Abstract
Neuroimmune communication plays a crucial role in maintaining homeostasis and promptly responding to any foreign insults. Sympathetic nerve fibres are innervated into all the lymphoid organs (bone marrow, thymus, spleen, and lymph nodes) and provide a communication link between the central nervous system (CNS) and ongoing immune response in the tissue microenvironment. Neurotransmitters such as catecholamines (epinephrine and norepinephrine) bind to adrenergic receptors present on most immune and non-immune cells, establish a local neuroimmune-communication system, and help regulate the ongoing immune response. The activation of these receptors varies with the type of receptor-activated, target cell, the activation status of the cells, and timing of activation. Activating adrenergic receptors, specifically β-adrenergic signalling in immune cells leads to activation of the cAMP-PKA pathway or other non-canonical pathways. It predominantly leads to immune suppression such as inhibition of IL-2 secretion and a decrease in macrophages phagocytosis. This review discusses the expression of different adrenergic receptors in various immune cells, signalling, and how it modulates immune cell function and contributes to health and diseases. Understanding the neuroimmune communication through adrenergic receptor signalling in immune cells could help to design better strategies to control inflammation and autoimmunity.
Keywords: SNS, Sympathetic nervous system; L-DOPA, L-dihydroxyphenylalanine; AC, Adenylate cyclase; GRK, G protein-coupled receptor kinase; CNS, Central Nervous System; DCs, Dendritic cells; LPS, Lipopolysaccharide; TNF, Tumor necrosis factor; PKA, Protein kinase A; PDE, Phosphodiesterase; cAMP, Cyclic adenosine monophosphate
Keywords: Adrenaline, Adrenergic receptors, Epinephrine, Nerve-driven immunity, Neuroimmune communication, Neurotransmitters, Norepinephrine
Graphical abstract
Highlights
-
•
Primary and secondary lymphoid organs are innervated with sympathetic nerve fibres.
-
•
Adrenergic receptor expression on immune and non-immune cells establishes a local neuroimmune communication system.
-
•
Adrenergic receptor signalling in immune cells controls the differentiation and function of various immune cells.
-
•
Modulating adrenergic receptor signalling with a specific agonist or antagonist also affect the immune response.
1. Introduction
The sympathetic nervous system (SNS) plays a vital role in maintaining the homeostasis of the body by secreting different neurotransmitters like catecholamines, acetylcholine, glutamine, etc. Among all the neurotransmitters, catecholamine has a very important and diversified role in the various organs. Catecholamines contain a catechol (3,4 dihydroxyphenyl) group along with an amine group. John Jacob Abel, in 1897 first obtained a crystalline substance from the adrenal gland of sheep in an impure form that can regulate blood pressure, and he named it epinephrine (Greek epi and nephros mean ‘on the kidney’) (Scanzano and Cosentino, 2015). But in 1900, Jokichi Takamine obtained a pure crystalline form of epinephrine. He patented it with the name adrenalin (Latin ad and Renes means “near the kidney”) and marketed by Parke, Davis & Company (Wang et al., 2009). British Approved Name (BAN) introduced the name “adrenaline” in the United Kingdom and British Commonwealth. Still, United States Approved Name (USAN) used the term “epinephrine” for this neurotransmitter in the USA. To avoid such controversy, Recommended International Nonproprietary Name (rINN) formulated some standard names for all drugs, but as an exception, adrenaline and noradrenaline are still being used rather than their rINN name epinephrine and norepinephrine (Schedler, 2006). Soon after discovering epinephrine, norepinephrine was synthesized, but on July 7, 1946, Ulf von Euler pointed out that norepinephrine has a sympathomimetic role in the body (Shampo and Kyle, 1995). Chromaffin cells of the adrenal medulla secrete both epinephrine and norepinephrine, which are directly secreted into the bloodstream after stimulation with sympathetic nerve fibre. Some parts of the central nervous system, like the amygdala region, are also known to secrete epinephrine as a neurotransmitter forming the locus coeruleus-noradrenergic system or LC-NA system, which regulates arousal, attention, and stress response (Benarroch, 2009). The hypothalamic-pituitary-adrenal axis and sympathoadrenergic fibres establish a major neuroimmune communication pathway to regulate the immune response.
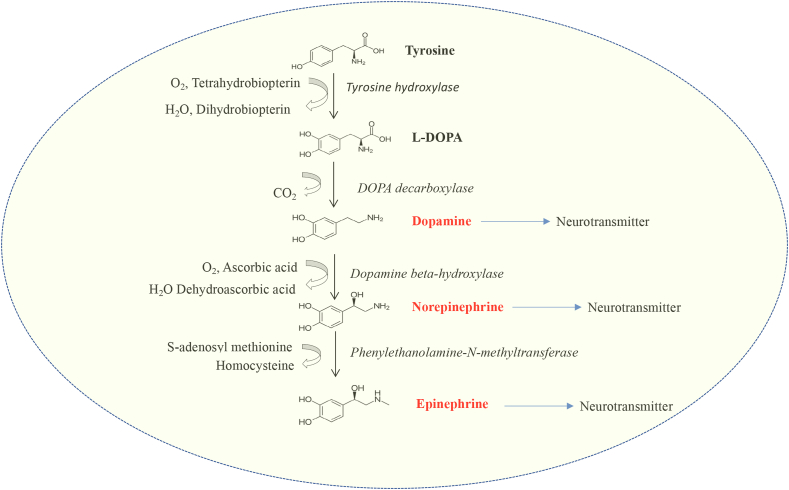
Adrenaline is synthesized from its precursor amino acid tyrosine; it is also synthesized from hepatic hydroxylation of another amino acid, phenylalanine. Synthesis of catecholamine begins with the rate-limiting step governed by the enzyme tyrosine hydroxylase, which converts tyrosine into L-DOPA (L-dihydroxyphenylalanine). Subsequently, L-DOPA is converted to dopamine and then to norepinephrine by DOPA decarboxylase and dopamine beta-hydroxylase, respectively (Fig. 1). Dopamine beta-hydroxylase is a copper-containing enzyme and requires ascorbic acid for its function. In the chromaffin cells of the adrenal medulla, norepinephrine is then converted to epinephrine by the enzyme phenyl ethanolamine-N-methyltransferase. The synthesis of adrenaline is controlled by glucocorticoids, which enter the chromaffin cells and stimulate the enzyme phenyl ethanolamine-N-methyl transferase (PNMT) (Wurtman et al., 1972). Acetylcholine also drives catecholamine secretion by nicotinic and muscarinic acetylcholine receptors (Wakade and Wakade, 1983).
Fig. 1.
Synthesis of catecholamines (dopamine, norepinephrine, and epinephrine). The various steps, enzymes and co-factors required for the synthesis are depicted.
Both epinephrine and norepinephrine stimulate common receptors named adrenergic receptors. Structurally these receptors have seven hydrophobic transmembrane regions and an intracellular C-terminal domain and an extracellular N-terminal domain, along with 3 intracellular and extracellular loops. The N-terminal domain contains sites for N-linked glycosylation. (Strosberg, 1993). Based on specificity to epinephrine, adrenergic receptors are broadly divided into three categories, α1, α2, and β adrenergic receptors (Roth et al., 1991). The α1 and α2 are again subdivided into α1A, α1B, α1D and α2A, α2B, and α2C, respectively. β adrenergic receptor is divided into β1, β2, and β3. These receptors and their tissue distribution are listed in Table 1. These receptors resemble a serpentine structure, but they vary in the intracellular C terminal region (Roth et al., 1991; Strosberg, 1993).
Table 1.
Tissue distribution of adrenergic receptors and their associated G proteins.
| Receptor types | Associated G proteins | Tissues | References |
|---|---|---|---|
| α1A | Gq/11(Gq) | Cerebral cortex, cerebellum, heart, liver, prostate, lymphocytes, heart | (O'Connell et al., 2014; Scanzano and Cosentino, 2015) |
| α1B | Gq/11(Gq) | Spleen, kidney, endothelial cells, osteoblast, lymphocytes, heart | (O'Connell et al., 2014; Scanzano and Cosentino, 2015) |
| α1D | Gq/11(Gq) | Cerebral cortex, aorta, blood vessel, lymphocytes, heart | (O'Connell et al., 2014; Scanzano and Cosentino, 2015) |
| α2A | Gi/Go | Brain, spleen, kidney, lung, liver | Scanzano and Cosentino (2015) |
| α2B | Gi/Go | Kidney, liver, brain, heart, cardiac muscle | Scanzano and Cosentino (2015) |
| α2C | Gi/Go | Brain, kidney, heart, spleen | Scanzano and Cosentino (2015) |
| β1 | Gs | Brain, kidney, lungs, spleen, liver, muscles | Scanzano and Cosentino (2015) |
| β2 | Gs | Brain, lung, lymphocyte, skin, liver, heart | Scanzano and Cosentino (2015) |
| β3 | Gs | Adipose tissues, stomach, gall bladder | Scanzano and Cosentino (2015) |
2. Types of adrenergic receptor
Radioligand binding assays suggested the presence of nine different types of adrenergic receptors, and all of them have a different affinity towards epinephrine and norepinephrine. Each of these nine receptors has been discussed in detail in the following paragraphs.
2.1. Alpha (α) 1 adrenergic receptor
2.1.1. α1A adrenergic receptor
Formerly known as the α1C adrenergic receptor, this receptor contains 466 amino acid residues identified in the bovine brain (Langer, 1998). They are profoundly expressed in the brain, heart, kidney, prostate, smooth muscles, etc. (Cotecchia, 2010). A small group of adrenergic receptors identified in the prostrate has a low affinity towards prazosin an α-blocker than the other α1 adrenergic receptors (Docherty, 2019). α1A adrenergic receptor helps contract different tissues like the aorta, vas deference, lower urinary tract, heart, etc., in various animals (Docherty, 2019).
2.1.2. α1B adrenergic receptor
α1B is the first alpha1 adrenergic receptor identified, and it is predominantly expressed in the brain, heart, spleen, etc. (Cotecchia, 2010). Ventricular myocytes of the mouse heart express α1B receptor, which has been shown to have a vital role during cardiac hypertrophy (Cotecchia et al., 2015). α1B adrenergic receptor has an important role in cell cycle progression and can induce transformation in sensitive cell lines (Gonzalez-Cabrera et al., 2004).
2.1.3. α1D adrenergic receptor
This receptor contains 560 amino acid residues, and it is mainly expressed in arteries, heart, kidney, spleen, etc. Norepinephrine has higher potency towards the α1D receptor than α1A and α1B (Docherty, 2019). It is shown that α1D adrenergic receptor oligomerizes with α1B adrenergic receptor acting as a single unit in response to norepinephrine. This oligomerization increases its response towards the ligand (Cotecchia et al., 2015).
2.2. α2 adrenergic receptors
2.2.1. α2A adrenergic receptor
α2A adrenergic receptor is mostly found in the central nervous system and helps suppress pain perception, sedation, etc. (Ruffolo et al., 1993). Some other organs like the pancreas and spleen also express α2 adrenergic receptors (Adefurin et al., 2016). It is found that Caucasians express three variants of α2A adrenergic receptor, which are associated with stress-induced hyperglycemia after acute myocardial infarction (Adefurin et al., 2016). The activation of the α2A adrenergic receptor leads to activation of inhibitory G protein Gi, inhibiting adenylate cyclase and suppressing voltage-gated calcium channels (Pao and Benovic, 2005).
2.2.2. α2B adrenergic receptor
α2B adrenergic receptor is mostly expressed in the central and peripheral nervous system, and it is also shown to activate inhibitory G protein hence inhibiting adenylate cyclase (Pao and Benovic, 2005). Internalization of α2B adrenergic receptor is mediated by binding of β−arrestin 3, which bind to two discrete regions in the intracellular loop (DeGraff et al., 2002). It is also found the interaction of tubulin with the α2B adrenergic receptor helps in its localization from the endoplasmic reticulum to the cell surface (Duvernay et al., 2011). α2B adrenergic receptor present in the postsynaptic nerve helps in vasoconstriction (Muszkat et al., 2005).
2.2.3. α2C adrenergic receptor
Both central and peripheral nervous systems express α2C adrenergic receptors, but some other organs like the heart and kidney also express α2C adrenergic receptors. This receptor also activates inhibitory G protein and inhibits the release of norepinephrine (Small et al., 2006). α2C adrenergic receptor has been shown to produce homodimer. It also forms a heterodimer with α2A adrenergic receptor. Their heterodimeric state impairs phosphorylation by GRK2 and recruitment of arrestin and hence influences receptor internalization (Small et al., 2006).
2.3. Beta (β) adrenergic receptor
2.3.1. β1 adrenergic receptor
β1 adrenergic receptor is found in many organs like the heart, kidney, adipose tissues (Rasmussen et al., 2005), salivary gland, etc. Another atypical β-adrenergic receptor called β4 adrenergic receptor is also found in adipose tissue and the heart, and it is regarded as a novel state of β1 adrenergic receptor. β1 adrenergic receptor activates stimulatory G protein and subsequently cyclic AMP. β1 adrenergic receptor down-regulates COX2 and promotes degradation of COX2 (Brender and Barki-Harrington, 2014).
2.3.2. β2 adrenergic receptor
β2 adrenergic receptor is the most common and widely studied among all adrenergic receptors. It is highly expressed in different tissues, including brain, kidney, heart, lungs, liver, etc. Activation of β2 adrenergic receptor stimulates G protein, which helps in cAMP production, leading to the activation of different protein kinases (Billington et al., 2017). Due to its widespread function, β2 adrenergic receptor has several therapeutic roles in various diseases. More about this receptor and its function in various diseases will be discussed in further chapters.
2.3.3. β3 adrenergic receptor
This 408 amino acid-containing receptor is present mainly in the brain, eye, heart, adipose tissue, etc. In adipose tissue, it helps in lipid mobilization and lipolysis (Ferrer-Lorente et al., 2005). β3 adrenergic receptor present in the bladder helps in the relaxation of the bladder, which is mediated by activating cAMP and Ca+ activated K+ channel. Rodent retinal blood vessels also contain β3 adrenergic receptor, which helps in the relaxation of these vessels.
3. Mechanism of action of epinephrine
All adrenergic receptors work through different G proteins like Gs, Gi, etc. β2 adrenergic receptor is the most abundant and most widely studied adrenergic receptor, and their mechanism of action has been well illustrated. Signalling via adrenergic receptors leads to the generation of two pathways: canonical, or G protein-dependent, and non-canonical or G protein-independent.
3.1. Canonical pathway
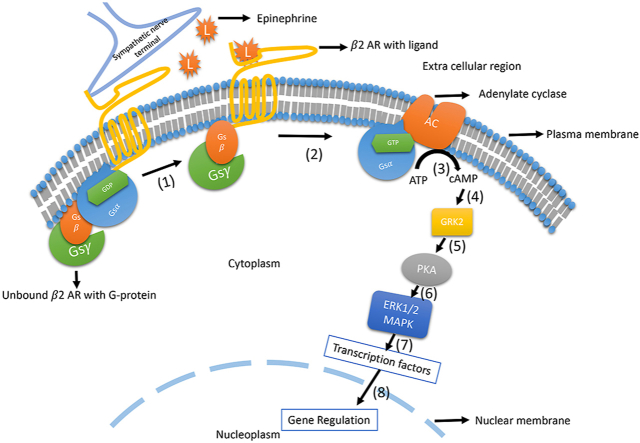
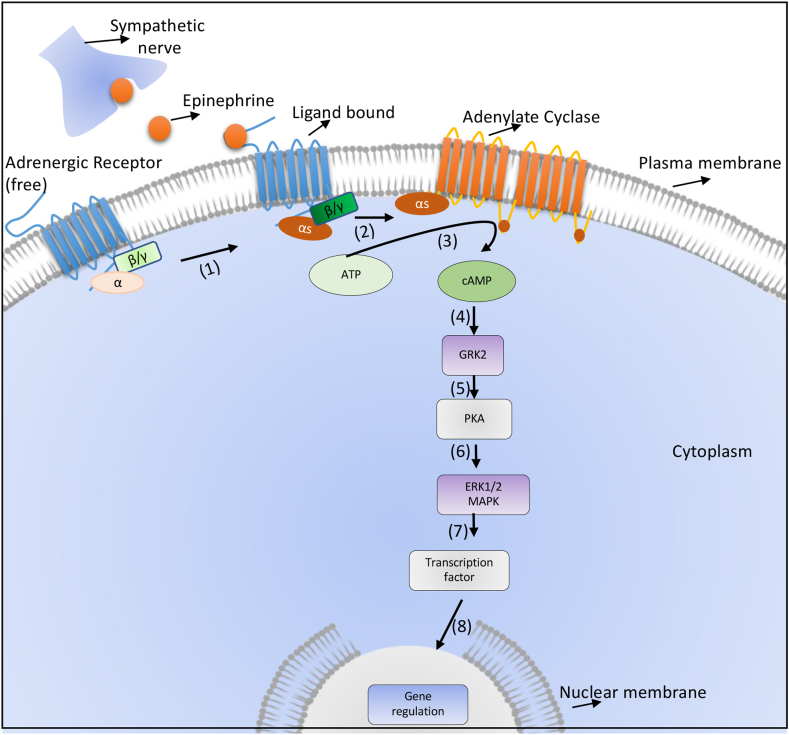
In the canonical pathway, epinephrine binds to its receptor, which helps in the exchange of GDP to GTP, which leads to dissociation of GαS and Gβϒ subunits. Gαs then stimulate another membrane-bound enzyme, adenylate cyclase (AC), which converts ATP to cAMP (Alcántara-Hernández and Hernández-Méndez, 2018). About ten different adenylate cyclases have been shown to exist in various organisms, out of which nine are membrane-bound and one in the soluble form (Simpson et al., 2019). AC7 is highly expressed in immune cells, but AC3 also shows expression at very low levels (Duan et al., 2010). All these ACs are found in different locations of the cell membrane and destined for various functions, which somehow explains the varied role played by these receptors in a different state of the body (Lorton and Bellinger, 2015). cAMP generated by AC binds to the regulatory subunit of PKA (protein kinase A), releasing the catalytic subunit that helps transfer a terminal phosphate group to the serine or threonine residue on the target molecules, which can be a transcription factor or a cytoplasmic enzyme (Fig. 2). Two different types of PKA like PKARI and PKARII, have been reported to exist in various cells. RI and RII are further subdivided into RIα, R1β, and RIIα and RIIβ, respectively (Lorton and Bellinger, 2015).
Fig. 2.
Canonical β2 adrenergic receptor signalling. Binding of norepinephrine secreted by the sympathetic nerve stimulate the β-adrenergic receptor. Due to conformation change by replacement of GDP with GTP, αs, and βγ subunits of G protein separates from each other, and then αs activates the enzyme adenylate cyclase, which converts ATP to cAMP, which further phosphorylates GRK2; which in turn activates protein kinase A (PKA). Activated PKA then activation of ERK1/2, MAPK pathway leading to transcription regulation of several important genes.
3.2. Regulation of canonical pathway
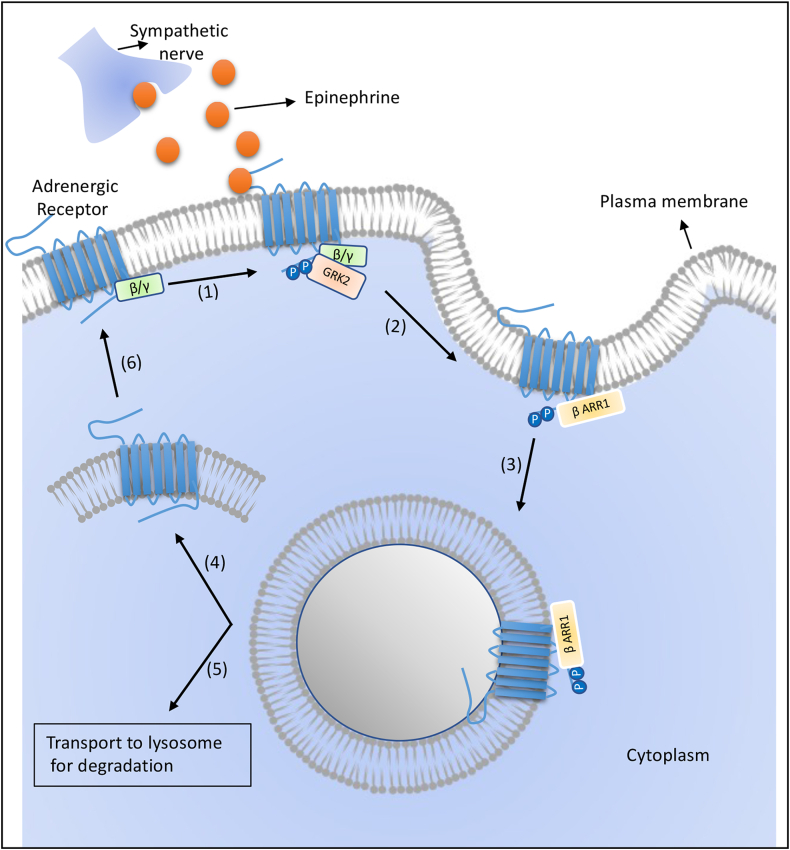
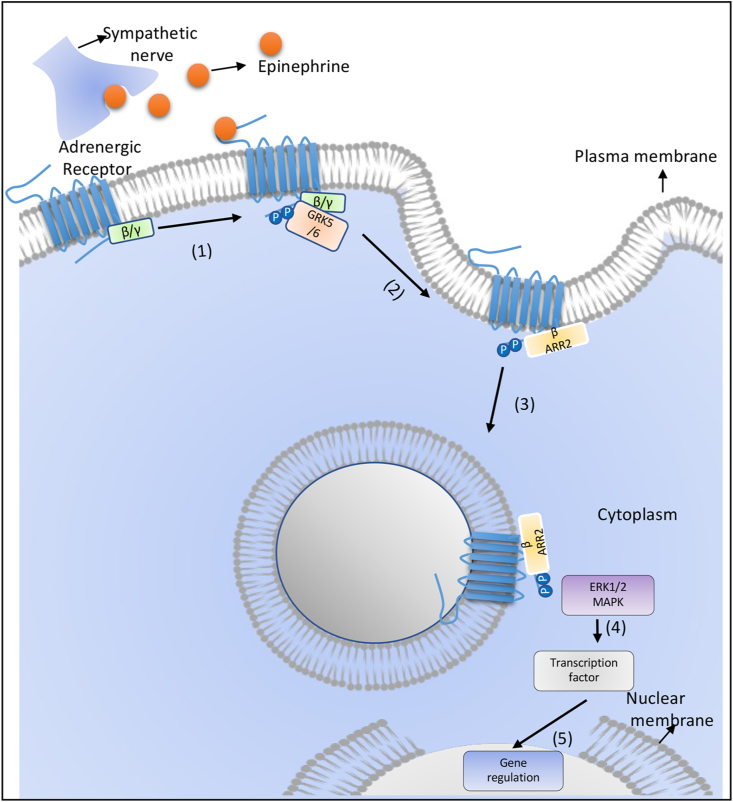
During any antigenic insult, pro-inflammatory cytokines increase SNS activity, leading to an increase in norepinephrine, ultimately regulating the immune cells on the target region (MacNeil et al., 1996; Meltzer et al., 2003). A high concentration of norepinephrine induces PKA to phosphorylate adrenergic receptor, which is phosphorylated by G protein-coupled receptor kinase (GRK), creating a binding site for β-arrestin. About seven different GRKs have been reported, yet GRK2 is most widely studied (Pitcher et al., 1998). The binding of β-arrestin leads to the internalization of the receptor. This internalized receptor may be recycled back or degraded in the lysosome to maintain normal homeostasis (Fig. 3). Various diseases like heart failure (Goodman et al., 1996) and asthma are linked to an imbalance in receptor desensitization.
Fig. 3.
Canonical pathways induced by adrenergic receptor signalling. The higher ligand concentration leads to phosphorylation of adrenergic receptors by GRK2. Phosphorylated adrenergic receptors bind to β-arrestin 1. This drives the receptor internalization and which either may get dephosphorylated and recycled back the adrenergic receptor at the plasma membrane or degraded in the lysosome.
It is found that phosphorylation of the receptor by PKA leads to a conformational change that decreases the affinity of the receptor to Gs (stimulatory) but increases its activity towards Gi (inhibitory) (Lorton and Bellinger, 2015). The binding of the receptor with Gi inhibits cAMP synthesis. The binding of beta-arrestin to receptor recruits another enzyme called phosphodiesterase (PDE), which hydrolyze cAMP and convert it to 5′-monophosphate, decreasing the concentration of the second messenger (Bjørgo et al., 2010; Giembycz, 1996). About eleven different PDEs have been reported, but PDE4 has been found in higher concentration during the inflammatory condition, and immune cells inhibition of PDE leads to decrease cAMP breakdown, so it is used as a tool to regulate inflammation (Page and Spina, 2011).
Immune cells also express GRK2 as well as other types of GRKs. The expression of mRNA of GRK2 in lymphocytes and heart increases when rats are treated with β2 adrenergic receptor agonists (Oyama et al., 2005). It is also found that hypersensitive patients express a high concentration of GRK2 in their PBMCs (Gros et al., 1997). GRK2 level is reported to be reduced in an animal model of EAE and adjuvant-induced arthritis and some autoimmune diseases like arthritis and multiple sclerosis in humans (Lombardi et al., 1999).
3.3. Non-canonical pathway
The non-canonical pathway depends upon β-arrestin, and the signal cascade is different from the canonical pathway (Watari et al., 2014). The subtype of GRK has an important role in deciding whether the internalized receptor will undergo desensitization or generate a non-canonical pathway for signal transduction (Nobles et al., 2011). Agonist concentration has played an important role in deciding which subtype of GRK like GRK2 or GRK5/6 will phosphorylate the receptor (Lorton and Bellinger, 2015). When GRK2 phosphorylates the receptor, it leads to desensitization, whereas phosphorylation by GRK5/6 leads to signalling through β-arrestin (Nobles et al., 2011). β-arrestin has been reported to have four subtypes, and β-arrestin-1 and -2 are exclusively reported in mammalian cells (Pierce and Lefkowitz, 2001), and both have the property to desensitize or signal transduction, but β-arrestin 1 generates cAMP-PKA signalling, and β-arrestin 2 generates MAPK signalling cascade and sustain ERK1/2 signalling (Fig. 4).
Fig. 4.
Non-canonical pathways induced by adrenergic receptor signalling. GRK5/6 phosphorylates the adrenergic receptor and then recruits β-arrestin 2. This leads to receptor internalization and generation of second signalling by activating ERK1/2, MAPK, which leads to activation of transcription factors and regulation of gene transcription.
4. Expression of adrenergic receptors on various immune cells
There are ample evidence suggesting the expression of different adrenergic receptors in both primary and secondary lymphoid organs (Lubahn et al., 2014). Immune cells in these organs express various adrenergic receptors both in mice and humans (Bergquist et al., 1994; Cosentino et al., 2007; Elenkov et al., 2000; Josefsson et al., 1996; Kenney and Ganta, 2014; Kohm and Sanders, 2001; Marino et al., 1999) and are listed in Table 2. Both innate and adaptive immune systems are controlled by the sympathetic nervous system signalling through adrenergic receptors. Expression of β2 adrenergic receptor has been well established in all immune cells (except Th2 cells) (Sanders, 2012). The expression of other receptors in various immune cells is well documented (Emeny et al., 2007; Kavelaars, 2002a, Kavelaars, 2002b). This review discusses immune cells expressing adrenergic receptors and their role in immune response and various diseases.
Table 2.
Cellular distribution of adrenergic receptors.
| Cell types | Adrenergic receptors | Functions | References |
|---|---|---|---|
| CD4+ T cells | α1, α2, β | Reduce IL-2 production, enhance suppressive function of Treg cells | (Cosentino et al., 2007; Guereschi et al., 2013; Lubahn et al., 2014; Pilipović et al., 2019; Yuki, 2021) |
| CD8+ T cells | α1, α2, β | Inhibit cytotoxicity | (Grisanti et al., 2011; Jetschmann et al., 1997) |
| Macrophages | α1, α2, β | Decrease phagocytic activity, | (Grisanti et al., 2011; Marino and Cosentino, 2013; Miksa et al., 2009; Yuki, 2021) |
| Monocytes | α1, α2, β | Immunosuppressive, downregulate TNF-α | (Kavelaars, 2002a, Kavelaars, 2002b; Marino and Cosentino, 2013; Yuki, 2021) |
| Dendritic cells | α1, α2, β | Enhance IL-6, IL-10 expression, decrease-IL-12 production, influence migration | (Grisanti et al., 2011; Maestroni, 2000; Maestroni and Mazzola, 2003) |
| Neutrophils | α1, α2, β | Reduce CD11b, prevent netosis | (Jaime García-Prieto et al., 2017; Kim et al., 2014a; Marino et al., 2018; Yuki, 2021) |
| NK cells | α1, α2, β | Inhibit migration, suppress NK cell cytotoxicity | (Gan et al., 2002; Grisanti et al., 2011; Jetschmann et al., 1997; Shakhar and Ben-Eliyahu, 1998) |
| B cells | β | Hamper antibody production | (Sanders, 2012; Silberman et al., 2003) |
4.1. Dendritic cells (DCs)
DCs are potent antigen-presenting cells and an important secretor of cytokines; hence they represent an important cell during the immune reaction. DCs have been shown to express α1, α2, and β-adrenergic receptors. In both skin-derived and bone marrow-derived DCs, norepinephrine reduces IL-12 production and enhances IL-10 production. It has been reported that the migration of dendritic cells is stimulated by α1 adrenergic receptor and inhibited by the β2 adrenergic receptor (Maestroni, 2004). It is also suggested that β2 adrenergic receptor signalling in CD40 stimulated human dendritic cells enhance the cAMP level and decrease IL-12 level, altering Th1/Th2 balance (Panina-Bordignon et al., 1997). It is found that epinephrine injection reduces the number of DCs in pigs (Reiske et al., 2020). Lipopolysaccharides (LPS)-challenged DCs, when stimulated by β2 adrenergic receptor, secrete twice as much IL-23 as IL-12p70, which impacts the differentiation of T cells and secretion of lower IFN-γ and higher IL-17 (Takenaka et al., 2016). IL-33 production increases in LPS-challenged DCs when stimulated by either epinephrine or norepinephrine involving the cAMP-PKA pathway, which suggests the association of DCs with the stress-related disorder by promoting the differentiation of Th2 cells (Yanagawa et al., 2011).
A β2 adrenergic receptor agonist, salbutamol, increases IL-6 production in NOD2 (Nucleotide-binding oligomerization domain-containing protein 2) ligand muramyl dipeptide-treated DCs. When injected intradermally with Pam3CysSK4+muramyl dipeptide, Norepinephrine enhances Th17 response in mice (Manni et al., 2011). β2 adrenergic receptor has been found to prevent antigen cross-presentation between DCs and CD8+ T cells by affecting phagosomal antigen degradation. Both CTLA4 and β2 adrenergic receptor signalling pathways were found to crosstalk with each other at the level of NF-kB (Hervé et al., 2013). When the β2 adrenergic receptor is activated with clenbuterol, a β2 adrenergic receptor agonist inhibits monocyte differentiation to DCs in humans (Giordani et al., 2015). When given to differentiated DCs, Isoproterenol reduces CD86 and MHC class II molecules and enhances antigen uptake (Wu et al., 2016). TLR2 activation and β2 adrenergic receptor antagonist at the site of intradermal cancer vaccination either increase the anti-tumour function or show tolerogenic function, depending upon the maturation status of DCs (Botta and Maestroni, 2008). Given the importance of adrenergic receptor signalling on the phenotype and function of DCs, suggesting its pharmacological intervention in vaccination and anti-tumour immune responses.
4.2. Monocytes and macrophages
Monocytes and macrophages represent the major antigen-presenting cells, and they also secrete various cytokines. Macrophages are known for their phagocytic activity. Both ligand binding and flow cytometry-based studies have proved the expression of adrenergic receptors in human monocytes (Fragala et al., 2011; Ratge et al., 1988). The role of β-adrenergic receptor on monocyte is mostly showing immunosuppressive and anti-inflammatory properties such as downregulation of TNF-α expression. The up-regulation of β-adrenergic receptor reduces the phagocytosis in Candida albicans (Borda et al., 1998; Guirao et al., 1997) and inhibits IL-18 and IL-12 production in LPS-treated monocyte (Mizuno et al., 2005). In severely burned children, the number of circulating immunosuppressive M2b monocytes is reduced by β-blocker propranolol so that increased susceptibility to opportunistic infections are controlled (Kobayashi et al., 2011). In a stressful condition like myocardial infarction, monocyte catecholamine directly stimulates human cytomegalovirus immediate-early enhancer/promoter by β2 adrenergic receptor. It creates an active infection in a latent patient, and epinephrine also enhances HCMV gene expression in infected THP-1 cells (Prösch et al., 2000). In an animal model, immune cells deficient in β2-adrenergic receptor show reduced infiltration of immune cells such as proinflammatory macrophages and lymphocytes, leading to fewer cardiac injuries with low cardiomyocyte death, interstitial fibrosis, and hypertrophy (Tanner et al., 2021).
Epinephrine helps attach monocyte to laminin and oxidizes low-density lipoprotein phagocytosis, which is considered pro-inflammatory and atherogenic (Sarigianni et al., 2011). In certain conditions, activation of the adrenergic receptors in monocyte leads to pro-inflammatory functions like increased production of IL-18 (Takahashi et al., 2003) and up-regulation of IL-4-induced CD23 expression (Mencia-Huerta et al., 1991). Surface expression of L-selectin is increased in monocytes after treatment with epinephrine (Rainer et al., 1999). Both immunoblot and radioligand binding assay suggest increased LPS induced IL-1β production in isoprenaline treated THP-1 cells through β1 adrenergic receptor (Grisanti et al., 2010). The culture of human monocyte with β2 agonist terbutaline stimulates expression α1B and α1D adrenergic receptor mRNA expression (Rouppe van der Voort et al., 1999). LPS treatment also helps in the expression of α1B and α1D adrenergic receptor mRNA by activating ERK2 in human peripheral mononuclear cells (Rouppe van der Voort et al., 2000). In vitro differentiation of human monocyte to macrophage leads to loss of responsiveness of β-adrenergic receptor even having functional adenylate system (Baker and Fuller, 1995). TNF-α production is not hindered by an agonist of the adrenergic receptor (Ezeamuzie et al., 2011). In monocyte-derived macrophages, β2 adrenergic receptor signalling reduces IL-6, IL-1β, and TNF-α production (Ağaç et al., 2018). In macrophages, norepinephrine stimulates phagocytic activities are controlled by both α- and β-adrenergic receptors, whereas chemotactic function is governed by α-adrenergic receptors (García et al., 2003a, b). Activation of β2 adrenergic receptor may dampen the polarization of pro-inflammatory M1 macrophage (Bacou et al., 2017) and promote anti-inflammatory or regulatory M2 macrophage induction (Grailer et al., 2014). Increased β2 adrenergic signalling after ischemic stroke onset leads to a reduction in up-regulation of both pro- and anti-inflammatory cytokines in microglia and monocyte-derived macrophage, hence increasing stroke size (Lechtenberg et al., 2019). In the resting stage, α1 blockade enhances phagocytic activity of macrophages through NOS2 and HSP70 expression (da Silva Rossato et al., 2014). Several reports suggest that monocytes and macrophages endogenously produce epinephrine and norepinephrine, indicating the existence of autocrine signalling (Chou et al., 1998; Nguyen et al., 2011). Together, it suggests the role of the β2-adrenergic receptor in the anti-inflammatory response and α-adrenergic receptor in both pro- and anti-inflammatory response. However, a more detailed study of how each receptor type signalling affects the phenotype and function in a specific disease and tissues still needs to be investigated.
4.3. Natural killer (NK) cells
NK cells are well known for their anti-viral and anti-tumour response (Paul et al., 2016; Paul and Lal, 2017). It has been shown that NK cells do express adrenergic receptors. Epinephrine and norepinephrine via β-adrenergic receptor inhibit NK cell cytotoxicity (Sun et al., 2018) and cytokine production (Ruiz-Medina et al., 2018), and at lower concentrations, it may also stimulate its cytotoxicity (Hellstrand et al., 1985). It has been reported that distinct and highly differentiated NK cells undergo tissue-specific relocalization induced by epinephrine (Graff et al., 2018b). Human CD16+NK cells express α1, α2, and β2 adrenergic receptor, and epinephrine infusion reduces the expression of all the receptors (Jetschmann et al., 1997). IL-2 stimulated NK cells upon exposure to norepinephrine show inhibition of IFN-γ and TNF-α production and prevented NK cell maturation into cytotoxic cells (Gan et al., 2002). Migration of NK cells is inhibited when nadolol, a β-blocker, is given during exercise, implying that a β2 adrenergic receptor help to mobilize differentiated NK cells (Graff et al., 2018a).
β-adrenergic receptors are known to cause a decrease in NK cell activity and contribute to cancer progression under stress conditions (Page and Ben-Eliyahu, 2000). Adrenergic receptor antagonist propranolol reverses the number of NK cells in the lungs and blood due to acute stress (Page and Ben-Eliyahu, 2000). In many cancer models, blocking adrenergic signalling in NK cells has played a beneficiary role (Ricon et al., 2019). Mindful-based stress reduction (MBSR) techniques enhance NK cell activity in breast cancer and HIV infection patients (Kenne Sarenmalm et al., 2017). The immunostimulatory role of IL-12 on NK cells in rats is disrupted by continuous administration of adrenergic agonists (Levi et al., 2011). Mice treated with β2 adrenergic receptors show higher susceptibility to MCMV (murine cytomegalovirus) infection (Wieduwild et al., 2020). The absence of β2 adrenergic receptor impairs remarkably NK cell expansion and memory formation during MCMV infection. Hence, β2 adrenergic signalling has an important role in the normal function of NK cells during MCMV infection (Diaz-Salazar et al., 2020). It was suggested that β2 adrenergic receptor antagonists might help in reducing the tumour progression and metastasis, such as melanoma (De Giorgi et al., 2011) and breast cancer (De Giorgi et al., 2011; Powe et al., 2010). However, it is not very clear that the antagonist works directly on NK cells or acts on cancer cells to show its anti-tumour activity, and it needs to be further investigated.
4.4. Neutrophils
Neutrophils are the major component of the innate immune system. Plenty of evidence exists suggesting the expression of different adrenergic receptors on neutrophils (Nicholls et al., 2018; Scanzano et al., 2015). β2 adrenergic receptor expression is the significantly higher amount as compared to other adrenergic receptors (Nicholls et al., 2018). Isoproterenol, prevent respiratory burst in human neutrophil (Nielson, 1987), and later on, its function was attributed to β2 adrenergic receptor (Scanzano and Cosentino, 2015). IL-8 was shown to be insensitive to epinephrine in physiological concentration in the resting neutrophil, whereas when the concentration of epinephrine was increased, IL-8 slightly altered the expression of adhesion molecules like CD15, CD44, and CD54 molecules (Matthias Wahle et al., 2005). On further investigation, epinephrine and norepinephrine had been shown to reduce expression of CD11b/CD18, ROS production, and migration of neutrophils without affecting IL-8 (Scanzano et al., 2015). Further antagonizing α1 and α2 did not make any changes. When β antagonism was done, the ROS generation was reverted, which explains the β adrenergic receptor playing a key role in the modulation of ROS production (Scanzano et al., 2015). It was also noted that both α1 and α2 exert an opposite role in CD11b expression, such as α1-adrenergic receptor increases expression and α2-adrenergic receptor decreases expression of CD11b (Kanashiro et al., 2020; Scanzano et al., 2015). Adrenaline also prevents stimulus-induced NET (neutrophil extracellular traps) formation, most probably through β-adrenergic receptor stimulation (Marino et al., 2018). β-adrenergic receptor activation in neutrophils is mainly attributed to the pathway involving cAMP and PKA (Bazzoni et al., 1991; Gibson-Berry et al., 1993; Marino et al., 2018). Expression of adrenergic receptor varies in different conditions such as in post-traumatic stage disorder leads to increase in expression of β-adrenergic receptor on neutrophils show higher β-adrenergic receptor (Gurguis et al., 1999), whereas its expression decreases in hypertension (Corradi et al., 1981), juvenile type 1 diabetes mellitus (Schwab et al., 1993), and strenuous physical exercise (Ratge et al., 1988; Schwab et al., 1993). Overexpression of α2B adrenergic receptor in monosodium urate (MSU) induced inflammatory condition increases the migration of neutrophils (Duan et al., 2019). β-blocker propranolol can decrease circulating neutrophils and suppress neutrophil infiltration to colonic tissues and attenuate the damage in inflammatory bowel disease (IBD) (Deng et al., 2016). Adrenergic receptors' action on neutrophils also depends on the sex of the individual. The binding site for β2 adrenergic receptors is more in female neutrophils than males. Isoprenaline causes nondirectional chemokinesis neutrophils in females but not in males, etc. (de Coupade et al., 2004).
Neutrophils are also shown to have catecholamine degrading enzyme-like monoamine oxidase (Balsa et al., 1989). Epinephrine alters the migration of neutrophils during wound healing, and through the β2 adrenergic receptor, it impairs wound healing by upregulating IL-6 (Kim et al., 2014b). β-adrenergic receptor modulated neutrophil migration requires activation of the nicotinic receptor and the integrity of the spleen (Silva et al., 2016). Meteropol, a β1 adrenergic receptor antagonist, prevents the interaction of neutrophils and platelet in acute myocardial infarction patients by targeting neutrophils (García-Prieto et al., 2017). Adrenergic receptors are well-known end target for several autonomic dysregulations, so exploiting the importance of these receptors on neutrophils can open new possibilities in the treatment of these diseases.
4.5. B cells
B cells represent a major arm of the adaptive immune response. The expression of adrenergic receptors on B cells has been well documented in generating an antibodies-mediated immune response (Cross et al., 1986; Fuchs et al., 1988). Antigen-specific B cells have been shown to express adrenergic receptors (Kohm and Sanders, 1999). Chronic stress in BALB/c leads to impaired isotype switching in IgGs production but does not affect IgM production (Silberman et al., 2003). In vivo murine model suggested that the depletion of norepinephrine in mice shows confusing results that either decrease or increase T cell-dependent IgG and IgM response (Kohm and Sanders, 2001; Sanders and Munson, 1985). The reason for such confusion in the results might be that the drugs used for epinephrine depletion might create an initial epinephrine burst before epinephrine depletion. To address this, antigen-specific Th2 and B cells were adoptively transferred to severe combined immunodeficiency mice (SCID) already depleted of norepinephrine with 6-OHDA (Kohm and Sanders, 1999). This leads to lowering the level of IgM and IgG in serum, which can be reverted by adding a β-specific agonist. This finding proves that at the early stage, norepinephrine helps in T cell-dependent IgG and IgM production. IgG1 production was shown to be delayed when these mice were subjected to secondary immunization. It is also found that this depletion leads to a decrease in follicular expansion and germinal centre formation.
In vitro culture of B cells with polyclonal activating stimuli and IL-4 along with stimulating β2 adrenergic receptor leads to an increase in production of IgG1 (Podojil and Sanders, 2003). β2 adrenergic signalling in CD40 induced activated B cells leads to an increase in IgG1 in two different pathways, the first one is direct activation of PKA (Podojil and Sanders, 2003) and the second one elevating the expression of another costimulatory molecule, CD86, which when get stimulated, leads to a distinct pathway that helps in the production of IgG1 antibody in mice (Kasprowicz et al., 2000). Stimulation of both β2 adrenergic receptor and CD86 leads to an increase in IgG1 secretion through a transcription factor Oct2 and its coactivator OCA-B in human B cells (Podojil and Sanders, 2005). In humans, activation of the β-adrenergic receptor leads to a decrease in the proliferation of peripheral B cells (Faisy et al., 2010). It is also reported that epinephrine enhances the antibody response of B cells upon immune challenge (Simkins et al., 2014).
4.6. T cells
T cells are an important component of the adaptive immune system. Both CD4 and CD8 T cells have been shown to express different adrenergic receptors (Araujo et al., 2019; Grisanti et al., 2011; Sanders, 2006). β2 is the most widely studied adrenergic receptor in T cells due to its high expression and functional implication in different disease conditions (Sanders, 2006). It is found that the expression of β2 adrenergic receptor is suppressed in Th2 cells due to epigenetic modification in T cells (Sanders, 2012). Adrenergic signalling controls the T cell by directly regulating thymocytes. It has been reported that β-adrenergic signalling in stressed mice activates the p38 mitogen-activated pathway and then up-regulation of Fas ligand, which promotes negative selection in the thymus hence decreasing the number of T cells (Lajevic et al., 2011). Chronic unpredictable stress decreases the number of double negative cells in the thymus Activation of β2 adrenergic receptor in CD4+ T cells in the presence of IL-12 leads to elevated secretion IFN-γ. The secretion of IFN-γ is directly dependent on the time of adrenergic engagement to cell activation; if the engagement occurs before, during and after cell activation, it leads to less, unchanged or higher secretion of IFN-γ by Th1 cells, respectively (Sanders, 2012). Engagement of adrenergic receptors in Th2 cells cultured with a lower level of IL-4 enhances IL-4 secretion by these cells, but when cultured with moderate or high IL-4, the secretion pattern remains normal. It is also observed that stimulation of adrenergic receptors in anti-CD3 and anti-CD28 activated CD4+ T cells freshly isolated from splenocyte decrease the secretion of IL-2 (Ramer-Quinn et al., 2000). It is found that when DCs were stimulated with β2 adrenergic receptor, they changed the normal ratio of cytokine production, i.e., from more IL-12p70 than IL-23 to low IL-12p70 and high IL-23, and upon TCR engagement with CD4+ T cells produce high IL-17 and lower IFN-γ (Takenaka et al., 2016). β2 adrenergic receptor has been shown to enhance the suppressive function of CD4+FOXP3+ Treg by enhancing CTLA-4 in a PKA-dependent pathway (Guereschi et al., 2013).
As adrenergic signalling mostly plays a suppressive role in T cells, adrenergic signalling is well studied in various diseases like cancer and other autoimmune diseases. Propranolol, a β-blocker, has been found to increase vaccine efficacy in a murine model by blocking adrenergic signalling in naïve CD8+ T cells (Daher et al., 2018). It is also demonstrated that inhibition of adrenergic signalling decreases the number of PD-1 in CD8+ T cells and helps in checkpoint inhibition therapy (Bucsek et al., 2017). Activated T cells require glucose as a sole energy source, and the absence of glucose makes them anergic. It is found that adrenergic signalling in CD8+ T cells reduces the expression of GLUT1 receptors and interferes with glucose uptake. Hence β-adrenergic blockers can be used to increase the anti-tumour response of CD8+ T cells in controlling tumour progression (Qiao et al., 2019). It has been demonstrated that the sympathetic nervous system restrains the anti-viral immunity of CD8+ T cells by adrenergic receptors. When these receptors are antagonized, CD8+ T cells generate robust anti-viral immunity against influenza (Grebe et al., 2009).
4.7. Other immune cells
Adrenaline signalling in eosinophil is studied mainly with regards to its role in allergy. Radioligand binding shows an expression of the different adrenergic receptors in eosinophils (Barnes, 1999). Earlier it was reported that norepinephrine reduces the number of circulating eosinophils in the blood (Humphreys and Raab, 1950). β-adrenergic agonist formoterol prevents the adhesion of neutrophils and eosinophils and prevents their traffic into the airway (Barnes, 1999). It is also suggested that β-agonists have an indirect effect on eosinophil survival (Barnes, 1999). In the presence of phosphodiesterase inhibitors, beta-agonist show an inhibitory role against immunoglobulin-induced degranulation of eosinophils (Kita et al., 1991). Basophils also show the expression of different adrenergic receptors (Miadonna et al., 1989). Clonidine, a α2 agonist, inhibits histamine secretion during allergy (Miadonna et al., 1989). Mast cells also show the expression of different adrenergic receptors (Scanzano and Cosentino, 2015). Immunoglobulin-mediated histamine release is inhibited by β-adrenergic signalling in mast cells (Masini et al., 1982). Gamma-delta (γδ) T cells play an important role in immunity, autoimmunity, and cancer (Giri and Lal, 2021; Paul et al., 2014, Paul et al., 2015). Adrenaline is reported to increase the mobilization of γδ T cells in circulation and provide protection from invading pathogens (Dimitrov et al., 2010). However, a detailed expression of adrenergic receptors and their impact on γδ T cells phenotype and function need to be investigated.
5. Clinical role of epinephrine and norepinephrine in various diseases
Given the importance of adrenergic signalling, several agonists and antagonists are in clinical trials in several diseases and pathophysiological conditions. These agonists and antagonists are listed in Table 3. It has been reported exercise, along with β-adrenergic receptor signalling controls the distribution of the immune cells in different peripheral organs, mitochondrial biogenesis, immunometabolism and immune response to cancer, viral diseases and autoimmunity (Simpson et al., 2021). Direct β2-adrenergic input controls the lymph node expansion and function (Chen et al., 2021), which may impact the ongoing immune response. We discussed some of the diseases and the role of adrenergic signalling below-
Table 3.
Adrenergic receptor agonists and antagonists and their therapeutic usage.
| Adrenergic receptor | Agonists/Antagonists | Functions | National Clinical Trial number | Stage of clinical trials | Disease | References | |
|---|---|---|---|---|---|---|---|
| α1 | Agonists | Oxymetazoline | Vasoconstrictor | NCT01847131 | Phase 4 | Nasal obstruction | Druce et al. (2018) |
| NCT03228914 | Phase 4 | Endoscopic sinus surgery | |||||
| Phenylephrine | Vasoconstrictor | NCT02323399 | Phase 4 | Hypotension | (Xu et al., 2019) | ||
| NCT03702400 | Phase 2 | Hypotension | |||||
| Methoxamine | Vasoconstrictor | NCT01656720 | Phase 2 | Faecal incontinence | Siproudhis et al. (2014) | ||
| Antagonists | Tamsulosin | Benign prostatic hyperplasia | NCT04232683 | Early phase 1 | Urinary Retention | Dunn et al. (2002) | |
| NCT04597372 | Phase 2 | Urinary Retention post-operative | |||||
| Phentolamine | Vasodilator | NCT03740386 | Anesthesia, local | Becker and Reed (2012) | |||
| NCT04024891 | Phase 2 | Mydriasis, Dilation | |||||
| Risperidone | Anti-psychotic | NCT03978832 | Phase 4 | Schizophrenia | Corena-McLeod (2015) | ||
| NCT01726335 | Phase 4 | Schizophrenia | |||||
| α2 | Agonists | Dexmedetomidine | Sedative | NCT04027829 | Phase 2 | Postoperative care | Lee (2019) |
| NCT03799783 | Phase 2 | Procedural sedation, Behavior disorders | |||||
| Clonidine | Anti-hypertensive | NCT02769390 | Phase 2 | Postoperative pain | Krieger et al. (2018) | ||
| NCT03065933 | Phase 4 | Bipolar disorder, Mania | |||||
| Brimonidine | Ocular hypertension | NCT03825081 | Early phase 1 | Presbyopia, Pseudophakia | Lusthaus and Goldberg (2017) | ||
| NCT02761174 | Phase 4 | Telangiectasias | |||||
| Antagonists | Lisuride | Anti-Parkinson | NCT00408915 | Phase 3 | Parkinson's disease | Clarke and Speller (2000) | |
| NCT00089622 | Phase 2 | Parkinson's disease | |||||
| Yohimbine | Erectile dysfunction | NCT00975325 | Phase 4 | Erectile dysfunction | |||
| NCT04346394 | Early phase 1 | Parkinson's disease | |||||
| Phentolamine | Vasodilator | NCT04024891 | Phase 2 | Mydriasis dilation | Majid et al. (1971) | ||
| NCT04004507 | Phase 2 | Decrease in night vision, disturbance, vision loss | |||||
| β | Agonists | Isoprenaline | Treatment of bradycardia and heart block | NCT00624416 | Phase 1 | Lipoma | Redman et al. (2011) |
| NCT00226551 | Phase 2 | Coronary disease | |||||
| Salmeterol | Bronchodilator | NCT03238482 | Phase 1 | Asthma | Jentzsch et al. (2019) | ||
| NCT01395849 | Respiratory disorder | ||||||
| Salbutamol | Bronchodilator | NCT01903785 | Phase 4 | Bronchoconstriction | (Morfin-Maciel and Castillo-Morfin, 2011) | ||
| NCT03044938 | Phase 4 | Asthma, fast heart rate | |||||
| Antagonists | Propranolol | Anti-hypertensive | NCT04518124 | Phase 2 | Angiosarcoma | Gandhi et al. (2021) | |
| NCT02962947 | Phase 2 | Melanoma | |||||
| Phase 3 | |||||||
| Metoprolol | Anti-hypertensive | NCT04457323 | Phase 4 | Hypertension | Papadopoulos and Papademetriou (2009) | ||
| NCT02737891 | Phase 2 | Type 2 diabetes mellitus | |||||
| Carvedilol | Anti-hypertensive | NCT03879629 | Phase 2 | Breast cancer | Guglin et al. (2019) | ||
| NCT02832089 | Phase 3 | Atrial fibrillation | |||||
5.1. Cancer
Cancer has been the research in focus due to its fatality and versatility. Stress and cancer both come as a guest simultaneously, so the role of stress modulating factors in cancer development has been well documented (Krizanova et al., 2016). An early study in different carcinoma proves that various tumours like melanoma (Valles et al., 2013), breast cancer (Vandewalle et al., 1990), pituitary cancer (Reisine et al., 1983), and pancreatic cancer (Reisine et al., 1983; Zhang et al., 2011) do express functional adrenergic receptors. The expression of receptors on these tumours helps them to survive and proliferate. Tumour secretes neural growth factors to attract innervation of the autonomic nervous system and angiogenesis (Iñigo-Marco and Alonso, 2019). When the β-adrenergic receptor is blocked in pancreatic cancer, it leads to induction of apoptosis and hence a better response to therapy. The β-blocker suppresses the activity of NF-kB, down-regulates Bcl2 and upregulates Bax protein to promote apoptosis (Jansen et al., 2014; Zhang et al., 2011). Most of the function of the β-adrenergic receptor is mediated via the cAMP-PKA pathway; other protein activated by cAMP is exchange protein activated by cAMP (EPAC) and cyclic nucleotide-gated ion channel. It has been shown that the expression pattern of adrenergic receptors changes during metastasis of tumours. Knockdown of adrenergic receptor β2 has an inductive effect on molecules like vimentin, N-cadherin, β-catenin, as well as integrin β4, suggesting epithelial to mesenchymal transition, and these knockdown cells show increased migratory as well as invasive properties (Yu et al., 2007). It is also found that blocking β1 or β2-adrenergic receptors inactivate proteins like CREB, AP-1, NF-kB, and its target gene like MMP-9, MMP-2, VEGF, etc., ultimately inhibiting cancer cell migration and proliferation (Zhang et al., 2010). Adrenergic receptors are also known to inhibit epithelial to mesenchymal transition in some cancers like colorectal cancer, breast cancer etc. (Haldar et al., 2020)
A nonselective agonism of β-adrenergic receptors in B cell lymphoma in the murine model had supported the immunosuppressive function of these receptors, suppressing CD8+ T cells response by inhibiting its proliferation and cytotoxicity. Selective agonism of β2-adrenergic receptors had been reported to suppress NK cell cytotoxicity, promoting FoxP3+ T cells (Nissen et al., 2018; Shakhar and Ben-Eliyahu, 1998). Even selective agonism of β1-adrenergic receptors had been shown to promote Treg population, and antagonizing β3-adrenergic receptor promoted CD8+ T cells and cytotoxicity of NK cells (Calvani et al., 2019; Xu et al., 2019). β-adrenergic receptor signalling reduces antigen cross-presentation by dendritic cells and maturation of these cells, subsequently affecting the function of CD8+ T cells. Even elevated β-adrenergic receptor signalling during immunotherapy treatment reduces IFN-γ and cytotoxicity of CD8+ T cells (Nissen et al., 2018). Adrenergic receptor activation elevates frequency and suppresses the function of myeloid-derived suppressor cells (MDSCs) in the tumour microenvironment, spleen and blood. It also helps in the survival of MDSCs by activating STAT3, along with regulating the expression of Fas/FasL (Mohammadpour et al., 2019). Recently it has been shown that b2-adrenergic signalling controls several metabolic pathways in MDSCs, such as decreased glycolysis and increased oxidative phosphorylation and fatty acid oxidation and affect the function of these cells in the tumour microenvironment (Mohammadpour et al., 2021). It is reported that pan-β-blocker propranolol or mice deficient of β2-adrenergic receptor show reduced tumour growth due to few infiltrations exhausted T cells in the tumour microenvironment (Qiao et al., 2021).
In melanoma, adrenergic receptor helps in promoting tumour growth and metastasis. Norepinephrine enhances the production of different cytokines like VEGF, IL-8, and IL-6 in human melanoma, which help in the progression of the tumour. Blocking β-adrenergic receptors by antagonists like propranolol leads to a reduction in myeloid suppressive cells and enrichment of cytotoxic lymphoid cells (Jean Wrobel et al., 2016). Antagonizing the nonselective β-adrenergic receptor in melanoma has been shown to promote apoptosis of cancer cells (Moretti et al., 2013). Propranolol had gained a significant attain in the treatment of melanocarcinoma. It had been reported to block tumour angiogenesis and affect other cells in the tumour microenvironment (Filippi et al., 2020). Propranolol is also reported as a mediator of cell cycle arrest leading to apoptosis by AKT/MAPK pathway in melanoma cells (Zhou et al., 2016). Hence, beta-blockers have been used as a suitable target drug for melanoma treatment (Kokolus et al., 2018). It is also known that melanocytes and keratinocytes express different enzymes for catecholamine synthesis, depicting autocrine signalling in melanoma for tumour survival and growth (Schallreuter et al., 1992). So adrenergic signalling has been beneficial to tumour growth and metastasis and blocking the signal from this receptor can be used as an add-on therapy during cancer treatment.
5.2. Rheumatoid arthritis (RA)
Rheumatoid arthritis is a long-lasting autoimmune disorder that primarily affects the joints. There is some evidence about β-adrenergic receptor polymorphism at codon 16 and human leukocyte antigen HLA-DRB1, which make susceptibility to RA (Malysheva et al., 2008). Expression of β-adrenergic receptor decreases in both peripheral blood lymphocyte (PLF), and synovial fluid lymphocytes (SFL) in RA (Wahle et al., 2002). Activation of adrenergic receptors in secondary lymphoid organs leads to inhibition of IL-2, hence inhibiting lymphocyte proliferation in adjuvant-induced arthritis, further sympathectomy in secondary lymphoid organs intensify the disease condition through the promotion of type-1 immune reaction as well as downregulating IL-4 and IL-10 (Lorton et al., 1999; Straub and Härle, 2005). Some studies noted that α2-adrenergic receptor could induce proliferation of synovial fibroblast of RA patients by activating phospholipase C, PKC and MAPK (Mishima et al., 2001; Straub and Härle, 2005). In chronic conditions, the number of sympathetic nerve fibre decreases with lower adrenaline production in such cases, synovial macrophage fulfils the production of adrenaline, and this loss in sympathetic fibres uncoupled synovial tissue from the hypothalamic autonomic axis which intensifies the severity of the disease (Miller et al., 2000). It is also found that activation of the adrenergic receptor leads to the promotion of humoral immunity and inhibition of cell-mediated immunity by regulating phenotypic differentiation of CD4+ T helper cells. This occurs when the β2 adrenergic receptor is activated in Th0/Th1 cells, leading to the accumulation of cAMP that inhibits IFN-γ production and promotes IL-4 production in Th2 cells. In early-stage stimulation of β-adrenergic receptor has a protective role by inducing T cells to secrete IFN-γ (Wu et al., 2018) but in other immune cells, it shows inflammatory role such as in B cell it helps in generation of autoantigen, in macrophage, it helps in the production of inflammatory cytokines like IL-1β and TNF-α. In dendritic cells, it helps process autoantigen and the production of different cytokines like TNF-α, IL-12, and IL-6 (Wu et al., 2018).
5.3. Multiple sclerosis (MS)
Multiple sclerosis results from the degradation of the myelin sheath that covers nerves in both the brain and spinal cord by the immune system resulting in communication problems between the brain and other body parts. Higher expression of the β-adrenergic receptor in PBMCs, including lymphocytes, is well documented in multiple sclerosis patients (Zoukos et al., 1992, 1994). This high expression of the β-adrenergic receptor is mainly related to disease activity and is mostly specific for CD8+CD28− T cell population (Karaszewski et al., 1991, 1993). In circulating PBMCs, the gene expression of β-adrenergic receptor and its responsiveness towards its agonist isoprenaline is less in untreated patients than the one treated with IFN-β (Giorelli et al., 2004). The outcome of the disease is grossly affected by multiple comorbidities like cardiovascular dysfunction, which is mostly mediated by the hyperactivity of the adrenergic system. Adrenergic hyperactivity also influences blood pressure which becomes another comorbidity (Habek et al., 2020). Increased levels of IL-12 had been correlated to disease activity. Drugs that downregulate IL-12 were considered beneficial for the progression of multiple sclerosis (Comabella et al., 1998) and in a study, it is found that albuterol, a β-adrenergic receptor agonist reduces IL-12 production in multiple sclerosis patients, hence can be used as an add on treatment with glatiramer acetate therapy (Khoury et al., 2010). Another agonist, fenoterol, can potentially reduce the risk of developing the disease (Tsai et al., 2014). IFN-γ had been shown to negatively control the synthesis of catecholamine in human lymphocytes. Still, the trend is opposite in the case of IFN-β, mostly increasing the expression of tyrosine hydroxylase (Zaffaroni et al., 2008). It has been reported that triggers such as early life trauma are associated with higher relapse rates in multiple sclerosis patients, and β1-adrenergic receptor agonist abrogates such changes at least in the experimental model in mice (Khaw et al., 2021). Th17-specific deficiency of tyrosine hydroxylase in mice does not significantly alter the EAE disease score compared to wild-type mice but shows increased neutrophil infiltration in the CNS tissues (Yang et al., 2021).
5.4. Inflammatory bowel disease (IBD)
IBD, which includes ulcerative colitis and Crohn's disease, is a chronic and relapsing gut condition (Sun et al., 2019). Stress from different sources disturbs the brain-gut axis, leading to various inflammatory conditions like IBD in the gut. A complex relationship exists between stress and the progression of inflammatory bowel disease (Sun et al., 2019). It is found that activation of α- and β-adrenergic receptors in immune cells increases peripheral and central inflammatory cytokines and results in the NF-kB pathway (Johnson et al., 2005). In the murine model of colitis, it is found that mononuclear cells of lamina propria secrete catecholamine that activates α2 adrenergic receptor and hence, facilitate the progress of colitis, and when α2-blockers are given to the mice, it down-regulate inflammatory response (Bai et al., 2009). It is also demonstrated that when activated by adrenergic receptors, murine macrophages show an anti-inflammatory response in RAG1−/− mice (Willemze et al., 2019). Signalling through adrenergic receptor upregulates chemokines secreted from neutrophils and helps migrate these cells into colonic tissue, promoting colonic neutrophil infiltration (Deng et al., 2016).
5.5. COVID19 infection
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) is an RNA virus causing COVID19 showing a broad spectrum of signs and symptoms, from mild flu-like to multi-organ failure. Some of the patients with this disease have been shown to have cytokine storm syndrome, a hyperinflammatory condition releasing lots of inflammatory cytokines like IL-6, IL-2R, TNF-α, and G-CSF, etc., which mostly occurs during autoimmune condition or CAR-T cell therapy, etc. (ÇOpur et al., 2020; Konig et al., 2020). Catecholamines play an important role in increasing the production of IL-6 through α1 adrenergic receptor, so it is proposed that antagonizing this receptor may help reduce the lethality of this disease. In an animal models of hyperinflammation/sepsis, blocking of catecholamine sysnthesis with a-methyltyrosine (metyrosine, MTR) or a1-adrenergic signalling with prazosin prevents the cytokine storm and death (Staedtke et al., 2018). It is seen that the chances of the requirement of mechanical ventilator and chances of death are less in patients who have taken α-adrenergic receptor antagonists (Konig et al., 2020; Rose et al., 2021). It is also proposed that as β-adrenergic receptors regulate the level of renin, which ultimately regulate angiotensin-converting enzyme (ACE), the receptor for the entry of the SARS-CoV2 virus, so it is hypothesized that blocking beta-adrenergic receptors may have a beneficial role in treating COVID19 patients (Vasanthakumar, 2020).
6. Conclusions
Recently, neuroimmune communication in modulating the adaptive and innate immune response is getting much attention in different inflammatory and autoimmune diseases and cancer (Cardoso et al., 2021; Halder and Lal, 2021; Karmakar and Lal, 2021; Mishra and Lal, 2021). The sympathetic nervous system (SNS) controls T cell responses induced during viral and parasitic infection as well as in anti-tumour immunity (Araujo et al., 2019; Devi et al., 2021). Most of the immune cells are known to express adrenergic receptors, and the function of these receptors plays an important role in inflammation. In most cases, activation of β2 adrenergic receptor shows an immune-suppressive function which may vary in different disease conditions. The sympathetic nervous system controls both the immune system's innate and adaptive branches via these adrenergic receptors. Pharmacological interventions showed that adrenergic receptors play a significant role in modulating the immune response. The direction of the adrenergic system in specific cell types, in specific tissues and how it controls the cross talk among the various immune cells in shaping the immune response needs a systematic and well-defined experimental strategy. The adrenergic signalling is mostly studied in the vasodilation and blood flow in the tissues. Recently, it was found that noradrenaline administration promoted the constriction of post-capillary venules and artrioles in the lymph nodes, which had impact on immune cell interaction in the secondary lymphoid organs and immune response (Devi et al., 2021). As adrenergic signalling has an immense role in different diseases, specific pharmacological agonists and antagonists targeting a defined adrenergic receptor in specific diseases could be explored for better clinical outcomes. A better understanding of the cellular and molecular mechanisms of adrenergic receptors in various immune cells and their importance will help explore the therapeutic repurposing of known agonists and antagonists.
Funding supports
GL received Swarna Jayanti Fellowship (DST/SJF/LSA-01/2017–18) from the Department of Science and Technology, Ministry of Science and Technology, Government of India. SC received a Junior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India.
CRediT authorship contribution statement
Sushanta Chhatar: Conceptualization, Writing – review & editing, prepared the Tables and the Figures. Girdhari Lal: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adefurin A., Darghosian L., Okafor C., Kawai V., Li C., Shah A., Wei W.Q., Kurnik D., Stein C.M. Alpha2A adrenergic receptor genetic variation contributes to hyperglycemia after myocardial infarction. Int. J. Cardiol. 2016;215:482–486. doi: 10.1016/j.ijcard.2016.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ağaç D., Gill M.A., Farrar J.D. Adrenergic signaling at the interface of allergic asthma and viral infections. Front. Immunol. 2018;9:736. doi: 10.3389/fimmu.2018.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcántara-Hernández R., Hernández-Méndez A. [Adrenergic signaling molecular complexes] Gac. Med. Mex. 2018;154:223–235. doi: 10.24875/GMM.18002390. [DOI] [PubMed] [Google Scholar]
- Araujo L.P., Maricato J.T., Guereschi M.G., Takenaka M.C., Nascimento V.M., de Melo F.M., Quintana F.J., Brum P.C., Basso A.S. The sympathetic nervous system mitigates CNS autoimmunity via beta2-adrenergic receptor signaling in immune cells. Cell Rep. 2019;28:3120–3130. doi: 10.1016/j.celrep.2019.08.042. e3125. [DOI] [PubMed] [Google Scholar]
- Bacou E., Haurogné K., Allard M., Mignot G., Bach J.M., Hervé J., Lieubeau B. β2-adrenoreceptor stimulation dampens the LPS-induced M1 polarization in pig macrophages. Dev. Comp. Immunol. 2017;76:169–176. doi: 10.1016/j.dci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- Bai A., Lu N., Guo Y., Chen J., Liu Z. Modulation of inflammatory response via alpha2-adrenoceptor blockade in acute murine colitis. Clin. Exp. Immunol. 2009;156:353–362. doi: 10.1111/j.1365-2249.2009.03894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A.J., Fuller R.W. Loss of response to beta-adrenoceptor agonists during the maturation of human monocytes to macrophages in vitro. J. Leukoc. Biol. 1995;57:395–400. doi: 10.1002/jlb.57.3.395. [DOI] [PubMed] [Google Scholar]
- Balsa M.D., Gómez N., Unzeta M. Characterization of monoamine oxidase activity present in human granulocytes and lymphocytes. Biochim. Biophys. Acta. 1989;992:140–144. doi: 10.1016/0304-4165(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. Effect of beta-agonists on inflammatory cells. J. Allergy Clin. Immunol. 1999;104:S10–S17. doi: 10.1016/s0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- Bazzoni G., Dejana E., Del Maschio A. Adrenergic modulation of human polymorphonuclear leukocyte activation. Potentiating effect of adenosine. Blood. 1991;77:2042–2048. [PubMed] [Google Scholar]
- Becker D.E., Reed K.L. Local anesthetics: review of pharmacological considerations. Anesth. Prog. 2012;59:90–103. doi: 10.2344/0003-3006-59.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. The locus ceruleus norepinephrine system. Funct. organ. potential clinical significance. 2009;73:1699–1704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- Bergquist J., Tarkowski A., Ekman R., Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington C.K., Penn R.B., Hall I.P. β(2) agonists. Handb. Exp. Pharmacol. 2017;237:23–40. doi: 10.1007/164_2016_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørgo E., Solheim S.A., Abrahamsen H., Baillie G.S., Brown K.M., Berge T., Okkenhaug K., Houslay M.D., Taskén K. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Mol. Cell Biol. 2010;30:1660–1672. doi: 10.1128/MCB.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda E.S., Tenenbaum A., Sales M.E., Rumi L., Sterin-Borda L. Role of arachidonic acid metabolites in the action of a beta adrenergic agonist on human monocyte phagocytosis. Prostagl. Leukot. Essent. Fat. Acids. 1998;58:85–90. doi: 10.1016/s0952-3278(98)90145-4. [DOI] [PubMed] [Google Scholar]
- Botta F., Maestroni G.J. Adrenergic modulation of dendritic cell cancer vaccine in a mouse model: role of dendritic cell maturation. J. Immunother. 2008;31:263–270. doi: 10.1097/CJI.0b013e318160995e. (Hagerstown, Md. : 1997) [DOI] [PubMed] [Google Scholar]
- Brender S., Barki-Harrington L. β1-Adrenergic receptor downregulates the expression of cyclooxygenase-2. Biochem. Biophys. Res. Commun. 2014;451:319–321. doi: 10.1016/j.bbrc.2014.07.123. [DOI] [PubMed] [Google Scholar]
- Bucsek M.J., Qiao G., MacDonald C.R., Giridharan T., Evans L., Niedzwecki B., Liu H., Kokolus K.M., Eng J.W., Messmer M.N., Attwood K., Abrams S.I., Hylander B.L., Repasky E.A. β-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639–5651. doi: 10.1158/0008-5472.CAN-17-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani M., Bruno G., Dal Monte M., Nassini R., Fontani F., Casini A., Cavallini L., Becatti M., Bianchini F., De Logu F., Forni G., la Marca G., Calorini L., Bagnoli P., Chiarugi P., Pupi A., Azzari C., Geppetti P., Favre C., Filippi L. β(3) -Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br. J. Pharmacol. 2019;176:2509–2524. doi: 10.1111/bph.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F., Klein Wolterink R.G.J., Godinho-Silva C., Domingues R.G., Ribeiro H., da Silva J.A., Mahu I., Domingos A.I., Veiga-Fernandes H. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature. 2021;597:410–414. doi: 10.1038/s41586-021-03830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.S., Weber J., Holtkamp S.J., Ince L.M., de Juan A., Wang C., Lutes L., Barnoud C., Kizil B., Hergenhan S.M., Salvermoser J., Lasch M., Deindl E., Schraml B., Baumjohann D., Scheiermann C. Loss of direct adrenergic innervation after peripheral nerve injury causes lymph node expansion through IFN-gamma. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R.C., Dong X.L., Noble B.K., Knight P.R., Spengler R.N. Adrenergic regulation of macrophage-derived tumor necrosis factor-alpha generation during a chronic polyarthritis pain model. J. Neuroimmunol. 1998;82:140–148. doi: 10.1016/s0165-5728(97)00196-3. [DOI] [PubMed] [Google Scholar]
- Clarke C.E., Speller J.M. Lisuride versus bromocriptine for levodopa-induced complications in Parkinson's disease. Cochrane Database Syst. Rev. 2000;1999 doi: 10.1002/14651858.CD001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comabella M., Balashov K., Issazadeh S., Smith D., Weiner H.L., Khoury S.J. Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J. Clin. Invest. 1998;102:671–678. doi: 10.1172/JCI3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÇOpur S., Kanbay A., AfŞAr B., ElsÜRer AfŞAr R., Kanbay M. Pathological features of COVID-19 infection from biopsy and autopsy series. Tuberk Toraks. 2020;68:160–167. doi: 10.5578/tt.69611. [DOI] [PubMed] [Google Scholar]
- Corena-McLeod M. Comparative pharmacology of risperidone and paliperidone. Drugs R. 2015;15:163–174. doi: 10.1007/s40268-015-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi L., Negri F., Parini A., Partesana N., Finardi G. Decreased beta-adrenoceptors in polymorphonucleates in essential hypertension. Bollettino della Societa italiana di biologia sperimentale. 1981;57:1766–1770. [PubMed] [Google Scholar]
- Cosentino M., Fietta A.M., Ferrari M., Rasini E., Bombelli R., Carcano E., Saporiti F., Meloni F., Marino F., Lecchini S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- Cotecchia S. The α1-adrenergic receptors: diversity of signaling networks and regulation. J. Recept. Signal Transduct. Res. 2010;30:410–419. doi: 10.3109/10799893.2010.518152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S., Del Vescovo C.D., Colella M., Caso S., Diviani D. The alpha1-adrenergic receptors in cardiac hypertrophy: signaling mechanisms and functional implications. Cell. Signal. 2015;27:1984–1993. doi: 10.1016/j.cellsig.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Cross R.J., Jackson J.C., Brooks W.H., Sparks D.L., Markesbery W.R., Roszman T.L. Neuroimmunomodulation: impairment of humoral immune responsiveness by 6-hydroxydopamine treatment. Immunology. 1986;57:145–152. [PMC free article] [PubMed] [Google Scholar]
- da Silva Rossato J., Krause M., Fernandes A.J., Fernandes J.R., Seibt I.L., Rech A., Homem de Bittencourt P.I., Jr. Role of alpha- and beta-adrenoreceptors in rat monocyte/macrophage function at rest and acute exercise. J. Physiol. Biochem. 2014;70:363–374. doi: 10.1007/s13105-013-0310-3. [DOI] [PubMed] [Google Scholar]
- Daher C., Vimeux L., Stoeva R., Peranzoni E., Bismuth G., Donnadieu E., Bercovici N., Trautmann A., Feuillet V. Blockade of β-adrenergic receptor signaling improves cancer vaccine efficacy through its effect on naive CD8+ T-cell priming. bioRxiv. 2018 doi: 10.1158/2326-6066.CIR-18-0833. [DOI] [PubMed] [Google Scholar]
- de Coupade C., Gear R.W., Dazin P.F., Sroussi H.Y., Green P.G., Levine J.D. Beta 2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br. J. Pharmacol. 2004;143:1033–1041. doi: 10.1038/sj.bjp.0705972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi V., Grazzini M., Gandini S., Benemei S., Lotti T., Marchionni N., Geppetti P. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch. Intern. Med. 2011;171:779–781. doi: 10.1001/archinternmed.2011.131. [DOI] [PubMed] [Google Scholar]
- DeGraff J.L., Gurevich V.V., Benovic J.L. The third intracellular loop of alpha 2-adrenergic receptors determines subtype specificity of arrestin interaction. J. Biol. Chem. 2002;277:43247–43252. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- Deng Q., Chen H., Liu Y., Xiao F., Guo L., Liu D., Cheng X., Zhao M., Wang X., Xie S., Qi S., Yin Z., Gao J., Chen X., Wang J., Guo N., Ma Y., Shi M. Psychological stress promotes neutrophil infiltration in colon tissue through adrenergic signaling in DSS-induced colitis model. Brain Behav. Immun. 2016;57:243–254. doi: 10.1016/j.bbi.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Devi S., Alexandre Y.O., Loi J.K., Gillis R., Ghazanfari N., Creed S.J., Holz L.E., Shackleford D., Mackay L.K., Heath W.R., Sloan E.K., Mueller S.N. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity. 2021;54:1219–1230. doi: 10.1016/j.immuni.2021.03.025. e1217. [DOI] [PubMed] [Google Scholar]
- Diaz-Salazar C., Bou-Puerto R., Mujal A.M., Lau C.M., von Hoesslin M., Zehn D., Sun J.C. Cell-intrinsic adrenergic signaling controls the adaptive NK cell response to viral infection. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S., Lange T., Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010;184:503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- Docherty J.R. The pharmacology of α(1)-adrenoceptor subtypes. Eur. J. Pharmacol. 2019;855:305–320. doi: 10.1016/j.ejphar.2019.04.047. [DOI] [PubMed] [Google Scholar]
- Druce H.M., Ramsey D.L., Karnati S., Carr A.N. Topical nasal decongestant oxymetazoline (0.05%) provides relief of nasal symptoms for 12 hours. Rhinology. 2018;56:343–350. doi: 10.4193/Rhin17.150. [DOI] [PubMed] [Google Scholar]
- Duan B., Davis R., Sadat E.L., Collins J., Sternweis P.C., Yuan D., Jiang L.I. Distinct roles of adenylyl cyclase VII in regulating the immune responses in mice. J. Immunol. 2010;185:335–344. doi: 10.4049/jimmunol.0903474. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., Chen J., Razavi M., Wei Y., Tao Y., Rao X., Zhong J. Alpha2B-Adrenergic receptor regulates neutrophil recruitment in MSU-induced peritoneal inflammation. Front. Immunol. 2019;10:501. doi: 10.3389/fimmu.2019.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C.J., Matheson A., Faulds D.M. Tamsulosin: a review of its pharmacology and therapeutic efficacy in the management of lower urinary tract symptoms. Drugs Aging. 2002;19:135–161. doi: 10.2165/00002512-200219020-00004. [DOI] [PubMed] [Google Scholar]
- Duvernay M.T., Wang H., Dong C., Guidry J.J., Sackett D.L., Wu G. Alpha2B-adrenergic receptor interaction with tubulin controls its transport from the endoplasmic reticulum to the cell surface. J. Biol. Chem. 2011;286:14080–14089. doi: 10.1074/jbc.M111.222323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Emeny R.T., Gao D., Lawrence D.A. Beta1-adrenergic receptors on immune cells impair innate defenses against Listeria. J. Immunol. 2007;178:4876–4884. doi: 10.4049/jimmunol.178.8.4876. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
- Ezeamuzie C.I., Shihab P.K., Al-Radwan R. Loss of surface beta-2 adrenoceptors accounts for the insensitivity of cultured human monocytes to beta-2 adrenoceptor agonists. Int. Immunopharm. 2011;11:1189–1194. doi: 10.1016/j.intimp.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Faisy C., Pinto F.M., Blouquit-Laye S., Danel C., Naline E., Buenestado A., Grassin Delyle S., Burgel P.R., Chapelier A., Advenier C., Candenas M.L., Devillier P. beta2-Agonist modulates epithelial gene expression involved in the T- and B-cell chemotaxis and induces airway sensitization in human isolated bronchi. Pharmacol. Res. 2010;61:121–128. doi: 10.1016/j.phrs.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Ferrer-Lorente R., Cabot C., Fernández-López J.A., Alemany M. Combined effects of oleoyl-estrone and a beta3-adrenergic agonist (CL316,243) on lipid stores of diet-induced overweight male Wistar rats. Life Sci. 2005;77:2051–2058. doi: 10.1016/j.lfs.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Filippi L., Bruno G., Domazetovic V., Favre C., Calvani M. Current therapies and new targets to fight melanoma: a promising role for the β3-adrenoreceptor. Cancers. 2020;12 doi: 10.3390/cancers12061415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragala M.S., Kraemer W.J., Mastro A.M., Denegar C.R., Volek J.S., Häkkinen K., Anderson J.M., Lee E.C., Maresh C.M. Leukocyte β2-adrenergic receptor expression in response to resistance exercise. Med. Sci. Sports Exerc. 2011;43:1422–1432. doi: 10.1249/MSS.0b013e31820b88bc. [DOI] [PubMed] [Google Scholar]
- Fuchs B.A., Campbell K.S., Munson A.E. Norepinephrine and serotonin content of the murine spleen: its relationship to lymphocyte beta-adrenergic receptor density and the humoral immune response in vivo and in vitro. Cell. Immunol. 1988;117:339–351. doi: 10.1016/0008-8749(88)90123-2. [DOI] [PubMed] [Google Scholar]
- Gan X., Zhang L., Solomon G.F., Bonavida B. Mechanism of norepinephrine-mediated inhibition of human NK cytotoxic functions: inhibition of cytokine secretion, target binding, and programming for cytotoxicity. Brain Behav. Immun. 2002;16:227–246. doi: 10.1006/brbi.2000.0615. [DOI] [PubMed] [Google Scholar]
- Gandhi S., Pandey M.R., Attwood K., Ji W., Witkiewicz A.K., Knudsen E.S., Allen C., Tario J.D., Wallace P.K., Cedeno C.D., Levis M., Stack S., Funchain P., Drabick J.J., Bucsek M.J., Puzanov I., Mohammadpour H., Repasky E.A., Ernstoff M.S. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 2021;27:87–95. doi: 10.1158/1078-0432.CCR-20-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García J.J., del Carmen Sáez M., De la Fuente M., Ortega E. Noradrenaline and its end metabolite 3-methoxy-4-hydroxyphenylglycol inhibit lymphocyte chemotaxis: role of alpha- and beta-adrenoreceptors. Mol. Cell. Biochem. 2003;254:305–309. doi: 10.1023/a:1027349904589. [DOI] [PubMed] [Google Scholar]
- García J.J., del Carmen Sáez M., De la Fuente M., Ortega E. Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol. Cell. Biochem. 2003;254:299–304. doi: 10.1023/a:1027345820519. [DOI] [PubMed] [Google Scholar]
- García-Prieto J., Villena-Gutiérrez R., Gómez M., Bernardo E., Pun-García A., García-Lunar I., Crainiciuc G., Fernández-Jiménez R., Sreeramkumar V., Bourio-Martínez R., García-Ruiz J.M., Del Valle A.S., Sanz-Rosa D., Pizarro G., Fernández-Ortiz A., Hidalgo A., Fuster V., Ibanez B. Neutrophil stunning by metoprolol reduces infarct size. Nat. Commun. 2017;8 doi: 10.1038/ncomms14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson-Berry K.L., Whitin J.C., Cohen H.J. Modulation of the respiratory burst in human neutrophils by isoproterenol and dibutyryl cyclic AMP. J. Neuroimmunol. 1993;43:59–68. doi: 10.1016/0165-5728(93)90075-a. [DOI] [PubMed] [Google Scholar]
- Giembycz M.A. Phosphodiesterase 4 and tolerance to beta 2-adrenoceptor agonists in asthma. Trends Pharmacol. Sci. 1996;17:331–336. [PubMed] [Google Scholar]
- Giordani L., Cuzziol N., Del Pinto T., Sanchez M., Maccari S., Massimi A., Pietraforte D., Viora M. β2-Agonist clenbuterol hinders human monocyte differentiation into dendritic cells. Exp. Cell Res. 2015;339:163–173. doi: 10.1016/j.yexcr.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Giorelli M., Livrea P., Trojano M. Post-receptorial mechanisms underlie functional disregulation of beta2-adrenergic receptors in lymphocytes from Multiple Sclerosis patients. J. Neuroimmunol. 2004;155:143–149. doi: 10.1016/j.jneuroim.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Giri S., Lal G. Differentiation and functional plasticity of gamma-delta (gammadelta) T cells under homeostatic and disease conditions. Mol. Immunol. 2021;136:138–149. doi: 10.1016/j.molimm.2021.06.006. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabrera P.J., Shi T., Yun J., McCune D.F., Rorabaugh B.R., Perez D.M. Differential regulation of the cell cycle by α1-adrenergic receptor subtypes. Endocrinology. 2004;145:5157–5167. doi: 10.1210/en.2004-0728. [DOI] [PubMed] [Google Scholar]
- Goodman O.B., Jr., Krupnick J.G., Santini F., Gurevich V.V., Penn R.B., Gagnon A.W., Keen J.H., Benovic J.L. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Graff R.M., Kunz H.E., Agha N.H., Baker F.L., Laughlin M., Bigley A.B., Markofski M.M., LaVoy E.C., Katsanis E., Bond R.A., Bollard C.M., Simpson R.J. beta2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018;74:143–153. doi: 10.1016/j.bbi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Graff R.M., Kunz H.E., Agha N.H., Baker F.L., Laughlin M., Bigley A.B., Markofski M.M., LaVoy E.C., Katsanis E., Bond R.A., Bollard C.M., Simpson R.J. β(2)-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 2018;74:143–153. doi: 10.1016/j.bbi.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Grailer J.J., Haggadone M.D., Sarma J.V., Zetoune F.S., Ward P.A. Induction of M2 regulatory macrophages through the β2-adrenergic receptor with protection during endotoxemia and acute lung injury. J. Innate Immun. 2014;6:607–618. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe K.M., Hickman H.D., Irvine K.R., Takeda K., Bennink J.R., Yewdell J.W. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5300–5305. doi: 10.1073/pnas.0808851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L.A., Evanson J., Marchus E., Jorissen H., Woster A.P., DeKrey W., Sauter E.R., Combs C.K., Porter J.E. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol. Immunol. 2010;47:1244–1254. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L.A., Perez D.M., Porter J.E. Modulation of immune cell function by α(1)-adrenergic receptor activation. Curr. Top. Membr. 2011;67:113–138. doi: 10.1016/B978-0-12-384921-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R., Benovic J.L., Tan C.M., Feldman R.D. G-protein-coupled receptor kinase activity is increased in hypertension. J. Clin. Invest. 1997;99:2087–2093. doi: 10.1172/JCI119381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guereschi M.G., Araujo L.P., Maricato J.T., Takenaka M.C., Nascimento V.M., Vivanco B.C., Reis V.O., Keller A.C., Brum P.C., Basso A.S. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur. J. Immunol. 2013;43:1001–1012. doi: 10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- Guglin M., Krischer J., Tamura R., Fink A., Bello-Matricaria L., McCaskill-Stevens W., Munster P.N. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J. Am. Coll. Cardiol. 2019;73:2859–2868. doi: 10.1016/j.jacc.2019.03.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao X., Kumar A., Katz J., Smith M., Lin E., Keogh C., Calvano S.E., Lowry S.F. Catecholamines increase monocyte TNF receptors and inhibit TNF through beta 2-adrenoreceptor activation. Am. J. Physiol. 1997;273:E1203–E1208. doi: 10.1152/ajpendo.1997.273.6.E1203. [DOI] [PubMed] [Google Scholar]
- Gurguis G.N., Andrews R., Antai-Otong D., Vo S.P., Blakeley J.E., Orsulak P.J., Rush A.J. Neutrophil beta2-adrenergic receptor coupling efficiency to Gs protein in subjects with post-traumatic stress disorder and normal controls. Psychopharmacology. 1999;143:131–140. doi: 10.1007/s002130050928. [DOI] [PubMed] [Google Scholar]
- Habek M., Pucić D., Mutak T., Crnošija L., Lovrić M., Krbot Skorić M. The association between the adrenergic hyperactivity and blood pressure values in people with multiple sclerosis. Neurol. Sci. 2020;41:3157–3164. doi: 10.1007/s10072-020-04432-3. [DOI] [PubMed] [Google Scholar]
- Haldar R., Ricon-Becker I., Radin A., Gutman M., Cole S.W., Zmora O., Ben-Eliyahu S. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer. 2020;126:3991–4001. doi: 10.1002/cncr.32950. [DOI] [PubMed] [Google Scholar]
- Halder N., Lal G. Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.660342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand K., Hermodsson S., Strannegård O. Evidence for a beta-adrenoceptor-mediated regulation of human natural killer cells. J. Immunol. 1985;134:4095–4099. (Baltimore, Md. : 1950) [PubMed] [Google Scholar]
- Hervé J., Dubreil L., Tardif V., Terme M., Pogu S., Anegon I., Rozec B., Gauthier C., Bach J.M., Blancou P. β2-Adrenoreceptor agonist inhibits antigen cross-presentation by dendritic cells. J. Immunol. 2013;190:3163–3171. doi: 10.4049/jimmunol.1201391. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
- Humphreys R.J., Raab W. Response of circulating eosinophils to nor-epinephrine, epinephrine and emotional stress in humans. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1950;74:302–303. doi: 10.3181/00379727-74-17884. (New York, N.Y.) [DOI] [PubMed] [Google Scholar]
- Iñigo-Marco I., Alonso M.M. Destress and do not suppress: targeting adrenergic signaling in tumor immunosuppression. J. Clin. Invest. 2019;129:5086–5088. doi: 10.1172/JCI133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen L., Hoffmeister M., Arndt V., Chang-Claude J., Brenner H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer. 2014;120:1178–1186. doi: 10.1002/cncr.28546. [DOI] [PubMed] [Google Scholar]
- Jean Wrobel L., Bod L., Lengagne R., Kato M., Prévost-Blondel A., Le Gal F.A. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016;7:77825–77837. doi: 10.18632/oncotarget.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentzsch N.S., Silva G.C.G., Mendes G.M.S., Brand P.L.P., Camargos P. Treatment adherence and level of control in moderate persistent asthma in children and adolescents treated with fluticasone and salmeterol. J. Pediatr. 2019;95:69–75. doi: 10.1016/j.jped.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Jetschmann J.U., Benschop R.J., Jacobs R., Kemper A., Oberbeck R., Schmidt R.E., Schedlowski M. Expression and in-vivo modulation of alpha- and beta-adrenoceptors on human natural killer (CD16+) cells. J. Neuroimmunol. 1997;74:159–164. doi: 10.1016/s0165-5728(96)00221-4. [DOI] [PubMed] [Google Scholar]
- Johnson J.D., Campisi J., Sharkey C.M., Kennedy S.L., Nickerson M., Greenwood B.N., Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Josefsson E., Bergquist J., Ekman R., Tarkowski A. Catecholamines are synthesized by mouse lymphocytes and regulate function of these cells by induction of apoptosis. Immunology. 1996;88:140–146. doi: 10.1046/j.1365-2567.1996.d01-653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanashiro A., Hiroki C.H., da Fonseca D.M., Birbrair A., Ferreira R.G., Bassi G.S., Fonseca M.D., Kusuda R., Cebinelli G.C.M., da Silva K.P., Wanderley C.W., Menezes G.B., Alves-Fiho J.C., Oliveira A.G., Cunha T.M., Pupo A.S., Ulloa L., Cunha F.Q. The role of neutrophils in neuro-immune modulation. Pharmacol. Res. 2020;151:104580. doi: 10.1016/j.phrs.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaszewski J.W., Reder A.T., Anlar B., Arnason G.W. Increased high affinity beta-adrenergic receptor densities and cyclic AMP responses of CD8 cells in multiple sclerosis. J. Neuroimmunol. 1993;43:1–7. doi: 10.1016/0165-5728(93)90068-a. [DOI] [PubMed] [Google Scholar]
- Karaszewski J.W., Reder A.T., Anlar B., Kim W.C., Arnason B.G. Increased lymphocyte beta-adrenergic receptor density in progressive multiple sclerosis is specific for the CD8+, CD28- suppressor cell. Ann. Neurol. 1991;30:42–47. doi: 10.1002/ana.410300109. [DOI] [PubMed] [Google Scholar]
- Karmakar S., Lal G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics. 2021;11:5296–5312. doi: 10.7150/thno.55986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprowicz D.J., Kohm A.P., Berton M.T., Chruscinski A.J., Sharpe A., Sanders V.M. Stimulation of the B cell receptor, CD86 (B7-2), and the beta 2-adrenergic receptor intrinsically modulates the level of IgG1 and IgE produced per B cell. J. Immunol. 2000;165:680–690. doi: 10.4049/jimmunol.165.2.680. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav. Immun. 2002;16:799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of α-1 adrenergic receptors in the immune system. Brain Behav. Immun. 2002;16:799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Kenne Sarenmalm E., Mårtensson L.B., Andersson B.A., Karlsson P., Bergh I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer medicine. 2017;6:1108–1122. doi: 10.1002/cam4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney M.J., Ganta C.K. Autonomic nervous system and immune system interactions. Comprehensive Physiol. 2014;4:1177–1200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw Y.M., Majid D., Oh S., Kang E., Inoue M. Early-life-trauma triggers interferon-beta resistance and neurodegeneration in a multiple sclerosis model via downregulated beta1-adrenergic signaling. Nat. Commun. 2021;12:105. doi: 10.1038/s41467-020-20302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury S.J., Healy B.C., Kivisäkk P., Viglietta V., Egorova S., Guttmann C.R., Wedgwood J.F., Hafler D.A., Weiner H.L., Buckle G., Cook S., Reddy S. A randomized controlled double-masked trial of albuterol add-on therapy in patients with multiple sclerosis. Arch. Neurol. 2010;67:1055–1061. doi: 10.1001/archneurol.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Gorouhi F., Ramirez S., Granick J.L., Byrne B.A., Soulika A.M., Simon S.I., Isseroff R.R. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2-adrenergic receptor-mediated upregulation of IL-6. J. Invest. Dermatol. 2014;134:809–817. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.H., Gorouhi F., Ramirez S., Granick J.L., Byrne B.A., Soulika A.M., Simon S.I., Rivkah Isseroff R. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2-adrenergic receptor-mediated upregulation of IL-6. J. Invest. Dermatol. 2014;134:809–817. doi: 10.1038/jid.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Abu-Ghazaleh R.I., Gleich G.J., Abraham R.T. Regulation of Ig-induced eosinophil degranulation by adenosine 3',5'-cyclic monophosphate. J. Immunol. 1991;146:2712–2718. (Baltimore, Md. : 1950) [PubMed] [Google Scholar]
- Kobayashi M., Jeschke M.G., Asai A., Kogiso M., Yoshida S., Herndon D.N., Suzuki F. Propranolol as a modulator of M2b monocytes in severely burned patients. J. Leukoc. Biol. 2011;89:797–803. doi: 10.1189/jlb.1010553. [DOI] [PubMed] [Google Scholar]
- Kohm A.P., Sanders V.M. Suppression of antigen-specific Th2 cell-dependent IgM and IgG1 production following norepinephrine depletion in vivo. J. Immunol. 1999;162:5299–5308. (Baltimore, Md. : 1950) [PubMed] [Google Scholar]
- Kohm A.P., Sanders V.M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol. Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- Kokolus K.M., Zhang Y., Sivik J.M., Schmeck C., Zhu J., Repasky E.A., Drabick J.J., Schell T.D. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. OncoImmunology. 2018;7 doi: 10.1080/2162402X.2017.1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M.F., Powell M., Staedtke V., Bai R.Y., Thomas D.L., Fischer N., Huq S., Khalafallah A.M., Koenecke A., Xiong R., Mensh B., Papadopoulos N., Kinzler K.W., Vogelstein B., Vogelstein J.T., Athey S., Zhou S., Bettegowda C. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J. Clin. Invest. 2020;130:3345–3347. doi: 10.1172/JCI139642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger E.M., Drager L.F., Giorgi D.M.A., Pereira A.C., Barreto-Filho J.A.S., Nogueira A.R., Mill J.G., Lotufo P.A., Amodeo C., Batista M.C., Bodanese L.C., Carvalho A.C.C., Castro I., Chaves H., Costa E.A.S., Feitosa G.S., Franco R.J.S., Fuchs F.D., Guimarães A.C., Jardim P.C., Machado C.A., Magalhães M.E., Mion D., Jr., Nascimento R.M., Nobre F., Nóbrega A.C., Ribeiro A.L.P., Rodrigues-Sobrinho C.R., Sanjuliani A.F., Teixeira M., Krieger J.E. Spironolactone versus clonidine as a fourth-drug therapy for resistant hypertension: the ReHOT randomized study (resistant hypertension optimal treatment) Hypertension. 2018;71:681–690. doi: 10.1161/HYPERTENSIONAHA.117.10662. [DOI] [PubMed] [Google Scholar]
- Krizanova O., Babula P., Pacak K. Stress, catecholaminergic system and cancer. Stress. 2016;19:419–428. doi: 10.1080/10253890.2016.1203415. [DOI] [PubMed] [Google Scholar]
- Lajevic M.D., Suleiman S., Cohen R.L., Chambers D.A. Activation of p38 mitogen-activated protein kinase by norepinephrine in T-lineage cells. Immunology. 2011;132:197–208. doi: 10.1111/j.1365-2567.2010.03354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S.Z. Nomenclature and state of the art on alpha1-adrenoceptors. Eur. Urol. 1998;2:2–6. doi: 10.1159/000052227. [DOI] [PubMed] [Google Scholar]
- Lechtenberg K.J., Meyer S.T., Doyle J.B., Peterson T.C., Buckwalter M.S. Augmented β2-adrenergic signaling dampens the neuroinflammatory response following ischemic stroke and increases stroke size. J. Neuroinflammation. 2019;16:112. doi: 10.1186/s12974-019-1506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Dexmedetomidine: present and future directions. Korean J. Anesthesiol. 2019;72:323–330. doi: 10.4097/kja.19259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi B., Benish M., Goldfarb Y., Sorski L., Melamed R., Rosenne E., Ben-Eliyahu S. Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behav. Immun. 2011;25:727–735. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi M.S., Kavelaars A., Schedlowski M., Bijlsma J.W., Okihara K.L., Van de Pol M., Ochsmann S., Pawlak C., Schmidt R.E., Heijnen C.J. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. Faseb. J. : official publication of the Federation of American Societies for Experimental Biology. 1999;13:715–725. doi: 10.1096/fasebj.13.6.715. [DOI] [PubMed] [Google Scholar]
- Lorton D., Bellinger D.L. Molecular mechanisms underlying beta-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int. J. Mol. Sci. 2015;16:5635–5665. doi: 10.3390/ijms16035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorton D., Lubahn C., Klein N., Schaller J., Bellinger D.L. Dual role for noradrenergic innervation of lymphoid tissue and arthritic joints in adjuvant-induced arthritis. Brain Behav. Immun. 1999;13:315–334. doi: 10.1006/brbi.1999.0564. [DOI] [PubMed] [Google Scholar]
- Lubahn C.L., Lorton D., Schaller J.A., Sweeney S.J., Bellinger D.L. Targeting α- and β-adrenergic receptors differentially shifts Th1, Th2, and inflammatory cytokine profiles in immune organs to attenuate adjuvant arthritis. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusthaus J.A., Goldberg I. Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation. Expet Opin. Drug Saf. 2017;16:1071–1078. doi: 10.1080/14740338.2017.1346083. [DOI] [PubMed] [Google Scholar]
- MacNeil B.J., Jansen A.H., Greenberg A.H., Nance D.M. Activation and selectivity of splenic sympathetic nerve electrical activity response to bacterial endotoxin. Am. J. Physiol. 1996;270:R264–R270. doi: 10.1152/ajpregu.1996.270.1.R264. [DOI] [PubMed] [Google Scholar]
- Maestroni G.J. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J. Immunol. 2000;165:6743–6747. doi: 10.4049/jimmunol.165.12.6743. [DOI] [PubMed] [Google Scholar]
- Maestroni G.J. Modulation of skin norepinephrine turnover by allergen sensitization: impact on contact hypersensitivity and T helper priming. J. Invest. Dermatol. 2004;122:119–124. doi: 10.1046/j.0022-202X.2003.22132.x. [DOI] [PubMed] [Google Scholar]
- Maestroni G.J., Mazzola P. Langerhans cells beta 2-adrenoceptors: role in migration, cytokine production, Th priming and contact hypersensitivity. J. Neuroimmunol. 2003;144:91–99. doi: 10.1016/j.jneuroim.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Majid P.A., Sharma B., Taylor S.H. Phentolamine for vasodilator treatment of severe heart-failure. Lancet. 1971;2:719–724. doi: 10.1016/s0140-6736(71)92098-8. [DOI] [PubMed] [Google Scholar]
- Malysheva O., Pierer M., Wagner U., Wahle M., Wagner U., Baerwald C.G. Association between beta2 adrenergic receptor polymorphisms and rheumatoid arthritis in conjunction with human leukocyte antigen (HLA)-DRB1 shared epitope. Ann. Rheum. Dis. 2008;67:1759–1764. doi: 10.1136/ard.2007.083782. [DOI] [PubMed] [Google Scholar]
- Manni M., Granstein R.D., Maestroni G. β2-Adrenergic agonists bias TLR-2 and NOD2 activated dendritic cells towards inducing an IL-17 immune response. Cytokine. 2011;55:380–386. doi: 10.1016/j.cyto.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino F., Cosentino M. Adrenergic modulation of immune cells: an update. Amino Acids. 2013;45:55–71. doi: 10.1007/s00726-011-1186-6. [DOI] [PubMed] [Google Scholar]
- Marino F., Cosentino M., Bombelli R., Ferrari M., Lecchini S., Frigo G. Endogenous catecholamine synthesis, metabolism storage, and uptake in human peripheral blood mononuclear cells. Exp. Hematol. 1999;27:489–495. doi: 10.1016/s0301-472x(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Marino F., Scanzano A., Pulze L., Pinoli M., Rasini E., Luini A., Bombelli R., Legnaro M., de Eguileor M., Cosentino M. β(2) -Adrenoceptors inhibit neutrophil extracellular traps in human polymorphonuclear leukocytes. J. Leukoc. Biol. 2018;104:603–614. doi: 10.1002/JLB.3A1017-398RR. [DOI] [PubMed] [Google Scholar]
- Masini E., Blandina P., Mannaioni P.F. Mast cell receptors controlling histamine release: influences on the mode of action of drugs used in the treatment of adverse drug reactions. Klin. Wochenschr. 1982;60:1031–1038. doi: 10.1007/BF01716967. [DOI] [PubMed] [Google Scholar]
- Meltzer J.C., MacNeil B.J., Sanders V., Pylypas S., Jansen A.H., Greenberg A.H., Nance D.M. Contribution of the adrenal glands and splenic nerve to LPS-induced splenic cytokine production in the rat. Brain Behav. Immun. 2003;17:482–497. doi: 10.1016/s0889-1591(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Mencia-Huerta J.M., Paul-Eugène N., Dugas B., Petit-Frère C., Gordon J., Lagente V., Cairns J., Braquet P. Beta-2-adrenoceptor agonists up-regulate the in vitro Fc epsilon receptor II/CD23 expression on, and release from, the promonocytic cell line U937 and human blood monocytes. Int. Arch. Allergy Appl. Immunol. 1991;94:91–92. doi: 10.1159/000235334. [DOI] [PubMed] [Google Scholar]
- Miadonna A., Tedeschi A., Leggieri E., Parravicini P., Lorini M., Zanussi C. Clonidine inhibits IgE-mediated and IgE-independent in vitro histamine release from human basophil leukocytes. Int. J. Immunopharm. 1989;11:473–477. doi: 10.1016/0192-0561(89)90176-8. [DOI] [PubMed] [Google Scholar]
- Miksa M., Das P., Zhou M., Wu R., Dong W., Ji Y., Goyert S.M., Ravikumar T.S., Wang P. Pivotal role of the α2a-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.E., Jüsten H.P., Schölmerich J., Straub R.H. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. Faseb. J. 2000;14:2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- Mishima K., Otani H., Tanabe T., Kawasaki H., Oshiro A., Saito N., Ogawa R., Inagaki C. Molecular mechanisms for alpha2-adrenoceptor-mediated regulation of synoviocyte populations. Jpn. J. Pharmacol. 2001;85:214–226. doi: 10.1254/jjp.85.214. [DOI] [PubMed] [Google Scholar]
- Mishra A., Lal G. Neurokinin receptors and their implications in various autoimmune diseases. Curr. Res. Immunol. 2021;2:66–78. doi: 10.1016/j.crimmu.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Takahashi H.K., Iwagaki H., Katsuno G., Kamurul H.A., Ohtani S., Mori S., Yoshino T., Nishibori M., Tanaka N. Beta2-adrenergic receptor stimulation inhibits LPS-induced IL-18 and IL-12 production in monocytes. Immunol. Lett. 2005;101:168–172. doi: 10.1016/j.imlet.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Mohammadpour H., MacDonald C.R., McCarthy P.L., Abrams S.I., Repasky E.A. beta2-adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour H., MacDonald C.R., Qiao G., Chen M., Dong B., Hylander B.L., McCarthy P.L., Abrams S.I., Repasky E.A. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 2019;129:5537–5552. doi: 10.1172/JCI129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti S., Massi D., Farini V., Baroni G., Parri M., Innocenti S., Cecchi R., Chiarugi P. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab. Invest. 2013;93:279–290. doi: 10.1038/labinvest.2012.175. [DOI] [PubMed] [Google Scholar]
- Morfin-Maciel B.M., Castillo-Morfin B.M. [Angioedema and paradoxical bronchoconstriction secondary to ipratropium bromide/salbutamol administered by metered dose inhaler in patients with soy allergy] Rev. Alerg. Mex. 2011;58:219–223. [PubMed] [Google Scholar]
- Muszkat M., Sofowora G.G., Xie H.G., Wood A.J., Stein C.M. Alpha2B adrenergic receptor 301-303 deletion polymorphism and vascular alpha2 adrenergic receptor response. Pharmacogenetics Genom. 2005;15:23–28. doi: 10.1097/01213011-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Nguyen K.D., Qiu Y., Cui X., Goh Y.P., Mwangi J., David T., Mukundan L., Brombacher F., Locksley R.M., Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls A.J., Wen S.W., Hall P., Hickey M.J., Wong C.H.Y. Activation of the sympathetic nervous system modulates neutrophil function. J. Leukoc. Biol. 2018;103:295–309. doi: 10.1002/JLB.3MA0517-194RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson C.P. Beta-adrenergic modulation of the polymorphonuclear leukocyte respiratory burst is dependent upon the mechanism of cell activation. J. Immunol. 1987;139:2392–2397. (Baltimore, Md. : 1950) [PubMed] [Google Scholar]
- Nissen M.D., Sloan E.K., Mattarollo S.R. β-Adrenergic signaling impairs antitumor CD8(+) T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res. 2018;6:98–109. doi: 10.1158/2326-6066.CIR-17-0401. [DOI] [PubMed] [Google Scholar]
- Nobles K.N., Xiao K., Ahn S., Shukla A.K., Lam C.M., Rajagopal S., Strachan R.T., Huang T.Y., Bressler E.A., Hara M.R., Shenoy S.K., Gygi S.P., Lefkowitz R.J. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci. Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell T.D., Jensen B.C., Baker A.J., Simpson P.C. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol. Rev. 2014;66:308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama N., Urasawa K., Kaneta S., Sakai H., Saito T., Takagi C., Yoshida I., Kitabatake A., Tsutsui H. Chronic beta-adrenergic receptor stimulation enhances the expression of G-Protein coupled receptor kinases, GRK2 and GRK5, in both the heart and peripheral lymphocytes. Circ. J. : official J. Jpn. Circulation Soc. 2005;69:987–990. doi: 10.1253/circj.69.987. [DOI] [PubMed] [Google Scholar]
- Page C.P., Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb. Exp. Pharmacol. 2011:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- Page G.G., Ben-Eliyahu S. Natural killer cell activity and resistance to tumor metastasis in prepubescent rats: deficient baselines, but invulnerability to stress and beta-adrenergic stimulation. Neuroimmunomodulation. 2000;7:160–168. doi: 10.1159/000026434. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Mazzeo D., Lucia P.D., D'Ambrosio D., Lang R., Fabbri L., Self C., Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C.S., Benovic J.L. Structure/function analysis of alpha2A-adrenergic receptor interaction with G protein-coupled receptor kinase 2. J. Biol. Chem. 2005;280:11052–11058. doi: 10.1074/jbc.M412996200. [DOI] [PubMed] [Google Scholar]
- Papadopoulos D.P., Papademetriou V. Metoprolol succinate combination in the treatment of hypertension. Angiology. 2009;60:608–613. doi: 10.1177/0003319708326450. [DOI] [PubMed] [Google Scholar]
- Paul S., Kulkarni N., Shilpi Lal G. Intratumoral natural killer cells show reduced effector and cytolytic properties and control the differentiation of effector Th1 cells. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2016.1235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Shilpi, Lal G. Role of gamma-delta (gammadelta) T cells in autoimmunity. J. Leukoc. Biol. 2015;97:259–271. doi: 10.1189/jlb.3RU0914-443R. [DOI] [PubMed] [Google Scholar]
- Paul S., Singh A.K., Shilpi Lal G. Phenotypic and functional plasticity of gamma-delta (gammadelta) T cells in inflammation and tolerance. Int. Rev. Immunol. 2014;33:537–558. doi: 10.3109/08830185.2013.863306. [DOI] [PubMed] [Google Scholar]
- Pierce K.L., Lefkowitz R.J. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat. Rev. Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- Pilipović I., Vujnović I., Stojić-Vukanić Z., Petrović R., Kosec D., Nacka-Aleksić M., Jasnić N., Leposavić G. Noradrenaline modulates CD4+ T cell priming in rat experimental autoimmune encephalomyelitis: a role for the α(1)-adrenoceptor. Immunol. Res. 2019;67:223–240. doi: 10.1007/s12026-019-09082-y. [DOI] [PubMed] [Google Scholar]
- Pitcher J.A., Freedman N.J., Lefkowitz R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Podojil J.R., Sanders V.M. Selective regulation of mature IgG1 transcription by CD86 and beta 2-adrenergic receptor stimulation. J. Immunol. 2003;170:5143–5151. doi: 10.4049/jimmunol.170.10.5143. (Baltimore, Md. : 1950) [DOI] [PubMed] [Google Scholar]
- Podojil J.R., Sanders V.M. CD86 and beta2-adrenergic receptor stimulation regulate B-cell activity cooperatively. Trends Immunol. 2005;26:180–185. doi: 10.1016/j.it.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Powe D.G., Voss M.J., Zanker K.S., Habashy H.O., Green A.R., Ellis I.O., Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prösch S., Wendt C.E., Reinke P., Priemer C., Oppert M., Krüger D.H., Volk H.D., Döcke W.D. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology. 2000;272:357–365. doi: 10.1006/viro.2000.0367. [DOI] [PubMed] [Google Scholar]
- Qiao G., Bucsek M.J., Winder N.M., Chen M., Giridharan T., Olejniczak S.H., Hylander B.L., Repasky E.A. β-Adrenergic signaling blocks murine CD8(+) T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. : CII. 2019;68:11–22. doi: 10.1007/s00262-018-2243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao G., Chen M., Mohammadpour H., MacDonald C.R., Bucsek M.J., Hylander B.L., Barbi J.J., Repasky E.A. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer immunology research. 2021;9:651–664. doi: 10.1158/2326-6066.CIR-20-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer T.H., Lam N., Cocks R.A. Adrenaline upregulates monocyte L-selectin in vitro. Resuscitation. 1999;43:47–55. doi: 10.1016/s0300-9572(99)00121-5. [DOI] [PubMed] [Google Scholar]
- Ramer-Quinn D.S., Swanson M.A., Lee W.T., Sanders V.M. Cytokine production by naive and primary effector CD4+ T cells exposed to norepinephrine. Brain Behav. Immun. 2000;14:239–255. doi: 10.1006/brbi.2000.0603. [DOI] [PubMed] [Google Scholar]
- Rasmussen M., Belza A., Almdal T., Toubro S., Bratholm P., Astrup A., Christensen N.J. Change in beta1-adrenergic receptor protein concentration in adipose tissue correlates with diet-induced weight loss. Clin. Sci. 2005;108:323–329. doi: 10.1042/CS20040238. [DOI] [PubMed] [Google Scholar]
- Ratge D., Wiedemann A., Kohse K.P., Wisser H. Alterations of beta-adrenoceptors on human leukocyte subsets induced by dynamic exercise: effect of prednisone. Clin. Exp. Pharmacol. Physiol. 1988;15:43–53. doi: 10.1111/j.1440-1681.1988.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Redman L.M., Moro C., Dobak J., Yu Y., Guillot T.S., Greenway F.L. Association of β-2 adrenergic agonist and corticosteroid injection in the treatment of lipomas. Diabetes Obes. Metabol. 2011;13:517–522. doi: 10.1111/j.1463-1326.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T.D., Heisler S., Hook V.Y., Axelrod J. Activation of beta 2-adrenergic receptors on mouse anterior pituitary tumor cells increases cyclic adenosine 3':5'-monophosphate synthesis and adrenocorticotropin release. J. Neurosci. : the official journal of the Society for Neuroscience. 1983;3:725–732. doi: 10.1523/JNEUROSCI.03-04-00725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiske L., Schmucker S., Pfaffinger B., Weiler U., Steuber J., Stefanski V. Intravenous infusion of cortisol, adrenaline, or noradrenaline alters porcine immune cell numbers and promotes innate over adaptive immune functionality. J. Immunol. 2020;204:3205–3216. doi: 10.4049/jimmunol.2000269. [DOI] [PubMed] [Google Scholar]
- Ricon I., Hanalis-Miller T., Haldar R., Jacoby R., Ben-Eliyahu S. Perioperative biobehavioral interventions to prevent cancer recurrence through combined inhibition of β-adrenergic and cyclooxygenase 2 signaling. Cancer. 2019;125:45–56. doi: 10.1002/cncr.31594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose L., Graham L., Koenecke A., Powell M., Xiong R., Shen Z., Mench B., Kinzler K.W., Bettegowda C., Vogelstein B., Athey S., Vogelstein J.T., Konig M.F., Wagner T.H. The association between alpha-1 adrenergic receptor antagonists and in-hospital mortality from COVID-19. Front. Med. 2021;8 doi: 10.3389/fmed.2021.637647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N.S., Lefkowitz R.J., Caron M.G. Structure and function of the adrenergic receptor family. Adv. Exp. Med. Biol. 1991;308:223–238. doi: 10.1007/978-1-4684-6015-5_20. [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C., Kavelaars A., van de Pol M., Heijnen C.J. Neuroendocrine mediators up-regulate alpha1b- and alpha1d-adrenergic receptor subtypes in human monocytes. J. Neuroimmunol. 1999;95:165–173. doi: 10.1016/s0165-5728(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C., Kavelaars A., van de Pol M., Heijnen C.J. Noradrenaline induces phosphorylation of ERK-2 in human peripheral blood mononuclear cells after induction of alpha(1)-adrenergic receptors. J. Neuroimmunol. 2000;108:82–91. doi: 10.1016/s0165-5728(00)00253-8. [DOI] [PubMed] [Google Scholar]
- Ruffolo R.R., Jr., Nichols A.J., Stadel J.M., Hieble J.P. Pharmacologic and therapeutic applications of alpha 2-adrenoceptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1993;33:243–279. doi: 10.1146/annurev.pa.33.040193.001331. [DOI] [PubMed] [Google Scholar]
- Ruiz-Medina B.E., Cadena-Medina D.A., Esparza E., Arrieta A.J., Kirken R.A. Isoproterenol-induced beta-2 adrenergic receptor activation negatively regulates interleukin-2 signaling. Biochem. J. 2018;475:2907–2923. doi: 10.1042/BCJ20180503. [DOI] [PubMed] [Google Scholar]
- Sanders V.M. Adrenergic receptors on T and B lymphocytes: evidence, function, and clinical implications. Clin. Neurosci. Res. 2006;6:34–41. [Google Scholar]
- Sanders V.M. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav. Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders V.M., Munson A.E. Norepinephrine and the antibody response. Pharmacol. Rev. 1985;37:229–248. [PubMed] [Google Scholar]
- Sarigianni M., Bekiari E., Tsapas A., Konstantinidis D., Kaloyianni M., Koliakos G., Paletas K. Effect of epinephrine and insulin resistance on human monocytes obtained from lean and obese healthy participants: a pilot study. Angiology. 2011;62:38–45. doi: 10.1177/0003319710371616. [DOI] [PubMed] [Google Scholar]
- Scanzano A., Cosentino M. Adrenergic regulation of innate immunity: a review. Front. Pharmacol. 2015;6:171. doi: 10.3389/fphar.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzano A., Schembri L., Rasini E., Luini A., Dallatorre J., Legnaro M., Bombelli R., Congiu T., Cosentino M., Marino F. Adrenergic modulation of migration, CD11b and CD18 expression, ROS and interleukin-8 production by human polymorphonuclear leukocytes. Inflamm. Res. 2015;64:127–135. doi: 10.1007/s00011-014-0791-8. [DOI] [PubMed] [Google Scholar]
- Schallreuter K.U., Wood J.M., Lemke R., LePoole C., Das P., Westerhof W., Pittelkow M.R., Thody A.J. Production of catecholamines in the human epidermis. Biochem. Biophys. Res. Commun. 1992;189:72–78. doi: 10.1016/0006-291x(92)91527-w. [DOI] [PubMed] [Google Scholar]
- Schedler D. Drug discovery: a history (sneader, walter) Journal of Chemical Education - J CHEM EDUC. 2006;83 [Google Scholar]
- Schwab K.O., Bartels H., Martin C., Leichtenschlag E.M. Decreased beta 2-adrenoceptor density and decreased isoproterenol induced c-AMP increase in juvenile type I diabetes mellitus: an additional cause of severe hypoglycaemia in childhood diabetes? Eur. J. Pediatr. 1993;152:797–801. doi: 10.1007/BF02073373. [DOI] [PubMed] [Google Scholar]
- Shakhar G., Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J. Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- Shampo M.A., Kyle R.A. Ulf von Euler--norepinephrine and the nobel prize. Mayo Clin. Proc. 1995;70:273. doi: 10.4065/70.3.273. [DOI] [PubMed] [Google Scholar]
- Silberman D.M., Wald M.R., Genaro A.M. Acute and chronic stress exert opposing effects on antibody responses associated with changes in stress hormone regulation of T-lymphocyte reactivity. J. Neuroimmunol. 2003;144:53–60. doi: 10.1016/j.jneuroim.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Silva R.L., Castanheira F.V., Figueiredo J.G., Bassi G.S., Ferreira S.H., Cunha F.Q., Cunha T.M., Kanashiro A. Pharmacological beta-adrenergic receptor activation attenuates neutrophil recruitment by a mechanism dependent on nicotinic receptor and the spleen. Inflammation. 2016;39:1405–1413. doi: 10.1007/s10753-016-0372-9. [DOI] [PubMed] [Google Scholar]
- Simkins T., Crawford R.B., Goudreau J.L., Lookingland K.J., Kaplan B.L. Enhanced humoral immunity in mice lacking CB1 and CB2 receptors (Cnr1-/-/Cnr2-/- mice) is not due to increased splenic noradrenergic neuronal activity. J. Neuroimmune Pharmacol. : the official journal of the Society on NeuroImmune Pharmacology. 2014;9:544–557. doi: 10.1007/s11481-014-9549-x. [DOI] [PubMed] [Google Scholar]
- Simpson J., Pálvölgyi A., Antoni F.A. Direct stimulation of adenylyl cyclase 9 by the fungicide imidazole miconazole. N. Schmied. Arch. Pharmacol. 2019;392:497–504. doi: 10.1007/s00210-018-01610-1. [DOI] [PubMed] [Google Scholar]
- Simpson R.J., Bosslau T.K., Weyh C., Niemiro G.M., Batatinha H., Smith K.A., Kruger K. Exercise and adrenergic regulation of immunity. Brain Behav. Immun. 2021;97:303–318. doi: 10.1016/j.bbi.2021.07.010. [DOI] [PubMed] [Google Scholar]
- Siproudhis L., Jones D., Shing R.N., Walker D., Scholefield J.H. Libertas: rationale and study design of a multicentre, Phase II, double-blind, randomised, placebo-controlled investigation to evaluate the efficacy, safety and tolerability of locally applied NRL001 in patients with faecal incontinence. Colorectal Dis. 2014;16(Suppl. 1):59–66. doi: 10.1111/codi.12546. [DOI] [PubMed] [Google Scholar]
- Small K.M., Schwarb M.R., Glinka C., Theiss C.T., Brown K.M., Seman C.A., Liggett S.B. Alpha2A- and alpha2C-adrenergic receptors form homo- and heterodimers: the heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry (Mosc.) 2006;45:4760–4767. doi: 10.1021/bi052074z. [DOI] [PubMed] [Google Scholar]
- Staedtke V., Bai R.Y., Kim K., Darvas M., Davila M.L., Riggins G.J., Rothman P.B., Papadopoulos N., Kinzler K.W., Vogelstein B., Zhou S. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564:273–277. doi: 10.1038/s41586-018-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R.H., Härle P. Sympathetic neurotransmitters in joint inflammation. Rheum. Dis. Clin. N. Am. 2005;31:43–59. doi: 10.1016/j.rdc.2004.09.003. viii. [DOI] [PubMed] [Google Scholar]
- Strosberg A.D. Structure, function, and regulation of adrenergic receptors. Protein Sci. : a publication of the Protein Society. 1993;2:1198–1209. doi: 10.1002/pro.5560020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Li L., Xie R., Wang B., Jiang K., Cao H. Stress triggers flare of inflammatory bowel disease in children and adults. Frontiers in Pediatrics. 2019;7 doi: 10.3389/fped.2019.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Hou D., Liu S., Fu W., Wang J., Liang Z. Norepinephrine inhibits the cytotoxicity of NK92-MI cells via the β2-adrenoceptor/cAMP/PKA/p-CREB signaling pathway. Mol. Med. Rep. 2018;17:8530–8535. doi: 10.3892/mmr.2018.8872. [DOI] [PubMed] [Google Scholar]
- Takahashi H.K., Morichika T., Iwagaki H., Yoshino T., Tamura R., Saito S., Mori S., Akagi T., Tanaka N., Nishibori M. Effect of beta 2-adrenergic receptor stimulation on interleukin-18-induced intercellular adhesion molecule-1 expression and cytokine production. J. Pharmacol. Exp. Therapeut. 2003;304:634–642. doi: 10.1124/jpet.102.042622. [DOI] [PubMed] [Google Scholar]
- Takenaka M.C., Araujo L.P., Maricato J.T., Nascimento V.M., Guereschi M.G., Rezende R.M., Quintana F.J., Basso A.S. Norepinephrine controls effector T cell differentiation through β2-adrenergic receptor–mediated inhibition of NF-κB and AP-1 in dendritic cells. J. Immunol. 2016;196:637–644. doi: 10.4049/jimmunol.1501206. [DOI] [PubMed] [Google Scholar]
- Tanner M.A., Maitz C.A., Grisanti L.A. Immune cell beta2-adrenergic receptors contribute to the development of heart failure. Am. J. Physiol. 2021;321:H633–H649. doi: 10.1152/ajpheart.00243.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C.P., Lin F.C., Lee C.T. Beta2-adrenergic agonist use and the risk of multiple sclerosis: a total population-based case-control study. Mult. Scler. 2014;20:1593–1601. doi: 10.1177/1352458514528758. [DOI] [PubMed] [Google Scholar]
- Valles S.L., Benlloch M., Rodriguez M.L., Mena S., Pellicer J.A., Asensi M., Obrador E., Estrela J.M. Stress hormones promote growth of B16-F10 melanoma metastases: an interleukin 6- and glutathione-dependent mechanism. J. Transl. Med. 2013;11:72. doi: 10.1186/1479-5876-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle B., Revillion F., Lefebvre J. Functional beta-adrenergic receptors in breast cancer cells. J. Cancer Res. Clin. Oncol. 1990;116:303–306. doi: 10.1007/BF01612908. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar N. Can beta-adrenergic blockers be used in the treatment of COVID-19? Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle M., Greulich T., Baerwald C., Häntzschel H., Kaufmann A. Influence of catecholamines on cytokine production and expression of adhesion molecules of human neutrophils in vitro. Immunobiology. 2005;210:43–52. doi: 10.1016/j.imbio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wahle M., Krause A., Pierer M., Hantzschel H., Baerwald C.G. Immunopathogenesis of rheumatic diseases in the context of neuroendocrine interactions. Ann. N. Y. Acad. Sci. 2002;966:355–364. doi: 10.1111/j.1749-6632.2002.tb04235.x. [DOI] [PubMed] [Google Scholar]
- Wakade A.R., Wakade T.D. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983;10:973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]
- Wang W.C., Mihlbachler K.A., Brunnett A.C., Liggett S.B. Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway beta2-adrenergic receptor physiologic signaling. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15007–15012. doi: 10.1073/pnas.0906034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari K., Nakaya M., Kurose H. Multiple functions of G protein-coupled receptor kinases. J. Mol. Signal. 2014;9:1. doi: 10.1186/1750-2187-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieduwild E., Girard-Madoux M.J., Quatrini L., Laprie C., Chasson L., Rossignol R., Bernat C., Guia S., Ugolini S. beta2-adrenergic signals downregulate the innate immune response and reduce host resistance to viral infection. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemze R.A., Welting O., van Hamersveld P., Verseijden C., Nijhuis L.E., Hilbers F.W., Meijer S.L., Heesters B.A., Folgering J.H.A., Darwinkel H., Blancou P., Vervoordeldonk M.J., Seppen J., Heinsbroek S.E.M., de Jonge W.J. Loss of intestinal sympathetic innervation elicits an innate immune driven colitis. Molecular medicine (Cambridge, Mass. 2019;25:1. doi: 10.1186/s10020-018-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Chen J., Song S., Yuan P., Liu L., Zhang Y., Zhou A., Chang Y., Zhang L., Wei W. β2-adrenoceptor signaling reduction in dendritic cells is involved in the inflammatory response in adjuvant-induced arthritic rats. Sci. Rep. 2016;6:24548. doi: 10.1038/srep24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Tai Y., Hu S., Zhang M., Wang R., Zhou W., Tao J., Han Y., Wang Q., Wei W. Bidirectional role of β2-adrenergic receptor in autoimmune diseases. Front. Pharmacol. 2018;9:1313. doi: 10.3389/fphar.2018.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman R.J., Pohorecky L.A., Baliga B.S. Adrenocortical control of the biosynthesis of epinephrine and proteins in the adrenal medulla. Pharmacol. Rev. 1972;24:411–426. [PubMed] [Google Scholar]
- Xu S., Shen X., Liu S., Yang J., Wang X. Efficacy and safety of norepinephrine versus phenylephrine for the management of maternal hypotension during cesarean delivery with spinal anesthesia: a systematic review and meta-analysis. Medicine (Baltim.) 2019;98 doi: 10.1097/MD.0000000000014331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Wu Y., Wang L., Bai Y., Du Y., Li Y., Cao N., Zhao Y., Zhang Y., Liu H. Autoantibody against β(1)-adrenoceptor promotes the differentiation of natural regulatory T cells from activated CD4(+) T cells by up-regulating AMPK-mediated fatty acid oxidation. Cell Death Dis. 2019;10:158. doi: 10.1038/s41419-018-1209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y., Matsumoto M., Togashi H. Adrenoceptor-mediated enhancement of interleukin-33 production by dendritic cells. Brain Behav. Immun. 2011;25:1427–1433. doi: 10.1016/j.bbi.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Yang P., Tian H., Zou Y.R., Chambon P., Ichinose H., Honig G., Diamond B., Kim S.J. Epinephrine production in Th17 cells and experimental autoimmune encephalitis. Front. Immunol. 2021;12:616583. doi: 10.3389/fimmu.2021.616583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Cao Q., Mehra R., Laxman B., Yu J., Tomlins S.A., Creighton C.J., Dhanasekaran S.M., Shen R., Chen G., Morris D.S., Marquez V.E., Shah R.B., Ghosh D., Varambally S., Chinnaiyan A.M. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Yuki K. The immunomodulatory mechanism of dexmedetomidine. Int. Immunopharm. 2021;97 doi: 10.1016/j.intimp.2021.107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffaroni M., Marino F., Bombelli R., Rasini E., Monti M., Ferrari M., Ghezzi A., Comi G., Lecchini S., Cosentino M. Therapy with interferon-β modulates endogenous catecholamines in lymphocytes of patients with multiple sclerosis. Exp. Neurol. 2008;214:315–321. doi: 10.1016/j.expneurol.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Zhang D., Ma Q., Wang Z., Zhang M., Guo K., Wang F., Wu E. β2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol. Cancer. 2011;10:146. doi: 10.1186/1476-4598-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ma Q.Y., Hu H.T., Zhang M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol. Ther. 2010;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- Zhou C., Chen X., Zeng W., Peng C., Huang G., Li X., Ouyang Z., Luo Y., Xu X., Xu B., Wang W., He R., Zhang X., Zhang L., Liu J., Knepper T.C., He Y., McLeod H.L. Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget. 2016;7:68314–68327. doi: 10.18632/oncotarget.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukos Y., Kidd D., Woodroofe M.N., Kendall B.E., Thompson A.J., Cuzner M.L. Increased expression of high affinity IL-2 receptors and beta-adrenoceptors on peripheral blood mononuclear cells is associated with clinical and MRI activity in multiple sclerosis. Brain : J. Neurol. 1994;117(Pt 2):307–315. doi: 10.1093/brain/117.2.307. [DOI] [PubMed] [Google Scholar]
- Zoukos Y., Leonard J.P., Thomaides T., Thompson A.J., Cuzner M.L. beta-Adrenergic receptor density and function of peripheral blood mononuclear cells are increased in multiple sclerosis: a regulatory role for cortisol and interleukin-1. Ann. Neurol. 1992;31:657–662. doi: 10.1002/ana.410310614. [DOI] [PubMed] [Google Scholar]