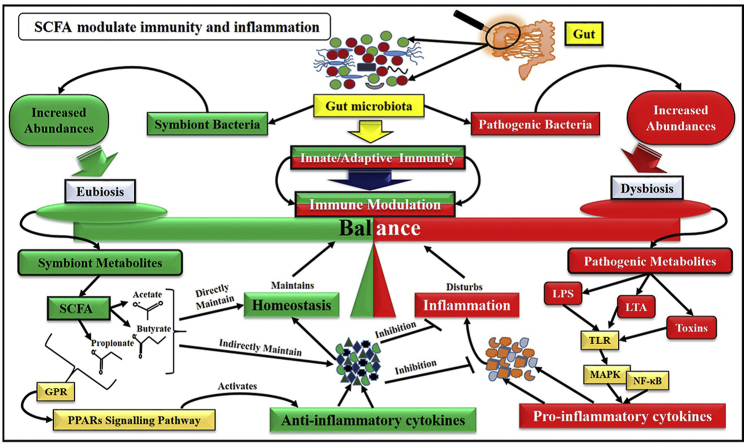
Abstract
Gut inflammation is a challenging concern in humans and animals, which disturbs normal growth and leads to severe bowel diseases. Short chain fatty acids (SCFA) are the gut microbiota metabolites produced from fermentation of non-digestible carbohydrates, and have been reported to modulate gut inflammation. SCFA have been implicated as the potential therapeutic bioactive molecules for gut inflammatory diseases, and could be an alternative to antibiotic growth promoters (AGP). In this review, the existing knowledge about the types of SCFA, the related gut microbes producing SCFA, the roles of SCFA in maintaining gut homeostasis, and how SCFA modulate gut inflammation is summarized. The therapeutic application of SCFA in the treatment of inflammatory bowel disease (IBD) is also highlighted.
Keywords: Gut microbiota, Short chain fatty acid, Gut inflammation, Gut homeostasis, Inflammatory bowel disease
Graphical abstract
1. Introduction
Gut inflammation contributes to epithelial barrier defects, disturbs beneficial microbiota, and dysregulates immune response (Garcia-Carbonell et al., 2019). It also causes maldigestion and malabsorption of nutrients and reduces host growth and development (Nii et al., 2020). The usage of antimicrobial agents to prevent inflammation and/or as growth promoters has led to the evolution of antibiotic resistance among pathogenic bacteria (Nhung et al., 2017), which is causing public health concern around the globe (McEwen and Collignon, 2018). Over the past few years, nearly all produced antibiotics are gradually losing their inhibitory activity against pathogenic microbes (Kromann et al., 2017). Increasing antibiotic resistance has instigated the exploration of novel antimicrobial compounds (Yang et al., 2019).
Gut microbiota is a complex consortium of microbial communities consisting of trillions of fungi, viruses, archaea, micro-eukaryotes, and especially bacteria. Most of them are beneficial microbes, but some are pathogens. Gut microbiota significantly influences human and animal health, and during gut diseases, the normal microbial composition is impaired (Barko et al., 2018; Sarin et al., 2019). It is reported that gut microbiota is crucial in controlling gut inflammation (Khosravi et al., 2014). Short chain fatty acids (SCFA) are gut microbiota-produced fermentation products of dietary fibres, which could reshape the gut ecology, induce immune modulation and antibiotic activity, and mediate inflammatory signalling cascade during gut inflammation (McHardy et al., 2013). Emerging literature has recognized the dynamic roles of SCFA in the host immunity, metabolism, and inflammation, and their potential profile for gut immunity regulation has also been described substantially (Holscher, 2017; Beukema et al., 2020). The gut microbes producing SCFA have also been identified. The role of SCFA in immune modulation during gut inflammation, particularly via G protein-coupled receptors (GPR)/Toll-like receptors (TLR)-mediated underlying signalling cascade has become an emerging matter of interest in the modern era. Thus, SCFA could be the potential bioactive molecules which would be used in preventing gut inflammation.
Further, due to high incident rate of antibiotic resistance, SCFA could also be an effective choice to counter gut inflammation. That's why, it is also speculated that SCFA might be a viable alternative source that could potentially modulate host immune regulation and efficiently mediate inflammatory responses during gut inflammation. Therefore, the aim of this review is to compile the existing knowledge panoramically and elucidate the potential immunomodulatory impact of SCFA on host immune regulation to mitigate gut inflammation.
2. Types of SCFA
SCFA, being saturated in nature, contain (1 to 6) carbon (C) atoms. Formate, acetate, propionate, butyrate, valerate, and caproate are the members of SCFA comprising C1, C2, C3, C4, C5, and C6 atoms, respectively. Following the international union of pure and applied chemistry (IUPAC) criteria, SCFA are the aliphatic carboxylic acids with less than 6 carbons atoms in their aliphatic tails, and can be divided on the number of carbon atoms they contain. The IUPAC names for these SCFA, i.e., formic acid (C1), acetic acid (C2), propionic acid (C3), isobutyric acid (C4), butyric acid (C4), isovaleric acid (C5), and valeric acid (C5), are methanoic acid, ethanoic acid, propanoic acid, 2-methylpropanoic acid, butanoic acid, 3-methylbutanoic acid, and pentanoic acid respectively. Additionally, 2-methylbutanoic acid (C5) is a SCFA, which has no common name yet (Layden et al., 2013). Formic acid is the smallest SCFA with just 1 carbon atom with a minor role in human physiology. However, during methanol metabolism, formic acid is produced as a by-product and causes issues relating to metabolic acidosis (Layden et al., 2013). Acetic acid is a main component of vinegar, and several anaerobic bacteria produce it in the human gut. It binds with co-enzyme A and is involved in fat and carbohydrate metabolic pathways in cells. Compared with other SCFA, higher concentrations of acetic acid are found in blood circulation and colon lumen (Shimazu et al., 2010). Likewise, propionic acid is also formed as a fermentation by-product of gut microbiota, and it decreases hepatic cholesterol synthesis and improves fat metabolism. It has anti-inflammatory and antibacterial properties and also protects the human gut from pathogens (Markowiak-Kopeć and Śliżewska, 2020). Butyric acid also acts as an energy source for colonocytes, regulates multiple gut functions, i.e., immune modulation, gut development, cell differentiation, gene expression, reduces oxidative stress and inflammation by controlling pathogens, and improves growth (Bedford and Gong, 2018). Among these SCFA, a comparatively small production of formate, valerate, and caproate has been reported, whereas acetate, propionate, and butyrate are produced abundantly. Diet dependent fibre contents are responsible for the production of these SCFA in the gut. Similarly, the transit time, microbiota composition, and substrate type also alter SCFA relative proportion (Layden et al., 2013).
3. Gut microbiota are the main producers of SCFA (acetate, propionate and butyrate)
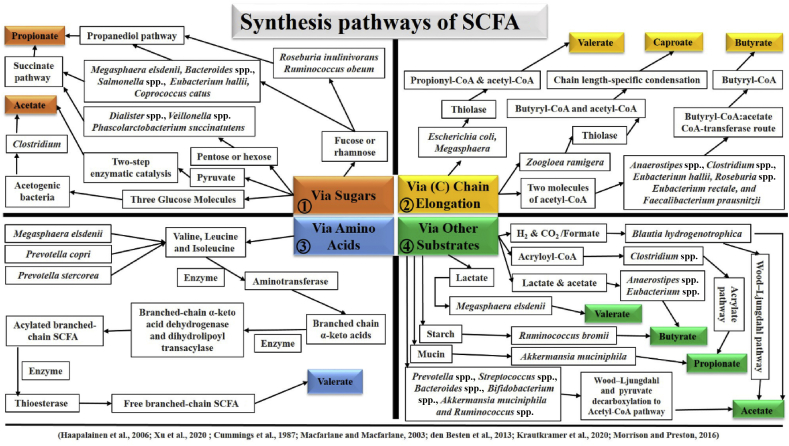
Gut microbiota consisting of a complex consortium of abundant microbial communities is substantially established in several metabolites production, of which SCFA are the potential metabolites essential for host normal physiology (Rooks and Garrett, 2016). SCFA are the energy metabolites for the host, which yield almost 10% of daily caloric requirements for humans. Host colonocytes metabolize SCFA from the gut lumen up to the hepatic vein with a higher metabolic activity for butyrate than acetate and propionate. Host liver also actively takes part in SCFA uptake (den Besten et al., 2013a, den Besten et al., 2013b). Carbohydrate metabolism during glycolysis, and amino acid or organic acid metabolism produces propionate and butyrate. Acetate is derived from acetyl-CoA in glycolysis, and can also be converted to butyrate using butyryl-CoA: acetyl-CoA transferase. In the colon lumen, almost 90% to 95% of produced SCFA are absorbed in the gut mucosa (Parada Venegas et al., 2019). The gut lumen absorbs SCFA by exchanging Cl− with HCO3− ions. The absorbed SCFA are usually transported to the liver through the portal vein and metabolized by hepatocytes. SCFA are metabolized in the mitochondrial matrix and fuel different tissues, i.e., liver, heart, and skeletal muscles, as an energy source. Further, SCFA also modulate the lipid and carbohydrate metabolism in the liver and play a supportive role in glucose synthesis from pyruvate/lactate (Schönfeld and Wojtczak, 2016). SCFA production depends upon gut microbiota, which synthesizes these bioactive compounds via fermenting non-digestible carbohydrate, and resulting the principal end products, particularly acetate, butyrate, and propionate. For instance, Akkermansia muciniphila is recognized as a fundamental bacterium for propionate production which helps to degrade the mucin. Similarly, in the colon, Ruminococcus bromii significantly contributes to producing butyrate via fermenting resistant starch. Even more, Faecalibacterium prausnitzii, Eubacterium hallii and Eubacterium rectale are considered as chief butyrate producers (Morrison and Preston, 2016). Several studies have reported that a considerable variety of other gut microbiota is associated with producing a large variety of SCFA. For instance, Prevotella spp., Streptococcus spp., Bacteroides spp., Bifidobacterium spp., Clostridium spp., A. muciniphila, Blautia hydrogenotropphica and Ruminococcus spp. produce acetate as their SCFA metabolite via the Wood–Ljungdahl pathway and pyruvate decarboxylation to Acetyl–CoA pathways. Further, Phascolarctobacterium succinatutens, Megasphaera elsdenii, Ruminococcus obeum, Bacteroides. spp., Veillonella spp., Salmonella spp., Dialister spp., Roseburia inulinivorans, E. hallii and Coprococcus catus are recognized to produce propionate as their effective metabolite via following succinate, propanediol and acrylate pathways. Furthermore, E. hallii, Roseburia spp., Anaerostipes spp., Coprococcus comes, F. prausnitzii, E. rectale, C. catus and Coprococcus eutactus produce butyrate via following exogenous acetate and butyrate kinase pathways (Cummings et al., 1987; Macfarlane and Macfarlane, 2003; den Besten et al., 2013a, den Besten et al., 2013b; Krautkramer et al., 2020; Xu et al., 2020). It is also reported that Prevotella stercorea, M. elsdenii, and Prevotella copri produce valerate using valine, leucine and isoleucine with aminotransferase, thioesterase, dehydrogenase, and transacylase enzymes. Zoogloea ramigera, Megasphaera, and Escherichia coli process butyryl-CoA, propionyl-CoA, and acetyl-CoA to produce valerate and caproate using thiolase. Likewise, Acidobacteria and Megasphaera massiliensis MRx0029 also produce valerate (Yuille et al., 2018; Liu et al., 2019a, Liu et al., 2019b). Acetogenic bacteria, i.e., Clostridium uses 3 glucose molecules to produce acetate (Haapalainen et al., 2006; Xu et al., 2020). The synthesis pathways of different SCFA are also represented in Fig. 1. Additionally, Roseburia hominis and F. prausnitzii have been reported as butyrate-producing bacteria in humans, whose production is severely influenced during gut dysbiosis in inflammatory bowel disease (IBD). It is suggested that butyrate administration might have some beneficial effects in IBD patients (Lavelle and Sokol, 2020). Moreover, formate is also a by-product produced as a result of anaerobic fermentation by gut bacteria (Pietzke et al., 2020); however, limited studies are available to date. Pham and co-workers reported that E. hallii has the ability to produce formate by consuming glucose (Pham et al., 2017). The important SCFA, their types, common and synthetic names, and sources are also listed in Table 1. The reported literature cited above suggested the direct/indirect causal roots between host and gut microbiota through SCFA. Thus, gut microbiota and its derived SCFA are worth assessing in normal host physiology.
Fig. 1.
The synthesis pathways of different SCFA. SCFA synthesis depends upon the type of substrate, and the preference of every bacterium for available substrates is probably distinct. Based on substrate type, SCFA synthesis might be classified into 4 categories. Different bacteria use sugars (as demonstrated in section 1), carbon chain elongation (section 2), amino acids (section 3) or other substrates (section 4) for SCFA synthesis following different pathways. Propanediol and succinate pathways are represented in section 1 (Top left) of the figure. Thiolase-mediated butyryl-CoA, propionyl-CoA, and acetyl–CoA pathways are represented in section 2 (Top right) of the figure. Aminotransferase, thioesterase, dehydrogenase, and transacylase enzymes pathway using different amino acids as a basic source is represented in section 3 (Bottom left) of the figure. Wood–Ljungdahl and pyruvate decarboxylation to Acetyl–CoA pathways, and other types of substrates are represented in section 4 (Bottom right) of the figure. SCFA = short chain fatty acids; C = carbon; CoA = coenzyme A.
Table 1.
Important short chain fatty acids, their types, common and synthetic names, and sources.
| Number of carbon (C) | Common name | Synthetic name | Sources | References |
|---|---|---|---|---|
| C1 | Formic acid | Methanoic acid | Eubacterium hallii | Pham et al. (2017) |
| C2 | Acetic acid | Ethanoic acid | Prevotella spp., Streptococcus spp., Bacteroides spp., Bifidobacterium spp., Clostridium spp., Akkermansia muciniphila, Blautia hydrogenotropphica, Ruminococcus spp. | Xu et al. (2020); Macfarlane and Macfarlane (2003); Krautkramer et al. (2020); Cummings et al. (1987) |
| C3 | Propionic acid | Propanoic acid | Akkermansia muciniphila, Phascolarctobacterium succinatutens, Megasphaera elsdenii, Ruminococcus obeum, Bacteroides spp., Veillonella spp., Salmonella spp., Dialister spp., Roseburia inulinivorans, Eubacterium hallii and Coprococcus catus | Morrison and Preston (2016); den Besten et al., 2013a, den Besten et al., 2013b; Xu et al. (2020) |
| C4 | Butyric acid | Butanoic acid | Ruminococcus bromii, Faecalibacterium prausnitzii, Eubacterium hallii, Eubacterium rectale, Roseburia spp., Anaerostipes spp., Coprococcus comes, Coprococcus catus and Coprococcus eutactus | Lavelle and Sokol (2020); den Besten et al., 2013a, den Besten et al., 2013b; Xu et al. (2020); Morrison and Preston (2016) |
| C5 | Valeric acid | Pentanoic acid | Acidobacteria, Megasphaera massiliensis MRx0029 |
Liu et al., 2019a, Liu et al., 2019b Yuille et al. (2018) |
| C6 | Caproic Acid | Hexanoic acid | Caproiciproducens galactitolivorans BS-1T | Bengelsdorf et al. (2019) |
4. SCFA maintain gut homeostasis via regulating host immunity and inflammation
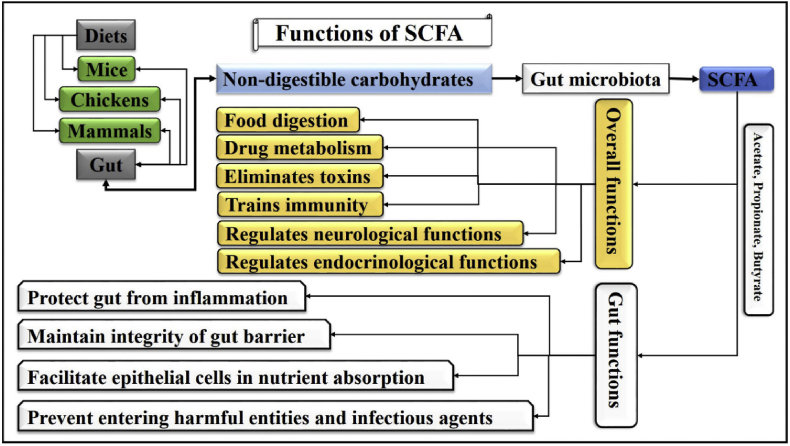
It has been established that gut homeostasis depends upon the interaction between gut microbiota-produced SCFA and the host. Although gut microbiota directly alter host functional profile, modern researchers are investigating their role through SCFA, which has been recognized as a popular metabolite for immune response regulation during infection (Sencio et al., 2021). Therefore, exploring the potential roles of SCFA in different metabolic and infectious conditions has become an interesting concern in the recent decade. Several studies described that SCFA could modulate host metabolic functions at different organ sites (Frampton et al., 2020). They help in food digestion, modify drug physiological metabolism, regulate neurological and endocrinological functions, eliminate toxins, and train immunity (Fan and Pedersen, 2020). The salient functions of SCFA are represented in Fig. 2. These SCFA are also involved in the development and survival of leukocytes and can lower the colonic pH, which promotes beneficial bacterial growth, i.e., Lactobacillus and Bifidobacterium. As these bacteria are significant producers of SCFA, they play a vital role to maintain effective immune responses during inflammation (McLoughlin et al., 2017).
Fig. 2.
Overall and gut-specified functions of SCFA. In humans and animals, after ingestion, the feed interacts with several types of digestive enzymes/gastric secretions, which help in nutrients digestion. However, some proportion of the nutrients remains undigested. Gut microbiota helps digest the remaining undigested carbohydrates and produces SCFA, i.e., acetate, propionate, and butyrate. Overall, these SCFA help in nutrients digestion, drug metabolism, toxin elimination, immunity training, and neurological/endocrinological functions regulation. Specifically, these SCFA protect the gut from inflammation, maintain gut barrier integrity, facilitate epithelial cells in nutrient absorption, and prevent entry of harmful entities and infectious agents. SCFA = short chain fatty acids.
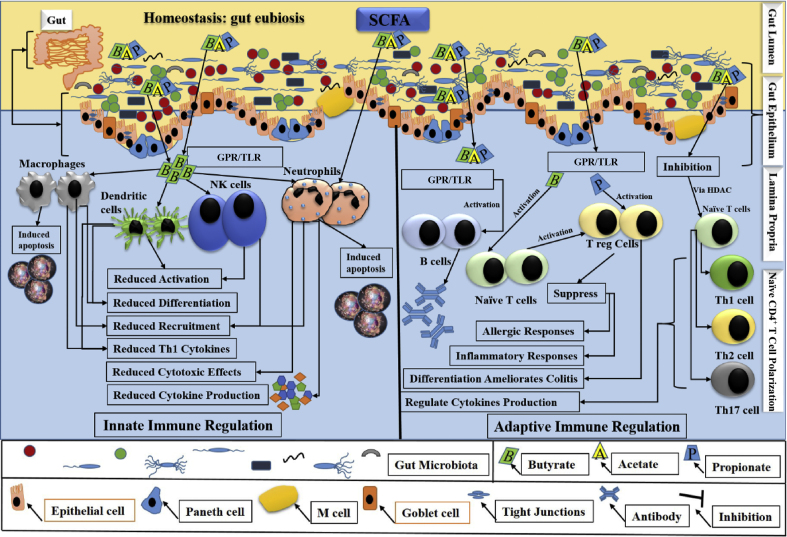
Current studies recognize that host gut homeostasis is fundamentally controlled by gut microbiota and/or its derived SCFA, which is essential for a smooth functioning of an immune system. For instance, butyrate regulates the mature production, proper trafficking and an adequate functioning of innate and adaptive immune cells in IBD, indicating its role in gut inflammation (Gonçalves et al., 2018). Further, a study reported that SCFA-exposed pre-adipocytes elicited an innate immune response, suggested an optimum status of SCFA in immune regulation during inflammatory diseases (Garland, 2011). On the other hand, gut microbiota dysbiosis is the structural and functional perturbation of gut microbiota, which enhances the pathogenesis of IBD, and alters the production and proper functioning of SCFA (Khan et al., 2019). Dysbiosis leads to an impaired gut barrier integrity, which initiates pathogens intrusion in deep mucosal layers and causes spontaneous intestinal cell (goblet and Paneth) death, ultimately results in barrier dysfunction and defects in innate immunity. Enteric pathogen translocation has been described as a driving force in the etiopathogenesis of IBD, which also develops an adaptive immune response (Khan et al., 2019). Thus, reversing dysbiosis maintains cell survival and minimizes inflammation pathogenesis, which is largely dependent on SCFA. Instead of an energy source as adenosine triphosphate (ATP), SCFA link the microbiota and immune system via intestinal epithelial cells (IEC), which establish a physical protective barrier against a microorganism's entry and hence, are the dynamic components of the host defensive system as these cells sense the invasion of pathogenic microbes (Parada Venegas et al., 2019). These reports highlight the role of SCFA in assisting IEC to maintain gut barrier integrity, which might be targeted to prevent IBD pathogenesis. The schematic presentation of gut eubiosis and homeostasis in the regulation of innate and adaptive immune response is represented in Fig. 3, which illustrates a potential contribution of immune cells in immunity.
Fig. 3.
Schematic presentation of gut eubiosis and homeostasis. Intact gut epithelium maintains eubiosis and homeostatic balance. Most of the pathogens remained in the gut lumen, whereas the commensal bacteria elicited the underlying mechanisms via producing SCFA and triggering innate and adaptive immune regulation. SCFA via their GPR and (correlated) TLR modulate the immune regulation. During innate immune regulation (the left grey colour representation in the figure), SCFA, especially butyrate, induced apoptosis via activating the neutrophils and macrophages. On the other hand, activation of natural killer and dendritic cells, differentiation of macrophages and dendritic cells, and recruitment of neutrophils and macrophages were reduced. Th1-mediated cytokine productions from macrophages and dendritic cells, and neutrophilic induced cytotoxic effects and cytokine secretion were also reduced. During adaptive immune regulation (the right grey colour representation in the figure), SCFA, via GPR/TLR receptors activated B cells, Naïve T cells, and T regulatory cells (Treg). Treg were either directly activated by propionate or were indirectly activated by butyrate via Naïve T cells. B cells produced IgA, which inhibits bacterial invasion through the gut epithelium. Treg suppressed inflammatory and allergic responses, and its differentiation ameliorated colitis. Additionally, butyrate via HDAC modulated proliferation and recruitment of T cells (Th1, Th2, Th17), induced apoptosis, thus regulated cytokine production. SCFA properly maintain the gut eubiosis and homeostasis via both innate and adaptive immune regulation. SCFA = short chain fatty acids; GPR = G protein-coupled receptors; TLR = Toll-like receptors; Th1 = T helper type 1 cells; Th2 = T helper type 2 cells; Th17 = T helper type 17 cells; Treg = T regulatory cells; IgA = immunoglobulin A; HDAC = histone deacetylases.
It is demonstrated that the immune system in the gastrointestinal tract (GIT) is continually confronted with a variety of antigens, and therefore, tolerable or harmful antigens must be distinguished in a timely manner. Inflammation studies in mice, chickens, and other mammals described the dynamic immune cells interaction to distinguish the destructive entities, and uncovered the potential roles of SCFA to regulate the immune system (Cait et al., 2018; Leone et al., 2019). During normal circumstances, a dynamic regulation is maintained in the components of the tight junctions. However, an infection or sustained inflammation, i.e., IBD, may cause dysregulation, which further leads to barrier breaching and microbe's invasion (Pott and Hornef, 2012; Odenwald and Turner, 2017). Microbial invasion triggers neutrophils infiltration, which is the first line of defence in innate immune response at an inflammation site. On-site, these cells efficiently mount a response against invaders and initially orchestrate the activation and subsequent recruitment of macrophages. Interestingly, SCFA interact with neutrophils, actively participate in their recruitment and modulate their chemotaxis, and other physiological functions and survival in various tissues (Vinolo et al., 2011; Corrêa-Oliveira et al., 2016). Further, butyrate and/or propionate enhanced the neutrophil transmigration to the inflammatory cite by increasing the secretion of cytokine-induced neutrophil chemoattractant 2αβ (CINC-2αβ) (Vinolo et al., 2009). Thus, SCFA markedly modulate the neutrophilic functions during inflammation and regulate immunity. Furthermore, butyrate modulates the intestinal macrophage functions and significantly inhibits the lipopolysaccharide (LPS) induced pro-inflammatory mediators, i.e., interleukin (IL)-6, IL-12 and nitric oxide (NO) (Sun et al., 2017). Interestingly, butyrate also improved macrophages' antimicrobial activity during its differentiation (monocyte to macrophage) (Schulthess et al., 2019), indicating immunomodulatory effects of SCFA during increased macrophages influx, which exacerbates the inflammation.
Moreover, in inflamed tissues, dendritic cells sample different antigens and then migrate towards lymph nodes. Here, they present these antigens to the T cells and produce different cytokines as an immune response, and influence T cells polarization into Th1, Th2, or Th17 cells (Nastasi et al., 2015). Butyrate effectively regulated the functions and development of dendritic cells, lowered T cells stimulating capacity of dendritic cells and reduced pro-inflammatory (IFN-γ, IL-12 p40) cytokines production (Liu et al., 2012). Another study reported that higher SCFA concentrations inhibited histone deacetylases (HDAC) in dendritic cells via reducing the differentiation of these cells, thus decreasing pro-inflammatory cytokines production (Corrêa-Oliveira et al., 2016). Additionally, subsequent production, differentiation and functioning of T cells depend upon the balance between gut eubiosis and dysbiosis. These studies demonstrated how SCFA alter dendritic and T cells functions to minimize inflammation. Some evidence in modern studies also indicated that SCFA exert immunomodulating effects for both B and T lymphocytes as an adaptive immune response in IBD (Trompette et al., 2018; Russo et al., 2019). For instance, lamina propria is the underlying intestinal epithelium rich in T and B lymphocytes. Peyer's patches provide an inductive site where lymphocytes priming occurs, and lamina propria acts as an effector site where activated lymphocytes respond to an appropriate stimulus. Thus, in lamina propria, T cells activate CD25 and CD69 for immune regulation during inflammation (McDermott and Huffnagle, 2014). A recent study reported that butyrate at the rate of 1.0 mmol/L constrains T cell proliferation, and at > 2.0 mmol/L induces apoptosis in stimulated T cells. On the other hand, propionate at the rate of 2 to 5 mmol/L constrains mitogens-stimulated T cell proliferation, and 250 to 500 μmol/L inhibits the immune response of Th1-type in peripheral blood mononuclear cells of humans (Trompette et al., 2014; Ohira et al., 2017). Indeed, Arpaia et al. demonstrated that SCFA significantly influenced the balance of pro/anti-inflammatory cells by a profound communication between the immune system and gut microbial communities (Arpaia et al., 2013), highlighting the major role of SCFA in inflammation and immune regulation. The overall representation of these immune cells with their relevant functional profile during inflammation and gut dysbiosis was potentially proposed and is presented in Fig. 4.
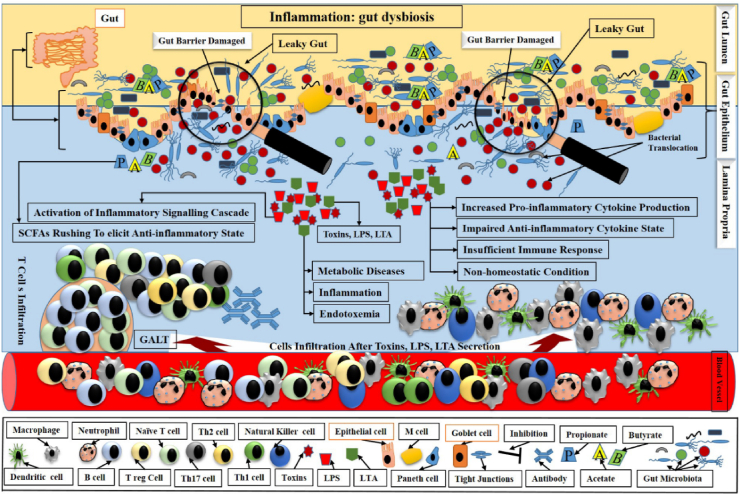
Fig. 4.
Schematic outlining of the main players in gut dysbiosis and inflammation. Gut dysbiosis depicts a decreased biodiversity and is accompanied by loss of beneficial bacteria, with defective barrier functions leading to leaky gut, impaired anti-microbial response, and enhanced bacterial adhesion and translocation. After damaging gut barrier, the gut became a leaky gut, which allowed the pathogens’ intrusion into lamina propria via crossing damaged barriers. Here, these pathogens produced toxins and secreted LPS and LTA. These toxins, LPS and LTA, together initiated inflammation, endotoxemia and thus caused metabolic diseases. These factors increased pro-inflammatory cytokines production, impaired anti-inflammatory cytokine secretion, and hence insufficient immune response led to the non-homeostatic condition. These factors also triggered the underlying cell signalling cascade. Although SCFA rushed to overcome the dysbiosis, however, SCFA could not elicit a proper anti-inflammatory state due to sudden invasion of pathogens. Gut-associated lymphoid tissue (GALT) also interactively released T cells and collaboratively competed with an intruded pathogenic storm. LPS = lipopolysaccharide; LTA = lipoteichoic acid; SCFA = short chain fatty acids.
In short, all defensive cells, i.e., neutrophils, monocytes, macrophages, dendritic cells, Treg cells, Th cells, and lymphocytes correlate with SCFA to modulate the picture of inflammatory cytokines in gut inflammation.
5. SCFA regulate immune response and inflammation via GPR/TLR following underlying signalling cascade
It is well-known that SCFA play a substantial role in initiating an immune response and immune regulatory mechanisms via their receptors during inflammation. Thus, stimulation of an immune response depends on the stimuli, the environment, and the receptor-type, i.e., G protein-coupled receptors (GPR) and/or TLR. SCFA can influence host physiology via interactions with GPR and HDAC, and exert their mediational role through modulating immunity directly or indirectly. Thus, different SCFA, i.e., butyrate, propionate, or acetate, bind with and efficiently activate different kinds of receptors; free fatty acid receptors (FFAR), i.e., GPR41 (FFAR3) or GPR43 (FFAR2). On the other hand, only niacin and butyrate can activate GPR109A, which is also called hydroxycarboxylic acid receptor 2 (HCAR2) (Chen et al., 2017; Dalile et al., 2019). Several recent studies have demonstrated the beneficial effects of SCFA dependent on their vital receptors. SCFA are well-known to protect gut epithelial integrity and have useful effects against gut inflammation, i.e., in IBD (Taggart et al., 2005; Chen et al., 2019). The receptors and their interaction with related SCFA are listed in Table 2.
Table 2.
Receptors for common SCFA and their related interaction.
| Receptors | Interaction | References |
|---|---|---|
| GPR41/FFAR3 | It has more affinity with propionate and least with acetate. | Koh et al. (2016) |
| GPR43/FFAR2 | It has more affinity with propionate and acetate and least affinity with butyrate. | Mandaliya and Seshadri (2019) |
| GPR109A/HCAR2 | Butyrate has attraction with GPR109A or HCAR2. | Dalile et al. (2019) |
SCFA = short chain fatty acids; GPR = G protein-coupled receptor; FFAR = free fatty acid receptor; HCAR2 = hydroxycarboxylic acid receptor 2.
Recent data suggested that GPR influenced the leukocytes activation, intestinal barrier integrity, microbial defence, and production of inflammatory cytokines as pathophysiological mechanisms, which are all associated with IBD (Zeng et al., 2020). Thus, the role of GPR in SCFA functionality is well-established. For instance, Citrobacter rodentium-induced infection in GPR41−/−, GPR43−/− mice significantly reduced inflammatory response, indicating GPR as crucial inflammatory mediators (Kim et al., 2013). Other researchers demonstrated that SCFA initiate an immune response via GPR41 in bovine rumen epithelial cells (Zhan et al., 2019), and via GPR41/GPR43 in different porcine tissues, i.e., in the spleen, ileum, adipose tissue and colon (Li et al., 2014). Similarly, these SCFA through GPR modulate different enzymatic activities, transcription factors of hypoxia-inducible factor and HDAC, and histone acetyltransferase by reducing inflammation (Corrêa-Oliveira et al., 2016). Other studies reported that SCFA via GPR also modulate different hormonal secretion, i.e., insulin, glucagon, incretin, and leptin, which regulates immune response and energy haemostasis (Xiong et al., 2004; Vinolo et al., 2012). Thus, GPR has been proven to be a SCFA gateway to host metabolism, which might be a good therapeutic target in infection prevention. Furthermore, an alteration in microbial diversity and GPR are also interlinked with gut inflammation levels. Several research reports also demonstrated a significant modulation of underlying inflammatory signalling cascade with SCFA via GPR (Li et al., 2018a, Li et al., 2018b; Luu and Visekruna, 2019). For instance, Wang and co-workers found that Lactobacillus acidophilus treated mice significantly increased gut microbiota diversity, and analysis of mRNA expression revealed a positive correlation of GPR41 and GPR43 with serum interferon- γ (IFN-γ) and transforming growth factor-β (TGF-β), whereas it showed a negative correlation with serum IL-6, IL-4, and IL-17 levels (Wang et al., 2019). The substantial roles of SCFA in the modulation of anti-inflammatory cytokines to regulate the immune system in inflammation are also critical. For instance, TGF-β regulates CD8+ T cells, natural regulatory T cells, and natural killer T cells development. Similarly, IL-10 is critical in immune system homeostasis by limiting the inflammatory response and reducing tissues damage during gut inflammation, and IL-22 also potentially maintains the epithelial barrier (Sanjabi et al., 2009; Brockmann et al., 2017). It is also reported that butyrate enhanced the production of IL-10 in T cells of IBD patients (Sun et al., 2018). Sodium butyrate also exhibited anti-inflammatory potential in IBD of in-vivo (mice) and in-vitro (RAW 264.7 cells) models (Chen et al., 2018), indicating how SCFA prevent inflammation severity and protect the intestinal epithelium. Likewise, both propionate and acetate also increased the production of IL-10 (Mandaliya et al., 2021). Recently, an in-vivo study demonstrated that Lactobacillus plantarum ZS2058 significantly reduced Salmonella-induced pathogenicity by enhancing the faecal propionic acid level and producing mucin-2 in mouse colon following IL-22/IL-23 and IL-17/IL-23 dependent pathways (Liu et al., 2019a, Liu et al., 2019b). The findings of these studies highlighted how SCFA stimulated anti-inflammation factors to protect a host from infections. On the other hand, the mechanisms of how gut microbiota-derived SCFA suppress pro-inflammatory mediators to modulate the inflammation and immunity have become of considerable interest. For instance, the level of IL-6 is considered to be a diagnostic marker in a variety of inflammatory diseases (Czepiel et al., 2014). TNF-α participates in adhesion of leukocyte to the epithelium, in edema formation, and in vasodilatation, and regulates the blood coagulation, hence, induces fever indirectly (Zelová and Hošek, 2013; Akhtar et al., 2020). In monocytes, propionate and butyrate reduced the expression of LPS-induced NO synthase and TNF-α via FFA2/FFA3 receptor's activation or HDAC inhibition (Li et al., 2018a, Li et al., 2018b). In another in-vitro study, sodium butyrate remarkably reduced the IL-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) production and significantly inhibited mitogen activated protein kinase (MAPK) phosphorylation in association with nuclear factor-kappa B (NF-κB) activity, thus attenuated inflammation (Ohira et al., 2013). SCFA-induced GPR41 and GPR43 interlinked dysbiosis-mediated metabolic endotoxemia, which consequently provoked an immune response and other inflammatory signalling mechanisms (Brar and Kohn, 2019). Additionally, SCFA are associated with the differentiation of adipose tissues, and regulation of immune responses and inflammation in farm animals (Mielenz, 2017). The findings of these studies indicated how SCFA reduced pro-inflammation and enhanced anti-inflammation factors to modulate inflammatory conditions and regulate immunity.
Instead of GPR/FFAR, pathogens initiated immune response could also be triggered by pattern-recognition receptors (PRR), i.e., TLR, which are the chief players during inflammation. Analysis of nearest–neighbour correlation revealed that TLR2 and TLR4, considered essential in innate immunity, are regulated with GPR (Maslowski et al., 2009). For instance, gut dysbiosis influences TLR expression and contributes to unbalancing Th17/Treg, which is correlated with functional and compositional changes in mucosal microbiota (Chen et al., 2017). Similarly, myeloid differentiation primary response protein 88 (MyD88)-interlinked TLR interaction with dendritic cells on mucosa and in sub-epithelial layer triggers the immune modulatory MAPK and NF-κB pathways (Kobyliak et al., 2016). Another study reported that IL-1β production and NLRP3 inflammasome activation are mainly mediated by TLR4, which regulates intestinal immune homeostasis (Lötscher and Balmer, 2019). Further, butyrate significantly increased the expression of TLR4 and CD14 in human (SW480) cells and mouse (CT26) cells, and induced phosphorylation of p-38, ERK, and JNK, thus attenuated MAPK signalling (Xiao et al., 2018), indicating inflammation suppression role of SCFA. In male Boer goats, 1% sodium butyrate decreased the mRNA expression levels and phosphorylation of MAPK, and inhibited protein kinase C (PKC) in the ruminal epithelium, and increased the expression of zona occludin-1, occludin, claudin-1, and claudin-4 by mitigating the ruminal epithelial damage (Zhang et al., 2018), suggesting that SCFA protects gut barrier and inhibits inflammation. Butyrate with a dose rate of 100 and 500 μmol/L significantly attenuated NF-κB and its relevant cytokine levels (Lee et al., 2017). TLR1 and TLR6 are the central players of innate immune dysfunction in IBD's pathogenesis, and are considered as effective targets to treat IBD (Lu et al., 2018). Interestingly, in IBD, the anti-inflammatory characteristics of butyrate were also described via inhibiting the NF-κB pathway and inducing the expression of intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) (Zapolska-Downar et al., 2004; Chen et al., 2018). These studies indicated the inhibitory role of SCFA in inflammation following TLR core pathways. Hence, TLR and gut microbiota-associated SCFA act as a bridge to initiate the host metabolism and immunologic processes (Spiljar et al., 2017). Larraufie et al. reported that butyrate increased the peptide YY (Pyy) gene expression, and TLR3/TLR4 are directly linked with barrier function regulation and degranulation of Paneth cells (Larraufie et al., 2017). In addition, up-regulated TLR10 is related to the increased absorption and production of SCFA to promote immune tolerance in goat's ruminal epithelium (Shen et al., 2016). Similarly, a reduced level of TLR9 gene expression in the Peyer's patches in the ileum and mesenteric lymph nodes indicated a reduced inflammation intensity in the intestine (Jang et al., 2016). These reports highlighted the substantial role of TLR in association with SCFA in inflammation reduction and immune regulation. Furthermore, SCFA also modulate the expression of peroxisome proliferator-activated receptors (PPAR). In a murine colitis model, N-(1-carbamoyl-2-phenylethyl) butyramide (FBA), a butyrate derivative, suppressed histone deacetylase-9, re-established acetylation of H3 histone, exerted a potent anti-inflammatory influence via PPAR-γ up-regulation, and modulated the host immune response during gut inflammation (Simeoli et al., 2017). In another study, PPAR-γ signalling restricted the growth of pathogenic bacteria, i.e., Salmonella and Escherichia, hence prevented gut dysbiosis (Byndloss et al., 2017). Similarly, propionate regulated gut lipid metabolism and immune regulation via inducing the expression of PPAR-α (Higashimura et al., 2015). These studies revealed that pathogen-associated molecular patterns (PAMP) i.e., LPS, LTA or toxins elicited TLR to initiate the inflammation, whereas SCFA, i.e., acetate, butyrate and propionate triggered the immune responsive cells, which further activated either innate immunity or adaptive immunity. Thus, SCFA via immune regulation maintained gut epithelial integrity, and protected the host from gut inflammation.
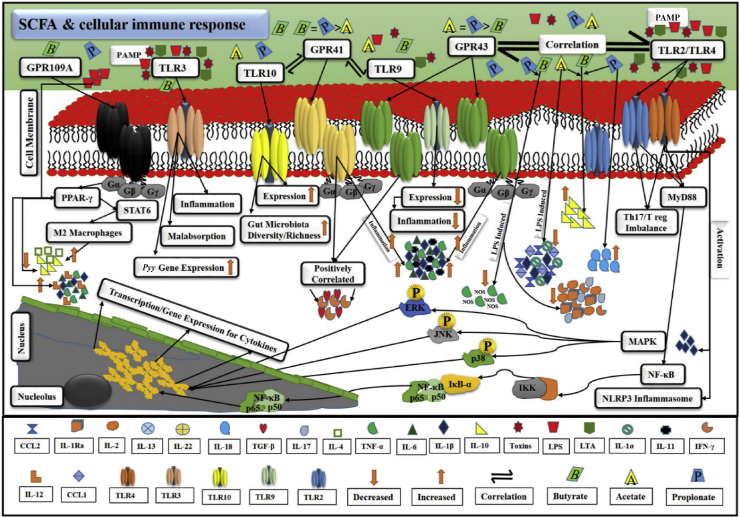
All these accumulating shreds of evidence suggested a potential role of SCFA-mediated underlying signalling cascade in immune regulation and distinct inflammatory responses. SCFA-mediated cellular immune responses and the demonstration of pro-/anti-inflammatory cytokines secretions via GPR/TLR following NF-κB/MAPK/PPAR signalling cascades are represented in Fig. 5.
Fig. 5.
A probable cellular mechanism of SCFA-related immune response in gut homeostasis. GPR and TLR work in appropriate correlation. Bacterial PAMP, i.e., toxins, LPS and LTA, stimulated TLR and underlying signalling pathways. They promoted the secretion of pro-inflammatory cytokines, i.e., IL-1β, TNF-α, IL-6, etc. As a result, inflammation is initiated. SCFA, i.e., acetate, propionate, and butyrate, intricately correlate with TLR, i.e., TLR2, TLR3, TLR4, TLR9 and TLR10 via GPR, i.e., GPR41, GPR43, and GPR109A. GPR are inter-connected with SCFA via G proteins, which are heterotrimeric and comprise 3 subunits, alpha (α), beta (β), and gamma (γ). This demonstration explains the stimulation of TLR by PAMP, activation of GPR by SCFA, correlation and trans-membrane signalling activities of GPR and TLR, initiation of pro-inflammatory cytokines-mediated inflammation and other malfunctions, stimulation of anti-inflammatory activity to overcome inflammation, and activation of PPAR, MAPK, NF-κB, and NLRP3 inflammasome pathways and relevant transcription/genes expression in overall immune regulation via SCFA. GPR = G protein-coupled receptors; TLR = toll-like receptors; PAMP = pathogen-associated molecular patterns; LPS = lipopolysaccharide; LTA = lipoteichoic acid; IL-1β = interleukin 1β; TNF-α = tumor necrosis factor α; IL-6 = interleukin 6; SCFA = short chain fatty acids; TLR2 = toll-like receptor 2; TLR3 = toll-like receptor 3; TLR4 = toll-like receptor 4; TLR9 = toll-like receptor 9; TLR10 = toll-like receptor 10; GPR41 = G protein-coupled receptor 41; GPR43 = G protein-coupled receptor 43; GPR109A = G protein-coupled receptor 109 A; PPAR = peroxisome proliferator-activated receptors; MAPK = mitogen activated protein kinase; NF-κB = nuclear factor-kappa B; NLRP3 = NOD (nucleotide-binding oligomerization domain) LRR (leucine-rich repeat) and pyrin domain-containing 3.
6. Therapeutic applications of SCFA
It has been reported that SCFA possess substantial anti-microbial activities against several microbes. Therefore, they are widely applied in agriculture, food, and medicinal industries (Arora et al., 2011). Several studies reported the therapeutic applications of SCFA. For instance, a study described that low concentrations of SCFA could inactivate bacterial cells without hazardous effects on the host (Dewulf et al., 2011). Further, acetate, butyrate, propionate, and formate have proved to be effective anti-microbial compounds in inhibiting oral microbes (Alva-Murillo et al., 2012). SCFA effectively blocked the type III secretion systems (T3SS), which is a primary mechanism for pathogenicity of Salmonella, and regulated the expression of its virulence genes, thus preventing gut inflammation (Rauf et al., 2021). Similarly, butyrate markedly inhibits Shigella infection in humans by reducing its pathogenicity via up-regulating the cathelicidin (Raqib et al., 2006). In Campylobacter jejuni infection, acetate, butyrate and propionate mixture could slightly reduce the probable ABC transporter ATP-binding protein PEB1C (peb1c) gene expression in the chick gut to inhibit its pathogenesis and inflammation (Luethy et al., 2017). However, further research is still required to explore the mechanisms of Shigella and C. jejuni infections. Sodium acetate and sodium propionate could suppress the chromosomal locus for enterocyte effacement (LEE) gene expression to prevent enterohemorrhagic E. coli pathogenicity (Nakanishi et al., 2009). Although Listeria monocytogenes could survive in acidic conditions, higher butyrate concentrations in the gut can resist its attachment and colonization, and could significantly reduce its infection (Sun et al., 2012). Furthermore, in neurological disorders, SCFA, via inhibiting HDAC, have proved to be potential modulators in learning and memory processes (Rauf et al., 2021). Depression impairs human behaviour and causes social issues. Decreased fecal SCFA concentration is observed in depressed patients, whereas butyrate acts as an anti-depressant and normalizes behavioural changes (Sun and Buys, 2016; Burokas et al., 2017). Additionally, SCFA also perform roles in metabolic disorders. Butyrate might increase insulin response (Sanna et al., 2019), whereas impaired propionate production might enhance the risk of type-2 diabetes (Kaczmarczyk et al., 2012). Moreover, many studies described the anti-inflammatory effects of SCFA by substantially inhibiting the pro-inflammatory cytokine secretion to reduce inflammation (Tedelind et al., 2007; Li et al., 2018a, Li et al., 2018b). IBD is also an inflammatory disorder which might have multiple etiological factors, i.e., environmental, genetics, microbiological and immunological. However, dysbiosis is a common factor in IBD patients as compositional or functional alterations of gut microbiota in these patients are common. Disturbed gut microbiota is unable to ferment the undigested food components in the host gut, thus fail to produce SCFA, and functionalities of the immune system are compromised, which further exacerbates the inflammation (Parada Venegas et al., 2019; Rauf et al., 2021). SCFA exhibits immunomodulatory actions by nourishing host colonocytes in IBD patients (Plichta et al., 2019). The literature cited above has demonstrated that SCFA have potential therapeutic applications in different pathologies and could be considered alternatives to antibiotic growth promoters (AGP) as well.
7. Conclusion and perspectives
Recently, attention has been paid to the functional profiling of SCFA associated with immunoregulatory impact in gut inflammation. Gut microbiota, its derivatives SCFA, and the host immune system are implicated together in intricate crosstalk. Innumerable host-associated environmental cues within the body influence the functional profile of SCFA. SCFA have been increasingly recognized as having profound impacts on immune-related host functions/dysfunctions to assess the co-metabolism in human physiology and pathology.
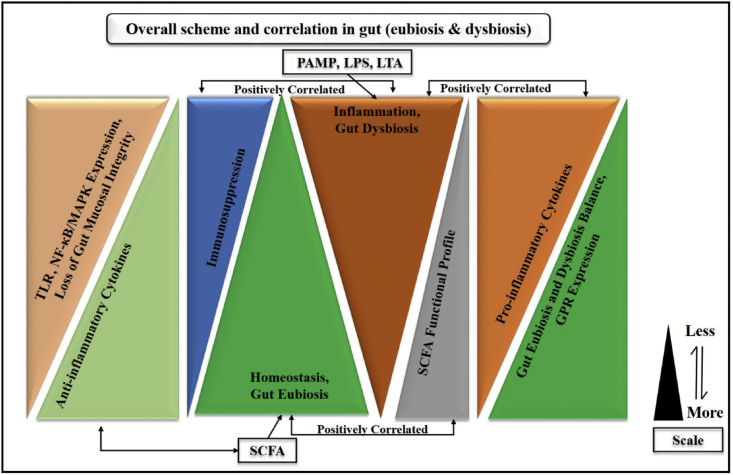
For an optimal host functioning, the holobiont idea might ease the understanding of host and microbes' interdependency for a common concern. Herein, this review summed up that SCFA via their GPR-associated TLR modulated the innate and adaptive immune response during inflammation. Gut dysbiosis was implicated in PAMP-mediated gut inflammation, which triggered the influx of neutrophils, macrophages, natural killer cells, epithelial cells, and dendritic cells; indeed, it is an innate immune response. Further, systemic inflammation was characterized by dysbiosis via an overwhelming storm of pro-inflammatory cytokines IL-1β, TNF-α, IL-6, etc., followed by proliferation of T and B lymphocytes, the polarization of Th1, Th2, and Th17 cells, which was speculated as an adaptive immune response. Conversely, in eubiosis, SCFA inhibited the pro-inflammatory cytokines-mediated NF-κB/MAPK/PPAR cascades via reducing leukocyte infiltration and promoting the anti-inflammatory cytokines underlying the same signalling cascade. Overall, these findings suggested that SCFA potentially modulated the GPR/TLR-mediated NF-κB/MAPK/PPAR cascades via its relevant pro-/anti-inflammatory cytokine secretion to properly regulate the immune responses during inflammation. Additionally, we also speculated that SCFA might be effective agents to replace antibiotics, and could be used against several pathologies. The overall scheme and correlation of all possible inflammation factors (gut dysbiosis) and homeostasis (gut eubiosis) are presented in Fig. 6.
Fig. 6.
Overall scheme and correlation of all possible factors in inflammation (gut dysbiosis) and homeostasis (gut eubiosis). PAMP = pathogen-associated molecular patterns; LPS = lipopolysaccharide; LTA = lipoteichoic acid; SCFA = short chain fatty acids; TLR = Toll-like receptors; MAPK = mitogen activated protein kinase; NF-κB = nuclear factor-kappa B.
As this review has demonstrated the dynamic and functional capabilities of SCFA in the gut associated with immune response regulation during inflammation, these findings might have profound implications for host health, and might positively support human health affecting metabolic and immune responsive traits. Although many mice models and in-vitro trials remarkably revealed the potential impacts of SCFA, explaining their roles in host gut physiology and pathology, the actual picture for human health remains. Further, a quantitative approach for SCFA measurement in colonocytes would be extremely desireable to screen the potential roles of SCFA, especially for eubiosis/dysbiosis balance in immune response regulations. We speculate that emerging technologies might have an enormous potential to utilize SCFA as potential therapeutic agents to maintain eubiosis and homeostasis, thus promote human health and reduce gut inflammation.
Author contributions
Muhammad Akhtar: Drafted the original manuscript, conceptualization, preparation, formal analysis, creation and/or presentation of the published work, visualization/data presentation, software, writing/reviewing and editing. Yan Chen: Software, writing, resources, formal analysis, reviewing & editing. Ziyu Ma: Resources, formal analysis, writing/reviewing & editing. Xiaolong Zhang: Formal analysis, writing/reviewing & editing. Deshi Shi: Conceptualization, visualization/data presentation, writing, reviewing & editing. Jawaria Ali Khan: Resources, formal analysis, writing/reviewing & editing. Huazhen Liu: Funding acquisition, project administration, conceptualization, preparation, formal analysis, presentation of the published work, visualization, data presentation, software analysis, writing/review and editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgement
We are sincerely grateful to Xinyun Li for his valuable suggestions on this manuscript. The National Key Research and Development Program of China (2017YFE0113700), the Hubei Provincial Natural Science Foundation of China (2017CFB514), and the National Natural Science Foundation of China (30800808) supported this work.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Akhtar M., Shaukat A., Zahoor A., Chen Y., Wang Y., Yang M., et al. Hederacoside-c inhibition of staphylococcus aureus-induced mastitis via tlr2 & tlr4 and their downstream signaling nf-κb and mapks pathways in vivo and in vitro. Inflammation. 2020;43:579–594. doi: 10.1007/s10753-019-01139-2. [DOI] [PubMed] [Google Scholar]
- Alva-Murillo N., Ochoa-Zarzosa A., López-Meza J.E. Short chain fatty acids (propionic and hexanoic) decrease staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet Microbiol. 2012;155:324–331. doi: 10.1016/j.vetmic.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Arora T., Sharma R., Frost G. Propionate. Anti-obesity and satiety enhancing factor? Appetite. 2011;56:511–515. doi: 10.1016/j.appet.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., et al. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barko P.C., McMichael M.A., Swanson K.S., Williams D.A. The gastrointestinal microbiome: a review. J Vet Intern Med. 2018;32:9–25. doi: 10.1111/jvim.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengelsdorf F.R., Poehlein A., Daniel R., Dürre P. Genome sequence of the caproic acid-producing bacterium caproiciproducens galactitolivorans bs-1(t) (jcm 30532) Microbiol Resour Announc. 2019;8 doi: 10.1128/MRA.00346-19. e00346-00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukema M., Faas M.M., de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp Mol Med. 2020;52:1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar P.C., Kohn B. Use of the microbiome in the management of children with type 2 diabetes mellitus. Curr Opin Pediatr. 2019;31:524–530. doi: 10.1097/MOP.0000000000000781. [DOI] [PubMed] [Google Scholar]
- Brockmann L., Giannou A.D., Gagliani N., Huber S. Regulation of t(h)17 cells and associated cytokines in wound healing, tissue regeneration, and carcinogenesis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatr. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- Byndloss M.X., Olsan E.E., Rivera-Chávez F., Tiffany C.R., Cevallos S.A., Lokken K.L., et al. Microbiota-activated ppar-γ signaling inhibits dysbiotic enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cait A., Hughes M.R., Antignano F., Cait J., Dimitriu P.A., Maas K.R., et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11:785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- Chen B., Sun L., Zhang X. Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J Autoimmun. 2017;83:31–42. doi: 10.1016/j.jaut.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Chen G., Ran X., Li B., Li Y., He D., Huang B., et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a tnbs-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Sun M., Wu W., Yang W., Huang X., Xiao Y., et al. Microbiota metabolite butyrate differentially regulates th1 and th17 cells' differentiation and function in induction of colitis. Inflamm Bowel Dis. 2019;25:1450–1461. doi: 10.1093/ibd/izz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa-Oliveira R., Fachi J.L., Vieira A., Sato F.T., Vinolo M.A. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel J., Biesiada G., Brzozowski T., Ptak-Belowska A., Perucki W., Birczynska M., et al. The role of local and systemic cytokines in patients infected with clostridium difficile. J Physiol Pharmacol. 2014;65:695–703. [PubMed] [Google Scholar]
- Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- den Besten G., Lange K., Havinga R., van Dijk T.H., Gerding A., van Eunen K., et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305:G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf E.M., Cani P.D., Neyrinck A.M., Possemiers S., Van Holle A., Muccioli G.G., et al. Inulin-type fructans with prebiotic properties counteract gpr43 overexpression and pparγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011;22:712–722. doi: 10.1016/j.jnutbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2020;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- Frampton J., Murphy K.G., Frost G., Chambers E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metabol. 2020;2:840–848. doi: 10.1038/s42255-020-0188-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Carbonell R., Yao S.-J., Das S., Guma M. Dysregulation of intestinal epithelial cell ripk pathways promotes chronic inflammation in the ibd gut. Front Immunol. 2019;10:1094. doi: 10.3389/fimmu.2019.01094. 1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland S.H. Short chain fatty acids may elicit an innate immune response from preadipocytes: a potential link between bacterial infection and inflammatory diseases. Med Hypotheses. 2011;76:881–883. doi: 10.1016/j.mehy.2011.02.041. [DOI] [PubMed] [Google Scholar]
- Gonçalves P., Araújo J.R., Di Santo J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:558–572. doi: 10.1093/ibd/izx029. [DOI] [PubMed] [Google Scholar]
- Haapalainen A.M., Meriläinen G., Wierenga R.K. The thiolase superfamily: condensing enzymes with diverse reaction specificities. Trends Biochem Sci. 2006;31:64–71. doi: 10.1016/j.tibs.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Higashimura Y., Naito Y., Takagi T., Uchiyama K., Mizushima K., Yoshikawa T. Propionate promotes fatty acid oxidation through the up-regulation of peroxisome proliferator-activated receptor α in intestinal epithelial cells. J Nutr Sci Vitaminol. 2015;61:511–515. doi: 10.3177/jnsv.61.511. [DOI] [PubMed] [Google Scholar]
- Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S., Sun J., Chen P., Lakshman S., Molokin A., Harnly J.M., et al. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles, and intestinal gene expression in pigs. J Nutr. 2016;146:673–680. doi: 10.3945/jn.115.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk M.M., Miller M.J., Freund G.G. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61:1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I., Ullah N., Zha L., Bai Y., Khan A., Zhao T., et al. Alteration of gut microbiota in inflammatory bowel disease (ibd): cause or consequence? Ibd treatment targeting the gut microbiome. Pathogens. 2019;8:126. doi: 10.3390/pathogens8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi A., Yáñez A., Price Jeremy G., Chow A., Merad M., Goodridge Helen S., et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Kang S.G., Park J.H., Yanagisawa M., Kim C.H. Short-chain fatty acids activate gpr41 and gpr43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.e391-310. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- Kobyliak N., Virchenko O., Falalyeyeva T. Pathophysiological role of host microbiota in the development of obesity. Nutr J. 2016;15:43. doi: 10.1186/s12937-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Krautkramer K.A., Fan J., Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2020;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- Kromann S., Kudirkiene E., Li L., Thoefner I., Daldorph E., Christensen J.P., et al. Treatment with high-dose antidepressants severely exacerbates the pathological outcome of experimental escherichia coli infections in poultry. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larraufie P., Doré J., Lapaque N., Blottière H.M. Tlr ligands and butyrate increase pyy expression through two distinct but inter-regulated pathways. Cell Microbiol. 2017;19 doi: 10.1111/cmi.12648. [DOI] [PubMed] [Google Scholar]
- Lavelle A., Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- Layden B.T., Angueira A.R., Brodsky M., Durai V., Lowe W.L., Jr. Short chain fatty acids and their receptors: new metabolic targets. Transl Res. 2013;161:131–140. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Lee C., Kim B.G., Kim J.H., Chun J., Im J.P., Kim J.S. Sodium butyrate inhibits the nf-kappa b signaling pathway and histone deacetylation, and attenuates experimental colitis in an il-10 independent manner. Int Immunopharm. 2017;51:47–56. doi: 10.1016/j.intimp.2017.07.023. [DOI] [PubMed] [Google Scholar]
- Leone R.D., Zhao L., Englert J.M., Sun I.M., Oh M.H., Sun I.H., et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Su H., Zhou Z., Yao W. Identification of the porcine g protein-coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., He J., Xin X., Wang M., Xu J., Zhang J. Enhanced bioproduction of short-chain fatty acids from waste activated sludge by potassium ferrate pretreatment. Chem Eng Trans. 2018;332:456–463. [Google Scholar]
- Li M., van Esch B., Wagenaar G.T.M., Garssen J., Folkerts G., Henricks P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–59. doi: 10.1016/j.ejphar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Liu J., Gu Z., Song F., Zhang H., Zhao J., Chen W. Lactobacillus plantarum zs2058 and lactobacillus rhamnosus gg use different mechanisms to prevent salmonella infection in vivo. Front Microbiol. 2019;10:299. doi: 10.3389/fmicb.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li L., Min J., Wang J., Wu H., Zeng Y., et al. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277:66–73. doi: 10.1016/j.cellimm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Liu S., Li E., Sun Z., Fu D., Duan G., Jiang M., et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9:287. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötscher J., Balmer M.L. Sensing between reactions - how the metabolic microenvironment shapes immunity. Clin Exp Immunol. 2019;197:161–169. doi: 10.1111/cei.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li X., Liu S., Zhang Y., Zhang D. Toll-like receptors and inflammatory bowel disease. Front Immunol. 2018;9:72. doi: 10.3389/fimmu.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethy P.M., Huynh S., Ribardo D.A., Winter S.E., Parker C.T., Hendrixson D.R. Microbiota-derived short-chain fatty acids modulate expression of campylobacter jejuni determinants required for commensalism and virulence. mBio. 2017;8 doi: 10.1128/mBio.00407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu M., Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of t cells. Eur J Immunol. 2019;49:842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- Mandaliya D.K., Patel S., Seshadri S. The combinatorial effect of acetate and propionate on high-fat diet induced diabetic inflammation or metaflammation and t cell polarization. Inflammation. 2021;44:68–79. doi: 10.1007/s10753-020-01309-7. [DOI] [PubMed] [Google Scholar]
- Mandaliya D.K., Seshadri S. Short chain fatty acids, pancreatic dysfunction and type 2 diabetes. Pancreatology. 2019;19:280–284. doi: 10.1016/j.pan.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Markowiak-Kopeć P., Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor gpr43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A.J., Huffnagle G.B. The microbiome and regulation of mucosal immunity. Immunology. 2014;142:24–31. doi: 10.1111/imm.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen S.A., Collignon P.J. Antimicrobial resistance: a one health perspective. Microbiol Spectr. 2018;6 doi: 10.1128/microbiolspec.arba-0009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy I.H., Goudarzi M., Tong M., Ruegger P.M., Schwager E., Weger J.R., et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1:17. doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin R.F., Berthon B.S., Jensen M.E., Baines K.J., Wood L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:930–945. doi: 10.3945/ajcn.117.156265. [DOI] [PubMed] [Google Scholar]
- Mielenz M. Invited review: nutrient-sensing receptors for free fatty acids and hydroxycarboxylic acids in farm animals. Animal. 2017;11:1008–1016. doi: 10.1017/S175173111600238X. [DOI] [PubMed] [Google Scholar]
- Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Tashiro K., Kuhara S., Hayashi T., Sugimoto N., Tobe T. Regulation of virulence by butyrate sensing in enterohaemorrhagic escherichia coli. Microbiology (Read) 2009;155:521–530. doi: 10.1099/mic.0.023499-0. [DOI] [PubMed] [Google Scholar]
- Nastasi C., Candela M., Bonefeld C.M., Geisler C., Hansen M., Krejsgaard T., et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhung N.T., Chansiripornchai N., Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens: a review. Front Vet Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T., Bungo T., Isobe N., Yoshimura Y. Intestinal inflammation induced by dextran sodium sulphate causes liver inflammation and lipid metabolism disfunction in laying hens. Poultry Sci. 2020;99:1663–1677. doi: 10.1016/j.psj.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H., Fujioka Y., Katagiri C., Mamoto R., Aoyama-Ishikawa M., Amako K., et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atherosclerosis Thromb. 2013;20:425–442. doi: 10.5551/jat.15065. [DOI] [PubMed] [Google Scholar]
- Ohira H., Tsutsui W., Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atherosclerosis Thromb. 2017;24:660–672. doi: 10.5551/jat.RV17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., et al. Short chain fatty acids (scfas)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V.T., Lacroix C., Braegger C.P., Chassard C. Lactate-utilizing community is associated with gut microbiota dysbiosis in colicky infants. Sci Rep. 2017;7:11176. doi: 10.1038/s41598-017-11509-1. 11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzke M., Meiser J., Vazquez A. Formate metabolism in health and disease. Mol Metabol. 2020;33:23–37. doi: 10.1016/j.molmet.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta D.R., Graham D.B., Subramanian S., Xavier R.J. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell. 2019;178:1041–1056. doi: 10.1016/j.cell.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J., Hornef M. Innate immune signalling at the intestinal epithelium in homeostasis and disease. EMBO Rep. 2012;13:684–698. doi: 10.1038/embor.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R., Sarker P., Bergman P., Ara G., Lindh M., Sack D.A., et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A. 2006;103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A., Khalil A.A., Rahman U.U., Khalid A., Naz S., Shariati M.A., et al. Recent advances in the therapeutic application of short-chain fatty acids (scfas): an updated review. Crit Rev Food Sci Nutr. 2021:1–21. doi: 10.1080/10408398.2021.1895064. [DOI] [PubMed] [Google Scholar]
- Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E., Giudici F., Fiorindi C., Ficari F., Scaringi S., Amedei A. Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front Immunol. 2019;10:2754. doi: 10.3389/fimmu.2019.02754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S., Zenewicz L.A., Kamanaka M., Flavell R.A. Anti-inflammatory and pro-inflammatory roles of tgf-beta, il-10, and il-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna S., van Zuydam N.R., Mahajan A., Kurilshikov A., Vich Vila A., Võsa U., et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600–605. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S.K., Pande A., Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol. 2019;70:260–272. doi: 10.1016/j.jhep.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Schönfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57:943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulthess J., Pandey S., Capitani M., Rue-Albrecht K.C., Arnold I., Franchini F., et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50:432–445.e437. doi: 10.1016/j.immuni.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sencio V., Machado M.G., Trottein F. The lung–gut axis during viral respiratory infections: the impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021;14:296–304. doi: 10.1038/s41385-020-00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Lu Z., Chen Z., Wu Y., Shen Z. Rapid fermentable substance modulates interactions between ruminal commensals and toll-like receptors in promotion of immune tolerance of goat rumen. Front Microbiol. 2016;7:1812. doi: 10.3389/fmicb.2016.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T., Hirschey M.D., Huang J.Y., Ho L.T., Verdin E. Acetate metabolism and aging: an emerging connection. Mech Ageing Dev. 2010;131:511–516. doi: 10.1016/j.mad.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Simeoli R., Mattace Raso G., Pirozzi C., Lama A., Santoro A., Russo R., et al. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br J Pharmacol. 2017;174:1484–1496. doi: 10.1111/bph.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiljar M., Merkler D., Trajkovski M. The immune system bridges the gut microbiota with systemic energy homeostasis: focus on tlrs, mucosal barrier, and scfas. Front Immunol. 2017;8:1353. doi: 10.3389/fimmu.2017.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Buys N.J. Glucose- and glycaemic factor-lowering effects of probiotics on diabetes: a meta-analysis of randomised placebo-controlled trials. Br J Nutr. 2016;115:1167–1177. doi: 10.1017/S0007114516000076. [DOI] [PubMed] [Google Scholar]
- Sun M., Wu W., Chen L., Yang W., Huang X., Ma C., et al. Microbiota-derived short-chain fatty acids promote th1 cell il-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, gpcr, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wilkinson B.J., Standiford T.J., Akinbi H.T., O'Riordan M.X.D. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J Bacteriol. 2012;194:5274–5284. doi: 10.1128/JB.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart A.K., Kero J., Gan X., Cai T.Q., Cheng K., Ippolito M., et al. (d)-beta-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor puma-g. J Biol Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13:2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J., et al. Dietary fiber confers protection against flu by shaping ly6c(-) patrolling monocyte hematopoiesis and cd8(+) t cell metabolism. Immunity. 2018;48:992–1005.e1008. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Hirabara S.M., Curi R. G-protein-coupled receptors as fat sensors. Curr Opin Clin Nutr Metab Care. 2012;15:112–116. doi: 10.1097/MCO.0b013e32834f4598. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Hatanaka E., Hebeda C.B., Farsky S.H., Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci (Lond) 2009;117:331–338. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Hatanaka E., Sato F.T., Sampaio S.C., Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Wang J.J., Zhang Q.M., Ni W.W., Zhang X., Li Y., Li A.L., et al. Modulatory effect of lactobacillus acidophilus klds 1.0738 on intestinal short-chain fatty acids metabolism and gpr41/43 expression in β-lactoglobulin-sensitized mice. Microbiol Immunol. 2019;63:303–315. doi: 10.1111/1348-0421.12723. [DOI] [PubMed] [Google Scholar]
- Xiao T., Wu S., Yan C., Zhao C., Jin H., Yan N., et al. Butyrate upregulates the tlr4 expression and the phosphorylation of mapks and nk-κb in colon cancer cell in vitro. Oncol Lett. 2018;16:4439–4447. doi: 10.3892/ol.2018.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Miyamoto N., Shibata K., Valasek M.A., Motoike T., Kedzierski R.M., et al. Short-chain fatty acids stimulate leptin production in adipocytes through the g protein-coupled receptor gpr41. Proc Natl Acad Sci U S A. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhu Y., Li X., Sun B. Dynamic balancing of intestinal short-chain fatty acids: the crucial role of bacterial metabolism. Trends Food Sci Technol. 2020;100:118–130. [Google Scholar]
- Yang Y., Ashworth A.J., Willett C., Cook K., Upadhyay A., Owens P.R., et al. Review of antibiotic resistance, ecology, dissemination, and mitigation in u.S. Broiler poultry systems. Front Microbiol. 2019;10:2639. doi: 10.3389/fmicb.2019.02639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuille S., Reichardt N., Panda S., Dunbar H., Mulder I.E. Human gut bacteria as potent class i histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolska-Downar D., Siennicka A., Kaczmarczyk M., Kołodziej B., Naruszewicz M. Butyrate inhibits cytokine-induced vcam-1 and icam-1 expression in cultured endothelial cells: the role of nf-kappab and pparalpha. J Nutr Biochem. 2004;15:220–228. doi: 10.1016/j.jnutbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Zelová H., Hošek J. Tnf-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Mukherjee A., Varghese A.P., Yang X.-L., Chen S., Zhang H. Roles of g protein-coupled receptors in inflammatory bowel disease. World J Gastroenterol. 2020;26:1242–1261. doi: 10.3748/wjg.v26.i12.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan K., Gong X., Chen Y., Jiang M., Yang T., Zhao G. Short-chain fatty acids regulate the immune responses via g protein-coupled receptor 41 in bovine rumen epithelial cells. Front Immunol. 2019;10:2042. doi: 10.3389/fimmu.2019.02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Meng M., Gao L., Tu Y., Bai Y. Sodium butyrate improves high-concentrate-diet-induced impairment of ruminal epithelium barrier function in goats. J Agric Food Chem. 2018;66:8729–8736. doi: 10.1021/acs.jafc.8b03108. [DOI] [PubMed] [Google Scholar]