Abstract
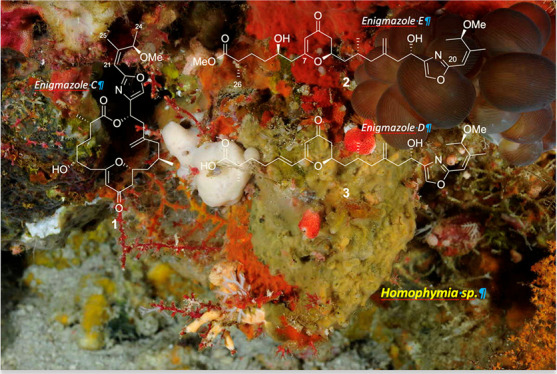
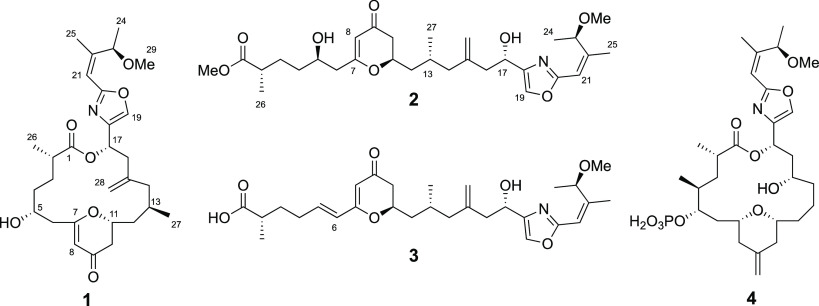
A new macrolide, enigmazole C (1), and two additional analogues, enigmazoles E (2) and D (3), were obtained from a new species of the Homophymia genus as part of an ongoing discovery program at PharmaMar to study cytotoxic substances from marine sources. The structures were fully characterized by cumulative analyses of NMR, IR, and MS spectra, along with density functional theory computational calculations. All three of the new compounds feature an unusual 2,3-dihydro-4H-pyran-4-one moiety, but only enigmazoles C (1) and D (3) showed cytotoxic activity in the micromolar range against A-549 (lung), HT-29 (colon), MDA-MB-231 (breast), and PSN-1 (pancreas) tumor cells.
Marine sponges represent a prolific source of structurally unique macrolides possessing promising biological activities, including cytotoxic, anticancer, and neuroprotective properties, thus suggesting their potential value for the development of leads in drug discovery.1 Particularly, sponges from the Neopeltidae family have not been extensively chemically investigated, with relatively few structures being described in the literature from each of the three genera Homophymia, Callipelta, and Daedalopelta. By way of illustration, the high molecular weight peptides homophymines A–E/A1–E1,2,3 homophymamide A,4 pipecolidepsins A–C,5−7 and callipeltins A and B,8−10 have all been reported to have significant cytotoxic and anti-HIV activities. Others metabolites isolated from Neopeltidae sponges that were assigned in 2013 to the suborder Astrophorina11,12 are the bioactive tetramic acid glycoside aurantoside C13 and the macrocycles callipeltosides A–C.14,15 Furthermore, only two structures belonging to the Daedalopelta genus have been described, the cytotoxic cyclodepsipeptide daedophamide16 and the 14-membered macrolide neopeltolide,17 which has been extensively studied and synthesized because it is a potent cytochrome bc1 complex inhibitor, as well as a cytotoxic compound with activity against A-549 human lung adenocarcinoma, NCI/ADR-Res ovarian sarcoma, and P388 murine leukemia cell lines.18−21
During continuing efforts at PharmaMar to discover new cytotoxic compounds from marine natural sources, we have evaluated a new sponge species of the Homophymia genus (Vacelet & Vasseur, 1971) collected off the coast of Gorontalo, Indonesia. In this paper, we describe the isolation of a new macrocyclic compound as well as two open-chain analogues, all isolated from a specimen of this sponge collected in Indonesia. Despite the fact that the new macrocycle shows structural similarities to neopeltolide, a clear resemblance to the macrolide enigmazole A isolated by Oku et al. in 2010 from the marine sponge Cynachyrella enigmatica,22 has led us to designate the three new compounds as enigmazoles C (1), E (2), and D (3). The configurations of these enigmazoles have been solved using a combination of microscale chemical conversions and the use of an elegant J-based configurational analysis based on capillary NMR measurements.23
Chart 1. Structures of Enigmazoles C (1), E (2), and D (3) and Known Enigmazole A (4).
A methanolic extract of the Homophymia sponge specimen showed cytotoxic activity against A-549 (lung), HT-29 (colon), MDA-MB-231 (breast), and PSN-1 (pancreas) tumor cells and was hence selected for a more detailed bioassay-guided chemical investigation.
Careful fractionation of the MeOH extract led to isolation of the new macrolide enigmazole C (1, 2.9 mg), and two open-chain derivatives, enigmazole D (3, 0.4 mg) and the methanolic adduct enigmazole E (2, 1.4 mg), which were purified by semipreparative HPLC.
Enigmazole C (1) was isolated as a colorless amorphous solid and showed a [M + H]+ ion at m/z 516.2964 (calcd for C29H42NO7m/z 516.2956) in its (+)-HR-ESITOF-MS spectrum. The presence of the sodium adduct at m/z 538.2785 (calcd for C29H41NO7Na m/z 538.2775) confirmed the molecular formula and 10 degrees of unsaturation required for this compound.
NMR experiments of 1 were carried out in CD3CN because a chemical transformation was observed using CD3OD. 1H, 13C, and edited-HSQC NMR analysis of 1 revealed the presence of 29 carbons assigned to seven sp2 nonprotonated carbons (δC 193.6, 175.6, 175.5, 161.2, 152.7, 143.9, and 141.7), three sp2 methine carbons (δH/δC 7.63/135.9, 6.20/113.6, and 5.30/106.8), a disubstituted exomethylene moiety (δH/δC 4.91; 4.86/113.9), six sp3 methine carbons (δH/δC 5.92/66.7, 5.20/75.5, 4.48/77.8, 3.74/69.5, 2.49/39.9, and 2.20/25.8), seven sp3 methylene carbons (δH/δC 2.72; 2.62/42.2, 2.49; 2.27/44.6, 2.49; 1.59/40.2, 2.41; 2.23/42.2, 1.81; 1.46/42.9, 1.69; 1.63/30.8 and 1.43; 1.31/34.5), and five methyl groups (δH/δC 3.15/56.6 (OMe), 1.86/17.7, 1.23/19.5, 1.10/17.6, and 0.95/21.2).
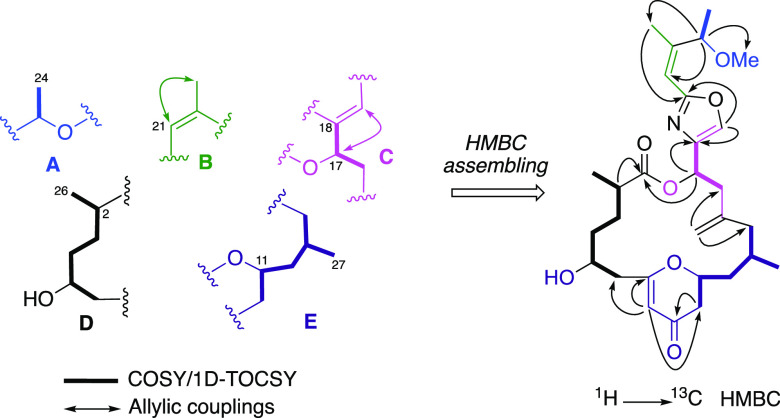
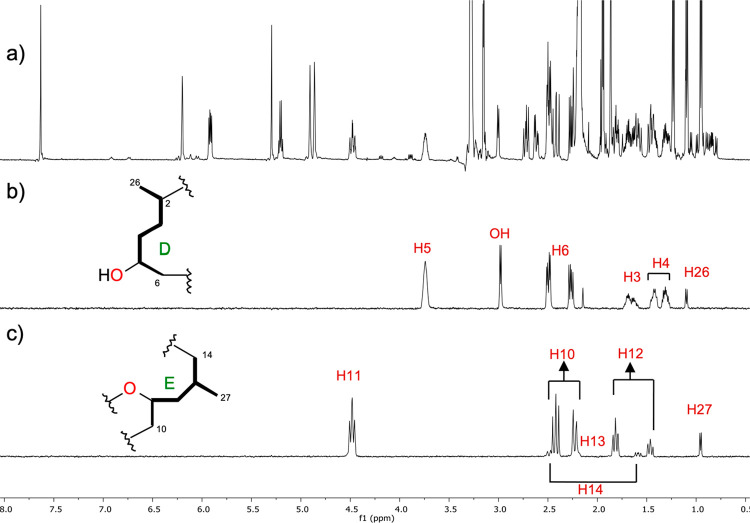
Two-dimensional COSY, 2D-TOCSY, and selective 1D-TOCSY experiments of 1 allowed us to identify five different spin systems, fragments A (C23–C24), B (C21–C25), C (C16–C19), D (C2–C6, C26), and E (C10–14, C27) (Figure 1). Special attention was paid to fragments D and E due to the presence of four stereogenic centers. Thus, the spin system D was identified by selective 1D-TOCSY from selective irradiation of proton H-5 at δH 3.74, which gave responses to the methylene protons at δH 1.69/1.63 (H2-3), δH 1.43/1.31 (H2-4), and δH 2.49/2.27 (H2-6), as well as the methine at δH 2.49 (H-2), the methyl group at δH 1.10 (H3-26), and a broad signal at δH 3.00 assigned to a hydroxy proton (Figure 2b).
Figure 1.
Spin systems deduced by COSY and 1D-TOCSY experiments and HMBC assembling in 1.
Figure 2.
(a) NMR spectrum of enigmazole C (1) in CD3CN at 500 MHz. Selective 1D-TOCSY irradiation experiments at (b) δH 3.74 (H-5) and (c) δH 4.48 (H-11) to identify fragments D and E.
In the same way, fragment E was deduced by selective 1D-TOCSY irradiation of H-11 at δH 4.48, which showed coupling responses to three methylenes at δH 2.41/2.23 (H2-10), δH 1.81/1.46 (H2-12), and δH 2.49/1.59 (H2-14), the methine H-13 (δH 2.20), and the methyl group δH 0.95 (H3-27) (Figure 2c).
The interconnection between the determined fragments A–E was achieved by using an HMBC experiment as follows:
-
(a)
The methine at δH 5.20 (H-23) showed long-range correlation with three methyl groups at δC 19.5 (C-24), δC 17.7 (C-25), and δC 56.6 (C-29), along with the sp2 methine carbon at δC 113.6 (C-21), implying the position of the methoxy group on C-23 and the connection between fragments A and B.
-
(b)
The HMBC cross-peak observed from H-21 (δH 6.20) to C-22 (δC 152.7) placed a nonprotonated carbon in the double bond. On the other hand, the singlet at δH 7.63 (δC 135.9) presented a typical chemical shift and 1JCH of 210 Hz of a 2,4-disubstituted oxazole ring, which was completed by inspection of the HMBC correlations observed between the oxazolic proton H-19 to carbons C-18 (δC 141.7) and C-20 (δC 161.2). The connection between fragments B and C was undoubtedly determined by the HMBC cross-peak from H-25 (δH 1.86) to C-20 (δC 161.2).
-
(c)
Fragments D and E were connected through a six-membered ring by the cross-peaks observed from δH 5.30 (H-8) to δC 44.6 (C-6), δC 42.2 (C-10), and to a nonprotonated oxygenated sp2 carbon at δC 175.5 (C-7). A dihydropyranone ring was deduced by the observation of the HMBC correlations from both diastereotopic protons at H-10a (δH 2.41) and H10b (δH 2.23) to an α,β-unsaturated carbonyl signal at δC 193.6.
-
(d)
Fragments C and E were linked by a disubstituted exomethylene group as the HMBC correlations observed of H-28 (δH 4.86) with C-14 (δC 40.2) and C-16 (δC 42.2).
-
(e)
A cross-peak from H-17 (δH 5.92) to C-1 (δC 175.6) allowed us to connect fragments C and D, therefore assembling the cyclic macrolactone planar structure for 1 as is drawn in Figure 1.
At this point, the resemblance of 1 to the known compound enigmazole A (4) was clear, as some structural features are common in both compounds: a disubstituted oxazole ring, a pyran ring, the presence of an exomethylene double bond, and a similar macrolactone size. Once the planar structure of 1 was established, the relative and absolute configuration were determined by ROESY experiments, derivatization reactions, and computational calculations.
First, the Z configuration of the C-21/C-22 double bond was deduced from the ROESY correlation between H3-25 (δH 1.86) and H-21 (δH 6.20).
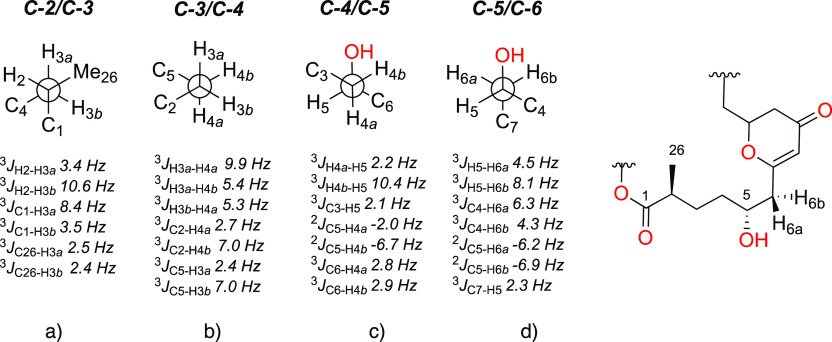
To deduce the relative configuration of the entire spin system D, we were nicely able to relate the two stereogenic centers at C-2 and C-5 located three carbon–carbon bonds away through a J-based configurational analysis (JBCA).24 To make this possible, we had to change the NMR solvent to acetone-d6, because it gave us a 1H NMR spectrum where the two diastereotopic pairs at C-3 (H-3a and H-3b) and C-4 (H-4a and H-4b) are well separated and fully resolved.
Not many approaches of this kind have been applied to a natural compound with two sp3 methylenes surrounded by two stereogenic centers, even though this approximation is a very good tool when the diastereotopic protons can be unequivocally assigned with their corresponding chemical shifts and their sets of proton–proton and carbon–proton coupling constants. Therefore, 13C–1H-HSQC-TOCSY-HECADE and J-HMBC experiments were needed to measure key small coupling constants for 3JC26–H3a and 3JC26–H3b to place H3-26 in a gauche disposition to both H-3 diastereotopic protons at the C-2/C-3 single bond (Figure 3a). These two experiments along with selective 1H NMR irradiation spectra were satisfactory to deduce relationships from diastereotopic protons H-3a/H-3b and H-4a/H-4b to C-2 and C-5 as is drawn Figure 3b) for the C-3/C-4 bond. The hydroxylated carbon at C-5 helped us to deduce the relative dispositions for H-4a and H-4b to the OH group with regard to the C-4/C-5 bond (Figure 3c), as well as H-6a and H-6b to the mentioned OH relative to the C-5/C-6 bond (Figure 3d).
Figure 3.
C-2 to C-6 fragment deduced by J-based configurational analysis.
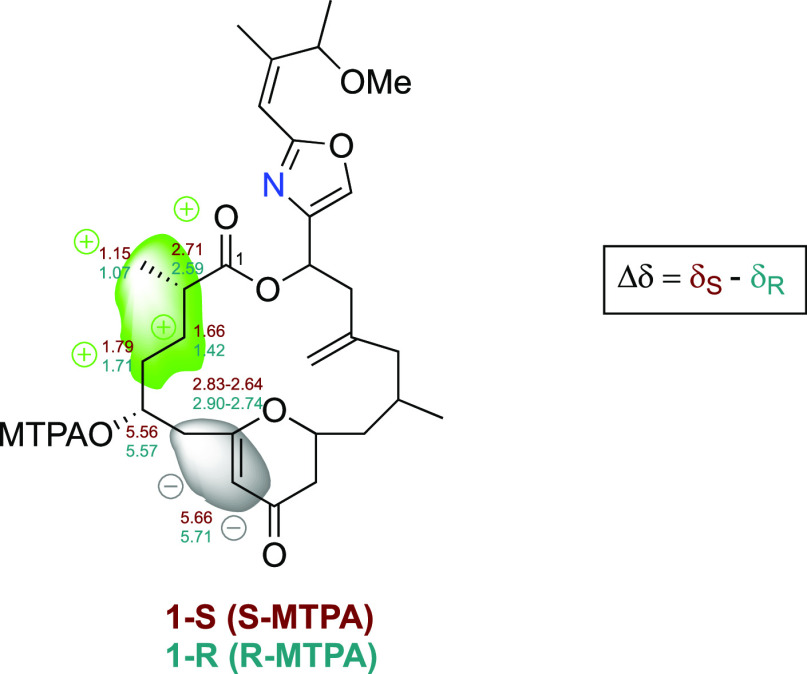
The absolute configuration at position C-5 was elucidated by the application of the modified Mosher’s method (MMM).25,26 The derivatization of the secondary alcohol with R- and S-MTPA-Cl was completed directly in pyridine-d5 in an NMR tube to give compounds 1-S and 1-R, respectively. Comparison of the Δδ values (δS – δR) obtained from the MTPA esters (Figure 4) indicated that the absolute configuration of C-5 was R, which implies a C-2S configuration.
Figure 4.
Mosher’s derivatives of enigmazole C (1).
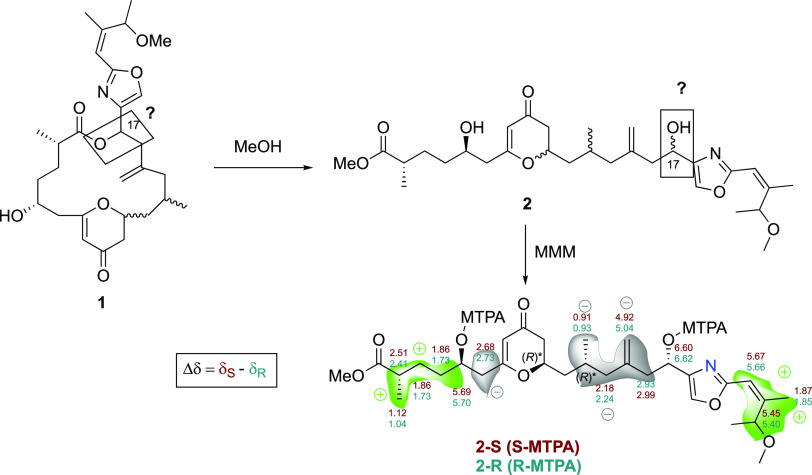
During the process of purification of enigmazole C, the use of MeOH in the HPLC separation procedure induced a macrolactone ring-opening that produces the methyl ester adduct 2, which we have named as enigmazole E.
The (+)-HR-ESIMS-TOF data of 2 at m/z 548.3249 [M + H]+ (calcd for C30H46NO8, m/z 548.3218) and the 1H NMR of 2 revealed the opening of the macrolactone ring.
Thus, the 1H NMR spectrum of 2 displays a singlet signal at δH 3.61 (s, 3H) that was assigned to a methoxy group, which shows an HMBC correlation to an carbonyl carbon at δC 176.6 assigned to the new carbonyl ester at C-1. Moreover, the proton chemical shift at δH 5.92, assigned to H-17 in 1, was clearly shifted to δH 4.72 in 2 as expected due to the lack of the lactone moiety. The presence of a hydroxy group at C-17 in 2 due to the macrolactone ring opening of 1 could be used to determine the absolute configuration at this position by the application of the MMM using the α-methoxy-α-trifluoromethylphenylacetic (MTPA) esters.
In this case, the comparison between the proton chemical shifts of both MTPA esters at C-17 (δ2-S – δ2-R) established the absolute configuration of C-17 as S (see Figure 5). In addition, the esterification of 2 with MTPA-Cl generated also the MTPA esters at C-5; the comparison of proton chemical shift values confirmed again the configuration of this stereogenic center as R.
Figure 5.
Modified Mosher methodology (MMM) applied to enigmazole E (2) to deduce the absolute configuration at C-17 and confirming the absolute configuration at C-5.
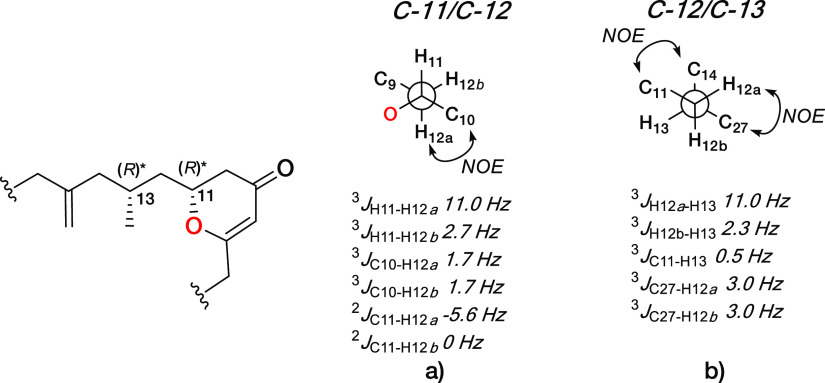
To establish the configuration between C-11 and C-13 at 1, and therefore of 2, homonuclear coupling constants of H-11/H-12b, H-11/H-12a, H-12b/H-13, and H-12a/H-13 were extracted from selective 1D-TOCSY experiments (Figure 2c). The multiplicity observed for H-11 as a dddd with two large and two small couplings (3JH11–H10a 14.1 Hz, 3JH11–H10b 2.7 Hz, 3JH11–H12b 11.0 Hz, and 3JH11–H12a 2.6 Hz), the heteronuclear coupling constants (3JC10–H12a 1.7 Hz, 3JC10–H12b 1.7, 2JC11–H12a −5.6 Hz 2JC11–H12b 0 Hz), and the ROESY correlation observed between H-12b and H-10 allowed us to deduce the presence of the rotamer represented for C-11/C-12 bonds in Figure 6a.
Figure 6.
Rotamers along C-11/C-12 (a) and C-12/C-13 (b) bonds in enigmazole C (1).
In the same way, a large proton–proton coupling constant of 11.0 Hz between H-13 and H-12a fixed these protons in an antiperiplanar disposition, making necessary the use of ROESY correlations between the pairs H-12a/H-27 and H-11/H-14 and the long-range 13C–1H coupling constants 3JC11–H13 0.5 Hz, 3JC27–H12a 3.0 Hz, and 2JC27–H12a 3.0 Hz to determine the presence of the rotamer drawn in Figure 6b. Once H-12a and H-12b are interrelated to conformers 6a and 6b, we were able to determine the relative configuration of the two stereogenic centers C-11 and C-13 as R* and R*.
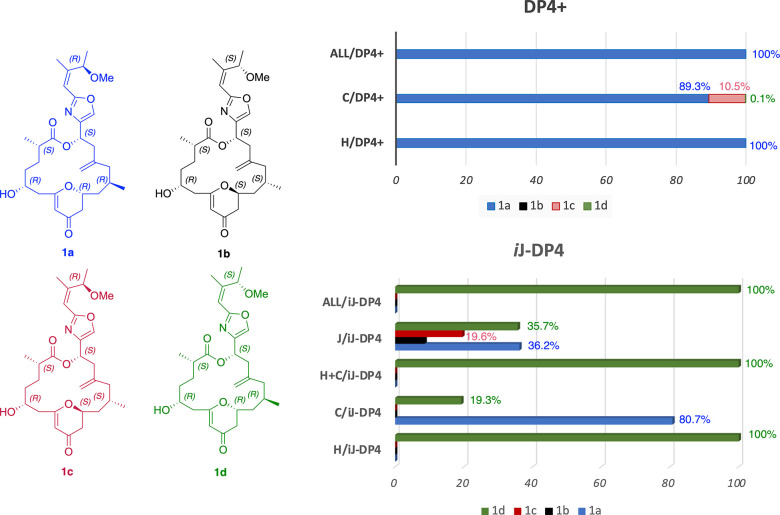
While the absolute configurations of C-2, C-5, and C-17 are already known as S, R, and S, respectively, the relative configurations of C-11 and C-13 as R* and R* along with the possible configuration at C-23 of either R or S give us four possible enigmazole C diastereomers (1a–d) that can be discriminated by an NMR–density functional theory (DFT) approximation. Initially we submitted all diastereoisomers to a conformational search with the Macromodel program using the protocol of Daranas, Sarotti, et al.27 Thus, 58 conformers for 1a-(2S,5R,11R,13R,17S,23R), 64 for 1b-(2S,5R,11S,13S,17S,23S), 60 for 1c-(2S,5R,11S,13S,17S,23R), and 62 for 1d-(2S,5R,11R,13R,17S,23S) were found within a 5.0 kcal/mol window, which were further classified by energy and frequencies using the B3LYP/6-31G(d) functional. Once the duplicates and conformers with imaginary frequencies were removed, a combination of MPW1PW91/6-31G(d,p) and the polarizable continuum model was used for proton and carbon chemical shift calculations using MeCN as solvent. The sets of 1H and 13C chemical shifts were compared by the statistical DP4+ parameter developed by Sarotti and co-workers.27,28 A 100% probability DP4+ value for all chemical shifts was in agreement with diastereoisomer 1a-(2S,5R,11R,13R,17S,23R) (Figure 7).
Figure 7.
DP4+ and iJ-DP4 statistical correlations of diastereoisomers 1a-(2S,5R,11R,13R,17S,23R), 1b-(2S,5R,11S,13S,17S,23S), 1c-(2S,5R,11S,13S,17S,23R), and 1d-(2S,5R,11R,13R,17S,23S) of enigmazole C.
However, when the iJ-DP4 was computed using the suggested combination of the MMFF-5 kcal/mol conformational search and B3LYP/6-31G(d) for chemical shifts and coupling constants (just the contact Fermi contribution), no clear discrimination between isomers 1a-(2S,5R,11R,13R,17S,23R) and 1d-(2S,5R,11R,13R,17S,23S) was achieved when six 3JHH constraints were introduced.27 Clearly both DP4+ and iJ-DP4 were able to discriminate 11R,13R diastereoisomers 1a and 1d from both 11S,13S-1b and 11S,13S-1c. At this point just C-23 remains to be assigned, and since the fragment C-18/C-25 showed similar NMR values (see chemical shifts in CD3OD in the Supporting Information) to that for enigmazole A (4),22 we propose the final structure for enigmazole C (1) as 2S,5R,11R,13R,17S,23R (diastereoisomer 1a). In comparison with enigmazole A, 1 presents the same configurations of the stereogenic centers at the C-2, C-5, C-11, C-13, C-17, and C-23 positions.
Along with 1, an analogue of 3, enigmazole D, was also found during the process of isolation and purification. Its HR-ESITOF-MS data showed the [M + H]+ adduct at m/z 516.2932, which was in perfect agreement for a molecular formula of C29H42NO7. Although the formula of 3 matches that found for enigmazole C, its HPLC retention time and 1H NMR spectrum were different. Three new 1H NMR signals corresponding to two vinylic protons (δH 6.58 and 6.00) and one oxygenated methine at δH 4.74, in addition to the absence of the signals for H-17 (δH 5.92), H-5 (δH 3.74), and the OH group at δH 3.00 in 1, lead us to suspect the opening of the macrolactone ring accompanied by an elimination of H2O. COSY correlations of the sp2 methine group at δH 6.58 with δH 6.00 and 2.21 and two extra signals observed from sp2 methines, with the loss of the hydroxy group, clearly suggested the formation of a new double bond in 3. The movement of the signal at δH 5.92 (H-17) found in 1 to a lower value of δH 4.74 in 3 along with the expected chemical shift of the new methine proton is in agreement with the opening of the lactone ring. With all these data on hand, we were able to assign the structure of enigmazole E to 3, a metabolite resulted from the opening of enigmazole C and a further dehydration at position C-5. It is important to notice that this new double bond has an E configuration, based on the 15.6 Hz coupling constant found between H-5 and H-6.
Compounds 2 and 3 can be considered derivatives of enigmazole C, and therefore we assumed the absolute configurations at positions C-2, C-5 (just for compound 2), C-11, C-13, C-17, and C-23 were the same as for 1; thus (2S,5R,11R,13R,17S,23R) for enigmazole E (2) and (2S,11R,13R,17S,23R) for enigmazole D (3) were determined.
All of the new compounds were tested in a panel of four human cancer cell lines: A-549 (lung), HT-29 (colon), MDA-MB-231 (breast), and PSN-1 (pancreas). Enigmazole C (1) showed activity on the order of micromolar range with a GI50 of 9.9 μM against the A-549 cell line (Table 2). Although enigmazole D (3) is the open form with an additional double bond of compound 1, cytotoxicity was observed in all cell lines (Table 3), while the methyl ester 2 of the open form of 1 renders the compound totally devoid of cytotoxicity.
Table 2. Cytotoxic Activities for Enigmazole C (1).
| cell line | GI50 | TGI | LC50 |
|---|---|---|---|
| A-549 | 9.9 μM | >19 μM | >19 μM |
| HT-29 | 18 μM | >19 μM | >19 μM |
| MDA-MB-231 | >19 μM | >19 μM | >19 μM |
| PSN-1 | 87 μM | >19 μM | >19 μM |
Table 3. Cytotoxic Activities for Enigmazole D (3).
| cell line | GI50 | TGI | LC50 |
|---|---|---|---|
| A-549 | 1.4 μM | >19 μM | >19 μM |
| HT-29 | 1.0 μM | 3.7 μM | >19 μM |
| MDA-MB-231 | 4.1 μM | >19 μM | >19 μM |
| PSN-1 | 1.1 μM | >19 μM | >19 μM |
In summary, the investigation of the Homophymia sp. sponge led to the isolation of one new macrolide lactone, enigmazole C (1), along with two open form congeners, enigmazoles E (enigmazole C seco acid methyl ester) (2) and enigmazole D (3). Spectroscopic data, chemical derivatization, and NMR-DFT methods were necessary to deduce the configurations of the six stereogenic centers present in their carbon skeletons. Notably, compounds 1 and 3 exhibited cytotoxic activities against A-549 (lung), HT-29 (colon), MDA-MB-231 (breast), and PSN-1 (pancreas) cell lines.
Experimental Section
General Experimental Procedures
Optical rotations were measured on a JASCO DIP-1000 polarimeter, with a Na (589 nm) lamp and filter. UV spectra were measured on a JASCO V-650 spectrophotometer. IR spectra were measured on a FTIR Bruker Vector 22 spectrometer. 1H, 13C, and 2D NMR spectra were recorded on a Varian “Unity 500” (500 MHz for 1H and 125 MHz for 13C), Varian “Unity 400” (400 MHz for 1H and 100 MHz for 13C), and Bruker Avance 500 (500 MHz for 1H and 125 MHz for 13C) with a dual cryoprobe or a BBI probe. CD3OD, CD3CN, C5D5N, and acetone-d6 were used as deuterated solvents. Chemical shifts are reported in δ scale relative to methanol-d4 (δ 3.31 ppm for 1H NMR, δ 49.0 ppm for 13C NMR), acetonitrile-d3 (δ 1.94 ppm for 1H NMR, δ 1.32 ppm for 13C NMR), acetone-d6 (δ 2.05 ppm for 1H NMR, δ 29.84 ppm for 13C NMR), and pyridine-d5 (δ 8.74 ppm for 1H NMR, δ 150.35 ppm for 13C NMR). HSQC-TOCSY-HECADE experiments were acquired with 32 scans and 256 increments with a mixing time of 60 ms and 4K points. J-HMBC was run with 16 scans, 200 increments, and 2 K points in F2.
LRESIMS and HRESIMS experiments were performed on the Applied Biosystems QSTAR Elite system or on an Agilent 6230 TOF LC/MS. HPLC separation was performed on an Agilent 1100 or 1200 using reversed-phase chromatographic columns.
Animal Material
The sponge belongs to the genus Homophymia Vacelet and Vasseur, 1971. It was collected by hand using a rebreather diving system in Gorontalo, Indonesia (01° 19.836′ S/122° 45.022′ E) at depths ranging between 40 and 80 m. The animal material was identified by Dr. María Jesús Uriz (Center for Avanced Studies of Blanes). A sample of the specimen was deposited in the Center for Advanced Studies of Blanes in Girona, Spain, with the reference code GORO-093. In addition, a voucher specimen (ORMA136284) was deposited at PharmaMar.
Description: Thickly globular sponge (3 cm high, 4 cm wide, 2 cm tick), smooth without conulous projections. Hard consistency, white color, and small single oscule in apical position. Megascleres: fine monoactines spicules broken in their preparations. Desmas pseudotetraclones with very tuberculated zygomes. Ectosomal megascleras are smooth pesudophyllotriaenes with different irregular pseudocladis tuberculated in the same specimen. Microscleres consist of a single type of microspinose amphiasters occurring in different size classes: the smaller 10–15 μm long, the larger 12–25 μm long.
Extraction and Isolation
A specimen of Homophymia (33 g) was triturated and exhaustively extracted with MeOH/CH2Cl2 (50:50, 3 × 500 mL). The combined extracts were concentrated to yield a mass of 2.5 g, which was subjected to VLC on Lichroprep RP-18 (Merck KGaA) with a stepped gradient from H2O to MeOH and then CH2Cl2. The fraction eluting with MeOH (96 mg) was subjected to semipreparative HPLC (Ascentis C18, 5 μm, 410 × 150 mm, isocratic H2O/MeCN (60:40) for 3 min, gradient H2O/MeCN from 50% to 65% MeCN in 25 min, UV detection, flow 1 mL/min, tr: 19.5 min to give compound 1 (2.9 mg), 12.0 min to give compound 2 (1.4 mg), and 13.0 min to give compound 3 (0.4 mg)).
Enigmazole C (1):
amorphous, yellow oil; [α]D +56 (c 0.03, MeOH); UV (MeOH) λmax 261 nm; IR (ATR) νmax 3378, 2966, 2311, 1675, 1140 cm–1; 1H NMR (500 MHz) and 13C NMR (125 MHz), Table 1; (+)-HRESI-TOFMS m/z 516.2964 [M + H]+ (calcd for C29H42NO7, 516.2956); m/z 538.2785, [M + Na]+ (calcd for C29H41NO7Na).
Table 1. NMR Data of Enigmazole C (1) in CD3CN and Acetone-d6.
| CD3CN δH, m (J in Hz) |
acetone-d6 |
|||
|---|---|---|---|---|
| pos. | δC, type | δH, m (J in Hz) | δC | δH, m (J in Hz) |
| 1 | 175.6, C | 174.9 | ||
| 2 | 39.9, CH | 2.49, m | 39.7 | 2.53 ddq (10.6, 6.9, 3.4) |
| 3 | 30.8, CH2 | a: 1.69, m | 30.8 | a: 1.79, dddd (13.8, 9.9, 5.4, 3.4) |
| b: 1.63, m | b: 1.68, dddd (13.8, 10.6, 5.3, 5.3) | |||
| 4 | 34.5, CH2 | a: 1.43, m | 34.5 | a: 1.51, dddd (14.4, 9.9, 5.3, 2.2) |
| b: 1.31, m | b: 1.38, dddd (14.4, 10.4, 5.4, 5.3) | |||
| 5 | 69.5, CH | 3.74, ddddd (10.4, 7.6, 5.5, 5.5, 2.5) | 69.6 | 3.83, ddddd (10.4, 8.1, 5.8, 4.5, 2.2) 2.2) |
| 6 | 44.6, CH2 | a: 2.49, dd (13.2, 5.5) | 44.9 | a: 2.59, dd (13.1, 4.5) |
| b: 2.27, dd (13.2, 7.6) | b: 2.31, dd (13.1, 8.1) | |||
| 7 | 175.5, C | 174.8 | ||
| 8 | 106.8, CH | 5.30, d (1.0) | 106.8 | 5.28, bs (1.0) |
| 9 | 193.6, C | 192.4 | ||
| 10 | 42.2, CH2 | a: 2.41, dd (16.7, 14.0) | 42.3 | a: 2.41, dd (16.7, 14.0) |
| b: 2.23, dd (16.7, 2.6) | b: 2.26, dd (16.7, 2.7) | |||
| 11 | 77.8, CH | 4.48, ddt (14.0, 11.0, 2.6, 2.6) | 77.4 | 4.53 ddt (14.0, 11.0, 2.7, 2.7) |
| 12 | 42.9, CH2 | a: 1.81, ddd (14.2, 10.9, 2.6) | 43.1 | a: 1.85, ddd (14.1, 11.0, 2.7) |
| b: 1.46, dd (14.2, 11.0) | b: 1.54, ddd (14.2, 11.1, 2.3) | |||
| 13 | 25.8, CH | 2.20, m | 25.6 | 2.29, m |
| 14 | 40.2, CH2 | a: 2.49, dd (15.8, 1.5) | 40.0 | a: 2.27, dd (16.0, 2.7) |
| b: 1.59, dd (15.8, 10.7) | b: 1.61, dd (16.0, 11.0) | |||
| 15 | 143.9, C | 143.8 | ||
| 16 | 42.2, CH2 | a: 2.72, dd (13.7, 9.6) | 42.3 | a: 2.75, dd (13.8, 9.7) |
| b: 2.62, dd (13.7, 5.2) | b: 2.67, dd (13.8, 5.1) | |||
| 17 | 66.7, CH | 5.92, ddd (9.4, 5.2, 0.8) | 66.3 | 6.00, dd (9.7, 4.9) |
| 18 | 141.7, C | 142.0 | ||
| 19 | 135.9, CH | 7.63, d (0.7) | 135.6 | 7.77, s |
| 20 | 161.2, C | 161.0 | ||
| 21 | 113.6, CH | 6.20, dd (1.5, 0.8) | 113.5 | 6.21, bs |
| 22 | 152.7, C | 152.4 | ||
| 23 | 75.5, CH | 5.20, dq (6.5, 0.8) | 75.3 | 5.29, bq (6.5) |
| 24 | 19.5, CH3 | 1.23, d (6.5) | 19.4 | 1.24, d (6.5) |
| 25 | 17.7, CH3 | 1.86, d (1.5) | 17.5 | 1.88, d (1.6) |
| 26 | 17.6, CH3 | 1.10, d (6.9) | 17.6 | 1.11, d (6.9) |
| 27 | 21.2, CH3 | 0.95, d (6.7) | 21.2 | 0.98, d (6.6) |
| 28 | 113.9, CH2 | a: 4.91, s | 113.5 | a: 4.94, s |
| b: 4.86, s | b: 4.88, s | |||
| 29 | 56.6, CH3 | 3.15, s | 56.5 | 3.17, s |
| OH | - | 3.00, d (6.0) | - | 3.90 d (5.8) |
Enigmazole E (enigmazole C seco acid methyl ester, 2):
yellow oil; UV (MeOH) λmax 265 nm; 1H and 13C NMR, Table S1; (+)-HRESI-TOFMS m/z 548.3249 [M + H]+ (calcd for C30H46NO8, 548.3218).
Enigmazole D (3):
yellow oil; UV (MeOH) λmax 254, 277, 286 nm; 1H and 13C NMR, Table S2; (+)-HRESI-TOFMS m/z 516.2932 [M + H]+ (calcd for C29H42NO7, 516.2956).
Computational Calculations
Conformational searches were performed by using the corresponding module implemented in the Maestro Quantum mechanical software. The MMFF force field with acetonitrile as solvent was used, and torsional enhanced sampling with 10 000 steps was fixed using an energy window of 5 kcal/mol.
DP4+
Molecular geometry optimizations were performed for 1a–d at the DFT theoretical level using the Gaussian 16W package first at the B3LYP/6-31G(d) level for energy and frequency calculations. After removing redundant conformers and those with imaginary frequencies, theoretical Boltzmann energy population-weighted 1H and 13C NMR were calculated by using the combination MPW1PW91/6-31G(d,p). Results were input in Sarotti’s and Darana’s Excel spreadsheet27 to calculate the best fit.
iJ-DP4
MMFF conformers at 5 kcal/mol were filtered by six proton–proton vicinal coupling restriction: 3JH11H12a, 3JH11H12b, 3JH5H4b, 3JH5H4a, 3JH2H3b, and 3JH2H3a. Those conformers were chosen to calculate the population-weighted Boltzmann MMFF energy. 1H and 13C NMR and 1H–1H coupling constants (just the Fermi’s contact contribution) were then calculated by the combination B3LYP/6-31G(d). Results were input in Sarotti’s and Darana’s Excel spreadsheet27 to calculate the best fit.
Preparation of the (S)-MTPA Ester of Enigmazole C (1-S)
R-(−)-MTPA chloride (10 μL) was added to a solution of 1 (1.0 mg) in 0.5 mL of pyridine-d5 in an NMR tube. The resulting mixture was allowed to stand at room temperature (rt) for 8 h to yield the (R)-MTPA ester of 1, which was monitored by recording 1H NMR spectra at 500 MHz. 1H NMR (500 MHz, C5D5N) δ 5.66 (s, H-8), 5.56 (m, H-5), 2.83 (dd, J = 13.7, 5.1 Hz, H-6a), 2.71 (m, H2), 2.64 (dd, J = 13.7, 7.2 Hz, H-6b), 1.79 (m, H-4), 1.66 (m, H-3), 1.15 (d, J = 6.8 Hz, H-26).
Preparation of the (R)-MTPA Ester of Enigmazole C (1-R)
Treatment of 1 (1.0 mg) in the same manner as before with S-(+)-MTPA chloride (10 μL) gave the (R)-MTPA ester of 1. 1H NMR (500 MHz, C5D5N) δ 5.71 (s, H-8), 5.57 (m, H-5), 2.90 (dd, J = 13.8, 5.0 Hz, H-6a), 2.59 (m, H-2), 2.74 (dd, J = 13.8, 7.0 Hz, H-6b), 1.71 (m, H-4), 1.42 (m, H-3), 1.07 (d, J = 6.8 Hz, H-26).
Preparation of the (S)-MTPA Ester of Enigmazole E (2-S)
R-(−)-MTPA chloride (10 μL) was added to a solution of 2 (0.4 mg) in 0.5 mL of pyridine-d5 in an NMR tube. The resulting mixture was allowed to stand at rt for 8 h to yield the (S)-MTPA ester of 2, which was monitored by recording 1H NMR spectra at 500 MHz. 1H NMR (500 MHz, C5D5N) δ 6.60 (dd, J = 7.2, 7.2 Hz, H-17), 5.69 (m, H-5), 5.67 (s, H-21), 5.45 (d, J = 6.4 Hz, H-23), 4.92 (s, H-28), 2.93 (m, H-16), 2.68 (m, H-6), 2.51 (m, H-2), 2.18 (m, H-14), 1.87 (s, H-25), 1.86 (m, H-3), 1.86 (m, H-4), 1.12 (d, 7.0 Hz, H-26), 0.91 (d, 5.9 Hz, H-27).
Preparation of the (R)-MTPA Ester of Enigmazole E (2-R)
Treatment of 2 (0.4 mg) in the same manner as before with S-(+)-MTPA chloride (5 μL) gave the (R)-MTPA ester of 2. 1H NMR (500 MHz, C5D5N) δ 6.62 (dd, J = 7.1, 7.1 Hz, H-17), 5.70 (m, H-5), 5.66 (s, H-21), 5.40 (d, J = 6.3 Hz, H-23), 5.04 (s, H-28), 2.99 (m, H-16), 2.73 (m, H-6), 2.41 (m, H-2), 2.24 (m, H-14), 1.85 (s, H-25), 1.73 (m, H-3), 1.73 (m, H-4), 1.04 (d, 7.0 Hz, H-26), 0.93 (d, 5.9 Hz, H-27).
Biological Assays
The cytotoxic activities of compounds 1, 2, and 3 were tested against A-549 human lung carcinoma cells, MDA-MB-231 human breast adenocarcinoma cells, HT-29 human colorectal carcinoma cells, and PSN-1 pancreatic adenocarcinoma cells. The concentration giving 50% inhibition of cell growth (GI50) was calculated according to the procedure described in the literature. Cell survival was estimated using the National Cancer Institute (NCI) algorithm. Three dose–response parameters were calculated for 1, 2, and 3.
Acknowledgments
We gratefully acknowledge the Expeditions, Collection and Cell Biology Departments of PharmaMar S.A.U. We also thank S. Bueno and Dra. M. J. Uriz (CEAB-Centro de Estudios Avanzados de Blanes, Spain) for determining the sponge taxonomy and S. Munt for revision of the manuscript. PharmaMar also acknowledges the Udayana University of Bali, Indonesia. J.R. and C.J. acknowledge Xunta de Galicia and CESGA for the computational resources.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jnatprod.1c01179.
1H, 13C, and 2D NMR data of the new compounds and J configurational analysis of 1 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Radjasa O. K.; Vaske Y. M.; Navarro G.; Vervoort H. C.; Tenney K.; Linington R. G.; Crews P.. Highlights of Marine Invertebrate-Derived Biosynthetic Products: Their Biomedical Potential and Possible Production by Microbial Associants. Bioorg. Med. Chem. 2011, 19, pp 6658–6674 10.1016/j.bmc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampella A.; Sepe V.; Luciano P.; Bellotta F.; Chiara Monti M.; Valeria D’Auria M.; Jepsen T.; Petek S.; Adeline M.-T.; Laprévôte O.; Aubertin A.-M.; Debitus C.; Poupat C.; Ahond A. Homophymine A, an Anti-HIV Cyclodepsipeptide from the Sponge Homophymia Sp. J. Org. Chem. 2008, 73 (14), 5319–5327. 10.1021/jo800583b. [DOI] [PubMed] [Google Scholar]
- Zampella A.; Sepe V.; Bellotta F.; Luciano P.; D’Auria M. V.; Cresteil T.; Debitus C.; Petek S.; Poupat C.; Ahond A. Homophymines B-E and A1-E1, a Family of Bioactive Cyclodepsipeptides from the Sponge Homophymia Sp. Org. Biomol. Chem. 2009, 7 (19), 4037–4044. 10.1039/b910015f. [DOI] [PubMed] [Google Scholar]
- Kanki D.; Nakamukai S.; Ogura Y.; Takikawa H.; Ise Y.; Morii Y.; Yamawaki N.; Takatani T.; Arakawa O.; Okada S.; Matsunaga S. Homophymamide A, Heterodetic Cyclic Tetrapeptide from a Homophymia Sp. Marine Sponge: A Cautionary Note on Configurational Assignment of Peptides That Contain a Ureido Linkage. J. Nat. Prod. 2021, 84 (6), 1848–1853. 10.1021/acs.jnatprod.1c00336. [DOI] [PubMed] [Google Scholar]
- Coello L.; Reyes F.; Martín M. J.; Cuevas C.; Fernández R. Isolation and Structures of Pipecolidepsins A and B, Cytotoxic Cyclic Depsipeptides from the Madagascan Sponge Homophymia Lamellosa. J. Nat. Prod. 2014, 77 (2), 298–303. 10.1021/np400888e. [DOI] [PubMed] [Google Scholar]
- Albericio F.; García-Ramos Y.; Martín-López M. J.; Pelay Gimeno M.; Tulla-Puche J.. Synthetic Process for the Manufacture of Pipecolidepsin Compounds. WO2014108526A1, 2014.
- Pelay-Gimeno M.; García-Ramos Y.; Jesús Martin M.; Spengler J.; Molina-Guijarro J. M.; Munt S.; Francesch A. M.; Cuevas C.; Tulla-Puche J.; Albericio F.. The First Total Synthesis of the Cyclodepsipeptide Pipecolidepsin A. Nat. Commun. 2013, 4, 10.1038/ncomms335 article number: 2352. [DOI] [PubMed] [Google Scholar]
- Bassarello C.; Zampella A.; Monti M. C.; Gomez-Paloma L.; D’Auria M. V.; Riccio R.; Bifulco G. Quantum Mechanical Calculation of Coupling Constants in the Configurational Analysis of Flexible Systems: Determination of the Configuration of Callipeltin A. Eur. J. Org. Chem. 2006, 2006, 604–609. 10.1002/ejoc.200500740. [DOI] [Google Scholar]
- Kikuchi M.; Konno H. Cytotoxic Evaluation of Natural and Synthetic Callipeltins: A Revision of Cytotoxicity of Callipeltin B. Biosci., Biotechnol., and Biochem. 2016, 80 (6), 1066–1069. 10.1080/09168451.2016.1148581. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy R.; Vazquez-Serrano L. D.; Turk J. A.; Kowalski J. A.; Benson A. G.; Breaux N. T.; Lipton M. A. Solid-Phase Total Synthesis and Structure Proof of Callipeltin B. J. Am. Chem. Soc. 2006, 128 (48), 15392–15393. 10.1021/ja0666250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker R. W.; Hill A. L.; Hill M. S.; Redmond N. E.; Collins A. G.; Morrow C. C.; Lori Spicer ô; Carmack C. A.; Zappe M. E.; Pohlmann D.; Hall C.; Diaz M. C.; Bangalore P. V.; Francisco S.. Nearly Complete 28S RRNA Gene Sequences Confirm New Hypotheses of Sponge Evolution At. Integrative Comparative Biol. 2013, 53, 373. 10.1093/icb/ict071-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond N. E.; Morrow C. C.; Thacker R. W.; Diaz M. C.; Boury-Esnault N.; Cárdenas P.; Hajdu E.; Lôbo-Hajdu G.; Picton B. E.; Pomponi S. A.; Kayal E.; Collins A. G. Phylogeny and Systematics of Demospongiae in Light of New Small-Subunit Ribosomal DNA (18S) Sequences. Integrative and Comparative Biol. 2013, 53 (3), 388–415. 10.1093/icb/ict078. [DOI] [PubMed] [Google Scholar]
- Wolf D.; Schmitz F. J.; Qiu F.; Kelly-Borges M. Aurantoside C, a New Tetramic Acid Glycoside from the Sponge Homophymia Conferta. J. Nat. Prod. 1999, 62 (1), 170–172. 10.1021/np980283x. [DOI] [PubMed] [Google Scholar]
- Zampella A.; D’Auria M. V.; Minale L.; Debitus C.; Roussakis C. Callipeltoside A: A Cytotoxic Aminodeoxy Sugar-Containing Macrolide of a New Type from the Marine Lithistida Sponge Callipelta Sp. J. Am. Chem. Soc. 1996, 118 (45), 11085–11088. 10.1021/ja9621004. [DOI] [Google Scholar]
- Zampella A.; Valeria D’auria M.; Minale L.; Debitus C. Callipeitosides B and C, Two Novel Cytotoxic Glycoside Macrolides from a Marine Lithistida Sponge Callipelta sp. Tetrahedron 1997, 53, 3243–3248. 10.1016/S0040-4020(97)00035-5. [DOI] [Google Scholar]
- Urda C.; Fernández R.; Rodríguez J.; Pérez M.; Jiménez C.; Cuevas C.. Daedophamide, a Cytotoxic Cyclodepsipeptide from a Daedalopelta Sp. Sponge Collected in Indonesia. J. Nat. Prod. 2017, 80 ( (1), ), 3054. 10.1021/acs.jnatprod.7b00678. [DOI] [PubMed] [Google Scholar]
- Wright A. E.; Botelho J. C.; Guzmán E.; Harmody D.; Linley P.; McCarthy P. J.; Pitts T. P.; Pomponi S. A.; Reed J. K. Neopeltolide, a Macrolide from a Lithistid Sponge of the Family Neopeltidae. J. Nat. Prod. 2007, 70 (3), 412–416. 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- Fuwa H.; Naito S.; Goto T.; Sasaki M. Total Synthesis of (+)-Neopeltolide. Angew. Chem., Int. Ed. 2008, 47 (25), 4737–4739. 10.1002/anie.200801399. [DOI] [PubMed] [Google Scholar]
- Zhu X. L.; Zhang R.; Wu Q. Y.; Song Y. J.; Wang Y. X.; Yang J. F.; Yang G. F. Natural Product Neopeltolide as a Cytochrome Bc 1 Complex Inhibitor: Mechanism of Action and Structural Modification. J. Agric. Food Chem. 2019, 67 (10), 2774–2781. 10.1021/acs.jafc.8b06195. [DOI] [PubMed] [Google Scholar]
- Li J.; Preinfalk A.; Maulide N. Diastereo- and Enantioselective Access to Stereotriads through a Flexible Coupling of Substituted Aldehydes and Alkenes. Angew. Chem., Int. Ed. 2019, 58 (18), 5887–5890. 10.1002/anie.201900801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M. Q.; Chen T.; Wang Y. X.; Zhu X. L.; Yang G. F. Design and Synthesis of Potent Inhibitors of Bc1 Complex Based on Natural Product Neopeltolide. Bioorg. Med. Chem. Lett. 2020, 30 (16), 127324–127328. 10.1016/j.bmcl.2020.127324. [DOI] [PubMed] [Google Scholar]
- Oku N.; Takada K.; Fuller R. W.; Wilson J. A.; Peach M. L.; Pannell L. K.; McMahon J. B.; Gustafson K. R. Isolation, Structural Elucidation, and Absolute Stereochemistry of Enigmazole a, a Cytotoxic Phosphomacrolide from the Papua New Guinea Marine Sponge Cinachyrella Enigmatica. J. Am. Chem. Soc. 2010, 132 (30), 10278–10285. 10.1021/ja1016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinski T. F.; Morinaka B. I. Integrated Approaches To the Configurational Assignment of Marine Natural Products. Tetrahedron 2012, 68 (46), 9307–9343. 10.1016/j.tet.2011.12.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori N.; Kaneno D.; Murata M.; Nakamura H.; Tachibana K. Stereochemical Determination of Acyclic Structures Based on Carbon-Proton Spin-Coupling Constants. A Method of Configuration Analysis for Natural Products. J. Org. Chem. 1999, 64 (3), 866–876. 10.1021/jo981810k. [DOI] [PubMed] [Google Scholar]
- Hoye T. R.; Jeffrey C. S.; Shao F. Mosher Ester Analysis for the Determination of Absolute Configuration of Stereogenic (Chiral) Carbinol Carbons. Nat. Protoc. 2007, 2 (10), 2451–2458. 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- Seco J. M.; Quiñoá E.; Riguera R. The Assignment of Absolute Configuration by NMR. Chem. Rev. 2004, 104, 17–117. 10.1021/cr000665j. [DOI] [PubMed] [Google Scholar]
- Grimblat N.; Gavín J. A.; Hernández Daranas A.; Sarotti A. M. Combining the Power of J Coupling and DP4 Analysis on Stereochemical Assignments: The J-DP4 Methods. Org. Lett. 2019, 21 (11), 4003–4007. 10.1021/acs.orglett.9b01193. [DOI] [PubMed] [Google Scholar]
- Grimblat N.; Zanardi M. M.; Sarotti A. M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80 (24), 12526–12534. 10.1021/acs.joc.5b02396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.