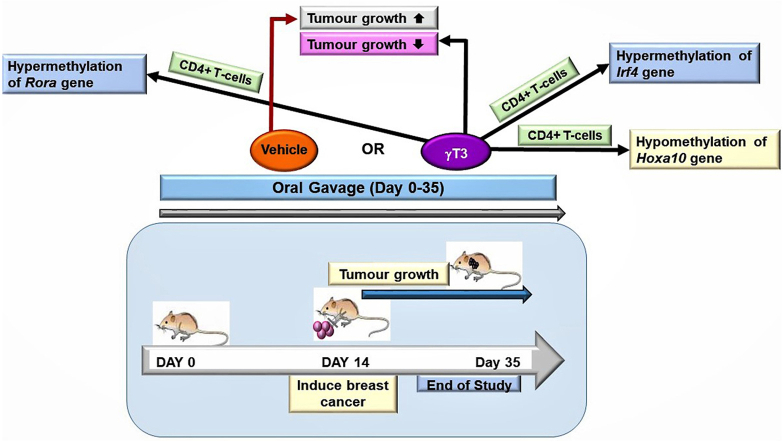
Abstract
DNA methylation plays a crucial role in polarising naïve lymphocytes towards their various sub-populations to fight against many immune challenges including establishment of tumour. Gamma-tocotrienol (γT3) is a natural form of vitamin E, reported to possess anticancer and immunomodulatory effects. This study reports the anticancer effects of γT3 through modulation of DNA methylation in several genes in CD4+ T-lymphocytes using a syngeneic mouse model of breast cancer. Female BALB/c mice were fed with γT3 or vehicle (soy oil) for two-weeks via oral gavage before they were inoculated with 4T1 mouse mammary cancer cells. Supplementation continued until the mice were sacrificed. At autopsy, blood was collected via cardiac puncture and CD4+ T-cells were isolated for DNA extraction. The DNA was analysed using the EpiTech Methyl II mouse T-helper cell differentiation PCR array. γT3 supplementation reduced tumour growth in the tumour-induced animals and modulated host immune system by inducing changes in DNA methylation patterns of the HOXA10, IRF4 and RORα genes, which are involved in differentiation and clonal expansion of CD4+ T-cells. Results suggest that γT3 may enhance cell-mediated immune response in mice with breast cancer by inducing changes in DNA methylation pattern.
Keywords: Gamma-tocotrienol, DNA methylation, Breast cancer, CD4+ T-lymphocytes
Abbreviations: γT3, gamma-tocotrienol; HOXA10, Homeobox A10; IRF4, Interferon Regulatory Factor 4; RORα, Receptor-related orphan receptor-alpha
Graphical abstract
Highlights
-
•
γT3 supplementation reduced tumour growth in a syngeneic mouse model of breast cancer.
-
•
Dietary γT3 decreased DNA methylation in Hoxa10 gene in the CD4+ T-cells from tumour-laden mice.
-
•
Dietary γT3 increased DNA methylation in Irf4 and RORα genes in the CD4+ T-cells from tumour-laden mice.
1. Introduction
Tocotrienols (T3) belong to the vitamin E family exists naturally in four isoforms i.e. alpha (α), beta (β), delta (δ) and gamma (γ) (Wong and Radhakrishnan, 2012). Natural sources include in vegetable oils such as palm oil, rice bran oil and annatto beans (Liu et al., 2008; Moraes et al., 2015). Several studies using cell-based and animal models have shown that T3 possess anti-cancer effects through various mechanisms (Abraham et al., 2019; Montagnani Marelli et al., 2019). We have previously reported that tocotrienols possess anticancer (Loganathan et al., 2021; Ramdas et al., 2019, Ramdas et al., 2020), and immunomodulatory (Subramaiam et al., 2021; Radhakrishnan et al., 2013) effects in cell-based and animal models. In addition, we also found that daily supplementation of palm vitamin E generated tumour-specific cytotoxic T-lymphocytes (CTL) in a syngeneic mouse model of breast cancer (BC) (Hafid et al., 2013) as well as augmented immune response to tetanus toxoid vaccine (Radhakrishnan et al., 2013; Mahalingam et al., 2011). Recently, we reported on the immunomodulatory effects of gamma-T3 supplementation in a syngeneic mouse model (Subramaniam et al., 2021) where we reported that daily supplementation of gamma-T3 reduced infiltration of T-regulatory cells in the tumours.
DNA methylation refers to the addition of a methyl group (CH3) to cytosine-guanine (CG) residues at the 5’ end of double-stranded DNA (dsDNA) strands (Petryk et al., 2021), which could be a vital process to stabilise gene expression and enhance the plasticity of naïve lymphocytes differentiating into different subpopulations. This process is reported to help increase the flexibility and functionality of the lymphocytes and may be integral to their ability to respond to various immune challenges and the generation of immunological responses (Morales-Nebreda et al., 2019; Omilusik and Goldrath, 2019). Unusual DNA methylation patterns have been associated with various immune disorders (Suarez-Alvarez et al., 2012). Hence, a balance in regulating DNA methylation may be required to maintain a stable environment to minimise the occurrence of these immune diseases. However, epigenetic modifications are reported to be reversible events (Herman and Baylin, 2003).
Recent studies suggest a vital link between nutrition and DNA methylation (Zhang, 2015; Lim and Song, 2012). For instance, bioactive compounds were reported to modulate DNA methylation of genes involved in carcinogenesis (Stefanska et al., 2012; Meeran et al., 2010). To date, the effect of dietary γT3 in modulating DNA methylation of any genes in murine CD4+ T-lymphocytes has not been described. The present study describes modifications to DNA methylation levels of gene involved in T-helper differentiation following dietary γT3 using a syngeneic mouse model of breast cancer.
2. Methods
Ethics approval
The Joint Committee approved all experimental procedures involving animals for Research and Ethics of the International Medical University (IMU) (IMU-R113-2013). The study complied with the Animal Ethics Guidelines of the IMU, which complies with the ARRIVE guidelines. The study was carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.1. Experimental animals
Female BALB/c mice (five-week-old) were purchased from a commercial source in Kuala Lumpur, Malaysia (Chenur Suppliers, Selangor, Malaysia) and housed at the Animal House Facilities (AHF) at the IMU, Kuala Lumpur, Malaysia. The animals were allowed to acclimatise for seven-days before they were used in this study.
2.2. Cell culture
The 4T1 murine breast cancer cell line (ATCC CRL-2539) was purchased from the American Type Culture Collection (ATCC, Rockville, USA). The tumour formed from 4T1 cell inoculation in BALB/c is reported to mimic stage IV of human breast cancer (Tao et al., 2008). The 4T1 cells were maintained in complete medium [RPMI 1640 medium, 10% FBS, 1% penicillin-streptomycin, 1% sodium pyruvate and 1% HEPES (Gibco UK)] at 37 °C in a humidified 5% CO2 incubator.
2.3. Test compound and administration
Gamma-tocotrienol (γT3) was provided by Davos Life Sciences, Pte Ltd, Singapore. Soy oil (Soya Lite, Malaysia) was used as vehicle to feed γT3 to the experimental animals via oral gavage.
2.4. Syngeneic mouse model of breast cancer
The mice (n = 24) were randomly assigned into two groups i.e. fed with 50 μL of vehicle (soy oil) (n = 12) or 0.5 mg γT3 in soy oil (n = 12) twice daily by oral gavage for 14-days. Then, half (n = 6) of the mice in each group were injected with 4T1 cells (103 cells in 50 μL) in their right second thoracic mammary fat pad to induce breast cancer (Selvaduray et al., 2010). The same supplement was continued till the end-point of the study. Throughout the study, the mice from each treatment group were housed together with three mice per cage. The cages were maintained in the same room and rack, with no relocation. We did not pool samples from separate experiments. Tumour volume (V) was calculated using the formula: V = 0.52 × L2 × W (Selvaduray et al., 2010). The perpendicular diameters referred to length (L) and width (W) were measured seven days using a digital calliper. The mice were monitored daily to minimise any suffering and were culled on day 35 of the study.
2.5. Isolation of CD4+ T-lymphocytes
At autopsy, peripheral blood obtained via cardiac puncture was collected in heparinised tubes. The CD4+ T-lymphocytes were isolated from whole blood using the MagniSort® Mouse CD4 Positive Selection Kit as recommended by the manufacturer (eBioscience, San Diego, CA). Briefly, CD4+ T-lymphocytes bind magnet bead-labelled CD4-antibody in a tube. The tube was placed in a magnet, which binds the beads bound to the CD4+ T-cells. The CD4+ T-cells can then be separated from other blood cells by discarding any unbound material.
2.6. DNA methylation of T-Helper cell differentiation
Genomic DNA was extracted from the isolated CD4+ T-lymphocytes using the QIAGEN DNeasy Blood and Tissue mini kit as recommended by the manufacturer's protocol (QIAGEN, USA). After checking for the quality and quantity of DNA, the genomic DNA was processed for DNA methylation analysis. The EpiTech methyl II DNA restriction kit (SABioscience, USA) was used to prepare the reaction mix with the DNA samples before these were loaded into the respective well of the EpiTech Methyl II signature PCR plate that was specially to study DNA methylation patterns of 22 genes related to T-helper differentiation. The EpiTech Methyl II Mouse T-Helper Cell Differentiation PCR Array (SABiosciences, USA) uses a real-time PCR system designed to analyse methylation patterns of these 22 genes (Table 1) reported to be involved in mouse T-helper cell differentiation.
Table 1.
Functional grouping of genes annotated in the DNA methylation array for mouse T Helper Cell Differentiation.
| GROUPS | GENES |
|---|---|
| Th1 Cells | Eomes, Tbx21 |
| Th2 Cells | Gata3, Il13, Pparg |
| Th17 Cells | Rora |
| Inducible & Natural Regulatory T (iTreg & nTreg) Cells | Fosl1, Irf4, Irf8, Myb, Nr4a3, Pou2f2, Rel, Relb, Tgif1, Tnfsf11 |
| Conventional Versus Regulatory T Cells | Chd7, Gata4, Hoxa10, Id2, Lrrc32, Perp |
2.7. Statistical analysis
Statistical analysis was carried out for all the results obtained. Student T-test was performed for all the studies by means of the treated groups and control was compared for significance with paired T-test. In all cases, significance level was set at p < 0.05 and p < 0.01. Data presented in texts, as well as figures were represented as means ± standard deviation (SD).
3. Results
3.1. Tumour growth
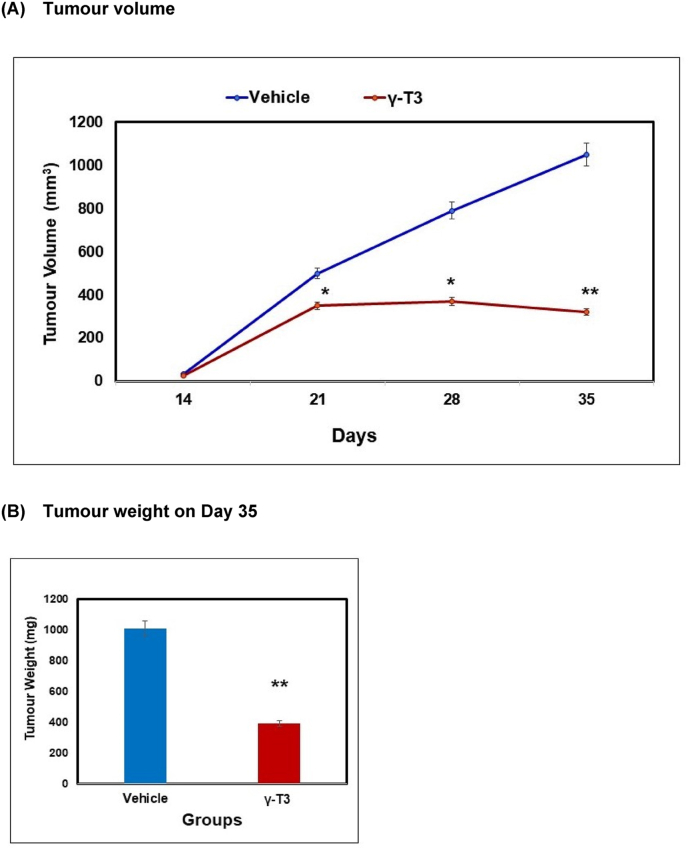
There was a marked reduction (P < 0.05) of tumour volume observed in the tumour-induced animals fed with γT3 compared to the vehicle-fed group (Fig. 1).
Fig. 1.
(A) Tumour volume was measured once every seven-day after the tumour was palpable using a digital calliper. Tumour volume was calculated using a previously reported formula (28). (B) Tumour weight on day 35. Data is represented as mean tumour vol/wt ± standard deviation (SD) calculated from six independent mice per group. [*p < 0.05 versus vehicle treated on day 14, **P < 0.01 versus vehicle treated on day 14].
3.2. Changes in DNA methylation pattern
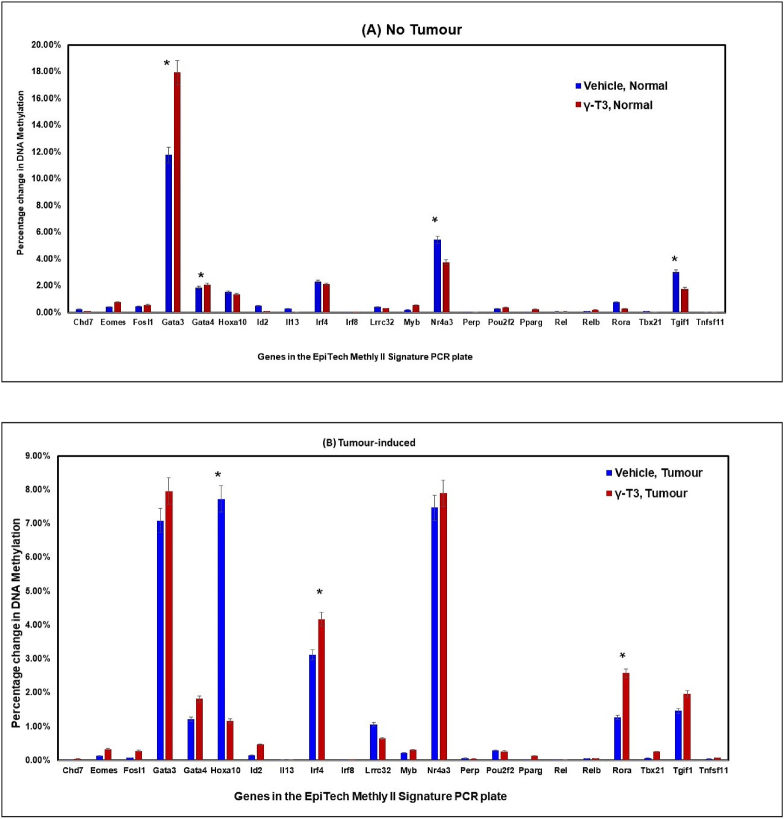
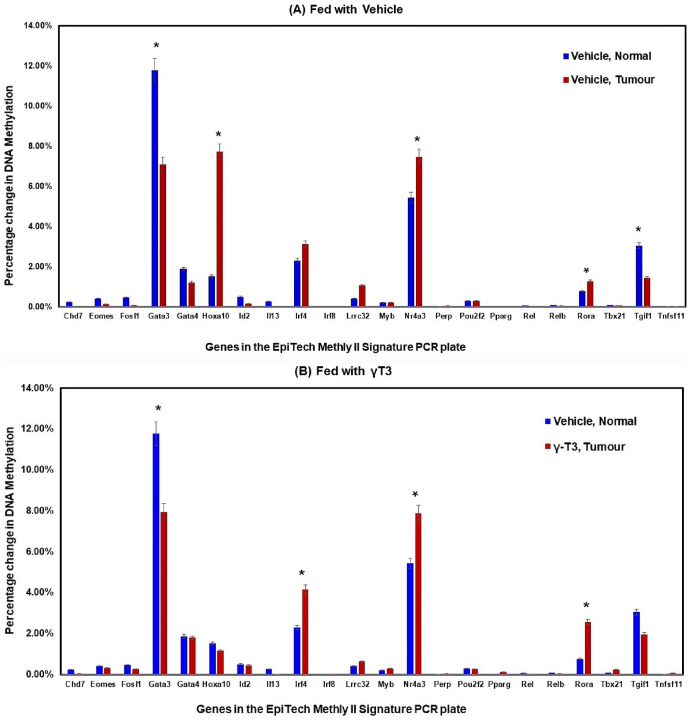
Daily supplementation with γT3 caused significant (p < 0.05) changes in the DNA methylation patterns of Gata3, Gata4, Nr4a3 and Tgif1 genes (Fig. 2A) in their CD4+ T-lymphocytes when compared to mice fed with vehicle. In the Gata3 and Gata4 genes, there was increased level of methylation in the Gata3 and Gata4 genes and reduced levels in Nr4a3 and Tgif1 genes. In the mice induced with BC and fed with γT3, there was significant (p < 0.05) changes in the Hoxa10, Irf4 and Rora genes in their CD4+ T-lymphocytes when compared to mice fed with vehicle (Fig. 2B). In this group, the percentage of DNA methylation was reduced in Hoxa10 gene but increased in Irf4 and Rora genes. There was significant (p < 0.05) changes in the DNA methylation pattern of five genes (Gata3, Hoxa10, Nr4a3, Rora and Tgif1) out of the 22 genes in the DNA methylation array between vehicle-fed mice induced with tumour compared to those without tumour (Fig. 3A). In γT3-fed animal, there was significant (p < 0.05) changes in the DNA methylation pattern of four genes (Gata3, Irf4, Nr4a3, and Rora) out of the 22 genes in the DNA methylation array in tumour-induce mice compared to no tumour group (Fig. 3B).
Fig. 2.
Comparing percentage of DNA methylation in CD4+ T-lymphocytes from (A) control (no tumour) or (B) tumour-induced mice fed with (A) vehicle (soy oil) or (B) γT3 (γT3 in soy oil). Genomic DNA was extracted from CD4+ T-lymphocytes (QIAGEN DNeasy Blood and Tissue mini kit) isolated from peripheral blood at autopsy. The DNA was analysed for modification in the DNA methylation of the 22 genes that were annotated in the commercial DNA methylation array (EpiTech Methyl II signature PCR for murine T-helper differentiation array plate, SABioscience, USA). Data is represented as mean percentage of methylation ± standard deviation (SD) and is representative of at least three independent mice. (*P < 0.05 versus vehicle, tumour).
Fig. 3.
Comparing percentage of DNA methylation in CD4+ T-lymphocytes from tumour-induced mice fed with (A) vehicle (soy oil) or (B) γT3 (γT3 in soy oil). Genomic DNA was extracted from CD4+ T-lymphocytes (QIAGEN DNeasy Blood and Tissue mini kit) isolated from peripheral blood at autopsy. The DNA was analysed for modification in the DNA methylation of the 22 genes that were annotated in the commercial DNA methylation array (EpiTech Methyl II signature PCR for murine T-helper differentiation array plate, SABioscience, USA). Data is represented as mean percentage of methylation ± standard deviation (SD) and is representative of at least three independent mice. (*P < 0.05 versus vehicle, tumour).
4. Discussion
Supplementation with γT3 caused significant reduction in tumour volume and metastasis compared to vehicle-fed mice, which is in agreement with some of the published literature with regards to the anti-cancer effects of T3. For instance, supplementation of T3 from annatto beans, which contain δT3 (90%) and γT3 (10%) was shown to inhibit growth of mammary tumour (Pierpaoli et al., 2013). In another study, γT3 supplementation caused marked inhibition tumour growth in prostate cancer (Yap et al., 2010) and gastric cancer (Manu et al., 2012). In our recent paper, we provided evidence that showed daily supplementation with γT3 modulated host immune response in this same syngeneic mouse model of breast cancer (Subramaniam et al., 2021). Daily supplementation with γT3 reduced infiltration of Treg into the tumours, which correlate with decreased tumour growth and metastasis as well as regulated gene expression that supported Th1 responses.
Apart from anticancer activities, T3 also induced immune-enhancing activities. For example, TRF supplementation boosted host immune response to vaccine (Mahalingam et al., 2011; Radhakrishnan et al., 2013) as well as proceed tumour-specific cytotoxic T-lymphocytes following dendritic cell immunotherapy in a syngeneic mouse model of BC (Hafid et al., 2013).
A stable regulation of DNA methylation is required for the plasticity of the CD4+ T-lymphocytes to allow flexible immune responses (Omilusik et al., 2019). Recently, it was reported that T3 can induce epigenetics changes in cancer cells (Aggarwal et al., 2019; Huang et al., 2017). To date, there are no reports on γT3 supplementation causing immunomodulatory effects through changes in DNA methylation of genes related to immune response. In the present study, we found that γT3 supplementation caused changes to the DNA methylation levels of several genes associated with immune response.
There was an increase (P < 0.05) in methylation levels of the GATA3 gene in CD4+ T-cells isolated from γT3 supplemented animals. GATA binding protein 3 (GATA3) gene is reported to be a master regulator of the Th2 subsets differentiation (Wan, 2014). The Th2 cells play a vital role to eliminate extracellular parasites and secrete cytokines that suppress Th1 immune responses (Coffman, 2006). Hypermethylation means that the expression of the GATA3 gene will be reduced. So, these findings suggest γT3 supplementation may promote Th1 immune responses by hypermethylation of the GATA3 genes; thereby suppressing development of Th2 cells.
The nuclear receptor subfamily 4, group A, (Nr4a) gene family consist of Nr4a1, Nr4a2 as well as Nr4a3 receptors (Sekiya et al., 2013). Studies have shown that the Nr4a receptors play important roles in the development of T-reg cells by activating the master transcription factor Foxp3 (Bandukwala and Rao, 2013). We found that γT3 supplementation reduced methylation levels (P < 0.05) of the Nr4a gene in CD4+ T-cells in healthy mice compared to the vehicle-fed; suggesting that γT3 may support maintenance of immunological tolerance under normal circumstances through activation of the Nr4a gene.
The expression of the homeobox A10 (HOXA10) gene is down-regulated during T-cell maturation and development (Taghon et al., 2003). In tumour-induced mice, the was a marked (p < 0.05) increase in the methylation level of the Hoxa10 gene in their CD4+ T-cells when compared to normal mice. This suggest that expression of the Hoxa10 gene was reduced in tumour-laden mice. However, in γT3-fed mice with, reduced (p < 0.05) methylation level of the Hoxa10 gene was observed, which suggest that expression of this gene may be restored in these animals.
Increased (P < 0.05) levels of methylation was observed in the interferon-regulatory factor-4 (IRF4) gene from CD4+ T-lymphocytes isolated from tumour-induced mice fed with γT3 compared to vehicle-fed mice, which suggest that γT3 hypermethylated the IRF4 gene. The IRF4 gene is the master regulator of the CD4+ Th9, Th2, Th17 and T-follicular helper cells (Huber and Lohoff, 2014). The Th9 cells have an ambivalent role in the tumour microenvironment (Schmitt and Bopp, 2012).
Hypermethylation (P < 0.05) of the retinoic acid receptor-related orphan receptor alpha (RORα) gene was observed in CD4+ T-cells obtained from the tumour-laden mice fed with γT3 compared to vehicle-fed. The RORα gene is a regulator of Th17 differentiation (Luckheeram et al., 2012). Recent studies have found that the Th17 cells may play dual roles in a tumour microenvironment.
5. Conclusion
Supplementation of γT3 to tumour-inoculated mice twice a day for two-weeks before induction of BC showed significant (p < 0.05) reduction in tumour volume. Supplementation of γT3 to healthy mice caused significant change to DNA methylation patterns of three (GATA3, NR4A3 and TGIF1) genes compared to those fed with vehicle. However, in tumour-induced mice fed with γT3, there was significant change in DNA methylation patterns of the HOXA10, RORα as well as IRF4 genes. These findings suggest that γT3 supplementation can regulate the DNA methylation patterns of CD4+ T-lymphocytes, which in turn may have an impact on their ability to regulate induce anti-tumour immune responses in the tumour-induced animals and boost immunity in healthy mice.
Author contributions
AKR: conceptualised, designed and supervised the study; and wrote the manuscript; JSAR: a postgraduate student (MSc), performed the animal studies and the DNA methylation work; analysed data and prepared figures; SS helped to monitor the animals and supported laboratory work; PR was involved in data analysis of the DNA methylation studies and edited the manuscript;
Funding
This work was supported by grant from the Fundamental Research Grant Scheme (FRGS) awarded by the Ministry of Higher Education, Malaysia [Grant number [FRGS/1/2013/SG05/IMU/01/1].
CRediT authorship contribution statement
Ammu K. Radhakrishnan: Conceptualization, Supervision, Writing – original draft, designed and supervised the study; and wrote the manuscript. Jeya Seela Anandha Rao: Formal analysis, a postgraduate student (MSc), performed the animal studies and the DNA methylation work; analysed data and prepared figures. Shonia Subramaniam: helped to monitor the animals and supported laboratory work. Premdass Ramdas: Data curation, Formal analysis, was involved in data analysis of the DNA methylation studies and edited the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ammu Kutty Radhakrishnan reports financial support and equipment, drugs, or supplies were provided by Malaysia Ministry of Higher Education. The second author (Jeya Seela Anandha Rao) was employed as a graduate research assistant using funds from this grant. The findings from this project was used in her Master of Science thesis. The third author (Dr Shonia Subramaniam) was a PhD student who assisted the second author with the experimental work. She received a stipend from the Malaysian Palm Oil Board (MPOB); the MPOB was not part of this study, which is why they are not acknowledged in the study. The other authors are academic staff employed at the International Medical University (IMU) and have no conflict of interest to declare. The first author (Ammu Radhakrishnan) was also employed by the IMU as an academic staff at the time this study was conducted. She has since moved to another university.
Acknowledgements
The authors would like to thank the International Medical University for providing the research and animal holding facilities to conduct this study.
Footnotes
Footnote: This work was carried out at the International Medical University, which is cited as the main author's primary affiliation. The main author, who is also the corresponding author, has since moved to another institution, which is included as the last affiliation and is also reflected in the corresponding author information.
References
- Abraham A., Kattoor A.J., Saldeen T., Mehta J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019;59:2831–2838. doi: 10.1080/10408398.2018.1474169. [DOI] [PubMed] [Google Scholar]
- Aggarwal V., Kashyap D., Sak K., Tuli H.S., Jain A., Chaudhary A., Garg V.K., Sethi G., Yerer M.B. Molecular mechanisms of action of tocotrienols in cancer: recent trends and advancements. Int. J. Mol. Sci. 2019;20:656. doi: 10.3390/ijms20030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandukwala H.S., Rao A. Nurr'ishing T reg cells: Nr4a transcription factors control Foxp3 expression. Nat. Immunol. 2013;14(3):201–203. doi: 10.1038/ni.2546. https://www.nature.com/articles/ni.2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R.L. Origins of the TH 1-TH 2 model: a personal perspective. Nat. Immunol. 2006;7(6):539–541. doi: 10.1038/ni0606-539. https://www.nature.com/articles/ni0606-539 [DOI] [PubMed] [Google Scholar]
- Hafid S.R.A., Chakravarthi S., Nesaretnam K., Radhakrishnan A.K. Tocotrienol-adjuvanted dendritic cells inhibit tumor growth and metastasis: a murine model of breast cancer. PLoS One. 2013;8(9):e74753. doi: 10.1371/journal.pone.0074753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.G., Baylin S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. https://www-nejm-org.ezproxy.lib.monash.edu.au/doi/full/10.1056/NEJMra023075 [DOI] [PubMed] [Google Scholar]
- Huang Y., Wu R., Su Z.Y., Guo Y., Zheng X., Yang C.S., Kong A.N. A naturally occurring mixture of tocotrienols inhibits the growth of human prostate tumor, associated with epigenetic modifications of cyclin-dependent kinase inhibitors p21 and p27. JNB (J. Nutr. Biochem.) 2017;40:155–163. doi: 10.1016/j.jnutbio.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Huber M., Lohoff M. IRF4 at the crossroads of effector T‐cell fate decision. Eur. J. Immunol. 2014;44:1886–1895. doi: 10.1002/eji.201344279. [DOI] [PubMed] [Google Scholar]
- Lim U., Song M.A. Springer Science + Business Media, LLC; 2012. Dietary and Lifestyle Factors of DNA Methylation. Cancer Epigenetics, Chapter 23; pp. 359–376.https://link.springer.com/protocol/10.1007/978-1-61779-612-8_23 [DOI] [PubMed] [Google Scholar]
- Liu D., Shi J., Posada L.R., Kakuda Y., Xue S.J. Separating tocotrienols from palm oil by molecular distillation. Food Rev. Int. 2008;24:376–391. doi: 10.1080/87559120802303840. [DOI] [Google Scholar]
- Loganathan R., Selvaduray K.R., Nesaretnam K., Radhakrishnan A.K. Palm tocotrienols cause cleavage of poly-(adp)-ribose polymerase enzyme and down-regulation of cyclooxygenase-2 protein level in human breast cancer cells. J. Oil Palm Res. 2021;33(1):119–128. [Google Scholar]
- Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4+ T cells: differentiation and functions. Clin. Dev. Immunol. 2012:925135. doi: 10.1155/2012/925135. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam D., Radhakrishnan A.K., Amom Z., Ibrahim N., Nesaretnam K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 2011;65:63–69. doi: 10.1038/ejcn.2010.184. https://www.nature.com/articles/ejcn2010184 [DOI] [PubMed] [Google Scholar]
- Manu K.A., Shanmugam M.K., Ramachandran L., Li F., Fong C.W., Kumar A.P., Tan P., Sethi G. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin. Cancer Res. 2012;18:2220–2229. doi: 10.1158/1078-0432.CCR-11-2470. https://clincancerres.aacrjournals.org/content/18/8/2220.short [DOI] [PubMed] [Google Scholar]
- Meeran S.M., Ahmed A., Tollefsbol T.O. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin. Epigenet. 2010;1(3–4):101–116. doi: 10.1007/s13148-010-0011-5. https://link.springer.com/content/pdf/10.1007/s13148-010-0011-5.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani Marelli M., Marzagalli M., Fontana F., Raimondi M., Moretti R.M., Limonta P. Anticancer properties of tocotrienols: a review of cellular mechanisms and molecular targets. J. Cell. Physiol. 2019;234:1147–1164. doi: 10.1002/jcp.27075. [DOI] [PubMed] [Google Scholar]
- Moraes M.N., Zabot G.L., Meireles M.A. Extraction of tocotrienols from annatto seeds by a pseudo continuously operated SFE process integrated with low-pressure solvent extraction for bixin production. J. Supercrit. Fluids. 2015;96:262–271. doi: 10.1016/j.supflu.2014.09.007. [DOI] [Google Scholar]
- Morales-Nebreda L., McLafferty F.S., Singer B.D. DNA methylation as a transcriptional regulator of the immune system. Transl. Res. 2019;204:1–18. doi: 10.1016/j.trsl.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omilusik K.D., Goldrath A.W. Remembering to remember: T cell memory maintenance and plasticity. Curr. Opin. Immunol. 2019;58:89–97. doi: 10.1016/j.coi.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryk N., Bultmann S., Bartke T., Defossez P.A. Staying true to yourself: mechanisms of DNA methylation maintenance in mammals. Nucleic Acids Res. 2021;49(6):3020–3032. doi: 10.1093/nar/gkaa1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli E., Viola V., Barucca A., Orlando F., Galli F., Provinciali M. Effect of annatto-tocotrienols supplementation on the development of mammary tumors in HER-2/neu transgenic mice. Carcinogenesis. 2013;34:1352–1360. doi: 10.1093/carcin/bgt064. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A.K., Mahalingam D., Selvaduray K.R., Nesaretnam K. BioMed Research International; 2013. Supplementation with Natural Forms of Vitamin E Augments Antigen-specific TH1-type Immune Response to Tetanus Toxoid. Article ID 782067, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdas P., Radhakrishnan A.K., Abdu Sani, Abdul-Rahman P.S. Tocotrienols modulate breast cancer secretomes and affect cancer-signaling pathways in MDA-MB-231 cells: A label-free quantitative proteomic analysis. Nutr. Cancer. 2019;71(8):1263–1271. doi: 10.1080/01635581.2019.1607407. [DOI] [PubMed] [Google Scholar]
- Ramdas P., Radhakrishnan A.K., Abdu Sani A.A., Kumari M., Anandha Rao, Abdul-Rahman P.S. Advancing the role of gamma-tocotrienol as proteasomes inhibitor: A quantitative proteomic analysis of MDA-MB-231 human breast cancer cells. Biomolecules. 2020;10(1):19. doi: 10.3390/biom10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt E., Bopp T. Amazing IL-9: revealing a new function for an “old” cytokine. J. Clin. Invest. 2012;122:3857–3859. doi: 10.1172/JCI65929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Kashiwagi I., Yoshida R., Fukaya T., Morita R., Kimura A., Ichinose H., Metzger D., Chambon P., Yoshimura A. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013;14:230–237. doi: 10.1038/ni.2520. https://www.nature.com/articles/ni.2520 [DOI] [PubMed] [Google Scholar]
- Selvaduray K.R., Radhakrishnan A.K., Kutty M.K., Nesaretnam K. Palm tocotrienols inhibit proliferation of murine mammary cancer cells and induce expression of interleukin-24 mRNA. J. Interferon Cytokine Res. 2010;30:909–916. doi: 10.1089/jir.2010.0021. [DOI] [PubMed] [Google Scholar]
- Stefanska B., Karlic H., Varga F., Fabianowska‐Majewska K., Haslberger A.G. Epigenetic mechanisms in anti‐cancer actions of bioactive food components–the implications in cancer prevention. Br. J. Pharmacol. 2012;167:279–297. doi: 10.1111/j.1476-5381.2012.02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Alvarez B., Rodriguez R.M., Fraga M.F., López-Larrea C. DNA methylation: a promising landscape for immune system-related diseases. Trends Genet. 2012;28:506–514. doi: 10.1016/j.tig.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Anandha Rao J.S., Ramdas P., Ng M.H., Kannan Kutty M., Selvaduray K.R., Radhakrishnan A.K. Reduced infiltration of T‐regulatory cells in tumours from mice fed daily with gamma‐tocotrienol supplementation. Clin. Exp. Immunol. 2021 doi: 10.1111/cei.13650. (online print) May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghon T., Thys K., De Smedt M., Weerkamp F., Staal F.J.T., Plum J., Leclercq G. Homeobox gene expression profile in human hematopoietic multipotent stem cells and T-cell progenitors: implications for human T-cell development. Leukemia. 2003;17:1157–1163. doi: 10.1038/sj.leu.2402947. https://www.nature.com/articles/2402947 [DOI] [PubMed] [Google Scholar]
- Tao K., Fang M., Alroy J., Sahagian G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:1–19. doi: 10.1186/1471-2407-8-228. https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-8-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.Y. GATA3: a master of many trades in immune regulation. Trends Immunol. 2014;35(6):233–242. doi: 10.1016/j.it.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S., Radhakrishnan A.K. Tocotrienol research: past into present. Nutr. Rev. 2012;70:483–490. doi: 10.1111/j.1753-4887.2012.00512.x. [DOI] [PubMed] [Google Scholar]
- Yap W.N., Zaiden N., Luk S.Y., Lee D.T.W., Ling M.T., Wong Y.C., Yap Y.L. In vivo evidence of γ-tocotrienol as a chemosensitizer in the treatment of hormone-refractory prostate cancer. Pharmacology. 2010;85:248–258. doi: 10.1159/000278205. [DOI] [PubMed] [Google Scholar]
- Zhang N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Animal Nutrition. 2015;1:144–151. doi: 10.1016/j.beem.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]