Abstract
Background
Studies assessing whether heart failure (HF) is associated with cancer and cancer-related mortality have yielded conflicting results.
Objectives
This study assessed cancer incidence and mortality according to pre-existing HF in a community-based cohort.
Methods
Among individuals ≥50 years of age from the Puglia region in Italy with administrative health data from 2002 to 2018, no cancer within 3 years before the baseline evaluation, and ≥5-year follow-up, the study matched 104,020 subjects with HF at baseline with 104,020 control subjects according to age, sex, drug-derived complexity index, Charlson comorbidity index, and follow-up duration. Cancer incidence and mortality were defined based on International Classification of Diseases-Ninth Revision codes in hospitalization records or death certificates.
Results
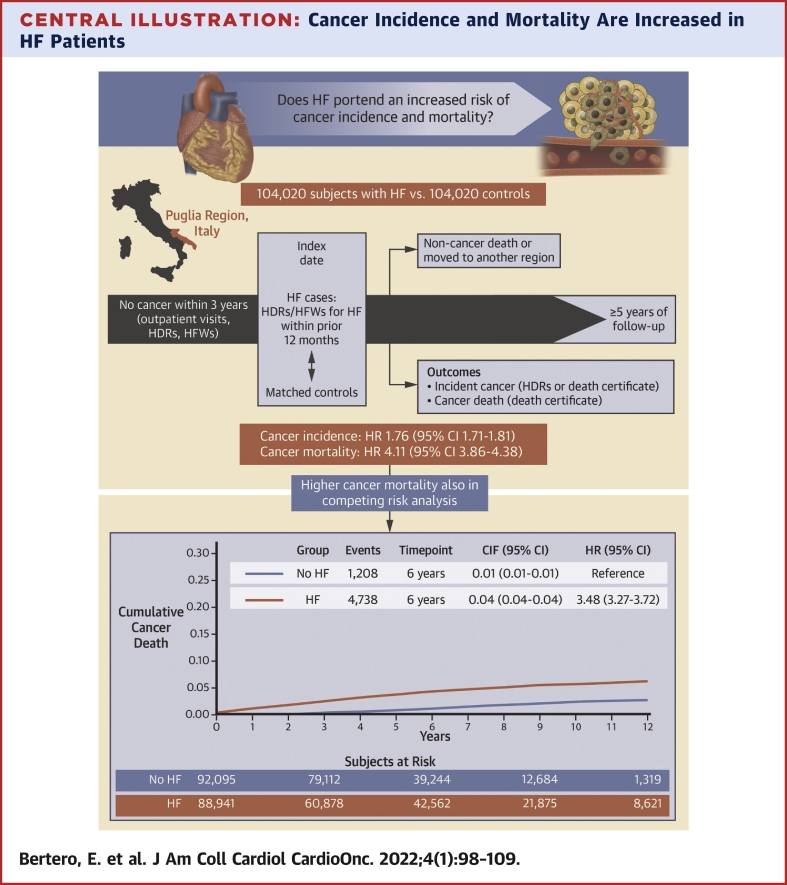
The incidence rate of cancer in HF patients and control subjects was 21.36 (95% CI: 20.98-21.74) and 12.42 (95% CI: 12.14-12.72) per 1000 person-years, respectively, with the HR being 1.76 (95% CI: 1.71-1.81). Cancer mortality was also higher in HF patients than control subjects (HR: 4.11; 95% CI: 3.86-4.38), especially in those <70 years of age (HR: 7.54; 95% CI: 6.33-8.98 vs HR: 3.80; 95% CI: 3.44-4.19 for 70-79 years of age; and HR: 3.10; 95% CI: 2.81-3.43 for ≥80 years of age). The association between HF and cancer mortality was confirmed in a competing risk analysis (subdistribution HR: 3.48; 95% CI: 3.27-3.72). The HF-related excess risk applied to the majority of cancer types. Among HF patients, prescription of high-dose loop diuretic was associated with higher cancer incidence (HR: 1.11; 95% CI: 1.03-1.21) and mortality (HR: 1.35; 95% CI: 1.19-1.53).
Conclusions
HF is associated with an increased risk of cancer and cancer-related mortality, which may be heightened in decompensated states.
Key Words: cardio-oncology, comorbidity, cancer, heart failure, mortality
Abbreviations and Acronyms: ATC, Anatomical Therapeutic Chemical; CCI, Charlson comorbidity index; DDCI, drug-derived complexity index; DP, drug prescription; HDR, hospital discharge record; HF, heart failure; HFW, health care cost-related fee waiver; ICD-9-CM, International Classification of Diseases-Ninth Revision-Clinical Modification; IR, incidence rate; SHR, subdistribution HR
Central Illustration
The association between heart failure (HF) and cancer has gained increasing attention in the last years.1, 2, 3 The coexistence of these 2 entities is increasingly common caused by the progressive aging of the population and the growth in risk factors predisposing to both conditions, posing a substantial clinical and economic burden.
The complex and bidirectional nature of the interaction between HF and cancer has been recently addressed by epidemiological and preclinical studies.2 On the one hand, cardiovascular disease occurs more commonly in patients with malignancies, compared with those without,4 and is more frequent in cancer survivors than in the general population, regardless of the age at which tumors are diagnosed and treated.5,6 On the other hand, HF patients might have a higher risk of incident cancer compared with individuals without HF,7, 8, 9, 10, 11 and emerging evidence suggests that HF represents an oncogenic condition.12,13 However, other studies have questioned this observation, proposing that the excess risk of cancer in HF patients is mainly driven by comorbidities.14,15 Moreover, the burden of cancer in HF has also increased as a result of the abatement of cardiovascular mortality attained by the advances in HF treatment.16
Thus, the interconnection between cancer and HF is increasingly recognized, but it remains unclear whether HF is associated with an excess risk of cancer and cancer-related mortality. In this context, we assessed cancer incidence and mortality in a large cohort of HF patients compared with matched control subjects.
Methods
We conducted a retrospective community-based cohort study using anonymized administrative health care records of Puglia, a region in southern Italy with a population of ∼4 million inhabitants. No Ethics Committee approval was required by our institution for the analysis of such data.
Data sources
The data sources for this study consisted of drug prescriptions (DPs), outpatient visit reports, hospital discharge records (HDRs), health care cost-related fee waivers (HFWs), and death certificates. In the Italian health care system, DPs are coded according to the Anatomical Therapeutic Chemical (ATC) classification and diagnoses are coded according to the International Classification of Diseases-9th Revision-Clinical Modification (ICD-9-CM).17 HDRs provide information on primary diagnoses and up to 5 coexisting conditions, procedures performed during the hospitalization, dates of admission and discharge, and in-hospital death. The reliability of these sources and of record linkage for the purpose of epidemiological investigations was previously validated.18, 19, 20, 21, 22, 23, 24
Selection of cases and control subjects
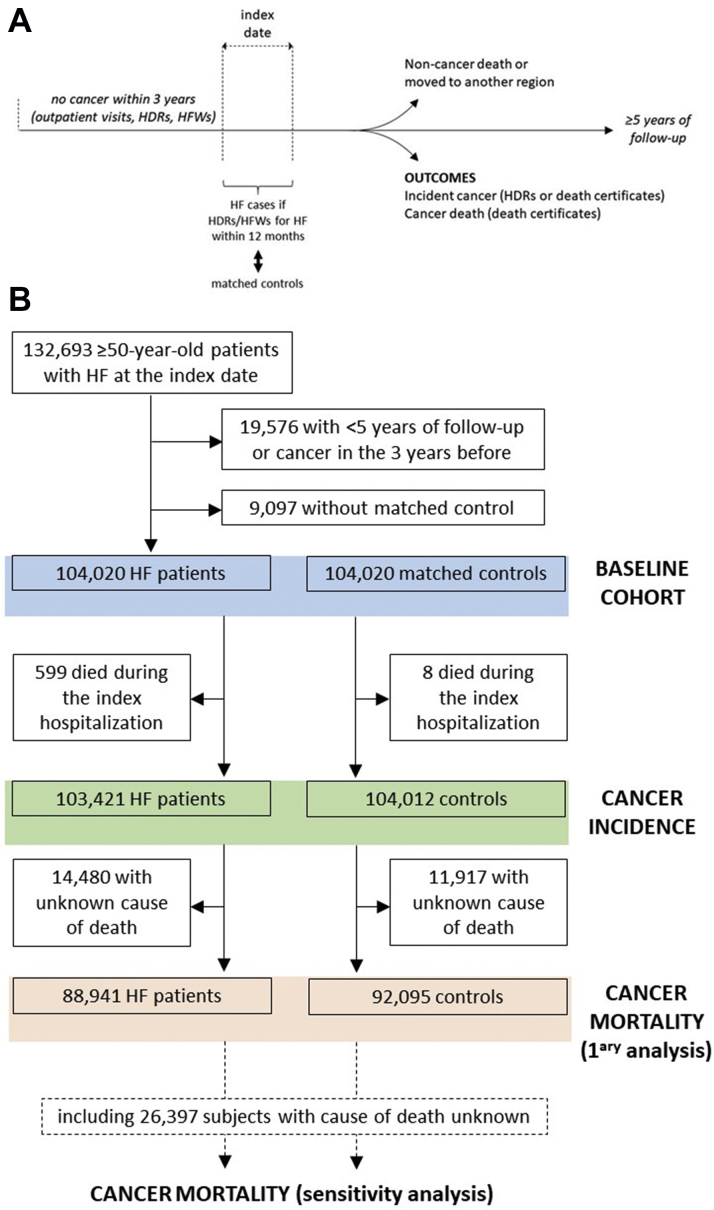
The study included individuals who were ≥50 years of age between January 1, 2005, and December 31, 2013, did not have a history of cancer in the 3 years before inclusion, and had a follow-up of ≥5 years unless they were diagnosed with cancer, died, or moved to another region (Figure 1). Cancer was assessed by ICD-9-CM or ATC codes assigned in outpatient visits, HDRs, and HFWs (Supplemental Table 1).
Figure 1.
Study Design
(A) Cancer incidence and mortality were evaluated among individuals who were ≥50 years of age at the index date, did not have a history of cancer in the 3 years before inclusion, and had a follow-up of ≥5 years unless they were diagnosed with cancer, died, or moved to another region. (B) Selection of heart failure (HF) cases and control subjects for the assessment of the study outcomes. HDR = hospital discharge record; HFW = health care cost-related fee waiver.
We identified as HF cases those subjects who had a HDR with a diagnosis of HF or received a HFW for HF within the previous 12 months (Supplemental Table 2); the date of the hospital admission or HWF was considered as the index date. This approach, which was previously validated,25,26 relies on the fact that the Italian health care system provides coverage to virtually the entire resident population, and thus any person with HF leading to hospital admission (including emergency department visits with subsequent discharge) or needing drug prescription is identified.
Control subjects were selected by excluding HF according to the same criteria and were matched 1:1 to cases based on age, sex, drug-derived complexity index (DDCI), and follow-up duration (Figure 1). In order to minimize the potential confounding effect of the socioeconomic status, we also performed a case-control matching within each of the 43 health care districts (urban and/or industrialized and rural) of the Puglia region. Individuals who were admitted to hospital at the index date were also matched based on Charlson comorbidity index (CCI). CCI and DDCI were computed as previously described.27
Baseline characteristics
Use of HF medications in the 12 months preceding the index date was determined based on ATC codes as listed in Supplemental Table 3: these drugs included beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, renin inhibitors, sacubitril-valsartan, diuretics, cardiac glycosides, antiarrhythmics, cardiac or peripheral vasodilators, and calcium-channel blockers. Prescription of loop diuretic agents in the 12 months before the index date was also assessed, as the daily dose of furosemide (ATC codes C03CA01, C03CB01, and C03EB01) and the double of the daily dose of torsemide (ATC code C03CA04). Furthermore, implantation of an electronic device or a left ventricular assist device within the previous 36 months was ascertained based on HDRs (Supplemental Table 3).
Outcome variables
The study outcomes were cancer incidence and mortality (Figure 1). Incident cancer consisted of a new diagnosis of cancer or death from cancer during follow-up, as inferred by the HDRs and death certificates, respectively. Time to incident cancer was calculated from the index date to the earliest diagnosis of cancer during follow-up, or to death from cancer if no diagnosis was made before. Cancer mortality was assessed based on death certificates with ICD-9-CM codes 140.x-208.x and 209.0-209.3, which include all most common types of solid and hematologic malignancies.
The outcomes were also evaluated by cancer type, as defined according to the ICD-9-CM codes listed in Supplemental Table 4.
Statistical analysis
Data are presented as mean ± SD or as count and percentage. Descriptive analyses were performed by Student’s t test, and chi-square test, as appropriate. Incidence rates (IRs) with 95% CIs per 1000 person-years were estimated for the outcomes in the case and control groups using Poisson regression with exact CI. Cause-specific HRs and 95% CIs were then obtained using Cox regression comparing cases with control subjects to study the etiology of the disease. A model using time-dependent explanatory variables was used to assess proportional hazards assumption. All-cause mortality was also evaluated by Kaplan-Meier analysis. Furthermore, cancer-specific mortality cumulative incidence rates were estimated using Fine and Gray's regression model (eg, subdistribution hazards) to better predict an individual's risk, considering death for noncancer causes as a competing event.
To investigate whether HF severity influenced the study outcomes, HF patients who had been prescribed ≥80 mg/d of furosemide equivalent for ≥30 days in the year before the index date (high-dose group) were compared with those who had been prescribed less or no loop diuretic.
Results were considered statistically significant when P < 0.05. All statistical analyses were performed using SAS Software Release 9.4 (SAS Institute).
Results
Baseline characteristics of the study population
We identified 132,693 patients ≥50 years of age with HF at baseline. Of these, we excluded 19,576 patients with duration of follow-up <5 years or a history of cancer in the 3 years preceding the index date, and 9,097 cases without a matched control subject, leaving a sample of 104,020 HF patients and 104,020 control subjects.
The baseline characteristics and cardiovascular medications of the study cohort according to HF status and age at the index date are presented in Table 1 and Supplemental Table 5. Overall, the mean age was 76 ± 10 years. The proportion of males progressively decreased in both cases and control subjects across the age groups (<70, 70 to 79, and ≥80 years of age), while the DDCI increased (Supplemental Table 5). As expected, follow-up was longer for younger individuals. At any age, prescription of cardiovascular medications as well as cardiac device therapy was significantly more common in HF patients than in control subjects (Table 1).
Table 1.
Baseline Characteristics of the Study Population by HF Status and Age Group
| <70 Years of Age (n = 57,328) |
70-79 Years of Age (n = 70,156) |
≥80 Years of Age (n = 80,556) |
Total (N = 208,040) |
|||||
|---|---|---|---|---|---|---|---|---|
| HF Patients (n = 28,664) | Control Subjects (n = 28,664) | HF Patients (n = 35,078) | Control Subjects (n = 35,078) | HF Patients (n = 40,278) | Control Subjects (n = 40,278) | HF Patients (n = 104,020) | Control Subjects (n = 104,020) | |
| Male | 17,585 (61.3) | 17,585 (61.3) | 16,592 (47.3) | 16,592 (47.3) | 14,476 (35.9) | 14,476 (35.9) | 48,653 (46.8) | 48,653 (46.8) |
| Follow-up, y | 8.5 (5.8-11.3) | 8.3 (6.3-10.0) | 6.2 (2.9-9.0) | 6.0 (4.8-7.3) | 2.6 (0.4-5.6) | 3.3 (2.5-4.1) | 5.6 (1.8-8.8) | 5.3 (3.6-7.4) |
| Cardiovascular risk factors | ||||||||
| Hypertension | 23,023 (80.3) | 19,780 (69.0) | 30,879 (88.0) | 29,277 (83.5) | 35,385 (87.8) | 34,111 (84.7) | 89,287 (85.8) | 83,168 (80.0) |
| Dyslipidemia | 12,012 (41.9) | 6,501 (22.7) | 15,378 (43.8) | 10,011 (28.5) | 12,624 (31.3) | 8,988 (22.3) | 40,014 (38.5) | 25,500 (24.5) |
| Diabetes mellitus | 8,917 (31.1) | 7,871 (27.5) | 12,484 (35.6) | 11,022 (31.4) | 11,184 (27.8) | 10,271 (25.5) | 32,585 (31.3) | 29,164 (28.0) |
| Cardiovascular diseases | ||||||||
| Stroke | 3,894 (13.6) | 1,348 (4.7) | 7,796 (22.2) | 3,453 (9.8) | 10,374 (25.8) | 5,168 (12.8) | 22,064 (21.2) | 9,969 (9.6) |
| Myocardial infarction | 5,695 (19.9) | 1,072 (3.7) | 5,993 (17.1) | 1,325 (3.8) | 5,424 (13.5) | 1,167 (2.9) | 17,112 (16.4) | 3,564 (3.4) |
| Stable CAD | 8,783 (30.6) | 2,403 (8.4) | 11,365 (32.4) | 3,841 (10.9) | 11,167 (27.7) | 3,981 (9.9) | 31,315 (30.1) | 10,225 (9.8) |
| Atrial fibrillation | 6,160 (21.5) | 1,149 (4.01) | 11,363 (32.4) | 2,623 (7.5) | 15,025 (37.3) | 3,335 (8.3) | 32,548 (31.3) | 7,107 (6.8) |
| PCI | 4,005 (14.0) | 1,124 (3.9) | 3,554 (10.1) | 1,367 (3.9) | 1,931 (4.8) | 742 (1.8) | 9,490 (9.1) | 3,233 (3.1) |
| Peripheral artery disease | 2,603 (9.1) | 630 (2.2) | 3,745 (10.7) | 1,080 (3.1) | 2,933 (7.3) | 983 (2.4) | 9,281 (8.9) | 2,693 (2.6) |
| Pulmonary embolism | 147 (0.5) | 23 (0.1) | 255 (0.7) | 66 (0.2) | 360 (0.9) | 95 (0.2) | 762 (0.7) | 184 (0.2) |
| Chronic kidney disease | 2,106 (7.3) | 353 (1.2) | 4,418 (12.6) | 771 (2.2) | 7,942 (19.7) | 1,591 (3.9) | 14,466 (13.9) | 2,715 (2.6) |
| Cardiovascular drugs | ||||||||
| Cardiac glycosides | 4,552 (15.9) | 1,304 (4.5) | 8,509 (24.3) | 4,361 (12.4) | 13,245 (32.9) | 10,079 (25.0) | 26,306 (25.3) | 15,744 (15.1) |
| Antiarrhythmics | 3,996 (13.9) | 1,771 (6.2) | 6,707 (19.1) | 4,105 (11.7) | 7,145 (17.7) | 5,319 (13.2) | 17,848 (17.2) | 11,195 (10.8) |
| Cardiac vasodilators | 5,156 (18.0) | 2,224 (7.8) | 9,153 (26.1) | 4,761 (13.6) | 11,619 (28.8) | 7,621 (18.9) | 25,928 (24.9) | 14,606 (14.0) |
| Peripheral vasodilators | 0 (0.0) | 2 (0.0) | 0 (0.0) | 4 (0.0) | 43 (0.1) | 71 (0.2) | 43 (0.0) | 77 (0.1) |
| Diuretics | 17,995 (62.8) | 13,392 (46.7) | 27,332 (77.9) | 22,978 (65.5) | 32,850 (81.6) | 28,763 (71.4) | 78,177 (75.2) | 65,133 (62.2) |
| Beta-blockers | 10,813 (37.7) | 8,008 (27.9) | 12,099 (34.5) | 9,665 (27.5) | 10,461 (26.0) | 7,798 (19.4) | 33,373 (32.1) | 25,471 (24.5) |
| Calcium-channel blockers | 10,131 (35.3) | 7,959 (27.8) | 16,589 (47.3) | 13,806 (39.4) | 18,548 (46.0) | 15,878 (39.4) | 45,268 (43.5) | 37,643 (36.2) |
| ACE inhibitors | 15,079 (52.6) | 12,118 (42.3) | 21,654 (61.7) | 19,084 (54.4) | 24,716 (61.4) | 22,683 (56.3) | 61,449 (59.1) | 53,885 (51.8) |
| ARBs | 10,017 (34.9) | 7,777 (27.1) | 15,279 (43.6) | 12,473 (35.6) | 16,914 (42.0) | 14,064 (34.9) | 42,210 (40.6) | 34,314 (33.0) |
| Renin inhibitors | 41 (0.1) | 12 (0.0) | 65 (0.2) | 19 (0.1) | 57 (0.1) | 10 (0.0) | 163 (0.2) | 41 (0.0) |
| Sacubitril-valsartan | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Device therapy | 2,224 (7.8) | 205 (0.7) | 3,466 (9.9) | 665 (1.9) | 4,089 (10.1) | 1,286 (3.2) | 9,779 (9.4) | 2,156 (2.1) |
Values are n (%) or median (interquartile range). Within each age group, all comparisons of the frequency of cardiovascular drugs between HF patients and control subjects were significant with P < 0.001, with the exception of peripheral vasodilators, for which P = 0.157 for <70 years; P = 0.045 for 70-79 years; P = 0.009 for ≥80 years, and P = 0.002 for any age.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; CAD = coronary artery disease; HF = heart failure; PCI = percutaneous coronary intervention.
Incident cancer
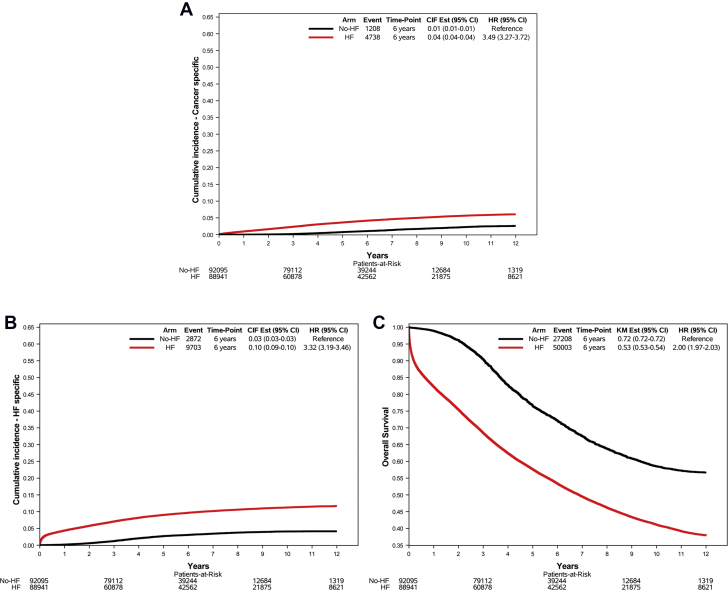
Because 599 cases and 8 control subjects were hospitalized at the index date and eventually died in the hospital, cancer incidence could be assessed in 103,421 cases and 104,012 control subjects (Figure 1). We identified a total of 12,036 new diagnoses of cancer in HF patients and 7,045 in control subjects over a median follow-up of 5 (IQR: 3.1-8.0) years. The IR of cancer was 21.36 (95% CI: 20.98-21.74) per 1000 person-years in patients with HF and 12.42 (95% CI: 12.14-12.72) per 1000 person-years in control subjects, corresponding to an HR of 1.76 (95% CI: 1.71-1.81). Table 2 and Supplemental Table 6 show the IRs and HRs for cancer according to cancer type and age at the index date. While the IR of all types of cancer increased with age, the HF-related excess risk was consistent across all age groups. In subjects <70 years of age, the IR of cancer was 16.32 (95% CI: 15.81-16.86) in those with HF and 9.72 (95% CI: 9.32-10.14) in those without (HR: 1.66; 95% CI: 1.58-1.75). In the group 70-79 years of age, the IR was 24.06 (95% CI: 23.40-24.74) in HF patients and 14.64 (95% CI: 14.13-15.17) in control subjects (HR: 1.69; 95% CI: 1.61-1.77). Finally, in individuals ≥80 years of age, the IR of cancer was 25.73 (95% CI: 24.88-26.61) in HF cases and 13.61 (95% CI: 12.99-14.25) in control subjects (HR: 2.07; 95% CI: 1.95-2.19).
Table 2.
Incidence Rates per 1000 Person-Years in HF Patients vs Control Subjects for the Most Common Cancers
| <70 Years of Age (n = 57,261) |
70-79 Years of Age(n = 70,022) |
≥80 Years of Age(n = 80,150) |
Total (N = 207,433) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF Patients |
Control Subjects |
HR (95% CI) | HF Patients |
Control Subjects |
HR (95% CI) | HF Patients |
Control Subjects |
HR (95% CI) | HF Patients |
Control Subjects |
HR (95% CI) | |
| IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | |||||
| Any cancer | 16.32 (15.8-16.86) | 9.72 (9.32-10.14) | 1.66 (1.58-1.75)a | 24.06 (23.40-24.74) | 14.64 (14.13-15.17) | 1.69 (1.61-1.77)a | 25.73 (24.88-26.61) | 13.61 (12.99-14.25) | 2.07 (1.95-2.19)a | 21.36 (20.98-21.74) | 12.42 (12.14-12.72) | 1.76 (1.71-1.81)a |
| Colorectal | 2.17 (1.99-2.37) | 1.43 (1.28-1.59) | 1.50 (1.31-1.73)a | 3.21 (2.98-3.47) | 2.45 (2.25-2.67) | 1.35 (1.20-1.52)a | 3.97 (3.65-4.33) | 2.69 (2.43-2.98) | 1.64 (1.43-1.88)a | 2.98 (2.84-3.12) | 2.10 (1.98-2.22) | 1.46 (1.35-1.57)a |
| Lung | 2.89 (2.68-3.12) | 0.58 (0.49-0.68) | 5.02 (4.16-6.06)a | 4.14 (3.87-4.43) | 1.04 (0.91-1.19) | 4.16 (3.58-4.84)a | 3.57 (3.26-3.90) | 0.83 (0.69-1.00) | 4.89 (3.97-6.03)a | 3.50 (3.35-3.66) | 0.81 (0.74-0.88) | 4.49 (4.06-4.98)a |
| Female breast | 2.59 (2.28-2.95) | 2.31 (2.02-2.65) | 1.10 (0.91-1.33) | 3.09 (2.78-3.43) | 2.96 (2.66-3.29) | 1.08 (0.92-1.25) | 2.73 (2.40-3.09) | 2.87 (2.53-3.24) | 1.00 (0.83-1.20) | 2.83 (2.64-3.03) | 2.73 (2.55-2.93) | 1.05 (0.95-1.15) |
| Male reproductive system | 2.67 (2.41-2.96) | 3.57 (3.27-3.90) | 0.75 (0.65-0.86)a | 4.59 (4.17-5.05) | 5.73 (5.26-6.24) | 0.86 (0.75-0.98)c | 6.65 (5.94-7.46) | 5.21 (4.58-5.92) | 1.39 (1.16-1.66)a | 3.97 (3.74-4.22) | 4.56 (4.32-4.82) | 0.90 (0.83-0.98)c |
| Lymphoma | 0.59 (0.50-0.70) | 0.29 (0.22-0.36) | 2.00 (1.48-2.70)a | 0.66 (0.56-0.78) | 0.43 (0.35-0.53) | 1.58 (1.20-2.07)b | 0.58 (0.46-0.73) | 0.28 (0.21-0.39) | 2.31 (1.56-3.44)a | 0.61 (0.55-0.68) | 0.34 (0.29-0.39) | 1.84 (1.54-2.20)a |
| Multiple myeloma | 0.42 (0.34-0.51) | 0.19 (0.14-0.26) | 2.16 (1.50-3.11)a | 0.76 (0.65-0.89) | 0.38 (0.30-0.47) | 2.08 (1.58-2.75)a | 0.95 (0.80-1.13) | 0.37 (0.28-0.49) | 2.87 (2.05-4.02)a | 0.67 (0.60-0.74) | 0.30 (0.26-0.35) | 2.33 (1.94-2.80)a |
HF = heart failure; IR = incidence rate.
P < 0.001.
P < 0.05.
P < 0.005.
The increased risk of incident cancer in HF patients applied to the majority of solid malignancies, especially for cancers arising in the lung (HR: 4.49; 95% CI: 4.06-4.98), pancreas (HR: 4.64; 95% CI: 3.71-5.81), liver (HR: 3.78; 95% CI: 3.30-4.34), and nervous system (HR: 3.85; 95% CI: 2.91-5.08). HF was also associated with an excess risk of hematologic malignancies, specifically multiple myeloma (HR: 2.33; 95% CI: 1.94-2.80), leukemia (HR: 2.17; 95% CI: 1.85-2.54), and lymphoma (HR: 1.84; 95% CI: 1.54-2.20). In contrast, the risk of melanoma, breast cancer, and neoplasms of the endocrine system did not differ between cases and control subjects over the whole study period (Table 2, Supplemental Table 6).
The overall and type-specific risks of cancer were similar in men and women (Supplemental Table 7). However, male patients with HF had a lower incidence of cancer of the reproductive system, including prostate cancer, compared with control subjects (HR: 0.90; 95% CI: 0.83-0.98).
Cancer mortality
There were a total of 103,608 deaths, of which 64,483 were in the HF group and 39,125 were in the control group. Information on cause of death was missing for 14,480 cases and 11,917 control subjects (Figure 1). Among the 77,211 deaths with known cause (74.5% of all deaths), 5,946 were caused by cancer (4,738 in subjects with HF and 1,208 in those without) and 12,575 were caused by HF (9,703 in subjects with HF and 2,872 in those without).
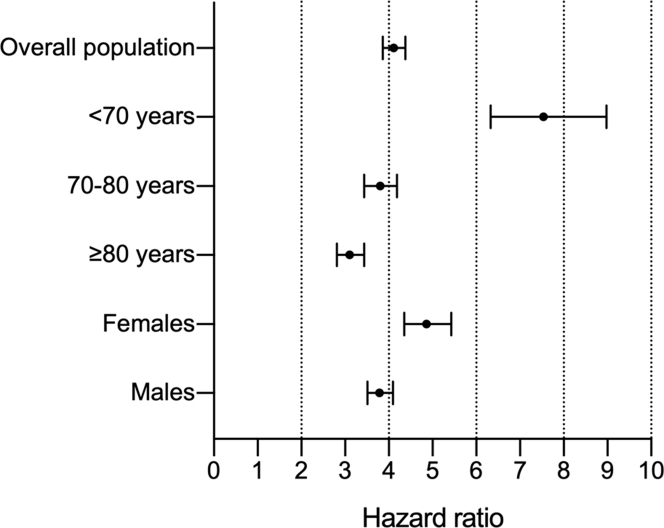
HF patients died secondary to cancer more frequently than control subjects (Figure 2). The HR for cancer mortality in subjects with vs without HF at baseline was 4.11 (95% CI: 3.86-4.38; P < 0.001) (Figure 3). The excess risk of cancer death applied to all age groups but declined with age: subjects <70-year-old with HF had an almost 8-fold higher risk of cancer mortality than those without HF (HR: 7.54; 95% CI: 6.33-8.98), whereas in the 70-79-year-old and ≥80-year-old age groups the HRs for cancer death were 3.80 (95% CI: 3.44-4.19) and 3.10 (95% 2.81-3.43), respectively (Figure 3, Table 3, Supplemental Table 8). Indeed, there was a significant interaction between HF and age on cancer mortality (P < 0.001). The association of HF with cancer mortality was confirmed also when taking into account the competing risk of death for other causes, both in the whole cohort (subdistribution HR [SHR]: 3.48; 95% CI: 3.27-3.72) and across age groups (<70 years of age, SHR: 6.65; 95% CI: 5.60-7.94; 70-80 years of age, SHR: 3.14; 95% CI: 2.84-3.48; and ≥80 years of age, SHR: 2.81; 95% CI: 2.55-3.10).
Figure 2.
Mortality in HF Patients vs Control Subjects
Cumulative incidence and overall survival curves showing mortality for (A) cancer, (B) heart failure (HF), and (C) any cause, as assessed by International Classification of Diseases-9th Revision-Clinical Modification codes, in inhabitants of the Puglia region in Italy, without a history of cancer in the 3 years before the index date and with (red line) or without (black line) HF at baseline. CIF = cumulative incidence function.
Figure 3.
Cancer Death in Heart Failure Patients According to Age and Sex
Cause-specific HRs (point) and 95% CIs (bars) for cancer mortality in subjects with heart failure vs control subjects in the whole study population and across age and sex groups.
Table 3.
Mortality Rates per 1000 Person-Years in HF Patients vs Control Subjects for the Most Common Cancers
| <70 Years of Age (n = 53,611) |
70-79 Years of Age (n = 60,806) |
≥80 Years of Age (n = 66,619) |
Total (N = 181,036) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF Patients |
Control Subjects |
HR (95% CI) | HF Patients |
Control Subjects |
HR (95% CI) | HF Patients |
Control Subjects |
HR (95% CI) | HF Patients |
Control Subjects |
HR (95% CI) | |
| IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | IR (95% CI) | |||||
| Any death | 30.94 (30.22-31.68) | 4.34 (4.07-4.62) | 7.33 (6.85-7.83)a | 85.44 (84.12-86.78) | 32.81 (32.00-33.63) | 2.48 (2.41-2.56)a | 241.25 (238.40-244.13) | 174.05 (171.66-176.49) | 1.31 (1.29-1.34)a | 96.13 (95.29-96.98) | 51.05 (50.45-51.66) | 2.00 (1.97-2.03)a |
| Any cancer | 4.71 (4.44-5.01) | 0.63 (0.54-0.75) | 7.54 (6.33-8.98)a | 10.35 (9.89-10.82) | 2.68 (2.46-2.93) | 3.80 (3.44-4.19)a | 15.67 (14.95-16.41) | 4.81 (4.42-5.23) | 3.10 (2.81-3.43)a | 9.11 (8.85-9.37) | 2.27 (2.14-2.40) | 4.11 (3.86-4.38)a |
| Colorectal | 0.46 (0.38-0.56) | 0.09 (0.06-0.14) | 5.16 (3.19-8.35)a | 1.00 (0.86-1.15) | 0.36 (0.29-0.46) | 2.58 (1.95-3.42)a | 2.06 (1.81-2.35) | 0.83 (0.68-1.01) | 2.21 (1.73-2.84)a | 1.00 (0.92-1.09) | 0.35 (0.30-0.40) | 2.92 (2.47-3.46)a |
| Lung | 1.42 (1.27-1.58) | 0.12 (0.09-0.18) | 11.83 (8.03-17.42)a | 2.62 (2.40-2.86) | 0.41 (0.33-0.51) | 6.71 (5.28-8.54)a | 2.23 (1.97-2.52) | 0.39 (0.29-0.53) | 6.32 (4.58-8.71)a | 2.02 (1.91-2.15) | 0.28 (0.24-0.33) | 7.41 (6.24-8.79)a |
| Female breast | 0.26 (0.17-0.40) | 0.07 (0.03-0.15) | 3.92 (1.60-9.65)a | 0.74 (0.60-0.94) | 0.34 (0.25-0.47)b | 1.95 (1.30-2.93)b | 1.40 (1.16-1.70) | 0.77 (0.60-1.00) | 1.43 (1.01-2.01)c | 0.77 (0.67-0.89) | 0.37 (0.30-0.45) | 2.03 (1.59-2.58)a |
| Male reproductive system | 0.24 (0.17-0.34) | 0.09 (0.05-0.15) | 2.52 (1.29-4.91)b | 1.30 (1.08-1.57) | 0.81 (0.64-1.02) | 1.36 (0.99-1.86) | 4.22 (3.62-4.92) | 2.08 (1.68-2.59) | 1.71 (1.29-2.26)b | 1.19 (1.07-1.34) | 0.62 (0.53-0.73) | 1.94 (1.60-2.35)a |
| Lymphoma | 0.14 (0.10-0.19) | 0.01 (0.00-0.04) | 15.59 (3.72-65.34)a | 0.28 (0.21-0.37) | 0.09 (0.06-0.14)c | 2.65 (1.51-4.66)b | 0.27 (0.19-0.39) | 0.09 (0.05-0.16) | 2.84 (1.35-5.94)b | 0.22 (0.18-0.26) | 0.05 (0.04-0.08) | 3.92 (2.60-5.90)a |
| Multiple myeloma | 0.12 (0.08-0.18) | 0.01 (0.00-0.03) | 26.43 (3.58-194.80)a | 0.29 (0.22-0.38) | 0.08 (0.05-0.13) | 3.48 (1.94-6.23)a | 0.50 (0.38-0.64) | 0.12 (0.07-0.21) | 4.04 (2.22-7.37)a | 0.26 (0.22-0.31) | 0.06 (0.04-0.08) | 4.70 (3.16-6.99)a |
HF = heart failure; IR = incidence rate.
P < 0.001.
P < 0.005.
P < 0.05.
The increased risk of death caused by cancer was detected for all types of solid malignancies including lung cancer (HR: 7.41; 95% CI: 6.24-8.79), colorectal cancer (HR: 2.92; 95% CI: 2.47-3.46), and female breast cancer (HR: 2.03; 95% CI: 1.59-2.58), as well as for hematologic malignancies such as multiple myeloma (HR: 4.70; 95% CI: 3.16-6.99) and leukemia (HR: 4.27; 95% CI: 3.08-5.93) (Table 3, Supplemental Table 8).
Having HF at baseline portended an increased risk of cancer mortality both in men (HR: 3.79; 95% CI: 3.51-4.10) and in women (HR: 4.86; 95% CI: 4.35-5.43) (Figure 3, Supplemental Table 9).
Given the notable proportion of deaths caused by unknown cause, we performed a sensitivity analysis in which these deaths were arbitrarily attributed to either HF or cancer. When all deaths of unknown cause were classified as cancer related, the HR for cancer death decreased but remained higher in patients with HF compared with control subjects (HR: 1.35; 95% CI: 1.32-1.38; SHR for competing risk analysis: 1.21; 95% CI: 1.19-1.24). Similarly, cancer mortality remained higher in HF patients compared with control subjects when missing causes of deaths were attributed to HF (HR: 1.58; 95% CI: 1.54-1.61; SHR for competing risk analysis: 1.44; 95% CI: 1.41-1.47).
Incident cancer and cancer death in HF patients according to consumption of loop diuretics
Among patients with HF at baseline, prescription of high-dose loop diuretic was associated with an older age, female sex, higher DDCI, and a higher frequency of cardiovascular DPs and device therapy (Supplemental Table 10). Furthermore, use of high-dose loop diuretic implied an increased risk of mortality (Supplemental Figure 1). HF patients with a high consumption of loop diuretic agents also had higher cancer incidence (HR: 1.11; 95% CI: 1.03-1.21) (Supplemental Table 11) and mortality (HR: 1.35; 95% CI: 1.19-1.53) (Supplemental Table 12). However, this increased risk was driven by a few solid malignancies (Supplemental Tables 11 and 12).
Incident cancer and cancer death according to number of hospitalizations
The increased risk of cancer in the HF population could be explained—at least in part—by a surveillance bias (ie, HF patients undergoing more frequent medical evaluation, thus increasing the likelihood of cancer diagnosis). To address this potential confounder, we stratified the study population based on the number of hospitalizations during follow-up and assessed cancer incidence and mortality in HF patients compared with control subjects with the same number of hospital admissions. The number of hospitalizations was higher in HF patients than in control subjects (Supplemental Table 13). Nevertheless, after accounting for the number of hospitalizations, cancer incidence and mortality remained higher in subjects with HF than in those without HF (Supplemental Tables 14 and 15, respectively). Another approach to minimize the possibility of a surveillance bias is to exclude cancer diagnoses made early after the onset of HF, when patients receive more intensive clinical observation.7 After excluding cancer diagnoses or deaths occurring up to 1 year after the HDRs or HFWs defining HF cases, the risk of cancer remained higher in HF patients compared with control subjects (Supplemental Table 16).
Discussion
In this community-based cohort study, we found increased cancer incidence and mortality in patients with HF compared with matched control subjects (Central Illustration). The excess risk applied to several common types of solid and hematologic malignancies, and appeared to be correlated with the severity of HF as estimated by exposure to loop diuretics.
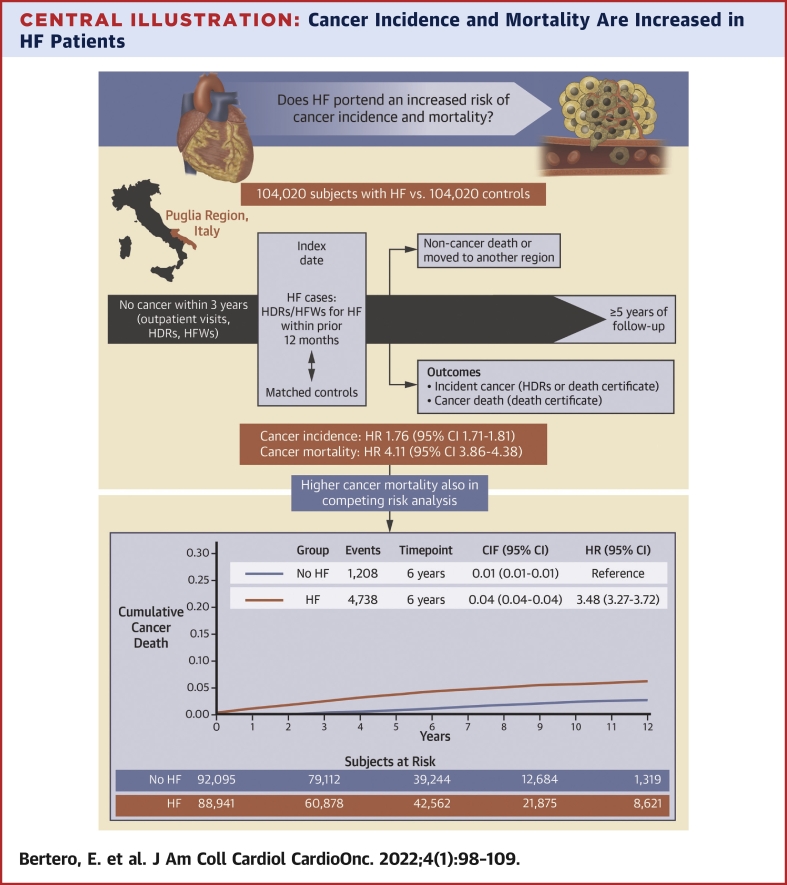
Central Illustration.
Cancer Incidence and Mortality Are Increased in HF Patients
This community-based study included individuals ≥50 years of age from the Puglia region in Italy, without cancer within 3 years before the baseline evaluation, and ≥5-year follow-up. After matching for age, sex, drug-derived complexity index, health care district, Charlson comorbidity index, and follow-up duration, subjects with heart failure (HF) had a higher risk of cancer incidence and mortality compared with subjects without HF. CIF = cumulative incidence function; HDR = hospital discharge record; HFW = health care cost-related fee waiver.
HF and associated with an increased risk of cancer
HF and cancer commonly coexist, and there is an increasing appreciation that shared risk factors and common biologic pathways can promote the development of both conditions.2,28,29 In fact, traditional cardiovascular disease risk factors such as age, sex, and smoking are independently associated with the risk of cancer.30,31 In this context, the question whether cancer is more likely to occur in subjects with HF has relevant implications for the management of these patients.1 However, population studies addressing the issue of cancer in HF yielded conflicting results, possibly because of the heterogeneity of the samples evaluated and differences in the methodologies employed.
An increased risk of cancer in patients with HF was initially reported by 3 independent groups.7, 8, 9, 10, 11 A recent study confirmed the association of HF with cancer in postmenopausal women, and found that it was stronger for patients with HF with preserved ejection fraction compared with those with reduced ejection fraction.11 In contrast, 2 reports did not find a higher risk of cancer in HF patients, proposing that the association of HF and cancer is mostly driven by the presence of comorbidities,14,15 and another study reported that the risk of breast cancer is not affected by the presence of HF in postmenopausal women.32
This study supports the notion that HF does portend an increased risk of cancer, regardless of sex and age, although the incidence rate of cancer was markedly higher in males and increased with age. It is notable that the findings presented here were obtained after matching HF patients and control subjects based on DDCI and, secondarily, CCI, thus at least in part controlling for the potential confounding effect of comorbidities. While we acknowledge that this and the previously published observational analyses cannot conclusively establish whether and to what extent other concomitant diseases influence the relationship between HF and cancer, we believe that attributing the association of HF with cancer only to comorbidities is limiting.
In our work as well as in prior reports, malignancies of the respiratory tract were among the cancer types whose incidence was most affected by HF.7,8,11,15 On the other hand, <80-year-old male HF patients had a lower risk of prostate cancer, which is also in line with some earlier investigations.7,14 This observation might reflect a less frequent screening for prostate cancer by measuring prostate-specific antigen in HF patients.7,14 Furthermore, urinary symptoms that often lead to the detection of prostate cancer may go unrecognized because of diuretic therapy.
The epidemiological observation of a higher likelihood of cancer in HF is in line with biological data indicating that HF represents an oncogenic condition.2,28 This concept is supported by preclinical studies showing that the failing heart secretes oncogenic factors,12,33 and that neurohormonal activation, a hallmark of HF with reduced ejection fraction, promotes tumor development and progression.34 Another possibility is that both HF and cancer are more likely to occur in the presence of systemic low-grade inflammation.2
Cancer mortality in patients with HF
Noncardiovascular causes of death have become increasingly common in patients with HF over the past 2 decades, and cancer is the most frequent one, accounting for up to 15% of noncardiovascular mortality in HF.35,36 Cancer mortality has often been overlooked in HF clinical trials; however, when assessed, it accounted for 6% to 14% of all deaths in HF with reduced ejection fraction16,37 and 10% to 13% in HF with preserved ejection fraction.38 Individuals with both HF and cancer have a markedly worse survival than subjects with only 1 of the 2 conditions.7,9 Of note, this holds true for patients with malignancies deemed not to be aggressive by clinicians.37
In the current study, patients with HF died of cancer more frequently than control subjects. The increase in mortality was observed for several solid and hematologic malignancies, and was highest for lung cancer. When stratifying the study population by age, we observed a progressive age-dependent reduction in the HF-related excess cancer mortality. This decline might be attributable to elderly HF patients dying earlier than control subjects, therefore making cancer death more likely in subjects without HF.
Overall, death caused by cancer was also higher in patients with more severe HF, as inferred by consumption of diuretic agents.39,40 The analysis by cancer types revealed that only some malignancies were associated with this risk, making the strength of the finding questionable. Nonetheless, it remains possible that oncological therapies are inappropriately withdrawn in subjects with more severe HF, or that these patients have worse global conditions and, thus, are more vulnerable and eventually prone to succumb to cancer.
Study limitations
The observational design is the main limitation of the present study. However, the large size and broad representation of the cohort, the unbiased selection process by which it was obtained, and the long follow-up duration buttress our results. The diagnosis of HF was assigned based on health care records. Although ascertainment of HF by a clinician would be more accurate, the methods used here were validated in previous publications.18, 19, 20, 21, 22, 23, 24 Similarly, the reliability of the DDCI and CCI-based matching strategy we adopted has already been demonstrated.27 Clearly, we cannot exclude residual confounding, with unrecognized factors actually mediating the association with cancer outcomes, but this shortcoming is intrinsic to analysis such as the present one regardless of the way cases and control subjects are matched. Third, information on left ventricular ejection fraction was not available, and thus we could not distinguish between HF with reduced or preserved ejection fraction. Furthermore, we did not have access to data about cancer treatment and, thereby, we cannot reject the hypothesis that the reason for higher cancer mortality in HF patients is less intensive cancer treatment; however, this would not explain the higher incidence of cancer in HF patients. Finally, the cohort we evaluated was made of ≥50-year-old individuals, leaving unanswered the question of whether HF in the young is also associated with an increased risk of cancer. However, the prevalence of HF in the population <50 years of age is low, and large cohorts should be examined to answer this question.
Conclusions
In this community-based cohort, individuals with HF had higher cancer incidence and mortality compared with matched control subjects. The excess risk applied to both males and females and virtually all types of solid and hematologic malignancies. Furthermore, our analysis raises the question of whether the risk of cancer may be higher in patients with decompensated, more severe HF, who require high consumption of loop diuretics.
Perspectives.
COMPETENCY IN CLINICAL KNOWLEDGE: HF is associated with an increased risk of cancer and cancer-related mortality, which may be heightened in decompensated states. The cardiologist should be aware that cancer is a major comorbidity in HF, and close collaboration between cardiologists and oncologists is required to guide the optimal management of patients with both HF and cancer.
TRANSLATIONAL OUTLOOK: The interconnection between HF and cancer represents a nascent area of cardio-oncology. Further studies are required to assess the prognostic impact of cancer in the HF population and to elucidate potential mechanistic links between HF and cancer.
Funding Support and Author Disclosures
This research was supported by the Italian Ministry of Health (GR-2018-12365661 - CHANGE Study, principal investigator Pietro Ameri) and is part of the work covered by the 2015-2020 Collaboration between the Regional Healthcare Agency of Puglia Region (AReSS Puglia) and the Istituto di Ricerche Farmacologiche Mario Negri IRCCS. Dr Maack was supported by the Barth Syndrome Foundation, the German Research Foundation (Ma 2528/7-1, SFB 894, TRR-219), and the German Federal Agency for Education and Research (01EO1504). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank the Director of the Regional Health Agency of Puglia, Giovanni Gorgoni, for providing the analyzed data.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Ameri P., Canepa M., Anker M.S., et al. Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail. 2018;20:879–887. doi: 10.1002/ejhf.1165. [DOI] [PubMed] [Google Scholar]
- 2.Bertero E., Canepa M., Maack C., Ameri P. Linking heart failure to cancer. Circulation. 2018;138:735–742. doi: 10.1161/CIRCULATIONAHA.118.033603. [DOI] [PubMed] [Google Scholar]
- 3.de Boer R.A., Meijers W.C., van der Meer P., van Veldhuisen D.J. Cancer and heart disease: associations and relations. Eur J Heart Fail. 2019;21(12):1515–1525. doi: 10.1002/ejhf.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duarte C.W., Lindner V., Francis S.A., Schoormans D. Visualization of cancer and cardiovascular disease co-occurrence with network methods. JCO Clin Cancer Inform. 2017;1:1–12. doi: 10.1200/CCI.16.00071. [DOI] [PubMed] [Google Scholar]
- 5.Chao C., Xu L., Bhatia S., et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol. 2016;34:1626–1633. doi: 10.1200/JCO.2015.65.5845. [DOI] [PubMed] [Google Scholar]
- 6.Armenian S.H., Xu L., Ky B., et al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banke A., Schou M., Videbaek L., et al. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail. 2016;18:260–266. doi: 10.1002/ejhf.472. [DOI] [PubMed] [Google Scholar]
- 8.Hasin T., Gerber Y., Weston S.A., et al. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016;68:265–271. doi: 10.1016/j.jacc.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasin T., Gerber Y., McNallan S.M., et al. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto M., Hasegawa T., Asakura M., et al. Does the pathophysiology of heart failure prime the incidence of cancer? Hypertens Res. 2017;40:831–836. doi: 10.1038/hr.2017.45. [DOI] [PubMed] [Google Scholar]
- 11.Leedy D.J., Reding K.W., Vasbinder A.L., et al. The association between heart failure and incident cancer in women: An analysis of the Women’s Health Initiative. Eur J Heart Fail. 2021;23:1712–1721. doi: 10.1002/ejhf.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijers W.C., Maglione M., Bakker S.J.L., et al. Heart failure stimulates tumor growth by circulating factors. Circulation. 2018;138:678–691. doi: 10.1161/CIRCULATIONAHA.117.030816. [DOI] [PubMed] [Google Scholar]
- 13.Koelwyn G.J., Newman A.A.C., Afonso M.S., et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. 2020;26(9):1452–1458. doi: 10.1038/s41591-020-0964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvaraj S., Bhatt D.L., Claggett B., et al. Lack of association between heart failure and incident cancer. J Am Coll Cardiol. 2018;71:1501–1510. doi: 10.1016/j.jacc.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz B., Schou M., Gislason G.H., Køber L., Torp-Pedersen C., Andersson C. Prevalence and incidence of various cancer subtypes in patients with heart failure vs matched control subjects. Int J Cardiol. 2020;316:209–213. doi: 10.1016/j.ijcard.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Tini G., Bertero E., Signori A., et al. Cancer mortality in trials of heart failure with reduced ejection fraction: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.016309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.InternationalClassification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). U.S. Centers for Disease Control and Prevention; 1999.
- 18.Macchia A., Monte S., Pellegrini F., et al. Omega-3 fatty acid supplementation reduces one-year risk of atrial fibrillation in patients hospitalized with myocardial infarction. Eur J Clin Pharmacol. 2008;64:627–634. doi: 10.1007/s00228-008-0464-z. [DOI] [PubMed] [Google Scholar]
- 19.Macchia A., Romero M., D'Ettorre A., Mariani J., Tognoni G. Temporal trends of the gaps in post-myocardial infarction secondary prevention strategies of co-morbid and elderly populations vs. younger counterparts: an analysis of three successive cohorts between 2003 and 2008. Eur Heart J. 2012;33:515–522. doi: 10.1093/eurheartj/ehr410. [DOI] [PubMed] [Google Scholar]
- 20.Monte S., Macchia A., Romero M., D'Ettorre A., Giuliani R., Tognoni G. Antidepressants and cardiovascular outcomes in patients without known cardiovascular risk. Eur J Clin Pharmacol. 2009;65:1131–1138. doi: 10.1007/s00228-009-0692-x. [DOI] [PubMed] [Google Scholar]
- 21.Macchia A., Monte S., Romero M., D'Ettorre A., Tognoni G. The prognostic influence of chronic obstructive pulmonary disease in patients hospitalised for chronic heart failure. Eur J Heart Fail. 2007;9:942–948. doi: 10.1016/j.ejheart.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 22.De Berardis G., D'Ettorre A., Graziano G., et al. The burden of hospitalization related to diabetes mellitus: a population-based study. Nutr Metab Cardiovasc Dis. 2012;22:605–612. doi: 10.1016/j.numecd.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 23.De Berardis G., Lucisano G., D'Ettorre A., et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–2294. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 24.Winkler W.E. 1992. Comparative analysis of record linkage decision rules. [Google Scholar]
- 25.Corrao G., Ghirardi A., Ibrahim B., Merlino L., Maggioni A.P. Burden of new hospitalization for heart failure: a population-based investigation from Italy. Eur J Heart Fail. 2014;16:729–736. doi: 10.1002/ejhf.105. [DOI] [PubMed] [Google Scholar]
- 26.Corrao G., Ghirardi A., Ibrahim B., Merlino L., Maggioni A.P. Short- and long-term mortality and hospital readmissions among patients with new hospitalization for heart failure: a population-based investigation from Italy. Int J Cardiol. 2015;181:81–87. doi: 10.1016/j.ijcard.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Robusto F., Lepore V., D'Ettorre A., et al. The Drug Derived Complexity Index (DDCI) predicts mortality, unplanned hospitalization and hospital readmissions at the population level. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijers W.C., de Boer R.A. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115(5):844–853. doi: 10.1093/cvr/cvz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau E.S., Paniagua S.M., Liu E., et al. Cardiovascular risk factors are associated with future cancer. J Am Coll Cardiol CardioOnc. 2021;3:48–58. doi: 10.1016/j.jaccao.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van 't Klooster C.C., Ridker P.M., Cook N.R., et al. Prediction of lifetime and 10-year risk of cancer in individual patients with established cardiovascular disease. J Am Coll Cardiol CardioOnc. 2020;2:400–410. doi: 10.1016/j.jaccao.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lam P.H., Barac A., Nohria A., et al. Temporal associations and outcomes of breast cancer and heart failure in postmenopausal women. J Am Coll Cardiol CardioOnc. 2020;2:567–577. doi: 10.1016/j.jaccao.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avraham S., Abu-Sharki S., Shofti R., et al. Early cardiac remodeling promotes tumor growth and metastasis. Circulation. 2020;142:670–683. doi: 10.1161/CIRCULATIONAHA.120.046471. [DOI] [PubMed] [Google Scholar]
- 34.Cole S.W., Nagaraja A.S., Lutgendorf S.K., Green P.A., Sood A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moliner P., Lupon J., de Antonio M., et al. Trends in modes of death in heart failure over the last two decades: less sudden death but cancer deaths on the rise. Eur J Heart Fail. 2019;21(10):1259–1266. doi: 10.1002/ejhf.1569. [DOI] [PubMed] [Google Scholar]
- 36.Conrad N., Judge A., Canoy D., et al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86000 individuals. JAMA Cardiol. 2019;4(11):1102–1111. doi: 10.1001/jamacardio.2019.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ameri P., Canepa M., Luigi Nicolosi G., et al. Cancer in chronic heart failure patients in the GISSI-HF trial. Eur J Clin Invest. 2020;50 doi: 10.1111/eci.13273. [DOI] [PubMed] [Google Scholar]
- 38.Vaduganathan M., Claggett B.L., Jhund P.S., et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. doi: 10.1016/S0140-6736(20)30748-0. [DOI] [PubMed] [Google Scholar]
- 39.Kapelios C.J., Bonou M., Malliaras K., et al. Association of loop diuretics use and dose with outcomes in outpatients with heart failure: a systematic review and meta-analysis of observational studies involving 96,959 patients. Heart Fail Rev. 2022;27(1):147–161. doi: 10.1007/s10741-020-09995-z. [DOI] [PubMed] [Google Scholar]
- 40.Laszczynska O., Severo M., Frioes F., et al. Prognostic effect of the dose of loop diuretic over 5 years in chronic heart failure. J Card Fail. 2017;23:589–593. doi: 10.1016/j.cardfail.2017.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.