Abstract
The short-chain fatty acids (SCFAs) are metabolites originated from the fermentation of dietary fibers and amino acids produced by the bacteria of the intestinal microbiota. The most abundant SCFAs, acetate, propionate, and butyrate, have been proposed as a treatment for inflammatory bowel diseases (IBDs) due to their anti-inflammatory properties. This work aimed to analyze the effects of the treatment of three combined SCFAs in TNBS-induced intestinal inflammation in zebrafish larvae. Here, we demonstrated that SCFAs significantly increased the survival of TNBS-exposed larvae, preserved the intestinal endocytic function, reduced the expression of inflammatory cytokines and the intestinal recruitment of neutrophils caused by TNBS. However, SCFAs treatment did not appear to avoid TNBS-induced tissue damage in the intestinal wall and did not restore the number of mucus-producing goblet cells. Finally, exposure to TNBS induced dysbiosis of the microbiota with an increase in Betaproteobacteria and Actinobacteria, while the treatment with SCFAs maintained these population levels similar to control. Thus, we demonstrate that the treatment of three combined SCFAs presented anti-inflammatory properties previously seen in mammals, opening an opportunity to use zebrafish to explore the potential benefit of these and other metabolites to treat inflammation.
Keywords: Intestinal inflammation, TNBS, Short-chain fatty acids, Microbiota, Larval zebrafish
Graphical abstract
Highlights
-
•
Short-chain fatty acids reduce intestinal inflammation in larval zebrafish.
-
•
Survival is increased but intestinal tissue damage is not restored.
-
•
Microbiota dysbiosis caused by intestinal inflammation is similar to mammals.
-
•
Short-chain fatty acids are capable to regulate dysbiosis.
-
•
Zebrafish is an excellent model to study the effect of microbiota metabolites.
1. Introduction
Short-chain fatty acids (SCFAs) are metabolites produced by gut microbiota as a result of anaerobic fermentation of dietary amino acids and fiber (den Besten et al., 2013). SCFAs are organic acids from one to six carbons of which the most abundant in the human intestine are acetate (C2), propionate (C3), and butyrate (C4), present in concentrations between 50 and 200 mM (Louis et al., 2007; Louis and Flint, 2017). Butyrate is mainly used as an energy source by colonocytes, while propionate and acetate are absorbed and taken up by the liver to participate in gluconeogenesis and the metabolism of cholesterol, fatty acids, and glutamine/glutamate, respectively (den Besten et al., 2013). SCFAs have been extensively related to host health, metabolism, and regulation of inflammation (Tan et al., 2014). In intestinal epithelial cells (IECs) SCFAs have increased the expression of antimicrobial peptides and regulated the production of IL-18, a cytokine related to epithelial integrity, as well as an increase of mucin production and expression of tight junctions (Raqib et al., 2006; Peng et al., 2009; Kelly et al., 2015). SCFAs effects on immune cells include the regulation of recruitment, effector function, and survival of neutrophils (Rodrigues et al., 2016), modulation of migration, production of cytokines and prostaglandin E2 (PGE2) on monocytes and macrophages (Cox et al., 2009; Maa et al., 2010; Liu et al., 2012; Vinolo et al., 2012b), inhibition of maturation and expression of activation markers and cytokines in dendritic cells (DCs) (Millard et al., 2002; Liu et al., 2012) that had led to a decreased capacity of stimulating T-cells (Liu et al., 2012). SCFAs also modulate T-cells activation and effector response, inducing T-regulatory (Treg) cells indirectly, through the effect on DCs and macrophages, and directly on the generation of extra-thymic Treg Foxp3+ cells through histone deacetylases (HDACs) inhibition (Singh et al., 2010, 2014; Arpaia et al., 2013; Furusawa et al., 2013; Gurav et al., 2015).
The therapeutic potential of SCFAs has been of great interest to the clinic as their immunomodulatory effects could be used for the treatment of inflammatory diseases, in particular inflammatory bowel diseases (IBDs) (Parada Venegas et al., 2019). IBDs comprise a series of chronic and recurrent inflammatory conditions of the intestine, including Ulcerative Colitis (UC) and Crohn's Disease (CD) (Ko and Auyeung, 2014). IBDs are multifactorial diseases influenced by genetics, immunological, microbial, and environmental factors (Ko and Auyeung, 2014). Dysbiosis is one of the main characteristics present in IBDs, creating an inflammatory environment in the gastrointestinal tract (Joossens et al., 2011; Nishida et al., 2018). Interestingly, IBD patients have reduced concentrations of SCFAs in the intestine and a lower abundance of SCFAs-producing bacteria compared with healthy individuals (Wang et al., 2014; Takahashi et al., 2016; Zhuang et al., 2019). Nowadays, researchers have taken advantage of genetic and chemically-induced IBD models to study different aspects of these diseases (Kiesler et al., 2015; Mizoguchi et al., 2020). Common induced murine models of IBD include the administration of chemicals on the drinking water of the animals such as dextran sodium sulfate (DSS) (Eichele and Kharbanda, 2017) or by intrarectal administration of oxazolone or 2,4,6-trinitrobenzene sulfonic acid (TNBS) (Antoniou et al., 2016; Weigmann and Neurath, 2016). The SCFAs have been used as a treatment in murine models of colitis and patients with IBDs, with different outcomes (Tominaga et al., 2018; Parada Venegas et al., 2019). For example, treatment with butyrate alone reduced mucosal damage and expression of inflammatory cytokines in the intestine of mice with DSS-induced colitis (Ji et al., 2016), however, this was not effective in mice treated by antibiotics (Chang et al., 2014). Another study showed that butyrate alone was less efficient in reducing inflammation in TNBS-induced colitis mice when compared to the treatment with the supernatant of/or live Faecalibacterium prausnitzii, a known SCFA-producing bacterium (Sokol et al., 2008). This demonstrates that the concentration of SCFAs, and the duration and route of administration of the treatment, as well as the type of inflammation during colitis/IBDs, can influence the outcome of the treatment. Extensive details about the effects of SCFAs in animal models and patients can be found in the literature (Vinolo et al., 2012a; den Besten et al., 2013; Corrêa-Oliveira et al., 2016), however little is known about the effect of SCFAs in non-mammalian systems.
To better understand the complexity of IBD and find effective treatments, it is crucial to conduct robust reliable studies, preferably reproducible in different pre-clinical animal models. Thus, we choose an emerging model in the immunology field, the zebrafish (Danio rerio) to investigate the role of SCFAs as a treatment for intestinal inflammation. This small tropical fish has become a versatile model in scientific research because of its characteristics such as size, high fecundity, and fast development, which make it an optimal model for visualization techniques and drug testing (Bradford et al., 2017). Additionally, the external fertilization, the availability of a sequenced genome and the fact that the zebrafish shares 70% of orthologous genes with humans, make it a great model for genetic manipulation (Howe et al., 2013). Moreover, the zebrafish presents a differentiated innate and adaptive immune system, the former being functional at 48 h post-fertilization (hpf) while the latter is mature around four to six weeks after fertilization (Trede et al., 2004). Zebrafish presents most of the immune cells seen in mammals with conserved developmental processes (Willett et al., 1997; Palis and Yoder, 2001; Trede et al., 2004; Lam et al., 2002; Langenau et al., 2004; Paik and Zon, 2010; Page et al., 2013). These data demonstrate that the zebrafish is an excellent model to study immune-mediated diseases.
The zebrafish are stomachless animals, as the intestine is directly connected to the esophagus (Wallace et al., 2005). The zebrafish intestine is a compartmentalized tubular structure with three regions: the bulb or anterior intestine, the mid-intestine and the posterior intestine. It is formed by columnar absorptive enterocytes, goblet cells, and enteroendrocrine cells, but lacks Paneth cells (Wallace et al., 2005). This organ is completely functional around 5 days post-fertilization (dpf) and shares gastrointestinal functions analogous to mammals in the liver, pancreas, and gallbladder (Wallace et al., 2005; Brugman, 2016). Nowadays, different models of genetic and chemically-induced intestinal inflammation have already been validated in zebrafish and showed parallel inflammatory features with human IBD (Morales Fénero et al., 2016; Uyttebroek et al., 2020). Induced protocols in larvae have the advantage of applying chemicals directly on the water avoiding the invasiveness of the mouse models (Morales Fénero et al., 2016). The most recognized model of intestinal inflammation in zebrafish is induced by TNBS, which has demonstrated dose-dependent effects related to the presence of the microbiota (Fleming et al., 2010; Oehlers et al., 2011b; He et al., 2013). In this sense, zebrafish have also been important for the study of the microbiota and its interactions with the host. The development of germ-free (GF) animals has allowed the discovery of the microbiota influence on intestinal development, immune cell development, and metabolism (Rawls et al., 2004; Bates et al., 2006; Milligan-Myhre et al., 2011; Melancon et al., 2017; Stagaman et al., 2020). Although there is a difference between the abundance of gut bacteria populations in humans and zebrafish, the main phyla are shared: Proteobacteria, Bacteroidetes, and Firmicutes (Roeselers et al., 2011). The production of SCFAs has been detected in the intestines of adult zebrafish, and recently, researchers have shown that the microbiota of this teleost is capable of producing acetate, butyrate, and propionate ex vivo (Zhang et al., 2019; Cholan et al., 2020).
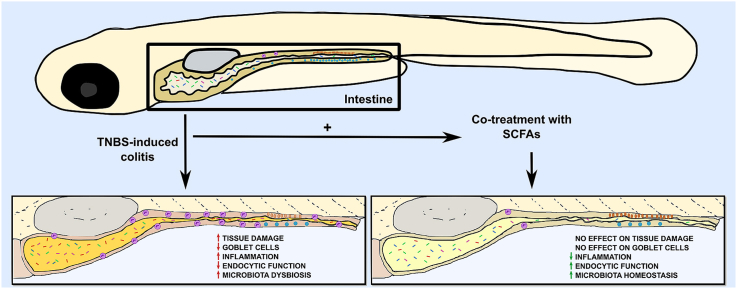
To our knowledge, no other study has shown that SCFAs can be used as a treatment for intestinal inflammation in zebrafish, although an anti-inflammatory effect of butyrate has been reported in a model of tail-fin cut (Cholan et al., 2020). Hence, here we aim to investigate the effect of three SCFAs: acetate, butyrate, and propionate, on a zebrafish intestinal inflammatory model induced by TNBS. Our results showed that using a combination of SCFAs ameliorated the inflammation, maintained intestinal function, and increased the survival of TNBS-exposed larvae but did not recover mucus production, goblet cell numbers nor prevent tissue damage. Besides this, SCFAs were capable of restoring TNBS-induced dysbiosis. Thus, we concluded that the anti-inflammatory properties of SCFAs seen in mammalian models are conserved in the zebrafish although the exact mechanisms of action need further investigation.
2. Materials and methods
2.1. Ethics statement
All procedures were submitted and approved by the Ethics Committee for the Use of Animals of the Institute of Biomedical Sciences of the University of São Paulo (nº: 119/2013 and 9351280818), following the legislation of the regulation of the use of animals in teaching and scientific research in Brazil. All procedures involving euthanasia were performed between 8 and 10 dpf with an overdose (0.3 mg/mL) of MS-222 (E10521, Sigma-Aldrich) and placement of the tube containing the larvae on ice for 20 min (Wilson et al., 2009; Matthews and Varga, 2012).
2.2. Animal housing
Zebrafish (Danio rerio) larvae from AB and Tg(lysC:DsRed2)nz50 strains were obtained by natural spawn, collected in 1x E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, pH 7.2) and kept in Petri dishes at 28 °C incubator with 14/10 dark-light cycle with daily medium changes until 3 dpf (Westerfield, 2007). Fishes were provided by the Zebrafish Facility of the Institute of Bioscience (IB) at the University of São Paulo (USP). Experiments were performed at the Institute of Biomedical Sciences (ICB) – USP.
2.3. SCFAs preparation
Stock solutions of 1M of Acetic acid (71251, Supelco), Propionic acid (402907, Sigma-Aldrich), and Butyric acid (B103500, Sigma-Aldrich) were prepared in 40 mL of sterile 1X PBS. Then, adjusted to pH 7.4, filter-sterilized, and stored at −20 °C in 1 mL aliquots in Eppendorf tubes until use.
2.4. SCFAs treatment
Larvae were kept in groups of 100 in Petri dishes with 40 mL of 1X E3 medium from spawning/fertilization day until day 3. On day 3 post-fertilization groups of 20 larvae were randomly separated into 6 well plates with 10 mL of liquid. Controls were transferred to 6 well plates with new 1X E3. Mixtures of 3, 6, and 9 mM SCFAs were prepared from the combination of 1M acetic acid, 1M propionic acid, and 1M butyric acid, diluted in 1X E3 medium. Then, 10 mL of this mixture was added to the wells, at least in triplicates.
2.5. TNBS-induced inflammation
A 2,4,6-trinitrobenzene sulfonic acid (TNBS) solution (P2297, Sigma-Aldrich) was prepared directly from the stock the day of the experiment, using concentrations of 50–75-100 μg/mL. The total quantity of TNBS to be used was prepared in 1X E3 medium and 10 mL of this solution was added to 20 larvae at 7 dpf, at least in triplicates, and maintained until the day of analysis.
2.6. Histology
At 10 dpf, larvae were euthanized as mentioned in 2.1., collected in groups of 10 in Eppendorf tubes, and fixed in 4% paraformaldehyde (PFA) overnight at 4 °C. The next day, fixed larvae were washed 3 times with 1X PBS and immersed in 2% agarose until solid. Blocks of agarose were processed for histology and embedded in paraffin. Paraffin blocks were cut in sections of 5 μm thickness and stained with Hematoxylin and Eosin. Pictures were taken in the microscope Nikon Eclipse Ti–S and analyzed using ImageJ.
2.7. Neutral red staining
At 9 dpf, Neutral red solution was added to the medium of live larvae to a final concentration of 5 μg/mL (Stock solution 2.5 mg/mL) and incubated overnight at 28 °C. The next day, the excess dye was washed with repeated changes of 1X E3 medium. Then, larvae were anesthetized with 0.168 mg/mL MS-222 (Matthews and Varga, 2012) and mounted in 1% agarose covered with anesthetic solution. Images were taken on ZEISS AxioZoom.V16 microscope and the stained area was delimited manually using the "Color Threshold" selection option from ImageJ in a double-blind analysis. Larvae were euthanized after the procedure.
2.8. Alcian blue staining
At 10 dpf, larvae were euthanized, collected in groups of 10 in Eppendorf tubes, and fixed in 4% PFA overnight at 4 °C. The next day, fixed larvae were washed with acid ethanol (1% HCl/ 70% ethanol), incubated in slow agitation in Alcian blue solution (0.1% Alcian blue, 80% ethanol, and 20% glacial acetic acid) for 3 h at room temperature. Then, stained larvae were washed repeatedly with acid ethanol until all blue background was washed away. Next, stained larvae were washed and transferred to 1X PBS to image taking on ZEISS AxioZoom.V16 microscope. Goblet cells were counted manually using the "cell counter" plug-in from ImageJ in a double-blind analysis.
2.9. Cytokine quantification
The relative expression of genes related to inflammation was assessed using qPCR. At 8, 9 and 10 dpf (24, 48 and 72 h after TNBS 50 μg/mL exposure, respectively), groups of 20 larvae were euthanized with an overdose of Tricaine (0.3 mg/mL) and collected in Precellys® tubes. Then, the liquid was discarded and total RNA extraction was performed by TRIzol™ method (Rio et al., 2010). The total RNA was quantified, and 2 μg was used to synthesize cDNA using the M-MLV enzyme. Samples were diluted at 1:10. Primers were obtain by online available TaqManTMGene Expression Assay (FAM) sequences (ThermoFisher Scientific): il1b (Dr03114371_m1), tnfa (Dr03126850_m1), il6 (Dr03431399_m1) and eef1a1 1 (Dr03432748_m1). qPCR was perform using TaqMan™ Gene Expression Master Mix (Applied Biosystems) in a QuantStudio 12k Flex PCR System (Applied Biosystems). Data analysis was made using 2(−ΔΔCT) formula, using eef1a1 1 as the reference gene and normalizing by the mean of the controls. Outliers were excluded before statistical analyses were performed.
2.10. Neutrophil visualization and quantification
For this experiment, the Tg(lysC:DsRed2)nz50 strain was used (Hall et al., 2007). This strain has red fluorescent protein labeling the lysozyme promoter, which is expressed mainly in neutrophils. At 10 dpf, larvae were euthanized as in 2.1., oriented to the side over a glass slide and photographed using the ZEISS AxioZoom.V16 microscope. Next, the intestine was separated from the body using micro dissecting needles and the fluorescent cells were directly counted.
2.11. Microbiota phylum analysis
At 10 dpf, larvae were euthanized, as previously described (2.1) and the intestines were dissected using micro dissecting needles. For DNA extraction of the microbiota, pools of 40 intestines were placed in Eppendorf tubes with Extraction Buffer (10 mM Tris pH 8.0, 10 mM EDTA, 200 mM NaCl, 0.5% SDS, 200 μg/mL Proteinase K) and incubated at 55 °C for 3 h with intermittent vortex mixing. Then, an equal volume of Phenol: Chloroform (1:1) was added, vortexed for 2 min, and centrifuged. The upper phase was recovered in a new tube with 3M sodium acetate and isopropanol, and incubated overnight at −20 °C. The next day, the samples were centrifuged and washed with 70% ethanol, air-dried, and suspended in 20 μL of nuclease-free water. DNA was quantified, diluted and 10 ng were used to perform the qPCR reaction. Phylum-specific primers were used to identify bacteria groups (Table 1). qPCR was perform using Power SYBR® Green Master Mix (ThermoFisher Scientific) in a QuantStudio 12k Flex PCR System (Applied Biosystems). Data analysis was made using relative expression formula, normalized by primer efficiency, using Total Bacteria (16S) as the reference gene (Pfaffl, 2001).
Table 1.
Bacteria phylum-specific primers.
| Phylum | Primer Sequences | Reference | |
|---|---|---|---|
| Total Bacteria (16S) | Forward Reverse |
TGGCTCAGGACGAACGCTGGCGGC CCTACTGCTGCCTCCCGTAGGAGT |
Gómez-Hurtado et al. (2011) |
| Fusobacteria | Forward Reverse |
CGAGGAACCTTACCAGCGTT ATCTCACGACACGAGCTGAC |
This study |
| Firmicutes | Forward Reverse |
CAGCAGTAGGGAATCTTC ACCTACGTATTACCGCGG |
Pfeiffer et al. (2014) |
| Bacteroidetes | Forward Reverse |
CRAACAGGATTAGATACCCT GGTAAGGTTCCTCGCGTAT |
Bacchetti De Gregoris et al. (2011) |
| Alphaproteobacteria | Forward Reverse |
CIAGTGTAGAGGTGAAATT CCCCGTCAATTCCTTTGAGTT |
Bacchetti De Gregoris et al. (2011) |
| Betaproteobacteria | Forward Reverse |
CGAAAAACCTTACCTACC GTATGACGTGTGAAGCC |
Pfeiffer et al. (2014) |
| Gammaproteobacteria | Forward Reverse |
CCATGCCGCGTGTGTGAA ACTCCCCAGGCGGTCAACTTA |
Mühling et al. (2008) |
| Actinobacteria | Forward Reverse |
GAGACTGCCGGGGTCAACT TCTGCGATTACTAGCGAC |
Pfeiffer et al. (2014) |
2.12. Statistical analysis
Graphical data are presented with mean and standard deviation. Survival curves were analyzed by Log-rank (Mantel-Cox) test. The difference between the experimental groups was determined by One-way ANOVA with Tukey's multiple comparisons and Two-way ANOVA with Tukey's multiple comparisons when more than one time point were evaluated. Values of p considered significant were expressed as: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. All graphs and statistical analysis were performed using the GraphPad PRISM® 7 program.
3. Results
3.1. Administration of SCFAs increased survival of TNBS-exposed larvae
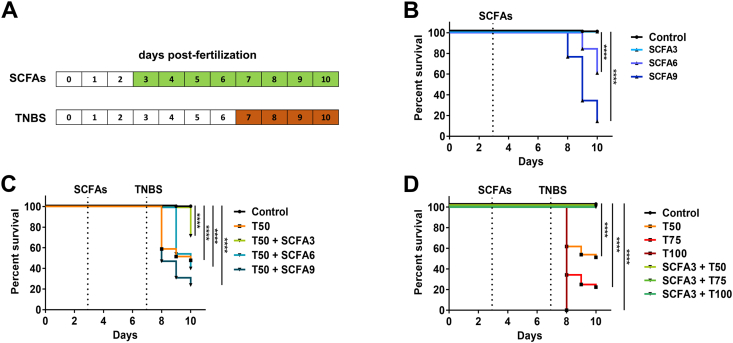
SCFAs are bacterial products from fermentation with known immunomodulatory effects. Reports have shown that the concentrations of SCFAs in the human large intestine range between 50 and 200 mM (Louis and Flint, 2017). However, at the beginning of this study, there were no reports of known physiological concentrations of SCFAs in the intestine of the zebrafish, furthermore, previous experiments in our laboratory showed human physiological concentrations (50–200 mM) to be toxic for zebrafish survival (data not shown). Thus, we tested a low-dose combination of the three main intestinal SCFAs on the water of larvae from day 3 post-fertilization to day 10 (Fig. 1A - Green). Initially, acetate, propionate, and butyrate were combined and administered in three different concentrations: 3, 6, and 9 mM (from now on SCFA3/6/9). A survival analysis of these animals showed a decrease in survival from day 8 on SCFAs with a concentration of 6 and 9 mM but not at 3 mM, which remained alive until the end of the experiment (Fig. 1B).
Fig. 1.
SCFAs increase survival in TNBS-exposed larvae. A. Experimental design of SCFAs and TNBS treatment. Larvae were treated with SCFAs from 3 to 10 dpf with a change of medium at day 7. TNBS was added to the medium at 7 dpf and kept until 10 dpf. B. Percent survival of larvae exposed to SCFAs at 3 mM (SCFA3), 6 mM (SCFA6) and 9 mM (SCFA9), from 3 to 10 dpf. Log-rank (Mantel-Cox), ****p < 0.0001 compared to control. N = 90–130. C. Percent survival of larvae exposed to TNBS 50 μg/mL (T50) from 7 to 10 dpf and treated with SCFA 3, 6 and 9 mM from 3 to 10 dpf. Log-rank (Mantel-Cox), ****p < 0.0001 compared to control. N = 100–136. D. Percent survival of larvae exposed to TNBS 50 μg/mL (T50), 75 μg/mL (T75) and 100 μg/mL (T100) from 7 to 10 dpf and treated with SCFA 3 mM from 3 to 10 dpf. Log-rank (Mantel-Cox), ****p < 0.0001 compared to control. N = 70–80.
TNBS has been used by other groups as an efficient inducer of intestinal inflammation in zebrafish (Fleming et al., 2010; Oehlers et al., 2011b; He et al., 2013). Based on this we tested the effect of 50 μg/mL TNBS (from now on T50) from day 7–10 (Fig. 1A - Orange) and compared the survival of animals with a treatment of SCFAs (Fig. 1C). T50 alone induced a decrease in survival 24 h after application, with total mortality of 52.2% at the end of 10 days (Fig. 1C - Orange). Interestingly, the treatment with SCFA9 worsened the survival in comparison with T50 alone (Fig. 1C – Dark Green). SCFA6 had little effect on T50 animal survival, delaying the larval mortality by one day (Fig. 1C – Medium Green). However, SCFA3 had a protective effect on T50 survival, decreasing death to 28.3% at the end of day 10 (Fig. 1C – Light Green). Thus, we selected the dose of SCFA3 as treatment for TNBS-induced colitis. To further assess the hypothesis that SCFA3 was protective, we tested increased concentrations of TNBS in treatment with SCFA3. The concentrations of 50, 75, and 100 μg/mL (T50, T75, and T100) induced death of 38.2%, 65.8%, and 100%, respectively, of the total larvae 24 h after application (Fig. 1D). SCFA3 treatment was capable of maintaining survival in all TNBS concentrations until day 10 (Fig. 1D). This demonstrates the capacity of SCFAs to maintain the survival of animals subjected to lethal levels of TNBS.
3.2. Treatment with SCFAs did not ameliorate TNBS-induced histological changes
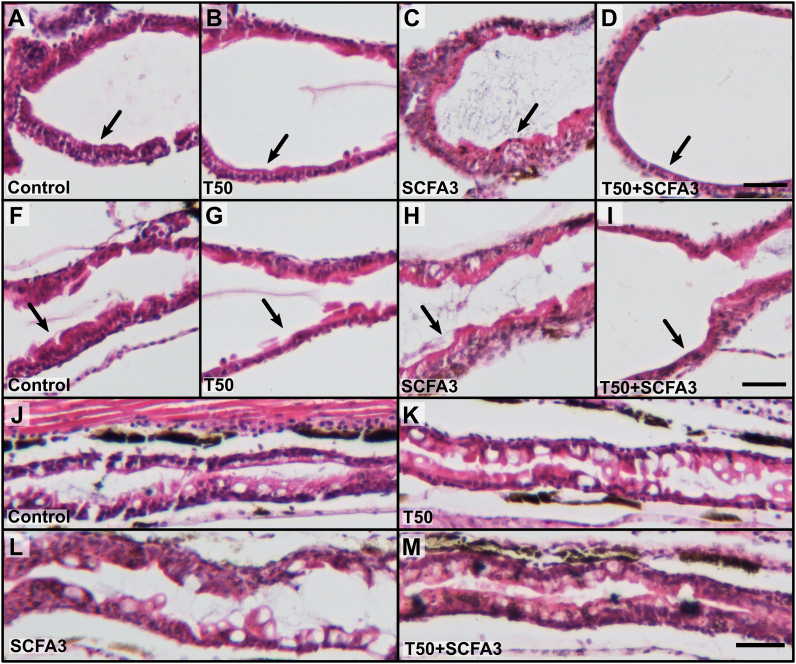
In murine models, TNBS is usually administrated intrarectally, having a direct effect on the intestine (Antoniou et al., 2016). However, in zebrafish larvae this chemical is applied to the water, thus having the chance to induce global effects. As our main focus was to investigate the effect of TNBS in the intestine, we analyzed the histology of this organ in animals exposed to TNBS as well as the effect of SCFAs treatment at 10 dpf. The analysis of the intestinal segments of T50-exposed larvae showed an increase of the lumen in the anterior region and a decrease in the thickness of the intestinal wall on the bulb (Fig. 2B, black arrow) and at the junction of anterior and mid-intestine (Fig. 2G, black arrow) in comparison to the controls (Fig. 2A and F). This thinning of the intestinal wall was not seen in SCFA3-treated animals (Fig. 2C and H). However, treatment of T50 with SCFA3 did not avoid the erosive effects caused by TNBS on the intestinal wall (Fig. 2D and I, black arrows). Analysis of the posterior segment of T50-exposed animals (Fig. 2K) did not show any apparent morphological changes compared to controls (Fig. 2J). This was also consistent in larvae administered with SCFA3 alone or T50 treated with SCFA3 (Figs. 2L and M, respectively). These results demonstrate that the TNBS is affecting mainly the first portion of the intestine and although SCFA3 increase the survival of T50 larvae its effects do not seem to reconstitute the intestinal wall from T50 damage.
Fig. 2.
SCFAs do not restore TNBS-induced erosion damage. Representative histology images stained with Hematoxylin and Eosin from the intestine of the zebrafish, focusing on the bulb (A–D), anterior and mid-intestine junction (F–I), and posterior intestine (J–M) from larvae of 10 dpf. Rostral to the left and dorsal up. Black arrow showing thickness of the intestinal wall. Scale bar 500 μm. N = 10.
3.3. SCFAs maintained endocytic intestinal function during TNBS-exposure
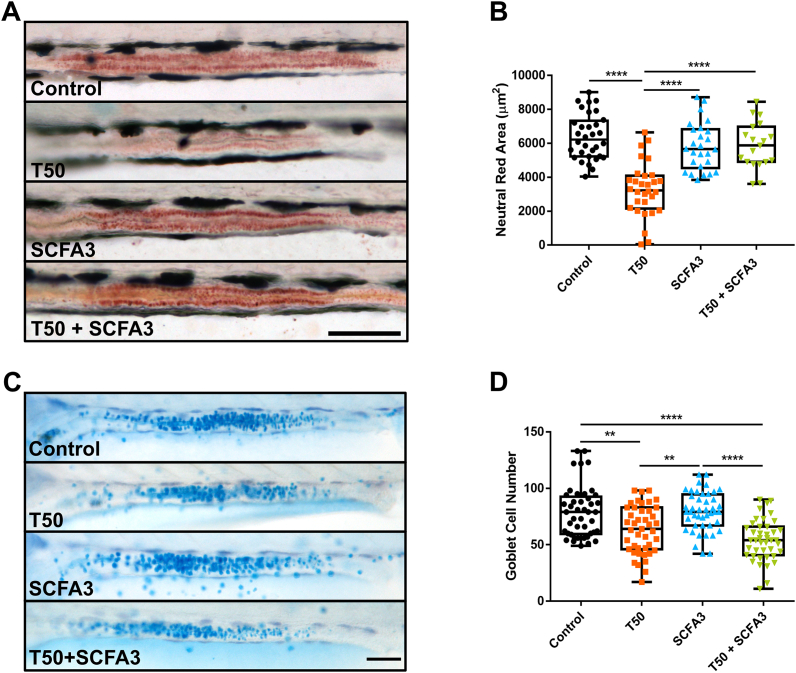
After verifying that TNBS was effectively causing damage in the intestine but that SCFA treatment did not restore the tissue damage caused on the intestinal wall, we decided to analyze other parameters of intestinal function. The mid-intestine of the zebrafish presents a highly endocytic epithelium with lysosomes-rich enterocytes (LREs) (Oehlers et al., 2011b; Park et al., 2019). We used a Neutral Red uptake assay as a way of estimate the viability and the endocytic capacity of these cells by incorporation of the dye on lysosomes (Repetto et al., 2008). Thus, in control animals, we observed an extended portion of the red dye captured by IECs (Fig. 3A, Control). The intensity and extension of the dye were decreased in TNBS-exposed animals (Fig. 3A, T50) and were recovered in a treatment with SCFA3 (Fig. 3A, T50 + SCFA3). As expected the administration of SCFA3 alone did not affect the capacity of the intestine of capturing the dye (Fig. 3A, SCFA3). This data was confirmed by the quantification of the area of neutral red staining (Fig. 3B), demonstrating that SCFAs maintain the endocytic capacity of intestinal epithelial cells even in presence of a colitis inductor.
Fig. 3.
SCFAs restore endocytic function but not mucus production. A. Representative images of the mid-intestine of larvae at 10 dpf exposed to Neutral Red vital staining. Rostral to the left and dorsal up. Scale bar 100 μm. B. Graph representing the area of neutral red staining in the mid-intestine of 10 dpf larvae. One-way ANOVA with multiple comparisons. ****p < 0.0001. N = 19–32. C. Representative images of the mid-intestine of larvae at 10 dpf stained with Alcian Blue. Rostral to the left and dorsal up. Scale bar 100 μm. D. Graph representing the number of goblet cells stained by Alcian Blue at the mid-intestine of 10 dpf larvae. One-way ANOVA with Tukey's multiple comparisons. **p < 0.01; ****p < 0.0001. N = 42–45. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. SCFAs did not recover the goblet cell number affected by TNBS
One of the protective mechanisms of SCFAs is to increase the production of mucus in the intestine through the expression of MUC genes (Gaudier et al., 2004). To analyze the number of intestinal goblet cells we performed a whole larva staining with the acid mucin dye Alcian Blue. In the zebrafish, goblet cells are spread through the intestine but a major accumulation of these cells is present in the mid-intestine (Wallace et al., 2005; Chen et al., 2012). Considering this anatomic characteristic, we focused our examination of goblet cells on the midgut section. Visualization of the intestine did not show any apparent difference in mucus production between the different groups (Fig. 3C). Nonetheless, the exposure to T50 significantly decreased the number of goblet cells in larvae compared with untreated controls (Fig. 3D). The treatment with SCFA3 alone did not have any effect on goblet cell number compared with the control, and the treatment with SCFA3 in T50 larvae did not show recovery of the number of these cells (Fig. 3D). These results indicate that the regeneration of goblet cells is not part of SCFAs protective mechanisms.
3.5. SCFAs reduced inflammation in TNBS-exposed larvae
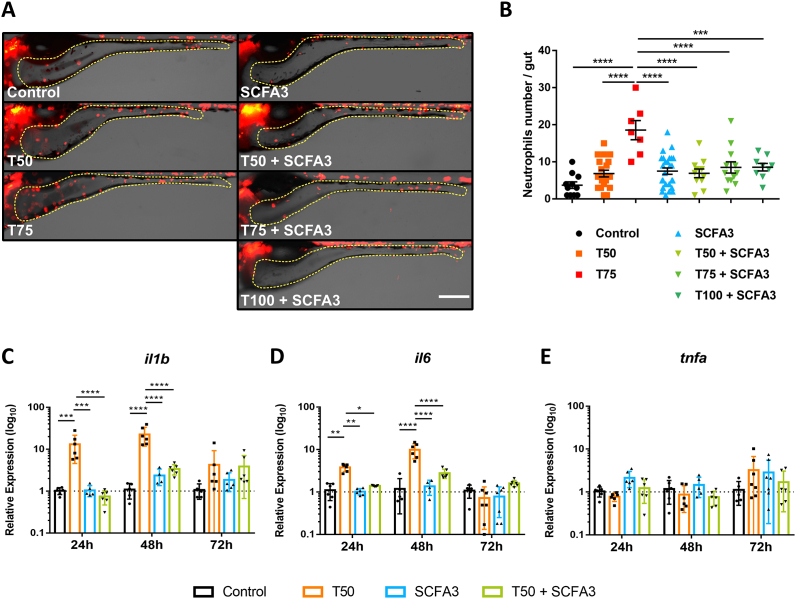
After analyzing the impact of SCFAs treatment on morphology and physiology of the intestine in TNBS-exposed larvae, we decided to study the immunomodulatory effects of SCFAs in this model. SCFAs are known to act as a chemoattractant to neutrophils in vitro but induce different responses depending on the inflammatory context in vivo, either increasing the recruitment or reducing the migration to the inflammatory site (Vinolo et al., 2009, 2011a, 2011b, 2011c). Thus, to verify the effect of SCFAs on intestinal inflammation, we observed the neutrophils present in the intestine of Tg(lysC:DsRed2)nz50 larvae after 72h of TNBS exposure and treated with SCFAs (Fig. 4A and B). The direct visualization of fluorescent neutrophils in the intestine was apparently increasing with T50 and T75 (Fig. 4A). T100 exposed larvae were not able to survive the experiment. Yet, the treatment with SCFA3 seemed to reduce the infiltration of neutrophils in the gut, even in the T100 + SCFA3 group (Fig. 4A). To corroborate this, we performed the quantification of neutrophils in the whole gut. Although T50 had a slight increase in neutrophil infiltration, this was not significant, however, the exposure to T75 was able to increase the infiltration of neutrophils in the whole intestine (Fig. 4B). The treatment with SCFA3 was able to keep the inflammation at control levels in increasing concentrations of TNBS including T50, T75, and T100 (Fig. 4B). SCFA3 alone did not affect the number of neutrophils in the gut.
Fig. 4.
SCFAs decrease TNBS-induced inflammation. A. Representative images of the intestinal area (yellow dotted line) of Tg(lysC:DsRed2)nz50 transgenic larvae after 72h (10 dpf) of 50 μg/mL (T50) and 75 μg/mL (T75) TNBS exposure and treated with 3 mM of SCFAs (SCFA3). Rostral to the left and dorsal up. Scale bar 200 μm. B. Graphical representation of the number of lysC: DsRed + cells (neutrophils) in the intestine of 10 dpf larvae. One-way ANOVA with Tukey's multiple comparisons. ****p < 0.0001. N = 7–26. C-E. Relative expression of il1b(C), il6(D), and tnfa(E), in whole larvae after 24, 48 and 72h of TNBS 50 μg/mL exposure and treated with SCFA 3 mM. Two-way ANOVA with Tukey's multiple comparisons. *p < 0.05; **p < 0.01; ***p < 0.001; ***p < 0.0001. N = 4–7. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Another effect of SCFAs on human and mouse cells is the modulation of the production of cytokines (Blais et al., 2007; Park et al., 2007; Tedelind et al., 2007; Cox et al., 2009; Vinolo et al., 2011b, 2011c). To know whether the expression of cytokines is modulated by SCFAs after TNBS exposure, we analyzed the kinetics of whole-body expression of the cytokines il1b, il6 and tnfa. At 24h after T50 exposure (8 dpf) we observed a significant increase in il1b and il6, but no difference was found on tnfa expression (Fig. 4C–E, 24h). The same was seen at 48h (9 dpf), where the expression of il1b and il6 was even higher in T50 group compared to 24h, while tnfa was not affected (Fig. 4C-E, 48h). In both cases, SCFA3 was able to reduce the expression of il1b and il6 (Fig. 4C and D, 24h). At 72h after T50 exposure (10 dpf) there were not evident changes in any of the cytokines, although il1b showed a tendency to increase that was not significant (Fig. 4C-E, 72h). The treatment with SCFAs also did not significantly modify the expression of any cytokine (Fig. 4C-E, 72h). Interestingly, tnfa did not show changes in expression in any of the time points analyzed. These results demonstrate that SCFAs can modulate inflammation in the zebrafish, properties already seen in mammals.
3.6. The microbiota dysbiosis caused by TNBS is recovered by SCFAs treatment
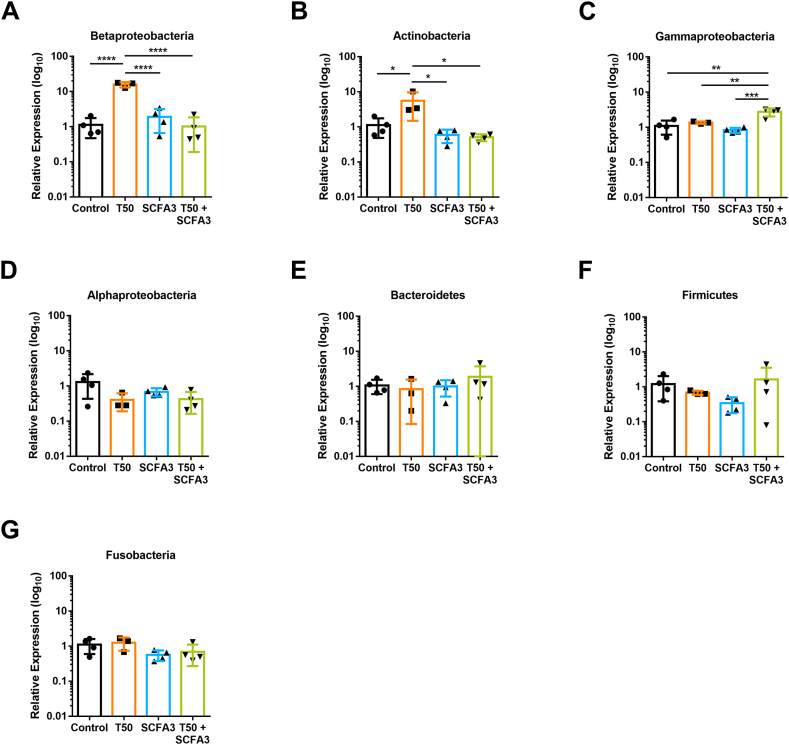
SCFAs are known for maintaining the homeostasis of the intestine, including the homeostasis of the microbiota populations. Thus, using specific phylum-primers of the 16S rRNA we analyzed the different groups that compose the microbiota of the zebrafish. The exposure to T50 significantly increased the population of Betaproteobacteria (Fig. 5A) and Actinobacteria (Fig. 5B), which was maintained comparable to control levels with the SCFA3 treatment. Curiously, the combination of T50 and SCFA3 induced an increase in the population of Gammaproteobacteria (Fig. 5C), which was not observed on controls or T50 and SCFA3 isolated exposition. The phyla of Alphaproteobacteria, Bacteroidetes, Firmicutes, and Fusobacteria did not show any significant variations (Fig. 5D–G). These results demonstrate that the changes in the microbiota populations caused by TNBS are reversible by the treatment with SCFAs.
Fig. 5.
Microbiota changes induced by TNBS and SCFAs-exposed animals. Relative expression of 16S rRNA phylum-specific genes representatives of Betaproteobacteria (A), Actinobacteria (B), Gammaproteobacteria (C), Alphaproteobacteria (D), Bacteroidetes (E), Firmicutes (F), and Fusobacteria (G), from 10 dpf larvae. One-way ANOVA with Tukey's multiple comparisons, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. N = 3–4.
4. Discussion
Intestinal inflammation studies in zebrafish have shown they share similar disease features with human IBDs (Brugman, 2016; Morales Fénero et al., 2016; Hanyang et al., 2017). For instance, mutations in NODs genes, which encode intracellular bacterial recognition receptors, have been related to a higher risk for the development of UC (NOD1) and CD (NOD2) (Mukherjee et al., 2019). In zebrafish, nod1 and nod2 genes are expressed in IECs and neutrophils and have been related to defense against infection and inflammation (Oehlers et al., 2011a). Moreover, zebrafish knockout studies involving the adaptor molecule myeloid differentiation primary response 88 (MyD88) have shown the importance of this molecule in the response against bacterial infections and for the expression of immune-related transcription factors, such as NFκB and AP-1 (Stockhammer et al., 2009; van der Vaart et al., 2013; Warner and Núñez, 2013). IBDs in zebrafish have not only been studied in a genetic approach but also have been modeled using chemicals such as TNBS, DSS, and oxazolone (Brugman et al., 2009; Fleming et al., 2010; Oehlers et al., 2011b, 2013; Geiger et al., 2013; He et al., 2013). In these models, signs of disease are displayed in different morphological features that appear within hours to days, affecting the epithelial integrity, the thickness of the intestinal epithelial wall, the conformation of intestinal folds, and the number of goblet cells, in different proportions. However, all of them induce an inflammatory reaction with immune cells infiltrating the intestine and high expression of pro-inflammatory cytokines, such as IL-1β and TNFα (Brugman et al., 2009; Fleming et al., 2010; Oehlers et al., 2011b, 2013; Geiger et al., 2013; He et al., 2013). Moreover, zebrafish intestinal inflammation models have shown a strong microbiota influence, as antibiotic treatment animals have decreased inflammation and increased survival (Brugman et al., 2009; Oehlers et al., 2011a, 2012). This correlates with the microbiota dysbiosis seen in IBD patients (Mirsepasi-Lauridsen et al., 2018). Thus, we decided to focus on microbiota-derived metabolites with anti-inflammatory properties to treat intestinal inflammation in zebrafish.
Recently, Cholan (2020) analyzed the microbiota of zebrafish using an ex vivo culture approach and demonstrated the production of acetate, propionate, and butyrate by the gut commensals of this teleost (Cholan et al., 2020). Other authors have managed to measure the production of SCFAs in adult zebrafish intestines (Ma et al., 2020). However, there are no reports of the production of SCFAs in the zebrafish larvae. In this work, we used a combination of three SCFAs as a treatment in a TNBS-induced intestinal inflammation model in zebrafish larvae. To our knowledge, this is the first time that these microbiota metabolites are used to treat this inflammation model in zebrafish. Our results demonstrated that low doses of SCFAs (3 mM) are enough to increase the survival of larvae immersed in TNBS concentrations of 50, 75, and 100 μg/mL. However, concentrations of SCFAs higher than 3 mM caused mortality in larvae, probably due to toxic effects but acidification of the medium could be another possibility, as SCFAs have shown to decrease intestinal luminal pH (den Besten et al., 2013). Cholan (2020) also tested acetate, propionate, or butyrate in the water of zebrafish larvae in an isolated treatment with a concentration 10 times higher (30 mM) and in a shorter period (6 h) (Cholan et al., 2020). The survival of animals at this concentration was not mentioned in the study as the aim was to analyze the anti-inflammatory effects of SCFAs in a fin tail cut model (Cholan et al., 2020). This indicates that the concentration of SCFAs diluted in zebrafish medium can have different effects depending on the time of exposure, however, the bases for future research can be established by Cholan's and this study.
Next, we analyzed the morphology of the intestine under TNBS-induced inflammation and SCFAs treatment. Studies have shown that TNBS aggravates enterocolitis score in a dose-dependent way in zebrafish, with features that include epithelial disruption, expansion of the lumen, loss of villi and clefts reduction (Fleming et al., 2010; Oehlers et al., 2011b; He et al., 2013). These changes are usually observed in the anterior region (bulb) of the gut. In this study, was observed loss of the thickness of the epithelial wall and an increase of the lumen in the anterior region. However, there was no recovery of the erosion damage caused by TNBS with the SCFAs treatment. This has also been reported in rats with TNBS-induced colitis, where the treatment with SCFAs enemas did not decrease the intestinal damage (Tarrerias et al., 2002), and in diversion colitis patients, where the use of SCFA enemas did not change endoscopic and histologic lesions (Guillemot et al., 1991). This could mean that SCFAs have limiting effects on the regeneration of the intestinal tissue.
The posterior mid-intestine of the zebrafish gut has been described to have a highly endocytic epithelium with lysosome-rich enterocytes (LREs) (Oehlers et al., 2011b; Chuang et al., 2019; Park et al., 2019). Neutral red staining is captured by lysosomes inside the cell indicating healthy organelles. Hence, the decrease in Neutral red staining indicates low endocytic capacity and unhealthy LREs (Chuang et al., 2019; Park et al., 2019). We showed that TNBS exposure induced a loss of Neutral red staining in the mid-intestinal region indicating a decrease in endocytic function. This phenomenon was also observed by Oehlers (2011b) when describing the TNBS-induced colitis model (Oehlers et al., 2011b). SCFAs treatment avoided the loss of neutral red staining seen in TNBS-exposed larvae, demonstrating the capacity of these metabolites to maintain intestinal endocytic function, however, the mechanisms involved in this protection need further investigation. On the other hand, the analysis of goblet cells at 10 dpf with an exposure of 50 μg/mL TNBS showed a decrease in the number of these cells. In this sense, other studies have shown different results: Fleming (2010) observed an increase in goblet cell number at 8 dpf with a TNBS exposure of 75 μg/mL for 6 days (Fleming et al., 2010). He (2013) also observed an increase of these cells at 6 and 8 dpf with 25, 50, and 75 μg/mL TNBS, and at 4 dpf with 75 μg/mL TNBS only, applied from day 3 (He et al., 2013). However, Oehlers (2011b) tested these same concentrations and did not find any changes in goblet cell numbers (Oehlers et al., 2011b). One possible explanation for this is that goblet cells could be releasing their mucus content upon proinflammatory stimulation so the Alcian blue staining is not staying inside the cells, decreasing the appearance of visible goblet cells (Dartt and Masli, 2014; Grondin et al., 2020). Another explanation could be that goblet cells differentiated around 100 hpf (~4 dpf), so these differences could be attributed to the stage of larval development when the TNBS exposure occurs (Ng et al., 2005; Wallace et al., 2005). Thus, exposure of goblet cells to TNBS during an early differentiation stage, such as 3 dpf (Fleming et al., 2010; Oehlers et al., 2011b; He et al., 2013), might lead to a different outcome than when they are completely differentiated (7 dpf, this study). Nonetheless, the treatment with SCFAs did not change the mucus production nor recover goblet cell numbers in TNBS-exposed larvae. Although SCFAs, especially butyrate, have been demonstrated to induce MUC-2 expression and increase the production of mucus in the intestine, there are no reports of induction of new goblet cells (Shimotoyodome et al., 2000; Willemsen et al., 2003; Blaak et al., 2020).
One of the most important features of the SCFAs treatment is the modulation of the immune system. Studies in humans and mice have demonstrated the effects of SCFAs in neutrophils and macrophages migration, regulation of the production of chemokines and cytokines, changes in the production of reactive oxygen species (ROS), modulation of phagocytic capacity, and response and activation of lymphocytes (Vinolo et al., 2011c; Corrêa-Oliveira et al., 2016). Reports in teleosts using SCFAs as food additives have shown improvement in growth, digestibility, survival rate, immune responses, resistance to diseases, and enhanced intestinal mucosal immunity (Safari et al., 2016; Hoseinifar et al., 2017; Tran et al., 2020). However, there is currently one report in zebrafish testing the SCFAs in an inflammation model. In order to study the effects of SCFAs on a zebrafish intestinal inflammation model, we analyzed the number of neutrophils in the intestine after 72h of TNBS exposure. We demonstrated that the SCFAs treatment reduced the recruitment of neutrophils to this organ, an anti-inflammatory property reported in zebrafish in a tail fin cut model, where they observed a decrease in neutrophil migration in the larvae treated with butyrate (Cholan et al., 2020). Other studies have demonstrated that the effect of SCFAs in immune cells varies depending on the experimental context. The administration of tributyrin, a pro-drug of butyrate, reduced the recruitment of neutrophils to the peritoneum after the injection of a glycogen solution (Vinolo et al., 2011b). In another study by the same authors, the migration of neutrophils was increased when SCFAs were injected in a sterile air pouch in rats (Vinolo et al., 2009).
In vitro experiments with mouse neutrophils demonstrated that SCFAs increase the chemotaxis of these cells through activation of the G-protein coupled receptor (GPR) 43 and increased expression of L-selectin (Vinolo et al., 2009, 2011a). SCFAs are sensed by GPR43 and GPR41, also known as free fatty acid receptor (FFAR)-2 and −3, respectively, as well as by GPR109a and the Olfactory receptor 78 (Olfr78), expressed in the intestinal epithelium and immune cells (Sina et al., 2009; Thangaraju et al., 2009; Pluznick, 2014; Nastasi et al., 2015; Agus et al., 2016; Ang et al., 2016). In zebrafish, there are no studies describing GPR43, GPR41, GPR109a, or Olfr78 as SCFAs receptors, although orthologs of the three first receptors are present in the zebrafish genome (EMBL-EBI, 2021a, b, c). Different studies have described GPR81 (hcar1) as lactate and butyrate receptor in the zebrafish, with high expression in neutrophils and the gut (Kuei et al., 2011; Athanasiadis et al., 2017; Rougeot et al., 2019; Cholan et al., 2020). Moreover, the effect of butyrate on decreasing neutrophil migration after a tail fin cut was inhibited in hcar1-depleted embryos but did not affect the effect of butyrate in macrophages and TNFα expression (Cholan et al., 2020).
To further analyze the capacity of SCFAs to modulate the immune response we performed kinetics of cytokine expression analyzing at 24, 48 and 72 h after TNBS exposure. The expression of il1b and il6 followed a pattern where T50 induced their increase at 24h and a higher expression at 48h, but then this expression was decreased at 72h to levels not significantly different compared with the control group. Interestingly, studies in mammals have associated the IL-6 expression to be induced by IL-1β, through the activation of NF-κB transcription factor or a pathway involving PI3K/AKT/IKKαT23 upstream of AP-1 (Tosato and Jones, 1990; Libermann and Baltimore, 1990; Cahill and Rogers, 2008). This was also seen in other teleost fish but the mechanisms are still not known (Varela et al., 2012). In both cases, the increased expression of il1b and il6 induced by TNBS was decreased by SCFAs treatment, a phenomenon also seen in mammals (Blais et al., 2007; Vinolo et al., 2011c). However, we did not observe differences in gene expression of tnfa in any time point, while other groups have described increased protein expression using TNBS after 2–5 days of exposure (Fleming et al., 2010; Oehlers et al., 2011b; He et al., 2013). This could mean we are not seeing differences in the transcript because the rate of protein transcription is too high or the transcripts are released earlier than the time points we measured, as has been demonstrated by other immunological challenges (Oehlers et al., 2010). In any case, SCFAs did also not change the expression of tnfa. These results demonstrate the capacity of SCFAs to modulate inflammation in the zebrafish.
Finally, TNBS exposure increased the presence of Proteobacteria and Actinobacteria phyla. He (2013) observed an increase in the Proteobacteria population and a decrease of Firmicutes in TNBS-induced colitis in zebrafish (He et al., 2013). Moreover, mouse models of colitis and patients with IBD, have also shown an increase in these two bacteria phyla, demonstrating the correlation and conservation that these groups have in the development and maintenance of inflammatory bowel diseases (Gophna et al., 2006; Frank et al., 2007; Carvalho et al., 2012; Maharshak et al., 2013; Selvanantham et al., 2016; Rizzatti et al., 2017). Interestingly, the treatment with SCFA3 was enough to decrease Proteobacteria and Actinobacteria markers to control levels. However, the combination of TNBS with SCFAs increased the phylum of Gammaproteobacteria. Although this phylum is characteristic of patients with Crohn's disease and mouse models of TNBS, and it has known members that are pathogens, such as Legionellales, Pseudomonadacea, Aeromonadales, and Vibrionales, many of the main members of the zebrafish commensal microbiota belongs to this group (Sokol and Seksik, 2010; Berman, 2012; Stephens et al., 2016; Bauer and Thiele, 2018; Wiles et al., 2018; Busbee et al., 2020). Moreover, gamma-proteobacteria are the most abundant class of commensals on zebrafish larvae and as they live in an aquatic system we probably would see some differences in affected populations in comparison to terrestrial mammals (Stephens et al., 2016). Nonetheless, we are aware that qPCR is not the best method to measure these differences and that sequencing techniques can address our questions in a better setting. Overall, it is difficult to deduce the real effect of microbiome modulation in this model once we are not seeing the effect of SCFAs without the microbiota (gnotobiotic model). Thus, the effect of SCFAs could be happening directly on host cells through the activation of a SCFA receptor, such as HCAR1, which has been already shown to be present in the zebrafish and activated by butyrate (Kuei et al., 2011; Cholan et al., 2020). A second option is the action of SFCAs as inhibitors of HDACs (Singh et al., 2010, 2014; Arpaia et al., 2013; Furusawa et al., 2013; Gurav et al., 2015), increasing epithelial barrier function. On the other side, an indirect effect could be mediated by unknown microbiota by-products or microbiota competition driven by the low pH in presence of SCFAs (Louis and Flint, 2009). Nonetheless, the results presented here demonstrate that SCFAs have effects that counteract the dysbiotic changes induced by TNBS in zebrafish, which are in some cases shared with mammals. We do not expect these changes to be exactly the same as in humans because zebrafish live in an aquatic environment and present different proportions of the microbiota populations. Therefore, we think is important to continue this work with a deeper analysis of the microbiome at the genus and species level, and also include the evaluation of changes induced by SCFAs in gnotobiotic larvae.
5. Conclusions
In this study, we demonstrated for the first time that treatment with SCFAs in a model of induced intestinal inflammation in zebrafish is effective. We confirmed that SCFAs have positive effects on survival, immune cells and cytokines regulation, and conservation of the epithelial integrity related to endocytic function. Importantly, SCFA modulated the microbiota of TNBS-exposed larvae, maintaining a balance of bacteria similar to healthy fish. A more in-depth study of the mechanisms involved in this protection by SCFA could clarify whether the effects of these metabolites are shared between zebrafish and mammals.
Funding
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) Process Numbers: 2012/02270-2; 2013/07467-1; 2015/21644-9; 2017/05264-7, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), financial code 001.
CRediT authorship contribution statement
Camila Morales Fénero: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Mariana Abrantes Amaral: Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Izabella Karina Xavier: Validation, Investigation. Barbara Nunes Padovani: Validation, Investigation, Writing – review & editing. Lais Cavalieri Paredes: Validation, Investigation, Writing – review & editing. Tatiana Takiishi: Validation, Investigation. Mônica Lopes-Ferreira: Resources, Funding acquisition. Carla Lima: Resources, Funding acquisition. Alicia Colombo: Resources. Niels Olsen Saraiva Câmara: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank our collaborators from the Zebrafish Facility at the Immunoregulation Unit of the Laboratory of Applied Toxinology (CeTICs) at the Butantan Institute; to the Laboratory of Maria Rita dos Santos e Passos-Bueno and the Zebrafish Facility of the Genetics and Evolutionary Biology Department at the Bioscience Institute of the University of São Paulo. We also thank Prof. Dr. Alicia Colombo Flores for managing the acquisition and donation of Tg(lysC:DsRed2)nz50 strain originally from Phil Crosier Lab at the University of Auckland. We kindly thank the Laboratory of Prof. Dr. Clarice Fujihara for histological processing.
Contributor Information
Camila Morales Fénero, Email: ci.moralesfe@alumni.usp.br.
Niels Olsen Saraiva Câmara, Email: niels@icb.usp.br.
References
- Agus A., Denizot J., Thévenot J., Martinez-Medina M., Massier S., Sauvanet P., Bernalier-Donadille A., Denis S., Hofman P., Bonnet R., Billard E., Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016;6:19032. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang Z., Er J.Z., Tan N.S., Lu J., Liou Y.C., Grosse J., Ding J.L. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci. Rep. 2016;6:34145. doi: 10.1038/srep34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou E., Margonis G.A., Angelou A., Pikouli A., Argiri P., Karavokyros I., Papalois A., Pikoulis E. The TNBS-induced colitis animal model: an overview. Ann. Med. Surg. (Lond). 2016;11:9–15. doi: 10.1016/j.amsu.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., Rudensky A.Y. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiadis E.I., Botthof J.G., Andres H., Ferreira L., Lio P., Cvejic A. Single-cell RNA-sequencing uncovers transcriptional states and fate decisions in haematopoiesis. Nat. Commun. 2017;8:2045. doi: 10.1038/s41467-017-02305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti De Gregoris T., Aldred N., Clare A.S., Burgess J.G. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J. Microbiol. Methods. 2011;86:351–356. doi: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Bates J.M., Mittge E., Kuhlman J., Baden K.N., Cheesman S.E., Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 2006;297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Bauer E., Thiele I. From metagenomic data to personalized in silico microbiotas: predicting dietary supplements for Crohn's disease. NPJ Syst. Biol. Appl. 2018;4:27. doi: 10.1038/s41540-018-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.J. Taxonomic Guide to Infectious Diseases. Elsevier BV; 2012. Gamma proteobacteria; pp. 37–47. [Google Scholar]
- Blaak E.E., Canfora E.E., Theis S., Frost G., Groen A.K., Mithieux G., Nauta A., Scott K., Stahl B., van Harsselaar J., van Tol R., Vaughan E.E., Verbeke K. Short chain fatty acids in human gut and metabolic health. Benef. Microbes. 2020;11:411–455. doi: 10.3920/BM2020.0057. [DOI] [PubMed] [Google Scholar]
- Blais M., Seidman E.G., Asselin C. Dual effect of butyrate on IL-1beta--mediated intestinal epithelial cell inflammatory response. DNA Cell Biol. 2007;26:133–147. doi: 10.1089/dna.2006.0532. [DOI] [PubMed] [Google Scholar]
- Bradford Y.M., Toro S., Ramachandran S., Ruzicka L., Howe D.G., Eagle A., Kalita P., Martin R., Taylor Moxon S.A., Schaper K., Westerfield M. Zebrafish models of human disease: gaining insight into human disease at ZFIN. ILAR J. 2017;58:4–16. doi: 10.1093/ilar/ilw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S., Liu K.Y., Lindenbergh-Kortleve D., Samsom J.N., Furuta G.T., Renshaw S.A., Willemsen R., Nieuwenhuis E.E. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology. 2009;137:1757–1767. doi: 10.1053/j.gastro.2009.07.069. e1. [DOI] [PubMed] [Google Scholar]
- Brugman S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Busbee P.B., Menzel L., Alrafas H.R., Dopkins N., Becker W., Miranda K., Tang C., Chatterjee S., Singh U., Nagarkatti M., Nagarkatti P.S. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. JCI Insight. 2020;5 doi: 10.1172/jci.insight.127551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill C.M., Rogers J.T. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho F.A., Koren O., Goodrich J.K., Johansson M.E., Nalbantoglu I., Aitken J.D., Su Y., Chassaing B., Walters W.A., González A., Clemente J.C., Cullender T.C., Barnich N., Darfeuille-Michaud A., Vijay-Kumar M., Knight R., Ley R.E., Gewirtz A.T. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Lu Y.F., Li I.C., Hwang S.P. Zebrafish Agr2 is required for terminal differentiation of intestinal goblet cells. PloS One. 2012;7 doi: 10.1371/journal.pone.0034408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholan P.M., Han A., Woodie B.R., Watchon M., Kurz A.R., Laird A.S., Britton W.J., Ye L., Holmes Z.C., McCann J.R., David L.A., Rawls J.F., Oehlers S.H. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microb. 2020;12:1–11. doi: 10.1080/19490976.2020.1824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang L.S., Morrison J., Hsu N.Y., Labrias P.R., Nayar S., Chen E., Villaverde N., Facey J.A., Boschetti G., Giri M., Castillo-Martin M., Thin T.H., Sharma Y., Chu J., Cho J.H. Zebrafish modeling of intestinal injury, bacterial exposures and medications defines epithelial in vivo responses relevant to human inflammatory bowel disease. Dis. Model. Mech. 2019;12 doi: 10.1242/dmm.037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa-Oliveira R., Fachi J.L., Vieira A., Sato F.T., Vinolo M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunology. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M.A., Jackson J., Stanton M., Rojas-Triana A., Bober L., Laverty M., Yang X., Zhu F., Liu J., Wang S., Monsma F., Vassileva G., Maguire M., Gustafson E., Bayne M., Chou C.C., Lundell D., Jenh C.H. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009;15:5549–5557. doi: 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt D.A., Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2014;14:464–470. doi: 10.1097/ACI.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele D.D., Kharbanda K.K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017;23:6016–6029. doi: 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMBL-EBI Gene: CU855821.1 ENSDARG00000079661. 2021. http://www.ensembl.org/Danio_rerio/Gene/Summary?g=ENSDARG00000079661 accessed February 18.
- EMBL-EBI Gene: hcar1-3 ENSDARG00000062874. 2021. http://www.ensembl.org/Danio_rerio/Gene/Summary?g=ENSDARG00000062874 accessed February 18.
- EMBL-EBI Gene: si:dkey-211g8.6 ENSDARG00000063088. 2021. http://www.ensembl.org/Danio_rerio/Gene/Summary?g=ENSDARG00000063088 accessed February 18.
- Fleming A., Jankowski J., Goldsmith P. In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: a feasibility study. Inflamm. Bowel Dis. 2010;16:1162–1172. doi: 10.1002/ibd.21200. [DOI] [PubMed] [Google Scholar]
- Frank D.N., St Amand A.L., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N.N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J.M., Topping D.L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gaudier E., Jarry A., Blottière H.M., de Coppet P., Buisine M.P., Aubert J.P., Laboisse C., Cherbut C., Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- Geiger B.M., Gras-Miralles B., Ziogas D.C., Karagiannis A.K., Zhen A., Fraenkel P., Kokkotou E. Intestinal upregulation of melanin-concentrating hormone in TNBS-induced enterocolitis in adult zebrafish. PloS One. 2013;8 doi: 10.1371/journal.pone.0083194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gophna U., Sommerfeld K., Gophna S., Doolittle W.F., Veldhuyzen van Zanten S.J. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J. Clin. Microbiol. 2006;44:4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin J.A., Kwon Y.H., Far P.M., Haq S., Khan W.I. Mucins in intestinal mucosal defense and inflammation: learning from clinical and experimental studies. Front. Immunol. 2020;11:2054. doi: 10.3389/fimmu.2020.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F., Colombel J.F., Neut C., Verplanck N., Lecomte M., Romond C., Paris J.C., Cortot A. Treatment of diversion colitis by short-chain fatty acids. Prospective and double-blind study. Dis. Colon Rectum. 1991;34:861–864. doi: 10.1007/BF02049697. [DOI] [PubMed] [Google Scholar]
- Gurav A., Sivaprakasam S., Bhutia Y.D., Boettger T., Singh N., Ganapathy V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J. 2015;469:267–278. doi: 10.1042/BJ20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Hurtado I., Santacruz A., Peiró G., Zapater P., Gutiérrez A., Pérez-Mateo M., Sanz Y., Francés R. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PloS One. 2011;6 doi: 10.1371/journal.pone.0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Flores M.V., Storm T., Crosier K., Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyang L., Xuanzhe L., Xuyang C., Yujia Q., Jiarong F., Jun S., Zhihua R. Application of zebrafish models in inflammatory bowel disease. Front. Immunol. 2017;8:501. doi: 10.3389/fimmu.2017.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Wang L., Wang F., Wang C., Tang C., Li Q., Li J., Zhao Q. Microbial fingerprinting detects intestinal microbiota dysbiosis in Zebrafish models with chemically-induced enterocolitis. BMC Microbiol. 2013;13:289. doi: 10.1186/1471-2180-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseinifar S.H., Safari R., Dadar M. Dietary sodium propionate affects mucosal immune parameters, growth and appetite related genes expression: insights from zebrafish model. Gen. Comp. Endocrinol. 2017;243:78–83. doi: 10.1016/j.ygcen.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L., McLaren S., Sealy I., Caccamo M., Churcher C., Scott C., Barrett J.C., Koch R., Rauch G.J., White S., Chow W., Kilian B., Quintais L.T., Guerra-Assunção J.A., Zhou Y., Gu Y., Yen J., Vogel J.H., Eyre T., Redmond S., Banerjee R., Chi J., Fu B., Langley E., Maguire S.F., Laird G.K., Lloyd D., Kenyon E., Donaldson S., Sehra H., Almeida-King J., Loveland J., Trevanion S., Jones M., Quail M., Willey D., Hunt A., Burton J., Sims S., McLay K., Plumb B., Davis J., Clee C., Oliver K., Clark R., Riddle C., Elliot D., Eliott D., Threadgold G., Harden G., Ware D., Begum S., Mortimore B., Mortimer B., Kerry G., Heath P., Phillimore B., Tracey A., Corby N., Dunn M., Johnson C., Wood J., Clark S., Pelan S., Griffiths G., Smith M., Glithero R., Howden P., Barker N., Lloyd C., Stevens C., Harley J., Holt K., Panagiotidis G., Lovell J., Beasley H., Henderson C., Gordon D., Auger K., Wright D., Collins J., Raisen C., Dyer L., Leung K., Robertson L., Ambridge K., Leongamornlert D., McGuire S., Gilderthorp R., Griffiths C., Manthravadi D., Nichol S., Barker G., Whitehead S., Kay M., Brown J., Murnane C., Gray E., Humphries M., Sycamore N., Barker D., Saunders D., Wallis J., Babbage A., Hammond S., Mashreghi-Mohammadi M., Barr L., Martin S., Wray P., Ellington A., Matthews N., Ellwood M., Woodmansey R., Clark G., Cooper J., Tromans A., Grafham D., Skuce C., Pandian R., Andrews R., Harrison E., Kimberley A., Garnett J., Fosker N., Hall R., Garner P., Kelly D., Bird C., Palmer S., Gehring I., Berger A., Dooley C.M., Ersan-Ürün Z., Eser C., Geiger H., Geisler M., Karotki L., Kirn A., Konantz J., Konantz M., Oberländer M., Rudolph-Geiger S., Teucke M., Lanz C., Raddatz G., Osoegawa K., Zhu B., Rapp A., Widaa S., Langford C., Yang F., Schuster S.C., Carter N.P., Harrow J., Ning Z., Herrero J., Searle S.M., Enright A., Geisler R., Plasterk R.H., Lee C., Westerfield M., de Jong P.J., Zon L.I., Postlethwait J.H., Nüsslein-Volhard C., Hubbard T.J., Roest Crollius H., Rogers J., Stemple D.L. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Shu D., Zheng M., Wang J., Luo C., Wang Y., Guo F., Zou X., Lv X., Li Y., Liu T., Qu H. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016;6:24838. doi: 10.1038/srep24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joossens M., Huys G., Cnockaert M., De Preter V., Verbeke K., Rutgeerts P., Vandamme P., Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A., Weir T.L., Ehrentraut S.F., Pickel C., Kuhn K.A., Lanis J.M., Nguyen V., Taylor C.T., Colgan S.P. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler P., Fuss I.J., Strober W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.K., Auyeung K.K. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr. Pharmaceut. Des. 2014;20:1082–1096. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]
- Kuei C., Yu J., Zhu J., Wu J., Zhang L., Shih A., Mirzadegan T., Lovenberg T., Liu C. Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol. Pharmacol. 2011;80:848–858. doi: 10.1124/mol.111.074500. [DOI] [PubMed] [Google Scholar]
- Lam S.H., Chua H.L., Gong Z., Wen Z., Lam T.J., Sin Y.M. Morphologic transformation of the thymus in developing zebrafish. Dev. Dynam. 2002;225:87–94. doi: 10.1002/dvdy.10127. [DOI] [PubMed] [Google Scholar]
- Langenau D.M., Ferrando A.A., Traver D., Kutok J.L., Hezel J.P., Kanki J.P., Zon L.I., Look A.T., Trede N.S. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327-2334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li L., Min J., Wang J., Wu H., Zeng Y., Chen S., Chu Z. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell. Immunol. 2012;277:66–73. doi: 10.1016/j.cellimm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Louis P., Scott K.P., Duncan S.H., Flint H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- Ma C., Guo H., Chang H., Huang S., Jiang S., Huo D., Zhang J., Zhu X. The effects of exopolysaccharides and exopolysaccharide-producing Lactobacillus on the intestinal microbiome of zebrafish (Danio rerio) BMC Microbiol. 2020;20:300. doi: 10.1186/s12866-020-01990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa M.C., Chang M.Y., Hsieh M.Y., Chen Y.J., Yang C.J., Chen Z.C., Li Y.K., Yen C.K., Wu R.R., Leu T.H. Butyrate reduced lipopolysaccharide-mediated macrophage migration by suppression of Src enhancement and focal adhesion kinase activity. J. Nutr. Biochem. 2010;21:1186–1192. doi: 10.1016/j.jnutbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Maharshak N., Packey C.D., Ellermann M., Manick S., Siddle J.P., Huh E.Y., Plevy S., Sartor R.B., Carroll I.M. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microb. 2013;4:316–324. doi: 10.4161/gmic.25486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M., Varga Z.M. Anesthesia and euthanasia in zebrafish. ILAR J. 2012;53:192–204. doi: 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- Melancon E., Gomez De La Torre Canny S., Sichel S., Kelly M., Wiles T.J., Rawls J.F., Eisen J.S., Guillemin K. Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods Cell Biol. 2017;138:61–100. doi: 10.1016/bs.mcb.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard A.L., Mertes P.M., Ittelet D., Villard F., Jeannesson P., Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin. Exp. Immunol. 2002;130:245–255. doi: 10.1046/j.0009-9104.2002.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan-Myhre K., Charette J.R., Phennicie R.T., Stephens W.Z., Rawls J.F., Guillemin K., Kim C.H. Study of host-microbe interactions in zebrafish. Methods Cell Biol. 2011;105:87–116. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsepasi-Lauridsen H.C., Vrankx K., Engberg J., Friis-Møller A., Brynskov J., Nordgaard-Lassen I., Petersen A.M., Krogfelt K.A. Disease-specific enteric microbiome dysbiosis in inflammatory bowel disease. Front. Med. 2018;5:304. doi: 10.3389/fmed.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi E., Low D., Ezaki Y., Okada T. Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models. Int. Res. 2020;18:151–167. doi: 10.5217/ir.2019.09154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales Fénero C.I., Colombo Flores A.A., Câmara N.O.S. Inflammatory diseases modelling in zebrafish. World J. Exp. Med. 2016;6:9–20. doi: 10.5493/wjem.v6.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T., Hovingh E.S., Foerster E.G., Abdel-Nour M., Philpott D.J., Girardin S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019;670:69–81. doi: 10.1016/j.abb.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Mühling M., Woolven-Allen J., Murrell J.C., Joint I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2008;2:379–392. doi: 10.1038/ismej.2007.97. [DOI] [PubMed] [Google Scholar]
- Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., Biagi E., Andersen M.H., Brigidi P., Ødum N., Litman T., Woetmann A. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A.N., de Jong-Curtain T.A., Mawdsley D.J., White S.J., Shin J., Appel B., Dong P.D., Stainier D.Y., Heath J.K. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 2005;286:114–135. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Nishida A., Inoue R., Inatomi O., Bamba S., Naito Y., Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- Oehlers S.H., Flores M.V., Hall C.J., O'Toole R., Swift S., Crosier K.E., Crosier P.S. Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev. Comp. Immunol. 2010;34:352–359. doi: 10.1016/j.dci.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Oehlers S.H., Flores M.V., Hall C.J., Swift S., Crosier K.E., Crosier P.S. The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis. Model. Mech. 2011;4:832–841. doi: 10.1242/dmm.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers S.H., Flores M.V., Okuda K.S., Hall C.J., Crosier K.E., Crosier P.S. A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev. Dynam. 2011;240:288–298. doi: 10.1002/dvdy.22519. [DOI] [PubMed] [Google Scholar]
- Oehlers S.H., Flores M.V., Hall C.J., Crosier K.E., Crosier P.S. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis. Dis. Model. Mech. 2012;5:457–467. doi: 10.1242/dmm.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers S.H., Flores M.V., Hall C.J., Okuda K.S., Sison J.O., Crosier K.E., Crosier P.S. Chemically induced intestinal damage models in zebrafish larvae. Zebrafish. 2013;10:184–193. doi: 10.1089/zeb.2012.0824. [DOI] [PubMed] [Google Scholar]
- Page D.M., Wittamer V., Bertrand J.Y., Lewis K.L., Pratt D.N., Delgado N., Schale S.E., McGue C., Jacobsen B.H., Doty A., Pao Y., Yang H., Chi N.C., Magor B.G., Traver D. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood. 2013;122:e1–11. doi: 10.1182/blood-2012-12-471029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik E.J., Zon L.I. Hematopoietic development in the zebrafish. Int. J. Dev. Biol. 2010;54:1127–1137. doi: 10.1387/ijdb.093042ep. [DOI] [PubMed] [Google Scholar]
- Palis J., Yoder M.C. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Corrigendum: short chain fatty acids (SCFAs)-Mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:1486. doi: 10.3389/fimmu.2019.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Lee E.J., Lee J.C., Kim W.K., Kim H.S. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappaB and ERK signaling pathways. Int. Immunopharm. 2007;7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Park J., Levic D.S., Sumigray K.D., Bagwell J., Eroglu O., Block C.L., Eroglu C., Barry R., Lickwar C.R., Rawls J.F., Watts S.A., Lechler T., Bagnat M. Lysosome-rich enterocytes mediate protein absorption in the vertebrate gut. Dev. Cell. 2019;51:7–20. doi: 10.1016/j.devcel.2019.08.001. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S., Pastar M., Mitter B., Lippert K., Hackl E., Lojan P., Oswald A., Sessitsch A. Improved group-specific primers based on the full SILVA 16S rRNA gene reference database. Environ. Microbiol. 2014;16:2389–2407. doi: 10.1111/1462-2920.12350. [DOI] [PubMed] [Google Scholar]
- Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microb. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Sarker P., Bergman P., Ara G., Lindh M., Sack D.A., Nasirul Islam K.M., Gudmundsson G.H., Andersson J., Agerberth B. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J.F., Samuel B.S., Gordon J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto G., del Peso A., Zurita J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008;3:1125–1131. doi: 10.1038/nprot.2008.75. [DOI] [PubMed] [Google Scholar]
- Rio D.C., Ares M., Hannon G.J., Nilsen T.W. Purification of RNA using TRIzol (TRI reagent) Cold Spring Harb. Protoc. 2010 doi: 10.1101/pdb.prot5439. 2010, pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. BioMed Res. Int. 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues H.G., Takeo Sato F., Curi R., Vinolo M.A.R. Fatty acids as modulators of neutrophil recruitment, function and survival. Eur. J. Pharmacol. 2016;785:50–58. doi: 10.1016/j.ejphar.2015.03.098. [DOI] [PubMed] [Google Scholar]
- Roeselers G., Mittge E.K., Stephens W.Z., Parichy D.M., Cavanaugh C.M., Guillemin K., Rawls J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011;5:1595–1608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeot J., Torraca V., Zakrzewska A., Kanwal Z., Jansen H.J., Sommer F., Spaink H.P., Meijer A.H. Corrigendum: RNAseq profiling of leukocyte populations in zebrafish larvae reveals a cxcl11 chemokine gene as a marker of macrophage polarization during mycobacterial infection. Front. Immunol. 2019;10:2720. doi: 10.3389/fimmu.2019.02720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari R., Hoseinifar S.H., Kavandi M. Modulation of antioxidant defense and immune response in zebra fish (Danio rerio) using dietary sodium propionate. Fish Physiol. Biochem. 2016;42:1733–1739. doi: 10.1007/s10695-016-0253-z. [DOI] [PubMed] [Google Scholar]
- Selvanantham T., Lin Q., Guo C.X., Surendra A., Fieve S., Escalante N.K., Guttman D.S., Streutker C.J., Robertson S.J., Philpott D.J., Mallevaey T. NKT cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J. Immunol. 2016;197:4464–4472. doi: 10.4049/jimmunol.1601410. [DOI] [PubMed] [Google Scholar]
- Shimotoyodome A., Meguro S., Hase T., Tokimitsu I., Sakata T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2000;125:525–531. doi: 10.1016/s1095-6433(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Sina C., Gavrilova O., Förster M., Till A., Derer S., Hildebrand F., Raabe B., Chalaris A., Scheller J., Rehmann A., Franke A., Ott S., Häsler R., Nikolaus S., Fölsch U.R., Rose-John S., Jiang H.P., Li J., Schreiber S., Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009;183:7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- Singh N., Thangaraju M., Prasad P.D., Martin P.M., Lambert N.A., Boettger T., Offermanns S., Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J. Biol. Chem. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., Lee J.R., Offermanns S., Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.J., Blugeon S., Bridonneau C., Furet J.P., Corthier G., Grangette C., Vasquez N., Pochart P., Trugnan G., Thomas G., Blottière H.M., Doré J., Marteau P., Seksik P., Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Seksik P. The intestinal microbiota in inflammatory bowel diseases: time to connect with the host. Curr. Opin. Gastroenterol. 2010;26:327–331. doi: 10.1097/MOG.0b013e328339536b. [DOI] [PubMed] [Google Scholar]
- Stagaman K., Sharpton T.J., Guillemin K. Zebrafish microbiome studies make waves. Lab. Anim. 2020;49:201–207. doi: 10.1038/s41684-020-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens W.Z., Burns A.R., Stagaman K., Wong S., Rawls J.F., Guillemin K., Bohannan B.J. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2016;10:644–654. doi: 10.1038/ismej.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhammer O.W., Zakrzewska A., Hegedûs Z., Spaink H.P., Meijer A.H. Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 2009;182:5641–5653. doi: 10.4049/jimmunol.0900082. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nishida A., Fujimoto T., Fujii M., Shioya M., Imaeda H., Inatomi O., Bamba S., Sugimoto M., Andoh A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn's disease. Digestion. 2016;93:59–65. doi: 10.1159/000441768. [DOI] [PubMed] [Google Scholar]
- Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- Tarrerias A.L., Millecamps M., Alloui A., Beaughard C., Kemeny J.L., Bourdu S., Bommelaer G., Eschalier A., Dapoigny M., Ardid D. Short-chain fatty acid enemas fail to decrease colonic hypersensitivity and inflammation in TNBS-induced colonic inflammation in rats. Pain. 2002;100:91–97. doi: 10.1016/s0304-3959(02)00234-8. [DOI] [PubMed] [Google Scholar]
- Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraju M., Cresci G.A., Liu K., Ananth S., Gnanaprakasam J.P., Browning D.D., Mellinger J.D., Smith S.B., Digby G.J., Lambert N.A., Prasad P.D., Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Canc. Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K., Kamimura K., Takahashi K., Yokoyama J., Yamagiwa S., Terai S. Diversion colitis and pouchitis: a mini-review. World J. Gastroenterol. 2018;24:1734–1747. doi: 10.3748/wjg.v24.i16.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosato G., Jones K.D. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990;75:1305–1310. [PubMed] [Google Scholar]
- Tran N.T., Li Z., Wang S., Zheng H., Aweya J.J., Wen X., Li S. Progress and perspectives of short-chain fatty acids in aquaculture. Rev. Aquacult. 2020;12:283–298. doi: 10.1111/raq.12317. [DOI] [Google Scholar]
- Trede N.S., Langenau D.M., Traver D., Look A.T., Zon L.I. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- Uyttebroek L., Pype C., Hubens G., Timmermans J.P., Van Nassauw L. Effect of TNBS-induced colitis on enteric neuronal subpopulations in adult zebrafish. Eur. J. Histochem. 2020;64 doi: 10.4081/ejh.2020.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart M., van Soest J.J., Spaink H.P., Meijer A.H. Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis. Model. Mech. 2013;6:841–854. doi: 10.1242/dmm.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M., Dios S., Novoa B., Figueras A. Characterisation, expression and ontogeny of interleukin-6 and its receptors in zebrafish (Danio rerio) Dev. Comp. Immunol. 2012;37:97–106. doi: 10.1016/j.dci.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Hatanaka E., Hebeda C.B., Farsky S.H., Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. (Lond.) 2009;117:331–338. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Ferguson G.J., Kulkarni S., Damoulakis G., Anderson K., Bohlooly-Y M., Stephens L., Hawkins P.T., Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PloS One. 2011;6 doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Hatanaka E., Sato F.T., Sampaio S.C., Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011;22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo M.A., Hirabara S.M., Curi R. G-protein-coupled receptors as fat sensors. Curr. Opin. Clin. Nutr. Metab. Care. 2012;15:112–116. doi: 10.1097/MCO.0b013e32834f4598. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Festuccia W.T., Crisma A.R., Alves V.S., Martins A.R., Amaral C.L., Fiamoncini J., Hirabara S.M., Sato F.T., Fock R.A., Malheiros G., dos Santos M.F., Curi R. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endocrinol. Metab. 2012;303:E272–E282. doi: 10.1152/ajpendo.00053.2012. [DOI] [PubMed] [Google Scholar]
- Wallace K.N., Akhter S., Smith E.M., Lorent K., Pack M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wang W., Chen L., Zhou R., Wang X., Song L., Huang S., Wang G., Xia B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014;52:398–406. doi: 10.1128/JCM.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner N., Núñez G. MyD88: a critical adaptor protein in innate immunity signal transduction. J. Immunol. 2013;190:3–4. doi: 10.4049/jimmunol.1203103. [DOI] [PubMed] [Google Scholar]
- Weigmann B., Neurath M.F. Oxazolone-induced colitis as a model of Th2 immune responses in the intestinal mucosa. Methods Mol. Biol. 2016;1422:253–261. doi: 10.1007/978-1-4939-3603-8_23. [DOI] [PubMed] [Google Scholar]
- Westerfield M. fifth ed. Univ. of Oregon Press; Eugene: 2007. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) [Google Scholar]
- Wiles T.J., Wall E.S., Schlomann B.H., Hay E.A., Parthasarathy R., Guillemin K. Modernized tools for streamlined genetic manipulation and comparative study of wild and diverse proteobacterial lineages. mBio. 2018;9 doi: 10.1128/mBio.01877-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen L.E., Koetsier M.A., van Deventer S.J., van Tol E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett C.E., Cherry J.J., Steiner L.A. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics. 1997;45:394–404. doi: 10.1007/s002510050221. [DOI] [PubMed] [Google Scholar]
- Wilson J.M., Bunte R.M., Carty A.J. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio) J. Am. Assoc. Lab. Anim. Sci. 2009;48:785–789. [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Ran C., Ding Q.W., Liu H.L., Xie M.X., Yang Y.L., Xie Y.D., Gao C.C., Zhang H.L., Zhou Z.G. Ability of prebiotic polysaccharides to activate a HIF1α-antimicrobial peptide axis determines liver injury risk in zebrafish. Commun. Biol. 2019;2:274. doi: 10.1038/s42003-019-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X., Li T., Li M., Huang S., Qiu Y., Feng R., Zhang S., Chen M., Xiong L., Zeng Z. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2019;25:1751–1763. doi: 10.1093/ibd/izz188. [DOI] [PubMed] [Google Scholar]