Abstract
The Centers for Disease Control and Prevention’s 2016 Guideline for Prescribing Opioids for Chronic Pain aimed to reduce unsafe opioid prescribing. It is unknown whether the guideline influenced prescribing in the target population: patients with chronic, noncancer pain, who may be at particular risk for opioid-related harms. To study this question, we used 2014–18 data from a commercial claims database to examine associations between the release of the guideline and opioid dispensing in a national cohort of more than 450,000 patients with four common chronic pain diagnoses. We also examined whether any reductions associated with the guideline were larger for diagnoses for which there existed stronger expert consensus against opioid prescribing. Overall, the guideline was associated with substantial reductions in dispensing opioids, including a reduction in patients’ rate of receiving at least one opioid prescription by approximately 20 percentage points by December 2018 compared with the counterfactual, no-guideline scenario. However, the reductions in dispensing did not vary by the strength of expert consensus against opioid prescribing. These findings suggest that although voluntary guidelines can drive changes in prescribing, questions remain about how clinicians are tailoring opioid reductions to best benefit patients.
More than 20 percent of US adults, or approximately fifty million people, experience chronic pain;1 efforts to ensure safe and effective pain management in this population are critical. In March 2016 the Centers for Disease Control and Prevention (CDC) released the Guideline for Prescribing Opioids for Chronic Pain, providing twelve recommendations to promote safe opioid prescribing for patients with chronic, noncancer pain.2 The guideline emphasizes treating chronic pain with nonpharmacologic and nonopioid options when possible; starting opioids at the lowest effective dose; avoiding increasing the dose to 90 morphine milligram equivalents if possible; and avoiding concurrent prescribing of opioids and benzodiazepines, which can further increase opioid risk. Initial evidence suggests that this guideline has contributed to a reduction in the quantity of opioids prescribed and dispensed in the overall US population,3 but its effect on prescribing to the intended patient population—patients with chronic noncancer pain—is not yet known.
Patients with chronic, noncancer pain may be at particularly high risk for prescription opioid–related harms. Although the efficacy of opioids in treating chronic pain remains in question,4,5 patients frequently receive long-term opioid therapy to treat it.6–8 Long-term opioid therapy, in turn, is associated with increased risk for opioid use disorder and overdose.9–11 In addition, patients with chronic pain frequently experience comorbid mental health conditions corresponding to increased risks for opioid dependence; concurrent opioid/benzodiazepine use; and, in turn, overdose.12,13 Strategies for minimizing opioid-related harms in patients with chronic pain, while ensuring sufficient pain management and access to treatment for opioid dependence or addiction, are needed.
Clinical guidelines offer one potential tool for achieving these goals. Adherence to the 2016 CDC guideline is entirely voluntary, which is not the case with prescription drug monitoring programs, opioid prescribing limits, and most other legal interventions for reducing excessive opioid prescribing. Instead of imposing hard limits, the guideline highlights the clinician’s role in tailoring opioid prescribing decisions for individual patients, weighing their likelihood of benefiting from a given regimen against the likelihood and magnitude of potential harms. Given the guideline’s voluntary nature, it is unclear whether it has actually influenced prescribing to patients with chronic pain and whether any impacts have varied by the patient’s likelihood of benefiting from opioids.
We therefore estimated the association between the release of the 2016 CDC guideline and the frequency and intensity of opioid dispensing (as a proxy for prescribing) for chronic pain, using 2014–18 data for a national cohort of patients with chronic, noncancer pain. In addition, we explored whether provider response to the guideline varied by the consistency of expert consensus regarding opioid prescribing at the time. That is, we investigated whether any guideline-associated reductions in opioid dispensing were greater in patients diagnosed with fibromyalgia or chronic headache compared to those diagnosed with osteoarthritis or chronic back or neck pain. In line with recommendations from other countries, the guideline concluded that the expected benefits of initiating opioids for fibromyalgia and chronic headache are unlikely to outweigh the risks.2,14,15 In contrast, the guideline did not make an explicit recommendation about the use of opioids for osteoarthritis or chronic back/neck pain, despite naming these conditions. Moreover, some other clinical guidelines at the time recommended opioids for certain patients with osteoarthritis16 and chronic low back pain17 who do not respond to first-line treatments. (More recent research has found that on average, opioids are not superior to nonopioid medications for treating osteoarthritis and chronic back pain.)5 A sharper guideline-related reduction in opioid dispensing in patients with fibromyalgia or headache would provide preliminary evidence that prescribers accounted for the perceived likelihood of benefit when reducing opioid prescribing for chronic pain.
Study Data And Methods
We used interrupted time series analysis to compare postguideline opioid dispensing for chronic pain with an estimated counterfactual scenario in which the guideline had not been released.
DATA AND SAMPLE
We examined opioid dispensing in three cohorts of commercially insured adults younger than age sixty-five with documented chronic, noncancer pain: patients with fibromyalgia or chronic headache (the group for whom expert consensus against opioid prescribing was strongest), patients with osteoarthritis or chronic back/neck pain, and patients with any of the four diagnoses.We used 2014–18 deidentified outpatient, inpatient, and pharmacy claims data from Optum’s Clinformatics Data Mart database; these data are comparable to the entire US commercially insured population.18,19 To ensure a consistent denominator before and after the release of the CDC guideline, we used closed cohorts of people continuously enrolled in commercial health plans for twenty-four months or more. Individuals were included in a given cohort if they received a relevant diagnosis at least twelve months before the release of the CDC guideline (see online appendix A for details about cohort creation).20 We concentrated on adults younger than age sixty-five because they experience greater risk for nonmedical prescription opioid use and overdose than older adults.9,21
The final guideline was released in March 2016 after a draft release in December 2015. Our analyses examined a twenty-one-month baseline period (March 2014–November 2015) and an eighteen-month postintervention period (July 2016–December 2018). To account for potential anticipatory and lagged effects of the guideline, we excluded December 2015–June 2016, the three months before and after guideline release (the “implementation period”); details about these periods are in appendix exhibit 1.20
THE GUIDELINE AND OPIOID DISPENSING MEASURES
We evaluated four primary outcomes that map to the guideline recommendations, as well as three secondary outcomes. Primary outcomes were receipt of at least one prescription opioid; average daily morphine-equivalent dose, given one or more opioid fills; receipt of a prescription of 90 morphine milligram equivalents or more daily, given one or more opioid fills (“high dose”); and receipt of concurrent opioid and benzodiazepine prescriptions, given one or more opioid fills. High-dose receipt and concurrent opioid/benzodiazepine receipt are frequently considered indicators of high-risk opioid prescribing, as they are associated with increased risk for respiratory suppression.9,22 The three secondary outcomes were total opioid days supplied; total number of opioid fills obtained; and number of concurrent opioid/benzodiazepine days supplied, given at least one concurrent day (measured as counts). Appendix B details the ascertainment of opioid fills and average daily dose.20
ANALYTIC STRATEGY
We used interrupted time series analysis with generalized estimating equations to estimate, at the month level, the association between the guideline and opioid dispensing in the three cohorts. Each month, a patient was included in the analyses if they received at least one diagnosis of interest during a clinical encounter. This enabled comparison of opioid dispensing among patients who had the opportunity to receive a prescription associated with a pain-related visit. The coefficients of interest were the estimated level change (or postguideline change in the mean of the outcome), if any, and trend change (or postguideline change in the slope of the outcome over time), if any.
We controlled for month-level secular trends to help account for other changes influencing opioid dispensing between 2014 and 2018; we used Pan’s quasi-likelihood model fit statistics to determine whether a linear trend alone or both a linear and a quadratic trend term was most appropriate in a given model.23,24 We also used these statistics to select the model link function and error distribution family. Appendix D provides further details about our model selection strategy.20
To address the risk of confounding as a result of temporal changes in sample composition, we controlled for a number of individual-level clinical and sociodemographic indicators that could influence opioid dispensing and whose distribution in the sample may vary over time. Appendix D provides details on the included covariates.20 The clinical covariates, all time varying, included monthly number of relevant pain-related visits, receipt of any other pain-related diagnoses in the given month, cumulative burden of illness (measured using a modified Elixhauser Comorbidity Index),25,26 and history of diagnosis with a mental health condition or substance use disorder. Sociodemographic variables included patient age, sex, race and ethnicity, and census division. Race and ethnicity data are obtained from the patient at the time of insurance plan enrollment; where it is not provided, Optum imputes race and ethnicity information (see appendix C for more detail).20 In addition, we included month fixed effects to account for potential seasonality in dispensing.
When possible, we used a maximally flexible correlation structure (“unstructured”) to account for likely correlation in outcomes within individuals over time; in cases of model nonconvergence, we used the exchangeable structure.
Based on the regression results, we generated predicted means for each outcome in the observed scenario (with guideline) and the counterfactual scenario (as if no guideline had been released, all else equal) for participants in the data set in December 2018 (the last month observed). These estimates reflect a combination of any level or trend changes in a given outcome associated with the guideline.We used preguideline secular trends to estimate the predicted means in the counterfactual scenario. We compared the predicted means in the two scenarios to estimate the difference in each outcome associated with the guideline.
NEGATIVE CONTROL ANALYSIS
Because the CDC guideline was applied at the national level, there is no “control” group of patients with chronic pain not affected by the guideline. Instead, we conducted a negative control analysis of the effect of the guideline on the rate of one or more benzodiazepine fills in patients with diagnosed anxiety and no pain-related diagnosis at any time during the analytic period.We selected benzodiazepines as a negative control because of their regulatory similarity with opioids as a controlled substance but without a similar change in national clinical guidelines. We would expect benzodiazepine dispensing to be affected by many of the same nonguideline forces that affect opioid dispensing and could be alternative explanations for our main results, such as prescription drug monitoring program implementation and increased general concern about prescription drug overdose risk.
SUPPLEMENTARY ANALYSES
In a supplementary analysis we assessed whether changes in dispensing rates were concentrated in acute versus longer-term prescriptions. In another we truncated the data set at December 2017 and conducted the main regressions to investigate whether our results were influenced by nonrandom selection of patients remaining in the cohort through 2018 or by confounding by any changes to opioid dispensing occurring in 2018.
LIMITATIONS
This study had several limitations. First, the observed reductions in opioid dispensing could have been caused by something other than the guideline. Although our negative control analysis accounted for forces that would influence both opioid and benzodiazepine dispensing, it could not account for forces that would affect only opioid dispensing. However, we were unable to identify national policies or events that occurred during the same period and that would be expected to have caused the observed changes; in addition, our modeling technique enabled us to control for preexisting secular trends. Moreover, truncating the data set at December 2017 did not change the substantive results, suggesting that our findings were not confounded by events occurring thereafter. Second, and relatedly, the predicted means for the counterfactual (no-guideline) scenario were based on the preguideline trend, which might not have held constant through 2018 absent the guideline. This would affect the estimated differences between the guideline and no-guideline scenarios in December 2018.
Third, we examined opioid dispensing in commercially insured adults younger than age sixty-five; the findings might not reflect changes experienced by Medicaid, Medicare, and Veterans Affairs patients. Fourth, our findings might not be generalizable to chronic pain conditions not examined here. However, the four conditions we studied are among the most common diagnoses of chronic pain, which afflicts at least forty million US adults; for example, nearly twenty-seven million people experience chronic low back pain alone, nearly thirty million people experience joint pain, and nearly thirteen million experience severe headache or migraine.1,27
Fifth, we were unable to capture prescriptions paid for out of pocket; it is possible that some patients with chronic pain sought opioid prescriptions from alternative cash payment sources if they received fewer insurance-covered prescriptions after the release of the guideline. Sixth, our measures of opioid dispensing intensity might not equal the intensity of actual use by the patient; however, declines in dispensing are important to assess, as they may reflect lower use as well as lower risk for diversion of unused opioids. Seventh, this study investigated heterogeneity in the effects of the guideline by diagnosis, but heterogeneity may also exist over time or by region, physician type or practice, and patient characteristics such as race. Finally, we were unable to assess the appropriateness of opioid dispensing or unintended consequences of reduced dispensing on patients’ pain level, pain-related functioning, or mental health.
Study Results
SAMPLE CHARACTERISTICS
Exhibit 1 provides sample characteristics. The combined cohort comprised 454,569 people who contributed 4,071,382 monthly observations. A total of 361,515 people met the criteria for osteoarthritis or chronic back/neck pain (3,958,329 person-months of observation), and 169,487 met the criteria for fibromyalgia or chronic headache (983,988 person-months of observation). A total of 76,609 people (16.8 percent) were eligible for both disaggregated cohorts, primarily because patients with fibromyalgia were likely to experience chronic back/neck pain. A given month was only included in an analysis if the patient received a diagnosis relevant to that particular cohort in that month.
EXHIBIT 1.
Characteristics of the study sample of commercially insured nonelderly US adults with chronic noncancer pain and of filled opioid prescriptions, 2014–18
| Combined cohorts | Osteoarthritis or back/neck pain | Fibromyalgia or headache | |
|---|---|---|---|
| Distinct individuals | 454,569 | 361,515 | 169,487 |
| Total observations | 4,071,382 | 3,958,329 | 983,988 |
| Mean age, years | 47.5 | 48.8 | 44.7 |
| Female, % | 58.7 | 56.5 | 68.9 |
| Race and ethnicity, % | |||
| White | 71.6 | 72.3 | 70.8 |
| Black | 9.0 | 9.1 | 8.6 |
| Hispanic | 10.1 | 9.5 | 10.8 |
| Asian | 3.3 | 3.2 | 3.6 |
| Missing | 6.0 | 5.9 | 6.1 |
| No. of months with at least 1 pain-related visit | 11.2 | 12.4 | 6.4 |
| No. of pain-related visits per month | 1.7 | 1.7 | 1.5 |
| Pain diagnosis of interest, % | |||
| Osteoarthritis | 27.9 | 36.2 | —a |
| Back/neck pain | 58.7 | 68.9 | —a |
| Fibromyalgia | 20.3 | —a | 68.2 |
| Chronic headache | 13.5 | —a | 37.1 |
| Other pain-related diagnosis in month, % | 28.7 | 25.3 | 36.7 |
| Elixhauser Comorbidity Indexb | 0.5 | 0.5 | 0.5 |
| Diagnosis in present or prior month, % | |||
| Mental health | |||
| Anxiety, PTSD | 11.4 | 10.9 | 15.5 |
| Depression | 11.1 | 11.0 | 14.7 |
| Psychosis, schizophrenia, or bipolar | 2.5 | 2.5 | 3.2 |
| Substance use disorder | 7.4 | 7.8 | 8.4 |
| Any opioid prescription fill, % | 29.9 | 31.6 | 24.3 |
| No. of fills per month, if any | 2.0 | 2.0 | 2.0 |
| No. of days supplied per month, if any | |||
| 0–14 | 17.8 | 16.2 | 22.1 |
| 15–31 | 57.2 | 58.2 | 53.6 |
| >31 | 24.9 | 25.6 | 24.3 |
| Average dose (MME), if any | 64.6 | 66.3 | 59.4 |
| Receiving >90 MME, if any, % | 19.8 | 20.6 | 17.4 |
| Concurrent opioids/benzodiazepines, if any opioid | |||
| Any overlap, % | 25.7 | 25.6 | 31.8 |
| Days of overlap, if any | 27.0 | 27.4 | 26.4 |
SOURCE Authors’ analysis of data from Optum’s Clinformatics Data Mart, 2014–18.
NOTES Sociodemographic, pain visit, comorbidity, and mental health or substance use disorder estimates refer to the person’s first month in the cohort. “Pain diagnosis of interest” refers to the distribution of diagnoses qualifying a person for a given cohort in the first observed month; in some cases, patients received multiple qualifying diagnoses. “Other pain-related diagnosis” refers to those not included in the given cohort. Prescription fill estimates refer to all analyzed months in which a visit for at least one of the relevant pain conditions occurred. A version of this table with associated confidence intervals is in appendix exhibit 6 (see note 20 in text). MME is morphine milligram equivalents. PTSD is posttraumatic stress disorder.
Not applicable.
The Elixhauser Comorbidity Index indicates the number of distinct comorbidities for which an individual had a visit in the month of interest. Twenty-six standard comorbidities were assessed; depression, psychoses, and substance use disorders were excluded from the index because they are captured in separate covariates.
Women made up 57 percent of patients in the osteoarthritis or back/neck pain cohort and 69 percent of those in the fibromyalgia or headache cohort. In all three cohorts, people were predominantly White (71–72 percent), whereas approximately 9 percent of patients in each cohort were Black, approximately 10–11 percent were Hispanic, and approximately 3–4 percent were Asian. Mean age across cohorts was 45–49 years; the fibromyalgia or headache cohort was slightly younger.
Patients filled at least one opioid prescription in nearly one-third of months with a relevant pain diagnosis; the rate of one or more fills was higher in patients with osteoarthritis or back/neck pain than in patients with fibromyalgia or headache. Mean daily dose of opioid prescriptions ranged from 59 to 66 morphine milligram equivalents. Daily dose exceeded 90 morphine milligram equivalents in 17–21 percent of prescriptions across cohorts. A concurrent benzodiazepine prescription was filled in 26–32 percent of months with opioid prescriptions.
REGRESSION RESULTS
Appendix exhibit 7 depicts scatterplots of means in each outcome.20 Across cohorts, the guideline was associated with a reduction in the level, trend, or both of most of the opioid dispensing outcomes (appendix exhibits 8–10).20 Associations between covariates and outcomes were qualitatively similar across pain cohorts (appendix C).20
PREDICTED MEANS
Exhibit 2 depicts the predicted rate of receiving one or more opioid prescription fills, by cohort, in the no-guideline (counterfactual) and guideline (observed) scenarios over time; exhibits 3 and 4 present predicted means for patients observed in December 2018, holding all other predictors constant (see appendix exhibit 12 for estimates with 95% confidence intervals).20 Overall, there were apparent reductions in all primary outcomes (one or more fills, average dose, high-dose receipt, and concurrent opioid/benzodiazepine receipt) in all cohorts, with the exception of concurrent opioid/benzodiazepine receipt in the fibromyalgia or headache cohort (exhibit 3). For some cohort-outcome pairs, the estimated trend change coefficient was negative but not statistically significant, whereas the estimated difference between the predicted means in the guideline and no-guideline scenarios for December 2018 was statistically significant; this was due to the accumulation of the negative trend change over time. Contrary to our hypothesis, reductions associated with the guideline did not appear larger in patients with fibromyalgia or headache than in those with osteoarthritis or back/neck pain; indeed, point estimates were often larger in the osteoarthritis or back/neck pain cohort, although confidence intervals overlapped.
EXHIBIT 2.
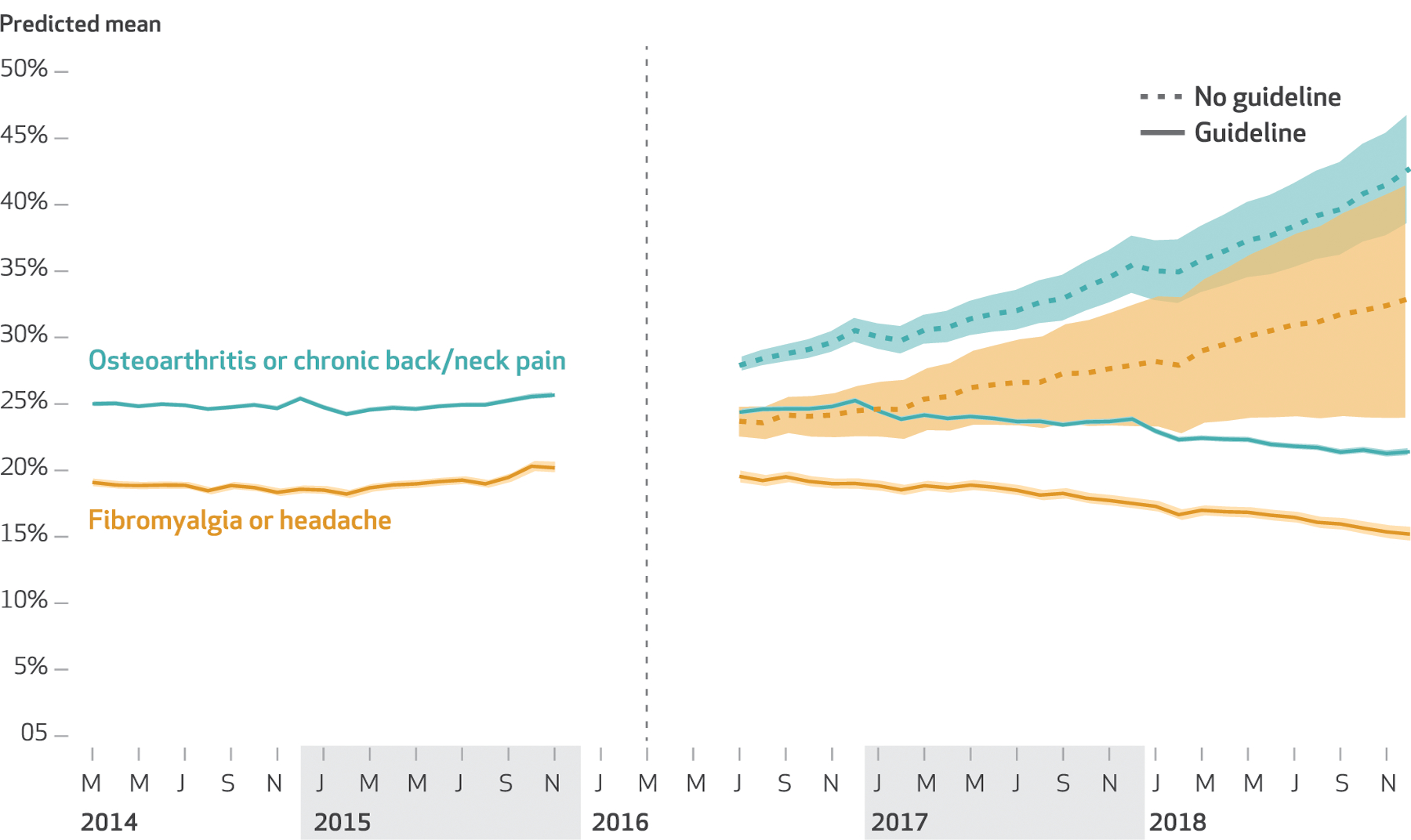
Predicted rate of one or more opioid prescription fills among commercially insured nonelderly US adults with selected noncancer pain with and without the Centers for Disease Control and Prevention guideline (observed versus counterfactual), by cohort, 2014–18
SOURCE Authors’ analysis of data from Optum’s Clinformatics Data Mart, 2014–18. NOTE The vertical line indicates the release of the guideline, and the ungraphed portion indicates the implementation period between November 2015 and July 2016, which was not modeled.
EXHIBIT 3.
Predicted means of any opioid prescription fill, dosage, and concurrent opioid/benzodiazepine receipt in December 2018 among commercially insured nonelderly US adults with selected noncancer pain, with and without the Centers for Disease Control and Prevention guideline (observed versus counterfactual)
| Conditions | One or more fills | Average dose | High dose | Concurrent opioids/benzodiazepines |
|---|---|---|---|---|
| ALL CONDITIONS | ||||
| Number | 40,897 | 11,853 | 11,853 | 11,867 |
| No guideline | 39.7% | 47.7 MME | 16.8% | 31.4% |
| Guideline | 19.3% | 32.6 MME | 6.5% | 12.8% |
| Difference (95% CI) | 20.4a (16.41, 24.39) | 15.1 MME (10.74,19.35) | 10.3a (9.40, 11.39) | 18.6a (11.46, 25.66) |
| OSTEOARTHRITIS OR BACK/NECK PAIN | ||||
| Number | 36,627 | 11,016 | 11,016 | 11,033 |
| No guideline | 42.9% | 49.0 MME | 16.7% | 43.9% |
| Guideline | 21.4% | 33.4 MME | 9.1% | 14.3% |
| Difference (95% CI) | 21.5a (17.38, 25.52) | 15.6 MME (11.00, 20.16) | 7.6a (6.39, 8.81) | 29.6a (21.44, 37.72) |
| FIBROMYALGIA OR HEADACHE | ||||
| Number | 6,465 | 1,554 | 1,554 | 1,555 |
| No guideline | 33.0% | 41.2 MME | 14.6% | 23.5% |
| Guideline | 15.2% | 30.6 MME | 7.1% | 19.8% |
| Difference (95% CI) | 17.8a (8.84, 26.70) | 10.6 MME (1.73, 19.35) | 7.5a (5.11, 10.01) | 3.7a (−4.03, 11.36) |
SOURCE Authors’ analysis of data from Optum’s Clinformatics Data Mart, 2014–18.
NOTES Average and high doses are defined in the text. Sample sizes refer to individuals present in the data set in December 2018. The no-guideline (counterfactual) scenario is that in which the guideline was not released but all other predictors were as observed. A version of this table with corresponding confidence intervals is in appendix exhibit 12 (see note 20 in text). MME is morphine milligram equivalents.
Percentage points.
EXHIBIT 4.
Predicted number of opioid prescription fills and days supplied in December 2018 among commercially insured nonelderly US adults with selected noncancer pain, with and without the CDC guideline (observed versus counterfactual)
| Conditions | Fills | Days supplied | Days of concurrent opioids/benzodiazepines |
|---|---|---|---|
| ALL CONDITIONS | |||
| Number | 11,867 | 11,867 | 2,229 |
| No guideline | 2.4 | 24.9 | 26.2 |
| Guideline | 1.5 | 20.6 | 19.8 |
| Difference (95% CI) | 0.9 (−5.12, 6.81) | 4.3 (3.56, 5.01) | 6.4 (−1.36, 14.07) |
| OSTEOARTHRITIS OR BACK/NECK PAIN | |||
| Number | 11,011 | 11,011 | 2,012 |
| No guideline | 2.3 | 24.7 | 22.0 |
| Guideline | 1.5 | 20.9 | 20.3 |
| Difference (95% CI) | 0.8 (0.30, 1.22) | 3.8 (3.16, 4.60) | 1.7 (−1.97, 5.32) |
| FIBROMYALGIA OR HEADCHE | |||
| Number | 1,555 | 1,555 | 397 |
| No guideline | 1.6 | 25.7 | 16.8 |
| Guideline | 1.5 | 21.6 | 19 |
| Difference (95% CI) | 0.1 (−0.01, 0.23) | 4.1 (2.45, 5.86) | −2.2 (−5.63, 1.27) |
SOURCE Authors’ analysis of data from Optum’s Clinformatics Data Mart, 2014–18.
NOTES The no-guideline (counterfactual) scenario is that in which the guideline was not released but all other predictors were as observed. Sample sizes refer to individuals present in the data set in December 2018. A version of this table with all corresponding confidence intervals is in appendix exhibit 12 (see note 20 in text).
By December 2018 the guideline was associated with a 20.4-percentage-point reduction in the rate of one or more opioid fills (exhibit 3). The estimated effect was larger in the osteoarthritis or back/neck pain cohort (21.5 percentage points), but confidence intervals overlapped. The estimated effect was smaller in the fibromyalgia or headache cohort (17.8 percentage points).
The guideline was associated with lower average dose across cohorts by December 2018. It was associated with a reduction in the combined cohort of 15.1 morphine milligram equivalents, a reduction in the osteoarthritis or back/neck pain cohort of 15.6 morphine milligram equivalents, and a reduction in the fibromyalgia or headache cohort of 10.6 morphine milligram equivalents. In the combined cohort, the guideline was associated with a reduction in high-dose dispensing of 10.3 percentage points by December 2018. The cohort-specific declines in high-dose receipt were similar: 7.6 percentage points in patients with osteoarthritis or back/neck pain and 7.5 percentage points in those with fibromyalgia or headache (exhibit 3).
In the combined cohort and the osteoarthritis or back/neck pain cohort, the guideline was associated with reduced rates of concurrent opioid/benzodiazepine prescriptions by December 2018 compared with the counterfactual: 18.6 percentage points in the combined cohort and 29.6 percentage points in the osteoarthritis or back/neck pain cohort (exhibit 3). The guideline was also associated with a reduction of 3.8–4.3 days supplied across cohorts (exhibit 4).There was a small reduction in the number of opioid fills per month in patients with osteoarthritis or back/neck pain; however, there was limited evidence of a decrease in the number of days of concurrent opioids/benzodiazepines supplied in any cohort.
SUPPLEMENTARY ANALYSES
Separate examination of receipt of one or more opioid fills lasting 1–14, 15–31, and more than 31 days suggested that the decrease in receipt of one or more fills associated with the guideline was concentrated in prescriptions of 31 days or fewer (appendix exhibit 13).20 Conducting the regressions through 2017 rather than 2018 did not influence our main conclusions.
NEGATIVE CONTROL ANALYSIS
We found no evidence that there was a decrease in benzodiazepine dispensing in the comparison cohort of patients with diagnosed anxiety (appendix exhibit 14)20 after the CDC guideline release.
Discussion
This study found that release of the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain was associated with lower frequency and intensity of opioid dispensing in its target population: patients with chronic, noncancer pain. In our combined cohort of patients with four common chronic pain conditions (osteoarthritis, back/neck pain, fibromyalgia, or chronic headache), the guideline was associated with reductions in the percentage receiving any opioids, average dose prescribed, percentage receiving high-dose prescriptions (that is, 90 morphine milligram equivalents or greater), percentage receiving concurrent opioid/benzodiazepine prescriptions, and number of opioid days supplied.
By December 2018, thirty-three months after the release of the guideline, the percentage of patients receiving at least one opioid fill in a given month was roughly 20 percentage points lower than we estimated it would have been absent the guideline. The average opioid dose received was roughly 15 morphine milligram equivalents lower than we estimated it would have been without the guideline. We also observed a reduction in the percentage of patients receiving high-dose opioid prescriptions and concurrent opioid/benzodiazepine prescriptions—which may increase risk of opioid-related harms, and which the guideline discourages2—of roughly 10 and 19 percentage points, respectively. The average number of days supplied of concurrent opioid/benzodiazepine prescriptions was approximately four days lower. The association between the guideline and the reduced rate of at least one opioid fill was concentrated in prescriptions lasting thirty-one days or fewer.
Together, these results suggest that clinical guidelines can result in substantial shifts in some prescribing behavior even when guideline-concordant care is entirely voluntary. The CDC guideline may have reduced the risk for adverse outcomes in patients with four common sources of chronic, noncancer pain, who are at particularly high risk for high-dose opioid receipt and overdose death.9,28 Still, a cross-sectional analysis of health care claims data from outpatient settings nationwide found that in 2017 patients with fibromyalgia, chronic back pain, and other conditions frequently received opioid prescriptions not in line with clinical guidelines, suggesting that some patients continue to receive opioids for which the benefits fail to outweigh the risks.29
The nationwide nature of the CDC guideline, combined with the considerable publicity and clinical outreach surrounding its release,3,30 may have reinforced its potential to have substantial impact. Clinicians’ awareness of the guideline’s recommendations may therefore have been greater than for some condition-specific guidelines and state-level legislative changes. Lack of awareness is a key reason for nonadherence to clinical guidelines, and some evidence suggests that multipronged educational efforts, similar to those used to raise awareness of the CDC guideline, are associated with better uptake.31
To balance the need for effective pain management with the risks of opioid-related harms, the guideline emphasizes the clinician’s role in tailoring opioid prescribing decisions to the individual patient’s needs, weighing their likelihood of benefiting from opioids against the associated risks. Such an approach may enable more nuanced efforts to address excessive opioid prescribing than permitted by universal prescribing limits, but whether such tailoring is occurring is not known. To preliminarily investigate whether any effects of the guideline varied by patients’ likelihood of benefiting from opioids, we disaggregated the analysis by type of pain diagnosis (osteoarthritis or chronic back/neck pain and fibromyalgia or chronic headache). Because during our analytic period the expert consensus against opioid prescribing for fibromyalgia and chronic headache was stronger and more consistent than that regarding osteoarthritis and back/neck pain, we hypothesized that guideline-associated reductions in opioid dispensing would be greater in patients with fibromyalgia or chronic headache. This would provide preliminary evidence that prescribers took into account the perceived likelihood of benefit when reducing opioid prescribing for chronic pain. In general, however, the estimated guideline-associated reductions in dispensing were not larger in the fibromyalgia or headache cohort; indeed, the point estimates were often larger in the osteoarthritis or back/neck pain cohort, although confidence intervals overlapped. It is thus not clear what factors are driving reduced opioid dispensing and whether those factors correspond to patients’ likelihood of benefiting from prescription opioids; further research is needed.
Importantly, we were unable to assess whether patients were able to gain access to effective nonopioid strategies for pain management after the guideline and whether, for patients who experienced reductions in opioid receipt, opioid tapering occurred safely. The observed declines could translate into fewer opioid-related harms but could also reflect increases in unmanaged pain and greater risk of shifting to illicit opioid use.32,33 Black and Hispanic patients, as well as women, are at particular risk for undertreatment of pain and disproportionate opioid sparing when it comes to prescriptions.34,35 Indeed, some have expressed concern about potential misinterpretation of the guideline among certain prescribers, states, and private insurers. This includes state policies imposing hard dosage or duration limits, insurers restricting coverage to limit dosage, and increased frequency of abrupt opioid tapering or dismissal from care, which are not in line with the guideline.30,36 Such consequences, which prompted the guideline authors to issue a clarification in 2019,30 may have contributed to the associations observed in this study. Further research thus is needed to better understand the appropriateness and safety of the opioid dispensing declines we report.
This study suggests that the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain specifically contributed to a substantial reduction in the frequency and intensity of opioid dispensing to patients with four common sources of chronic noncancer pain, who are at elevated risk for opioid-related harms.7,9 Further research is needed to examine the extent to which the observed opioid sparing is appropriate and safe and whether these reductions were accompanied by improved patient access to, and use of, effective nonopioid strategies for pain management.
Supplementary Material
Acknowledgments
Tarlise Townsend received Grant No. 5T32DA007233-37 from the National Institute on Drug Abuse. She acknowledges funding from the Rory Meyers College of Nursing, New York University, in New York, New York. This article was conceived and drafted when Rebecca Haffajee was employed at the RAND Corporation, and the findings and views in this article do not necessarily reflect the official views or policy of her current employer, the Department of Health and Human Services, or the United States government.
Contributor Information
Tarlise Townsend, postdoctoral fellow in the Department of Population Health, NYU Grossman School of Medicine, in New York, New York. She was a PhD student in the Department of Health Management and Policy, University of Michigan School of Public Health, in Ann Arbor, Michigan, when this work was conducted..
Magdalena Cerdá, Department of Population Health, NYU Grossman School of Medicine, in New York, New York..
Amy Bohnert, Department of Anesthesiology, University of Michigan..
Pooja Lagisetty, Division of General Internal Medicine, University of Michigan..
Rebecca L. Haffajee, Department of Health and Human Services, in Washington, D.C..
NOTES
- 1.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36): 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15): 1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohnert ASB, Guy GP Jr, Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169(6):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–86. [DOI] [PubMed] [Google Scholar]
- 5.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9): 872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeMik DE, Bedard NA, Dowdle SB, Burnett RA, McHugh MA, Callaghan JJ. Are we still prescribing opioids for osteoarthritis? J Arthroplasty. 2017;32(12):3578–3582.e1. [DOI] [PubMed] [Google Scholar]
- 7.Janakiram C, Fontelo P, Huser V, Chalmers NI, Lopez Mitnik G, Brow AR, et al. Opioid prescriptions for acute and chronic pain management among Medicaid beneficiaries. Am J Prev Med. 2019;57(3):365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzcharles M-A, Ste-Marie PA, Gamsa A, Ware MA, Shir Y. Opioid use, misuse, and abuse in patients labeled as fibromyalgia. Am J Med. 2011;124(10):955–60. [DOI] [PubMed] [Google Scholar]
- 9.Bohnert ASB, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–21. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010; 152(2):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boscarino JA, Rukstalis M, Hoffman SN, Han JJ, Erlich PM, Gerhard GS, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10): 1776–82. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen S, Lintzeris N, Bruno R, Campbell G, Larance B, Hall W, et al. Benzodiazepine use among chronic pain patients prescribed opioids: associations with pain, physical and mental health, and health service utilization. Pain Med. 2015;16(2): 356–66. [DOI] [PubMed] [Google Scholar]
- 14.Häuser W, Schug S, Furlan AD. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Rep. 2017;2(3):e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–28. [DOI] [PubMed] [Google Scholar]
- 16.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–74. [DOI] [PubMed] [Google Scholar]
- 17.Chou R, Qaseem A, Snow V, Casey D, Cross JT Jr, Shekelle P, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007; 147(7):478–91. [DOI] [PubMed] [Google Scholar]
- 18.Jeffery MM, Hooten WM, Henk HJ, Bellolio MF, Hess EP, Meara E, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007–16: retrospective cohort study. BMJ. 2018;382: k2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.University of Michigan, Institute for Healthcare Policy and Innovation. OptumInsight data seminar. Ann Arbor (MI): University of Michigan; 2016. [Google Scholar]
- 20.To access the appendix, click on the Details tab of the article online.
- 21.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017; 167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 22.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui J QIC program and model selection in GEE analyses. Stata J. 2007;7(2):209–20. [Google Scholar]
- 24.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1): 120–5. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 26.Manitoba Centre for Health Policy. Concept: Elixhauser Comorbidity Index [Internet]. Winnipeg: University of Manitoba; 2019. [last updated 2020 Nov 5; cited 2021 Aug 31]. Available from: http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1436 [Google Scholar]
- 27.Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20(2): 146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7): 686–91. [DOI] [PubMed] [Google Scholar]
- 29.Mikosz CA, Zhang K, Haegerich T, Xu L, Losby JL, Greenspan A, et al. Indication-specific opioid prescribing for US patients with Medicaid or private insurance, 2017. JAMA Netw Open. 2020;3(5):e204514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24): 2285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans WN, Lieber EMJ, Power P. How the reformulation of OxyContin ignited the heroin epidemic. Rev Econ Stat. 2019;101(1):1–15. [Google Scholar]
- 33.Alpert A, Powell D, Pacula RL. Supply-side drug policy in the presence of substitutes: evidence from the introduction of abuse-deterrent opioids. Am Econ J Econ Policy. 2018;10(4):1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2): 150–74. [DOI] [PubMed] [Google Scholar]
- 35.Buonora M, Perez HR, Heo M, Cunningham CO, Starrels JL. Race and gender are associated with opioid dose reduction among patients on chronic opioid therapy. Pain Med. 2019;20(8):1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, et al. Challenges with implementing the Centers for Disease Control and Prevention opioid guideline: a consensus panel report. Pain Med. 2019;20(4):724–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


