Abstract
Alopecia areata (AA) is an autoimmune disorder resulting in hair loss. It has numerous variants or patterns, including diffuse type, patchy type, AA totalis, AA universalis, and more. In this graphical review, we provide an overview of AA immunopathogenesis, highlighting loss of immune privilege in the hair follicle as well as key immune cell types, cytokines and chemokines that drive autoimmune attack of the hair follicle. We also summarize recent literature identifying agents that block these pathways that could serve as new immunomodulatory treatments for AA.
Keywords: Alopecia, Alopecia areata, Autoimmune, Hair loss, Immunopathogenesis
1. Introduction & clinical presentations of alopecia areata
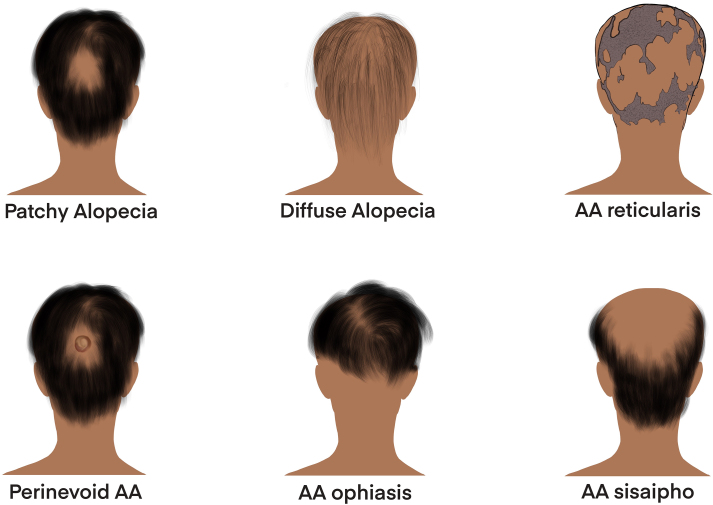
Alopecia areata (AA) is an autoimmune disorder that results in the damage of hair follicles leading to hair loss (Pratt et al., 2017). Hair loss can present in various patterns and with varying severity. AA variants include alopecia totalis, which is total scalp hair loss, and alopecia universalis, which is complete body hair loss. Other clinical forms include patchy AA, diffuse AA, AA reticularis, AA ophiasis, AA sisaipho, and perinevoid AA (Fig. 1) (Finner, 2011). A recent study identified differential gene expression patterns in alopecia totalis and patchy phenotypes, demonstrating that the immunopathogenesis of these clinical variants has unique aspects lending to these distinct patterns of hair loss (Jabbari et al., 2016).
Fig. 1.
Clinical classifications of alopecia areata variants. Patchy Alopecia involves hair loss localized to one or more patches on the head. Diffuse alopecia involves hair loss throughout the head. AA reticularis involves hair loss that is in a reticular pattern with no distinguishable distinct bald patches. Perinevoid AA is patchy hair loss with a melanocytic nevus found in the hairless patch. AA ophiasis is hair loss localized to the back and sides of the scalp. AA sisaipho hair loss spares the back and sides of the scalp; it is thought of as the opposite of AA ophiasis, and is actually ophiasis said backwards. Created with Procreate.
AA affects people of all ages, with an estimated lifetime risk of 2.1% in the general United States population (Pratt et al., 2017). The disease course is more severe and less responsive to treatments when patients have risk factors such as female gender, allergic disease (atopy), onset before age 16, and concurrent autoimmune conditions. An estimated 2.2% of AA cases in the United States are of individuals under the age of 18 (Benigno et al., 2020). There is a genetic basis to AA as well, as there is an association with positive family history, and genome-wide association studies (GWAS) suggest that AA is a complex polygenic disease with linkage on many genes encoding components of both the adaptive and innate immune system. Current medical therapies for AA, namely steroids and retinoids, are not consistently effective, although there have been promising case studies of patients treated with Janus kinase (JAK) inhibitors (Xing et al., 2014). AA can have harmful psychosocial effects on patients of all ages, including anxiety and depression (Colón et al., 1991).
2. AA pathogenesis
AA is believed to be a multifactorial disease, with genetic, environmental, and autoimmune facets. Here, we describe each of these aspects, including the intersections of these facets in the context of the hair follicle, with a particular focus on immunopathogenesis of AA.
2.1. Genetic predisposition
Several immune-related GWAS hits have been identified in AA, including Human Leukocyte Antigen (HLA) class II loci, UL16-binding proteins 3/6 loci, cytotoxic T lymphocyte-associated protein 5 (CTLA-4), IL-2/IL-21 locus, IL-2RA locus, and Eos locus (Petukhova, 2016; Petukhova et al., 2010; Betz et al., 2015). These genes play roles in T cell activation and/or survival, thereby potentially increasing the possibility of autoreactive cells to escape anergy or other peripheral tolerance mechanisms.
2.2. The hair growth cycle
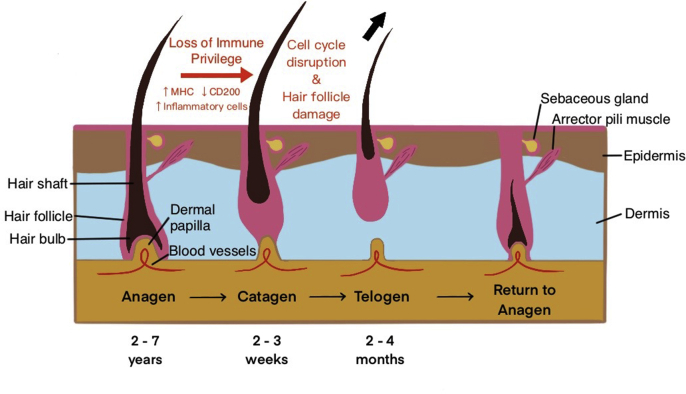
The hair growth cycle normally consists of the anagen (active growth) phase, the catagen (controlled apoptosis of epithelial cells) phase, and the telogen (resting) phase (Fig. 2) (Paus and Cotsarelis, 1999). Hair follicle immune privilege, a microenvironment where a structure or organ is protected from autoimmune reactions, is maintained during the anagen phase but lost during the telogen and catagen phases (Christoph et al., 2000). In AA, inflammation leads to a dystrophic anagen phase and a premature launch into the telogen phase (Fig. 2) (Paus and Bertolini, 2013). During the catagen phase, elevated leukocyte presence suggests increased apoptosis. The inflammation spares the stem cell component of the hair follicle, leading to hair loss that is usually reversible (Ito et al., 2004).
Fig. 2.
Hair growth cycle. The normal hair growth cycle consists of the anagen (active growth) phase, the catagen (controlled apoptosis) phase, the telogen (resting) phase, and the return to anagen (also called exogen) phase. In the anagen phase, the hair follicle is attached to the dermal papilla and able to receive nourishment from the blood vessels that run through the papilla. The hair is actively growing upwards out of the scalp. Anagen lasts about 2–7 years, and 88–90% of hairs on the head are in anagen at any given time. During catagen, the hair follicle begins to separate from the dermal papilla due to controlled apoptosis of epithelial cells. Catagen lasts about 2–4 weeks and 2% of hairs are in catagen at any given time. During telogen, the follicle detaches from the dermal papilla and thus its source of nutrients. Without its nutrient source, the hair dies and falls out of the follicle (indicated with the black arrow). The follicle is in its resting stage. Telogen lasts about 2–4 months and 8–10% of hair is in telogen at any given time. As the cycle returns to the anagen phase, the dermal papilla reunite with the hair follicle and the hair matrix begins to form a new hair. In AA, the normal hair growth cycle is disrupted (indicated with red text). Immune privilege is lost during the anagen phase due to increased MHC I activity and decreased CD200 immunoregulatory presence. The hair follicle is prematurely launched into the telogen phase with increased inflammation and destruction during the catagen phase. Created with Procreate.
2.3. Loss of immune privilege in the hair follicle: evidence of environmental insults
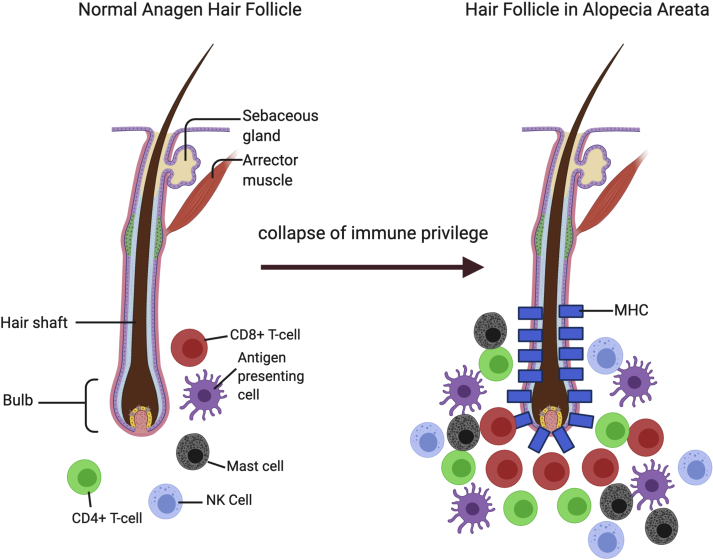
The anagen hair follicle immune privilege state is thought to be maintained due to a lack of major histocompatibility complex (MHC) class I, and presence of CD200, in the outer root sheath (Ito et al., 2004). AA likely results from the collapse of the hair follicle immune privilege causing inflammatory cells to swarm and attack the hair bulb in what is known as the “swarm of bees” (Fig. 3). It is believed that AA hair follicle cells and dendritic cells have increased MHC class I and MHC class II activity, presenting autoantigens to CD8+ T-cells, CD4+ T-cells, and Natural Killer (NK) cells (Ito et al., 2004) (Fig. 4). The autoantigens are likely melanogenesis-associated peptides from hair follicles that produce melanin, as evidenced by humanized mouse models in which these peptides were able to confer hair loss to grafted skin (Gilhar et al., 2001). However, MHC:peptide elution studies isolated from AA hair follicles to identify presented antigens via mass spectrometry have not yet been performed.
Fig. 3.
“Swarm of Bees” appearance of inflammatory infiltrate. In the AA hair follicle, it is believed that increased activity of MHC class I and MHC class II is associated with leukocyte recruitment to the hair bulb. This image depicts collapse of the hair follicle immune privilege, leading to a swarm of CD8+ T-cells, CD4+ T-cells, antigen-presenting cells, and mast cells surrounding the follicle. These immunoinflammatory cells cause hair growth cycle disruption and follicle damage. Created with BioRender.com.
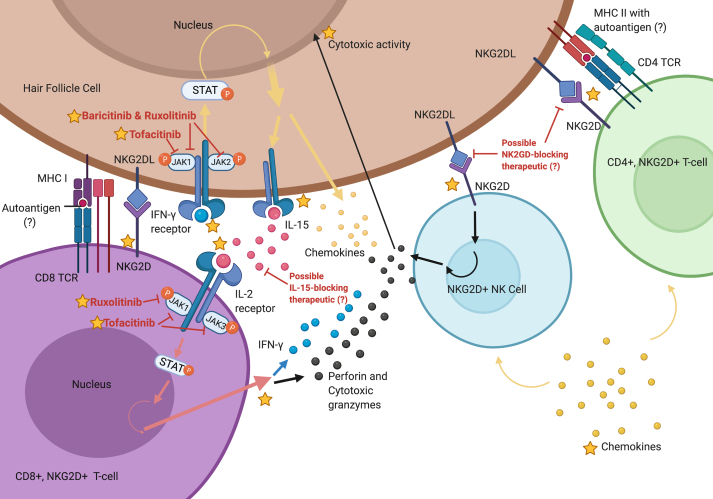
Fig. 4.
Immunopathogenesis of AA. Schematic diagram depicting the autoimmune process of AA. The hair follicle presents autoantigen on MHC class I and MHC class II to CD8+ and CD4+ T cells, which presumably interact with these cells during immunosurveillance, and/or in response to inflammatory signals released in response to environmental stimuli. Inflammatory cytokines and chemokines produced by these T cell populations act on the hair follicle cells, causing them to release more cytokines and chemokines that further recruit T cells and NK cells. IFN-γ, a key mediator of AA pathogenesis, upregulates IL-15 and inflammatory chemokines, such as CXCL9/10/11, in the hair follicle via the JAK-STAT pathway. In CD8+ T cells, IL-15 increases production of perforin and cytotoxic granzymes, also via the JAK-STAT pathway. NKG2D + NK cells and CD4+ T cells can bind with NKG2D ligands on the hair follicle, triggering further release of perforin and cytotoxic granzymes and resulting in apoptosis of hair follicle cells. Potential immunomodulatory treatments, such as JAK inhibitors, are denoted in red, and signaling pathways are denoted by yellow, pink, and black arrows. Published information is marked with a star, and speculative information is marked with a question mark (?). Created with BioRender.com.
2.4. Positive feedback loops driving inflammation & AA immunopathogenesis
During or following immune privilege collapse, the hair follicle cells release cytokines and chemokines that further recruit CD4+ T-cells and NK cells (Xing et al., 2014). Interferon (IFN) signatures and the interferon-inducible chemokines (CXCR3 ligands) appear to be prominent drivers of leukocyte recruitment to the follicle (Ito et al., 2013; Dai et al., 2016; McPhee et al., 2012), though some studies have observed Th2-type cytokine and chemokine responses as well, indicating mixed immune responses (Suárez-Fariñas et al., 2015; Fuentes-Duculan et al., 2016). Binding of the IFN receptors results in increased production of inflammatory signaling molecules, including interleukin-15 (IL-15) and IFN-gamma (IFN-γ), and increased release of cytotoxic granzymes including Granzyme B. IL-15 activates the JAK-STAT pathway in the CD8+ T-cells, causing them to produce more IFN-γ and creating a positive feedback loop. These CD8 T cells become clonally expanded within the follicles (de Jong et al., 2018). The accumulation of cytotoxic granules and IFN-γ results in destruction of hair follicle cells and disruption of the hair growth cycle (Fig. 4). IL-15 has also been shown to serve as a serum biomarker of AA (Ebrahim et al., 2019), and is important for establishing resident memory T cells in the context of vitiligo, another autoimmune skin disease with similar immunopathogenesis (Richmond et al., 2018).
3. Current & future AA treatments
AA can be difficult to treat with varying results. The most common treatment for AA is local steroid injections at the site of alopecia, combined with topical retinoids (Pratt et al., 2017). For pediatric patients, topical cream steroids are preferred to steroid injections. JAK-inhibitors have shown promising results in case studies (Fig. 4), though these are not durable and patients often experience relapse after cessation of treatment (Harris et al., 2016; Kennedy Crispin et al., 2016). Blockade of other immune molecules and their regulators, such as IL-15 and natural killer group 2D (NKG2D), may provide future avenues of treatment for AA. For example, case studies of mesenchymal stem cell transplant for AA treatment have shown efficacy for AA, with a presumed mechanism involving NKG2D inhibition (Byun et al., 2017).
4. Conclusions and future directions
In the past several years, great strides have been made in understanding AA immunopathogenesis, and research has yielded new treatments for patients. Future research will hopefully identify specific danger-associated molecular patterns (DAMPs) associated with hair follicle immune privilege loss, and aspects of innate immunity involved in the initial triggering event, as well as durable therapeutic options for AA.
CRediT authorship contribution statement
Jadesola (Jadé) Temitope Olayinka: Conceptualization, Writing – original draft, preparation, Writing – review & editing, Visualization. Jillian M. Richmond: Conceptualization, Writing – original draft, preparation, Writing – review & editing, Supervision.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
Fig. 1, Fig. 2 were created with Procreate, and Fig. 3, Fig. 4 were created with BioRender.com. JMR is supported by a Career Development Award from the Dermatology Foundation.
Contributor Information
Jadesola (Jadé) Temitope Olayinka, Email: jadesola.md@gmail.com.
Jillian M. Richmond, Email: jillian.richmond@umassmed.edu.
References
- Benigno M., Anastassopoulos K.P., Mostaghimi A., Udall M., Daniel S.R., Cappelleri J.C., Chander P., Wahl P.M., Lapthorn J., Kauffman L., et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin. Cosmet. Invest. Dermatol. 2020;13:259–266. doi: 10.2147/CCID.S245649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz R.C., Petukhova L., Ripke S., Huang H., Menelaou A., Redler S., Becker T., Heilmann S., Yamany T., Duvic M., et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat. Commun. 2015;6:5966. doi: 10.1038/ncomms6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun J.W., Kim H.J., Na K., Ko H.S., Song H.J., Song S.U., Jeon M.-S., Choi G.S. Bone marrow-derived mesenchymal stem cells prevent alopecia areata development through the inhibition of NKG2D expression: a pilot study. Exp. Dermatol. 2017;26:532–535. doi: 10.1111/exd.13255. [DOI] [PubMed] [Google Scholar]
- Christoph T., Müller-Röver S., Audring H., Tobin D.J., Hermes B., Cotsarelis G., Rückert R., Paus R. The human hair follicle immune system: cellular composition and immune privilege. Br. J. Dermatol. 2000;142:862–873. doi: 10.1046/j.1365-2133.2000.03464.x. [DOI] [PubMed] [Google Scholar]
- Colón E.A., Popkin M.K., Callies A.L., Dessert N.J., Hordinsky M.K. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr. Psychiatr. 1991;32:245–251. doi: 10.1016/0010-440x(91)90045-e. [DOI] [PubMed] [Google Scholar]
- Dai Z., Xing L., Cerise J., Wang E.H.C., Jabbari A., de Jong A., Petukhova L., Christiano A.M., Clynes R. CXCR3 blockade inhibits T cell migration into the skin and prevents development of alopecia areata. J. Immunol. 2016;197:1089–1099. doi: 10.4049/jimmunol.1501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A., Jabbari A., Dai Z., Xing L., Lee D., Li M.M., Duvic M., Hordinsky M., Norris D.A., Price V., et al. High-throughput T cell receptor sequencing identifies clonally expanded CD8+ T cell populations in alopecia areata. JCI Insight. 2018;3 doi: 10.1172/jci.insight.121949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim A.A., Salem R.M., El Fallah A.A., Younis E.T. Serum interleukin-15 is a marker of alopecia areata severity. Int. J. Trichol. 2019;11:26–30. doi: 10.4103/ijt.ijt_80_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finner A.M. Alopecia areata: clinical presentation, diagnosis, and unusual cases. Dermatol. Ther. 2011;24:348–354. doi: 10.1111/j.1529-8019.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- Fuentes-Duculan J., Gulati N., Bonifacio K.M., Kunjravia N., Zheng X., Suárez-Fariñas M., Shemer A., Guttman-Yassky E., Krueger J.G. Biomarkers of alopecia areata disease activity and response to corticosteroid treatment. Exp. Dermatol. 2016;25:282–286. doi: 10.1111/exd.12918. [DOI] [PubMed] [Google Scholar]
- Gilhar A., Assy B., Shalaginov R., Serafimovich S., Landau M., Kalish R.S. Melanocyte-associated T cell epitopes can function as autoantigens for transfer of alopecia areata to human scalp explants on prkdcscid mice. J. Invest. Dermatol. 2001;117:1357–1362. doi: 10.1046/j.0022-202x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Harris J.E., Rashighi M., Nguyen N., Jabbari A., Ulerio G., Clynes R., Christiano A.M. Mackay-Wiggan J: rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA) J. Am. Acad. Dermatol. 2016;74:370–371. doi: 10.1016/j.jaad.2015.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Ito N., Bettermann A., Tokura Y., Takigawa M., Paus R. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am. J. Pathol. 2004;164:623–634. doi: 10.1016/S0002-9440(10)63151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Hashizume H., Shimauchi T., Funakoshi A., Ito N., Fukamizu H., Takigawa M., Tokura Y. CXCL10 produced from hair follicles induces Th1 and Tc1 cell infiltration in the acute phase of alopecia areata followed by sustained Tc1 accumulation in the chronic phase. J. Dermatol. Sci. 2013;69:140–147. doi: 10.1016/j.jdermsci.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Jabbari A., Cerise J.E., Chen J.C., Mackay-Wiggan J., Duvic M., Price V., Hordinsky M., Norris D., Clynes R., Christiano A.M. Molecular signatures define alopecia areata subtypes and transcriptional biomarkers. EBioMedicine. 2016;7:240–247. doi: 10.1016/j.ebiom.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy Crispin M., Ko J.M., Craiglow B.G., Li S., Shankar G., Urban J.R., Chen J.C., Cerise J.E., Jabbari A., Winge M.C.G., et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1 doi: 10.1172/jci.insight.89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee C.G., Duncan F.J., Silva K.A., King L.E., Jr., Hogenesch H., Roopenian D.C., Everts H.B., Sundberg J.P. Increased expression of Cxcr3 and its ligands, Cxcl9 and Cxcl10, during the development of alopecia areata in the mouse. J. Invest. Dermatol. 2012;132:1736–1738. doi: 10.1038/jid.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R., Bertolini M. The role of hair follicle immune privilege collapse in alopecia areata: status and perspectives. J. Invest. Dermatol. Symp. Proc. 2013;16:S25–S27. doi: 10.1038/jidsymp.2013.7. [DOI] [PubMed] [Google Scholar]
- Paus R., Cotsarelis G. The biology of hair follicles. N. Engl. J. Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Petukhova L. Christiano AM: functional interpretation of genome-wide association study evidence in alopecia areata. J. Invest. Dermatol. 2016;136:314–317. doi: 10.1038/JID.2015.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova L., Duvic M., Hordinsky M., Norris D., Price V., Shimomura Y., Kim H., Singh P., Lee A., Chen W.V., et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C.H., King L.E., Jr., Messenger A.G., Christiano A.M., Sundberg J.P. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J.M., Strassner J.P., Zapata L., Jr., Garg M., Riding R.L., Refat M.A., Fan X., Azzolino V., Tovar-Garza A., Tsurushita N., et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aam7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Fariñas M., Ungar B., Noda S., Shroff A., Mansouri Y., Fuentes-Duculan J., Czernik A., Zheng X., Estrada Y.D., Xu H., et al. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J. Allergy Clin. Immunol. 2015;136:1277–1287. doi: 10.1016/j.jaci.2015.06.032. [DOI] [PubMed] [Google Scholar]
- Xing L., Dai Z., Jabbari A., Cerise J.E., Higgins C.A., Gong W., de Jong A., Harel S., DeStefano G.M., Rothman L., et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]