Abstract
Background
Giant ovarian tumors are rarely seen with severe obesity. There are few reports of perioperative management of giant ovarian tumors and severe obesity. Here, we report the perioperative management of physiological changes in massive intraabdominal tumors in a patient with severe obesity.
Case presentation
A 46-year-old Japanese woman (height 166 cm, weight 193.2 kg; body mass index 70.1 kg/m2) was scheduled to undergo laparotomy for a giant ovarian tumor. The patient was placed in the ramp position. Preoxygenation was performed using a high-flow nasal cannula, and awake tracheal intubation was performed using a video laryngoscope. Mechanical ventilation using a limited tidal volume with moderate positive end-expiratory pressure was applied during the surgical procedure. The aspiration speed for 15 L of tumor aspirate was set to under 1 L/minute, and the possibility of reexpansion pulmonary edema was foreseen by conventional monitoring.
Conclusions
We successfully completed anesthetic management in a patient with concomitant severe obesity and giant ovarian tumors.
Keywords: Severe obesity, Giant ovarian tumor, Nasal high flow cannula, Reexpansion pulmonary edema
Introduction
Obesity is associated with a variety of complications in general anesthesia, including apnea, hypoventilation [1], and difficulties in intubation [2]. In addition, giant ovarian tumors are very rare in current medical practice, and anesthesiologists are expected be familiar with the physiological changes caused by large tumors. Furthermore, changes in respiratory and circulatory dynamics associated with airway maintenance and large intraabdominal tumors that occur during perioperative complications in patients with both diseases are not well understood. Here, we report a case of anesthetic management and intraoperative changes in respiratory and circulatory dynamics associated with severe obesity and giant ovarian tumors.
Case presentation
A 46-year-old Japanese woman (height 166 cm, weight 193.2 kg; body mass index 70.1 kg/m2) was scheduled to undergo laparotomy for a giant ovarian tumor. Abdominal computed tomography showed a tensed 35-cm cystic mass that arose from the left ovary and occupied the entire abdomen (Fig. 1). Preoperative pulmonary function tests indicated a restrictive impairment. The patient had no obstructive sleep apnea; therefore, bilevel positive airway pressure was not required. However, she was assessed as bedridden and had a below 2 metabolic equivalent of tasks because she could manage to eat and use the toilet in a sitting position. We predicted difficulty in airway management due to Mallampati III, severe obesity, limited thyromental distance, age (46 years), and thickness of the neck [3]. Acid reduction prophylaxis was not administered the night prior to induction of general anesthesia.
Fig. 1.
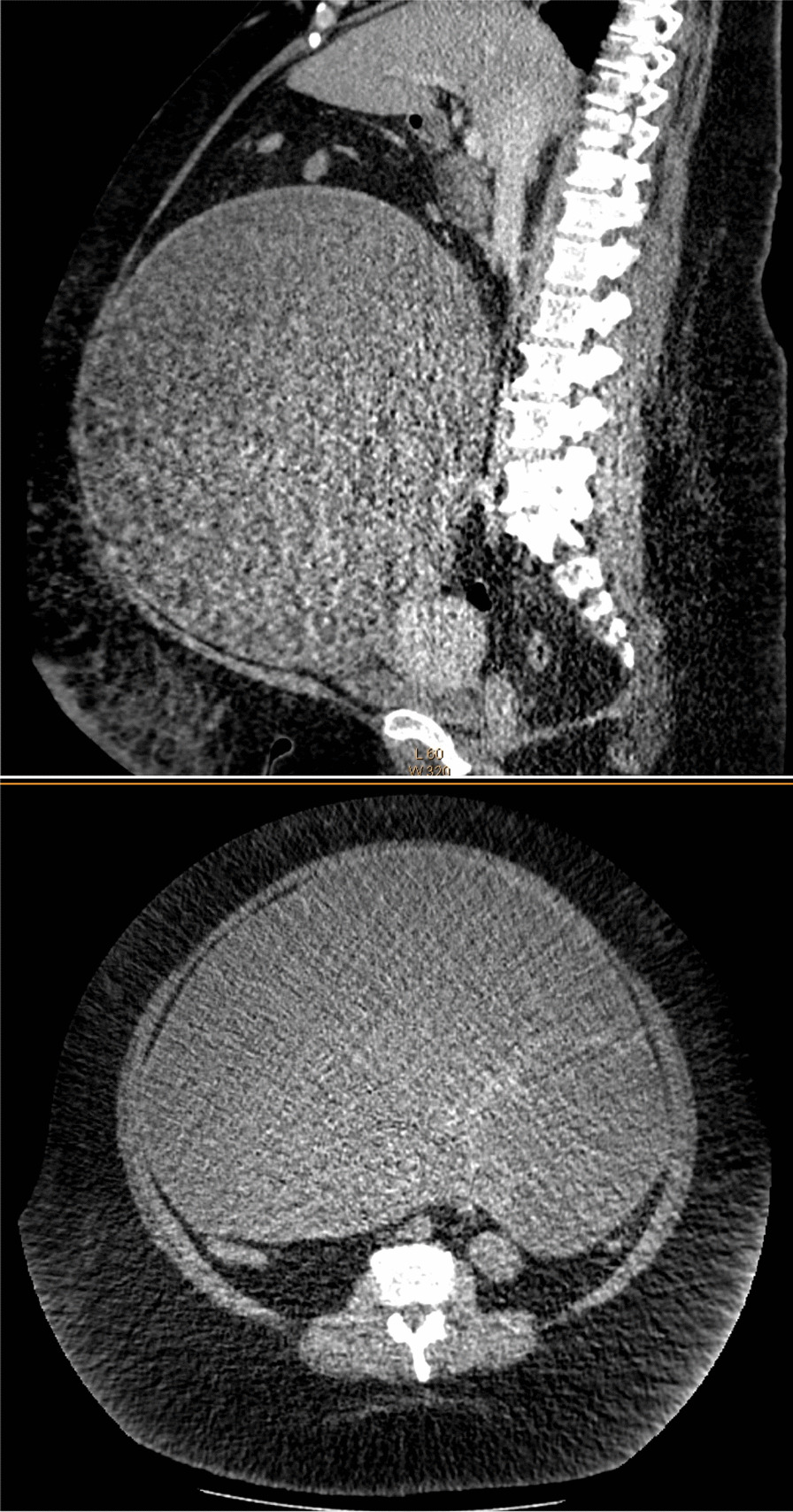
Preoperative abdominal CT shows a giant cystic tumor. Sagittal plane and transverse plane
During the induction of anesthesia, in consideration of her discomfort, the patient was placed in the ramp position and preoxygenated with a high-flow nasal cannula (HFNC) at 30 L/minute at an inspired oxygen fraction (FiO2) of 1.0. After spraying about 4 ml 2% lidocaine into the pharyngolarynx of the patient, a videolaryngoscope (McGRATH MAC®, Aircraft Medical, UK) was inserted while the patient was conscious. Topical airway block was not selected. The patient did not complain of pain, and the glottis could be observed visually. A tracheal tube with inner diameter of 7.0 mm was subsequently intubated. In addition to peripheral insertion of a central venous catheter before surgery, the invasive arterial pressure measurement and peripheral venous path were secured under ultrasonic guidance. Intraoperative anesthesia was maintained by continuous infusion of desflurane using the bispectral index (BIS) target (MAC 1.0); rocuronium bromide was added intermittently to obtain a clinically adequate depth of anesthesia, and remifentanil was administered continuously at 0.1–0.3 μg/kg/minute (ideal body weight 60 kg). After induction of anesthesia, the FiO2 was 0.4. The tidal volume (TV) was set at 8 ml/kg predicted body weight and positive end-expiratory pressure (PEEP) of 8–10 mmH2O. After an abdominal incision, a total of 15,750 ml of tumor content was carefully aspirated at under 1 L/minute. With aspiration, airway pressure and central venous pressure decreased markedly, and blood pressure stabilized without catecholamine in the infusion load (Fig. 2). Various drugs have been terminated with the completion of surgery, the patient awoke 15 min after discontinuing inhaled agents. After surgery, the patient was extubated with HFNC at 30 L/minute and FiO2 of 1.0. The patient’s respiratory condition after extubation was stable, and she was returned to the intensive care unit (ICU) under administration of 6 L/minute of oxygen. Wound infiltration anesthesia was performed in the operative field with 60 ml levobupivacaine (0.25%) because postoperative pain control, epidural anesthesia, and abdominal trunk nerve block were judged to be difficult to perform by prescan using ultrasonic equipment. The operation time was 266 minutes, and the anesthesia time was 404 minutes. The patient lost a small amount of blood, and fluid volume was 1550 ml. The patient was discharged from the ICU on postoperative day 38. Her discharge from the hospital was delayed due to catheter-related infections, diet, weight control, adjustment of antihypertensive and diuretic medications, rehabilitation, and pressure ulcers.
Fig. 2.

Clinical course before and after tumor removal, anesthetic chart. BIS bispectral index, ABP arterial blood pressure, HR heart rate, SpO2 peripheral blood oxygen saturation, CVP central venous pressure, PIP peak inspiratory pressure, EtCO2 end-tidal CO2, PEEP positive end-expiratory pressure
Discussion
We performed a preoperative evaluation of airway maintenance in a patient with severe obesity, and found that it is possible to safely introduce anesthesia by combining awake tracheal intubation and HFNC. It was also performed at the time of extubation. We were able to perform perioperative management without any complications in response to changes in respiratory and circulatory dynamics associated with the patient’s giant ovarian tumor resection and severe obesity.
Airway management in patients with severe obesity and giant ovarian tumors has been discussed from the respective viewpoints. As a result, the patient was placed in the ramp position, with preoxygenation being performed under the use of HFNC, and awake tracheal intubation being performed using a video laryngoscope. Obese patients develop hypoxemia within 2–4 minute after apnea, even with adequate preoxygenation [4–6]. In this case, severe obesity and intubation difficulties during preoperative airway evaluation were anticipated. Previous studies have shown that head-up [7, 8] or beach-chair [9] positioning improves safe apnea time before the occurrence of significant hypoxemia. Furthermore, NHFC is effective for increasing apnea time under general anesthesia in obese patients and preventing a decrease in peripheral blood oxygen saturation (SpO2) [10]. Our patient had a giant ovarian tumor, which might have caused aspiration due to increased abdominal pressure at the time of anesthesia induction. Since there is a possibility of aspiration due to increased abdominal pressure caused by a giant ovarian tumor at the time of induction of anesthesia, induction in lateral decubitus position [11], intubation under consciousness, and induction after suction of ovarian contents under consciousness [12] have been reported. Acid reduction prophylaxis should have been considered for use in this case with material risk factors [13]. In our case, there was a risk of aspiration caused by increased abdominal pressure due to the giant ovarian tumor and severe obesity. Moreover, we believe that combining awake tracheal intubation with NHFC makes airway management safer for severe obesity and giant ovarian tumors. Topical airway block may be advantageous for awake tracheal intubation, but no one could perform it.
When anesthesia with muscle relaxation is introduced after preoxygenation with 100% O2, the end-expiratory lung volume is further reduced by about 50% when a PEEP of 5 cmH2O is used after the start of ventilation [14]. Thus, the main mechanism of impaired gas exchange in obese patients is shunting (atelectasis). During invasive ventilation, obese patients are more prone to lung collapse and require higher PEEP to avoid it [15]. In this case, we chose to use pressure-assisted ventilation and were able to manage the patient without pressure trauma resulting from increased airway pressure due to positive pressure ventilation.
We were able to perform perioperative management in response to changes in the respiratory and circulatory dynamics associated with tumor resection in a patient with a giant ovarian tumor. The aspiration speed of a total of 15 L of tumor was set to under 1 L/minute, and the possibility of reexpansion pulmonary edema was foreseen by conventional monitoring.
Preoperative aspiration of the tumor is useful in preventing rapid hemodynamic changes and reexpansion of pulmonary edema during the induction of general anesthesia [16]. Intraoperative drainage is often performed at a slow rate of 0.5–1 L/minute [17, 18]. Furthermore, there have been reports of the use of FloTrack® sensors [19], central veins, and transesophageal echocardiography monitors to control the circulatory system. Although reexpansion pulmonary edema might have developed, hypoxia suggestive of reexpansion pulmonary edema did not occur during or after ovarian aspiration. In our case, we gradually reduced the TV to prevent pulmonary edema. During the operation, the patient’s airway pressure and CVP decreased, and her blood pressure stabilized. The possibility of reexpansion pulmonary edema (RPE) due to a rapid decrease in pulmonary thoracic compliance and right heart failure due to the release of pressure on the inferior vena cava before and after tumor resection was foreseen, and efforts were made to stabilize respiration and circulatory dynamics. Peritoneal dissemination of tumor cells was suspected by percutaneous puncture. Alternatively, a double-balloon catheter could have been used preoperatively [13]. It was found that drainage under local anesthesia was difficult because of severe obesity.
It is possible to safely carry out anesthesia introduction and extubation by using HFNC in airway maintenance during anesthesia management in patients with severe obesity. We were able to perform perioperative management in response to changes in circulatory dynamics associated with tumor resection in a patient with a giant ovarian tumor. Furthermore, it has been reported that there are fewer perioperative complications in robot-assisted root surgery than in open surgery, and anesthetic management of obese patients in robotic surgery is also predicted to increase [20].
Conclusions
Anesthetic management of giant ovarian tumor extraction in a severely obese patient was performed. Our report suggests that, for patients with both of these rare diseases, a combination of conscious intubation and NHFC is necessary, and perioperative management is required to respond to changes in the slow rate of tumor aspiration, mechanical ventilation, and circulatory dynamics in severely obese patients before and after removal of the giant ovarian tumor.
Acknowledgements
Not applicable.
Abbreviations
- HFNC
High-flow nasal cannula
- FiO2
Inspired oxygen fraction
- BIS
Bispectral index
- TV
Tidal volume
- PEEP
Positive end-expiratory pressure
- RPE
Reexpansion pulmonary edema
- ICU
Intensive care unit
- SpO2
Peripheral blood oxygen saturation
Authors’ contributions
SY and MK contributed to the anesthesia management of the patient, conceptualization of the case report, and writing of the original draft. MS edited the manuscript. TS was the overall supervisor of this case. All authors read and approved the final manuscript.
Funding
The authors declare no conflicts of interest associated with this manuscript. This manuscript has no funding, but all the authors are employed by Kyoto Prefectural University of Medicine.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests associated with this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shoko Yamochi, Email: y-syoko@koto.kpu-m.ac.jp.
Mao Kinoshita, Email: mao6515@koto.kpu-m.ac.jp.
Teiji Sawa, Email: anesth@koto.kpu-m.ac.jp.
References
- 1.Shah U, Wong J, Wong DT, Chung F. Preoxygenation and intraoperative ventilation strategies in obese patients: a comprehensive review. Curr Opin Anaesthesiol. 2016;29:109–118. doi: 10.1097/ACO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 2.Juvin P, Lavaut E, Dupont H, Lefevre P, Demetriou M, Dumoulin JL, et al. Difficult tracheal intubation is more common in obese patients than in lean patients. Anesth Analg. 2003;97:595–600. doi: 10.1213/01.ANE.0000072547.75928.B0. [DOI] [PubMed] [Google Scholar]
- 3.JSA airway management guideline 2014. Improving the safety of anesthesia induction. Japanese Society of Anesthesiologists. J Anesth. 2014;28(4):482–93. [DOI] [PubMed]
- 4.Ramachandran SK, Cosnowski A, Shanks A, Turner CR. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized controlled trial of nasal oxygen administration. J Clin Anesth. 2010;22:164–168. doi: 10.1016/j.jclinane.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Baraka AS, Taha SK, Siddik-Sayyid SM, Kanazi GE, El-Khatib MF, Dagher CM, et al. Supplementation of pre-oxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia. 2007;62:769–773. doi: 10.1111/j.1365-2044.2007.05104.x. [DOI] [PubMed] [Google Scholar]
- 6.Berthoud MC, Peacock JE, Reilly CS. Effectiveness of preoxygenation in patients with morbid obesity. Br J Anaesth. 1991;67:464–466. doi: 10.1093/bja/67.4.464. [DOI] [PubMed] [Google Scholar]
- 7.Altermatt FR, Muoz HR, Delfino AE, Cortinez LI. Preoxygenation in obese patients: effects of position on apnoea tolerance. Br J Anaesth. 2005;95:706–709. doi: 10.1093/bja/aei231. [DOI] [PubMed] [Google Scholar]
- 8.Dixon BJ, Dixon JB, Carden JR, Burn AJ, Schachter LM, Playfair JM, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized trial. Anesthesiol. 2005;102(6):1110–1115. doi: 10.1097/00000542-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Couture EJ, Provencher S, Somma J, Lellouche F, Marceau S, Bussires JS. Effect of position and positive pressure ventilation on functional residual capacity in morbidly obese patients: a randomized trial. Can J Anaesth. 2018;65:522–528. doi: 10.1007/s12630-018-1050-1. [DOI] [PubMed] [Google Scholar]
- 10.Wong DT, Dallaire A, Singh KP, Madhusudan P, Jackson T, Singh M, Wong J, Chung F. High-flow nasal oxygen improves safe apnea time in morbidly obese patients undergoing general anesthesia: a randomized controlled trial. Anesth Analg. 2019;129(4):1130–1136. doi: 10.1213/ANE.0000000000003966. [DOI] [PubMed] [Google Scholar]
- 11.Shinohara H, Ishii H, Kakuyama M, Fukuda K. Morbidly obese patient with a huge ovarian tumor who was intubated while awake using airway scope in lateral decubitus position. Masui. 2010;59(5):625–628. [PubMed] [Google Scholar]
- 12.Imada Y, Egi M, Kubota K, Kitahara J, Izuta S, Mizobuchi S. Preoperative percutaneous drainage using a double-balloon catheter with measurement of pressure in a patient with giant ovarian tumor. Masui. 2020;69:319–323. [Google Scholar]
- 13.Clark K, Lam LT, Gibson S, Currow D. The effect of ranitidine versus proton pump inhibitors on gastric secretions: a meta-analysis of randomised control trials. Anaesthesia. 2009;2009(64):652–657. doi: 10.1111/j.1365-2044.2008.05861.x. [DOI] [PubMed] [Google Scholar]
- 14.Nestler C, Simon P, Petroff D, Hammermuller S, Kamrath D, Wolf S, Dietrich A, Camilo LM, Beda A, Carvalho AR, Giannella-Neto A, Reske AW, Wrigge H. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth. 2017;119:1194–1205. doi: 10.1093/bja/aex192. [DOI] [PubMed] [Google Scholar]
- 15.De Jong A, Wrigge H, Hedenstierna G, Gattinoni L, Chiumello D, Frat J-P, Ball L, Schetz M, Pickkers P, Jaber S. How to ventilate obese patients in the ICU. Intensive Care Med. 2020;46:2423–2435. doi: 10.1007/s00134-020-06286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YT, Kim JW, Choe BH. A case of huge ovarian cyst of 21-year-old young woman. J Obstet Gynaecol Res. 1999;25:275–359. doi: 10.1111/j.1447-0756.1999.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 17.Einenkel J, Alexander H, Schotte D, Stumpp P, Horn LC. Giant ovarian cysts: is a pre and intraoperative drainage an advisable procedure? Int J Gynecol Cancer. 2006;16:2039–2043. doi: 10.1111/j.1525-1438.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda T, Kurasako N, Nishitani K, Okada S, Arai T. Anesthetic management for removal of a giant ovarian tumor using FloTracTM VigileoTM monitoring system. Masui. 2014;63:439–442. [PubMed] [Google Scholar]
- 19.Akazawa M, Saito T, Nagayama R, Ariyoshi K, Okadome M. Management of a giant ovarian tumor more than 30 kg: a case report and review of the literature. J Gynecol Surg. 2018;34:243–247. doi: 10.1089/gyn.2018.0008. [DOI] [Google Scholar]
- 20.Ishida Y, Nakazawa K, Okada T, Tsuzuki Y, Kobayashi T, Yamada R, Uchino H. Anesthetic management of a morbidly obese patient with endometrial cancer during robot-assisted laparoscopic surgery. JA Clin Rep. 2021;7(1):30. doi: 10.1186/s40981-021-00434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


