H3K4me3 binding and H3K9me1/2 demethylation. Nat Struct Mol Biol 2010;17:445–50. [DOI] [PubMed] [Google Scholar]
- [44].Kim SN, Jung YS, Kwon HJ, Seong JK, Granneman JG, Lee YH. Sex differences in sympathetic innervation and browning of white adipose tissue of mice. Biol Sex Differ 2016;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]


