Abstract
Eosinophils have multiple relevant biological functions, including the maintenance of homeostasis, host defense against infectious agents, innate immunity activities, immune regulation through Th1/Th2 balance, anti-inflammatory, and anti-tumorigenic effects. Eosinophils also have a main role in tissue damage through eosinophil-derived cytotoxic mediators that are involved in eosinophilic inflammation, as documented in Th2-high asthma and other eosinophilic-associated inflammatory conditions.
Recent evidence shows that these multiple and apparently conflicting functions may be attributed to the existence of different eosinophil subtypes (i.e.: tissue resident and inducible eosinophils). Therapeutic intervention with biological agents that totally deplete tissues and circulating eosinophils or, vice versa, maintain a minimal proportion of eosinophils, particularly the tissue-resident ones, could therefore have a very different impact on patients, especially when considering the administration of these therapies for prolonged time. In addition, the characterization of the predominant pathway underlying eosinophilic inflammation by surrogate biomarkers (circulating eosinophils, organ-specific eosinophils levels such as eosinophil count in sputum, bronchoalveolar lavage, tissue biopsy; total circulating IgE levels, or the use of FeNO) in the single patient with an eosinophilic-associated inflammatory condition could help in choosing the treatment.
These observations are crucial in light of the increasing therapeutic armamentarium effective in modulating eosinophilic inflammation through the inhibition in different, yet complementary ways of eosinophil pathways, such as the interleukin-5 one (with mepolizumab, benralizumab, reslizumab) or the interleukin-4/13 one (with dupilumab and lebrikizumab), in severe T2-high asthma as well as in other systemic eosinophilic associated diseases, such as eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome.
Keywords: Eosinophils, Homeostasis, Inflammation, Asthma, Type 2 phenotype, Eosinophilic granulomatosis with polyangiitis, Hypereosinophilic syndrome, Mepolizumab, Benralizumab, Reslizumab, Dupilumab
Graphical abstract
Highlights
-
•
Recent evidence pointed out the existence of different eosinophil subtypes, i.e. tissue resident and inducible eosinophils, with different and apparently conflicting functions.
-
•
Biological therapies with different mechanisms can deplete completely tissues and circulating eosinophils or maintain a minimal proportion of eosinophils, particularly the tissue-resident ones, and this could therefore have a different impact on patients, especially when considering the administration of these therapies for prolonged time.
-
•
The identification of the predominant pathway underlying eosinophilic inflammation by surrogate biomarkers (circulating eosinophils, organ-specific eosinophils levels such as eosinophil count in sputum, bronchoalveolar lavage, tissue biopsy; total circulating IgE levels, or the use of FeNO) should be sought in the single patient with an eosinophilic-associated inflammatory condition.
-
•
These considerations may help in choosing the best anti-eosinophilic treatment, considering the increasing therapeutic armamentarium effective in modulating eosinophilic inflammation through the inhibition of the interleukin-5 one (with mepolizumab, benralizumab, reslizumab) or the interleukin-4/13 one (with dupilumab and lebrikizumab)
1. Background
In 1879 Paul Ehrlich identified blood eosinophils in several mammals (rabbits, dogs, and humans) by eosin staining (Kay, 2015; Lombardi, 1996). After this first observation, several studies have reported on the multiple biological functions of eosinophils. Indeed, the intrinsic roles of eosinophils are much more complex, including the maintenance of homeostasis, host defense against infectious agents, innate immunity activities, immune regulation through Th1/Th2 balance, anti-inflammatory, and anti-tumorigenic effects (Kanda et al., 2021). Moreover, eosinophils have a main role in tissue damage through eosinophil-derived cytotoxic mediators that are involved in eosinophilic inflammation, as documented in Th2-high asthma and other eosinophilic-associated diseases (Choi et al., 2020; Januskevicius et al., 2020). Recently, it has been hypothesized that these multiple and apparently conflicting effects may be attributed to the existence of different eosinophil subtypes (i.e.: tissue resident and inducible eosinophils) (Kanda et al., 2021; Mesnil et al., 2016). These observations are crucial since we have now treatments very effective in modulating eosinophilic inflammation in different fashions through the inhibition of interleukin(IL)-5 activity (i.e. mepolizumab, benralizumab, reslizumab) or targeting IL4/13 activity (dupilumab, lebrikizumab) (Riggioni et al., 2020; Bagnasco et al., 2017). These eosinophil-targeted therapeutic strategies may impact on Th2-infilammation with different, yet complementary mechanisms, potentially with different application within the therapeutic armamentarium of eosinophilic-associated disorders, as Th2-asthma, as well as in other systemic eosinophilic associated diseases, such as eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome.
In this review, we discuss the recent literature evidence on the role of eosinophil in health and diseases. We also discussed about the potential impact of recent biological anti-IL-5 and IL4/13 therapies on these different eosinophil subtypes.
2. Biology of eosinophils
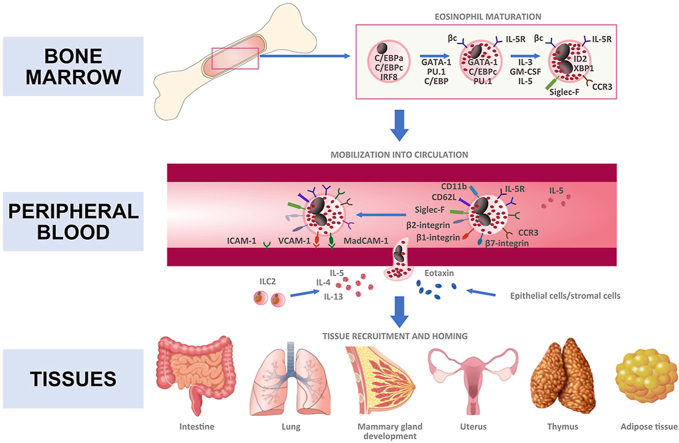
Eosinophils are terminally differentiated leukocytes approximately accounting for 1–5% of peripheral blood leukocytes in healthy individuals (Kim and Jung, 2020; Jung and Rothenberg, 2014; Jung, 2015). Eosinophils are produced in the bone marrow and thus they migrate into the peripheral circulation as mature cells, identified as Siglec-F+ CCR3+ F4/80+ CD62L+. Here they have a relatively short half-life, approximately 18 h (Fig. 1). They migrate to the peripheral tissues under homeostatic conditions, or to inflammatory sites in response to recruitment signals, primarily IL-5 and eotaxin-1 (CCL11) (Rothenberg and Hogan, 2006; Rosenberg et al., 2013). GATA-1 is the most important transcription factor for eosinophil maturation, as observed in mice with targeted deletion of the high-affinity GATA-1 binding site in the Gata1 promoter (Yu et al., 2002). Further eosinophils evolutionary states (differentiation and proliferation) are also regulated by IL-5, IL-3 and GM-CSF (Hara and Miyajima, 1996).
Fig. 1.
Development of eosinophils expressing various types of functional cell surface molecules: from bone marrow to tissues.
IL-5 is the most specific cytokine released for inducing the selective differentiation and mobilization of eosinophils from the bone marrow during inflammation, as well as for the eosinophil homing into various tissues in the steady state (Mould et al., 1997). During allergic inflammation, in addition to Th2 cells group 2, innate lymphoid cells (ILC2s) are a major non-T cell source of IL-5 (Klose and Artis, 2016). Proliferation and tissue accumulation of eosinophils is modulated by a complex interaction between pro-survival signaling factors (GM-CSF, IL-3, IL-5) and inhibitory receptor signaling (Kim and Jung, 2020). Notably, the tissue distribution of eosinophils may be IL-5 independent, as demonstrated by the observation of residual tissue eosinophils in IL-5-deficient mice (Mould et al., 1997). Furthermore, eotaxin promotes selective recruitment of eosinophils into eotaxin-expressing tissues cooperatively with IL-5 via an IL-5-independent manner (Weller and Spencer, 2017). Beside IL-5 and eotaxin, eosinophil trafficking into inflamed tissues also involves IL-4 and IL-13 (with IL-5, Th2 cytokine pattern), and a number of cytokines, chemokines, and adhesion molecules (i.e., α4β7 and α4β1 integrin) that contribute in different ways to eosinophil homing (Fig. 1).
2.1. Eosinophils as contributors to tissue homeostasis and regulators of host defence
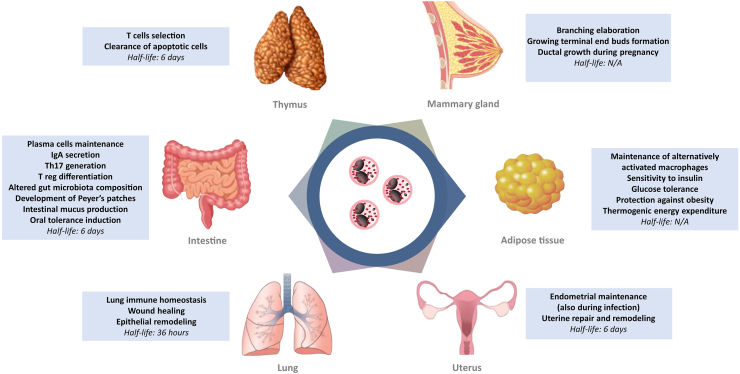
Eosinophils have a wide range of pleotropic activities including protective immunity and anti-microbic activity, but are also responsible of several physiological responses, such as organ development and metabolism. Although eosinophils are normally considered circulating blood cells, a fraction of eosinophils reside stably in various tissues (Fig. 2). In particular, eosinophils have been shown to be present in the thymus, adipose tissue, gastrointestinal (GI) tract, lungs and female reproductive system during homeostatic conditions (Kim and Jung, 2020). The GI tract harbors the largest number of tissue-resident eosinophils in the body (about 20%–30% of the total number of resident leukocytes), whereas eosinophils in the adipose tissue and the lung constitute only ≤4% of the stromal/vascular fraction and ≤1% of total leukocytes, respectively (Wu et al., 2011; Mesnil et al., 2016). Tissue-resident eosinophils also can be found in the mammary gland and uterus tissues in homeostatic and various inflamed tissues. Tissue-specific microenvironmental signals have significant roles in the phenotypic and functional properties of eosinophils.
Fig. 2.
Schematic overview of the homeostatic function of eosinophils in the individual organ tissues.
In some of these organs, a reference value to establish an excess of eosinophils have been establish in humans. For instance, eosinophils ≥2% of cells in sputum as well as ≥3% in bronchoalveolar lavage are usually considered pathologic. For gastrointestinal tract, for eosinophilic esophagitis ≥15 eosinophils per high-power microscopy field (HPF) are required, while the cut off may change for colitis from >50/HPF in the right colon, >35/HPF in the transverse colon and >25 per HPF in the left colon (Impellizzeria et al., 2019).
Tissue-resident eosinophils are primarily found in several mucosal sites where they contribute to various homeostatic and tissue-protective functions (Shah et al., 2020). Eosinophils play a beneficial role in regulating and modulating immune responses. Indeed, it has become clear that eosinophils also play important non-inflammatory activities in the generation and maintenance of adaptive immune responses. Eosinophils, being a major source of the plasma cell survival factor APRIL (activation and proliferation-induced ligand), are essential not only for the long-term survival of plasma cells in the bone marrow, but also for the maintenance of these cells in the lamina propria underling the gut epithelium (Berek, 2015). Furthermore, data suggest that eosinophils are required for tissue integrity and are necessary for tissue remodeling (Lee et al., 2010). It has been demonstrated also the role of these cells in the generation of IgA expressing B cells, and thus in the development of the IgA plasma cells which reside in the lamina propria and contribute to immune defense at mucosal surfaces (Chu et al., 2014). Innate immune activities of eosinophils include expression of pattern recognition receptors, such as Toll-like receptors 1–5, 7, and 9, nucleotide oligomerization domains 1 and 2, Dectin-1, and receptor for advanced glycation end products, which recognize pathogen-associated molecular patterns or danger-associated molecular patterns (Jacobsen et al., 2012). In recent years, eosinophils have also been recognized as having numerous immuno-modulatory functions toward B cell linage maturation or maintenance: a) in T cell-dependent immune responses, eosinophils enhance early B cell activation; b) eosinophils and megakaryocytes are required for efficient homing of plasma cells to bone marrow; c) eosinophils are the main source of plasma cell survival factors; and d) eosinophils are required for the maintenance of plasma cells in the lamina propria (Berek, 2015).
It is also well known that eosinophils have anti-microbial activity. In particular, eosinophil numbers are often elevated in the course of parasitic infections and represent important effector cells in helminth infections (Simon et al., 2020). Furthermore, recent studies demonstrating anti-bacterial activities for eosinophils in the context of eosinophil extracellular traps (EET) formation, and less recently it has already been shown that MBP and ECP have bactericidal properties of MBP and ECP in vitro. Data in vivo seem to support a direct anti-bacterial role of eosinophils, as the adoptive transfer of eosinophils has been found to be sufficient to protect against bacterial septic shock (Lee et al., 2010; Yousefi et al., 2008). Eosinophils may also exhibit anti-viral properties. Granule protein eosinophil-derived neurotoxin (EDN) has been shown to have anti-viral activity in vitro (Rugeles et al., 2003). In experimental models, hypereosinophilic mice clear respiratory syncytial virus more effectively than wild-type mice (Phipps et al., 2007) and can prevent infection with the natural rodent pathogen pneumonia virus of mice (Percopo et al., 2014). In guinea pigs, allergen-induced eosinophilia has been associated with a decreased viral load during parainfluenza virus infection (Adamko et al., 1999). Both human and murine eosinophils produce NO via inducible NO synthase, which can have direct antiviral effects on parainfluenza virus and RSV (Drake et al., 2016). Although the role of eosinophils in COVID-19 has not been elucidated yet, it has been shown that SARS-CoV2 infection can be associated with profound eosinopenia and that persistent eosinopenia may be associated with clinical deterioration and increased risk of mortality (Roca et al., 2020).
2.2. Eosinophils in inflammatory processes
Eosinophils can regulate local immune and inflammatory responses, and their accumulation in the blood and tissue is associated with several allergic, rheumatologic, infectious, neoplastic, and rare idiopathic disorders. Although eosinophils can contribute to tissue homeostasis in steady-state conditions, many studies have trended toward focusing on the contribution of eosinophils in the pathogenesis of eosinophil-associated diseases. Indeed, eosinophils may exert their biological effects via cytotoxic mediators such as type 2 cytokines (IL-4, IL-5, IL-9, IL-13, and IL-25), type 1 cytokines (IL-12, IFN-γ), acute proinflammatory cytokines (TNF-a, IL-1b, IL-6, and IL-8), chemokines, and lipid mediators (PAF and LTC4) (Table 1) (Kanda et al., 2021). A significant association was established between eosinophils and a number of disease conditions characterized by an inflammatory state (Lombardi and Passalacqua, 2003). Activation of eosinophils and release of proinflammatory lipid mediators, cytokines, free oxygen radicals, highly charged cationic proteins contribute to the onset and maintenance of tissue inflammation. Furthermore, eosinophil accumulation in blood and tissues has been related to a defect in their apoptotic death (Shen and Malter, 2015).
Table 1.
Mediators of eosinophils.
| CLASS OF MEDIATORS | EXAMPLES |
|---|---|
| Granule-associated proteins | Major Basic Protein (MBP), Eosinophil Peroxidase(EPX), Eosinophil Cationic Protein (ECP), Eosinophil-Derived Neurotoxin(EDN), Charcot-Leyden Crystal (CLC) protein |
| Cytokines | IL-1β, IL-1Rα, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-16, IL-17, IFN-γ, GM-CSF, TGF-β, TNF-α |
| Chemokines | CCL-1/eotaxin-1, CCL-1/MCP-4, CCL2, CCL3, CCL5, CCL7, CCL8, CCL11, CCL13, CXCL1, CXCL10, CXCL12 |
| Growth factors | Vascular Endothelial Growth Factor (VEGF), Platelet-Derived-Growth Factor (PDGF), Proliferation-Inducing Ligand (APRIL), Epidermal Growth Factor (EGF), Stem Cell Factor (SCF) |
| Neuromediators | Substance P, Vasoactive Intestinal Peptide (VIP), Nerve Growth Factor (NGF) |
| Lipid mediators | Leukotrienes (LTD4-LTE4), Prostaglandines (PGE1,PGE2) |
| Enzymes | Matrix metallopeptidase-9 (MMP-9), acid phosphatase, collagenase, histaminase, phospholipase D, catalase, arylsulphatase B |
| Adhesion molecules | β1-integrin, β2-integrin, CD62L, CD49f, CD49d, CD11a, CD11b, CD11c |
Nowadays the scientific community is changing perspective, recognizing the increasingly paramount role of eosinophils in the pathogenesis of non-allergic inflammatory diseases, beside the well-known importance in the pathogenesis of the allergic ones. Indeed, eosinophilic inflammation can involve locomotor, urinary, cardiovascular, nervous, gastrointestinal systems and other mucosal surfaces, such inflammation also can accompany tissue trauma, foreign-body reactions, and necrotic or granulomatous processes (Gonlugur and Gonlugur, 2006).
Eosinophils may also play a role in tissue repair and regeneration; for example, muscle damage promotes rapid recruitment of eosinophils in the inflammatory foci and eosinophils play a key role in muscle regeneration during muscle injury as a major source of IL-4. IL-4 produced by eosinophils activates muscle resident fibrocyte-adipocyte progenitors (FAPs), which induce regeneration of injured muscles (Aoki et al., 2021). Eosinophils can induce angiogenesis by the production of pre-formed pro-angiogenic mediators, among others the vascular endothelial growth factor (VEGF). Eosinophil-derived IL-4 is also required for liver regeneration. Eosinophil-derived IL-4 induces the proliferation of quiescent hepatocytes and regulates the regeneration of the liver (Li and Hua, 2017). Furthermore, eosinophils confer protection following myocardial infarction (Lavine, 2020). Thus, eosinophils play a profound role in tissue repair and regeneration in various organs. The study of various types of eosinophilic inflammation may increase our understanding of the biological responses of eosinophil leukocytes to different inflammatory stimuli and recent research suggests that eosinophils may have additional roles in these settings that are related to control and resolution of inflammation (Strandmark et al., 2016).
2.3. Eosinophils subpopulations in health and diseases
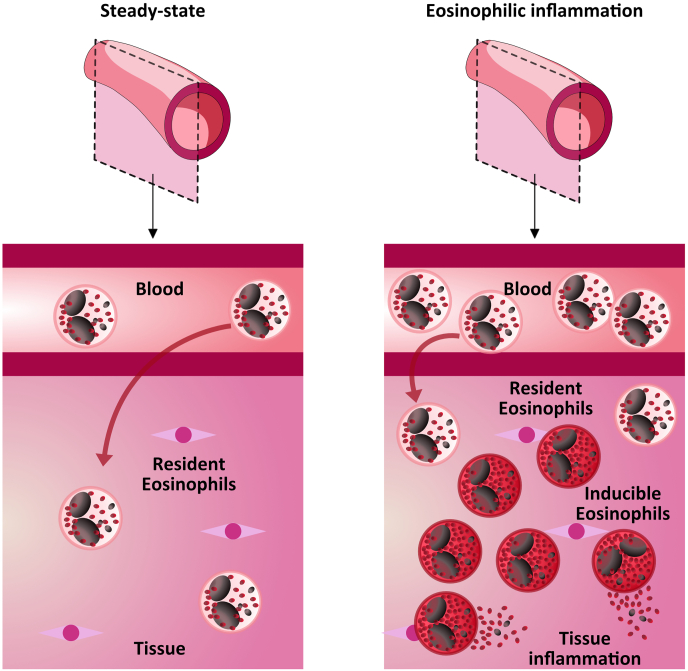
Eosinophils are a heterogeneous cell population with different functional characteristics depending on the site of residence. The function exerted is likely controlled by the local milieu, that can induce the upregulation of pro- or anti-inflammatory eosinophils or the conversion of inflammatory eosinophils into anti-inflammatory ones or vice versa (Yang et al., 2017). It would be worthwhile to phenotype different eosinophil subpopulations by membrane surface markers, in order to distinguish homeostatic versus inflammatory eosinophils. A functional distinction has been drawn between eosinophils recruited from circulation in response to tissue pathology (e.g. allergies or parasitic infection) and those that maintain residence and are present in tissues at homeostasis (Fig. 3). Like T and B cells, ILC, and dendritic cells, a new classification system that defines eosinophil subpopulations on the basis of surface antigen expression, tissue localization, content, and function, has been presented for mouse eosinophils: eosinophilic precursors, steady states eosinophils, type 1 and type 2 eosinophils (Simon et al., 2020; Abdala-Valencia et al., 2018). Eosinophil Progenitors are immature eosinophils or committed precursors undergoing in situ hematopoiesis; steady state eosinophils are true resident eosinophils in quiescent tissues, with nonsegmented “donut-shape” nuclear morphology and eosin staining. Type 1 eosinophils are found in interstitium or stroma in “transient” morphogenetic contexts and during Type 1 immune activation, featuring segmented nuclear morphology but lacking vacuolarization. Type 2 eosinophils are found within the epithelium and during Type 2 immune inflammation, characterized by highly segmented nuclei and the presence of vacuoles (Abdala-Valencia et al., 2018). Phenotypically, type 1 eosinophils are CD101low, CD62L, Siglec-Fmed, while type 2 eosinophils have higher CD101 and Siglec-F, while lacking expression of CD62. In addition, mouse eosinophils exhibited a proinflammatory gene expression signature that was lost upon initiation of the repair phase (Reichman et al., 2017).
Fig. 3.
Eosinophilic subpopulations. Left panel: steady state with resident eosinophils (rEOSs); Right panel: eosinophilic pathological condition with inducible eosinophils (iEOSs) tissue accumulation” (modified from Kanda et al. (Kanda et al., 2021)).
In humans, the intestinal mucosa of subjects with eosinophil esophagitis included both CD25 positive and negative eosinophils (Straumann et al., 2005). In eosinophilic skin diseases, populations of eosinophils expressing unique subsets of cytokines were evaluated (Roth et al., 2011). Classically, they have been divided into 2 different types: normodense and hypodense eosinophils, which are indicated by normal density and lower density, respectively (Prin et al., 1983). In asthmatic patients, an increased number of hypodense eosinophils in the peripheral blood was correlated with clinical severity and airway hyperresponsiveness, and inhaled corticosteroids significantly decreased hypodense eosinophils (Kuo et al., 1994). Furthermore, increased number of hypodense eosinophils was observed in bronchoalveolar lavage eosinophils following an antigen-challenge (Kroegel et al., 1994). In patients with atopic dermatitis and peripheral eosinophilia, a correlation between hypodense eosinophil levels and disease severity has also been demonstrated (Miyasato et al., 1996).
More recently, it has also been shown that eosinophils can be phenotypically distinguished into subtypes in mice lungs. Indeed, Mesnil et al. found 2 phenotypically distinct subtypes of eosinophils in the lung tissue under steady-state and house dust mites-induced allergic inflammation: resident eosinophils (rEos; Siglec-Fint CD62L + CD101low), which are IL-5 independent parenchymal cells with a ring-shaped nucleus, and inducible eosinophils (iEos; Siglec-Fhigh CD62L- CD101high), which are IL-5 dependent peribronchial cells with a segmented nucleus (Kanda et al., 2021; Mesnil et al., 2016). Lung rEos were located in the parenchyma, which contrasts with the localization of eosinophils in asthma, in which eosinophils are classically within the peribronchial area (Barnes, 2008). In particular, it was observed that rEos expressed genes implicated in the negative regulation of immune responses (e.g., Anxa1, Nedd4, Runx3, Serpinb1a, and Ldlr) in contrast to iEos that highly express several proinflammatory genes (e.g., Slc3a2, Tlr4, C3ar1, Il13ra1, and Il6) (Mesnil et al., 2016). Kanda et al. have also shown the presence of phenotypically distinct rEos and iEos in the lungs from naïve and OVA-induced allergic asthma mice, and these rEos and iEos were characterized by normodense and hypodense eosinophils, respectively (Kanda et al., 2021). However, a recent study analyzing gene expression of human or murine eosinophils at steady-state conditions in vivo and after stimulation in vitro, showed that restriction of IL5 availability did not elicit any detectable transcriptional response in steady-state residual eosinophils, and influenced only a few genes in their response to in vitro stimulation. Ultimately, this seems to suggest that targeting the IL5 pathways spares a pool of circulating residual eosinophils largely resembling those of healthy individuals (Von Hulst, 2021).
In contrast with mice, rEos and iEos has not been fully characterized in human tissue. In humans, the modulation of surface expression levels in a large variety of receptors on eosinophils depends on environmental factors. The upregulation of several surface receptors reflecting activation markers (i.e., CD11b, CD11c, CD13, CD18, CD25, CD29, CD58, CD63, CD66e, CD67, CD108, CD123, HLA-DR, and TSLPR) was observed on eosinophils in the BAL and/or induced sputum of patients with allergic disease, which leads to increased inflammation (Johansson, 2014; Metcalfe et al., 2016). Furthermore, activated eosinophils from the nasal polyps of patients with eosinophilic chronic rhinosinusitis with polyposis expressed CD69 whereas eosinophils from the peripheral blood of the same patients did not (Yun et al., 2020). There is experimental and clinical evidence that only activated eosinophils can express MHC class II (Hansel et al., 1991; Duez et al., 2004). Moreover, Januskevicius et al. found out that eosinophil subtypes differ in their adhesive properties and survivability (Januskevicius et al., 2020). Finally, in contrast with iEos, human rEos do not evoke inflammatory reactions or tissue damage (Straumann et al., 2005).
Altoghether, rEos and iEos appear to be functionally different for multiple reasons: 1) lung rEos are parenchimal Siglec-Fint CD125int cells with a ring-shaped nucleus; 2) lung rEos are not affected by allergic inflammation and differ from iEOS; 3) lung rEos and iEos have distinct gene expression profiles; 4) expression of CD62L and CD101 discriminates between mouse rEos and iEos; 5) rEos are independent of IL-5 for their presence in the lung; 6) rEos, but not iEos, can inhibit the development of Th2 responses to house dust mites and the pro-Th2 potential of dedritic cells; and 6) lung parenchymal eosinophils exist in humans and are phenotypically distinct from iEos found in asthmatic airways.
3. Eosinophilic-associated inflammatory conditions
The causes of eosinophilia are multiple. It is essential to systematically approach patients who present with unexplained eosinophilia, because the only a correct and timely diagnosis lead to a potentially resolutive treatment. Regarding the common causes of blood eosinophilia, there are significant geographical differences, with reported parasitic infections in tropical settings and allergic diseases or drug hypersensitivity reactions in more developed countries (Lombardi, 1996; Lombardi and Passalacqua, 2003). Many other hematologic (primary) and non-hematologic (reactive or secondary) conditions associated with eosinophilia have been described (Table 2). Only after an appropriate diagnostic work up to rule out of the more common causes, the diagnosis of an eosinophilic-associated inflammatory condition should be made.
Table 2.
Differential diagnosis of peripheral eosinophilia.
| CLASS | CONDITIONS |
|---|---|
| INFECTIONS |
|
| |
| |
| |
| DRUG HYPERSENSITIVITY |
|
| |
| |
| |
| |
| HEMATOLOGIC/NEOPLASTIC DISORDERS |
|
| |
| |
| IMMUNE DYSREGULATIONS |
|
| |
| |
| |
| |
| |
| COMMON ALLERGIC DISORDERS |
|
| |
| OTHERS |
Eosinophilic-associated inflammatory conditions are a heterogeneous and often overlapping group of different diseases accumunated by high tissue infiltrating and/or circulating eosinophils, potentially affecting different organs, such as upper and lower respiratory tract or digestive system, without a known cause (Table 3).
Table 3.
Eosinophilic-associated inflammatory conditions.
| Systemic eosinophilic-associated conditions | Organ-limited eosinophilic-associated conditions |
|---|---|
| Eosinophilic granulomatosis with polyangiitis (EGPA) | Eosinophilic Asthma |
| Hypereosinophilic syndrome (HES) | Chronic Rhinosinusitis (CRS)±Nasal Polyps (CRSwNP) |
| Other systemic eosinophilic-associated conditions | Eosinophilic Esophagitis/Gastroenteritis/colitis |
| Gleich syndrome | Eosinophilic Pneumonia |
| Kimura Disease | Eosinophilic Cystitis |
| Others | Eosinophilic Fasciitis |
3.1. Eosinophilic type 2 asthma
At least three major determinants have been identified in asthma: allergy (allergic vs non-allergic asthma), age of onset (early versus late onset) and prevalent type of airway inflammation (T2-high or T2-low, eosinophilic/neutrophilic/paucigranulocytic/mixed).
These features combined themselves to form clinically overlapping phenotypes. The phenotype of severe type 2 asthma is characterized by Th2 inflammation and activation of innate lymphoid cells-2 (ILC-2). The identification of T2-high patients is facilitated by the use of several non-invasive biomarkers, as elevated peripheral blood eosinophil count and elevated circulating IgE, which are used in clinical practice because of their diffuse availability, easy interpretation and reproducibility (Lim and Nair, 2018). The measurement of eosinophils in induced sputum is an extensively studied validated biomarker, but it is investigative, not available to all centers and it is more expensive. However, it is important to remember that blood eosinophil count is subject to wide variability over time and may not always accurately reflect the cellular pattern of the airways in asthmatics (Petsky et al., 2018). Measurement of fractional exhaled nitric oxide (FENO) is a noninvasive, safe, and simple method of quantifying airway inflammation, that reflects eosinophilic inflammation of the airways. Of note, inducible nitric oxide synthase (iNOS) is induced by a variable range of cytokines and mediators, but more specifically by IL-13 (Locksley, 2010; Amelink et al., 2013; de Groot et al., 2016).
The pathobiology of T2-high early-onset allergic asthma has been well established in animal and human studies. In patients who are genetically predisposed to an allergic immune response, the inhalation of an environmental stimuli such as the aeroallergen triggers the epithelial cells to release cytokines (IL-25, IL-33, thymic stromal lymphopoietin) and initiate a series of downstream events that differentiate naïve T cells into mature Th2 lymphocytes, that together with ILC-2, are capable of producing the classic Th2 cytokine pattern: IL-4, IL-5 and IL-13. The release of the Th2 cytokine IL-4 stimulates the cellular B isotype switch leading to IgE synthesis, a hallmark of allergic inflammation (Yu et al., 2014). In the event of re-exposure to allergens to which the patient is sensitized, IgE attached to mast cells and basophils leads to the release of inflammatory mediators (histamines, prostaglandins and leukotrienes). Through their effects on airway smooth muscle, these mediators are responsible for the clinical asthma syndrome that characterizes the initial response to allergen exposure. In addition, through their systemic effects on other organs and systems, inflammatory mediators can lead to the development of comorbidities, such as rhinitis and polyposis.
The late-onset variant of severe asthma is characterized by pathophysiological mechanisms different from childhood allergic asthma, typically presenting in the fourth or fifth decade of life. In this phenotype, T2-high inflammation is demonstrable by increased airway eosinophils, which in a remarkable fraction of patients tends to persist despite corticosteroid therapy. Frequent asthmatic exacerbations occur with significant dependence on oral corticosteroids. Chronic rhinosinusitis and nasal polyposis with or without sensitivity to acetylsalicylic acid (ASA) may also be present in these patients. Although late-onset eosinophilic asthma is characterized by T2-high inflammation, as in early-onset, experimental evidence suggests that “allergen-independent” signals involving ILCs producing IL-5 and IL-13 are activated (Smith et al., 2016). These observations have therefore led to the distinction in adult eosinophilic asthma between an allergic form from a non-allergic form. Some evidence has demonstrated that persistent eosinophilia in upper and lower airway mucosa contributes to asthma severity by producing various mediators including cytokines, chemokines and granule proteins. Moreover, extracellular traps released from eosinophils have been revealed to enhance type 2 inflammation in patients with severe asthma (Choi et al., 2020). Alarmin-like cytokines (IL-33 and thymic stromal lymphopoietin (TSLP), released from airway epithelium, are involved in the development of severe asthma (Li et al., 2018; Mitchell and O'Byrne, 2017). IL-33 activates the ILC to exacerbate airway inflammation but stimulates eosinophils as well (Stolarski et al., 2010). TSLP is known to be important for inducing type 2 cytokine production, leading to the activation of eosinophils (Wong et al., 2010). Persistent airway inflammation induced by eosinophils leads to constant tissue damage, resulting in smooth muscle thickening, goblet cell hyperplasia and extracellular matrix protein deposition called airway remodeling (Tagaya and Tamaoki, 2007).
3.2. Eosinophilic granulomatosis with polyangiits
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare primary systemic necrotizing vasculitis affecting the small vessels, often occurring in patients with late-onset asthma and sustained peripheral blood eosinophilia (Berti et al., 2020a). EGPA presents with upper airway tract and lung involvement in virtually all cases, including rhinosinusitis, asthma and migratory pulmonary infiltrates; peripheral neuropathy, cardiac involvement and skin lesions (Churg and Strauss, 1951; Groh et al., 2015). Anti-neutrophil cytoplasmic antibodies (ANCA) are usually directed against myeloperoxidase (MPO) are present in up to 40% of patients affected (Sinico et al., 2005).
Genetic background supports the distinction between MPO-ANCA positive and negative patients. MPO-ANCA positive EGPA is an eosinophilic autoimmune disease sharing HLA-DQ association with MPO-ANCA positive ANCA associated vasculitis (e.g. certain forms of microscopic polyangiitis and granulomatosis with polyangiitis), while ANCA-negative EGPA may instead have a mucosal/barrier dysfunction origin (Lyons et al., 2019). EGPA is characterized by a Type 2 immune response with elevation of circulating IgG4, which could be mediated by IL-10, and total IgE, mediated by IL-4 (Berti et al., 2020a). In comparison to eosinophilic asthma, elevation of eosinophils are significantly higher, usually above 1.5 × 109/L or 10% of the leukocyte fraction.
EGPA typically develops in three partially overlapping phases: a prodromal phase potentially lasting for years characterized by respiratory tract symptoms, asthma and rhinosinusitis; a second phase with blood eosinophilia, eosinophilic tissue infiltration and eosinophil-induced organ inflammation; and a third phase dominated by systemic necrotizing vasculitis (Groh et al., 2015). Interestingly, the presence of respiratory atopy the year before the diagnosis of vasculitis was more often associated with severe asthma and a greater use of oral corticosteroids (Berti et al., 2018). When the diagnosis of EGPA is established, the clinical course can range from an acute, self-limited process to progressive multiorgan dysfunction with significant morbidity and mortality (Samson et al., 2013).
Surrogates of a vasculitic process include purpura, peripheral nervous system involvement (e.g. mononeuritis multiplex), scleritis, alveolar hemorrhage and glomerulonephritis. In addition to the vasculitic manifestations, other signs and symptoms due to eosinophil-related inflammation are reported as well, including pulmonary infiltrates, pleural effusion, urticarial papules, eosinophilic tubulointerstitial nephritis and eosinophilic myocarditis (Groh et al., 2015). International criteria exists to classify the diagnosis, that needs to be applied in patients with vasculitis (Lanham criteria, ACR criteria, CHCC definition, ERS and GERM“O”P criteria, MIRRA trial criteria), summarized in (Berti et al., 2020a).
Data reported in the main series of patients highlighted EGPA clinical heterogeneity, suggesting that ANCA-test might distinguish different phenotypes, with MPO-positivity correlating with a “vasculitic” phenotype and an increased relapse rate, while the prognosis of ANCA-negative “eosinophilic” patients seems to be poorer, due to more frequent cardiomyopathy and cardiac death (Samson et al., 2013; Durel et al., 2016; Guillevin et al., 2011; Comarmond et al., 2013). Yet, this dichotomization between “vasculitic” vs. “eosinophilic” phenotypes is perhaps too simplistic, and it is likely that some disease manifestations could rather be due to both pathophysiological processes.
Recent and less recent cohort studies showed a high rate of sequelae and poor long-term outcomes after the resolution of vasculitis, regardless the treatment chosen (Samson et al., 2013; Comarmond et al., 2013). The main sequelae are chronic airway obstruction (65–85%), neurologic damage and especially peripheral neuropathy (40%), chronic rhinitis (35%) and chronic sinusitis (19%), severe lung disease (17%), chronic kidney disease (0–13%) and chronic heart failure (10%) (Rothenberg and Hogan, 2006). In particular, long-term poorly controlled asthma and persistent airflow obstruction may be present in more than 40% of EGPA, thereby leading to significant glucocorticoid-related side effects which negatively affect patient global health status and quality of life (Berti et al., 2020b).
3.3. Hypereosinophilic syndromes
Hypereosinophilic syndrome (HES) is a heterogeneous group of conditions, characterize by persistent eosinophilia that is associated with damage to multiple organs (Rosenberg et al., 2013). More specifically, HES can be defined as: hypereosinophilia (circulating eosinophil count >1.5 × 109/mL documented on at least 2 occasions) or marked tissue eosinophilia, and clinical manifestations directly attributable to the eosinophilia or presumed to be consequence of eosinophilia and for which no alternative cause can be identified (Klion, 2015a).
This definition is broad and it captures all patients with clinical manifestations due to eosinophilia regardless of the underlying etiology. The following classification categories have been proposed (Kay, 2015): myeloproliferative HES, including in FIPIL1-PDGFR-A associated eosinophilic myeloproliferative neoplasms (Lombardi, 1996); lymphocytic variant HES, in which an aberrant or clonal lymphocyte population drives eosinophilia through the production of eosinophilopoietic cytokines (Kanda et al., 2021); overlap HES restricted to a single organ (Choi et al., 2020); secondary HES in the setting of a distinct diagnosis (i.e., parasitic helminth infection, drug hypersensitivity and primary immunodeficiency) in which eosinophilia has been described in a subset of affected patients (Januskevicius et al., 2020); familial HES, a rare autosomal dominant disorder; and (Mesnil et al., 2016) idiopathic HES (Klion, 2015b). This classification system slightly differs from with the 2008 WHO guidelines, which include myeloid and lymphoid neoplasms in the same category, on the basis of a variety of genetic, histopathologic, and clinical criteria.
Regardless this etiological heterogeneity, clinical signs and symptoms gtreatly overlaps with those defined as “manifestations due to eosinophil-related inflammation” abovementioned for EGPA, thus being often difficult to distinguish between HES and EGPA. Features helping to differentiate between EGPA and HES are a lower prevalence of asthma and a higher prevalence of splenomegaly and lymph nodes enlargement in HES. In a retrospective analysis of 166 patients with blood eosinophilia >1.0 × 109/L and systemic manifestations, serum C-reactive protein levels could be a reliable biomarker in patients with eosinophilic asthma and systemic manifestations, able to distinguish between EGPA and idiopathic HES. therefore, in the absence of confounding factors (infection, venous thrombosis, malignancy, …) low C-reactive protein levels (i.e. < 36 mg/L) are suggestive of idiopathic HES rather than EGPA (Leurs et al., 2019).
3.4. Other eosinophilic-associated inflammatory conditions
Other less common eosinophilic-associated inflammatory disorders are eosinophilic chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis or gastroenteritis or colitis, eosinophilic pneumonia, eosinophilic cystitis, and eosinophilic fasciitis (Rosenberg et al., 2013; O'Sullivan and Bochner, 2018; Furuta and Katzka, 2015). These disorders are less recognized as separated eosinophilic clinical entities, being often complications of other diseases (e.g. allergies), but when possible causes or confounders are excluded, this definition applies, making these conditions potentially eligible for eosinophilic targeted treatments.
4. Implications for biological therapies of eosinophilic-associated inflammatory conditions
The development of biological agents targeting IL-5, IL-4 and IL-13 has provided opportunities to treat patients with eosinophilic-associated inflammatory conditions whose severity or symptoms are driven by eosinophil biology (Table 4). Most studies evaluating these biologics were conducted initially in asthmatic patients by selecting those with raised blood eosinophil numbers (Koenderman et al., 2000). In peripheral blood, eosinophils constitute less than 5% of all leukocytes (Brigden and Graydon, 1997). In absolute counts, blood eosinophils are reported to range from 0.05–0.5 × 109 cells/L (or 5–500 cells/μL), and eosinophilia is traditionally defined by values above the upper normal values (Valent et al., 2012). Large epidemiological study now identifies normal blood eosinophil counts in health to be around 100 cells/μL, once risk factors excluded. In most of these studies on healthy subjects, as many as 75% of the individuals studied had an eosinophil count below 210 cells/μL (Hartl et al., 2020; Vedel-Krogh, 2020).
Table 4.
Biological agents that directly or indirectly target crucial eosinophil pathways, discussed in this article.
| Biological Agent | Specifics | Molecular Target(s) | Approved Indications |
|---|---|---|---|
| Mepolizumab | Humanized IgG1 given subcutaneously | IL-5 | Asthma (age 12 and older) EGPA (age 6 and older) HES (age 12 and older) CRSwNP (age 18 and older) |
| Reslizumab | Humanized IgG4 given intravenously | IL-5 | Asthma age 18 and older (and others) |
| Benralizumab | Humanized afucosylated IgG1 given subcutaneously | IL-5Rα | Asthma (age 12 and older) |
| Dupilumab | Human IgG4 given subcutaneously | IL-4Rα, blocking both IL-4 and IL-13 | Atopic dermatitis (age 12 and older) Asthma (age 12 and older) CRSwNP (age 12 and older) |
| Lebrikizumab | Humanized IgG4 given intravenously | IL-13 | Ongoing studies for Atopic Dermatitis |
Abbreviations: IL = interleukin; Rα = receptor subunit alpha; EGPA = eosinophilic granulomatosis with polyangiitis; HES = hypereosinophilic syndrome; CRSwNP = chronic rhinosinusitis with nasal polyps.
First, the existence of distinct eosinophils subtypes that are differently involved in these conditions could give important data for better disease management. Importantly, only rEos were described as IL-5 independent cells (Von Hulst, 2021). The data suggest that basal levels of eosinophils left after absolute IL-5 depletion (mepolizumab or reslizumab) are a steady-state rEos population, and anti-IL-5 treatment affected eosinophils are in inflammatory processes involved iEos (Januskevicius et al., 2020; Mesnil et al., 2016; Abdala-Valencia et al., 2016, 2018). A deeper depletion of eosinophils, eliminating also the rEos population might disturb lung homeostasis. In the field of precision medicine, the different biology of eosinophil subtypes should be taken into account, and a truly targeted therapeutic intervention should be ideally able to distinguish between rEos and iEos. Second, a better characterization of the predominant pathway underlying the eosinophilic inflammation in the single patient could help: there are cases in which asthmatic patients have a modest hypereosinophilia and increased FeNO, or a normalization of peripheral eosinophils during anti-IL-5 treatment with suboptimal asthma control and increased FeNO, suggesting a predominance of IL-13 activation that might respond better to IL-13 targeted treatment (Erzurum and Gaston, 2012). Similarly, high circulating IgE levels (in addition to eosinophilia) in asthmatic patients, may suggest an hyperactivation of IL-4 pathway. Third, markers of type 2 inflammation don't always predict responsiveness to corticosteroids. For example, in patients with eosinophilic asthma, the persistence of sputum eosinophilia despite high dose of corticosteroid identifies a subgroup of patients that despite Th2 inflammation are unresponsive to corticosteroids (17% of the totals in a recent study) (Berthon et al., 2017; Dunican and Fahy, 2017). More in general, these type of patients with persistent circulating or organ-limited eosinophilia despite corticosteroid treatment, can be those that better benefit of anti-eosinophil targeted treatments. In addition, the availability of different agents with different ways of administrations, half-life, and mechanisms of actions is another element that can be weighed to tailor the choice of the anti-eosinophil targeted treatment on the single patient.
Several of these anti-eosinophil targeted treatments have been already approved (especially for asthma), while others are pending. It has been documented that the impact of anti-IL5 biological agents on blood and tissue levels of eosinophils is not equal, likely depending on the different mechanism of action. In recent years two monoclonal antibodies (mepolizumab and reslizumab) directed against IL-5 and one monoclonal antibody directed against the alpha-subunit of the IL-5 receptor (IL-5R) (benralizumab) have been developed.
4.1. ANTI-IL5-RECEPTOR agents
Benralizumab is a monoclonal antibody that binds to IL-5R via its Fab domain, blocking the binding of IL-5 to its receptor and resulting in inhibition of eosinophil differentiation and maturation in bone marrow. In addition, benralizumab is able to bind through its afucosylated Fc domain to the RIIIa region of the Fcγ receptor on NK cells, macrophages, and neutrophils, thus strongly inducing antibody-dependent, cell-mediated cytotoxicity in both rEos and iEos. This double function of benralizumab induces almost complete fast and maintained depletion of eosinophils that is much greater than that induced by other monoclonal antibodies targeting the IL-5 pathway, such as mepolizumab and reslizumab (Kelly et al., 2017). Laviolette et al. documented that in asthmatics treated with the subcutaneous benralizumab (patients randomized to 100 mg or 200 mg, for 3 months) eosinophil counts decrease with a median value of 95.8% in the airways (day 84; placebo, 46.7%; P = 0.06), 89.9% in sputum (day 28) and a 100% in blood counts (day 84), even if the extent of decrease in tissue eosinophil showed individual variability (Laviolette et al., 2013). Moreover, eosinophils were not detectable in bone marrow of benralizumab-treated subjects (day 28, n = 4) (Laviolette et al., 2013). In addition, a mechanistic study showed that lung eosinophils of bronchoalveolar lavage of patients downregulate IL-5R, therefore it remains unclear if antibody dependent cytotoxicity (ADCC) can occur in lung tissue (Kelly et al., 2017). A deep and persistent depletion of blood eosinophils after chronic administration of benralizumab was observed also in patients with severe, uncontrolled asthma in the BORA study (Busse et al., 2019). Similar results were observed more recently with the approved sub-cutaneous dose of benralizumab (30 mg), resulting in an almost complete depletion of eosinophils in both blood and sputum after 12 weeks of treatment (Sehmi et al., 2018; Nair et al., 2017).
Of note, benralizumab, has also been investigated in EGPA in a phase II study, facilitating oral corticosteroid reduction and reducing exacerbations in EGPA, while being well tolerate. These positive signals were probably due to its ability to induce profound eosinophil depletion (antibody-dependant cytotoxicity of IL-5Rα-bearing cells) (Guntur et al., 2021). Larger controlled trials are warranted to further evaluate the role of benralizumab in EGPA, currently ongoing.
4.2. ANTI-IL5 agents
Reslizumab, adiminstred intravenously at a dose of 3 mg/kg with some adjustment every 4 weeks, has been approved for severe eosinophilic asthma thanks to two multicenter large phase III trials on uncontrolled asthma with elevated blood eosinophil counts (Castro et al., 2015). In both studies, a significant reduction of asthma exacerbation with reslizumab was observed (rate ratio study 1 0.50 [95% CI 0.37–0.67]; study 2: 0.41 [0.28–0.59]; both p < 0·0001) compared with those receiving placebo.
Mepolizumab is a fully humanized monoclonal antibody IgG1 specific for human IL-5. Mepolizumab blocks the binding of human IL-5, impairing its ligation to the a-chain of the IL-5R–a complex, which is mainly expressed on the eosinophil cell surface. Flood Page et al. already demonstrated in a 2003 paper that mepolizumab produced a median decrease from baseline of 55% for airway eosinophils, 52% for bone marrow eosinophils, and 100% for blood eosinophils in twenty-four patients with mild asthma that received three intravenous doses of either 750 mg of mepolizumab or placebo in a randomized, double-blind, parallel-group fashion over 20 weeks (Flood-Page et al., 2003). Therefore, the administration at high doses of mepolizumab does not deplete airway or bone marrow eosinophils. Blood pharmacodynamic responses showed that a subcutaneous dose of 100 mg of mepolizumab administered subcutaneously provides the desired pharmacology to neutralize serum IL-5 levels and reduce blood eosinophil counts to approximately 40 cells/mL in asthmatic patients, which corresponds to a low normalized blood eosinophil count (Yancey et al., 2017). Indeed, similar data have also been observed in the multicenter, open-label, long-term, Phase IIIb COSMEX study. In this study patients received mepolizumab 100 mg subcutaneously every 4 weeks as add-on therapy for up to 172 weeks with eosinophils blood levels constantly detected at levels of about 50 cells/μL) (Geometric Mean Blood Eosinophil Count) (Khurana et al., 2019). Pavord et al. demonstrated in the DREAM study that mepolizumab, at the 750-mg intravenous dose, has determined comparable reductions of 88% in blood and sputum eosinophil counts; while for the 250-mg intravenous dose, the reduction in blood eosinophil counts was 86% compared with 65% for sputum, and for the 75 mg intravenous dose, the reduction in blood eosinophil counts was 78% compared with 32% for sputum (Pavord et al., 2012). A similar trend on the decrease of eosinophils in induced sputum was also observed in the study of Pouliquen et al. (2015). Furthermore, mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis (Conus et al., 2010). Mepolizumab had no effect on the duodenal infiltration of eosinophils, T cells, and mast cells in subjects participating in the first placebo-controlled clinical study performed in adult patients with eosinophilic esophagitis (Straumann et al., 2010). Whereas ‘‘inflammatory’’ eosinophils at the site of inflammation are reduced by approximately 50%, physiologic eosinophil infiltration, at least in the duodenum, is not affected, even at the high 1500 mg dose level used in this study. In addition, the anti–IL-5 therapy did not change the expression levels of IL-5Ra and the eosinophil activation markers CD25 and IL-13, suggesting that the relative proportions of potential functionally different eosinophil subgroups also did not change. Finally, in another study that assessed outcomes after cessation of mepolizumab in severe eosinophilic asthmatic patients, the blood eosinophils count resulted higher in 0–3 months (p < 0.001) in treated patients, demonstrating that the inhibitory effect of mepolizumab on eosinophils is rapidly reversible upon its cessation (Haldar et al., 2014). Overall, this indicates that Mepolizumab, which targets the IL-5 molecule does not impact on tissue eosinophils as strong as benralizumab, which target the IL-5 receptor.
Having demonstrated efficacy in severe asthma, mepolizumab have been tested in patients with nasal polyposis with or without asthma, showing to reduce nasal and sinus nasal polyp burden monitored by nasal endoscopy and to improve nasal symptoms. The study SYNAPSE enrolled 407 patients were randomized to 100 mg mepolizumab subcutaneously or placebo once every 4 weeks, in addition to standard of care. This study demonstrate efficacy in larger patient populations with current, refractory severe chronic rhinosinusitis with nasal polyps, leading to the registration for the indication nasal polyps (Han et al., 2021).
Mepolizumab has been approved for the treatment of refractory or relapsing EGPA by EMA in September 2021. One of the most important studies involved the subcutaneous administration of 300 mg every four weeks, i.e. 3 times higher than the asthma dose (Wechsler et al., 2017). Interestingly, no specific dose-findings studies were performed for EGPA. This multicenter, randomized controlled trial enrolled 136 subjects with stable disease on a stable prednisolone or prednisone dose along with standard EGPA care. Treatment with mepolizumab led to a greater likelihood of having 24 weeks of disease remission (28% on active drug versus 3% on placebo, with similar proportions at 48 weeks), which was the primary endpoint, and an average daily dose of prednisolone or prednisone of ≤4 mg per day during the last four weeks of the 52 of observations was achieved in 44% of the mepolizumab group versus 7% of placebo. This highlights the steroid sparing potential of this treatment in EGPA. Even if better than placebo, remission did not occur in 47% of the participants in the mepolizumab group versus 81% of those in the placebo group. Only 13% of patients in both arms were ANCA positive at enrollment (19% were ANCA-positive at some time in the course of the disease), which clearly represents a limitation of this study. In addition, none of the patients received mepolizumab as first-line therapy, and overall, mepolizumab's ability to curb vasculitis manifestations remains unclear.
Similarly, a phase III trial let to mepolizumab approval by EMA for FIP1-like-1-platelet-derived growth factor receptor α-negative HES, given subcutaneously at a dose of 300 mg every 4 weeks versus placebo, and added on HES therapy (Roufosse et al., 2020). While as safe as standard of care, the proportion of patients experiencing 1 or more flares/withdrawing from the study was 50% lower with mepolizumab versus placebo, and the open-label extension of the trial confirmed long-term control of disease flares, blood eosinophil counts, plus reductions in corticosteroid use (Gleich et al., 2021).
4.3. ANTI-IL4/IL13 agents
Dupilumab is a recombinant human IgG4 antibody to the IL-4 receptor. There are 2 types of IL-4 receptors: the type 1 receptor, which is composed of the IL-4 chain (IL-4Rα) and a γ chain (γC), and the type 2 receptor, which is composed of the IL-4Rα chain and the α1 chain of the IL-13 receptor (IL-13Rα1) (Sastre and Dávila, 2018). The type 1 receptor can be activated by IL-4 and the type 2 receptor can be activated by both IL-4 and IL-13. By blocking the IL-4R alpha subunit, dupilumab inhibits IL-4 and IL-13 cytokine-induced responses, including the release of proinflammatory cytokines, chemokines, and immunoglobulin E. Transient eosinophilia is a known side effect of dupilumab and transient increases in eosinophils have been observed in dupilumab clinical studies. The treatment with dupilumab in asthmatic subjects was associated with an increase in blood eosinophils with a maximum increase occurring at approximately 16–20 weeks after starting therapy, and the mean percent increase of approximately 10% and fifty-two of 1264 treated subjects (4.1%) developed a treatment emergent eosinophilia (Castro et al., 2018). All subjects with an eosinophil response recovered, some of them with systemic corticosteroid therapy and dupilumab discontinuation and others improving despite continuation of the dupilumab. In subjects who remained on dupilumab, the eosinophil count generally decreased to baseline by 28–52 weeks. In a French multicentre, retrospective real‐life cohort study (treated patients: 64) a hypereosinophilia ≥1500/mm3 was observed at least once during follow‐up in 16 patients (25%), persisting after 6 months in 8 (14%) of them. Increase in blood eosinophil count did not modify the clinical response during the study period (Dupin et al., 2020). Transient eosinophilia is felt to be self-limited due to the drug's effect on the Th2 immune response and its subsequent effect on blunting migration of eosinophils into target tissues. As a result, it is not commonly thought to cause eosinophilic disease processes, due to the subsequent sequestration of eosinophils in the blood and inhibition of their migration into target tissues. Two theories have been proposed for this finding of increased eosinophilia in response to dupilumab: (a) eosinophilia could represent a transient rebound elevation in response to eosinophil-promoting mediators such as IL-5, as a result of blocking IL-4 and IL-13 (Chung, 2016), and (b) as IL-4 and IL-13 recruit and facilitate eosinophil migration into tissues, and their inhibition may cause eosinophil accumulation in the peripheral blood (Darveaux and Busse, 2015; Barranco et al., 2017). However, there have been some cases of marked increase of eosinophilia and tissue accumulation of eosinophils during dupilumab therapy. One patient developed hypereosinophilic syndrome; the treatment was discontinued, and corticosteroid therapy was administered with immediate improvement (Wenzel et al., 2013). It has been also reported a case of eosinophilic pneumonia with sustained hypereosinophilia (maximum eosinophil count of 2080/μL) associated with the use of dupilumab (Menzella et al., 2019).
The dupilumab trial findings on uncontrolled asthma are in contrast to those of two large international phase 3 studies assessing lebrikizumab (LAVOLTA I and II), in which patients were randomized to receive placebo or lebrikizumab (Hanania et al., 2016). Overall primary endpoint was not met, i.e. the reduction in exacerbations over a one-year period in subjects with high blood eosinophil counts or periostin levels: one study reached statistical significance while the other one did not. Taken all together, one could speculate that in asthma targeting both IL-4 and IL-13 as dupilumab does is better than targeting IL-13 alone as done by lebrikizumab, or alternatively that targeting the receptor alpha rather than the cytokine could impact more or eosinophil biology.
5. Conclusions and future perspectives
Overall, data suggest that eosinophils are heterogeneous and that different eosinophil subpopulations exist in vivo, i.e. rEos and iEos. Besides their roles in Th2-oriented diseases, eosinophils also regulate homeostatic processes at steady state, thereby challenging the exclusive paradigm of the eosinophil as a destructive and inflammatory cell (Mesnil et al., 2016). These findings may thus have an impact on the efficacy of eosinophil-targeted therapy.
Distinctive eosinophil subpopulations exert different functions and the identification of functional subgroups and specific markers is a mandatory research objective. Application of precision medicine by proteomics, transcriptomics, and metabolomics to eosinophil biology may help to promote further advances in this research field.
Therapeutic intervention with biological agents that totally deplete tissues and circulating eosinophils or, vice versa, maintain a minimal proportion of eosinophils, particularly those residents in tissues or rEos, could therefore have a very different impact, especially when considering the administration of these therapies for prolonged periods. Similarly, a wider use of routine biomarkers of Type 2 inflammation (circulating eosinophils, and organ-specific eosinophils levels such as eosinophil count in sputum, bronchoalveolar lavage, tissue biopsy; total circulating IgE levels or the use of FeNO) can help in to choose the best eosinophil-targeted approach among the increasing therapeutic armamentarium of biological agents.
The identification of predictors for the efficacy of anti-eosinophil treatments is therefore a key objective and might further result in the identification of new functional eosinophil subsets as well as new categories of eosinophil-associated clinical disorders.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
Writing the original draft, investigation, literature analysis, conceptualization, figures: CL. Contribution to writing and conceptual framing of the article: AB. All the Authors critically review and approved the final manuscript.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdala-Valencia H., Loffredo L., Misharin A., Berdnikovs S. Phenotypic plasticity and targeting of Siglec-F high CD 11clow eosinophils to the airway in a murine model of asthma. Allergy. 2016;71:267–271. doi: 10.1111/all.12776. [DOI] [PubMed] [Google Scholar]
- Abdala-Valencia H., Coden M.E., Chiarella S.E., Jacobsen E.A., Bochner B.S., Lee J.J., et al. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 2018 Jul;104(1):95–108. doi: 10.1002/JLB.1MR1117-442RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamko D.J., Yost B.L., Gleich G.J., Fryer A.D., Jacoby D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 1999 Nov;190(10):1465–1478. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelink M., de Groot J.C., de Nijs S.B., et al. Severe adult-onset asthma: a distinct phenotype. J. Allergy Clin. Immunol. 2013;132:336–341. doi: 10.1016/j.jaci.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Aoki A., Hirahara K., Kiuchi M., Nakayama T. Eosinophils: cells known for over 140 years with broad and new functions. Allergol. Int. 2021 Jan;70(1):3–8. doi: 10.1016/j.alit.2020.09.002. [DOI] [PubMed] [Google Scholar]
- Bagnasco D., Ferrando M., Varricchi G., et al. Anti-interleukin 5 (IL-5) and IL-5Ra biological drugs: efficacy, safety, and future perspectives in severe eosinophilic Asthma. Front. Med. 2017;4:135. doi: 10.3389/fmed.2017.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- Barranco P., Phillips-Angles E., Dominguez-Ortega J., Quirce S. Dupilumab in the management of moderate-to severe asthma: the data so far. Therapeut. Clin. Risk Manag. 2017;13:1139–1149. doi: 10.2147/TCRM.S125964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C. Eosinophils: important players in humoral immunity. Clin. Exp. Immunol. 2015;183:57–64. doi: 10.1111/cei.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon B.S., Gibson P.G., Wood L.G., et al. A sputum gene expression signature predicts oral corticosteroid response in asthma. Eur. Respir. J. 2017;49:1700180. doi: 10.1183/13993003.00180-2017. [DOI] [PubMed] [Google Scholar]
- Berti A., Volcheck G.W., Cornec D., Smyth R.J., Specks U., Keogh K.A. Severe/uncontrolled asthma and overall survival in atopic patients with eosinophilic granulomatosis with polyangiitis. Respir Med [Internet] 2018;142:66–72. doi: 10.1016/j.rmed.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Berti A., Boukhlal S., Groh M., Cornec D. Eosinophilic granulomatosis with polyangiitis: the multifaceted spectrum of clinical manife-stations at different stages of the disease- Expet Rev. Clin. Immunol. 2020;16(1):51–61. doi: 10.1080/1744666X.2019.1697678. [DOI] [PubMed] [Google Scholar]
- Berti A., Cornec D., Casal Moura M., Smyth R.J., Dagna L., Specks U., Keogh K.A. Eosinophili Granuloma-tosis with polyangiitis: clinical predictors of long-term Asthma severity. Chest. 2020;157(5):1086–1099. doi: 10.1016/j.chest.2019.11.045. May. Epub 2020 Jan 17. [DOI] [PubMed] [Google Scholar]
- Brigden M., Graydon C. Eosinophilia detected by automated blood cell counting in ambulatory North American outpatients. Incidence and clinical significance. Arch. Pathol. Lab Med. 1997;121:963–967. [PubMed] [Google Scholar]
- Busse W.W., Bleecker E.R., FitzGerald J.M., Ferguson G.T., Barker P., Sproul S., et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. The Lancet Respiratory. 2019;7(Issue 1):46–59. doi: 10.1016/S2213-2600(18)30406-5. [DOI] [PubMed] [Google Scholar]
- Castro M., Zangrilli J., Wechsler M.E., Bateman E.D., Brusselle G.G., Bardin P., Murphy K., Maspero J.F., O'Brien C., Korn S. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015 May;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9. Epub 2015 Feb 23. [DOI] [PubMed] [Google Scholar]
- Castro M., Corren J., Pavord I.D., et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N. Engl. J. Med. 2018;378:2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- Choi Y., Sim S., Park H.-S. Distinct functions of eosinophils in severe asthma with type 2 phenotype: clinical implications. Korean J. Intern. Med. (Korean Ed.) 2020;35:823–833. doi: 10.3904/kjim.2020.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu V.T., Beller A., Rausch S., et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Chung K.F. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3–4. doi: 10.1016/S0140-6736(16)30311-7. [DOI] [PubMed] [Google Scholar]
- Churg J., Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am. J. Pathol. 1951;27(2):277–301. 1951/03/01. [PMC free article] [PubMed] [Google Scholar]
- Comarmond C., Pagnoux C., Khellaf M., Cordier J.F., Hamidou M., Viallard J.F., et al. Eosinophilic granulo- matosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term follow-up of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270–281. doi: 10.1002/art.37721. 2012/10/10. [DOI] [PubMed] [Google Scholar]
- Conus S., Straumann A., Bettler E., Simon H.-U. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010;126(1):175–177. doi: 10.1016/j.jaci.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Darveaux J., Busse W.W. Biologics in asthma – the next step toward personalized treatment. J. Allergy Clin. Immunol. Pract. 2015;3(2):152–160. doi: 10.1016/j.jaip.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J.C., Storm H., Amelink M., et al. Clinical profile of patients with adult-onset eosinophilic asthma. ERJ Open Res. 2016;2(2) doi: 10.1183/23120541.00100-2015. 00100-2015-100-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M.G., Bivins-Smith E.R., Proskocil B.J., Nie Z., Scott G.D., Lee J.J., et al. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am. J. Respir. Cell Mol. Biol. 2016;55:387–394. doi: 10.1165/rcmb.2015-0405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez C., Dakhama A., Tomkinson A., Marquillies P., Balhorn A., Tonnel A.B., et al. Migration and accumulation of eosinophils toward regional lymphnodes after airway allergen challenge. J. Allergy Clin. Immunol. 2004;114:820–825. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Dunican E.M., Fahy J.V. Asthma and corticosteroids: time for a more precise approach to treatment. Eur. Respir. J. 2017 Jun 29;49(6):1701167. doi: 10.1183/13993003.01167-2017. [DOI] [PubMed] [Google Scholar]
- Dupin C., Belhadi D., Guilleminault L., Gamez A.-S., Berger P., De Blay F., et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real‐life French multi‐centre adult cohort. Clin. Exp. Allergy. 2020 Jul;50(7):789–798. doi: 10.1111/cea.13614. Epub 2020 May 29. [DOI] [PubMed] [Google Scholar]
- Durel C.A., Berthiller J., Caboni S., Jayne D., Ninet J., Hot A. Long-term followup of a multicenter cohort of 101 patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss) Arthritis Care Res. 2016;68(3):374–387. doi: 10.1002/acr.22686. 2015/09/01. [DOI] [PubMed] [Google Scholar]
- Erzurum S.C., Gaston B.M. Biomarkers in asthma: a real hope to better manage asthma. Clin. Chest Med. 2012 Sep;33(3):459–471. doi: 10.1016/j.ccm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood-Page P.T., Menzies-Gow A.N., Kay A.B., Robinson D.S. Eosinophil's role remains uncertain as anti–interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- Furuta G.T., Katzka D.A. Eosinophilic esophagitis. N. Engl. J. Med. 2015 Oct 22;373(17):1640–1648. doi: 10.1056/NEJMra1502863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G.J., Roufosse F., Chupp G., Faguer S., Walz B., Reiter A., Yancey S.W., Bentley J.H., Steinfeld J., HES Mepolizumab Study Group J. Allergy Clin. Immunol. Pract. 2021 Aug 10;(21) doi: 10.1016/j.jaip.2021.07.050. S2213-2198. 00891-6. [DOI] [PubMed] [Google Scholar]
- Gonlugur U., Gonlugur T.E. Non-allergic eosinophilic inflammation. Immunol. Invest. 2006;35(1):29–45. doi: 10.1080/08820130500496779. [DOI] [PubMed] [Google Scholar]
- Groh M., Pagnoux C., Baldini C., Bel E., Bottero P., Cottin V., et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur. J. Intern. Med. 2015;26(7):545–553. doi: 10.1016/j.ejim.2015.04.022. 2015/05/15. [DOI] [PubMed] [Google Scholar]
- Guillevin L., Pagnoux C., Seror R., Mahr A., Mouthon L., Le Toumelin P. The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Med. 2011;90(1):19–27. doi: 10.1097/MD.0b013e318205a4c6. 2011/01/05. [DOI] [PubMed] [Google Scholar]
- Guntur V.P., Manka L.A., Denson J., Dunn R.M., Dollin Y.T., Gill M., Kolakowski C., Strand M.J., Wechsler M.E. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. Pract. 2021 Mar;9(3):1186–1193. doi: 10.1016/j.jaip.2020.09.054. Epub 2020 Oct 14. [DOI] [PubMed] [Google Scholar]
- Haldar P., Brightling C.E., Singapuri A., Hargadon B., Gupta S., Monteiro W., et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J. Allergy Clin. Immunol. 2014;133(3):921–923. doi: 10.1016/j.jaci.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Han J.K., Bachert C., Fokkens W., Desrosiers M., Wagenmann M., Lee S.E., Smith S.G., Martin N., Mayer B., Yancey S.W., Sousa A.R., Chan R., Hopkins C. SYNAPSE study investigators. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021 Oct;9(10):1141–1153. doi: 10.1016/S2213-2600(21)00097-7. Epub 2021 Apr 16. PMID: 33872587. [DOI] [PubMed] [Google Scholar]
- Hanania N.A., Korenblat P., Chapman K.R., Bateman E.D., Kopecky P., Paggiaro P., et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016;4:781–796. doi: 10.1016/S2213-2600(16)30265-X. [DOI] [PubMed] [Google Scholar]
- Hansel T.T., Braunstein J.B., Walker C., Blaser K., Bruijnzeel P.L., Virchow J.C., Jr., et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin. Exp. Immunol. 1991;86:271–277. doi: 10.1111/j.1365-2249.1991.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Miyajima A. Function and signal transduction mediated by the interleukin 3 receptor system in hematopoiesis. Stem Cell. 1996;14:605–618. doi: 10.1002/stem.140605. [DOI] [PubMed] [Google Scholar]
- Hartl S., Breyer M.-K., Burghuber O.C., et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur. Respir. J. 2020;55:1901874. doi: 10.1183/13993003.01874-2019. [DOI] [PubMed] [Google Scholar]
- Impellizzeria G., Marascoa G., Eusebia L.H., Salfib N., Bazzolia F., Zagari R.M. Eosinophilic colitis: a clinical review. Dig. Liver Dis. 2019 Jun;51(6):769–773. doi: 10.1016/j.dld.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Jacobsen E.A., Helmers R.A., Lee J.J., Lee N.A. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120(19):3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januskevicius A., Jurkeviciute E., Janulaityte I., Kalinauskaite -Zukauske V., Miliauskas S., Malakauskas K. Blood eosinophils subtypes and their survivability in asthma patients. Cells. 2020;9:1248–1264. doi: 10.3390/cells9051248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.W. Activation states of blood eosinophils in asthma. Clin. Exp. Allergy. 2014;44:482–498. doi: 10.1111/cea.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y. Comparative analysis of dibutyric cAMP and butyric acid on the differentiation of human eosinophilic leukemia EoL-1 cells. Immune Netw. 2015;15:313–318. doi: 10.4110/in.2015.15.6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Rothenberg M.E. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J. Immunol. 2014;193:999–1005. doi: 10.4049/jimmunol.1400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda A., Yun Y., Van Bui D., Nguyen L.M., Kobayashi Y., Suzuki K., et al. The multple functions and subpopulations of eosinophils in tissues under steady-state and pathological conditions. Allergol. Int. 2021 Jan;70(1):9–18. doi: 10.1016/j.alit.2020.11.001. [DOI] [PubMed] [Google Scholar]
- Kay A.B. The early history of the eosinophil. Clin. Exp. Allergy. 2015;45:575–582. doi: 10.1111/cea.12480. [DOI] [PubMed] [Google Scholar]
- Kelly K.A., Esnault S., Liu L.Y., Evans M.D., Johansson M.W., Mathur S., Mosher D.F., Denlinger L.C., Jarjour N.N. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am. J. Respir. Crit. Care Med. 2017 Dec 1;196(11):1385–1395. doi: 10.1164/rccm.201611-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S., Brusselle G.G., Bel E.H., FitzGerald, Masoli M., Korn S., Kato M., Albers F.C., Bradford E.S., Gilson M.J., Price R.G., Humbert M. Long-term safety and clinical benefit of mepolizumab in patients with the most severe eosinophilic asthma: the COSMEX study. Clin. Therapeut. 2019;41(Number 10):2041–2056. doi: 10.1016/j.clinthera.2019.07.007. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Jung Y.J. The emerging role of eosinophils as multifunctional leukocytes in health and disease. Immune netw. 2020;20(3):e24. doi: 10.4110/in.2020.20.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klion A. Eosinophilia: a pragmatic approach to diagnosis and treatment. Hematology Am. Soc. Hematol Educ. Prog. 2015;2015:92–97. doi: 10.1182/asheducation-2015.1.92. [DOI] [PubMed] [Google Scholar]
- Klion A.D. How I treat hypereosinophilic syndromes. Blood. 2015;126(9):1069–1077. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C.S., Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- Koenderman L., Hassani M., Mukherjee M., Nair P. Monitoring eosinophils to guide therapy with biologics in asthma: does the compartment matter? Allergy. 2000 doi: 10.1111/all.14700. dec. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroegel C., Liu M.C., Hubbard W.C., Lichtenstein L.M., Bochner B.S. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J. Allergy Clin. Immunol. 1994;93:725–734. doi: 10.1016/0091-6749(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Kuo H.P., Yu T.R., Yu C.T. Hypodense eosinophil number relates to clinical severity, airway hyperresponsiveness and response to inhaled corticosteroids in asthmatic subjects. Eur. Respir. J. 1994;7:1452–1459. doi: 10.1183/09031936.94.07081452. [DOI] [PubMed] [Google Scholar]
- Lavine K.J. Eosinophils confer protection following myocardial infarction. JACC (J. Am. Coll. Cardiol.): Basic Translat. Sci. 2020;5(7):682–684. doi: 10.1016/j.jacbts.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette M., Gossage D.L., Gauvreau G., Leigh R., Olivenstein R., Katial R., Busse W.W., Wenzel S., Wu Y., Datta V., Kolbeck R., Molfino N.A. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J. Allergy Clin. Immunol. 2013 Nov;132(5):1086–1096. doi: 10.1016/j.jaci.2013.05.020. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Jacobsen E.A., McGarry M.P., Schleimer R.P., Lee N.A. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurs A., Chenivesse C., Lopez B., Gibier J.-B., Clément G., Groh M., et al. C-Reactive protein as a diagnostic tool in differential diagnosis of hypereosinophilic syndrome and antineutrophil cytoplasmic antibody–negative eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. Pract. 2019;7(4):1347–1351. doi: 10.1016/j.jaip.2018.10.002. Apr. [DOI] [PubMed] [Google Scholar]
- Li N., Hua J. Immune cells in liver regeneration. Oncotarget. 2017 Jan 10;8(2):3628–3639. doi: 10.18632/oncotarget.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang W., Lv Z., et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J. Immunol. 2018;200:2253–2262. doi: 10.4049/jimmunol.1701455. [DOI] [PubMed] [Google Scholar]
- Lim H.F., Nair P. Airway inflammation and inflammatory biomarkers. Semin. Respir. Crit. Care Med. 2018;39:56–63. doi: 10.1055/s-0037-1606217. 3. Licari A, Castagnoli R, Brambilla I, et al. Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol 2018;31:44-5. [DOI] [PubMed] [Google Scholar]
- Locksley R.M. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi C. first ed. Città Studi Edizioni; 1996. Eosinofili ed eosinofilie. Fisiopatologia e nosografia clinica. 88-251-0108-9. [Google Scholar]
- Lombardi C., Passalacqua G. Eosinophilia and diseases: clinical revision of 1862 cases. Arch. Intern. Med. 2003 Jun 9;163(11):1371–1373. doi: 10.1001/archinte.163.11.1371-b. [DOI] [PubMed] [Google Scholar]
- Lyons P.A., Peters J.E., Alberici F., Liley J., Coulson R.M.R., Astle W., et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nature Communications. 2019;10(1):5120. doi: 10.1038/s41467-019-12515-9. Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzella Francesco, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Therapeut. Clin. Risk Manag. 2019;15:869–871. doi: 10.2147/TCRM.S207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnil C., Raulier S., Paulissen G., Xiao X., Birrell M.A., Pirottin D., et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Invest. 2016;126:3279–3295. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe D.D., Pawankar R., Ackerman S.J., Akin C., Clayton F., Falcone F.H., et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ J. 2016;9:7. doi: 10.1186/s40413-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.D., O'Byrne P.M. Epithelial-derived cytokines in asthma. Chest. 2017;151:1338–1344. doi: 10.1016/j.chest.2016.10.042. [DOI] [PubMed] [Google Scholar]
- Miyasato M., Tsuda S., Nakama T., Kato K., Kitamura N., Nagaji J., et al. Serum levels of eosinophil cationic protein reflect the state of in vitro degranulation of blood hypodense eosinophils in atopic dermatitis. J. Dermatol. 1996;23:382–388. doi: 10.1111/j.1346-8138.1996.tb04038.x. [DOI] [PubMed] [Google Scholar]
- Mould A.W., Matthaei K.I., Young I.G., Foster P.S. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J. Clin. Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P., Wenzel S., Rabe K.F., Bourdin A., Lugogo N.L., Kuna P., et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N. Engl. J. Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J.A., Bochner B.S. Eosinophils and eosinophil-associated diseases: an update. J. Allergy Clin. Immunol. 2018 Feb;141(2):505–517. doi: 10.1016/j.jaci.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N., Ortega H., Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- Percopo C.M., Dyer K.D., Ochkur S.I., Luo J.L., Fischer E.R., Lee J.J., et al. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014 Jan;123(5):743–752. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsky H.L., Cates C.J., Kew K.M., et al. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax. 2018;73:1110–1119. doi: 10.1136/thoraxjnl-2018-211540. [DOI] [PubMed] [Google Scholar]
- Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F., et al. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007 Sep;110(5):1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- Pouliquen I.J., Kornmann O., Barton S.V., Price J.A., Ortega H.G. Characterization of the relationship between dose and blood eosinophil response following subcutaneous administration of mepolizu-mab. Int. J. Clin. Pharm. Ther. 2015;53(12):1015–1027. doi: 10.5414/CP202446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prin L., Capron M., Tonnel A.B., Bletry O., Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int. Arch. Allergy Appl. Immunol. 1983;72:336–346. doi: 10.1159/000234893. [DOI] [PubMed] [Google Scholar]
- Reichman H., Moshkovits I., Itan M., Pasmanik-Chor M., Vogl T., Roth J., et al. Transcriptome profiling of mouse colonic eosinophils reveals a key role for eosinophils in the induction of s100a8 and s100a9 in mucosal healing. Sci. Rep. 2017 Aug;7(1):7117. doi: 10.1038/s41598-017-07738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggioni C., Comberiati P., Giovannini M., Agache I., Akdis M., Alves-Correia M., et al. A compendium answering 150 questions on COVID-19 and SARS-CoV-2. Allergy. 2020;75:2503–2541. doi: 10.1111/all.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca E., Ventura L., Zattra C.M., Lombardi C. Eosinopenia: an early, effective, and relevant COVID-19 biomarker? QJM. 2020 Sep 2 doi: 10.1093/qjmed/hcaa259. hcaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H.F., Dyer K.D., Foster P.S. Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N., Städler S., Lemann M., Hösli S., Simon H.U., Simon D. Distinct eosinophil cytokine expression patterns in skin diseases - the possible existence of functionally different eosinophil subpopulations. Allergy. 2011 Nov;66(11):1477–1486. doi: 10.1111/j.1398-9995.2011.02694.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg M.E., Hogan S.P. The eosinophil. Annu. Rev. Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Roufosse F., Kahn J.-E., Rothenberg M.E., Wardlaw A.J., Klion A.D., Kirby S.Y., Gilson M.J., Bentley J.H., Bradford E.S., Yancey S.W., Steinfeld J., Gleich G.J. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2020;146(6):1397–1405. doi: 10.1016/j.jaci.2020.08.037. Epub 2020 Sep. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugeles M.T., Trubey C.M., Bedoya V.I., Pinto L.A., Oppenheim J.J., Rybak S.M., et al. Ribonuclease is partly responsible for the HIV-1 inhibitory effect activated by HLA alloantigen recognition. AIDS. 2003 Mar;17(4):481–486. doi: 10.1097/00002030-200303070-00002. [DOI] [PubMed] [Google Scholar]
- Samson M., Puechal X., Devilliers H., Ribi C., Cohen P., Stern M., et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) enrolled in two prospective trials. J. Autoimmun. 2013;43:60–69. doi: 10.1016/j.jaut.2013.03.003. 2013/04/18. [DOI] [PubMed] [Google Scholar]
- Sastre J., Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig. Allergol. Clin. Immunol. 2018;28(3):139–150. doi: 10.18176/jiaci.0254. [DOI] [PubMed] [Google Scholar]
- Sehmi R., Lim H.F., Mukherjee M., Huang C., Radford K., Newbold P., et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J. Allergy Clin. Immunol. 2018;141:1529–15232 e8. doi: 10.1016/j.jaci.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Shah K., Ignacio A., McCoy K.D., Harris N.L. The emerging roles of eosinophils in mucosal homeostasis. Mucosal Immunol. 2020;13:574–583. doi: 10.1038/s41385-020-0281-y. [DOI] [PubMed] [Google Scholar]
- Shen Z.J., Malter J.S. Determinants of eosinophil survival and apoptotic cell death. Apoptosis. 2015 Feb;20(2):224–234. doi: 10.1007/s10495-014-1072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H.-U., Yousefi S., Germic N., Arnould I.C., Haczku A., Karaulov A.V., Simon D., Rosemberg H.F. The cellular functions of eosinophils: collegium Internationale Allergologicum (CIA) update 2020. Int. Arch. Allergy Immunol. 2020;181:11–23. doi: 10.1159/000504847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinico R.A., Di Toma L., Maggiore U., Bottero P., Radice A., Tosoni C., et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005;52(9):2926–2935. doi: 10.1002/art.21250. Sep; [DOI] [PubMed] [Google Scholar]
- Smith S.G., Chen R., Kjarsgaard M., et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016;137:75–86. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Stolarski B., Kurowska-Stolarska M., Kewin P., Xu D., Liew F.Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- Strandmark J., Rausch S., Hartmann S. Eosinophils in homeostasis and their contrasting roles during inflammation and helminth infections. Crit. Rev. Immunol. 2016;36(3):193–238. doi: 10.1615/CritRevImmunol.2016018726. [DOI] [PubMed] [Google Scholar]
- Straumann A., Kristl J., Conus S., Vassina E., Spichtin H.P., Beglinger C., et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm. Bowel Dis. 2005 Aug;11(8):720–726. doi: 10.1097/01.mib.0000172557.39767.53. [DOI] [PubMed] [Google Scholar]
- Straumann A., Conus S., Grzonka P., Kita H., Kephart G., Bussmann C., et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomized, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- Tagaya E., Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol. Int. 2007;56:331–340. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]
- Valent P., Klion A.D., Horny H.P., et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 2012;130:607–612. doi: 10.1016/j.jaci.2012.02.019. e609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedel-Krogh S. The search for the “healthy” blood eosinophil count. Eur Respir J. 2020;55 doi: 10.1183/13993003.00473-2020. [DOI] [PubMed] [Google Scholar]
- Von Hulst G. Anti-IL5 mepolizumab minimally influences residual blood eosinophils in severe asthma. Eur. Respir. J. 2021 sept;2:2100935. doi: 10.1183/13993003.00935-2021. [DOI] [PubMed] [Google Scholar]
- Wechsler M.E., Akuthota P., Jayne D., Khoury P., Klion A., Langford C.A., Merkel P.A., Moosig F., Specks U., Cid M.C., Luqmani R., Brown J., Mallett S., Philipson R., Yancey S.W., Steinfeld J., Weller P.F., Gleich G.J., EGPA Mepolizumab Study Team Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N. Engl. J. Med. 2017;376(20):1921–1932. doi: 10.1056/NEJMoa1702079. May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P.F., Spencer L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017;17:746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F., et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- Wong C.K., Hu S., Cheung P.F., Lam C.W. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- Wu D., Molofsky A.B., Liang H.E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K., Chawla A., Locksley R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey S.W., Keene, Albers F.C., Ortega H., Bates S., Bleecker E.R., Pavord I. Biomarkers for severe eosinophilic asthma. J. Allergy Clin. Immunol. 2017;140:1509–1518. doi: 10.1016/j.jaci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Yang B.G., Seoh J.Y., Jang M.H. Regulatory eosinophils in inflammation and metabolic disorders. Immune Network. 2017;17(No. 1):41–47. doi: 10.4110/in.2017.17.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Gold J.A., Andina N., Lee J.J., Kelly A.M., Kozlowski E., et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008 Sep;14(9):949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- Yu C., Cantor A.B., Yang H., Browne C., Wells R.A., Fujiwara Y., Orkin S.H. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Kim H.Y., Chang Y.J., et al. Innate lymphoid cells and asthma. J. Allergy Clin. Immunol. 2014;133:943–950. doi: 10.1016/j.jaci.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Yun Y., Kanda A., Kobayashi Y., Van Bui D., Suzuki K., Sawada S., et al. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol. Int. 2020;69:232–238. doi: 10.1016/j.alit.2019.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.