Abstract
The content of tumor-derived extracellular vesicles (EVs) can regulate the tumor microenvironment and functionally acts in favor of cancer aggressiveness. To better elucidate the role of EVs in the interplay between immune system and tumor microenvironment, the purpose of this study was to analyze the effect of head and neck squamous cells carcinoma (HNSCC)-derived EVs on the modulation of inflammasomes - mediators of pyroptosis and secretion of inflammatory factors by macrophages. Our results showed that macrophages treated with the Vesicular Secretome Fraction (VSF) isolated from patient-derived HNSCC presented a reduction in the secretion of mature IL-1β and caspase-1 without affecting cell viability. An analysis of the protein content of HNSCC-derived VSF by antibody array revealed that some of the most expressed proteins share a correlation with Transforming Growth Factor-beta (TGF-β) activity. Since TGF-β is related to the inhibition of the NF-kB-related pathways, including those required for the priming phase of the inflammasomes, we sought to evalute the interference of the VSF in the induction of inflammasome components. In fact, HNSCC-derived VSF inhibited the induction of pro-IL-1β and pro-caspase-1 proteins and NLRP3 gene expression during the priming phase of inflammasome activation. Thus, our findings contribute to a better understanding of how tumor-derived EVs modulate inflammatory response by demonstrating their role in inhibiting NLRP3 inflammasomes.
Keywords: NLRP3, Extracellular vesicles, Exosomes, Inflammasomes, Head and neck cancer, TGF-β
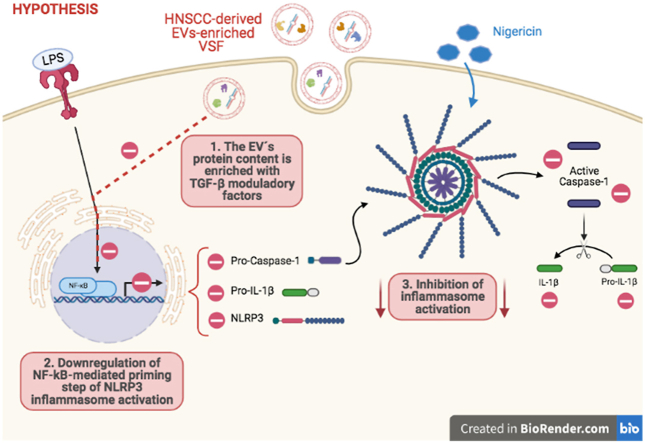
Graphical abstract
Highlights
-
•
Vesicular Secretome Fraction (VSF) from HNSCC inhibits macrophage responses to the NLRP3 inflammasomes agonists.
-
•
HNSCC-derived VSF is enriched with proteins correlated with the Transforming Growth Factor-b pathway.
-
•
HNSCC-derived VSF affects the priming phase of inflammasome activation.
1. Introduction
The interactions between cancer cells and their microenvironment are crucial for the tumor fate (Quatromoni and Eruslanov, 2012; Schiavoni et al., 2013). Cell to cell communication can be mediated through growth factors, hormones, cytokines, adhesion molecules, or by extracellular vesicles (EVs)(Marar et al., 2021). EVs are lipid bilayer membrane vesicles secreted by a great diversity of cells which content includes nucleic acids, lipids, and proteins. The content of EVs shed by cancer cells can play significant roles in the recipient cells, by inducing tumor progression (Bebelman et al., 2018; Yang et al., 2011; Zhang et al., 2015), metastatic spread (Bebelman et al., 2018; Karp and Zwicker, 2014; Wu et al., 2016; Mcgarty, 2013), and drug resistance (Rodrigues-Junior et al., 2019a). Due to their presence in body fluids and their key role in cell communication (Bebelman et al., 2018; Karp and Zwicker, 2014; Mcgarty, 2013; Raposo and Stoorvogel, 2013), EVs have been considered an emerging hallmark of cancer, with the potential to reveal new tumor biomarkers (Rodrigues-Junior et al., 2019b). Additionally, tumor-derived EVs also participate in several pro-tumorigenic strategies of immune evasion - including manipulation of immune cells phenotype and effector mechanisms (Bebelman et al., 2018; Karp and Zwicker, 2014; Mcgarty, 2013).
Inflammation has long been associated with tumor development and can be triggered by a variety of immune cells, including macrophages (Coussens and Werb, 2002). A central mechanism to drive inflammation on those cells is the assemble and activation of multimeric cytosolic complexes termed inflammasomes (Karki et al., 2017). One of the best-characterized inflammasomes is the NLRP3 (Nucleotide-binding Oligomerization Domain (NOD)-, Leucine-rich repeat–containing Receptors (NLRs) family Pyrin domain containing 3), that acts through the adapter molecule ASC (Apoptosis-associated Speck-like protein containing CARD (Caspase Activating and Recruitment Domain)) for the recruitment of caspase-1 (Malik and Kanneganti, 2017). Complete activation of NLRP3 inflammasome requires two steps: (i) a priming phase induced by an activating stimulus of the nuclear factor-kB (NF-kB) transcription factor for the expression of inflammasome components such as pro-interleukin (IL)-1β, pro-caspase-1 and NLRP3, and (ii) a agonist-triggered stimuli inducing the platform oligomerization and caspase-1 cleavage and activation (Swanson et al., 2019). Upon its assembly, inflammasomes activate caspase-1, leading to the maturation of pro-inflammatory cytokines IL-1β and IL-18 and the cleavage of Gasdermin D, the effector of pyroptosis (Swanson et al., 2019; Shi et al., 2015; Kayagaki et al., 2015).
The NLRP3 inflammasome is activated by a wide variety of unrelated stimuli. Its activation has already been reported during bacterial, protozoan, viral, and fungal infections, as well as in sterile inflammation mediated by endogenous signals and exposure to environmental irritants. A unifying factor of NLRP3 activators is that all of them induce cellular stress, suggesting that the NLRP3 is activated indirectly, reflecting the homeostatic nature of the cell (Swanson et al., 2019). Multiple signals upstream NLRP3 activation have already been proposed, including potassium (Muñoz-Planillo et al., 2013) and chloride ions (Tang et al., 2017) efflux, lysosomal disruption (Hornung et al., 2008), mitochondrial dysfunction (Groß et al., 2016; Iyer et al., 2013), metabolic changes (Sanman et al., 2016; Moon et al., 2015), release of oxidized DNA (Shimada et al., 2012), and trans-Golgi disassembly (Chen and Chen, 2018). Nevertheless, the precise molecular mechanism responsible for NLRP3 activation remains to be elucidated.
The interaction between macrophages and inflammasomes with tumor cells represents an often-controversial scenario due to the enormous macrophages plasticity and the various effects mediated by inflammasomes, which can be related to the better or worse prognosis of cancer patients (Kolb et al., 2014; Liss et al., 2001). The NLRP3 inflammasome has been described to contribute to the progression of gastric cancer, lung cancer, prostate cancer, breast cancer among others (Karki et al., 2017; He et al., 2018; Faria et al., 2021). However, inflammasome activation was also demonstrated to be protective in the context of melanoma and the production of IL-18 was critically involved in the protection against colorectal cancer (Karki et al., 2017). Moreover, the IL-1β signaling axis has been shown to lead an effective adaptive immune response against dying tumor cells, driving an effective immune response against transplantable tumor cells (Ghiringhelli et al., 2009).
As aforementioned, EVs and inflammasomes activation can have significant roles in the tumor microenvironment (Bebelman et al., 2018; Karp and Zwicker, 2014; Mcgarty, 2013; Kolb et al., 2014) and both interact in multiple scenarios, with EVs acting as positive or negative regulators of inflammasomes activation (Wang et al., 2019, 2020; Chen et al., 2019; Huang et al., 2020; Dai et al., 2020; Wu et al., 2020; Zhang et al., 2019; Kohli et al., 2016). Nevertheless, it is still unknown how head and neck squamous cell carcinoma (HNSCC)- derived EVs can affect macrophages effector mechanisms, especially those mediated by inflammasomes. Therefore, we decided to evaluate the modulation of NLRP3 inflammasome by HNSCC-derived EVs. Here we show that treatment of macrophages with HNSCC-derived VSF - which is enriched in EVs – induced the inhibition of the NLRP3 inflammasome. HNSCC-derived VSF downregulates pro-caspase-1, pro-IL-1β, and NLRP3 expression, thus affecting the priming phase of NLRP3 inflammasome activation.
2. Material and methods
2.1. Cell culture and animals
The primary HNSCC (NCC–HN19) and the HEK293T cell lines were grown in a suitable culture medium (RPMI-1640, ThermoFisher) supplemented with 10% fetal bovine serum (FBS, ThermoFisher), 1% Penicillin/Streptomycin (Gibco) and maintained at 37 °C in the presence of 5% CO2.
C57BL/6 mice with 6–8 weeks of age were provided by the Center for Development of Experimental Models for Medicine and Biology (CEDEME) from Federal University of São Paulo (UNIFESP). All animals were maintained in specific pathogen-free conditions in microisolators with free access to water and feed. The development of this project was approved by the UNIFESP Ethics Committee on Animal Use (CEUA, #9957100217). The mice were inoculated intraperitoneally with 2 mL of 1.5% potato starch (Sigma) diluted in 1X PBS for the enrichment of the peritoneum by macrophages. After 96h, the mice were euthanized in a closed chamber by Halothane inhalation (Cristalia). After euthanasia, 5 mL of sterile and cold PBS (1X) were injected into the peritoneal cavity to obtain cell suspension. These cells were centrifuged at 500×g for 5 min and resuspended with 5 mL of complete RPMI1640 medium (ThermoFisher) supplemented with 3% FBS (LGC).
Further, according to the assays, cells were seeded and incubated for 4h at 37 °C in an atmosphere containing 5% CO2, to enrich the macrophage population by adhesion. PBS was used to remove non-adherent cells. All the stimuli used (LPS, nigericin and VSF treatment) were prepared using OptMem medium (Life Technologies) to avoid FBS interference in the results.
2.2. Vesicular Secretome Fraction
The NCC-HN19 and HEK293T cells-derived VSF was obtained as previously described (Rodrigues-Junior et al., 2019a). The cells were incubated for 72h in phenol red-free DMEM (Gibco), supplemented with 5% Insulin-Transferrin-Selenium-Ethanolamine (ThermoFisher), 10 mM of Non-Essential Amino Acids (ThermoFisher), 500 μg of fibroblast growth factor-basic (ThermoFisher), 100 mM of Sodium Pyruvate and 55 mM of 2-Mercaptoethanol (ThermoFisher). After incubation, the culture medium was collected and centrifuged at 250×g for 5 min to remove dead cells. Then, the supernatant was filtered on a 0.22 μm filter and further concentrated 20X by tangential flow filtration on a 50 kDa Ultra-15 Centrifugal Filter (Millipore) by centrifugation at 1200×g. This concentrated conditioned medium, denominated as Vesicular Secretome Fraction (VSF) is enriched in functional EVs (Rodrigues-Junior et al., 2019a; Lai et al., 2010).
Hence, in order to characterize the EVs present in the VSF, first the size distribution was measured using the NanoSight system (NTA 3.1 analytic software; Malvern Panalytical) for nanoparticle tracking analysis (NTA). The concentration, mean and modal average diameter of the nanoparticles were used for statistical analysis. The EVs morphology present in the VSF was visualized by transmission electron microscopy (TEM). Briefly, the VSF from NCC-HN19 and HEK293T were incubated with 50 μl of uranyl-oxalate solution for 5 min and washed four times with H2O. Images were acquired on a JEM 1200 EX II (JEOL) at 80 kV at the UNIFESP Electron Microscopy Center (CEME) (Théry et al., 2006). Furthermore, the expression of EVs markers (ALIX and CD9) were also idenfied as detailed below in the immunoblotting section.
2.3. Cell culture and stimulation
To assess the role of HNSCC-derived VSF on peritoneal macrophages, the cells (5 × 105 cells/well) isolated from wild-type C57BL/6 mice were treated with different VSF concentrations overnight at 37 °C. The concentrations used in the experiments were 1:5 dilution (corresponding to one part of VSF diluted in four parts of medium), 1:20 dilution (corresponding to one part of VSF for nineteen parts of medium) and 1:50 dilution (corresponding to one part of VSF for forty-nine parts of medium). The concentration of nanoparticles/ml obtained by NTA was used to estimate the concentration of nanoparticles in each treatment. The VSF dilutions were performed using the phenol red-free medium used to isolate EVs (EVs-free culture medium). Then, the macrophages were treated with 500 ng/mL of Lipopolysaccharide (LPS) (InvivoGen) for 3h and stimulated with 10 μM nigericin (InvivoGen) for 2h.
2.4. Cell viability
For cell viability evaluation, 3 × 105 macrophages were cultured in the presence of 5 nM Sytox Green (ThermoFisher) which can be incorporated by dead cells. The images were obtained using the Incucyte Zoom microscope and the frequency of Sytox Green positive cells was analyzed using IncuCyte® ZOOM Live-Cell Analysis system software (Essen BioScience). Cell incorporation of ethidium bromide and acridine orange (Sigma-Aldrich) was performed to evaluate cell death by fluorescence microscopy. Cell viability was also evaluated through lactate dehydrogenase (LDH) release, using comercial kits (Sigma-Aldrich), according to manufacturer's instructions.
2.5. Cytokine measurment
IL-1β and IL-6 quantification in macrophages culture supernatants (5 × 105) was performed by capture sandwich ELISA (eBioscience), according to the manufacturer's instructions. Absorbance from ELISA plates were read with a Spectra Max M2e microplate reader (Molecular Devices) using Soft-Max Pro Version 5.4 software (Molecular Devices).
2.6. Immunoblotting
Western Blotting assays were performed as previously described (Buzzo et al., 2017). Antibodies anti-ALIX and anti-CD9 were purchased from Santa Cruz Biotechnology; anti-IL-1β from R&D Systems and anti-β-actin from Sigma-Aldrich. Caspase-1 antibody was kindly provided by Dr. Vishva Dixit from Genentech. Image J software (Image J, NIH) was used to determine densitometric quantification, according to β-actin expression.
2.7. Antibody array
The VSF isolated from NCC-HN19 cells were lysed with cell lysis buffer (#K269; Biovision) and 100 μL of the protein lysate was analyzed using the RayBio L-Series Human Antibody Array 1000 Glass Slide Kit (#AAH-BLG-1000-4, RayBiotech), according to manufacturer's instructions. After the immune reaction, the array was scanned and normalized using Gene-Pix Pro 7 software (Molecular Devices), the analysis were performed as previously described (Rodrigues-Junior et al., 2019b). The Fold Change >0.5 was used as a cut-off to identify the altered proteins in the samples, according to their expression. The specific and differentially expressed proteins present in the VSF isolated from NCC-HN19 were functionally clustered using the STRING algorithm (Search Tool for the Retrieval of Interacting Genes/Proteins - https://string-db.org/). STRING was also used to identify the predicted biological networks associated with the VSF related proteins.
2.8. Real time PCR
NLRP3 expression was evaluated by real-time PCR. Macrophages were treated with the VSF and LPS-stimulated as previously described. Cellular RNA was isolated using TRIzol (ThermoFisher Scientific, Inc). The concentration and purification of mRNA were analyzed by spectrophotometry (NanoDrop 2000c – ThermoFisher Scientific, Inc). cDNA was generated from 300 ng of total RNA, using M-MLV Reverse Transcriptase (Invitrogen), according to manufacturer's instructions. cDNA (50 ng) was homogenized with TaqMan Universal PCR Master Mix (Applied BioSystems) and standardized assays of NLRP3 expression (Mm_00840904_m1) were carried out. NLRP3 expression levels were normalized using the expression level of beta-actin, as an endogenous control (Mm02619580_g1). Reactions were conducted in the Real-time PCR System QuantumStudio 6 Flex (Applied BioSystems).
2.9. Statistical analyzes
The statistical analyzes were carried out with the aid of Graphpad Prism software (GraphPad Software Incorporation, version 6.0). The statistic test used was two-way ANOVA, followed by Sidak's or Bonferroni's test. The value of p < 0.05 was considered statistically significant.
3. Results
3.1. VSF characterization
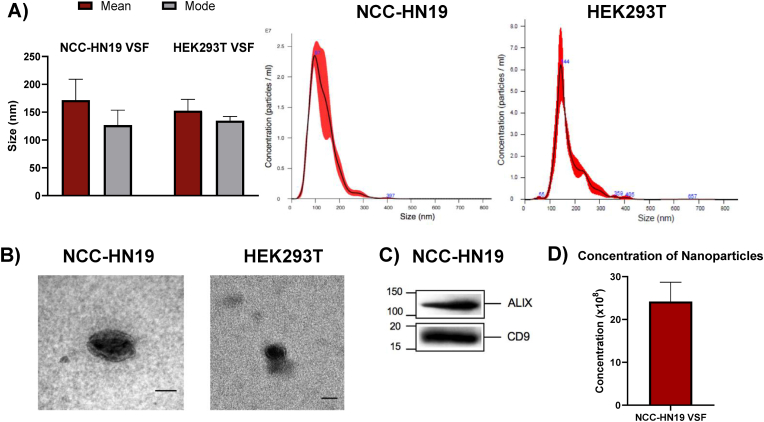
To characterize the EVs secreted by NCC-HN19 and HEK293T cell lines, the size of nanoparticles presented in their respective VSF was first determined by NTA. Our analysis showed that NCC-HN19-derived VSF is composed mainly of nanoparticles up to 200 nm. The average mean and modal sizes of EVs from NCC-HN19 present in the VSF were 171.63 ± 30.52 nm and 126.80 nm ± 21.98, respectively (Fig. 1A). HEK293T is a non-cancerigenous cell line that was used in some analysis as a control. Similar to NCC-HN19, HEK293T-derived VSF is composed mainly of nanoparticles up to 200 nm. In concern of this cell line, the average mean and modal sizes of EVs from HEK293T present in the VSF were 152.56 ± 17.65 nm and 134.66 nm ± 6.7, respectively (Fig. 1A). The TEM analysis of the VSF derived from NCC-HN19 and HEK293T cell lines revealed spheroidal and rounded structures, as expected (Fig. 1B). Moreover, CD9 and ALIX, classical EVs markers, were found highly expressed in the VSF isolated from NCC-HN19, confirming the enrichment of EVs (Fig. 1C). Finally, the concentration of nanoparticles/ml present in NCC-HN19-derived VSF was determined through NTA. Since 24,2 × 108 ± 4,48 × 108 particles/ml were found in the VSF (Fig. 1D), it was possible to estimate the concentration of nanoparticles present in each treatment. Thus, the treatment of 5 × 105 cells with 200 μl of 1:5 VSF-diluted medium (40μl of concentrated VSF and 160 μl of pure medium) contains around 0,968 × 108 nanoparticles (or ≅193,6 nanoparticles per cell). Same logic applies to 1:20 and 1:50 concentrations, which correspond to ≅48,4 and ≅19,3 nanoparticles/cell, respectively.
Fig. 1.
Characterization of EVs present in the VSF. (A) The particle size analysis performed by NTA showed that VSF is composed mainly of small particles up to 200 nm. The graph represents the mean and mode sizes obtained in the three independent assays. The values obtained indicated the modal particle size was 126 nm, while the mean size was 171 nm. (B) Transmission electron microscopy of EVs present in the VSF of NCC-HN19 and HEK293T, scales bar = 50 nm. (C) Western Blotting assay of VSF isolated from the NCC-HN19 cells for two different classical EVs markers (ALIX; 95 kDa, and CD9; 24 kDa). (D) The nanoparticles concentration values obtained through NTA showed that the VSF is enriched with approximately 24,2 × 108 nanoparticles/ml.
3.2. HNSCC-VSF treatment reduces the secretion of mature forms of IL-1β and caspase-1 by macrophages
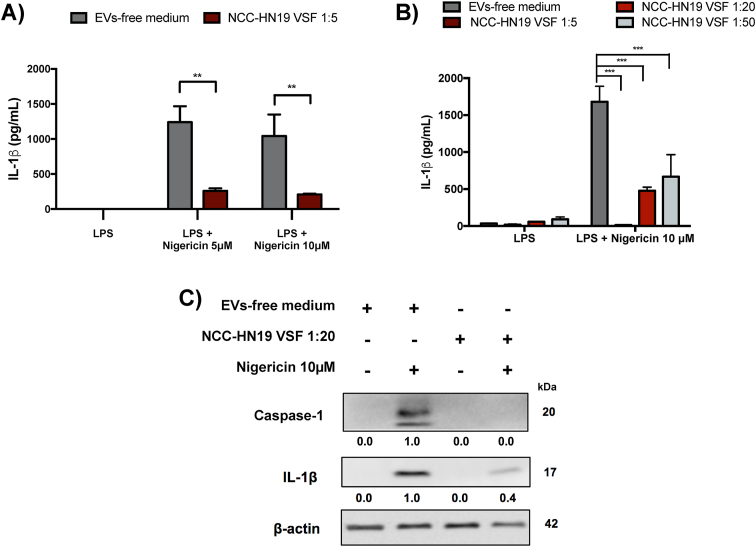
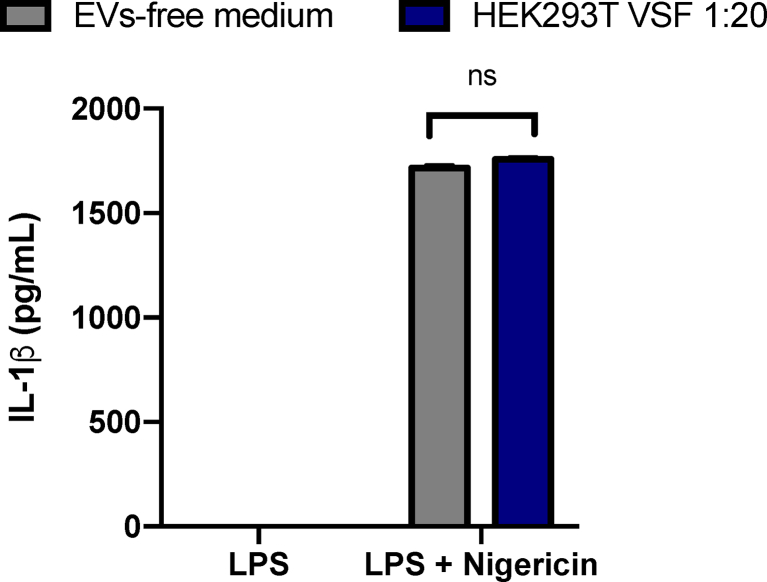
Next, we sought to evaluate the VSF impact in the regulation of inflammasomes. To this end, peritoneal macrophages were cultured in the presence or absence of HNSCC-derived VSF prior to the LPS priming. After 3h of LPS priming, cells were stimulated with 5 μM or 10 μM of nigericin for 2h to induce inflammasome activation. VSF treatment led to a reduction of IL-1β levels secreted by nigericin-stimulated macrophages (Fig. 2A) in a concentration-dependent manner (Fig. 2B). It is possible to note that the lowest dilution of the VSF (1:5, corresponding to 193 nanoparticles/cell) was more efficient in inhibiting inflammation, but other dilutions (1:20 and 1:50) were also capable of inducing inflammasome inhibition. Based on this, the 1:20 dilution was standardized for further assays. The inflammasome activation was also assessed through the presence of the mature forms of IL-1β and caspase-1 by WB. Our data showed a 60% reduction of mature IL-1β (p17) and a total inhibition of caspase-1 (p20) in the culture supernatant of VSF-treated macrophages in comparison to those treated in the absence of the vesicles (Fig. 2C), demonstrating that HNSCC-derived VSF inhibits inflammasome activation. Preliminary data obtained by our group showed that treating the peritoneal macrophages with the non-cancerigenous cell line HEK293T-derived VSF did not induce a decrease in IL-1β secretion, raising the possibility that the observed inhibition could be restricted to tumor-derived VSF (Supplementary Fig. 1).
Fig. 2.
Treatment of macrophages with VSF obtained from HNSCC cells reduces IL-1β and caspase-1 secretion. Peritoneal macrophages from wild-type C57BL/6 mice were initially treated overnight with VSF (1:5) (A) or VSF (1:5, 1:20, and 1:50) (B). Then the macrophages were primed with LPS (500 ng/mL) for 3h and stimulated with nigericin (10 μM) for 2h. Secretion of IL-1β was analyzed by ELISA. For the three analyzes, **p < 0.005 and ***p < 0.0005 when compared to control (EVs-free culture medium). Data are representative of four independent experiments with n = 3. (C) Protein expression of the active forms of IL-1β and caspase-1 in culture supernatant were evaluated by WB. β-actin was used as an endogenous control. Data are representative of three independent experiments with n = 3. Below each band, the numbers represent the respective densitometry quantification normalized in relation to β-actin expression. To determine the magnititude of reduction or enhancement of protein expression, positive controls were defined as 1.0 (100%) and were compared to VSF treatments.
3.3. HNSCC-VSF does not interfere in cell viability
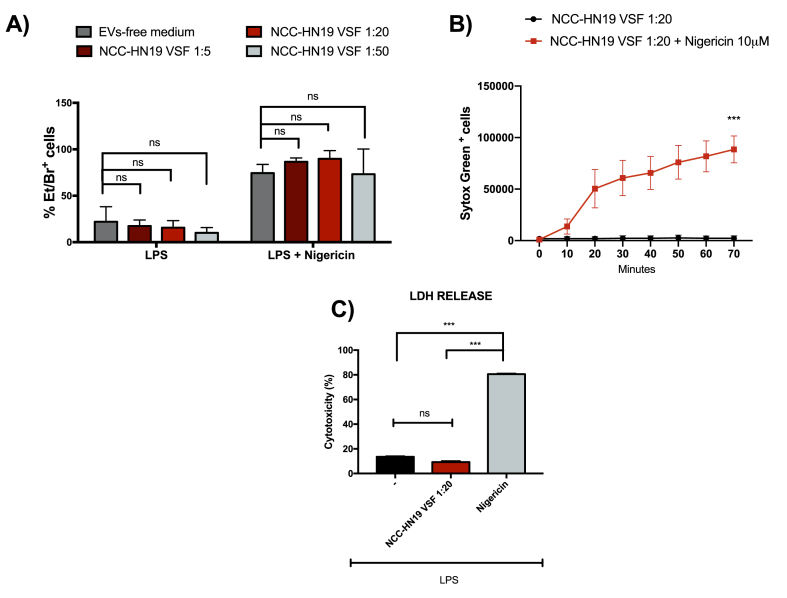
To further evaluate the impact of VSF treatment on the inhibition of inflammasome signaling and to discard the effect of VSF-induced cell death on reduced cytokine production, we evaluated the cell viability by the uptake of ethidium bromide (Et/Br) and the incorporation of SytoxGreen. Our data indicated that VSF treatment did not affect the uptake of Et/Br compared to cells cultured in the presence of EVs-free culture medium, even in the presence of nigericin which promotes loss of membrane integrity and consequent cell death (Fig. 3A).
Fig. 3.
HNSCC-VSF does not induce cell death. For the viability assays, peritoneal macrophages from wild-type C57BL/6 mice were treated overnight with VSF or EVs-free medium followed by priming with LPS (500 ng/mL) for 3h and stimulation with nigericin (10 μM) for 120 min (A and C) or 70 min (B). (A) Frequency of positive cells for ethidium bromide (Et/Br) was determined by fluorescence microscopy. (B) Incorporation of Sytox Green by permeable cells was determined in a Incucyte Zoom microscope. (C) The LDH release was measured using commercial kits, according to the manufacturer's instructions ***p < 0,0001 in comparison to the control. Data are representative of three independent experiments with n = 3. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
To further confirm the data obtained with the Et/Br incorporation assay and to perform a real-time analysis of cell viability, we also observed the incorporation of SytoxGreen by permeable cells with the aid of the Incucyte Zoom microscope. This assay confirmed that treatment with the VSF does not induce cell death by itself (Fig. 3B) or alters the frequency of nigericin-induced pyroptosis (Fig. 3A). Finally, no significant levels of LDH release were observed in macrophages treated in the presence or absence of HNSCC-derived VSF, rulling out the cytotoxic potential of VSF treatment, in contrast to that caused by nigericin (Fig. 3C). The data obtained by multiple viability assays corroborate the hypothesis that the inhibition of inflammasome activation by the VSF treatment occurs independently of cell death.
3.4. The protein content of the HNSCC-derived VSF is enriched in TGF-β modulatory factors
Tumor-derived EVs carry a variety of cargo that functions in immune activation and suppression (Marar et al., 2021). To evaluate the HNSCC-derived VSF protein content, the total protein was tested against a commercial 1000-immobilized antibody array. Interestingly, some of the most expressed proteins share a common correlation with Transforming Growth Factor-beta (TGFβ) activity (Table 1). Among the top-15 most expressed proteins we could identify Thrombospondin-1 (THBS1), Transferrin receptor (TRFC), and Plasminogen (PLG). Noteworthy, TGF-β is a pleiotropic cytokine with established anti-inflammatory effects that can act as an inhibitor of the NF-kB pathway, downregulating pro-inflammatory cytokine production (Ruscetti et al., 1992; Lee et al., 2011; Shiou et al., 2013; Cho et al., 2006). Our previous findings suggest that HNSCC derived EVs can carry TGFβ isoforms (Rodrigues-Junior et al., 2019a), and the results of this study allow us to speculate a potential new role for the protein content of HNSCC-derived VSF during the observed inhibition of the inflammasome complex.
Table 1.
List of the 15 mostly expressed proteins in the Vesicular Secretome Fraction isolated from NCC-HN19 cell line.
| Gene Name | Full Protein Name | Swissprot | Expression Level |
|---|---|---|---|
| ApoC3 | Apolipoprotein C-III | P02656 | 4,8003 |
| THBS1 | Trombospondin-1 | P07996 | 3,0147 |
| TFRC | Transferrin receptor protein 1 | P02786 | 2,2542 |
| PLG | Plasminogen | P00747 | 2,2003 |
| GZMA | Granzyme A | P12544 | 1,4665 |
| MMP-10 | Stromelysin-2 | P09238 | 1,4326 |
| FN1 | Fibronectin | P02751 | 1,4228 |
| CLU | Clusterin | P10909 | 1,3740 |
| MMP-12 | Macrophage metalloelastase | P39900 | 1,3367 |
| TGFBI | Transforming growth factor-beta-induced protein ig-h3 | Q15582 | 1,2812 |
| CXCL1 | Growth-regulated alpha protein | P09341 | 1,2385 |
| CXCL9 | C-X-C motif chemokine 9 | Q07325 | 1,1936 |
| IL6 | Interleukin-6 | P05231 | 1,1257 |
| IL13 | Interleukin-13 | P35225 | 1,1010 |
| TXNIP | Thioredoxin-interacting protein | Q9H3M7 | 1,0851 |
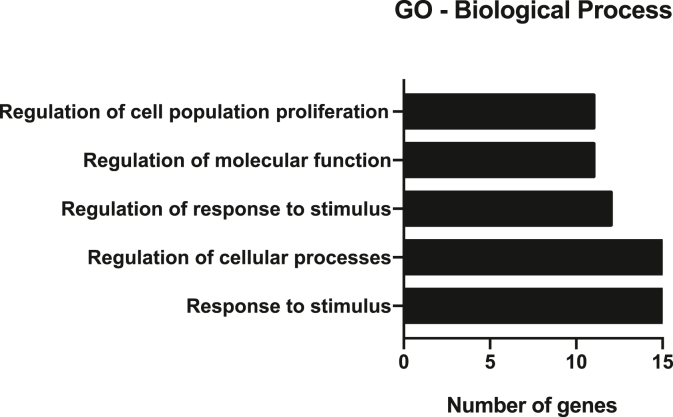
The top-15 most expressed proteins detected in this screening step were functionally clustered using the STRING algorithm to determine their contribution to biological processes. The most represented biological processes associated with the VSF protein cargo are response to stimulus, regulation of cellular processes, regulation of response to stimulus, regulation of molecular function, and regulation of cell population proliferation (Fig. 4). So, it is possible to suggest that those proteins might also be involved in the NLRP3 inflammasome inhibition.
Fig. 4.
The majority of VSF protein content is involved with regulation of cellular processes. Gene-Ontology based mostly enriched Biological Process related to the fifteen-mostly expressed VSF proteins identified through STRING algorithm.
3.5. HNSCC-derived VSF treatment interferes with the induction of NLRP3 inflammasome components, thus impacting its priming phase
Activation of NLRP3 inflammasome requires two distinct steps. The first step – the priming – is associated with two main functions: (i) the upregulation of NLRP3 and other inflammasome components and (ii) the induction of post-translational modifications of NLRP3 (Swanson et al., 2019; Kelley et al., 2019). The transcriptional upregulation can be induced through the recognition of diverse pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) that engage pattern recognition receptors (PRRs) and lead to NF-kB activation and gene transcription (Swanson et al., 2019). The agonist-triggered second step induces the full inflammasome assembly and activation. The stimuli that activate NLRP3 are diverse and unrelated but converge in the fact that they all induce cellular stress (Malik and Kanneganti, 2017; Swanson et al., 2019).
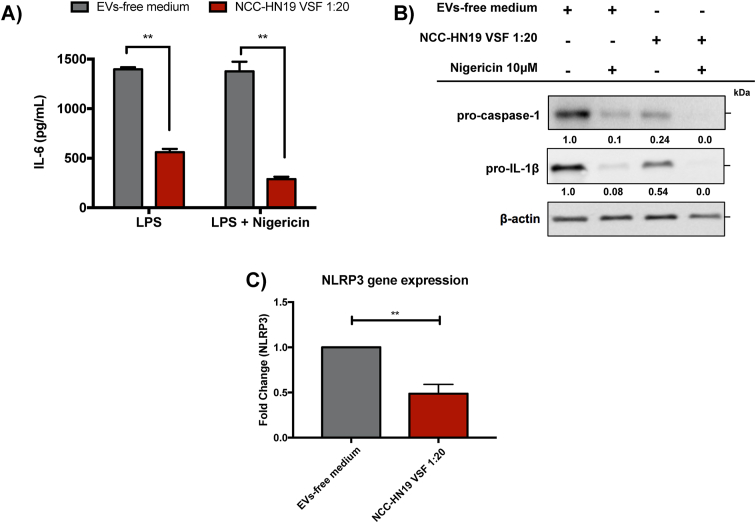
The activation of NF-kB pathway that characterizes the priming step of inflammasomes activation is widely associated with the upregulation of pro-inflammatory genes involved in several physiological processes. To explore the VSF mechanism of action during inflammasome inhibition we first evaluated the secretion of IL-6, a pro-inflammatory cytokine. HNSCC-derived VSF led to diminished secretion of IL-6 (Fig. 5A) in comparison to the macrophages cultured in the absence of EVs, thus suggesting a possible downregulation of NF-kB signaling pathway by TGF-β-related molecules.
Fig. 5.
HNSCC-derived VSF inhibits the induction of inflammasomes components. Peritoneal macrophages (5 × 10 (Yang et al., 2011) cells/well for ELISA and 1 × 10 (Zhang et al., 2015) for WB assays) from wild-type C57BL/6 mice were initially treated with the HNSCC-derived VSF (1:20) overnight. Then the macrophages were primed with 500 ng/mL LPS for 3h and stimulated with 10 μM nigericin for 2h. (A) The secretion of IL-6 was evaluated by ELISA. (B) Protein expression of the precursor forms of IL-1β and caspase-1 in cell lysates were analyzed by WB. β-actin was used as endogenous control. Below each band, the numbers represent the respective densitometry quantification normalized in relation to β-actin expression. To determine the magnititude of reduction or enhancement of protein expression, positive controls were defined as 1.0 (100%) and were compared to VSF treatments. (C) NLRP3 gene expression in VSF-treated macrophages in comparison to non-treated cells was determined by RT-PCR. All values were normalized using the β-actin as an endogenous control. Data are representative of independent experiments with n = 3. **p < 0.005 compared to the control.
For a deeper comprehension of VSF influence on the modulation of inflammasome activation and signaling, we evaluated the impact of VSF treatment on the induction of precursor forms. Intersentingly, the presence of HNSCC derived-VSF induced a 55% and 75% reduction in the pro-IL-1β and pro-caspase-1, respectively (Fig. 5B). Moreover, a significant reduction in the NLRP3 gene expression was found after the treatment with HNSCC derived-VSF (Fig. 5C), supporting the idea that HNSCC derived-VSF treatment affects the priming phase of NLRP3 inflammasome activation.
4. Discussion
Numerous studies have identified EVs as an essential means of intercellular communication that plays a role in physiological or biological important processes (Rashed et al., 2017). EVs are key players in cancer progression, being able to induce both pro-and anti-tumor responses (Bebelman et al., 2018; Mcgarty, 2013; Dörsam et al., 2018). For instance, cancer-derived EVs are carriers of tumor-antigens that can mediate antitumor immunity (Bebelman et al., 2018), as reported by Wolfers et al. (2001). It was also demonstrated that DC-derived EVs can directly induce apoptosis in various tumor cell lines and promote the proliferation of natural killer cells (Viaud et al., 2009). On the other hand, EVs are also capable of impairing the cytotoxic activity of TCD8+ lymphocytes (Liu et al., 2013) and increase the proliferation of regulatory T cells (Treg) (Wieckowski et al., 2009), “educating” the innate immune components towards a pro-tumorigenic phenotype (Boyiadzis and Whiteside, 2015) and resulting in an immunoprivileged status for tumor cells (Bebelman et al., 2018; Karp and Zwicker, 2014; Raposo and Stoorvogel, 2013; Dörsam et al., 2018).
Inflammation is a classic hallmark of cancer. Although there are numerous studies on the involvement of TLRs or interferon pathways in the chronic inflammation that affects tumor development, the role of inflammasomes is controversial (Kantono and Guo, 2017). The interaction between inflammasomes and EVs is cell and context-dependent. In the literature, EVs have already been associated with both activation and inhibition of inflammasomes. For example, EVs from cyclic stretch-exposed periodontal ligament cells inhibited NLRP3 activation in macrophages through inhibition of NK-kB signaling patway (Wang et al., 2019). Similarly, in a model of myocardial ischemia/reperfusion (MI/R), it was demonstrated that M2 macrophage-derived EVs inhibited NLRP3 activation through the downregulation of thioredoxin-interacting protein (TXNIP) signaling pathway (Dai et al., 2020).
On the other hand, a recent work from Lee et al. demonstrated that EVs released by Staphylococcus aureus induced NLRP3 activation in THP-1 cells and human macrophages (Wang et al., 2020). Also, the ability of plasma-derived EVs from acute pancreatitis (AP) mice to trigger NLRP3-dependent pyroptosis of alveolar macrophages was described in 2020 (Wu et al., 2020). Furthermore, it was demonstrated that the injection of pregnant mice with endothelial cells-derived EVs lead to the development of characteristic hallmarks of preeclampsia (PE), with NLRP3 activation in trophoblast cells (Kohli et al., 2016).
Based on the above discussion, the precise role of EVs in inflammasome modulation is not fully elucidated. Despite the involvement of inflammasomes and EVs in both genesis and control of tumor progression (Kolb et al., 2014; Dörsam et al., 2018), there were no records in the literature about a possible interaction between those two mechanisms in HNSCC, which is one of the most common cancers, with more than 633,000 new cases diagnosed per year worldwide (Bray et al., 2018). As far as we are concerned, this is the first time that inflammasome modulation induced by tumor-derived VSF was explored in the context of HNSCC. Our data demonstrated that HNSCC-derived EVs-enriched VSF treatment resulted in inflammasome inhibition, as indicated by the reduction in mature caspase-1 and IL-1β secretion in response to nigericin, a classic agonist of NLRP3 inflammasome. Of importance, the reduction in the secretion of mature forms of caspase-1 and IL-1β could not be assigned to a cytotoxic effect of VSF since the treatment by itself did not impact cell viability and did not affect the nigericin-induced cell death. Conversely, HNSCC-derived VSF treatment seems to prevent the priming step of NLRP3 inflammasome activation.
The bioactive components of tumor-derived EVs play key roles in mediating tumor microenvironment reprogramming (Xiao et al., 2019), drug resistance (Samuel et al., 2017), promoting cell migration and invasion (Li et al., 2016), stimulating tumor innervation (Madeo et al., 2018) and metastasis (Sento et al., 2016; Li et al., 2018), and inducing M1-like polarization of tumor-associated macrophages (Chen et al., 2018). Of note, out of 772 proteins that were identified in the HNSCC-derived VSF by antibody array, the five most abundant were Apolipoprotein C3 (ApoC3), THBS1, TRFC, PLG, and Granzyme A (GZMA) and the majority of them can be identified as TGF-β activity facilitators. For instance, THBS1 is known as the major activator of TGF-β (Crawford et al., 1998; Daniel et al., 2004). THBS1 interacts with the N-terminal region of the latency-associated peptide (LAP), leading to conformational changes that will release TGF-β from the latent form, allowing the interaction with its receptor and all the subsequent signaling events (Schultz-Cherry et al., 1995; Khalil, 1999). Also, a study using neural crest cells showed that the ablation of TRFC was associated with the development of craniofacial defects with concomitant suppression of TGF-β signaling in mandibular tissues, suggesting that TRFC may act as a facilitator for the activation of this pathway (Lei et al., 2016). Similar to THBS1, there are some reports about the activation of TGF-β by PLG and the components of its activation system as plasmin and urokinase plasminogen activator (Yee et al., 1993; Chu and Kawinski, 1998; Lyons et al., 1990).
TGF-β is known to mediate the inhibition of NF-kB pathway (Lee et al., 2011; Shiou et al., 2013; Cho et al., 2006). As aforementioned, the NF-kB pathway is required to the induction of precursor forms of IL-1β and IL-18 thus being indispensable for the inflammasomes activation. Thus, the observed downregulation in the induction of pro-IL-1β, pro-caspase-1 precursors, and NLRP3 gene expression could be accounted by the TGF- β-related protein cargo found in tumor-derived VSF. Of importance, TGF-β along with programmed cell death-ligand 1 (PD-L1) and Fas ligand (FasL) are important players in the immunosuppressive interactions mediated by tumor-derived EVs (Marar et al., 2021). TGF-β carried by hypoxia-induced EVs is involved with NK cell supression (Berchem et al., 2016). Also, depletion of TGF-β in leukemia or colon cancer-derived EVs resulted in enhanced antitumor immune response (Huang et al., 2017; Rossowska et al., 2019), highlighting the relevance of this molecule in the tumor immunobiology.
Although the data shown here is promising, further studies are needed to elucidate the precise mechanism by which EV contents interfere with the activity of inflammasomes. Even showing that the HNSCC-derived VSF plays a key role in modulating immune responses, it is impossible to claim each VSF molecule's specific contribution for the observed modulation yet. Unrevealing the protein content of the VSF brings important insights into the immunomodulatory role of EVs. Nevertheless, it is essential to keep in mind that EVs carry a diverse range of molecules including sugars, lipids, and RNAs besides proteins. Therefore, to better understand the whole contribution of vesicles secreted by HNSCC cells in the modulation of inflammasomes, these other molecules should also be examined in the future. Moreover, the use of a unique patient-derived HNSCC cell line, considering the cell plasticity, makes it hard to predict if the observed induction of an inflammasome inhibitory behavior would be phenocopied by EVs from distinct HNSCC cells.
Throughout the literature on tumor immunomodulation, it is already well established that vesicles derived from tumor cells usually take advantage of the plasticity of immune cells to shape the immunosuppressive population that best suits them, supporting the tumor development. Thus, it is possible to assume that inhibiting inflammasome activation could be one of the approaches taken during this manipulation. Considering that the activation of inflammasomes represents a highly inflammatory response leading to the recruitment of several immune cells it's is possible to hypothesize that, in a co-evolutionary manner, the inhibition of inflammasomes caused by the HNSCC-EVs could be a suitable manipulation of the microenvironment to maintain tumor progression, evading host defense.
Because tumor cells are the major EVs producers, therapeutical strategies based on those nanoparticles are increasingly being proposed. The main step to conclusively succeed in this search is to elucidate the mechanisms triggered upon EVs incorporation, mainly on immune cells. In this context, understanding the impact of EVs in the activation and regulation of inflammasomes is of extreme importance for the development of appropriate therapies for HNSCC treatment. In addition, exploring the behavior, composition, and biogenesis of EVs in different tumor models promotes the search for successful therapies based on their activity and structure.
5. Conclusions
Our results demonstrated that HNSCC-derived VSF inhibits the priming phase of inflammasome activation, which could represent an escape mechanism during the complex interplay between tumor environment and immune system.
Ethics statement
All experimental procedures involving mice were carried out in accordance to the Brazilian National Law 11.794 (2008), the Guide for the Care and Use of Laboratory Animals of the Brazilian National Council of Animal Experimentation (CONCEA) and the ARRIVE guidelines. This study was approved by the Institutional Animal Care and Use Committees (IACUC) of the Federal University of São Paulo (UNIFESP) under the protocol #9957100217.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo [FAPESP, grant numbers 2017/25942-0 and 2015/09182-0 to K.R.B. and A.L.V.], Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq, grant number 402100/2016-6 to K.R.B.], the Instituto Nacional de Ciência e Tecnologia de Vacinas (INCTV/CNPq). N.G.I. acknowledges the National Medical Research Council of Singapore Clinician Scientist Award (NMRC/CSA-INV/011/2016). The sponsors had no role in the study design, collection and analysis, interpretation of the data, decision to publish, or writing the manuscript. L.Z.M.F.B. and L.M.B. received fellowships from FAPESP/CNPq. D.M.R.Jr. received fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/FAPESP.
CRediT authorship contribution statement
Luiza Zainotti Miguel Fahur Bottino: Investigation, Methodology, Formal analysis, Validation, Writing – original draft, Visualization. Dorival Mendes Rodrigues-Junior: Investigation, Methodology, Validation, Writing – review & editing. Ingrid Sancho de Farias: Investigation, Methodology, Validation. Laura Migliari Branco: Investigation, Methodology, Validation, Writing – review & editing. N. Gopalakrishna Iyer: Methodology, Resources. Gabriela Estrela de Albuquerque: Methodology, Validation. André Luiz Vettore: Conceptualization, Methodology, Resources, Writing – review & editing, Visualization, Supervision, Project administration. Karina Ramalho Bortoluci: Conceptualization, Methodology, Resources, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank FAPESP, CNPq and CAPES for funding. The Graphical Abstract was created with Biorender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crimmu.2021.10.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
References
- Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018;188:1–11. doi: 10.1016/j.pharmthera.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Berchem G., et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyiadzis M., Whiteside T.L. Information transfer by exosomes: a new frontier in hematologic malignancies. Blood Rev. 2015 doi: 10.1016/j.blre.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Buzzo C.D.L., et al. Epigenetic regulation of nitric oxide synthase 2, inducible (Nos2) by NLRC4 inflammasomes involves PARP1 cleavage. Sci. Rep. 2017;7 doi: 10.1038/srep41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Chen Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–76. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Xiao M., Zhang J., Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2018;37:1–15. doi: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhou Y., Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol. Pharm. 2019;16:2690–2699. doi: 10.1021/acs.molpharmaceut.9b00246. [DOI] [PubMed] [Google Scholar]
- Cho M. La, et al. Transforming growth factor beta 1(TGF-β1) down-regulates TNFα-induced RANTES production in rheumatoid synovial fibroblasts through NF-κB-mediated transcriptional repression. Immunol. Lett. 2006 doi: 10.1016/j.imlet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Chu T.M., Kawinski E. Plasmin, substilisin-like endoproteases, tissue plasminogen activator, and urokinase plasminogen activator are involved in activation of latent TGF-β1 in human seminal plasma. Biochem. Biophys. Res. Commun. 1998 doi: 10.1006/bbrc.1998.9760. [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002 doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S.E., et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998 doi: 10.1016/S0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Dai Y., et al. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- Daniel C., et al. Thrombospondin-1 is a major activator of TGF-β in fibrotic renal disease in the rat in vivo. Kidney Int. 2004 doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Dörsam B., Reiners K.S., von Strandmann E.P. Cancer-derived extracellular vesicles: friend and foe of tumour immunosurveillance. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:4–10. doi: 10.1098/rstb.2016.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria S.S., et al. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J. Biomed. Sci. 2021;28 doi: 10.1186/s12929-021-00724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F., et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1Β-dependent adaptive immunity against tumors. Nat. Med. 2009 doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Groß C.J., et al. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45:761–773. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- He Q., Fu Y., Tian D., Yan W. The contrasting roles of inflammasomes in cancer. Am. J. Cancer Res. 2018;8(4):566–583. [PMC free article] [PubMed] [Google Scholar]
- Hornung V., et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Wan J., Hu W., Hao S. Enhancement of anti-leukemia immunity by leukemia-derived exosomes via downregulation of TGF-β1 expression. Cell. Physiol. Biochem. 2017;44:240–254. doi: 10.1159/000484677. [DOI] [PubMed] [Google Scholar]
- Huang J.H., et al. Extracellular vesicles derived from epidural fat-mesenchymal stem cells attenuate NLRP3 inflammasome activation and improve functional recovery after spinal cord injury. Neurochem. Res. 2020;45:760–771. doi: 10.1007/s11064-019-02950-x. [DOI] [PubMed] [Google Scholar]
- Iyer S.S., et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantono M., Guo B. Inflammasomes and cancer: the dynamic role of the inflammasome in tumor development. Front. Immunol. 2017;8:1–9. doi: 10.3389/fimmu.2017.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Man S.M., Kanneganti T.D. Inflammasomes and cancer. Cancer Immunol. Res. 2017 doi: 10.1158/2326-6066.CIR-16-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp R., Zwicker J. Extracellular Vesicles In Health And Disease 317–336. Pan Stanford Publishing; 2014. Microvesicles and exosomes in cancer. [DOI] [Google Scholar]
- Kayagaki N., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil N. TGF-β: from latent to active. Microb. Infect. 1999 doi: 10.1016/S1286-4579(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Kohli S., et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood. 2016;128:2153–2164. doi: 10.1182/blood-2016-03-705434. [DOI] [PubMed] [Google Scholar]
- Kolb R., Liu G.H., Janowski A.M., Sutterwala F.S., Zhang W. Inflammasomes in cancer: a double-edged sword. Protein and Cell. 2014;5:12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R.C., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., et al. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat. Commun. 2011 doi: 10.1038/ncomms1469. [DOI] [PubMed] [Google Scholar]
- Lei R., et al. Transferrin receptor facilitates TGF-β and BMP signaling activation to control craniofacial morphogenesis. Cell Death Dis. 2016 doi: 10.1038/cddis.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-1625. [DOI] [PubMed] [Google Scholar]
- Li Y. yin, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018 doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss C., Fekete M.J., Hasina R., Lam C.D., Lingen M.W. Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int. J. Cancer. 2001;93:781–785. doi: 10.1002/ijc.1407. [DOI] [PubMed] [Google Scholar]
- Liu Z.M., Wang Y. Bin, Yuan X.H. Exosomes from murine-derived Gl26 cells promote glioblastoma tumor growth by reducing number and function of CD8+T cells. Asian Pac. J. Cancer Prev. APJCP. 2013 doi: 10.7314/APJCP.2013.14.1.309. [DOI] [PubMed] [Google Scholar]
- Lyons R.M., Gentry L.E., Purchio A.F., Moses H.L. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J. Cell Biol. 1990 doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo M., et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018 doi: 10.1038/s41467-018-06640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Kanneganti T.-D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017;130:3955–3963. doi: 10.1242/jcs.207365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marar C., Starich B., Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021;22:560–570. doi: 10.1038/s41590-021-00899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgarty T.P. vols. 1–18. 2013. (Exosomes and Cancer. Draft White Pap). [DOI] [Google Scholar]
- Moon J.S., et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J. Clin. Invest. 2015;125:665–680. doi: 10.1172/JCI78253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Muñoz-Planillo R., et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatromoni J.G., Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. JCB (J. Cell Biol.) 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed M.H., et al. Exosomes: from garbage bins to promising therapeutic targets. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Junior D.M., et al. Circulating extracellular vesicle-associated TGFβ3 modulates response to cytotoxic therapy in head and neck squamous cell carcinoma. Carcinogenesis. 2019 doi: 10.1093/carcin/bgz148. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Junior D.M., et al. A preliminary investigation of circulating extracellular vesicles and biomarker discovery associated with treatment response in head and neck squamous cell carcinoma. BMC Cancer. 2019 doi: 10.1186/s12885-019-5565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossowska J., et al. Antitumor potential of extracellular vesicles released by genetically modified murine colon carcinoma cells with overexpression of interleukin-12 and shRNA for TGF-β1. Front. Immunol. 2019;10:211. doi: 10.3389/fimmu.2019.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F.W., Dubois C.M., Jacobsen S.E.W., Keller J.R. Transforming growth factor β and interleukin-1: a paradigm for opposing regulation of haemopoiesis. Baillieres. Clin. Haematol. 1992 doi: 10.1016/S0950-3536(11)80013-2. [DOI] [PubMed] [Google Scholar]
- Samuel P., Fabbri M., Carter D.R.F. Mechanisms of drug resistance in cancer: the role of extracellular vesicles. Proteomics. 2017 doi: 10.1002/pmic.201600375. [DOI] [PubMed] [Google Scholar]
- Sanman L.E., et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. Elife. 2016;5 doi: 10.7554/eLife.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G., Gabriele L., Mattei F. The tumor microenvironment: a pitch for multiple players. Front. Oncol. 2013;3:90. doi: 10.3389/fonc.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S., et al. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995 doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Sento S., Sasabe E., Yamamoto T. Application of a persistent heparin treatment inhibits the malignant potential of oral squamous carcinoma cells induced by tumor cell-derived exosomes. PLoS One. 2016 doi: 10.1371/journal.pone.0148454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Shimada K., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiou S.R., et al. Oral administration of transforming growth Factor-β1 (TGF-β1) protects the immature gut from injury via smad proteindependent suppression of epithelial nuclear factor κb (NF-κB) signaling and proinflammatory cytokine production. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.503946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Viaud S., et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Rα. PLoS One. 2009 doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., et al. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1β production through the inhibition of the NF-κB signaling pathway in macrophages. Front. Immunol. 2019;10:1–17. doi: 10.3389/fimmu.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Eagen W.J., Lee J.C. Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proc. Natl. Acad. Sci. U.S.A. 2020;117:3174–3184. doi: 10.1073/pnas.1915829117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski E.U., et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8 + T lymphocytes. J. Immunol. 2009 doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers J., et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001 doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- Wu L., et al. Exosomes derived from gastric cancer cells activate NF-??B pathway in macrophages to promote cancer progression. Tumor Biol. 2016;37:12169–12180. doi: 10.1007/s13277-016-5071-5. [DOI] [PubMed] [Google Scholar]
- Wu X.B., et al. Plasma-derived exosomes contribute to pancreatitis-associated lung injury by triggering NLRP3-dependent pyroptosis in alveolar macrophages. Biochimica et Biophysica Acta - Molecular Basis of Disease 1866. 2020;1866(5):165685. doi: 10.1016/j.bbadis.2020.165685. [DOI] [PubMed] [Google Scholar]
- Xiao C., et al. Exosomes in head and neck squamous cell carcinoma. Front. Oncol. 2019;9:1–13. doi: 10.3389/fonc.2019.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011 doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J.A., Yan L., Dominguez J.C., Allan E.H., Martin T.J. Plasminogen‐dependent activation of latent transforming growth factor beta (TGFβ) by growing cultures of osteoblast‐like cells. J. Cell. Physiol. 1993 doi: 10.1002/jcp.1041570312. [DOI] [PubMed] [Google Scholar]
- Zhang L., et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015 doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., et al. Exosomes mediate hippocampal and cortical neuronal injury induced by hepatic ischemia-reperfusion injury through activating pyroptosis in rats. Oxid. Med. Cell. Longev. 2019;2019:3753485. doi: 10.1155/2019/3753485. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]