Abstract
Background
Breast cancer (BC) survivors experience an increased burden of long-term comorbidities, including heart failure (HF). However, there is limited understanding of the risk for the development of HF subtypes, such as HF with preserved ejection fraction (HFpEF), in BC survivors.
Objectives
This study sought to estimate the incidence of HFpEF and HF with reduced ejection fraction (HFrEF) in postmenopausal BC survivors and to identify lifestyle and cardiovascular risk factors associated with HF subtypes.
Methods
Within the Women’s Health Initiative, participants with an adjudicated diagnosis of invasive BC were followed to determine the incidence of hospitalized HF, for which adjudication procedures determined left ventricular ejection fraction. We calculated cumulative incidences of HF, HFpEF, and HFrEF. We estimated HRs for risk factors in relation to HF, HFpEF, and HFrEF using Cox proportional hazards survival models.
Results
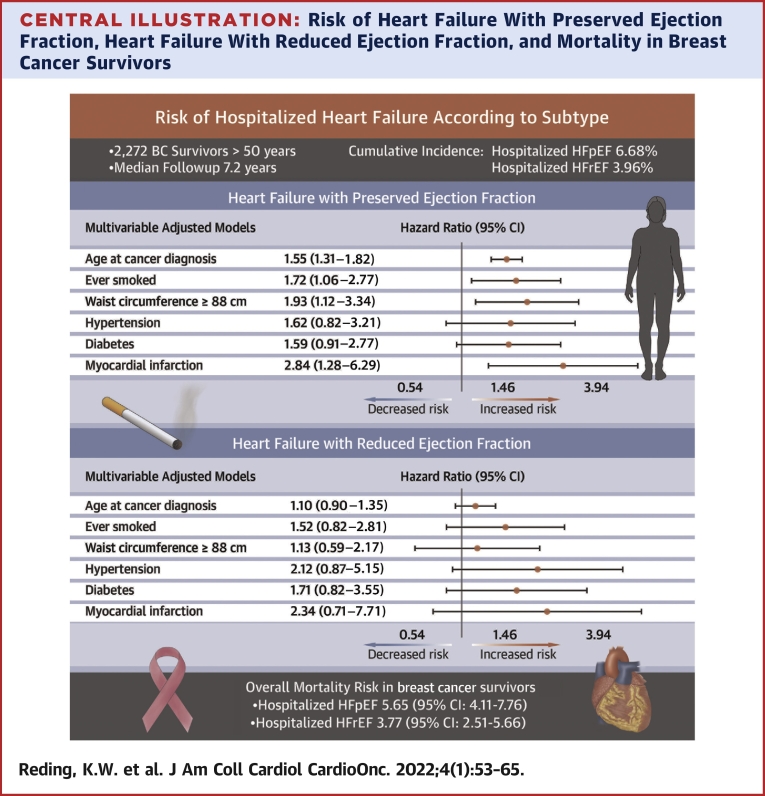
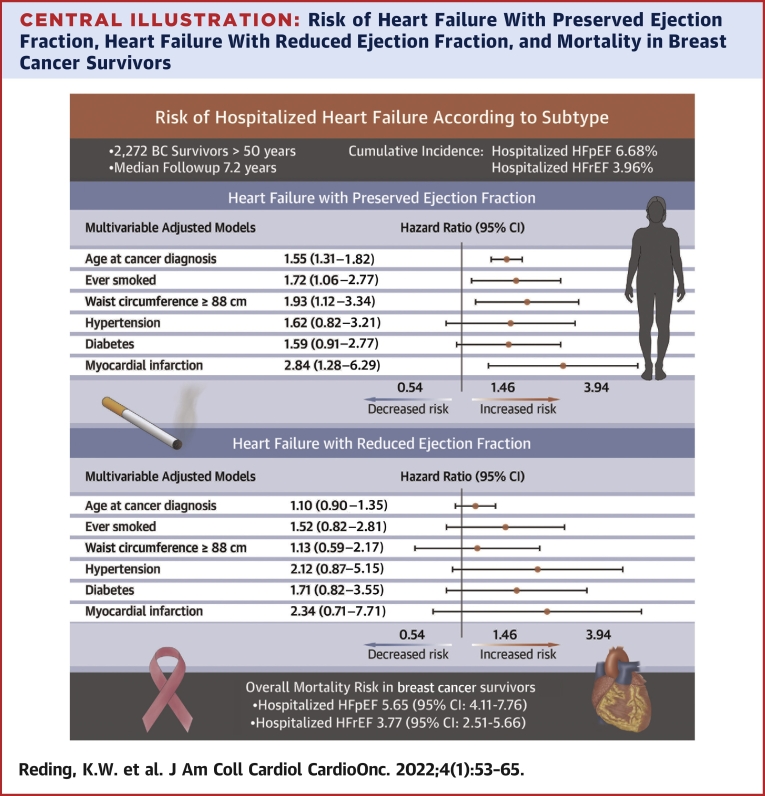
In 2,272 BC survivors (28.6% Black and 64.9% White), the cumulative incidences of hospitalized HFpEF and HFrEF were 6.68% and 3.96%, respectively, over a median of 7.2 years (IQR: 3.6-12.3 years). For HFpEF, prior myocardial infarction (HR: 2.83; 95% CI: 1.28-6.28), greater waist circumference (HR: 1.99; 95% CI: 1.14-3.49), and smoking history (HR: 1.65; 95% CI: 1.01-2.67) were the strongest risk factors in multivariable models. With the exception of waist circumference, similar patterns were observed for HFrEF, although none were significant. In relation to those without HF, the risk of overall mortality in BC survivors with hospitalized HFpEF was 5.65 (95% CI: 4.11-7.76), and in those with hospitalized HFrEF, it was 3.77 (95% CI: 2.51-5.66).
Conclusions
In this population of older, racially diverse BC survivors, the incidence of HFpEF, as defined by HF hospitalizations, was higher than HFrEF. HF was also associated with an increased mortality risk. Risk factors for HF were largely similar to the general population with the exception of prior myocardial infarction for HFpEF. Notably, both waist circumference and smoking represent potentially modifiable factors.
Key Words: breast cancer, cancer survivorship, cardio-oncology, heart failure, obesity
Abbreviations and Acronyms: BC, breast cancer; BMI, body mass index; CVD, cardiovascular disease; ER, estrogen receptor; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PR, progesterone receptor; WHI, Women’s Health Initiative
Central Illustration
An estimated 3.8 million breast cancer (BC) survivors are living in the United States.1 Treatment advances have led to increased survival,1 which is offset by increased risk of long-term comorbidities, including heart failure (HF).2,3 To date, cardio-oncology research has focused predominantly on HF with reduced ejection fraction (HFrEF) due to well-recognized associations between cancer treatment and left ventricular ejection fraction (LVEF) declines.4, 5, 6 However, an understudied component is the development of HF with preserved ejection fraction (HFpEF) in BC survivors. There is a paucity of data on HFpEF in BC survivors despite HFpEF being more common than HFrEF in older women7,8 and BC and HFpEF sharing multiple risk factors (eg, obesity and hypertension).7 Currently, data are lacking on both the incidence of HFpEF and the risk factors associated with HFpEF in BC survivors.
In the general population, risk factors differ between HFpEF and HFrEF.8 For HFpEF, strong risk factors include obesity, particularly central adiposity, and obesity-related comorbidities such as type 2 diabetes mellitus, dyslipidemia, and hypertension.9 For HFrEF, the strongest risk factors are prior myocardial infarction (MI) and hypertension.8 Both HF subtypes are associated with smoking and low physical activity.8,10,11 Currently, it is unclear the extent to which differences in risk factors for HF subtypes hold true for BC survivors in whom cancer treatment may alter the causal pathways of traditional risk factors.12 For example, elevated body mass index (BMI) and increased central adiposity are associated with LVEF declines in cancer survivors13,14 despite no relationship between obesity and LVEF in the general population.15 Moreover, cancer treatments have been shown to cause changes in body composition, specifically leading to increased central adiposity and reduced fat-free mass.16 Determining the relative incidence of HF subtypes and elucidating the associated risk factors could guide providers in risk stratification and inform recommendations for HF surveillance after BC.
In this study, we leveraged the Women’s Health Initiative (WHI) in which HF hospitalizations were adjudicated as HFpEF or HFrEF through a validated approach8 to: 1) estimate the incidence of HFpEF and HFrEF in a racially diverse sample of postmenopausal BC survivors; 2) investigate associations between lifestyle factors, including BMI, central adiposity, and smoking, and HFpEF and HFrEF in BC survivors; and 3) explore associations between BC treatment and HFpEF.
Methods
The WHI enrolled 161,808 postmenopausal women aged 50 to 79 years at 40 clinical centers across the United States. between 1993 and 1998.17 Participants were followed through March 2005 and were subsequently invited to continue follow-up in two extension studies (through 2020). Cancer diagnoses were updated annually.18 Consent was obtained for all women at enrollment and at the beginning of each extension study. Study procedures were approved by the Institutional Review Board at the Fred Hutchinson Cancer Center.
The present study was limited to a subcohort of 44,174 participants in the WHI Medical Records Cohort, which included all participants of the menopausal hormone therapy randomized trials and all women self-reporting as African American or Hispanic. In this subcohort study, adjudication of HF sought to determine LVEF (henceforth referred to as the subcohort) such that, when available, LVEF was used to classify HFpEF and HFrEF.8 For the present study, we included WHI participants in the subcohort who had a confirmed diagnosis of incident, invasive BC from July 21, 1994, through March 1, 2019. Patients with pre-existing HF (n = 51) or <2 weeks of follow-up (n = 4) were excluded from the analysis, resulting in 2,272 women for our analytic cohort (Figure 1).
Figure 1.
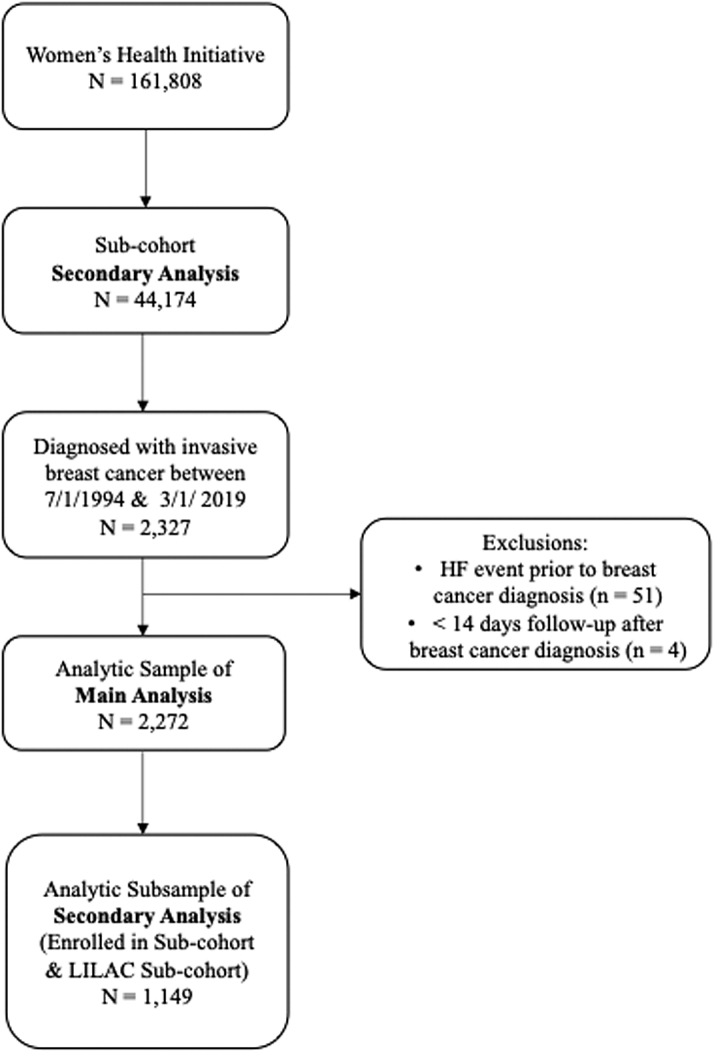
Study Flow Diagram
An illustration of the inclusion and exclusion criteria and the resulting sample size of the analytic data set. HF = heart failure.
Cancer adjudication
Participant reports of BC were verified by centrally trained adjudicators.18 Clinical information about BC diagnosis and characteristics included staging (using Surveillance Epidemiology and End Results classification, which incorporated tumor size and lymph node status), estrogen receptor (ER), progesterone receptor (PR), HER2 expression, and pathology review using SEER criteria.
The LILAC (Life and Longevity After Cancer) study, a cancer survivorship subcohort within the WHI, collected treatment data on women diagnosed with cancer.19 Women with no prior cancer diagnosis at WHI enrollment were eligible if they had a confirmed cancer diagnosis during WHI follow-up.19 Half (50.6%) of the women from the main analysis were enrolled in LILAC. A subset analysis limited to the LILAC subcohort was conducted in order to explore associations with BC treatment (n = 1,149).
HF adjudication
Self-reports of hospitalization for incident HF in the subcohort were confirmed by trained physician adjudicators at the University of North Carolina.8 Briefly, upon the first report of a hospitalization for acute HF, the WHI central adjudication committee obtained medical records. The abstracted data contained evidence of new onset of symptoms, HF history, general medical history, diagnostic tests, physical examination, signs and symptoms, biomarkers (eg, brain natriuretic peptide and cardiac troponins), and medications. Physician adjudicators reviewed this information for evidence of acute decompensated HF and recorded LVEF at the time of the hospitalization or the most recent known LVEF as determined by echocardiography, nuclear imaging, cardiac magnetic resonance, or other cardiac imaging technique.
We studied the first adjudicated event of acute HF hospitalization (definite or possible decompensated) after BC. Subtypes of HF were classified as HFrEF for LVEF <50% and HFpEF for LVEF ≥50%, a cut point consistent with the 2013 American College of Cardiology Foundation and American Heart Association and the 2016 European Society of Cardiology guidelines.20,21 We also classified HFrEF using a 40% cut point in order to investigate whether this more restricted definition altered our findings. HF subtype was not defined when LVEF could not be determined (n = 26).
Statistical analyses
Upon WHI enrollment, women self-reported age, race and ethnicity, attained education, family income, family history of cardiovascular disease (CVD), and alcohol intake. During follow-up, women also self-reported smoking status, leisure-time physical activity, and medications including antihypertensive medications and diabetes medications. Anthropometric variables, including BMI calculated from weight and height (kg/m2) and waist and hip circumference, were measured using a standardized protocol at the enrollment clinic visit and during follow-up. Blood pressure measurements were obtained at clinic visits using a standardized protocol. A history of MI was established through the WHI central adjudication committee. For variables collected at multiple time points, data at the time point closest and before BC diagnosis were used. Covariates were analyzed as categoric variables, except age at BC diagnosis, which was analyzed in 5-year intervals as a continuous variable.
HRs and 95% CI were estimated by Cox proportional hazards survival models. The follow-up time was from BC diagnosis to the first acute hospitalized HF event. Otherwise, follow-up was censored at the time of the last documented follow-up contact, death, or March 1, 2019, whichever occurred first. For estimation of HRs associated with specific HF subtypes, follow-up was censored for participants with acute hospitalized HF other than the subtype being analyzed.
We conducted age-adjusted models for lifestyle factors, demographics, and BC characteristics. We selected variables for inclusion in a multivariable model based on associations observed in age-adjusted models (for P < 0.10) while taking into consideration correlations between variables in the model. Thus, anthropometric variables were considered one at a time, and waist circumference, the variable with the greatest association with HF, was retained. Tests for linear trend were conducted by modeling the categoric covariate as a continuous variable. To verify the proportional hazards assumption, we tested interactions of the covariates with the time variable. None of the interaction terms in these models was significant, indicating that the assumption of proportionality was satisfied.
We reported the number of HF overall and HF subtypes diagnosed over the median follow-up and list the IQR to demonstrate quartiles 1 and 3. We also calculated cumulative incidence functions under competing risk assumptions according to the method of Fine and Gray.
We considered the potential for collider bias because this analysis was conditional on a BC diagnosis (ie, smoking and waist circumference are both associated with BC, and both are associated with HF). Therefore, we investigated the removal of smoking from the multivariable model.22 We conducted additional prespecified sensitivity analyses limited to the LILAC data set (in which cancer treatment data were available) to examine cancer treatment in the multivariable model. We also examined age- and multivariable-adjusted subdistribution HRs from Fine and Gray competing risk models.
Among the 2,272 participants in the primary analysis, we evaluated the associations of hospitalized HFpEF and HFrEF with CVD mortality and BC mortality. HF subtype was treated as a time-dependent covariate. We used a Cox regression model to jointly estimate the associations of the HF outcomes with CVD mortality and BC mortality treated as competing risks. The model was adjusted for race and ethnicity, age at BC diagnosis (continuous in 5-year intervals), cancer stage, ER/PR status, and history of MI. Homogeneity of HR estimates for CVD death or BC death, in relation to each HF outcome, was assessed by the statistical significance of interaction terms between the HF variables and a variable defining specific cause of death (CVD or BC). We also accounted for differences in risks of CVD death and BC death in relation to age at BC diagnosis and cancer stage by including interaction terms between these 2 covariates and the cause of death variable. HRs and 95% CIs for all-cause mortality were estimated from a separate Cox regression model that included HF subtype as a time-dependent covariate, race and ethnicity, age at BC diagnosis (continuous in 5-year intervals), cancer stage, ER/PR status, and MI history.
To evaluate the association of invasive BC with subsequent outcomes of acute hospitalized HF (overall, HFpEF, and HFrEF), we conducted additional Cox proportional hazards survival models among the full subcohort (N = 44,174), excluding 310 with no follow-up time after WHI baseline, resulting in 43,864. Invasive BC was treated as a time-dependent covariate. Selected risk factors for HF and BC, as ascertained at WHI baseline, were included in the models. These included age, self-reported race and ethnicity, history of hypertension, history of treated diabetes, leisure-time physical activity, and waist circumference. The time metric was from WHI baseline to the first acute hospitalized HF event. To compare risk factors for participants with and without invasive BC, we extended the models by including interaction terms between the time-dependent invasive BC covariate and all other covariates in the model. Differences in risk factors between the BC subgroup and the noninvasive BC subgroup were based on P values for the significance of the interaction terms.
We used SAS version 9.4 and Stata version 15. All tests were 2-sided; P values were considered significant at 0.05.
Results
Characteristics of the study population
In this population of 2,272 BC survivors >50 years of age at enrollment, there were 138 adjudicated incident hospitalized HF events diagnosed during a median (quartile 1, quartile 3) follow-up of 7.2 (IQR: 3.6-12.3) years post-BC diagnosis. Of those, 70 (50.7%) were classified as HFpEF and 42 (30.4%) as HFrEF; the remainder were unable to be classified (n = 26). The average age at BC diagnosis was 73.4 ± 7.8 years for those with subsequent HF and 71.7 ± 8.2 years for those without subsequent HF. BC survivors experienced a greater incidence of HFpEF than HFrEF, with annualized incidence rates of HFpEF and HFrEF of 0.73% and 0.37%, respectively, translating to respective cumulative incidences of 6.68% and 3.96% at the end of follow-up (Figure 2).
Figure 2.
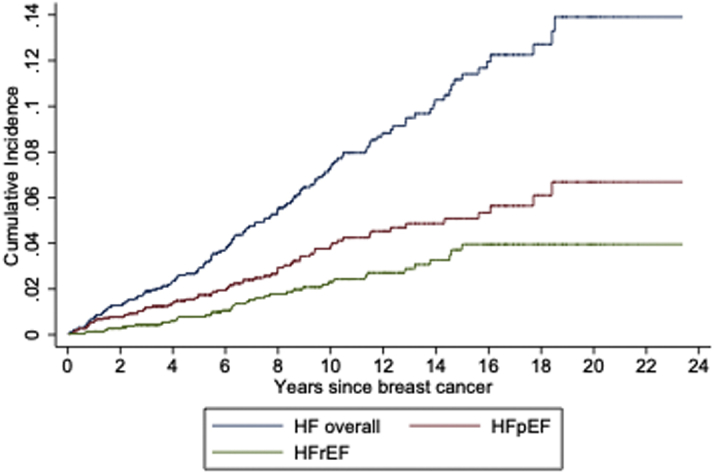
HF Hospitalizations After Breast Cancer
The cumulative incidence of hospitalized heart failure (HF) (blue), heart failure with preserved ejection fraction (HFpEF) (red), and heart failure with reduced ejection fraction (HFrEF) (green) in breast cancer survivors during the Women’s Health Initiative study (a maximum and median follow-up of 24 and 7.2 years, respectively).
The majority of the study population was ≥70 years of age (62.7%) and was diagnosed with BC in the decade of 2000 to 2009 (54.3%) (Table 1). Black women comprised 28.6% of the data set, and women of Hispanic ethnicity comprised 10.3%. The majority of women were nonsmokers, and nearly a majority were obese (49.5% with BMI ≥30 kg/m2). Nearly three-quarters were diagnosed with localized stage, and approximately three-fifths were diagnosed with ER+/PR+ BC. When stratifying by BMI, we observed that women with obesity were more likely to report low physical activity, not consume alcohol, have a lower family income, and a younger age at BC diagnosis. Women with obesity were also more likely to have a history of hypertension and diabetes, more advanced BC stage, and ER+/PR+ disease (Supplemental Table 1). Compared with all BC cases in WHI, the BC cases in this subcohort were more diverse racially and ethnically, had a lower family income and percent with a college degree, higher BMI, lower physical activity, higher prevalence of hypertension and diabetes, and higher proportion of ER−/PR− tumors relative to the entire WHI BC cohort.
Table 1.
Demographic, Lifestyle, and Clinical Characteristics of the Study Sample of Invasive Breast Cancer Survivors (N = 2,272)
| Age at breast cancer diagnosis, y | |
| 51-64 | 462 (20.3) |
| 65-69 | 386 (17.0) |
| 70-74 | 580 (25.5) |
| 75-79 | 444 (19.5) |
| 80-99 | 400 (17.6) |
| Year of breast cancer diagnosis | |
| 1994-1999 | 416 (18.3) |
| 2000-2004 | 744 (32.7) |
| 2005-2009 | 490 (21.6) |
| 2010-2014 | 416 (18.3) |
| 2015-2018 | 206 (9.1) |
| Characteristics at enrollment in the Women’s Health Initiative | |
| Race | |
| American Indian/Alaska Native | 8 (0.4) |
| Asian/Pacific Islander | 27 (1.2) |
| Black/African American | 650 (28.6) |
| White | 1475 (64.9) |
| More than one race | 42 (1.8) |
| Unknown/not reported | 70 (3.1) |
| Hispanic/Latina ethnicity | |
| No | 2033 (89.5) |
| Yes | 234 (10.3) |
| Unknown/not reported | 5 (0.2) |
| Education | |
| High school diploma/GED or less | 526 (23.2) |
| School after high school | 934 (41.1) |
| College degree or higher | 794 (34.9) |
| Unknown | 18 (0.8) |
| Family income | |
| <$20,000 | 391 (17.2) |
| $20,000-$49,999 | 1,063 (46.8) |
| $50,000-$74,999 | 422 (18.6) |
| ≥$75,000 | 274 (12.1) |
| Unknown | 122 (5.4) |
| Family history of myocardial infarction or stroke | |
| No | 730 (32.1) |
| Yes | 1,477 (65.0) |
| Unknown | 65 (2.9) |
| Alcohol intake | |
| Nondrinker/past drinker | 730 (32.1) |
| <7 drinks per week | 1,273 (56.0) |
| 7+ drinks per week | 238 (10.5) |
| Unknown | 31 (1.4) |
| Antihypertensive use | |
| ACE inhibitor/ARB use | 191 (8.4) |
| Beta blocker use | 134 (5.9) |
| Calcium channel blocker use | 253 (11.1) |
| Diuretic use | 359 (15.8) |
| Characteristics assessed before breast cancer diagnosisa | |
| Ever smoked | |
| No | 1,166 (51.3) |
| Yes | 1,104 (48.6) |
| Unknown | 2 (0.1) |
| Body mass index, kg/m2 | |
| <25 | 390 (17.2) |
| 25-<30 | 755 (33.2) |
| ≥30 | 1,124 (49.5) |
| Unknown | 3 (0.1) |
| Waist circumference ≥88 cm | |
| No | 932 (41.0) |
| Yes | 1,338 (58.9) |
| Unknown | 2 (0.1) |
| Waist-to-hip ratio >0.85 | |
| No | 1,418 (62.4) |
| Yes | 852 (37.5) |
| Unknown | 2 (0.1) |
| Leisure time physical activity, MET h/wk | |
| 0 | 490 (21.6) |
| >0-<9.0 | 866 (38.1) |
| ≥9.0 | 872 (38.4) |
| Unknown | 44 (1.9) |
| History of statin use | 607 (26.7) |
| History of nonsteroidal anti-inflammatory drug use | 1,265 (55.7) |
| History of hypertensionb | 1,718 (75.6) |
| History of treated diabetes | 422 (18.6) |
| History of myocardial infarction | 76 (3.3) |
| Tumor characteristics | |
| Summary stage | |
| Localized | 1,649 (72.6) |
| Regional/distant | 587 (25.8) |
| Unknown | 36 (1.6) |
| Hormone receptor status | |
| ER+/PR+ | 1,407 (61.9) |
| ER−/PR− | 343 (15.1) |
| Mixed | 362 (15.9) |
| Unknown | 160 (7.0) |
Values are n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor block; ER = estrogen receptor; MET = metabolic equivalent; PR = progesterone receptor.
For variables collected at multiple time points, the data presented were collected at the time point most proximal and before breast cancer.
A history of hypertension was ascertained through any of the following: study measurement of blood pressure at clinic visits, self-report of hypertension diagnosis, or report of antihypertensive treatment
Risk of HF associated with lifestyle and cardiovascular factors
With the exception of age at BC diagnosis, demographic variables were not associated with hospitalized HF risk in this cohort (Table 2). HF risk increased in relation to anthropometric variables; in age-adjusted models, elevated BMI and high waist circumference were both associated with a nearly 2-fold higher HF risk (Table 2). As expected, a history of smoking, hypertension, diabetes, and MI were associated with higher HF risk, ranging from 1.64 to 3.01. Whereas increasing leisure-time physical activity (P trend = 0.042) and moderate alcohol consumption were associated with a lower HF risk. No relationship was detected between BC tumor characteristics, namely stage or hormone receptor status, and HF risk.
Table 2.
Risk of Acute, Hospitalized HF Among invasive Breast Cancer Survivors
| N | HF Risk |
||||
|---|---|---|---|---|---|
| HF | HR | 95% CI | P Value | ||
| Age at breast cancer diagnosis, 5-y intervals | 2,722 | 138 | 1.44 | (1.28-1.61) | <0.001 |
| Year of breast cancer diagnosis | |||||
| 1994-1999 | 416 | 29 | 1.00 | Reference | 0.32a |
| 2000-2004 | 744 | 58 | 1.13 | (0.71-1.80) | |
| 2005-2009 | 490 | 29 | 0.77 | (0.44-1.35) | |
| 2010-2014 | 416 | 17 | 0.78 | (0.39-1.55) | |
| 2015-2018 | 206 | 5 | 0.97 | (0.34-2.79) | |
| Demographics at enrollment in the Women’s Health Initiative | |||||
| Race | |||||
| White | 1,475 | 93 | 1.00 | Reference | 0.64 |
| Asian/Pacific Islander | 27 | 3 | 1.61 | (0.51-5.09) | |
| Black/African American | 650 | 38 | 1.25 | (0.85-1.83) | |
| Education | |||||
| High school diploma/GED or less | 526 | 30 | 1.00 | Reference | 0.61a |
| School after high school | 934 | 60 | 1.11 | (0.71-1.72) | |
| College degree or higher | 794 | 48 | 0.92 | (0.58-1.45) | |
| Family income | |||||
| <$20,000 | 391 | 33 | 1.00 | Reference | 0.52a |
| $20,000-$49,999 | 1,063 | 56 | 0.57 | (0.37-0.88) | |
| $50,000-$74,999 | 422 | 22 | 0.62 | (0.36-1.06) | |
| ≥$75,000 | 274 | 18 | 0.84 | (0.47-1.49) | |
| Risk factors at enrollment in the Women’s Health Initiative | |||||
| Family history of myocardial infarction or stroke | |||||
| No | 730 | 37 | 1.00 | Reference | 0.42 |
| Yes | 1,477 | 96 | 1.17 | (0.80-1.71) | |
| Alcohol intake | |||||
| Nondrinker/past drinker | 730 | 54 | 1.00 | Reference | 0.024 |
| <7 drinks per week | 1,273 | 63 | 0.61 | (0.42-0.88) | |
| 7+ drinks per week | 238 | 18 | 0.89 | (0.52-1.52) | |
| Characteristics assessed before breast cancer diagnosisb | |||||
| Ever smoked | |||||
| No | 1,166 | 57 | 1.00 | Reference | 0.004 |
| Yes | 1,104 | 81 | 1.64 | (1.17-2.30) | |
| Body mass index, kg/m2 | |||||
| <25 | 390 | 19 | 1.00 | Reference | <0.001a |
| 25-<30 | 755 | 35 | 1.00 | (0.57-1.74) | |
| ≥30 | 1,124 | 84 | 1.92 | (1.16-3.16) | |
| Waist circumference ≥88 cm | |||||
| No | 932 | 39 | 1.00 | Reference | <0.001 |
| Yes | 1,338 | 99 | 1.99 | (1.37-2.88) | |
| Waist-to-hip ratio >0.85 | |||||
| No | 1,418 | 67 | 1.00 | Reference | <0.001 |
| Yes | 852 | 71 | 1.79 | (1.28-2.51) | |
| Leisure time physical activity, MET h/wk | |||||
| 0 | 490 | 37 | 1.00 | Reference | 0.042a |
| >0-<9.0 | 866 | 51 | 0.76 | (0.50-1.16) | |
| ≥9.0 | 872 | 47 | 0.63 | (0.41-0.98) | |
| Nonsteroidal anti-inflammatory drug use | |||||
| No | 1,007 | 55 | 1.00 | Reference | 0.35 |
| Yes | 1,265 | 83 | 1.18 | (0.83-1.67) | |
| History of hypertensionc | |||||
| No | 554 | 18 | 1.00 | Reference | 0.001 |
| Yes | 1,718 | 120 | 2.25 | (1.37-3.71) | |
| History of treated diabetes | |||||
| No | 1,850 | 104 | 1.00 | Reference | <0.001 |
| Yes | 422 | 34 | 1.97 | (1.33-2.91) | |
| History of myocardial infarction | |||||
| No | 2,196 | 126 | 1.00 | Reference | <0.001 |
| Yes | 76 | 12 | 3.01 | (1.66-5.45) | |
| Tumor characteristics | |||||
| Summary stage | |||||
| Localized | 1,649 | 105 | 1.00 | Reference | 0.79 |
| Regional/distant | 587 | 32 | 1.06 | (0.71-1.57) | |
| Hormone receptor status | |||||
| ER+/PR+ | 1,407 | 85 | 1.00 | Reference | 0.53 |
| ER−/PR− | 343 | 21 | 1.30 | (0.81-2.11) | |
| Mixed | 362 | 24 | 1.13 | (0.72-1.78) | |
Values are n unless otherwise indicated.
Data were not presented for cell sizes ≤2. HRs were adjusted for linear age at breast cancer diagnosis grouped by 5-year intervals.
HF = heart failure; other abbreviations as in Table 1.
P value for linear trend.
For variables collected at multiple time points, the data presented were collected at the time point most proximal and before breast cancer.
A history of hypertension was ascertained through any of the following: study measurement of blood pressure at clinic visits, self-report of hypertension diagnosis, or report of antihypertensive treatment.
Risk of HF subtypes associated with lifestyle and cardiovascular risk factors
With respect to anthropometric variables by HF subtype, an increased risk of hospitalized HFpEF was associated with an elevated waist circumference and an elevated waist-to-hip ratio and marginally so for BMI in age-adjusted models (Table 3). For hospitalized HFrEF, the risk estimates associated with BMI were similar to those observed with HFpEF, whereas no association was observed for waist circumference or waist-to-hip ratio.
Table 3.
Risk of Hospitalized HFpEF and HFrEF Among Invasive Breast Cancer Survivors
| Age Adjusted |
Multivariable Adjusteda |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HFpEF |
HFrEF |
HFpEF |
HFrEF |
|||||||
| n | HR | 95% CI | n | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age at breast cancer diagnosis, 5-y intervals | 70 | 1.56 | (1.33-1.83) | 42 | 1.14 | (0.93-1.39) | 1.55 | (1.31-1.82) | 1.10 | (0.90-1.35) |
| Alcohol intake | ||||||||||
| Nondrinker/past drinker | 25 | 1.00 | Reference | 17 | 1.00 | Reference | ||||
| <7 drinks per week | 36 | 0.75 | (0.45-1.25) | 18 | 0.56 | (0.29-1.09) | ||||
| 7+ drinks per week | 8 | 0.85 | (0.38-1.88) | 7 | 1.13 | (0.47-2.72) | ||||
| Ever smoked | ||||||||||
| No | 28 | 1.00 | Reference | 18 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Yes | 42 | 1.74 | (1.08-2.81) | 24 | 1.50 | (0.81-2.76) | 1.72 | (1.06-2.77) | 1.52 | (0.82-2.81) |
| Body mass index, kg/m2 | ||||||||||
| <25 | 10 | 1.00 | Reference | 5 | 1.00 | Reference | ||||
| 25-<30 | 17 | 0.92 | (0.42-2.01) | 11 | 1.16 | (0.40-3.35) | ||||
| ≥30 | 43 | 1.89 | (0.95-3.77) | 26 | 2.10 | (0.80-5.48) | ||||
| Waist circumference ≥88 cm | ||||||||||
| No | 18 | 1.00 | Reference | 15 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Yes | 52 | 2.26 | (1.32-3.87) | 27 | 1.38 | (0.73-2.60) | 1.93 | (1.12-3.34) | 1.13 | (0.59-2.17) |
| Waist-to-hip ratio >0.85 | ||||||||||
| No | 35 | 1.00 | Reference | 25 | 1.00 | Reference | ||||
| Yes | 35 | 1.67 | (1.05-2.68) | 17 | 1.17 | (0.63-2.17) | ||||
| Leisure time physical activity, MET h/wk | ||||||||||
| <9.0 | 46 | 1.00 | Reference | 25 | 1.00 | Reference | ||||
| ≥9.0 | 22 | 0.68 | (0.41-1.13) | 16 | 0.90 | (0.48-1.68) | ||||
| Nonsteroidal anti-inflammatory drug use | ||||||||||
| No | 25 | 1.00 | Reference | 22 | 1.00 | Reference | ||||
| Yes | 45 | 1.34 | (0.81-2.19) | 20 | 0.78 | (0.42-1.44) | ||||
| History of hypertensionb | ||||||||||
| No | 10 | 1.00 | Reference | 6 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Yes | 60 | 1.90 | (0.97-3.74) | 36 | 2.25 | (0.94-5.37) | 1.62 | (0.82-3.21) | 2.12 | (0.87-5.15) |
| History of treated diabetes | ||||||||||
| No | 52 | 1.00 | Reference | 32 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Yes | 18 | 2.00 | (1.16-3.43) | 10 | 1.97 | (0.96-4.05) | 1.59 | (0.91-2.77) | 1.71 | (0.82-3.55) |
| History of myocardial infarction | ||||||||||
| No | 63 | 1.00 | Reference | 39 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Yes | 7 | 3.33 | (1.52-7.29) | 3 | 2.71 | (0.83-8.83) | 2.84 | (1.28-6.29) | 2.34 | (0.71-7.71) |
HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; MET = metabolic equivalent.
Variables in the multivariate model are those included in this column, namely age, smoking, waist circumference, history of hypertension, history of diabetes, and history of myocardial infarction.
A history of hypertension was ascertained through any of the following: study measurement of blood pressure at clinic visits, self-report of hypertension diagnosis, or report of antihypertensive treatment.
Additionally, in age-adjusted models, HFpEF risk was associated with smoking history (HR: 1.74), diabetes (HR: 2.00), and MI history (HR: 3.33), and a borderline significant risk was associated with hypertension in the expected direction (HR: 1.90). For HFrEF, a suggestion of higher risk was observed in relation to hypertension (HR: 2.25), diabetes (HR: 1.97), and prior MI (HR: 2.71) and to a lesser degree with smoking history (Table 3), although the lack of statistical significance may be due to power.
There was no association for either HFpEF or HFrEF with increasing metabolic equivalents/week of leisure-time physical activity, but for HFpEF the risk estimates appeared to be a nonsignificant reduced risk. There was no evidence of an association between HFpEF or HFrEF risk and BC characteristics.
In models adjusted simultaneously for anthropometric, lifestyle, and cardiometabolic disorders (Central Illustration), we observed associations to persist for a higher HFpEF risk in relation to smoking and elevated waist circumference; this was true both when waist circumference was examined with a cut point at 88 cm (Table 3) and when modeled by quartile (P trend = 0.048; data not shown). For HFrEF, in multivariable models, a history of smoking had a similar point estimate as with HFpEF, but waist circumference was not associated with an elevated risk when dichotomized at the cut point (Table 3) or in quartiles (P = 0.184; data not shown).
Central Illustration.
Risk of Heart Failure With Preserved Ejection Fraction, Heart Failure With Reduced Ejection Fraction, and Mortality in Breast Cancer Survivors
HRs, displayed on the logarithmic scale, and corresponding 95% CIs of selected risk factors in relation to hospitalized (top) heart failure with preserved ejection fraction and (bottom) heart failure with reduced ejection fraction. Risk factors were selected if associated with either heart failure subtype in age-adjusted models. All risk factors shown are included simultaneously in the multivariable model underlying the HRs shown. The risk between heart failure subtypes and mortality is also shown.
Sensitivity analyses
In sensitivity analysis, we investigated a cut point for LVEF of 40%. These risk estimates were largely similar to the estimates when HFrEF was defined as LVEF <50% (data not shown). Additionally, we did not detect any evidence of smoking inducing collider bias. Lastly, there was no appreciable difference in our results when accounting for competing risks using the Fine and Gray approach.
Exploratory analyses
When restricted to the LILAC subcohort (in whom BC treatment data were available; n = 1,149), in age-adjusted analysis, we detected a significant difference in risk between HFrEF and HFpEF associated with anthracycline treatment (P = 0.046) but did not detect a difference for left-sided radiation (P = 0.16). In multivariable analysis, anthracycline chemotherapy was associated with a marginally significant 2.47-fold increased risk of HFrEF (95% CI: 0.94-6.46) that was not observed for HFpEF.
HF subtypes and mortality
In relation to those without HF, the risk of overall mortality in BC survivors with hospitalized HFpEF was 5.65 (95% CI: 4.11-7.76), and in those with hospitalized HFrEF was 3.77 (95% CI: 2.51-5.66). When investigated for cause-specific mortality, BC survivors who developed HFpEF were at a 12.56-fold (95% CI: 7.68-20.56) increased risk of CVD-specific mortality (which included HF) and a 1.98-fold (95% CI: 0.69-5.73) increased risk of BC-specific mortality in relation to those without HF (P homogeneity = 0.002), and those who developed HFrEF were at 10.42-fold (95% CI: 5.96-18.22) and 2.18 (95% CI: 0.80-5.98) increased risks of CVD-specific and BC-specific mortalities, respectively (P homogeneity = 0.004).
HF risk in relation to BC diagnosis
In the larger data set of all WHI participants comprising women with and without invasive BC in the subcohort (n = 43,864), we investigated HF risk associated with a BC diagnosis. We observed BC patients to be at a 1.19-fold increased risk of HF overall (95% CI: 1.00-1.42) (Supplemental Table 2). The risks of HFpEF and HFrEF associated with BC diagnosis were similar (HR: 1.15 and 1.14, respectively) but were not statistically significant.
Discussion
In this prospective cohort of racially diverse postmenopausal BC survivors, these results demonstrate the relatively higher rate of hospitalized HFpEF than hospitalized HFrEF. This analysis demonstrated that anthropometric factors, a prior history of cardiometabolic disorders, and smoking were associated with an elevated risk of HFpEF, and broadly the same characteristics were suggestive of elevated HFrEF risk, indicating that BC survivors are affected by conventional risk factors for HF subtypes. In the literature to date, minimal attention has been given to HFpEF in BC survivors,7 potentially due to the diagnosis of HF largely being based on LVEF measures that may miss cases of HFpEF, and associated symptoms of HFpEF such as shortness of breath may be attributed to side effects of chemotherapy/radiation therapy or deconditioning rather than HFpEF. In the majority of BC trials and cardioprotection clinical trials, the focus of monitoring during treatment has relied on LVEF,6 rather than a more holistic view of cardiac function that incorporates diastolic parameters in addition to systolic function.
Similar to observations of a higher incidence of HFpEF than HFrEF in older women in the general population,7,8 we observed a higher incidence of HFpEF in this older population of BC survivors. Despite the known elevated HFpEF risk in older women, it remains an understudied component of HF after BC. However, HFpEF contributes to an equal proportion of hospitalizations as HFrEF and an equally increased risk for long-term mortality after diagnosis.23,24 Hence, it is critical to understand the incidence of HFpEF after BC, and in this population, it is important to identify women at greatest risk. In this data set with adjudicated HF events containing LVEF obtained from a medical record review, we were able to assess the incidence of HFpEF, finding that in postmenopausal women HFpEF incidence was higher relative to HFrEF during the entire follow-up after BC diagnosis. As such, it places into context the risks associated with each diagnosis for postmenopausal women after a BC diagnosis and emphasizes the need to monitor for HF even in the presence of normal LVEF.
This analysis investigated risk factors separately for hospitalized HFpEF and hospitalized HFrEF. In the general population, there exists a stronger relationship for obesity with HFpEF than with HFrEF.8 Eaton et al8 demonstrated in a WHI cohort that obesity was associated with HFpEF but not HFrEF risk. Specifically, women with a BMI of 30 to <35 were at a 1.4-fold higher HFpEF risk and those with BMI ≥35 a 2.2-fold higher HFpEF risk compared with HFrEF risk of 1.0 and 0.87, respectively. This contrast was not observed between HFpEF and HFrEF in BC survivors because BMI appeared related to both HF subtypes to a similar degree. As a prior meta-analysis showed, obesity (BMI ≥30) at BC diagnosis was associated with an increased risk of anthracycline-induced cardiotoxicity (defined as LVEF decline of ≥10% or decline below 50%),14 and elevated central adiposity was associated with LVEF declines of 5% in cancer survivors.13 Thus, it is plausible that obesity confers a greater risk of HFrEF in a BC population than in the general population because of the interaction with cancer treatment.
Additional lifestyle factors and a history of cardiometabolic disorders were associated with a higher risk of HF overall8,10,11 and HFpEF in our study. These findings largely align with observations within the general population.8,10,11 Only waist circumference, MI history, and smoking remained significantly associated with HFpEF in multivariable models. Central adiposity has been suggested to play an important role in HFpEF etiology,25 which is supported by findings in this cohort of BC survivors. In particular, a high waist circumference emerged as the strongest lifestyle factor for HFpEF.
Although the 2 HF subtypes have similarities, including the hallmark symptom of shortness of breath upon exertion, there are clear distinctions. The physiologic mechanisms underlying ventricular remodeling, a defining feature of HFpEF,26,27 involve increased left ventricular afterload due to hypertension28 and a proinflammatory milieu due to obesity and metabolic disorders.29,30 The role of central adiposity in particular is supported by the finding that increased visceral adiposity is associated with elevations in systemic inflammation.25 Thus, a link between waist circumference and HFpEF in our data set may indicate that similar mechanisms involving central adiposity play a role for BC survivors. Investigation of central adiposity in HFpEF within cancer survivors has received minimal attention in the literature. A prospective study at Kaiser Permanente showed a 1.4-fold increased CVD risk in relation to the highest tertile of visceral adiposity; however, this analysis did not investigate HF independently or stratify by HF subtype.31
Our finding that smoking is a salient risk factor for hospitalized HF in BC survivors parallels findings from large cohort studies in noncancer populations. The biological mechanisms of HFrEF are better understood than HFpEF. Smoking has been associated with atherosclerotic changes and cardiac remodeling, reduced systolic LV function, and HFrEF32,33 and has been associated with impaired left ventricular diastolic function and ventricular remodeling, both of which are associated with HFpEF.34, 35, 36 It is likely that multiple pathways are activated with smoking contributing to both HFpEF and HFrEF.
Interestingly, we found a significant association between MI history and HFpEF, which was not observed in previous studies in the general population. However, a substantial minority (30%) of individuals with HFpEF report a history of MI.37 It is plausible that a history of MI before cancer treatment may be a marker of increased inflammatory milieu, which may interact with treatment to create the conditions for HFpEF. Additionally, shared risk factors may predispose to MI, cancer, and HFpEF.38 A history of diabetes was significantly associated with HFpEF, and a history of hypertension was of borderline significance, although these relationships did not persist after adjustment for multiple variables. In the general population, diabetes and hypertension have been associated with the risk of both HFrEF and HFpEF and have been linked to pathophysiologic changes associated with HFrEF and HFpEF.8,39,40 The lack of statistically significant findings with respect to HFrEF likely is due to the relative rarity of HFrEF after BC in this cohort.
Additionally, we observed BC itself to be a risk factor for HF when analysis was expanded to the larger WHI population of both BC survivors and women without BC. Although the point estimates were similar for HFpEF and HFrEF compared with HF overall (HR: 1.15, 1.14, and 1.19, respectively), the elevated risk associated with BC was not significant for HFpEF and HFrEF. This requires investigation in a data set in which greater numbers of BC survivors with HF subtypes have been determined.
We conducted an exploratory analysis to investigate the relationship between HF subtypes and cancer treatment for which associations with LVEF decline have been documented.4, 5, 6 As anticipated, we found a significant difference in anthracycline-associated risks between HFrEF and HFpEF, with an increased risk observed only for HFrEF.
Study limitations
A limitation of our study was lack of sufficient power to investigate cancer treatment in relation to HFpEF and HFrEF because of the need to restrict the sample to participants who were in both the subcohort (in which HF subtypes were determined) and the LILAC subcohort (in which BC treatment was ascertained). The relationship between specific cancer treatments and differential risks for HFpEF and HFrEF requires interrogation in a larger data set. In addition, the lack of statistically significant results for some of the associations investigated in relation to HFrEF likely was driven by the relative rarity of HFrEF because only 42 HFrEF cases arose during follow-up. Lastly, our study only included older, postmenopausal women, so the generalizability of our findings to younger, premenopausal women is unknown. A strength of our study was the large, racially diverse sample of women with BC in whom HF was adjudicated over long-term follow-up. An additional strength is the use of a well-validated method for adjudicating acute hospitalized HF and classifying HF subtypes.
Conclusions
Our study is notable because it puts into context the increased risk for HFpEF in postmenopausal BC survivors, which has historically been under-recognized and understudied. HFpEF may be a contributor to long-term morbidity and mortality in these patients, particularly because our data suggest a higher overall mortality for BC survivors with subsequent HFpEF. This indicates a need to monitor for clinical HF overall and suggests that LVEF assessment alone is insufficient for monitoring BC survivors in the long-term. Importantly, we observed similar elevations in the risk of HFpEF and HFrEF in relation to MI history. We also demonstrate a robust association between central adiposity and subsequent HFpEF in this cohort of BC survivors, whereas we found no evidence of an association with HFrEF. Because of the biological plausibility for its role in HFpEF risk, further examination of the role of central adiposity as measured by visceral adiposity is warranted. Importantly, this line of research may provide actionable findings that inform interventions targeting central adiposity as a means of mitigating risk of HFpEF.41
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Our study demonstrates that HFpEF was more common than HFrEF in postmenopausal (age ≥50 years) BC survivors. The results highlight the need to monitor for clinical HF overall and that LVEF assessment alone is insufficient for monitoring BC survivors in the long-term. Risk factors for HFpEF largely corresponded to those of the general population and include older age, prior MI, central adiposity, history of smoking, hypertension, and diabetes. Compared with those without HF, the risk of mortality in BC survivors with hospitalized HFpEF or HFrEF was increased.
TRANSLATIONAL OUTLOOK: Future studies are needed: 1) to inform optimal monitoring strategies for BC survivors for the development of HFpEF and not only HFrEF; and 2) to develop effective strategies to target modifiable factors, such as smoking and central adiposity, to reduce the risk of HF. In addition, additional mechanistic and clinical data are needed to better understand how BC may represent an independent risk factor for HF overall because our data show an increased risk of HF, even after consideration for risk factors such as hypertension and tobacco use.
Funding Support and Author Disclosures
The WHI program is funded by National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts, HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors wish to thank the participants of WHI for their involvement and the WHI staff for their dedication to this study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.American Cancer Society . American Cancer Society, Inc; 2019. Breast Cancer Facts and Figures 2019-2020. [Google Scholar]
- 2.Bradshaw P.T., Stevens J., Khankari N., Teitelbaum S.L., Neugut A.I., Gammon M.D. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27:6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardinale D., Colombo A., Bacchiani G., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 5.Du X.L., Xia R., Liu C.C., et al. Cardiac toxicity associated with anthracycline-containing chemotherapy in older women with breast cancer. Cancer. 2009;115:5296–5308. doi: 10.1002/cncr.24621. [DOI] [PubMed] [Google Scholar]
- 6.Lin K.J., Lengacher C.A. Anthracycline chemotherapy–induced cardiotoxicity in breast cancer survivors: a systematic review. Oncol Nurs Forum. 2019;46:E145–E158. doi: 10.1188/19.ONF.E145-E158. [DOI] [PubMed] [Google Scholar]
- 7.Haykowsky M.J., Beaudry R., Brothers R.M., Nelson M.D., Sarma S., La Gerche A. Pathophysiology of exercise intolerance in breast cancer survivors with preserved left ventricular ejection fraction. Clin Sci. 2016;130:2239–2244. doi: 10.1042/2Fcs20160479. [DOI] [PubMed] [Google Scholar]
- 8.Eaton C.B., Pettinger M., Rossouw J., et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. 2016;9:139–148. doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy Y.N., Borlaug B.A. Heart failure with preserved ejection fraction. Curr Probl Cardiol. 2016;41:145–188. doi: 10.1016/j.cpcardiol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Sandesara P.B., Samman-Tahhan A., Topel M., Venkatesh S., O’Neal W.T. Effect of cigarette smoking on risk for adverse events in patients with heart failure and preserved ejection fraction. Am J Cardiol. 2018;122:400–404. doi: 10.1016/j.amjcard.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson M., Dardari Z., Kianoush S., et al. Relation between cigarette smoking and heart failure (from the Multiethnic Study of Atherosclerosis) Am J Cardiol. 2019;123(12):1972–1977. doi: 10.1016/j.amjcard.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saiki H., Petersen I.A., Scott C.G., et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135(15):1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reding K.W., Ghemigian K., Carbone S., et al. The relationship between abdominal fat and change in left ventricular ejection fraction in cancer patients. Obes Sci Pract. 2021;7:82–90. doi: 10.1002/osp4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenancia C., Lefebvre A., Cardinale D., et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2016;34(26):3157–3165. doi: 10.1200/JCO.2016.67.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel V.G., Gupta D.K., Terry J.G., et al. Left ventricular function across the spectrum of body mass index in African Americans: the Jackson Heart Study. J Am Coll Cardiol HF. 2017;5(3):182–190. doi: 10.1016/j.jchf.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman R.J., Aziz N., Albanes D., et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89(5):2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 17.Anderson G.L., Manson J., Wallace R., et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 18.Curb J.D., Mctiernan A., Heckbert S.R., et al. Outcomes ascertainment and adjudication methods in the women’s health initiative. Ann Epidemiol. 2003;13(suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 19.Paskett E.D., Caan B.J., Johnson L., et al. The women’s health initiative (WHI) life and longevity after cancer (LILAC) study: description and baseline characteristics of participants. Cancer Epidemiol Biomarkers Prev. 2018;27(2):125–137. doi: 10.1158/1055-9965.EPI-17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 21.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Caan B.J., Feliciano E.M., Kroenke C.H. The importance of body composition in explaining the overweight paradox in cancer-counterpoint. Cancer Res. 2018;78:1906–1912. doi: 10.1158/2F0008-5472.can-17-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancy C.W., Lopatin M., Stevenson L.W., De Marco T., Fonarow G.C. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol. 2006;47(1):76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow G.C., Stough W.G., Abraham W.T., et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. A Report From the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50(8):768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 25.Kitzman D.W., Shah S.J. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68(2):200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Aurigemma G.P., Zile M.R., Gaasch W.H. Contractile behavior of the left ventricle in diastolic heart failure: With emphasis on regional systolic function. Circulation. 2006;113(2):296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 27.Aurigemma G.P., Gaasch W.H. Clinical practice. Diastolic heart failure. N Engl J Med. 2004;351(11):1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obokata M., Reddy Y.N., Pislaru S.V., Melenovsky V., Borlaug B.A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy Y.N., Lewis G.D., Shah S.J., et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: A RELAX trial ancillary study. Mayo Clin Proc. 2019;94(7):1199–1209. doi: 10.1016/j.mayocp.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Cespedes Feliciano E.M., Chen W.Y., Bradshaw P.T., et al. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J Clin Oncol. 2019;37(28):2528–2536. doi: 10.1200/JCO.19.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEvoy J.W., Nasir K., Defilippis A.P., et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):1002–1010. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Csordas A., Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10(4):219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 34.Nadruz W., Claggett B., Gonçalves A., et al. Smoking and cardiac structure and function in the elderly: the ARIC study (Atherosclerosis Risk in Communities) Circ Cardiovasc Imaging. 2016;9(9) doi: 10.1161/CIRCIMAGING.116.004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah A.M. Ventricular remodeling in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):341–349. doi: 10.1007/s11897-013-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heckbert S.R., Post W., Pearson G.D.N., et al. Traditional Cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham J.W., Vaduganathan M., Claggett B.L., et al. Myocardial infarction in heart failure with preserved ejection fraction: pooled analysis of 3 clinical trials. J Am Coll Cardiol HF. 2020;8(8):618–626. doi: 10.1016/j.jchf.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 38.de Boer R.A., Aboumsallem J.P., Bracun V., et al. A new classification of cardio-oncology syndromes. Cardiooncology. 2021;7(1):24. doi: 10.1186/s40959-021-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devereux R.B., Roman M.J., Paranicas M., et al. Impact of diabetes on cardiac structure and function: the Strong Heart study. Circulation. 2000;101(19):2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson M.J., Zadourian A., Taub P.R. Heart Failure and diabetes mellitus: defining the problem and exploring the interrelationship. Am J Cardiol. 2019;124(suppl 1):S3–S11. doi: 10.1016/j.amjcard.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 41.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:E997–E1012. doi: 10.1161/2Fcir.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.